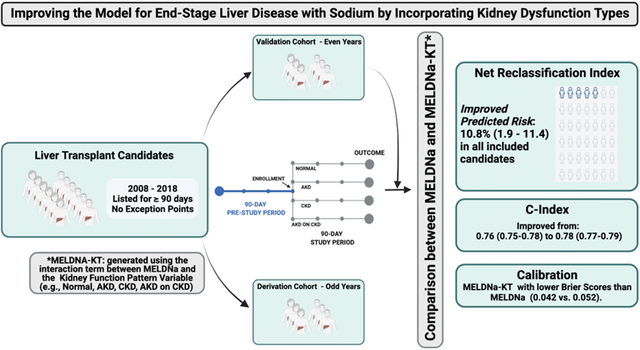
Abstract
Background:
We investigated the impact of the inclusion of kidney dysfunction type on the discrimination and calibration of the model for end-stage liver disease with sodium (MELDNa) score.
Methods:
We included all adults listed for ≥90 days without exception points from January 1, 2008, through December 31, 2018. We defined kidney dysfunction types as follows: Acute Kidney Disease (AKD) (an increase of ≥0.3 mg/dL or ≥50% in serum creatinine in the last 7 days or fewer than 72 days of hemodialysis), Chronic Kidney Disease (CKD) (an estimated glomerular filtration rate <60 ml/min/1.73 m2 for 90 days or ≥72 days of hemodialysis), AKD on CKD (met both definitions), or None (met neither definition). We then developed and validated a multivariable survival model with follow-up beginning at the first assessment after 90 days from waitlist registration and ending at the time of death, waitlist removal, or 90 days from enrollment in this study. The predictor variables were MELDNa and the derived MELDNa-Kidney Dysfunction Type (MELDNa-KT) model.
Results:
In the derivation cohort, kidney dysfunction type was significantly associated with waitlist mortality after controlling for MELDNa. There was a significant linear interaction between kidney dysfunction type and MELDNa score. In the validation cohort, we saw an improvement in the discrimination of the model with an increase in the c index from 0.76 with MELDNa to 0.78 with MELDNa-KT(p=0.002) and a net reclassification index of 10.8%(95CI 1.9–11.4%). The newly derived MELDNa-KT model had lower Brier scores (MELDNa-KT:0.042 v. MELDNa: 0.053).
Discussion:
This study demonstrates the feasibility and the potential for objectively-defined kidney dysfunction types to enhance the prognostication of waitlist mortality provided by the MELDNa score.
Keywords: Acute Kidney Injury, Chronic Kidney Disease, Cirrhosis, Frailty, Cirrhosis, Mortality, Liver Transplant
Graphical Abstract
INTRODUCTION
Currently, liver transplant prioritization depends on the Model for End-Stage Liver Disease with serum sodium score (MELDNa).1 This metric incorporates kidney function as a single serum creatinine (sCr) value. Effectively, this assumes that a given sCr value imparts the same risk for all patients; however, previous studies have demonstrated that this is not the case — kidney dysfunction types are associated with waitlist mortality.2–4 For patients with the same sCr values, those with acute kidney disease (AKD) or AKD on chronic kidney disease (AKD on CKD) have a 2–3 times higher risk of waitlist mortality compared to those with stable CKD, even after controlling for demographics and severity of illness.3 Despite this finding, how to incorporate these kidney dysfunction types and their impact on MELDNa score discrimination and calibration is unknown.
The MELDNa score represents a scoring system to prioritize patients for liver transplant by identifying those at greatest risk for waitlist mortality. It is a proven metric, but to fulfill the goals of the final rule, the regulatory framework for the structure and operations of the organ procurement and transplantation network, the MELDNa score should be continuously updated to improve its discrimination and calibration, particularly as the demographics of the liver transplant population continue to change.5 In recent years the accuracy of the MELDNa score has been decreasing.6 One possible explanation is because of the recent demographic changes in the liver transplant population—the emergence of nonalcoholic fatty liver disease (NAFLD) and its associated co-morbidities (e.g., diabetes, hypertension) coupled with an aging population—the distribution and burden of kidney dysfunction types among liver transplant candidates has changed. As a result, a single sCr measurement, as included in the MELDNa score, is decreasingly capturing the risk of waitlist mortality.7,8 Given these trends, we hypothesized that incorporating kidney dysfunction types (i.e., normal, AKD, CKD, and AKD on CKD), an objective and longitudinal assessment of sCr, in the MELDNa score would improve the model’s performance.
Herein, we demonstrate that the inclusion of kidney dysfunction types, which account for longitudinal changes in sCr, into the MELDNa score leads to a model that is well-calibrated and improves discrimination over the MELDNa score in predicting 90-day mortality.
METHODS
Patients
All patients listed for liver transplant in the UNOS/OPTN registry from January 1st, 2008 through December 31st, 2018, representing a time frame of relatively stable liver allocation policy, were evaluated for inclusion in this study. Patients who were less than 18 years old or listed as Status 1, including those with fulminant hepatic failure, were excluded. Those who received exceptions points or underwent a living donor liver transplant were also excluded, as these patients have a likelihood of receiving a liver transplant that is independent of their liver function. Because we could not know whether patients listed for < 90 days had acute or chronic kidney disease, we excluded those who spent < 90 days on the waitlist (49%). We then used listing year – either even or odd – to divide the cohort into model development (odd) and validation datasets (even).
Covariates
Data were obtained from the UNOS/OPTN registry as of December 6th, 2019. Demographic data and transplant date were collected at listing. Race was self-reported by study participants, and race categories (White and Black) were defined by investigators based on participant self-report to the UNOS/OPTN registry. Similarly, sex was self-reported by study participants, and sex categories (Male and Female) were defined based on report to the UNOS/OPTN registry. The following data were collected at listing, each follow-up, and at the end of follow-up: serum creatinine, serum sodium, total bilirubin, international normalized ratio (INR), presence of hepatic encephalopathy (HE), and presence of ascites. Cutoffs deemed to be implausible were as follows: total bilirubin ≤ 0 mg/dL, INR ≤ 0, and creatinine ≤ 0 mg/dL (9). Observations with implausible values or missing data were dropped—this represented <1% of the cohort. The Model for End-Stage Liver Disease including Serum Sodium (MELDNa) score (10) was calculated and capped at 6 and 40, per current liver allocation policy. Listing diagnoses were grouped into the following common diagnostic categories: hepatitis C virus (HCV), nonalcoholic fatty liver disease (NAFLD, including cryptogenic cirrhosis and nonalcoholic steatohepatitis), alcohol-related cirrhosis (ALD), autoimmune etiologies (including primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis), and other etiologies of cirrhosis (any other listing code that met inclusion criteria). Listing and receipt of a simultaneous-liver kidney transplant (SLKT) were determined at the time of listing. As outlined in UNOS protocol, these patients received no additional prioritization for liver transplant.
Kidney Function
Serum creatinine and estimated glomerular filtration rate (eGFR) were determined longitudinally from the time of listing for liver transplant to removal from the liver transplant waitlist. Kidney function was assessed, as dictated by transplant policy. This was every 7 days if MELDNa ≥ 25, every 30 days if MELDNa 19 – 24, every 3 months if MELDNa 11 – 18, and yearly if MELDNa ≤ 10. When a patient had more than one serum creatinine for the same 7-day period, the first test result was used. To define AKD, each serum creatinine was compared to the serum creatinine seven or more days earlier; a cut-off of either a 0.3 mg/dL increase in sCr or a ≥ 50% from the previous value was chosen according to the International Club of Ascites definition.9 Because we used sCr measurements at ≥ 7-day intervals, we used the terminology of AKD, rather than acute kidney injury (AKI).10 We defined CKD according to KDIGO guidelines: an eGFR < 60 ml/min/1.73 m2 for ≥ 90 days.11 We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine-based equation.12,13 We chose this equation because understanding the limitations of the GFR estimations that can be used with the data available in the UNOS/OPTN registry, the CKD-EPI creatinine-based equation most closely estimates GFR relative to measured GFR in patients with cirrhosis.14–17 Those on hemodialysis were treated as having an eGFR <15 ml/min/1.73 m2 and were not included in the descriptive statistics for serum creatinine and eGFR. Patients with fewer than two observations of kidney function were treated as having normal kidney function unless they were on hemodialysis, which was treated as AKD or CKD, as above.
Kidney Dysfunction Types Definitions
We defined kidney dysfunction types as follows:
AKD: an increase in serum creatinine by either a ≥ 0.3 mg/dL, ≥ 50%, or < 72 days of hemodialysis
CKD: an eGFR<60 ml/min/1.73 m2 for 90 days or ≥ 72 days of hemodialysis
AKD on CKD: meeting both AKD and CKD definitions
Normal Kidney Function: meeting neither definition
Outcomes
The primary outcome was waitlist mortality, defined as death or removal from the waitlist for sickness. Patient follow-up began at their first assessment after 90 days from waitlist registration and ended at the time of death, removal from the waitlist, or 90 days from enrollment in this study (Figure 1). Only data from the enrollment assessment for this study were analyzed. We chose 90-day outcomes to replicate previous studies on MELDNa score in liver transplant.1,18 Patients removed from the waitlist for adequate hepatic reserve, social reasons, or liver transplant were censored at the time of their removal.
Figure 1. Schematic Diagram of Study Design.
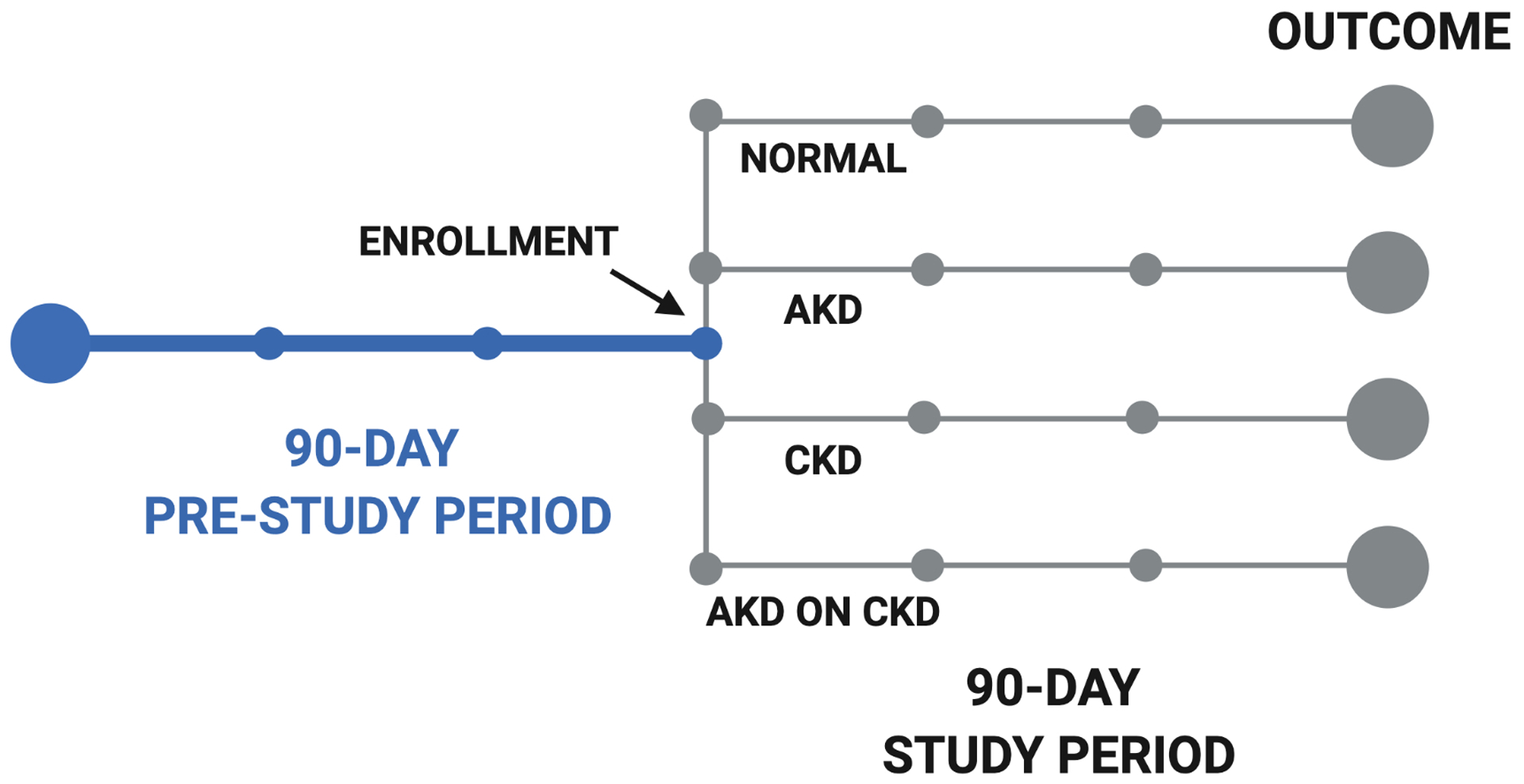
Acute Kidney Disease (AKD); Chronic Kidney Disease (CKD);
Statistical analysis
Continuous variables were compared between derivation and validation cohorts by Wilcoxon rank-sum. Categorical variables were compared between derivation and validation cohorts by chi-squared test.
Model Building
We hypothesized that there was a link between kidney dysfunction types and our outcomes of interest. To test this hypothesis, we utilized Cox regression analyses and Kaplan-Meier methods to determine the risk of AKD and waitlist mortality associated with each variable. We chose standard Cox models instead of competing risks models because we were interested in using models that have stronger etiological interpretations.19 Our approach was similar to that of prior studies investigating changes in MELD score.1,18,20
Using the model development cohort, the initial step was to ensure there were no significant non-linear interactions between MELDNa score and kidney dysfunction type. We did this by testing for the interactions between the kidney dysfunction type and MELDNa score after 3-, 5-, and 7-knot cubic splines. Because we did not find any nonlinear interactions, we utilized a linear interaction between the MELDNa score and the kidney dysfunction type. This model was used to convert the impact of kidney dysfunction type into a change in MELDNa points.
Model Testing
Using the validation cohort, we compared the discrimination and calibration between the current MELDNa model and our adjusted model, MELDNa-Kidney Dysfunction Type (MELDNa-KT).21 We tested the discrimination of the model by measuring the c-indices of a MELDNa vs. MELDNa-KT model. These were calculated using Harrell’s C method. These were compared using Bootstrap resampling with 1000 iterations and using a nonparametric analytical approach for two correlated c-indices that estimates the variance of the C estimator and the covariance of two C estimators.22 We determined the time trends in the discrimination ability of the two models by varying the truncation time-point based on previously described techniques that rely on the inverse probability of censoring weighted estimators.23 In other words, we utilized inverse probability of censoring weighted estimators to determine the c-index of the models to predict waitlist outcomes at 10-day intervals (i.e., Day 10, Day 20, […] Day 90). Additionally, we performed a net reclassification analysis to determine the number of patients, the number of cases (deaths), and the number of controls that would be assigned either greater or lower risk with model adjustment. We constructed 5 × 5 tables based on their predicted risk at 20% risk intervals (reported as MELDNa scores and risk percentiles) for all patients, cases, and controls. We report the number of all patients, all cases, and all controls that were reclassified. We report the net reclassification index (NRI) to demonstrate the percentage of patients that have any improvement in their predicted risk with the addition of kidney dysfunction types. We again used bootstrap methods to determine 95% confidence intervals for these proportions with 1000 iterations. We tested the calibration of the model by applying the Hosmer and Lemeshow goodness of fit test to survival data using the Nam and D’Agostino method and by generating Brier scores.24 We generated calibration plots by comparing the proportion of observed mortality to predicted mortality from both MELDNa and MELDNa-KT.
Sensitivity Analysis
A priori, we planned to complete two sensitivity analyses to evaluate if these changes would differentially impact sub-populations. We repeated all analyses by sex and race. Given reports that the MELDNa has decreased in accuracy in recent years, we performed a third sensitivity analysis where we stratified our analysis in the validation cohort by two eras (Era 1: 2008, 2010, 2012, 2014; Era 2: 2016, 2018).25 We first analyzed patients listed in 2008, 2010, 2012, and 2014. We then repeated these analyses in those listed from 2016 and 2018. We additionally stratified patients by listing year and calculated the c-index and 95% confidence intervals for each model. We present the c-indices, NRIs, Brier scores, and calibration plots for all sub-groups.
Significance, Software, Disclosures
Two-sided p-values <0.05 were considered statistically significant. Analyses were performed using R Studio statistical software (R 3.6.2, Vienna, Austria) and the following packages: survival, haven, compareC, dplyr, ggplot2, nricens, pec, stdca, GND.calib, and kmdec. This study was approved by the institutional review board at the University of California, San Francisco.
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
RESULTS
During the study period, 36,123 participants were awaiting liver transplant and were included in our analyses—16,611 in the derivation cohort and 19,512 in the validation cohort. Characteristics of the patients in the derivation and validation sets are shown in Table 1. These groups were statistically similar for all variables, except for black race, where the validation cohort had slightly greater representation (8 vs. 7%, p=0.01).
Table 1.
Demographics of Derivation and Validation Cohorts.
| Derivation (N=16,611) |
Validation (N=19,512) |
p-value1 | |
|---|---|---|---|
| Kidney Function Pattern, n (%) | 0.38 | ||
| AKD | 754 (4.5) | 840 (4.3) | |
| AKD on CKD | 1225 (7.4) | 1506 (7.7) | |
| CKD | 4418 (27) | 5118 (26) | |
| Normal | 10214 (61) | 12048 (62) | |
| Age (Years), Median (IQR) | 56 (49 – 62) | 56 (49 – 61) | 0.87 |
| Female Sex, n (%) | 6421 (39) | 7648 (39) | 0.29 |
| Self-Identified Black, n (%) | 1297 (7.8) | 1391 (7.1) | 0.01 |
| Etiology of Cirrhosis, n (%) | 0.21 | ||
| Alcohol-Related | 3652 (22) | 4318 (22) | |
| Autoimmune-Related | 2305 (14) | 2649 (14) | |
| HCV | 5116 (31) | 6125 (31) | |
| NAFL | 4019 (24) | 4557 (23) | |
| Other | 1519 (9.1) | 1863 (10) | |
| MELDNa (Points), Median (IQR) | 18 (13 – 23) | 18 (13 – 23) | 0.81 |
| Ascites, n (%) | 6564 (40) | 7867 (40) | 0.12 |
| Hepatic Encephalopathy (HE), n (%) | 2201 (13) | 2702 (14) | 0.10 |
| Waitlist Outcome, n (%) | 0.43 | ||
| DDLT | 6355 (38) | 7524 (39) | |
| Death | 4806 (29) | 5710 (29) | |
| Waiting | 5450 (33) | 6278 (32) |
Acute Kidney Disease (AKD), Chronic Kidney Disease (CKD), interquartile range (IQR), Hepatitis C (HCV), Non-alcoholic Fatty Liver Disease (NAFL), Deceased Donor Liver Transplant (DDLT)
Pearson’s Chi-squared test; Wilcoxon rank sum test
In the derivation cohort, the 90-day Kaplan-Meier survival rate was 92%. After adjusting for MELDNa at enrollment and as compared to those with normal kidney function, those with AKD (adjusted hazard ratio [aHR] 1.72, 95% CI 1.44 – 2.08) and those with AKD on CKD (aHR 1.48, 95CI 1.26 – 1.75) had higher rates of waitlist mortality, while those with CKD (aHR 0.80, 95CI 0.70 – 0.92) had lower rates of waitlist mortality.
Additionally, we found a significant interaction between MELDNa score and Kidney Dysfunction type (as compared to the interaction between normal+MELDNa, AKD+MELDNa: coefficient (coef) 0.035, p=0.01; CKD+MELDNa: coef 0.038, p<0.001; AKDonCKD+MELDNa: coef 0.036, p<0.001). Because of the interaction, the impact of kidney dysfunction type depends on the MELDNa score, and therefore we made the following calculations to derive MELDNa score equivalents:
AKD: (MELDNa × 0.035480 – 0.318060)/(0.134426+0.035480)
CKD: (MELDNa × 0.038190 – 1.087994)/(0.134426+0.038190)
AKD on CKD: (MELDNa × 0.036395 – 0.488997)/(0.134426+0.036395)
For example, if a patient has a MELDNa score of 20, then the impact of AKD is equivalent to adding 2.30 (20 × 0.035480 – 0.318060)/(0.134426+0.035480) points to the MELDNa score (Table 2).
Table 2.
The change to MELDNa score based on kidney dysfunction type at 5 point MELDNa intervals
| At a MELDNa of*: | |||||||
|---|---|---|---|---|---|---|---|
| 10 | 15 | 20 | 25 | 30 | 35 | 40 | |
| +0.22 | +1.26 | +2.30 | +3.35 | +4.39 | +5.44 | +6.48 | |
| CKD | −4.09 | −2.98 | −1.88 | −0.77 | +0.33 | +1.44 | +2.55 |
| AKD on CKD | −0.73 | +0.33 | +1.40 | +2.46 | +3.53 | +4.59 | +5.66 |
AKD (Acute Kidney Disease); CKD (Chronic Kidney Disease)
These were calculated using the following equations:
AKD: (MELDNa × 0.035480 – 0.318060)/(0.134426+0.035480);
CKD: (MELDNa × 0.038190 – 1.087994)/(0.134426+0.038190);
AKD on CKD: (MELDNa × 0.036395 – 0.488997)/(0.134426+0.036395
+ indicate points that were added to the MELDNa score; - indicate points that were deducted from the MELDNa score
In the validation cohort, we found that MELDNa-KT was significantly associated with waitlist mortality (HR 1.15, 95CI 1.15 – 1.16). To test the model’s discrimination, we next tested the c-indices of both the MELDNa-KT and MELDNa models. These were 0.78 (95CI 0.77 – 0.79) and 0.76 (95CI 0.75 – 0.78), respectively. Although these c-indices were numerically similar, the difference was statistically significant (p=0.002). Additionally, the c-index for the MELDNa-KT model was greater than the c-index for the MELDNa model at predicting waitlist mortality at each time point of follow-up (Figure 2). To compare the discrimination of the MELDNa-KT model with a different method, we performed a net reclassification analysis. We found that moving to a MELDNa-KT model improved the predicted risk in 10.8% (95CI 1.9 – 11.4%) overall. This included 11.9% (95CI 1.5 – 12.8%) in cases and −1.0% (95CI −1.2 – 2.3%) of controls. We demonstrate the number of participants reclassified by overall, cases, and controls in Tables 3a–3c.
Figure 2.
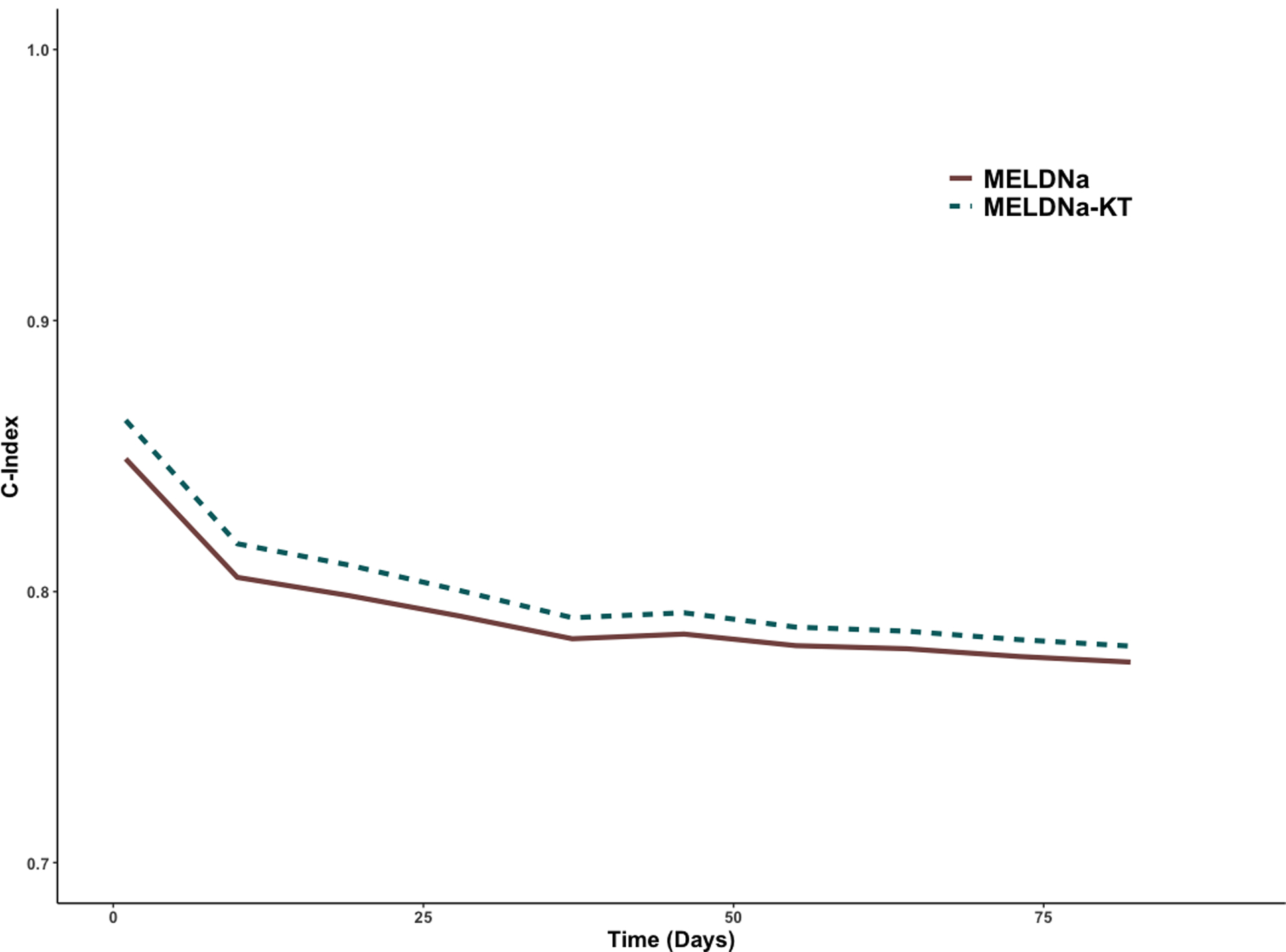
Concordance Statistics of MELDNa and MELDNa-KT Models at Predicting Waitlist Mortality at 10-day intervals of Follow-up
Tables 3A-C.
Reclassification of Liver Transplant Candidates Between MELDNa and MELDNa-KT in the Validation Set.
| Percentile of Risk and MELDNa-KT Category | |||||||
|---|---|---|---|---|---|---|---|
| Percentile of Risk with MELDNa (%) and MELDNa Category | <20 | 20–39 | 40–59 | 60–79 | >80 | ||
| 6–26 | 27–31 | 31–34 | 35–38 | >38 | |||
| <20 | 6–26 | 16763 | 253 | 0 | 0 | 0 | |
| 20–39 | 27–31 | 173 | 1345 | 246 | 0 | 0 | |
| 40–59 | 31–34 | 0 | 60 | 238 | 104 | 0 | |
| 60–79 | 35–38 | 0 | 0 | 49 | 80 | 89 | |
| >80 | >38 | 0 | 0 | 0 | 11 | 101 | |
| Percentile of Risk and MELDNa-KT Category | |||||||
| <20 | 20–39 | 40–59 | 60–79 | >80 | |||
| Percentile of Risk with MELDNa (%) | 6–26 | 27–31 | 31–34 | 35–38 | >38 | ||
| <20 | 6–26 | 831 | 44 | 0 | 0 | 0 | |
| 20–39 | 27–31 | 22 | 276 | 69 | 0 | 0 | |
| 40–59 | 31–34 | 0 | 12 | 79 | 50 | 0 | |
| 60–79 | 35–38 | 0 | 0 | 15 | 27 | 52 | |
| >80 | >38 | 0 | 0 | 0 | 5 | 61 | |
| Percentile of Risk and MELDNa-KT Category | |||||||
| <20 | 20–39 | 40–59 | 60–79 | >80 | |||
| Percentile of Risk with MELDNa (%) | 6–26 | 27–31 | 31–34 | 35–38 | >38 | ||
| <20 | 6–26 | 15942 | 209 | 0 | 0 | 0 | |
| 20–39 | 27–31 | 151 | 1069 | 177 | 0 | 0 | |
| 40–59 | 31–34 | 0 | 48 | 159 | 54 | 0 | |
| 60–79 | 35–38 | 0 | 0 | 34 | 53 | 37 | |
| >80 | >38 | 0 | 0 | 0 | 6 | 40 | |
Green demarcation indicates areas of up-scoring (MELDNa-KT category higher than MELDNa); Red demarcation indicates areas of down-scoring
We next tested the calibration of the MELDNa-KT model as compared to the MELDNa model. We found that both the MELDNa-KT and MELDNa models both significantly had a lack of fit for the validation cohort (p<0.001, for both). We demonstrate the calibration plot in Figure 3. The Brier scores for the MELDNa-KT and MELDNa models, where lower scores signify better calibration, were 0.042 and 0.052, respectively.
Figure 3.
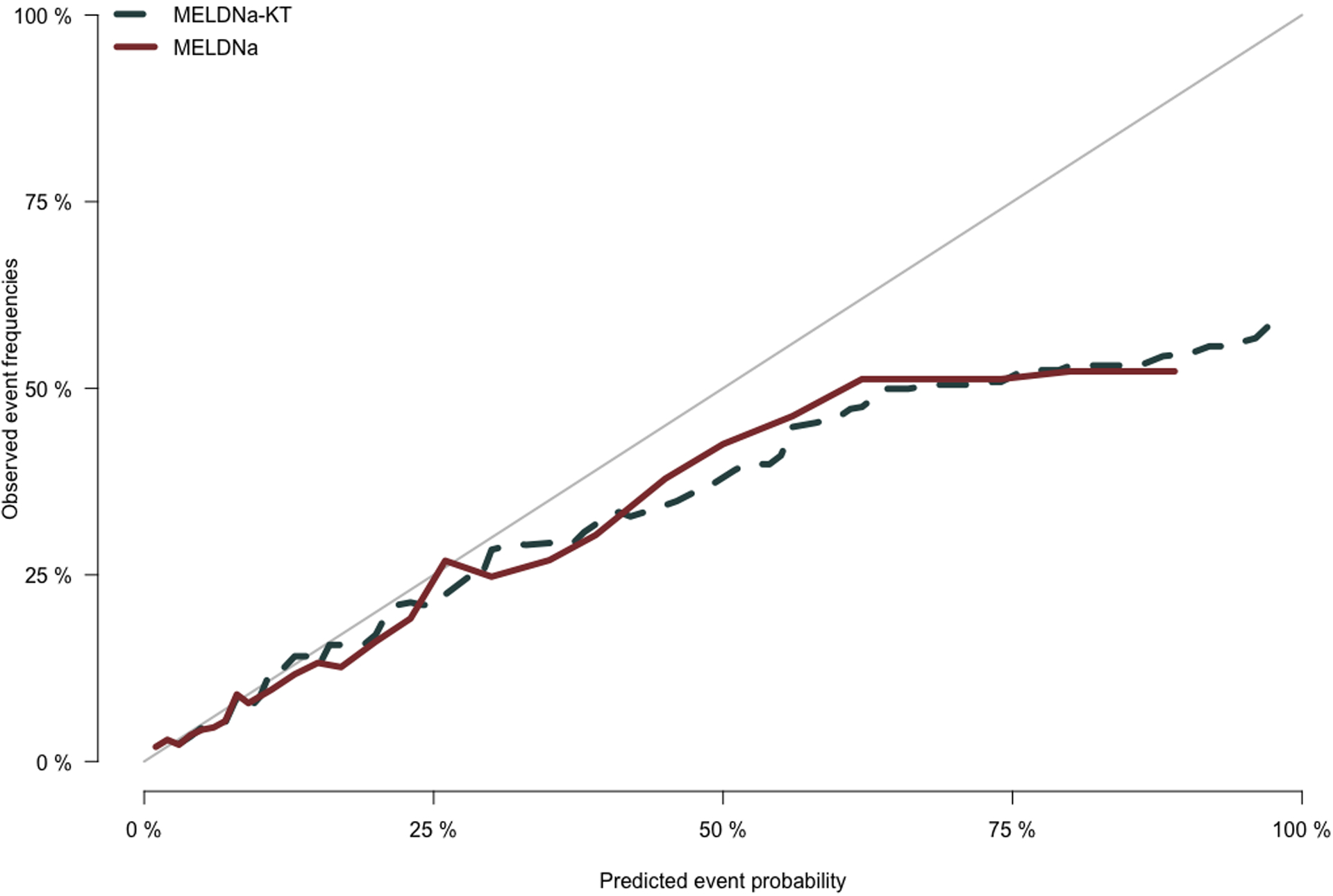
Calibration Plot Comparing Actual to Predicted Percent Waitlist Mortality
Model Performance in Women
Among the 7,648 women in the validation cohort, the Kaplan-Meier survival was 92%. We found that the MELDNa-KT model had a significantly higher c-index for waitlist mortality among women than the MELDNa model (MELDNa-KT: 0.79, 95CI 0.77 – 0.81 vs. MELDNa: 0.77, 95CI 0.76 – 0.80; p=0.03). We did not find a significant net reclassification in women: Overall: 2.9%, 95CI −2.3 – 10.8; Cases: 1.3%, 95CI −3.7 – 10.1%; Controls: 1.5%, 95CI −0.1 – 3.0%. Among women, we found that the Brier scores were lower for the MELDNa-KT model, as compared to the MELDNa (0.043 v. 0.055, respectively).
Model Performance in Self-Identified Blacks
Among the 1,391 Blacks in the validation cohort, the Kaplan-Meier survival was 91%. We found that there was no significant difference among Blacks between the c-indices for the MELDNa-KT and MELDNa models (MELDNa-KT: 0.79, 95CI 0.74 – 0.84 vs. MELDNa: 0.78, 95CI 0.74 – 0.83, p=0.32). We found no significant improvement in the reclassification of Blacks with the MELDNa-Kidney model: Overall: 5.7%, 95CI −9.7 – 15.6%; Cases: 4.7%, 95CI −11.4 – 14.7%; Controls: 1.0%, 95CI −1.3 – 3.6%. We found that the Brier scores were lower for the MELDNa-KT model, as compared to the MELDNa (0.042 v. 0.051, respectively).
Model Performance by Era
In 2008, 2010, 2012, and 2014, we had 14392 patients in the validation cohort. We found that the c-indices for the MELDNa-KT and MELDNa models were similar (MELDNa-KT: 0.80, 95CI 0.78 – 0.81 vs. MELDNa: 0.79, 95CI 0.77 – 0.81, p=0.15). Among those listed in 2008, 2010, 2012, and 2014, we did not find a significant improvement in the reclassification in patients – Overall: 2.7%, 95CI −1.3 – 8.7%; Cases: 1.1%, 95CI −2.8% – 8.2%; Controls: 1.6%, 95CI −0.4% – 2.0%. However, in the 5,120 patients in the validation cohort listed in 2016 and 2018, we saw a significant increase in the c-index for the MELDNa-KT model as compared to the MELDNa model (MELDNa-KT: 0.73, 95CI 0.71 – 0.75 vs. MELDNa: 0.70, 95CI 0.68 – 0.73, p<0.001). Likewise, we saw a significant improvement in the reclassification of patients: Overall: 8.8%, 95CI 4.6 – 17.2%; Cases: 9.6%, 95CI 5.1 – 18.2%; Controls: −0.7%, 95CI −2.4 – 1.1%. We then measured the c-indices stratified by listing year in Figure 4.
Figure 4. Calculated C-Indices and Confidence Intervals for Each Model when Patients Stratified by Listing Year.
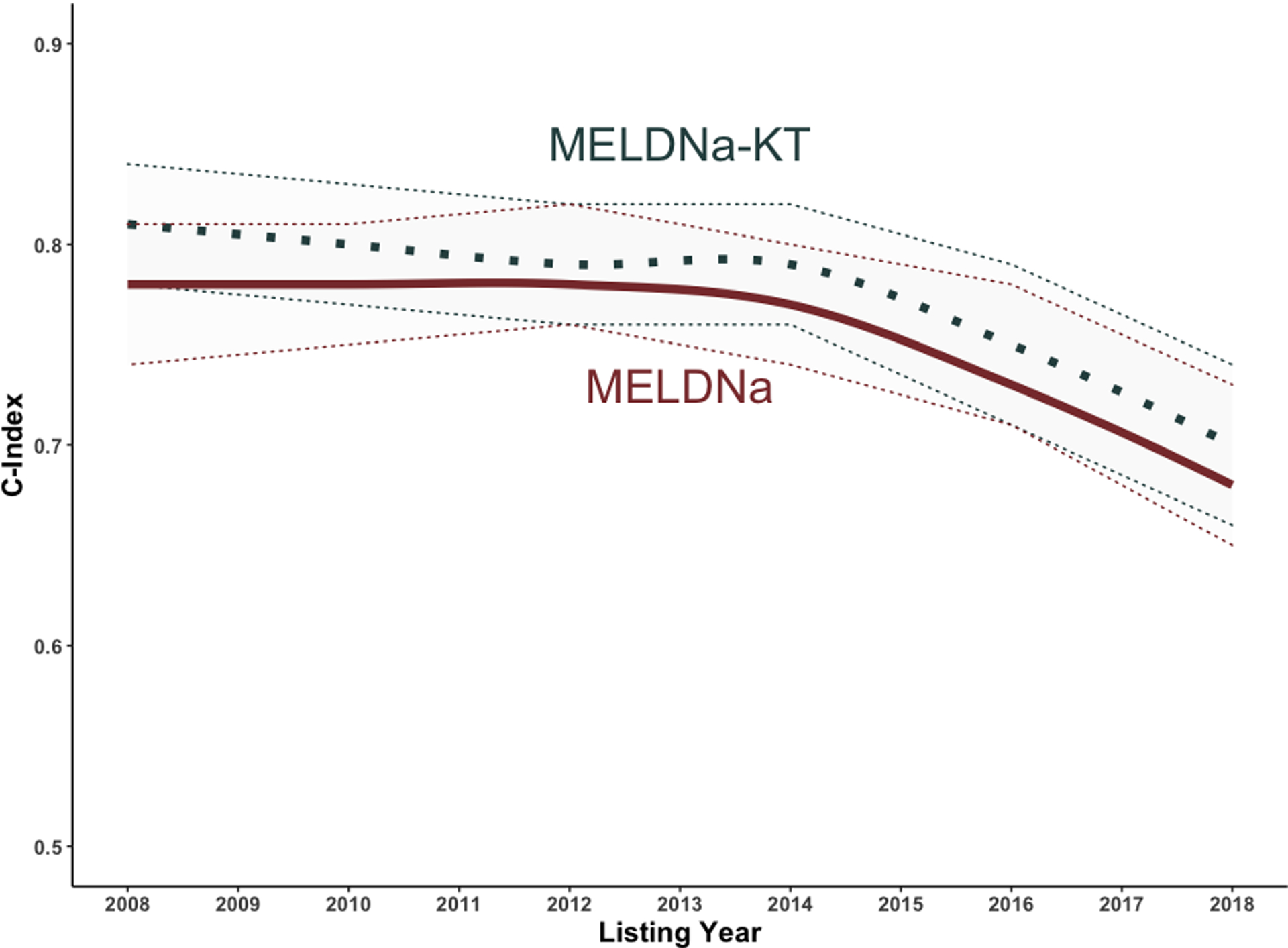
Solid Line = C Index; Dotted Line = Upper and Lower 95% Confidence Intervals
DISCUSSION
In this study, we highlight the feasibility and impact on the model performance of including kidney dysfunction types in the MELDNa score—MELDNa-KT. In a selected cohort, we demonstrate that a simple adjustment to MELDNa score with the inclusion of objectively defined kidney dysfunction types (i.e., normal, AKD, CKD, AKD on CKD) can improve the discrimination and calibration of the MELDNa score without introducing any unexpected biases by race or sex. Transition to the MELDNa-KT score, as compared to the MELDNa score, improves the discrimination by 2% and ultimately appropriately reclassifies ~11% of all patients listed for >90 days without exception points. Importantly, we found that the MELDNa-KT would increase the predicted risk of waitlist mortality in ~12% of those who died waiting for a liver transplant, increasing their prioritization for transplant and possibly allowing for a timelier organ offer.
What might explain these findings? We hypothesize that the inclusion of kidney dysfunction types provides greater “clinical context” to the MELDNa score. We suspect that those patients who experience AKD or AKD on CKD likely had a decompensating event (e.g., infection, bleeding, hypotension). These events, particularly when associated with acute-on-chronic liver failure or a rapid increase in MELDNa score, inherently carry a greater risk of waitlist mortality.26,27 Therefore, we are accounting for that risk by including the AKD and AKD on CKD types with an interaction term that increases this contribution at higher MELDNa scores. Conversely, patients with CKD have an added advantage of an increased MELDNa score without deterioration in their hepatic function. By accounting for these kidney dysfunction types, we can improve the ability of the MELDNa score to execute its function—identifying those patients with decompensated cirrhosis at the greatest risk of dying.
Why is this important? The rising prevalence of CKD among liver transplant candidates is widely reported—the burden of CKD has risen ~200% from 2002 – 2017, and the utilization for simultaneous liver-kidney transplant has increased from ~300% from 2002 – 2017.7,28,29 These trends highlight two weaknesses in the current application of the MELDNa score: 1. The MELD score was developed in a cohort of patients without CKD;18 2. The MELD and MELDNa scores were derived and validated in cohorts with a significantly lower burden of CKD.1,18 Our data demonstrate that although the c-indices of MELDNa and MELDNa-KT decreased over time, there was a greater decrease with the MELDNa score. This suggests that the inclusion of kidney dysfunction types may abrogate some of the deterioration in MELDNa performance. Incorporating kidney dysfunction types in the MELDNa score provides an opportunity to modify the MELDNa score to align with current trends in the liver transplant population.
Additionally, one needs to be cognizant of any unexpected impact of changes to the MELDNa score. To evaluate for unintended effects, we studied the changes in MELDNa discrimination and calibration by race and sex. Women have historically been under-served by the MELDNa score.30–33 For women, we saw that the MELDNa-KT significantly improved the c-index, and although not significant, the net reclassification index was numerically positive. Likewise, the MELDNa-KT appears to have improved calibration, as compared to the MELDNa model (Brier Scores: 0.043 v. 0.054, respectively). For Blacks, the transition to the MELDNa-KT score did not significantly impact the discrimination, as compared to the MELDNa score. We did see a numerically greater calibration with the MELDNa-KT, as compared to the MELDNa score (Brier Scores 0.043 v. 0.055, respectively). These findings highlight that a transition to a MELDNa-KT model should not harm these populations; however, our data demonstrate that the inclusion of kidney dysfunction types may not address the known sex-based disparities in liver transplantation. For this, it is likely that a more accurate calculation of kidney function (i.e., cystatin C, Glomerular Filtration Rate Assessment in Liver Disease (GRAIL), or Royal Free Hospital cirrhosis GFR) may be needed.15,31,34
This study has several limitations. First, our findings do not apply to the entire transplant population. Instead, these data represent just those patients listed without exception points for ≥ 90 days. This concession was needed to avoid the introduction of either a lead-time or an immortal-time bias, as there was no accurate method to identify the kidney function pattern among those listed for <90 days. Although a significant proportion (51%) of patients are listed for ≥ 90 days, this limits the application of kidney dysfunction types into policy; however, it does provide increasing evidence for the inclusion of data regarding the duration, severity, and etiology of kidney dysfunction in transplant databases. Second, patients in the STAR file have varying intervals between sCr measurements. This may introduce an ascertainment bias such that kidney dysfunction types may be misclassified because of either insufficient data or more frequent assessments. That being said, the interval between blood draws is protocolized by the MELDNa score. Given that all adjustments to the MELDNa score were made taking into account the MELDNa score, this bias should be limited. Third, as always with an observational study, the results could be confounded by factors we did not include in our analysis. However, since our previous study demonstrated that the impact of kidney function pattern was independent of etiology, age, sex, race, body mass index, ascites status, hepatic encephalopathy status, diabetes mellitus status, functional status, hemodialysis status, ventilation status, albumin level, and MELDNa score, we do not think such confounding to be likely.3 Finally, as with any analysis of UNOS registry data, our results are limited by the accuracy of the registry inputs. We minimized any impact input errors may have had in our results by focusing on objective data (e.g., laboratory values).
Despite these limitations, our study demonstrates the feasibility and potential benefit of including kidney dysfunction types in the MELDNa score. The adjusted MELDNa score generated, MELDNa-KT, improved the discrimination and calibration of the MELDNa score by including objectively defined criteria. The generalizability of these findings, particularly in those listed for <90 days, warrants further study. Nevertheless, these data are further evidence of the potential kidney dysfunction types have to enhance prognostication of mortality and prioritization for liver transplant among patients with decompensated cirrhosis.
Financial Support:
This study was funded by NIH K23AG048337 (Lai), NIH R01AG059183 (Lai), and the UCSF Liver Center P30 DK-026743 (Cullaro, Lai). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Disclosures:
The authors of this manuscript have conflicts of interest to disclose: Giuseppe Cullaro – Research Support: Mallinckrodt Pharmaceuticals. Elizabeth C. Verna – Salix, Inc. (research support). Jennifer C. Lai – Axcella Health, Inc. (research support; site PI, clinical trial), Lipocine (site PI, clinical trial), Novo Nordisk (advisory board).
Abbreviations:
- AKI
Acute Kidney Injury
- CKD
Chronic Kidney Disease
- MELD
Model for End-Stage Liver Disease
REFERENCES
- 1.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and Mortality among Patients on the Liver-Transplant Waiting List. New Engl J Medicine 2008;359(10):1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullaro G, Rubin JB, Fortune BE, et al. Association Between Kidney Dysfunction Types and Mortality Among Hospitalized Patients with Cirrhosis. Digest Dis Sci 2021;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cullaro G, Verna EC, Lai JC. Association Between Renal Function Pattern and Mortality in Patients with Cirrhosis. Clin Gastroenterol H 2019;17(11):2364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mindikoglu AL, Hernaez R, Liu Y, et al. Renal Trajectory Patterns Are Associated With Postdischarge Mortality in Patients With Cirrhosis and Acute Kidney Injury. Clin Gastroenterol H 2020;18(8):1858–1866.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.eCFR :: 42 CFR Part 121 -- Organ Procurement and Transplantation Network [Internet]. [cited 2021 Oct 19];Available from: https://www.ecfr.gov/current/title-42/chapter-I/subchapter-K/part-121
- 6.Godfrey EL, Malik TH, Lai JC, et al. The decreasing predictive power of MELD in an era of changing etiology of liver disease. Am J Transplant 2019;19(12):3299–307. [DOI] [PubMed] [Google Scholar]
- 7.Cullaro G, Verna EC, Lee BP, Lai JC. Chronic Kidney Disease in Liver Transplant Candidates: A Rising Burden Impacting Post–Liver Transplant Outcomes. Liver Transplant 2020;26(4):498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullaro G, Rubin JB, Mehta N, Lai JC. Differential Impact of Age Among Liver Transplant Candidates With and Without Hepatocellular Carcinoma. Liver Transplant 2020;26(3):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in Pathophysiology, Definition and Classification of Hepatorenal Syndrome: a step beyond the International Club of Ascites (ICA) Consensus document. J Hepatol 2019;71(4):811–22. [DOI] [PubMed] [Google Scholar]
- 10.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017;13(4):241–57. [DOI] [PubMed] [Google Scholar]
- 11.Inker LA, Astor BC, Fox CH, et al. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am J Kidney Dis 2014;63(5):713–35. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Becker C, Inker LA. Glomerular Filtration Rate and Albuminuria for Detection and Staging of Acute and Chronic Kidney Disease in Adults: A Systematic Review. Jama 2015;313(8):837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inker LA, Schmid CH, Tighiouart H, et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. New Engl J Medicine 2012;367(1):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francoz C, Nadim MK, Baron A, et al. Glomerular filtration rate equations for liver‐kidney transplantation in patients with cirrhosis: Validation of current recommendations. Hepatology 2014;59(4):1514–21. [DOI] [PubMed] [Google Scholar]
- 15.Kalafateli M, Wickham F, Burniston M, et al. Development and validation of a mathematical equation to estimate glomerular filtration rate in cirrhosis: The royal free hospital cirrhosis glomerular filtration rate. Hepatology 2017;65(2):582–91. [DOI] [PubMed] [Google Scholar]
- 16.Mindikoglu AL, Dowling TC, Weir MR, Seliger SL, Christenson RH, Magder LS. Performance of chronic kidney disease epidemiology collaboration creatinine‐cystatin C equation for estimating kidney function in cirrhosis. Hepatology 2014;59(4):1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: Problems and pitfalls. Am J Kidney Dis 2003;41(2):269–78. [DOI] [PubMed] [Google Scholar]
- 18.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33(2):464–70. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016;133(6):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim WR, Mannalithara A, Heimbach JK, et al. MELD 3.0: The Model for End-stage Liver Disease Updated for the Modern Era. Gastroenterology 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook NR, Ridker PM. Advances in Measuring the Effect of Individual Predictors of Cardiovascular Risk: The Role of Reclassification Measures. Ann Intern Med 2009;150(11):795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right‐censored survival outcome: a one‐shot nonparametric approach. Stat Med 2015;34(4):685–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerds TA, Kattan MW, Schumacher M, Yu C. Estimating a time‐dependent concordance index for survival prediction models with covariate dependent censoring. Stat Med 2013;32(13):2173–84. [DOI] [PubMed] [Google Scholar]
- 24.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness‐of‐fit in the survival setting. Stat Med 2015;34(10):1659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godfrey EL, Malik TH, Lai JC, et al. The decreasing predictive power of MELD in an era of changing etiology of liver disease. Am J Transplant 2019;19(12):3299–307. [DOI] [PubMed] [Google Scholar]
- 26.Cullaro G, Sharma R, Trebicka J, Cárdenas A, Verna EC. Precipitants of Acute‐on‐Chronic Liver Failure: An Opportunity for Preventative Measures to Improve Outcomes. Liver Transplant 2020;26(2):283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massie AB, Luo X, Alejo JL, Poon AK, Cameron AM, Segev DL. Higher Mortality in registrants with sudden model for end‐stage liver disease increase: Disadvantaged by the current allocation policy. Liver Transplant 2015;21(5):683–9. [DOI] [PubMed] [Google Scholar]
- 28.Singal AK, Ong S, Satapathy SK, Kamath PS, Wiesner RH. Simultaneous liver kidney transplantation. Transplant Int 2019;32(4):343–52. [DOI] [PubMed] [Google Scholar]
- 29.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol H 2019;18(12):2650–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen AM, Heimbach JK, Larson JJ, et al. Reduced Access to Liver Transplantation in Women. Transplantation 2018;102(10):1710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asrani SK, Jennings LW, Kim WR, et al. MELD‐GRAIL‐Na: Glomerular Filtration Rate and Mortality on Liver‐Transplant Waiting List. Hepatology 2020;71(5):1766–74. [DOI] [PubMed] [Google Scholar]
- 32.Lai JC, Ganger DR, Volk ML, et al. Association of Frailty and Sex With Wait List Mortality in Liver Transplant Candidates in the Multicenter Functional Assessment in Liver Transplantation (FrAILT) Study. Jama Surg 2021;156(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verna EC, Lai JC. Time for Action to Address the Persistent Sex-Based Disparity in Liver Transplant Access. Jama Surg 2020;155(7):545–7. [DOI] [PubMed] [Google Scholar]
- 34.Singapura P, Ma T, Sarmast N, et al. Estimating Glomerular Filtration Rate in Cirrhosis Using Creatinine‐Based and Cystatin C–Based Equations: Systematic Review and Meta‐Analysis. Liver Transplant 2021;27(11):1538–52. [DOI] [PubMed] [Google Scholar]


