Abstract
Due to the emergence of various new infectious (viral/bacteria) diseases, the remote surveillance of infected persons has become most important, especially if hospitals need to isolate infected patients to prevent the spreading of pathogens to health care personnel. Therefore, we develop a remote health monitoring system by integrating a stretchable asymmetric supercapacitor (SASC) as a portable power source with sensors that can monitor the human physical health condition in real-time and remotely. An abnormal body temperature and breathing rate could indicate a person’s sickness/infection status. Here we integrated FePS3@graphene-based strain sensor and SASC into an all-in-one textile system and wrapped it around the abdomen to continuously monitor the breathing cycle of the person. The real body temperature was recorded by integrating the temperature sensor with the SASC. The proposed system recorded physiological parameters in real-time and when monitored remotely could be employed as a screening tool for monitoring pathogen infection status.
Subject terms: Electronic properties and materials, Electronic properties and devices
Introduction
Many infectious diseases are connected with several physiological changes that can be monitored using wearable health sensors1,2. An abnormal body temperature, breathing rate, and diastolic blood pressure could indicate a patient’s infection status. Recently the world faced a pandemic by SARS-CoV-2 virus and many health care personnel were infected by the direct contact with infected patients during their physiological parameters assessment. Demonstrating that it is necessary to maintain social distance and quarantine to decrease the risk of spreading the infection to others. In this situation, a remote health monitoring system (RHMS) has proven to be the safest method for health monitoring of infected patients3,4. RHMS can be defined as the delivery of healthcare services over a distance. RHMS typically comprises three main components: a sensing device, data transmission device, and power device5. The sensing device detects various physiological parameters like body temperature, heart rate, and blood pressure, and converts them into electrical signals that are transmitted via a wireless connection to a smartphone or the cloud for further analysis. Both the sensing and data transfer processes necessitate the use of an energy storage source.
In comparison to other typical electronic gadgets, a wearable health monitoring system has unique power source requirements6. First, the energy storage device as well as the sensing device must be stretchable, mechanically and electrically stable to enable a conformable shape with the body for constant detection performance. Second, the device often comes into contact with human body parts, thus, the energy storage and sensing devices must be biocompatible to minimize undesirable immune responses and other negative effects. With regard to the above points, we fabricated a proof-of-concept wearable integrated healthcare monitoring system that employs a stretchable asymmetric supercapacitor (SASC) and strain sensor. Where SASC was fabricated using two different types of 2D materials such as transition metal carbides (e.g., Ti3C2) and metal phosphorus chalcogen@ reduced graphene oxide composite (e.g., FePS3@rGO). While strain sensor was assembled using the same FePS3@rGO composite.
Emerging family of layered materials, metal phosphorous chalcogenides with a common formula of MPXy where M represents a transition metals, X presents a chalcogen (S or Se) and can be 3 or 4 possess superior electronic, magnetic and anisotropy properties. They have been applied in a wide variety of applications such as optoelectronics, spintronic, sensing, hydrogen adsorption, catalysis as well as batteries electrochemical properties7–11. Recently, considerable attention has been focused on utilizing MPS3 (M = Iron (Fe), Nickel (Ni), tin (Sn), cobalt (Co), etc.) materials for lithium-ion batteries12–14. Specifically, FePS3, NiPS3, and MnPS3 have been identified as promising cathode materials in batteries, with greater capacity and longer operational periods than the carbon and metal oxide electrodes investigated previously13–15. Moreover, in our earlier study, we stated that some of the MPS3 materials (e.g., FePS3, NiPS3, and CoPS3) showed remarkable performance as electrocatalysts for hydrogen and oxygen production16. However, MPXy has not yet received much interest as a flexible and portable energy source for wearable electronic applications. On the other hand, the Ti3C2Tx (MXenes) are one of the most frequently researched owing to their inexpensive cost and range of benefits17–20. Therefore, Ti3C2Tx can be easily incorporated into fabrics, showing promise in health sensors and energy storage applications21–23.
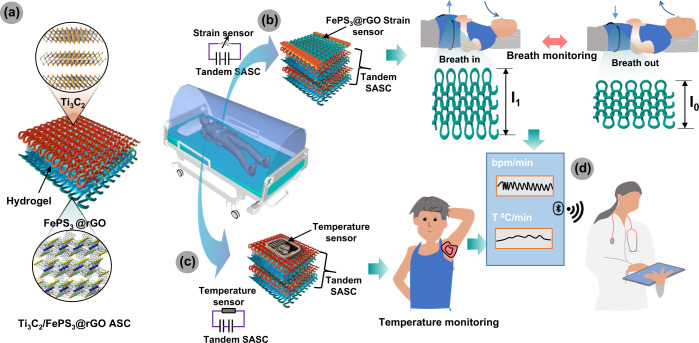
In this study, we report on the fabrication of a textile-based remote health monitoring system comprising a high-performance stretchable asymmetric supercapacitor (SASC) and strain sensor (Fig. 1). A composite based on FePS3 and rGO was used as an electrode of SASC and strain sensor coated on stretchable fabric. The second electrode of SASC is based on Ti3C2Tx. This unique integrated system can be attached directly to the human body (abdomen) to monitor accurately a patient’s breathing rate. In addition, SASC was used to power temperature sensor attached to the armpit and monitor the body temperature as well. This system enables patients to keep track of these health indicators without having direct contact with health care personnel. Most importantly, the data from real-time monitoring of breathing and body temperature can be sent via wireless communication to the hospital cloud system for clinical assessment.
Fig. 1. Telemedicine platform for remotely health monitoring by integrated 2D FePS3@rGO and Ti3C2 based wearable devices.
a Schematic illustration of the Ti3C2/FePS3@rGO SASC. b Integrated FePS3@rGO-based strain sensor with series-connected two SASC for real-time breath monitoring. c Integrated temperature sensor with series-connected two SASC for real-time body temperature monitoring. d Bluetooth or Wi-Fi signal sources used to transfer breathing and temperature data to a healthcare provider.
Result and discussion
Remotely healthcare system may become a basic necessity for monitoring patients with infections illnesses (e.g., SARS-CoV-2), especially when they are under quarantine. Thus, the purpose of this work was to develop a lightweight and wearable health monitoring system that could monitor critical physiological parameters during all stages of infection diseases. Intending to design a remote health monitoring system, we integrated strain sensor and temperature sensor with series-connected two SASCs into all in one textile system. This all-in-one wearable health system represents a potential application for monitoring the physiological parameters of patients and wirelessly relaying data to the health care provider. The fabrication procedures for the SASC and strain sensor are schematically depicted in Supplementary Fig. 1 We assembled a fabric-based SASC by combining negative Ti3C2 and positive FePS3@rGO electrode in a polymeric hydrogel. The strain sensor is made of the same FePS3@rGO materials. Also, presented a scalable fast deposition process for spray-coating Ti3C2 and FePS3@rGO onto a wearable fabric substrate for SASC and strain sensor applications. To showcase a real-world application, we first must examine the morphology and electrochemical performance of the active 2D materials.
Morphology characterization of Ti3C2/FePS3@rGO SASC
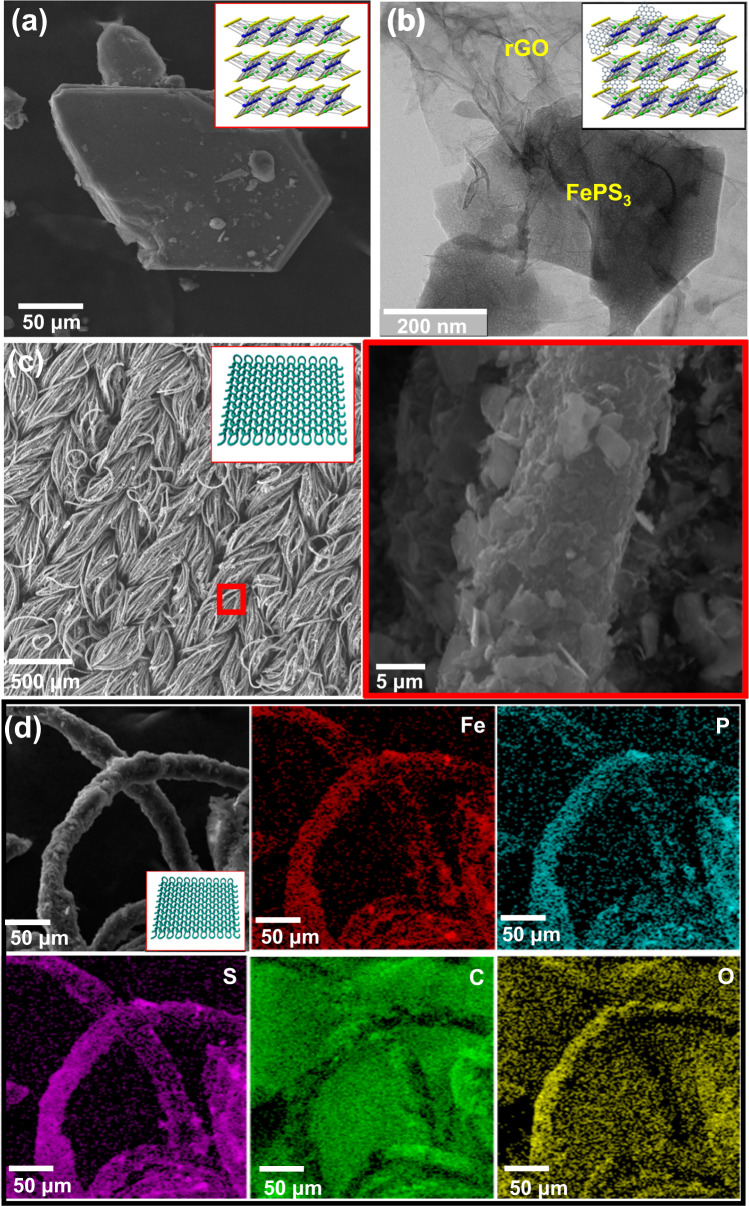
A typical scanning electron microscopy (SEM) image of the bulk FePS3 exhibits a large polygonal stack (Fig. 2a), which is consistent with prior results24. To obtain FePS3 nanosheets, physical exfoliation was carried out using a strong sonication bath. After 2 h of sonication, a colloidal solution was obtained (Supplementary Fig. 2a). Figure 2b shows a transmission electron microscopy (TEM) image of FePS3@rGO colloidal solution (Supplementary Fig. 2b), where clear exfoliation is observed. Small FePS3 nanosheets are scattered on large rGO sheets, yielding a hybrid scaffold that can be seen by the contrast between two components. As revealed in SEM images of the FePS3@rGO-coated on cotton fabric (Fig. 2c), the nanosheets are uniformly and tightly wrapped around the entire fabric matrix. Figure 2c (right pannel), d shows a high-resolution SEM image of FePS3 coated fabric as well as the corresponding elemental mapping and energy-dispersive X-ray (EDS) spectrum (Supplementary Fig. 3a). The distribution of Fe, P, C, S, and O on the fabric surface is uniform, suggesting that the fabric is tightly wrapped by a layer of FePS3@rGO. The crystal structure of FePS3 is determined using X-ray diffraction (XRD). The XRD patterns of FePS3@rGO are shown in Supplementary Fig. 4a, with peak characteristics at 15.9°, 26.82°, 30.23°, 35.48°, 47.59°, and 55.49° on the 2θ scale corresponding to the (001), (002), (−201), (131), (202), and (−331) planes of FePS3 phase (1998-JCPDS 78–496)25. The sharpness of the different peaks indicates that FePS3 is highly crystalline26, meaning that FePS3 has no impurities. To investigate more about surface properties, nitrogen adsorption-desorption was measured for pristine FePS3 and FePS3@rGO composite (Supplementary Fig. 4b). The specific surface area (SSA) of pristine FePS3 is 12.6 m2 g−1 due to the serious restacking structure that obstructs accessibility of FePS3 to electrolyte ions. On the other hand, SSA of FePS3@rGO is obtained as 78 m2 g−1, which is significantly higher. This is due to the rGO having a large surface area on account of nanopores on the surface27,28. Furthermore, the introduction of rGO into the FePS3 can aid in the reduction of serious restacking and establish an inter-conductive path is suitable for accelerating transport and the diffusion of electrolyte ions in SC applications that require rapid charge-discharge cycles.
Fig. 2. Morphology study of FePS3, FePS3@rGO and FePS3@rGO coated fabric.
a SEM image of bulk FePS3. b TEM image of FePS3@rGO. c Low- and high-magnification SEM images of FePS3@rGO-coated fabric. d EDS mapping of FePS3@rGO-coated fabric.
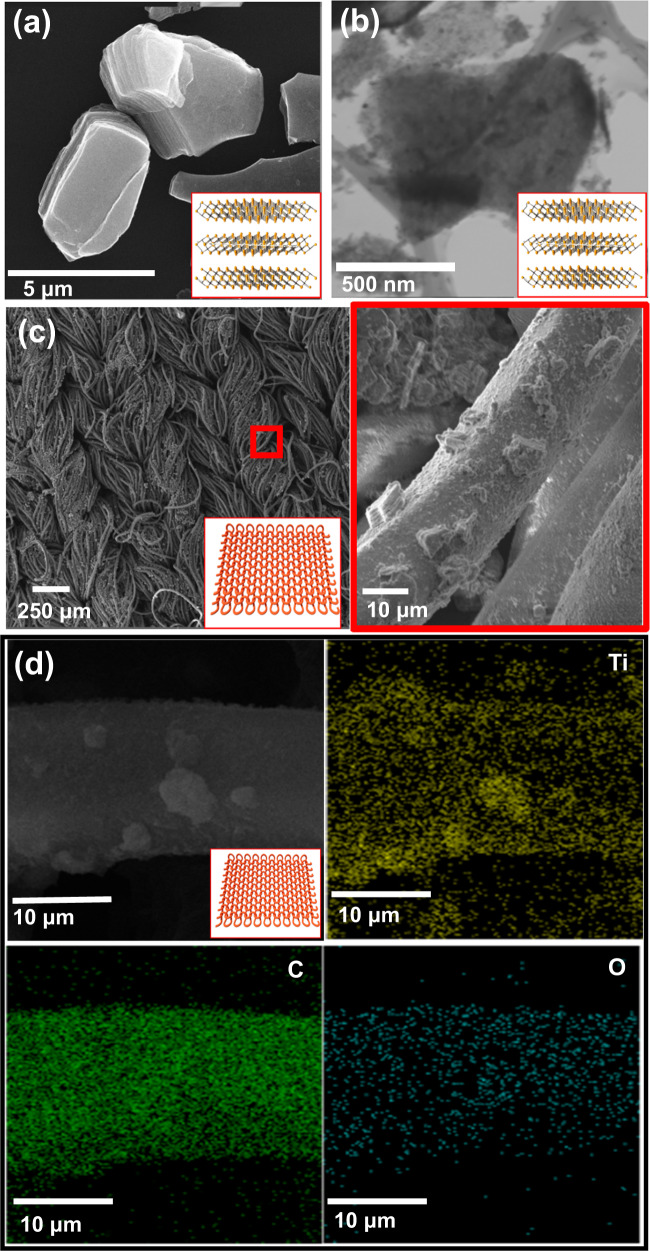
Figure 3a displays the SEM image of pristine Ti3C2 with a multi-layered structure. These multi-layered structures intercalated with water molecules yielded a single layer Ti3C2 (Fig. 3b). As stated earlier, the exfoliated Ti3C2 can be easily dispersed in water due to a large number of oxygen-containing groups on the surface of Ti3C2 (Supplementary Fig. 2c)29–31. Thus, the homogeneous Ti3C2 dispersion shows excellent stability and typical colloidal nature, making it appropriate for a spray-coating technique (Fig. 3c). The majority of the Ti3C2 is deposited on the fabric surface without aggregation. Moreover, the high-magnification SEM image demonstrates the individual Ti3C2 attached to the fabric (right panel of Fig. 3c). Figure 3d and Supplementary Fig. 3b shown the Ti3C2 elemental distribution and EDS spectrum, respectively on the CF, revealing that Ti, C, and O are evenly dispersed across the entire fabric surface. The XRD peak observed at angles 9.69° and 20.4° correspond to the (002) and (004) planes, respectively, representing Ti3C2 (Supplementary Fig. 4a)32,33.
Fig. 3. Morphology study of Ti3C2 and Ti3C2 coated fabric.
a, b SEM and TEM images of Ti3C2. c low- and high-magnification SEM images of Ti3C2 coated fabric. d EDS mapping of Ti3C2 coated fabric.
Based on the above results, we suggest that FePS3@rGO composite could be promising electrode material for energy storage devices. To confirm the suitable potential window for SASC, FePS3@rGO composite and Ti3C2 were deposited on glassy carbon electrodes as a working electrode and the electrochemical performance was measured by a three-electrode configuration using 1 M H2SO4 as the electrolyte.
Supplementary Fig. 5a depicts the CV curves of the pristine FePS3 and varying amounts of rGO (0.6–2.4 wt%) in FePS3 composites at a scan rate of 50 mV s−1. A pair of wide redox peaks can be seen throughout the CV cycles, revealing that the capacitance is mostly obtained from the pseudocapacitance based on the reversible oxidation state of Fe atoms13,14. The CV integration area of the FePS3@rGO0.6 electrode is significantly larger than that of pristine FePS3. This is because the incorporation of rGO into the FePS3 can effectively enhance the interlayer space and reduce FePS3 restacking, resulting in a large surface area available for electrolyte ions. When the 1.8 wt% rGO is introduced into the FePS3, the relevant CV integration area reaches the maximum and provides the highest Csp values. However, the CV integration area of FePS3@rGO2.4 is smaller than FePS3@rGO1.8 because the large amount of rGO can reduce the pseudocapacitive reaction and conductivity of the electrode. Thus, we decided to undertake a comprehensive electrochemical study with optimum FePS3@rGO1.8 composite. Supplementary Fig. 5b shows the CV curves of FePS3@rGO1.8 electrode at different scan rates from 10–100 mV s−1. The CV curve retains anodic and cathodic peaks without distortion at a high scan rate, implying an excellent rate capability and capacitive nature.
In Supplementary Fig. 5c, the GCD curves of the FePS3@rGO1.8 electrode at current densities ranging from 1 to 10 A g−1, revealed a non-symmetrical shape of charge/discharge curves. It was found that the discharge time was monotonically declined as a function of current density. This could be due to the low absorption of ions onto the surface of nanosheets at a rapidly changing potential. The FeS3@rGO exhibited a high Csp of 175 F g−1 at 1.0 A g−1 and still maintained Csp retention above 44.5% (78 F g−1) even at a high current density (Supplementary Fig. 5d). To further investigate the kinetics of ion transport of the pristine FePS3 and FePS3@rGO1.8 electrodes, electrochemical impedance spectroscopy (EIS) was performed (Supplementary Fig. 5e). The Nyquist plot exhibits lower equivalent series resistance (ESR) and interfacial charge-transfer resistance (Rct) of FePS3@rGO1.8 compared to pristine FePS3, indicating that the conductivity and ion transport of FePS3@rGO1.8 was increased after introducing rGO. Furthermore, the vertical slope of FePS3@rGO1.8 is much higher than pristine FePS3, implying that FePS3@rGO1.8 has a lower diffusion resistance34,35.
To meet the outstanding performance of the FePS3@rGO positive electrode, a suitable negative electrode is necessary to find a high-performance ASC device. Supplementary Fig. 5f–h depicts the electrochemical performance of Ti3C2. CV curves in Supplementary Fig. 5f show a pair of distinct redox peaks at 100 mV s−1, indicating a quick and reversible redox reaction of Ti atoms36,37. The GCD curves (Supplementary Fig. 5g) of Ti3C2 are nonlinear, showing its pseudocapacitive nature38,39. In comparison to Csp of 153 F g−1 at a current density of 1.0 A g−1, the Ti3C2 retained 55% of its Csp at high current density, proving its high rate capability (Supplementary Fig. 5h).
Electrochemical performance evaluation
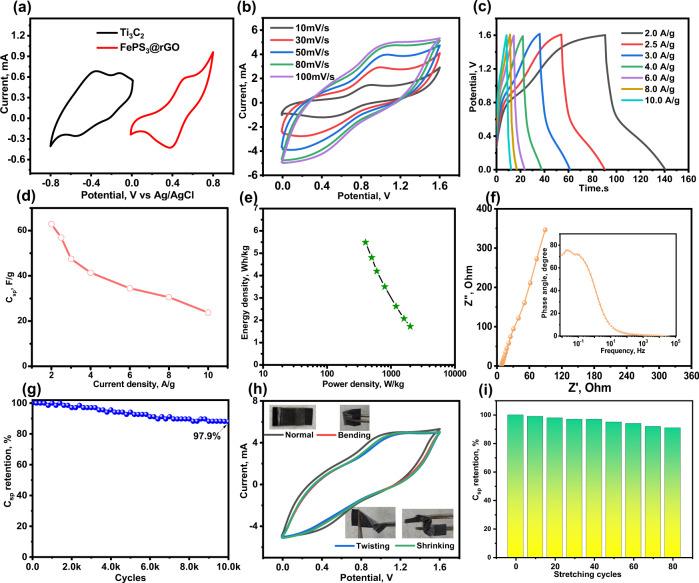
We further fabricated SASC device employing Ti3C2 as the negative electrode and FePS3@rGO as the positive electrode in polymeric hydrogel electrolyte. The charge balance between Ti3C2 NS and FePS3@rGO electrodes to maximize the potential window of the Ti3C2/FePS3@rGO SASC is obtained by balancing the mass loading before device fabrication. The mass ratio of FePS3@rGO to Ti3C2 was maintained at 0.89 to charge both electrodes. Figure 4a shows the potential windows of the Ti3C2 (0 to −0.8 V) and FePS3 (0 to 0.8 V) at the scan rate of 50 mV s−1, demonstrating that each electrode stored a similar charge. Finally, a working window of up to 1.6 V is attained for the SASC, which can be delivered with higher capacitance, power, and energy density than the individual electrode devices. Figure 4b shows the CV curves of the Ti3C2/FePS3@rGO SASC at a scan rate of 10–100 mV s−1. It exhibits a stable operating window of 1.6 V as well as a pair of redox peaks. There was no significant distortion at a high scan rate of 100 mV s−1, indicating that the electrodes possess low resistance and rapid and reversible charge/discharge capabilities. In addition, the GCD curves of SASC at various current densities (2–10 A g−1) are approximately symmetric with a plateau that corresponds to the CV results (Fig. 4c). The Csp values calculated from GCD plots as a function of current densities (Fig. 4d) show that the SASC exhibited a high gravimetric Csp of 62.9 F g−1 at 1.0 A g−1. Notably, the SASC also demonstrated impressive rate performance with 23.7 F g−1 Csp at a high current density of 10 A g−1.
Fig. 4. Electrochemical performance of Ti3C2/FePS3@rGO SASC.
a CV curves of Ti3C2 and FePS3 at scan rate 50 mV s−1. b CV curves of Ti3C2/FePS3@rGO SASC at different scan rates. c GCD plots as function of current densities. d Csp at different current densities. e Ragone plot: power density versus energy density. f Nyquist plot (inset display bode plot). g Long-term cycling stability. h CV curves of the device measured at various bending modes. i Csp retention versus 30% stretching over 80 cycles.
The Ragone plot (Fig. 4e) shows the power (P) and energy (E) densities of the SASC. It exhibited a high E of 5.59 Wh kg−1 at P of 400 W kg−1 and retained E of 2.1 Wh kg−1 at P of 2 KW kg−1. A Ragone plot comparing the performance of Ti3C2/FePS3@rGO SASC with different reported supercapacitors is shown in Supplementary Table 1. Notably, E of SASC comparable to the pristine rGO, Ti3C2Tx, NiPS3 and their composites. Whereas, SASC shows three fold higher power densities. These results indicate that SASC should be able compete with micro batteries in a wide range of health care applications. Further, EIS was used to estimate the charge transfer and internal resistance of the SASC device (Fig. 4f). The values of ESR and Rct calculated from the equivalent circuit are 7.8 and 2.5 Ω, respectively. The low Rct value can be ascribed to the high interfacial conductivity of both electrodes. Inset of Fig. 4f shows the phase angle of −72° at low frequency is very close to the ideal capacitors (−90°)33,40. Next, the cycling stability of SASC was conducted at a high current density (10 A g−1) for 10,000 charge/discharge cycles. As seen in Fig. 4g, Csp retention after 10,000 cycles is 97.9% of its initial Csp, demonstrating outstanding cycling stability.
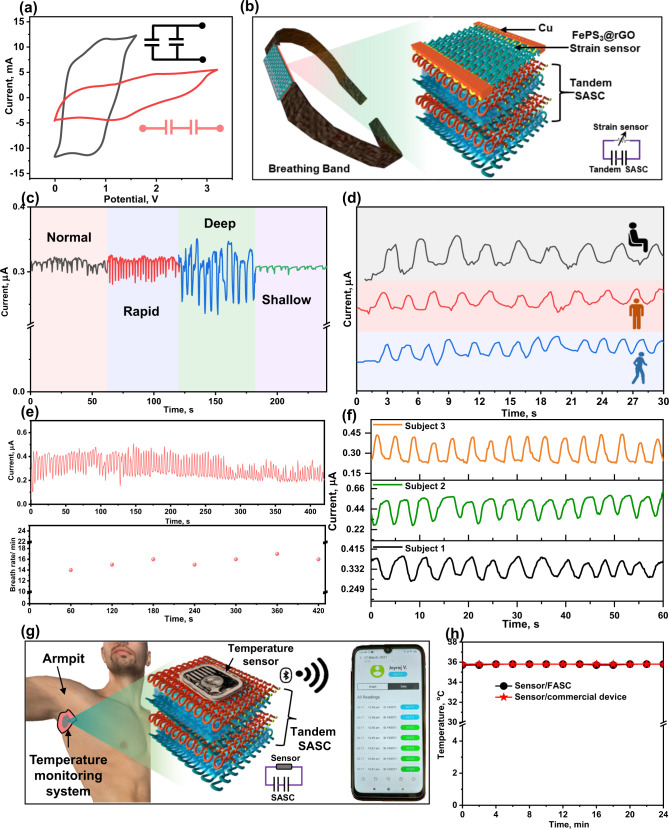
As studied above, the SASC exhibited a wide potential window, excellent capacitance, rate capability, cycling stability, and power density. To demonstrate the SASC as a portable power source in healthcare applications, it is important to investigate device mechanical properties and power output. Figure 4h displays that the CV curves are identical in different bending modes (i.e., bending, twisting, and shrinking), demonstrating the satisfactory mechanical stability and robustness of the SASC for realistic wearable applications. The CV profiles of the SASC under various applied tensile strains (Supplementary Fig. 6) exhibit negligible alterations, indicating that the device performance remains unchanged even when subjected to 30% tensile strain. Likewise, the electrochemical performance demonstrates outstanding durability when subjected to several stretching cycles. The stretching study of SASC resulted in 91% Csp retention after 80 cycles (Fig. 4i). These findings demonstrate that our SASC can withstand high mechanical deformation while still delivering outstanding energy storage performance. The performance of this device was normal when compared to other ASC, but their exceptional mechanical features inspired the construction of a portable power source for the healthcare monitoring system. In real applications, the voltage and capacitance requirements are variable; hence, the SASCs should be coupled in parallel or in series to satisfy the needs. For example, when two SASCs are connected in series, the output voltage is increased to 3.2 V. While two SASCs are connected in parallel, the enclosed area of CV curves can be double that of a single SASC (Fig. 5a).
Fig. 5. Remotely monitoring physiological parameters of human.
a CV profile of the SASC connected in parallel and in series. b Schematic illustration of breathing band containing integrated FePS3@rGO strain sensor with tandem SASC. c Breathing signals recorded at normal, rapid, deep, and shallow breathings. d Breathing pattern as a function of activity such as sitting, walking, and running. e Multiple breathing cycles and corresponding breathing rate per minute. f Breathing rate of three volunteer subjects. g Integrated temperature sensor with tandem Ti3C2/FePS3@rGO SASC to monitor the user’s body temperature and wirelessly transmit data to the mobile phone. h Real temperature as a function of time as measured by the integrated temperature sensor operated by Ti3C2/FePS3@rGO SASC and bias of 1.5 V.
Remotely monitoring physiological parameters
Further, we developed a wearable breathing band composed of the tandem SASC and FePS3@rGO-based stain sensor (Fig. 5b). This integrated system can detect human breathing in real-time. Fabrication details for the FePS3@rGO strain sensor are found in the supporting information. To investigate the FePS3@rGO suitability for usage in wearable strain sensor, the FePS3@rGO was coupled in a closed circuit with a red-light emitting diode (LED). The red LED glow faded dramatically when the FePS3@rGO was stretched, showing a change of electrical conductivity (or resistance) with stretching (Supplementary Movie 1). This ability to translate mechanical distortion into a detectable electrical signal could be used for strain sensing. SEM measurements were used to demonstrate how the morphology of FePS3@rGO strain sensor changes upon stretching. The digital photographs and SEM images of the FePS3@rGO strain sensor with and without strain are shown in Supplementary Fig. 7. We can observe that the contact between meandering loops remains when being expanded in the y-direction. Therefore, we assembled the FePS3@rGO strain sensor and SASC in the same direction.
To evaluate the stretch properties of the strain sensor, the difference in relative resistance was measured as a function of tensile strain (Supplementary Fig. 8a). The relative resistance is defined as ΔR/R0, where ΔR = R − R0 and R0 and R are the resistance before and after the applied strain, respectively41. Applied tensile strain is calculated by ε = (L − L0)/L0 × 100, where L0 is initial length and L is length after being stretched42. It was found that the FePS3@rGO strain sensor resistance increased linearly with a corresponding gauge factor of 38.7 (R2 = 0.96) at 40% strain. The obtained gauge factor value was superior to that previously reported for some of the 2D materials and carbon-coated textile strain sensors43–46. The repeatability of the FePS3@rGO strain sensor was then examined using step and hold strain cycles, and the results are displayed in Supplementary Fig. 8b. The sensor resistance achieved a steady plateau and stayed unchanged during the holding and repeating strain cycles. Then, a response time for the stretching and releasing process was determined to be ~2.38 s at 1% strain (Supplementary Fig. 8c).
Based on satisfactory results of SASC and strain sensor were integrated into one textile and fabricated breathing monitoring band. Typically, the normal breathing rate for the adult is around 16–20 breaths per minute47,48. When individuals are infected with viruses such as COVID 19, they can get severe breathing syndrome and have trouble breathing normally49,50. In this situation, remotely monitored breathing is required to provide real-time measurements and alerts to quick action, particularly for those with moderate symptoms51,52. Current breathing monitoring systems are too costly and inconvenient to be used for prolonged periods. As a result, a low cost and lightweight breathing monitoring system is proposed. The fabricated breathing monitoring band is wrapped around the middle part of the abdomen, which can continuously monitor breath cycles of the individual and relay data to a mobile device via wireless communication (Supplementary Figs. 9a and 10a). As we know, inhalation and exhalation comprise one complete breathing cycle. When a user inhales and exhales, the abdomen expands and contracts, respectively, causing the FePS3@rGO strain sensor to stretch and release, resulting in electrical (or resistance) signals. Using the breathing monitoring band, a real-time breathing pattern of an individual was obtained in 60 s with four distinct breathing states: normal, fast, slow, and shallow (Fig. 5c). The rate obtained for normal breathing is around 14 breaths per minute (bpm) while the rate for fast breathing is 23 bpm. However, slow and shallow breathing states calculated rates are 12 and 9 bpm, respectively.
Further, breathing patterns were monitored in real-time when sitting, walking, and running (Fig. 5d). The current signal obtained by the breathing band can clearly differentiate between the various physical activities. For instance, the number of breathing cycles when running (~26 bpm) is higher than when walking (~20 bpm) and sitting (~15 bpm). A long-term breathing cycle and corresponding calculated breathing rate is presented in Fig. 5e. It can be clearly seen that the current signals of repeated inhalations and exhalations remain steady with a breathing rate of 14–16 bpm. We also measured the breathing rate of three male volunteers. The current responses generated by their breathing cycles are shown in Fig. 5f. Subjects’ 1, 2, and 3 average breathing rate was 13, 14, and 16 bpm, respectively, which demonstrates good reliability of the breath monitoring system. All of these demonstrations indicate that our integrated FePS3@rGO strain sensor is capable of continuously measuring breathing cycles in real time. Supplementary Movie 2 shows the real-time remote sensing performance of the integrated FePS3@rGO strain sensor in response to repeated breathing strain when connected to a wireless transmitter: the response is quick and repeatable. The signal can alert the user or a healthcare facility if a situation emerges when the breathing rate exceeds or goes below the typical range. As a result, medical personnel can take the necessary actions such as dispatching an ambulance to the patient’s location.
Following the satisfactorily integrated FePS3@rGO strain sensor performance, a wearable temperature monitoring system was further developed that can detect abnormal body temperatures (such as fever) and be worn on a smartphone via wireless communication. Figure 5g shows the temperature sensor was linked with the tandem SASC. The Bluetooth module enables the recorded temperature sensor data to be sent to a smartphone app (Supplementary Figs. 9b and 10b). Bluetooth have been used widely to transfer data from medical devices to common consumer devices (smartphones, smartwatches and laptops). Moreover, Bluetooth is relatively biocompatible, user friendly and very affordable53.
The self-discharge profile of the tandem Ti3C2/FePS3@rGO SASC (Supplementary Fig. 6b) exhibits ~53.0% (1.7 V) of the initial charge potential after 2000s of self-discharge. This result demonstrates that the temperature sensor can be driven for long periods by a tandem SASC device. Figure 5h depicts the recorded body temperature as a function of time, demonstrating that the Ti3C2/FePS3@rGO SASC can generate a steady power supply to the integrated temperature sensor. Likewise, a constant voltage of 1.5 V (control experiment) was used to measure real body temperature by the integrated temperature sensor. The obtained results of the temperature sensor driven by Ti3C2/FePS3@rGO SASC and constant voltage demonstrated the possibility of using SASC to power the temperature sensor instead of a commercial energy source. Real-time temperature detection in the human body (early identification of fever) aids in illness mitigation. Most importantly, this wearable system assists in identifying whether or not a user is infected with SARS-CoV-2 or other viroid diseases and allows for a quick response.
In summary, we successfully developed a wearable health monitoring system using integrated sensors with SASC to monitor real-time physiological parameters of remote individuals for the early identification of viral infection or emergencies. The spray-coating deposition method was used to prepare Ti3C2 and FePS3@rGO-based stretchable electrodes and the Ti3C2/FePS3@rGO SASC was assembled using polymer gel electrolyte. The assembled SASC device exhibits high electrochemical performance in terms of Csp, cycling stability, power density, rate capability, bending, and stretching stability. Further, FePS3@rGO-based energy storage device and strain sensors to be fabricated as a flexible and stretchable power source and a sensor for monitoring real-time breathing cycles. We successfully monitored live breathing patterns as a function of activity in volunteers. Lastly, we integrated the Ti3C2/FePS3@rGO SASC with a temperature sensor that can be affixed to a user’s skin and accurately monitor a person’s body temperature in real time and wirelessly relay data to a smartphone. The wireless devices developed in this work, can be used in the emergence room to monitor of various infectious (viral/bacteria) diseases, especially if hospitals need to isolate infected patients to prevent the spreading of pathogens to health care personnel. Moreover, this study paves the way for the development of innovative wearable e-health monitoring systems based on flexible and stretchable energy storage devices.
Methods
Materials
Lithium tetrafluoroborate (LiBF4) and pluronic polymer (F108) were purchased from Sigma Aldrich. Conductive carbon ink was obtained from DuPont. A wearable temperature sensor was purchased from Amazon in the Czech Republic. Stretchable cotton fabric was purchased at a local shop in Prague, Czech Republic.
Preparation of FePS3@rGO and Ti3C2 based fabric electrodes
Cotton fabric (CF) was chosen as the substrates for coating FePS3@rGO and Ti3C2 materials due to their abundance of oxygen-containing functional groups. In addition, fabric has higher hydrophilicity than synthetic textiles, which aids in functional material adsorption. Carbon ink was used to make fabric conductive before active 2D materials were deposited on its surface. The carbon ink coated stretchable fabrics were dried at 50 °C for 30 min in the electronic oven. Afterwards, the FePS3@rGO colloidal solution was sprayed onto the fabric substrate. Additionally, four different FePS3@rGO suspensions were made with rGO weight percentages of 0.6, 1.2, 1.8 and 2.4 in the mixture donated as FePS3@rGO0.6, FePS3@rGO1.2, FePS3@rGO1.8, and FePS3@rGO2.4, respectively. The height between the spray nozzle and CF substrate remains constant during the spray-coating process. A similar process was used to make Ti3C2 coated fabric. Finally, all electrodes were dried at 55 °C to ensure water elimination.
Fabrication of SASC and integrated healthcare system
Ti3C2/FePS3@rGO SASC: The SASC was fabricated by sandwiching the F108@LiBF4 gel electrolyte between the positive (FePS3@rGO-coated fabric) and negative (Ti3C2 coated fabric) electrodes. The F108@LiBF4 gel electrolyte is made up of 35 % w/w pluronic polymer (Pluronic®-F108, PEO122-PPO50-PEO133) dissolved in 1 M LiBF4 aqueous solution.
Wearable temperature monitoring system: The temperature sensor was connected with tandem Ti3C2/FePS3@rGO SASC using copper tap to assemble the integrated temperature sensor. The temperature sensor is directly attached to the user’s armpit and monitors real-time body temperature while wirelessly transmitting data to the mobile phone.
Wearable breath monitoring system: The FePS3@rGO strain sensor and tandem Ti3C2/FePS3@rGO SASC were interconnected using silver ink and copper tap. The energy stored in the Ti3C2/FePS3@rGO SASC can be used to power the strain sensor.
Materials characterizations and electrochemical measurements
The morphology and structural properties of the FePS3 and Ti3C2 were analyzed using scanning electron microscopy (SEM, JEOL 7600F, Japan), transmission electron microscopy (TEM), energy-dispersive spectroscopy (EDS, SDD detector X-MaxN 80TS), X-ray powder diffraction (XRD, Bruker, D8, Germany) and Brunauer-Emmett–Teller (BET, Quantachrome Instrument) method. The electrochemical performance of SASC was evaluated by cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS) measurements on an electrochemical workstation (Autolab PGSTAT204, Netherlands) using two and three-electrode configurations. Three electrode configurations, glassy carbon coated FePS3@rGO, Ag/AgCl and platinum disk were used as working, reference and counter electrodes, respectively in 1 M H2SO4 aqueous solution. EIS measurements were performed in the frequency range between 10−2 and 105 Hz with AC amplitude of 10 mV and open-circuit voltage.
The charge balancing mechanism was used to calculate the loading mass of active materials on negative and positive electrodes using Eq. (1). The gravimetric specific capacitance (Csp, F g−1), power density (P, W kg−1) and energy density (E, Wh kg−1) were calculated based on the total mass of the active materials according to the following Eqs. (2), (3) and (4).
| 1 |
| 2 |
| 3 |
| 4 |
Where, Δt, V, I, and m are the total discharge time, operating voltage window, discharge current and total mass of the two electrodes, respectively.
Supplementary information
Acknowledgements
The authors thank the project Advanced Functional Nanorobots (Reg. no. CZ.02.1.01/0.0/0.0/15_003/0000444 financed by the EFRR) for support.
Author contributions
J.V.V., C.C.M.-M., and M.P.: devised the project. J.V.V.: methodology, investigation, and writing the original draft; C.C.M.-M.: revised original draft; M.P.: initiated and oversaw the project. All authors contributed to writing the manuscript. Thanks to volunteers Dr. David Kasprzak (Subject 2) and Dr. Jan Vyskocil (Subject 3) for real-time breathing monitoring.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41528-022-00208-1.
References
- 1.Islam, T. & Mukhopadhyay, S. C. Chapter 1: sensors for physiological parameters measurement: physics, characteristics, design and applications. In Wearable sensors applications, designs and implementation; wearable. 1–31 (IOP Publishing Ltd, 2017).
- 2.Wu L, Li Z, Li M. Portable human physiological parameters detection system. Int. J. Multimed. Ubiquitous Eng. 2016;11:1–8. [Google Scholar]
- 3.Al Bassam N, Hussain SA. IoT based wearable device to monitor the signs of quarantined remote patients of COVID-19. Inform. Med. Unlocked. 2021;24:100588. doi: 10.1016/j.imu.2021.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20:1193. doi: 10.1186/s12889-020-09301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rida, J. F. A. Development of a remote health care wireless sensor network based on wireless spread spectrum communication networks. Mater. Today: Proc. 10.1016/j.matpr.2021.02.534 (2021).
- 6.Mahbub, I., Pullano, S. A., Shamsir, S., Kamrul, S. I. & Pullano, S. A. Chapter 2. Low-power wearable and wireless sensors for advanced healthcare monitoring. In IoT and low-power wireless circuits, architectures, and techniques. 2nd edn, (CRC Press, 2018).
- 7.Cheng Z, et al. High-yield production of monolayer FePS3 quantum sheets via chemical exfoliation for efficient photocatalytic hydrogen evolution. Adv. Mater. 2018;30:1707433. doi: 10.1002/adma.201707433. [DOI] [PubMed] [Google Scholar]
- 8.Gusmão R, Sofer Z, Pumera M. Exfoliated layered manganese trichalcogenide phosphite (MnPX3, X = S, Se) as electrocatalytic van der waals materials for hydrogen evolution. Adv. Funct. Mater. 2019;29:1805975. doi: 10.1002/adfm.201805975. [DOI] [Google Scholar]
- 9.Gusmão R, Sofer Z, Pumera M. Metal phosphorous trichalcogenides (MPCh3): from synthesis to contemporary energy challenges. Angew. Chem. Int. Ed. 2019;58:9326–9337. doi: 10.1002/anie.201810309. [DOI] [PubMed] [Google Scholar]
- 10.Brec R, Ouvrard G, Rouxel J. Relationship between structure parameters and chemical properties in some MPS3 layered phases. Mater. Res. Bull. 1985;20:1257–1263. doi: 10.1016/0025-5408(85)90118-7. [DOI] [Google Scholar]
- 11.Joy PA, Vasudevan S. Magnetism in the layered transition-metal thiophosphates MPS3 (M = Mn, Fe, and Ni) Phys. Rev. B. 1992;46:5425–5433. doi: 10.1103/PhysRevB.46.5425. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, et al. An exfoliated iron phosphorus trisulfide nanosheet with rich sulfur vacancy for efficient dinitrogen fixation and Zn-N2 battery. Nano Energy. 2021;81:105613. doi: 10.1016/j.nanoen.2020.105613. [DOI] [Google Scholar]
- 13.Glass DE, Jones JP, Shevade AV, Bugga RV. Transition metal phosphorous trisulfides as cathode materials in high temperatures batteries. J. Electrochem. Soc. 2020;167:110512. doi: 10.1149/1945-7111/aba0d4. [DOI] [Google Scholar]
- 14.Wang M, Tang K. A Facile synthesis of FePS3@C nanocomposites and their enhanced performance in lithium-ion batteries. Dalton Trans. 2019;48:3819–3824. doi: 10.1039/C8DT04866E. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, et al. Facile synthesis of FePS3 nanosheets@MXene composite as a high-performance anode material for sodium storage. Nano-Micro Lett. 2020;12:54. doi: 10.1007/s40820-020-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayorga-Martinez CC, et al. Layered metal thiophosphite materials: magnetic, electrochemical, and electronic properties. ACS Appl. Mater. Interfaces. 2017;9:12563–12573. doi: 10.1021/acsami.6b16553. [DOI] [PubMed] [Google Scholar]
- 17.Vyskočil J, et al. 2D stacks of MXene Ti3C2 and 1T-Phase WS2 with enhanced capacitive behavior. ChemElectroChem. 2019;6:3982–3986. doi: 10.1002/celc.201900643. [DOI] [Google Scholar]
- 18.Garg R, Agarwal A, Agarwal M. A review on MXene for energy storage application: effect of interlayer distance. Mater. Res. Express. 2020;7:022001. doi: 10.1088/2053-1591/ab750d. [DOI] [Google Scholar]
- 19.Jolly S, Paranthaman MP, Naguib M. Synthesis of Ti3C2Tz MXene from low-cost and environmentally friendly precursors. Mater. Today Adv. 2021;10:100139. doi: 10.1016/j.mtadv.2021.100139. [DOI] [Google Scholar]
- 20.Vaghasiya JV, Mayorga-Martinez CC, Pumera M. Smart energy bricks: Ti3C2@polymer electrochemical energy storage inside bricks by 3D printing. Adv. Funct. Mater. 2021;31:2106990. doi: 10.1002/adfm.202106990. [DOI] [Google Scholar]
- 21.Vaghasiya JV, Mayorga-Martinez CC, Vyskocil J, Sofer Z, Pumera M. Integrated biomonitoring sensing with wearable asymmetric supercapacitors based on Ti3C2 MXene and 1T-Phase WS2 nanosheets. Adv. Funct. Mater. 2020;30:2003673. doi: 10.1002/adfm.202003673. [DOI] [Google Scholar]
- 22.Levitt A, Zhang J, Dion G, Gogotsi Y, Razal JM. MXene-based fibers, yarns, and fabrics for wearable energy storage devices. Adv. Funct. Mater. 2020;30:2000739. doi: 10.1002/adfm.202000739. [DOI] [Google Scholar]
- 23.Qin S, et al. Development and applications of MXene-based functional fibers. ACS Appl. Mater. Interfaces. 2021;13:36655–36669. doi: 10.1021/acsami.1c08985. [DOI] [PubMed] [Google Scholar]
- 24.Latiff NM, et al. Cytotoxicity of layered metal phosphorus chalcogenides (MPXY) nanoflakes; FePS3, CoPS3, NiPS3. FlatChem. 2018;12:1–9. doi: 10.1016/j.flatc.2018.11.003. [DOI] [Google Scholar]
- 25.Ismail N, El-Meligi AA, Temerk YM, Madian M. Synthesis and characterization of layered FePS3 for hydrogen uptake. Int. J. Hydrog. Energy. 2010;35:7827–7834. doi: 10.1016/j.ijhydene.2010.05.061. [DOI] [Google Scholar]
- 26.Ruiz León D, Manríquez Castro V, Kasaneva J, Ávila RE. Insertion of trivalent cations in the layered MPS3 (Mn, Cd) materials. Mater. Res. Bull. 2002;37:981–989. doi: 10.1016/S0025-5408(02)00719-5. [DOI] [Google Scholar]
- 27.Shao L, et al. MXene/RGO composite aerogels with light and high-strength for supercapacitor electrode materials. Compos. Commun. 2020;19:108–113. doi: 10.1016/j.coco.2020.03.006. [DOI] [Google Scholar]
- 28.Fan Z, et al. Modified MXene/holey graphene films for advanced supercapacitor electrodes with superior energy storage. Adv. Sci. 2018;5:1800750. doi: 10.1002/advs.201800750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YZ, et al. MXene printing and patterned coating for device applications. Adv. Mater. 2020;32:1908486. doi: 10.1002/adma.201908486. [DOI] [PubMed] [Google Scholar]
- 30.Abdolhosseinzadeh S, Jiang X, Zhang H, Qiu J, Zhang C. Perspectives on solution processing of two-dimensional MXenes. Mater. Today. 2021;48:214–240. doi: 10.1016/j.mattod.2021.02.010. [DOI] [Google Scholar]
- 31.Abdolhosseinzadeh S, Heier J, Zhang C. Printing and coating MXenes for electrochemical energy storage devices. J. Phy. Energy. 2020;2:031004. doi: 10.1088/2515-7655/aba47d. [DOI] [Google Scholar]
- 32.Chia HL, et al. MXene titanium carbide-based biosensor: strong dependence of exfoliation method on performance. Anal. Chem. 2020;92:2452–2459. doi: 10.1021/acs.analchem.9b03634. [DOI] [PubMed] [Google Scholar]
- 33.Vaghasiya JV, Mayorga-Martinez CC, Sofer Z, Pumera M. MXene-based flexible supercapacitors: influence of an organic ionic conductor electrolyte on the performance. ACS Appl. Mater. Interfaces. 2020;12:53039–53048. doi: 10.1021/acsami.0c12879. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, et al. Engineering 3D ion transport channels for flexible mxene films with superior capacitive performance. Adv. Funct. Mater. 2019;29:1900326. doi: 10.1002/adfm.201900326. [DOI] [Google Scholar]
- 35.Chepkasov IV, Ghorbani-Asl M, Popov ZI, Smet JH, Krasheninnikov AV. Alkali metals inside bi-layer graphene and MoS2: insights from first-principles calculations. Nano Energy. 2020;75:104971. doi: 10.1016/j.nanoen.2020.104927. [DOI] [Google Scholar]
- 36.Xie X, et al. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy. 2016;26:513. doi: 10.1016/j.nanoen.2016.06.005. [DOI] [Google Scholar]
- 37.Yan J, et al. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017;27:1701264. doi: 10.1002/adfm.201701264. [DOI] [Google Scholar]
- 38.Hu M, et al. Emerging 2D MXenes for supercapacitors: status, challenges and prospects. Chem. Soc. Rev. 2020;49:6666–6693. doi: 10.1039/D0CS00175A. [DOI] [PubMed] [Google Scholar]
- 39.Tang H, et al. MXene-2D layered electrode materials for energy storage. Prog. Nat. Sci. Mater. Int. 2018;28:133–147. doi: 10.1016/j.pnsc.2018.03.003. [DOI] [Google Scholar]
- 40.Manikandan R, et al. Electrochemical behaviour of lithium, sodium and potassium ion electrolytes in a Na0.33V2O5 symmetric pseudocapacitor with high performance and high cyclic stability. ChemElectroChem. 2018;5:101–111. doi: 10.1002/celc.201700923. [DOI] [Google Scholar]
- 41.Feng E, et al. Long-term anti-freezing active organohydrogel based superior flexible supercapacitor and strain sensor. ACS Sustain. Chem. Eng. 2021;9:7267–7276. doi: 10.1021/acssuschemeng.1c01209. [DOI] [Google Scholar]
- 42.Vo TT, Lee HJ, Kim SY, Suk JW. Synergistic effect of graphene/silver nanowire hybrid fillers on highly stretchable strain sensors based on spandex composites. Nanomater. 2020;10:2063. doi: 10.3390/nano10102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai G, et al. Flexible and wearable strain sensing fabrics. Chem. Eng. J. 2017;325:396–403. doi: 10.1016/j.cej.2017.05.091. [DOI] [Google Scholar]
- 44.Kim SJ, et al. High durability and waterproofing rGO/SWCNT-fabric-based multifunctional sensors for human-motion detection. ACS Appl. Mater. Interfaces. 2018;10:3921–3928. doi: 10.1021/acsami.7b15386. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, et al. High-performance wearable strain sensor based on MXene@cotton fabric with network structure. Nanomater. 2021;11:889. doi: 10.3390/nano11040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y-Z, et al. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018;4:eaat0098. doi: 10.1126/sciadv.aat0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrett, K. E., Barman, S. M. & Boitano, S. Ganong’s review of medical physiology, 26th edn, (McGraw-Hill Education, 2010).
- 48.Zhao Z, et al. Machine-washable textile triboelectric nanogenerators for effective human respiratory monitoring through loom weaving of metallic yarns. Adv. Mater. 2016;28:10267–10274. doi: 10.1002/adma.201603679. [DOI] [PubMed] [Google Scholar]
- 49.Maveddat A, et al. Severe acute respiratory distress syndrome secondary to coronavirus 2 (SARS-CoV-2) Int J. Occup. Environ. Med. 2020;11:157–178. doi: 10.34172/ijoem.2020.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massaroni C, Nicolò A, Schena E, Sacchetti M. Remote respiratory monitoring in the time of COVID-19. Front Physiol. 2020;11:635. doi: 10.3389/fphys.2020.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicari S, Rizzardi A, Coen-Porisini A. Home quarantine patient monitoring in the era of COVID-19 disease. Smart Health. 2022;23:100222. doi: 10.1016/j.smhl.2021.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, Jiang S, Li Z, Lo B. A pervasive respiratory monitoring sensor for COVID-19 pandemic. IEEE open J. Eng. Med. Biol. 2021;2:11–16. doi: 10.1109/OJEMB.2020.3042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christoe MJ, Yuan J, Michael A, Kalantar-Zadeh K. Bluetooth signal attenuation analysis in human body tissue analogues. IEEE Access. 2021;9:85144–85150. doi: 10.1109/ACCESS.2021.3087780. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.