Abstract
The objective of the study was to assess the efficacy and safety of mepivacaine compared with lidocaine used in local anaesthesia in dentistry. Medline, Cochrane Central Register of Controlled Trials, EMBASE, Chinese BioMedical Literature Database, China National Knowledge Infrastructure and WHO International Clinical Trials Registry Platform were searched electronically. Relevant journals and references of studies included were hand-searched for randomised controlled trials comparing mepivacaine with lidocaine in terms of efficacy and safety. Twenty-eight studies were included, of which 15 had low risk of bias and 13 had moderate risk of bias. In comparison with 2% lidocaine with 1:100,000 adrenaline, 3% mepivacaine showed a lower success rate (P = 0.05), a shorter onset time of pulpal anaesthesia (P = 0.0005), inferior pain control during injection phase and superior inhibition of heart rate increase (P < 0.0001). In contrast, 2% mepivacaine with 1:100,000 adrenaline gave a higher success rate (P < 0.00001), a similar onset time of pulpal anaesthesia (P = 0.34) and superior pain control during injection phase (P < 0.0001); 2% mepivacaine with 1:20,000 levonordefrin had the same success rate (P = 0.69) and similar onset time of pulpal anaesthesia (P = 0.90). In addition, 3% mepivacaine had shorter onset time (P = 0.004), same level of success rate (P = 0.28) and similar pain control during injection and postinjection compared with 2% lidocaine with 1:50,000 adrenaline. Given the efficacy and safety of the two solutions, 2% mepivacaine with vasoconstrictors is better than 2% lidocaine with vasoconstrictors in dental treatment. Meanwhile, 3% plain mepivacaine is better for patients with cardiac diseases.
Key words: Mepivacaine, lidocaine, local anaesthesia, meta-analysis, dentistry
INTRODUCTION
Pain occurs throughout dental treatment, so good management of pain and anxiety is a key issue that can win a patient’s trust1. An epidemiological study revealed that more than 50% of Americans avoid dental treatments because of fear of pain, and a similar figure was reported among Brazilians2., 3.. About 14% of 4- to 11-year-old Dutch children are dentally anxious, and the strongest fear is associated with pain4. Local anaesthesia is a principal way of preventing pain and discomfort in dental treatment5.
Mepivacaine was first introduced into dentistry in 1960 as a 2% solution containing synthetic vasopressor levonordefrin, and in 1961 as a 3% solution without any vasoconstrictor. Lidocaine which was introduced first in 1943 has high efficacy, low allergenicity and minimal toxicity proven through clinical use and research; it shows higher anaesthetic efficacy only when combined with vasoconstrictors6. Mepivacaine has the same anaesthetic potency as lidocaine7, but also has milder vasodilating ability, which leads to a longer duration of anaesthesia without a vasoconstrictor8. Mepivacaine is the third most widely used solution in dentistry only after articaine and lidocaine in some parts of the world9. In dentistry, mepivacaine is always available as a 3% solution without any vasoconstrictors or as a 2% solution with vasoconstrictors such as 1:20,000 levonordefrin and 1:100,000 adrenaline; lidocaine is always available as a 2% solution with 1:100,000 or 1:50,000 adrenaline10.
Although several trials have been conducted to compare mepivacaine with lidocaine in dental treatment, their conclusions are somewhat controversial. Therefore, it is necessary to combine the results to obtain more precise evidence on the efficacy and safety of mepivacaine in comparison with lidocaine.
METHODS
A protocol that specified the method of the review was established in advance. Study selection, risk of bias assessment, and data extraction were conducted in duplicate by two trained reviewers. Disagreement between them was resolved through discussion, and the unresolved issues were brought to a third reviewer for consensus.
Inclusion criteria
Those trials that met the following criteria would be included:
-
•
Randomised controlled or quasi-randomised designs which explore the efficacy and safety of mepivacaine solutions, including 3% mepivacaine, 2% mepivacaine with 1:20,000 levonordefrin and 2% mepivacaine with 1:100,000 adrenaline compared with lidocaine solutions, including 2% lidocaine with 1:100,000 adrenaline or 1:50,000 adrenaline in local anaesthesia of dentistry
-
•
The outcome variables would include at least one of the following: success rate of anaesthesia (SRA), onset time of pulpal anaesthesia (OTP), pain ratings at injection phase (PTI), pain ratings at postinjection phase (PTP) and adverse events (AE).
Exclusion criteria
The following trials would be excluded:
-
•
Review articles, cohort studies and other kinds of studies
-
•
Trials involving participants who were hypersensitive to mepivacaine or lidocaine, or were pregnant, lactating, unreliable and unable to return for follow-up
-
•
Trials involving participants who had a history of significant medical conditions, or who took any medication that may affect anaesthetic assessment
-
•
Repetitive publications (only the best-described one was included).
Search strategy and study inclusion
The Sichuan University Electronic Databases including Medline (1946 to July 2013), Cochrane Central Register of Controlled Trials (CENTRAL, July 2013), EMBASE (via OVID, 1984 to July 2013) were searched without language limitation. The Chinese BioMedical Literature Database (1978 to July 2013) and China National Knowledge Infrastructure (1994 to July 2013) were searched for the related Chinese literatures which were not indexed in the above databases. Chinese and English journals related to local anaesthesia collected by the Medical Library of the University were hand-searched. The references of the studies included were also retrieved. The World Health Organisation (WHO) International Clinical Trials Registry Platform was searched to trace ongoing clinical trials. Some experts in this field were communicated with by letter.
The searching strategies included MeSH terms such as ‘mepivacaine’, ‘lidocaine’, ‘Anaesthesia, Local’ and free text words. The Cochrane Highly Sensitive Search Strategies were combined to identify randomised trials.
Risk of bias assessment
Risk of bias assessment was performed using Cochrane Collaboration’s tool, 2011, on the following seven domains: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting and (7) other bias11. A trial would be considered as ‘low risk’ of bias if all the seven domains were judged as ‘low risk’, ‘moderate risk’ if any of the seven items was judged as ‘unclear risk’ or ‘high risk’ if any item was judged as ‘high risk’.
The meta-analysis results were further assessed by GRADE which is short for Grading of Recommendations Assessment, Development and Evaluation on GRADEprofiler which is a software used to grade the quality of the evidence in the systematic reviews, and were scored as high, moderate, low, or very low12.
Data extraction
A customised data extraction form was developed, including the following items: study designs, method of randomisation, concealment and blinding, demographic data, usage of the drugs, anaesthesia techniques, losses to follow-up and the reasons and the final outcomes.
Data analysis
review manager 5.1 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was chosen for data analysis. The pooled results were expressed as relative risks (RRs) and its 95% confidence intervals (95% CIs) for dichotomous data or as mean difference (MD) along with its 95% CI for continuous data. The statistical significance of hypothesis test was set at α = 0.05 (two-tailed z-tests). Heterogeneity was explored and if it was significant, causes of heterogeneity were analysed. A random-effects model instead of a fixed-effect model was be used and subgroup analysis conducted. Sensitivity analysis was used to test the stability of the results. Any data unable to be pooled was just described. Publication bias was detected by using funnel plots if there were about 10 studies13., 14.. For crossover or split-mouth trials, the carry-over/carry-across effect was assessed15. If carry-over/carry-across effect was considered a problem, the analysis was based on the first period; if not, we attempted to approximate a paired analysis following the handbook. If no crossover or split-mouth designs exist, the first period and second period were mixed and pooled with parallel groups expressed as odds ratio (OR) and 95% CI15.
RESULTS
Results of the search and study inclusion
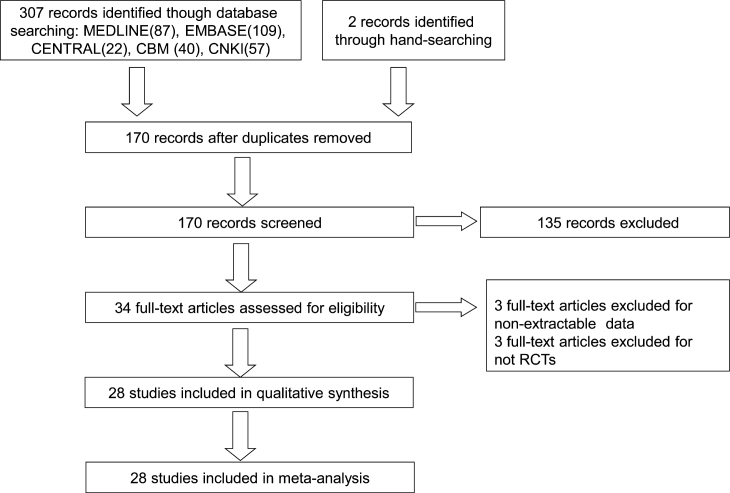
One hundred and seventy citations were obtained through the extensive searching. Screening of the titles and abstracts yielded 34 eligible studies and their full texts were retrieved. In total, 28 studies were included – 15 in English5., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29. and 13 in Chinese30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42. (Figure 1).
Figure 1.
Flow diagram of the study inclusion. RCTs, randomised controlled trials.
Characteristics of the studies included
Of the 28 studies included, 16 were parallel designs16., 17., 18., 19., 20., 21., 22., 23., 24., 28., 29. and 12 were crossover designs5., 25., 26., 27., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42.. The washout periods of crossover designs were 1–3 weeks. The success of anaesthesia was defined in 10 studies5., 16., 17., 18., 19., 20., 21., 22., 23., 24.as equal or over 80 readings obtained with electronic pulpal tester (EPT) after injection, or as no pain or mild pain evaluated by visual analogue scale (VAS) or operators in other studies25., 26., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42.. The pain ratings at injection and postinjection phases were evaluated by pain grades of four-point scale as no, mild, moderate and severe in seven studies16., 19., 20., 23., 28., 38., 39. using (VAS)20., 28., 38., 39. or rating scales 0–316., 19., 23.. The other characteristics are presented in Table 1.
Table 1.
Characteristics of studies included
| Study ID | Study design | Country | Sex (male/female) | Age | Methods (comparison) | Anaesthesia technique | Dosage (experiment/control) | Recording time | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Abdulwahab5 | Crossover | USA | 6/12 | 18–65 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline in mandibular first molars | Infiltration | 0.9 ml | Every minute for the first 20 minutes after injection using EPT | SRA |
| Schleder16 | Crossover | USA | 40/35 | 20–31 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline in mandibular first premolar | Periodontal ligament injection | 1.82 ml | 2,4,10,30,45 minutes using EPT | SRA, PTI, AE |
| Mason17 | Crossover | USA | 31/29 | 19–43 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline/2% lidocaine + 1:50,000 adrenaline in maxillary lateral incisor and first molars | Infiltration | 1.8 ml | 3-min cycles for 60 minutes at 1 minutes after injection using EPT | SRA, OTP |
| Lawary18 | Crossover | USA | 30/30 | 21–31 | 2% Mepivacaine + 1:20,000 levonordefrin with 2% lidocaine + 1:100,000 adrenaline in maxillary teeth | Infiltration | 1.8 ml | 2-minute cycles for 60 minutes at 1 minute after injection using EPT | SRA |
| Berberich19 | Crossover | USA | 34/6 | 23–33 | 3% mepivacaine with 2% lidocaine + 1:100,000 adrenaline/2% lidocaine + 1:50,000 adrenaline in maxillary teeth | Intraoral, infraorbital nerve block | Unclear | 4-minute cycles for 60 min at 1–4 min after injection using EPT | SRA, PTI, PTP, OTP |
| Forloine20 | Crossover | USA | 27/23 | 18–57 | 3% mepivacaine with 2% lidocaine + 1:100,000 adrenaline in maxillary teeth | Maxillary block | 3.6 ml | 4-minute cycles for 60 minutes at 1–4 minutes after injection using EPT | SRA, PTI, PTP, AE |
| McLean21 | Crossover | USA | 24/6 | 24–43 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline in mandibular teeth | IANB | 1.8 ml | 3-minute cycles for 50 minutes at 1–3 minutes after injection using EPT | SRA, OTP |
| Hinkley22 | Crossover | USA | 19/11 | 23–42 | 2% Mepivacaine + 1:20,000 levonordefrin with 2% lidocaine + 1:100,000 adrenaline in mandibular teeth | IANB | 1.8 ml | 3-minute cycles for 50 minutes at 1–3 minutes after injection using EPT | SRA, OTP |
| Replogle23 | Crossover | USA | 25/17 | 18–39 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline in mandibular molars | IO | 1.8 ml | 2-minute cycles for 60 minutes at 1–2 minutes after injection using EPT | SRA, PTI, PTP |
| Burns24 | Cross-over | USA | 20/20 | 19–47 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline in maxillary central incisors, lateral incisors and canines using computer-assisted injection system | P-ASA | 1.4 ml | 4-minute cycles for 72 minutes at 1–4 minutes after injection using EPT | SRA |
| Bradley25 | Parallel | Australia | 131/128 | 5–14 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth | Infiltration and IANB | 1.8 ml & 0.8–3.6 ml | Unclear | SRA |
| Cohen26 | Parallel | USA | Unclear | Unclear | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline in mandibular teeth diagnosed with irreversible pulpitis | IANB | 1.8 ml | 1 minute after injection using DDM | SRA |
| Kramp27 | Parallel | USA | Totally 150 | Unclear | 2% Mepivacaine + 1:20,000 levonordefrin with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth | Infiltration and IANB | 0.3–0.6 ml for infiltration and 1.8 ml for IANB | Unclear | PTI |
| Nusstein28 | Crossover | USA | 20/20 | 18–65 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth using the Wand Plus system | P-ASA | 1.4 ml | 4-minute cycles for 72 minutes after injection using the EPT | PTI, PTP, AE |
| Replogle29 | Crossover | USA | 25/17 | 18–39 | 3% Mepivacaine with 2% lidocaine + 1:100,000 adrenaline | IO | 1.8 ml | 2-minute cycles for 30 minutes after injection using EPT | AE |
| Shi30 | Parallel | China | 55/72 | 20–65 | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth | Infiltration | 1.8 ml | 1, 3, 5 minutes after injection using the EPT | SRA, AE, OTP |
| Chen31 | Parallel | China | 11/7 | 18–60 | Compared 2% mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary teeth diagnosed with pulpitis or deep caries | Infiltration | 1.8 ml | At 1, 3, 5 minutes after injection using the EPT | SRA, AE, OTP |
| Wu32 | Parallel | China | 17/43 | 12–70 | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth diagnosed with irreversible pulpitis | Infiltration and IANB | 1.8 ml/5.0 ml | Unclear | SRA |
| Li33 | Parallel | China | 38/32 | 60–72 | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth diagnosed with irreversible pulpitis | Infiltration | 1.8 ml/5.0 ml | 7 minutes after injection | SRA, AE |
| Dong34 | Parallel | China | 73/134 | Mean 37.4 | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary molars diagnosed with acute pulpitis, chronic pulpitis. | Infiltration and IANB | 1.8 ml/5.0 ml | Unclear | SRA |
| Liu35 | Parallel | China | 39/41 | 23–62 | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth that need pulp therapy or extraction | Infiltration and IANB | 1.7 ml/5.0 ml | Unclear | SRA |
| Xing36 | Parallel | China | Unclear | Unclear | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth diagnosed with irreversible pulpitis or periodontal disease | Infiltration and IANB | 0.9–1.8 ml/5.0 ml | At 1, 2, 5 minutes after injection | SRA |
| Luo37 | Parallel | China | 372/0 | 20–40 | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary molars that need extraction | Infiltration and maxillary block | 1.8 ml/5.0 ml | Unclear | SRA |
| Ge38 | Parallel | China | Totally 57 | Unclear | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in mandibular molars diagnosed with chronic pulpitis | Infiltration and IANB | 1.8 ml/5.0 ml | Unclear | SRA, PTI |
| Xuan39 | Parallel | China | 51/45 | 20–60 | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth that need prosthodontic therapy | Infiltration and IANB | 1.8 ml/4.0 ml | Unclear | SRA, PTI |
| He40 | Parallel | China | 151/241 | 12–70 | 2% Mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth diagnosed with irreversible pulpitis | Infiltration and IANB | 1.8 ml/5.0 ml | Unclear | SRA, AE |
| Zhou41 | Parallel | China | 43/57 | 23–76 | 2% mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in mandibular teeth that need pulp therapy or extraction | IANB | 1.5 ml/5.0 ml | Unclear | SRA |
| Jin42 | Parallel | China | Totally 360 | 6–78 | 2% mepivacaine + 1:100,000 adrenaline with 2% lidocaine + 1:100,000 adrenaline in maxillary and mandibular teeth diagnosed with pulpitis or tooth trauma | Infiltration and IANB | 1.8 ml/5.0 ml | Unclear | SRA |
IANB, inferior alveolar nerve block; SRA, success rate of anaesthesia; OTP, onset time of pulpal aesthesia; PTI, pain ratings for injection phase; PTP, pain ratings for postinjection phase; AE, adverse events; IO, intraosseous injection; P-ASA, palatalanterior superior alveolar injection.
Risk of bias of studies included
Among the studies included, 15 had low risk of bias5., 16., 17., 18., 19., 20., 21., 22., 23., 24., 27., 28., 29., 30., 31. and the other 13 had moderate risk of bias25., 26., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42. (Table 2).
Table 2.
Risk of bias assessment of studies included
| Study ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Risk of bias of studies |
|---|---|---|---|---|---|---|---|---|
| Abdulwahab5 | L | L | L | L | L | L | L | L |
| Schleder16 | L | L | L | L | L | L | L | L |
| Mason17 | L | L | L | L | L | L | L | L |
| Lawary18 | L | L | L | L | L | L | L | L |
| Berberich19 | L | L | L | L | L | L | L | L |
| Forloine20 | L | L | L | L | L | L | L | L |
| McLean21 | L | L | L | L | L | L | L | L |
| Hinkley22 | L | L | L | L | L | L | L | L |
| Replogle23 | L | L | L | L | L | L | L | L |
| Burns24 | L | L | L | L | L | L | L | L |
| Bradley25 | U | U | L | U | L | L | U | M |
| Cohen26 | U | U | U | U | L | L | U | M |
| Kramp27 | L | L | L | L | L | L | L | L |
| Nusstein28 | L | L | L | L | L | L | L | L |
| Replogle29 | L | L | L | L | L | L | L | L |
| Shi30 | L | L | L | L | L | L | L | L |
| Chen31 | L | L | L | L | L | L | L | L |
| Wu32 | L | U | U | U | U | U | U | M |
| Li33 | U | U | U | U | U | U | U | M |
| Dong34 | L | U | U | U | U | L | U | M |
| Liu35 | U | U | U | U | U | U | U | M |
| Xing36 | U | U | U | U | U | U | U | M |
| Luo37 | U | U | U | U | U | U | U | M |
| Ge38 | U | U | U | U | U | U | U | M |
| Xuan39 | U | U | U | U | U | U | U | M |
| He40 | U | U | U | U | U | U | U | M |
| Zhou41 | L | U | U | U | U | U | U | M |
| Jin42 | U | U | U | U | U | U | U | M |
L, means low risk of bias; U, means unknown risk of bias; M, means moderate risk of bias.
Efficacy and safety of mepivacaine compared with lidocaine
The parallel and crossover studies were mixed for meta-analyses because none of the 12 crossover studies reported the data of the first and second periods separately and none of them was suitable for a paired analysis14.
Success rate
3% mepivacaine versus 2% lidocaine with 1:100,000 adrenaline
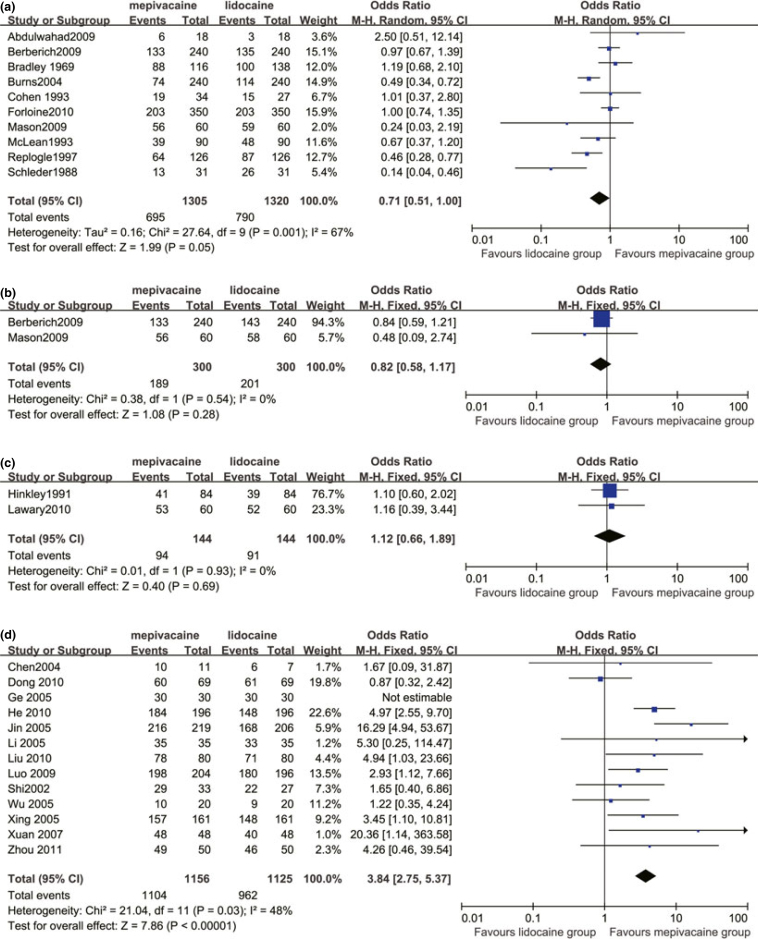
Ten studies compared 3% mepivacaine and 2% lidocaine with 1:100,000 adrenaline5., 16., 17., 19., 20., 21., 23., 24., 25., 26.. The results showed that 3% mepivacaine was significantly inferior to 2% lidocaine with 1:100,000 adrenaline (OR 0.71, 95% CI 0.51–1.00, P = 0.05) (Figure 2a). When anaesthetic techniques were considered, the OR was 0.94 (95% CI 0.14–6.29, P = 0.95) for infiltrations5., 17. and 0.78 (95% CI 0.57–1.05, P = 0.10) for nerve blocks19., 20., 21., 24., 26.. When jaws were considered, the OR was 0.83 (95% CI 0.57–1.20; P = 0.31) for maxillary regions17., 19., 20., 24., 25. and 0.69 (95% CI 0.39–1.21, P = 0.20) for mandibular regions5., 16., 21., 23., 25., 26..
Figure 2.
Meta-analyses of success rate of local anaesthesia in comparing 3% mepivacaine with 2% lidocaine with 1:100,000 adrenaline (a), 3% mepivacaine with 2% lidocaine with 1:50,000 adrenaline (b), 2% mepivacaine with 1:20,000 levonordefrin with 2% lidocaine with 1:100,000 adrenaline (c) and 2% mepivacaine with 1:100,000 adrenaline with 2% lidocaine with 1:100,000 adrenaline (d).
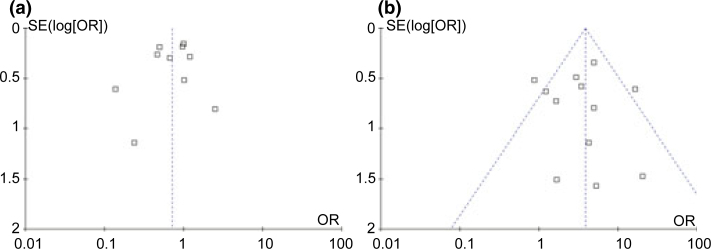
The funnel plot was symmetrical, indicating a low possibility of publication bias for this meta-analysis (Figure 3a). The strength of evidence of this outcome was moderate.
Figure 3.
Funnel plots for comparing 3% mepivacaine with 2% lidocaine with 1:100,000 adrenaline (a) and 2% mepivacaine with 1:100,000 adrenaline with 2% lidocaine with 1:100,000 adrenaline (b) in success rate of local anaesthesia.
3% mepivacaine versus 2% lidocaine with 1:50,000 adrenaline
Two studies compared 3% mepivacaine and 2% lidocaine with 1:50,000 adrenaline17., 19.. The outcome showed no significant difference between the two groups (OR 0.82, 95% CI 0.58–1.17, P = 0.28) (Figure 2b).
2% mepivacaine with 1:20,000 levonordefrin versus 2% lidocaine with 1:100,000 adrenaline
Two studies compared 2% mepivacaine with 1:20,000 levonordefrin and 2% lidocaine with 1:100,000 adrenaline18., 22.. There was no statistical significance between the two groups (OR 1.12, 95% CI 0.66–1.89, P = 0.69) (Figure 2c).
2% mepivacaine with 1:100,000 adrenaline versus 2% lidocaine with 1:100,000 adrenaline
Thirteen studies compared 2% mepivacaine with 1:100,000 adrenaline and 2% lidocaine with 1:100,000 adrenaline30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42.. Mepivacaine was significantly superior to lidocaine when both were combined with 1:100,000 adrenaline (OR 3.84, 95% CI 2.75–5.37, P < 0.00001) (Figure 2d). When anaesthetic techniques were considered, OR was 2.07 (95% CI 0.65–6.58, P = 0.22) for infiltrations21., 30., 33. and 4.26 (95% CI 0.46–39.54, P = 0.20) for block41. When jaws were considered, OR was 1.76 (95% CI 0.93–3.34, P = 0.08) for maxillary regions31., 33., 34., 37. and was 0.77 (95% CI 0.38–1.56, P = 0.47) for mandibular regions33., 38., 41..
The funnel plot
The funnel plot is also symmetrical and thus bias of publication was unlikely for the meta-analysis (Figure 3b). The strength of this outcome was also moderate.
Onset time of pulpal anaesthesia
In comparison with 2% lidocaine plus 1:100,000 adrenaline, 3% plain mepivacaine had a shorter onset time of pulpal anaesthesia while 2% mepivacaine with 1:20,000 levonordefrin or with 1:100,000 adrenaline both had similar time of onset. In comparison with 2% lidocaine with 1:50,000 adrenaline, 3% plain mepivacaine had shorter onset time of pulpal anaesthesia (Table 3).
Table 3.
Meta-analysis outcomes in onset time of pulpal anaesthesia
| Comparison | Number of studies included | Mean difference (95% CI) | P | Heterogeneity (I2) % |
|---|---|---|---|---|
| 3% Plain mepivacaine versus 2% lidocaine with 1:100,000 adrenaline | 317., 19., 21. | −1.13 (−1.77–0.49) | 0.0005 | 0 |
| 3% Plain mepivacaine versus 2% lidocaine with 1:50,000 adrenaline | 217., 19. | −0.83 (−1.40–0.26) | 0.004 | 0 |
| 2% Mepivacaine with 1:20,000 levonordefrin versus 2% lidocaine with 1:100,000 adrenaline | 122 | 0.20 (−2.87–3.27) | 0.90 | _ |
| 2% Mepivacaine with 1:100,000 adrenaline versus 2% lidocaine with 1:100,000 adrenaline | 230., 31. | −0.19 (−0.57–0.20) | 0.34 | 0 |
Pain ratings for injection phase
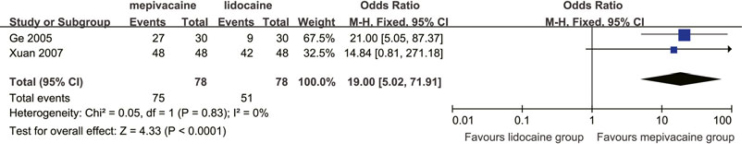
Five studies used this variable and divided the injection phase into three sections (i.e. needle insertion, needle placement and solution deposition)16., 19., 20., 23., 28.. The combined results showed that 3% mepivacaine was inferior to 2% lidocaine with 1:100,000 adrenaline in terms of the percentage of patients who felt no pain or mild pain during needle insertion and solution deposition, but there was no intergroup difference during needle placement (Table 4). In addition, there was no significant difference between 3% mepivacaine and 2% lidocaine with 1:50,000 adrenaline in terms of the percentage of patients who felt no pain or mild pain during solution deposition19. Another two studies38., 39. comparing 2% mepivacaine with 1:100,000 adrenaline with 2% lidocaine with 1:100,000 adrenaline were pooled and the outcome showed that the results in the mepivacaine group were superior to the lidocaine group in terms of the percentage of patients who felt no pain or mild pain during injection phase (Figure 4).
Table 4.
Meta-analysis outcomes comparing 3% mepivacaine with 2% lidocaine with 1:100,000 adrenaline in pain ratings of injection phase and postinjection phase
| Time | Number of studies included | Odds ratio (95% CI) | P | Heterogeneity (I2) % | |
|---|---|---|---|---|---|
| Injection phase | Needle insertion | 416., 20., 23., 28. | 0.50 (0.26 0.96) | 0.04 | 0 |
| Needle placement | 220., 28. | 1.20 (0.67 2.15) | 0.55 | 0 | |
| Solution deposition | 516., 19., 20., 23., 28. | 0.50 (0.29 0.87) | 0.01 | 0 | |
| Postinjection phase | Day 0 | 419., 20., 23., 28. | 1.00 (0.53 1.89) | 1.00 | 0 |
| Day 1 | 419., 20., 23., 28. | 0.78 (0.39 1.56) | 0.48 | 0 | |
| Day 2 | 419., 20., 23., 28. | 1.86 (0.61 5.73) | 0.28 | 0 | |
| Day 3 | 419., 20., 23., 28. | 1.00 (0.22 4.48) | 1.00 | 0 |
Figure 4.
Meta-analysis comparing 2% mepivacaine with 1:100,000 adrenaline with 2% lidocaine with 1:100,000 adrenaline in pain ratings during injection phase.
Pain ratings for postinjection phase
Pain on postinjection phase was recorded in day 0, day 1, day 2 and day 3 after injection in four studies19., 20., 23., 28.. The pooled results of these studies showed no intergroup difference in the percentage of patients who felt no pain or mild pain on day 0 to day 3 in those receiving 3% mepivacaine or 2% lidocaine with 1:100,000 adrenaline (Table 4). In addition, there was no statistically significant difference in the percentage of patients who felt no pain or mild pain during the postinjection phase with either 3% mepivacaine or 2% lidocaine with 1:50,000 adrenaline19.
Adverse events
Eight studies16., 20., 28., 29., 30., 31., 33., 40. reported adverse events, which were evenly distributed in both groups except for a significant increase in heart rate in those receiving 2% lidocaine with 1:100,000 adrenaline compared with those receiving 3% mepivacaine (OR 0.01, 95% CI, 0.00–0.09, P < 0.0001)20., 29. (Table 5).
Table 5.
Percentage, number and odds ratio of subjects who experienced adverse events
| Adverse events | Studies included | Percentage and number of events in mepivacaine group % | Percentage and number of events in lidocaine group % | Odds ratio (95% CI) | P |
|---|---|---|---|---|---|
| Diplopia | Forloine20 | 16 (8/50) | 12 (6/50) | 1.40 (0.45 4.37) | 0.57 |
| Increase in heart rate | Forloine20 | 0 (0/50) | 30 (15/50) | 0.01 (0.00 0.09) | <0.0001 |
| Replogle29 | 0 (0/42) | 67 (28/42) | |||
| Mandibular lip numbness | Forloine20 | 26 (13/50) | 32 (16/50) | 0.75 (0.31 1.78) | 0.51 |
| Incisive papilla swelling or soreness | Nusstein28 | 20 (8/40) | 28 (11/40) | 0.66 (0.23 1.86) | 0.43 |
| Temporary anaesthesia/paraesthesia of incisive papilla | Nusstein28 | 12 (5/40) | 18 (7/40) | 0.67 (0.19 2.33) | 0.53 |
| Thermal pulpal sensitivity | Nusstein28 | 2 (1/40) | 2 (1/40) | 1.00 (0.06 16.56) | 1.00 |
| Ulcerations | Nusstein28 | 5 (2/40) | 0 (0/40) | 5.26 (0.24 113.11) | 0.29 |
| Hyperaemia and soreness of injection site | Chen31 | 9 (1/11) | 0 (0/7) | 2.14 (0.08 60.17) | 0.65 |
| Dizzy and syncope | Shi30 | 2 (1/66) | 2 (1/61) | 0.48 (0.09 2.66) | 0.59 |
| Li33 | 0 (0/35) | 3 (1/35) | |||
| He40 | 0 (0/196) | 1 (1/196) | |||
| Herpes-like lesions | Schleder16 | 3 (1/31) | 10 (3/31) | 0.31 (0.03 3.17) | 0.32 |
| Haematoma of interproximal papillae | Schleder16 | 3 (1/31) | 0 (0/31) | 3.10 (0.12 79.04) | 0.49 |
DISCUSSION
When the results involving 1:100,000 adrenaline were combined in both groups, the success rate of mepivacaine increased dramatically and exceeded that of lidocaine (3.84 vs. 1). The presence of adrenaline in local anaesthetic solutions was confirmed to be beneficial with regard to duration, depth of anaesthesia, reduction of bleeding and systemic toxicity of the anesthetics43. Mepivacaine has milder vasodilating ability than lidocaine, which might explain why with the same vasoconstrictor, mepivacaine has dramatically superior effect to that of lidocaine. This advantage leads to broad application of mepivacaine as a local anaesthetic in dentistry in China, while it has seldom been used in Western dentistry up to now.
In addition, the milder vasodilating ability of mepivacaine does facilitate the usage of higher concentrations of plain mepivacaine. Although the success rate of 3% plain mepivacaine was nearly 30% lower in comparison with lidocaine with 1:100,000 adrenaline, the depth of local anaesthesia could satisfy the demand for pain control in those with cardiovascular diseases. Although the meta-analysis shows that 3% mepivacaine had a similar success rate to that of 2% lidocaine with 1:50,000 adrenaline, this was mainly because of data insufficiency.
Mepivacaine with 1:20,000 levonordefrin had the same level of success rate in comparison with 2% lidocaine with 1:100,000 adrenaline. In Western clinical practice, levonordefrin is commonly used as a vasoconstrictor with mepivacaine in a concentration of 1:20,000 in local anaesthesia10., 18.. It is believed from molecular structures and clinical observations that adrenaline at 1/100,000 had similar effect on local anaesthesia as did levonordefrin at 1/20,00010. Nevertheless, a conclusion derived from this review was that mepivacaine with levonordefrin and lidocaine with adrenaline showed the same level of effect. This might be ascribed to a chance effect because only two studies were included. In terms of safety, Guilielmo et al44 demonstrated no significant difference in increasing heart rate between 2% mepivacaine with 1:20,000 levonordefrin and 2% lidocaine with 1:100,000 adrenaline after intra-osseous injection of 1.8 ml in both groups. That is, 1:20,000 levonordefrin may have similar efficacy and safety as 1:100,000 adrenaline.
What should be mentioned is the difference in the methods of assessment of the success of anaesthesia used in the trials. Because the anaesthesia of soft tissues is not a stable predictor for profound pulpal anaesthesia and painless operations45, we focused on trials in which the presence or absence of pulpal anaesthesia was evaluated by using EPT, VAS and patients‘ feelings and expressions. In terms of teeth with vital pulp, anaesthetic success is often defined as the percentage of participants who achieve two consecutive EPT readings of 80 within 15 minutes after administration of anaesthetic and sustain this lack of responsiveness continuously for 60 minutes. However, as for symptomatic teeth, a lack of response to EPT may not guarantee that a tooth is experiencing profound pulpal anaesthesia, mainly because of the complex mechanism of neuroinflammatory and neuropulpal interactions, which still need to be clarified46. Therefore, VAS and the patients‘ intraoperative feelings and expressions are used to evaluate the success of an anaesthetic.
The 3% mepivacaine had shorter onset time in pulpal anaesthesia than 2% lidocaine with epinephrine, which was in agreement with other reports47. The time of onset of anaesthesia is directly related to the rate of epineural diffusion correlated with the percentage of drug in the base form, which is proportional to the pKa of that agent. The 3% plain mepivacaine had pKa of 7.6, while lidocaine with epinephrine had pKa of 7.910.
Evidence in this review shows that in the injection phase and postinjection phase, the 3% mepivacaine groups suffered more pain than the 2% lidocaine with 1:100,000 epinephrine group during needle insertion and solution deposition, but there was no significant difference during needle placement or from day 0 to day 3 postinjection. During the injection, pain could result from mechanical trauma of needle during insertion, or from the sudden distension of the tissues caused by a rapid insertion of the solution and the chemical stimulation of the first few drops of the local anesthetics48., 49.. The lipid solubility of lidocaine is four times as high as mepivacaine10. That is, lidocaine, with slightly higher lipid solubility, is regarded as an effective topical anaesthetic and used widely in various operations in dentistry10. Therefore, lidocaine can be infiltrated to regional mucosa more easily than mepivacaine50. During the injection phase, the first few drops were penetrated more rapidly when lidocaine was used, and the regional tissue was anesthetised more rapidly in the lidocaine group while the sudden stress of regional space was lower. This might explain why the lidocaine group experienced less pain than the mepivacaine group during injection. In clinical practice some improvements could be made to relieve pain during the injection of mepivacaine, such as use of a smaller syringe needle, slower injection velocity, use of binding cartridges upon injection of mepivacaine or the use of the topical anaesthesia before injection. Furthermore, computer-controlled local anaesthetic delivery (C-CLAD), which has a slow and stable rate of delivering agents, might be used for ensuring less pain and more comfort in local anaesthesia in dentistry10. However, the 2% mepivacaine with 1:100,000 epinephrine groups suffered from less pain than the 2% lidocaine with 1:100,000 epinephrine groups during the injection phase, probably because of the presence of adrenaline in the mepivacaine, which is proved to have the functions of pain control and increasing depth of anaesthesia10., 43..
In terms of safety, results from two studies20., 29. indicated that 3% mepivacaine was superior to 2% lidocaine with 1:100,000 epinephrine in inhibiting an increase in heart rate. This can be explained by the fact that adrenaline as a vasoconstrictor in lidocaine can stimulate the cardiac and central nervous systems and may increase heart rate especially when it is used excessively, which may be related to the β-activity of adrenaline10. The anaesthetic techniques used in the two studies were maxillary nerve block and intraosseous injection, respectively. In addition, 3% mepivacaine was also superior to 2% lidocaine with 1:100,000 epinephrine when the two anaesthetic techniques were considered separately. The maximum adrenaline dose used in local anaesthesia of dentistry is recommended to be 0.2 mg per appointment for a normal healthy patient (American Society of Anesthesiologists Physical Status classification system 1: ASA 1), or 0.04 mg per appointment for patients with clinically significant cardiovascular disease (ASA 3 or 4).10 With increased levels of adrenaline in the blood, cardiac dysrhythmias become more common. There was no evidence showing any significant difference in other adverse events between the two solutions. In conclusion, 3% plain mepivacaine was safer than 2% lidocaine with 1:100,000 epinephrine.
Although the studies included had low to moderate risk of bias, there was still some bias in the reviewing process. The number of studies included in some meta-analyses was small, which might lead to bias of outcome. Parallel trials and crossover trials were mixed in meta-analysis because of imperfect data reporting in the studies, which might cause deviation in the outcomes. The diversity of the studies, small sample size and unexplained statistical heterogeneity limit the overall conclusions, which call for future studies to obtain more stable outcomes.
We hope that more higher-quality studies comparing 2% mepivacaine and epinephrine with 2% lidocaine and epinephrine, 2% mepivacaine and levonordefrin with 2% lidocaine and 1:100,000 epinephrine), and 3% mepivacaine with 2% lidocaine and 1:50,000 epinephrine can be conducted to further evaluate the efficacy and pain control during injection phase and postinjection phase between mepivacaine and lidocaine. In addition more randomised controlled trials could be done to evaluate any adverse events.
In summary the clinical evidence tells us that:
-
•
2% mepivacaine with 1:100,000 adrenaline is superior in increasing the success rate of local anaesthesia and pain control during the injection phase and has similar onset time of pulpal anaesthesia and safety in comparison with 2% lidocaine with 1:100,000 epinephrine
-
•
2% mepivacaine with 1:20,000 levonordefrin has the same level of success and a similar onset time of pulpal anaesthesia compared with 2% lidocaine with 1:100,000 epinephrine
-
•
3% mepivacaine has shorter onset time of pulpal anaesthesia and greater safety, but is inferior in increasing success rate and pain control during injection, in comparison with 2% lidocaine with 1:100,000 epinephrine, especially for patients with cardiovascular diseases
-
•
3% mepivacaine has the same level of success, similar pain control during injection and postinjection phases and shorter onset time of pulpal anaesthesia, compared with 2% lidocaine with 1:50,000 epinephrine.
In conclusion, given the efficacy and safety of the two solutions, 2% mepivacaine with vasoconstrictors is better than 2% lidocaine with epinephrine in dental treatment, and 3% plain mepivacaine is better for patients with cardiac diseases. However, more studies are still needed to make a definitive conclusion.
Conflicts of interest
None declared.
REFERENCES
- 1.Hargreaves KM, Cohen S, Berman LH. 10th ed. Mosby; Saint Louis: 2011. Pathways of the Pulp; pp. 691–705. [Google Scholar]
- 2.Peretz B, Moshonov J. Dental anxiety among patients undergoing endodontic treatment. J Endod. 1998;24:435–437. doi: 10.1016/S0099-2399(98)80028-9. [DOI] [PubMed] [Google Scholar]
- 3.Claffey E, Reader A, Nusstein J, et al. Anesthetic efficacy of articaine for inferior alveolar nerve blocks in patient with irreversible pulpitis. J Endod. 2004;30:568–571. doi: 10.1097/01.don.0000125317.21892.8f. [DOI] [PubMed] [Google Scholar]
- 4.Berge TM, Veerkamp JS, Hoogstraten J, et al. Childhood dental fear in the Netherlands: prevalence and normative data. Community Dent Oral Epidemiol. 2002;30:101–107. doi: 10.1034/j.1600-0528.2002.300203.x. [DOI] [PubMed] [Google Scholar]
- 5.Abdulwahab M, Boynes S, Moore P, et al. The efficacy of six local anesthetic formulations used for posterior mandibular buccal infiltration anesthesia. JADA. 2009;140:1018–1024. doi: 10.14219/jada.archive.2009.0313. [DOI] [PubMed] [Google Scholar]
- 6.Poorni S, Veniashok B, Senthikumar AD. Anesthetic efficacy of four percent articaine for pulpal anesthesia by using inferior alveolar nerve block and buccal infiltration techniques in patients with irreversible pulpitis: a prospective randomized double-blind clinical trial. J Endod. 2011;37:1603–1607. doi: 10.1016/j.joen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Bromage PR. Pharmacologic basis for use of local anesthetics. Physiol Pharmacol Physicians. 1966;1:1–4. [PubMed] [Google Scholar]
- 8.Sadove MS, Vernino D, Lock F, et al. An evaluation of mepivacaine hydrochloride. J Oral Surg Anesth Hosp Dent Serv. 1962;20:399–404. [PubMed] [Google Scholar]
- 9.Gaffen AS, Haas DA. Survey of local anesthetic use by Ontario dentists. J Can Dent Assoc. 2009;75:649. [PubMed] [Google Scholar]
- 10.Malamed SF. 6th ed. Mosby; Saint Louis: 2012. Handbook of Local Anesthesia; pp. 1–122. [Google Scholar]
- 11.Higgins JPT, Green S. In: Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 version. Higgins JPT, Altman DG, editors. John Wiley& Sons; Chichester: 2011. Assessing risk of bias in included studies; pp. 182–234. [Google Scholar]
- 12.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JAC, Egger M, Moher D. In: Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 version. Higgins JPT, Green S, editors. John Wiley& Sons; Chichester: 2011. Addressing reporting biases; pp. 279–311. [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. In: Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 version. Higgins JPT, Deeks JJ, Altman DG, editors. John Wiley& Sons; Chichester: 2011. Special topics in statistics; pp. 429–467. [Google Scholar]
- 16.Schleder JR, Reader A, Beck M, et al. The periodontal ligament injection: a comparison of 2% lidocaine, 3% mepivacaine, and 1:100,000 epinephrine to 2% lidocaine with 1:100,000 epinephrine in human mandibular premolars. J Endod. 1988;14:397–404. doi: 10.1016/S0099-2399(88)80124-9. [DOI] [PubMed] [Google Scholar]
- 17.Mason R, Drum M, Reader A, et al. A prospective randomized, double-blind comparison of 2% lidocaine with 1:100,000 and 1:50,000 epinephrine and 3% mepivacaine for maxillary infiltrations. J Endod. 2009;35:1173–1177. doi: 10.1016/j.joen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Lawaty I, Drum M, Reader A, et al. A prospective, randomized, double-blind comparison of 2% mepivacaine with 1:20,000 levonordefrin versus 2% lidocaine with 1:100,000 epinephrine for maxillary infiltrations. Anesth Prog. 2010;57:139–144. doi: 10.2344/0003-3006-57.4.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berberich G, Reader A, Drum M, et al. A prospective, randomized, double-blind comparison of the anesthetic efficacy of two percent lidocaine with 1:100,000 and 1:50,000 epinephrine and three percent mepivacaine in the intraoral, infraorbital nerve block. J Endod. 2009;35:1498–1504. doi: 10.1016/j.joen.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Forloine A, Drum M, Reader A, et al. A prospective, randomized, double-blind comparison of the anesthetic efficacy of two percent lidocaine with 1:100,000 epinephrine and three percent mepivacaine in the maxillary high tuberosity second division nerve block. J Endod. 2010;36:1770–1777. doi: 10.1016/j.joen.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 21.McLean C, Reader A, Beck M, et al. An evaluation of 4% prilocaine and 3% mepivacaine compared with 2% lidocaine (1:100,000 epinephrine) for inferior alveolar nerve block. J Endod. 1993;19:146–150. doi: 10.1016/s0099-2399(06)80510-8. [DOI] [PubMed] [Google Scholar]
- 22.Hinkley SA, Reader A, Beck M. An evaluation of 4% prilocaine with 1:200,000 epinephrine and 2% mepivacaine with 1:20,000 levonordefrin compared with 2% lidocaine with 1:100,000 epinephrine for inferior alveolar nerve block. Anesth Prog. 1991;38:84–89. [PMC free article] [PubMed] [Google Scholar]
- 23.Replogle K, Reader A, Nist R, et al. Anesthetic efficacy of the intraosseous injection of 2% lidocaine (1:100,000 epinephrine) and 3% mepivacaine in mandibular first molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:30–37. doi: 10.1016/s1079-2104(97)90087-1. [DOI] [PubMed] [Google Scholar]
- 24.Burns Y, Reader A, Nusstein J, et al. Anesthetic efficacy of the palatal-anterior superior alveolar injection. JADA. 2004;135:1269–1276. doi: 10.14219/jada.archive.2004.0402. [DOI] [PubMed] [Google Scholar]
- 25.Bradley DJ, Martin ND. Clinical evaluation of mepivacaine and lidocaine. Australian Dent J. 1969;14:377–381. doi: 10.1111/j.1834-7819.1969.tb02291.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen HP, Cha BY, Spangberg LSW. Endodontic Anesthesia in mandibular molars: a clinical study. J Endod. 1993;19:370–373. doi: 10.1016/S0099-2399(06)81366-X. [DOI] [PubMed] [Google Scholar]
- 27.Kramp LF, Eleazer PD, Scheetz JP. Evaluation of prilocaine for the reduction of pain associated with transmucosal anesthetic administration. Anesth Prog. 1999;46:52–55. [PMC free article] [PubMed] [Google Scholar]
- 28.Nusstein J, Burns Y, Reader A, et al. Injection pain and postinjection pain of the palatal-anterior superior alveolar injection, administered with the Wand plus system, comparing 2% lidocaine with 1:100,000 epinephrine to 3% mepivacaine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:164–172. doi: 10.1016/j.tripleo.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Replogle K, Reader A, Nist R, et al. Cardiovascular effects of intraosseous injections of 2 percent lidocaine with 1:100,000 epinephrine and 3 percent mepivacaine. JADA. 1999;130:649–657. doi: 10.14219/jada.archive.1999.0274. [DOI] [PubMed] [Google Scholar]
- 30.Shi ZD, Wang XY, Cheng ZY, et al. Multi-center randomized double-blinded controlled clinical trial on local anesthetic efficacy of scandonest 2% special and its safety. Chin J Evid-based Med. 2002;2:86–91. [Google Scholar]
- 31.Chen XM, Shi ZD, Huang DM, et al. Anesthetic efficacy of 2% mepivacaine in conservative dentistry. West China J Stomatol. 2004;22:390–392. [PubMed] [Google Scholar]
- 32.Wu YN, Yang SQ, Zhu QP, et al. Effects of three local anesthesia agents during procedures of pulpectomy. Stomatology. 2005;25:291–292. [Google Scholar]
- 33.Li SY, Liu Y, Liu RS. Clinical evaluation of the anesthetic effect of mepivacaine hydrochloride and adrenaline injection during pulpal treatment in the elderly. Chin J Geriatr Dent. 2005;3:78–80. [Google Scholar]
- 34.Dong HD, Liu Q. The pulpal anesthetic efficacy of the scandonest on maxillary pulpitis molars. J Pract Stomatol. 2010;26:609–611. [Google Scholar]
- 35.Liu MZ. Application of mepivacaine hydrochloride injection in tooth extraction and endodontic treatment. Chin J Nurse Educ. 2010;7:378–379. [Google Scholar]
- 36.Xing XY, Xing WB, Zhang QS. Clinical evaluation of the effect of scandonest (mepivacaine hydrochloride) used in dentistry. J Tianjin Med Univ. 2005;11:243–245. [Google Scholar]
- 37.Luo LC, Li H. Comparison of anesthetic effect between scandonest and lidocaine in the extraction of the upper molar. Hainan Med J. 2009;20:204–205. [Google Scholar]
- 38.Ge XJ, Li X, Wang XY, et al. Comparison between scandonest and lidocaine in the endodontic treatment of the mandibular posterior teeth. Chin Remedies Clin. 2005;15:956–957. [Google Scholar]
- 39.Xuan GJ. Application of scandonest in teeth preparation of vital pulp. J Clin Stomatol. 2007;123:190–191. [Google Scholar]
- 40.He P, Wang JR. Application of scandonest in endodontic treatment. Stomatology. 2010;30:511–512. [Google Scholar]
- 41.Zhou Q. Observation of mepivacaine hydrochloride with epinephrine solutions in inferior alveolar nerve block anesthesia. Mod Pract Med. 2011;23:1257–1259. [Google Scholar]
- 42.Jin XD, Wang GL. Efficacy of mepivacaine hydrochloride with epinephrine in endodontic treatment. Guangdong Med J. 2005;26:694–695. [Google Scholar]
- 43.Knoll-Kohler E, Fortsch G. Pulpal anesthesia dependent on epinephrine dose in 2% lidocaine. A randomized controlled double-blind crossover study. Oral Surg Oral Med Oral Pathol. 1992;73:537–540. doi: 10.1016/0030-4220(92)90091-4. [DOI] [PubMed] [Google Scholar]
- 44.Guglielmo A, Reader A, Nist R, et al. Anesthetic efficacy and heart rate effects of the supplemental intraosseous injection of 2% mepivacaine with 1:20.000 levonordefrin. Oral Surg Oral Med Oral Pathol Radiol Endod. 1999;87:284–293. doi: 10.1016/s1079-2104(99)70210-6. [DOI] [PubMed] [Google Scholar]
- 45.Hannan L, Reader A, Nist R, et al. The use of ultrasound for guiding needle placement for inferior alveolar nerve blocks. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:658–665. doi: 10.1016/s1079-2104(99)70156-3. [DOI] [PubMed] [Google Scholar]
- 46.Hargreaves KM, Goodis HE, Seltzer S. 1st ed. Quintessence Pub Co Inc; Chicago: 2002. Seltzer and Bender’s Dental Pulp; pp. 1–106. [Google Scholar]
- 47.Cox B, Durieux ME, Marcus MA. Toxicity of local anesthetics. Best Pract Res Clin Anaesthesiol. 2003;17:111–136. doi: 10.1053/bean.2003.0275. [DOI] [PubMed] [Google Scholar]
- 48.Ballard BE. Biopharmaceutical considerations in subcutaneous and intramuscular drug administration. J Pharm Sci. 1968;57:357–378. doi: 10.1002/jps.2600570301. [DOI] [PubMed] [Google Scholar]
- 49.Keller BJ. Comparison of the effectiveness of two topical anesthetics and a placebo in reducing injection pain. Hawaii Dent J. 1985;16:10–11. [PubMed] [Google Scholar]
- 50.Qiu WL, Zhang ZK, Zhang ZY. In: Oral and Maxillofacial Surgery. 6th ed. Zhao YF, editor. People’s Medical Publishing House; Beijing: 2010. Anesthesia, analgesia and intensive care of oral and maxillofacial surgery; pp. 19–49. [Google Scholar]