Key Points
Question
Is self-monitoring of blood pressure using an enhanced device that pairs with a connected smartphone application more effective in reducing systolic blood pressure than self-monitoring using a standard device?
Findings
In this randomized clinical trial of 2101 patients with uncontrolled blood pressure, patients were randomly assigned to standard or enhanced self-monitoring of their blood pressure and mailed a self-monitoring device, after which usual care and in-person clinic blood pressure measurements from ambulatory visits during 6 months of follow-up were used to compare changes from baseline. The mean (SD) change in systolic blood pressure was −10.8 (18) mm Hg vs −10.6 (18) mm Hg in enhanced vs standard groups.
Meaning
This randomized clinical trial found that enhanced self-monitoring of blood pressure using a device paired with a connected smartphone application is not more effective than standard self-monitoring.
Abstract
Importance
Self-measured blood pressure (SMBP) with commercially available connected smartphone applications may help patients effectively use SMBP measurements.
Objective
To determine if enhanced SMBP paired with a connected smartphone application was superior to standard SMBP for blood pressure (BP) reduction or patient satisfaction.
Design, Setting, and Participants
This randomized clinical trial was conducted among 23 health systems participating in PCORnet, the National Patient-Centered Clinical Research Network, and included patients who reported having uncontrolled BP at their last clinic visit, a desire to lower their BP, and a smartphone. Enrollment and randomization occurred from August 3, 2019, to December 31, 2020, which was followed by 6 months of follow-up for each patient. Analysis commenced shortly thereafter.
Interventions
Eligible participants were randomly assigned to enhanced SMBP using a device that paired with a connected smartphone application (enhanced) or a standard device (standard). Participants received their device in the mail, along with web-based educational materials and phone-based support as needed. No clinician engagement was undertaken, and the study provided no special mechanisms for delivering measurements to clinicians for use in BP management.
Main Outcomes and Measures
Reduction in systolic BP, defined as the difference between clinic BP at baseline and the most recent clinic BP extracted from electronic health records at 6 months.
Results
Enrolled participants (1051 enhanced [50.0%] vs 1050 standard [50.0%]; 1191 women [56.7%]) were mostly middle-aged or older (mean [SD] age, 58 [13] years), nearly a third were Black or Hispanic (645 [31%]), and most were relatively comfortable using technology (mean [SD], 4.1 [1.1] of 5). The mean (SD) change in systolic BP from baseline to 6 months was −10.8 (18) mm Hg vs −10.6 (18) mm Hg (enhanced vs standard: adjusted difference, −0.19 mm Hg; 95% CI, −1.83 to 1.44; P = .81). Secondary outcomes were mostly null, except for documented attainment of BP control to lower than 140/<90 mm Hg, which occurred in 32% enhanced vs 29% standard groups (odds ratio, 1.15; 95% CI, 1.01-1.34). Most participants were very likely to recommend their SMBP device to a friend (70% vs 69%).
Conclusions and Relevance
This randomized clinical trial found that enhanced SMBP paired with a smartphone application is not superior to standard SMBP for BP reduction or patient satisfaction.
Trial Registration
ClinicalTrials.gov Identifier: NCT03796689
This randomized clinical trial explores if enhanced self-measured blood pressure (SMBP) paired with a connected smartphone application is superior to standard SMBP for blood pressure reduction or patient satisfaction.
Introduction
Uncontrolled blood pressure (BP) contributes to more than 500 000 deaths per year in the US.1 Self-measurement of BP (SMBP) between office visits can aid in diagnosing hypertension and titration of BP-lowering medication between visits. National and international guidelines recommend SMBP,2,3,4,5,6 and its importance for hypertension management is likely to grow in future years as telehealth-based care increasingly replaces in-person office visits.7,8
By itself, standard SMBP has minimal effect on BP control.3,9,10 To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions,3,9 and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.10 For example, SMBP with telehealth-based medication management by a pharmacist may be highly effective11; education and support programs for patients and clinicians can overcome barriers to medication titration12,13; and technology integration that transmits SMBP measurements directly to clinical teams may also result in improved BP control.14 However, these approaches require substantial programmatic investments by health systems in technology infrastructure, personnel, or clinical workflow redesign.
Devices that enhance standard SMBP with additional digital support from a paired and connected smartphone application are commercially available. These devices transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders (to measure BP and/or take medications), recommendations (for lifestyle interventions, medication adherence, or to discuss their BP with their clinician), and communications (eg, emailing a summary to a family member or clinician).15 Use of this functionality by patients does not require health system investment, and the devices are only slightly more expensive than standard SMBP devices.
However, it is unclear whether enhanced SMBP with a connected smartphone application is superior to standard SMBP. To inform clinicians and patients initiating SMBP about the decision of whether to purchase and learn how to use a standard device or a device enhanced with a connected smartphone application, we designed a randomized clinical trial to compare these 2 options.
Methods
Overview and Study Design
The PCORnet Blood Pressure Home Monitoring Study (BP Home) is a large simple pragmatic randomized clinical trial16 designed to generate real-world evidence17 comparing the effectiveness of SMBP with a standard BP monitor vs a BP monitor enhanced with connectivity to a smartphone application in adults with uncontrolled BP (Supplement 1). Outcomes were reduction in clinic systolic BP (SBP) from electronic health records (EHRs) analysis, and a patient-reported Net Promoter Score. The BP Home trial was conducted in the PCORnet BP Control Laboratory18 using PCORnet, the National Patient-Centered Clinical Research Network,19 and the Eureka Research Platform18,20 for electronic informed consent, randomization, and surveys. The study protocol was approved by the institutional review board at the University of California, San Francisco, and registered at ClinicalTrials.gov (NCT03796689). The target sample size (n = 2000) was determined to provide 80% power to detect a small treatment effect (0.125 standardized effect size) and modest heterogeneity across prespecified subgroups. No interim analyses were planned or conducted. A study protocol modification that was approved on August 30, 2019, clarified eligibility requirements and the primary outcome definition. We followed Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Study Sample
Patients meeting screening criteria were invited to participate by mail, email, phone, patient portal message, or in person and were directed to the online study portal for eligibility assessment. Adults 18 years or older were eligible if they made at least 1 ambulatory visit at a participating study site during the past year, self-reported an SBP greater than 145 mm Hg at their most recent clinic visit and a commitment to “work on lowering [their] blood pressure by 10 points or more,” had an email address, owned a smartphone (either Android [Google] or iOS [Apple]), and could complete online surveys in English. Participants were excluded if they owned and used a functioning SMBP device during the last 3 months or had an arm circumference of less than 22 cm or more than 42 cm as estimated with their age, sex, height, and weight.21 Eligible consenting participants were enrolled after completing baseline surveys and then randomized 1:1 to 2 intervention groups, with stratification by clinical site and block sizes randomly varying between 2 and 4. Neither participants, treating clinicians, nor study staff were masked to randomization assignment. Randomization commenced August 3, 2019, and ended December 31, 2020, after enrollment goals were met.
Interventions
The BP Home trial was designed to compare the effectiveness of 2 SMBP device types, which were delivered by mail in standard commercial packaging and with minimal additional training and support. Participants randomly assigned to the standard SMBP group were mailed an OMRON BP monitor (BP785N or BP7200), along with a set of instructional videos made by study staff and links to online SMBP resources. On request, study staff assisted participants by phone with use of their device.
Participants randomly assigned to the enhanced SMBP monitor with smartphone application group were mailed an OMRON BP monitor (BP786N or BP7250) with similar instructions. They also received instructions and support to install the OMRON Connect smartphone application and sync their device with their smartphone application periodically to transmit measurements. OMRON Connect features reminders to measure BP, BP measurement tracking, interpretation, annotation and visualization tools, and support for emailing a summary of their SMBP measurements. We did not provide any special connectivity with EHR systems or deliver BP measurements in any other way to clinicians. Individual clinicians were not masked, but the study did not directly provide them any information about patients’ enrollment in BP Home, randomization assignments, or SMBP measurements.
Measurements
Baseline surveys were used to collect self-reported age, sex assigned at birth, race and ethnicity (“select all that apply” categories), subjective social status (MacArthur Scale22), comfort using technology, and satisfaction with BP management (overall treatment, health care clinician, and BP medications). Follow-up surveys collected information about use and satisfaction with their SMBP device, and quality of shared decision-making adapted from the CollaboRATE survey.23
Electronic health record data through September 29, 2021, were extracted, in the PCORnet Common Data Model24 format, for enrolled participants who did not subsequently withdraw from the study. Electronic health record data from health care delivered before randomization were used to assess baseline comorbidities, smoking, and medication use. Electronic health record data from follow-up were used for BP outcomes and to describe processes relevant to BP control. Only BP measurements from ambulatory visits were included, and implausible measurements (SBP <70 mm Hg, SBP >250 mm Hg, DBP <50 mm Hg, or diastolic BP [DBP] >150 mm Hg) were excluded. When multiple BP measurements were recorded during a single ambulatory visit, the lowest SBP and the lowest DBP measurements were used.25
The primary BP outcome was reduction in SBP, defined as the difference between clinic SBP self-reported in the eligibility survey (patients were provided with their last available clinic SBP during enrollment) and clinic SBP at the most recent ambulatory visit during the 6-month follow-up; if the participant made no ambulatory visits with BP measurements within 6 months, the baseline measurement was carried forward (ie, the reduction was 0). The primary patient satisfaction outcome was the Net Promoter Score, defined by asking the likelihood the participant would recommend their device to a friend interested in managing their BP, with answers ranging from 0 (“not at all likely”) to 10 (“extremely likely”). As per published methods,26,27 persons answering 9 or 10 were “promoters”; 7 or 8 were “passives”; and <7 were “detractors,” and the score was calculated by subtracting the percentage of detractors from the percentage of promoters. Secondary outcomes included reduction in DBP, BP control (to <140/<90 mm Hg, and to <130/<80 mm Hg), and patient-reported survey outcomes at 6 months.
Statistical Analysis
We described characteristics at baseline, and BP-related health care delivered during follow-up and calculated standardized mean differences. We described BP trajectories by plotting a simple average of office SBP measurements collected from EHR data extraction by group and week since randomization.
For primary outcome analyses, we used multiple imputation, which was conducted simultaneously for continuous, binary, nominal, and ordinal variables using iterative chained equations under the assumption that the data were missing-at-random conditional on observed covariates and outcomes.28,29 The BP outcomes were treated as missing only if no EHR data were successfully extracted for a given participant; for participants with successfully extracted EHR data, the last measurement was carried forward.
Regression modeling with robust standard errors was used to account for site-level clustering and adjust for site participation in a concurrently running cluster-randomized quality improvement trial.18 Linear regression was used for continuous and Likert-type outcomes and logistic for binary outcomes. Multinomial logistic regression was used for the 3-level categorized primary patient satisfaction outcome (promoter, passive, or detractor); expected proportions by treatment assignment were then obtained using regression standardization and used to estimate the between-group difference in Net Promoter Scores.
Prespecified and post hoc subgroups were analyzed (and labeled as such). Statistical significance was set at P < .05; no adjustments for multiple hypothesis testing were applied. Complete case and as-treated analyses, EHR data–only analyses of the primary outcome, and a linear mixed modeling approach to analysis of clinic BP measurements were conducted as sensitivity analyses. SAS, version 9.4 (SAS Institute), was used for data management and simple statistics, and Stata, version 16.1 (StataCorp), was used for multiple imputation and outcome analyses.
Results
All randomized participants were enrolled and analyzed with 1 exception (Figure 1). Enrolled participants (n = 2101) were mostly middle-aged or older (mean [SD] age, 58 [13] years) and less often male (448 [43%] and 462 [44%]), nearly a third were non-Hispanic Black or Hispanic/Latinx individuals (645 [31%]), and most were comfortable using technology (mean [SD], 4.1 [1.1] of 5). Linked EHR data, which were available for nearly all participants (2062 [98%]), showed that diagnosis with comorbid conditions and prior use of BP medications were common. Characteristics were generally well balanced between groups (Table 1).
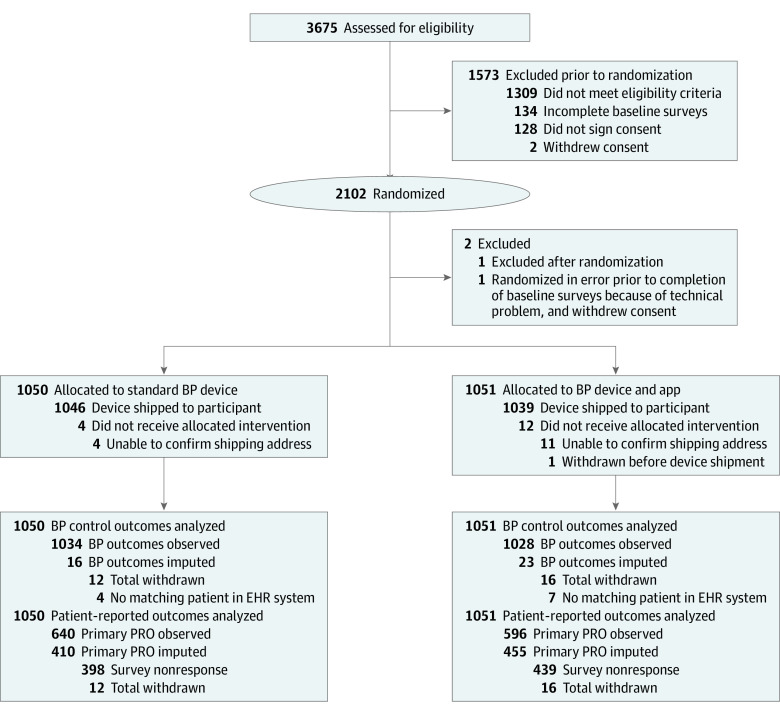
Figure 1. CONSORT Diagram.
All randomized participants were analyzed besides 1 that was randomized in error before completion of baseline surveys, and who withdrew consent before study staff could intervene. Per protocol, multiple imputation was used to impute missing clinic blood pressure (BP) measurements for 39 participants (1.9%) for whom linked electronic health record (EHR) data were not available, and to impute missing responses for the primary patient-reported outcome (PRO) (Net Promoter Score) for 865 participants (41%). App indicates smartphone application; SMBP, self-measured blood pressure.
Table 1. Characteristics of BP Home Participants, by Study Arm.
| Characteristic | SMBP, No. (%) | Standardized mean difference | |
|---|---|---|---|
| Standard | Enhanced with smartphone application | ||
| No. | 1050 | 1051 | NA |
| Self-reported | |||
| Age, mean (SD), y | 58 (13) | 59 (13) | 0.04 |
| Female sex | 602 (57) | 589 (56) | 0.03 |
| Male sex | 448 (43) | 462 (44) | 0.03 |
| Race and ethnicity | 0.12 | ||
| Hispanic/Latinx, any race | 101 (10) | 79 (8) | .12 |
| Non-Hispanic | |||
| Asian | 22 (2) | 6 (0.6) | |
| Black | 226 (22) | 239 (23) | |
| White | 666 (63) | 676 (64) | |
| Other/multiraciald | 35 (3) | 51 (5) | |
| Subjective social status, mean (SD)a | 6.2 (2.2) | 6.2 (2.2) | 0 |
| Eligibility BP, mean (SD), mm Hg | |||
| Systolic | 158 (12) | 157 (11) | −0.06 |
| Diastolic | 88 (12) | 88 (11) | −0.04 |
| Satisfaction with BP management, 1-5 scale, mean (SD)b | |||
| Overall | 3.7 (1.2) | 3.8 (1.1) | 0.02 |
| Your health care clinician | 4.3 (1.0) | 4.3 (1.1) | −0.02 |
| Your BP medications | 3.7 (1.2) | 3.7 (1.2) | −0.01 |
| Comfort using technology like a computer or smartphone, 1-5 scale, mean (SD) | 4.1 (1.1) | 4.1 (1.1) | −0.04 |
| From electronic health records c | |||
| Total with linked medical record data | 1034 (98) | 1028 (98) | −0.05 |
| Smoking status | |||
| Current | 218 (21) | 208 (20) | 0.03 |
| Past | 305 (29) | 306 (30) | |
| Never | 460 (44) | 463 (45) | |
| Missing or unclear in EHR data | 51 (5) | 51 (5) | |
| Diabetes | 200 (19) | 217 (21) | 0.04 |
| Congestive heart failure | 30 (2.9) | 33 (3.2) | 0.02 |
| Coronary artery disease | 75 (7.3) | 93 (9.1) | 0.07 |
| Chronic obstructive pulmonary disease | 31 (3.0) | 46 (4.5) | 0.08 |
| End stage kidney disease | 16 (1.6) | 12 (1.2) | −0.03 |
| No. of current BP medication classes of medications prescribed during the past year | |||
| 0 | 338 (33) | 307 (30) | 0.08 |
| 1 | 239 (23) | 249 (24) | |
| 2 | 218 (21) | 234 (23) | |
| 3 | 134 (13) | 128 (12) | |
| ≥4 | 105 (10) | 110 (11) | |
| BP medication class prescribed in the past year | |||
| Thiazide or thiazide-like diuretic | 295 (29) | 261 (25) | −0.07 |
| ACE inhibitor | 304 (29) | 282 (27) | −0.04 |
| ARB | 184 (18) | 201 (20) | 0.05 |
| Calcium channel blocker | 298 (29) | 304 (30) | 0.02 |
| β-Blocker | 272 (26) | 309 (30) | 0.08 |
| α-Blocker | 18 (1.7) | 28 (2.7) | 0.07 |
| Aldosterone inhibitor | 28 (2.7) | 38 (3.7) | 0.06 |
| K-sparing diuretic | 18 (1.7) | 12 (1.2) | −0.05 |
| Centrally acting | 49 (4.7) | 45 (4.4) | −0.02 |
| Renin antagonist | 0 | 0 | 0 |
| Vasodilator | 46 (4.5) | 66 (6.4) | 0.09 |
| Loop diuretic | 61 (5.9) | 79 (7.7) | 0.07 |
| Fixed-dose combination medication prescribed during past year | 85 (8.2) | 71 (6.9) | −0.05 |
| No. of ambulatory visits with BP measured during the past year | |||
| 0 | 56 (5.4) | 43 (4.2) | 0.10 |
| 1 | 205 (20) | 209 (20) | |
| 2 | 186 (18) | 174 (17) | |
| 3 | 162 (16) | 143 (14) | |
| ≥4 | 425 (41) | 459 (45) | |
| Self-reported eligibility SBP/DBP vs past clinic SBP/DBP | |||
| Exact match at most recent visit | 635 (61) | 631 (61) | 0 |
| Exact match, not at most recent visit | 205 (20) | 206 (20) | |
| No exact match | 194 (19) | 191 (19) | |
| Lowest BP at most recent encounter, mean (SD), mm Hg | |||
| Systolic | 150 (16) | 150 (17) | −0.03 |
| Diastolic | 85 (12) | 85 (12) | −0.01 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; EHR, electronic health record; NA, not applicable; SMBP, self-measured blood pressure.
MacArthur Subjective Social Scale, 1-10 scale.22 One participant was missing data for this baseline characteristic because of a technical malfunction.
Participants were allowed to choose NA if, for example, they were not currently being treated for hypertension; these responses were excluded from calculations of the mean, SD, and P value. A total of 165 (7.9%) chose NA for “overall,” 74 (3.5%) chose NA for “your health care clinician,” and 423 (20.1%) chose NA for “your BP medications.”
EHR data were missing for 39 participants (Figure 1). Descriptive statistics from EHR data in the table exclude these 39 participants.
For descriptive purpose, participants choosing "other" or multiple categories were grouped together.
An SMBP device was shipped to 98% of participants; 1390 (66%) confirmed receipt and 440 (42%) of the enhanced group actively confirmed use of the smartphone application (Table 2). Health care utilization patterns during the 6 months after randomization were similar by group. Approximately one-quarter of participants recorded an ambulatory visit each month (average, 2 visits total), and half of participants received a prescription for a BP medication. The SBP trajectories were similar for standard and enhanced groups (Figure 2); in both groups, average clinic SBP rose before enrollment, fell quickly after randomization, and then stabilized through the end of 6 months.
Table 2. Blood Pressure–Related Health Care Utilized During 6 Months of Follow-up by Study Arm.
| Health care process | SMBP, No. (%) | P value | |
|---|---|---|---|
| Standard | Enhanced with smartphone application | ||
| No. | 1050 | 1051 | NA |
| Self-reported | |||
| Confirmeda | NA | ||
| Receipt of study device | 702 (67) | 688 (65) | .50 |
| Use of smartphone application | NA | 440 (42) | NA |
| From EHRsb | |||
| Ambulatory visits with BP measured, total No. | |||
| 0 | 398 (38) | 382 (37) | .14 |
| 1 | 233 (23) | 248 (24) | |
| 2 | 155 (15) | 124 (12) | |
| ≥3 | 248 (24) | 274 (27) | |
| Ambulatory visits with BP measured, moc | |||
| Any in month 1 after randomization | 278 (27) | 295 (29) | .36 |
| Any in month 2 | 254 (25) | 242 (24) | .59 |
| Any in month 3 | 249 (24) | 261 (25) | .49 |
| Any in month 4 | 248 (24) | 252 (25) | .78 |
| Any in month 5 | 243 (24) | 234 (23) | .69 |
| Any in month 6 | 229 (22) | 231 (22) | .86 |
| Total, mean (SD) | 2.0 (3.1) | 2.0 (3.1) | .94 |
| Days from randomization to last observed BP measurement during 6 mo follow-up, mean (SD) | 71 (70) | 73 (71) | .71 |
| Total clinic BP measurements | |||
| Mean (SD) | 3.9 (15) | 4.3 (25) | .63 |
| Median (IQR) | 1 (0-3) | 1 (0-3) | .64 |
| No. of BP medication prescription eventsd | |||
| 0 | 528 (51) | 470 (46) | .09 |
| 1 | 155 (15) | 166 (16) | |
| 2 | 137 (13) | 143 (14) | |
| ≥3 | 214 (21) | 249 (24) | |
| Any new class of BP medications prescribed | 192 (19) | 237 (23) | .01 |
| Most recent clinic BP measurements | |||
| Participants with any valid clinic BP measurements during 6-mo follow-up | 636 (62) | 646 (63) | .53 |
| Systolic, mean (SD)e | 140 (19) | 140 (19) | .53 |
| Diastolic, mean (SD)e | 81 (12) | 81 (11) | .99 |
Abbreviations: BP, blood pressure; EHR, electronic health record; NA, not applicable; SMBP, self-measured blood pressure.
Some participants actively confirmed that they had received a study device (by responding to a survey), and some in the enhanced group were able to donate BP measurements from their smartphone to the study database (which we requested), so we know they used their smartphone application at least once. For participants who did not provide these confirmations, we do not know whether they used their device or smartphone application.
EHR data were missing for 39 participants (Figure 1). Descriptive statistics in the table from EHR data exclude these 39 participants.
We analyzed visits appearing to be face-to-face ambulatory visits from the PCORnet Common Data Model, but these may also represent telehealth visits or other visits during which BP was recorded. At telehealth visits, patients may have been asked to take their BP at home and self-report it to their clinician.
We extracted medication prescribing orders, including medication name, but had inconsistent information about medication dose.
These descriptive statistics do not include the 780 participants with EHR data1 but without a valid clinic-based BP measurement during follow-up (ie, without carry forward of baseline data).
Figure 2. Average Clinic Systolic Blood Pressure (BP) by Week.
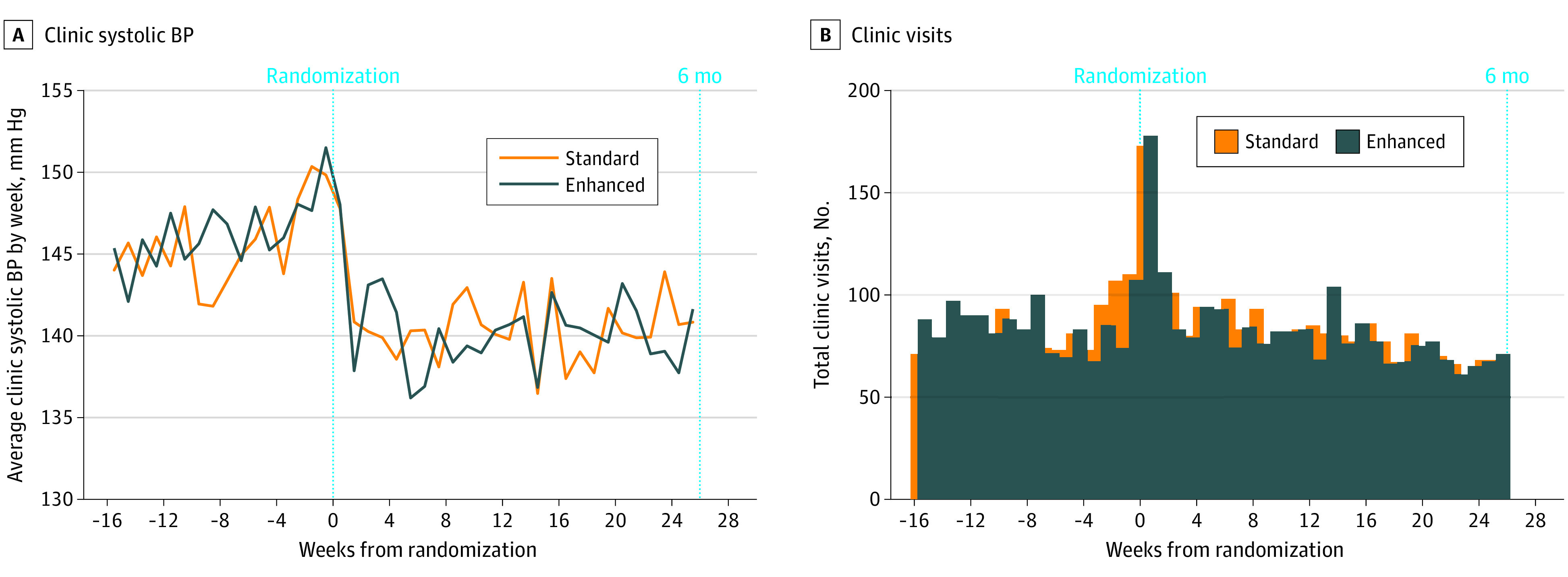
Average clinic systolic blood pressures (BPs) recorded in the electronic health record (A) and total number of clinic visits (B) by week for standard (orange) and enhanced (blue) groups. The excess clinic visits occurring in week 0 are explained by visits on the day of randomization (in-clinic recruitment).
The mean (SD) change in SBP, with carry forward for participants without any clinic visits during follow-up (primary BP outcome), was −10.6 (18) mm Hg in the standard group and −10.8 (18) mm Hg in the enhanced group (difference, –0.19 mm Hg; 95% CI, −1.83 to 1.44; P = .81). Other BP control outcomes were also mostly null, except for documented attainment of BP control to less than 140/90 mm Hg, which occurred in 32% vs 29% (enhanced vs standard: odds ratio, 1.15; 95% CI, 1.01-1.34; nominal P = .03) (Table 3).
Table 3. Study Outcomes by Study Arm With Multiple Imputation of Missing Values.
| Outcome | SMBP, mean (SD) | Comparing enhanced with standard, difference (95% CI) | P valuea | |
|---|---|---|---|---|
| Standard | Enhanced with smartphone application | |||
| No. | 1050 | 1051 | NA | |
| Blood pressure control outcomes b | ||||
| SBP change at 6 mo (primary blood pressure control outcome), mean (SD)c | −10.6 (18) | −10.8 (18) | −.19 (−1.83 to 1.44) | .81 |
| DBP change at 6 mo, mean (SD)c | −4.2 (10) | −4.3 (10) | −.13 (−1.10 to 0.84) | .79 |
| SBP reduction >10 mm Hg at 6 mo, %c | 41 | 40 | OR, 0.98 (0.88 to 1.09) | .72 |
| BP control to <140/<90 mm Hg at 6 mo, % | 29 | 32 | OR, 1.17 (1.01 to 1.34) | .03 |
| BP control to <130/<80 mm Hg at 6 mo, % | 12 | 13 | OR, 1.06 (0.76 to 1.48) | .74 |
| Patient-reported outcomes d | ||||
| How likely are you to recommend (device) to a friend (0-10), % | ||||
| 0-6 (detractor) | 12 | 11 | NA | NA |
| 7-8 (passive) | 19 | 19 | NA | NA |
| 9-10 (promoter) | 69 | 70 | NA | NA |
| Net Promoter Score (primary patient satisfaction outcome)e | .57 | .59 | .02 (−0.05 to 0.08) | .63 |
| Use of device during last month, % | ||||
| Never | 5 | 5 | NA | NA |
| Less than once a week | 14 | 12 | NA | NA |
| About once a week | 26 | 20 | NA | NA |
| 2-3 Times a week | 24 | 21 | NA | NA |
| 4 Or more times a week | 31 | 42 | OR of being in a higher category, 1.44 (1.10 to 1.90) | .01 |
| Shared measurements with your physician during last month, shared, % | 48 | 44 | OR, 0.85 (0.67 to 1.10) | .22 |
| How satisfied are you with (1-5 scale), mean (SD)f | ||||
| Your overall treatment | 4.4 (1.0) | 4.3 (1.0) | −.10 (−0.23 to 0.02) | .10 |
| Your health care clinician | 4.3 (1.1) | 4.3 (1.1) | −0.04 (−0.16 to 0.09) | .51 |
| Your blood pressure medication(s) | 4.1 (1.2) | 4.1 (1.1) | −0.03 (−0.14 to 0.09) | .60 |
| How much do you agree with the following statements about your home BP monitoring device (1-7 scale), mean (SD) | ||||
| My device is easy to use | 6.4 (1.4) | 6.4 (1.4) | −0.03 (−0.20 to 0.14) | .70 |
| Using my device improves my ability to manage my BP | 6.1 (1.5) | 6.0 (1.5) | −0.12 (−0.27 to 0.03) | .11 |
| I find my device useful for managing my blood pressure | 6.0 (1.5) | 5.9 (1.6) | −0.15 (−0.35 to 0.06) | .13 |
| My health care clinician thinks I should regularly use my deviceg | 5.7 (1.9) | 5.7 (1.8) | 0 (−0.24 to 0.24) | .99 |
| Overall, I am satisfied with my experience using my device | 6.3 (1.4) | 6.3 (1.5) | −0.04 (−0.20 to 0.12) | .59 |
| Quality of shared decision-making score (CollaboRATE-5)h | ||||
| Mean (SD) | 3.5 (0.9) | 3.6 (0.9) | 0.08 (−0.05 to 0.22) | .19 |
| No. with the top score | 5.5 | 6.6 | OR, 1.20 (0.81 to 1.78) | .36 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; EHR, electronic health record; NA, not applicable; OR, odds ratio; SBP, systolic blood pressure; SMBP, self-measured blood pressure.
Summary measures of association and P values were calculated using regression models that included a fixed effect for a clinic’s participation and randomization assignment in a concurrent clinic-level quality improvement intervention,18 and accounting for clustering by clinical site with robust standard errors (see Methods). For continuous measurements, a linear model was used and differences calculated; for dichotomous outcomes, a logistic model was used and ORs calculated; for ordinal variables, ordinal logistic regression was used, and ORs for being in the next higher category were calculated.
Multiple imputation was used to impute a follow-up BP measurement for 39 participants with missing EHR data (see Methods) at the 6-month point. For patients with available EHR data (n = 2066), the last clinic measurement was carried forward. For patients with no clinic measurements during follow-up, the baseline measurement was carried forward (ie, the reduction was 0; see Methods).
Difference in BP between self-reported most recent BP measurements at baseline (at the time of randomization) and the most recent office BP measurement from EHR data within 6 months (183 days) of randomization. If no clinic BP measurements were made during follow-up, the documented reduction was 0.
Multiple imputation was used to impute survey responses at the 6-month point (see Methods). A total of 865 participants required imputation for the Net Promoter Score, 1059 for use of device during the last month, 1057 for shared measurements with a physician during the last month, 1078 for “how satisfied were you with” survey questions, 1065 for “how much do you agree” survey about home BP monitoring device, and 1594 for the quality of shared decision-making score.
The Net Promoter Score is calculated as the proportion of promoters minus the proportion of detractors.26,27
Satisfaction scores additionally excluded persons answering NA (7% for “overall treatment,” 9% for “your health care clinician,” and 20% for “your blood pressure medications.”
Additionally excludes persons answering “don’t know” (23%).
Quality of shared decision-making uses responses to 3 questions (1-5 each) adapted from the CollaboRATE-5.23 We calculated the mean score and the proportion with the top score (answering the top of the scale on all 3 items) according to published methods.
Most participants were very likely to recommend their device to a friend (70% enhanced vs 69% standard), with very little difference between groups in the primary patient satisfaction outcome (Net Promoter Score, 0.59 vs 0.57; difference, 0.02; 95% CI, −0.05 to 0.09; P = .58). Participants in the enhanced group were more likely to report higher levels of SMBP device use during the last month, but participants were no more likely to have shared their measurements with their physician during the last month, and did not otherwise appear to find more satisfaction with their device, BP management, or clinician (Table 3).
No significant heterogeneity in primary outcomes (BP or satisfaction) was detected in subgroup analyses (eFigure 1 in Supplement 2). Complete case analyses (eTables 1 and 2 in Supplement 2), as-treated analyses (eTables 3 and 4 in Supplement 2), EHR data–only analyses (eTable 5 in Supplement 2), and an analysis using linear mixed modeling of clinic BP measurements (eTable 6 in Supplement 2) showed similarly null results. eFigure 2 in Supplement 2 describes SMBP measurements over time in a subset of the enhanced group.
Discussion
We conducted a large simple pragmatic randomized clinical trial to compare the effectiveness of 2 currently available strategies for managing uncontrolled BP: SMBP using a standard device, or SMBP using an enhanced device with a connected smartphone application. The study sample was large, with representation of Black and Hispanic/Latinx patients. Using clinic BP measurements from EHR data for follow-up, we found no difference in SBP reduction; both groups had an apparent reduction of approximately 11 mm Hg from their baseline self-reported office SBP to their most recent office BP measurement. Much of this reduction appeared quickly after randomization, presumably in part because of regression to the mean.30,31
Not all participants responded to surveys after randomization, and we could only confirm receipt of the device among two-thirds of participants. The results, with and without imputation of missing responses, and an as-treated analysis among only patients confirming receipt of the study device, showed no significant differences between groups. Most respondents in both arms liked their assigned device and were very likely to recommend it to a friend.
This study’s primary findings are consistent with prior literature. A series of large systematic reviews with meta-analyses in the last decade have analyzed the association of SMBP with BP outcomes.9,10,32,33,34 Each identified modest average effects with significant heterogeneity in the effect size that appeared at least partially explained by the presence and type of other support (cointerventions) provided to patients. Tucker et al10 conducted an individual participant meta-analysis, categorizing the intensity of cointervention as minimal additional contact (level 1), automated feedback or support (level 2), active intervention (level 3), or significant tailored support (level 4); they found that the degree of reduction in SBP was directly associated with the cointervention intensity level, with no significant association with SBP-lowering from levels 1 or 2. Enhanced SMBP with a smartphone application, the active intervention we tested in BP Home, could be categorized as a level 2 cointervention; despite our hopes that advances in smartphone technology and application design might engage patients and provide an efficient and effective mechanism for supporting SMBP, it appears to have had no greater effect on SBP reduction than SMBP with other level 2 cointerventions or SMBP alone (level 1).
We found a modest difference between arms in an important secondary outcome (attainment of BP control to <140/90 mm Hg) that was nominally significant without adjustment for multiple comparisons. Tucker et al10 reported heterogeneity in this end point for level 2 SMBP interventions with a summary relative risk for being uncontrolled of 0.90 (95% CI, 0.69-1.15); the results of the present study are consistent with this finding (the comparable statistic in BP Home would be 0.96 [95% CI, 0.71-0.68 from Table 3]), but they are also consistent with chance given the number of secondary outcomes we analyzed in BP Home.
Four recent randomized clinical trials of SMBP interventions are worth discussing more specifically. TASMINH435 reported a small statistically significant benefit from self-monitoring vs usual care at 13 months, but no clear additional benefit from telemonitoring. The Smart Hypertension Control Study36 found no significant benefits in SBP reduction at 6 months from an artificial intelligence–enhanced conversational smartphone application vs a regular smartphone application similar to the enhanced arm of BP Home. HOME BP12 (not to be confused with BP Home) found a small but significant benefit in SBP reduction at 12 months from an active digital SMBP intervention (vs usual care) that included active motivational education for patients and clinicians and development of an individualized stepwise drug titration plan for each patient and then alerted clinicians when SMBP measurements merited drug titration. HERB-DH137 found a small benefit from an intensive digital intervention with an integrated web application for clinicians vs standard lifestyle modification alone. Taken together, these studies, along with the meta-analyses and the results of the current study, consistently support the premise that SMBP itself produces small reductions in SBP that are not augmented much by simple digitally mediated cointerventions without clinician-engaged support.
Limitations
The BP Home trial is a large simple pragmatic trial16 with several limitations. In contrast to many BP control intervention trials, we did not conduct in-person research visits to measure BP via a research protocol or initiate SMBP with dedicated in-person education or device setup, and we had limited information about use of the device and fidelity to recommended SMBP regimens (we could not even confirm device or smartphone application use for many participants). Instead, we mailed commercially available devices to participants with web-based instruction materials and provided phone-based setup support when needed, which was a feasibly scalable approach to delivering SMBP that could be delivered to large populations of patients with uncontrolled BP without disruptions to clinical workflow. We used clinic BPs recorded during usual health care delivery, which are likely to be subject to more measurement error than protocolized research visit measurements, do not occur on a fixed schedule, and do not represent physiologic BP as accurately as research measurements. Average follow-up time from baseline to the last observed BP measurement was less than 3 months. We may have missed clinic visits and BP measurements that occurred in a different health system if participants received care elsewhere. However, the outcome definition is pragmatic: demonstration of BP control using BP measurements recorded by clinicians during an ambulatory visit is how quality of care for BP management is measured (National Quality Form 0018: Controlling High Blood Pressure25). Immediate reactions to elevated BP in both groups and regression to the mean30,31 may have made it harder to detect a benefit from enhanced SMBP. We evaluated a single smartphone application that, while an industry standard, may not be the most effective of the many currently available on the market.15 Much of this study was conducted during the COVID-19 pandemic when SMBP use patterns may have been unusual. Neither BP Home participants nor clinicians were masked to treatment assignment.
Conclusions
The results of this randomized clinical trial provide a definitive answer that BP Home provides a definitive answer to the simple, pragmatic question posed by the patient advisory board: should patients with uncontrolled BP be directed to purchase (or be provided) an enhanced SMBP device, spend the time to download and connect their device to a smartphone application, and learn how to use the application to track and use their SMBP measurements, or should they simply pursue standard SMBP? The answer from BP Home is clear: there is no significant benefit from enhanced vs standard SMBP when delivered without additional cointerventions or support. Enhanced SMBP does not provide any additional reduction in BP, and patients would not recommend an enhanced SMBP device to their peers more than a standard device. Future research should continue to evaluate novel technologies, which may yet provide a scalable and affordable approach to achieving better population-level BP control.
Trial protocol
eFigure 1. Subgroup analyses
eTable 1. Complete case analysis of blood pressure control outcomes, by study arm, among only participants without missing electronic health record data
eTable 2. Complete case analysis of patient-reported outcomes by study arm, among only participants without missing survey data
eTable 3. As-treated analysis of blood pressure control outcomes, by study arm, among only participants confirming receipt of the study device
eTable 4. As-treated analysis of patient-reported outcomes by study arm, among only participants without missing survey data
eTable 5. EHR data only analysis of the primary blood pressure control outcome, by study arm
eTable 6. Linear mixed modeling approach using EHR data only of the primary blood pressure control outcome, by study arm
eFigure 2. Average clinic BP and SMBP measurements, by week, in a subset of the enhanced group
Data sharing statement
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35-e66. doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimbo D, Artinian NT, Basile JN, et al. ; American Heart Association and the American Medical Association . Self-measured blood pressure monitoring at home: a joint policy statement from the American Heart Association and American Medical Association. Circulation. 2020;142(4):e42-e63. doi: 10.1161/CIR.0000000000000803 [DOI] [PubMed] [Google Scholar]
- 5.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi: 10.1097/HJH.0000000000002453 [DOI] [PubMed] [Google Scholar]
- 6.Andraos J, Munjy L, Kelly MS. Home blood pressure monitoring to improve hypertension control: a narrative review of international guideline recommendations. Blood Press. 2021;30(4):220-229. doi: 10.1080/08037051.2021.1911622 [DOI] [PubMed] [Google Scholar]
- 7.Tuckson RV, Edmunds M, Hodgkins ML. Telehealth. N Engl J Med. 2017;377(16):1585-1592. doi: 10.1056/NEJMsr1503323 [DOI] [PubMed] [Google Scholar]
- 8.Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Trends in outpatient care delivery and telemedicine during the COVID-19 pandemic in the US. JAMA Intern Med. 2021;181(3):388-391. doi: 10.1001/jamainternmed.2020.5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185-194. doi: 10.7326/0003-4819-159-3-201308060-00008 [DOI] [PubMed] [Google Scholar]
- 10.Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389. doi: 10.1371/journal.pmed.1002389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46-56. doi: 10.1001/jama.2013.6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McManus RJ, Little P, Stuart B, et al. ; HOME BP investigators . Home and Online Management and Evaluation of Blood Pressure (HOME BP) using a digital intervention in poorly controlled hypertension: randomised controlled trial. BMJ. 2021;372:m4858. doi: 10.1136/bmj.m4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312(8):799-808. doi: 10.1001/jama.2014.10057 [DOI] [PubMed] [Google Scholar]
- 14.Stoddart A, Hanley J, Wild S, et al. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): cost and cost-effectiveness analysis of a randomised controlled trial. BMJ Open. 2013;3(5):e002681. doi: 10.1136/bmjopen-2013-002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alessa T, Hawley MS, Hock ES, de Witte L. Smartphone apps to support self-management of hypertension: review and content analysis. JMIR Mhealth Uhealth. 2019;7(5):e13645. doi: 10.2196/13645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The National Academies Collection . Large Simple Trials and Knowledge Generation in a Learning Health System: Workshop Summary. National Academies Press; 2013. [PubMed] [Google Scholar]
- 17.US Food and Drug Administration . Real-world evidence. Accessed February 25, 2022. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence
- 18.Pletcher MJ, Fontil V, Carton T, et al. The PCORnet Blood Pressure Control Laboratory: a platform for surveillance and efficient trials. Circ Cardiovasc Qual Outcomes. 2020;13(3):e006115. doi: 10.1161/CIRCOUTCOMES.119.006115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forrest CB, McTigue KM, Hernandez AF, et al. PCORnet® 2020: current state, accomplishments, and future directions. J Clin Epidemiol. 2021;129:60-67. doi: 10.1016/j.jclinepi.2020.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.University of California, San Francisco . Eureka: we build online medical research studies. Accessed May 12, 2022. https://info.eurekaplatform.org/
- 21.Nwankwo T, Ostchega Y, Zhang G, Hughes JP. Validating prediction equations for mid-arm circumference measurements in adults: National Health and Nutrition Examination Survey, 2001-2012. Blood Press Monit. 2015;20(3):157-163. doi: 10.1097/MBP.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19(6):586-592. doi: 10.1037/0278-6133.19.6.586 [DOI] [PubMed] [Google Scholar]
- 23.Forcino RC, Barr PJ, O’Malley AJ, et al. Using CollaboRATE, a brief patient-reported measure of shared decision making: results from three clinical settings in the United States. Health Expect. 2018;21(1):82-89. doi: 10.1111/hex.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patient-Centered Outcomes Research Institute . PCORnet Common Data Model (CDM). Accessed July 20, 2022. https://pcornet.org/pcornet-common-data-model/ [PMC free article] [PubMed]
- 25.National Quality Forum . 0018: Controlling high blood pressure. Accessed January 29, 2021. https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-Measures/2020_Measure_236_MIPSCQM.pdf
- 26.Hamilton DF, Lane JV, Gaston P, et al. Assessing treatment outcomes using a single question: the net promoter score. Bone Joint J. 2014;96-B(5):622-628. doi: 10.1302/0301-620X.96B5.32434 [DOI] [PubMed] [Google Scholar]
- 27.Krol MW, de Boer D, Delnoij DM, Rademakers JJ. The Net Promoter score—an asset to patient experience surveys? Health Expect. 2015;18(6):3099-3109. doi: 10.1111/hex.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 29.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. Springer; 2011. [Google Scholar]
- 30.Morton V, Torgerson DJ. Effect of regression to the mean on decision making in health care. BMJ. 2003;326(7398):1083-1084. doi: 10.1136/bmj.326.7398.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N, Atkins ER, Salam A, Moore MN, Sharman JE, Rodgers A. Regression to the mean in home blood pressure: analyses of the BP GUIDE study. J Clin Hypertens (Greenwich). 2020;22(7):1184-1191. doi: 10.1111/jch.13933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bray EP, Holder R, Mant J, McManus RJ. Does self-monitoring reduce blood pressure? meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42(5):371-386. doi: 10.3109/07853890.2010.489567 [DOI] [PubMed] [Google Scholar]
- 33.Sheppard JP, Tucker KL, Davison WJ, et al. Self-monitoring of blood pressure in patients with hypertension-related multi-morbidity: systematic review and individual patient data meta-analysis. Am J Hypertens. 2020;33(3):243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shantharam SS, Mahalingam M, Rasool A, et al. Systematic review of self-measured blood pressure monitoring with support: intervention effectiveness and cost. Am J Prev Med. 2022;62(2):285-298. doi: 10.1016/j.amepre.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McManus RJ, Mant J, Franssen M, et al. ; TASMINH4 investigators . Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391(10124):949-959. doi: 10.1016/S0140-6736(18)30309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persell SD, Peprah YA, Lipiszko D, et al. Effect of home blood pressure monitoring via a smartphone hypertension coaching application or tracking application on adults with uncontrolled hypertension: a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200255. doi: 10.1001/jamanetworkopen.2020.0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kario K, Nomura A, Harada N, et al. Efficacy of a digital therapeutics system in the management of essential hypertension: the HERB-DH1 pivotal trial. Eur Heart J. 2021;42(40):4111-4122. doi: 10.1093/eurheartj/ehab559 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eFigure 1. Subgroup analyses
eTable 1. Complete case analysis of blood pressure control outcomes, by study arm, among only participants without missing electronic health record data
eTable 2. Complete case analysis of patient-reported outcomes by study arm, among only participants without missing survey data
eTable 3. As-treated analysis of blood pressure control outcomes, by study arm, among only participants confirming receipt of the study device
eTable 4. As-treated analysis of patient-reported outcomes by study arm, among only participants without missing survey data
eTable 5. EHR data only analysis of the primary blood pressure control outcome, by study arm
eTable 6. Linear mixed modeling approach using EHR data only of the primary blood pressure control outcome, by study arm
eFigure 2. Average clinic BP and SMBP measurements, by week, in a subset of the enhanced group
Data sharing statement