Abstract
Disuse and aging are known risk factors associated with bone mass and quality deterioration, resulting in increased fracture risk. Indeed, current and emerging evidence implicates a large number of shared skeletal manifestations between disuse and aging scenarios. This review provides a detailed overview of current preclinical models of musculoskeletal disuse and the clinical scenarios they seek to recapitulate. We also explore and summarize the major similarities between bone loss after extreme disuse and advanced aging at multiple length scales, including at the tissue, cellular, and molecular level. Specifically, shared structural and material alterations of bone loss are presented between disuse and aging, including preferential loss of bone at cancellous sites, cortical thinning, and loss of bone strength due to enhanced fragility. At the cellular level bone loss is accompanied, during disuse and aging, by increased bone resorption, decreased formation, and enhanced adipogenesis due to altered gap junction intercellular communication, WNT/β-catenin and RANKL/OPG signaling. Major differences between extreme short-term disuse and aging are discussed, including anatomical specificity, differences in bone turnover rates, periosteal modeling, and the influence of subject sex and genetic variability. The examination also identifies potential shared mechanisms underlying bone loss in aging and disuse that warrant further study such as collagen cross-linking, AGE-RAGE signaling, ROS and NF-κB signaling, cellular senescence and altered lacunar-canalicular connectivity (mechanosensation). Understanding the shared structural alterations, changes in bone cell function, and molecular mechanisms common to both extreme disuse and aging are paramount to discovering therapies to combat both age-related and disuse-induced osteoporosis.
Keywords: Aging, Preclinical Studies, Molecular Pathways – Remodeling, Bone Disorders and Diseases - Osteoporosis, Cells of Bone - Osteocytes
Introduction
Bone deterioration leading to osteoporosis from disuse and/or aging is a prevalent and debilitating clinical disease. Osteoporosis, defined by the World Health Organization (WHO) as a bone mineral density (BMD) 2.5 standard deviations (SD) below the average of a healthy adult, is prevalent in over 200 million adults worldwide and is a major risk factor for fragility fracture.1
The most at-risk populations for osteoporosis include individuals undergoing extended disuse and those of advanced age. For example, disuse from extended bedrest, immobilization, paralysis, and spaceflight-induced unloading is associated with low bone mass and increased risk of fracture at the organ level.2, 3 These extreme examples of disuse result in rapid and continued bone loss, especially in the postural spinal, hip and lower limb regions, that can be highly variable based on the individual. For example, upwards of 0.4–1.8% of areal bone mineral density (aBMD) at the femoral hip can be lost per month in some individuals, ultimately resulting in a 0.6–5.0% loss of estimated bone strength over the same period.4 The aging of the skeletal system is also associated with reduced bone mass and strength in humans. For example, aged men (mean 75 years) at the femoral hip show a 0.2–0.4% loss of BMD and an estimated loss of 1.1–1.7% femoral strength yearly.5–7 These same studies demonstrate that average yearly decreases in bone loss and strength in women are usually 2-fold greater compared to men. Similar to disuse induced bone loss, exercise regimens and anti-resorptive therapy can partially mitigate detrimental bone changes associated with aging.7–10 However structural variations may persist with continued skeletal disuse and the longterm ramifications of these changes in relation to fracture risk and bone quality are not fully understood. In contrast to disuse induced bone loss at weight-bearing sites, aging also leads to significant bone loss including at non-weight bearing skeletal sites that are also prone to fracture, such as the distal radius.6 These observations, coupled with the fact that physical activity declines with aging in humans11, 12 suggests that at least some of the bone changes associated with disuse may be shared with advanced aging, particularly at weight-bearing skeletal sites. The identification of similar phenotypic bone changes common to both disuse and aging will shed light on which bone processes are potentially reversible with mechanical intervention or could be simultaneously targeted by therapeutics. Improved understanding of the risk factors and mechanisms leading to disuse and age-associated bone loss are paramount. There are more than 2 million fragility fractures that occur annually in the US alone that result in significant loss of independence, increased financial burden, and mortality.13 With a predicted 5-fold increase in the number of hip fractures by 2050 with increased life expectancy, a better understanding of the risk factors and potential interventions to treat osteoporotic patients is desperately needed.14–16
The overall goal of this review is to summarize and inform new areas of interdisciplinary research and discovery to alleviate skeletal deterioration due to both aging and disuse. Therefore, this review highlights the similarities and differences between skeletal changes that occur with disuse and bone aging. PubMed indexed articles and Google scholarly works published from the year 2000 onward pertaining to bone disuse and aging (in humans and rodents) are included to provide a comprehensive overview of the current scientific literature to date. Major keywords included “hindlimb unloading”, “bone disuse”, bone aging”, “spaceflight”, “bedrest”, “preclinical model” “cellular and molecular” “bone structure” “bone strength” “genetics” “sexual dimorphism”. In this review, first, the major preclinical models used to study clinically relevant skeletal disuse are presented with their strengths and limitations. Second, skeletal changes from these disuse models and their relation to skeletal aging at the structure and strength level are discussed in detail. Third, the cellular dynamics likely underpinning these shared and different structural alterations between skeletal disuse and aging, are presented. Fourth, potential shared molecular mechanisms underlying skeletal changes in disuse and aging, along with limitations of disuse models to recapitulate some hallmarks of aging, are discussed. Finally, biological processes studied in detail in the context of aging but not skeletal disuse are discussed as opportunities for future research.
Preclinical Models of Moderate to Extreme Skeletal Disuse
The development of reliable models to simulate disuse requires several important and sequential steps. First, the physiological response(s) that the model intends to mimic must be defined and the system utilized to study those aspects. The data accumulated with the particular model must be compared and contrasted with data from human studies. As with any model, the studies should target selected aspects of disuse in humans, since it is unlikely that any model can fully recapitulate all the physiologic consequences of disuse. Rodents are a more simplistic model of human bone disuse due to their lack of osteonal remodeling, continued sex steroid production and long-bone modeling (albeit decreasing) throughout the animal’s lifetime.17 As such, mice, which are nocturnal, quadrupedal and quite physically active, may not accurately predict all components of skeletal disuse in humans, especially regarding periods of voluntary activity. Models of reduced or increased physical activity do exist in rodents, such as reduced cage size, deconditioning after exercise (treadmill or jump training) or variable access to voluntary rotary wheel running. Although these physical activity models remain increasingly important for modeling the human disuse continuum, most studies employing these inactivity models extensively report only the effects on skeletal muscle and/or energy metabolism.18–22 The few that do report on skeletal parameters suggest highly variable changes in cortical and cancellous bone architecture following exercise compared to normal cage activity.23–30 These variable bone changes are highly dependent on mouse strain, age, and exercise type. Due to this variation, only preclinical models of extreme to moderate skeletal disuse that have reproducible and convergent skeletal phenotypes depicting bone loss are presented. Models of extreme to moderate disuse rely on direct invasive (physical/chemical) restraint and or surgical manipulation to alter or diminish the loading environment on the rodent skeleton. Nonetheless, these models are highly utilized and remain critical for uncovering the structural, cellular, and molecular mechanisms of disuse-induced osteopenia in humans. The following section presents the most widely used preclinical models of extreme to moderate disuse. Specifically, some of the basic musculoskeletal manifestations and technical considerations are discussed for each preclinical model. A visual summary of these preclinical models and the human disuse conditions each model seeks to recapitulate are presented in Figure 1.
Figure 1. Models of Musculoskeletal Disuse.
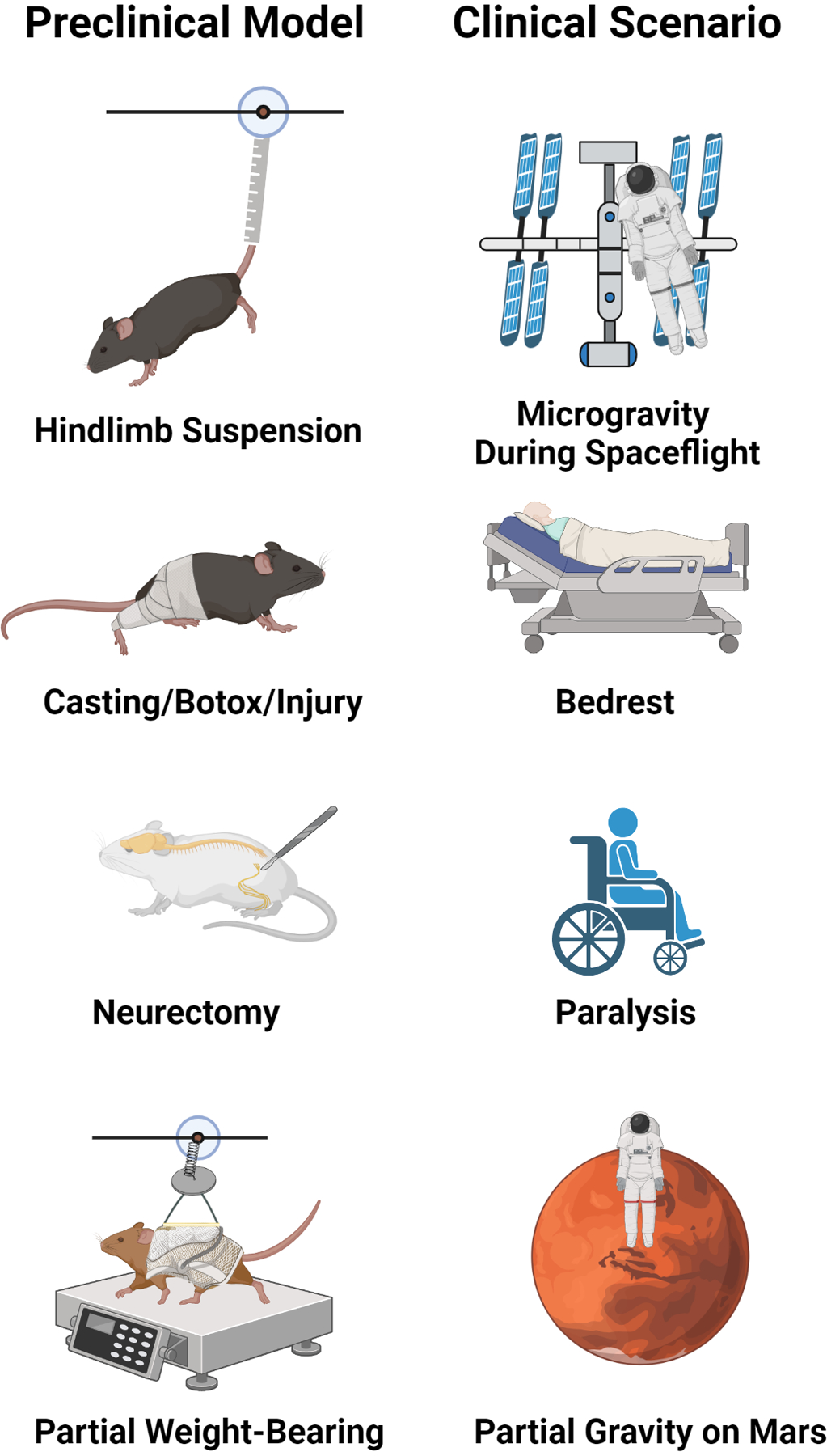
Hindlimb unloading induces bone loss and other systemic effects due to cardiovascular deconditioning and is a widely used analog of microgravity during spaceflight. Single limb immobilization by casting, Botox treatment, and or mechanical injury creates disuse-induced bone loss in the affected versus contralateral limb and recapitulates aspects of limb trauma or developmental injuries. Sciatic nerve resection (neurectomy) induces permanent disuse by loss of neural input to the affected leg and models injury-induced paralysis. Partial weight bearing by harness/pulley system depicts partial gravitational environments (Moon, Mars) and potential clinical scenarios of walker/cane ambulation after fracture, stroke, etc. Figure was created with Biorender.com.
Hindlimb Unloading (HLU)
Created by scientists at the National Aeronautics and Space Administration (NASA) in the mid-1970s, laboratories around the world have adopted the rodent hindlimb unloading model (HLU), typically by tail suspension, HLU to simulate weightlessness and study various aspects of musculoskeletal unloading.31 In the setting of HLUHLU, the primary physiological responses of vertebrates modeling spaceflight include cephalic fluid shifts, repositioning of certain organs, musculoskeletal unloading, and lack of stimulus to the vestibular system.
HLU exposes the hindlimbs to skeletal disuse through unloading by elevating the tail or pelvis off the ground using an appropriate restraint device attached to the cage ceiling (Figure 1).32 Hallmarks of the model include rapid loss of bone, especially in the cancellous regions of the long bones of the hindlimbs of mice (C57BL/6J) and rats (Sprague-Dawley), with significant bone outcomes 2–3 weeks after initiating HLU.33, 34 Bone loss following HLU is mostly reversible since reloading (by allowing reambulation after periods of HLU) has been shown to rescue the majority of the bone loss and all the muscle loss observed in young and adult rodents.35, 36However, the response to HLU-induced bone loss appears to be age- and rodent strain-dependent, with multiple studies showing that aged Fisher 344/Brown Norway rats have reduced responsiveness and osteogenic potential when mechanically unloaded.37, 38 In addition, HLU causes systemic effects that impact the musculoskeletal system, including transient activation of inflammation, lower limb hypoxia, vascular deconditioning, and increases in stress hormones.32 Some of these systemic effects, such as immune cell dynamics and increases in stress, appear to also be influenced by housing conditions. For example, a recent report demonstrated that single versus pair-housed C57BL/6N mice had significantly increased adrenal weight (marker of animal stress) and decreased peripheral T-cell numbers relative to controls.39 Therefore, housing conditions must be kept constant for HLU studies and should be considered when interpreting data. Limitations of HLU include increased sample size estimates because of the necessity of a separate age matched ground control group (not experiencing HLU) and that rodents undergoing HLU usually show significant weight loss and health complications compared to controls.34 Therefore, caution must be exercised and ethical practices noted when subjecting animals to long periods of HLU (> 3 weeks) or using rodents with underlying metabolic conditions (such as aged and or diabetic). Rodents with pre-existing comorbidities may not be able to survive the physical rigors of HLU, as noted above.
Immobilization and Paralysis
Other models to elicit bone loss due to mechanical disuse include single-limb immobilization (SLI) by casting, paralysis (chemical/surgical), and noninvasive joint injury (Figure 1). In the immobilization model recently developed by our lab and others,40, 41 a single hindlimb of the mouse is immobilized by casting in an open 1.5 ml micro-centrifuge tube. Extensive characterization of the SLI model has demonstrated that growing (10 weeks) and skeletally mature (16 weeks) male C57BL/6J casted mice have normal cage activity, food/water intake, and use of the contralateral limb.40–42 SLI in these mice induces femoral and tibial bone loss preferentially in the epiphyseal and metaphyseal cancellous sites and loss of lower limb musculature compared to contralateral limbs.40, 43 Clinical reports of lower extremity fractures in adolescents and adults treated by casting immobilization also demonstrate preferential loss of lower limb bone mineral density (particularly at distal tibia and proximal femur) compared to uninjured limbs and healthy controls.44, 45 Therefore, SLI effectively mimics lower limb bone loss seen in casted/immobilized patients following unilateral and lower extremity trauma. One potential advantage of the SLI method over HLU is the use of the contralateral limb as an internal control, which allows for decreasing sample size estimates for studies. However, the SLI model in 10-week-old C57BL/6J mice showed significant decreases in bone architectural indices in the casted and contralateral (noncasted) limb versus limbs in sham treated age-matched animals.40 This bone difference between sham controls suggests that casting alters gait and limb loading compared to age-matched naïve C57BL/6J mice and is a potential limitation of the SLI model.
Similar to SLI, noninvasive mechanical overloading of the rodent knee to cause ACL rupture has emerged as both a noninvasive injury-induced osteoarthritis model and a trabecular disuse-induced bone loss model. In the mechanical overloading model, 10 week old C57BL/6N female mice showed upwards of 35–45% loss of epiphyseal trabecular bone just one week after injury, similar to those undergoing HLU.46 However, this mechanical overloading model, unlike SLI and HLU, elicits only small increases in hindlimb muscle atrophy.46
Other common models of bone loss by mechanical disuse use either chemically induced muscle paralysis by botulinum toxin A (Botox) injection or permanent muscle paralysis by sciatic nerve resection (Figure 1). In the Botox model, the calf and quadriceps of mice are injected with Botox, which blocks presynaptic acetylcholine release in the neuromuscular junction, leading to partial limb paralysis.47 This partial limb paralysis in skeletally mature female C57BL/6J mice and Sprague-Dawley rats results in a rapid muscle and bone mass loss in the affected limb versus control, a response that may be more extreme than HLU alone.48–50 Similar to chemical-induced paralysis, lower limb unilateral denervation by sciatic nerve resection mimics paraplegia and the resulting disuse-induced bone loss.. Sham-operated rodents or a contralateral, unaffected limb typically serve as controls. C57BL/6J mice and Sprague-Dawley rats undergoing unilateral sciatic neurectomy typically show rapid cancellous bone loss within weeks on the order of 50–60% compared to sham-operated controls or 20–30% compared to contralateral limbs.51, 52 However, chemical induced muscle paralysis is not readily reversible and therefore the Botox model may not be useful for studying physical rehabilitation of the affected limb following disuse. Another potential confounding variable in the sciatic nerve model is that, due to innervation’s strong role in bone homeostasis, it is often difficult to determine whether bone loss is due directly to disuse or simply due to loss of neural impulses to the bone.53 This latter point remains somewhat controversial. Nonetheless, neurectomy remains an excellent model when studying musculoskeletal changes due to nerve injury and paralysis. Despite these newer models of mechanical disuse, few direct comparisons between models of disuse exist and HLU remains the most employed disuse model for musculoskeletal studies.
Partial Weight-Bearing Model (PWB)
To model less-extreme forms of disuse, across a continuum of load-levels, the partial weight-bearing (PWB) model was developed and characterized first in young female BALB/cBYJ mice54 and then young Wistar rats.55 The PWB model is practical for studying changes in the musculoskeletal system in partial gravitational environments (Moon, Mars) and potentially clinical scenarios of partial hindlimb unloading such as crutch and/or walker ambulation after fracture, and stroke.56 In the PWB model, rodents are placed in a harness connected to a variable spring that can be adjusted to achieve the desired level of PWB (20% – 100% Body Weight (BW)) across all four limbs. Female C57BL/6J mice (skeletally mature) experiencing unloading by PWB demonstrated bone and muscle changes over 21 days that were linearly proportional to the degree of partial weight-bearing.57 Furthermore, unloading to 20% resulted in cortical and cancellous bone changes similar to HLU, although muscle changes were less severe, suggesting that certain thresholds of partial physical unloading can mimic full disuse in mice. Another study utilizing the same model in skeletally mature female BALB/cBYJ demonstrated that mice exhibited dose-dependent reductions in skeletal architecture as well.58 However, unloading up to 33% BW resulted in musculoskeletal changes similar to HLU (0% BW in BALB/cBYJ mice. Although BALB/cBYJ and C57BL/6J mice have different basal bone phenotypes59, these unloading results in the PWB suggests that disuse-induced mechanosensitivity may differ by mouse genetics similar to load-induced bone formation.60, 61 The role of genetics in the response to disuse, although outside the scope of this review, will be briefly discussed in the cortical and cancellous section. Importantly, the PWB model has been reported to not induce a chronic stress state in female C57BL/6J mice or Wistar rats (14 weeks, male), indicated by plasma corticosteroid levels, or significantly altered hindlimb blood-flow, which are readily apparent in HLU.32, 57, 62 Due to these differences, the PWB method, when used in conjunction with a HLU group, may be a powerful tool for decoupling the role of stress and vascular changes in disuse-induced bone changes.
Bone Changes with Prolonged Disuse and Aging
Introduction
Aging, a process accompanied by DNA and tissue damage accumulation over time as well as increased susceptibility to cell death or decay,63–65 is complex and variable between individuals and across species. Although various models of accelerated aging in rodents have been developed via specific gene mutations,66, these models do not fully recapitulate all aspects of aging.67 Therefore, this review is not a discussion of the pros and cons of aging models, rather it will focus solely on the significant phenotypic changes that occur during disuse and whether they are also observed during aging. In particular, bone changes occurring after skeletal maturity (attainment of peak adult bone mass) into advanced age will be emphasized. In humans, skeletal maturity occurs by 25–30 years of age and in rodents typically occurs by 12–16 weeks of age, with alterations due to subject sex and genetics.68–70
In the next sections, the key bone structural and cellular changes occurring during extended disuse and aging are presented with similarities and potential differences noted (Figure 2). Most of the preclinical findings, especially those utilizing transgenic mice, come from disuse studies utilizing the C57BL/6J mouse. Due to the low degree of genetic diversity used currently in bone disuse studies, many findings may have limited translatability to the human population. Therefore, special attention is placed on the variation of disuse-induced structural alterations seen in preclinical models due to mouse sex and genetic background. Furthermore, the extent to which preclinical models accurately reflect the variation recorded in disuse and age-induced bone changes in the human population is highlighted. Once these structural changes are summarized, a brief overview of different cellular and molecular processes in the bone microenvironment that accompany these structural alterations with disuse and aging are included as avenues of future research.
Figure 2. Tissue and Cellular Changes in Bone with Extreme Disuse and Advanced Aging.
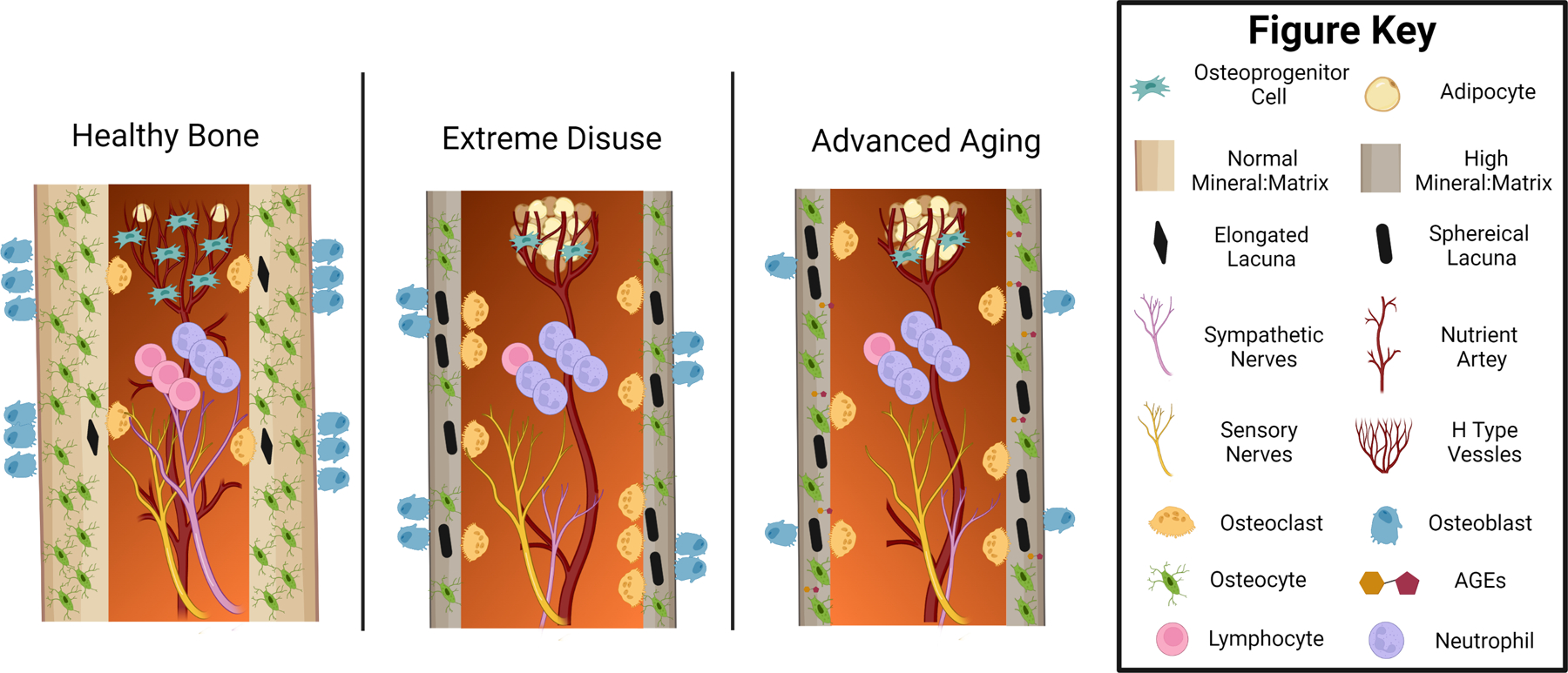
Healthy and skeletally mature bone has a rich supply of branching blood vessels and sympathetic and sensory nerves innervating the marrow and cortical bone. Near sites of sinusoidal vessels, there are rich osteoprogenitor cell niches with few adipocytes. The surrounding cortical bone is dense, has a normal mineral to matrix ratio, and high osteocyte density. The few existing apoptotic osteocytes have an elongated lacunar shape that is rapidly remodeled by balanced bone turnover through the coordinated action of osteoclasts and osteoblasts. In contrast, extreme disuse and aging are marked by a rapid loss of marrow vascularity and sympathetic nerve density. The number of osteoprogenitor cells and H type metaphyseal sinusoids are reduced and replaced by a large number of marrow adipocytes. Increased osteocyte apoptosis and lacunar osteolysis stimulate increased osteoclast activity and decreased osteoblast activity, which is also accompanied by increased neutrophil to lymphocyte immune cell recruitment and low-level inflammation. Overall, these shared changes lead to increased diaphyseal cortical thinning, increased intracortical porosity, and increased bone tissue mineral:matrix ratio that can result in skeletal fragility. Tissue and cellular level changes associated with aging but not yet studied in terms of disuse include an increased cortical abundance of advanced glycation end products (AGEs) and loss of osteocytic dendritic connections. Not drawn to scale or with a full depiction of neurovascular changes in the intracortical space and periosteum. Figure was created with Biorender.com.
Organ Level Changes and Bone Microarchitecture
Cortical and Cancellous - Structure
Bone is a highly dynamic organ that is structurally tuned to the mechanical environment. As a result, extended periods of disuse result in prominent and long-lasting changes at the whole organ level, including bone mass, geometry (microarchitecture), and strength. For example 3 weeks of HLU in young male and female C57BL/6J mice (3–4 months) induces a loss of 20–30% of cancellous bone volume fraction in the tibial and femoral metaphyses.34, 49, 71 These changes in cancellous bone appear more dramatic with Botox treatment in C57BL/6J mice (as severe as 50% loss of femoral BV/TV) and are largely driven by losses in trabecular thickness (Tb.Th) and connectivity by micro-CT analysis, similar to that seen in human spaceflight or bedrest data.72, 73 In addition, these same studies and others demonstrated that tibial and femoral diaphyseal cortical area fraction changes were less dramatic than cancellous changes, with decreases around 5–10%.50 These cortical changes following disuse appear to be due to increased marrow area and decreased periosteal modeling, resulting in decreased cortical thickness (Ct.Th) and moment of inertia (MOI). Partial weight-bearing in female BALB/cByJ and C57BL/6J mice also demonstrated average dose-dependent decreases in similar femoral cancellous (BV/TV, Tb.Th) and diaphyseal cortical indices (cortical area (Ct.Ar), MOI, and Ct.Th), suggesting that these structural parameters also change proportionally to the level of disuse.54, 57
In C57BL/6J mice, there are disuse-induced cancellous and cortical bone changes that are similar to changes that occur with aging. For example, there is a near-total loss of cancellous bone volume fraction in the distal femur and proximal tibia between 2 to 20 months of age, although this bone loss is more pronounced in female (−94%) versus male mice (−56%).69 Similarly, age-related changes in C57BL/6J cortical bone are similar to extreme disuse studies in that they are lower in magnitude than cancellous changes and usually result in increased marrow area.69, 74, 75 However, unlike extreme disuse studies in C57BL/6J, cancellous bone loss in aging is typically driven by changes in trabecular number (Tb.N) and to a lesser extent Tb.Th. Furthermore, cortical periosteal modeling and MOI of long bones continue to increase throughout aging, resulting in decreases in cortical thickness (Ct.Th) compared to disuse, especially in male mice, over the animal’s entire lifetime.69, 76 Thus, within the C57BL/6J mouse strain, phenotypic microarchitecture changes, including increased cancellous bone loss and cortical endosteal resorption leading to cortical thinning, are common to both disuse and aging, although at different magnitudes and timescales.
Although the C57BL/6J mouse is widely used, it is important to note that the C57BL/6J mouse strain shows enhanced bone loss due to disuse compared to other mouse strains,43, 77 and therefore may not accurately reflect all aspects of disuse-induced bone loss in humans. Bedrest and spaceflight studies in humans show wide variation, with changes in trabecular volumetric bone mineral density (Tb.vBMD) being anywhere from +0.5 to − 3% per month, driven by both changes in Tb.N and Tb.Th.73, 78, 79 This variation in human disuse induced bone loss is also reflected in the cortical compartment, with these studies illustrating cortical vBMD of the proximal tibia changing by 0 to −1.5%. Thus, like C57BL/6J mice, human data suggests that cancellous bone mass over cortical bone mass is preferentially lost with extreme disuse.80 However, unlike C57BL/6J mice, the response to disuse and aging is highly variable in humans, with some humans appearing largely unaffected by disuse induced by spaceflight or bedrest.73, 78, 79 Work by our group and others suggests that the variable response to skeletal disuse may be partially contributed to genetic factors.43, 81, 82 For example, our lab demonstrated that immobilization by casting among 8 genetically diverse inbred mouse strains led to high variability among disuse-induced cancellous and cortical changes.43 Among the mouse inbred strains, differences between diaphyseal cortical area fraction of immobilized and control limbs ranged from +0.62 to −7.42% with the greatest decrease in C57BL/6J and NOD/ShiLtJ mice. Differences between trabecular BV/TV of immobilized and control limbs ranged from −36.5 to −8.3%, with the greatest decrease in CAST/EiJ and C57BL/6J mice. This wide variation in bone loss is similar to a smaller study using HLU among skeletally mature C57BL/6J, C3H/HeJ, and BALB/cByJ mice.82 In this study, there was −59.5% to −8.5% change in trabecular BV/TV and a −11 to +1.3% change in diaphyseal cortical area (Ct.Ar), with BALB/cByJ showing the greatest decrease, followed by C57BL/6J. Thus, bone changes in the BALB/cByJ and C57BL/6J inbred strains may represent the higher extreme of bone loss that is to be seen in the human population with disuse, with most individuals experiencing significantly less detrimental bone changes
The variation in bone loss in different inbred mouse strains and humans may be driven by alterations in mechanosensitivity attributable to different individual genetics. Mechanosensation, or the process by which bone’s sense mechanical forces, can be influenced by changes in bone biomechanics as well as cellular and molecular elements within the bone itself. These biomechanical and cellular/molecular factors have been shown to be regulated by genetics in inbred mice. For example, C57BL/6J and BALB/cBYJ mice have previously been shown to have significantly different postnatal femoral cortical density and geometry when housed under similar conditions.59 Emerging evidence suggests that C57BL/6J and BALB/cBYJ mice also show intrinsic differences in immune cell populations (macrophages) that are becoming increasingly recognized as important mediators of skeletal adaptation.83, 84 The crucial role genetics plays in coordinating molecular mechanisms pertinent to disuse and age-related bone loss is outside the scope of this review. However, future preclinical disuse experiments should better elucidate the role of genetics in mechanosensation mechanisms. In all, these data highlight the need for utilization of multiple different inbred strains or outbred mice concurrently in disuse studies. Using a wide array of mice with differing basal bone phenotypes is critical for more accurately capturing the genetic induced variations of bone loss seen in humans.
Unfortunately, most rodent studies, especially those utilizing C57BL/6J mice, have used a single mouse sex and therefore inference of sexually dimorphic disuse-induced structural alterations of bone remain elusive. Clinical studies have not offered much additional insight, with conflicting reports and inconclusive evidence as regards to any large sex-effect on the response of bone to disuse.78, 85–87 In contrast, with aging in rodents and humans, the large effect of individual sex on bone mass changes is well-established. For example, as previously mentioned, C57BL/6J mice show a nearly 2-fold greater bone loss in the distal femoral metaphysis from skeletal maturity onward in female versus male mice.69 Similarly, clinical data shows an approximate 5–7.5% loss in cancellous BMD and a 3–5% decrease in cortical bone mass from middle (50 years) to advanced age (85 years) in women, with lower rates of loss in men.88, 89 Therefore, an unexplored difference between aging and disuse is the relative role of sex-differences in potentiating a catabolic response. Furthermore, in aging, significant bone loss also occurs in non-weight-bearing skeletal sites, such as the distal radius, in humans, but not in rodents.80, 90 Thus, aging appears to have more systemic and sex-linked effects across the skeleton than disuse.
Tissue Level Changes
Bone Matrix – Mechanical and Chemical Composition
At the tissue level, bone is a highly heterogeneous, hydrated, and porous tissue consisting of an organic phase and an inorganic phase. The organic phase primarily consists of collagen type 1 and, to a lesser extent, non-collagenous proteins. The inorganic phase of bone consists primarily of calcium and phosphate in the form of hydroxyapatite crystals. These organic and inorganic phases have been shown to contribute to the mechanical integrity of bone differentially, and changes in either phase may alter whole bone mechanical strength and fracture resistance. Higher mineral:matrix ratios and crystallinity are correlated with increased bone material stiffness, but increased brittleness and fracture risk at the organ level.91–93 Newly formed bone mineral contains high levels of phosphate that is eventually substituted with carbonate. Thus, the high carbonate:phosphate ratio is generally seen as a marker of aged or damaged bone in humans.94, 95 Preclinical models of extreme disuse such as HLU, casting, and sciatic neurectomy across multiple rodent strains demonstrate variable decreases in structural (stiffness, ultimate load) and material properties (modulus, ultimate stress) accompanied by increases in mineral:matrix, crystallinity, and carbonate:phosphate, compared to controls.43, 75, 76, 96, 97 Similarly, aged 19–20-month-old C57BL6/J and BALB/cByJ mice displayed increased mineral:matrix, crystallinity, and carbonate:phosphate compared to 5-month-old controls and inferior mechanical properties (stiffness, energy to fracture).75, 76, 98, 99 Importantly, multiple studies using the PWB model in C57BL/6J and BALB/cByJ suggest structural, but not material, level alterations with moderate disuse.57, 58 Overall, these data suggest that extreme disuse, similar to aging, leads to detrimental mechanical changes in bone. The aforementioned data also suggests that the increase in bone matrix mineralization and crystallinity seen with disuse, which mimics old or damaged bone, may be a key determinant in reduced mechanical competency. Although moderate disuse leads to less material-level changes, it still shows similarities to aging, such as decreases in ultimate load and stiffness that can predispose bone to fragility fracture, the main clinical outcome of osteopenia. Unfortunately, data regarding bone chemical changes in humans with disuse remains sparse, although aged human bone demonstrates increased mineral content, crystallinity, and carbonate substitution.100 Interestingly, like bone structural changes, material level bone changes due to extreme disuse also appear to be influenced by genetics. For example, casting for three weeks increased crystallinity in C57BL/6J and NZO/HiLtJ strains, and the carbonate:phosphate ratios in A/J mice.43 These preclinical findings add to the complexity of the disuse scenario and suggest that the propensity for disuse-induced bone mineralization changes has a genetic component.
In contrast to bone mineral, type-1-collagen (Col1) is the predominant organic molecule in bone and imparts ductility and energy absorbing ability (toughness).101 The role of Col1 in bone strength is extensively reviewed elsewhere.102–104 In brief, Col1 comprises a trimeric molecule organized into fibrils that are cross-linked to each other enzymatically, marked by mature cross-links (mature cross-links pyridinoline (PYP) and deoxypyridinoline (DPD)) for increased stability.103 Col1 can also be cross-linked nonenzymatically, in the presence of sugar, to form advanced glycation end-products (AGEs).105 Preclinical studies removing enzymatic cross-links with β-aminopropionitrile, which prevents LOX activity, show significant decreases in ultimate stress and energy to failure in rodent long bones.106 Clinically, lower enzymatic and higher nonenzymatic Col1 cross-links are found in fractured osteoporotic bone versus age-matched controls.107 In all, these studies suggest that enzymatic cross-linking imparts beneficial effects on bone strength and energy absorption, while non-enzymatic cross-links are detrimental. With bone disuse, Col1 breakdown by-products such as Collagen 1 N-telopeptide (NTX) and C-telopeptide (CTX) as well as PYP and DPD cross-links are readily increased and correlate with bone loss.73, 108 The loss of overall Col1 content in bone with extended disuse mirrors the rapid decline of absolute bone Col1 with advanced aging.104 However, what is not well known is how Col1 cross-linking (enzymatic and non-enzymatic) changes with disuse. Multiple studies have shown that HLU in rodents (BALB/cBYJ; Sprague-Dawley) decreased mature Col1 cross-links by decreasing the PYP:DPD ratio and increasing immature cross-links, which is contrary to what is seen in aged bone compared to healthy, skeletally mature human samples..104, 109–111 The decrease in mature cross-links in these disuse studies is attributed to increased collagen breakdown and decreased processing and mineralization of new collagen, due to inhibition of osteoblastogenesis seen with disuse. Although it is known that AGEs increase in aged bone and contribute to fragility via loss of bone toughness,105, 112 AGE accumulation in bone undergoing extreme levels of disuse remains largely undefined. More research investigating AGE accumulation during skeletal disuse is warranted, especially since bone cells (osteoclasts, osteoblasts, and osteocytes) are all known to express the receptor for advanced glycation end products (RAGE), the activation of which results in bone catabolism.113, 114 In all, these findings provide strong evidence that extreme disuse and unloading result in similar changes in bone mineral chemical composition, particularly the inorganic phase, to that seen with aging.
Cellular and Molecular Level Changes in Bone Microenvironment
Shared structural, material, and mechanical changes with disuse and aging suggest that some, but not all, of the cellular functions and activities of bone may be similarly affected between the two conditions. Osteoblasts and osteoclasts, which produce bone matrix and resorb bone, respectively, play key roles in maintaining bone’s structural integrity via the coordinated activity of bone remodeling. The coordinated activity of these cells is now widely recognized to be primarily controlled by osteocytes, which are post-mitotic, fully differentiated osteoblast lineage cells embedded in bone matrix.115 The mineralized bone matrix encases the bone marrow, which houses hematopoietic stem cells and mesenchymal progenitor cells that collectively give rise to immune cells and osteoblast lineage cells, respectively. Interspersed within the mineralized bone matrix and marrow are sensory neurons and blood vessels, allowing for nutrient exchange and thereby providing a conduit for endocrine action in bone. Some of the shared and distinct cellular changes likely driving these structural and mechanical changes with aging and disuse are highlighted in Figure 2, with potential shared underlying molecular mechanisms highlighted in Figure 3.
Figure 3. Potential Shared Molecular Mechanisms of Bone Loss with Extreme Disuse and Skeletal Aging.
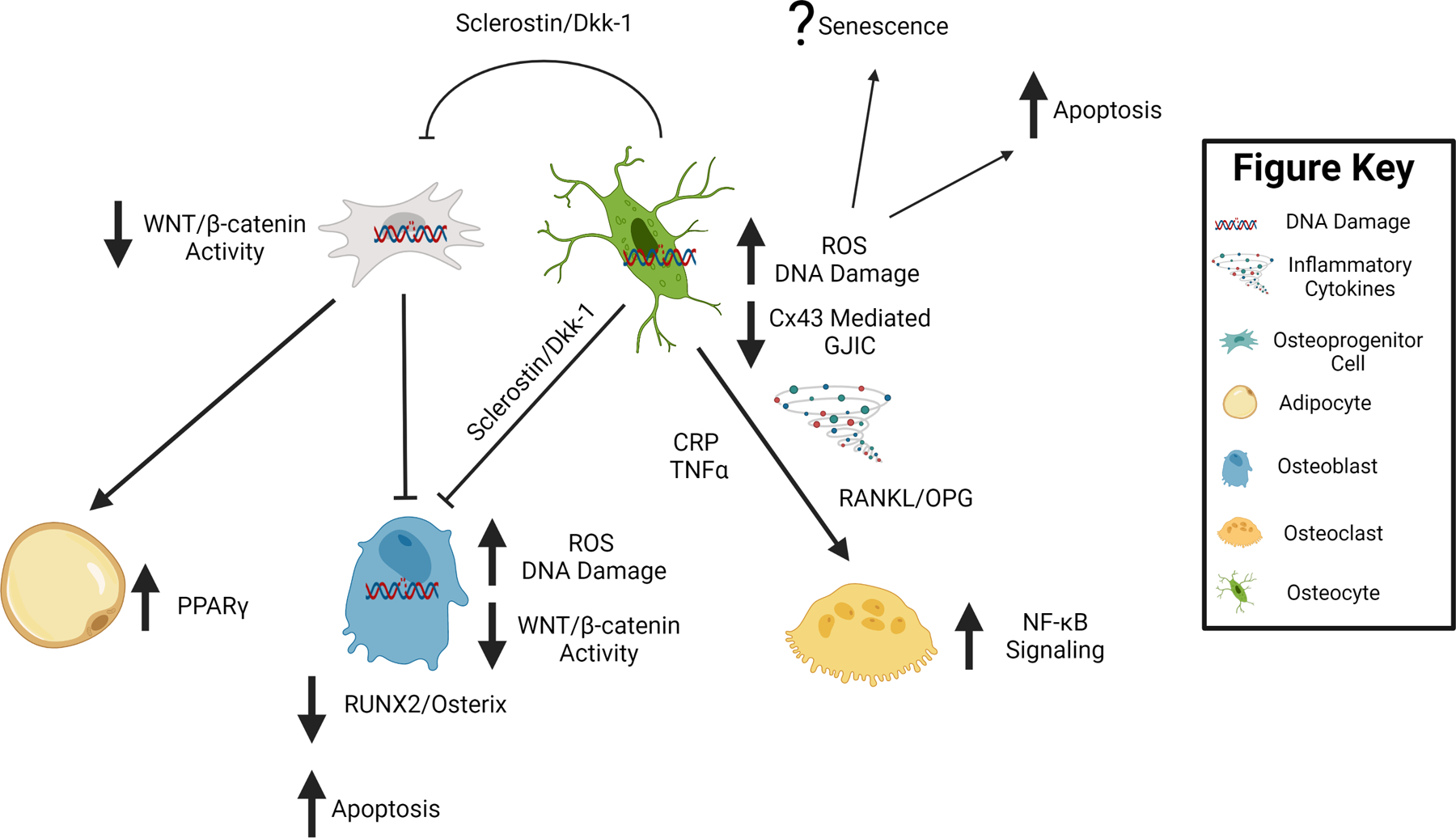
Potential shared molecular changes associated with extreme disuse and advanced aging are demonstrated by block arrows next to and connecting to the cell type of interest. In summary, extreme disuse and advanced aging are associated with increased reactive oxygen species (ROS), inflammation and DNA damage. Furthermore, there is a decrease in Connexin 43 (Cx43) mediated gap junctional intercellular communication (GJIC) in osteocytes, all of which results in bone cell apoptosis and potentially increased cellular senescence. These changes are accompanied by an increase in NF-κB mediated osteoclastogenesis and a decrease in WNT/β-catenin mediated osteoblastogenesis during bone disuse and aging. Figure was created with Biorender.com.
Osteoblasts and Osteoclasts
In healthy individuals, the activities of osteoblasts and osteoclasts are coupled, resulting in equal amounts of bone formation and resorption, respectively. However, moderate to extreme disuse in animals and humans appears to be driven by a decoupling of these two cellular processes, with increases in osteoclast activity and decreases in osteoblast activity. For example, HLU, spaceflight, and Botox immobilization in skeletally mature, female C57BL/6J mice all promote osteoclast activity and decreased osteoblast-based bone formation on both the endosteal and periosteal surface.48, 71, 116, 117 These findings of enhanced resorption are also corroborated in humans undergoing disuse by the large linear average increases in bone resorption markers (CTX and NTX) that occurs in subjects undergoing spaceflight or bedrest.118, 119 Evidence of decreased bone formation during clinical settings of disuse remains limited as multiple studies show conflicting results in serum markers of bone formation (PINP, osteocalcin).73, 118, 120 Overall, however skeletal disuse data suggest overall enhanced remodeling favoring resorption due to the decreased cancellous BV/TV and trabecular and cortical thinning, mentioned previously. The enhanced resorption causing bone loss with disuse also mirrors early aging to some degree and appears, in both disuse and aging scenarios, to be partially dependent on receptor activator of nuclear factor Kappa B ligand (RANKL) signaling. For example, HLU in C67BL/6J mice and spaceflight in humans result in a significant increase in serum RANKL/OPG expression or bone TNFSF11/TNFRSF11B expression (encodes RANKL and OPG, respectively) that is strongly correlated with bone loss.108, 117 Similar increases in TNFSF11 expression and osteoclast activity are also observed in the aged versus young skeleton¸ where there is an approximately 4-fold higher TNFSF11 expression in young versus aged C57BL/6J mice (24 months), and are strongly correlated with significant decreases in cancellous bone volume.121 In another report, bone marrow cells taken from humans and cultured in vitro displayed a doubling of TNFSF11 expression per decade of donor age.122 Similar approaches have also demonstrated that short-term spaceflight induces marrow cells to differentiate into osteoclasts more effectively.123 To combat the increased osteoclastogenesis, RANKL pharmacological inhibitors such as OPG-Fc and denosumab appear to be able to decrease bone loss associated with disuse and aging.124–126 Thus, disuse and aged bone appear primed to promote osteoclastogenesis, and targeting RANKL can ameliorate bone loss in both conditions. The enhanced osteoclastogenesis, coupled with the fact that aged bone in rodents and humans shows reduced osteoblast numbers,127 supports the net balance favoring resorption and decrease in bone mass with disuse and aging.
Even though increased bone remodeling is observed with disuse, it is important to note that the overall accelerated bone turnover seen acutely in disuse is not replicated in rodent or human studies of advanced aging. Omitting the menopausal transition period in which disruption of the hypothalamic-pituitary-gonadal-skeletal axis leads to increased bone turnover,128 advanced aging is typically associated with slower overall rates of turnover compared to younger subjects. For example, aged male and female C57BL/6J mice (31 months) demonstrate lower levels of bone turnover than young mice (4 months), marked by overall decreases in osteoblast and osteoclast numbers.129 In addition, men and women (premenopausal) see a decline in both CTX and PINP levels with aging, suggesting reduced bone turnover.130 The decline in bone turnover with aging would explain the greater microdamage rates and crystallinity seen in aged bone,95 and is similar to other bone scenarios where remodeling is suppressed by use of bisphosphonates.131 Thus, a limitation of disuse in modeling aging is the expedited bone turnover that occurs due to the transient and extreme nature of the stimulus. However, it appears that individuals with higher basal rates of bone turnover are more likely to lose bone under osteoporotic conditions such as advanced aging and spaceflight.73, 132 Thus, within an individual, bone turnover is a useful surrogate to predict future bone changes in both the context of disuse and aging.
Osteocytes and Gap Junctional Intercellular Communication (GJIC)
Osteocytes, which are terminally differentiated osteoblasts, reside in lacunae throughout the skeleton and are connected to each other and distant bone cells via dendritic processes that transverse through small (~150–300nm diameter) channels termed canaliculi.133 Osteocytes are recognized as the main drivers of bone remodeling and mechanosensation via signals to osteoblasts and osteoclasts, as well as for the release of exosomes as reviewed elsewhere.115 One of the predominant molecules regulating osteocyte signal transduction within the lacunar-canalicular system (LCS) is the gap junction protein connexin 43 (Cx43).134 This section will compare key changes in osteocyte vitality, LCS structure, and GJIC with disuse and aging.
Osteocyte cell death by apoptosis has been identified as a hallmark of bone tissue that has undergone extended periods of disuse or aging.135–137 For example, preclinical models of unloading, such as HLU, paralysis, and spaceflight, across various rodent strains all reveal increases in osteocyte apoptosis and changes in lacunar density, especially along the endosteum where bone resorption later occurs.50, 136, 138, 139 In these same studies, viable osteocytes and osteocyte lacunar density decrease by as much as 20–30% in response to unloading, with lacunae becoming more spherical in shape. Similar changes in osteocyte integrity are often seen in reports of bone from aged humans and C57BL/6J rodents.140–143 These osteocytic changes include a decrease in osteocyte density, shape, and size with age (~30% decrease in lacunar density and size) from skeletal maturity to median lifespan, which correlates with increases in bone porosity and microdamage.140–143 It is now becoming apparent that dramatic changes at even smaller length scales in the osteocyte may occur with disuse and aging. For instance, a recent study using 3D multiplexed confocal imaging demonstrated that the number of dendrites on an osteocyte linearly decreases with age and precedes the decline in osteocyte apoptosis.143 Furthermore, osteocyte apoptosis showed regional and sex-specific differences, with higher age-related osteocyte death in the midshaft of long bones in C57BL/6J male mice.143 Overall, these results suggest that loss of osteocytic dendrites may be a cause of eventual osteocyte death, and the mechanisms for such may differ between anatomical location and subject sex. These novel osteocyte connectivity findings have not been thoroughly investigated in models of disuse. Nonetheless, pharmacologically blocking osteocyte apoptosis has been shown to block unloading-induced bone loss and preserve bone integrity with aging in C57BL/6J mice.135, 144 These results strongly suggest that osteocyte apoptosis is at least part of the shared mechanism leading to disuse- and age-associated bone loss.
One critical way to modulate osteocyte vitality and bone remodeling that our lab has investigated is via connexin 43 (Cx43) mediated gap junctional communication (GJIC) (Figure 3). Cx43 is the predominant gap junction protein in bone cells and is highly expressed by osteocytes and osteoblasts.134 Cx43 deletion disrupts GJIC and increases osteocyte apoptosis via the AKT/P27/Caspase-3 pathway in vitro.145 Furthermore, mature osteoblast and osteocyte Cx43 deficiency in vivo results in osteocyte apoptosis and Cx43 is necessary for the anti-apoptotic effects of bisphosphonates and parathyroid hormone on osteoblast lineage cells.146 Therefore, the role of Cx43 in osteoblast and osteocyte apoptosis is presumably related to the function of gap junctions in the intercellular transport of, or binding with, key molecules such as Ca+2, nitric oxide (NO), cyclic adenosine monophosphate (cAMP), adenosine triphosphate (ATP), and prostaglandin E2 (PGE2) and or β-catenin and β-arrestin.147–149 The reported changes in Cx43 expression and function with disuse and aging therefore may alter the activity of these important factors, thereby increasing osteocyte apoptosis. In support of altered Cx43 levels with disuse, numerous in vitro models of unloading, in which cells experiencing apparent weightlessness via parabolic flight and random positioning machines (RPM), have demonstrated decreased Cx43 protein expression, especially at the cell surface, resulting in aberrant GJIC.150, 151 Conversely, Cx43 levels and GJIC increase when osteocytes (MLO-Y4 cells) undergo loading by fluid flow-induced shear stress.152, 153 These results demonstrate that Cx43 levels in osteocytes are mechanosensitive and that disuse-induced osteocyte apoptosis may be, in part, be a direct consequence of diminished Cx43 levels and disrupted GJIC. These observations mirror the phenotype observed during aging of the mouse skeleton, which also demonstrates reduced Cx43 levels in cortical osteocytes and increased cellular apoptosis.145 Furthermore, the selective deletion of Cx43 in osteoblasts and osteocytes (using osteocalcin Cre) in young C57BL/6J mice leads to a cortical phenotype resembling some components of advanced aging, including increased osteocyte apoptosis, endocortical resorption, and significant cortical thinning.154 We have shown that these same osteoblast and osteocyte Cx43 deficient bones display attenuated bone loss with unloading, a phenotype recapitulated in aged rodents or transgenic osteocyte ablated mice.37, 155, 156 Thus, decreased Cx43 expression in bone during disuse accurately reflects the same Cx43 decrease seen in the aged skeleton, suggesting similar shared mechanisms of Cx43 modulation. Furthermore, the overexpression of Cx43 prevents osteocyte apoptosis and preserves bone quality during aging.144 These data support the hypothesis that Cx43 is a major mechanism controlling postnatal bone mass, and that decreased expression in osteocytes is a consistent feature of both reduced cortical microarchitecture with extreme disuse and advanced aging.
Blood Vessels and Hypoxia
Bone is a highly vascularized tissue and blood flow to bone is extremely important for promoting bone formation, remodeling, and repair. The principal source of blood flow in long bones is via a main medullary nutrient artery, which is fed by smaller periosteal arteries.157 These arteries drain into the medullary arterio-venous sinusoids, near sites of hematopoietic stem cell development,158 before exiting the marrow via a small set of veins that run through the bone cortex. Thus, the blood supply to bone is largely centrifugal, or emitting outward from the marrow and largely controlled by vessel size (vasodilation) and number.159 To track blood flow, radiolabeled fluorescent microspheres, laser doppler flowmetry (LDF), and positron emission spectroscopy (PET) have all been employed.160, 161 A detailed analysis of the pros and cons of each technique can be found in Tomlinson et al.159 Blood flow is extremely important in regard to disuse because it drives bone interstitial fluid transport and pressure, which are the primary components driving osteocyte mechanosensation.162 For example, numerous preclinical models show decreased vessel number and blood flow in hindlimbs following HLU and paralysis in C57BL/6J mice and Fischer F344 rats.163–165 Similarly, Fisher F344 rats and humans also show a stark decrease in long bone blood flow with aging due to decreased vessel number and increases in vascular resistance.166, 167 At the cellular level, these changes are marked by preferential loss of metaphyseal H type vessels, named due to their high colocalization with key vascular markers endomucin and CD31.168 For example, a recent study in young C57BL/6N mice showed that H type vessels, which colocalize with osteoprogenitor cells marked by Osterix (Osx+), are decreased by nearly 50% following short-term HLU.169 Similarly, these H type vessels are largely reduced in bone from aged C57BL/6J mice (70 weeks) and humans (60–75 years).168, 170 Thus, the preferential loss of H type vessels occurs with both extreme short-term disuse and advanced skeletal aging. Expression of the angiogenic factor, hypoxia inducer factor-1α (HIF-1α), may drive the shared similarities between disuse and aging. For example, diaphyseal endosteal osteoblasts show increased HIF-1α expression following disuse.135, 171 HIF1α expression is increased in numerous tissues with aging172, however, the differential expression of HIF1α in young versus aged osteoblast lineage cells remains to be determined. These findings suggest that increasing interstitial bone fluid pressure and or promoting H type vessels may be a means to combat bone loss from extreme disuse and aging simultaneously. In support of this, Kwon et al. demonstrated that dynamic pressurization of the femoral marrow in vivo, which leads to increased interstitial fluid flow but no tissue-level strains, augments bone loss during HLU in C57BL/6J mice.173 Activation of HIF-1α either by genetic or systemic means (Deferoxamine; Panax quinquefolium saponin) increases H type vessel number and ameliorates bone loss and regeneration impairments with disuse and aging in C57BL/6J or C57BL/6N mice and F344 Fischer rats.168, 169, 174 Collectively, these data suggest that extreme disuse models the decline in bone marrow blood flow and interstitial bone pressure seen with advanced aging. Furthermore, extreme disuse causes an accelerated decrease in the prevalence of H type vessels, which are heavily characteristic of bone aging and associated with decreased bone mass.
Neural Cells
Heavily colocalized with the bone vasculature are autonomic nervous system fibers, which are emerging as important regulators of bone remodeling.175 These nerves penetrate cortical bone via the periosteum and enter the marrow and intracortical space via the Volkman’s and Haversian canals.53 Sympathetic (tyrosine hydrolase (TH+)) and peptidergic sensory (Calcitonin gene–related peptide (CGRP+)) nerves play a crucial role in bone pain and skeletal homeostasis, as surgical or pharmacological nerve destruction decreases local bone mass and skeletal adaptation.176, 177 Murine models of aging in C57BL/6J mice show decreased sympathetic fibers but not sensory fibers in the bone cortices and marrow with aging.178, 179 In fact, sympathetic nerve inhibition using propranolol, a competitive blocker of the β1 and β2 adrenergic receptors (Adrβ1, Adrβ2), attenuates bone loss observed with aging in humans and hindlimb unloading in numerous mouse strains.180, 181 This suggests that beta blockers, which decrease the effects of epinephrine, may protect against age- and disuse-induced bone loss by regulating sympathetic nervous tone. Similarly, Adrβ2 deletion in osteoblast lineage cells with2.3kb Col1a1 Cre activation leads to a high bone mass phenotype in C57BL/6J mice due to osteoclast inhibition.182 Conversely, systemic administration of dobutamine (β1 receptor agonist) mitigates unloading-induced cancellous bone loss in Sprague-Dawley rats by decreasing osteocyte apoptosis and boosting osteoblastic bone formation.183 However, under basal conditions, C57BL/6J mice lacking the β1 adrenergic receptor globally have a basal low bone mass phenotype that does not respond to the anabolic stimulus elicited by tibial loading.184 Therefore, global Adrβ1 appears to be a necessary component for skeletal adaption to mechanical loading. Double Adrβ1 and Adrβ2 knockout mice have a low bone-mass phenotype, suggesting the catabolic effects of Adrβ1 deletion supersede the anabolic effects of Adrβ2 deletion.184 Thus, Adrβ2 appears to be constitutively expressed and blocks osteoclastogenesis, whereas Adrβ1 appears to be anabolic and mechanoresponsive. These results suggest that age- and disuse-induced (non-paralytic) changes in skeletal bone mass could be augmented by simultaneously blocking Adrβ2 and increasing Adrβ1 signaling in osteoblasts and osteocytes. Additional research to understand the underlying cellular and molecular mechanisms by which β1/β2 adrenergic receptor signaling may mediate bone loss, especially in the context of extreme disuse and advanced aging, is warranted.
Adipocytes
Bone marrow adipocytes are cells in close contact with nerves and vasculature in bone and are strongly implicated in bone metabolism. For example, increased bone marrow adiposity is found in patients with osteoporosis and fragility fractures.185, 186 Therefore, understanding the mechanism(s) underlying the increased bone marrow adiposity and the relationship to fragility during extreme disuse and advanced aging is paramount. Advanced imaging methods demonstrate that extreme disuse and aging are accompanied by an increase in bone marrow adipose tissue (bMAT). For example, a study using young adult females showed a 2.5% increase in marrow fat accumulation, as assessed by MRI, in the vertebrae following 60 days of skeletal disuse by 6-degree head tilted downward (HDT) bedrest.187 In Wistar rats and C57Bl/6J mice, unloading models such as spaceflight and HLU have resulted in a nearly a 2–3-fold increase in bMAT within 2 weeks.188–190 Importantly, bMAT accumulation from these studies appears to occur due to increases in both adipocyte number and size, with preferential increases in the cancellous regions of bone. The disuse-induced change in marrow adiposity has strong parallels with skeletal aging. For example, both C57BL/6J and C3H/HeJ rodent strains show an age-related 2–3-fold increase in bMAT in the distal and proximal tibia, to differing extents, that occurs between skeletal maturity and 1 year of age.191 In humans, from skeletal maturity to middle age, marrow fat increases by 5–10% per decade in both sexes before rapidly increasing in females due to menopause.192, 193 The exact reason that disuse and aging preferentially increase marrow adiposity at cancellous versus cortical sites is unknown, but it is likely related to the more rapid decrease in vascularity and progenitor cell pool at these sites.168, 194, 195 These studies suggest that morphological and site-specific increases in bone marrow adiposity are both a hallmark of short-term extreme disuse and skeletal aging, although absolute changes in marrow adiposity are also dependent on the individual’s sex and genetics. One potential mechanism connecting increased adipogenesis and bone loss during extreme disuse and advanced aging is a decrease in WNT/β-catenin signaling. Particularly, WNT/β-catenin signaling in bone marrow progenitors and osteoblasts lineage cells (Figure 3). In support of the WNT/β-catenin pathway’s role in pathologies related to disuse and aging, WNT/β-catenin signaling activation via WNT10b in vitro increases osteoblastogenesis at the expense of adipogenesis in bone marrow progenitor cells and early osteoblasts.196, 197 The decreased adipogenesis appears to result from suppressed expression of peroxisome proliferator-activated receptor γ (PPARγ), a major adipogenic transcription factor, and increased expression of RUNX Family Transcription Factor 2 (RUNX2), a transcription factor necessary for osteoblastogenesis.198 Furthermore, the WNT/β-catenin antagonist, sclerostin is upregulated in the serum of humans experiencing disuse and or aging and is decreased by physical activity.199–201 Furthermore, treatment with a sclerostin neutralizing antibody rescues bone loss in young skeletally mature C57BL/6J mice undergoing hindlimb unloading and in aged osteoporotic individuals.202, 203 In addition, treating C57BL/6J mice with sclerostin neutralizing antibody decreases bMAT by reducing adipocyte size and number.204 Collectively, these studies suggest that decreases in WNT/β-catenin signaling in bone marrow progenitor cells induce increases in adipogenesis and bone loss during extreme disuse and aging. However, whether increased bone marrow adiposity, once formed, may also protect or exacerbate bone loss with aging and disuse is still under study.
Potential Shared Mechanisms between Bone Loss with Disuse and Aging
The shared skeletal similarities (structural, material, and cellular) between short-term extreme disuse and advanced aging suggest that some phenotypic changes between the two states may be due to underlying shared systemic mechanisms. Underlying processes shown to be associated with bone aging205–207 that may play a role in disuse-induced bone loss include, but are not limited to, mitochondrial damage/oxidative stress, chronic low-grade inflammation, cellular senescence, and, as previously mentioned, GJIC and altered WNT/β-catenin signaling (in osteocyte and adipocyte section). Although a full overview of these molecular mechanisms in bone is beyond the scope of this review, emerging data suggest that many of these aging mechanisms may also play a key role in mediating bone loss to extreme disuse as well (Figure 3). Briefly, each potential mechanism and associated findings in the context of disuse and aging are presented.
Oxidative Stress
Reactive oxygen species (ROS) and their metabolites are associated with mitochondrial dysfunction and damage to cellular DNA.208 ROS can be produced due to various external stimuli such as UV light, ionizing radiation, inflammatory cytokines, toxins, and fatty acid oxidation.209, 210 Cells fortunately have means to eliminate ROS, mainly through the activity of enzymatic catalases (glutathione reductase; superoxide dismutase (SOD)) and the Forkhead box O (FoxO) family of transcription factors.211 ROS metabolites have been shown to increase, while protective mechanisms decrease, with age in bone. For example, Almedia et al. showed that glutathione reductase shows an approximate 50–75% reduction in activity from skeletal maturity to advanced age (25 months) in the bone marrow of female and male C57BL6_J mice.129 At these same time points, ROS was significantly upregulated in the bone marrow and was accompanied by a significant decrease in cortical and cancellous BMD. These results suggest that some of age-related bone loss may be due to increased ROS in bone cells. Interestingly, SOD2 deficiency in osteocytes decreases bone structure and strength in young but not aged C57BL/6J rodents via osteocyte apoptosis and LCS disruption212. This is consistent with previous studies examining rodent aging effects on the LCS.143 Therefore, ROS induced mechanisms of bone loss appear to be driven by changes in bone LCS and osteocyte viability. Furthermore, osteocyte apoptosis has been a consistent finding in unloaded bone71, 135, 136, suggesting a potential role for ROS during disuse. Similar to aging, rodents and humans undergoing unloading as a result of spaceflight or HLU show increased ROS levels in bone and decreased antioxidant enzymes. For example, astronauts show upregulated ROS metabolites in urine213, 214 and increased ROS levels inbone marrow and osteoblast lineage cells.215 In addition, SOD1 global deletion exacerbates HLU induced bone loss215 but administration of antioxidants known to ameliorate ROS (Vitamin C and Curcumin) attenuates HLU-induced bone loss in C57BL/6J mice and Sprague-Dawley rats, respectively.216, 217 These results suggest that ROS is more prevalent in bone following disuse and aging and that ROS accumulation, likely due partially to reduced catalytic enzymatic activity, contributes to some of the negative effects of disuse, and aging on the skeleton.
Inflammation
A parallel feature to ROS accumulation with aging, which may be a shared mechanism of disuse, includes heightened low-grade inflammation systemically and in bone. In the aging field, increasing low grade chronic inflammation, termed “inflammaging”218, is increasingly recognized for its role in tissue pathologies. In aged humans and rodents, there are increased levels of inflammatory cytokines (C-reactive protein, TNFα) and neutrophil to lymphocyte cell ratios (termed NLR), that correlate with osteoporosis severity.219–224 Similarly, peripheral neutrophil counts and TNFα levels (systemic and in bone osteocytes) are upregulated in disuse models, including human spaceflight and bedrest, and in murine models of hindlimb unloading.33, 39, 225–227 These inflammatory cytokines (C-reactive protein, TNFα) induce RANKL expression and nuclear factor-κB (NF-κB) pathway activation, thereby leading to increased osteoclastogenesis (via promoting osteoclast differentiation and survival) and decreased osteoblastogenesis (via inhibiting osteoblast cell maturation).228–230 Therefore, NF-κB pathway activity may be a shared mechanism between bone disuse and aging that can control bone loss in both conditions. In support of NF-κB’s therapeutic potential, NF-κB global null mice are protected against bone loss by HLU.231 Furthermore, pharmacological inhibition of NF-κB in aging mice (52 weeks) is able to reduce some of the impaired osteogenic potential of skeletal stem cells. These data suggest that NF-κB pathway modulation could potentially restore bone loss with aging and disuse by affecting both arms of bone remodeling.
Cellular Senescence
Another consequence of increased oxidative stress and inflammation, and resulting DNA damage in cells, is increased cellular senescence. Once created, these senescent cells take on a senescence-associated secretory phenotype (SASP), upregulating secretion of pro-inflammatory cytokines and chemokines, that can induce adverse effects on surrounding cells. Due to their post-mitotic long-lived nature, osteocytes are especially susceptible to DNA damage and a SASP like phenotype. For example, Farr et al., demonstrated that senescence-associated distension of satellites (SADS), a marker of centromere unraveling and cellular senescence, was increased in osteocytes and myeloid cells in C57BL/6J old vs young mice (24 versus 6 months).232 Furthermore, anti-senolytic agents dasatinib and quercetin, when given to aged mice (20 months) for 4 months, cleared 50% of senescent osteocytes and attenuated bone loss in both males and females.233 These results suggest that senescent cell accumulation in bone with aging is a potential mechanism of age-related bone loss. However little data exists to suggest the potential of senescent cells to accumulate and contribute to disuse induced bone loss. A recent manuscript by Parker et al. demonstrated that fibro-adipogenic progenitors in skeletal muscle undergo senescence as a result of HLU.234 Skeletal disuse by unloading, as previously mentioned, increases ROS, inflammatory cytokine signaling, and various other DNA damaging stimuli shown to promote senescence. These data suggest that cellular senescence is a potential mechanism of disuse induced bone loss that warrants further study.
Conclusions and Future Directions
This review summarizes current evidence supporting the idea that extreme disuse results in local bone tissue, cellular, and molecular changes that are similar to changes seen in aged bone, albeit at an accelerated rate. These shared bone responses (Figure 4) occurring in both extreme to moderate disuse as well as advanced aging, include loss of cancellous bone (by trabecular thinning) and cortical bone (by endosteal resorption, cortical thinning). At the tissue-level, shared material property alterations between disuse and aging include loss of collagen content and alterations in the inorganic phase of bone mineral (enhanced crystallinity), which potentially lead to decreased mechanical competence and bone fragility. At the cellular and molecular level, the shared phenotypic similarities leading to bone loss are even more striking and include increases in osteocyte apoptosis, RANKL and sclerostin secretion by osteocytes, ROS accumulation in bone tissue, and enhanced marrow adiposity with localized decreases in blood flow and sympathetic tone. Overall, these well-orchestrated changes manifest in a global reduction in bone mass, deteriorated microarchitecture and decreased bone quality that decreases bone strength, leading to increased fracture risk. Importantly, the majority of these changes are detectable in a matter of weeks to months in models of extreme disuse versus the years and decades associated with bone loss due to aging. We posit that phenotypic similarities in the loss of bone tissue inexorably link mechanisms of bone loss with disuse to accelerated aging, especially in terms of the most clinically relevant outcome, i.e. fracture risk. However, many of these well-defined aging mechanisms, such as ROS accumulation, inflammaging, and cellular senescence are less well defined in bone disuse pathologies. It is important to note that both aging and skeletal disuse appear to be highly influenced by genetics, and thus utilizing multiple inbred strains or outbred strains simultaneously in research studies will more faithfully recapitulate the human condition. Furthermore, the partial weight-bearing model has the ability to test disuse across a continuum, not just extreme forms, and therefore will be a very important unloading model moving forward. Utilization of the partial weight-bearing model has improved the recapitulation of clinical conditions, such as physical inactivity, fracture/stroke rehabilitation, and extraterrestrial habitation. In all, the current shared similarities in bone loss with disuse and aging highlight the potential of overarching mechanisms warranting further study, including but not limited to, cellular senescence, altered GJIC and WNT/β-catenin signaling, low grade inflammation and oxidative DNA damage. These shared processes underscore the need for increased or improved physical activity regimens to combat age and disuse-induced bone loss. However, extreme disuse does not fully recapitulate the aging phenotype. For example, age-related bone changes occur gradually across the skeleton, not just at weight-bearing sites, and are accompanied by continued periosteal expansion (Figure 4). Furthermore, aging is accompanied by sex steroid deficiency,235 and therefore skeletal differences among males and females appear more pronounced in aging than with short-term extreme disuse. Future disuse preclinical and clinical studies utilizing an adequate number of male and female participants are needed to uncover sexually dimorphic mechanisms of bone loss. In addition, traits of skeletal aging that have yet to be examined in the context of skeletal disuse include collagen cross-linking (enzymatic and nonenzymatic), AGE-RAGE signaling, and altered lacunar-canalicular connectivity that may affect cell signaling and mechanosensation. Furthermore, more research examining the primary cell types contributing to disuse-induced increases in RANKL-OPG signaling, oxidative stress, and decreases in neural-vascularity is required. We anticipate that further elucidation of these fundamental cellular details is forthcoming, aided by next generation analyses (spatial transcriptomics, single cell RNA-sequencing) and fluorescent cell reporter lines undergoing in vivo disuse. It is anticipated that the elucidation of these common mechanistic changes will result in new therapies to combat disuse and aging-induced bone loss and help eliminate the wide incidence and burden of fractures that afflict patients with osteoporosis.
Figure 4. Venn Diagram of Phenotypic Changes Observed with Bone Loss in Extreme Skeletal Disuse and Advanced Aging.
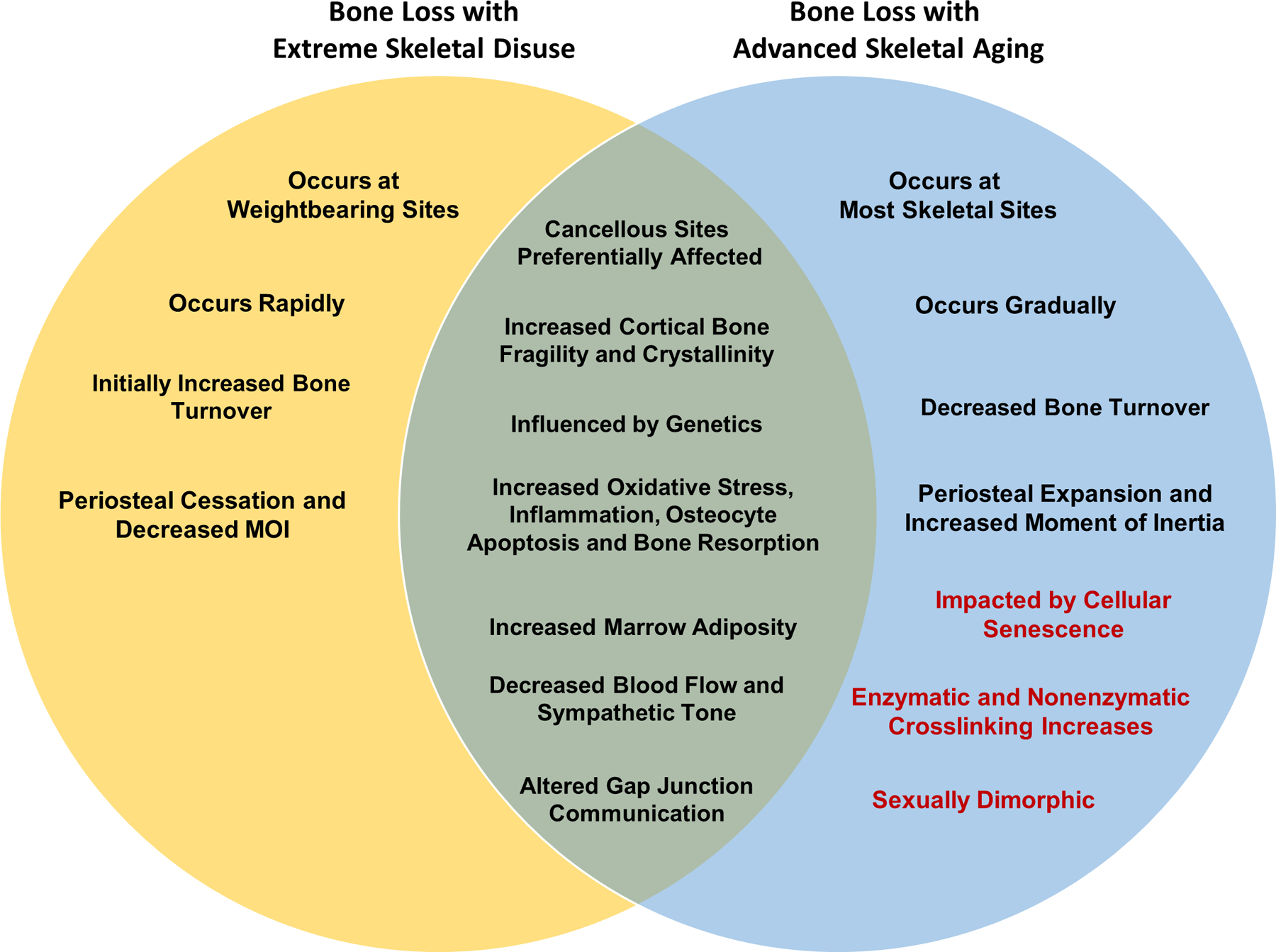
Items shown in black text represent well-documented similarities and differences observed with either extreme skeletal disuse and or advanced aging. Items in red text represent findings well-documented in skeletal aging literature that have yet to be thoroughly explored in skeletal disuse.
Acknowledgements:
Drs. Evan Buettmann and Michael Friedman were supported by the Translational Research Institute for Space Health through Cooperative Agreement with NASA (NNX16AO69A). Dr. Henry Donahue, Galen Goldscheitter, and Gabriel Hoppock were supported by NIH (NIAMS) grant R01 (AR068132), NASA grant 80NSSC18K1473, and National Space Biological Research Institute grant NSBRI/NASA, (MA02802). Dr. Larry J. Suva is supported by R01HD102909 and 4R37 AA018282 from NIH. Figures 1–3 were created with Biorender.com.
References:
- 1.Pisani P, et al. , Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J Orthop, 2016. 7(3): p. 171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBlanc AD, et al. , Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact, 2007. 7(1): p. 33–47. [PubMed] [Google Scholar]
- 3.Cosman F, et al. , Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int, 2014. 25(10): p. 2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keyak JH, et al. , Reduction in proximal femoral strength due to long-duration spaceflight. Bone, 2009. 44(3): p. 449–53. [DOI] [PubMed] [Google Scholar]
- 5.Keaveny TM, et al. , Age-dependence of femoral strength in white women and men. J Bone Miner Res, 2010. 25(5): p. 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannan MT, et al. , Risk Factors for Longitudinal Bone Loss in Elderly Men and Women: The Framingham Osteoporosis Study. Journal of Bone and Mineral Research, 2000. 15(4): p. 710–720. [DOI] [PubMed] [Google Scholar]
- 7.Berger C, et al. , Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. Cmaj, 2008. 178(13): p. 1660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shackelford LC, et al. , Resistance exercise as a countermeasure to disuse-induced bone loss. Journal of Applied Physiology, 2004. 97(1): p. 119–129. [DOI] [PubMed] [Google Scholar]
- 9.Marques EA, Mota J, and Carvalho J, Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Dordr), 2012. 34(6): p. 1493–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeBlanc AD, et al. , Alendronate as an effective countermeasure to disuse induced bone loss. J Musculoskelet Neuronal Interact, 2002. 2(4): p. 335–43. [PubMed] [Google Scholar]
- 11.McPhee JS, et al. , Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology, 2016. 17(3): p. 567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiPietro L, Physical Activity in Aging: Changes in Patterns and Their Relationship to Health and Function. The Journals of Gerontology: Series A, 2001. 56(suppl_2): p. 13–22. [DOI] [PubMed] [Google Scholar]
- 13.Curtis EM, et al. , The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone, 2017. 104: p. 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brauer CA, et al. , Incidence and mortality of hip fractures in the United States. Jama, 2009. 302(14): p. 1573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschfeld HP, Kinsella R, and Duque G, Osteosarcopenia: where bone, muscle, and fat collide. Osteoporosis International, 2017. 28(10): p. 2781–2790. [DOI] [PubMed] [Google Scholar]
- 16.Hida T, et al. , Managing sarcopenia and its related-fractures to improve quality of life in geriatric populations. Aging Dis, 2014. 5(4): p. 226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jilka RL, The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci, 2013. 68(10): p. 1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh J and Ladiges W, Voluntary Wheel Running in Mice. Curr Protoc Mouse Biol, 2015. 5(4): p. 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White Z, et al. , Voluntary resistance wheel exercise from mid-life prevents sarcopenia and increases markers of mitochondrial function and autophagy in muscles of old male and female C57BL/6J mice. Skelet Muscle, 2016. 6(1): p. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMullan RC, et al. , Long-term exercise in mice has sex-dependent benefits on body composition and metabolism during aging. Physiol Rep, 2016. 4(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmassani ZS, et al. , Absence of MyD88 from Skeletal Muscle Protects Female Mice from Inactivity-Induced Adiposity and Insulin Resistance. Obesity, 2020. 28(4): p. 772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie AI, et al. , Pharmacological inhibition of TLR4 ameliorates muscle and liver ceramide content after disuse in previously physically active mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2020. 318(3): p. R503–R511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauer JT, et al. , Muscle-bone properties after prolonged voluntary wheel running in a mouse model of dominant severe osteogenesis imperfecta. J Musculoskelet Neuronal Interact, 2021. 21(4): p. 517–527. [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman MA, Szczepankiewicz RP, and Kohn DH, Combined mineral-supplemented diet and exercise increases bone mass and strength after eight weeks and maintains increases after eight weeks detraining in adult mice. PLoS One, 2018. 13(9): p. e0204470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honda A, et al. , Bones benefits gained by jump training are preserved after detraining in young and adult rats. Journal of Applied Physiology, 2008. 105(3): p. 849–853. [DOI] [PubMed] [Google Scholar]
- 26.Mustafy T, et al. , High Impact Exercise Improves Bone Microstructure and Strength in Growing Rats. Scientific Reports, 2019. 9(1): p. 13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudreaux RD, et al. , Increased resistance during jump exercise does not enhance cortical bone formation. Med Sci Sports Exerc, 2014. 46(5): p. 982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avin KG, et al. , Voluntary Wheel Running Has Beneficial Effects in a Rat Model of CKD-Mineral Bone Disorder (CKD-MBD). J Am Soc Nephrol, 2019. 30(10): p. 1898–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirkes RK, et al. , Voluntary Wheel Running Partially Compensates for the Effects of Global Estrogen Receptor-α Knockout on Cortical Bone in Young Male Mice. Int J Mol Sci, 2021. 22(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzanares G, Brito-da-Silva G, and Gandra PG, Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res, 2018. 52(1): p. e7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morey-Holton ER and Globus RK, Hindlimb unloading rodent model: technical aspects. Journal of Applied Physiology, 2002. 92(4): p. 1367–1377. [DOI] [PubMed] [Google Scholar]
- 32.Globus RK and Morey-Holton E, Hindlimb unloading: rodent analog for microgravity. Journal of Applied Physiology, 2016. 120(10): p. 1196–1206. [DOI] [PubMed] [Google Scholar]
- 33.Metzger CE, et al. , Hindlimb unloading causes regional loading-dependent changes in osteocyte inflammatory cytokines that are modulated by exogenous irisin treatment. npj Microgravity, 2020. 6(1): p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd SA, et al. , Interdependence of muscle atrophy and bone loss induced by mechanical unloading. J Bone Miner Res, 2014. 29(5): p. 1118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen MR, Hogan HA, and Bloomfield SA, Differential bone and muscle recovery following hindlimb unloading in skeletally mature male rats. J Musculoskelet Neuronal Interact, 2006. 6(3): p. 217–25. [PubMed] [Google Scholar]
- 36.Miller BF, et al. , Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle, 2019. 10(6): p. 1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrien DS, et al. , Aging alters the skeletal response to disuse in the rat. Am J Physiol Regul Integr Comp Physiol, 2007. 292(2): p. R988–96. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham HC, et al. , Age-dependent bone loss and recovery during hindlimb unloading and subsequent reloading in rats. BMC Musculoskelet Disord, 2018. 19(1): p. 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tahimic CGT, et al. , Influence of Social Isolation During Prolonged Simulated Weightlessness by Hindlimb Unloading. Frontiers in Physiology, 2019. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman MA, et al. , Single limb immobilization model for bone loss from unloading. J Biomech, 2019. 83: p. 181–189. [DOI] [PubMed] [Google Scholar]
- 41.Lang SM, et al. , Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLoS One, 2012. 7(6): p. e38910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman MA, et al. , Genetic Variability affects the Skeletal Response to Unloading. bioRxiv, 2020: p. 2020.06.26.174326. [Google Scholar]
- 43.Friedman MA, et al. , Genetic variability affects the skeletal response to immobilization in founder strains of the diversity outbred mouse population. Bone Reports, 2021. 15: p. 101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veitch SW, et al. , Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos Int, 2006. 17(3): p. 364–72. [DOI] [PubMed] [Google Scholar]
- 45.Ceroni D, et al. , Effects of cast-mediated immobilization on bone mineral mass at various sites in adolescents with lower-extremity fracture. J Bone Joint Surg Am, 2012. 94(3): p. 208–16. [DOI] [PubMed] [Google Scholar]
- 46.Anderson MJ, et al. , Contribution of mechanical unloading to trabecular bone loss following non-invasive knee injury in mice. J Orthop Res, 2016. 34(10): p. 1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dolly O, Synaptic transmission: inhibition of neurotransmitter release by botulinum toxins. Headache, 2003. 43 Suppl 1: p. S16–24. [DOI] [PubMed] [Google Scholar]
- 48.Warner SE, et al. , Botox induced muscle paralysis rapidly degrades bone. Bone, 2006. 38(2): p. 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellman R, et al. , Combined effects of botulinum toxin injection and hind limb unloading on bone and muscle. Calcif Tissue Int, 2014. 94(3): p. 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatti V, et al. , Botox-induced muscle paralysis alters intracortical porosity and osteocyte lacunar density in skeletally mature rats. Journal of Orthopaedic Research, 2019. 37(5): p. 1153–1163. [DOI] [PubMed] [Google Scholar]
- 51.Ma X, et al. , Naringin ameliorates bone loss induced by sciatic neurectomy and increases Semaphorin 3A expression in denervated bone. Scientific Reports, 2016. 6(1): p. 24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monzem S, et al. , Sciatic neurectomy-related cortical bone loss exhibits delayed onset yet stabilises more rapidly than trabecular bone. Bone Rep, 2021. 15: p. 101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brazill JM, et al. , Nerves in Bone: Evolving Concepts in Pain and Anabolism. Journal of Bone and Mineral Research, 2019. 34(8): p. 1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner EB, et al. , Partial weight suspension: a novel murine model for investigating adaptation to reduced musculoskeletal loading. J Appl Physiol (1985), 2010. 109(2): p. 350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mortreux M, et al. , A novel partial gravity ground-based analog for rats via quadrupedal unloading. Journal of Applied Physiology, 2018. 125(1): p. 175–182. [DOI] [PubMed] [Google Scholar]
- 56.Hustedt JW, et al. , Current advances in training orthopaedic patients to comply with partial weight-bearing instructions. Yale J Biol Med, 2012. 85(1): p. 119–25. [PMC free article] [PubMed] [Google Scholar]
- 57.Ellman R, et al. , Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. Journal of Bone and Mineral Research, 2013. 28(4): p. 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swift JM, et al. , Partial weight bearing does not prevent musculoskeletal losses associated with disuse. Med Sci Sports Exerc, 2013. 45(11): p. 2052–60. [DOI] [PubMed] [Google Scholar]
- 59.Beamer WG, et al. , Genetic variability in adult bone density among inbred strains of mice. Bone, 1996. 18(5): p. 397–403. [DOI] [PubMed] [Google Scholar]
- 60.Fritton JC, et al. , Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone, 2005. 36(6): p. 1030–8. [DOI] [PubMed] [Google Scholar]
- 61.Silva MJ, et al. , Tibial Loading Increases Osteogenic Gene Expression and Cortical Bone Volume in Mature and Middle-Aged Mice. PLOS ONE, 2012. 7(4): p. e34980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mortreux M, et al. , The partial weight-bearing rat model using a pelvic harness does not impact stress or hindlimb blood flow. Acta Astronautica, 2020. 168: p. 249–255. [Google Scholar]
- 63.Chen J-H, Hales CN, and Ozanne SE, DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Research, 2007. 35(22): p. 7417–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harman D, The aging process. Proc Natl Acad Sci U S A, 1981. 78(11): p. 7124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gensler HL and Bernstein H, DNA damage as the primary cause of aging. Q Rev Biol, 1981. 56(3): p. 279–303. [DOI] [PubMed] [Google Scholar]
- 66.Hambright WS, et al. , Murine models of accelerated aging and musculoskeletal disease. Bone, 2019. 125: p. 122–127. [DOI] [PubMed] [Google Scholar]
- 67.Miller RA, ‘Accelerated aging’: a primrose path to insight? Aging Cell, 2004. 3(2): p. 47–51. [DOI] [PubMed] [Google Scholar]
- 68.Lu J, et al. , Peak Bone Mass and Patterns of Change in Total Bone Mineral Density and Bone Mineral Contents From Childhood Into Young Adulthood. J Clin Densitom, 2016. 19(2): p. 180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glatt V, et al. , Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res, 2007. 22(8): p. 1197–207. [DOI] [PubMed] [Google Scholar]
- 70.Papageorgiou M, et al. , Age- and Strain-Related Differences in Bone Microstructure and Body Composition During Development in Inbred Male Mouse Strains. Calcified Tissue International, 2020. 106(4): p. 431–443. [DOI] [PubMed] [Google Scholar]
- 71.Lloyd SA, et al. , Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res, 2012. 27(11): p. 2359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vico L, et al. , Cortical and Trabecular Bone Microstructure Did Not Recover at Weight-Bearing Skeletal Sites and Progressively Deteriorated at Non-Weight-Bearing Sites During the Year Following International Space Station Missions. J Bone Miner Res, 2017. 32(10): p. 2010–2021. [DOI] [PubMed] [Google Scholar]
- 73.Gabel L, et al. , Pre-flight exercise and bone metabolism predict unloading-induced bone loss due to spaceflight. British journal of sports medicine, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferguson VL, et al. , Bone development and age-related bone loss in male C57BL/6J mice. Bone, 2003. 33(3): p. 387–98. [DOI] [PubMed] [Google Scholar]
- 75.Willinghamm MD, et al. , Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int, 2010. 86(6): p. 470–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferguson VL, et al. , Bone development and age-related bone loss in male C57BL/6J mice. Bone, 2003. 33(3): p. 387–398. [DOI] [PubMed] [Google Scholar]
- 77.Lodberg A, et al. , Immobilization induced osteopenia is strain specific in mice. Bone Reports, 2015. 2: p. 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aubert AE, et al. , Towards human exploration of space: the THESEUS review series on cardiovascular, respiratory, and renal research priorities. npj Microgravity, 2016. 2(1): p. 16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belavy DL, et al. , Bone structure and density via HR-pQCT in 60d bed-rest, 2-years recovery with and without countermeasures. J Musculoskelet Neuronal Interact, 2011. 11(3): p. 215–26. [PubMed] [Google Scholar]
- 80.Macdonald HM, et al. , Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res, 2011. 26(1): p. 50–62. [DOI] [PubMed] [Google Scholar]
- 81.Judex S, et al. , Genetic and tissue level muscle-bone interactions during unloading and reambulation. J Musculoskelet Neuronal Interact, 2016. 16(3): p. 174–82. [PMC free article] [PubMed] [Google Scholar]
- 82.Judex S, et al. , Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res, 2004. 19(4): p. 607–13. [DOI] [PubMed] [Google Scholar]
- 83.Deng R, et al. , Periosteal CD68(+) F4/80(+) Macrophages Are Mechanosensitive for Cortical Bone Formation by Secretion and Activation of TGF-β1. Adv Sci (Weinh), 2022. 9(3): p. e2103343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Depke M, et al. , Bone marrow-derived macrophages from BALB/c and C57BL/6 mice fundamentally differ in their respiratory chain complex proteins, lysosomal enzymes and components of antioxidant stress systems. J Proteomics, 2014. 103: p. 72–86. [DOI] [PubMed] [Google Scholar]
- 85.Ploutz-Snyder L, et al. , Effects of sex and gender on adaptation to space: musculoskeletal health. J Womens Health (Larchmt), 2014. 23(11): p. 963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ellman R, Sibonga JD, and Bouxsein ML. Male Astronauts Have Greater Bone Loss and Risk of Hip Fracture Following Long Duration Spaceflights than Females. 2010.
- 87.Smith SM, et al. , Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J Bone Miner Res, 2012. 27(9): p. 1896–906. [DOI] [PubMed] [Google Scholar]
- 88.Chen H, et al. , Age- and gender-dependent changes in three-dimensional microstructure of cortical and trabecular bone at the human femoral neck. Osteoporos Int, 2010. 21(4): p. 627–36. [DOI] [PubMed] [Google Scholar]
- 89.Warming L, Hassager C, and Christiansen C, Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int, 2002. 13(2): p. 105–12. [DOI] [PubMed] [Google Scholar]
- 90.Khosla S, et al. , Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res, 2006. 21(1): p. 124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gourion-Arsiquaud S, et al. , Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res, 2009. 24(9): p. 1565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCreadie BR, et al. , Bone tissue compositional differences in women with and without osteoporotic fracture. Bone, 2006. 39(6): p. 1190–5. [DOI] [PubMed] [Google Scholar]
- 93.Yerramshetty JS and Akkus O, The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone, 2008. 42(3): p. 476–482. [DOI] [PubMed] [Google Scholar]
- 94.Ruppel ME, Burr DB, and Miller LM, Chemical makeup of microdamaged bone differs from undamaged bone. Bone, 2006. 39(2): p. 318–324. [DOI] [PubMed] [Google Scholar]
- 95.Osterhoff G, et al. , Bone mechanical properties and changes with osteoporosis. Injury, 2016. 47 Suppl 2(Suppl 2): p. S11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peres-Ueno MJ, et al. , Model of hindlimb unloading in adult female rats: Characterizing bone physicochemical, microstructural, and biomechanical properties. PLoS One, 2017. 12(12): p. e0189121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishimaru Y, et al. , Raman Spectroscopic Analysis to Detect Reduced Bone Quality after Sciatic Neurectomy in Mice. Molecules, 2018. 23(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raghavan M, et al. , Age-specific profiles of tissue-level composition and mechanical properties in murine cortical bone. Bone, 2012. 50(4): p. 942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mumtaz H, et al. , Age-related and sex-specific effects on architectural properties and biomechanical response of the C57BL/6N mouse femur, tibia and ulna. Bone Rep, 2020. 12: p. 100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boskey AL and Coleman R, Aging and bone. J Dent Res, 2010. 89(12): p. 1333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burr DB, The contribution of the organic matrix to bone’s material properties. Bone, 2002. 31(1): p. 8–11. [DOI] [PubMed] [Google Scholar]
- 102.Viguet-Carrin S, Garnero P, and Delmas PD, The role of collagen in bone strength. Osteoporosis International, 2006. 17(3): p. 319–336. [DOI] [PubMed] [Google Scholar]
- 103.Garnero P, The contribution of collagen crosslinks to bone strength. Bonekey Rep, 2012. 1: p. 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saito M and Marumo K, Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporosis International, 2010. 21(2): p. 195–214. [DOI] [PubMed] [Google Scholar]
- 105.Vashishth D, Advanced Glycation End-products and Bone Fractures. IBMS Bonekey, 2009. 6(8): p. 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paschalis EP, et al. , Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone, 2011. 49(6): p. 1232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saito M, et al. , Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporosis International, 2006. 17(7): p. 986–995. [DOI] [PubMed] [Google Scholar]
- 108.Smith SM, et al. , Bone metabolism and renal stone risk during International Space Station missions. Bone, 2015. 81: p. 712–720. [DOI] [PubMed] [Google Scholar]
- 109.Shiiba M, et al. , Alterations of collagen matrix in weight-bearing bones during skeletal unloading. Connect Tissue Res, 2001. 42(4): p. 303–11. [DOI] [PubMed] [Google Scholar]
- 110.Shiiba M, et al. , Regional Alterations of Type I Collagen in Rat Tibia Induced by Skeletal Unloading. Journal of Bone and Mineral Research, 2002. 17(9): p. 1639–1645. [DOI] [PubMed] [Google Scholar]
- 111.Yang P-F, et al. , Disuse Impairs the Mechanical Competence of Bone by Regulating the Characterizations of Mineralized Collagen Fibrils in Cortical Bone. Frontiers in Physiology, 2019. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong XN, et al. , In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone, 2011. 49(2): p. 174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tanaka K, et al. , Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun, 2015. 461(2): p. 193–9. [DOI] [PubMed] [Google Scholar]
- 114.McCarthy AD, et al. , Non-enzymatic glycosylation of a type I collagen matrix: effects on osteoblastic development and oxidative stress. BMC Cell Biol, 2001. 2: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bellido T, Osteocyte-driven bone remodeling. Calcif Tissue Int, 2014. 94(1): p. 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blaber EA, et al. , Microgravity Induces Pelvic Bone Loss through Osteoclastic Activity, Osteocytic Osteolysis, and Osteoblastic Cell Cycle Inhibition by CDKN1a/p21. PLOS ONE, 2013. 8(4): p. e61372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lloyd SA, et al. , Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone, 2013. 57(1): p. 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buehlmeier J, et al. , Markers of bone metabolism during 14 days of bed rest in young and older men. J Musculoskelet Neuronal Interact, 2017. 17(1): p. 399–408. [PMC free article] [PubMed] [Google Scholar]
- 119.Gabel L, et al. , Pre-flight exercise and bone metabolism predict unloading-induced bone loss due to spaceflight. British Journal of Sports Medicine, 2021: p. bjsports-2020-103602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith SM, et al. , Effects of artificial gravity during bed rest on bone metabolism in humans. J Appl Physiol (1985), 2009. 107(1): p. 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cao J, et al. , Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J Bone Miner Res, 2003. 18(2): p. 270–7. [DOI] [PubMed] [Google Scholar]
- 122.Chung PL, et al. , Effect of age on regulation of human osteoclast differentiation. J Cell Biochem, 2014. 115(8): p. 1412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Blaber EA, et al. , Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res, 2014. 13(2): p. 181–201. [DOI] [PubMed] [Google Scholar]
- 124.Kostenuik PJ, et al. , Denosumab, a Fully Human Monoclonal Antibody to RANKL, Inhibits Bone Resorption and Increases BMD in Knock-In Mice That Express Chimeric (Murine/Human) RANKL. Journal of Bone and Mineral Research, 2009. 24(2): p. 182–195. [DOI] [PubMed] [Google Scholar]
- 125.Lloyd SA, et al. , Osteoprotegerin is an effective countermeasure for spaceflight-induced bone loss in mice. Bone, 2015. 81: p. 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cummings SR, et al. , Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. New England Journal of Medicine, 2009. 361(8): p. 756–765. [DOI] [PubMed] [Google Scholar]
- 127.Zhou S, et al. , Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell, 2008. 7(3): p. 335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perrien DS, et al. , Bone Turnover across the Menopause Transition: Correlations with Inhibins and Follicle-Stimulating Hormone. The Journal of Clinical Endocrinology & Metabolism, 2006. 91(5): p. 1848–1854. [DOI] [PubMed] [Google Scholar]
- 129.Almeida M, et al. , Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem, 2007. 282(37): p. 27285–27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Michelsen J, et al. , Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone, 2013. 57(2): p. 399–404. [DOI] [PubMed] [Google Scholar]
- 131.Mashiba T, et al. , Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res, 2000. 15(4): p. 613–20. [DOI] [PubMed] [Google Scholar]
- 132.Melton LJ 3rd, et al. , Relative contributions of bone density, bone turnover, and clinical risk factors to long-term fracture prediction. J Bone Miner Res, 2003. 18(2): p. 312–8. [DOI] [PubMed] [Google Scholar]
- 133.You LD, et al. , Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol, 2004. 278(2): p. 505–13. [DOI] [PubMed] [Google Scholar]
- 134.Lloyd SA, et al. , Shifting paradigms on the role of connexin43 in the skeletal response to mechanical load. J Bone Miner Res, 2014. 29(2): p. 275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cabahug-Zuckerman P, et al. , Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs. J Bone Miner Res, 2016. 31(7): p. 1356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aguirre JI, et al. , Osteocyte Apoptosis Is Induced by Weightlessness in Mice and Precedes Osteoclast Recruitment and Bone Loss. Journal of Bone and Mineral Research, 2006. 21(4): p. 605–615. [DOI] [PubMed] [Google Scholar]
- 137.Qiu S, et al. , Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone, 2002. 31(2): p. 313–318. [DOI] [PubMed] [Google Scholar]
- 138.Gerbaix M, et al. , One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Scientific Reports, 2017. 7(1): p. 2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Britz HM, et al. , Prolonged unloading in growing rats reduces cortical osteocyte lacunar density and volume in the distal tibia. Bone, 2012. 51(5): p. 913–9. [DOI] [PubMed] [Google Scholar]
- 140.Vashishth D, et al. , Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone, 2000. 26(4): p. 375–80. [DOI] [PubMed] [Google Scholar]
- 141.Busse B, et al. , Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell, 2010. 9(6): p. 1065–75. [DOI] [PubMed] [Google Scholar]
- 142.Carter Y, et al. , Femoral osteocyte lacunar density, volume and morphology in women across the lifespan. Journal of Structural Biology, 2013. 183(3): p. 519–526. [DOI] [PubMed] [Google Scholar]
- 143.Tiede-Lewis LM, et al. , Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging (Albany NY), 2017. 9(10): p. 2190–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Davis HM, et al. , Cx43 overexpression in osteocytes prevents osteocyte apoptosis and preserves cortical bone quality in aging mice. JBMR Plus, 2018. 2(4): p. 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davis HM, et al. , Disruption of the Cx43/miR21 pathway leads to osteocyte apoptosis and increased osteoclastogenesis with aging. Aging Cell, 2017. 16(3): p. 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Plotkin LI, et al. , Connexin 43 Is Required for the Anti-Apoptotic Effect of Bisphosphonates on Osteocytes and Osteoblasts In Vivo. Journal of Bone and Mineral Research, 2008. 23(11): p. 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Buo AM and Stains JP, Gap junctional regulation of signal transduction in bone cells. FEBS Letters, 2014. 588(8): p. 1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bivi N, et al. , Connexin43 interacts with βarrestin: A pre-requisite for osteoblast survival induced by parathyroid hormone. Journal of Cellular Biochemistry, 2011. 112(10): p. 2920–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moorer MC, et al. , Defective signaling, osteoblastogenesis and bone remodeling in a mouse model of connexin 43 C-terminal truncation. J Cell Sci, 2017. 130(3): p. 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Di SM, et al. , Graviresponses of osteocytes under altered gravity. Advances in Space Research, 2011. 48(6): p. 1161–1166. [Google Scholar]
- 151.Xu H, et al. , Biological responses of osteocytic connexin 43 hemichannels to simulated microgravity. J Orthop Res, 2017. 35(6): p. 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cheng B, et al. , Expression of Functional Gap Junctions and Regulation by Fluid Flow in Osteocyte-Like MLO-Y4 Cells. Journal of Bone and Mineral Research, 2001. 16(2): p. 249–259. [DOI] [PubMed] [Google Scholar]
- 153.Alford AI, Jacobs CR, and Donahue HJ, Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism☆. Bone, 2003. 33(1): p. 64–70. [DOI] [PubMed] [Google Scholar]
- 154.Zhang Y, et al. , Enhanced Osteoclastic Resorption and Responsiveness to Mechanical Load in Gap Junction Deficient Bone. PLOS ONE, 2011. 6(8): p. e23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tatsumi S, et al. , Targeted Ablation of Osteocytes Induces Osteoporosis with Defective Mechanotransduction. Cell Metabolism, 2007. 5(6): p. 464–475. [DOI] [PubMed] [Google Scholar]
- 156.Cunningham HC, et al. , Age-dependent bone loss and recovery during hindlimb unloading and subsequent reloading in rats. BMC Musculoskeletal Disorders, 2018. 19(1): p. 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marenzana M and Arnett TR, The Key Role of the Blood Supply to Bone. Bone Res, 2013. 1(3): p. 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tamma R and Ribatti D, Bone Niches, Hematopoietic Stem Cells, and Vessel Formation. Int J Mol Sci, 2017. 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tomlinson RE and Silva MJ, Skeletal Blood Flow in Bone Repair and Maintenance. Bone Research, 2013. 1(1): p. 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tomlinson RE, Silva MJ, and Shoghi KI, Quantification of skeletal blood flow and fluoride metabolism in rats using PET in a pre-clinical stress fracture model. Mol Imaging Biol, 2012. 14(3): p. 348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Prinzen FW and Bassingthwaighte JB, Blood flow distributions by microsphere deposition methods. Cardiovasc Res, 2000. 45(1): p. 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fritton SP and Weinbaum S, Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu Rev Fluid Mech, 2009. 41: p. 347–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Colleran PN, et al. , Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J Appl Physiol (1985), 2000. 89(3): p. 1046–54. [DOI] [PubMed] [Google Scholar]
- 164.Ding WG, et al. , Difference in intraosseous blood vessel volume and number in osteoporotic model mice induced by spinal cord injury and sciatic nerve resection. J Bone Miner Metab, 2012. 30(4): p. 400–7. [DOI] [PubMed] [Google Scholar]
- 165.Spier SA, et al. , Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol, 2004. 556(Pt 3): p. 947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Prisby RD, et al. , Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res, 2007. 22(8): p. 1280–8. [DOI] [PubMed] [Google Scholar]
- 167.Dinenno FA, et al. , Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol, 2001. 531(Pt 2): p. 573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kusumbe AP, Ramasamy SK, and Adams RH, Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature, 2014. 507(7492): p. 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liang S, et al. , The coupling of reduced type H vessels with unloading-induced bone loss and the protection role of Panax quinquefolium saponin in the male mice. Bone, 2021. 143: p. 115712. [DOI] [PubMed] [Google Scholar]
- 170.Wang L, et al. , Human type H vessels are a sensitive biomarker of bone mass. Cell Death & Disease, 2017. 8(5): p. e2760–e2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gross TS, et al. , Selected Contribution: Osteocytes upregulate HIF-1α in response to acute disuse and oxygen deprivation. Journal of Applied Physiology, 2001. 90(6): p. 2514–2519. [DOI] [PubMed] [Google Scholar]
- 172.Alique M, et al. , Hypoxia-Inducible Factor-1α: The Master Regulator of Endothelial Cell Senescence in Vascular Aging. Cells, 2020. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kwon RY, et al. , Microfluidic enhancement of intramedullary pressure increases interstitial fluid flow and inhibits bone loss in hindlimb suspended mice. J Bone Miner Res, 2010. 25(8): p. 1798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Matsumoto T and Sato S, Stimulating angiogenesis mitigates the unloading-induced reduction in osteogenesis in early-stage bone repair in rats. Physiological Reports, 2015. 3(3): p. e12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Elefteriou F, Impact of the Autonomic Nervous System on the Skeleton. Physiol Rev, 2018. 98(3): p. 1083–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tomlinson RE, et al. , NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proceedings of the National Academy of Sciences, 2017. 114(18): p. E3632–E3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cardozo CP, et al. , Nandrolone slows hindlimb bone loss in a rat model of bone loss due to denervation. Ann N Y Acad Sci, 2010. 1192: p. 303–6. [DOI] [PubMed] [Google Scholar]
- 178.Chartier SR, et al. , The Changing Sensory and Sympathetic Innervation of the Young, Adult and Aging Mouse Femur. Neuroscience, 2018. 387: p. 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maryanovich M, et al. , Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med, 2018. 24(6): p. 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lary CW, et al. , Association of Beta Blocker Use With Bone Mineral Density in the Framingham Osteoporosis Study: A Cross-Sectional Study. JBMR Plus, 2020. 4(9): p. e10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kondo H, et al. , Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J Biol Chem, 2005. 280(34): p. 30192–200. [DOI] [PubMed] [Google Scholar]
- 182.Kajimura D, et al. , Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med, 2011. 208(4): p. 841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Swift JM, et al. , Beta-1 Adrenergic Agonist Treatment Mitigates Negative Changes in Cancellous Bone Microarchitecture and Inhibits Osteocyte Apoptosis during Disuse. PLOS ONE, 2014. 9(9): p. e106904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pierroz DD, et al. , Deletion of β-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res, 2012. 27(6): p. 1252–62. [DOI] [PubMed] [Google Scholar]
- 185.Yeung DKW, et al. , Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. Journal of Magnetic Resonance Imaging, 2005. 22(2): p. 279–285. [DOI] [PubMed] [Google Scholar]
- 186.Patsch JM, et al. , Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. Journal of Bone and Mineral Research, 2013. 28(8): p. 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Trudel G, et al. , Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol (1985), 2009. 107(2): p. 540–8. [DOI] [PubMed] [Google Scholar]
- 188.Ozcivici E, et al. , Low-Level Vibrations Retain Bone Marrow’s Osteogenic Potential and Augment Recovery of Trabecular Bone during Reambulation. PLOS ONE, 2010. 5(6): p. e11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Turner RT, Invited Review: What do we know about the effects of spaceflight on bone? Journal of Applied Physiology, 2000. 89(2): p. 840–847. [DOI] [PubMed] [Google Scholar]
- 190.Ahdjoudj S, et al. , Transforming Growth Factor β2 Inhibits Adipocyte Differentiation Induced by Skeletal Unloading in Rat Bone Marrow Stroma. Journal of Bone and Mineral Research, 2002. 17(4): p. 668–677. [DOI] [PubMed] [Google Scholar]
- 191.Scheller EL, et al. , Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun, 2015. 6: p. 7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ambrosi TH, et al. , Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell, 2017. 20(6): p. 771–784.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Griffith JF, et al. , Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging, 2012. 36(1): p. 225–30. [DOI] [PubMed] [Google Scholar]
- 194.Shi Y, et al. , Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat Commun, 2017. 8(1): p. 2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mizoguchi T, et al. , Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell, 2014. 29(3): p. 340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bennett CN, et al. , Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A, 2005. 102(9): p. 3324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Song L, et al. , Loss of wnt/β-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res, 2012. 27(11): p. 2344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Choi JY, et al. , Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci U S A, 2001. 98(15): p. 8650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Spatz JM, et al. , Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab, 2012. 97(9): p. E1736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mödder UI, et al. , Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res, 2011. 26(2): p. 373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sharma-Ghimire P, et al. , Sclerostin and Dickkopf-1 Characteristics According to Age and Physical Activity Levels in Premenopausal Women. Journal of Clinical Densitometry, 2021. [DOI] [PubMed] [Google Scholar]
- 202.Spatz JM, et al. , Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. Journal of Bone and Mineral Research, 2013. 28(4): p. 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cosman F, et al. , FRAME Study: The Foundation Effect of Building Bone With 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab. J Bone Miner Res, 2018. 33(7): p. 1219–1226. [DOI] [PubMed] [Google Scholar]
- 204.Fairfield H, et al. , The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol, 2018. 233(2): p. 1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Almeida M, Aging mechanisms in bone. Bonekey Rep, 2012. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.López-Otín C, et al. , The hallmarks of aging. Cell, 2013. 153(6): p. 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liguori I, et al. , Oxidative stress, aging, and diseases. Clin Interv Aging, 2018. 13: p. 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ryter SW, et al. , Mechanisms of cell death in oxidative stress. Antioxid Redox Signal, 2007. 9(1): p. 49–89. [DOI] [PubMed] [Google Scholar]
- 209.Mittal M, et al. , Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal, 2014. 20(7): p. 1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Azzam EI, Jay-Gerin JP, and Pain D, Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett, 2012. 327(1–2): p. 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Portal-Núñez S and Esbrit P, Role of Oxidative Stress in Bone Ageing, in Studies on Arthritis and Joint Disorders, Alcaraz MJ,Gualillo O, and Sánchez-Pernaute O, Editors. 2013, Springer New York: New York, NY. p. 109–123. [Google Scholar]
- 212.Kobayashi K, et al. , Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Scientific Reports, 2015. 5(1): p. 9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rai B, et al. , Effect of simulated microgravity on salivary and serum oxidants, antioxidants, and periodontal status. J Periodontol, 2011. 82(10): p. 1478–82. [DOI] [PubMed] [Google Scholar]
- 214.Smith SM, et al. , The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J Nutr, 2005. 135(3): p. 437–43. [DOI] [PubMed] [Google Scholar]
- 215.Morikawa D, et al. , Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. J Bone Miner Res, 2013. 28(11): p. 2368–80. [DOI] [PubMed] [Google Scholar]
- 216.Xin M, et al. , Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporos Int, 2015. 26(11): p. 2665–76. [DOI] [PubMed] [Google Scholar]
- 217.Morikawa D, et al. , Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. Journal of Bone and Mineral Research, 2013. 28(11): p. 2368–2380. [DOI] [PubMed] [Google Scholar]
- 218.Franceschi C and Campisi J, Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci, 2014. 69 Suppl 1: p. S4–9. [DOI] [PubMed] [Google Scholar]
- 219.Ishii S, et al. , C-reactive protein, bone strength, and nine-year fracture risk: data from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res, 2013. 28(7): p. 1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bruunsgaard H, et al. , Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol, 2000. 121(2): p. 255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Öztürk ZA, et al. , Inverse relationship between neutrophil lymphocyte ratio (NLR) and bone mineral density (BMD) in elderly people. Arch Gerontol Geriatr, 2013. 57(1): p. 81–5. [DOI] [PubMed] [Google Scholar]
- 222.Li J, et al. , Neutrophil-to-Lymphocyte Ratio Positively Correlates to Age in Healthy Population. J Clin Lab Anal, 2015. 29(6): p. 437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Scheidt-Nave C, et al. , Serum Interleukin 6 Is a Major Predictor of Bone Loss in Women Specific to the First Decade Past Menopause*. The Journal of Clinical Endocrinology & Metabolism, 2001. 86(5): p. 2032–2042. [DOI] [PubMed] [Google Scholar]
- 224.Josephson AM, et al. , Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proceedings of the National Academy of Sciences, 2019. 116(14): p. 6995–7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wilson JM, et al. , Comparison of hindlimb unloading and partial weight suspension models for spaceflight-type condition induced effects on white blood cells. Adv Space Res, 2012. 49(2): p. 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Paul AM, et al. , Neutrophil-to-Lymphocyte Ratio: A Biomarker to Monitor the Immune Status of Astronauts. Front Immunol, 2020. 11: p. 564950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jurdana M, et al. , Impact of 14-day bed rest on serum adipokines and low-grade inflammation in younger and older adults. Age (Dordr), 2015. 37(6): p. 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Novack DV, Role of NF-κB in the skeleton. Cell Research, 2011. 21(1): p. 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Luo G, et al. , TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol Med Rep, 2018. 17(5): p. 6605–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kim KW, et al. , Role of C-reactive protein in osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther, 2015. 17(1): p. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nakamura H, et al. , Disruption of NF-κB1 prevents bone loss caused by mechanical unloading. J Bone Miner Res, 2013. 28(6): p. 1457–67. [DOI] [PubMed] [Google Scholar]
- 232.Farr JN, et al. , Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res, 2016. 31(11): p. 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Farr JN, et al. , Targeting cellular senescence prevents age-related bone loss in mice. Nat Med, 2017. 23(9): p. 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Parker E, et al. , Hindlimb Immobilization Increases IL-1β and Cdkn2a Expression in Skeletal Muscle Fibro-Adipogenic Progenitor Cells: A Link Between Senescence and Muscle Disuse Atrophy. Frontiers in Cell and Developmental Biology, 2022. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Horstman AM, et al. , The Role of Androgens and Estrogens on Healthy Aging and Longevity. The Journals of Gerontology: Series A, 2012. 67(11): p. 1140–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]


