Abstract
Background
The number of mobile health (mHealth) apps released for musculoskeletal (MSK) injury treatment and self-management with home exercise programs (HEPs) has risen rapidly in recent years as digital health interventions are explored and researched in more detail. As this number grows, it is becoming increasingly difficult for users to navigate the market and select the most appropriate app for their use case. It is also unclear what features the developers of these apps are harnessing to support patient self-management and how they fit into clinical care pathways.
Objective
The objective of this study was to scope the current market of mHealth apps for MSK rehabilitation and to report on their features, claims, evidence base, and functionalities.
Methods
A cross-sectional study of apps for MSK rehabilitation was performed across the iTunes App Store and Google Play Store. Four search terms were used, namely, physiotherapy rehabilitation, physical therapy rehabilitation, rehabilitation exercise, and therapeutic exercise to identify apps, which were then cross-referenced against set selection criteria by 4 reviewers. Each reviewer, where possible, downloaded the app and accessed supplementary literature available on the product to assist in data extraction.
Results
A total of 1322 apps were identified. After applying the inclusion and exclusion criteria and removing duplicates, 144 apps were included in the study. Over half (n=81, 56.3%) of the included apps had been released within the past 3 years. Three quarters (n=107, 74.3%) of the apps made no reference to evidence supporting the design or efficacy of the app, with only 11.1% (n=16) providing direct citations to research. Most of the apps did utilize exercise pictures (n=138, 95.8%) or videos (n=97, 67.4%); however, comparatively few harnessed additional features to encourage engagement and support self-management, such as an adherence log (n=66, 45.8%), communication portal (n=32, 22.2%), patient-reported outcome capture (n=36, 25%), or direct feedback (n=57, 39.6%). Of note and concern, many of these apps prescribed generic exercises (n=93, 64.6%) in the absence of individualized input to the user, with few providing specific patient education (n=43, 34%) and safety advice or disclaimers (n=38, 26.4%).
Conclusions
The cohort of apps included in this study contained a large heterogeneity of features, so it is difficult for users to identify the most appropriate or effective app. Many apps are missing the opportunity to offer key features that could promote exercise adherence and encourage self-management in MSK rehabilitation. Furthermore, very few developers currently offering products on the market are providing evidence to support the design and efficacy of their technologies.
Keywords: mHealth, musculoskeletal rehabilitation, app, home exercise program, home exercise, telehealth, mobile health, connected health
Introduction
An injury to the musculoskeletal (MSK) system involves damage to 1 or more components of the locomotor system and its associated tissues. These injuries account for the greatest proportion of noncancer persistent pain conditions [1]. The World Health Organization (WHO) estimates that between 20% and 33% of people across the world live with a painful MSK condition, and it is the highest contributor to global disability, with low back pain the single leading cause of disability worldwide [1,2] The burden of MSK conditions on societal and personal well-being is escalating, resulting in a reduction of quality of life, mental well-being, and function [3]. Additionally, MSK conditions account for 25% of overall costs of illness globally, placing a significant burden on health care resources [4]. Exercise as treatment for MSK conditions is widely accepted [5], with clinical guidelines advocating the promotion of physical activity and the use of exercise programs [6,7].
The prescription of home exercise programs (HEPs) encourages patients to take responsibility and self-manage their condition to mitigate limitations in physical function, a hallmark consequence of MSK conditions [8]. Adherence is considered an important prerequisite for the success of HEPs and has a direct link to improved patient outcomes [9]. However, in a study by Bassett et al [10], nonadherence to HEPs was estimated to be as high as 50%. Therefore, solutions to improve adherence and support self-management are required to optimize the efficacy of MSK treatment [11,12]. It has been suggested that mobile apps and connected health technologies can incorporate design features to maximize adherence, encourage self-management, and bridge the gap between the clinic and home [11,13].
Mobile health (mHealth) is defined by the WHO Global Observatory for eHealth as a “medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices” [14]. The current capabilities and ubiquity of mobile devices make them a valuable tool for improving the delivery of health care services and providing scalable, low-cost interventions [15]. Today, 3.8 billion people worldwide own a smartphone [16], posing an opportunity for health care providers to make health care accessible to a large proportion of the population [17,18]. With this rise in accessibility of mHealth comes a surge in the choices of apps, with at least 318,000 health apps available worldwide [19]. Other areas of health, including diabetes and hypertension management, have reported promising results in favor of the use of apps for improving several clinical, behavioral, knowledge, and psychosocial outcomes [20,21]. With the mHealth app market growing exponentially, the employment of such apps in various clinical contexts correlates with this growth. Clinicians in both cardiac and neurorehabilitation/palliative care adopting mHealth apps into their practices have reported similar, clinically relevant successful outcomes [22,23].
One clear use case that mHealth affords health care professionals is the opportunity to provide interactive and engaging access to self-management programs for MSK rehabilitation, incorporating features such as goal setting, coaching, remote monitoring, and exercise tracking [11,24]. Therefore, these systems have the potential to increase self-efficacy, optimize quality of life, and reduce the burden of MSK conditions [25,26]. However, caution must be taken with this opportunity, as there is a need for better standardization and regulation of mHealth apps to ensure proper integration and identification of beneficial and safe apps [27,28]. With over 200 health apps being added to the iOS and Google Play app stores each day [17], the integrity, in terms of quality and safety, of mHealth apps is questionable. Despite the iTunes App Store and Google Play Store categorizing apps (health, well-being), searches on the stores yield millions of results of indeterminate quality [29,30], making the search and selection of health care apps challenging for clinicians and patients alike [31]. There is a large body of qualitative research looking at the potential of mHealth to improve adherence and the role that digital technology can play in exercise rehabilitation [32,33]. However, research examining the current state of mHealth apps for exercise rehabilitation is limited. A recent systematic review found that approximately one-third of the 102 studies included evaluated the clinical efficacy of an intervention, with the remainder assessing the functionality of the app or patient engagement with the app [34]. To our knowledge, there has been no research to date exploring the overall scope of the market.
Given the exponential rise in mHealth apps and the limited research into their effectiveness and acceptance, the aim of this study was to investigate the current state of the mHealth app market targeted at assisting patients with MSK exercise rehabilitation. Recent innovations in health care provision can help improve the delivery and efficacy of physiotherapy to this cohort of patients [35]. The aim of this paper is to scope the current market of mHealth apps for MSK rehabilitation and describe which features exercise rehabilitation apps currently offer, document the accessibility of the app, and explore the evidence supporting each individual app.
Methods
Study Design
A cross-sectional study of MSK rehabilitation apps was performed to identify apps from 2 major smartphone app stores: iTunes App Store and Google Play Store, which together represent 98.9% of the smartphone app market share [36]. Building on the approach by Giunti et al [27], a systematic search strategy was developed that attempted to identify all relevant apps, followed by a synthesis of the characteristics of the apps.
Setting
On October 28, 2020, 4 reviewers searched both stores from the Republic of Ireland using 4 different search terms: “physiotherapy rehabilitation,” “physical therapy rehabilitation,” “rehabilitation exercise,” and “therapeutic exercise.” The iTunes App Store is a digital distribution platform developed and maintained by Apple Inc for mobile apps on iOS with 1.96 million apps available [16]. Google Play store (originally the Android Market) serves as the official app store for the Android operating system and contains over 2.86 million apps [16].
Selection Criteria
Apps were included if they were available in English, focused on exercise interventions for MSK injuries or general MSK physiotherapy rehabilitation, and were available for use on smartphone devices. Apps that were determined to be general well-being/fitness apps without reference to physiotherapy or rehabilitation were excluded. A full list of inclusion and exclusion criteria can be found in Textbox 1.
Inclusion and exclusion criteria.
Inclusion criteria
Title or description makes reference to musculoskeletal (MSK) physiotherapy/physical therapy rehabilitation
Title or description makes reference to exercise interventions for specific or general MSK conditions
Patient-centered app
Includes exercise prescription
Exclusion criteria
Title or description does not make reference to MSK physiotherapy/physical therapy rehabilitation
Title or description does not make reference to exercise interventions for specific or general MSK conditions
Description is not written in English
Duplicates from the same store
Clinician-focused app
Women’s health apps such as pelvic floor center apps
General fitness apps with no mention of physiotherapy/physical therapy or MSK conditions
After the search was completed, the resultant apps were screened by 1 of 4 reviewers (authors SR, NNC, SOH, and DC) for eligibility against the inclusion and exclusion criteria. A small random sample (5%) was independently reviewed by 2 reviewers who evaluated the eligibility of the apps against the selection criteria. To assess the clarity of the selection criteria, interrater reliability was assessed using Cohen kappa coefficient. If any conflicts or disagreements arose, the app in question was discussed between the 4 reviewers until they came to an agreement. In line with common practice, different versions of the same app (basic/premium, iOS/Android) were included separately due to version capabilities or store submission processes [37]. A cohort of identical apps from the same developer was classed as “white labeled” by the authors, with the underlying app being identical but branded for different health care providers. During selection, this cohort was represented by 1 randomly selected app per developer from each store.
Data Extraction
All apps included for data extraction were split evenly between the 4 reviewers. The data were extracted from the app description on the stores, screenshots on the stores, and the app website (link provided on app stores). Data extracted on the apps included year of release, developer, charging models, targeted body part, features, and evidence. If information on the app features was unavailable or unclear in the description, screenshots, and website, the app was downloaded to decipher the remaining features. If a reviewer was unsure of any data, a discussion was held between the 4 reviewers until a resolution was reached. A list of parameters that were used for data extraction and a description of each is included in Multimedia Appendix 1.
Results
App Selection
A total of 1322 apps (326 iTunes App Store and 996 Google Play Store) were identified using the described search strategy. After screening, a total of 641 apps (246 from iTunes App Store and 395 from Google Play Store) met the inclusion/exclusion criteria. Duplicates were then removed, bringing the total to 343 apps. During data extraction from the apps and their associated websites, a further 36 apps were excluded because further investigation revealed that they did not meet the selection criteria. White-label app duplicates were then removed, resulting in a total of 144 apps for data analysis (40 from iTunes App Store and 104 from Google Play Store). Interrater reliability on data screening was tested by calculating the kappa coefficient, resulting in a value of 0.876. Figure 1 presents the flowchart of the app selection process.
Figure 1.
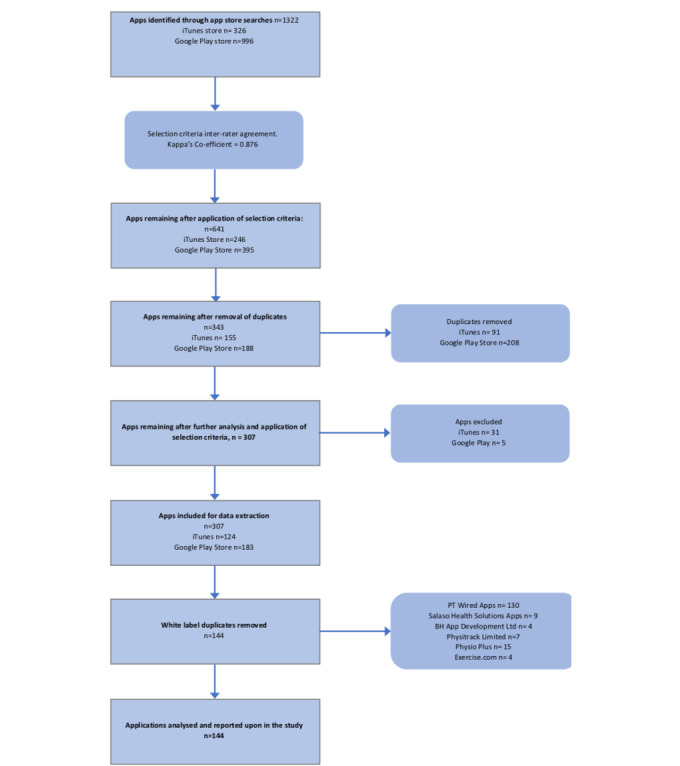
Study Flow.
App Analysis Results
General Characteristics of Included Apps
The content analysis of the included apps is shown in Table 1. Despite the existence of both app stores since 2008, the past 5 years account for 84% of all app releases, with a notable increase between 2017 and 2019.
Table 1.
Results of review of apps for musculoskeletal rehabilitation (N=144).
| Characteristics | Value, n (%) | |
| Year of app release | ||
|
|
2006 | 3 (2.1) |
|
|
2008 | 3 (2.1) |
|
|
2012 | 2 (1.4) |
|
|
2013 | 2 (1.4) |
|
|
2014 | 13 (9.0) |
|
|
2015 | 8 (5.6) |
|
|
2016 | 16 (11.1) |
|
|
2017 | 16 (11.1) |
|
|
2018 | 23 (16.0) |
|
|
2019 | 32 (22.2) |
|
|
2020 | 26 (18.1) |
| Charging model type | ||
|
|
Free to download and no in-app purchase | 52 (36.8) |
|
|
Clinic charged | 42 (29.9) |
|
|
Free to download with in-app purchase | 33 (23.6) |
|
|
Download charge for patient | 10 (6.9) |
|
|
Multiple | 3 (2.1) |
|
|
Unable to determine | 4 (2.7) |
| Evidence base | ||
|
|
No research highlighted | 107 (74.3) |
|
|
References provided to relevant research | 16 (11.1) |
|
|
Evidence based claims but no reference | 21 (14.6) |
| Method of exercise prescription | ||
|
|
Generic | 93 (64.6) |
|
|
Tailored to user requirements | 44 (30.6) |
|
|
Both | 7 (4.7) |
| Targeted body part | ||
|
|
Shoulder | 6 (4.2) |
|
|
Neck and shoulder | 2 (1.4) |
|
|
Neck | 5 (3.5) |
|
|
Knee and hip | 2 (1.4) |
|
|
Knee and back | 1 (0.1) |
|
|
Knee | 21 (14.6) |
|
|
Hand and wrist | 1 (0.1) |
|
|
Hand | 3 (2.1) |
|
|
Back and neck | 2 (1.4) |
|
|
Back and knee | 1 (0.1) |
|
|
Back and hip | 1 (0.1) |
|
|
Back and core | 1 (0.1) |
|
|
Back | 18 (12.5) |
|
|
Ankle | 2 (1.4) |
|
|
Tailored | 73 (50.7) |
| Presence of design enhancing features | ||
|
|
Pictures | 138 (95.8) |
|
|
Videos | 97 (67.4) |
|
|
Self-reported log | 66 (45.8) |
|
|
Adherence reminders | 49 (34.0) |
| Patient-reported outcomes | ||
|
|
Standardized instruments | 21 (14.6) |
|
|
Response to targeted questions | 2 (1.4) |
|
|
Present—unable to determine method | 1 (0.1) |
|
|
Free text | 1 (0.1) |
|
|
Multiple | 11 (7.6) |
|
|
None | 108 (75.0) |
| Communication features | ||
|
|
Video conferencing | 6 (4.2) |
|
|
Two-way messaging | 8 (5.6) |
|
|
Robotic messaging | 1 (0.1) |
|
|
Messaging—unable to differentiate | 2 (1.4) |
|
|
Instant messaging | 4 (2. 8) |
|
|
Multiple sources | 11 (7.6) |
|
|
None | 112 (77.8) |
| Feedback to patients | ||
|
|
Automated | 5 (3.5) |
|
|
Progress tracking | 25 (17.4) |
|
|
Gamification | 5 (3.5) |
|
|
Direct feedback from physiotherapist | 5 (3.5) |
|
|
Multiple | 17 (11.8) |
|
|
None | 87 (60.4) |
| Clinical specificity | ||
|
|
Clinic-specific | 18 (19.4) |
|
|
Public access | 116 (80.6) |
The predominant revenue model was a fully cost-free business-to-consumer approach (n=53, 36.8%), with revenue presumably derived from advertising and marketing. A further 23.6%
(n=34) of apps were free to download but offered in-app purchases. This included paying for additional exercises, exercise progression, or other features such as exercise logging. Many other developers (n=43, 29.9%) have pursued a business-to-business model whereby clinics pay for the platform and then provide it in their service to patients.
Almost three quarters (n=107, 74.3%) of apps made no reference to research or an evidence base for their interventions, nor did they make scientific claims about their apps. Meanwhile, 11.1% (n=16) of the apps did provide research evidence to support the clinical relevance of their platform, marketing claims, or features of the app. The remaining 14.6% (n = 21) of apps made evidence-based claims but failed to reference or supply links to the relevant research.
The majority (n=116, 80.6%) of apps were available to the general population, with the remainder being restricted to a specific clinic and requiring patients to log in to access the features available. Table S1 in Multimedia Appendix 2 shows the general characteristics of the apps.
Prevalence of Exercise Prescription and Assistance Features
Most (n=93, 64.6%) of the apps used automated exercise prescription to generate a generic HEP, while only 30.6% (n=44) of the apps prescribed exercises selected by a health care professional to the patient post assessment (Table 1). The remaining 4.7% (n=7) utilized both methods of exercise prescription. Just under half (71/144, 49.3%) of the apps only targeted the rehabilitation of a specific body part. The knee (n=21, 14.6%) was the most commonly featured body part, followed by the back (n=18, 12.5%) and shoulder (n=6, 4.2%). The remainder (n=73, 50.7%) of the apps did not target a specific body part or tailor the HEP to the specific needs of the individual patient.
Over two-thirds (n=97, 67.4%) of the apps included videos to illustrate the exercise and assist with technique, while the vast majority (n=138, 95.8%) incorporated static pictures in their HEPs. Less than half (n=66, 45.8%) of the apps utilized a self-reported exercise log, although adherence reminders were more frequently used, featuring in 66.7% (n=96) of the apps (Table 1). Table S1 in Multimedia Appendix 2 shows the exercise prescriptions and assistance features of the apps.
Prevalence of Communication and Feedback Features
Only 25% (n=36) of the apps offered patient-reported outcome (PRO) features supporting self or remote monitoring. Even fewer (n=32, 22.2%) had any direct personalized communication feature. Less than half (39.6%, n=57) included a feature for feedback from the app to the patient. Only 34% (n=49) contained patient education on their app, with fewer (n=38, 26.4%) featuring any safety advice or warnings.
In the apps containing PROs, standardized instruments like a visual analogue scale or a Likert scale (n=21, 14.6%) were the most common. Only 1.4% (n=2) included specific questions for the patient to respond to, and 0.7% (n=1) included free-text boxes for the patients. Meanwhile, 7.6% (n=11) used more than 1 of these features. It was not possible to determine whether PRO features were used in 0.7% (n=1) of the included apps.
The most common (5.6%, n=8) communication feature was 2-way text messaging between health care professionals and patients (Table 1), followed by video conferencing (n=6, 4.2%), instant messaging (n=4, 2.8%), and robotic automated messages (n=1 0.7%). More than 1 type of communication feature was seen in 7.6% (n=11) of the apps. In 1.4% (n=2) of the apps, it was not possible to identify which communication features were present or if there were any at all.
Of the apps that did include a feature to enable feedback to the patient, progress tracking was the most prevalent (n=25, 17.4%). This is where the patient could track the exercises or workouts they had completed on a calendar. Gamification was utilized in 3.5% (n=5) of the apps, where awards or badges were given. The same percentage of apps supplied direct feedback on progress from the health care provider and included automated feedback, meaning they would receive feedback on their progress through automated messages or emails. Overall, 11.8% (n=17) of the apps included 1 or more of the above feedback-supporting features. Table S2 in Multimedia Appendix 2 shows the additional features of the apps.
Discussion
Principal Findings
The sheer volume of mHealth apps available for exercise rehabilitation proves the popularity and prospects of technology in physical medicine. Yet, the acceptance of mHealth apps into routine clinical practice lags behind [38], as clinicians struggle to identify and select appropriate evidence-based apps. This study is the first to complete an in-depth analysis of exercise rehabilitation apps to help elucidate the state of the mHealth app market and investigate the relevance, design, and accessibility of the apps currently available in the iTunes and Google app stores. Despite the prevalence of these apps, many fail to offer individualized HEPs or harness design features available in mHealth systems to encourage self-management and adherence [11,39]. Going forward, app developers should focus on the inclusion of features that can be specific and customized to the end user for the capabilities of mHealth to be capitalized upon in rehabilitative medicine.
This study reveals a lack of evidence supporting the use of these apps, with only 11.1% (n=16) providing supporting research in their marketing material. Perhaps most concerning is the 14.6% (n=21) of apps that make claims relating to being evidence based but fail to cite any research; the absence of accessible evidence in any of the marketing material makes it difficult to appraise each offering. This might explain why most physiotherapists report only using apps for administrative purposes and not routinely recommending them to support patients’ HEPs in MSK rehabilitation [40]. Health care professionals must feel confident in the evidence base supporting the app to enhance their clinical judgement in their app selection and encourage adoption [41]. The National Institute for Health and Care Excellence (NICE), in collaboration with the National Health Service (NHS) in the United Kingdom, recently published an Evidence Standards Framework for digital and care technologies [42]. This framework contains a comprehensive list of evidence criteria required for such technologies to be adopted into the UK health system, including both minimum and best practice standards. Such frameworks create an awareness among developers, clinicians, and end users of the various types of evidence required for the effective development and implementation of technology in health care.
Communication is a cornerstone of the patient-physiotherapist relationship; a discrepancy in this alliance is a decisive indicator of nonadherence to HEPs, with poor physician communication increasing the risk of nonadherence by up to 19% [43]. Digital health technologies have a variety of communication methods to employ, from telehealth consultations (offered by only 6 of the included apps) to real-time messaging platforms, such as SMS text messaging, emails, or instant messaging (15 apps). The incorporation of such features may encourage the uptake of mHealth apps by clinicians and deviate patients from the more generic “back pain” or “shoulder pain” apps that provide automated programs in the absence of clinician input. The findings from this study are consistent with other research, as physiotherapists expressed concerns about app quality, patient safety, and knowledge base of mHealth apps [38]. A good HEP considers the individual it aims to help, which is fundamental to positively impacting adherence [44]. The literature makes a clear stance in favor of frequent and clear 2-way communication between the therapist and the patient [45], yet less than a quarter of the apps included in this study facilitated communication between the therapist and the patient.
Facilitating 2-way feedback (patient to clinician and clinician to patient), although challenging, is key to ensure that the clinician is readily equipped with data that can improve clinical decision making [11]. Consumer adoption of digital technology presents an opportunity to continuously capture feedback from patients through clinically approved PROs [46]. A variety of PROs, including standardized instruments, have been developed and validated to use as part of patient management [47], and such features improve communication and enhance clinical decision making [48]. Standardized PROs were featured in less than 15% (n=21) of the apps in this study, something that potentially contradicts the purpose of these “patient-centered” apps. Equally, the delivery of feedback to patients provides an opportunity for the therapist to reassure and educate the patient. The information a patient receives and the beliefs they hold about their condition influence their decision making and thus their adherence [49]. App developers may potentially be adopting the rationale that the inclusion of communication and feedback features may raise concerns regarding patient data security and privacy, with unencrypted communication and third-party data hosting common in general apps in the Google Play Store [50]. Numerous studies have identified the increasing amount of sensitive data handled by mHealth apps as new developments in the industry emerge [51], and this poses challenges to developers and regulators alike.
The idea of using an app in exercise rehabilitation is not to replace the therapist but rather to be seen as a facilitator [52]. mHealth apps have the capacity to send adherence reminders and notifications directly to the device, but the results of this study indicate that this is an area that developers are slow to take advantage of, with just over one-third of the apps featuring adherence reminders. Technology has the potential to affect the outcomes of HEPs by improving the accuracy, adherence, and quality of exercises performed by the patient through multimedia versions of a program (pictures and videos). The inclusion of pictures in a HEP is common in clinical practice [53], although providing patients with videos is slightly more difficult without the use of an app. Remarkably, one-third of the apps failed to incorporate videos into their HEPs [54]. The significant absence of these features, which have shown to increase levels of patient adherence [55], is an area we identified as an underutilization of the resources offered by mHealth apps.
Health care apps have become an industry in themselves for developers, investors, and health care professionals alike [56]. The findings in this study suggest that for these apps to be used in routine MSK practice, greater efforts need to be made by app developers to engage with academic research and stakeholders. Both health care providers and organizations have quality and validity concerns when it comes to choosing an app to recommend [57]. The absence of features proven to enhance adherence to HEPs, along with no real-time clinician input, leads to the information provided on these apps remaining static [27]. The findings in this study are consistent with those of apps to improve a patient's adherence to medications, with the majority lacking desirable features and considered to be of low quality [58]. There is a wide selection of tools to assess the quality of health-related websites; however, the same cannot be said when it comes to assessing and evaluating mHealth apps [57]. The Mobile App Rating Scale (MARS) is a tool for classifying and assessing the quality of mobile health apps. Further work should look at developing similar tools with a specific relevance to certain areas of health care such as rehabilitation [59]. It would be beneficial for future work to offer stakeholders an informative repository evaluating mHealth apps.
It was beyond the scope of this study to obtain access to the cohort of apps requiring payment to download or private subscriptions. In such cases, data extraction was completed via the app store through analysis of the available screenshots and developer websites. Where evidence of a feature could not be found using this method, it was stated that the feature was absent for this app. As highlighted by Giunti et al [27], while it is possible that an app’s features may only be disclosed to registered app users, this seems unlikely to be a common occurrence as the app store’s description and screenshots serve as major selling points to potential users.
Only apps available in the Republic of Ireland were included in this study. Hence, it is possible that there exists a cohort of eligible apps that have not been included due to geographical limitations. We also decided to exclude white labeled apps. Apps were considered to be white labeled if they were identified as identically structured apps provided by a single developer to multiple different companies. Given that the only discrepancy identified was in accessibility (customers must be linked to the specific private practice or company selling the app to gain access), we felt that the inclusion of such apps would provide a less relevant data set with heavily skewed results. These limitations aside, the data set reported upon reflects the most accurate depiction of the currently available apps for MSK rehabilitation across the 2 major app stores.
It is not surprising that as the capabilities of technology in health care grow, the number of apps coming onto the mHealth app market correlates. Just under 85% (n=122) of the apps that met the inclusion criteria were released into the respective app stores from 2015. The change in outpatient service delivery from traditional face-to-face patient contact to remote management has accelerated rapidly in response to the COVID-19 pandemic [60]. This shift toward technology was reflected in our findings, with 18% (26 apps) of the apps coming to the market in 2020. The pandemic has provided an opportunity for clinicians to embrace innovation and redesign their services to enhance their efficacy beyond the immediate crisis [61-63]. With the rapid proliferation of apps being brought to the market, the findings of this review highlight an opportunity is not being embraced to its full extent. Further research is required to investigate which digital health care features have a meaningful effect on adherence to HEPs. A framework to guide clinician and patient selection of mHealth apps in MSK rehabilitation could help navigate through the overwhelming number of apps available in the respective stores.
Conclusions
This study analyzed a large number of MSK rehabilitation apps available to consumers. Most of the apps were designed to provide HEPs and empower patients with the aim of improving adherence to HEPs and bridging the gap between the clinic and home. With the emerging capabilities and developments of mHealth, the use of apps in clinical practice is becoming more widely accepted. However, this study identified several missed opportunities by app developers to offer key features that promote adherence and self-management. There was a significant absence of properly cited sourced material or references in the apps included in this study. With the capabilities of mHealth underutilized in physical medicine, this review raises questions about the efficacy and quality of MSK rehabilitation apps, indicating that the current ecosystem of mHealth apps available do not lend well to evidence-based clinical practice. The paucity of evidence in this field reiterates the need for high-quality research and presents an opportunity to all stakeholders involved to develop and enhance these patient-facing apps to further bridge the gap between the clinic and the home.
Acknowledgments
This project was partly funded by Science Foundation Ireland (12/RC/2289_P2).
Abbreviations
- HEP
home exercise program
- MARS
Mobile App Rating Scale
- mHealth
mobile health
- MSK
musculoskeletal
- NHS
National Health Service
- NICE
National Institute for Health and Care Excellence
- PRO
patient-reported outcome
- WHO
World Health Organization
Featured descriptors.
Additional features.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Briggs AM, Woolf AD, Dreinhöfer K, Homb N, Hoy DG, Kopansky-Giles D, Åkesson K, March L. Reducing the global burden of musculoskeletal conditions. Bull World Health Organ. 2018 Apr 12;96(5):366–368. doi: 10.2471/blt.17.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James Sl, Abate D, Abate Kh, Abay Sm, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I, Abdulkader Rs, Abebe Z, Abera Sf, Abil Oz, Abraha Hn, Abu-Raddad Lj, Abu-Rmeileh Nme, Accrombessi Mmk, Acharya D, Acharya P, Ackerman In, Adamu Aa, Adebayo Om, Adekanmbi V, Adetokunboh Oo, Adib Mg, Adsuar Jc, Afanvi Ka, Afarideh M, Afshin A, Agarwal G, Agesa Km, Aggarwal R, Aghayan Sa, Agrawal S, Ahmadi A, Ahmadi M, Ahmadieh H, Ahmed Mb, Aichour An, Aichour I, Aichour Mte, Akinyemiju T, Akseer N, Al-Aly Z, Al-Eyadhy A, Al-Mekhlafi Hm, Al-Raddadi Rm, Alahdab F, Alam K, Alam T, Alashi A, Alavian Sm, Alene Ka, Alijanzadeh M, Alizadeh-Navaei R, Aljunid Sm, Alkerwi A, Alla F, Allebeck P, Alouani Mml, Altirkawi K, Alvis-Guzman N, Amare At, Aminde Ln, Ammar W, Amoako Ya, Anber Nh, Andrei Cl, Androudi S, Animut Md, Anjomshoa M, Ansha Mg, Antonio Cat, Anwari P, Arabloo J, Arauz A, Aremu O, Ariani F, Armoon B, Ärnlöv J, Arora A, Artaman A, Aryal Kk, Asayesh H, Asghar Rj, Ataro Z, Atre Sr, Ausloos M, Avila-Burgos L, Avokpaho Efga, Awasthi A, Ayala Quintanilla Bp, Ayer R, Azzopardi Ps, Babazadeh A, Badali H, Badawi A, Bali Ag, Ballesteros Ke, Ballew Sh, Banach M, Banoub Jam, Banstola A, Barac A, Barboza Ma, Barker-Collo Sl, Bärnighausen Tw, Barrero Lh, Baune Bt, Bazargan-Hejazi S, Bedi N, Beghi E, Behzadifar M, Behzadifar M, Béjot Y, Belachew Ab, Belay Ya, Bell Ml, Bello Ak, Bensenor Im, Bernabe E, Bernstein Rs, Beuran M, Beyranvand T, Bhala N, Bhattarai S, Bhaumik S, Bhutta Za, Biadgo B, Bijani A, Bikbov B, Bilano V, Bililign N, Bin Sayeed Ms, Bisanzio D, Blacker Bf, Blyth Fm, Bou-Orm Ir, Boufous S, Bourne R, Brady Oj, Brainin M, Brant Lc, Brazinova A, Breitborde Njk, Brenner H, Briant Ps, Briggs Am, Briko An, Britton G, Brugha T, Buchbinder R, Busse R, Butt Za, Cahuana-Hurtado L, Cano J, Cárdenas R, Carrero Jj, Carter A, Carvalho F, Castañeda-Orjuela Ca, Castillo Rivas J, Castro F, Catalá-López F, Cercy Km, Cerin E, Chaiah Y, Chang Ar, Chang H, Chang J, Charlson Fj, Chattopadhyay A, Chattu Vk, Chaturvedi P, Chiang Pp, Chin Kl, Chitheer A, Choi Jj, Chowdhury R, Christensen H, Christopher Dj, Cicuttini Fm, Ciobanu Lg, Cirillo M, Claro Rm, Collado-Mateo D, Cooper C, Coresh J, Cortesi Pa, Cortinovis M, Costa M, Cousin E, Criqui Mh, Cromwell Ea, Cross M, Crump Ja, Dadi Af, Dandona L, Dandona R, Dargan Pi, Daryani A, Das Gupta R, Das Neves J, Dasa Tt, Davey G, Davis Ac, Davitoiu Dv, De Courten B, De La Hoz Fp, De Leo D, De Neve J, Degefa Mg, Degenhardt L, Deiparine S, Dellavalle Rp, Demoz Gt, Deribe K, Dervenis N, Des Jarlais Dc, Dessie Ga, Dey S, Dharmaratne Sd, Dinberu Mt, Dirac Ma, Djalalinia S, Doan L, Dokova K, Doku Dt, Dorsey Er, Doyle Ke, Driscoll Tr, Dubey M, Dubljanin E, Duken Ee, Duncan Bb, Duraes Ar, Ebrahimi H, Ebrahimpour S, Echko Mm, Edvardsson D, Effiong A, Ehrlich Jr, El Bcheraoui C, El Sayed Zaki M, El-Khatib Z, Elkout H, Elyazar Irf, Enayati A, Endries Ay, Er B, Erskine He, Eshrati B, Eskandarieh S, Esteghamati A, Esteghamati S, Fakhim H, Fallah Omrani V, Faramarzi M, Fareed M, Farhadi F, Farid Ta, Farinha Cses, Farioli A, Faro A, Farvid Ms, Farzadfar F, Feigin Vl, Fentahun N, Fereshtehnejad S, Fernandes E, Fernandes Jc, Ferrari Aj, Feyissa Gt, Filip I, Fischer F, Fitzmaurice C, Foigt Na, Foreman Kj, Fox J, Frank Td, Fukumoto T, Fullman N, Fürst T, Furtado Jm, Futran Nd, Gall S, Ganji M, Gankpe Fg, Garcia-Basteiro Al, Gardner Wm, Gebre Ak, Gebremedhin At, Gebremichael Tg, Gelano Tf, Geleijnse Jm, Genova-Maleras R, Geramo Ycd, Gething Pw, Gezae Ke, Ghadiri K, Ghasemi Falavarjani K, Ghasemi-Kasman M, Ghimire M, Ghosh R, Ghoshal Ag, Giampaoli S, Gill Ps, Gill Tk, Ginawi Ia, Giussani G, Gnedovskaya Ev, Goldberg Em, Goli S, Gómez-Dantés H, Gona Pn, Gopalani Sv, Gorman Tm, Goulart Ac, Goulart Bng, Grada A, Grams Me, Grosso G, Gugnani Hc, Guo Y, Gupta Pc, Gupta R, Gupta R, Gupta T, Gyawali B, Haagsma Ja, Hachinski V, Hafezi-Nejad N, Haghparast Bidgoli H, Hagos Tb, Hailu Gb, Haj-Mirzaian A, Haj-Mirzaian A, Hamadeh Rr, Hamidi S, Handal Aj, Hankey Gj, Hao Y, Harb Hl, Harikrishnan S, Haro Jm, Hasan M, Hassankhani H, Hassen Hy, Havmoeller R, Hawley Cn, Hay Rj, Hay Si, Hedayatizadeh-Omran A, Heibati B, Hendrie D, Henok A, Herteliu C, Heydarpour S, Hibstu Dt, Hoang Ht, Hoek Hw, Hoffman Hj, Hole Mk, Homaie Rad E, Hoogar P, Hosgood Hd, Hosseini Sm, Hosseinzadeh M, Hostiuc M, Hostiuc S, Hotez Pj, Hoy Dg, Hsairi M, Htet As, Hu G, Huang Jj, Huynh Ck, Iburg Km, Ikeda Ct, Ileanu B, Ilesanmi Os, Iqbal U, Irvani Ssn, Irvine Cms, Islam Sms, Islami F, Jacobsen Kh, Jahangiry L, Jahanmehr N, Jain Sk, Jakovljevic M, Javanbakht M, Jayatilleke Au, Jeemon P, Jha Rp, Jha V, Ji Js, Johnson Co, Jonas Jb, Jozwiak Jj, Jungari Sb, Jürisson M, Kabir Z, Kadel R, Kahsay A, Kalani R, Kanchan T, Karami M, Karami Matin B, Karch A, Karema C, Karimi N, Karimi Sm, Kasaeian A, Kassa Dh, Kassa Gm, Kassa Td, Kassebaum Nj, Katikireddi Sv, Kawakami N, Karyani Ak, Keighobadi Mm, Keiyoro Pn, Kemmer L, Kemp Gr, Kengne Ap, Keren A, Khader Ys, Khafaei B, Khafaie Ma, Khajavi A, Khalil Ia, Khan Ea, Khan Ms, Khan Ma, Khang Y, Khazaei M, Khoja At, Khosravi A, Khosravi Mh, Kiadaliri Aa, Kiirithio Dn, Kim C, Kim D, Kim P, Kim Y, Kim Yj, Kimokoti Rw, Kinfu Y, Kisa A, Kissimova-Skarbek K, Kivimäki M, Knudsen Aks, Kocarnik Jm, Kochhar S, Kokubo Y, Kolola T, Kopec Ja, Kosen S, Kotsakis Ga, Koul Pa, Koyanagi A, Kravchenko Ma, Krishan K, Krohn Kj, Kuate Defo B, Kucuk Bicer B, Kumar Ga, Kumar M, Kyu Hh, Lad Dp, Lad Sd, Lafranconi A, Lalloo R, Lallukka T, Lami Fh, Lansingh Vc, Latifi A, Lau Km, Lazarus Jv, Leasher Jl, Ledesma Jr, Lee Ph, Leigh J, Leung J, Levi M, Lewycka S, Li S, Li Y, Liao Y, Liben Ml. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The burden of musculoskeletal disorders on Americans - opportunities for action. American Academy of Orthopaedic Surgeons. 2016. [2020-10-23]. https://www.boneandjointburden.org/docs/BMUSExecutiveSummary2016.pdf .
- 4.Walsh NE, Brooks P, Hazes JM, Walsh RM, Dreinhöfer Karsten, Woolf AD, Akesson Kristina, Lidgren L, BoneJoint Decade Task Force for Standards of Care for AcuteChronic Musculoskeletal Pain Standards of care for acute and chronic musculoskeletal pain: the Bone and Joint Decade (2000-2010) Arch Phys Med Rehabil. 2008 Sep;89(9):1830–45. doi: 10.1016/j.apmr.2008.04.009.S0003-9993(08)00393-6 [DOI] [PubMed] [Google Scholar]
- 5.Lewis R, Gómez Álvarez Constanza B, Rayman M, Lanham-New S, Woolf A, Mobasheri A. Strategies for optimising musculoskeletal health in the 21 century. BMC Musculoskelet Disord. 2019 Apr 11;20(1):164. doi: 10.1186/s12891-019-2510-7. https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-019-2510-7 .10.1186/s12891-019-2510-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallis R, Franklin B, Joy L, Ross R, Sabgir D, Stone J. Strategies for promoting physical activity in clinical practice. Prog Cardiovasc Dis. 2015 Jan;57(4):375–86. doi: 10.1016/j.pcad.2014.10.003.S0033-0620(14)00165-0 [DOI] [PubMed] [Google Scholar]
- 7.Fernandes Linda, Hagen Kåre B, Bijlsma JW, Andreassen Oyvor, Christensen Pia, Conaghan Philip G, Doherty Michael, Geenen Rinie, Hammond Alison, Kjeken Ingvild, Lohmander L Stefan, Lund Hans, Mallen Christian D, Nava Tiziana, Oliver Susan, Pavelka Karel, Pitsillidou Irene, da Silva José Antonio, de la Torre Jenny, Zanoli Gustavo, Vliet Vlieland Theodora P M, European League Against Rheumatism (EULAR) EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013 Jul 19;72(7):1125–35. doi: 10.1136/annrheumdis-2012-202745. https://ard.bmj.com/lookup/pmidlookup?view=long&pmid=23595142 .annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 8.Hagen KB, Dagfinrud H, Moe RH, Østerås Nina, Kjeken I, Grotle M, Smedslund G. Exercise therapy for bone and muscle health: an overview of systematic reviews. BMC Med. 2012 Dec 19;10(1):167. doi: 10.1186/1741-7015-10-167. https://bmcmedicine.biomedcentral.com/articles/10.1186/1741-7015-10-167 .1741-7015-10-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beinart NA, Goodchild CE, Weinman JA, Ayis S, Godfrey EL. Individual and intervention-related factors associated with adherence to home exercise in chronic low back pain: a systematic review. Spine J. 2013 Dec;13(12):1940–50. doi: 10.1016/j.spinee.2013.08.027.S1529-9430(13)01474-5 [DOI] [PubMed] [Google Scholar]
- 10.Bassett S. Measuring patient adherence to physiotherapy. J Nov Physiother. 2012;02(07) doi: 10.4172/2165-7025.1000e124. [DOI] [Google Scholar]
- 11.Argent R, Daly A, Caulfield B. Patient involvement with home-based exercise programs: can connected health interventions influence adherence? JMIR Mhealth Uhealth. 2018 Mar 01;6(3):e47. doi: 10.2196/mhealth.8518. http://mhealth.jmir.org/2018/3/e47/ v6i3e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert TE, Harvey LA, Avdalis C, Chen LW, Jeyalingam S, Pratt CA, Tatum HJ, Bowden JL, Lucas BR. An app with remote support achieves better adherence to home exercise programs than paper handouts in people with musculoskeletal conditions: a randomised trial. J Physiother. 2017 Jul;63(3):161–167. doi: 10.1016/j.jphys.2017.05.015. https://linkinghub.elsevier.com/retrieve/pii/S1836-9553(17)30067-X .S1836-9553(17)30067-X [DOI] [PubMed] [Google Scholar]
- 13.Aitken Dawn, Buchbinder R, Jones G, Winzenberg Tania. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Aust Fam Physician. 2015;44(1-2):39–42. http://www.racgp.org.au/afp/2015/januaryfebruary/interventions-to-improve-adherence-to-exercise-for-chronic-musculoskeletal-pain-in-adults/ [PubMed] [Google Scholar]
- 14.58th World Health Assembly. Resolution WHA58. World Health Organization; [2020-10-14]. https://apps.who.int/gb/ebwha/pdf_files/WHA58-REC1/english/A58_2005_REC1-en.pdf . [Google Scholar]
- 15.Free C, Phillips G, Watson L, Galli L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis. PLoS Med. 2013 Jan;10(1):e1001363. doi: 10.1371/journal.pmed.1001363. http://dx.plos.org/10.1371/journal.pmed.1001363 .PMEDICINE-D-12-00641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Internet usage worldwide. Statista. [2020-11-03]. https://www.statista.com/topics/1145/internet-usage-worldwide/
- 17.Deloitte’s 2019 global mobile consumer survey. Deloitte. 2019. [2020-11-05]. https://www2.deloitte.com/us/en/insights/industry/telecommunications/global-mobile-consumer-survey-2019.html .
- 18.Wallace S, Clark M, White J. 'It's on my iPhone': attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012 Aug 24;2(4):e001099. doi: 10.1136/bmjopen-2012-001099. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=22923627 .bmjopen-2012-001099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The growing value of digital health in the United Kingdom. IQVIA. [2020-11-08]. https://www.iqvia.com/insights/the-iqvia-institute/reports/the-growing-value-of-digital-health .
- 20.Cotter AP, Durant N, Agne AA, Cherrington AL. Internet interventions to support lifestyle modification for diabetes management: a systematic review of the evidence. J Diabetes Complicat. 2014 Mar;28(2):243–51. doi: 10.1016/j.jdiacomp.2013.07.003. http://europepmc.org/abstract/MED/24332469 .S1056-8727(13)00165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, Xie Z, Or CK. Effectiveness of mobile app-assisted self-care interventions for improving patient outcomes in type 2 diabetes and/or hypertension: systematic review and meta-analysis of randomized controlled trials. JMIR Mhealth Uhealth. 2020 Aug 4;8(8):e15779. doi: 10.2196/15779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worringham C, Rojek A, Stewart I. Development and feasibility of a smartphone, ECG and GPS based system for remotely monitoring exercise in cardiac rehabilitation. PLoS One. 2011 Feb 09;6(2):e14669. doi: 10.1371/journal.pone.0014669. https://dx.plos.org/10.1371/journal.pone.0014669 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bienfait F, Petit M, Pardenaud R, Guineberteau C, Pignon A. Applying m-health to palliative care: a systematic review on the use of m-health in monitoring patients with chronic diseases and its transposition in palliative care. Am J Hosp Palliat Care. 2020 Jul 27;37(7):549–564. doi: 10.1177/1049909119885655. [DOI] [PubMed] [Google Scholar]
- 24.Wykes T, Schueller S. Why reviewing apps is not enough: transparency for trust (T4T) principles of responsible health app marketplaces. J Med Internet Res. 2019 May 02;21(5):e12390. doi: 10.2196/12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keogh Edmund, Rosser B, Eccleston C. e-Health and chronic pain management: current status and developments. Pain. 2010 Oct;151(1):18–21. doi: 10.1016/j.pain.2010.07.014.00006396-201010000-00008 [DOI] [PubMed] [Google Scholar]
- 26.Brosseau L, Wells G, Brooks-Lineker S, Bennell K, Sherrington C, Briggs A, Sturnieks D, King J, Thomas R, Egan M, Loew L, De Angelis G, Casimiro L, Toupin April K, Cavallo S, Bell M, Ahmed R, Coyle D, Poitras S, Smith C, Pugh A, Rahman P. Internet-based implementation of non-pharmacological interventions of the "people getting a grip on arthritis" educational program: an international online knowledge translation randomized controlled trial design protocol. JMIR Res Protoc. 2015 Feb 03;4(1):e19. doi: 10.2196/resprot.3572. https://www.researchprotocols.org/2015/1/e19/ v4i1e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giunti G, Giunta D, Guisado-Fernandez E, Bender J, Fernandez-Luque L. A biopsy of breast cancer mobile applications: state of the practice review. Int J Med Inform. 2018 Feb;110:1–9. doi: 10.1016/j.ijmedinf.2017.10.022. https://linkinghub.elsevier.com/retrieve/pii/S1386-5056(17)30402-1 .S1386-5056(17)30402-1 [DOI] [PubMed] [Google Scholar]
- 28.Ventola C Lee. Mobile devices and apps for health care professionals: uses and benefits. P T. 2014 May;39(5):356–64. http://europepmc.org/abstract/MED/24883008 . [PMC free article] [PubMed] [Google Scholar]
- 29.Paglialonga A, Lugo A, Santoro E. An overview on the emerging area of identification, characterization, and assessment of health apps. J Biomed Inform. 2018 Jul;83:97–102. doi: 10.1016/j.jbi.2018.05.017. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(18)30104-7 .S1532-0464(18)30104-7 [DOI] [PubMed] [Google Scholar]
- 30.Gan SK, Koshy C, Nguyen P, Haw Y. An overview of clinically and healthcare related apps in Google and Apple app stores: connecting patients, drugs, and clinicians. Sci Phone Appl Mob Devices. 2016 Jul 19;2(1) doi: 10.1186/s41070-016-0012-7. [DOI] [Google Scholar]
- 31.Baxter C, Carroll J, Keogh B, Vandelanotte C. Assessment of mobile health apps using built-in smartphone sensors for diagnosis and treatment: systematic survey of apps listed in international curated health app libraries. JMIR Mhealth Uhealth. 2020 Feb 03;8(2):e16741. doi: 10.2196/16741. https://mhealth.jmir.org/2020/2/e16741/ v8i2e16741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan L, Kessie T, Caulfield B. Patient experiences of rehabilitation and the potential for an mhealth system with biofeedback after breast cancer surgery: qualitative study. JMIR Mhealth Uhealth. 2020 Jul 29;8(7):e19721. doi: 10.2196/19721. https://mhealth.jmir.org/2020/7/e19721/ v8i7e19721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Li F, Zhou C, Li J, Hong C, Tong Q. The effect of mobile applications for improving adherence in cardiac rehabilitation: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2019 Jul 12;19(1):166. doi: 10.1186/s12872-019-1149-5. https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-019-1149-5 .10.1186/s12872-019-1149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nussbaum R, Kelly C, Quinby E, Mac A, Parmanto B, Dicianno BE. Systematic review of mobile health applications in rehabilitation. Arch Phys Med Rehabil. 2019 Jan;100(1):115–127. doi: 10.1016/j.apmr.2018.07.439.S0003-9993(18)31175-4 [DOI] [PubMed] [Google Scholar]
- 35.Hurley M, Bearne LM. Physiotherapy for musculoskeletal conditions: more difficult than rocket science. Future Rheumatology. 2007 Apr;2(2):185–192. doi: 10.2217/17460816.2.2.185. [DOI] [Google Scholar]
- 36.36 The Statistic Portal. Global mobile OS market share in sales to end users from 1st quarter 2009 to 1st quarter 2018. Statista. [2020-11-09]. https://www.statista.com/statistics/266219/global-smartphone-sales-since-1st-quarter-2009-by-operating-system/
- 37.Bender JL, Yue RYK, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. 2013 Dec;15(12):e287. doi: 10.2196/jmir.2661. http://www.jmir.org/2013/12/e287/ v15i12e287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon WJ, Landman A, Zhang H, Bates DW. Beyond validation: getting health apps into clinical practice. NPJ Digit Med. 2020;3:14. doi: 10.1038/s41746-019-0212-z. http://europepmc.org/abstract/MED/32047860 .212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. JAHA. 2013 Nov 18;2(6):e000568–e000568. doi: 10.1161/jaha.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe M, Sauls B. The use of smartphone apps in clinical practice: A survey of South African physiotherapists. S Afr J Physiother. 2020 Apr 20;76(1):1327. doi: 10.4102/sajp.v76i1.1327. http://europepmc.org/abstract/MED/32391442 .SAJP-76-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berkowitz CM, Zullig LL, Koontz BF, Smith SK. Prescribing an app? Oncology providers’ views on mobile health apps for cancer care. JCO Clin Cancer Inform. 2017 Nov;(1):1–7. doi: 10.1200/CCI.17.00107. [DOI] [PubMed] [Google Scholar]
- 42.Unsworth H, Dillon B, Collinson L, Powell H, Salmon M, Oladapo T, Ayiku L, Shield G, Holden J, Patel N, Campbell M, Greaves F, Joshi I, Powell J, Tonnel A. The NICE Evidence Standards Framework for digital health and care technologies - Developing and maintaining an innovative evidence framework with global impact. Digit Health. 2021 Jun 24;7:20552076211018617. doi: 10.1177/20552076211018617. https://journals.sagepub.com/doi/10.1177/20552076211018617?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .10.1177_20552076211018617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zolnierek KBH, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009 Aug;47(8):826–34. doi: 10.1097/MLR.0b013e31819a5acc. http://europepmc.org/abstract/MED/19584762 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brewer BW, Cornelius AE, Van Raalte JL, Tennen H, Armeli S. Predictors of adherence to home rehabilitation exercises following anterior cruciate ligament reconstruction. Rehabil Psychol. 2013 Feb;58(1):64–72. doi: 10.1037/a0031297. http://europepmc.org/abstract/MED/23438001 .2013-06066-007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall AM, Ferreira P, Maher C, Latimer J, Ferreira ML. The influence of the therapist-patient relationship on treatment outcome in physical rehabilitation: a systematic review. Phys Ther. 2010 Aug;90(8):1099–110. doi: 10.2522/ptj.20090245.ptj.20090245 [DOI] [PubMed] [Google Scholar]
- 46.Martin SS, Feldman DI, Blumenthal RS, Jones SR, Post WS, McKibben RA, Michos ED, Ndumele CE, Ratchford EV, Coresh J, Blaha MJ. mActive: a randomized clinical trial of an automated mhealth intervention for physical activity promotion. J Am Heart Assoc. 2015 Nov 09;4(11) doi: 10.1161/JAHA.115.002239. https://www.ahajournals.org/doi/abs/10.1161/JAHA.115.002239?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .JAHA.115.002239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobrozsi Sarah, Panepinto J. Patient-reported outcomes in clinical practice. Hematology Am Soc Hematol Educ Program. 2015;2015:501–6. doi: 10.1182/asheducation-2015.1.501.2015/1/501 [DOI] [PubMed] [Google Scholar]
- 48.Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, Selby PJ. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. JCO. 2004 Feb 15;22(4):714–724. doi: 10.1200/jco.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 49.Taylor AH, May S. Threat and coping appraisal as determinants of compliance with sports injury rehabilitation: an application of Protection Motivation Theory. J Sports Sci. 1996 Dec;14(6):471–82. doi: 10.1080/02640419608727734. [DOI] [PubMed] [Google Scholar]
- 50.He D, Naveed M, Gunter C, Nahrstedt K. Security concerns in Android mHealth apps. AMIA Annu Symp Proc. 2014;2014:645–54. http://europepmc.org/abstract/MED/25954370 . [PMC free article] [PubMed] [Google Scholar]
- 51.Mosa ASM, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak. 2012;12:67. doi: 10.1186/1472-6947-12-67. http://www.biomedcentral.com/1472-6947/12/67 .1472-6947-12-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park S, Han KS, Kang C. Effects of exercise programs on depressive symptoms, quality of life, and self-esteem in older people: a systematic review of randomized controlled trials. Appl Nurs Res. 2014 Nov;27(4):219–26. doi: 10.1016/j.apnr.2014.01.004.S0897-1897(14)00032-9 [DOI] [PubMed] [Google Scholar]
- 53.Room J, Hannink E, Dawes H, Barker K. What interventions are used to improve exercise adherence in older people and what behavioural techniques are they based on? A systematic review. BMJ Open. 2017 Dec 14;7(12):e019221. doi: 10.1136/bmjopen-2017-019221. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=29247111 .bmjopen-2017-019221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peek K, Sanson-Fisher R, Mackenzie L, Carey M. Interventions to aid patient adherence to physiotherapist prescribed self-management strategies: a systematic review. Physiotherapy. 2016 Jun;102(2):127–35. doi: 10.1016/j.physio.2015.10.003.S0031-9406(15)03825-0 [DOI] [PubMed] [Google Scholar]
- 55.Park LG, Elnaggar A, Lee SJ, Merek S, Hoffmann TJ, Von Oppenfeld J, Ignacio N, Whooley MA. Mobile health intervention promoting physical activity in adults post cardiac rehabilitation: pilot randomized controlled trial. JMIR Form Res. 2021 Apr 16;5(4):e20468. doi: 10.2196/20468. https://formative.jmir.org/2021/4/e20468/ v5i4e20468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.mHealth app economics 2017. Research 2 Guidance. [2020-10-15]. https://research2guidance.com/product/connectivity-in-digital-health/
- 57.Nouri R, R Niakan Kalhori Sharareh, Ghazisaeedi M, Marchand G, Yasini M. Criteria for assessing the quality of mHealth apps: a systematic review. J Am Med Inform Assoc. 2018 Aug 01;25(8):1089–1098. doi: 10.1093/jamia/ocy050. http://europepmc.org/abstract/MED/29788283 .4996915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santo K, Richtering SS, Chalmers J, Thiagalingam A, Chow CK, Redfern J. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016 Dec 02;4(4):e132. doi: 10.2196/mhealth.6742. https://mhealth.jmir.org/2016/4/e132/ v4i4e132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stoyanov SR, Hides L, Kavanagh DJ, Zelenko O, Tjondronegoro D, Mani M. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015 Mar;3(1):e27. doi: 10.2196/mhealth.3422. http://mhealth.jmir.org/2015/1/e27/ v3i1e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peek N, Sujan M, Scott P. Digital health and care in pandemic times: impact of COVID-19. BMJ Health Care Inform. 2020 Jun 21;27(1):e100166. doi: 10.1136/bmjhci-2020-100166. https://informatics.bmj.com/lookup/pmidlookup?view=long&pmid=32565418 .bmjhci-2020-100166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lonergan PE, Washington Iii SL, Branagan L, Gleason N, Pruthi RS, Carroll PR, Odisho AY. Rapid utilization of telehealth in a comprehensive cancer center as a response to COVID-19. J Med Internet Res. 2020 Jun 21; doi: 10.2196/19322. doi: 10.2196/19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petracca F, Ciani O, Cucciniello M, Tarricone R. Harnessing digital health technologies during and after the COVID-19 pandemic: context matters. J Med Internet Res. 2020 Dec 30;22(12):e21815. doi: 10.2196/21815. https://www.jmir.org/2020/12/e21815/ v22i12e21815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eccleston C, Blyth FM, Dear BF, Fisher EA, Keefe FJ, Lynch ME, Palermo TM, Reid MC, Williams ACDC. Managing patients with chronic pain during the COVID-19 outbreak. Pain. 2020;161(5):889–893. doi: 10.1097/j.pain.0000000000001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Featured descriptors.
Additional features.


