Abstract
Purpose
Ocular involvement is frequent in autoimmune diseases and even can be the first manifestation. There are multiple descriptions in the literature around the world regarding this topic. However, we evidenced a lack of studies analyzing the relationship between the ocular manifestations and systemic biomarkers, especially in Latinamerica. Therefore, this study aimed to examine the relationship between the positivity of inflammatory biomarkers and the ocular manifestations in a Colombian cohort of rheumatological patients.
Patients and Methods
We conducted an observational, descriptive, non-comparative cross-sectional study in a rheumatology center, in Bogotá, Colombia, from 2013 to 2019. We calculated a sample size of 797 patients to assess the prevalence of ocular manifestations and inflammatory biomarkers. We performed univariate analyses for categorical and continuous variables and bivariate analyses using the Chi-square and Fisher’s exact test for categorical variables.
Results
Women represented 84% of the population, and the mean age was 54.61± 15.64 years. Of 797 patients, 21.45% reported one or more ophthalmological diagnoses, being keratoconjunctivitis sicca (KCS) the most common (15.93%), followed by uveitis, and cataract (1.38%, each one). Regarding ophthalmological symptoms, 35% presented at least one, being dry eye sensation (DE) the most common (30.86%), followed by ocular pain (2.76%), red eye, and decreased visual acuity (2.63%, each one). The antibodies or inflammatory biomarkers most frequently found were antinuclear antibodies (ANAs) (35.3%), C-reactive protein (28.7%), and rheumatoid factor (27.9%). We found statistical associations between consumption of complement 3, anti-CCP, anti-RO, and anti-LA antibodies with ocular manifestations such as photophobia, DE, conjunctivitis, KCS, uveitis, retinal vasculitis, and maculopathy.
Conclusion
Ocular manifestations are frequently found in patients with positive antibodies and inflammatory biomarkers. Our results suggest antibodies and inflammatory molecules could be biomarkers for ocular manifestations in patients with rheumatological diseases. This study provides the basis for future longitudinal studies.
Keywords: antibodies, inflammatory biomarker, rheumatological disease, ocular manifestations, ophthalmology, autoimmune diseases
Introduction
Autoimmune diseases (ADs) are chronic systemic disorders characterized by the activation of the immune system,1 mainly the adaptive response. This leads to the production of antibodies, which attack own tissues, generating destruction and dysfunction of different organs. One of the principal targets is the ocular tissue; this damage may present as the first manifestation of ADs.2
The prevalence and severity of ocular manifestations in ADs vary among diseases and are influenced by factors such as autoantibodies or inflammatory biomarkers positivity.3 Its prevalence range from 18% to 35% of patients.4 For example, in patients with rheumatoid arthritis (RA), the presence of anti-cyclic citrullinated peptide antibodies (Anti-CCP) and rheumatoid factor (RF) have been associated with vision-threatening complications, including scleritis, sclerosing keratitis, and peripheral ulcerative keratitis.5 Moreover, patients with anti-neutrophil cytoplasmic antibody-associated (ANCA) vasculitis may develop anterior segment or optic nerve involvement related to positive proteinase-3 ANCA (PR3-ANCA) and myeloperoxidase-ANCA (MPO-ANCA), respectively.6
Even though multiple studies have described the association between antibodies positivity and ocular manifestations in some ADs, more evidence is needed in most autoimmune and autoinflammatory diseases to guarantee early diagnosis and treatment.4,7 Additionally, there is a lack of studies in this field in Latinamerica.8 Therefore, this study aimed to examine the relationship between the positivity of inflammatory biomarkers and the ocular manifestations in Colombian patients with rheumatologic diseases.
Methodology
Design
We conducted an observational, descriptive, non-comparative cross-sectional study on patients who attended a rheumatology center in Bogotá, Colombia, from 2013 to 2019.
Study Population
Adult patients (>18 years old) who consulted the rheumatology center with a new or previous diagnosis of a rheumatological disease were included. A simple random sampling for finite populations was used to estimate the true proportion of adult patients with rheumatological diseases and ocular manifestations. Taking as reference what is reported in the literature, an expected proportion of 27%,2,9,10 a population of 13,763 patients treated at the rheumatology center, a confidence interval of 95% (taking a normal distribution critical point of 1.96), and an estimation error of 3%, a sample size of 793 medical records was obtained. Based on the information available, four additional cases were considered for a total of 797 medical records. The sample size calculation was done using R Software 4.0.4 sampling book package.11 All patients’ diagnoses included in this study were classified according to the International Classification of Disease, tenth edition (ICD-10).
Antibodies and Inflammatory Biomarkers Assessment
The database was validated with 38 antibodies and inflammatory biomarkers. All data were collected from the patients’ clinical records. We used the International Consensus on Antinuclear Antibodies (ANAs) Patterns recommendations to evaluate them (positive/negative, pattern, and antibody levels).12 For polyautoimmunity-associated antibodies, we considered the following as positive values: >300 for anti-double stranded DNA antibodies (anti-dsDNA), >20 IU for antibodies to extractable nuclear antigens (ENAs: Sm, U1-RNP, Ro/SS-A, La/SS-B), >40 IU for anti-cardiolipin (ACA IgG and IgM), and beta-2 glycoprotein 1 antibody (B2GPI IgG and IgM), >6 IU for RF, >60 IU for anti-CCP, >0.6 IU for autoantibodies directed against the thyroglobulin protein (anti-Tg), and >100 IU for anti-thyroperoxidase-antibodies (anti-TPO).13,14 For the other antibodies and biomarkers, laboratory reference values were used.
Data Collection
We elaborated and validated a database in Microsoft Excel (Microsoft Corp., Redmond, WA, USA) to record the information. Variables included were: type of rheumatologic diseases, antibodies, inflammatory biomarkers, ocular symptoms, and ocular diagnosis. Our trained personnel evaluated and extracted the electronic medical records information during four months.
Statistical Analysis
The results were reported as means and standard deviation for continuous variables and frequency distribution tables for categorical variables. In addition, to evaluate associations between ocular manifestations and positive antibodies and inflammatory biomarkers, categorical variables were assessed using the Chi-square independence test and Fisher’s exact test when indicated, with 95% and 99% confidence levels. All analyses were done in software R version 3.4.4.
Bias Control
Confusion bias was considered because some ocular manifestations could not be related to ADs but to other external causes (e.g. medications and systemic comorbidities). We controlled this bias by designing a temporality variable, which indicated the appearance of the ocular manifestation concerning ADs. After statistical analysis, we considered the onset moment to identify if ocular manifestations were explained by other systemic diseases or the use of medications, proposing hypotheses in the discussion section. Also, a selection bias was considered because patients who consulted were mainly from a high social level; nevertheless, some patients who attended were covered by the national health insurance. Finally, a random selection of the patients’ charts was performed to control the selection bias and ensure a heterogeneous sample.
Results
From a total of 13,763 medical records of patients with rheumatologic diseases, 797 patients were randomly included. Eighty-four percent of the population were women, and the mean age was 54.61 ± 15.64 years. Familial autoimmunity and smoking were the most frequent medical history. Demographic data are shown in Table 1.
Table 1.
Demographic Data
| Variable | Data n (%) |
|---|---|
| Female | 670 (84.06) |
| Age (mean, SD) | 54.61 SD: ± 15.64 years |
| Active smokers | 49 (6.14) |
| Past smokers | 178 (22.33) |
| Tattoos | 16 (2) |
| Silicon prosthesis | 22 (2.76) |
| Familial autoimunity | 211 (26.47) |
| Mean age of autoimmune disease diagnosis | Female: 45.88 years Male: 50.65 years |
Abbreviation: SD, standard deviation.
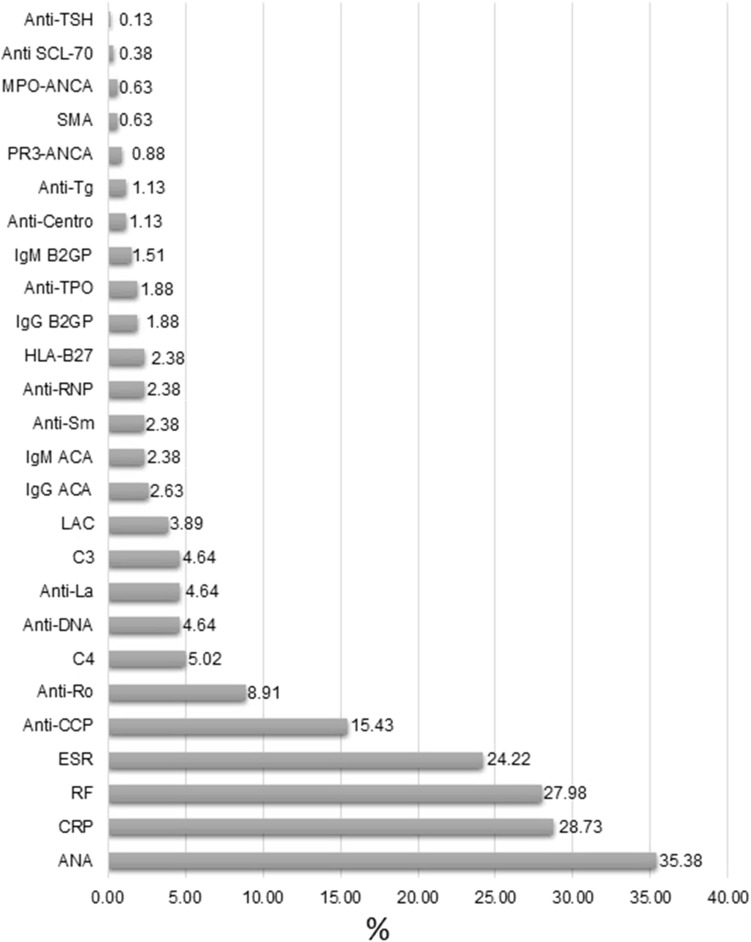
The most frequent positive biomarker was ANAs in 282 (35.38%) patients, followed by C-reactive protein in 229 (28.73%), RF in 223 (27.98%), and Erythrocyte Sedimentation Rate (ESR) in 193 (24.22%). More information is found in Figure 1.
Figure 1.
Frequency of positive autoantibodies and inflammatory biomarkers found in the rheumatological cohort.
Abbreviations: ANA, antinuclear antibodies; CRP, C-reactive protein; RF, rheumatoid factor; ESR, erythrocyte sedimentation rate; Anti CCP, anti-cyclic citrullinated peptide; Anti-Ro, anti-Sjögren’s syndrome-related antigen A; C4, Consumption of complement component 4; Anti-DNA, anti-double stranded DNA antibodies; Anti-La, Anti-Sjögren’s syndrome-related antigen B; C3, Consumption of complement component 3; LAC, lupus anticoagulant; IgG ACA, IgG anti-cardiolipin antibodies; IgM ACA, IgM anti-cardiolipin antibodies; Anti-Sm, anti-Smith antibodies; Anti-RNP, Anti-ribonucleoproteins antibodies; HLA-B27, human leukocyte antigen B27; IgG B2GP, IgG beta-2 glycoprotein 1; Anti-TPO, anti-thyroid peroxidase antibodies; IgM B2GP, IgM beta-2 glycoprotein 1; Anti-Centro, anti-centromere antibodies; Anti-Tg, anti-thyroglobulin antibodies; PR3-ANCA, Proteinase 3-antineutrophil cytoplasmic antibodies; SMA, anti-smooth muscle antibodies; MPO-ANCA, myeloperoxidase anti-neutrophil cytoplasmic antibodies; Anti SCL-70, anti-topoisomerase I antibodies; Anti-TSH, anti-thyroid stimulating hormone receptor antibodies.
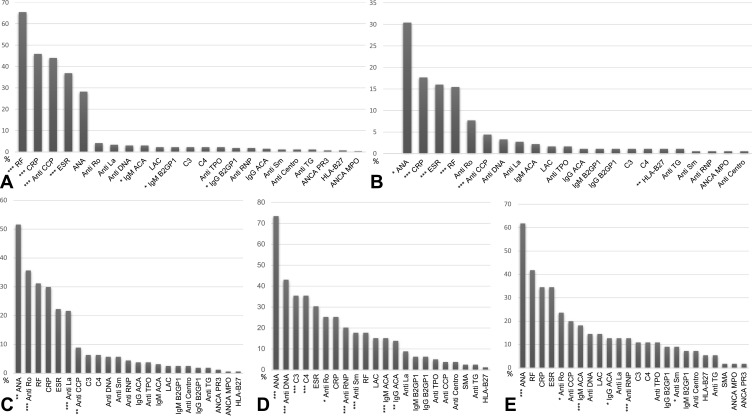
The distribution of antibodies by diseases had a usual pattern. However, we saw that in most cases, ANAs took an important role being significantly associated with most of the conditions (Figure 2) and [Supplementary Table 1]. In addition, ocular manifestations related to polyautoimmunity presented statistically significant associations with ANAs, anti-ribonucleoproteins antibodies (Anti RNP), IgM anti-cardiolipin antibodies (IgM ACA), IgG anti-cardiolipin antibodies (IgG ACA), anti-Sjögren syndrome-related antigen A (Anti-Ro), anti-Smith antibodies (Anti Sm) (Figure 2). More detailed information on diseases and laboratory findings is shown in [Supplementary Table 1].
Figure 2.
Antibodies and inflammatory markers associated with the most frequent rheumatological disease. (A) Rheumatoid Arthritis 266 (33.37%); (B) Fibromyalgia 181 (22.71%); (C) Sjögren Syndrome 157 (19.72%); (D) Systemic lupus erythematosus 79 (9.91%); (E) Polyautoimmunity 55 (6.9%). P-value is based on Chi-square Independence test and Fisher’s exact test with a 95% *and 99% **Confidence level. ***P-value asymptotically significant for a 95% confidence level.
Abbreviations: ANA, antinuclear antibodies; Anti DNA, anti-double stranded DNA antibodies; LAC, lupus anticoagulant; IgG ACA, IgG anti-cardiolipin antibodies; IgM ACA, IgM anti-cardiolipin antibodies; IgM B2GP, IgM beta-2 glycoprotein 1; IgG B2GP, IgG beta-2 glycoprotein 1; Anti-Ro, anti-Sjögren’s syndrome-related antigen A; Anti-La, anti-Sjögren’s syndrome-related antigen B; Anti Sm, anti Smith antibodies; Anti RNP, Anti ribonucleoproteins antibodies; SMA, anti-smooth muscle antibody; MPO-ANCA, myeloperoxidase anti-neutrophil cytoplasmic antibodies; PR3-ANCA, proteinase 3- antineutrophil cytoplasmic antibodies; RF, rheumatoid factor; Anti CCP, anti-cyclic citrullinated peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; C3, consumption of complement component 3; C4, consumption of complement component 4; Anti Centro, anti-centromere antibodies; HLA-B27, human leukocyte antigen B27; Anti-Tg, antibodies against thyroglobulin protein; Anti TPO, anti-thyroid peroxidase antibodies; Anti TRab, anti-thyrotropin receptor antibodies; Anti SCL-70, anti-topoisomerase I antibodies.
Thirty-five percent of the patients reported one or more ophthalmological symptoms, being dry eye (DE) sensation the most common in 246 (30.86%) patients, followed by ocular pain in 22 (2.76%), red eye, and decreased visual acuity (VA) in 21 (2.63%) each. We found statistically significant associations between DE and IgG B2GP (P=0.01), ANAs (P=0.002), Anti-Ro (P<0.0001), and anti-Sjögren syndrome-related antigen B (Anti-La) (P=0.0008). In the same way, we found statistically significant associations between photophobia and consumption of C3 (C3) (P=0.05) (Table 2). Data about ophthalmological symptoms and their association are shown in [Supplementary Table 2].
Table 2.
Ophthalmological Symptoms and Statistically Associated Antibodies Compared with the Literature
| Ocular Symptoms | Associated Antibodies in our Population | Associated Antibodies in the Literature |
|---|---|---|
| Ocular pain n=22 (2.76%) | – | Anti-MOG in patients with Optic neuritis48 AQP4 in patients with Optic neuritis48 TRab in patients with Graves orbitopathy49 |
| Photophobia n=14 (1.75%) | C3 (P=0.05***) | Anti-TRPM1 in patients with paraneoplastic retinopathy40 |
| Foreign body sensation n=5 (0.62%) | - | Lack of information |
| Tearing n=5 (0.62%) | - | Lack of information |
| Red-eye n=21 (2.63%) | - | PR3-ANCA and MPO in patients with AAV6 |
| Decreased VA n=21 (2.63%) | - | Anti-MOG in patients with Optic neuritis48 AQP4 in patients with Optic neuritis48 Anti-phospholipid antibodies17,28 |
| Pruritus n=4 (0.5%) | - | Lack of information |
| Burning n=6 (0.75%) | - | Lack of information |
| Floaters n=9 (1.12%) | - | Lack of information |
| Dry eye n=246 (30.86%) | IgG B2GP(P = 0.01**) ANA (P =0.002**) Anti-La (P =0.0008***)Anti-Ro (P <0.0001***) |
Anti-Ro in patients with SS44 Anti-La in patients with SS44 RF in patients with SS44 ANAS > o = 1:320 in patients with SS44 Anti-CCP in patients with AR3 Anti-dsDNA in patients with LES38 C3 in patients with LES38 SP1 in patients with SS36 Anti-CA6 in patients with SS37 M(3) mAChRs in patients with SS50 Anti-hK13 in patients with SS34 |
| Diplopia n=2 (0.25%) | - | LA; ACA; B2GP in patients with antiphospholipid syndrome17 PR3-ANCA and MPO-ANCA in patients with AAV6 mAChRs in patients with Myasthenia Gravis51 |
Notes: p-value is based on Chi-square independence test and Fisher’s exact test when indicated with a 99% **Confidence level. ***P-value asymptotically significant for a 95% confidence level.
Abbreviations: Anti-MOG, anti myelin oligodendrocyte glycoprotein antibody; AQP4, anti aquaporin-4 antibody; TRab, thyrotropin receptor autoantibodies; Anti-TRPM1, autoantibody against transient receptor potential cation channel, subfamily M, member 1; PR3-ANCA, Proteinase 3- antineutrophil cytoplasmic antibodies; MPO-ANCA, myeloperoxidase anti-neutrophil cytoplasmic antibodies; VA, visual acuity; LAC, lupus anticoagulant; ACA, anticardiolipin; B2GPI, anti-beta-2-glycoprotein I; ANAS, antinuclear antibody; Anti-Ro, anti–Sjögren’s syndrome-related antigen A autoantibodies; Anti-La, anti–Sjögren’s syndrome-related antigen B autoantibodies; RF, rheumatoid factor; Anti-CCP, anti-cyclic citrullinated peptide antibodies; Anti-dsDNA, anti-double-stranded DNA antibody; C3, consumption of complement component 3; SP1, Salivary protein 1; Anti-CA6, anti-carbonic anhydrase 6; mAChRs, autoantibodies against muscarinic acetylcholine receptors; Anti-hK13, autoantibodies against kallikrein 13; mAChRs, autoantibodies against muscarinic acetylcholine receptors; RA, rheumatoid Arthritis; SS, Sjögren Syndrome; SLE, systemic lupus erythematosus; ANCA, antineutrophil cytoplasmic antibodies.
On the other hand, 171 (21.45%) patients presented one or more ophthalmological diagnoses, being keratoconjunctivitis sicca (KCS) the most common, present in 127 (15.93%) of them, followed by cataract in 11 (1.38%), uveitis in 11 (1.38%), and scleritis in 10 (1.25%). We found statistically significant associations between KCS and Anti CCP (P= 0.03), ANAs (P= 0.03), lupus anticoagulant (LAC) (P=0.01), Anti-Ro (P<0.0001), and Anti-La (P<0.0001). Similarly, conjunctivitis was statistically significantly associated with C3 (P=0.03) and consumption of C4 (C4) (P=0.05). Also, relationships between retinal vasculitis and MPO-ANCA (P=0.05), uveitis and PR3-ANCA (P=0.04), and maculopathy and Anti CCP (P= 0.04) were found (Table 3). Ophthalmic diagnoses with laboratory findings are shown in [Supplementary Table 3].
Table 3.
Ophthalmological Diagnosis and Statistical Positive Association Antibodies Compared with Literature
| Ocular Diagnosis | Associated Antibodies in Our Population | Associated Antibodies in the Literature |
|---|---|---|
| Keratitis 8 (1%) | - | ANA in a patient with SLE52,53 |
| Conjunctivitis 4 (0.5) | C3 (P =0.03*) C4 (P = 0.05*) |
β4-integrin antibody in patients with OCP54,55 ANCA in patients with Granulomatosis with Polyangiitis33 |
| Keratoconjunctivitis Sicca 127 (15.93%) | Anti CCP (P = 0.03*) ANAs (P =0.03*) LAC (P =0.01*) Anti-Ro (P < 0.000***) Anti-La (P < 0.000***) |
Anti-Ro/SSA and Anti-La/SSB in patients with SS21 RF in patients with SS22 RF in patients with RA56 Anti-dsDNA and C3 in patients with SLE38 ANA in patients with SS22 anti-CA6 in patients with SS37 |
| OSSN 2 (0.25%) | - | Lack of information |
| PUK 2 (0.25%) | - | PR3-ANCA in patients with AAV6,7,27 |
| Corneal perforation 1 (0.12%) | - | Anti-dsDNA and ANA in patients with SLE57 |
| Cataract 11 (1.38%) | - | IAAs in a patient with T1DM58 |
| Uveitis 11 (1.38%) | PR3-ANCA (P = 0.04*) | HLA-B27 in patients with JIA31 HLA-B27 in patients with uveitis32,59 ANA in patients with JIA15 ARA were significantly associated in patients with uveitis30 |
| Anterior uveitis 7 (0.87%) | - | PR3-ANCA in patients with AAV7 HLA-B27 in patients with anterior uveitis60 |
| Posterior uveitis 1 (0.12%) | - | ACA in a patients with SLE28 Antiphospholipid antibodies in patients with APS17 |
| Panuveitis 1 (0.12%) | - | Anti-UACA in patients with VKH29 |
| Exophthalmos 2 (0.25%) | - | TRab in patients with Graves’ disease61–63 EMAb in hyperthyroid Graves’ disease64 |
| Glaucoma 7 (0.87%) | - | ACA and B2GP in patients with APS and normal-tension glaucoma65,66 Anti-Ro/SS-A in normal-pressure glaucoma67 Anti-rhodopsin antibody in normal-pressure glaucoma68 ARA in patient with optic nerve, and optic nerve head antigens in primary open-angle glaucoma and normal-tension glaucoma69 |
| Optic neuritis 5 (0.62%) | - | AQP4 and Anti-MOG48 |
| Retinal vasculitis 1 (0.12%) | MPO-ANCA (P =0.05*) | Antiphospholipid antibodies in patients with APS and LES17,28 ARA in patients with systemic inflammatory diseases70 P-ANCA positivity71 |
| Macular edema 1 (0.12%) | - | Antiretinal antibodies in retinitis pigmentosa72 Antiphospholipid antibodies in patients with APS17 |
| CRAO 1 (0.12%) | - | Lupus anticoagulant and ACA in SLE73 Antiphospholipid antibodies in APS17,74,75 MPO-ANCA in EGPA76 |
| Maculopathy 4 (0.5%) | Anti CCP (P =0.03*) | Anti-RPE antibodies in AEPVM77 Antiphospholipid antibodies in APS17 |
| Scleritis 10 (1.25%) | - | FR and Anti-CCP in RA78 MPO-ANCA and PR3-ANCA in AAV6,7 |
| Episcleritis 3 (0.37%) | - | Anti-CCP in RA3 MPO-ANCA and PR3-ANCA in patients with AAV6,7 |
| Blind eye 1 (0.12%) | - | Lack of information |
| Retinal detachment 1 (0.12%) | - | ACA in IDO28 Antiphospholipid antibodies in patients with APS17 |
Notes: p-value is based on the Chi-square independence test and Fisher’s exact test when indicated with a 95% *Confidence level. ***P-value asymptotically significant for a 95% confidence level.
Abbreviations: ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies; PR3-ANCA, Proteinase 3- antineutrophil cytoplasmic antibodies; MPO-ANCA, myeloperoxidase anti-neutrophil cytoplasmic antibodies; Anti-Ro, anti–Sjögren’s-syndrome-related antigen A autoantibodies; Anti-La, anti–Sjögren’s-syndrome-related antigen B autoantibodies; RF, rheumatoid factor; Anti-dsDNA, anti double-stranded DNA antibody; LAC, lupus anticoagulant; C3, consumption of complement component 3; anti-CA6, anti-carbonic anhydrase 6; P-ANCA, perinuclear antineutrophil cytoplasmic antibodies; IAAs, insulin autoantibodies; HLA-B27, human leukocyte antigen B27; ACA, anticardiolipin antibodies; Anti-UACA, anti-uveal autoantigen with coiled coil domains and ankyrin repeats; EMAb, eye‐muscle antibody; APSA, antiphosphatidylserine antibody; APL, antiphospholipid antibodies; ARA, antiretinal antibodies; anti-RPE, anti-retinal pigment epithelium; Anti-CCP, anti-cyclic citrullinated peptide antibody; Anti-TSH, anti-thyroid stimulating hormone receptor antibodies; SLE, systemic lupus erythematosus; OCP, ocular cicatricial pemphigoid; SS, Sjögren syndrome; RA, rheumatoid arthritis; AAV, ANCA-associated vasculitis; T1DM, type 1 diabetes mellitus; JIA, Juvenile Idiopathic Arthritis; JRA, Juvenile Rheumatoid Arthritis; VKH, Vogt-Koyanagi-Harada; APS, antiphospholipid syndrome; EGPA, eosinophilic granulomatosis with polyangiitis; AEPVM, acute exudative polymorphous vitelliform maculopathy; OSSN, Ocular Surface Squamous Neoplasia; PUK, peripheral ulcerative keratitis; JIA, Juvenile Idiopathic Arthritis.
Discussion
Although ocular manifestations and poor prognosis of ADs have been associated with antibodies positivity, most investigations focus on a common group of ADs; generally, ANCA-associated vasculitis (AAV), RA, antiphospholipid syndrome (APS), juvenile idiopathic arthritis (AIJ), Sjögren syndrome (SS), and Systemic lupus erythematosus (SLE).5,7,15–17 Therefore, we analyzed the relationship between ocular manifestations and the positivity of antibodies and inflammatory biomarkers in 45 immune-mediated diseases, as well as in polyautoimmunity phenomena, finding some novel and interesting associations that lead to new hypotheses about the role of these molecules as biomarkers in the phenotype of the ADs.
Keratoconjunctivitis Sicca
Currently, there is no validated definition or classification for KCS. Therefore, this varies depending on the study, making comparisons difficult.18 This condition strongly relates to SS and its autoimmune mechanism, especially in those with severe forms of the disease.19,20 Chung et al found that Anti-Ro and Anti-La autoantibodies concentrations were significantly higher in patients with primary SS who had moderate to severe KCS than those with mild KCS.21 Likewise, a cohort study in patients with primary SS found that patients with KCS with positive RF and ANAs were more likely to have abnormal Schirmer test values.22 Our results agree as we found a statistically significant association between KCS and seropositivity for Anti-Ro and Anti-La.
Contrarily to our study, no studies have shown a statistically significant association between LAC seropositivity and KCS. However, some studies have described a relationship between retinal involvement and LAC in patients with SLE due to immune complexes deposition in vessel walls.23 Based on our findings, more studies are needed to identify a possible mechanism for the development of KCS in the presence of LAC.
Conjunctivitis
The conjunctiva is the most immunologically active tissue in the external eye due to its anatomical location next to lymph nodes and vascular characteristics.24,25 Therefore, it can be affected by immunological responses mediated by antibodies or inflammatory biomarkers.25,26
Antibodies against the cytoplasmic domain of β4 integrin of the hemidesmosomes and anti-integrin antibodies have been associated with conjunctivitis in ocular cicatricial pemphigoid. In the same way, an association between conjunctivitis and ANCAs antibodies, especially in severe and recurrent cases, has been reported.6,27 Contrarily, we did not find any of these associations which could be attributed to the characteristics of our population. However, we found associations between C3 and C4 consumption and conjunctivitis, a finding that has not been previously described. Further studies are required to evaluate if this association is affected by the activity of diseases such as SLE or APS.
Maculopathy
Maculopathy can have different origins, among which the one with the most descriptions and associations with antibodies is cystoid macular edema, where it has been reported a possible relationship between LAC and ACA and macular edema in patients with LES and APS.17,28
Interestingly, our results showed an association between maculopathy and anti-CCP. All the patients with maculopathy had RA and anti-CCP with levels over 300 u/mL. However, this association could also be explained due to the previous use of hydroxychloroquine. Future longitudinal studies are necessary to clarify this possible association.
Uveitis and Retinal Vasculitis
A relationship between uveitis and HLA-B27, ANAs, anti-ACA, Uveal autoantigen with coiled-coil domains and ankyrin repeats (anti-UACA), PR3-ANCA, and anti-retinal antibodies (ARA) has been previously described. That is in line with our results; however, we emphasize that given the low number of patients with JIA in our population, the association with ANAs may not be seen.7,15,29–32 On the other hand, a close relation with antiphospholipid antibodies has been described when we talk about retinal vasculitis.17 In our study, we found an additional association with MPO-ANCAS. Although it is considered that these antibodies are related to scleral manifestations, in this case, we saw an interesting association with uveal manifestations.33 Nevertheless, this association could not be compared neck to neck with other studies cited due to the different methodologies and types of analyses.
Dry Eye
Dry eye is an ocular surface disease that presents as a consequence of chronic inflammation. This disease has been related to the presence of activated CD4+ T cells in ocular surface tissues, contributing to the idea that this is a self–antigen-driven autoimmune disease and that other autoreactive lymphocytes may be involved.34 Considering the difficulties in KCS and dry eye differentiation, we compared our results with those of patients with dry eye reported in the literature.18
Dry eye has been related to different systemic diseases and antibodies. For example, Anti-Ro positivity, ocular staining score, and Schirmer’s test are included as items in the 2016 American College of Rheumatology (ACR) Classification Criteria for SS.16,35 In the same way, Stern et al described the presence of autoantibodies against Klk13 and Klk1 in the serum of mice with dry eye and its contribution to ocular surface inflammation.34 Similarly, the “DREAM” Clinical Trial by Bunya et al described that patients with positive traditional SS autoantibodies had worse corneal and conjunctival staining and those with salivary protein-1 (SP-1) autoantibodies had a significantly higher dry eye prevalence.36 On the other hand, several autoantibodies for SS diagnosis, especially for early stages, have been studied. For example, carbonic anhydrase-6 antibody (CA6) is commonly abundant in lacrimal glands and helps to maintain tear film pH homeostasis to protect the epithelial cells of the cornea and conjunctiva from damage.37 Anti-CA6 was found to be associated with severe corneal and conjunctival staining in patients with aqueous-deficient dry eye.37 In our study, we did not evaluate anti-CA6.
Dry eye has also been described as an ocular manifestation of RA. Vignesh et al described the association between anti-CCP antibodies and the severity of ocular manifestations in RA. Additionally, anti-CCP-related dry eye was the most common manifestation, with a prevalence of 28%.3 Regarding antibodies and inflammatory biomarkers in patients with SLE and dry eye, Chen et al found that dry eye severity was correlated with positive anti-dsDNA and C3 consumption. However, there was no relationship with ESR, ANAs, and C4 consumption, previously associated with SLE activity.34,38 We describe a new association between IgG B2GP antibodies and dry eye. Furthermore, our study results support the association between Anti-Ro, Anti-La, ANAs, and dry eye.
Photophobia
Photophobia has been associated with different conditions.39 In 2019, Ueno et al studied photophobia through electroretinography in patients with paraneoplastic retinopathy; they found a relation between autoantibody transient receptor potential cation channel, subfamily M, member 1 (Anti-TRPM1), and photophobia.40 Due to the limited number of studies reporting this association, we present the first study describing a statistically significant association between inflammatory biomarkers with photophobia.
Polyautoimmunity and Antibodies
Rheumatological and autoimmune diseases are associated with multiple antibodies.41 It has been described that some antibodies can predispose to polyautoimmunity, as is the case of Anti-Ro antibodies in SLE and ANAs antibodies in SS.42,43 In 2001, Gilboe et al described that patients with poliautoimmunity (SLE and SS) and Anti-Ro/SSB antibodies presented worse outcomes on the visual analog fatigue scale (VAS).42
Although we corroborated previously described associations between antibodies and polyautoimmunity, we also present new associations [Supplementary Table 1]. Therefore, we suggest early ophthalmology referral for patients who present an autoimmune profile with these characteristics to prevent complications. However, more analytical studies are needed to support this suggestion and the use of antibodies in personalized medicine.44
Fibromyalgia and Antibodies
Although fibromyalgia (FM) is not classified as an autoimmune disease, it is considered a rheumatologic disease whose etiology remains unknown.45 In 2008, even though anti-polymer antibodies presented a low sensitivity in patients with FM, they were associated with more significant pain and fatigue.46 A recent study showed that FM pain was associated with IgG autoantibodies that sensitize peripheral nociceptive afferent neurons.47 Our results showed new associations between FM and antibodies and inflammatory biomarkers [Supplementary Table 1]. That could be explained by the mechanism of latent autoimmunity in these patients or also related to this disease. Further studies are needed to evaluate this atypical association.
Limitations
Information bias could be a limitation because the extensive autoantibodies panel was not ordered in all cases, as antibodies are generally requested on a case-by-case basis according to the clinical manifestations and the clinically suspected diagnosis, which could generate an under-recording and underestimation. In fact, in some cases, patients could have positive autoantibodies in a latent manner without overt poliautoimmunity clinical signs. In addition, a measurement bias could be present as antibody data were gathered by various specialists and analyzed retrospectively. However, to control this bias, we re-interpreted antibodies positivity based on interpretation guidelines and articles. Moreover, interviewer bias could also occur since the medical center doctors did not have the training to perform a detailed ophthalmological examination, but all of them were trained in rheumatology and internal medicine; therefore, high quality of information was ensured, and detection bias was prevented.
Also, a confusion bias may occur due to the study’s design, considering it is a cross-sectional study, where outcome and exposure are present simultaneously. In some cases, patients with systemic diseases developed ocular manifestations after diagnosing the autoimmune diseases that required treatment and, therefore, it could have an implication in the ocular manifestation. Further prospective studies should consider this bias. It is important to emphasize some of the associations that we found statistically significant were assessed with a low number of cases which implies that the determination is not conclusive.
Conclusion
Ocular manifestations are common in ADs. However, currently, there is weak evidence on the relation between ocular manifestations and autoantibodies and inflammatory biomarkers positivity. Nevertheless, searching these relations is a fundamental stage in personalized medicine that could allow prompt diagnosis, preventing severe complications and costs. We found that the most frequently positive antibody was ANAs. Also, statistically significant associations were found between consumption of complement 3, anti-CCP, anti-RO, and anti-LA antibodies with ocular manifestations such as photophobia, DE, conjunctivitis, KCS, uveitis, retinal vasculitis, and maculopathy. These results allow generating hypotheses about the biomarker role of these molecules in the ocular phenotype of patients with ADs. Nevertheless, this association was based on a few cases, so it must be interpreted carefully. More studies of these characteristics should be conducted to evaluate new antibodies and new patterns of association not previously described.
Acknowledgments
We are thankful to the Universidad del Rosario for financing the publication charges of this article and Nicolás Molano, statistician, for helping us with the statistical analysis.
Abbreviations
AAV, ANCA-associated vasculitis; ACA, anticardiolipin antibodies; AChR, acetylcholine receptor antibody; ACR, American College of Rheumatology; ADLT, Alejandra de-la-Torre; ADs, autoimmune diseases; AEPVM, acute exudative polymorphous vitelliform maculopathy; AIJ, Juvenile idiopathic arthritis; ANAS, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibodies; Anti Centro, anti-centromere antibodies; Anti DNA, anti-double stranded DNA antibodies; Anti RNP, anti ribonucleoproteins antibodies; Anti SCL-70, anti-topoisomerase I antibodies; Anti Sm, Anti Smith antibodies; Anti TPO, anti-thyroid peroxidase antibodies; Anti TRab, anti-thyrotropin receptor antibodies; Anti-CA6, anti-carbonic anhydrase 6; Anti-CCP, anti-cyclic citrullinated peptide antibodies; Anti-dsDNA, anti-double-stranded DNA antibody; Anti-hK13, autoantibodies against kallikrein 13; Anti-La, Anti–Sjögren’s syndrome-related antigen B autoantibodies; Anti-MOG, anti myelin oligodendrocyte glycoprotein antibody; Anti-Ro, anti–Sjögren’s syndrome-related antigen A autoantibodies; Anti-RPE, anti-retinal pigment epithelium; Anti-TRPM1, autoantibody against transient receptor potential cation channel, subfamily M, member 1; Anti-TSH, Anti-thyroid stimulating hormone receptor antibodies; Anti-Tg, anti-thyroglobulin antibodies; Anti-UACA, anti-uveal autoantigen with coiled coil domains and ankyrin repeats; APL, antiphospholipid antibodies; APS, antiphospholipid syndrome; APSA, antiphosphatidylserine antibody; AQP4, Anti Aquaporin-4 antibody; ARA, anti-retinal antibodies; ASDLR, Ana Sofía-De Los Ríos; B2GPI, Anti-beta-2-glycoprotein I; C3, consumption of complement component 3; C4, consumption of complement component 4; CA6, carbonic anhydrase-6 antibody; CCG, Carlos Cifuentes-González; CRP, C-reactive protein; DE, dry eye; EGPA, eosinophilic granulomatosis with polyangiitis; EMAb, eye‐muscle antibody; ESR, Erythrocyte sedimentation rate; FM, Fibromyalgia; HLA-B27, Human Leukocyte Antigen B27; HLA-B27, human leukocyte antigen B27; IAAs, insulin autoantibodies; IgG ACA, IgG Anti-cardiolipin antibodies; IgG B2GP, IgG beta-2 glycoprotein 1; IgM ACA, IgM Anti-cardiolipin antibodies; IgM B2GP, IgM beta-2 glycoprotein 1; IOD, inflammatory ocular disease; JIA, Juvenile Idiopathic Arthritis; JMO, Juliana Muñoz-Ortiz; JRA, Juvenile Rheumatoid Arthritis; JRG, Juliana Reyes-Guanes; KCS, keratoconjunctivitis sicca; LAC, Lupus anticoagulant; M(3) mAChRs, autoantibodies against M(3) muscarinic acetylcholine receptors; mAChRs, autoantibodies against muscarinic acetylcholine receptors; MPO-ANCA, myeloperoxidase-antineutrophil cytoplasmic antibodies; OCP, ocular cicatricial pemphigoid; OMG, ocular myasthenia; OSSN, ocular surface squamous neoplasia; P-ANCA, perinuclear antineutrophil cytoplasmic antibodies; PDVN, Paula Dora Victoria-Nova; PR3-ANCA, Proteinase 3- Antineutrophil cytoplasmic antibodies; PTMV, Paula Tatiana Muñoz-Vargas; PUK, peripheral ulcerative keratitis; PUR, Pilar Uribe-Reina; RA, Rheumatoid Arthritis; RDMH, Rubén Darío Mantilla-Hernández; RF, rheumatoid factor; SLE, systemic lupus erythematosus; SMA, anti-smooth muscle antibody; SP1, salivary protein 1; SS, Sjögren syndrome; T1DM, type 1 diabetes mellitus; TRab, thyrotropin receptor autoantibodies; VA, visual acuity; VAS, visual analog fatigue scale; VKH, Vogt-Koyanagi-Harada.
Data Sharing Statement
The datasets used and analyzed during the current study are available by the corresponding author on reasonable request.
Ethical Considerations
This study adheres to the ethical principles for human research established by the Helsinki Declaration, the Belmont Report, and Colombian Resolution 008430 of 1993. The confidentiality of the information has been preserved based on the Habeas data law (Organic Law 1581 of 2012). This investigation was presented to the research ethics committee of the Escuela Superior de Oftalmología del Instituto Barraquer de América, Bogotá, Colombia. However, as it is a retrospective study and according to the institution’s policies, it did require a registration process but did not require an ethics committee approval process.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Doria A, Zen M, Bettio S, et al. Autoinflammation and autoimmunity: bridging the divide. Autoimmun Rev. 2012;12(1):22–30. doi: 10.1016/j.autrev.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 2.Generali E, Cantarini L, Selmi C. Ocular involvement in systemic autoimmune diseases. Clin Rev Allergy Immunol. 2015;49:263–270. doi: 10.1007/s12016-015-8518-3 [DOI] [PubMed] [Google Scholar]
- 3.Vignesh APP, Srinivasan R. Ocular manifestations of rheumatoid arthritis and their correlation with anti-cyclic citrullinated peptide antibodies. Clin Ophthalmol. 2015;9:393–397. doi: 10.2147/OPTH.S77210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turk MA, Hayworth JL, Nevskaya T, Pope JE. Ocular manifestations in rheumatoid arthritis, connective tissue disease, and vasculitis: a systematic review and metaanalysis. J Rheumatol. 2021;48:25–34. doi: 10.3899/jrheum.190768 [DOI] [PubMed] [Google Scholar]
- 5.Itty S, Pulido JS, Bakri SJ, Baratz KH, Matteson EL, Hodge DO. Anti-cyclic citrullinated peptide, rheumatoid factor, and ocular symptoms typical of rheumatoid arthritis. Trans Am Ophthalmol Soc. 2008;106:75–81. [PMC free article] [PubMed] [Google Scholar]
- 6.Ungprasert P, Crowson CS, Cartin-Ceba R, et al. Clinical characteristics of inflammatory ocular disease in anti-neutrophil cytoplasmic antibody associated vasculitis: a retrospective cohort study. Rheumatology. 2017;56:1763–1770. doi: 10.1093/rheumatology/kex261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyanaga M, Takase H, Ohno-Matsui K. Anti-neutrophil cytoplasmic antibody-associated ocular manifestations in Japan: a review of 18 patients. Ocul Immunol Inflamm. 2020;29:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Carnero Contentti E, Daccach Marques V, Soto de Castillo I, et al. Clinical features and prognosis of late-onset neuromyelitis optica spectrum disorders in a Latin American cohort. J Neurol. 2020;267:1260–1268. doi: 10.1007/s00415-020-09699-2 [DOI] [PubMed] [Google Scholar]
- 9.Patel SJ, Lundy DC. Ocular manifestations of autoimmune disease. Am Fam Physician. 2002;66:991. [PubMed] [Google Scholar]
- 10.Zlatanović G, Veselinović D, Cekić S, Živković M, Jocić JĐ, Zlatanović M. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosn J Basic Med Sci. 2010;10(4):323–327. doi: 10.17305/bjbms.2010.2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manitz J, Hempelmann M, Kauermann G, et al. samplingbook: survey sampling procedures; 2021. Available from: https://CRAN.R-project.org/package=samplingbook. Accessed Mar 23, 2022.
- 12.Damoiseaux J, von Mühlen CA, Garcia-De La Torre I, et al. International consensus on ANA patterns (ICAP): the bumpy road towards a consensus on reporting ANA results. Autoimmun highlights. BioMed Central. 2016;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molano-González N, Rojas M, Monsalve DM, et al. Cluster analysis of autoimmune rheumatic diseases based on autoantibodies. New insights for polyautoimmunity. J Autoimmun. 2019;98:24–32. doi: 10.1016/j.jaut.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 14.Botello A, Herrán M, Salcedo V, Rodríguez Y, Anaya J-M, Rojas M. Prevalence of latent and overt polyautoimmunity in autoimmune thyroid disease: a systematic review and meta-analysis. Clin Endocrinol. 2020;93(4):375–389. doi: 10.1111/cen.14304 [DOI] [PubMed] [Google Scholar]
- 15.Berk AT, Koçak N, Ünsal E. Uveitis in juvenile arthritis. Ocul Immunol Inflamm. 2001;9(4):243–251. doi: 10.1076/ocii.9.4.243.3959 [DOI] [PubMed] [Google Scholar]
- 16.Karakus S, Baer AN, Agrawal D, Gurakar M, Massof RW, Akpek EK. Utility of novel autoantibodies in the diagnosis of Sjögren’s syndrome among patients with dry eye. Cornea. 2018;37:405–411. doi: 10.1097/ICO.0000000000001471 [DOI] [PubMed] [Google Scholar]
- 17.Franco AMM, Medina FMC, Balbi GGM, Levy RA, Signorelli F. Ophthalmologic manifestations in primary antiphospholipid syndrome patients: a cross-sectional analysis of a primary antiphospholipid syndrome cohort (APS-Rio) and systematic review of the literature. Lupus. 2020;29:1528–1543. doi: 10.1177/0961203320949667 [DOI] [PubMed] [Google Scholar]
- 18.Gonzales JA, Shiboski SC, Bunya VY, et al. Ocular clinical signs and diagnostic tests most compatible with keratoconjunctivitis sicca: a latent class approach. Cornea. 2020;39:1013–1016. doi: 10.1097/ICO.0000000000002311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu KC, Huynh K, Grubbs J, Davis RM. Autoimmunity in the pathogenesis and treatment of Keratoconjunctivitis Sicca. Curr Allergy Asthma Rep. 2014;14:403. doi: 10.1007/s11882-013-0403-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guimaraes de Souza R, Yu Z, Stern ME, Pflugfelder SC, de Paiva CS. Suppression of Th1-mediated Keratoconjunctivitis Sicca by lifitegrast. J Ocul Pharmacol Ther. 2018;34:543–549. doi: 10.1089/jop.2018.0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung JK, Kim MK, Wee WR. Prognostic factors for the clinical severity of keratoconjunctivitis sicca in patients with Sjögren’s syndrome. Br J Ophthalmol. 2012;96:240–245. doi: 10.1136/bjo.2011.202812 [DOI] [PubMed] [Google Scholar]
- 22.Koh J, Lee J, Chung S-H, Kwok S-K, Park S-H. Phenotypic features and predictors of the clinical severity of keratoconjunctivitis sicca and salivary gland dysfunction in patients with Sjögren’s syndrome: a longitudinal analysis of the Korean Initiative of primary Sjögren’s Syndrome (KISS) cohort. Scand J Rheumatol. 2019;48:198–206. doi: 10.1080/03009742.2018.1504982 [DOI] [PubMed] [Google Scholar]
- 23.de Andrade FA, Guimarães Moreira Balbi G, Bortoloti de Azevedo LG, et al. Neuro-ophthalmologic manifestations in systemic lupus erythematosus. Lupus. 2017;26:522–528. doi: 10.1177/0961203316683265 [DOI] [PubMed] [Google Scholar]
- 24.Akpek EK, Gottsch JD. Immune defense at the ocular surface. Eye. 2003;17:949–956. doi: 10.1038/sj.eye.6700617 [DOI] [PubMed] [Google Scholar]
- 25.Bielory L. Allergic and immunologic disorders of the eye. Part I: immunology of the eye. J Allergy Clin Immunol. 2000;106:805–816. doi: 10.1067/mai.2000.111029 [DOI] [PubMed] [Google Scholar]
- 26.Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16:5. doi: 10.1186/s13223-020-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohan S, Sivagurunathan PD, Khalid KHM, Raman P. Anti-neutrophil cytoplasmic antibody (ANCA)-associated scleritis: a diagnostic challenge and outcome. Egypt Rheumatol. 2020;42:79–82. doi: 10.1016/j.ejr.2019.04.002 [DOI] [Google Scholar]
- 28.Miserocchi E, Baltatzis S, Foster CS. Ocular features associated with anticardiolipin antibodies: a descriptive study. Am J Ophthalmol. 2001;131:451–456. doi: 10.1016/S0002-9394(00)00884-9 [DOI] [PubMed] [Google Scholar]
- 29.Yamada K, Senju S, Nakatsura T, et al. Identification of a novel autoantigen UACA in patients with panuveitis. Biochem Biophys Res Commun. 2001;280:1169–1176. doi: 10.1006/bbrc.2001.4189 [DOI] [PubMed] [Google Scholar]
- 30.Berge JCEM, Schreurs MWJ, Vermeer J, Meester-Smoor MA, Rothova A. Prevalence and clinical impact of antiretinal antibodies in uveitis. Acta Ophthalmol. 2016;94:282–288. doi: 10.1111/aos.12939 [DOI] [PubMed] [Google Scholar]
- 31.Zuber Z, Kania U, Król-Zdechlikiewicz A, et al. Analysis of clinical symptoms and laboratory profiles in children with Juvenile Idiopathic Arthritis in Malopolska Region (Poland) in the years 2007–2010. Open Access Maced J Med Sci. 2014;2:56–61. [Google Scholar]
- 32.Power WJ, Rodriguez A, Pedroza-Seres M, Foster CS. Outcomes in anterior uveitis associated with the HLA-B27 haplotype. Ophthalmology. 1998;105:1646–1651. doi: 10.1016/S0161-6420(98)99033-9 [DOI] [PubMed] [Google Scholar]
- 33.Kubal AA, Perez VL. Ocular manifestations of ANCA-associated vasculitis. Rheum Dis Clin North Am. 2010;36:573–586. doi: 10.1016/j.rdc.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 34.Stern ME, Schaumburg CS, Siemasko KF, et al. Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:2062–2075. doi: 10.1167/iovs.11-9299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiboski CH, Shiboski SC, Seror R, et al. American College of Rheumatology/European league against rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2016;2017(69):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunya VY, Ying G-S, Maguire MG, et al. Prevalence of novel candidate Sjogren syndrome autoantibodies in the Dry Eye Assessment and Management (DREAM) Study. Cornea. 2018;37:1425–1430. doi: 10.1097/ICO.0000000000001714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karakus S, Baer AN, Akpek EK. Clinical correlations of novel autoantibodies in patients with dry eye. J Immunol Res. 2019;2019:e7935451. doi: 10.1155/2019/7935451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen A, Chen H-T, Hwang Y-H, Chen Y-T, Hsiao C-H, Chen H-C. Severity of dry eye syndrome is related to anti-dsDNA autoantibody in systemic lupus erythematosus patients without secondary Sjogren syndrome: a cross-sectional analysis. Medicine. 2016;95:e4218. doi: 10.1097/MD.0000000000004218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol. 2016;61:466–477. doi: 10.1016/j.survophthal.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 40.Ueno S, Inooka D, Nakanishi A, et al. Clinical course of paraneoplastic retinopathy with Anti-Trpm1 autoantibody in Japanese cohort. Retina. 2019;39:2410–2418. doi: 10.1097/IAE.0000000000002329 [DOI] [PubMed] [Google Scholar]
- 41.Rojas M, Ramírez-Santana C, Acosta-Ampudia Y, et al. Polyautoimmunity clusters as a new taxonomy of autoimmune diseases; 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.08.15.21262029v1. Accessed August 8, 2021.
- 42.Gilboe I-M, Kvien TK, Uhlig T, Husby G. Sicca symptoms and secondary Sjögren’s syndrome in systemic lupus erythematosus: comparison with rheumatoid arthritis and correlation with disease variables. Ann Rheum Dis. 2001;60:1103–1109. doi: 10.1136/ard.60.12.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas-Villarraga A, Toro C-E, Espinosa G, et al. Factors influencing polyautoimmunity in systemic lupus erythematosus. Autoimmun Rev. 2010;9:229–232. doi: 10.1016/j.autrev.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 44.Ma W-T, Chang C, Gershwin ME, Lian Z-X. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: a comprehensive review. J Autoimmun. 2017;83:95–112. doi: 10.1016/j.jaut.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 45.Clauw DJ. Fibromyalgia: a Clinical Review. JAMA. 2014;311(15):1547–1555. doi: 10.1001/jama.2014.3266 [DOI] [PubMed] [Google Scholar]
- 46.Sarzi-Puttini P, Atzeni F, Di Franco M, et al. Anti-polymer antibodies are inversely correlated with pain and fatigue severity in patients with fibromyalgia syndrome. Autoimmunity. 2008;41(1):74–79. doi: 10.1080/08916930701620035 [DOI] [PubMed] [Google Scholar]
- 47.Goebel A, Gentry C, Cuhadar U, et al. Passive transfer of fibromyalgia pain from patients to mice; 2019:713495. Available from: https://www.biorxiv.org/content/10.1101/713495v1. Accessed July 27, 2022.
- 48.Ishikawa H, Kezuka T, Shikishima K, et al. Epidemiologic and clinical characteristics of optic neuritis in Japan. Ophthalmology. 2019;126:1385–1398. doi: 10.1016/j.ophtha.2019.04.042 [DOI] [PubMed] [Google Scholar]
- 49.Nicolì F, Lanzolla G, Mantuano M, et al. Correlation between serum anti-TSH receptor autoantibodies (TRAbs) and the clinical feature of Graves’ orbitopathy. J Endocrinol Invest. 2021;44:581–585. doi: 10.1007/s40618-020-01353-y [DOI] [PubMed] [Google Scholar]
- 50.Bacman S, Berra A, Sterin-Borda L, Borda E. Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjögren syndrome. Invest Ophthalmol Vis Sci. 2001;42:321–327. [PubMed] [Google Scholar]
- 51.Peeler CE, De Lott LB, Nagia L, Lemos J, Eggenberger ER, Cornblath WT. Clinical Utility of acetylcholine receptor antibody testing in ocular myasthenia gravis. JAMA Neurol. 2015;72:1170–1174. doi: 10.1001/jamaneurol.2015.1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbas N, Megalla M, Zhang LY, Meskin SW. Bilateral interstitial keratitis as the presenting manifestation of systemic lupus erythematosus in a child. Can J Ophthalmol. 2021;56:e77–e78. doi: 10.1016/j.jcjo.2020.10.017 [DOI] [PubMed] [Google Scholar]
- 53.Lin WV, Saumur M, Al-Mohtaseb Z. Scleritis, keratitis, and orbital cellulitis: isolated ocular manifestation of systemic lupus erythematosus. Lupus. 2018;27:1985–1988. doi: 10.1177/0961203318792365 [DOI] [PubMed] [Google Scholar]
- 54.Tyagi S, Bhol K, Natarajan K, Livir-Rallatos C, Foster CS, Ahmed AR. Ocular cicatricial pemphigoid antigen: partial sequence and biochemical characterization. Proc Natl Acad Sci. 1996;93:14714–14719. doi: 10.1073/pnas.93.25.14714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhol KC, Dans MJ, Simmons RK, Foster CS, Giancotti FG, Ahmed AR. The autoantibodies to α6β4 integrin of patients affected by ocular cicatricial pemphigoid recognize predominantly epitopes within the large cytoplasmic domain of human β4. J Immunol. 2000;165:2824–2829. doi: 10.4049/jimmunol.165.5.2824 [DOI] [PubMed] [Google Scholar]
- 56.Meek B, Kelder JC, Claessen AME, van Houte AJ, ter Borg E-J. Rheumatoid factor isotype and Ro epitope distribution in primary Sjögren syndrome and rheumatoid arthritis with keratoconjunctivitis sicca. Rheumatol Int. 2018;38:1487–1493. doi: 10.1007/s00296-018-4090-5 [DOI] [PubMed] [Google Scholar]
- 57.Chen H-CJ, Cheng J-C, Hsiao C-H, Ma DH-K. Systemic lupus erythematosus presenting as corneal perforation. Tzu Chi Med J. 2009;21:169–171. doi: 10.1016/S1016-3190(09)60032-X [DOI] [Google Scholar]
- 58.Papadimitriou DT, Bothou C, Skarmoutsos F, Alexandrides TK, Papaevangelou V, Papadimitriou A. The autoimmune hypothesis for acute bilateral cataract in type 1 diabetes. Diabetes Metab. 2016;42:386–387. doi: 10.1016/j.diabet.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 59.Rosenbaum JT. New developments in uveitis associated with HLA B27. Curr Opin Rheumatol. 2017;29:298–303. doi: 10.1097/BOR.0000000000000403 [DOI] [PubMed] [Google Scholar]
- 60.D’Ambrosio EM, La Cava M, Tortorella P, Gharbiya M, Campanella M, Iannetti L. Clinical features and complications of the HLA-B27-associated acute anterior uveitis: a metanalysis. Semin Ophthalmol. 2017;32:689–701. doi: 10.3109/08820538.2016.1170158 [DOI] [PubMed] [Google Scholar]
- 61.Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of graves’ orbitopathy. J Clin Endocrinol Metab. 2010;95:2123–2131. doi: 10.1210/jc.2009-2470 [DOI] [PubMed] [Google Scholar]
- 62.Eckstein AK, Plicht M, Lax H, et al. Thyrotropin receptor autoantibodies are independent risk factors for graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91(9):3464–3470. doi: 10.1210/jc.2005-2813 [DOI] [PubMed] [Google Scholar]
- 63.Khoo DH, Ho SC, Seah LL, et al. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in graves’ disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid. 1999;9:1175–1180. doi: 10.1089/thy.1999.9.1175 [DOI] [PubMed] [Google Scholar]
- 64.Chang TC, Huang KM, Chang TJ, Lin SL. Correlation of orbital computed tomography and antibodies in patients with hyperthyroid graves’disease. Clin Endocrinol. 1990;32:551–558. doi: 10.1111/j.1365-2265.1990.tb00897.x [DOI] [PubMed] [Google Scholar]
- 65.Kremmer S, Kreuzfelder E, Klein R, et al. Antiphosphatidylserine antibodies are elevated in normal tension glaucoma. Clin Exp Immunol. 2001;125:211–215. doi: 10.1046/j.1365-2249.2001.01578.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kremmer S, Kreuzfelder E, Bachor E, Jahnke K, Selbach JM, Seidahmadi S. Coincidence of normal tension glaucoma, progressive sensorineural hearing loss, and elevated antiphosphatidylserine antibodies. Br J Ophthalmol. 2004;88:1259–1262. doi: 10.1136/bjo.2003.040832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wax MB, Tezel G, Saito I, et al. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1998;125:145–157. doi: 10.1016/S0002-9394(99)80084-1 [DOI] [PubMed] [Google Scholar]
- 68.Romano C, Barrett DA, Li Z, Pestronk A, Wax MB. Anti-rhodopsin antibodies in sera from patients with normal-pressure glaucoma. Invest Ophthalmol Vis Sci. 1995;36:1968–1975. [PubMed] [Google Scholar]
- 69.Joachim SC, Pfeiffer N, Grus FH. Autoantibodies in patients with glaucoma: a comparison of IgG serum antibodies against retinal, optic nerve, and optic nerve head antigens. Graefes Arch Clin Exp Ophthalmol. 2005;243:817–823. doi: 10.1007/s00417-004-1094-5 [DOI] [PubMed] [Google Scholar]
- 70.Dumonde DC, Graham E, Kasp-Grochowska E, et al. Anti-retinal autoimmunity and circulating immune complexes in patients with retinal vasculitis. Lancet. 1982;320:787–792. doi: 10.1016/S0140-6736(82)92679-4 [DOI] [PubMed] [Google Scholar]
- 71.Gallagher MJ, Ooi KG-J, Thomas M, Gavin M. ANCA associated pauci-immune retinal vasculitis. Br J Ophthalmol. 2005;89:608–611. doi: 10.1136/bjo.2004.051177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heckenlively JR, Jordan BL, Aptsiauri N. Association of antiretinal antibodies and cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 1999;127:565–573. doi: 10.1016/S0002-9394(98)00446-2 [DOI] [PubMed] [Google Scholar]
- 73.Hua L, Patel K, Corbett JJ. Bilateral central retinal artery occlusion in a patient with systemic lupus erythematosus. J Stroke Cerebrovasc Dis. 2015;24:e139–e141. doi: 10.1016/j.jstrokecerebrovasdis.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 74.Zhu W, Wu Y, Xu M, et al. Antiphospholipid antibody and risk of retinal vein occlusion: a systematic review and meta-analysis. PLoS One. 2015;10:e0122814. doi: 10.1371/journal.pone.0122814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palmowski-Wolfe AM, Denninger E, Geisel J, Pindur G, Ruprecht KW. Antiphospholipid antibodies in ocular arterial and venous occlusive disease. Ophthalmologica. 2007;221:41–46. doi: 10.1159/000096521 [DOI] [PubMed] [Google Scholar]
- 76.Reddy AK, Lau MK, Sieck EG, Kolfenbach JR, Palestine AG. Retinal artery occlusion followed by contralateral amaurosis fugax in association with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). Am J Ophthalmol Case Rep. 2020;18:100683. doi: 10.1016/j.ajoc.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koreen L, He SX, Johnson MW, Hackel RE, Khan NW, Heckenlively JR. Anti–retinal pigment epithelium antibodies in acute exudative polymorphous vitelliform maculopathy: a new hypothesis about disease pathogenesis. Arch Ophthalmol. 2011;129:23–29. doi: 10.1001/archophthalmol.2010.316 [DOI] [PubMed] [Google Scholar]
- 78.Sahatçiu-Meka V, Rexhepi S, Manxhuka-Kërliu S, Rexhepi M. Extra-articular manifestations of seronegative and seropositive rheumatoid arthritis. Bosn J Basic Med Sci. 2010;10:26–31. doi: 10.17305/bjbms.2010.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]