Abstract
Background
The associations of time‐averaged cumulative blood pressure (BP) from midlife to late life with microvasculature expressed as retinal vessel diameters is not well studied. The aim of this study was to evaluate the association of cumulative systolic BP and diastolic BP (DBP) with retinal vessel calibers, focusing on race differences.
Methods and Results
The analysis included 1818 adults from the ARIC (Atherosclerosis Risk in Communities) study attending the fifth visit (2011–2013; age 77±5 years, 17.1% Black participants). Time‐averaged cumulative BPs were calculated as the sum of averaged BPs from adjacent consecutive visits (visits 1–5) indexed to total observation time (24±1 years). Summarized estimates for central retinal arteriolar equivalent and central retinal venular equivalent at the fifth visit represent average retinal vessel diameters. The arteriole:venule ratio was calculated. We tested for effect modification by race. Results from multiple linear regression models suggested that higher time‐averaged cumulative DBP (β [95% CI] per 1‐SD increase: −1.78 [−2.53, −1.02], P<0.001 and −0.005 [−0.009, −0.002], P=0.004, respectively) but not systolic BP (−0.52 [−1.30, 0.26], P=0.189 and 0.001 [−0.002, 0.005], P=0.485, respectively) was associated with smaller central retinal arteriolar equivalent and arteriole:venule ratio. The association between time‐averaged cumulative DBP and arteriole:venule ratio was strongest in White participants (interaction P=0.007). The association of cumulative systolic BP and DBP with central retinal venular equivalent was strongest in Black participants (interaction P=0.015 and 0.011, respectively).
Conclusions
Exposure to higher BP levels, particularly DBP, from midlife to late life is associated with narrower retinal vessel diameters in late life. Furthermore, race moderated the association of cumulative BP exposure with retinal microvasculature.
Keywords: blood pressure, microcirculation, race
Subject Categories: High Blood Pressure
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities Study
- AVR
arteriole:venule ratio
- CRAE
central retinal arteriolar equivalent
- CRVE
central retinal venular equivalent
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
Clinical Perspective.
What Is New?
Cumulative diastolic blood pressure but not systolic blood pressure from midlife to late life is associated with late‐life retinal arteriolar narrowing, and the importance of blood pressure as a risk factor differs between Black individuals and White individuals.
What Are the Clinical Implications?
The findings suggest that decreased retinal vessel diameters in late life may result from the cumulative effects of elevated blood pressure, particularly diastolic blood pressure, from midlife to late life and underscore the need for targeted interventions based on racial differences.
Histologically, retinal arterioles (measured as the central retinal arteriolar equivalent [CRAE]) and retinal venules (measured as the central retinal venular equivalent [CRVE]) offer a unique opportunity for the direct, noninvasive study of the human microvasculature. Retinal microvascular abnormalities have been shown to be prognostically important in a variety of diseases, including hypertension, 1 , 2 , 3 stroke, 4 , 5 , 6 , 7 and dementia 8 , 9 as well as diabetes, 10 peripheral artery disease, 11 and atherosclerotic cardiovascular disease (CVD) events. 12
Previous studies show that hypertension status and elevated blood pressure (BP) are risk factors for retinal vessel abnormalities. 13 , 14 , 15 , 16 , 17 Former studies have demonstrated that cumulative BP, an integrated assessment of long‐term severity and duration of BP exposure, was associated with incipient myocardial dysfunction 18 , 19 and increased urine albumin:creatinine ratio progression 20 as well as the risk of coronary heart disease 21 and heart failure. 21 , 22 However, limited data exist regarding the relationship of cumulative BP from midlife to late life with late‐life retinal vessel diameters. Furthermore, different BP components reflect distinctive hemodynamics and pathophysiologic mechanisms, 23 , 24 , 25 , 26 and which BP components plays a dominant role of the damage to microcirculation is poorly understood. Finally, it has been reported that both retinal vascular caliber and BP levels vary by race. 27 , 28 , 29 Racial differences in the impact of elevated BP on CVD and other end points have been shown in previous studies. 24 , 30 , 31 , 32 However, how race affects the relation of BP with microvasculature remains unclear.
Building on this background, in this study we examined the association of time‐averaged cumulative BP assessed for 25 years with late‐life microvasculature among 1885 adults in the Atherosclerosis Risk in Communities (ARIC) study. In addition, we compared the magnitude and strength of associations of cumulative systolic BP (SBP) with cumulative diastolic BP (DBP) and of White individuals with Black individuals.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The ARIC study's data and materials are publicly available to qualified investigators.
Study Population
The ARIC study is an ongoing, prospective observational study of CVD and atherosclerosis that recruited a total of 15 792 participants aged 45 to 64 years between 1987 and 1989 (visit 1) from 4 communities in 4 US centers (Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD). The second visit was accomplished in 1990 to 1992, the third in 1993 to 1995, the fourth in 1996 to 1998, and the fifth in 2011 to 2013. 33 Participants provided written informed consent, and the study's protocol was approved by the institutional review board of each participating institution.
Of the 6538 participants who attended the fifth visit, we evaluated participants who underwent complete BP measurement from visit 1 to visit 5 and had gradable retinal photography with >6 arteries and veins graded at visit 5 (n=2000). Participants missing data on covariates were excluded (n=115). We also excluded participants with retinal abnormalities in both eyes (retinopathy, macular edema, focal narrowing, arterio‐venous nicking, artery or vein occlusions, and other pathologies; n=67), leaving a sample size of 1818 for final analysis. Table S1 shows the characteristics of included and excluded participants. Compared with participants in the current study, participants excluded for missing or ungradable retinal data were more likely to be young, female, and Black participants who smoked and had high DBP levels.
Imaging of the Retinal Microvasculature
The retinal photography procedure and measurement of retinal vascular caliber were described in detail previously. 34 , 35 Digital, nonmydriatic retinal fundus photographs were taken and evaluated by trained graders at the ARIC Study Retinal Reading Center in the Department of Ophthalmology, University of Wisconsin–Madison. Using a semiautomatic method, the results from image analysis were summarized quantitatively as CRAE and CRVE, representing average arteriolar and venular diameter, respectively. 36 , 37 The arteriole:venule ratio (AVR) was CRAE divided by CRVE, which eliminates the magnification differences between photographs. For analysis, we averaged each participant's measurements at both eyes. If data were missing from 1 eye, data available from the other eye were selected. 16 , 38
BP and Time‐Averaged Cumulative BP
At each examination, BP was measured 3 times with standardized protocols after participants had been seated for 5 minutes and recorded by trained technicians. For all visits except the fourth, an average of the second and the third BP measurements was recorded with a random‐zero sphygmomanometer. 39
Time‐averaged cumulative BPs were calculated as the sum of averaged BPs from adjacent consecutive visits (visits 1–5) indexed to total observation time between visit 1 and 5 (mean time 24±1 years) as follows 22 (see also Figure S1):
where BP1, BP2, BP3, BP4, and BP5 indicate BP at visits 1, 2, 3, 4, and 5 and time1–2, time2–3, time3–4, and time4–5 indicate the participant‐specific time interval between consecutive visits 1 to 5 in years. Time1–5 means total time between visits 1 and 5.
Definition of Other Variables
All covariates were assessed at visit 5. Body mass index was calculated as body weight in kilograms divided by height in squared meters. Hypertension was defined as SBP ≥140 mm Hg and/or DBP ≥90 mm Hg, or BP medicine use in the past 2 weeks. Diabetes was defined as hemoglobin A1C value ≥6.5% or using medication for diabetes or self‐report diagnosis of diabetes. Information about age, race, sex, and smoking and drinking status was self‐reported.
Statistical Analysis
Continuous variables were expressed as mean and SD and compared using Student t tests. Categorical variables were expressed as number and percentage and compared using χ2 statistics. We also explored the relationship between tertiles of time‐averaged cumulative BP with retinal vessel diameters using ANCOVA.
We used multivariable linear regression models to assess the association between time‐averaged cumulative BP from midlife to late life and retinal vessel diameters at visit 5 (all as continuous variables). The primary exposures were measures of time‐averaged cumulative SBP and cumulative DBP (z scores). The primary outcome variables were CRAE, CRVE, and AVR. All analyses were performed in 2 models. In model 1, analyses were adjusted for age, sex, race, and body mass index at visit 5. In model 2, analyses were additionally adjusted for smoking status, drinking status, triglycerides, low‐density lipoprotein, high‐density lipoprotein, prevalence of diabetes, prevalence of hypertension, and antihypertensive medication at visit 5. These covariates were selected as potential confounders because they are known to be related to BP or retinal vessels. In addition, all analyses in model 2 were repeated using visit 1 or visit 5 BP levels as the independent variable to examine the associations of cumulative BP for 25 years compared with BP at a single time point. To test whether race moderates the association of cumulative BP with retinal vessels, we repeated all the analyses in model 2 and tested for interaction (race×cumulative exposure to BP). 40 Similarly, effect modifications by hypertension or diabetes were tested using interaction models.
To corroborate our analyses, multivariable logistic regression analysis was used to evaluate the association of cumulative BP with generalized retinal arteriolar narrowing defined as CRAE within the lowest quintile in the population. 17 , 38 , 41
All statistical analyses were conducted with IBM SPSS Statistics version 26.0, and a P<0.05 was considered statistically significant.
RESULTS
Population Characteristics
Among the 1818 study participants, the mean (SD) age at the time of visit 5 (2011–2013) was 76.6 (5.2) years, 56.1% were women, and 17.1% were Black participants. The mean (SD) time‐averaged cumulative exposure to SBP and DBP in the total cohort was 124.2 (12.7) mm Hg and 69.3 (7.4) mm Hg, respectively. Race‐specific clinical characteristics of the included participants are given in Table 1. The time‐averaged cumulative SBP, DBP, and mean arterial pressure; CRAE; CRVE; and prevalence of hypertension and diabetes were higher in Black people than in White people. However, age, current drinking status, triglycerides, and AVR were higher in White people than in Black people. In addition, time‐averaged cumulative pulse pressure, and smoking status were similar between races.
Table 1.
Clinical Characteristics of Study Cohort by Race at Visit 5 (2011–2013)
| Characteristics | White people (n=1507) | Black people (n=311) | P value |
|---|---|---|---|
| Age, y | 76.8 (5.2) | 75.9 (4.9) | 0.004 |
| Female sex, n (%) | 813 (53.9) | 204 (65.6) | <0.001 |
| BMI, kg/m2, mean (SD) | 28.2 (5.3) | 29.5 (6.5) | <0.001 |
| Visit center, mean (SD) | <0.001 | ||
| Forsyth County | 400 | 17 | |
| Jackson | 0 | 294 | |
| Minneapolis | 619 | 0 | |
| Washington County | 488 | 0 | |
| Smoking status, n (%) | 0.457 | ||
| Current | 70 (4.6) | 12 (3.9) | |
| Former | 785 (52.1) | 153 (49.2) | |
| Never | 652 (43.3) | 146 (46.9) | |
| Drinking status, n (%) | <0.001 | ||
| Current | 869 (57.7) | 53 (17.0) | |
| Former | 357 (23.7) | 141 (45.3) | |
| Never | 281 (18.6) | 117 (37.6) | |
| Diabetes, n (%) | 400 (26.5) | 127 (40.8) | <0.001 |
| Hypertension, n (%) | 1078 (71.5) | 263 (84.6) | <0.001 |
| Antihypertensive medication, n (%) | 1103 (73.2) | 267 (85.9) | <0.001 |
| SBP, mm Hg, mean (SD) | 128.9 (17.7) | 132.9 (18.3) | <0.001 |
| DBP, mm Hg, mean (SD) | 64.4 (10.4) | 69.2 (10.5) | <0.001 |
| Time‐averaged cumulative SBP, mm Hg, mean (SD) | 123.2 (12.4) | 129.3 (12.9) | <0.001 |
| Time‐averaged cumulative DBP, mm Hg, mean (SD) | 68.3 (7.1) | 74.2 (7.3) | <0.001 |
| Time‐averaged cumulative PP, mm Hg, mean (SD) | 54.9 (10.7) | 55.2 (10.3) | 0.677 |
| Time‐averaged cumulative MAP, mm Hg, mean (SD) | 86.6 (7.7) | 92.5 (8.2) | <0.001 |
| LDL, mg/dL, mean (SD) | 101.8 (34.4) | 108.2 (35.6) | 0.003 |
| HDL, mg/dL, mean (SD) | 51.6 (14.2) | 54.0 (13.5) | 0.007 |
| Triglycerides, mg/dL, mean (SD) | 129.2 (57.2) | 107.1 (43.5) | <0.001 |
| CRAE, μm, mean (SD) | 141.7 (14.6) | 144.2 (13.6) | 0.004 |
| CRVE, μm, mean (SD) | 201.0 (21.0) | 217.2 (22.9) | <0.001 |
| AVR, mean (SD) | 0.71 (0.07) | 0.67 (0.07) | <0.001 |
P values were calculated by unpaired t test or χ2 test. AVR indicates arteriolar:venular ratio; BMI, body mass index; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MAP, mean arterial pressure; PP, pulse pressure; and SBP, systolic blood pressure.
Associations Between Time‐Averaged Cumulative BP and Microvasculature
Results from the linear regression models to examine the association between time‐averaged cumulative BP exposure and microvasculature are summarized in Table 2. Higher exposure to cumulative DBP was associated with CRAE, CRVE, and AVR in all models. By contrast, cumulative exposure to SBP was not associated with CRAE, CRVE, and AVR in any of the models. Figure 1 shows the adjusted mean (SE) of retinal phenotypes in tertiles of cumulative SBP and DBP. Retinal vessel diameters decreased linearly with increasing cumulative DBP, but did not show an association with cumulative SBP. In multivariable logistic regression, time‐averaged cumulative DBP (adjusted odds ratio [OR; 95% CI] per 1‐SD increase, 1.25 [1.09, 1.43]; P=0.001), but not SBP (1.06 [0.92, 1.22], P=0.443), was associated with the odds of CRAE narrowing (Table S2).
Table 2.
Time‐Averaged Cumulative Systolic and Diastolic Blood Pressure in Relation to Retinal Phenotypes
| Blood pressure per 1 SD higher | CRAE, μm | CRVE, μm | AVR | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| Cumulative SBP | ||||||
| Model 1 | −0.51 (−1.20, 0.17) | 0.143 | −0.81 (−1.82, 0.21) | 0.119 | 0.0003 (−0.003, 0.004) | 0.835 |
| Model 2 | −0.52 (−1.30, 0.26) | 0.189 | −1.11 (−2.26, 0.04) | 0.059 | 0.001 (−0.002, 0.005) | 0.485 |
| Cumulative DBP | ||||||
| Model 1 | −1.84 (−2.56, −1.13) | <0.001 | −1.28 (−2.34, −0.22) | 0.018 | −0.005 (−0.008, −0.002) | 0.003 |
| Model 2 | −1.78 (−2.53, −1.02) | <0.001 | −1.17 (−2.28, −0.05) | 0.040 | −0.005 (−0.009, −0.002) | 0.004 |
β represents unstandardized regression coefficient. Model 1: adjusted for age, sex, race, body mass index, and visit center at visit 5. Model 2: adjusted for Model 1+smoking status, drinking status, triglycerides, low‐density lipoprotein, high‐density lipoprotein, prevalence of diabetes, prevalence of hypertension, and antihypertensive medication at visit 5. AVR indicates arteriolar:venular ratio; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Figure 1. Retinal phenotypes in tertiles of time‐averaged cumulative blood pressure.
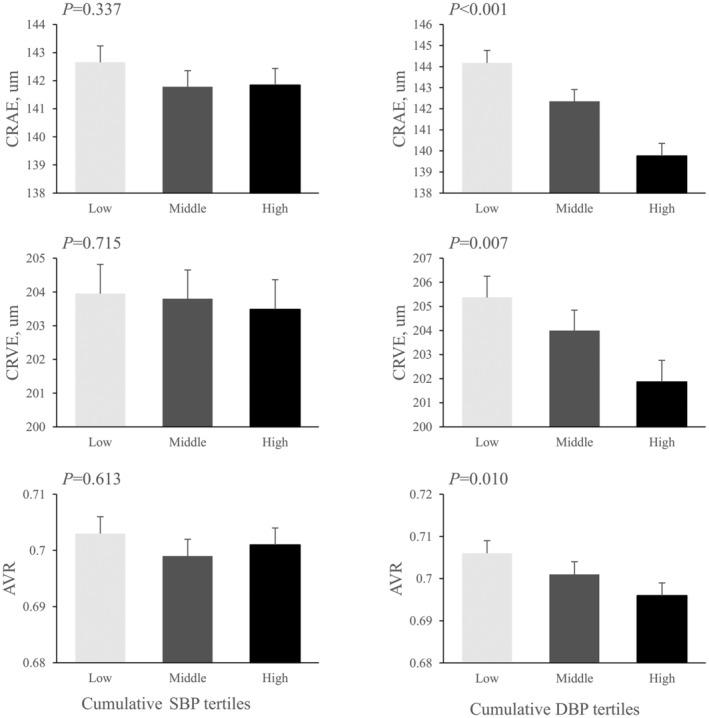
Bars represent means (SEs) calculated from ANCOVA after adjustment for age, race, sex, body mass index, and visit center at visit 5. P values show P for trends from ANCOVA. AVR indicates arteriolar:venular ratio; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Associations of Cumulative BP for 25 Years Compared With a Single BP Measurement
At baseline (visit 1), the mean age of the participants was 53±5 years, mean SBP was 116±16 mm Hg, and mean DBP was 73±10 mm Hg. The progression of BP with increasing age varied by race (Figure S2). Table 3 shows the associations of cumulative BP for 25 years compared with a single BP measurement (at baseline or visit 5) with retinal phenotypes at visit 5. Higher baseline SBP was not associated with any of the retinal phenotypes, and higher baseline DBP was only associated with narrower retinal arterioles. SBP measured at visit 5 was only associated with narrower retinal venule. DBP measured at visit 5 was associated with narrower CRAE and CRVE and showed no relation to AVR. Cumulative DBP for 25 years had stronger effects on retinal arterioles than baseline or visit 5 BP (unstandardized β in Table 3).
Table 3.
Relationships of Cumulative Exposure to Blood Pressure for 25 Years or Visit 1 or Visit 5 Blood Pressure With Retinal Phenotypes
| Blood pressure per 1 SD higher | CRAE, μm | CRVE, μm | AVR | |||
|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | |
| Cumulative SBP | −0.52 (0.40) | 0.189 | −1.11 (0.59) | 0.059 | 0.001 (0.002) | 0.485 |
| Visit 1 SBP | −0.11 (0.38) | 0.772 | −0.03 (0.56) | 0.957 | −0.0003 (0.002) | 0.880 |
| Visit 5 SBP | −0.58 (0.37) | 0.111 | −1.74 (0.54) | 0.001 | 0.003 (0.002) | 0.078 |
| Cumulative DBP | −1.78 (0.38) | <0.001 | −1.17 (0.57) | 0.040 | −0.005 (0.002) | 0.004 |
| Visit 1 DBP | −0.97 (0.38) | 0.011 | −0.33 (0.56) | 0.558 | −0.004 (0.002) | 0.030 |
| Visit 5 DBP | −1.38 (0.36) | <0.001 | −1.72 (0.53) | 0.001 | −0.001 (0.002) | 0.416 |
Analyses were adjusted for age, sex, race, body mass index, visit center, smoking status, drinking status, triglycerides, low‐density lipoprotein, high‐density lipoprotein, prevalence of diabetes, prevalence of hypertension, and antihypertensive medication at visit 5. β represents unstandardized regression coefficient. One SD was 13, 16, and 18 mm Hg, respectively, for time‐averaged cumulative SBP, visit 1 SBP, and visit 5 SBP and was 7, 10, and 11 mm Hg, respectively, for time‐averaged cumulative DBP, visit 1 DBP, and visit 5 DBP. AVR indicates arteriole:venule ratio; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Interaction Between Cumulative BP Exposure and Race
Figure 2 shows the association of cumulative BP with retinal phenotypes stratified by race. The association between cumulative exposure to BP and CRVE was strongest in Black participants (see also Figure S3), whereas the association between time‐averaged cumulative DBP and AVR was strongest in White participants (interaction P=0.007). The association between cumulative DBP and CRAE was also stronger in White participants, but the interaction was not statistically significant (interaction P=0.966). There was not significant interaction between cumulative SBP exposure and race, in relation to CRAE, or AVR (interaction P=0.383 and 0.100, respectively). Finally, the association between cumulative BP exposure and retinal vessels did not vary by diabetes or hypertension (Figures S4 and S5).
Figure 2. Association between cumulative blood pressure for 25 years with retinal phenotypes stratified by race.
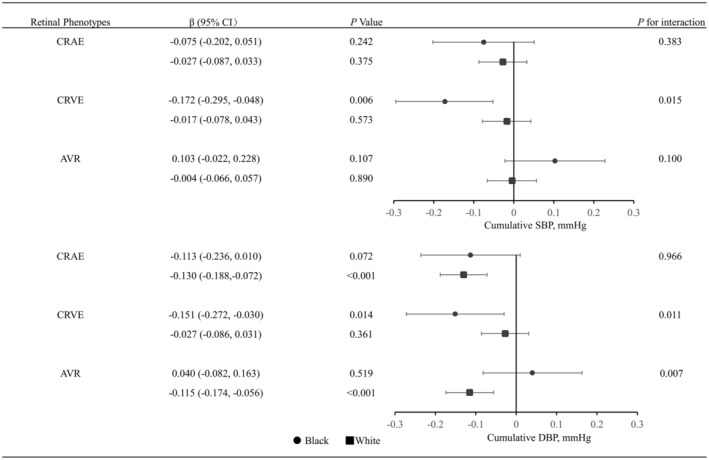
The β represents unstandardized regression coefficient. Retinal phenotypes were transformed to z scores. All analyses were adjusted for age, sex, race, body mass index, visit center, smoking status, drinking status, triglycerides, low‐density lipoprotein, high‐density lipoprotein, prevalence of diabetes, prevalence of hypertension, and antihypertensive medication at visit 5. AVR indicates arteriole:venule ratio; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
DISCUSSION
To our knowledge, our study is the first survey assessing the long‐term relationship of BP through midlife to late life with late‐life microvasculature. We report 2 novel findings. The main finding of this study is that exposure to higher BP starting in midlife is associated with narrower late‐life microvasculature, and cumulative DBP plays a dominant role of the damage to microcirculation, especially to the retinal arterioles. Furthermore, we found that higher cumulative DBP for 25 years was associated with narrower retinal arterioles in White participants but not Black participants, whereas higher cumulative BP was associated with narrower retinal venules in Black participants but not White participants.
Cumulative BP From Midlife to Late Life and Late‐Life Microvasculature
Previous data documented the independent effects of both current and past BP on small vessel caliber in the retina, suggesting that retinal vessel narrowing may result from the cumulative effects of long‐standing hypertension. 15 , 17 , 42 Consistent with prior studies, our study showed that time‐averaged cumulative BP from midlife to late life was associated with narrower retinal vessel caliber. However, past BP was obtained up to 10 years before retinal imaging and measured at a single time point in previous studies. 15 , 16 , 17 , 42 Our study first explored the impact of BP measured for 25 years (baseline BP) before retinal imaging on retinal vessel diameters as well as incorporated the severity and duration of BP exposure for risk assessment in the community.
Accumulating evidence indicates that different components of BP provide some distinctive information about the hemodynamic alterations and may be associated to varying degrees with different cardiovascular outcomes. 43 , 44 , 45 Our finding added to the evidence that cumulative DBP but not SBP from midlife to late life was associated with narrower retinal arterioles in late life. This finding was supported by another report of ARIC study, suggesting that higher DBP (OR [95% CI] per 1‐SD increase, 1.84 [1.04–3.24]), but not SBP (1.46 [0.90–2.39]), was associated with incident retinopathy for 10 years in participants without diabetes. 46
Potential mechanisms underlying the observed BP components differences may be as follows: DBP reflects a steady‐state load of BP, and SBP is an integrated measure of steady and pulsatile pressure load. 23 , 25 , 47 The impact of both SBP and DBP on CRVE suggests that both increased steady‐state load and pulsatile pressure load on vascular structures are important contributors to retinal venules narrowing. By contrast, DBP, but not SBP, was associated with smaller CRAE and AVR, suggesting that higher steady flow instead of pulsatile components of BP contributes to retinal arterioles narrowing. Our study underscores the importance of assessment and management of DBP through early stages of life to prevent and/or delay the progression of microvasculature abnormalities in later stages of life. Further investigations may help to clarify the mechanisms underlying our observations.
Racial Differences in Cumulative BP Exposure and Microvasculature
Harris et al 48 found that the effect of BP on risk of retinopathy differed between Black people and White people. The risk of retinopathy increased as BP increased among Black people, but did not reach statistical significance among White people. By contrast, data from the CARDIA (Coronary Artery Risk Development in Young Adults) study 24 suggested that DBP at baseline was associated with CVD risk in White people, but was unrelated to CVD risk in Black people. In our study, we observed a stronger association between BP and retinal venules in Black people than in White people, whereas the effect of DBP on retinal arterioles is more obvious in White people than in Black people. One potential mechanism may explain the observed racial differences. The diurnal pattern of BP differed between Black people and White people, and Black people showed less of a decline in BP during sleep than White people. 49 , 50 As a result, the importance of BP as a risk factor may differ between Black people and White people. Based on our study data, we hypothesized that retinal arteriolar and venular calibers were differentially associated with cardiovascular risk factors, such as race and high BP. This hypothesis was further supported by several previous studies. 51 , 52 , 53 The major systemic determinant of arteriolar diameters was BP, 51 , 52 whereas the venular calibers were also correlated with cigarette smoking, obesity, 51 and systemic inflammation. 51 , 53 Our study underscores the importance of more highly targeted interventions. For Black adults, both SBP and DBP could potentially serve as a marker of retinal venules narrowing. For White adults, DBP from midlife to late life is more related to retinal arterioles narrowing than SBP and could serve as a potential target for future therapies.
Limitations
There are limitations of the current study to consider. First, only 29% of our sample had gradable retinal photography and were included in the analyses. This could have introduced selection bias. However, participants analyzed and not analyzed had similar baseline characteristics, suggesting that this bias is likely nondifferential and would only attenuate our findings. Second, the results may not be generalizable to individuals from other race or ethnicity cohorts because the ARIC study only consisted of a population of Black and White participants. Although only 17% of our study were Black participants, we still found a stronger association between BP and CRVE in Black participants than in White participants, which only means that our results of interaction between BP and race are robust. Finally, follow‐up time between visits in the ARIC study is different, and the time interval between visits 4 and 5 is wider versus visits 1, 2, 3, and 4. However, time‐averaged cumulative BP was calculated as previous study described 22 so that BP measured at visits 4 and 5 would not give more weight to the cumulative BP exposure than BP at earlier visits.
CONCLUSIONS
Higher time‐averaged cumulative BP for 25 years from midlife to late life was independently associated with narrower microvasculature in late life. Of note, cumulative DBP load, rather than SBP, played a role in determining the risk of retinal arteriolar narrowing. Furthermore, the effect of BP on the risk of microvasculature narrowing differed between Black adults and White adults. The findings suggest that decreased retinal vessel diameters in late life may result from the cumulative effects of elevated BP, particularly DBP, from midlife to late life and underscore the need for targeted interventions based on racial differences.
Sources of Funding
The ARIC (Atherosclerosis Risk in Communities) study is performed as a collaborative trial supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This study was supported by the National Natural Science Foundation of China (81600206 to Dr Zhuang, 81870195 to Dr Liao), and Natural Science Foundation of Guangdong Province (2016A030310140 to Dr Zhuang, 20160903 to Dr Liao). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S5
Acknowledgments
We thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) study for their important contribution.
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Xiaodong Zhuang, Email: zhuangxd3@mail.sysu.edu.cn.
Xinxue Liao, Email: liaoxinx@mail.sysu.edu.cn.
References
- 1. Ding J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, Klein R, Klein BB, Cotch MF, Wang JJ, et al. Retinal vascular caliber and the development of hypertension: a meta‐analysis of individual participant data. J Hypertens. 2014;32:207–215. doi: 10.1097/HJH.0b013e32836586f4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kawasaki R, Cheung N, Wang JJ, Klein R, Klein BE, Cotch MF, Sharrett AR, Shea S, Islam FA, Wong TY. Retinal vessel diameters and risk of hypertension: the Multiethnic Study of Atherosclerosis. J Hypertens. 2009;27:2386–2393. doi: 10.1097/HJH.0b013e3283310f7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329:79. doi: 10.1136/bmj.38124.682523.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doubal FN, MacGillivray TJ, Hokke PE, Dhillon B, Dennis MS, Wardlaw JM. Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology. 2009;72:1773–1778. doi: 10.1212/WNL.0b013e3181a60a71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, de Jong PT, Breteler MM. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66:1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea [DOI] [PubMed] [Google Scholar]
- 6. Dumitrascu OM, Demaerschalk BM, Valencia Sanchez C, Almader‐Douglas D, O'Carroll CB, Aguilar MI, Lyden PD, Kumar G. Retinal microvascular abnormalities as surrogate markers of cerebrovascular ischemic disease: a meta‐analysis. J Stroke Cerebrovasc Dis. 2018;27:1960–1968. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.041 [DOI] [PubMed] [Google Scholar]
- 7. Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134–1140. doi: 10.1016/S0140-6736(01)06253-5 [DOI] [PubMed] [Google Scholar]
- 8. de Jong FJ, Schrijvers EM, Ikram MK, Koudstaal PJ, de Jong PT, Hofman A, Vingerling JR, Breteler MM. Retinal vascular caliber and risk of dementia: the Rotterdam Study. Neurology. 2011;76:816–821. doi: 10.1212/WNL.0b013e31820e7baa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deal JA, Sharrett AR, Albert M, Bandeen‐Roche K, Burgard S, Thomas SD, Gottesman RF, Knopman D, Mosley T, Klein B, et al. Retinal signs and risk of incident dementia in the Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2019;15:477–486. doi: 10.1016/j.jalz.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, Klein BE, Hubbard LD, Duncan BB. Retinal arteriolar narrowing and risk of diabetes mellitus in middle‐aged persons. JAMA. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528 [DOI] [PubMed] [Google Scholar]
- 11. Yang C, Kwak L, Ballew SH, Jaar BG, Deal JA, Folsom AR, Heiss G, Sharrett AR, Selvin E, Sabanayagam C, et al. Retinal microvascular findings and risk of incident peripheral artery disease: an analysis from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2020;294:62–71. doi: 10.1016/j.atherosclerosis.2019.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seidelmann SB, Claggett B, Bravo PE, Gupta A, Farhad H, Klein BE, Klein R, Di Carli M, Solomon SD. Retinal vessel calibers in predicting long‐term cardiovascular outcomes: the Atherosclerosis Risk in Communities Study. Circulation. 2016;134:1328–1338. doi: 10.1161/CIRCULATIONAHA.116.023425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–435. doi: 10.1016/S0140-6736(07)60198-6 [DOI] [PubMed] [Google Scholar]
- 14. Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–2317. doi: 10.1056/NEJMra032865 [DOI] [PubMed] [Google Scholar]
- 15. Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, Pinsky JL, Klein R. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997 [DOI] [PubMed] [Google Scholar]
- 16. Wei FF, Zhang ZY, Thijs L, Yang WY, Jacobs L, Cauwenberghs N, Gu YM, Kuznetsova T, Allegaert K, Verhamme P, et al. Conventional and ambulatory blood pressure as predictors of retinal arteriolar narrowing. Hypertension. 2016;68:511–520. doi: 10.1161/HYPERTENSIONAHA.116.07523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong TY, Hubbard LD, Klein R, Marino EK, Kronmal R, Sharrett AR, Siscovick DS, Burke G, Tielsch JM. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:1007–1013. doi: 10.1136/bjo.86.9.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kishi S, Teixido‐Tura G, Ning H, Venkatesh BA, Wu C, Almeida A, Choi EY, Gjesdal O, Jacobs DR Jr, Schreiner PJ, et al. Cumulative blood pressure in early adulthood and cardiac dysfunction in middle age: the CARDIA study. J Am Coll Cardiol. 2015;65:2679–2687. doi: 10.1016/j.jacc.2015.04.042 [DOI] [PubMed] [Google Scholar]
- 19. Vasconcellos HD, Moreira HT, Ciuffo L, Nwabuo CC, Yared GS, Ambale‐Venkatesh B, Armstrong AC, Kishi S, Reis JP, Liu K, et al. Cumulative blood pressure from early adulthood to middle age is associated with left atrial remodelling and subclinical dysfunction assessed by three‐dimensional echocardiography: a prospective post hoc analysis from the coronary artery risk development in young adults study. Eur Heart J Cardiovasc Imaging. 2018;19:977–984. doi: 10.1093/ehjci/jey086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zemaitis P, Liu K, Jacobs DR Jr, Cushman M, Durazo‐Arvizu R, Shoham D, Palmas W, Cooper R, Kramer H. Cumulative systolic BP and changes in urine albumin‐to‐creatinine ratios in nondiabetic participants of the Multi‐Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol. 2014;9:1922–1929. doi: 10.2215/CJN.02450314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nwabuo CC, Appiah D, Moreira HT, Vasconcellos HD, Yano Y, Reis JP, Shah RV, Murthy VL, Allen NB, Sidney S, et al. Long‐term cumulative blood pressure in young adults and incident heart failure, coronary heart disease, stroke, and cardiovascular disease: the CARDIA study. Eur J Prev Cardiol. 2021;28:1445–1451. doi: 10.1177/2047487320915342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teramoto K, Nadruz Junior W, Matsushita K, Claggett B, John JE, Skali H, Solomon S, Cheng S, Shah AM. Mid‐ to late‐life time‐averaged cumulative blood pressure and late‐life cardiac structure, function, and heart failure. Hypertension. 2020;76:808–818. doi: 10.1161/HYPERTENSIONAHA.120.14833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cecelja M, Keehn L, Ye L, Spector TD, Hughes AD, Chowienczyk P. Genetic aetiology of blood pressure relates to aortic stiffness with bi‐directional causality: evidence from heritability, blood pressure polymorphisms, and Mendelian randomization. Eur Heart J. 2020;41:3314–3322. doi: 10.1093/eurheartj/ehaa238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yano Y, Reis JP, Tedla YG, Goff DC Jr, Jacobs DR Jr, Sidney S, Ning H, Liu K, Greenland P, Lloyd‐Jones DM. Racial differences in associations of blood pressure components in young adulthood with incident cardiovascular disease by middle age: Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Cardiol. 2017;2:381–389. doi: 10.1001/jamacardio.2016.5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vlachopoulos C, O'Rourke M. Diastolic pressure, systolic pressure, or pulse pressure? Curr Hypertens Rep. 2000;2:271–279. doi: 10.1007/s11906-000-0010-6 [DOI] [PubMed] [Google Scholar]
- 26. Safar ME. Arterial aging‐‐hemodynamic changes and therapeutic options. Nat Rev Cardiol. 2010;7:442–449. doi: 10.1038/nrcardio.2010.96 [DOI] [PubMed] [Google Scholar]
- 27. Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi‐Ethnic Study of Atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH Jr, Hall WD, Jones WE, Kountz DS, Lea JP, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892 [DOI] [PubMed] [Google Scholar]
- 29. Mahal S, Strain WD, Martinez‐Perez ME, Thom SA, Chaturvedi N, Hughes AD. Comparison of the retinal microvasculature in European and African‐Caribbean people with diabetes. Clin Sci (Lond). 2009;117:229–236. doi: 10.1042/CS20080538 [DOI] [PubMed] [Google Scholar]
- 30. Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173:46–51. doi: 10.1001/2013.jamainternmed.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saad MF, Lillioja S, Nyomba BL, Castillo C, Ferraro R, De Gregorio M, Ravussin E, Knowler WC, Bennett PH, Howard BV, et al. Racial differences in the relation between blood pressure and insulin resistance. N Engl J Med. 1991;324:733–739. doi: 10.1056/NEJM199103143241105 [DOI] [PubMed] [Google Scholar]
- 32. Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Cushman M, McClure LA, Safford MM, Wadley VG. Blood pressure and cognitive decline over 8 years in middle‐aged and older black and white Americans. Hypertension. 2019;73:310–318. doi: 10.1161/HYPERTENSIONAHA.118.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 34. Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/S0161-6420(99)90525-0 [DOI] [PubMed] [Google Scholar]
- 35. Couper DJ, Klein R, Hubbard LD, Wong TY, Sorlie PD, Cooper LS, Brothers RJ, Nieto FJ. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002;133:78–88. doi: 10.1016/S0002-9394(01)01315-0 [DOI] [PubMed] [Google Scholar]
- 36. Parr JC, Spears GF. Mathematic relationships between the width of a retinal artery and the widths of its branches. Am J Ophthalmol. 1974;77:478–483. doi: 10.1016/0002-9394(74)90458-9 [DOI] [PubMed] [Google Scholar]
- 37. Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974;77:472–477. doi: 10.1016/0002-9394(74)90457-7 [DOI] [PubMed] [Google Scholar]
- 38. Meyer ML, Klein BE, Klein R, Palta P, Sharrett AR, Heiss G, Nambi V, Wong TY, Tanaka H. Central arterial stiffness and retinal vessel calibers: the Atherosclerosis Risk in Communities Study‐Neurocognitive Study. J Hypertens. 2020;38:266–273. doi: 10.1097/HJH.0000000000002252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu Y, Tang O, Brady TM, Miller ER III, Heiss G, Appel LJ, Matsushita K. Simplified blood pressure measurement approaches and implications for hypertension screening: the Atherosclerosis Risk in Communities Study. J Hypertens. 2021;39:447–452. doi: 10.1097/HJH.0000000000002682 [DOI] [PubMed] [Google Scholar]
- 40. Hayes AF, Rockwood NJ. Regression‐based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav Res Ther. 2017;98:39–57. doi: 10.1016/j.brat.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 41. Wang JJ, Mitchell P, Leung H, Rochtchina E, Wong TY, Klein R. Hypertensive retinal vessel wall signs in a general older population: the Blue Mountains Eye Study. Hypertension. 2003;42:534–541. doi: 10.1161/01.HYP.0000090122.38230.41 [DOI] [PubMed] [Google Scholar]
- 42. Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P. Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J Hypertens. 2004;22:1543–1549. doi: 10.1097/01.hjh.0000125455.28861.3f [DOI] [PubMed] [Google Scholar]
- 43. Cheng S, Gupta DK, Claggett B, Sharrett AR, Shah AM, Skali H, Takeuchi M, Ni H, Solomon SD. Differential influence of distinct components of increased blood pressure on cardiovascular outcomes: from the Atherosclerosis Risk in Communities Study. Hypertension. 2013;62:492–498. doi: 10.1161/HYPERTENSIONAHA.113.01561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, Levy D. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schillaci G, Pirro M, Mannarino E. Assessing cardiovascular risk: should we discard diastolic blood pressure? Circulation. 2009;119:210–212. doi: 10.1161/CIRCULATIONAHA.108.827931 [DOI] [PubMed] [Google Scholar]
- 46. Liew G, Campbell S, Klein R, Klein BE, Sharrett AR, Cotch MF, Wang JJ, Wong TY. Ten‐year longitudinal changes in retinal microvascular lesions: the Atherosclerosis Risk in Communities Study. Ophthalmology. 2011;118:1612–1618. doi: 10.1016/j.ophtha.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Franklin SS, Wt G, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.CIR.96.1.308 [DOI] [PubMed] [Google Scholar]
- 48. Harris EL, Feldman S, Robinson CR, Sherman S, Georgopoulos A. Racial differences in the relationship between blood pressure and risk of retinopathy among individuals with NIDDM. Diabetes Care. 1993;16:748–754. doi: 10.2337/diacare.16.5.748 [DOI] [PubMed] [Google Scholar]
- 49. Harshfield GA, Dupaul LM, Alpert BS, Christman JV, Willey ES, Murphy JK, Somes GW. Aerobic fitness and the diurnal rhythm of blood pressure in adolescents. Hypertension. 1990;15:810–814. doi: 10.1161/01.HYP.15.6.810 [DOI] [PubMed] [Google Scholar]
- 50. Harshfield GA, Hwang C, Grim CE. Circadian variation of blood pressure in blacks: influence of age, gender and activity. J Hum Hypertens. 1990;4:43–47. [PubMed] [Google Scholar]
- 51. Liew G, Sharrett AR, Wang JJ, Klein R, Klein BE, Mitchell P, Wong TY. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 2008;126:1404–1410. doi: 10.1001/archopht.126.10.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, de Jong PT. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390 [DOI] [PubMed] [Google Scholar]
- 53. Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S5


