Abstract
Heart failure (HF) with preserved ejection (HFpEF) constitutes a large and growing proportion of patients with HF around the world, and is now responsible for more than half of all HF cases in ageing societies. While classically described as a condition of elderly, hypertensive women, recent studies suggest heterogeneity in clinical phenotypes involving differential characteristics and pathophysiological mechanisms. Despite a paucity of disease-modifying therapy for HFpEF, an understanding of phenotypic similarities and differences among patients with HFpEF around the world provides the foundation to recognise the clinical condition for early treatment, as well as to identify modifiable risk factors for preventive intervention. This review summarises the epidemiology of HFpEF, its common clinical features and risk factors, as well as differences by age, comorbidities, race/ethnicity and geography.
Keywords: Heart failure with preserved ejection fraction, epidemiology, risk factors, comorbidities, cluster analysis, sex differences, prognosis
Heart failure (HF) with preserved ejection fraction (HFpEF) is well-recognised as a global public health problem. Its prevalence increases with age and elderly patients with HFpEF are known to have a high comorbidity burden. Common comorbidities associated with HFpEF include cardiovascular risk factors such as hypertension, diabetes, obesity and AF, as well as non-cardiovascular diseases such as chronic obstructive pulmonary disease (COPD) and chronic kidney disease. The high comorbidity burden is postulated to play a central pathophysiological role in HFpEF via systemic microvascular inflammation and coronary microvascular dysfunction, leading to myocardial stiffening, fibrosis and diastolic dysfunction.[1] Prior studies have described differences in prevalence, incidence and outcomes of HFpEF by sex, ethnicity and geography. More recently, cluster analyses have provided opportunities to identify groups of patients with specific combinations of these characteristics and comorbidities, postulated to represent patients with common pathophysiological mechanisms.[2–10]
This review aims to describe the epidemiology, common clinical features and phenotypic differences according to sex, ethnicity and geography that constitute the clinical syndrome of HFpEF. Throughout the review, it should be recognised that, even at the time of writing, the classification of HF and definition of HFpEF are changing.[11] Descriptions of the epidemiology and clinical features of patients will depend on the exact definition used. Numerous diagnostic algorithms have been proposed, a detailed discussion of which is beyond the scope of this review.[12–14] Instead, we broadly refer to HFpEF as the clinical syndrome of HF (manifested by typical symptoms and/or signs) caused by a structural and/or functional cardiac abnormality in the absence of overt reduction of left ventricular (LV) ejection fraction (LVEF), a broad definition most practically applied in epidemiological studies, and encompassing patients with HF and LVEF above 40%, 45% or 50% (depending on the study).
Prevalence and Incidence of HFpEF
Currently HF – regardless of LVEF – affects more than 64 million people worldwide.[15] The proportion of HFpEF among overall HF cases is further dependent on its definition and diagnostic precision as well as study settings. Community-based epidemiological studies and registries with LVEF assessments report the proportion of HFpEF anywhere between 19% and 55% of all HF cases (Table 1).[16–35]
Table 1: Proportion of HFpEF (out of Total Heart Failure Cases) in Community-based Studies and Registries.
| Study | Year Published | Years Conducted | Population Source | HFpEF Definition | Proportion of HFpEF (%) |
|---|---|---|---|---|---|
| Devereux et al.[16] | 2000 | 1993–1995 | Participants of Strong Heart Study | LVEF ≥55% | 53 (50/95) |
| Philbin et al.[17] | 2000 | 1995 and 1997 | Hospitalised patients for HF in 10 acute care community hospitals in New York, US | LVEF >50% | 24 (312/1,291) |
| Gottdiener et al.[18] | 2002 | 1989–1993 | Participants of Cardiovascular Health Study | LVEF ≥55% | 22 (60/269) |
| MacCarthy et al.[19] | 2003 | 1993–1995 | Participants of UK-HEART study | LVEF ≥50% | 31 (163/522) |
| Gustafsson et al.[20] | 2003 | 1993–1996 | Participants of DIAMOND-CHF | WMI >1.6 | 40 (2,218/5,491) |
| Redfield et al.[21] | 2003 | 1997–2000 | Randomly selected residents of Olmsted County, MN, US | LVEF ≥50% | 44 (20/45) |
| Lenzen et al.[22] | 2004 | 2000–2001 | Participants of EuroHeart Failure Survey | LVEF ≥40% | 46 (3,148/6,806) |
| Owan et al.[23] | 2005 | 1987–2001 | Hospitalised patients for decompensated HF at Mayo Clinic Hospitals in Olmsted County, Minnesota, US | LVEF ≥50% | 47 (2,167/4,596) |
| Bhatia et al.[24] | 2006 | 1999–2001 | Patients with HF admitted to 103 hospitals in Ontario, Canada | LVEF >50% | 31 (880/2,802) |
| Yancy et al.[25] | 2006 | 2001–2004 | Participants of ADHERE hospitalisation database | LVEF ≥40% | 50 (26,322/52,187) |
| Bursi et al.[26] | 2006 | 2003–2005 | Residents of Olmsted County, MN, US with incident or prevalent HF | LVEF ≥50% | 55 (308/556) |
| Gheorghiade et al.[27] | 2007 | 2003–2004 | Participants of OPTIMIZE-HF | LVEF ≥40% | 51 (21,149/41,276) |
| Lee et al.[28] | 2009 | 1981–2004 | Participants of Framingham Heart Study | LVEF >45% | 41 (220/534) |
| Ho et al.[29] | 2013 | 1981–2008 | Participants of Framingham Heart Study | LVEF >45% | 43* (196/457) |
| Gurwitz et al.[30] | 2013 | 2005–2008 | Patients with newly diagnosed HF from four sites from Cardiovascular Research Network | LVEF ≥50% | 52* (6,210/11,994) |
| Brouwers et al.[31] | 2013 | 1997–2010 | Participants of PREVEND study | LVEF ≥50% | 34* (125/374) |
| Cheng et al.[32] | 2014 | 2005–2011 | Participants of GWTG-HF registry | LVEF ≥50% | 47 (18,897/40,239) |
| Gerber et al.[33] | 2015 | 2000–2010 | Residents of Olmsted County, MN, US | LVEF ≥50% | 53* (1,089/2,074) |
| Alehagen et al.[34] | 2015 | 2000–2012 | Participants of Swedish Heart Failure Registry | LVEF ≥50% | 19 (9,140/46,959) |
| Ho et al.[35] | 2016 | 1979–2002 | Pooled data of four community-based cohorts (Framingham Heart Study, Cardiovascular Health Study, PREVEND, MESA) | LVEF >45% | 48* (909/1,891) |
*Based on incident rates. HF = heart failure; HFpEF = heart failure with preserved ejection fraction; LVEF = left ventricular ejection fraction; WMI = wall motion index. Adapted from Dunlay et al. 2017.[81] Used with permission from: Nature Publishing Group.
Trends in the prevalence and incidence of HFpEF over time are challenging to capture amid the evolution of its diagnostic criteria. Most epidemiological studies on trends report the proportion of total HF that constituted HFpEF over time. A study from Olmsted County in Minnesota showed a gradual increase in the proportion of HFpEF hospitalisation out of total HF hospitalisations from the late 1980s to early 2000s.[23] Participants in the Framingham Heart Study over three decades showed an increasing trend in the proportion of HF constituting HFpEF (LVEF ≥50%), from 41% in 1985–1994 to 56% in 2005–2014.[36] The trend in incidence of new-onset HFpEF is unclear, with some reporting no change or even a decline over the past decade, and others reporting an increase in the newly diagnosed cases of HF primarily driven by an increase in population size and age. A community-based study from Olmsted County reported a decline in the incidence of HFpEF from 2000 to 2010.[33] Conversely, a study of the Framingham Heart Study and Cardiovascular Health Study reported an increased trend in incident HFpEF over the two decades, from 4.7 per 1,000 persons in 1990–99 to 6.8 per 1,000 persons in 2000–09, standardised to age- and sex-specific 2010 (age 60–95 years) US population rates.[37]
To summarise, while the trend in absolute incidence of HFpEF is unclear, it is clear that HFpEF is constituting an increasing proportion of incident HF over the years. The prevalence of HFpEF, as a proportion of all HF, has also clearly been increasing over the past two decades. Population ageing, the increasing prevalence of HFpEF-related risk factors and comorbidities, heightened awareness and improvement in diagnostic precision as well as increased survival all potentially contribute to this increasing prevalence over time.
Clinical Features of HFpEF
Risk Factors and Comorbidities
The importance of risk factors and comorbidities in HFpEF is recognised in the strong (Class I) recommendation in the 2021 European Society of Cardiology guidelines to screen for and treat the aetiologies – as well as cardiovascular and non-cardiovascular comorbidities – in patients with HFpEF.[38] Cardiovascular risk factors and comorbidities include hypertension, AF, diabetes and obesity, whereas non-cardiovascular factors include chronic kidney disease and COPD.
Hypertension
Hypertension is a major risk factor for HFpEF. Among patients with HFpEF, the prevalence of hypertension ranges between 55% and 90%.[24,25,28] Consequently, appropriate blood pressure control remains central in the clinical management of hypertensive patients with HFpEF.[38] Hypertension causes LV hypertrophy, LV diastolic dysfunction, LV fibrosis, left atrial dilatation and macrovascular and microvascular stiffening, all of which are central and peripheral mechanisms involved in progression to HFpEF. A systemic proinflammatory state may also be triggered by hypertension together with other common comorbidities, contributing to HFpEF pathophysiology.[1]
Atrial Fibrillation
AF is a well-known risk factor and prognostic indicator in HFpEF. Many population-based and registry data report a high prevalence of AF in HFpEF, ranging from 15% to 40%, depending on the studied cohort and how AF was ascertained.[39] Importantly, AF is not only a comorbidity of HFpEF, but AF and HFpEF are also risk factors for each other. A study from Olmsted County found that among 450 patients with a new diagnosis of HFpEF, 32% developed AF during the median follow-up of 3.7 years, which corresponds to 69 cases per 1,000 patient-years.[40] Notably, diagnosis of HF can be complicated when AF coexists because both conditions share common clinical manifestations such as similar symptoms and elevated B-type natriuretic peptides. Hence, the diagnosis of HFpEF is challenging in the presence of AF. Finally, AF is an independent prognostic factor for mortality, HF hospitalisation and stroke or transient ischaemic attack in HFpEF.[41]
Diabetes
Diabetes is another important risk factor in patients with HFpEF. In the CHARM programme, 40% of patients with HFpEF had a diagnosis of diabetes at enrolment and another 22% were prediabetic with haemoglobin A1c between 6.0% and 6.4%.[42] Epidemiological studies also report that one-third of patients with HFpEF have a diagnosis of diabetes.[39] Oxidative stress, vascular inflammation and endothelial dysfunction play central roles in the pathophysiology of the development of HFpEF. In patients with diabetes, oxidative stress occurs via several mechanisms, increasing the production of reactive oxygen species (e.g. superoxide, hydrogen peroxide and hydroxyl radicals). Oxidative stress also reduces nitric oxide bioavailability, resulting in vascular inflammation and endothelial dysfunction. The endothelial dysfunction in HFpEF extends beyond peripheral endothelium to central cardiac endothelium consisting of coronary vessels, intramyocardial capillaries and intracardiac endocardium.[43]
The sodium–glucose cotransporter 2 inhibitors (SGLT2Is) canagliflozin, dapagliflozin, empagliflozin, ertugliflozin and sotagliflozin are recommended for patients with type 2 diabetes at risk of cardiovascular diseases to prevent cardiovascular events including hospitalisation for HF.[40] A meta-analysis of six trials investigating the efficacy of SGLT2Is for cardiovascular and kidney outcomes in patients with diabetes reported a 22% reduction of HF and cardiovascular hospitalisation by SGLT2Is.[44] Furthermore, dapagliflozin, empagliflozin and sotagliflozin are recommended in patients with HF with reduced ejection fraction (HFrEF) irrespective of the presence of type 2 diabetes.[38] The recommendation of SGLT2Is for patients with HFpEF varies by guidelines as the EMPEROR-Preserved trial showed the efficacy of empagliflozin in reducing the composite of cardiovascular death or HF hospitalisation by 21% in HFpEF, regardless of diabetic status.[45] Intensive promotion of diuresis and natriuresis without sympathetic activation is widely acknowledged as one of therapeutic mechanisms of SGLT2Is, but the full picture remains to be understood.
Obesity
Obesity and HFpEF share a complex relationship. Nearly 80% of patients with HFpEF are overweight or obese and obesity is a major risk factor of incident HFpEF.[46] A consortium of four large community cohorts (the Cardiovascular Health Study, PREVEND, Framingham Heart Study and MESA) assessing 22,681 individuals showed that every 1 SD increase in BMI was associated with 34% increase in incident HFpEF, consistent with other community studies.[23,29,47] The obese-HFpEF phenotype is increasingly recognised as a distinct inflammatory HF phenotype in which volume overload, pericardial restraint and reduced venous capacitance may also play a role.[48–50] Elevated epicardial and visceral fat are strongly associated with higher risk of HFpEF compared with elevated subcutaneous fat.[51] Of note, natriuretic peptide levels are lower in obese than non-obese patients with HFpEF and are frequently below standard diagnostic cut-off values.[52] Additionally, obese patients with HF are paradoxically protected against adverse outcomes compared with lean or underweight patients with HF, a phenomenon known as the obesity paradox[53] This paradox often does not hold true when other anthropometric indices of obesity (i.e. waist circumference, waist–to–hip ratio, body composition measures) are used, highlighting the shortcomings of BMI as a metric of adiposity.[53] Unfortunately, obese individuals have been systematically excluded from HF trials. More studies are needed to understand other pathophysiological pathways with which obesity may be robustly associated.
Chronic Kidney Disease
Patients with renal dysfunction and HFpEF constitute a particularly high-risk phenotype.[2] Depending on the adopted diagnostic criteria used and populations studied, the prevalence of renal disease is generally reported to range between 26% and 49% in HFpEF.[25] Studies on the association between renal dysfunction and the onset of incident HFpEF are limited, but it is reported that about one-third of those with excessive urinary albumin excretion from a community-based cohort were found to develop HFpEF over a median follow-up of 11.5 years.[31] Chronic kidney disease is also an independent prognostic factor for HFpEF, where mortality increases as estimated glomerular filtration rate decreases.[54] Worsening of renal function in HFpEF is commonly seen, and it is associated with increased risk of cardiovascular death or HF hospitalisation.[55]
The coexistence of HF and chronic kidney disease, commonly termed as cardiorenal syndrome, is common in HFpEF.[56] Cardiorenal syndrome is a bi-directional impairment of the heart and kidneys in which dysfunction in one organ may induce acute or chronic dysfunction in the other organ. It represents the convergence of heart–kidney interactions across several interfaces. There are multiple biological mechanisms driving cardiorenal syndrome. Elevated right atrial pressure due to increased sodium and fluid retention, renal anaemia, uremic toxin, and stimulation of renin–angiotensin–aldosterone and sympathetic systems are the common renal elements influencing cardiac function. Conversely, decreased kidney perfusion due to inadequate stroke volume and increased central venous pressure because of right HF are responsible for renal impairment. Common features such as endothelial dysfunction, inflammation and systemic and renal fibrosis are mutual consequences of diabetes, hypertension and dyslipidaemia, which can also be drivers of cardiorenal syndrome.
Other Risk Factors and Aetiologies
Other important risk factors and comorbidities of HFpEF include COPD, iron deficiency and anaemia. Although the prevalence of these risk factors may be lower than the others, they can be potential therapeutic targets because of their strong impact on the progression and outcomes of HFpEF. COPD plays a key role in the HFpEF paradigm, inducing proinflammatory state and triggering subsequent cascades leading to myocardial structural and functional alterations.[1] Iron deficiency (with or without anaemia) is highly prevalent in HFpEF and is associated with reduced effort tolerance and worse outcomes.[57] Anaemia is associated with a higher risk of death from malignancy, sudden cardiac death and aborted cardiac arrest in HFpEF.[58]
Recently, attention has turned to specific aetiologies that may masquerade as the clinical syndrome of HFpEF. As opposed to primary or ‘garden variety’ HFpEF related to conventional risk factors and comorbidities, specific secondary causes of the HFpEF syndrome include infiltrative restrictive cardiomyopathy (amyloidosis and Fabry disease) and restrictive cardiomyopathy related to endomyocardial fibrosis, radiation therapy, iron overload, toxicity due to recreational substance abuse, exposure to heavy metals and medications such as chloroquine, ergotamine, cytostatic drugs and immunomodulating drugs.[10] Such specific aetiologies are important to detect because effective treatments are available, for example tafamidis for cardiac amyloidosis.[59]
HFpEF Phenotypes
Novel analytical techniques, such as hierarchical clustering and latent class analysis, have been used to identify mutually exclusive data-driven HFpEF phenotypes. Clustering approaches and analysed features vary across studies (Supplementary Material Table 1).[2–9] Figure 1 shows that five phenotypes are commonly identified. A diabetes and obesity phenotype (gray in Figure 1) is most commonly identified and is highly prevalent in up to 30% of patients in some studies.[2,3,7] A phenotype AF and chronic kidney disease (blue) is also prevalent in most studies, with the worst outcomes in four out of eight studies. A phenotype younger patients with milder HF symptoms with fewer comorbidities (dark purple) is also prevalent in almost all studies. This phenotype had a better outcome than other phenotypes in most of the studies. The presence of AF characterised three out of the five common phenotypes (primarily men with AF [green], AF and chronic kidney disease with the worst outcome [blue] and elderly frail women with AF [light purple]) in eight studies. These data highlight the importance of AF and age in the phenotype of patients with HFpEF. The clinical utility of these HFpEF phenotypes has not been investigated in a prospective study. Future studies should focus on identifying phenotype-specific pathophysiological processes that can serve as treatment targets.
Figure 1: Common HFpEF Phenotypes Seen in Studies of Clustering Analysis.
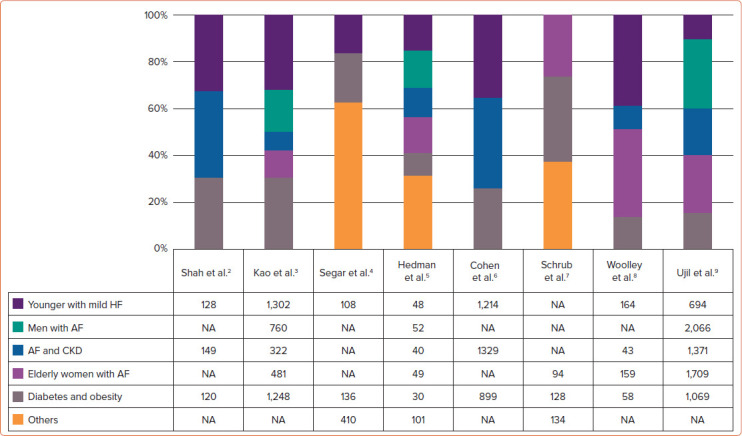
These common phenotypes are determined and classified by the authors based on common features found in each phenotype from eight major studies of clustering analysis in patients with HFpEF. (Refer to Supplementary Material Table 1 for detailed features of each cluster.) Numbers in the table represent the number of patients in each of the common phenotypes in each study. CKD = chronic kidney disease; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; NA = not applicable.
Accumulating evidence supports the existence of the inflammatory–metabolic phenotype of HFpEF, characterised by increased inflammatory biomarkers, accumulated epicardial fat and endothelial dysfunction, triggering myocardial inflammation and the subsequent derangement in cardiac function independent of other traditional risk factors like coronary artery disease.[60] Obesity, diabetes and metabolic syndrome, as well as systemic inflammatory disorders including rheumatoid arthritis and psoriasis, are associated with this inflammatory–metabolic phenotype. Women are more prevalent in this inflammatory–metabolic phenotype, owing to a greater susceptibility to haemodynamic and inflammatory stress as well as a higher preponderance for systemic inflammatory diseases. Recognition of the inflammatory–metabolic phenotype in HFpEF is critical as observational studies highlight the importance of rigorous treatments for these underlying systemic diseases.[61–63]
HFpEF by Age
Traditionally HFpEF has been perceived as a disease of the elderly. Studies from the late 1990s to early 2000s demonstrate an age-dependent increase in the prevalence of HFpEF. Several community-based studies and registries showed that ageing is one of the strongest risk factors of HFpEF.[18,26] An analysis of the PREVEND study showed that older age was strongly associated with new onset of HFpEF (HR 2.53; 95% CI [1.93–3.30], per 10 years).[31] In a recent individual participant data analysis of PREVEND, Framingham Heart Study and the MESA cohort suggested that common risk factors for HF, including hypertension, smoking and obesity, explained a greater proportion of the risk for future HFpEF in younger than older individuals.[64]
Studies in patients with prevalent HFpEF show important differences in risk factors and outcomes of patients with HFpEF. Particularly in Asia, up to 15% of patients are considered young (<55 years).[65] A post-hoc analysis of data from TOPCAT, I-Preserve and CHARM-Preserved showed a higher risk of cardiovascular death, particularly from sudden death in younger patients despite less comorbidities.[66] Together, these results highlight the significant burden and unique risk factor patterns of younger patients with HFpEF. Results from population studies suggest that common risk factors explain a larger proportion of the risk for incident HF in younger than older individuals. Therefore, preventative measures early in life in high-risk individuals might prove benefits to reduce the burden of HFpEF.
Sex Differences
Sex differences can be found in many aspects of HFpEF including definitions, prevalence, incidence, risk factors, haemodynamics, common pathophysiological mechanisms and outcomes. With the paucity of sex-specific studies, our knowledge is largely limited to differences in the prevalence and incidence of HFpEF. Women predominate in HFpEF.[16,26,30] Among incident HF cases between 2000 and 2010 in Olmsted County, the proportion of HFpEF increased over time (from 48% in 2000–2003 to 52% in 2008–2010), with women outnumbering men by 2:1.[33] Interestingly, the lifetime risk of HFpEF is reported to be similar between sexes, but women have a higher lifetime risk of HFpEF compared that of HFrEF.[67] Furthermore, women experienced less decline in the incidence of HFpEF compared to HFrEF over 10 years (-27 versus -61%, respectively).[33] Sex differences in the prevalence of risk factors of HFpEF differ depending on the studied population and its settings.
However, there are important differences in how risk factors common to both sexes confer risk differently. For example, women are more prone to augmented arterial pressure (i.e. hypertension) due to smaller vasculature and poorer diastolic reserve, resulting in greater arterial stiffness and more concentric LV hypertrophy. In a cohort of elderly patients with HFpEF in PURSUIT-HFpEF, female sex was independently associated with the presence of diastolic dysfunction and worse clinical outcomes.[68] While ischaemic heart disease and AF are more prevalent in men than women, obesity is more prevalent in women than men with higher risk of developing HFpEF.[47] Together with presence of diabetes, women are more predisposed to substantiate the inflammatory paradigm of HFpEF.[1,47]
Sex differences can also be found in common pathophysiological mechanisms of HFpEF where endothelial inflammation and microvascular dysfunction may be triggered by several female-specific risk factors, such as neurogenically triggered coronary microvascular dysfunction (i.e. takotsubo cardiomyopathy), peripartum cardiomyopathy, metabolic and autoimmune disorders (i.e. rheumatoid arthritis and systemic lupus erythematosus) and radiotherapy-induced cardiomyopathy for breast cancer treatment.[69] Women are more predisposed to systemic and pulmonary endothelial impairment that drive ventricular vascular uncoupling, also an important component of pathophysiological mechanism of HFpEF.
The prognosis of HFpEF is generally similar between sexes. However, recent findings from clinical trials of neurohormonal agents suggest potential benefit of candesartan, spironolactone and sacubitril/valsartan across a wider LVEF spectrum in women compared to men with HFpEF.[70] The recent investigation also challenges the lower threshold of LVEF for HFpEF, i.e. the LVEF cut-off point for normal LVEF and hence to define HFpEF, may be higher for women, the elderly and in some racial/ethnic groups.[7][1]
Racial/Ethnic and Geographical Differences
Regional variations are complexed, intertwined with racial/ethnicity factors and management gaps across different regions. Figure 2 illustrates regional variations of clinical characteristics and comorbidity burdens based on three major international HFpEF trials: I-Preserve, CHARM-Preserved and PARAGON-HF.[72,73] Patients from North America had a very high prevalence of diabetes and obesity and were at high risk of HF hospitalisation or cardiovascular death.[73] Patients from Latin America in the I-Preserve and PARAGON-HF trials were more likely to be women than those from other regions and had a relatively high prevalence of hypertension. Patients from western Europe were often older with a high prevalence of AF and modest risk of HF hospitalisation. Patients from eastern Europe were the youngest with a high prevalence of ischaemic heart disease, but had fewer coronary interventions. Those from Asia were significantly less obese yet with modest prevalence of diabetes with a high risk of HF hospitalisation. Importantly, these findings are based on very limited input including only the trial-based data, yet it appears that regional differences partly can be characterised by patient background and specific patterns of comorbidities. There is a paucity of data from African and Middle-Eastern countries.
Figure 2: Regional Characteristics of Patients with HFpEF.
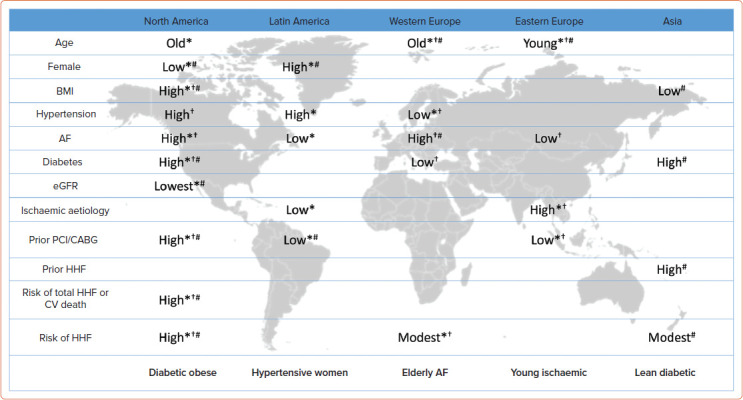
Demographics and relative prevalence of comorbidities and outcomes (high/low relative to the average of overall patients in trials) across geographical area from three major international clinical trials with HFpEF (I-Preserve,[72] CHARM-Preserved[72] and PARAGON-HF[73]) are presented. Note that regional characteristics of patients from Asia were not available from I-Preserve and CHARM-Preserved, and those from Latin America were not available from CHARM-Preserved. *I-Preserve; †CHARM-Preserved; #PARAGON-HF. CABG = coronary artery bypass graft; CV = cardiovascular; eGFR = estimated glomerular filtration rate; HFpEF = heart failure with preserved ejection fraction; HHF = hospitalisation for heart failure; PCI = percutaneous coronary intervention. Source: Reproduced with permission from Freepik Company.
Furthermore, socioeconomic, cultural/lifestyle and environmental disparities may differ within a specific region. For example, patients from southeast Asia had the highest burden of comorbidities, especially diabetes and chronic kidney disease, with the worst 1-year mortality compared to those from other parts of Asia.[7][4] More inclusive epidemiological studies are needed to understand region-specific factors of HFpEF.
Prognosis of HFpEF
Mortality
Clinical outcomes of HFpEF may vary depending on the study design and enrolment setting, as well as patients' demographic backgrounds, comorbidities and the stages of HF. Observational studies primarily enroling decompensated, hospitalised patients report 1-year mortality of HFpEF of approximately 20–30%.[23,24,33] Trial-based studies, primarily enroling chronic, ambulatory HF patients report rates of approximately 4–5%.[75,76] Both cardiac and non-cardiac risk factors similarly impact the prognosis between HFpEF and HFrEF. However, it is important to note that some non-cardiovascular disease such as COPD may have a more pronounced impact on mortality in HFpEF compared to HFrEF.
Causes and Modes of Death
Patients with HFpEF are more likely to experience non-cardiac death compared to patients with HFrEF. A study from Olmsted County reported that in HFpEF, 49% of deaths were non-cardiac.[77] The original and offspring cohort of the Framingham Heart Study also reported the proportion of non-cardiovascular death as 61% in men and 51% in women with HFpEF.[78] A more recent study highlighted sudden death as an important mode of death, accounting for nearly 25% of all-cause death in HFpEF.[79]
Hospitalisation
HFpEF has a greater risk of all-cause hospitalisation and emergency visits compared to HFrEF, primarily because of non-cardiovascular causes.[32,80]However, HF hospitalisation is still an important outcome in HFpEF. An observational study in more than 2,800 patients showed that 30-day and 1-year readmissions for HF were not different between HFpEF and HFrEF.[24] In fact, the proportion of hospitalisation for HFpEF among acute decompensated HF was increasing over time.[81]
Quality of Life
Many contemporary clinical trials and observational studies report the Kansas City Cardiomyopathy Questionnaire (KCCQ) or the Minnesota Living with Heart Failure Questionnaire (MLHFQ) as valid and reliable measures of patient-reported health status in HFpEF, consistently showing that the burden of symptoms and physical limitations are substantial in HFpEF. The KCCQ scores in HFpEF are reported to be similar or even worse than in HFrEF.[82,83] Additionally, sex differences have been reported in these patient-reported outcomes of HFpEF, whereby women tended to have a lower KCCQ score than men.[83] Despite no sex differences reported in MLHFQ score and 6-minute walk distance from a post hoc analysis of the RELAX and NEAT- trials, men and women had distinct clinical correlates of these scores where men had more comorbidities and LV hypertrophy that were associated with MLHFQ score while age and BMI were the only correlates of MLHFQ score in women.[84] Recent studies also suggest that different components/domains contribute to quality of life in HFpEF and HFrEF. In a single-centre observational study across a wide range of LVEF in patients with HF, those with higher LVEFs were more likely to perceive their HF to be primarily limited by non-cardiac medical or non-medical factors.[82]
Future Directions
Despite great strides taken in understanding of the epidemiology of HFpEF, knowledge gaps still exist. More research regarding sex/gender, racial/ethnic, geographical and socioeconomic variations in the prevalence, incidence, and outcomes are needed to address the global burden of HFpEF. Phenotypic subtyping approaches using artificial intelligence and other advanced analytical approaches may be useful in further identifying specific groups of patients with similar underlying pathophysiology and potential therapeutic options. In this way, a greater understanding of the epidemiology of HFpEF may open the door to deeper appreciation of the pathophysiological diversity of the condition, urgent unmet needs and sub-populations to target in dedicated clinical trials.
Conclusion
The prevalence of HFpEF is increasing worldwide. Multimorbidity involving both traditional cardiovascular as well as non-cardiovascular factors are important drivers of the pathophysiological mechanism of development and progression of HFpEF. While classically described as a condition of elderly hypertensive women, recent studies suggest heterogeneity in clinical phenotypes involving differential characteristics and pathophysiological mechanisms. There is a paucity of disease-modifying therapy for HFpEF, but understanding of phenotypic similarities and differences among patients with HFpEF around the world provides the foundation to recognise the clinical condition for early treatment, along with the identification of modifiable risk factors for preventive intervention.
Supplementary Material
References
- 1.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 2.Shah SJ, Katz DH, Selvaraj S et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–79. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao DP, Lewsey JD, Anand IS et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17:925–35. doi: 10.1002/ejhf.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segar MW, Patel KV, Ayers C et al. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur J Heart Fail. 2020;22:148–58. doi: 10.1002/ejhf.1621. [DOI] [PubMed] [Google Scholar]
- 5.Hedman ÅK, Hage C, Sharma A et al. Identification of novel pheno-groups in heart failure with preserved ejection fraction using machine learning. Heart. 2020;106:342–9. doi: 10.1136/heartjnl-2019-315481. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JB, Schrauben SJ, Zhao L et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020;8:172–84. doi: 10.1016/j.jchf.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrub F, Oger E, Bidaut A et al. Heart failure with preserved ejection fraction: a clustering approach to a heterogenous syndrome. Arch Cardiovasc Dis. 2020;113:381–90. doi: 10.1016/j.acvd.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Woolley RJ, Ceelen D, Ouwerkerk W et al. Machine learning based on biomarker profiles identifies distinct subgroups of heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:983–91. doi: 10.1002/ejhf.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uijl A, Savarese G, Vaartjes I et al. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:973–82. doi: 10.1002/ejhf.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorimachi H, Omote K, Borlaug BA. Clinical phenogroups in heart failure with preserved ejection fraction. Heart Fail Clin. 2021;17:483–98. doi: 10.1016/j.hfc.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Lam CSP, Solomon SD. Classification of heart failure according to ejection fraction: JACC review topic of the week. J Am Coll Cardiol. 2021;77:3217–25. doi: 10.1016/j.jacc.2021.04.070. [DOI] [PubMed] [Google Scholar]
- 12.Pieske B, Tschöpe C, de Boer RA et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020;22:391–412. doi: 10.1002/ejhf.1741. [DOI] [PubMed] [Google Scholar]
- 13.Reddy YNV, Carter RE, Obokata M et al. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–70. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozkurt B, Coats AJS, Tsutsui H et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–80. doi: 10.1002/ejhf.2115. [DOI] [PubMed] [Google Scholar]
- 15.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2017;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devereux RB, Roman MJ, Liu JE et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart study. Am J Cardiol. 2000;86:1090–6. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 17.Philbin EF, Rocco TA Jr, Lindenmuth NW et al. Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605–13. doi: 10.1016/s0002-9343(00)00601-x. [DOI] [PubMed] [Google Scholar]
- 18.Gottdiener JS, McClelland RL, Marshall R et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 19.MacCarthy PA, Kearney MT, Nolan J et al. Prognosis in heart failure with preserved left ventricular systolic function: prospective cohort study. BMJ. 2003;327:78–9. doi: 10.1136/bmj.327.7406.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafsson F, Torp-Pedersen C, Brendorp B et al. Long-term survival in patients hospitalized with congestive heart failure: relation to preserved and reduced left ventricular systolic function. Eur Heart J. 2003;24:863–70. doi: 10.1016/s0195-668x(02)00845-x. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC Jr et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Lenzen MJ, Scholte OP, Reimer WJ, Boersma E et al. Differences between patients with a preserved and a depressed left ventricular funciton: a report from the EuroHeart Failure survey. Eur Heart J. 2004;25:1214–20. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Owan TE, Hodge DO, Herges RM et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 24.Bhatia RS, Tu JV, Lee DS et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Lopatin M, Stevenson LW et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Bursi F, Weston SA, Redfield MM et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 27.Gheorghiade M, Abraham WT, Albert NM et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–26. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 28.Lee DS, Gona P, Vasan RS et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho JE, Lyass A, Lee DS et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–86. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurwitz JH, Magid DJ, Smith DH et al. Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med. 2013;126:393–400. doi: 10.1016/j.amjmed.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brouwers FP, de Boer RA, van der Harst P et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 32.Cheng RK, Cox M, Neely ML et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721–30. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Gerber Y, Weston SA, Redfield MM et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alehagen U, Benson L, Edner M et al. Association Between use of statins and mortality in patients with heart failure and ejection fraction of ≥50. Circ Heart Fail. 2015;8:862–70. doi: 10.1161/CIRCHEARTFAILURE.115.002143. [DOI] [PubMed] [Google Scholar]
- 35.Ho JE, Enserro D, Brouwers FP et al. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail. 2016;9:e003116. doi: 10.1161/CIRCHEARTFAILURE.115.003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasan RS, Xanthakis V, Lyass A et al. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging. 2018;11:1–11. doi: 10.1016/j.jcmg.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsao CW, Lyass A, Enserro D et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–85. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonagh TA, Metra M, Adamo M et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 39.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–93. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sartipy U, Dahlström U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail. 2017;5:565–74. doi: 10.1016/j.jchf.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Kristensen SL, Jhund PS, Lee MMY et al. Prevalence of prediabetes and undiagnosed diabetes in patients with HFpEF and HFrEF and associated clinical outcomes. Cardiovasc Drugs Ther. 2017;31:545–9. doi: 10.1007/s10557-017-6754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam CS. Diabetic cardiomyopathy: an expression of stage B heart failure with preserved ejection fraction. Diab Vasc Dis Res. 2015;12:234–8. doi: 10.1177/1479164115579006. [DOI] [PubMed] [Google Scholar]
- 44.McGuire DK, Shih WJ, Cosentino F et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–58. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anker SD, Butler J, Filippatos G et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 46.Kitzman DW, Brubaker P, Morgan T et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savji N, Meijers WC, Bartz TM et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6:701–9. doi: 10.1016/j.jchf.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabbah MS, Fayyaz AU, de Denus S et al. Obese-inflammatory phenotypes in heart failure with preserved ejection fraction. Circ Heart Fail. 2020;13:e006414. doi: 10.1161/CIRCHEARTFAILURE.119.006414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obokata M, Reddy YNV, Pislaru SV et al. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sorimachi H, Burkhoff D, Verbrugge FH et al. Obesity, venous capacitance, and venous compliance in heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:1648–58. doi: 10.1002/ejhf.2254. [DOI] [PubMed] [Google Scholar]
- 51.Rao VN, Fudim M, Mentz RJ et al. Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:1540–50. doi: 10.1002/ejhf.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176:611–7. doi: 10.1016/j.ijcard.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandramouli C, Tay WT, Bamadhaj NS et al. Association of obesity with heart failure outcomes in 11 Asian regions: a cohort study. PLoS Med. 2019;16:e1002916. doi: 10.1371/journal.pmed.1002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith DH, Thorp ML, Gurwitz JH et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013;6:333–42. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damman K, Perez AC, Anand IS et al. Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. J Am Coll Cardiol. 2014;64:1106–13. doi: 10.1016/j.jacc.2014.01.087. [DOI] [PubMed] [Google Scholar]
- 56.Agrawal A, Naranjo M, Kanjanahattakij N et al. Cardiorenal syndrome in heart failure with preserved ejection fraction – an under-recognized clinical entity. Heart Fail Rev. 2019;24:421–37. doi: 10.1007/s10741-018-09768-9. [DOI] [PubMed] [Google Scholar]
- 57.Yeo TJ, Yeo PS, Ching-Chiew Wong R et al. Iron deficiency in a multi-ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail. 2014;16:1125–32. doi: 10.1002/ejhf.161. [DOI] [PubMed] [Google Scholar]
- 58.Gupta K, Kalra R, Rajapreyar I et al. Anemia, mortality, and hospitalizations in heart failure with a preserved ejection fraction (from the TOPCAT trial). Am J Cardiol. 2020;125:1347–54. doi: 10.1016/j.amjcard.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maurer MS, Schwartz JH, Gundapaneni B et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–16. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 60.Packer M, Lam CSP, Lund LH et al. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020;22:1551–67. doi: 10.1002/ejhf.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sundström J, Bruze G, Ottosson J et al. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation. 2017;135:1577–85. doi: 10.1161/CIRCULATIONAHA.116.025629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation. 2005;112:357–63. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 63.Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford) 2005;44:677–80. doi: 10.1093/rheumatology/keh610. [DOI] [PubMed] [Google Scholar]
- 64.Tromp J, Paniagua SMA, Lau ES et al. Age dependent associations of risk factors with heart failure: pooled population based cohort study. BMJ. 2021;372:n461. doi: 10.1136/bmj.n461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tromp J, Teng TH, Tay WT et al. Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail. 2019;21:23–36. doi: 10.1002/ejhf.1227. [DOI] [PubMed] [Google Scholar]
- 66.Tromp J, Shen L, Jhund PS et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74:601–12. doi: 10.1016/j.jacc.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 67.Pandey A, Omar W, Ayers C et al. Sex and race differences in lifetime risk of heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Circulation. 2018;137:1814–23. doi: 10.1161/circulationaha.117.031622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sotomi Y, Hikoso S, Nakatani D et al. Sex differences in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e018574. doi: 10.1161/jaha.120.018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam CSP, Arnott C, Beale AL et al. Sex differences in heart failure. Eur Heart J. 2019;40:3859–68c. doi: 10.1093/eurheartj/ehz835. [DOI] [PubMed] [Google Scholar]
- 70.Dewan P, Jackson A, Lam CSP et al. Interactions between left ventricular ejection fraction, sex and effect of neurohumoral modulators in heart failure. Eur J Heart Fail. 2020;22:898–901. doi: 10.1002/ejhf.1776. [DOI] [PubMed] [Google Scholar]
- 71.Echocardiographic Normal Ranges Meta-Analysis of the Left Heart Collaboration. Echocardiographic normal ranges meta-analysis of the left heart collaboration. Ethnic-specific normative reference values for echocardiographic LA and LV size, LV mass, and systolic function: the EchoNoRMAL study. JACC Cardiovasc Imaging. 2015;8:656–65. doi: 10.1016/j.jcmg.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 72.Kristensen SL, Køber L, Jhund PS et al. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation. 2015;131:43–53. doi: 10.1161/CIRCULATIONAHA.114.012284. [DOI] [PubMed] [Google Scholar]
- 73.Tromp J, Claggett BL, Liu J et al. Global differences in heart failure with preserved ejection fraction: the PARAGON-HF trial. Circ Heart Fail. 2021;14:e007901. doi: 10.1161/CIRCHEARTFAILURE.120.007901. [DOI] [PubMed] [Google Scholar]
- 74.MacDonald MR, Tay WT, Teng TK et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction Across Asia: outcomes in the ASIAN-HF registry. J Am Heart Assoc. 2020;9:e012199. doi: 10.1161/JAHA.119.012199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Massie BM, Carson PE, McMurray JJ et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 76.Pitt B, Pfeffer MA, Assmann SF et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 77.Henkel DM, Redfield MM, Weston SA et al. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–7. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee DS, Gona P, Albano I et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaduganathan M, Claggett BL, Chatterjee NA et al. Sudden death in heart failure with preserved ejection fraction: a competing risks analysis From the TOPCAT trial. JACC Heart Fail. 2018;6:653–61. doi: 10.1016/j.jchf.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 80.Nichols GA, Reynolds K, Kimes TM et al. Comparison of risk of re-hospitalization, all-cause mortality, and medical care resource utilization in patients with heart failure and preserved Versus reduced ejection fraction. Am J Cardiol. 2015;116:1088–92. doi: 10.1016/j.amjcard.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 81.Steinberg BA, Zhao X, Heidenreich PA et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 82.Joyce E, Chung C, Badloe S et al. Variable contribution of heart failure to quality of life in ambulatory heart failure with reduced, better, or preserved ejection fraction. JACC Heart Fail. 2016;4:184–93. doi: 10.1016/j.jchf.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Chandra A, Vaduganathan M, Lewis EF et al. Health-related quality of life in heart failure with preserved ejection fraction: the PARAGON-HF trial. JACC Heart Fail. 2019;7:862–74. doi: 10.1016/j.jchf.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 84.Honigberg MC, Lau ES, Jones AD et al. Sex differences in exercise capacity and quality of life in heart failure with preserved ejection fraction: a secondary analysis of the RELAX and NEAT-HFpEF trials. J Card Fail. 2020;26:276–80. doi: 10.1016/j.cardfail.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


