Abstract
Purpose of Review
Review international efforts to build a global public health initiative focused on toxoplasmosis with spillover benefits to save lives, sight, cognition and motor function benefiting maternal and child health.
Recent Findings
Multiple countries’ efforts to eliminate toxoplasmosis demonstrate progress and context for this review and new work.
Summary
Problems with potential solutions proposed include accessibility of accurate, inexpensive diagnostic testing, pre-natal screening and facilitating tools, missed and delayed neonatal diagnosis, restricted access, high costs, delays in obtaining medicines emergently, delayed insurance pre-approvals and high medicare copays taking considerable physician time and effort, harmful shortcuts being taken in methods to prepare medicines in settings where access is restricted, reluctance to perform ventriculoperitoneal shunts promptly when needed without recognition of potential benefit, access to resources for care, especially for marginalized populations, and limited use of recent advances in management of neurologic and retinal disease which can lead to good outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40124-022-00268-x.
Keywords: congenital toxoplasmosis, diagnostic testing, prenatal screening, point-of-care testing, antiparasitic treatment, marginalized populations
Introduction
The earlier papers in this series presented studies by students and colleagues focused on educational tools and their efficacy in Panamá’, Colombia and the United States and on epidemiological aspects of Toxoplasma infection and toxoplasmosis in the USA, Europe, Morocco, Brazil, Panamá’ and Colombia. It is clear from earlier studies in the United States [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•], and the present studies that access to inexpensive, effective diagnostic tools and anti-parasitic treatment( and ultimately vaccines) [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 51•, 52, 53••, 54, 55••, 56••, 57•, 58••, 59•, 60••, 61, 62••, 63, 64.••••, 65, 66••, 67••, 68••, 69, 70, 71.••••, 72••, 73••, 74••, 75••, 76–78, 79••, 80••, 81••, 82, 83, 84••, 85••, 86••, 87••, 88••, 89••, 90, 91, 92••, 93, 94, 95, 96, 97, 98••, 99••, 100, 101••, 102••, 103••, 104••, 105••, 106••, 107••, 108••, 109••, 110, 111, 112, 113••, 114••, 115••, 116••, 117••, 118••, 119••, 120, 121, 122••, 123••, 124, 125••, 126••, 127••, 128••, 129••, 130••, 131••, 132, 133••, 134••, 135, 136••] are/will be critical to prevent this infection and its adverse sequelae. This is especially true for marginalized populations. This fourth paper reviews, considers and discusses results in the first three manuscripts and reviews and presents updates with additional data about solving ongoing problems. These are reviewed and summarized in order to emphasize the importance of, and steps to build, programs to address the clinical and public health challenges of preventing congenital toxoplasmosis. This analysis and discussion places work in the United States [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50, 52, 55••, 56••], Panama [59•] and Colombia [63, 65, 97], in the context of progress in other countries [51•, 52, 53••, 54, 55••, 56••, 57•, 58••, 59•, 60••, 61, 62••, 63, 64.••••, 65, 66••, 67••, 68••, 74••, 75••, 77, 92••, 100]. For the United States, in Part 1 there was a focus primarily on the work in the U.S. National Collaborative Chicago-based Congenital Toxoplasmosis Study ( NCCCTS, 1981 to present) as a whole with brief mention of and/or reference to some of the other US and European, as well as Brazilian studies and work [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 52, 55••, 57•, 58••, 68••, 76, 88••, 89••, 90, 91, 92••, 93, 94, 95, 116••, 117••].
Part IV considers briefly the foundation to which these studies contribute, providing a rationale and framework for extending the studies. This includes additional basic science and translational work and needed improvements in clinical care [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 51•, 52, 53••, 54, 55••, 56••, 57•, 58••, 64.••••, 68••, 88••, 89••, 90, 91, 92••, 93, 94, 118••, 124, 125••, 126••, 127••] and potential novel treatments and vaccines [84••, 85••, 86••, 87••, 88••, 98••, 99••, 100, 101••, 102••, 103••, 104••, 105••, 106••, 107••, 108••, 109••, 110, 111, 112, 113••, 114••, 115••, 116••, 117••, 118••, 119••, 120, 121, 122••, 123••, 124, 128••, 129••, 131••, 132, 133••, 134••, 135, 136••, 137••, 138••, 139••] including vaccines to prevent oocyst shedding by cats [95, 96]. Some of this work is ongoing in the Toxoplasmosis Center in Chicago, in other programs in the US and in other countries.
Approach
Overview and Summary
Initially, we consider the clinical manifestations and the benefits from optimizing clinical care pre- and postnatally. We compare approaches in several countries, individually considering strengths, and where problems have arisen/arise and present possible solutions. This analysis addresses available approaches, a « toolbox » of recent advances that can contribute to eliminating toxoplasmosis through optimizing care and preventable and therapeutic approaches. This includes a novel “toolbox” of diagnostic tools and potential preventative and therapeutic approaches. These developments and approaches are summarized in Tables 1, 2, 3, 4 and 5. These solutions have involved/ will involve a variety of skill sets and persons, « taking :a village » of many physicians, scientists, students and others in an ongoing global initiative.
Table 1.
Development of understanding of need for a WHO ASSURED * Criteria point-of-care testing functional access to correct medicines to initiate medicines immediately and promptly for this medical emergency in this disease in the US
| Date | Source of information | Information |
|---|---|---|
| 1950–1981 | France, Austria | Treatment appears beneficial, the earlier the better; laws passed in France and Austria mandating prenatal screening. |
| 1981–1990 | France, Austria, United States | Possible to treat continuously with pyrimethamine and sulfadiazine, Phase 1 clinical trial. |
| 1990–2008 | United States | Phase 2 and later phase trials. |
| 2010 | Illinois, United States | Attempt to introduce a legislation in Springfield, need for infrastructure, inexpensive test materials, and medicines. Identified cost-benefit macro-algorithm, spillover benefit identified, inexpensive testing tools identified. |
| 2014 | Worldwide | Global initiative begun. |
| 2015 | United States | Price of pyrimethamine increased from $0.01 ner pill to $750 per pill. ** |
| 2021 | France, United States, Morocco, Colombia, Panama | Improved inexpensive tests, meeting WHO/assured criteria, CE-marked criteria, FDA clearance pending. |
*Affordability, Sensitivity, User-friendly, Rapid and robust, Equipment-free and Deliverable to those who need it
**Cost of medicines and import restrictions have caused limitations in use in the US and Panamá. The problems with cost and access discussed in Part I of the manuscripts in this series may be resolving if the source material is of high quality and reliability. This is a new development in early 2022 and details are not yet available
Table 2.
Table contents for materials prepared for FDA review of 510k and CLIA waiver
Table 3.
Development of point-of-care (POC) and a revolutionary nearly 100% sensitive and specific test for acquisition of Toxoplasma gondii
| Concept | References |
|---|---|
| Development of test | [80••] |
| Application of test to serum | [43••] |
| Applicability to infections in the United States | [43••] |
| Meeting WHO ASSURED criteria with POC test | [44••] |
| Implementation and building a global initiative | [33••, 39••, 48••, 52, 55••, 58••, 60••, 61, 66••, 67••, 81••] |
| Scaling in Morocco, Panamá, Colombia, Brazil, Argentina, Europe, and the U.S. | [57•], In Progress |
| Multiplexing: Nano-Gold test | [82] |
Table 4.
Summary of evaluation performed using LDBIO Toxoplasma ICT IgG-IgM test
| Study | Published? | Sample | Npos | Nneg | Se | Sp | Comparative method |
|---|---|---|---|---|---|---|---|
| Black variant validation | No | Serum | 483 | 291 | 98.5% | 99.7% | CRS |
| El Mansouri, 2021 | Yes | Whole blood | 226 | 406 | 97% | 100% | CRS |
| Bichat/Chicagoa | Ongoing | Serum | 169 | 1215 | 98.2%a | 99.8%a | CRS |
| Chapey, 2016b | Yes | Serum | 109 | 291 | 97% | 96% | Architect |
| Mahinc, 2017b | Yes | Serum | 339 | 663 | 100% | 98.7% | CRS |
| Begeman, 2017b | Yes | Serum | 129 | 51 | 100% | 100% | CRS |
| Lykins, 2018 | Yes | Whole Blood | 101 | 143 | 100% | 100% | CRS |
| University of Chicago | No | Whole Blood | 5 | 35 | 100% | 100% | Bioplex ToRC |
| Gomez, 2018c | Yes | Serum | 170 | 140 | 100%c | 98.8%c | CRSc |
aAs the study is still under redaction, published performances may differ
bFormer pink variant CRS: composite reference standard, using more than a single test to determine status. See methodology of each study for details concerning the CRS used
cincluding CDC100 panel (100% Se/Sp)
Abbreviations: N number, pos presence of T.gondii specific antibody, neg absence of antibody to T.gondii, Se sensitivity, Sp specificity
Table 5.
Development of novel, improved medicines and vaccines to prevent, cure and eliminate Toxoplasma infection
| Approach to elimination with medicines, vaccines, environment approaches | References (Selected examples) | |
|---|---|---|
| Medicines | Novel use of established medicines | [19•] |
| New approaches to eliminating toxoplasma by small molecules and antisense | [85••, 86••, 87] | |
| Vaccines | Vaccines to prevent disease in humans | [88] |
| Novel approaches to using vaccines to prevent infection in humans and other animals | [100, 106, 132] | |
| Vaccines for cats to prevent oocyst shedding | [96, 97] | |
| Environmental Approaches | Novel approaches to prevention of contamination of the environment by oocysts | [49] |
| Water purification | [63] |
Clinical Manifestations of Ocular and Congenitally Infected Children and Treatment with Anti-parasitic Medicines
Panama
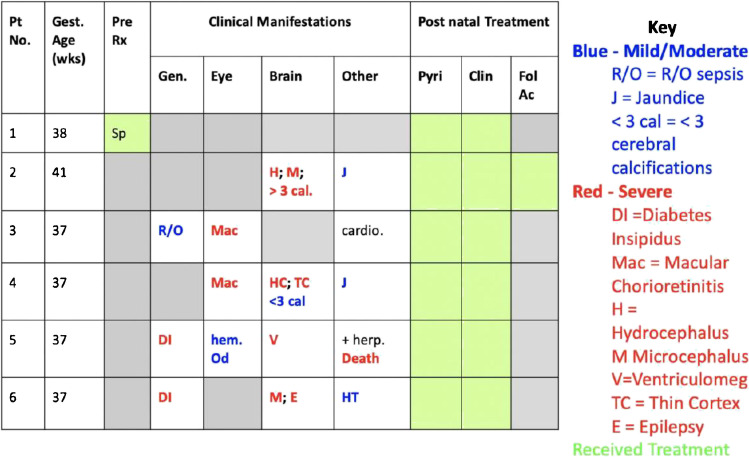
Details of the education and epidemiology programs were provided in Parts I to III of this series. They are a critical part of building this overall program. In this context, a physician (XN) and a visiting student (MR), initiated a retrospective chart review of patients who were identified through recognition of clinical symptoms and signs or were identified in a serologic screening program. The infants had a diagnosis of congenital toxoplasmosis made between 2016-2017 and were known to the Pediatric Infectious Diseases experts at Hospital del Niño, Panama City, Panamá. This work was performed with permission from the hospital’s bioethics committee. For each of six congenital toxoplasmosis cases identified, data regarding prenatal and postnatal treatment, gestational age at birth, clinical manifestations and laboratory tests were collected (Fig. 1; also See Ramirez et al. in the Supplement referring to serologic screening in Panamá, a glass half empty and a glass half full). These cases also were included in and formed the basis for a later, more extensive review [59•]. Furthermore, a perinatal healthcare center was established at Hospital del Niño and a program with skill in retinal disease and uveitis was developed. A gestational screening program to identify seroconverting pregnant women, facilitate initiation of treatment and prevent transmission to the fetus was established with an IRB protocol awaiting approval to provide the foundation for a study, which can be initiated when the complications associated with the SARS-CoVi-2 pandemic permit. This disease was recognized as a significant public health problem in Panamá’ and has become a focus for health care, public health and scientific initiatives in the country, extending the present studies described herein.
Fig. 1.
Results of characterization of the six congenitally infected children in Ciudad de Panama who presented for care at the new perinatal infections program in 2016-2017. The only asymptomatic child of the six (patient 1) corresponded to the only mother who was screened during gestation and given prenatal treatment with spiramycin. The other, unscreened mothers gave birth to children who had clinical manifestations such as hydrocephalus and diabetes insipidus
Colombia
Overview
In the spatial epidemiology analyses as described in Part I, additional ongoing studies were included [62••, 63, 64.••••, 65]. Guidelines for treatment nationwide were adopted and markedly reduced severe disease and prevalence of the infection were noted in a study extending from 2001 to 2019 [66••]. A new program to evaluate point of care testing in Armenia, Colombia was implemented [Gomez -Marin, In Progress 2022].
Pregnant Women and Congenital Toxoplasmosis
These studies include evaluations and treatment of seroconversion of seronegative pregnant women and their infants. A study of a point-of-care (POC) test developed in France, the United States and Morocco [43••, 44••, 57•, 58••] was also initiated. This is now being used in Europe with CE mark (European FDA equivalent) approval.
Ocular Toxoplasmosis in Adults
In addition, patients with known ocular toxoplasmosis have been identified and active infection treated. A free evaluation was offered in several areas in Quindío, Colombia, and patients who were seropositive with typical ocular lesions of toxoplasmosis were identified and referred for further care to a Center in Quindio, Armenia. This work was described in a recent separate paper [63].
United States, Europe and Morocco
Introduction of ICT-LDBIO Point of Care Test in Europe, the United States, Morocco, Colombia and Panamá’
In France, the Point of Care test was used primarily for sera but is now CE Marked. In the United States an ongoing longitudinal study called the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) was initiated in 1981 and continues into the present time was reviewed in Part I (1-150). This study has been central to characterizing and developing treatment for this disease. In Part IV we address newer work with Point of Care testing to make the diagnosis earlier so treatment of the pregnant woman can prevent infection or treat the fetus.
The LDBIO POC test was developed and first tested in France, then the United States, Morocco and then Panamá. Testing in Colombia has been initiated in the past month. This process and findings are described herein.
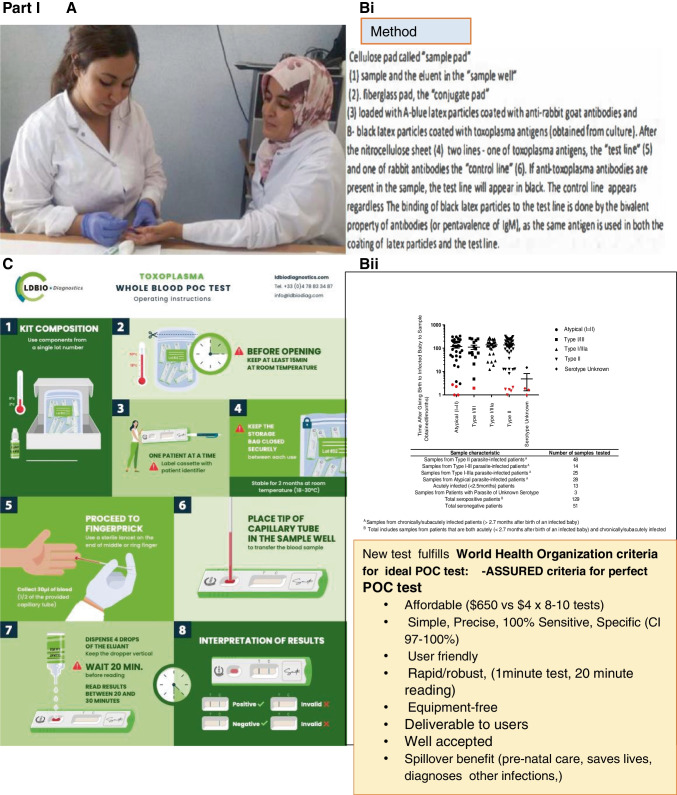
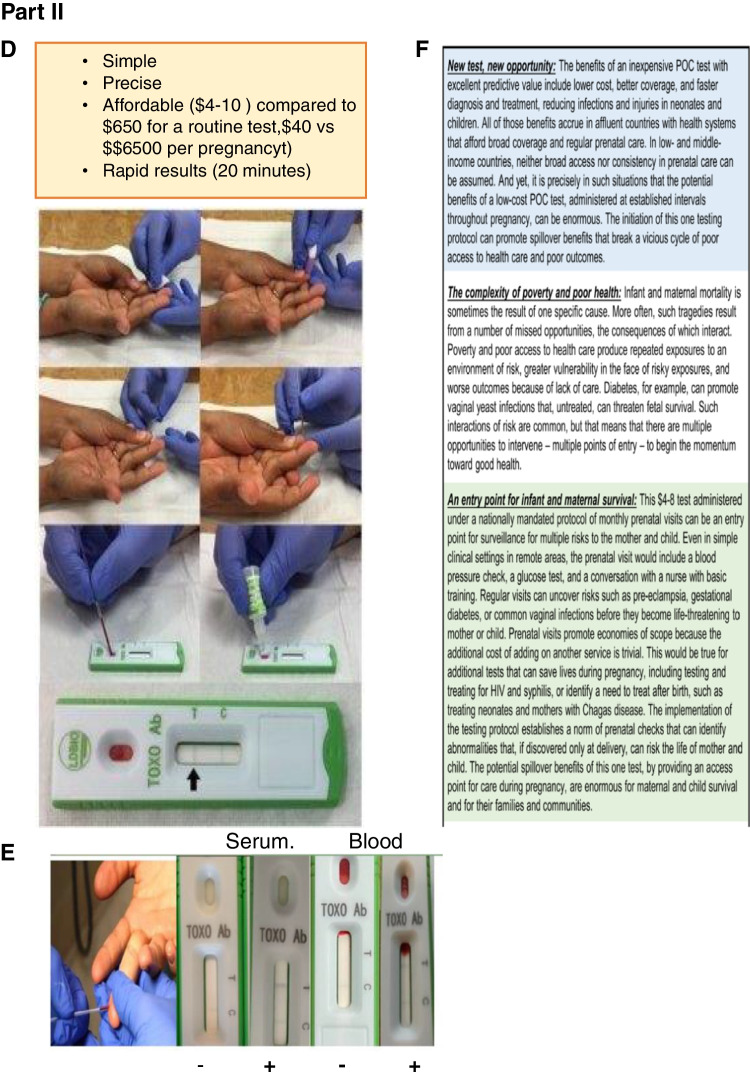
The test utilizes 15 microliters of sera or 30 microliters of whole blood placed in a well overlying filter paper. Four drops of elution buffer are added. Along the filter paper, there are black beads coated with Toxoplasma antigen that makes a double sandwich to capture human anti-Toxoplasma serum antibody and creates a black line when antibodies are captured. There is also a blue bead that is coated with goat anti-rabbit IgG that reacts with a line of rabbit IgG and serves as a migration control. This test detects both IgG and IgM antibodies specific for Toxoplasma.
The LDBIO lateral immunochromatography test was performed using sera as described in Begeman et al, and with whole blood from a finger prick by Lykins et al [43••, 44••]. In the first part of this analysis in Panamá’, one hundred sera were randomly selected from a larger number that were tested with the Roche IgG and IgM tests in Panamá’, as described in Part III of this series of manuscripts. These were randomly selected serum samples from the group of women who had serologic screening as described in Part III of this series of manuscripts at Hospital Santo Tomás, Panamá’ City, Panamá’. The investigators in this study randomly selected sera collected during this study that were sent to the United States’ Chicago center where the LDBIO test was performed by a visiting scholar (AG). These samples had been sent to the Toxoplasmosis Center at the University of Chicago and the Toxoplasmosis Research Institute where they were tested with the LDBIO Toxoplasma ICT IgG-IgM. A subset of the sample that were discrepant in the Roche and Chicago testing were also tested in Lyon France with the LDBIO Toxo II WB. LDBIO Toxo II WB, considered as a gold standard in Europe, was performed as per manufacturer’s instruction. The lateral immunochromatography test LDBio Toxoplasma IgG-IgM demonstrates nearly 100% specificity and sensitivity when tested compared with the Sabin - Feldman dye test or other comparable tests.
To introduce the use of this LDBIO chromatographic test that meets WHO-ASSURED (affordable, sensitive, specific, user-friendly. rapid and robust, equipment-free, deliverable), to Panamá’, the test was first introduced in an established study on wellness and aging in Panamá’ City, a high-priority program in which sera were obtained. The Panama Ethics Committee reviewed this study and gave permission to test the bio bank of sera using the LDBIO test. This second set of tests with sera in Panama was performed at the translational center at the Panama ‘ National Institutes of Health (INDICASAT). Sera were obtained from participants in the Panama Aging Research Institute (PARI) 1 and PARI 2 wellness and aging studies and had been stored at -80 degrees Fahrenheit. Participants were 65 years of age or older. A separate report will describe additional cognitive testing which was performed in that study (Britton, McLeod et al, In preparation in 2022.) Herein we only address prevalence of antibody using this now CE Marked test.
In the United States, France and Morocco, more than three thousand sera/persons have been tested using the LDBIO Toxoplasma ICT IgG-IgM test kit, with over one thousand of these also performed with whole blood from fingerstick testing, as described by Lykins et al [44••]. The results from Morocco have been published separately in an epidemiologic study that compared results with the LDBIO-POC test and the Pasteur Institute BIORAD ELISA [57•].
Anti-parasitic Treatment
As mentioned in Part I, pyrimethamine, sulfadiazine, leucovorin and spiramycin are essential for the treatment and prevention of congenital toxoplasmosis [17••, 33••, 46•, 48••, 53••, 60••, 65]. Through ongoing programs for care, the personal experience of the authors informed observations of availability of medicines in Panamá’ and the United States. These observations were made in the context of building programs in each of these countries.
Marginalized Populations
A challenge for delivery of care in all three countries—Panamá’, Colombia and the United States—involved considering how to reach out to, test and treat patients in marginalized populations. Recently, it is also clear that the same difficulties also exist in Canada, for example for the Inuit people in the Arctic. This has been especially problematic and evident during the COVID-19 pandemic in the United States and its territories. Poverty has made access to healthcare including medicines more difficult and has stressed the healthcare system, augmenting problems associated with access, availability of medicines and care that were substantial before.
In a complementary analysis, four students (two medical students and two social policy students) performed an analysis of how to develop suitable strategies for care of the Embera indigenous population in Panamá’. The Embera live in close proximity to soil, wild cats, potentially contaminated water, with an average lifespan of approximately thirty years, making substantial risks for acquisition of Toxoplasma. Some possible approaches considered included optimizing the roles of the public health system, MINSA, regular delivery of healthcare to remote areas by medical teams, developing systems to provide medicines and help patients reach areas with more sophisticated care, and systems to monitor treatment in such populations (Part IV. Supplemental Part A). This is also a consideration in indigenous populations in the United States and Canada, the problem including frequent infections for those in remote areas of the Arctic region.
Special Considerations
Clinical Manifestations of Congenitally Infected Children and Treatment with Medicines
Panama
For Panamá’, summaries from an initial study of the six children diagnosed with congenital toxoplasmosis at Hospital del Niño in 2016-2017, showed that five were severely affected (see Fig. 1). The only asymptomatic child corresponded to the only mother who was screened during gestation and given prenatal treatment with spiramycin. The other five mothers, who were not screened, did not receive any prenatal treatment, resulting in both severe clinical manifestations—such as hydrocephalus, microcephaly, diabetes insipidus, chorioretinitis involving the macula—and mild to moderate clinical manifestations, such as jaundice and cerebral calcifications. This is not an association between prenatal treatment and subclinical disease. Although prenatal treatment prevents sequelae, in this case, the way these patients were identified represents two completely different populations. Those symptomatic children were only identified because they had symptoms, while the asymptomatic infant was identified by prenatal screening. These data do not elucidate how many asymptomatic patients were born in the same period of time but had not been treated during prenatal care. Prenatal care would have benefited those babies with neurological disabilities. Also, many babies may have been born asymptomatic, to mothers who were contaminated by Toxoplasma in the last months of gestation, but were deprived of prenatal and postnatal treatment, which could have avoided sequelae, mainly ocular, that will only be noticed later in their life.
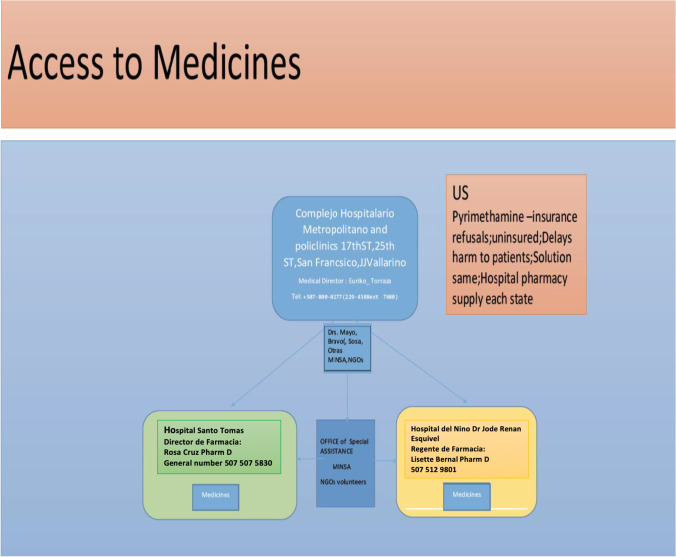
A medicine distribution strategy was created but is not yet implemented fully. This was because one area of difficulty noted when these early patients were identified in Panamá’ was obtaining medicines due to limitations with importation (Fig. 2).
Fig. 2.
Model of a distribution scheme to multiple hospitals with a central distribution site for medicines that can be used to treat toxoplasmosis. As distribution of medicines can be a significant issue for any country, this figure also addresses challenges with supplying medicines such as pyrimethamine in the United States
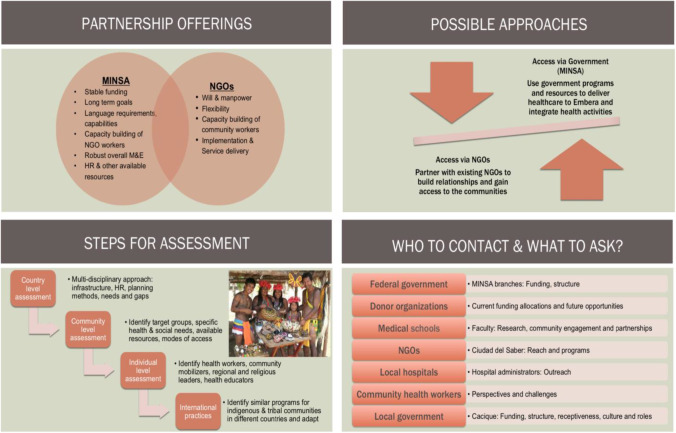
Considerations of ways in which government programs and NGOs could work to help indigenous and marginalized patients were considered by a group of medical students and public policy graduate students. The strengths of both the government agencies and NGOs were noted to be complementary (Fig. 3, Part IV Supplemental Part A).
Fig. 3.
Vishan Dhamsania, Nick Graves, Marci Kirchberg and Kopal Mathur considered international development strategies for bringing toxoplasmosis screening and treatment to the Embera, an indigenous group in Panamá. The student group evaluated what strengths and weaknesses of the Panamanian Ministry of Health (MINSA) and non-governmental organizations (NGOs) would be to implement screening programs for toxoplasmosis for the Embera. They considered the following criteria for successful implementation for a program: politics, economics, culture and sustainability. They found complementary strengths from MINSA and NGOs
Colombia
In an earlier manuscript [48••] the dramatic effect on reducing illness and disease in Colombia by introducing screening has demonstrated efficacy and utility of public health programs [48••]. Nonetheless, women in the health care system delivering infants at one hospital had little knowledge but easily learned relevant materials with educational materials as presented in Part II of this series of manuscripts.
Concerning bottled water and uncooked meat exposure, a reciprocal association was noted. In Guaduales de la Villa, the habits of drinking bottled water and eating undercooked meat were negatively associated with infection. At first this seemed counter intuitive and contrary to earlier studies. To better understand these results, a closer look at methodology, in the context of the results, present a possible set of explanations demonstrating possible relative risks and efficacy of public health measures: Eating undercooked meat was coded as 1 for 'yes' and O for 'no' . Presence of Toxoplasma specific IgG was coded as 1 and its absence was coded O.
This analysis used a logistic regression in R with seropositivity as the outcome and all variables used as covariates. In this model, undercooked meat had a p value of 0.034. In an analysis where undercooked meat is the only predictor and other variables are not accounted for, however, it was not significant with a p value of 0.06. The total model better adjusted for the effects of the other variables and the analyses in context of each other suggest that oocyst contamination of water is the most important source of infection, with both variables bottled water and less well cooked meat consumption perhaps influenced by socioeconomic status [57•]. Another separate statistical analysis of the same data set confirmed the latter finding. This analysis consisted of a simple analysis of the differences in proportions by using the chi-squared test or Fisher's exact test, as appropriate. Odds ratios and 95% confidence intervals were calculated. SPSS version 25 (SPSS Inc. IBM, Chicago, USA) statistical program was used to perform the analysis. P values <0.05 were considered statistically significant. As a single variable, the p value for undercooked meat was 0.057, similar to the analysis herein using R.
A previous study in Armenia concerning transmission by meat, suggests that it could be possible that people who prefer rare meat buy at stores where the meat is frozen [97]: A scoring system that ranked hygienic conditions and freezing of meat was utilized to compare meat stores. A score of 1 was least and a score of 3 was most. In stores that were most hygienic and froze meat, PCR for T. gondii DNA was least in meat samples. For chicken in the most hygienic stores, PCR detecting T. gondii DNA was significantly less than in the less hygienic stores. Another possible explanation could relate to socioeconomic status that allowed affording the more hygienic, less contaminated source of meat and also be a surrogate marker for another variable like adequate funds for bottled water.
United States
NCCCTS and Treatment
Results from work in the United States were reviewed in Part 1 of this series of papers [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 52, 55••, 56••] but not with an overview in some time. Thus this data set is included herein in a not previously published composite form in a table with comments that summarize some of the major findings in the context of others work (Part 1: Table 1; Fig. 4, 1-51). The cost benefit analyses emphasize that treatment brings not only reduction in individual suffering but also brings substantial cost savings for countries (e.g., 14-fold in Austria). The major role France has had in developing this improved approach that saves life, sight and cognition is represented also in Part 1: Fig. 4. As this has been addressed in earlier publications also included in the references, it is not considered in more depth here.
Fig. 4.
Testing of the Lateral Immunochromatography G and M test with whole blood from finger stick. Part I: A. Method used in study. B. Description of early results (from PLoS Neglected Tropical Diseases with permission). C. Brochure that will be the instructional material for when this is used in clinical practice, as in package insert shown with instructions for use of kit. Images show method used step by step. Part II: D. Representative results for serum or whole blood in this test. E. Example of obtaining blood by fingerstick, example of positive bands(T) and negative result (no T but C-control-band, C, sera , left, whole blood -right. F. Our expectation is that this global initiative to address the significant health problems toxoplasmosis presents, as a focus of the programs being built, will have spillover benefit to promote well-being, improve water supply reducing other water borne diseases, in addition to preventing devastating consequences of this infection. The opportunity for spillover benefit for diagnosing and treating other diseases, and the opportunity for understanding basic, translational aspects of the biology, developing vaccines and better medicines are another substantial benefit of this global initiative. Bii, D, F. With permission PloS Neglected Tropical Diseases, E. UChicago, article by John Easton
Updates
Screening Programs in the United States
Most diagnosis in the United States is made when there is symptomatic illness and obvious clinical manifestations and even then diagnosis is often missed or delayed. Screening during gestation occurs in small numbers of “high end” obstetrical practices but occurs only rarely on a larger scale or systematically in the United States. In contrast, this is usual now in France, Austria, Brazil, Colombia, Morocco among other countries.
Massachusetts and New Hampshire perform newborn screening [76]. Screening by obstetricians is infrequent and not systematic, with some higher end practices screening.
The early studies of the NCCCTS in the U.S. also confirm the findings in France [33••], that , although postnatal treatment confers considerable benefit, prompt prenatal treatment of seroconverting women was associated with the best outcomes and obviate the effect of parasite strain [33••].
Cost benefit analyses creating mathematical models that now have been applied in other countries were performed [56••]. Work with Point of Care (POC) testing grew from a parent motivated initiative. This occurred as follows: parents who understood the success of the screening initiatives in France and Austria aimed to prevent congenital toxoplasmosis through instituting a similar program in the US [51•, 52, 53••, 60••]. They sought to incentivize the legislature in Illinois to pass a law similar to the laws that were passed in France and Austria mandating serologic screening programs for acquisition of Toxoplasma by pregnant women. The coordinator at the Toxoplasmosis Center, M Sautter, worked with a legislator in Springfield, Illinois, to write a law that said obstetricians should inform their patients about Toxoplasma and offer testing [61]. A group of patients, their families and physicians involved with the NCCCTS traveled to Springfield, Illinois and met with the special Committee on Healthcare (Part IV Supplemental Part B). The legislators wanted to pass the law, while the Department of Public Health emphasized the importance of having an infrastructure in place as a public health program, while both the Obstetrical Society and the Department of Health sought a cost-benefit analysis to establish feasibility and assess possible costs versus benefits from such a program in the United States.
Some members of an organization concerned about terminations of pregnancies initially wanted to block this legislation but quickly realized that this was a program to diagnose and treat to prevent the infection and its serious sequelae rather than causing any loss of life. They became strong supporters of the legislation during and after the hearing. The Obstetrical Society felt that education programs rather than legislating what doctors should do was a better approach.
Prompted by this experience, cost-benefit analysis algorithms were developed [56••]. They have been applied in the United States, Austria and France as discussed in more detail below in the following section [51•, 52, 53••, 56••, 60••]. Educational materials were developed as presented herein in Part II of this series, as well as earlier in text books, scientific and clinical teaching presentations, and in many different publications, blogs, websites and public service announcements throughout the past 40 years. These are being made freely available on a not-for-profit website (Toxoplasmosis.org), in a University of California at RadioBio.net and now NSF sponsored podcast :(https://ucmerced.box.com/s/gtnu1gujkipfyc9fewmqi7rqy1nekjr2); (NSFScience360). This work has recently taken into consideration a new Randomized Control Trial in France by Mandelbrot et al showing benefit of pyrimethamine, sulfadiazine, leucovorin treatment after 14 weeks of gestation beginning dating gestation with onset of amenorrhea [60••]. The possible benefits of screening during pregnancy in the United States and practical aspects of its implementation are being adapted and considered in the United States at the Toxoplasmosis Center in Chicago and with support by the Thrasher Children’s Charity and the Kiphart Global Health Foundation [61]. Similar studies are taking place in Morocco and Colombia and planned for Panamá’ [57•, 59•] with interest in other countries. This POC test is now approved in Europe with CEMark (FDA equivalent for Europe) based on analyses in the U.S. France and Morocco (Tables 1, 2, 3, and 4). This work has been/will be submitted for consideration by the FDA for 510K and dual CLIA waiver in the United States (Summary in Table 4). This review will take place as soon as the extensive approval process for emergency use applications for SARS-CoV-2 no longer causes deferral of other types of FDA reviews in the United States.
Introduction of LDBIO IgG/IgM Lateral Chromatography Point of Care Test
Details of the use of this test have been described for France, the United States and Morocco. Using serum and whole blood, we have found high functioning of this lateral chromatography test with essentially 100% sensitivity and specificity, meeting the WHO-ASSURED criteria, with over 3,000 people now tested. [43••, 44••] (Tables 1, 2, 3, and 4).
Recent presentations were at International meetings, in teaching sessions, on a University of California (Merced) podcast, on a National Science Foundation (NSF) podcast, and as a plenary session keynote student presentation.
Links to International ToxoXV meeting in January 2021 are at toxoplasmosis.org (Dr. McLeod's Presentation: https://vimeo.com/showcase/8031816/video/502588615); (Dr. EL Bissati's Presentation: https://vimeo.com/showcase/8031816/video/502601087) and to the podcast discussed above at .https://ucmerced.box.com/s/gtnu1gujkipfyc9fewmqi7rqy1nekjr2 which include descriptions of this work.
Instructions are located in the kit, in publications, and are available at toxoplasmosis.org along with other educational materials herein (Fig. 4 and pamphlets).
Testing of more than 3000 serum samples in the United States, France and Morocco provided the results shown in Fig. 4 and Table 4. The LDBIO device demonstrates nearly 100% specificity and sensitivity when tested against gold standard Sabin-Feldman dye test or other comparable tests used with sera in these three geographic settings. This lateral chromatography test has become CE marked in Europe. Similarly, over 1000 sera were tested with whole blood obtained with a fingerstick in the United States and Morocco. Specificity and sensitivity demonstrated exceptionally high performance, as described above, for this test. In the United States, use of the POC test monthly beginning before conception, or early in the first trimester and continuing to the 6th week postpartum visit was extremely well received by patients, obstetricians and nurses, as demonstrated by questionnaires (Lykins, Leahy, Zhou, Siddiqui, Leong, Goodall, Romero, Ismael, Christmas, Peyron, Wallon, McLeod, in preparation, 2022). These data and others supported the CE Mark approval in Europe (parallel to the FDA in Europe), and data are waiting for review for 510(k) clearance and dual CLIA waiver with the FDA and CLIA in the United States. The FDA has put all reviews of diagnostic applications on hold to prioritize emergency use authorization application for COVID-19 diagnostics, vaccines and treatments. This material will be reviewed when clearance procedures can resume without the need to prioritize testing for reagents and methodology that will improve outcomes for SARS-CoV-2. This is anticipated to occur in 2022. The next step is to make certain that this high performing test can be utilized as reliably by less experienced practitioners. This is being prioritized currently in different demographics and at scale.
This test does solve the cost issues (between $5-10 per test meeting all WHO ASSURED criteria), specifically, and solving problems with false positive IgM results from commercial tests) (Grose, Wallon, Chapey, Leahy, Zhou, Piarroux, Limonne, Houze, Abraham, McLeod, In submission, 2022).
In working with the LDBIO Toxoplasma ICT IgG-IgM (LDBIO POC) in Lyon it was found that for 11 sera from patients with malaria there were no false positives. From 51 patients with leishmaniasis there were 6 false positives. These analyses were performed separately from those mentioned above in the United States, France and Morocco and were specifically sera from patients who were suffering from leishmaniasis in countries that are endemic for leishmania infections. As indicated in the Instructions for Use of the kit this could be a confounding factor for serodiagnosis for T.gondii with the LDBio for patients with leishmaniasis.
In Panamá, 100 sera from pregnant women were tested with the Roche test; 50% were positive. Testing of a small subset of these samples with the LDBIO test when the sera was shipped from Panama revealed three false positives of these samples when compared with Western blot, different than the prospective testing performed in the United States, France and Morocco where there was nearly 100% sensitivity and specificity. This latter result possibly was due to handling of the sera before it arrived in the United States or with a separate population which might result in separate concomitant diseases in Panama relative to the United States.
In an aging population of individuals over 65 years of age in Panama City, seroprevalence was found using the LDBIO test to be 85% with this CE Marked now approved test. Studies are underway to determine if T. gondii, this billion-brain parasite, contributes to neurodegeneration or other chronic diseases that are increased in aging populations (Britton, Villareyes, Perez, Naranjo-Galvis, Wroblewski, Karrison, Dogra, Ramirez, Dovgin, Dovgin, Fraczek, Lorenzi, Bennett, Wang, Kim, Funk, Zhou, Dodya, Ross, Piarroux, Limonne, McLeod, et al 2022, manuscript in preparation).
In Panamá, ethics committee materials are prepared and a prospective study of 200 pregnant women with monthly screening using the LDBio test is planned (Charter, Reyes, Ashti, Grose, McLeod, et al 2022, in progress), awaiting a diminution of cases of SARS-CoV-2 in Panamá’. This study was deferred because of high numbers of SARS-CoV-2 cases in Panamá’.
A similar study is being initiated in Colombia currently. Pregnant women are being tested monthly in a screening program to identify those who seroconvert and thus are at risk of transmitting the infection to their fetus. Identification of seroconverting women will allow prompt initiation of treatment to prevent the infection. This approach has already been found to reduce morbidity and mortality from this infection using standard commercial tests. The LDBIO POC, if sensitive and specific in this setting, can substantially reduce the cost of this type of program.
There are additional similar data from Morocco. In Morocco, a similar study was performed, and the findings are presented in Tables 1 and 4 [57•]. Specifically, in diverse settings, a total of 632 women were studied. Initially, for 283 women, sera were tested by Platellia ELISA IgG and IgM along with LDBIO POC fingerstick whole blood test. Then, for 349 women, a study was performed that compared POC testing with whole blood obtained by fingerstick and serum from contemporaneous venipuncture. Comparison of the results of the same sera tested with western blot also was performed. POC test sensitivity was 96.4% [IC95 90.6-98.9%] and specificity was 99.6% [IC95 97.3-99.9%]. Prevalence of Toxoplasma infection among women living in rural and mountainous areas, and in urban areas with lower educational levels, was high. Exposure to soil, agricultural work, well water and not washing fruits and vegetables prior to consuming them were risk factors for the 632 women within all the settings [57•]. Thus, in Morocco, sensitivity and specificity of the lateral chromatography test were again exceptional [57•].
In Armenia, Colombia, a prospective study to screen 200 pregnant women has been initiated with support and reagents approved for entry into the country (Gomez-Marin et al, unpublished 2022).
Anti-parasitic Treatments using Medicines Currently Available in Clinical Practice
In all three countries currently, accessing medicines presents challenges. The recent history concerning the availability of medicines has influenced care for toxoplasmosis in the United States, as discussed in Part I. This most recently has involved pyrimethamine and sulfadiazine.
In Panamá’, access to medicine is a challenge due to unavailability of medications. Pyrimethamine remains one of many essential medicines where importation is complicated. A hypothetical system was designed and created (Fig. 2) which centralizes and makes low-cost medicines available 24/7. It is hoped that along with creation of Centers with expertise in care, access to medicines will improve. There are some areas in Panamá, in Chiriquí for example (see Pirla et al in Supplemental in Building: Spatial Epidemiology Part III) or regionally within Panama City like San Miguelito where there is a particularly compelling need for availability of care due to the higher prevalence of contact with soil, poverty and dirt floors in dwellings.
In Colombia, similar difficulties with access are evident for certain marginalized populations. This is also confounded by oocyst contamination of water sources. The availability of bottled water is likely correlated with socioeconomic class.
Marginalized Populations
In Panamá,’ medical care for indigenous populations presents a particular challenge. Two medical students and two public policy students from the University of Chicago prepared a capstone course project (supplemental figure). One of the medical students worked for a year with the Ministry of Health (MINSA) in Panama on healthcare initiatives for the Embera people. Another of the students had worked to provide medical care in the region of Nigeria where Boca Haram was active while in the Military. Other students had worked with the Peace Corps, so each brought unique perspectives. Discussions included the Director of the Initiatives for Toxoplasmosis in Panama with legal background. Other discussants were the Director and Nurse in the Whiteriver Indian Health Service Programs in Arizona. The students performed an analysis of the strengths and weaknesses of public health policies and care algorithms when public agencies and not-for-profit agencies participated in addressing care for marginalized populations (Fig. 3; Supplemental A for Part IV). They concluded that each approach brought strengths, some overlapping. Some strengths from the governmental programs were: ability to uniformly assist with care in a broad, well-organized manner with less limitation of resources. Strengths for the NGOs included ability to assist families directly, flexibly and efficiently. These two differing approaches complement each other. The combination of both methods of supporting care could help to optimally deliver such care to marginalized populations who live in remote areas with more limited access to care such as to the Embera peoples in Panamá’. This analysis provided a roadmap for organizing such care (Fig. 2). A challenge arises from introducing a screening program without clear, immediate benefit for a population that has an average lifespan of 30 years. This is especially the case if they are fatalistic about illness in infants and what life can hold for a severely ill infant. This can make motivating screening programs seem irrelevant when there is no profound, clear, immediate evidence of benefit.
In the United States, although this is an infection affecting persons across all demographics, some of the populations of concern include people living in poverty [73••], individuals experiencing homelessness and some rural/agrarian populations.
There is considerable recent lay press concerning the poor record for maternal and child health and well-being, health care disparities, including for the sentinel clinical findings of toxoplasmosis, e.g., , prematurity, small for gestational age, intrauterine growth retardation, death in the first year of life, maternal morbidity and for marginalized indigenous and Latino populations. Titles from recent (November, 2021) lay press news articles emphasize this problem in the U.S.. These titles include: “U.S. Maternal And Infant Mortality: More Signs Of Public Health Neglect” (Forbes); “The U.S. Remains One of the Most Dangerous High-Income Countries for Childbirth, According to a New Report” (Health); Opinion (Apple News) | The lives and deaths of infants: America's disturbing disadvantage”.
Another example that prevalence varies by demographics is that seroprevalence in the Lancaster Amish area among those of childbearing age is approximately 50% with high risk and high exposure for those who are seronegative [32•] in contrast to other areas in the US where prevalence is <15%. Influence of other aspects of demographics is evident in the US in NCCCTS studies (e.g., 33) and in our large data analysis (45). Geography, ethnicity and socioeconomic status were associated with parasite serotype as was anatomy of hydrocephalous, prematurity and severity of illness at birth. Treatment in utero appeared to obviate those associations (33).
Need for Novel Medicines that Eliminate Encysted Bradyzoites and “Persister” Organisms, and Vaccines that Prevent this Infection in Humans and Oocyst Shedding by Cats
Recurrent disease demonstrates the great need for new and improved medicines which eliminate the dormant form of the parasite and vaccines to prevent acquisition in the first place. There is recent progress in these areas of research (Table 5 and Fig. 5) ( 64, 84- 135) with the hope that there will be resources that enable some of these approaches to treatment and prevention to reach the clinic resulting in definitive cure and prevention by vaccines. In animal models this appears to be feasible with very prompt treatment [84••, 85••, 86••]. One recent study suggests that there could be less new, recurrent retinal disease with prompt treatment of the acute acquired infection, at least in Colombia, and that a detectable allelic variation in a secreted Rhoptry protein, Rhoptry Protein 16 (ROP 16) is the responsible virulence factor at least in Colombia [97].
Fig. 5.
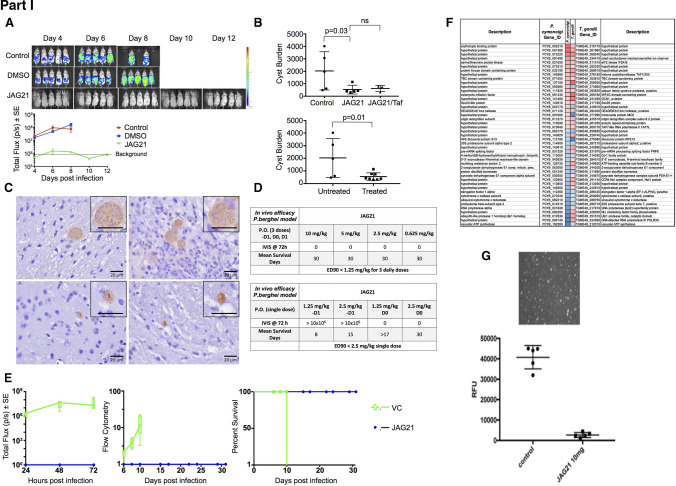
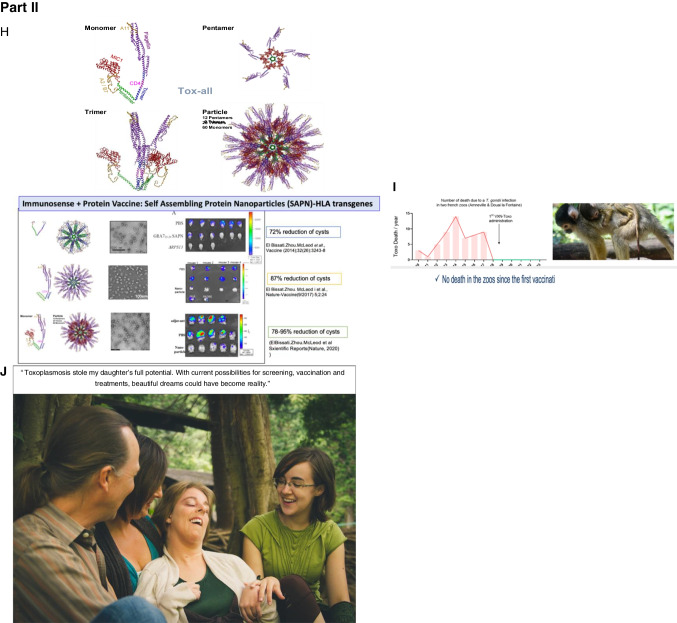
The Future. Recent studies in Chicago to develop medicines and vaccines, and an intranasal nano-vaccine and a dendrimer RNA vaccine developed by others (I). Part I. A potent tetrahydroquinolone effective against tachyzoites and bradyzoites in vitro, synergistic with atovaquone, eliminates >95% of encysted bradyzoites in vivo,, while active with a single oral dose of a nano formulation against tachyzoite infection and Plasmodium berghei’s three life cycle stages. Specifically reproduced with permission from Frontiers Cell Infection Microbiol : Figure and Legend directly from this paper. “JAG21 is a mature lead that protects against Toxoplasma gondii and Plasmodium berghei in vivo. (A) JAG21 treatment for 14 days protects against T. gondii tachyzoites in vivo. Tachyzoite challenge with Prugneaud luciferase parasites imaged with leuciferin using IVIS demonstrates that treatment with JAG21 eliminates leuciferase expressing parasites and leads to 100% survival of JAG21 treated infected mice. No cysts were found in brains of mice at 30 days after infection when they have been treated with JAG21 for the first 14 days after infection. There were 2 biological replicate experiments with 5 mice per group with similar results. (B) JAG21 and JAG21 plus tafenoquine markedly reduce Me49 strain brain cyst numbers in vivo in Balb/C mice at 30 days after infection. Parasites were quantitated by scanning the entire immunoperoxidase stained slide in an automated manner and by two observers blinded to the experimental treatment using microscopic evaluation. In each of two experiments, the numbers of mice per group were as follows: Experiment 1 had 4 diluent controls, 5 JAG21, 4 JAG21/Tafenoquine treated mice; and Experiment 2 had 5 diluent controls, 5 JAG21, 3 JAG21/Tafenoquine treated mice. Immunoperoxidase staining was performed. Parasite burden was quantitated using a positive pixel count algorithm of Aperio ImageScope software. Positive pixels were normalized to tissue area (mm2). Quantification was by counting positive pixels per square area. The entire brain in one section was scanned for each mouse. The parasite burden was quantitated as units of positive pixels per mm2. The average ± S.E.M. numbers of mm2 per slide quantitated was 30.2±1.6 mm2 per mouse for this quantification. Each high power field of view shown in C is ~0.02 mm2 per field of view. A representative single experiment is presented and the data from the two experiments analyzed together also demonstrated significant differences between the untreated and treated groups (p < 0.01; Supplemental Figure 1). (C) Microscopic evaluation of the slides reveal effect of JAG21 and JAG21 plus tafenoquine having the same pattern as the automated quantitation of immunoperoxidase stained material. There are usual appearing cysts in the DMSO control untreated mice as shown in the top panels, and rare cysts in the treated mice with most of the brown material appearing amorphous (bottom panels). (D) JAG21 nanoformulation dosages administered to P. berghei infected C57Bl6/albino mice compared with vehicle control. Design of single dose and 3 day dose experiments. (E) JAG21 nanoformulation cures P. berghei sporozoites (left panel), blood (middle panel) and liver stages, leading to 100% survival (right panel). This is with oral administration of a single dose of 2.5 mg/kg or 3 doses at 0.625 mg/kg. Single dose causal prophylaxis in 5 C57BL/6 albino mice at 2.5 mpk dosed on day 0, 1 h after intravenous administration of 10,000 P. bergheisporozoites. Shown is 3 dose causal prophylaxis treatment in 5 C57BL/6 albino mice at 0.625 mpk dosed on days −1, 0 and +1. Representative figure showing survival (right panel), luminescence (left panel) and parasitemia quantitated by flow cytometry (middle panel) for 5 mg/kg.” (F) Persisters of a RPS13 Λ strain of Toxoplasma can persist long times by exiting the cell cycle at G1 (G). The nanoformulation highly active in vivo against RH strain tachyzoites indicates that oral formulation in a conventional manner will likely be feasible. Biomarkers of illness, not shown, can also be a measure of efficacy in future studies, including circulating miRs, specific serum proteins and T2 weighted abnormalities in brain MRI. Part II. Vaccines on the horizon in pre-clinical studies. (H) Self assembling nanoparticle immunosense vaccine is potent in protection of mice (134, 137-9). RNA dendrimers also are promising in our vaccine work (Melo, Zhou, Weiss, Irvine, McLeod et al, In preparation, 2022). (I) Protection of primates eliminating death in French zoos with porous nanoparticles loaded with T.gondii lysate administered intranasally [https://www.inrae.fr/actualites/vaccine-contr-toxoplasmose-singes-saimiris]. Additional work of of others is referenced as well [100–135]. (J) Importance of screening and treating during gestation for those who are seronegative, new medicines and vaccines are emphasized in the words and image of J. Morel and her familly. Other images and Figure legend for Part I are reproduced with permission from Frontiers Cell Infect Microbiol, Nature Partners Journal Vaccines, and from Vaxinano
Discussion
Overall, in the United States, treatment algorithms for prenatal and postnatal care evolved in parallel with models for care from Europe and some South American countries. This experience, clinical studies and randomized control trial provided substantial insight into pathogenesis and presentation of toxoplasmosis [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 51•, 52, 53••, 54, 55••, 56••, 57•, 58••, 59•, 60••, 61, 62••, 63, 64.••••, 65, 66••, 67••, 68••, 69, 70, 71.••••, 72••, 73••, 74••, 75••]. This information, for the NCCCTS in the United States, was summarized in Part I Tables 1, 2, 3, 4, 5. Difficulties in obtaining optimal care was made more apparent by the pandemic and are highlighted herein. The difficulties with medicine access are in part related to cost and limited distribution and are summarized in Part I Tables 4 and 5. Guidelines for care in the United States are also presented in Part I.
In Panamá’, with respect to medical infrastructure and protocols, we started by creating a legal framework and an evidence-based clinical model for a preventive care approach to congenital toxoplasmosis; we then studied the national levels of compliance with these efforts. In October 2014, Decreto ejecutivo No. 1617 was passed in response to a study conducted by Hospital del Niño researchers at Hospital Santo Tomás; this study suggested seroprevalence of toxoplasmosis in Ciudad de Panama could be as high as 50 percent. The law passed in 2014 mandated that in Panamá, pregnant women be tested for congenital toxoplasmosis twice during gestation and there should be reporting of cases of infection to the Ministry of Health. However, our spatial analysis studies in 2016 and 2017 found that, even over two years after the law went into effect, screening rates varied from 0% to 60% regionally and were at about 39% nationwide. These findings led to investigations of why some areas are more compliant, as well as how these differences in compliance could inform educational approaches. Our later larger aggregate studies on Toxoplasma’s prevalence in Panamá—one of which used standard-of-care serologic testing for about 3500 pregnant women—enhanced national awareness of congenital toxoplasmosis and provided evidence for the considerable benefits of testing pregnant women consistently for seroconversion. In the meantime, as suggested by the low overall national screening rate, children with severe disease due to congenital toxoplasmosis are not uncommon in Panamá.
While increasing compliance is important, we also found that Panama could also benefit by implementing a more rigorous screening protocol than what Decreto ejecutivo No. 1617 stipulates. This is because—as our 2016 and 2017 studies showed—a Toxoplasma infection occurring between the two stipulated tests could remain undetected for a significant period of time. While 23 women were classified as IgG-/IgM- prior to these studies, we found in a second screening near the end of gestation that they had become IgG+, IgM-. We do not know whether these patients had false negative tests at first, or if their serologies missed the time when tests for acute infection were positive, suggesting that months had occurred after seroconversion. This means that these women could have been acutely infected at some point earlier in their gestation and thus could have transmitted the infection to their fetus when preventive therapy could have been implemented. These findings re-emphasize that—while Decreto ejecutivo No. 1617 is a good start—the optimal conditions for rapid diagnosis, prompt treatment and accurate tracking for seroconversion cannot depend only on two widely spaced screenings. Instead, studies in France, Austria and Colombia have suggested that identifying seronegative (and at-risk) mothers prior to gestation and then testing these women monthly for anti-Toxoplasma antibodies is a very effective and relatively cost-saving model for ensuring the fewest congenital toxoplasmosis -related adverse outcomes for infants [43••, 44••, 51•]. A longer delay between accurate diagnosis and appropriate treatment increases the likelihood that a congenitally infected child develops more severe illness or remains undiagnosed. As such, our study provides useful data for the Panamanian Ministry of Health about this problem and an opportunity to intervene. Even if there were universal compliance with Decreto ejecutivo No. 1617, testing women twice during gestation should be considered merely one early step toward a more comprehensive national gestational screening program.
With this ideal screening program in mind, we are hoping to implement POC tests for anti-Toxoplasma antibodies in specific practices in Panamá. We have already had some success with the LDBIO Toxoplasma ICT IgG-IgM test (LDBIO Diagnostic, Lyon, France), a test that meets World Health Organization’s ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid, Robust, Equipment-free, Deliverable) criteria [43••, 44••]. The high performance of this test, as documented in previous studies, made it appealing to pilot its use in early studies in Panama in 2019. Since then, the LDBIO device has made it possible to know when false positive results were generated by Roche IgG and IgM or other commercial tests. The LdBio performed relatively well on 100 sera from pregnant patients in Panamá. Since a positive test in a screening program will be confirmed by a second test that should not interfere with its use. All the negative test values were negative in both Roche and LdBio POC tests. This test was CE marked in Europe and an extension of CE approval including whole blood use was done in December 2020. If there is approval for use in the United States by the FDA, it could build the momentum to use the LDBIO Toxoplasma ICT IgG-IgM device on a larger scale in serologic screening programs during gestation in multiple countries, including in Panamá, the United States and Colombia. There are ongoing larger scale studies in Colombia and in Morocco in progress now and plans to begin other larger scale studies in the United States soon.
Our studies on seroprevalence and testing (discussed in more detail below) generated discussion at Hospital Santo Tomás and Hospital del Niño regarding the possibility of creating a more comprehensive congenital toxoplasmosis screening program. Researchers and physicians from both institutions recognized the considerable spillover benefit in having regular care with monthly screening that used a test like the LDBIO device. To make certain that there was an organized system to ensure high quality health care, Hospital del Niño founded a perinatal infectious disease outpatient program to support diagnosis and care for affected infants and children. Although Hospital Santo Tomás is now ready to implement a monthly screening program using the POC test, this was put on hold due to the COVID-19 pandemic. In the meantime, Hospital Santo Tomás’s program provides access to previously unavailable or difficult to obtain medicines for patients with Toxoplasma infection, and physicians in this program also follow the disease course of congenitally infected children. A proposal to assure availability and easy, prompt access to low-cost, POC testing is being developed currently for all of Panamá. The current and upcoming programs at Hospital Santo Tomás and Hospital del Niño offer a paradigm for improving this aspect of healthcare infrastructure in Panamá.
In addition, recent cost-benefit analyses of congenital toxoplasmosis screening in the United States and Austrian contexts should contribute to consideration of expanding these programs throughout the country [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 51•, 52, 53••, 54, 55••, 56••, 57•, 58••, 59•, 60••]. The value of addressing access to reliable rapid on-site diagnostic testing with spillover benefit was discussed in detail in Begeman et al [43••]. Herein we present new work to build such a program in the United States, Panamá, Colombia and enhance that in France and other countries. This is part of a toolbox of approaches to attempt to eliminate toxoplasmosis.
Not only is there potential from the work itself, but participating in the process of generating these materials has also proven useful for each group of students that has taken a part in our work—including the public health students who addressed how NGOs and MINSA could work together to initiate toxoplasmosis care in the Embera indigenous communities of eastern Panama (see Fig. 3 and Supplement: Dhamsania et al.), both in the countries and also for the United States’ students who were graciously hosted in Panama and Colombia. This work began with the work of the NCCCTS in 1981 with very brave families with afflicted children with congenital toxoplasmosis brave enough to try a new treatment, demonstration of efficacy and safety through a phase 1 clinical trial and then a randomized controlled trial of two doses of medicines [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•] transitioning into a longitudinal study of outcomes and biology of the infection and a reference center for care. Noting the extremely favorable outcomes for those treated in utero, an attempt was made to develop a program for screening in the United States [61]. This includes building supportive infrastructure and addressing cost benefit and developing ways to implement screening at low cost with very high-quality tests, a program has grown in the United States. This has moved forward in France, Austria and Slovenia, developed at scale in Morocco moving toward screening programs including 2,000 persons. In Colombia screening has been implemented and Panama is contemplating this as well.
Novel anti-parasitic treatments and vaccines and studies of pathogenesis informing understanding of consequences of this infection are also ongoing in the US, Europe, Colombia and Brazil among other countries [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 51•, 52, 53••, 54, 55••, 56••, 57•, 58••, 59•, 60••, 61, 62••, 63, 64.••••, 65, 66••, 67••, 68••, 69, 70, 71.••••, 72••, 73••, 74••, 75••, 76–78, 79••, 80••, 81••, 82, 83, 84••, 85••, 86••, 87••, 88••, 89••, 90, 91, 92••, 93, 94, 95, 96, 97, 98••, 99••, 100, 101••, 102••, 103••, 104••, 105••, 106••, 107••, 108••, 109••, 110, 111, 112, 113••, 114••, 115••, 116••, 117••, 118••, 119••, 120, 121, 122••, 123••, 124, 125••, 126••, 127••, 128••, 129••, 130••, 131••, 132, 133••, 134••, 135, 136••, 137••, 138••, 139••]. Some of this is summarized by McLeod et al in Weiss and Kim (Editors) [64.••••] very recently with some studies referenced herein [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 51•, 52, 53••, 54, 55••, 56••, 57•, 58••, 59•, 60••, 61, 62••, 63, 64.••••, 65, 66••, 67••, 68••, 69, 70, 71.••••, 72••, 73••, 74••, 75••, 76–78, 79••, 80••, 81••, 82, 83, 84••, 85••, 86••, 87••, 88••, 89••, 90, 91, 92••, 93, 94, 95, 96, 97, 98••, 99••, 100, 101••, 102••, 103••, 104••, 105••, 106••, 107••, 108••, 109••, 110, 111, 112, 113••, 114••, 115••, 116••, 117••, 118••, 119••, 120, 121, 122••, 123••, 124, 125••, 126••, 127••, 128••, 129••, 130••, 131••, 132, 133••, 134••, 135, 136••, 137••, 138••, 139••]. There are novel inhibitory compounds that appear effective against both active rapidly and slow growing Toxoplasma. There are very promising small molecule inhibitors [84••, 85••, 86••], antisense [109], promising vaccines [98, 99, 114] and greater understanding of pathogenesis and consequences of infection [1••, 2, 3••, 4••, 5••, 6••, 7••, 8••, 9••, 10••, 11••, 12, 13, 14••, 15••, 16••, 17••, 18•, 19•, 20•, 21•, 22•, 23, 24••, 25, 26, 27•, 28•, 29–30, 31•, 32•, 33••, 34•, 35•, 36, 37•, 38•, 39••, 40•, 41•, 42••, 43••, 44••, 45, 46•, 47••, 48••, 49, 50•, 51•, 52, 53••, 54, 55••, 56••, 57•, 58••, 59•, 60••, 61, 62••, 63, 64.••••, 65, 66••, 67••, 68••, 69, 70, 71.••••, 72••, 73••, 74••, 75••, 76–78, 79••, 80••, 81••, 82, 83, 84••, 85••, 86••, 87••, 88••, 89••, 90, 91, 92••, 93, 94, 95, 96, 97, 98••, 99••, 100, 101••, 102••, 103••, 104••, 105••, 106••, 107••, 108••, 109••, 110, 111, 112, 113••, 114••, 115••, 116••, 117••, 118••, 119••, 120, 121, 122••, 123••, 124, 125••, 126••, 127••, 128••, 129••, 130••, 131••, 132, 133••, 134••, 135, 136••, 137••]. This progress in these areas will contribute to eliminating toxoplasmosis as a human disease in the future.
This work has become part of a global initiative to save the lives, sight and cognition of fetuses, infants and children [48••]. It has also provided a framework to study the effect of this parasite in causing neurodegenerative diseases, epilepsy and other diseases later in life as this parasite remains in the brain of more than 2 billion people lifelong [138]. New compounds with potential for cure [84••, 85••, 86••] and approaches for development for vaccines to prevent this disease [84••, 98, 99, 101••, 102••, 103••, 104••, 120, 121, 132, 133••, 134••, 135, 136••, 137••, 138••, 139••] are also underway.
They are inspired by the observations of afflicted children in these programs and the needs to build strong programs to assist in their care and prevention of this disease, and discovery and development of novel medicines and vaccines to treat and prevent it even more effectively.
Supplementary Information
(PDF 1.01 MB)
(PDF 498 kb)
Acknowledgments
We gratefully acknowledge the following organizations and people for their financial, logistical and/or technical support during our work on this project.
Panamá: Thanks to the Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT), the Sistema Nacional de Investigación (SNI), the Institute for Scientific Research and High Technology Services of Panama (INDICASAT), Hospital del Niño, Hospital Santo Tomás, and Roche Diagnostics International Ltd. Special thanks to the medical staff of the high-risk pregnancy and infectious disease wings of Hospital Santo Tomás, especially Aris Caballero de Mendieta, Susana Frías, Dario Beneditto, Edwin Ortiz, Carlos Moreno, Ana Belen Arauz, Monica Pachar, Eyra Garcia, as well as to the nurses for all their logistical support.
We thank little Henry Arosemena, who was the impetus for starting the program in Panama and the Arosemena family for their support of educational initiatives in Panamá.
Colombia: Thanks to the Grupo de Investigación en Parasitología Molecular (GEPAMOL) at the University of Quindío, to Richard James Orozco González and to Hospital del Sur and Hospital San Juan de Dios in Armenia, Colombia.
United States: Thanks to Dr. Brian Callender, Dr. Jeanne Farnan, Dr. Olofunmilayo Olopade, Absera Melaku, MPH, Kristyna Hulland, MS, Dr. Susan Duncan and Dr. John Schneider for facilitating summer research through the Summer Research Program at the Pritzker School of Medicine at Chicago.
We appreciate Aaron Ponsler’s suggestion at a Thrasher Foundation site visit at Stanford University that the LDBio could do the most good if it were cleared by the FDA and waived by CLIA.
We thank all those in the National Collaborative Chicago Based Congenital Toxoplasmosis Study (NCCCTS) who helped build and sustain programs in the United States.
We especially thank the many families who participated in and/or encouraged these initiatives and their primary care physicians. We give special thanks to these participant families and their physicians without whom this NCCCTS could not have been performed.
We thank all those who traveled to Springfield to begin these in the United States, recognizing the importance this would have for their families, and who, in addition, participated in testing of the Point of Care test allowing the work that validated its use in the United States with genetically divergent Toxoplasma..
We thank those who worked with the NCCCTS in the earlier years including Marilyn Mets, William Mieler, Michael Grassi, Michael Kip, Hassan Shah, Diane Patton, Vicky Aithchison, Holly McGinnis, Randee Estes, Douglas Mack, Michael Kirisits, Adrian Esquivel, Toria Trendler, Simone Cezar, Jessica Coyne, Daniel Lee, Jane Babiarz, Veena Arun, Annie Kuo, Laura Phan, as well as Daniel Johnson, Adil Javed and others as reflected in the author lists in references 3–50. Their contributions to the initial phases of this work were substantial and valuable.
We thank additional authors and members of the NCCCTS group Barbara Danis, Mark Stein, Linda Pfiffner, Jeanne Perkins, Sanford Meyers, Balaji Gupta, Ahmed Abdelsalam, Huiyuan Zhang, John Marcinak, Saeid Mojatahedi, Dianna Bardo, Carina Yang, Delilah Burrowes, John Collins Marisha Humphries, Douglas Mack, Michael J. Kirisits, Diana Chamot, Ronald Thisted and (University of Chicago, Chicago, IL); Lazlo Stein (Northwestern University, Chicago; deceased); Andrew Suth (Argosy University, Chicago); Audrey Cameron (Mount Sinai Hospital, Chicago); Marie Weissbourd (Northwestern University Hospital, Chicago); Joyce Hopkins (Illinois Institute of Technology, Chicago); Dushyant Patel (Michael Reese Medical Center, Chicago); and others.
We gratefully acknowledge Kristen Wroblewski MS and Theodore Karrison PhD’s assistance with many aspects of this work with the NCCCTS and assisting with analyses.
We thank Jack Remington and those in the Remington serology laboratory who performed the serologic testing for participants in the NCCCTS establishing the diagnosis for each participant as part of their clinical care, allowing the NCCCTS study to be based on rigorous, meticulous diagnostic criteria.
We thank the Data Safety Monitoring Board members, John Flagherty, MD, Rchard Chappell PhD, David Mittelman MD, Dana Bradzunias MD.
We thank those working with us to establish gestational screening for perinatal infections and to develop and test WHO-compatible POC tests in the United States, France and Morocco, Mohamed Rhajaoui, Amina Barkat, Hua Cong and Bo Shiun Lai.
Additionally, we thank Shaun Carey, James Lynch, Millie Malekar, others in the IRB office, Jim Rago and Sam Campbell for photography, David G Goodman, Colin Shephard, Ribhi M.Shawar, Matthew Macgilvray and Steven Gitterman for their guidance with FDA processes, NIH DMID Program, Michael Gottlieb, F. Lee Hall, Stephanie James and John Rogers for many suggestions, Additional Morocco Group colleagues working alongside and with us, help and support in implementing prenatal screening for toxoplasmosis in Morocco. We thank Iris Ramirez for her contribution to screening. We thank Tom Lint for assistance with many aspects of the study.
France/Austria: Many thanks to our French and Austrian colleagues, who paved the way to and worked with us in understanding the importance of prompt gestational screening, treatment and prevention of congenital toxoplasmosis.
We thank and greatfully acknowledge our scientific colleagues working with us in our basic science, immunology, molecular biology, medicinal chemistry,systems biology, formulation, vaccine and medicine development programs as shown in Fig. 5 and also throughout the work as referenced.
We thank Dr. Lago, Dr. Gupta and Dr. Goldstein for their helpful suggestions.
Funding
Denis Limone Pharm D, is Chairman , shareholder and CEO and Raphael Piarroux Pharm D, PhD, is RandD Director and employee at LDBio Diagnostics. A patent application was submitted in the United States for the development of the whole blood point of care test with the scientists at the University of Chicago to insure its continued high quality performance and reproducibility of the results described herein. In the United States and Panama in the more recent studies the LDBio kit was provided for the studies without charge by D Limonne. The Roche kit was provided without charge in Panamá. For the predicate test costs for the comparison test for 70 consecutive persons for the feasibility clinical trial the cost of performing the Biorad IgG and IgM tests was provided by Global Health Kiphard Foundation Seed Funds. Kamal El Bissati is heading the initiatives to prevent toxoplasmosis in Morocco. Patents have been obtained for the medicine, anti-sense, vaccine, biomarker development work to facilitate their development toward clinical use and sustain availability if/when they are used in clinical practice. RMcLeod was reimbursed for time in performing a literature review concerning spiramycin by Sanofi Pasteur in accordance with Sunshine laws. RMcLeod (with her colleagues) shared first prize merit award in the Alzgerm initiative to identify the highest quality and rigorously developed and described work demonstrating that a pathogen can initiate, progress and contribute to neurodegenerative diseases, and that there can be effect of this chronic active infection on cognition, motor function and other disease processes. This monetary prize was used to further this ongoing work.
Additional thanks for funding from the Jeff Metcalf Internship Program, the Margaret P Thorp Scholarship Fund, the American College of Obstetricians and Gynecologists for their Medical Student Award, the National Institute of Diabetes and Digestive and Kidney Diseases for their Grant #T35DK062719-30, the American Society of Tropical Medicine and Hygiene for their Benjamin H. Kean Fellowship, the National Institutes of Health for their Division of Microbiology and Infectious Diseases Grants to RMc #R01 AI2753,RO1 16945, AI08749-01A1 BIOL-3 , U01 AI77887, U01 AI082180, TMP R01-AI071319, the Thrasher Research Fund for their E.W. “Al” Thrasher Award, the Kiphardt Global-Local Health Seed Fund Award, University of Chicago. SENACYT. Research to Prevent Blindness Foundation, and March of Dimes (6-528), FDA , MRI, Pillsbury Foundation. Michael Reese Physicians Research and Education Foundation, Fullbright Scholar Awards (Craig Roberts, Kamal ElBissati; Dorota Nowakowska), Pathological Society of Great Britain and Northern Ireland Travel Award, WHO DIF, Stanley Foundation, This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH (Michael Grigg)..
We are grateful to Toxoplasmosis Research Institute, Taking out Toxo, Network for Good, the Cornwell Mann Family Foundation, The Rodriguez family, The Samuel family and Running for Fin, The Buchanan Family Foundation, the Morel, Rooney, Mussalami, Kapnick, Taub, Engel, Harris, Quinn, Drago families, Pritzker, and the Hyatt Hotels, Foundation, United Friendly Skies, American, Southwest, Braniff, Angel Flight, Pan Am, Horizon Britt Canada and other airlines.
We gratefully acknowledge the Susan and Richard Kiphart Family Foundation for providing funding for open access charges.
Declarations
Conflict of Interest/Competing Interests
There are no other disclosures and no other competing interests.
Footnotes
Charles Swisher, Eileen Stillwaggon and Paul Meier are deceased. This paper is dedicated to his/her memory.
Mariangela Soberón Felín, Kanix Wang, Aliya Moreira, Andrew Grose, Karen Leahy, Ying Zhou, Fatima Alibana Clouser, Maryam Siddiqui, Nicole Leong, Perpetua Goodall, Mahmoud Ismail, Monica Christmas, Stephen Schrantz, Zuleima Caballero, Ximena Norero, Dora Estripeaut, David Ellis, Catalina Raggi, Catherine Castro, Davina Moossazadeh, Margarita Ramirez, Abhinav Pandey, Kevin Ashi, Samantha Dovgin, Ashtyn Dixon, Xuan Li, Ian Begeman, Sharon Heichman, Joseph Lykins, Delba Villalobos-Cerrud, Lorena Fabrega, José Luis Sanchez Montalvo, Connie Mendivil, Mario R. Quijada, Silvia Fernández-Pirla, Digna Wong, Mayrene Ladrón de Guevara, Carlos Flores, Jovanna Borace, Anabel García, Natividad Caballero, Claudia Rengifo-Herrera, Maria Theresa Moreno de Saez, Michael Politis, Stephanie Ross, Mimansa Dogra, Vishan Dhamsania, Nicholas Graves, Marci Kirchberg, Kopal Mathur, Ashley Aue, Carlos M. Restrepo, Alejandro Llanes, German Guzman, Arturo Rebellon, Kenneth Boyer, Peter Heydemann, A. Gwendolyn Noble, Charles Swisher, Peter Rabiah, Shawn Withers, Teri Hull, Chunlei Su, Paul Latkany, Ernest Mui, Daniel Vitor Vasconcelos-Santos, Alcibiades Villareal, Ambar Perez, Carlos Andrés Naranjo Galvis, Mónica Vargas Montes, Nestor Ivan Cardona Perez, Morgan Ramirez, Cy Chittenden, Edward Wang, Laura Lorena Garcia-López, Juliana Muñoz-Ortiz, Nicolás Rivera-Valdivia, María Cristina Bohorquez-Granados, Gabriela Castaño de-la-Torre, Guillermo Padrieu, Daniel Celis-Giraldo, John Alejandro Acosta Dávila, Elizabeth Torres, Manuela Mejia Oquendo, José Y. Arteaga-Rivera, Dan L Nicolae, Andrey Rzhetsky, Nancy Roizen, Eileen Stillwaggon, Larry Sawers, Francois Peyron, Martine Wallon, Emanuelle Chapey, Pauline Levigne, Carmen Charter, Migdalia De Frias, Jose Montoya, Cindy Press, Raymund Ramirez, Despina Contopoulos-Ioannidis, Yvonne Maldonado, Oliver Liesenfeld, Carlos Gomez, Kelsey Wheeler, Samantha Zehar, James McAuley, Denis Limonne, Raphael Piarroux, Vera Tesic, Kathleen Beavis, Ana Abeleda, Mari Sautter, Bouchra El Mansouri, Adlaoui El Bachir, Fatima Amarir, Kamal El Bissati, Alejandra de-la-Torre, Gabrielle Britton, Jorge Motta, Eduardo Ortega-Barria, Isabel Luz Romero, Michael Grigg, Paul Meier, Jorge Gomez-Marin, Jagannatha Rao Kosagisharaf, Xavier Saez LLorens, Osvaldo Reyes, Rima McLeod and other students and members of NCCCTS and have also contributed substantially to the foundation of this work, see under acknowledgments
This article is part of the Topical Collection on International Health
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mariangela Soberón Felín and Rima McLeod contributed equally, differently to this work.
Change history
11/6/2022
Open Access funding information has been added in the Funding Note.
Contributor Information
Jorge Gómez-Marín, Email: gepamol2@uniquindio.edu.co.
Jagannatha Rao Kosagisharaf, Email: jrao@indicasat.org.pa.
Xavier Sáez Llorens, Email: xsaezll@cwpanama.net.
Osvaldo Reyes, Email: oreyespanama@yahoo.es.
Rima McLeod, Email: rmcleod@uchicago.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Mcleod R, Mack DG, Boyer K, Mets M, Roizen N, Swisher C, Patel D, Beckmann E, Vitullo D, Johnson D, et al. Phenotypes and functions of lymphocytes in congenital toxoplasmosis. J Lab Clin Med. 1990;116(5):623–635. [PubMed] [Google Scholar]
- 2.McGee T, Wolters C, Stein L, Kraus N, Johnson D, Boyer K, Mets M, Roizen N, Beckman J, Meier P, Swisher C, Holfels E, Withers S, McLeod R. Absence of sensorineural hearing loss in treated infants and children with congenital toxoplasmosis. Otolaryngol Head Neck Surg. 1992;106(1):75–80. doi: 10.1177/019459989210600131. [DOI] [PubMed] [Google Scholar]
- 3.Mcleod R, Mack D, Foss R, Boyer K, Withers S, Levin S, Hubbell J. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Toxoplasmosis Study Group. Antimicrob Agents Chemother. 1992;36(5):1040–1048. doi: 10.1128/AAC.36.5.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAuley J, Boyer KM, Patel D, Mets M, Swisher C, Roizen N, Wolters C, Stein L, Stein M, Schey W, Remington J, Meier P, Johnson D, Heydemann P, Holfels E, Withers S, Mack D, Brown C, Patton D, McLeod R. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994;18(1):38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 5.•• Swisher CN, Boyer K, McLeod R. Congenital toxoplasmosis. In: Bodensteiner JB (ed). Sem Ped Neurol, 1994;1:4-25. Describes neurologic findings of congenital toxoplasmosis in the United States. [PubMed]
- 6.Roizen N, Swisher CN, Stein MA, Hopkins J, Boyer KM, Holfels E, Mets MB, Stein L, Patel D, Meier P, Withers S, Remington J, Mack D, Heydemann P, Patton D, McLeod R. Neurologic and developmental outcome in treated congenital toxoplasmosis. Pediatrics. 1995;95(1):11–20. [PubMed] [Google Scholar]
- 7.Patel DV, Holfels EM, Vogel NP, Boyer KM, Mets MB, Swisher CN, Roizen NJ, Stein LK, Stein MA, Hopkins J, Withers SE, Mack DG, Luciano RA, Meier P, Remington JS, McLeod RL. Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology. 1996;199(2):433–440. doi: 10.1148/radiology.199.2.8668790. [DOI] [PubMed] [Google Scholar]
- 8.•• Vogel N, Kirisits M, Michael E, Bach H, Hostetter M, Boyer K, Simpson R, Holfels E, Hopkins J, Mack D, Mets MB, Swisher CN, Patel D, Roizen N, Stein L, Stein M, Withers S, Mui E, Egwuagu C, et al. Congenital toxoplasmosis transmitted from an immunologically competent mother infected before conception. Clin Infect Dis. 1996;23:1055–60 Identifies that occasionally preconception infection can be transmitted to a fetus even when the pregnant women did not have known immune comprompromise. [DOI] [PubMed]
- 9.•• Mets MB, Holfels E, Boyer KM, Swisher CN, Roizen N, Stein L, Stein M, Hopkins J, Withers S, Mack D, Luciano R, Patel D, Remington JS, Meier P, McLeod R. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1996;122:309–24 Characterizes ocular toxoplasmosis with congenital infection, that it can be treated effectively without loss of vision in recurrences, and the timing of recurrences especially at school age entry, adolescence, and with significant stress. [DOI] [PubMed]
- 10.Roberts F, McLeod R. Pathogenesis of toxoplasmic retinochoroiditis. Parasitol Today. 1999;15:51–57. doi: 10.1016/S0169-4758(98)01377-5. [DOI] [PubMed] [Google Scholar]
- 11.Mack DG, Johnson JJ, Roberts F, Roberts CW, Estes RG, David C, Grumet FC, McLeod R. HLA-class II genes modify outcome of Toxoplasma gondii infection. Int J Parasitol. 1999;29:1351–1358. doi: 10.1016/S0020-7519(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 12.Lyons RE, Lyons K, McLeod R, Roberts CW. Construction and validation of a polycompetitor construct (SWITCH) for use in competitive RT-PCR to assess tachyzoite-bradyzoite interconversion in Toxoplasma gondii. Parasitology. 2001;123:433–439. doi: 10.1017/S003118200100868X. [DOI] [PubMed] [Google Scholar]
- 13.Brezin AP, Thulliez P, Couvreur J, Nobre R, McLeod R, Mets MB. Ophthalmic outcomes after prenatal and postnatal treatment of congenital toxoplasmosis. Am J Ophthalmol. 2003;135:779–784. doi: 10.1016/S0002-9394(02)01704-X. [DOI] [PubMed] [Google Scholar]
- 14.•• Boyer KM, Holfels E, Roizen N, Swisher C, Mack D, Remington J, Withers S, Meier P, McLeod R. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: Implications for prenatal management and screening. Am J Obstet Gynecol. 2005;192:564–71 Identifies risk factors and lack of recognition in congential toxoplasmosis in the US. [DOI] [PubMed]
- 15.•• McLeod R, Khan AR, Noble GA, Latkany P, Jalbrzikowski J, Boyer K. Severe sulfadiazine hypersensitivity in a child with reactivated congenital toxoplasmic chorioretinitis. Pediatr Infect Dis J, 2006. 25:270–2 Emphasizes that severe hypersensitivity with sulfonamides can occur with treatment of ocular toxoplasmosis, in this case DRESS syndrome which can be lethal. [DOI] [PubMed]
- 16.Roizen N, Kasza K, Karrison T, Mets M, Noble AG, Boyer K, Swisher C, Meier P, Remington J, Jalbrzikowski J, McLeod R, Kipp M, Rabiah P, Chamot D, Estes R, Cezar S, Mack D, Pfiffner L, Stein M, Danis B, Patel D, Hopkins J, Holfels E, Stein L, Withers S, Cameron A, Perkins J, Heydemann P. Impact of visual impairment on measures of cognitive function for children with congenital toxoplasmosis: implications for compensatory intervention strategies. Pediatrics. 2006;118:e379–e390. doi: 10.1542/peds.2005-1530. [DOI] [PubMed] [Google Scholar]
- 17.McLeod R, Boyer K, Karrison T, Kasza K, Swisher C, Roizen N, Jalbrzikowski J, Remington J, Heydemann P, Noble AG, Mets M, Holfels E, Withers S, Latkany P, Meier P. Outcome of treatment for congenital toxoplasmosis, 1981-2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis. 2006;42:1383–1394. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 18.Arun V, Noble AG, Latkany P, Troia RN, Jalbrzikowski J, Kasza K, Karrison T, Cezar S, Sautter M, Greenwald MJ, Mieler W, Mets MB, Alam A, Boyer K, Swisher CN, Roizen N, Rabiah P, Del Monte MA, McLeod R. Cataracts in congenital toxoplasmosis. J AAPOS. 2007;11:551–554. doi: 10.1016/j.jaapos.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benevento JD, Jager RD, Noble AG, Latkany P, Mieler WF, Sautter M, Meyers S, Mets M, Grassi MA, Rabiah P, Boyer K, Swisher C, McLeod R. Toxoplasmosis-associated neovascular lesions treated successfully with ranibizumab and antiparasitic therapy. Arch Ophthalmol. 2008;126:1152–1156. doi: 10.1001/archopht.126.8.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phan L, Kasza K, Jalbrzikowski J, Noble AG, Latkany P, Kuo A, Mieler W, Meyers S, Rabiah P, Boyer K, Swisher C, Mets M, Roizen N, Cezar S, Remington J, Meier P, McLeod R. Longitudinal study of new eye lesions in treated congenital toxoplasmosis. Ophthalmology. 2008;115:553–59.e8. doi: 10.1016/j.ophtha.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Phan L, Kasza K, Jalbrzikowski J, Noble AG, Latkany P, Kuo A, Mieler W, Meyers S, Rabiah P, Boyer K, Swisher C, Mets M, Roizen N, Cezar S, Sautter M, Remington J, Meier P, McLeod R. Longitudinal study of new eye lesions in children with toxoplasmosis who were not treated during the first year of life. Am J Ophthalmol. 2008;146:375–384. doi: 10.1016/j.ajo.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.• Jamieson SE, de Roubaix LA, Cortina-Borja M, Tan HK, Mui EJ, Cordell HJ, Kirisits MJ, Miller EN, Peacock CS, Hargrave AC, Coyne JJ, Boyer K, Bessieres MH, Buffolano W, Ferret N, Franck J, Kieffer F, Meier P, Nowakowska DE, et al. Genetic and epigenetic factors at COL2A1 and ABCA4 influence clinical outcome in congenital toxoplasmosis. PLoS One. 2008;3:e2285 Discovery of epigenetic influences on the development of congenital toxoplasmosis. [DOI] [PMC free article] [PubMed]
- 23.Jamieson SE, Cordell H, Petersen E, McLeod R, Gilbert RE, Blackwell JM. Host genetic and epigenetic factors in toxoplasmosis. Mem Inst Oswaldo Cruz. 2009;104:162–9 Discusses discovery of susceptibility genes that indicate imprinting and epigenetic influences. [DOI] [PMC free article] [PubMed]
- 24.•• Tan T, Mui E, Cong H, Witola W, Montpetit A, Muench S, Sidney J, Alexander J, Sette A, Grigg M, Reed S, Maewal A, Kim S, Boothroyd J, McLeod R. Identification of T. gondii epitopes, adjuvants, & host genetic factors that influence protection of mice & humans. Vaccine. 2010;28:3977–89 Identification of susceptibility gene in NCCCTS cohort, establishing MHC Class I pathway processing byERAAP. Parts of initial steps in development of a vaccine to prevent toxoplasmosis in humans which is ongoing with expanding approaches. Demonstration of powerful adjuvant effect of GLA-SE in HLA transgenic mice. [DOI] [PMC free article] [PubMed]
- 25.Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, Mui EJ, Witola WH, Coyne JJ, Hargrave AC, Jamieson SE, Blackwell JM, Wiley JS, Smith NC. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol. 2010;184:7040–7046. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamieson SE, Peixoto-Rangel AL, Hargrave AC, Roubaix LA, Mui EJ, Boulter NR, Miller EN, Fuller SJ, Wiley JS, Castellucci L, Boyer K, Peixe RG, Kirisits MJ, Elias Lde S, Coyne JJ, Correa-Oliveira R, Sautter M, Smith NC, Lees MP, Swisher CN, Heydemann P, Noble AG, Patel D, Bardo D, Burrowes D, McLone D, Roizen N, Withers S, Bahia-Oliveira LM, McLeod R, Blackwell JM. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010;11:374–383. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.• Noble G, Latkany P, Kusmierczyk J, Mets M, Rabiah P, Boyer K, Jalbrzikowski J, Wroblewski K, Karrison T, Swisher C, Mieler W, Meier P, McLeod R. Chorioretinal lesions in mothers of children with congenital toxoplasmosis in the National Collaborative Chicago-based, Congenital Toxoplasmosis Study. Sci Med. 2010;20:20–6 Finds 10 percent of mothers of infants in the US who have congenital toxoplasmosis have retinal lesions, emphasizing importance of performing eye examinations of mothers of children with congenital toxoplasmsosis. [PMC free article] [PubMed]
- 28.Witola WH, Mui E, Hargrave A, Liu S, Hypolite M, Montpetit A, Cavailles P, Bisanz C, Cesbron-Delauw MF, Fournié GJ, McLeod R. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun. 2011;79:756–766. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delair E, Latkany P, Noble AG, Rabiah R, McLeod R, Brezin A. Clinical Manifestations of Ocular Toxoplasmosis. Ocul Immunol Inflamm. 2011;2:91–102 Collates and presents clinical manifestations of ocular toxoplasmosis in a pictoral collection. [DOI] [PubMed]
- 30.Hill D, Coss C, Dubey JP, Wroblewski K, Sautter M, Hosten T, Munoz-Zanzi C, Mui E, Withers S, Boyer K, Hermes G, Coyne J, Jagdis F, Burnett A, McLeod P, Morton H, Robinson D, McLeod R. Toxoplasmosis Study Group. Identification of a sporozoite-specific antigen from Toxoplasma gondii. J Parasitol. 2011;97:328–37 Identifies antigen in sporozoites oocysts that elicits an antibody response present for the half life of that antibody, about 8 months in human serum following exposure. Also identifies high prevalence of infection in Lancaster Pennsylvania Amish family/persons. [DOI] [PMC free article] [PubMed]
- 31.• Stillwaggon E, Carrier CS, Sautter M, McLeod R. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis. 2011;5:e1333 Demonstrates parameters that lead to cost effective screening forToxoplasmainfection acquired in gestation. This suggests strategies important in this model for lowering costs for diagnosis and treatment to facilitate programs for screening. [DOI] [PMC free article] [PubMed]
- 32.• Boyer K, Hill D, Mui E, Wroblewski K, Karrison T, Dubey JP, Sautter M, Noble AG, Withers S, Swisher C, Heydemann P, Hosten T, Babiarz J, Lee D, Meier P, McLeod R. Toxoplasmosis Study Group. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin Infect Dis. 2011;53:1081–9 Identifies epidemics of acute acquiredToxoplasmainfection in NorthAmerica and that ingestion of oocysts may go unrecognized. [DOI] [PMC free article] [PubMed]
- 33.•• McLeod R, Boyer K, Lee D, Mui E, Wroblewski K, Karrison T, Noble AG, Withers S, Swisher CN, Heydemann PT, Sautter M, Babiarz J, Rabiah P, Meier P, Grigg M. Prematurity & Severity Associate with T. gondii Alleles (NCCCTS, 1981-2009). Clin Infect Dis. 2012;54:1595–605 Defines serotypes ofT. gondiiand their distribution in the United States, and associations ofToxoplasmagenotypes with phenotypes of illness including prematurity and severity of illness. [DOI] [PMC free article] [PubMed]
- 34.• Lai BS, Witola WH, El Bissati K, Zhou Y, Mui E, Fomovska A, McLeod R. Molecular target validation, antimicrobial delivery, and potential treatment of Toxoplasma gondii infections. Proc Natl Acad Sci U S A. 2012;109:14182–7 Identification or validation of molecular targets inToxoplasmaand that they can be targeted with antisense as potential for medicines. [DOI] [PMC free article] [PubMed]
- 35.Burrowes D, Boyer K, Swisher CN, Noble AG, Sautter M, Heydemann P, Rabiah P, Lee D, McLeod R. Toxoplasmosis Study Group. Spinal Cord Lesions in Congenital Toxoplasmosis Demonstrated with Neuroimaging, Including Their Successful Treatment in an Adult. J Neuroparasitology. 2012;3:1–8. doi: 10.4303/jnp/235533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Béla SR, Dutra MS, Mui E, Montpetit A, Oliveira FS, Oliveira SC, Arantes RM, Antonelli LR, McLeod R, Gazzinelli RT. Impaired Innate Immunity in Mice Deficient in Interleukin-1 Receptor-Associated Kinase 4 Leads to Defective Type 1 T Cell Responses, B Cell Expansion, and Enhanced Susceptibility to Infection with Toxoplasma gondii. Infect Immun. 2012;80:4298–4308. doi: 10.1128/IAI.00328-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witola W, Liu S, Montpetit A, Hypolite M, Zhou Y, Mui E, Cesbron-Delauw MF, Fournie G, Cavailles P, Bisanz C, Boyer K, Withers S, Noble AG, Swisher C, Heydemann P, Rabiah P, McLeod R. ALOX12 In Human Toxoplasmosis. Infect Immun. 2014;82(7):2670–2679. doi: 10.1128/IAI.01505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.• McLeod R. Editorial on Lago EG Utility and limitations of T. gondii-specific IgM serum antibodies in the diagnosis of congenital toxoplasmosis in Porto Alegre. J Pediatr (Rio J). 2014;90(4):329–31 Emphasizes that many infants with congenital toxoplasmosis have negative anti-ToxoplasmaIgM at birth. When present in the neonate, IgM usually becomes negative very early. [DOI] [PubMed]
- 39.McLeod R, Lykins J, Noble AG, Rabiah P, Swisher C, Heydemann P, McLone D, Frim D, Withers S, Clouser F, Boyer K. Management of Congenital Toxoplasmosis. Curr Pediatr Rep. 2014;2(3):166–194. doi: 10.1007/s40124-014-0055-7. [DOI] [Google Scholar]
- 40.• Hutson SL, Wheeler KM, McLone D, Frim D, Penn R, Swisher CN, Heydemann PT, Boyer KM, Noble AG, Rabiah P, Withers S, Montoya JG, Wroblewski K, Karrison T, Grigg ME, McLeod R. Patterns of Hydrocephalus Caused by Congenital Toxoplasma gondii Infection and Association with Parasite Strain. Clin Infect Dis. 2015;48:58 Pattern of hydrocephalus associates with parasite serotype although not completely suggesting different epitopes may be critical or that there may be multifactorial influences.
- 41.Contopoulos-Ioannidis D, Wheeler KM, Ramirez R, Press C, Mui E, Zhou Y, Van Tubbergen C, Prasad S, Maldonado Y, Withers S, Boyer KM, Noble AG, Rabiah P, Swisher CN, Heydemann P, Wroblewski K, Karrison T, Grigg ME, Montoya JG, McLeod R. Clustering of Toxoplasma gondii Infections Within Families of Congenitally Infected Infants. Clin Infect Dis. 2015;61(12):1815–1824. doi: 10.1093/cid/civ721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLeod R, Wheeler KM, Boyer K. Reply to Wallon and Peyron. Clin Infect Dis. 2016;62(6):812–814. doi: 10.1093/cid/civ1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.•• Begeman IJ, Lykins J, Zhou Y, Lai BS, Levigne P, El Bissati K, Boyer K, Withers S, Clouser F, Noble AG, Rabiah P, Swisher CN, Heydemann PT, Contopoulos-Ioannidis DG, Montoya JG, Maldonado Y, Ramirez R, Press C, Stillwaggon E, et al. Point-of-care testing for Toxoplasma gondii IgG/IgM using Toxoplasma ICT IgG-IgM test with sera from the United States and implications for developing countries. PLoS Negl Trop Dis. 2017;11:e0005670 All sera from persons NCCCTS including all serotypes and acute, subacute and chronic, detected with 100% sensitivity and specificity. Considers spillover benefit and clinical implications. [DOI] [PMC free article] [PubMed]
- 44.Lykins J, Li X, Levigne P, Zhou Y, El Bissati K, Clouser F, Wallon M, Morel F, Leahy K, El Mansouri B, Siddiqui M, Leong N, Michalowski M, Irwin E, Goodall P, Ismail M, Christmas M, Adlaoui EB, Rhajaoui M, Barkat A, Cong H, Begeman IJ, Lai BS, ContopoulosIoannidis DG, Montoya JG, Maldonado Y, Ramirez R, Press C, Peyron F, McLeod R. Rapid, inexpensive, fingerstick, whole-blood, sensitive, specific, point-of-care test for antiToxoplasma antibodies. PLOS Negl Trop Dis. 2018;12(8):e0006536. doi: 10.1371/journal.pntd.0006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lykins J, Wang K, Wheeler K, Clouser F, Dixon A, El Bissati K, Zhou Y, Lyttle C, Rzhetsky A, McLeod R. Understanding Toxoplasmosis in the United States through “Large Data” Analyses. Clin Infect Dis. 2016;63(4):468–75 Initial effort to understand the magnitude and implications ofToxoplasmainfection of humans in the U.S. Editorial by E.Wing. Editors choice. [DOI] [PMC free article] [PubMed]
- 46.• Peyron F, McLeod R, Ajzenberg D, Contopoulos-Ioannidis D, Kieffer F, Mandelbrot L, Sibley D, Pelloux H, Villena I, Wallon M, Montoya J. Congenital Toxoplasmosis in France and the United States: One Parasite, Two Diverging Approaches. PLoS Neglected Tropical Diseases. 2017;1(2):e0005222 Contrasts gestational screening in France facilitating early treatment with delays that are the norm in the United States. [DOI] [PMC free article] [PubMed]
- 47.Ngô HM, Zhou Y, Lorenzi H, Wang K, Kim TK, Zhou Y, El Bissati K, Mui E, Fraczek L, Rajagopala SV, Roberts CW, Henriquez FL, Montpetit A, Blackwell JM, Jamieson SE, Wheeler K, Begeman IJ, Naranjo-Galvis C, Alliley-Rodriguez N, Davis RG, Soroceanu L, Cobbs C, Steindler DA, Boyer K, Noble AG, Swisher CN, Heydemann PT, Rabiah P, Withers S, Soteropoulos P, Hood L, McLeod R. Toxoplasma Modulates Signature Pathways of Human Epilepsy, Neurodegeneration & Cancer. Sci Rep (Nature). 2017;7(1):11496. 10.1038/s41598-017-10675-6Using a systems biology analysis of genetics, transcriptomics, proteomics, serum and miR biomarkers, upstream regulators, protein association string, immunohistochemistry, and human MM6 (myeloid) and primary human brain neuronal stem cells, disease association analyses,it was found thatmodulates signature pathways of human epilepsy, neurodegeneration and cancer. This has important implications for the 2 billion people who have this parasite in their brains across lifetimes, including in Panama, Colombia and the United States, making the seroprevalence of 85% in Panama adults over the age of 65 years potentially of considerable significance for those later in life.
- 48.El Bissati K, Levigne P, Lykins J, Adlaoui EB, Barkat A, Berraho A, Laboudi M, El Mansouri B, Ibrahimi A, Rhajaoui M, Quinn F, Murugesan M, Seghrouchni F, Gómez Marín JE, Peyron F, McLeod R. Global initiative for congenital toxoplasmosis: an observational and international comparative clinical analysis. Emerging Microbes & Infections. 2018;7:165. doi: 10.1038/s41426-018-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguirre AA, Longcore T, Barbieri M, Dabritz H, Hill D, Klein PN, Lepczyk C, Lilly EL, McLeod R, Milcarsky J, Murphy CE, Su C, VanWormer E, Yolken R, Sizemore GC. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. Ecohealth. 2019. 10.1007/s10393-019-01405-7. ConsidersToxoplasmaas part of a “one health” initiative including cat excrement exposure and what could be done to improve water systems and eliminate contamination due to oocysts.
- 50.• McLone D, Frim D, Penn R, Swisher CN, Heydemann P, Boyer K, Noble AG, Rabiah P, Withers S, Wroblewski K, Karrison T, Hutson S, Wheeler K, Cohen W, Lykins J, McLeod R. Outcomes of hydrocephalus secondary to congenital toxoplasmosis. J Pediatric Neurosurg. 10.3171/2019.6.PEDS186842019Documents that prompt treatment including needed neurosurgery placement of ventriculoperitoneal shunts can result in favorable, and best of the outcomes. [DOI] [PubMed]
- 51.• Wallon M, Stillwaggon E, Sawyer L et al. Prevention of congenital toxoplasmosis in France using prenatal screening: A decision-analytic economic model PLOS ONE In Press 2022. Improved outcome with screening and treatmentin utero. [DOI] [PMC free article] [PubMed]
- 52.Mandelbrot L, Kieffer F, Wallon M, Winer N, Massardier J, Picone O, Fuchs F, Benoist G, Garcia-Meric P, L'Ollivier C, Paris L, Piarroux R, Villena I, Peyron F. Toxoplasmosis in pregnancy: Practical Management. Gynecol Obstet Fertil Senol. 2021;49(10):782-91. 10.1016/j.gofs.2021.03.003Presents a contrast of France with strong screening program and treatment. US clinicians and Scientists trying to build a comparable program. [DOI] [PubMed]
- 53.•• Prusa AR, Kasper DC, Sawers L, Walter E, Hayde M, Stillwaggon E. Congenital toxoplasmosis in Austria: prenatal screening for prevention is cost-saving. PLoS Negl Trop Dis. 2017;11(7):e0005648 Fourteen-fold cost savings, with efficacy that has transformed outcomes of congenital infection in Austria with use of pre-natal screening. Goal herein is to scale up such programs worldwide to benefit individual patients and societies with spill over benefit for maternal and child health. [DOI] [PMC free article] [PubMed]
- 54.Kosack CS, Pasge AL, Klatser PR. A guide to aid the selection of diagnostic tests. Bull World Health Organ. 2017;95(9):639–645. doi: 10.2471/BLT.16.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.•• Gomez CA, Budvytyte LN, Press C, Zhou L, McLeod R, Maldonado Y, Montoya JG, Contopoulos-Ioannidis DG. Evaluation of three point-of-care (POC) tests for detection of Toxoplasma immunoglobulin IgG and IgM in the United States: proof of concept and challenges. Open For Inf Dis. 2018. 10.1093/ofid/ofy215High sensitivity and specificity of LDBio used with sera. [DOI] [PMC free article] [PubMed]
- 56.•• Stillwaggon E, Carrier CS, Sautter M, McLeod R. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis. 201;5(9):e1333. Dec 10(1):1675-1682. 10.1080/22221751.2021.1948359. Supplement. Equations for determining cost efficacy of gestational screening for acquisition ofToxoplasmaduring gestation. This is to facilitate prompt treatment to prevent congenital infection and/or its sequelae. [DOI] [PMC free article] [PubMed]
- 57.• El Mansouri B, Amarir F, Peyron F, Adlaoui EB, Piarroux R, El Abassi M, Lykins J, Nekkal N, Bouhlal N, Makkaoui M, Barkat A, Lyaghfouri A, Zhou Y, Rais S, Daoudi F, Ismail Elkoraichi I, Zekri M, Nezha B, Hajar M, et al. Performance of a novel point-of-care blood test for Toxoplasma infection in women from diverse regions of Morocco. Microbes Infect. 2021;10(1):1675–82. 10.1080/22221751.2021.1948359 High prevalence ofToxoplasmainfection in Morocco, LDBio can be used for diagnosis with high sensitivity and specificity, infection was associated with poverty, well water, not washing fruits and vegetables.
- 58.•• Grose A, Wallon M, Chapey E, Leahy K, Zhou Y, Siddiqui M, Leong N, Goodall P, Tesic V, Beavis K, Houze S, Abraham S, Limonne D, Piarroux R, McLeod R, Eliminating Confounding False Positive IGMtests by Using a Lateral Immunochromatography Test that meets WHO ASSURED Criteria In submission, 2022. Demonstrates that LDBio can eliminate problems from false positive IgMs in serologic screening programs for congenital toxoplasmosis for those without antiT.gondii IgG antibody.
- 59.Flores C, Villalobos-Cerrud D, Borace J, Mcleod R, Fábrega L, Norero X, Sáez-Llorens X, Moreno MT, Restrepo CM, Llanes A, Mario Quijada RM, Ladrón De Guevara M, Guzmán G, de la Guardia V, García A, Wong D, Reyes O, Soberon M, Caballero EZ. Prevalence and risk factors associated with T. gondii infection in pregnant women and newborns from Panama. Pathogens. 2021;10(6):764. doi: 10.3390/pathogens10060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.•• Mandelbrot L, Kieffer F, Sitta R, Laurichesse-Delmas H, Winer N, Mesnard L, Berrebi A, Le Bouar G, Bory J-P, Cordier A-G, Ville Y, Perrotin F, Jouannic J-M, Biquard F, d’Ercole C, Houfflin-Debarge V, Villena I, Thiébaut R Toxoges. Prenatal therapy with pyrimethamine + sulfadiazine versus spiramycin to reduce placental transmission of toxoplasmosis: A multicenter, randomized trial. Am J Obstetr Gynecol. 2018. 10.1016/j.ajog.2018.05.031Demonstrates that outcomes can be highly favorable with serologic screening in gestation and prompt treatment, with slight improvement in outcomes with pyrimethamine and sulfadiazine compared to spiramycin treatment after 16 weeks gestation defined as 16 weeks of amennhorea.
- 61.McLeod R., Sautter M,, Rooney T, Morel J, Taub L, Taub D, Taub V, Taub JW, Tirado S, Latkany P, Boyer K, Hotez P, Lin E, McLone D, et al. Submission to the Committee on Public Health Illinois State Senate. Regarding: Prenatal and Neonatal Congenital Toxoplasmosis Prevention and Treatment Act, SB3667 in the context of the National Collaborative Chicago Based Congenital Toxoplasmosis Study. 2010. Introduction of a bill to have a law that obstetricians in Illinois offer their patients the opportunity to have serologic testing in gestation, in Illinois. This provided information from patients, families, physicians, scientists. Committee favored law.
- 62.Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Villena I. Congenital toxoplasmosis in humans: an update of worldwide rate of congenital infections. Parasitology. 2021;148:1406–1416. doi: 10.1017/S0031182021001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Marín JE, Munoz-Ortiz J, Mejía-Oquendo M, Arteaga-Rivera JY, Rivera-Valdivia N, Bohorquez-Granados MC, Velasco-Velasquez S, Castano-de-la-Torre G, Acosta-Davila JA, García-Lopez LL, Torres-Morales E, Vargas M, Valencia JD, Celis-Giraldo D, de-la-Torre A. High frequency of ocular toxoplasmosis in Quindío, Colombia and risk factors related to the infection. Heliyon. 2021;7:e06659 Identifies high prevalence of ocular toxoplasmosis in Quindio, Colombia and associated risk factors. Importance of drinking bottled water associated with prevention was identified. [DOI] [PMC free article] [PubMed]
- 64.••.McLeod R, Cohen W, Dovgin S, Finkelstein L, Boyer K. Human Toxoplasmosis. In: Weiss LM, Kim K, editors. The Model apicomplexan: Perspectives and Methods Third Edition. Chapter 4: Elsevier; 2020. p. 117–228. Comprehensive up to date consideration of human toxoplasmosis, including clinical aspects, treatment, prevention, other considerations of pathogenesis, unifying concepts and toward the future.
- 65.Mejia-Oquendo M, Marulanda-Ibarra E, Gomez-Marin JE. Evaluation of the impact of the first evidence-based guidelines for congenital toxoplasmosis in Armenia (Quindío) Colombia: An observational retrospective analysis. The Lancet Regional Health - Americas (In Press, 2021). Between 2001 and 2019, the national guideline has had a positive impact by improving early diagnosis and treatment of prenatal toxoplasmosis and reducing severe forms, as observed at the referral center. [DOI] [PMC free article] [PubMed]
- 66.Kieffer F, Wallon M, Garcia P, Thulliez P, Peyron F. Franck ?Risk factors for retinochoroiditis During the firat 2 years of life in infants with treated Congenital toxoplasmosis. J. Pediatr Infect Dis J. 2008;27(1):27–32. doi: 10.1097/INF.0b013e318134286d. [DOI] [PubMed] [Google Scholar]
- 67.Wallon M, et al. Congenital Toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin. Infect. Dis. 2013;56:1223–1231. doi: 10.1093/cid/cit032. [DOI] [PubMed] [Google Scholar]
- 68.Montoya JG, Laessig K, Sohail Fazeli M, Siliman G, Yoon SS, Drake-Shanahan E, Zhu C, Akbary A, McLeod R. A Fresh Look at the Role of Spiramycin in Preventing a Neglected Disease: Meta-analyses of Observational Studies. Eur J Med Res. 2021;I26(1):143. doi: 10.1186/s40001-021-00606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLeod R, Lee D, Clouser F, Boyer K (2015). Toxoplasmosis in the Fetus and Newborn Infant. In: Stevenson D, Sunshine P (eds) Neonatology: Clinical Practice and Procedures. 1st ed. New York: McGraw Hill, pp 821-76. Comprehensive review of data concerning toxoplasmosis in the fetus and newborn infant with images.
- 70.McLeod R, Lee D, Clouser F, Boyer K. Diagnosis of Congenital Toxoplasmosis. In: Stevenson D, Sunshine P, editors. Neonatology: Clinical Practice and Procedures. 1. New York: McGraw Hill; 2015. pp. 1297–1310. [Google Scholar]
- 71.••.McLeod R, Boyer K. Toxoplasmosis. In: Behrman RL, Kliegman R, Arvin AM, editors. In Nelson’s Textbook of Pediatrics, 2 2ndt Ed. Philadelphia: WB Saunders; 2021. [Google Scholar]
- 72.•• Remington JS, McLeod R, Thulliez P, Desmonts G, Toxoplasmosis. Infectious Diseases of the Fetus and Newborn Infant. Ch 31. Elsevier. 6th edition pp. 947-1091.2006. Detailed, thorough chapter that presents data and images of toxoplasmosis in the fetus and newborn infant.
- 73.•• Hotez PJ. Neglected Infections of Poverty in the United States of America P 2008;2(6):e2. PLoS Neglect Trop Dis. Demonstrates and emphasizes that the diseases noted to occur in developing countries also occur in the United States , especially in areas of poverty. These include toxoplasmosis, which affects persons of all demographics. [DOI] [PMC free article] [PubMed]
- 74.Lago EG, Endres MM, Scheeren MFDC, Fiori Ocular Ourcome of Brazilian Patients with. Congenital toxoplasmosis. Pediatr Infect Dis J. 2021;40(1):e21–e27. doi: 10.1097/INF.0000000000002931. [DOI] [PubMed] [Google Scholar]
- 75.•• Silva Machado A, et al. Biomarker Analysis Revealed Distinct Profiles of Innate and Adaptive immunity in Infants with ocular lesions of Congenital toxoplasmosis. Sci Rep. 2014:2020. Active retinal lesions associated with leukocytosis with monocytes and NK cells CD8+ T cells, possibly predicting morbidity. [DOI] [PMC free article] [PubMed]
- 76.Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med. 1994;330:1858 Newborn screening program in Massachusetts, New Hampshire and ermont detects congenital toxoplasmosis not otherwise recognized at birth with approximately half with retinal or central nervous system signs with careful evaluation. [DOI] [PubMed]
- 77.Dubey JP, Lago EG. Gennari SM. Su C, Jones JL. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. 10 July 2012 Comprehensive review of toxoplasmosis in Brazil. [DOI] [PubMed]
- 78.Chapey E, Wallon M, Peyron F. Evaluation of theLDBIO point of care test for the combined detection of toxoplasmic IgG and IgM. Clin Chim Acta. 2017;464:200–201. doi: 10.1016/j.cca.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 79.•• Pomares C, Zhang B, Arulkumar S, Gonfrier G, Marty P, Zhao S, Cheng S, Tang M, Dai H, Montoya JG. Validation of IgG, IgM multiplex plasmonic gold platform in French clinical cohorts for the serodiagnosis and follow-up of Toxoplasma gondii infection. Diagn Microbiol Infect Dis. 2017;87(3):213–8. 10.1016/j.diagmicrobio.2016.09.001Inexpensive serum and saliva nanotest diagnosesToxoplasma infection. [DOI] [PubMed]
- 80.Li X, Pomares C, Peyron F, Press CJ, Ramirez R, Gonfrier G, Cannavo I, Chapey E, Levigne P, Wallon M, Montoya JG, Hongjie DH. Plasmonic gold chips for the diagnosis of Toxoplasma gondii, CMV, and rubella infections using saliva with serum detection precision. Eur J Clin Microbiol Infect Disj. 2019;38(5):883–890. doi: 10.1007/s10096-019-03487-1. [DOI] [PubMed] [Google Scholar]
- 81.•• Abraham S, McLeod R, Houhou-Fidouh, Nicaise-Rolland P, Landraud L, Houze S. Performances of Toxoplasma ICT IgG-IgM in comparison with Vidas Toxo Competition and Toxoscreen, Lyon France European meeting. Lateral Chromatography test highly sensitive and specific when testing sera from persons in France and the USA. Also In submission 2022.
- 82.Ben-Abdallah R et al. Contribution of the Toxoplasma ICT IgG IGM test in determining the immune status of pregnant women against toxoplasmosis. 2021. 10.1002/jcla23749. Lyon France European meeting. ICTLateral immunochromatography test sensitive and specific with sera in Algeria.
- 83.Cardosa L, Piarroux R. Lopes APInterest du test Toxoplasma ICT IgG-IgM dans le diagnostic serologique de l’infection a Toxoplasma gondi chez le chat. Lyon Parasitology Meeting. In Press 2022. POC test also of use for detection of infection in cats but not as sensitive and specific as humans.
- 84.McPhillie M, Zhou Y, El Bissati K, Dubey J, Lorenzi H, Capper M, Lukens AK, Hickman M, Muench S, Verma SK, Weber CR, Wheeler K, Gordon J, Sanders J, Moulton H, Wang K, Kim TK, He Y, Santos T, Woods S, Lee S, Donkin D, Kim E, Fraczek L, Lykins J, Esaa F, Alibana-Clouser F, Dovgin S, Weiss L, Brasseur G, Wirth D, Kent M, Hood L, Meunieur B, Roberts C, Hasnain SS, Antonyuk SV, Fishwick C, McLeod R. New paradigms for understanding and step changes in treating active and chronic, persistent apicomplexan infections. Sci Rep. 2016;6:29179. doi: 10.1038/srep29179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McPhillie M, Zhou Y, Hickman M, Gordon J, Weber C, Li Q, Lee P, Amporndanai K, Johnson RH, Darby H, Woods S, Li ZH, Priestley R, Ristroph K, Biering GC, El Bissati K, Hwang S, Hakim FE, Dovgin S, Lykins J, Roberts L, Hargrave K, Cong H, Sinai A, Muench SP, Dubey JP, Prud’homme R, Lorenzi H, Biagini GC, Moreno S, Roberts CW, Atonyuk S, Fishwick CW, McLeod R. Potent Tetrahydroquinolone Eliminates Apicomplexan Parasites. Front Cell Infect Microbiol. 2020;10:203. doi: 10.3389/fcimb.2020.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.•• McPhillie M, ZhouY HM, Gordon J, Weber C, Li Q, Lee P, Amporndanai K, Johnson RH, Darby H, Woods S, Li ZH, Priestley R, Ristroph K, Biering GC, El Bissati K, Hwang S, Hakim FE, Dovgin S, Lykins J, et al. Potent Tetrahydroquinolone Eliminates Apicomplexan Parasites. Front Cell Infect Microbiol. 2020; Microbiol Ebook with editorial by de-la-Torre A and Gomez-Marin JE that states:” “Finally, one major finding reported in this Research Topic is the development of a powerful tetrahydroquinolone, JAG21, which can eliminate apicomplexan parasites in tissues (McPhillie et al.). This is an extraordinary achievement in in vivo models because, until now, there are no drugs that can eliminate tissue cysts of the parasite responsible for reactivations during the host’s period of life. The authors created a next generation lead compound with high in vitro and in vivo efficacy against T.gondii tachyzoites, bradyzoites, and established encysted organisms (McPhillie et al). This compound is promising and deserves further development through preparation of advanced formulations and testing in further studies of pharmacokinetics, efficacy, and safety. In summary, this Research Topic shows significant advances made in the study of T. gondii using in-vitro, in-vivo and animal models. The results presented here will illuminate the pathway to create an effective clinical response to this public health key issue.”.
- 87.••Lykins JD, Filippova EV, Halavaty AS, Minasov G, Zhou Y, Dubrovska I, Flores KJ, Shuvalova LA, Ruan J, El Bissati K, Dovgin S, Roberts CW, Woods S, Moulton JD, Moulton H, McPhillie MJ, Muench SP, Fishwick CWG, Sabini E, Shanmugam D, Roos DS, McLeod R, Anderson WF, Ngô HM. CSGID Solves Structures and Identifies Phenotypes for Five Enzymes in Toxoplasma gondii. Front Cell Infect Microbiol. 2018;8:352. 10.3389/fcimb.2018.00352. eCollection 2018. Crystalography pipeline identifies potential molecular targets (enzymes) inToxoplasma gondii;Vivo morpholinos and crispr cas 9 screen together demonstrate which is essential in tachyzoites. [DOI] [PMC free article] [PubMed]
- 88.••Eichenwald HF. A study of congenital toxoplasmosis with particular emphasis on clinical manifestations, sequelae and therapy. In: Human Toxoplasmosis, Siim JC (Ed), Munksgaard, Copenhagen 1959. Congenital toxoplasmosis with generalized or neurologic presentation in the US if untreated or treated only one month causes substantial neurologic and eye disease by the age of 4 years with significant morbidity.
- 89.Koppe JG, Loewer-Sieger DH, de Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;1:254. doi: 10.1016/S0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 90.Wilson CB, Remington JS, Stagno S, Reynolds DW. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics. 1980;66:767. doi: 10.1542/peds.66.5.767. [DOI] [PubMed] [Google Scholar]
- 91.Rostami A, et al. Acute Toxoplasma infection in pregnant women worldwide: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13(10):e0007807. doi: 10.1371/journal.pntd.0007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.••Lago EG. Risk factors for congenital toxoplasmosis are not always obvious. ACOG. 2006;194(3):907; author reply 907-8. 10.1016/j.ajog.2005.07.021. Risk fctors not always clear. [DOI] [PubMed]
- 93.Endres MM, Scheeren MFDC, Fiori HH. Ocular Outcome of Brazilian Patients With Congenital Toxoplasmosis. Pediatr Infect Dis J. 2021;40(1):e21–e27. doi: 10.1097/INF.0000000000002931. [DOI] [PubMed] [Google Scholar]
- 94.Ryning FW, Mcleod R, Maddox JC, Hunt S, Remington JS. Probable transmission of Toxoplasma gondii by organ transplantation. Ann Intern Med. 1979;90(1):47–9. Patient who received a cardiac transplant donated from a person who died in a motorcycle accident who had acute toxoplasmosis. The recipient of this infected donor organ developed brain abscesses and encephalitis due toToxoplasma gondii. His toxoplasmic brain disease responded to many months of treatment with pyrimethamine, sulfadiazine and leucovorin demonstrating this infection could be treated in such immune compromised persons when treatment was continued throughout the immune compromise until resolution of brain involvement. [DOI] [PubMed]
- 95.Levin M, Mcleod R, Young Q, Abrahams C, Chambliss M, Walzer P, Kabins SA. Pneumocystis pneumonia: importance of gallium scan for early diagnosis and description of a new immunoperoxidase technique to demonstrate Pneumocystis carinii. Am Rev Respir Dis. 1983;128(1):182–5 Initial patient with toxoplasmic encephalitis in the Midwest and eleventh in the United States with multiple opportunistic infections at the outset of the AIDS epidemic. His toxoplasmic encephalitis responded to treatment with pyrimethamine sulfadizune given with leucovorin. [DOI] [PubMed]
- 96.Dubey JP Schizogony and gametogony of oocyst-deficient T-263 strain of Toxoplasma gondi. Vet Parasitol. 2017;245:160-162. doi: 10.1016/j.vetpar.2017.05.024. T-263 cannot make life cycle stages beyond schizonts, male and female gametes. This chemically mutagenized parasite clone is an effective accine when administered orally in separate study eliminating endemic toxoplasmic infections on a pig farm in southern Illinois when administered to cats on this farm. Subsequent analysis of this clone provides a rosetta stone to merozoite development and capacitation preparing for Conception ofToxoplasma gondii(McLeod et al in submission, 2022). [DOI] [PubMed]
- 97.Ramakrishnan C, Maier S, Walker R, et al. An experimental genetically attenuated live vaccine to prevent transmission of Toxoplasma gondii by cats. Scientific Reports. 2019;9:1474 “RNA-Seq analysis of cat enteric stages ofT. gondiiuncovered genes expressed uniquely in microgametes and macrogametes. A CRISPR/Cas9 strategy was used to create aT. gondiistrain that exhibits defective fertilization, decreased fecundity and generates oocysts that fail to produce sporozoites. Inoculation of cats with this engineered parasite strain totally prevented oocyst excretion following infection with wild-typeT. gondii, demonstrating that this mutant is an attenuated, live, transmission-blocking vaccine.” Description from reference 97. [DOI] [PMC free article] [PubMed]
- 98.Alvarez C, de-la-Torre A, Vargas M, Herrera C, Uribe-Huertas LD, Lora F, Gómez-Marín JE. Striking Divergence in Toxoplasma ROP16 Nucleotide Sequences From Human and Meat Samples. JID. 2015;211(15 June):2006. doi: 10.1093/infdis/jiu833. [DOI] [PubMed] [Google Scholar]
- 99.••Hutson SL, Mui E, Kinsley K, Witola WH, Behnke MS, El Bissati K, Muench SP, Rohrman B, Liu SR, Wollmann R, Ogata Y, Sarkeshik A, Yates JR 3rd, McLeod R T. gondii RP promoters & knockdown reveal molecular pathways associated with proliferation and cell-cycle arrest. PLoS One. 2010;5:e14057 Gi arrested tetracycline regulated mutant RH strain parasite is a dormant persisting parasite when tetracycline regulating and leading to transcription of the the small ribosomal protein 13 (RPS13) gene is absent. This organism can recrudesce after very long times in tissue culture exceeding 6 months demonstrating effect of ribosomal stress secondary to protein starvation. This mutated parasite is a highly effective vaccine for mice. [DOI] [PMC free article] [PubMed]
- 100.Chu K-B, Quan F-S. Advances in Toxoplasma gondii Vaccines: Current Strategies and Challenges for Vaccine Development. Vaccines. 2021;9:413. 10.3390/vaccines9050413Excellent review of many current vaccine studies. [DOI] [PMC free article] [PubMed]
- 101.de Mourn L, Bahia-Oliveira G, Maria L, Wada MY, Jones JL, Tuboi SH, Carmo EH, Ramalho WM, Camargo NJ, Trevisan R, Graça RMT, da Silva AJ, Moura I, Dubey JP, Garrett DO. Waterborne Toxoplasmosis, Brazil, from Field to Gene. Emerg Infect Dis. 2006;12(2):326–329. doi: 10.3201/eid1202.041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.••Tan TG, Mui E, Cong H, Witola W, Montpetit A, Muench S, Sidney J, Alexander J, Sette A, Grigg M, Reed S, Maewal A, Kim S, Boothroyd J, McLeod R. Identification of T. gondii epitopes, adjuvants, & host genetic factors that influence protection of mice & humans. Vaccine. 2010;28:3977–89 Creation and understanding of vaccines foundational for creation of immunosense vaccines to preventToxoplasmainfection in humans. [DOI] [PMC free article] [PubMed]
- 103.••El Bissati K, Zhou Y, Paulillo S, Raman SK, Karch C, Roberts CW, Burkhard P, McLeod R. Novel protein nanovaccine confers robust immunity against Toxoplasma. Nature (NPJ) Vaccines. 2017;2:24. 10.1038/s41541-017-0024-6. eCollection 2017. Self assembling nanoparticle vaccine created using immunosense priciples creates a powerful vaccine tailored to protect humans but the principles can also incorporated full length proteins effective for immunizing other species. [DOI] [PMC free article] [PubMed]
- 104.••Zhou Y., Melo M, Weiss R, ElBissati K, Irvine D, McLeod R. RNA replicon vaccines protect against toxoplasmosis. Immunosense and SAG1 in a noel SAPN vaccine with a new Toxoplasma CD4 eliciting peptide. In preparation. 2022. RNA replicons created using immunosense principles effetively immunize againstT.gondiibut SAG1 is most critical when combined with RNA encoding the peptides, emphasizing importance of SAG1 as an immunogen in future vaccines.
- 105.••Zhou Y, El Bissati K, Reed S, Fox C, Alexander J, Sidney J, Sette A, Burkhard P, McLeod R. Adjuvanting designer SAPN vaccines with conventional adjvuants demonstrates superiority of GLA-SE and inability to combine the adjuvant CPG and GLA-SE. In preparation, 2022. Designer vaccines to protect humans against toxoplasmosis cannot be adjuvanted with cpg and gla-se togegether but are effective with one or the other adjuvants.
- 106.••Waldman, B.S., Schwarz, D., Wadsworth, M.H., Saeij, J.P.,Shalek, A.K., Lourido, S., 2019. Identification of a master regulator of differentiation in Toxoplasma. BioRxiv660753. Available from: 10.1101/660753. Regulator of bradyzoite stage switch discovered. [DOI] [PMC free article] [PubMed]
- 107.••Elbissati K, Raczy M, ZhouY, Weber C, Solaki A, Reed S, Fox C, Alexander J, Sidney J, Sette A, Burkhard P, Hubbel J, McLeod R. In preparation 2022. Creation of a “plug ad play” strategy to create designer vaccines to protect humans and other species. GLA-se and immunosense SAPN create a potent vaccine against toxoplasmosis in HLA transgenic mice.
- 108.••Chalal JS, et al Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondiichallenges with a single dose. 2016. 10.1073/pnas.1600299113. Venezuelan Equine Virus (VEEV)- based modified dendrimer nanoparticle RNA (MDNP RNA) vaccine protects against death due to Pru with HGXPRT strainToxoplasma gondii. RNA replicons encoding full length SAG1, SAG2A, AMA1, GRA6, ROP2A, and ROP18, protect C57Bl6 mice for 175 days post challenge against this strain when replicon is incorporated in polyethylene glycol lipid nanoparticle.
- 109.••Lai, B.-S., Witola, W.H., Bissati, K., El Zhou, Y., Mui, E. et al., 2012. Molecular target validation, antimicrobial delivery, and potential treatment of Toxoplasma gondii infections. Proc Natl Acad Sci USA. 109;(35):1418214187. Peptide or vivo morpholinos targeting essential parasite molecules such as DHFR, ENR, and Apetela 2 transcription factors can treatToxoplasmainfection. [DOI] [PMC free article] [PubMed]
- 110.Tipparaju SK, Muench SP, Mui EJ, Ruzheinikov SN, Lu JZ, Hutson SL, Kirisits MJ, Prigge ST, Roberts CW, Henriquez FL, Kozikowski AP, Rice DW, Mcleod RL. Identification and development of novel inhibitors of Toxoplasma gondii enoyl reductase. J Med Chem. 2010;53:6287–6300. doi: 10.1021/jm9017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mazumdar J, Wilson HE, Masek K, Hunter AC, Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc Natl Acad Sci USA. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Welsch ME, Zhou J, Gao Y, Yan Y, Porter G, Gautam Agnihotri G, Li Y, Lu H, Chen Z, Thomas SB. Discovery of Potent and Selective Leads against Toxoplasma gondii Dihydrofolate Reductase via Structure-Based Design ACS. Med Chem Lett. 2016;7(12): 1124–1129. Published online 2016. 10.1021/acsmedchemlett.6b00328. TargetingToxoplasmaDHFR creates a potent inhibitor ofT.gondiitachyzoites currently held by Vyera. [DOI] [PMC free article] [PubMed]
- 113.Mui EJ, Schiehser GA, Milhous WK, Hsu H, Roberts CW, Kirisits M, Muench S, Rice D, Dubey JP, Fowble JW, Rathod PK, Queener SF, Liu SR, Jacobus DP, McLeod R. Novel Triazine JPC-2067-B Inhibits Toxoplasma gondii In Vitro and In Vivo. PLoS Negl Trop Dis. 2008;2:e190. doi: 10.1371/journal.pntd.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Samuel BU, Hearn B, Mack D, Wender P, Rothbard J, Kirisits MJ, Mui E, Wernimont S, Roberts CW, Muench SP, Rice DW, Prigge ST, Law AB, McLeod R. Delivery of antimicrobials into parasites. Proc Natl Acad Sci U S A. 2003;100:14281–14286. doi: 10.1073/pnas.2436169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou Y, Fomovska A, Muench S, Lai BS, Mui E, Mcleod R. Spiroindolone that inhibits PfATPase4 is a Potent, Cidal Inhibitor of Toxoplasma gondii tachyzoites in vitro and in vivo. Antimicrob Agents Chemother. 2014;58(3):1789–1792. doi: 10.1128/AAC.02225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fomovska A, Huang Q, El Bissati K, Mui EJ, Witola WH, Cheng G, Zhou Y, Sommerville C, Roberts CW, Bettis S, Prigge ST, Afanador GA, Hickman MR, Lee PJ, Leed SE, Auschwitz JM, Pieroni M, Stec J, Muench SP, Rice DW, Kozikowski AP, McLeod R. Novel N-benzoyl-2-hydroxybenzamide disrupts unique parasite secretory pathway. Antimicrob Agents Chemother. 2012;56:2666–2682. doi: 10.1128/AAC.06450-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.••Smith D et al. Toxoplasma TgATG9 is critical for autophagy and long-term persistence in tissue cysts. eLife. 2021. Autophagy protein TgATG9 is critical for long term persistence ofT.gondiicysts. [DOI] [PMC free article] [PubMed]
- 118.••Huang KI, et al. CD8 T cells cry for help. Front Cell Infect Microbiol. 9:136. 10.3389/fcimb.2019.00136. BLIMP regulates 41BB mediated exhaustion of T cells that can be corrected by agonist antibody to 41BB. This eliminates encysted organisms. This then could make it possible to eliminate remaining tahyzoites by waking up protective immunologic mechanisms and targeting tachyzoites.
- 119.••Souza SP, Splitt SD, Alvarez JA, Wizzard S, Wilson JN, Luo Z, Baumgarth N, Jensen. KDC NFkBid is required 1 for immunity and antibody responses to Toxoplasma gondii bioRxiv preprint. 10.1101/2020.06.26.174151. A “collaborative cross” of mice demonstrates that hypervirulent South American parasite escapes protective immunological mechanisms effective against Type I,II,III parasites. Bumble mice with a difference in NFkBid also are not protected. These studies demonstrate the importance of NKcells and specific isotypes of antibody in protection against toxoplasmosis.
- 120.Borrmann S, et al. Fosmidomycin plus Clindamycin for Treatment of Pediatric Patients Aged 1 to 14 Years with Plasmodium falciparum Malaria. Antimicrob Agents Chemother. 2006;50(8):2713–8. 10.1128/AAC.00392-06Inhibitor of mevalonate pathway discovered by Jomaa and Soldati inhibitsToxoplasmaandPlasmodia. When administered with clindamycin -a delayed acting inhibitor is effective but has hematologic toxicity in humans. [DOI] [PMC free article] [PubMed]
- 121.Dimier-Poisson I et al. Mic1-3 Knockout of Toxoplasma gondii Is a Successful Vaccine against Chronic and Congenital Toxoplasmosis in Mice. Mic1-3 Knockout of Toxoplasma gondii Is a Successful Vaccine against Chronic and Congenital Toxoplasmosis in Mic October 2006. J Infect Dis. 194(8):1176-83. 10.1086/507706. Knockout of microneme proteins creates lie attenuate organism that functions well to protect mice against toxoplasmosis including congenital infection. [DOI] [PubMed]
- 122.••Dimier-Poisson I, Carpentier R, Nguyen T, Dahmani F, Ducournau C, Betbeder D. Prtous nanoparticle as delivery system of complex antigens for an effective vaccine against acute and chroni Toxoplasma gondii infection. Biomaterials. Remarkable formulation of RH parasite lysate in a nanoparticle administered intranasally protects sheep and primates in a zoo ending an epidemi of deaths due to toxoplasmosis in susceptible new world primate colonies in zoos across France creates a vaccine capable of protecting multiple species of mammals. Remarkable protection with practical proof in French zoos for primates.
- 123.El Bissati K, et al. Adjuvanted multi-epitope vaccines protect HLA-A*11:01 transgenic mice against Toxoplasma gondii. JCI Insight. 2016;1(15):e85955. doi: 10.1172/jci.insight.85955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lourido S, Jeschke GR, Turk BE, Sibley LD. Exploiting the unique ATP-binding pocket of toxoplasmacalcium-dependent protein kinase 1 to identifyits substrates. ACS Chem Biol. 2013;8(6):1155–1162. doi: 10.1021/cb400115y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karanovic D, Michelow IC, Hayward AR, DeRavin SS, Delmonte OM, Grigg ME, Dobbs AK, Niemela JE, Stoddard J, Alhinai Z, Rybak N, Hernandez N, Pittaluga S, Rosenzweig SD, Uzel G, Notarangelo LD. Disseminated and Congenital Toxoplasmosis in a Mother and Child with Toxoplasma Infections in Primary Immunodeficiencies. Front Immunol. 2019;10:77. doi: 10.3389/fimmu.2019.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.••Naranjo-Galvis CA, de-la-Torre A, Mantilla-Muriel LE, Beltrán-Angarita L, Elcoroaristizabal-Martín X, McLeod R, Alliey-Rodriguez N, Begeman IJ, López de Mesa C, Gómez-Marín JE, Sepúlveda-Arias JC. Genetic Polymorphisms in Cytokine Genes in Colombian Patients with Ocular Toxoplasmosis. Infect Immun. 2018;86(4):e00597-17. 10.1128/IAI.00597-17. Print 2018 Apr. PMID: Identifies importance of cytokine genes in protection against toxoplasmosis in Colombia. [DOI] [PMC free article] [PubMed]
- 127.••Perce-da-Silva DdeS, Thays EJ, Aleixo QdoC, Motta J, Ribeiro-Alves M, Oliveira Ferreira Jde, Porto CdeMSL, Banic DM, Reis AMR. Influence of Activating and Inhibitory Killer Immunoglobulin-Like Receptors (KIR) genes on the recurrence rate of ocular toxoplasmosis in Brazil. BioRx. 10.1101/2020.09.16.299446. Identifies natural killer cell receptors in protection against recurrence of ocular toxoplasmosis in humans in Brazil.
- 128.••Roberts F, Roberts CW, Johnson JJ, Kyle DE, Krell T, Coggins JR, Coombs GH, Milhous WK, Tzipori S, Ferguson DJ, Chakrabarti D, McLeod R. Evidence for the shikimate pathway in apicomplexan parasites. Nature. 1998:393–801-5 Plant-like metabolic pathways in apicomplexan parasites can be targeted. Apicomplexan parasites are mosaics of metabolic pathways identifying unique ways to target organellar and cytoplasmic pathways. [DOI] [PubMed]
- 129.••Zuther E, Johnson JJ, Haselkorn R, McLeod R, Gornicki P. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc NatL Acad Sci U S A. 1999;96:13387-92. Targetting the plant -like acetyl-CoA carboxylase with herbicides known to inhibit this enzyme in weeds identifies two classes of small molecules capable of inhibiting apicomplexan parasites through targeting enzymes stored in the chloroplast like organelle inherited from an ancient progenitor Copydelia demonstrating this plant-like endosymbiotic organelle can be targeted. This suggests the possibility of targeting other unique organellar constituents and phylogenetically genetically distinct molecules including plant-like transcription factors as in McPhillie reference above as “Achilles heels” where these parasites are susceptible to unique methods of inhibiting these parasites. [DOI] [PMC free article] [PubMed]
- 130.Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordón C, Charman SA, Katneni K, Schultz T, Burrows JN, Hinrichs DJ, Meunier B, Carruthers VB, Riscoe MK. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A. 2012;109:15936–15941. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.••Kortagere S, Mui E, McLeod R, Welsh WJ. Rapid discovery of inhibitors of Toxoplasma gondii using hybrid structure-based computational approach. J Comput Aided Mol Des. 2011; 25:403–411. 10.1007/s10822-011-9420-6. Targeting Myosin in Toxoplasma and using a computational approach with medicinal chemistry creates an inhibitor ofToxoplasmatachyzoites. [DOI] [PubMed]
- 132.Yang S, Parmley SF. Toxoplasma gondii expresses two distinct lactate dehydrogenase homologous genes during its life cycle in intermediate hosts. Gene. 1997;184:1–12. doi: 10.1016/S0378-1119(96)00566-5. [DOI] [PubMed] [Google Scholar]
- 133.Dando C, Schroeder ER, Hunsaker LA, Deck LM, Royer RE, Zhou X, Parmley SF, Vander Jagt DL. The kinetic properties and sensitivities to inhibitors of lactate dehydrogenases (LDH1 and LDH2) from Toxoplasma gondii: comparisons with pLDH from Plasmodium falciparum. Mol Biochem Parasitol. 2001;118:23–32. doi: 10.1016/S0166-6851(01)00360-7. [DOI] [PubMed] [Google Scholar]
- 134.••El Bissati K, Zhou Y, Paulillo SM, Raman SK, Karch CP, Reed S, Estes A, Estes A, Lykins J, Burkhard P, McLeod R. Engineering and characterization of a novel Self Assembling Protein for Toxoplasma peptide vaccine in HLA-A*11:01, HLA-A*02:01 and HLA-B*07:02 transgenic mice. Scientific Reports (Nature) September 2020. 10:16984. Online 2020 Oct 12. 10.1038/s41598-020-73210-0. Potent imunosense protein self-assembling nanoaccine adjuvanted with GLA-SE protects HLA transgenic mice demonstrating effect of peptides where genetic restriction demonstrates protection is operative for three HLA Class I supermotifs. [DOI] [PMC free article] [PubMed]
- 135.Del Valle Mojica C, Montoya JG, McGuire J, Palma KL, Shekdar KV, McLeod R … et al. Late diagnosis of congenital toxoplasmosis with macrocephaly in two dizygotic twins after incidental detection of leukocoria: a case report. J Peds Infect Dis. 2021. Late symptomatic infection is treated medically in dizygotic twins. [DOI] [PMC free article] [PubMed]
- 136.••Mack DG, McLeod R, Stark B. R NAse P of T. gondii. Mack D, McLeod R, Stark BC. Characterization of ribonuclease P from Toxoplasma gondiiEuropean Journal of Protistology. 32:96-103. 10.1016/S0932-4739(96)80044-7. RNA enzymatic activity inToxoplasma.
- 137.••Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, Sette A, Maewal A, El Bissati K, Zhou Y, Suzuki Y, Lee D, Woods S, Sommerville C, Henriquez FL, Roberts CW, McLeod R. Toxoplasma gondii HLA-B*0702-restricted GRA7(20-28) peptide with adjuvants and a universal helper T cell epitope elicits CD8(+) T cells producing interferon-γ and reduces parasite burden in HLA-B*0702 mice. Hum Immunol. 2012;73:1–10. Self assembling nanoparticle containing GRA7(peptide 20-29) elicits a protective immune response when administered with GLA-SE to HLA B0702 transgenic mice. [DOI] [PMC free article] [PubMed]
- 138.••Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, Sette A, Maewal A, McLeod R. Human immunome, bioinformatic analyses using HLA supermotifs and the parasite genome, binding assays, studies of human T cell responses and immunization of HLA-A*1101 transgenic mice including novel adjuvants provide a foundation for HLA-A03 restricted CD8+T cell epitope based, adjuvanted vaccine protective against Toxoplasma gondii. Immunome Res. 2010;6:12. Self assembling nanoparticle-peptides elicit protective immunity in HLA-A*1101 transgenic mice. [DOI] [PMC free article] [PubMed]
- 139.••Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, Sette A, Maewal A, McLeod R. Towards an immunosense vaccine to prevent toxoplasmosis: protective Toxoplasma gondii epitopes restricted by HLAA*0201. Vaccine. 2011;29:754–62. GLA-SE and HLA02 binding-peptides elicit protective immunity in HLA-A*0201 transgenic mice. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1.01 MB)
(PDF 498 kb)