Abstract
Introduction:
Alcohol-induced thickening of the gut mucosal layer, and increased expression of goblet cell gel forming mucins, such as mucin-2 (MUC2) are associated with disruptions to the gut barrier in alcohol liver disease (ALD). Interest in drugs that can target gut mucins in ALD has grown, however to date, no studies have examined the properties of drugs on expression of gut mucins in models of ALD. We previously demonstrated that at 10 mg/kg/day, the drug Fenretinide [N-(4-hydroxyphenyl) retinamide (Fen)], a synthetic retinoid, mitigates alcohol-associated damage to the gut barrier, and liver injury in a mouse model of ALD.
Methods:
In this study we specifically sough to examine the effects of Fen on gut goblet cells and expression of mucins, including MUC2 using a 25-day Lieber-DeCarli model of chronic alcohol intake.
Results:
Our results show that chronic alcohol intake increased gut mucosal thickening, goblet cell numbers, and mRNA and protein expression of MUC2 in both the ileum and colon. Alcohol intake was associated with marked decreases to ileal and colonic Notch signaling, levels of Notch ligands Dll1 and Dll4, and increases in the expression of Notch-associated genes indispensable for goblet cell specification, including Math1 and Spdef. Interestingly, ileal and colonic expression of KLF4, which is involved in terminal differentiation of goblet cells, was reduced in mice chronically fed alcohol. Co-administration of alcohol with Fen at 10 mg/kg/day significantly reduced alcohol associated increases in ileal and colonic mucosal thickening, ileal Muc2, colonic Muc2, Muc5ac and Muc6 mRNAs, and goblet cell numbers. We also found that Fen strongly prevented alcohol-mediated suppression of the Notch ligand Dll1, Notch signaling, and expression of Notch-associated goblet cell specification genes in both the ileum and colon. In the absence of alcohol, Fen treatments alone at 10 mg/kg/day had no effects on any of the goblet cell related endpoints.
Conclusion:
These data show for the first time that the drug Fen possesses mucosal layer-modulating properties in response to alcohol abuse. These data warrant further pre-clinical examination of Fen given emerging evidence of a role for intestinal goblet cell mucins in the progression of ALD.
Keywords: alcohol, Fenretinide, gut, mucin-2, goblet cells
INTRODUCTION
A convincing body of evidence shows that chronic alcohol intake has multiple negative effects on the gut (reviewed in [1]), including microbial dysbiosis, and disruption of the gut barrier that can promote alcoholic liver disease (ALD) [2, 3]. Gut mucins, synthesized by goblet cells, play an integral role in maintenance of barrier function and protection against gut pathogens (reviewed in [4]). For reasons that remain incompletely understood, alcohol intake alters gut mucin composition, increases mucosal thickening, which is associated with human and murine ALD [5–7]. Conversely, mice lacking intestinal MUC2, the major gel forming mucin [8, 9], are protected against alcohol disruptions to the gut barrier and development of ALD [5], suggesting that MUC2 itself may be involved in the progression of ALD. It was recently shown that mice lacking MUC2 are also protected against a related liver pathology, non-alcoholic fatty liver disease [10]. Given emerging roles for mucins in these pathology and others, including some cancers[11], interest has grown in the potential of gut MUC2, and goblet cells as novel drug targets for ALD other gut pathology[11, 12].
Fenretinide [N-(4-hydroxyphenyl) retinamide (Fen), is a synthetic retinoid known for its anti-cancer, and recently for its anti-diabetic properties [13–15]. We previously demonstrated that when concomitantly administered during chronic alcohol intake, Fen at 10 mg/kg/day prevents disruptions to gut barrier function, and mitigates endotoxemia and liver damage in a murine model of ALD [16]. We also reported, consistent with previous studies in models of type 2 diabetes [15, 17], that Fen at 10 mg/kg/day is non-toxic, and shows no adverse effects, or hepatotoxicity when administered to both untreated, and EtOH-treated mice [16]. Therefore, in this study, we sought to examine the effects of Fen at 10 mg/kg/day on expression of gut secretory mucins, including MUC2, in the distal intestine (ileum) and proximal colon of mice chronically fed alcohol for 25 days.
MATERIALS AND METHODS
Liquid Ethanol Diet.
Male Wild type (Wt) C57BL/6J mice (8–9-week-old) (n=20) were fed a Lieber-DeCarli liquid alcohol diet as descried [16].
Please see extended materials and methods for all experimental details.
RESULTS
Fenretinide reduces ileum and colon mucosal thickness and goblet cell numbers.
We used Periodic Acid Schiff (PAS) and the Alcian Blue (AB) staining to examine the gut mucosal layer thickness and mucin density, as previously described [18, 19]. We found that compared to pair-fed mice, EtOH-fed mice had ~2.5-fold increases in mucosal thickness in the ileum (Shown in Fig. 1A [b vs. a, red arrows], C), and ~2-fold increases in the colon (Shown in Fig. 1F [b vs. a), red arrows], H). Accordingly, we also found that in EtOH-treated compared to pair fed mice, villus PAS staining was 3-fold and 2.3-fold higher in ileum and colonic tissue respectively (Shown in Fig. 1D, I). Moreoever, AB staining, which stains for goblet cell total mucins, showed that ileum and colon goblet cell numbers (Shown in Fig. 1B and 1G [b vs. a, yellow arrows], E, J), and goblet cell mucin density increased by 2–2.5-fold (Show in Fig. 1E), and 3.2–3.7-fold (Shown in Fig. 1J), respectively, in EtOH-treated compared to pair-fed mice. In contrast, ileal mucosal thickness was reduced by 44% (Shown in Fig. 1A, [d vs. b, red arrows], C), and PAS staining (Shown in Fig. 1D), and goblet cell number, and goblet mucin intensity (Shown in Fig. 1E), were similarly reduced by 42%, 31% and 36%, respectively, in EtOH-Fen compared to EtOH-treated mice. We also found that EtOH + Fen mice showed reductions to colonic mucosal thickness by 34% (Shown in Fig. 1F, [d vs. b, red arrows], H), as well as reductions to PAS staining (Shown in Fig. 1I), and goblet cell numbers, and goblet cell mucin intensity, by 57%, 46% and 34% respectively (Shown in Fig. 1J), compared to EtOH-treated mice. We found no differences in mucosal thickness, PAS staining, goblet cell number or mucin intensity in pair-fed + Fen mice compared to the pair-fed group (Shown in Fig. 1A-J).
Fig. 1. Fenretinide reduces ileal and colonic mucosal thickness and goblet cell numbers.
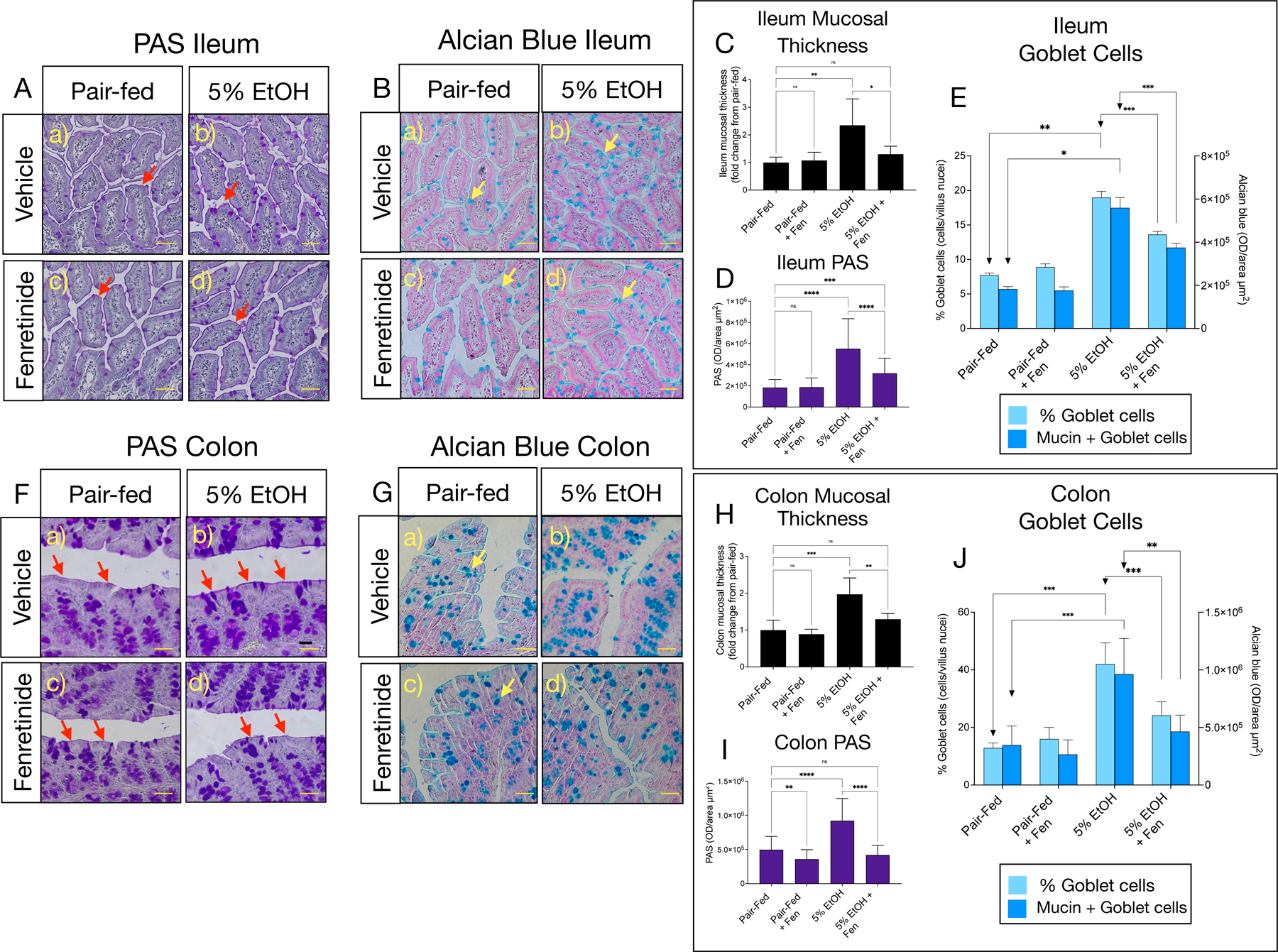
A) and B) Representative images of Periodic Acid Schiff (PAS), and Alcian blue stained goblet cells (yellow arrows) in the ileum, and F) and G) the colon. C) and D) Quantification of mucosal thickness (red arrows), and PAS staining in the ileum, and H) and I) the colon. E) and J) Quantification of goblet cell numbers (light blue bars), and Alcian blue optical intensity (dark blue bars) in the ileum and colon. All image magnification is ×100; scale bar = 50 μm. All data errors bars represent ±SD, with **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant.
Fenretinide reduces ileum and colon mucin mRNA and protein levels.
Mice express three secreted gut mucins, MUC2, MUC5ac and MUC6 [8, 9], and by qPCR we found that ileal mRNA transcripts of Muc2, and Muc6, but not Muc5ac (Shown in Fig. 2A-C), and colonic Muc2, Muc5ac and Muc6 (Shown in Fig. 2F-H), were significantly increased in EtOH-treated compared to pair-fed mice. However, we found that, in contrast, ileum Muc2 (Shown in Fig. 2A), and colonic Muc2 and Muc6 (Shown in Fig. 2F, H), were reduced by approximately 40% in EtOH-Fen mice compared to EtOH-treated mice. We detected no differences in mRNA transcript levels of Muc2, Muc5ac or Muc6 in the ileum or colon of pair-fed + Fen mice compared to any other group (Shown in Fig. 2A-C, F–H). We next assessed ileum and colonic MUC2 protein immunoreactivity by immunohistochemistry (IHC). We found, consistent with the mRNA increases, that ileal and colonic goblet-cell MUC2 protein immunoreactivity increased by 2.5-fold (Shown in Fig. 2D, E [b vs. a, yellow arrows]), and 3.4-fold (Shown in Fig. 2I, J [b vs. a, yellow arrows]), respectively in EtOH-treated mice compared to pair-fed mice. In contrast, we found that compared to EtOH-treated, that ileum and colonic MUC2 levels were reduced by 38% (Shown in Fig. 2D, E [d vs. b, yellow arrows]), and 55% (Shown in Fig. 2I, J [d vs. b, yellow arrows]) respectively, in EtOH-Fen treated mice. We did not detect any changes to ileal or colonic MUC2 immunoreactivity in pair-fed + Fen treated compared to pair-fed mice (Shown in Fig. 2E and J[c vs. a, yellow arrows]).
Fig. 2. Fenretinide reduces ileal and colonic mucin mRNA and protein levels.
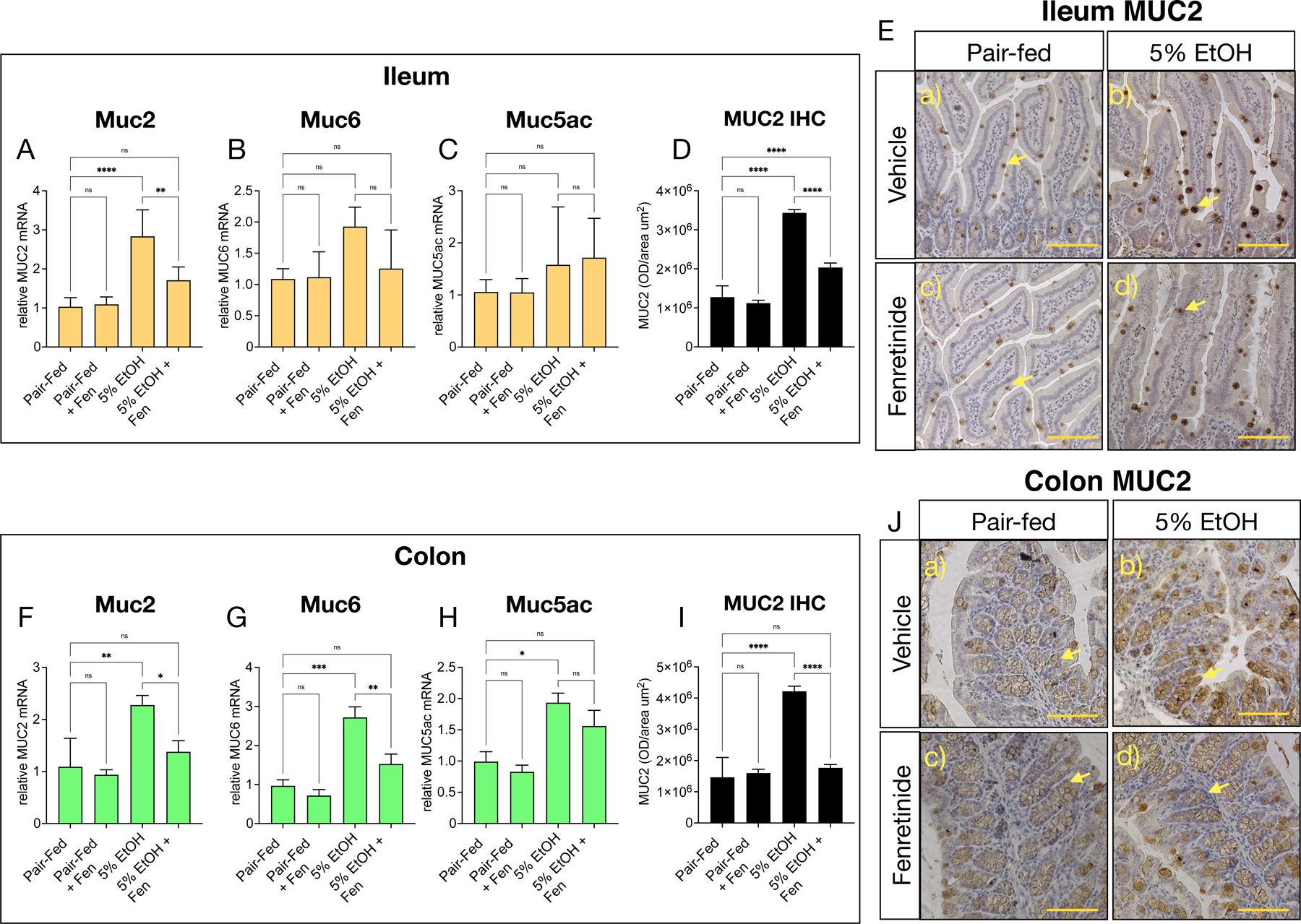
Quantitative PCR (qPCR) histograms of A-C) ileal, and F-H) colonic relative mRNA levels of the genes Muc2, Muc5ac, and Muc6. E) and J) Representative images, and D) and I), quantification of ileal, and) colonic MUC2 immunohistochemistry (IHC). Magnification: ×100; scale bar = 100 μm. All histogram errors bars represent ±SD, with *p< 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant. qPCR results represent gene fold changes relative to pair-fed mice normalized against the housekeeping gene 36B4.
Fenretinide prevents alcohol suppression of gut canonical Notch signaling.
Canonical Notch signaling is indispensable for the maintenance of a balance of gut absorptive cells (enterocyte) and secretory cells (i.e. goblet and Paneth cells) (reviewed in [20]). Therefore, we next determined the effects of EtOH and Fen treatments on ileal and colonic mRNA levels of Notch receptors, and genes involved in canonical Notch signaling. We found that ileal and colonic gene expression of Notch1 and Notch2 receptors were unchanged in all groups compared to pair-fed mice (Shown in Fig. 3A, B, G,H), however, mRNA levels of the Notch target genes, Hes1 and Hey1 [20], were reduced by 2-fold and 2.4-fold in the ileum (Shown in Fig. 3C, D), and 8-fold and 4.5-fold in colon (Shown in Fig. 3I, J) of EtOH-treated, versus pair-fed mice. In contrast, ileum and colonic mRNA levels of Hes1 and Hey1 were unchanged in EtOH + Fen-treated mice compared to pair-fed mice, and significantly higher than EtOH-treated mice (Shown in Fig. 3C, D, I, J). Given these findings, we next measured by IHC ileal and colonic protein expression of the Notch intracellular domain (NICD), which, upon Notch activation, travels to the nucleus to regulate expression of Notch associated genes, and therefore is an indicator of active Notch signaling [20]. Our IHC data showed strong ileal and colonic crypt cell expression, and nuclear localization of NICD in pair-fed mice, and pair-fed + Fen mice (Shown in Fig. 3E and K, yellow arrows). However, compared to pair-fed, EtOH-treated mice had 2–3-fold reductions to of Ileal and colonic NICD protein levels, which showed weak nuclear, and mostly sparse cytoplasmic immunoreactivity (Shown in Fig. 3E and K, b) vs. a), yellow arrows, F, L). In contrast to EtOH-treated, ileal and colonic protein levels of NICD were increased by ~62% and ~158% in EtOH + Fen treated mice, and unchanged compared to pair-fed mice (Shown in Fig. 3E and K, d) vs. b), yellow arrows, F, L).
Fig. 3. Fenretinide prevents alcohol suppression of gut canonical Notch signaling.
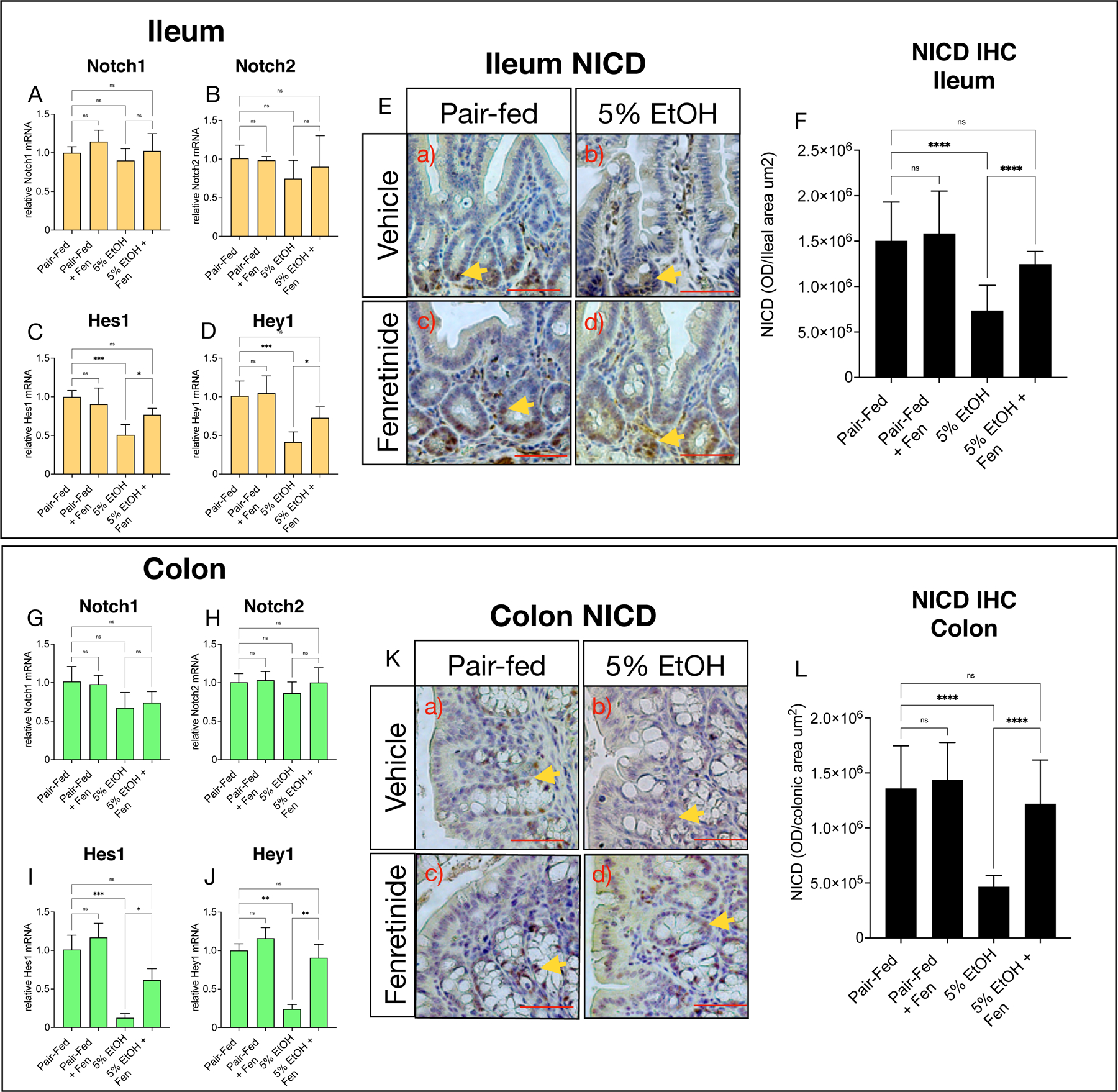
Quantitative PCR (qPCR) histograms of A-D) ileal, and G-J) colonic relative mRNA levels of genes involved in canonical Notch signaling, Notch1, Notch2, Hes1 and Hey1. E) and K) Representative images, and F) and L) quantification of ileal, and colonic Notch intra-cellular domain (NICD) immunohistochemistry (IHC) (yellow arrows). Magnification: ×200; scale bar = 100 μm. All histogram errors bars represent ±SD, with *p< 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant. qPCR results represent gene fold changes relative to pair-fed mice normalized against the housekeeping gene 36B4.
Along with Notch, intestinal Wnt signaling is another key pathway in maintenance of gut absorptive and secretory cells (for review [21]). However, we found mRNA expression of the canonical Wnt target genes Axin2, Tcf4, and Lgr5, an intestinal stem cell marker [22], unchanged in ileal tissue (Shown in Supplementary Fig. 1A-C). Although we found that colonic mRNA levels of Axin2 and Tcf4 were trending higher in EtOH-treated mice compared to pair-fed mice, our analysis found no significant mRNA changes to these genes, or Lgr5 in colonic tissue across all groups (Shown in Supplementary Fig. 1G-I).
Fenretinide opposes alcohol-mediated increases in gut goblet specification genes.
Evidence shows that Notch signaling regulates the gut pool of secretory cells through Hes1-mediated suppression of Math1 (Atoh1), and its downstream target genes involved in goblet cell development and maintenance in the gut [20, 23]. We next measured mRNA levels of Math1, and found that compared to pair-fed, ileal and colonic transcripts of Math1 were increased by ~2.5–3- fold in EtOH-treated mice, but comparatively reduced by 44% and 42% in ileal and colonic tissue respectively in EtOH + Fen mice (Shown in Fig. 4A, I). We next measured mRNA levels of the Math1 target genes, Creb3l1, Clca1, Agr2, and Spdef, which are involved in goblet cell differentiation [20, 23, 24]. We found, consistent with the increases in gut Math1 mRNA levels, that ileal and colonic expression of Creb3l1, and Clca1 were increased ~2–5 fold in the ileum and colon in EtOH-treated compared to pair-fed mice (Shown in Fig. 4B, C, J, K). However, in contrast to EtOH-treated mice, transcript levels of these genes were significantly reduced by ~30–50% in the ileum (Shown in Fig. 4B-D), and 45–54% in the colon (Shown in Fig. 4J-L) of EtOH + Fen-treated mice. Transcript levels of Agr2 were unchanged in ileal tissue of all groups (Shown in Fig. 4D), but increased by ~2-fold in both EtOH-treated and EtOH + Fen, compared to pair-fed mice (Shown in Fig. 4L).
Fig. 4. Fenretinide opposes alcohol-mediated increases in gut goblet specification genes.
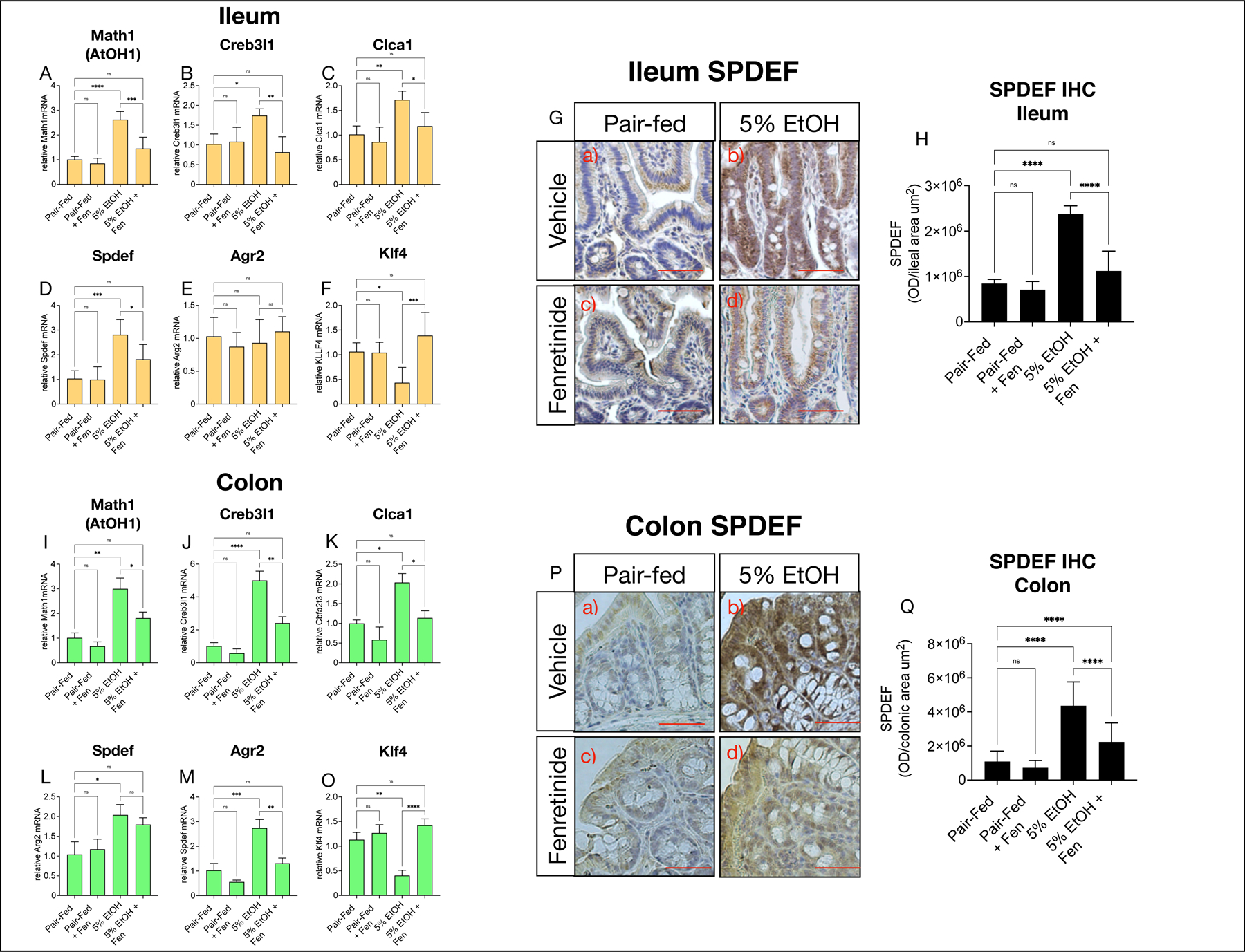
Quantitative PCR (qPCR) histograms of A-F) ileal, and I-O) colonic relative mRNA levels of the goblet specification genes Creb3l1, Clca1, Spdef, Agr2, Klf4. G) and P) Representative images, and H) and Q) quantification of ileal, and colonic SPDEF (SAM Pointed Domain Containing ETS Transcription Factor). Magnification: ×200; scale bar = 100 μm. All histogram errors bars represent ±SD, with *p< 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant. qPCR results represent gene fold changes relative to pair-fed mice normalized against the housekeeping gene 36B4.
Evidence shows that Spdef is required for the terminal differentiation of goblet cells [25, 26]. We found Spdef mRNA levels in ileal and colonic tissue of EtOH-treated mice were elevated approximately 3-fold compared pair-fed, but in comparison reduced ~35–50% in EtOH + Fen-treated mice (Shown in Fig. 4E, M). Consistent with this, we found, using IHC, SPDEF protein immunoreactivity levels elevated by ~2.8–4.2-fold in ileal and colonic tissue of EtOH-treated mice compared to pair-fed, and comparatively reduced by ~50% in EtOH + Fen-treated mice (Shown in Fig. 4G, H, P, Q).
Along with SPDEF, KLF4 is another transcription factor involved in terminal differentiation of goblet cells[27, 28], and is also negatively regulated by Notch[29]. However, we found, unlike Spdef, that ileal and colonic Klf4 mRNA levels were reduced ~2.5-fold in EtOH-treated mice, but unchanged in EtOH + Fen treated mice compared to pair-fed mice (Shown in Fig. 4F, N). Using immunofluorescence microscopy (IFM), we next examined KLF4 protein levels in the gut, and found that, compared to pair-fed mice, ileal and colonic tissue from EtOH-treated mice showed 2–3-fold reductions to KLF4 positive cells in villus regions with concomitantly higher levels of MUC2 positive goblet cells (Shown in Fig. 5A and C [ b vs. a, inset, white arrows], B, H). However, in contrast to EtOH-treated, ileal and colonic tissue from EtOH + Fen treated mice, had 46% and 78% higher levels of KLF4 in villus regions with comparatively lower levels of MUC2 positive goblet cells (Shown in Fig. 5A and C [ d vs. b, inset, white arrows], B, H). Compared to pair-fed, we did not detect changes to gut Math1, or Math1 target genes (Shown in Fig 4A-E, I–M), Klf4 mRNA (Shown in Fig. 4F, O), or percentages of KLF4 positive cells (Shown in Fig. 5A-D) in pair-fed + Fen mice.
Fig. 5. Fenretinide increases ileal and colonic KLF4 positive epithelial cells.
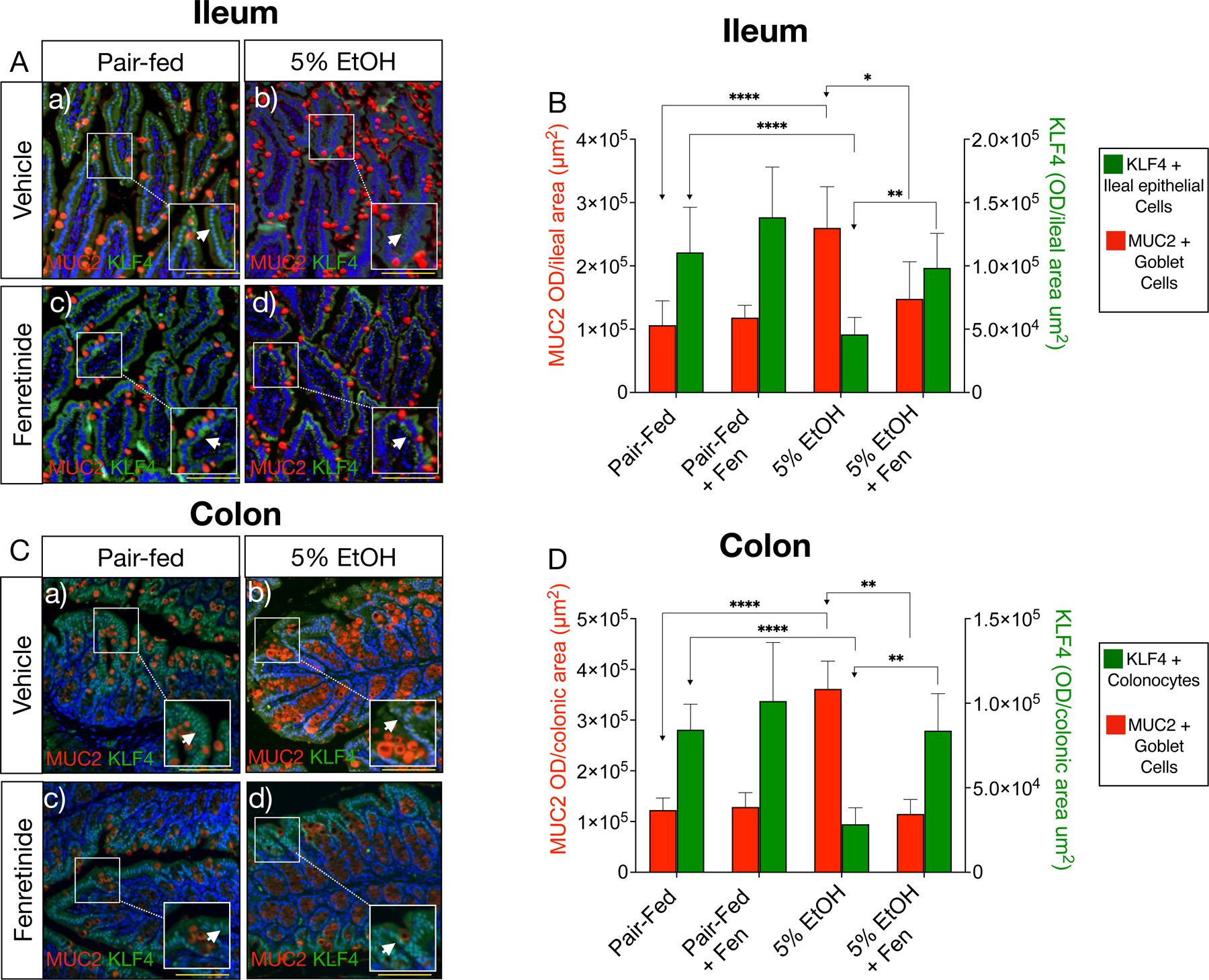
A) and C) Representative double immunofluorescence microscopy (IFM) images of ileal, and colonic tissue double stained with antibodies against MUC2 (red channel) and KLF4 (green channel, white arrows). Magnification: ×100 (inset 200X), scale bar = 100 μm; B) and D) Quantification of ileal and colonic IFM optical intensity for MUC2 (red bars) and KLF4 (green bars). All data error bars represent ±SD, with **p < 0.01, ****p < 0.0001 and ns = not significant.
Notch suppression of Math1 regulated pathways is also necessary for development and maintenance of absorptive enterocytes, and secretory Paneth cells [20, 25, 30]. However, we found that ileal and colonic mRNA levels of markers for enterocytes (Keratin 19 [Krt19])[31], and Paneth cells (lysozyme [Lyz1])[20], were unchanged across all experimental groups (Shown in Supplementary Fig. 1D, E, J, K). Collectively, these data demonstrate that among the intestinal cell types dependent on Notch signaling [20], that EtOH and EtOH + Fen disproportionally modulated Notch signaling and Notch-associated genes, and gene pathways involved in intestinal goblet cell specification.
Fenretinide prevents alcohol mediated reductions to gut Notch ligands, Dll1 and Dll4.
We next measured gut expression of known mediators of the Notch signaling in order to determine potential mechanisms behind EtOH and Fen modulation of Notch signaling in the gut. We first measured ileal and colonic mRNA levels of Psen1, the major subunit of membrane γ-secretase, which liberates NICD from the Notch receptor upon activation [20], and has been used to estimate γ-secretase and Notch signaling activity in cells and tissue [32]. Our qPCR data showed that, compared to pair-fed, ileal transcripts of Psen1 were reduced by approximately 40% in EtOH-treated mice, and unchanged in EtOH + Fen treated mice (Shown in Supplementary Fig. 1F), however these changes did not reach statistical significance. In contrast, colonic Psen1 mRNA levels were unchanged across all experimental groups (Shown in Supplementary Fig. 1L).
We next measured gut expression of Notch ligands, which evidence shows, among the five known ligands for Notch receptors, Jagged 1,2, and Delta-like ligands (Dll1, Dll3 and Dll4)[33], that Dll1 and Dll4 play a key role in development and maintenance of goblet cells in the gut [34]. Therefore, we next examined if EtOH or Fen treatments affected gut mRNA levels of Dll1 and Dll4. Our qPCR analysis showed that, compared to pair-fed, EtOH-treated mice had 47% and 34% reductions to ileal Dll1 and Dll4 respectively, however only the reductions to Dll1 were found to be significantly different (Shown in Supplementary Fig. 2A, B). Similarly, we found that colonic mRNA levels of Dll1 and Dll4 were reduced 62% and 51% respectively in EtOH-treated mice versus pair-fed mice (Shown in Fig. 3C, D). Ileal and colonic mRNA levels of Dll1 and Dll4, were unchanged in EtOH + Fen-treated mice compared to pair-fed (Shown in Supplementary Fig. 2A-D). Moreoever, compared to EtOH-treated, ileal Dll1, and both ileal and colonic Dll1 and Dll4 were significantly higher in ETOH+Fen treated mice (Shown in Supplementary Fig. 2A-D). Next, we measured DLL1 protein by IFM, and found, consistent with the patterns of NICD crypt staining (Shown in Fig. 3E, K), that ileal and colonic DLL1 protein was expressed primarily in crypt cells, (Shown in Supplementary Fig. 2E, F, yellow arrows). In agreement with the mRNA changes, ileal and colonic DLL1 protein was reduced by 2.5–3.2-fold in EtOH-treated mice compared to pair-fed, but comparatively increased by ~75% in EtOH + Fen-treated mice (Shown in Supplementary Fig. 2E-H).
DISCUSSION/CONCLUSIONS
Our data strongly suggest that gut mucosal thickening and increases in goblet cell numbers with chronic EtOH exposure involves reductions in gut canonical Notch signaling (Shown in Fig. 3E, K), and a relative increase in goblet cell specification genes suppressed by Notch, including Math1, Creb3l1 and Spdef (Shown in Fig 4A-D, I–L). Paradoxically, despite increases in goblet cell numbers, we found that EtOH intake led to marked decreases to gut levels of KLF4 (Shown in Fig. 5A-D), which along with SPDEF, is also involved in promoting terminal differentiation of goblet cells [20, 27]. These finding may be due to conflicting reports from a number of KLF4 −/− mutant mouse models on how dispensable KLF4 is on maintaining goblet cells in the adult gut [27, 28, 34, 35].
Two previous studies examining the effects of EtOH feeding on gut secretory cell types showed disparate results [36, 37]. A study by Forsythe et al. [36] reported that feeding mice 15% EtOH (v/v) for 56 days, resulted in suppression of gut canonical Notch signaling (i.e. decreases in Hes1), through inhibition of Notch 1 receptor. However, this study reported increases in gut markers for enterocytes and enteroendocrine cells, but no changes to goblet cells or Wnt signaling [36]. In contrast Park et al. [37] showed that mice fed 5% EtOH (v/v) for 10 days, had increased Wnt signaling in colonic organoids, but no changes to Notch signaling. Our findings are most similar to those by Forsythe et al.[36], however we did not detect reductions to either Notch 1 or Notch 2. Moreoever, our data show no evidence of modulation of Wnt, or to other intestinal cell types. The reasons for the difference across these studies and ours remain unclear, but as with other targets of EtOH exposure in ALD[2], including stem cells[38, 39], we suspect that the impact and mechanisms of EtOH modulation of gut Notch and Wnt, and shifts in gut cell specification, are likely dependent on dose and length of EtOH exposure. Therefore, future studies of EtOH on gut cell specification must consider time and dose of EtOH exposure.
When co-administered with EtOH, Fen at 10 mg/kg/day prevented EtOH-associated mucosal thickening(Shown Fig. 1A, D, E, H), and increases to MUC2 positive goblet cells (Shown in Fig. 3F, H) in both the ileum and colon. Mechanistically, Fen prevention of EtOH-mediated reductions to gut Notch ligands Dll1 and Dll4 (Shown in Supplementary Fig 2A-H), could be a key molecular aspect behind the lower levels of goblet-cell specification genes and mucosal thickening we observed in EtOH + Fen treated mice (Supplementary Fig. 4 model). This is supported by evidence that mice lacking gut DLL1, have suppressed gut Notch signaling, and increases in goblet cell numbers [34]. However, it is incompletely understood what signals modulate gut DLL1 and DLL4 positive intestinal epithelial cells (IEC), or how these IECs transmit their Notch ligands laterally Notch receptor positive IECs [40].
We are not aware of data demonstrating Notch or goblet cell modulating properties of Fen in the gut or in other cell types. The pharmacological effect of Fen on Notch in other cell types has have been limited to cancer cells, where evidence suggests Fen opposes Notch signaling through its growth arresting properties[41, 42]. Still, given that Fen treatments alone had no effect on Notch or any goblet-cell endpoint, suggests that the mechanisms of action of Fen on limiting the EtOH-induced increase in mucosal thickening are likely indirect, and instead involve pathways that are specifically affected by chronic EtOH exposure.
Regarding retinoid signaling, we found that mRNA levels of RARβ and Cyp26A1, two genes strongly regulated by retinoic acid receptors (RARs) [43, 44], were increased in ileum and colon by ~2–40 fold in EtOH-treated mice compared to pair-fed (Shown in Supplementary Fig. 3A-D). These findings are consistent with our previous data and others reporting marked increases in retinoid target genes in the livers of EtOH-fed mice[16, 45, 46]. There were no differences in ileal and colonic mRNA levels of RARβ and Cyp26A1 in EtOH + Fen mice versus EtOH-treated mice (Shown in Supplementary Fig. 3A-D), which suggests, and consistent with[47], RAR-independent pharmacological properties of Fen.
We recognize the limitations to these findings, and, therefore more experiments are needed, including ileal and colonic organoid isolation and ex-vivo studies in EtOH and Fen-treated mice at various time points. Moreoever, an in-dept analysis of gut microbiome, mucosal layer compositional changes, and turnover dynamics with EtOH and Fen are warranted, as evidence suggest that gut bacterial and EtOH can alter the mucosal glycosylation signature and stability[7], which may impact gut barrier functions[48]. Nevertheless, given the novel findings that excessive intestinal mucosa may be clinically relevant to the gut injury and progression of ALD[5–7], these preliminary findings suggest that more pre-clinical studies of Fen are warranted in chronic alcohol abuse.
Supplementary Material
Acknowledgments.
We would like to thank the Hunter College Animal Care Facility for their help in animal handling and care.
Funding Sources
This research was supported by the NIGMS 5SC2GM127206–0 to ST and KM, and by NIAA R21AA027637 and Weill Cornell funds to MM, X-HT and LG.
Footnotes
Statement of Ethics All animal studies and experimental procedures were conducted at Hunter College. This study protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC)/ ethics committee of Hunter College (protocol number approval #STRAR19). All animal experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health, Hunter College IACUC guidelines, and in accordance with the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines 2.0 for in vivo animal experiments [49].
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
REFERENCES
- 1.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol 2003;17(4):575–92. [DOI] [PubMed] [Google Scholar]
- 2.Osna NA, Donohue TM, Kharbanda KK. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res 2017;38(2):147–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Rao R Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009;50(2):638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015;3(1–2):e982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology 2013;58(1):108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur J Chronic ethanol feeding affects intestinal mucus lipid composition and glycosylation in rats. Ann Nutr Metab 2002;46(1):38–44. [DOI] [PubMed] [Google Scholar]
- 7.Grewal RK, Mahmood A. Ethanol effects on mucin glycosylation of mucins in rat intestine.. Ann Gastroenterol 2009;22:178–83. [Google Scholar]
- 8.Gouyer V, Leir SH, Tetaert D, Liu Y, Gottrand F, Harris A, et al. The characterization of the first anti-mouse Muc6 antibody shows an increased expression of the mucin in pancreatic tissue of Cftr-knockout mice. Histochem Cell Biol 2010;133(5):517–25. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Piñeiro AM, Bergström JH, Ermund A, Gustafsson JK, Schütte A, Johansson ME, et al. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am J Physiol Gastrointest Liver Physiol 2013;305(5):G348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann P, Seebauer CT, Mazagova M, Horvath A, Wang L, Llorente C, et al. Deficiency of intestinal mucin-2 protects mice from diet-induced fatty liver disease and obesity. Am J Physiol Gastrointest Liver Physiol 2016;310(5):G310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009;9(12):874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z, Zhong W. Targeting the gut barrier for the treatment of alcoholic liver disease. Liver Res 2017;1(4):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veronesi U, Mariani L, Decensi A, Formelli F, Camerini T, Miceli R, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol 2006;17(7):1065–71. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JP, Reynolds CP, Cho H, Kang MH. Clinical development of fenretinide as an antineoplastic drug: Pharmacology perspectives. Exp Biol Med (Maywood) 2017;242(11):1178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preitner F, Mody N, Graham TE, Peroni OD, Kahn BB. Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am J Physiol Endocrinol Metab 2009;297(6):E1420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang X-H, Melis M, Mai K, Gudas LJ, Trasino SE. Fenretinide Improves Intestinal Barrier Function and Mitigates Alcohol Liver Disease. Frontiers in Pharmacology 2021;12(205). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mcilroy GD, Delibegovic M, Owen C, Stoney PN, Shearer KD, McCaffery PJ, et al. Fenretinide treatment prevents diet-induced obesity in association with major alterations in retinoid homeostatic gene expression in adipose, liver, and hypothalamus. Diabetes 2013;62(3):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filipe MI. Mucins in the human gastrointestinal epithelium: a review. Invest Cell Pathol 1979;2(3):195–216. [PubMed] [Google Scholar]
- 19.Sakamoto K, Hirose H, Onizuka A, Hayashi M, Futamura N, Kawamura Y, et al. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J Surg Res 2000;94(2):99–106. [DOI] [PubMed] [Google Scholar]
- 20.Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol 2016;594(17):4791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 2009;71:241–60. [DOI] [PubMed] [Google Scholar]
- 22.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol 2009;174(3):715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo YH, Chung E, Li Z, Wan YW, Mahe MM, Chen MS, et al. Transcriptional Regulation by ATOH1 and its Target SPDEF in the Intestine. Cell Mol Gastroenterol Hepatol 2017;3(1):51–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YC, Lu YF, Li IC, Hwang SP. Zebrafish Agr2 is required for terminal differentiation of intestinal goblet cells. PLoS One 2012;7(4):e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res 2010;316(3):452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 2009;137(4):1333–45.e1–3. [DOI] [PubMed] [Google Scholar]
- 27.Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Krüppel-like factor 4 gene. Dev Biol 2011;349(2):310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu T, Chen X, Zhang W, Li J, Xu R, Wang TC, et al. Krüppel-like factor 4 regulates intestinal epithelial cell morphology and polarity. PLoS One 2012;7(2):e32492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H, Pritchard DM, Yang X, Bennett E, Liu G, Liu C, et al. KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 2009;296(3):G490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci U S A 2012;109(23):8965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Q, Toivola DM, Feng N, Greenberg HB, Franke WW, Omary MB. Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol Biol Cell 2003;14(7):2959–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azimi M, Le TT, Brown NL. Presenilin gene function and Notch signaling feedback regulation in the developing mouse lens. Differentiation 2018;102:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci 2013;126(Pt 10):2135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 2011;140(4):1230–40.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002;129(11):2619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forsyth CB, Shaikh M, Bishehsari F, Swanson G, Voigt RM, Dodiya H, et al. Alcohol Feeding in Mice Promotes Colonic Hyperpermeability and Changes in Colonic Organoid Stem Cell Fate. Alcohol Clin Exp Res 2017;41(12):2100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JH, Jung IK, Lee Y, Jin S, Yun HJ, Kim BW, et al. Alcohol stimulates the proliferation of mouse small intestinal epithelial cells via Wnt signaling. Biochem Biophys Res Commun 2021;534:639–45. [DOI] [PubMed] [Google Scholar]
- 38.Di Rocco G, Baldari S, Pani G, Toietta G. Stem cells under the influence of alcohol: effects of ethanol consumption on stem/progenitor cells. Cell Mol Life Sci 2019;76(2):231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serio RN, Laursen KB, Urvalek AM, Gross SS, Gudas LJ. Ethanol promotes differentiation of embryonic stem cells through retinoic acid receptor-γ. J Biol Chem 2019;294(14):5536–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schröder N, Gossler A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns 2002;2(3–4):247–50. [DOI] [PubMed] [Google Scholar]
- 41.Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, et al. Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene 2011;30(31):3454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohan N, Banik NL, Ray SK. Synergistic efficacy of a novel combination therapy controls growth of Bcl-x(L) bountiful neuroblastoma cells by increasing differentiation and apoptosis. Cancer Biol Ther 2011;12(9):846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gudas LJ. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim Biophys Acta 2012;1821(1):213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dollé P, Niederreither K. The retinoids : biology, biochemistry, and disease First edition. ed. Hoboken, New Jersey: Wiley Blackwell; 2015. xvii, 585 pages, 28 unnumbered pages of plates p. [Google Scholar]
- 45.Melis M, Tang XH, Attarwala N, Chen Q, Prishker C, Qin L, et al. A retinoic acid receptor β2 agonist protects against alcohol liver disease and modulates hepatic expression of canonical retinoid metabolism genes. Biofactors 2021. [DOI] [PMC free article] [PubMed]
- 46.Ferdouse A, Agrawal RR, Gao MA, Jiang H, Blaner WS, Clugston RD. Alcohol induced hepatic retinoid depletion is associated with the induction of multiple retinoid catabolizing cytochrome P450 enzymes. PLoS One 2022;17(1):e0261675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabichi AL, Xu H, Fischer S, Zou C, Yang X, Steele VE, et al. Retinoid receptor-dependent and independent biological activities of novel fenretinide analogues and metabolites. Clin Cancer Res 2003;9(12):4606–13. [PubMed] [Google Scholar]
- 48.Maccioni L, Leclercq IA, Schnabl B, Stärkel P. Host Factors in Dysregulation of the Gut Barrier Function during Alcohol-Associated Liver Disease. Int J Mol Sci 2021;22(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol 2020;18(7):e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9(7):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.


