Supplemental Digital Content is Available in the Text.
Key Words: video doctor intervention, viral suppression, retention in care
Background:
To determine whether Positive Health Check, a highly tailored video doctor intervention, can improve viral suppression and retention in care.
Setting:
Four clinics that deliver HIV primary care.
Methods:
A hybrid type 1 effectiveness-implementation randomized trial design was used to test study hypotheses. Participants (N = 799) who were not virally suppressed, were new to care, or had fallen out of care were randomly assigned to receive Positive Health Check or the standard of care alone. The primary endpoint was viral load suppression, and the secondary endpoint was retention in care, both assessed at 12 months, using an intention-to-treat approach. A priori subgroup analyses based on sex assigned at birth and race were examined as well.
Results:
There were no statistically significant differences between Positive Health Check (N = 397) and standard of care (N = 402) for either endpoint. However, statistically significant group differences were identified from a priori subgroup analyses. Male participants receiving Positive Health Check were more likely to achieve suppression at 12 months than male participants receiving standard of care adjusted risk ratio [aRR] [95% confidence interval (CI)] = 1.14 (1.00 to 1.29), P = 0.046}. For retention in care, there was a statistically significant lower risk for a 6-month visit gap in the Positive Health Check arm for the youngest participants, 18–29 years old [aRR (95% CI) = 0.55 (0.33 to 0.92), P = 0.024] and the oldest participants, 60–81 years old [aRR (95% CI) = 0.49 (0.30 to 0.81), P = 0.006].
Conclusions:
Positive Health Check may help male participants with HIV achieve viral suppression, and younger and older patients consistently attend HIV care.
Registry Name:
Positive Health Check Evaluation Trial. Trial ID: 1U18PS004967-01. URL: https://clinicaltrials.gov/ct2/show/NCT03292913.
INTRODUCTION
HIV transmission remains an urgent public health challenge. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million persons in the United States have HIV, with 34,800 new infections occurring in 2019.1 Because of advances in antiretroviral therapy (ART), which suppresses the plasma HIV-1 RNA viral load (VL), more people are managing HIV as a chronic health condition. Early initiation of and adherence to ART and retention in care (RIC) are critical prevention strategies, because people with HIV (PWH) who are adherent to ART and are virally suppressed have effectively no risk of transmitting HIV sexually.2–5 To improve HIV VL suppression rates, effective interventions are needed to engage PWH in regular health care that supports ART adherence and RIC.4
Digital interventions are a promising approach for improving ART adherence and VL suppression.6–10 A systematic review found that digital interventions can improve HIV outcomes, including VL suppression, self-care behaviors, and sexual risk reduction.11 However, more recent meta-analyses conclude that the few studies examining VL suppression and the risk of bias in these studies should be considered when examining benefits.12 Digital interventions that use video doctors are a promising approach because they mimic physician–patient interactions.7,13 These interventions can be designed to provide interactive and highly tailored information based on user input, which makes the information provided more relevant, patient-centered, and actionable.9,14
Despite the promise of video doctor interventions, previous studies had small sample sizes, relied on self-reported outcomes, and have not examined both VL suppression and retention-in-care outcomes.6,13,15 To address these issues, we conducted a randomized trial that relied on data extracted from participants' electronic health records (EHRs). Our a priori hypothesis was that, compared with patients in the standard of care (SOC) arm, patients in the intervention arm who received a highly tailored, video doctor intervention called Positive Health Check (PHC) in addition to their SOC would have both higher VL suppression rates (primary endpoint) and higher RIC rates (secondary endpoint) at the 12-month assessment.
METHODS
Design
We used a type 1 hybrid effectiveness-implementation randomized trial design and the Pragmatic-Explanatory Continuum Indicator Summary tool16 as a guide to develop this trial in favor of pragmatism over explanatory methods. This article presents the effectiveness outcomes. The companion article by Garner et al.17 examines the implementation outcomes that were assessed in this trial.
Study Setting
The study was conducted in 4 regionally and demographically diverse HIV primary care clinics in the United States: one in the south central, 2 in the southeast, and one in the northeast. The following Institutional Review Boards approved the study: RTI International, Emory University, Rutgers University, the Florida Department of Health, the University of South Florida, and the Atlanta VA Medical Center Research and Development Committee. The trial protocol was published previously.18
Participants
Participants were recruited from February 28, 2018, to March 1, 2019, from the four participating clinics. Eligibility criteria of the patients included the following: (1) 18 years of age or older; (2) HIV diagnosis; (3) English-speaking; (4) receiving care at a participating clinic; (5) meet at least one of the following subcriteria: (a) most recent VL laboratory result of ≥200 copies/mL; (b) new to care within the past 12 months (ie, new to the clinic or to HIV medical care); and (c) out of care (ie, last attended appointment at the clinic was more than 12 months ago); and (6) no other research study participation that could confound the trial results.
For those who were out of care, we used an adapted version of the Patients Unable to Follow-up Found re-engagement strategy.19 A total of 128 out-of-care participants were recruited and randomized into the study. All participants were compensated $50 at baseline and $50 when the final VL measure was collected.
Randomization and Blinding
We used an electronic, sealed envelope approach for randomization. We generated 500 study identifiers for each site, noting the order in which they were generated. We used sealedenvelope.com's “Create a randomization list” software20 to randomize the assignment study identifiers to either the intervention arm, which included PHC plus the participant's SOC, or the control arm, which was the SOC alone. Although this trial is registered as open label,21 to maintain the integrity of the primary analysis, the Principal Investigator (M.L.), Lead Statistician (C.B.), and trial staff—who interacted with clinic stakeholders (O.B., B.Z., A.O.)—who were implementing the protocol were masked to trial conditions. Clinicians at the study sites did not have access to participants' study arm assignments, and study staff with knowledge of participants' study arm assignments were not involved in data collection.
Intervention
The PHC intervention is an interactive, highly tailored intervention informed by multiple health behavior theories, including motivational interviewing,22 the Information-Behavioral-Motivation Model,23 and the Transtheoretical Model.24
The intervention consists of 7 core components: (1) participant-reported tailoring questions, which included 4 demographic questions, delivered by a video nurse, used to tailor and route a participant through the intervention, and 17 questions delivered by a video doctor, interspersed throughout the intervention to provide tailored information in 6 domains, including treatment readiness, medication adherence, RIC, sexual risk reduction, mother-to-child transmission, and injection drug use; (2) tailored content delivered in the 6 domains; (3) behavior change “tips” provided across the 6 domains; (4) four video doctor options [varying by race (Black, White) and sex (female, male)]; (5) library that autogenerated a list of tailored questions based on participant preferences that could be used during their clinical encounter; (6) patient handout, which could be printed on site, showing the behavior change tips and questions; and (7) an “Extra Info” microsite at the end of the intervention with additional resources and information, such as sexually transmitted infections, condom use, mental health, and transgender health.
Many intervention components, including actor selection, were developed with the input of a diverse panel of HIV primary care providers and PWH. The details of the formative research and technical development of PHC are described elsewhere.25,26 Those assigned to the intervention arm used PHC at baseline during an initial clinical visit when randomization occurred and could use PHC up to 2 more times over the study period. Participants chose the video doctor they would interact with as part of the PHC intervention experience. Tailored messaging and content were provided to participants based on responses to participant-reported tailoring questions. PHC was used in the clinic for this trial but can also be accessed via a link at home or in other locations. Participants used a study-supplied tablet with earbuds and a privacy screen to complete the intervention during their clinic visit. An online digital appendix (see Intervention Overview, Supplemental Digital Content, http://links.lww.com/QAI/B923) provides an overview of PHC.
Standard of Care
Participants in both study arms continued to receive routine care from their health care provider.
Measures
Outcome measures were abstracted from each participant's EHR over the 16-month study period. VL suppression, the primary endpoint, was defined as having <200 copies/mL by the end of each participant's 12 months of follow-up assessment (with a window from the start of 10 months through the end of 16 months postrandomization to accommodate the timing of clinic visits). An intention-to-treat approach was used for all analyses. Participants without VL values at follow-up were considered unsuppressed. There was no statistically significant difference in percentages of participants with missing VL values at follow-up for PHC (n = 74; 20%) compared with SOC (n = 88; 24%) (χ2 = 1.36; P = 0.243).
RIC, the secondary endpoint, was defined for the present study (ie, the PHC-defined measure) as a participant having at least one visit in each 6-month period within 12 months postrandomization, with the 2 visits separated by at least 2 months. Likewise, based on the Health Resources and Services Administration (HRSA) HIV/AIDS Bureau's definition of RIC, a comparable measure entailed having kept 2 postrandomization visits separated by at least 90 days.27,28 In addition, a measure of a 6-month visit gap was defined as having at least 189 days between 2 sequentially kept visits, postrandomization; participants with fewer than 2 postrandomization visits or more than one visit postrandomization but with a gap of 189 or more days were coded as having a visit gap. Demographic variables such as age, race, sex assigned at birth, and new to care were also abstracted from the participant's EHR.
Statistical Analyses
Demographic characteristics of participants in the 2 study arms were compared at baseline using χ2 tests. The percentages of participants in each study arm who achieved VL suppression at the 12-month assessment were calculated for the overall sample and separately by subgroups defined by background characteristics (sex, age, race, site, viral load suppression at baseline, and new to care). Similarly, the percentages of participants in each arm who were retained in care using the HRSA measure and the percentages of those who had a 6-month visit gap were computed.
Unadjusted comparisons of the outcomes (VL suppression and RIC) between the intervention and the control arms were performed using two-sided Fisher exact tests. Generalized linear models with the binomial distribution and the log link function were fit to examine differences in VL suppression across intervention arms, after controlling for sex, age, race, site, baseline VL values, and new to care.
To account for the nonnormality of the distribution of baseline VL values, a base 10 log transformation was applied, and values above 1,000,000 were truncated. Similar models were conducted to examine differences in RIC across the 2 intervention arms, controlling for sex, age, race, site, and new to care. Intervention effects were summarized using adjusted relative risks and 95% confidence intervals. Potential differential intervention impact was evaluated by fitting separate multivariable regression models for each subgroup based on the following variables: sex, age, race, site, baseline VL suppression, and new to care. These models adjusted for all covariates except for the variable used to delineate the subgroup (eg, models for male participants controlled for all variables except sex).
This study was designed to achieve 80% power for comparisons between the intervention and control arms on the primary endpoint (VL suppression), assuming rates of 62% and 50%, respectively, at the 5% significance level and accounting for 25% annual attrition; details of the power analysis are provided in the study by Lewis et al.18 Analyses were conducted using SAS software, version 9.4.29 Statistical tests were performed at the 5% significance level.
RESULTS
Sample Characteristics
Participants (N = 799) were randomized at the baseline clinic visit to receive either the PHC intervention (n = 397; intervention arm) or SOC only (n = 402; control arm) (Fig. 1). Of those participants, 740 (93%)—368 PHC and 372 SOC—had VL data at baseline and were included in the VL analyses. Of these 740 participants, 162 (22%) had missing viral load values at follow-up and were assumed to be unsuppressed. Of the participants with any visits during the 12-month follow-up period, 24.2% of the PHC group and 25.6% of the SOC group had only one follow-up visit.
Figure 1.
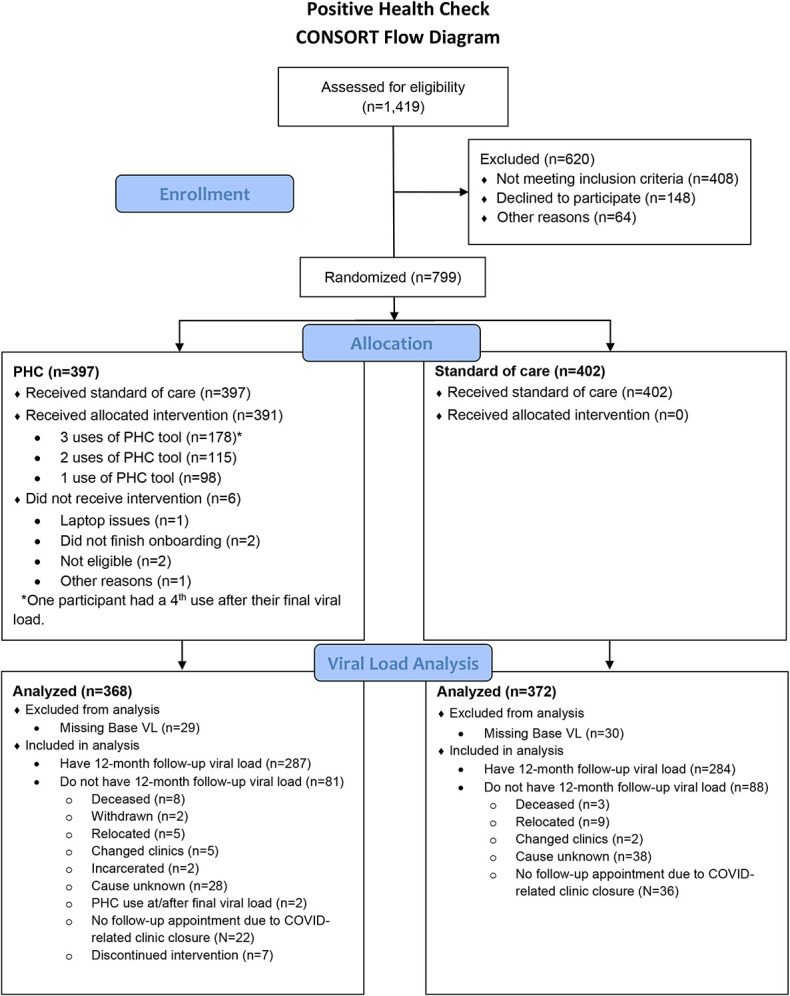
Positive health check trial consort diagram.
The sample was predominantly male (76%) and had a mean (SD) age of 44.9 (12.8) years. Approximately three-quarters (76%) of participants were Black, and roughly one-quarter of the sample was recruited from each of the 4 sites. More than half (56%) of the sample had VL suppression at baseline, and 42% were new to care. There were no statistically significant differences in participant characteristics between the 2 arms (Table 1).
TABLE 1.
Participant Characteristics by Study Arm (N = 799)
| Characteristic | All (N = 799) | PHC (N = 397) | SOC (N = 402) | PHC vs. SOC |
| n (%) | n (%) | n (%) | P | |
| Sex | ||||
| Male | 609 (76) | 303 (76) | 306 (76) | 0.997 |
| Female | 189 (24) | 94 (24) | 95 (24) | |
| Age, yrs | ||||
| 18–29 | 129 (16) | 58 (15) | 71 (18) | 0.368 |
| 30–39 | 177 (22) | 89 (22) | 88 (22) | |
| 40–49 | 172 (22) | 89 (22) | 83 (21) | |
| 50–59 | 223 (28) | 105 (27) | 118 (29) | |
| 60–81 | 96 (12) | 55 (14) | 41 (10) | |
| Race | ||||
| White | 173 (22) | 85 (22) | 88 (22) | 0.958 |
| Black | 600 (76) | 299 (76) | 301 (75) | |
| Other | 21 (3) | 11 (3) | 10 (3) | |
| Site | ||||
| A | 191 (24) | 95 (24) | 96 (24) | 0.998 |
| B | 205 (26) | 101 (25) | 104 (26) | |
| C | 203 (25) | 102 (26) | 101 (25) | |
| D | 200 (25) | 99 (25) | 101 (25) | |
| Baseline viral load | ||||
| Suppressed | 411 (56) | 207 (56) | 204 (55) | 0.699 |
| Unsuppressed | 329 (44) | 161 (44) | 168 (45) | |
| New to care | ||||
| Yes | 332 (42) | 163 (41) | 169 (42) | 0.778 |
| No | 467 (58) | 234 (59) | 233 (58) | |
| Returned to care | ||||
| Yes | 128 (16) | 64 (16) | 64 (16) | 0.938 |
| No | 671 (84) | 333 (84) | 338 (84) |
P-values were calculated using χ2 tests. The following variables have missing values: sex (n = 1), age (n = 2), race (n = 5), and baseline VL (n = 59).
VL Suppression
At baseline, 411 (56%) of study participants had VL values meeting the criteria for suppression, whereas at follow-up, 423 (57%) of study participants were virally suppressed. Of the 329 participants who were unsuppressed at baseline, a total of 69 (21%) were new to care. Percentages of participants with VL suppression at follow-up were the same between the intervention (57%) and control (57%) arms (P = 1.000). In addition, VL suppression at follow-up did not vary statistically significantly between the 2 study arms for any of the subgroups evaluated (Table 2).
TABLE 2.
Number (and Percentage) of Participants With Viral Load Suppression at Follow-up by Subgroup and Study Arm (N = 740)
| Subgroup | PHC (N = 368) | SOC (N = 372) | P |
| n (%) | n (%) | ||
| All participants | 210 (57) | 213 (57) | 1.000 |
| Sex | |||
| Male | 168 (60) | 163 (57) | 0.609 |
| Female | 42 (48) | 50 (57) | 0.288 |
| Age, yrs | |||
| 18–29 | 29 (52) | 35 (51) | 1.000 |
| 30–39 | 54 (64) | 53 (62) | 0.874 |
| 40–49 | 44 (52) | 35 (47) | 0.634 |
| 50–59 | 52 (54) | 67 (63) | 0.201 |
| 60–81 | 30 (65) | 22 (59) | 0.652 |
| Race | |||
| White | 53 (65) | 50 (61) | 0.627 |
| Black | 150 (55) | 157 (57) | 0.669 |
| Other | 6 (55) | 4 (40) | 0.670 |
| Site | |||
| A | 63 (77) | 55 (71) | 0.376 |
| B | 63 (64) | 60 (59) | 0.560 |
| C | 43 (47) | 51 (54) | 0.379 |
| D | 41 (42) | 47 (47) | 0.477 |
| Baseline viral load | |||
| Suppressed | 149 (72) | 142 (70) | 0.664 |
| Unsuppressed | 61 (38) | 71 (42) | 0.433 |
| New to care | |||
| Yes | 107 (69) | 104 (67) | 0.716 |
| No | 103 (48) | 109 (50) | 0.700 |
| Returned to care | |||
| Yes | 43 (69) | 48 (76) | 0.427 |
| No | 167 (55) | 165 (53) | 0.808 |
P-values are based on Fisher exact tests.
Among the overall sample, there was no statistically significant difference in VL suppression for the intervention arm compared with the control arm in regression analysis when controlling for sex, age, race, site, VL suppression at baseline, and new to care adjusted risk ratio (aRR) [95% confidence interval (CI)] =1.07 (0.96 to 1.21), P = 0.220}. Furthermore, there were no statistically significant intervention group differences in VL suppression after controlling for other factors in the subgroup analyses, except among male participants. Male participants receiving the PHC intervention were more likely to have VL suppression at follow-up than those receiving SOC only [aRR (95% CI) = 1.14 (1.00 to 1.29), P = 0.046] (Fig. 2).
Figure 2.
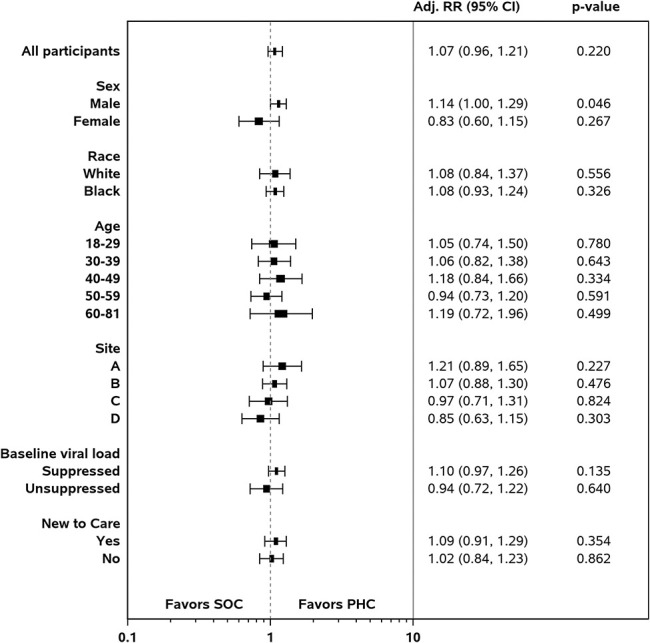
Adjusted relative risks of viral load suppression by study arm (PHC vs. SOC) among subgroups, n = 740.
Retention in Care
Next, we examined differences between the 2 groups based on the 3 measures used to assess RIC (PHC-defined measure, HRSA HAB measure, and visit gap). In the bivariate analyses, there were no significant differences between the PHC + SOC and the SOC groups for any of the 3 RIC measures (Table 3) among the sample as a whole. In the subgroup analyses, the PHC + SOC subgroup had statistically significantly better retention than the SOC group for all 3 measures among participants at one of the sites. Among older participants (ages 60–81 years), patients in the intervention arm had better retention based on the PHC-defined and visit gap measures compared with those in the control arm. Among the youngest participants (ages 18–29 years), patients in the intervention arm had a lower percentage with a visit gap as well.
TABLE 3.
Number (and Percentage) of Participants With Retention in Care by Subgroup and Measure (N = 799)
| Subgroup | PHC Measure (% Retained) | HRSA HAB Measure (% Retained) | 6-Month Visit Gap (% With Gap) | ||||||
| PHC n (%) |
SOC n (%) |
P | PHC n (%) |
SOC n (%) |
P | PHC n (%) |
SOC n (%) |
P | |
| All participants | 257 (65) | 247 (61) | 0.342 | 267 (67) | 264 (66) | 0.654 | 144 (36) | 164 (41) | 0.192 |
| Sex | |||||||||
| Male | 197 (65) | 181 (59) | 0.156 | 204 (67) | 192 (63) | 0.269 | 113 (37) | 132 (43) | 0.160 |
| Female | 60 (64) | 66 (69) | 0.443 | 63 (67) | 72 (76) | 0.200 | 31 (33) | 31 (33) | 1.000 |
| Age, yrs | |||||||||
| 18–29 | 33 (57) | 41 (58) | 1.000 | 37 (64) | 47 (66) | 0.853 | 17 (29) | 34 (48) | 0.046 |
| 30–39 | 60 (67) | 53 (60) | 0.350 | 58 (65) | 60 (68) | 0.750 | 37 (42) | 39 (44) | 0.762 |
| 40–49 | 53 (60) | 55 (66) | 0.430 | 59 (66) | 56 (67) | 1.000 | 36 (40) | 28 (34) | 0.430 |
| 50–59 | 71 (68) | 76 (64) | 0.672 | 72 (69) | 77 (65) | 0.670 | 38 (36) | 41 (35) | 0.889 |
| 60–81 | 39 (71) | 21 (51) | 0.058 | 40 (73) | 23 (56) | 0.128 | 16 (29) | 22 (54) | 0.020 |
| Race | |||||||||
| White | 50 (59) | 49 (56) | 0.759 | 48 (56) | 54 (61) | 0.539 | 36 (42) | 37 (42) | 1.000 |
| Black | 200 (67) | 192 (64) | 0.441 | 210 (70) | 205 (68) | 0.596 | 102 (34) | 120 (40) | 0.151 |
| Other | 6 (55) | 4 (40) | 0.670 | 8 (73) | 4 (40) | 0.198 | 4 (36) | 6 (60) | 0.395 |
| Site | |||||||||
| A | 63 (66) | 46 (48) | 0.013 | 65 (68) | 49 (51) | 0.018 | 36 (38) | 53 (55) | 0.020 |
| B | 67 (66) | 67 (64) | 0.883 | 70 (69) | 70 (67) | 0.767 | 32 (32) | 38 (37) | 0.556 |
| C | 73 (72) | 74 (73) | 0.875 | 72 (71) | 80 (79) | 0.196 | 30 (29) | 31 (31) | 0.879 |
| D | 54 (55) | 60 (59) | 0.568 | 60 (61) | 65 (64) | 0.662 | 46 (46) | 42 (42) | 0.569 |
| Baseline viral load | |||||||||
| Suppressed | 143 (69) | 130 (64) | 0.253 | 148 (71) | 139 (68) | 0.519 | 66 (32) | 76 (37) | 0.256 |
| Unsuppressed | 100 (62) | 102 (61) | 0.821 | 105 (65) | 109 (65) | 1.000 | 64 (40) | 73 (43) | 0.504 |
| New to care | |||||||||
| Yes | 102 (63) | 102 (60) | 0.735 | 111 (68) | 109 (64) | 0.562 | 63 (39) | 68 (40) | 0.823 |
| No | 155 (66) | 145 (62) | 0.386 | 156 (67) | 155 (67) | 1.000 | 81 (35) | 96 (41) | 0.153 |
| Returned to care | |||||||||
| Yes | 48 (75) | 47 (73) | 1.000 | 50 (78) | 49 (77) | 1.000 | 17 (27) | 19 (30) | 0.844 |
| No | 209 (63) | 200 (59) | 0.344 | 217 (65) | 215 (64) | 0.688 | 127 (38) | 145 (43) | 0.238 |
P-values are based on Fisher exact tests. For the PHC measure, retention is defined as having at least 1 visit in each 6-month period within 12 months, separated by 2 months or more, postrandomization. For the HRSA HAB measure, retention is defined as having 2 visits separated by ≥90 days postrandomization. For the 6-month visit gap measure, a gap is defined as having ≥189 days elapsed between sequential visits, postrandomization.
In the analysis of all participants, RIC did not differ statistically significantly between the PHC and SOC arms after controlling for sex, age, race, site, and new to care, based on the PHC-defined measure [aRR (95% CI) = 1.04 (0.94 to 1.15), P = 0.481] or the HRSA HAB-based measure [aRR (95% CI) = 1.00 (0.91 to 1.10), P = 0.966]. For the PHC-defined measure, retention by arm was PHC (65%) vs. SOC (61%). For the HRSA HAB measure, retention by arm was PHC (67%) vs. SOC (66%) (Table 3). Of participants in the PHC arm, 36% had a visit gap compared with 41% in the SOC arm (Table 3). The risk of having a 6-month visit gap did not differ statistically significantly between those receiving PHC and those receiving SOC, after controlling for other factors [aRR (95% CI) = 0.86 (0.72 to 1.03), P = 0.093] (Fig. 3).
Figure 3.
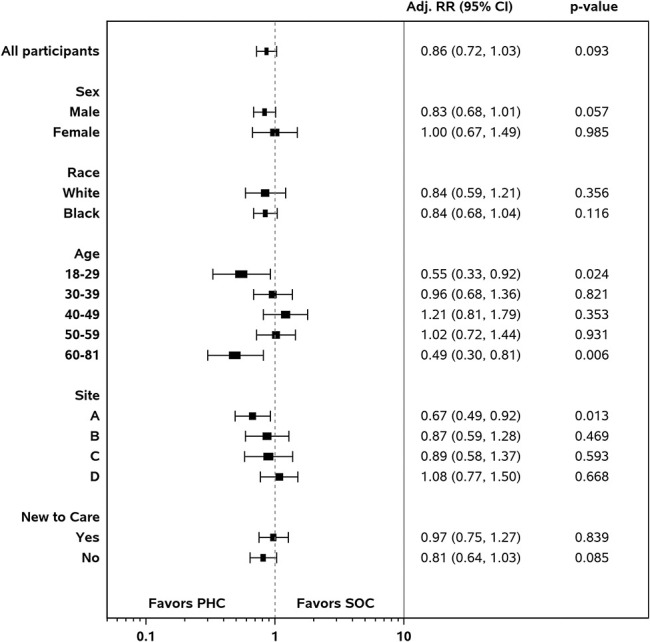
Adjusted relative risk of visit gap by study arm (PHC vs. SOC) among subgroups, n = 799.
In the subgroup analyses, statistically significantly lower risks for having a visit gap were found in the intervention arm than in the control arm, after controlling for covariates for those at the ends of the age continuum: 18–29 years old [aRR (95% CI) = 0.55 (0.33 to 0.92), P = 0.024] and 60–81 years old [aRR (95% CI) = 0.49 (0.30 to 0.81), P = 0.006]. Among the youngest age group, 29% receiving PHC and 48% receiving SOC had visit gaps, although these values were 29% and 54% among the oldest age group.
In addition, the intervention arm shows statistically significant lower risks for a visit gap at one site (site A). At this site, 38% of participants who received PHC had a visit gap, compared with 55% who received SOC [aRR (95% CI) = 0.67 (0.49 to 0.92), P = 0.013]. Participants at this site who received PHC were also more likely to be retained in care based on the PHC-defined measure [66% vs. 48%; aRR (95% CI) = 1.35 (1.05 to 1.72), P = 0.019] and HRSA measure [68% vs. 51%; aRR (95% CI) = 1.32 (1.05 to 1.67), P = 0.019]. No other statistically significant differences were found among the subgroups for the PHC-defined and HRSA measures.
Adverse Events
During the study, there were 14 participant deaths. Each death was reviewed by the respective site's Institutional Review Boards and deemed unrelated to the trial protocol.
DISCUSSION
Our results did not demonstrate statistically significant differences between study arms for our primary and secondary endpoints. However, in a priori defined subgroup analyses, adjusted results showed that PHC resulted in male participants achieving viral suppression at the 12-month assessment point. This finding extends previous research and meta-analyses that point to the potential importance of digital interventions to enhance HIV outcomes and the use of video doctors, specifically.7,12,13 We may have found differences for male participants because of their greater numbers in the study sample and thus there was greater power to detect statistically significant differences among them. Given the limited sample sizes in other subgroups, small differences between the percentages of participants in the PHC and SOC arms who had viral load suppression and various RIC outcomes generally could not be detected, and, conversely, substantial differences between these percentages could not be ruled out.
Beyond issues of statistical power, however, others have argued that because Black male participants experience significant inequities in health care, digital interventions may be helpful to them.30 These types of interventions may facilitate information exchange and shared accountability in health care decision making with clinicians by supporting better communication.30 In addition, it may be that information tailoring plays a particularly powerful role in empowering Black male participants to manage their chronic illness.31 Future research should explore these possibilities.
Our analyses examining the benefit of PHC for RIC did not yield statistically significant differences between study arms. Analysis of a priori defined subgroups found that PHC significantly lowered the risk of a 6-month visit gap for the youngest participants (ages 18–29 years) and oldest participants (ages 60–81 years). Research shows that all age groups are using digital technology at high levels, with older adults increasing their use the most over the last 10 years.32 Older people with HIV tend to have higher retention rates33; however, we cannot determine why the oldest group benefited more from the intervention than the second oldest group (ages 50–59 years). Most research shows that older adults face more barriers to using digital devices and do not feel confident about using digital devices like tablets.34 The extra support for how to use the tablet during the study may have provided the help needed for the oldest participants to benefit from PHC. Because being younger is a risk factor for poorer RIC,35 and many behaviorally based interventions do not help younger people,36 the finding that PHC benefited younger participants' RIC is particularly important.
Our RIC analysis examined 3 RIC measures because there is no established “gold standard.” Research studies conducted in clinical environments should use multiple measures because all measures have limitations.28 Future research testing interactive, tailored, and video doctor interventions would benefit from using multiple RIC measures to advance our understanding of RIC measurement. We operationalized our visit gap measure based on the approach of Mugavero et al.28 Because this measure uses a cut point of 6 months, we may have overestimated the number of study participants with a visit gap by not accounting for visits that occurred right outside this visit gap window. Future research should test if timing around variations in how the visit gap is measured, such as 1–3 weeks outside the window, result in meaningful differences in RIC. One contribution of the Positive Health Check Trial, however, is showing how different RIC measures may improve our understanding of RIC.
We used the Pragmatic-Explanatory Continuum Indicator Summary tool16 as a guide to design a trial that favored pragmatism over explanatory methods to answer the question, “Can a video doctor intervention be implemented under usual clinic conditions and provide a benefit?” In determining effectiveness for PHC, our pragmatic trial design, which embedded the trial in clinic workflows, imposed trade-offs for maximizing external validity. For example, participants completed PHC at a clinic visit—as compared with a specified trial visit—to gather effectiveness evidence while implementing PHC in the real-world of HIV primary care service delivery. Relying on clinic workflows also resulted in using multiple inclusion criteria that may have diluted the effect of the PHC intervention on our main outcome.
Additionally, after baseline, participants continued to complete PHC before an appointment. However, if needed, participants were also allowed to complete PHC after an appointment. Other design choices, such as relying on EHRs for clinical outcome measurement, increased the validity of the VL measurement but precluded collecting participant-reported outcomes. This approach limited our ability to test intervention mediators and collect consistent gender identity information. EHRs in 3 sites did not collect gender identity systematically; and one site did not collect it at all. We had to rely on sex assigned at birth as male or female to characterize the sample. Future studies should collect both EHR and patient-reported outcomes.
Digital interventions may benefit patient outcomes, such as linkage to care, ART adherence, and RIC, more than clinical outcomes, such as viral suppression, given recent advances in treatments for HIV. One benefit of digital interventions is that they can be rapidly modified to accommodate changes in clinical practice guidelines and scale up to home and other environments than other types of interventions.14 Future studies should investigate if interactive, tailored, video doctor interventions, like PHC, improve linkage to care and ART adherence because these outcomes could support a sustained relationship with the health care system. Health care systems provide highly effective treatments to prevent HIV transmission and support for PWH who manage HIV as a chronic health condition.
The results of this trial add to previous research examining the utility of video doctor interventions for other health issues not related to HIV. They improve dietary behaviors and physical activity among pregnant women.37 They also have decreased the number of cigarettes smoked and days of smoking and increased the number of discussions about intimate partner violence when combined with provider cues among pregnant women attending prenatal clinic visit.38,39 Previous studies also indicate that video doctor interventions are feasible and acceptable to patients.40 Many of these studies were conducted when computing power and digital strategies were far less powerful or sophisticated. Our findings and previous studies suggest that video doctors can be applied across a wide array of behaviors and health issues. Given the adaptability, effectiveness, and feasibility of these digitally based interventions, future research should examine whether they are effective in challenging communication contexts that may have rapidly changing evidence bases, such as COVID-19 vaccination. As digital technology advances, the potential for video doctors to assist health systems provide better care will increase as well.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Positive Health Check Team Collaborators: Theron Clark-Stuart, Nana Addo Padi-Adjirackor, Kathryn Meagley, Elena Morales, Julia Gallini, Eric Asencio, Marta Paez-Quinde, Christian Telleria, Pam Holm, Mable Chow, Surya Nagesh, Bomi Choi, Gabriela Plazarte, Paige Ricketts, and Nadine Conner.
Footnotes
V.C.M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences, and ViiV. V.C.M. has received funding from the Emory University Center for AIDS Research (NIH Grant 2P30-AI-050409). This research was supported by a Cooperative Agreement from the Centers for Disease Control and Prevention to Megan A. Lewis (U18PS004967). The other authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Camilla Harshbarger, Email: uzz9@cdc.gov.
Carla Bann, Email: cmb@rti.org.
Vincent C. Marconi, Email: vcmarco@emory.edu.
Charurut Somboonwit, Email: charurut@usf.edu.
Michelle Dalla Piazza, Email: mld229@njms.rutgers.edu.
Shobha Swaminathan, Email: swaminsh@njms.rutgers.edu.
Olivia Burrus, Email: oliviampburrus@gmail.com.
Carla Galindo, Email: fco4@cdc.gov.
Craig B. Borkowf, Email: uzz3@cdc.gov.
Gary Marks, Email: garysmarks@gmail.com.
Shawn Karns, Email: karns@rti.org.
Brittany Zulkiewicz, Email: bzulk@upenn.edu.
Alexa Ortiz, Email: amortiz@rti.org.
Iddrisu Abdallah, Email: xbw7@cdc.gov.
Bryan R. Garner, Email: bryan.garner@osumc.edu.
Cari Courtenay-Quirk, Email: afv2@cdc.gov.
REFERENCES
- 1.Centers for Disease and Control and Prevention. Core Indicators for Monitoring the Ending the HIV Epidemic Initiative (Early Release): National HIV Surveillance System Data Reported through December 2020; and Preexposure Prophylaxis (PrEP) Data Reported through September 2020. HIV Surveillance Data Tables. 2021:2. Available at: http://www.cdc.gov/hiv/library/reports/surveillance-data-tables/vol-2-no-2/index.html. Accessed June 20, 2021. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Fact Sheet. Evidence of HIV Treatment and Viral Suppression in Preventing the Sexual Vransmission of HIV. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hiv/pdf/risk/art/cdc-hiv-art-viral-suppression.pdf. Accessed March 20, 2019. [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crepaz N, Tang T, Marks G, et al. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012-2013. Clin Infect Dis. 2016;63:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393:2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hersch RK, Cook RF, Billings DW, et al. Test of a web-based program to improve adherence to HIV medications. AIDS Behav. 2013;17:2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurth AE, Spielberg F, Cleland CM, et al. Computerized counseling reduces HIV-1 viral load and sexual transmission risk: findings from a randomized controlled trial. J Acquir Immune Defic Syndr. 2014;65:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lustria ML, Cortese J, Noar SM, Glueckauf RL. Computer-tailored health interventions delivered over the Web: review and analysis of key components. Patient Educ Couns. 2009;74:156–173. [DOI] [PubMed] [Google Scholar]
- 9.Noar SM, Black HG, Pierce LB. Efficacy of computer technology-based HIV prevention interventions: a meta-analysis. AIDS. 2009;23:107–115. [DOI] [PubMed] [Google Scholar]
- 10.Pellowski JA, Kalichman SC. Recent advances (2011–2012) in technology-delivered interventions for people living with HIV. Curr HIV/AIDS Rep. 2012;9:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daher J, Vijh R, Linthwaite B, et al. Do digital innovations for HIV and sexually transmitted infections work? Results from a systematic review. BMJ Open. 2017;7:e017604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey JV, Wayal S, Aicken CR, et al. Interactive digital interventions for prevention of sexually transmitted HIV. AIDS. 2021;35:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert P, Ciccarone D, Gansky SA, et al. Interactive “Video Doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3:e1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noar SM. Computer technology-based interventions in HIV prevention: state of the evidence and future directions for research. AIDS Care. 2011;23:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claborn KR, Fernandez A, Wray T, et al. Computer-based HIV adherence promotion interventions: a systematic review. Transl Behav Med. 2015;5:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic–explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62:464–475. [DOI] [PubMed] [Google Scholar]
- 17.Garner BR, Burrus O, Ortiz A, et al. A longitudinal mixed-methods examination of Positive Health Check: implementation results from a type 1 effectiveness-implementation hybrid trial. J Acquir Immune Defic Syndr. 2022, May 18. Available at: 10.1097/QAI.000000000003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis MA, Harshbarger C, Bann C, et al. Positive Health Check evaluation: a type 1 hybrid design randomized trial to decrease HIV viral loads in patients seen in HIV primary care. Contemp Clin Trials. 2020;96:106097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitapati AM, Limneos J, Bonet-Vázquez M, et al. Retention: building a patient-centered medical home in HIV primary care through PUFF (Patients Unable to Follow-up Found). J Health Care Poor Underserved. 2012;23:81–95. [DOI] [PubMed] [Google Scholar]
- 20.Sealed Envelope Ltd. Create a Randomisation List. 2019. Available at: https://www.sealedenvelope.com/simple-randomiser/v1/lists. Accessed September 12, 2019. [Google Scholar]
- 21.RTI International. Positive Health Check Evaluation Trial (PHC). 2000. Available at: https://clinicaltrials.gov/ct2/show/NCT03292913?term=NCT03292913&rank=1. [Google Scholar]
- 22.Miller ER, Rollnick S. Motivational Interviewing. 3rd ed. New York, NY: Guildford Press; 2013. [Google Scholar]
- 23.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;111:455–474. [DOI] [PubMed] [Google Scholar]
- 24.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. [DOI] [PubMed] [Google Scholar]
- 25.Harshbarger C, Taylor O, Uhrig JD, et al. Positive health check: developing a web-based video counseling tool for HIV primary care clinics. J Commun Healthc. 2017;10:70–77. [Google Scholar]
- 26.Harshbarger C, Burrus O, Rangarajan S, et al. Challenges of and solutions for developing tailored video interventions that integrate multiple digital assets to promote engagement and improve health outcomes: tutorial. JMIR Mhealth Uhealth. 2021;9:e21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Health Resources and Services Administration (HRSA). HIV/AIDS Bureau Performance Measures. United States Department of Health and Human Services. Available at: https://hab.hrsa.gov/sites/default/files/hab/clinical-quality-management/coremeasures.pdf. Accessed November 13, 2019. [Google Scholar]
- 28.Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Institute. Inc. SAS 9.4 Statements: Reference. 2013. [Google Scholar]
- 30.Sherman LD, Grande SW. Building better clinical relationships with patients: an argument for digital health solutions with black men. Health Serv Insights. 2019;12:1178632919834315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grande SW, Sherman LD. Too important to ignore: leveraging digital technology to improve chronic illness management among black men. J Med Internet Res. 2018;20:e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson M. Mobile technology and home broadband 2019. Pew Research Center. Available at: https://www.pewresearch.org/internet/2019/06/13/mobile-technology-and-home-broadband-2019/. Accessed June 13, 2019.
- 33.Bhatta M, Nandi S, Dutta N, et al. HIV care among elderly population: systematic review and meta-analysis. AIDS Res Hum Retroviruses. 2020;36:475–489. [DOI] [PubMed] [Google Scholar]
- 34.Anderson M, Perrin A. Barriers to Adoption and Attitudes Towards Technology. Available at: https://www.pewresearch.org/internet/2017/05/17/barriers-to-adoption-and-attitudes-towards-technology/. Accessed May 17, 2017. [Google Scholar]
- 35.Bulsara SM, Wainberg ML, Newton-John TR. Predictors of adult retention in HIV care: a systematic review. AIDS Behavior. 2018;22:752–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurenzi CA, du Toit S, Ameyan W, et al. Psychosocial interventions for improving engagement in care and health and behavioural outcomes for adolescents and young people living with HIV: a systematic review and meta-analysis. J Int AIDS Soc. 2021;24:e25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson RA, Stotland NE, Caughey AB, et al. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Educ Couns. 2011;83:203–209. [DOI] [PubMed] [Google Scholar]
- 38.Humphreys J, Tsoh JY, Kohn MA, et al. Increasing discussions of intimate partner violence in prenatal care using Video Doctor plus Provider Cueing: a randomized, controlled trial. Womens Health Issues. 2011;21:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsoh JY, Kohn MA, Gerbert B. Promoting smoking cessation in pregnancy with Video Doctor plus provider cueing: a randomized trial. Acta Obstet Gynecol Scand. 2010;89:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerbert B, Berg-Smith S, Mancuso M, et al. Using innovative video doctor technology in primary care to deliver brief smoking and alcohol intervention. Health Promot Pract. 2003;4:249–261. [DOI] [PubMed] [Google Scholar]


