Abstract
Mitral valve prolapse (MVP) is a common condition present in 1–3% of the population. There has been evidence that a subset of MVP patients is at higher risk of sudden cardiac death. The arrhythmogenic mechanism is related to fibrotic changes in the papillary muscles caused by the prolapsing valve. ECG features include ST-segment depression, T wave inversion or biphasic T waves in inferior leads, and premature ventricular contractions arising from the papillary muscles and the fascicular system. Echocardiography can identify MVP and mitral annular disjunction, a feature that has significant negative prognostic value in MVP. Cardiac MRI is indicated for identifying fibrosis. Patients with high-risk features should be referred for further evaluation. Catheter ablation and mitral valve repair might reduce the risk of malignant arrhythmia. MVP patients with high-risk features and clinically documented ventricular arrhythmia may also be considered for an ICD.
Keywords: Arrhythmia, mitral valve prolapse, sudden cardiac death, mitral annular disjunction, premature ventricular contractions, papillary muscle fibrosis, echocardiography
Definitions
Mitral valve prolapse (MVP) has been conventionally defined as a superior displacement of the mitral leaflets of more than 2 mm above the annular plane in the long axis view into the left atrium during systole. This can affect one or both leaflets and can be due to disruption or elongation of the leaflets, chordae and papillary muscles. It is also characterised by fibromyxomatous degeneration of the leaflets with a maximal thickness of more than 5 mm during diastole (Barlow's valve). However, MVP without mitral valve thickening (<5 mm) has also been recognised.[1] A prodromal morphology of a familial form of MVP has also been described, characterised by an elongated posterior leaflet and an anteriorly displaced coaptation zone (towards the aorta) with no displacement of the leaflets in the left atrium.[2] This morphology shares, either completely or partially, the haplotype of complete MVP, thereby suggesting that it might be either a precursor or the result of incomplete penetrance of the MVP phenotype.[3]
Epidemiology
MVP is a common valve condition in developed countries, affecting 1–3% of the population.[1,4] MVP may lead to significant mitral regurgitation (MR), left ventricular dysfunction, infective endocarditis, thromboembolism and arrhythmias, such as AF and ventricular arrhythmias, even leading to sudden cardiac death (SCD).[5] Patients without significant MR have been shown to enjoy excellent survival rates.[6] However, there is a subset of patients with MVP that may incur a higher risk of ventricular tachycardia (VT) and mortality, regardless of the presence of MR.[7] In this study however, patients with coronary artery disease and severe MR were not excluded, which might confound VT and mortality outcomes.[8,9] The true incidence of SCD due to MVP varies depending on the methods used to evaluate the event (autopsy versus survivors) and the available clinical data (ECG, echocardiogram, cardiac MRI [CMR]) and therefore may be underestimated. In autopsy-based studies there is a wide variability in the incidence of MVP-related SCD because of the uncertain causative relationship between the finding of MVP at autopsy and arrhythmic SCD.[10] In a series of 339 autopsy-defined sudden arrhythmic deaths, MVP was identified in seven, while six more were identified by reviewing echocardiograms, indicating that MVP-related SCD may be underestimated even in post-mortem studies.[11]
Pathophysiological Mechanism
On the microscopic level, MVP is characterised by marked proliferation of the spongiosa, a glycosaminoglycan and proteoglycan-rich middle layer contained within a spongy elastin network. This causes interruption of the fibrosa, the underlying, collagen-rich lower layer located towards the ventricular side of the valve. Secondary effects include fibrotic changes on the mitral valve leaflets, thinning and elongation of the chordae tendinae, which is also caused by accumulation of glycosaminoglycans and ventricular friction lesions.[12] There is some evidence the MVP itself may be the cause of the papillary and peripapillary fibrotic changes.[13] The pathological systolic shortening and deformation may exert excessive traction on the papillary muscle, therefore inducing fibrotic change.[14,15] These fibrotic changes, friction lesions and myocardial stretching may give rise to ventricular arrhythmias either through re-entry or triggered activity.[16] Furthermore, there is evidence of increased sympathetic activity, coupled with decreased vagal activity and elevated catecholamine levels in MVP patients with a high ventricular arrhythmic load.[17] This autonomic dysfunction not only increases the frequency of ectopic activity but also predisposes the ventricular myocardium to ectopic activity.
To summarise, the pathogenesis of arrhythmia in MVP is best explained when we consider the myocardial hypertrophy and fibrosis as the substrate with the mechanical stretch being the trigger of the arrhythmia.[18]
Clinical and ECG Features of Mitral Valve Prolapse
MVP-related VT seems to occur more commonly in women – being as high as 70–90% in some studies.[19] There is no clear reason for this discrepancy and is probably multifactorial. A majority of patients with MVP-related SCD demonstrate T wave inversions, ST segment depressions, or biphasic T waves on the inferior leads (II, III, aVF) on ECG.[7,20] However, this finding alone is not specific enough to classify a patient's risk by itself. Premature ventricular contractions (PVC) are another common finding in MVP patients. Although MR patients exhibit more PVCs compared with non-MR patients, even non-MR patients exhibit pleomorphic PVCs more commonly than the general population.[7,21] These PVCs seem to arise from the papillary muscle, the fascicular system and the outflow tract, areas which are often found to be under mechanical stress and undergoing fibrosis in MVP (Figure 1 and Supplementary Material Figure 1).[22]
Figure 1: 12-Lead ECG with Premature Ventricular Contractions Arising Either from the Mitral Annulus or from the Posterior Papillary Muscle.
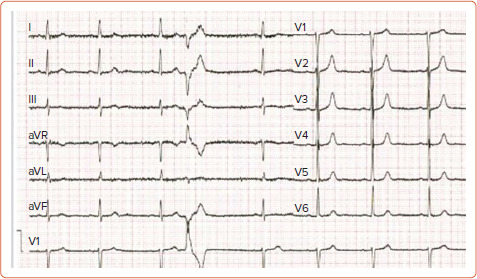
In a retrospective study that involved 25 MVP patients who underwent catheter ablation of PVCs, 27 PVCs originating from the papillary muscles were identified and they all demonstrated a right bundle branch block pattern. Of these, 17 arose from the posteromedial papillary muscle and demonstrated a superior axis with negative forces in leads II and III, while 10 originated from the anterolateral papillary muscle and demonstrated an inferiorly directed axis (n=5) or inferior lead discordance (n=5), with negative QRS in lead II and positive QRS in lead III. Furthermore, four patients presented with PVC-associated VT and a fifth patient, who died during follow-up, presented with PVC-mediated cardiomyopathy. ST-T wave changes were present in 15 patients, affecting the inferior leads in five patients, the lateral leads in four and both in six patients. In a series of 14 patients with MVP and ventricular ectopy, at least one of the ectopic foci originated from the papillary muscle or the Purkynje system and ablation at these foci resulted in symptom relief and a reduction in defibrillator shocks in 12 of these patients.[23] A cross-sectional study that examined 100 MVP patients and 100 healthy subjects detected early repolarisation phenomena (a notch in the descending arm of the QRS complex and ST elevation) in 74% of the patients (51% in inferior leads, 23% in I, aVL, and 8% in all three leads) while these phenomena were only observed in six subjects in the control group.[24]
Echocardiography
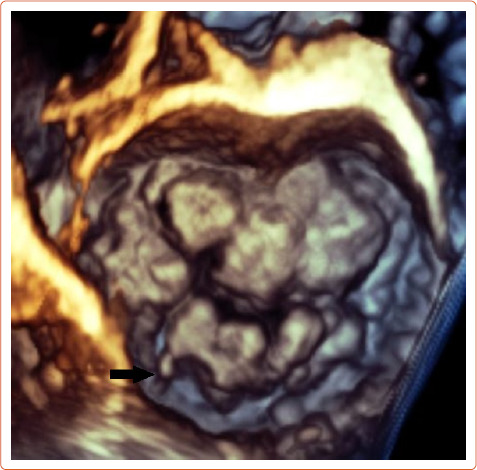
Echocardiography can easily recognise MVP in the parasternal long axis view and is defined as a superior displacement of the mitral leaflets of more than 2 mm into the left atrium during systole. Also leaflet length, redundancy and thickness are evaluated during diastole, and 3D echocardiography can also estimate the extent of the prolapsed segments (Figure 2).
Figure 2: Bileaflet Mitral Valve Prolapse with Flail (Black Arrow) Posterior Lateral Scallop Imaged with 3D Echocardiography.
The literature suggests that mitral annular disjunction (MAD) is a significant negative prognostic factor in MVP, characterised by a detachment of the ‘roots’ of the annulus from the ventricular myocardium. The posterior leaflet under the P1 and P2 segments of the valve is most commonly affected because the anterior leaflet is supported by the dense fibrous trigones of the cardiac skeleton. A frame-by-frame analysis of a 2D echocardiography in the parasternal long axis view usually confirms the diagnosis (Figure 3 and Supplementary Material Figures 2 and 3). MAD can also be recognised in the apical four-chamber view (Figure 4) and 3D echocardiography has shown that the disjunctive annulus exhibits paradoxical expansion and flattening during systole. MAD is not always present in patients who have MVP and it can be found in patients without MVP. In a Norwegian study which enrolled 116 patients with MAD, 34% of them presented with severe VT.[25] This study supported that papillary muscle fibrosis may be linked to severe arrhythmic events and showed an association among PVCs, arrhythmic events and MAD independently of the presence of MVP, indicating that MAD itself may be an arrhythmogenic entity. However, more studies are needed to prove and better define this.
Figure 3: Mitral Annular Disjunction Characterised by the Detachment of the Roots of the Posterior Part of the Mitral Annulus Under P1 and P2 Segments.
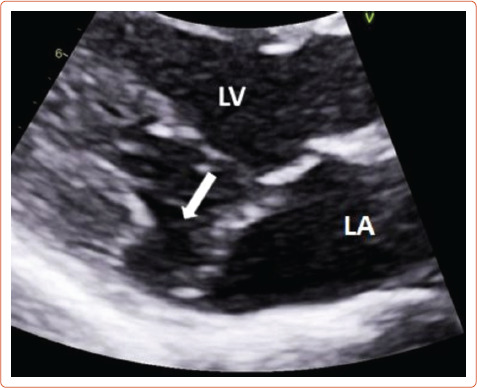
The white arrow shows detachment of the ‘roots’ of the annulus from ventricular myocardium at the parasternal long axis view transthoracic echocardiography. LA = left atrium; LV = left ventricle.
Figure 4: Presence of Mitral Annular Disjunction at the Four-chamber View on Transthoracic Echocardiography.
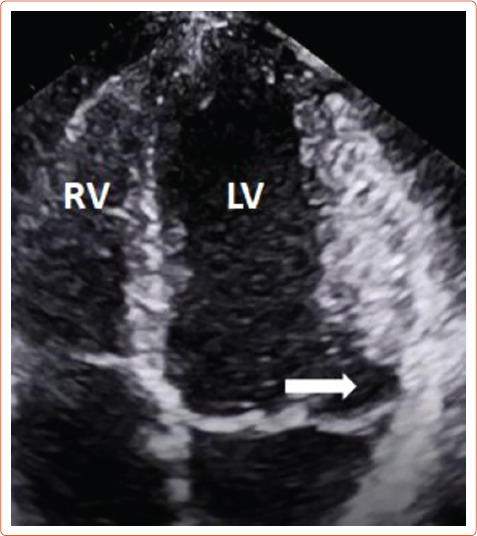
The white arrow shows detachment of the ‘roots’ of the annulus from ventricular myocardium. LV = left ventricle; RV = right ventricle.
MR frequently develops in patients with MVP. It is quantified by echocardiography based on the recommendations for the non-invasive evaluation of MR more frequently uses the proximal isovelocity surface area and vena contracta method. One study showed that severe MR is an independent predictor of ventricular arrhythmias.[26] However, in a recent systematic review of MVP and SCD most of the patients experienced SCD in the setting of non-severe MR. Even patients with mild or moderate MR are at risk of SCD.[27]
Finally, in a study of 21 patients with bileaflet MVP and MR, patients at a high risk (67% versus 22%; p<0.08) to develop a ventricular arrhythmia were identified by increased peak systolic lateral velocity of the lateral mitral annulus >1.6 m/s.[28] This so called Pickelhaube sign was not present in the low-risk group. However larger studies are required to confirm the validity of this sign.
Cardiac MRI
After identifying fibrotic changes at post-mortem, Basso et al. theorised that these changes can be identified in vivo by means of CMR. CMR is the ideal diagnostic test to identify specific arrhythmogenic fibrotic regions and also provide evidence of MAD (Figure 5).[29] In a series of 43 cases of SCD in young patients with MVP papillary muscle fibrosis (88%) or inferobasal fibrosis (93%) was identified and late gadolinium enhancement (LGE) distribution on CMR correlated with histopathological findings.[20] In another series of 62 cases, fibrosis of at least one papillary muscle or the adjacent left ventricular (LV) wall was identified.[30] In a study comparing the prevalence of LV fibrosis in MR patients with and without MVP, higher prevalence was identified in the MVP group (36.7% versus 6.7%; p<0.001).[31] The fibrotic pattern was most commonly found in the basal inferolateral (31.1%) and basal inferior (10.7%) wall. During follow-up, an arrhythmic event occurred in 4.5% (n=8) of MVP patients, with the highest event rate being recorded in MVP patients with replacement fibrosis. This study also suggests that MVP may promote fibrotic changes beyond those that follow the MR-associated volume overload.
Figure 5: Cardiac MRI with Evidence of Mitral Annular Disjunction (Yellow Line).
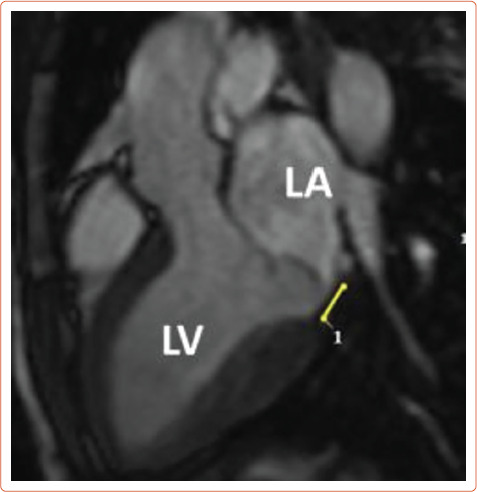
LA = left atrium; LV = left ventricle.
A forensic study of 17 MVP-SCD patients and 17 matched controls, showed that LV fibrosis was significantly higher in the posterior and lateral walls compared with the anterior wall and interventricular septum with a significant endocardial-to-epicardial fibrotic gradient, while the right ventricular fibrotic changes were comparable between the two groups, thereby suggesting that both localised and generalised fibrosis contributes to the pathogenesis of SCD.[32] However, the posteromedial and anterolateral papillary muscles, the inferobasal portion as well as leaflet thickness and annular disjunction were not evaluated, as these are areas of interest relating to localised fibrotic change. Also, inability to assess atrial enlargement does not exclude the suspicion of subclinical MR, especially in the context of increased cardiac mass.[33]
A prospective study of 400 MVP patients detected replacement myocardial fibrosis by LGE CMR in 110 patients, most commonly localised in the basal inferolateral wall and the papillary muscle. Fibrosis was detected in mild MR (13%), 28% in moderate and 37% in severe MR and was associated with a more dilated LV and more frequent ventricular arrhythmias (VAs) (45% versus 26%, p<0.0001).[34] In the subgroup of patients with mild MR, abnormal LV dilatation in the absence of volume overload was detected in 16% of patients, furthering the evidence of MVP-related fibrosis, and VAs in 25%. Replacement fibrosis was associated with worse 4-year cardiovascular event-free survival.
Another retrospective study of 41 patients with MVP indicates that even the presence of diffuse subclinical ventricular fibrosis was associated with a higher ventricular arrhythmic load and might be a precursor of focal papillary fibrosis.[35]
Finally, severe curling, defined as an unusual systolic motion of the posterior mitral ring >3.5 mm, has been associated with myocardial fibrosis and has been identified with LGE in a study of 54 MVP patients.[36] A 3.5 mm cut-off was the median in this study.
Risk Stratification
Risk stratification of MVP is not clear despite the plethora of risk factors associated with it because there is still no strong predictor of malignant arrhythmias. Additionally, it is not clear which combination of risk factors incurs the highest risk. Another challenge is that people with high risk exist among a much larger population who are traditionally perceived as low-risk patients. However, identification of features, such as complex ventricular ectopy, echocardiographic features of leaflet redundancy or MAD and repolarisation abnormalities on ECG should lead to further evaluation with a 24-hours, 7-day rhythm Holter monitoring of the VA load and also CMR evaluation of the LV regardless of the LV ejection fraction, the degree of MR and the LV end systolic diameter. Bileaflet MVP and leaflet thickening have been considered as high-risk features of SCD but prognostic significance of these features is unclear.[20,22,37] While it seems that there is a 42% prevalence of bileaflet MVP in young idiopathic out-of-hospital cardiac arrest survivors, bileaflet MVP without any additional risk factors did not significantly increase the risk of SCD or defibrillator implantation.[22,37]
The literature suggests that SCD more commonly occurs in younger women with MVP, however this has not been verified in all studies.[38,7] In patients with MVP and syncope it is imperative to determine the nature of the syncope. Arrhythmic syncope is suspected if it occurs either during strenuous exercise or during rest without any previous warning symptoms. For patients with MVP, suspected arrhythmic syncope and evidence of scarring, it is acceptable to consider an electrophysiology study. If it is unremarkable or not feasible, implantation of a loop recorder may be considered dependent on the amount of high-risk features present.[39] However, it should be mentioned that programmed electrical ventricular stimulation as a means to stratify the VA risk is of limited significance as the induction of polymorphic VT is a non-specific finding.[40] Patients with a truly positive study should undergo implantation of an ICD.
The American Heart Association and the European Society of Cardiology guidelines for VA and SCD prevention have no specific recommendation for MVP risk stratification.[5,41] The management of low-risk MVP patients with polymorphic VA consists of lifestyle modifications such as avoiding stimulants such as caffeine, tobacco, alcohol and recreational drugs, and the agents of choice are β-blockers in both symptomatic and asymptomatic, non-sustained or sustained VA. An ICD would be indicated for the secondary prevention of out-of-hospital cardiac arrest. There are no validated scores to help stratify risk and decisions should be shared with the patient after a frank, transparent and honest discussion. Role of family history of MVP, MAD and SCD can also help identify risk, but specificity is unknown. Family members of SCD patients who are identified as having MVP should undergo evaluation, but there is a lack of evidence to support this.
Catheter Ablation
Catheter ablation of scar-related malignant arrhythmias has been reported as an effective treatment.[23,42] PVC triggers, which are often located on the papillary muscles, can be mapped and ablation can be performed successfully, which also leads to a reduction in the need for ICD therapies.[23] Papillary muscle ablation can be technically demanding because of catheter instability, intramural origin and the need to ablate at the base of the papillary muscle.[43] However, in a study of 15 MVP patients that underwent ablation of high-burden PVCs, ventricular bigeminy, non-sustained or sustained VT, five patients developed haemodynamically significant VT or VF and underwent ICD implantation over 9 years of follow-up. In three of these five patients there was also appropriate ICD therapy.[44] This late recurrence can arise from arrhythmic foci not present at the time of ablation, highlighting the need for long-term follow-up of these patients.
Mitral Valve Repair and Exercise Recommendations
Mitral valve repair should reverse the deformed structure and relieve the papillary muscles, promoting positive remodeling and reducing the arrhythmic load. There is evidence that mitral valve surgery can be beneficial in younger patients (<42 years).[45–49] However, data are limited and surgery may not effectively reduce the rate of malignant arrhythmias.[50]
Due to the benign clinical course of the condition in most people, asymptomatic patients with mild or moderate MR can participate in competitive and informal sports. Exercise restrictions should only be recommended in athletes with MVP that present the following features: prior arrhythmogenic syncope, sustained or repetititve non-sustained VT, or complex VAs recorded on Holter monitoring, T wave inversions on inferior leads, long QT, basal inferoseptal wall fibrosis, bileaflet mitral valve prolapse, severe MR, LV systolic dysfunction (LV ejection fraction <50%), prior embolic event, or family history of MVP-related SCD. Athletes presenting with any of these features should participate only in low-intensity competitive sports such as golf, alternatively low-intensity aerobic exercise should be encouraged on the basis of maintaining general well-being.[51,52] The incidence of adverse cardiovascular events in patients without any high-risk features is 0.5%, according to one study.[53]
ICD
There are limited data supporting prophylactic ICD implantation in patients with MVP and high-risk features. In a study of 13 MVP patients who underwent ICD implantation as secondary prevention, nine had documented non-sustained VT, two had received anti-tachycardia pacing and two had received defibrillation.[54] Therefore, MVP patients with high-risk features should be closely monitored for malignant arrhythmias and may be considered for ICD implantation.
Lack of Solid Evidence
Due to the nature of the disease, most of the data regarding the incidence, diagnosis and possible treatment options come from inadequately powered studies either due to confounding factors or low sample number.[7–9,22–25,27,32,43–50] The scarcity of large-scale, randomised, double-blind studies on the subject should also be noted. Further studies will help clear doubts by adding solid data to the current knowledge on MVP.
Conclusion
There is an association between MVP and SCD. Identifying the most at‑risk individuals is a demanding task which requires careful evaluation of patients with clinical and echocardiographic features. The presence and history of PVCs play a prominent role and further evaluation with CMR, 24-hour ambulatory monitoring or invasive procedures may be indicated in selected patients. Further large-scale studies are required to clearly identify the risk factors that could lead to an accurate identification of high-risk individuals, paving the way for appropriate and successful primary prevention intervention.
Clinical Perspective
Mitral valve prolapse, albeit a common condition, is associated with ventricular arrhythmias and sudden cardiac death.
Electrocardiographic features include ST-segment depression, T wave inversion or biphasic T waves in the inferior leads, QT prolongation and premature ventricular complexes.
Echocardiography can identify mitral valve prolapse as well as mitral annular disjunction, a high-risk feature for ventricular arrhythmias, while late gadolinium enhancement cardiac MRI can detect the fibrotic arrhythmogenic regions in the papillary muscles and ventricular myocardium.
Patients with high-risk features should be referred for further evaluation, but risk stratification is challenging.
An ICD and/or mitral valve repair should be considered in truly high-risk patients.
Supplementary Material
References
- 1.Freed LA, Benjamin EJ, Levy D et al. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002;40:1298–304. doi: 10.1016/s0735-1097(02)02161-7. [DOI] [PubMed] [Google Scholar]
- 2.Delling FN, Rong J, Larson MG et al. Evolution of mitral valve prolapse: insights from the Framingham Heart Study. Circulation. 2016;133:1688–95. doi: 10.1161/CIRCULATIONAHA.115.020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesta F, Leyne M, Yosefy C et al. New locus for autosomal dominant mitral valve prolapse on chromosome 13: clinical insights from genetic studies. Circulation. 2005;112:2022–30. doi: 10.1161/CIRCULATIONAHA.104.516930. [DOI] [PubMed] [Google Scholar]
- 4.Nkomo VT, Gardin JM, Skelton TN et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Nishimura RA, Bonow RO et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2021;143:e35–71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 6.Avierinos JF, Gersh BJ, Melton LJ, 3rd et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106:1355–61. doi: 10.1161/01.cir.0000028933.34260.09. [DOI] [PubMed] [Google Scholar]
- 7.Essayagh B, Sabbag A, Antoine C et al. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2020;76:637–49. doi: 10.1016/j.jacc.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Alqarawi W, Cheung CC, Davies B, Krahn AD. Arrhythmic mitral valve prolapse: looking for a needle in a haystack. J Am Coll Cardiol. 2020;76:2688–9. doi: 10.1016/j.jacc.2020.08.085. [DOI] [PubMed] [Google Scholar]
- 9.Han HC, Teh AW, Hare DL et al. The clinical demographics of arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2020;76:2689–90. doi: 10.1016/j.jacc.2020.09.591. [DOI] [PubMed] [Google Scholar]
- 10.Oliva A, Brugada R, D’Aloja E et al. State of the art in forensic investigation of sudden cardiac death. Am J Forensic Med Pathol. 2011;32:1–16. doi: 10.1097/PAF.0b013e3181c2dc96. [DOI] [PubMed] [Google Scholar]
- 11.Delling FN, Aung S, Vittinghoff E et al. Antemortem and post-mortem characteristics of lethal mitral valve prolapse among all countrywide sudden deaths. JACC Clin Electrophysiol. 2021;7:1025–34. doi: 10.1016/j.jacep.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014;129:2158–70. doi: 10.1161/CIRCULATIONAHA.113.006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huttin O, Pierre S, Venner C et al. Interactions between mitral valve and left ventricle analysed by 2D speckle tracking in patients with mitral valve prolapse: one more piece to the puzzle. Eur Heart J Cardiovasc Imaging. 2017;18:323–31. doi: 10.1093/ehjci/jew075. [DOI] [PubMed] [Google Scholar]
- 14.Basso C, Calabrese F, Corrado D, Thiene G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001;50:290–300. doi: 10.1016/s0008-6363(01)00261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basso C, Perazzolo Marra M. Mitral annulus disjunction: emerging role of myocardial mechanical stretch in arrhythmogenesis. J Am Coll Cardiol. 2018;72:1610–2. doi: 10.1016/j.jacc.2018.07.069. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama T, Fukata M. Increased coupling interval variability – mechanistic, diagnostic and prognostic implication of premature ventricular contractions and underlying heart diseases. Circ J. 2015;79:2317–9. doi: 10.1253/circj.CJ-15-0963. [DOI] [PubMed] [Google Scholar]
- 17.Sniezek-Maciejewska M, Dubiel JP, Piwowarska W et al. Ventricular arrhythmias and the autonomic tone in patients with mitral valve prolapse. Clin Cardiol. 1992;15:720–4. doi: 10.1002/clc.4960151029. [DOI] [PubMed] [Google Scholar]
- 18.Basso C, Iliceto S, Thiene G, Perazzolo Marra M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. 2019;140:952–64. doi: 10.1161/CIRCULATIONAHA.118.034075. [DOI] [PubMed] [Google Scholar]
- 19.Zuppiroli A, Mori F, Favili S et al. Arrhythmias in mitral valve prolapse: relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. Am Heart J. 1994;128:919–27. doi: 10.1016/0002-8703(94)90590-8. [DOI] [PubMed] [Google Scholar]
- 20.Basso C, Perazzolo Marra M, Rizzo S et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–66. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 21.Kligfield P, Hochreiter C, Kramer H et al. Complex arrhythmias in mitral regurgitation with and without mitral valve prolapse: contrast to arrhythmias in mitral valve prolapse without mitral regurgitation. Am J Cardiol. 1985;55:1545–9. doi: 10.1016/0002-9149(85)90970-1. [DOI] [PubMed] [Google Scholar]
- 22.Sriram CS, Syed FF, Eric Ferguson ME et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–30. doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 23.Syed FF, Ackerman MJ, McLeod CJ et al. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol. 2016;9:e004005. doi: 10.1161/CIRCEP.116.004005. [DOI] [PubMed] [Google Scholar]
- 24.Peighambari MM, Alizadehasl A, Totonchi Z. Electrocardiographic changes in mitral valve prolapse syndrome. J Cardiovasc Thorac Res. 2014;6:21–3. doi: 10.5681/jcvtr.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dejgaard LA, Skjølsvik ET, Lie ØH et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72:1600–9. doi: 10.1016/j.jacc.2018.07.070. [DOI] [PubMed] [Google Scholar]
- 26.Turker Y, Ozaydin M, Acar G et al. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int J Cardiovasc Imaging. 2010;26:139–45. doi: 10.1007/s10554-009-9514-6. [DOI] [PubMed] [Google Scholar]
- 27.Han HC, Ha FJ, Teh AW et al. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc. 2018;7:e010584. doi: 10.1161/JAHA.118.010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthukumar L, Rahman F, Jan MF et al. The Pickelhaube sign: novel echocardiographic risk marker for malignant mitral valve prolapse syndrome. JACC Cardiovasc Imaging. 2017;10:1078–80. doi: 10.1016/j.jcmg.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Fulton BL, Liang JJ, Enriquez A et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol. 2018;29:146–53. doi: 10.1111/jce.13374. [DOI] [PubMed] [Google Scholar]
- 30.Sheppard MN, Steriotis AK, Sharma S. Letter by Sheppard et al regarding article ‘Arrhythmic mitral valve prolapse and sudden cardiac death’. Circulation. 2016;133:e458. doi: 10.1161/CIRCULATIONAHA.115.018775. [DOI] [PubMed] [Google Scholar]
- 31.Kitkungvan D, Nabi F, Kim RJ et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72:823–34. doi: 10.1016/j.jacc.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 32.Han HC, Parsons SA, Curl CL et al. Systematic quantification of histologic ventricular fibrosis in isolated mitral valve prolapse and sudden cardiac death. Heart Rhythm. 2021;18:570–6. doi: 10.1016/j.hrthm.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo S, Perazzolo Marra M, De Gaspari M, Basso C. The missing pieces in the puzzle of arrhythmic mitral valve prolapse: papillary muscles, mitral annulus dysjunction, and myocardial scarring. Heart Rhythm. 2021;18:577–8. doi: 10.1016/j.hrthm.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Constant Dit Beaufils AL, Huttin O, Jobbe-Duval A et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation. 2021;143:1763–74. doi: 10.1161/CIRCULATIONAHA.120.050214. [DOI] [PubMed] [Google Scholar]
- 35.Bui AH, Roujol S, Foppa M et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart. 2017;103:204–9. doi: 10.1136/heartjnl-2016-309303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perazzolo Marra M, Basso C, De Lazzari M et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9:e005030. doi: 10.1161/CIRCIMAGING.116.005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordhues BD, Siontis KC, Scott CG et al. Bileaflet mitral valve prolapse and risk of ventricular dysrhythmias and death. J Cardiovasc Electrophysiol. 2016;27:463–8. doi: 10.1111/jce.12914. [DOI] [PubMed] [Google Scholar]
- 38.Enriquez-Sarano M. Mitral annular disjunction: the forgotten component of myxomatous mitral valve disease. JACC Cardiovasc Imaging. 2017;10:1434–36. doi: 10.1016/j.jcmg.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Brignole M, Moya A, de Lange FJ et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883–948. doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- 40.Morady F, Shen E, Bhandari A et al. Programmed ventricular stimulation in mitral valve prolapse: analysis of 36 patients. Am J Cardiol. 1984;53:135–8. doi: 10.1016/0002-9149(84)90697-0. [DOI] [PubMed] [Google Scholar]
- 41.Priori SG, Blomström-Lundqvist C, Mazzanti A et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 42.Hong-Tao Y, Yang M, Zhong L et al. Ventricular premature contraction associated with mitral valve prolapse. Int J Cardiol. 2016;221:1144–9. doi: 10.1016/j.ijcard.2016.06.252. [DOI] [PubMed] [Google Scholar]
- 43.Van Herendael H, Zado ES, Haqqani H et al. Catheter ablation of ventricular fibrillation: importance of left ventricular outflow tract and papillary muscle triggers. Heart Rhythm. 2014;11:566–73. doi: 10.1016/j.hrthm.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 44.Marano PJ, Lim LJ, Sanchez JM et al. Long-term outcomes of ablation for ventricular arrhythmias in mitral valve prolapse. J Interv Card Electrophysiol. 2021;61:145–54. doi: 10.1007/s10840-020-00775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naksuk N, Syed FF, Krittanawong C et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol J. 2016;16:187–91. doi: 10.1016/j.ipej.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pocock WA, Barlow JB, Marcus RH, Barlow CW. Mitral valvuloplasty for life-threatening ventricular arrhythmias in mitral valve prolapse. Am Heart J. 1991;121:199–202. doi: 10.1016/0002-8703(91)90976-o. [DOI] [PubMed] [Google Scholar]
- 47.Vaidya VR, DeSimone CV, Damle N et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol. 2016;46:137–43. doi: 10.1007/s10840-015-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eriksson MJ, Bitkover CY, Omran AS et al. Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr. 2005;18:1014–22. doi: 10.1016/j.echo.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Newcomb AE, David TE, Lad VS et al. Mitral valve repair for advanced myxomatous degeneration with posterior displacement of the mitral annulus. J Thorac Cardiovasc Surg. 2008;136:1503–9. doi: 10.1016/j.jtcvs.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 50.Vohra J, Sathe S, Warren R et al. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol. 1993;16:387–93. doi: 10.1111/j.1540-8159.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 51.Pelliccia A, Sharma S, Gati S et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Rev Esp Cardiol (Engl Ed) 2021;74:545. doi: 10.1016/j.rec.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Maron BJ, Ackerman MJ, Nishimura RA et al. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol. 2005;45:1340–5. doi: 10.1016/j.jacc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Caselli S, Mango F, Clark J et al. Prevalence and clinical outcome of athletes with mitral valve prolapse. Circulation. 2018;137:2080–2. doi: 10.1161/CIRCULATIONAHA.117.033395. [DOI] [PubMed] [Google Scholar]
- 54.Bumgarner JM, Patel D, Kumar A et al. Management and outcomes in mitral valve prolapse with ventricular arrhythmias undergoing ablation and/or implantation of ICDs. Pacing Clin Electrophysiol. 2019;42:447–52. doi: 10.1111/pace.13613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


