Abstract
Individually, D1 and D3 dopamine receptors (D1R and D3R, respectively) have been implicated in L-DOPA-induced dyskinesia (LID). Of late, direct D1R-D3R interactions have been linked to LID yet remain enigmatic. Therefore, the current research sought to characterize consequences of putative D1R-D3R interactions in dyskinesia expression and in LID-associated downstream cellular signaling. To do so, adult male Sprague-Dawley hemi-parkinsonian rats were given daily L-DOPA (6 mg/kg; s.c.) for 2 weeks to establish stable LID, as measured via the abnormal voluntary movements (AIMs) scale. Thereafter, rats underwent dose-response AIMs testing for the D1R agonist SKF38393 (0, 0.3, 1.0, 3.0 mg/kg) and the D3R agonist, PD128907 (0, 0.1, 0.3, 1.0 mg/kg). Each agonist dose-dependently induced dyskinesia, implicating individual receptor involvement. More importantly, when threshold doses were co-administered, rats displayed synergistic exacerbation of dyskinesia. Interestingly, this observation was not mirrored in general locomotor behaviors, highlighting a potentially dyskinesia-specific effect. To illuminate the mechanisms by which D1R-D3R co-stimulation led to in vivo synergy, levels of striatal phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2) were quantified after administration of SKF38393 and/or PD128907. Combined agonist treatment synergistically drove striatal pERK1/2 expression. Together, these results support the presence of a functional, synergistic interaction between D1R and D3R that manifests both behaviorally and biochemically to drive dyskinesia in hemi-parkinsonian rats.
1. Introduction
For over half a century, dopamine (DA) replacement therapy by means of the DA precursor, L-DOPA, has been the most efficacious and non-invasive method of Parkinson’s Disease (PD) symptom management. However, chronic L-DOPA treatment often leads to the development of a spectrum of side effects including motor fluctuations and L-DOPA induced dyskinesia (LID) characterized by abnormal involuntary movements (AIMs; (Smith et al., 2012). About 90% of PD patients develop LID within their first decade of treatment and by year 15, a staggering 95% of patients display some LID (Ahlskog and Muenter, 2001; Hely et al., 2005). Understanding the mechanisms that portend LID is crucial in the pursuit of better pharmacotherapy with less aversive side effects.
The role of D1 receptors (D1R) in LID are particularly well characterized. D1R sensitized in LID and multiple techniques have demonstrated their role in LID (Darmopil et al., 2009; Guigoni et al., 2007; Perez et al., 2017; Westin et al., 2007). Unfortunately, strategies to suppress D1R signaling have been hindered by a reduction in the therapeutic efficacy of L-DOPA or global motor suppression, suggesting that intact D1R signaling likely plays a role in both the therapeutic and dyskinetic components of L-DOPA therapy (Chiken et al., 2015; Gomez-Mancilla and Bedard, 1991; Grondin et al., 1999).
Until more recently, the role of D3 receptors (D3R) in LID has been understudied. This is, in part, because within the intact dorsal striatum of the rat, D3R is sparsely expressed (Ridray et al., 1998). However, D3R is significantly upregulated within this area following L-DOPA administration where they co-localize with D1R expressing neurons (Bordet et al.,1997; Farre et al., 2015; Solis et al., 2015). This shift is of particular importance considering D3R displays the highest affinity for DA and consequently, expression changes can significantly influence dopaminergic tone (Sokoloff et al., 1990). In fact, overexpression of D3R in rats lacking DA lesions is sufficient to precipitate L-DOPA-induced dyskinesia (Cote et al., 2014; Cote and Kuzhikandathil, 2015). Furthermore, mice lacking D3R have tempered LID development (Cote et al., 2014; Solis et al., 2015). Although D3R is clearly involved in dyskinesia manifestation, pharmacological interrogation of its role has been both challenging and inconsistent. Some report that LID is resistant to D3R antagonism or interferes with L-DOPA efficacy (Hsu et al.,2004; Mela et al., 2010; Silverdale et al., 2004), whereas others report that normalizing D3R activity can attenuate LID expression (Bezard et al., 2003; Sebastianutto et al., 2016; Visanji et al., 2009). Fortunately, new DA receptor targets have emerged that have reinvigorated the field (Cortes et al., 2016). More specifically, direct interactions between G-protein-coupled receptors have emerged as promising therapeutic targets (Ferre et al., 2010, 2014). There is strong evidence that D1R and D3R interact as one of these complexes. Upregulation of D3R mRNA is induced by D1R agonist treatments alone and L-DOPA-induced changes in D3R expression are attenuated with the co-administration of a D1R antagonist, providing strong evidence of not only an interaction between these two receptors, but also a possible link between their activation states and cross receptor mediated effects (Bordet et al., 1997). More recently, in situ proximity ligation assay (PLA) showed D1R and D3R co-localization increased within the striatum of dyskinetic rats and monkeys (Farre et al., 2015). That D1R and D3R could form heteromers in the striatum was also suggested by previous coimmunoprecipitation and radioligand binding experiments (Fiorentini et al., 2008; Marcellino et al., 2008). In those studies, D1R-D3R heteromerization was demonstrated in transfected cells with bioluminescence and fluorescence resonance energy transfer techniques (BRET and FRET, respectively). Taken together, there is a substantial amount of evidence supporting the presence of striatal D1R-D3R cooperativity, potentially in the form of heteromers, and their role in LID expression, but precise behavioral consequences have yet to be characterized.
Despite compelling in vitro support for D1R-D3R interactions, in vivo work has been limited. Therefore, the present study utilized a unilateral 6-OHDA-lesioned rat model to investigate the behavioral consequences of D1R-D3R co-activation via receptor specific agonists. We also evaluated signaling changes in phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2), as enhanced pERK1/2 expression has been consistently observed in the dyskinetic striatum across multiple models of LID and PD (Fasano et al., 2010; Lindgren et al., 2009; Pavon et al., 2006; Santini et al., 2007; Westin et al., 2007). We hypothesized that D1R and D3R agonist coadministration would produce synergistic increases in dyskinesia expression. In line with previous in vitro work, we also predicted that animals co-administered both D1R and D3R agonists would display synergistic enhancement of pERK1/2 compared to the additive effect of those treated with D1R or D3R agonists alone. Ultimately, the present research provides in vivo support of D1R-D3R cooperativity and resultant synergistic behavioral and biochemical properties.
2. Methods and materials
2.1. Animals
Adult male Sprague-Dawley rats (300e400 g) were used throughout the experiments (N = 55). Animals were pair housed in plastic cages (22 × 45 × 23 cm) and were allowed free access to both food (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. Rats were maintained on a 12/12 light/dark cycle beginning at 07:00 h in a temperature-controlled room (22–23 °C). Rats were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the “Guide for the Care and Use of Laboratory Animals” (Institute for Laboratory Animal Research, National Academies Press, 2011).
2.2. 6-Hydroxydopamine lesion surgeries
All rats underwent a unilateral DA lesion with 6-hydroxydopamine hydrobromide (6-OHDA; Sigma) 6-OHDA; Sigma, St Louis, MO, USA) infused in the left medial forebrain bundle (MFB), a procedure previously described in (Conti et al., 2014; Meadows et al., 2017). In brief, rats were anesthetized with inhalant isoflurane (2–3%; Sigma) in oxygen (2.5 L/min) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) with the incisor bar positioned at 5.0mmbelow the interaural line. The targeted site, relative to bregma, was: AP, −1.8 mm; ML, +2.0 mm; DV, −8.6 mm. A 10 mL Hamilton syringe attached to a 26 gauge needle was lowered into the target through a small hole in the skull and then used to deliver 6-OHDA (3 mg/mL; Sigma) dissolved in 0.9% NaCl. 0.1% ascorbic acid at a rate of 2 mL/min, for a total volume of 4 mL. Five minutes later, the needle was withdrawn. To minimize pain and discomfort buprenex (buprenorphine HCl; 0.03 mg/kg, i.p.; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) was administered before surgery and the following day. After urgery, animals were pair-housed, placed in warmed, clean cages and monitored for a post-operative period of 10 days in which they received soft food, fruit, and fluid replacement as needed to facilitate recovery. All experiments began 3 weeks post-surgery to allow for sufficient recovery time.
2.3. Experimental design
2.3.1. Pharmacological treatments
Three weeks after surgery, all lesioned animals received a daily subcutaneous injection of L-DOPA methyl ester (6 mg/kg; Sigma). DL-serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride (benserazide; 15 mg/kg; Sigma), dissolved in vehicle (0.9% NaCl.0.1% ascorbic acid), for a 2-week priming period in order to establish stable LID expression (Fig. 1; (Lindgren et al., 2007; Putterman et al., 2007). D1R and/or D3R were stimulated individually by agonist administration. The D1R partial agonist, SKF38393 (SKF; Sigma) has been shown to induce dyskinesia individually, with selective D1R antagonists attenuating this effect (Monville et al., 2005). D3R agonist PD128907 (PD; Tocris) has been shown to bind to the D3R receptor preferentially over other DA subtypes. Most importantly, it displays higher D3R affinity compared to the pharmacologically similar DA D2 receptor (D2R; (Cote and Kuzhikandathil, 2014; Pugsley et al., 1995). Both agonists were dissolved in dH2O (vehicle) and administered at an injection volume of 1 ml/kg subcutaneously.
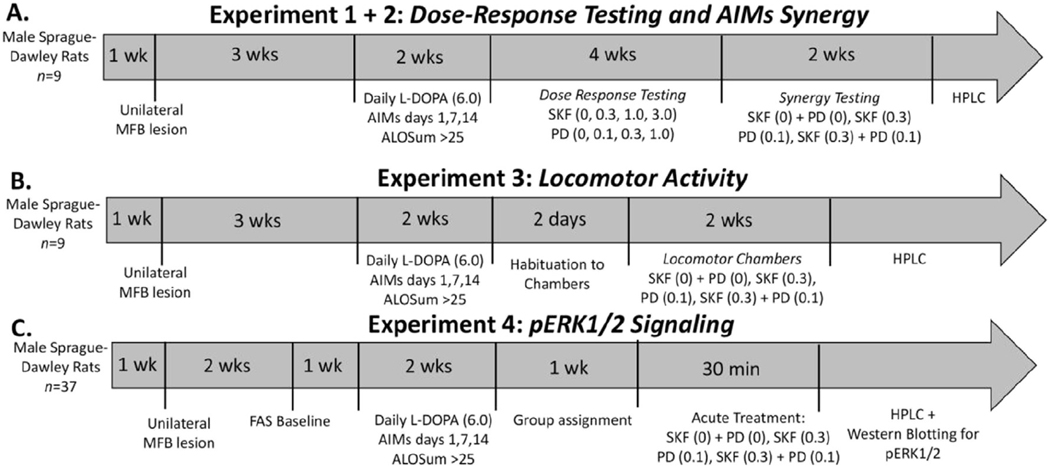
Fig. 1.
Experimental timeline and design. In all 4 experiments, rats were given a unilateral 6-OHDA lesion in the left medial forebrain bundle (MFB). After 2 weeks, lesion efficacy was assessed using the forepaw adjustment steps (FAS) test in Experiment 4. Three weeks post-surgery, rats began to receive daily L-DOPA (6 mg/kg) injections for a 2-week priming period to induce stable AIMs expression. Rats were rated for AIMs on days 1,7, and 14 and had to reach a cumulative ALO score >25 to continue to testing. In Experiment 1 (A), rats underwent dose response testing for both D1R agonist SKF38393 (SKF; 0, 0.3, 1.0, 3.0 mg/kg) and D3R agonist PD128907 (PD; 0, 0.1, 0.3, 1.0 mg/kg) in a fully counterbalanced, withinsubject design. ALO AIMs and rotations were evaluated for 180 min post injection. Threshold doses were chosen for synergy testing in Experiment 2. Rats were treated with either vehicle (SKF(0) + PD (0) mg/kg), SKF(0.3) mg/kg, PD (0.1)mg/kg, or both SKF (0.3) + PD (0.1) mg/kg and ALO AIMs were monitored. In Experiment 3(B) rats were treated with either vehicle (SKF(0) + PD (0) mg/kg), SKF(0.3) mg/kg, PD (0.1) mg/kg, or both SKF (0.3) + PD (0.1) mg/kg and locomotor activity was recorded beginning 10 min post-injection for a period of 60 min. In Experiment 4(C), 1 week after priming, rats were organized into equally dyskinetic groups. Rats were then treated with either vehicle (SKF(0) + PD (0) mg/kg), SKF(0.3) mg/kg, PD (0.1) mg/kg, or both SKF(0.3) mg/kg + PD (0.1) mg/kg. ALO AIMs were rated for 30 min post-injection at which point rats were sacrificed and striatal tissue was taken for HPLC analysis. At the conclusion of all experiments, left and right striata were collected for lesion confirmation via HPLC.
2.3.2. Experiment 1: establishment of a dose response for DA agonist-induced dyskinesia
The timeline of Experiment 1 is described in Fig. 1A. Following surgery, recovery, and priming, rats (n. 9) entered Experiment 1 to establish dose responses for each agonist. In order to establish a dose response curve for both SKF38393 and PD128907, rats were administered either SKF38393 (0, 0.3, 1.0, 3.0 mg/kg) or PD128907 (0, 0.1, 0.3, 1.0 mg/kg) in a within-subject, fully counterbalanced testing design. Importantly, all rats received all doses of each agonist to avoid the possibility of additional sensitization due to DA stimulation following the stabilization of LID. AIMs were rated on each day of treatment beginning 10 min post-injection. Washout periods of 2–3 days were in between each test day. During an individual testing session, an experimenter blind to treatment conditions rated dyskinesia using the Abnormal involuntary movements (AIMs; described below) scale and quantified drug-induced rotations. Based on the AIMs data, threshold doses that induced mild dyskinesia were selected and used throughout Experiments 2–4. At the conclusion of this experiment, rats were used for testing of behavioral synergy in Experiment 2.
2.3.3. Experiment 2: testing agonist-induced AIMs synergy with threshold doses
The timeline of Experiment 2 is described in Fig. 1A. After threshold doses were established from Experiment 1, rats were evaluated for potential drug-induced synergy of agonist coadministration (Fig. 1A). In a within-subjects counterbalanced design, rats from Experiment 1 (n = 9) were administered vehicle (SKF38393(0) + PD128907 (0) mgkg), SKF38393 (0.3 mg/kg), PD128907 (0.1 mg/kg), or both SKF38393 (0.3 mg/kg) + PD128907 (0.1 mg/kg). Beginning 10 min after injection, an experimenter blind to the treatment condition rated AIMs and quantified rotations. After a 3-day washout period, rats were sacrificed off treatment and left and right striata were collected for lesion confirmation.
2.3.4. Experiment 3: testing agonist-induced changes in generalized locomotor activity
The timeline of Experiment 3 is described in Fig. 1B. A new cohort of rats was tested for the presence of drug-induced synergy in generalized motor behaviors (Fig. 1B). Following two days of habituation in locomotor chambers, in a within-subjects counterbalanced design, rats (n = 10) were administered vehicle (SKF38393(0) + PD128907 (0) mgkg), SKF38393 (0.3 mg/kg; s.c.), PD128907 (0.1 mg/kg; s.c.), or both SKF38393 + PD128907. Beginning 10 min after injection, rats were placed in locomotor chambers to begin testing. After a 3-day washout period, rats were sacrificed off-treatment and left and right striata were collected for lesion confirmation.
2.3.5. Experiment 4: testing cellular synergy with threshold doses
The timeline of Experiment 4 is described in Fig. 1C. In the final experiment, a cohort of rats (n = 7–10/group) underwent surgery, recovery and priming. On day 14 of priming, rats were organized into 4 equally dyskinetic groups. In a between-subjects design, each group was administered either vehicle (SKF38393(0) + PD128907 (0) mg/kg; n = 9), SKF38393 (0.3 mg/kg; n = 8), PD128907 (0.1 mg/kg; n = 7) or both (SKF38393 (0.3 mg/kg) + PD128907 (0.1 mg/kg); (n = 9). Animals classified as non-responders (n = 4) were treated SKF38393 (0.3 mg/kg) + PD128907 (0.1 mg/kg) to evaluate differences in pERK1/2 regulation between lesioned rats that displayed dyskinesia and those that did not. All rats were rapidly decapitated 30 min post-treatment and left anterior dorsal striatal tissue was collected on ice and immediately frozen in −80 before undergoing whole cell tissue preparation (Westin et al., 2007; Santini et al., 2007). Posterior left and right striata were dissected for lesion confirmation.
2.4. Behavioral analyses
2.4.1. Abnormal involuntary movements (AIMs)
Rats were monitored for rodent dyskinesia using the AIMs rating scale which has been pharmacologically validated through the administration of known anti-dyskinetic compounds (Dekundy et al., 2007). Beginning 10 min after all injections, rats were placed in clear plexiglass cylinders (20 × 25) and a trained observer blind to experimental condition observed and rated axial, limb, and orolingual behaviors (ALO) for 60 s every 10 min for a total duration of 180 min, a procedure previously outlined in (Bishop et al., 2012; Dekundy et al., 2007). Axial AIMs are characterized by dystonic twisting of the trunk contralateral to lesion. Limb AIMs are excessive, uncontrollable movement of the fist and forelimb contralateral to lesion. Orolingual AIMs are categorized by tongue protrusions and jaw tremors. Each AIM is rated on a scale of 0–4: 0, behavior not present; 1, behavior present<30 s; 2, behavior present for 30 s; 3, behavior present for 60 s and able to be interrupted by a extraneous stimulus; 4 behavior present for 60 s an unable to be interrupted. All rats in all experiments went through the same 2-week priming period. On priming day 14, all animals were required to meet a cumulative ALO sum of >25 in order to continue throughout the study (Fig. 1; Taylor et al., 2005). This priming schedule results in the development of pronounced AIMs. In fact, on day 14, at the 60 min time point the median ALO AIMs score for all dyskinetic rats was “4”. Rats that were motor impaired and have confirmed lesions that did not develop a cumulative ALO sum >25 were categorized as non-responders and kept for western blot analysis in Experiment 4. Drug induced rotations were also quantified during the same assessment period. Contralateral rotations counted positively and ipsilateral rotations counted negatively to create a summed rotational score. Total ALO sums and rotational behavior were used for data analysis.
2.4.2. Forepaw adjusting steps (FAS)
The FAS test is a measure of rat akinesia in the forelimb to monitor clinical features of PD. Rats with >80% striatal DA depletion perform poorly on this test (Chang et al., 1999). In experiment 4, The FAS test was performed as described previously in (Conti et al., 2014; Meadows et al., 2017) to assess lesion-induced motor deficits. In brief, rats were held by an experimenter so that both hindlegs and one forelimb is secured. Rats were then moved laterally across a table at a speed of 90 cm/10s during which an additional experimenter recorded the number of steps taken on both the lesioned and intact paw in both the forehand and backhand direction.
2.4.3. Locomotor activity
To monitor drug-induced changes in generalized motor activity locomotor behaviors were assessed using activity chambers (Accuscan Instruments, Columbus, OH). Six Acrylic chambers (41 L x 41 W x 30.5 H cm) surrounded by infrared photocell arrays synched with Versamax and Versadat software were used to measure locomotor activity. Patterns of horizontal and vertical beam breaks were recorded and analyzed. Data were collected and analyzed every 10 min for a period of 60 min. Variables chosen for further analysis included: the total distance traveled (in centimeters); the number starts and stops separated by at least 1 s (“movement number”), and the number of repetitive, stereotypic movements within a given photocell array with no detection of ambulation (“stereotypy count”; (Bishop et al., 2005; Ostock et al., 2015).
2.5. Neurochemical analysis
2.5.1. High performance liquid chromatography (HPLC)
Lesions were confirmed in all rats via reverse phase HPLC evaluation of DA and 3,4-Dihydroxyphenylacetic acid (DOPAC) levels via electrochemical detection as described in (Conti et al., 2014, 2016). The limit of detection was 10−10 M for monoamines. Final oxidation current values were plotted on a standard curve with known concentrations varying from 10−6 to 10−9 M, and were adjusted according to tissue weights. Monoamine levels are expressed as pg of monoamine per mg of tissue.
2.5.2. Sample preparation and western blotting
Thirty minutes after drug administration in Experiment 4, left dorsal striatum was collected and frozen (Fig. 1C). Striatal tissue underwent a whole cell preparation method and was homogenized in the presence of 1 X Tris EDTA, Halt Protease inhibitor cocktail (Thermo Fisher), and phosphatase inhibitor cocktails 2 and 3 (Sigma) to prohibit degradation of the protein of interest. Protein concentration was quantified using a bicinchoninic acid assay (Thermo Fisher). Samples were normalized and underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis using Novex Tris-Glycine gels (8e16%) and were subsequently transferred to polyvinylidene difluoride membranes (Invitrogen, Carlsbad, CA, USA). Each membranewas first probed with an antibody for pERK1/2 (RRID: AB_2281741, #4370, Cell Signaling Technology, Danvers, MA, USA), mildly stripped with Restore stripping buffer (Thermofisher), and then reprobed for ERK1/2 (RRID: AB_2665488, #4696, Cell Signaling Technology, Danvers, MA, USA). Antibodies were diluted in BSA buffer (1:1000, Sigma). The primary antibodies corresponding with each protein of interest differed in their host species to prevent any false detection. To confirm this, a cross reactivity test was carried out (data not shown). Secondary antibodies were acquired from Thermo Fisher (1:10,000; Waltham, MA). Two bands were detected for both ERK1/2 and pERK1/2 at 42/44 kDa by chemiluminscent reaction (GE Healthcare, Piscataway, NJ, USA) and analyzed using NIH ImageJ software. Gels were counterbalanced, run in triplicate, and optical band densities were averaged.
2.5.3. Statistical analyses
AIMs data (expressed as medians + median absolute difference; M.A.D.) were analyzed by non-parametric statistics. For within-subjects designs, the Friedman test was employed with Wilcoxon post-hoc tests. For between-subjects designs, the Kruskal-Wallis ANOVA with Mann-Whitney U post-hoc test was used. Treatment-induced rotational data and locomotor data were assessed by ANOVAs with Fisher LSD post-hoc analyses. All HPLC monoamine and metabolite data (mean pg/mg of tissue ± S.E.M.) and western blot data (expressed as mean percent of control + S.E.M.) were analyzed using T-tests or ANOVAs with Fisher’s LSD post hoc tests. Analyses for all experiments were performed with SPSS software (Chicago, IL, USA) with alpha set at p < .05.
3. Results
3.1. Experiment 1: effects of D1R and D3R agonists on ALO AIMs and rotations
3.1.1. D1R agonist effects
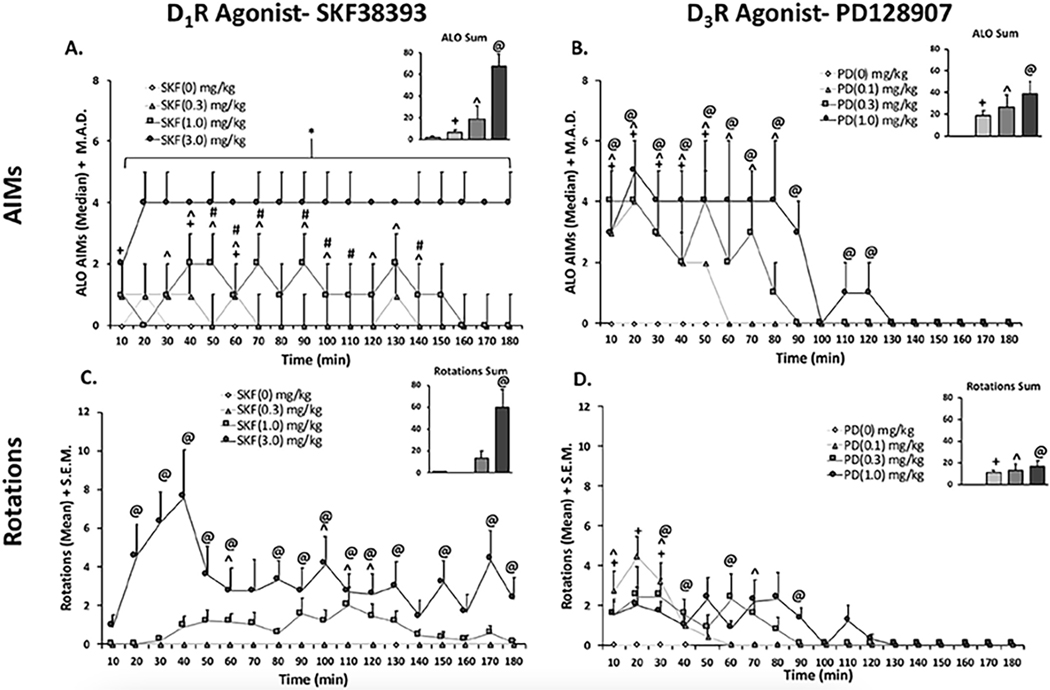
To establish a dose-response curve for D1R-mediated dyskinesia, animals (n = 9) were administered SKF38393 at four different doses (0, 0.3, 1.0, or 3.0 mg/kg, s.c.) in a within-subjects, counter-balanced design. Statistical analyses of total ALO AIMs showed differences between all treatments and a dose-dependent increase of SKF38393 on AIMs expression (bar graph, Fig. 2A; χ2 (3) = 26.103, all p < .05). Time course analyses revealed that the highest dose of SKF38393 (3.0 mg/kg) produced long-lasting, pronounced AIMs that persisted throughout the duration of observation. Treatment with the middle dose of SKF38393 (1.0 mg/kg) induced ALO AIMs significantly different from vehicle treatment during the 30–70 min time points as well as the 90, 100 and 120–140 min time points. The low dose of SKF38393 (0.3 mg/kg) produced only mild dyskinesia at the 10, 40 and 60 min time points (all p < .05) and was therefore chosen as a threshold dose in Experiments 2–4.
Fig. 2.
Effects of D1R Agonist SKF38393 and D3R Agonist PD128907 on ALO AIMs (2A + 2B) and rotations (2C + 2D). In a within-subjects, counterbalanced design rats were treated with either D1R agonist SKF38393 (SKF; 0, 0.3, 1.0, 3.0 mg/kg; Fig. A + C) or (PD; 0, 0.1, 0.3, 1.0 mg/kg; Fig. B + D). Animals were rated for ALO AIMs and rotations for 3 h post-injection. AIMs time course and ALO sums are expressed as medians (ALO . median absolute difference; M.A.D.) Rotations time course is expressed as means (rotations + standard error of the mean; S.E.M.). Significant AIMs differences were determined by non-parametric Friedman ANOVAs with Wilcoxon Match Pairs post-hoc tests. Significant rotational differences were determined using parametric repeated measures ANOVAs with LSD post-hoc tests (@p < .05 high vs. vehicle, ^p < .05 middle vs. vehicle, +p < .05 low vs. vehicle).
Rotational behavior was also recorded for the same observation period. In rotational sums, there was a main effect of treatment on total rotations with SKF38393 (3.0 mg/kg) producing significantly higher rotational behavior with respect to vehicle (bar graph; Fig. 2C; F(3,24) = 12.48, p < .05). To analyze time course data, a 4 (treatment) x 18 (time) repeated measures ANOVA was employed and revealed a main effect of treatment (Fig. 2C; F(3,24). = 13.504, p < .05), time (F(17,136) = 2.461, p < .05), and a treatment x time interaction (F(51,408) = 2.593, p < .05). Post-hoc analyses of the treatment x time interaction demonstrated that rotations were induced by SKF38393 (3.0 mg/kg) at the 20–60, 80–130, 150 and 180 min time points (all p < .05). SKF38393 (1.0 mg/kg) induced significant rotations from vehicle at 60, 100–120 time points (each p < .05), while SKF38393 (0.3 mg/kg) did not induce any rotations (Fig. 2C).
3.1.2. D3R agonist effects
To establish a dose response for PD128907, rats were administered PD128907 (0, 0.1, 0.3, or 1.0 mg/kg, s.c.) at four different doses in a within-subjects counterbalanced design. A similar dose-dependent effect was observed for PD128907 (Fig. 2B; bar graph; χ2 (3) = 22.733, p < .05). Time course analyses showed that the highest dose of PD128907 (1 mg/kg) produced the most severe, long-lasting ALO AIMs, relative to vehicle that persisted from the 10–120 min time points (all <0.05). The middle dose of PD128907 (0.3 mg/kg) produced AIMs significantly different vehicle from the 10–80 min time points (all p < .05). Lastly, treatment with the lowest dose of PD128907 (0.1 mg/kg) produced the lowest-grade AIMs with the shortest duration beginning at 10 min and subsiding by the 60-min time point (Fig. 2B; all p < .05). This dose was therefore selected as a threshold dose in Experiments 2–4.
For rotational sums, there was a main effect of treatment on total rotations (F(3,24). 4.264, p < .05), with all treatments significantly differing from vehicle (bar graph; Fig. 2D; p < .05). A 4 (treatment) x 18 (time) repeated measures ANOVA revealed a main effect of treatment (Fig. 2D; F(3,24) = 4.264, p < .05), time (F(17,136) = 8.099, p < .05), and a treatment by time interaction (F(51,408) = 3.095, p < .05). Although all three doses differed from vehicle, they did not differ from each other (bar graph; Fig. 2D; p < .05). Treatment with the high dose produced rotations significantly different from vehicle at the 30, 40, 60, and 90 min (all p < .05). At the middle dose (0.3 mg/kg), rotational behavior was significantly different from vehicle at the 10, 30, and 70 min time points (all p < .05). When rats were treated with the lowest dose (0.1 mg/kg), they had significantly higher rotations, in relation to vehicle, during the first 10e30 min time points (all p < .05).
3.2. Experiment 2: effects of D1R and D3R agonist co-administration on ALO AIMs and contralateral rotations
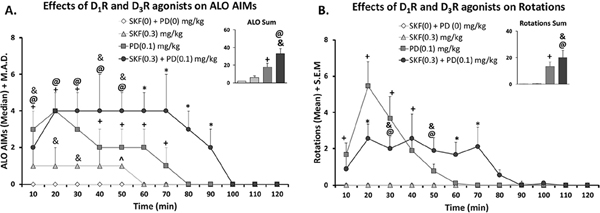
At the conclusion of Experiment 1, rats were tested for the presence of synergistic dyskinesia expression following D1R-D3R agonist co-administration in Experiment 2. Rats received either Vehicle (SKF38393(0) + PD128907 (0) mg/kg), SKF38393 (0.3 mg/kg), PD128907 (0.1 mg/kg), or both SKF38393 + PD128907 (Fig.1A). First total ALO AIMs were analyzed, revealing a significant effect of treatment (bar graph; Fig. 3A; χ2 (3) = 23.378, p < .05). Post-hocs demonstrated that rats displayed significantly higher ALO AIMsexpression when treated with either PD128907 or both SKF38393 + PD128907, with respect to vehicle (bar graph; Fig. 3A; p < .05). Time course analyses revealed several significant effects. When treated with PD128907 alone, rats had significantly higher ALO AIMs, with respect to vehicle from the 10e70 min time points. SKF38393 alone only induced ALO AIMs significantly different from vehicle at the 50 min time point. Most importantly, when rats received both PD128907 + SKF38393 they displayed exacerbated dyskinesia that manifested as heightened and protracted AIMs compared to all other treatments from the 60–90 min time points (Fig. 3A; all p < .05).
Fig. 3.
Effects of co-administration of D1R Agonist SKF38393 and/or D3R Agonist PD128907 on ALO AIMs, (4A) and rotations (4B). In a within-subject, counterbalanced design rats were treated with either vehicle (SKF(0) + PD (0) mg/kg), D1R agonist SKF38393 (SKF; 0.3 mg/kg), D3R agonist PD128907 (PD; 0.1 mg/kg), or both (SKF(0.3) + PD (0 0.1) mg/kg). Rats were rated for ALO AIMs and rotations for 3 h post-injection. The first 2 h are shown. AIMs time course and ALO sums are expressed as medians (ALO . median absolute difference; M.A.D.) Rotations time course is expressed as means (Rotations . standard error of the mean; S.E.M.) Significant AIMs differences were determined by non-parametric Friedman ANOVAs with Wilcoxon Match Pairs post-hoc tests. Significant rotational differences were determined using parametric repeated measures ANOVAs with LSD post-hoc tests (@p < .05 syn vs. vehicle, ^p < .05 SKF vs. vehicle, +p < .05 PD vs. vehicle, *p < .05 Syn vs. ALL, &p < .05 Syn vs. SKF).
In rotational sums, there was a main effect of treatment on rotational behavior (bar graph; Fig. 3B; F(3,24) = 10.83; p < .05). Rats had significantly higher rotations when treated with either PD128907 alone or with SKF38393 + PD128907. Rotations analyzed by a 4 (treatment) x 18 (time) repeated measures ANOVA also revealed a main effect of treatment (F(3,24) = 7.214, p < .05), time (F(17,136) = 13.966, p < .05), and a significant treatment by time interaction (F(51,255) = 4.627, p < .05). SKF38393 alone did not induce any rotations relative to vehicle (p < .05). When rats received PD128907 alone, they experienced higher peak rotations at the 20–30 min time points. (Fig. 3B; p < .05). Relative to vehicle, rats rotated significantly more when receiving PD128907 alone or PD128907 + SKF38393 (bar graph; Fig. 3B; p < .05) but total rotations between these two groups did not differ. However, when the rotations time course was evaluated, differences between these two groups were clear. Rats receiving PD128907 + SKF38393 rotated for a longer duration when compared to other treatments, displaying significantly more rotational behavior during the 60–70 min time points (Fig. 4B; p < .05).
Fig. 4.
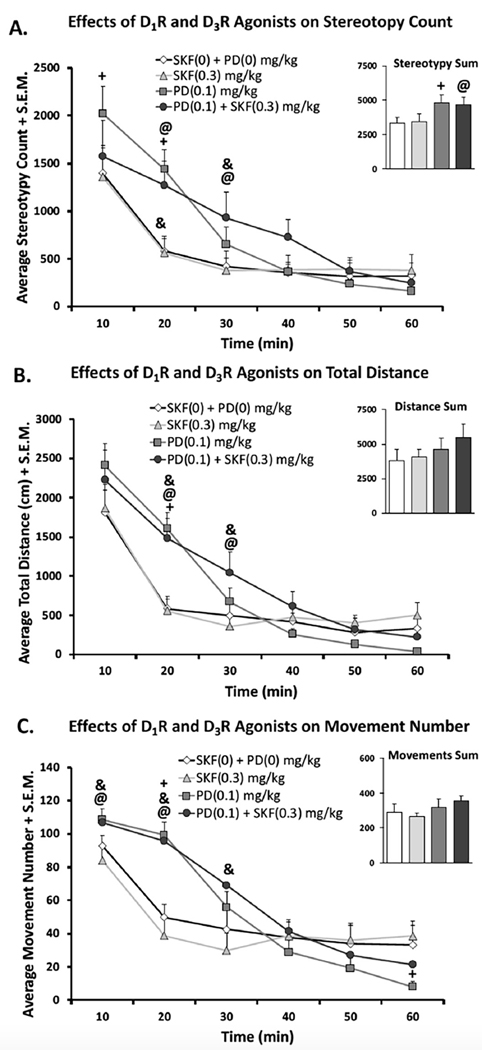
Effects of co-administration of D1R Agonist SKF38393 and/or D3R Agonist PD128907 on Stereotypy, (4A) Distance Traveled (4B) and Movement number (4C). In a within-subject, counterbalanced design rats were treated with either vehicle (SKF(0) + PD (0) mg/kg), D1R agonist SKF38393 (SKF; 0.3 mg/kg), D3R agonist PD128907 (PD; 0.1 mg/kg), or both (SKF(0.3) + PD (0 0.1) mg/kg). Rats were placed in locomotor chambers for 60 min. Time course and sums are expressed as means (Variable + standard error of the mean; S.E.M.) Significant differences were determined using parametric repeated measures ANOVAs with LSD post-hoc tests (@p < .05 syn vs. vehicle, ^p < .05 SKF vs. vehicle, +p < .05 PD vs. vehicle, *p < .05 Syn vs. ALL, &p < .05 Syn vs. SKF).
3.3. Experiment 3: effects of D1R and D3R agonist co-administration on locomotor activity
To determine if the synergistic alterations in ALO AIMs weremirrored in generalized motor movement, locomotor activity was assessed. Rats received either Vehicle (SKF38393(0) + PD128907 (0) mg/kg), SKF38393 (0.3 mg/kg), PD128907 (0.1 mg/kg), or both SKF38393 + PD128907 (Fig. 1B). First, sums for each measure of interest were analyzed via a repeated measures ANOVA. Second, time course analyses were performed via a 4 (treatment) x 6 (time) repeated measures ANOVA. Post-hoc analyses were performed as necessary.
In stereotypy count sums, there was a main effect of treatment (F(3,27) = 3.683, p < .05). Post-hoc analyses show that when rats received either PD128907 or SKF38393 + PD128907, they displayed stereotypy significantly difference from vehicle. Time course analyses revealed a main effect of treatment (F(3,27) = 55.517, p < .05), time (F(5,45) = 23.243, p < .05) and a treatment x time interaction (F(15, 135) = 10.425, p < .05). When rats received both PD128907 + SKF38393, they only displayed stereotypy significantly different from vehicle at the 20–30 min time points.
For total distance traveled, time course analyses of total distance revealed only a main effect of time (F(5,45) = 49.472, p < .05), and a significant treatment x time interaction (F(15,135) = 4.867, p < .05). Treatment differences only existed at the 20 and 30 min time points, with both PD128907 and PD128907 + SKF38393 differing significantly from vehicle (p < .05).
For movement number only time course analyses revealed a significant main effect of time (F(5,45) = 51.745, p < .05) and treatment time interaction (F(15,135) = 6.982, p < .05). Post-hoc analyses showed that when rats were treated with both PD128907 + SKF38393 they significantly differed from vehicle only during the 10–20 min time points (p < .05).
3.4. Experiment 4: effects of D1R and D3R agonist co-administration on ALO AIMs and pERK1/2 expression
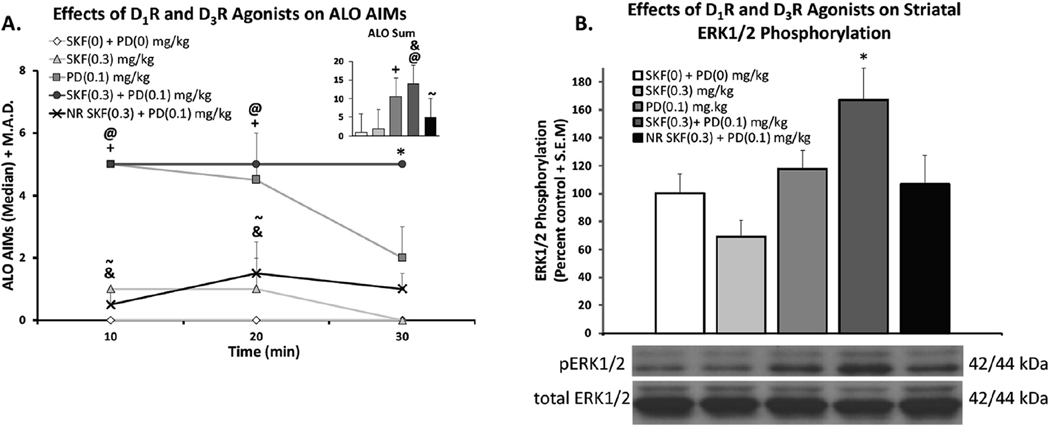
In order to assess how D1R-D3R agonist co-treatment modifies downstream cellular signaling, rats (n = 37) were organized into equally dyskinetic groups and assigned a treatment of either vehicle, SKF38393 (0.3 mg/kg; s.c.), PD128907 (0.1 mg/kg; s.c.), or both SKF38393 and PD128907. To track dyskinetic behaviors, rats were monitored for 30 min post-injection. Both PD128907 and SKF38393 + PD128907 groups displayed significantly higher ALO sums relative to vehicle (bar graph; Fig. 5A; χ2 (4) = 27.875; p < .05). Time course analyses revealed that, even within the first 30 min, significant differences emerged. At the 30-min time point, AIMs had declined for both individual agonist treatment groups while the co-administration group displayed significantly greater dyskinesia than all other groups (Fig. 5A; p < .05). Non-responders did not exhibit dyskinesia significantly different from vehicle-treated rats at any time point.
Fig. 5.
Effects of co-administration of D1R Agonist SKF38393 and/or D3R Agonist PD128907 on ALO AIMs (Fig. 5A) and striatal pERK1/2 expression (Fig. 5B). In a between-subjects design rats were treated with either vehicle (SKF(0) + PD (0) mg/kg), D1R agonist SKF38393 (SKF; 0.3 mg/kg), D3R agonist PD128907 (PD; 0.1 mg/kg), or both (SKF(0.3) + PD (0.1) mg/kg).Non-responders (NR) were also treated with both (SKF(0.3). PD (0.1) mg/kg). Rats were rated for ALO AIMs for 30 min post-injection. AIMs time course and ALO sums are expressed as medians (ALO +median absolute difference; M.A.D Significant AIMs differences were determined by non-parametric Kruskal Wallis ANOVAs with Mann Whitney U Pairs post-hoc tests as necessary. After 30 min, rats were sacrificed, dorsal striatum was collected and subsequently underwent whole cell Western Blot procedures. The ratio of pERK1/2 to total ERK1/2 is shown. Significant differences were detected with a one-way ANOVA and LSD post-hoc tests as appropriate (@p < .05 Syn vs. vehicle, *p < .05 Syn vs. ALL, &p < .05 Syn vs. SKF, ~p < .05 Syn vs. NR).
After the final AIMs rating, rats were sacrificed and left striata were collected and frozen for Western Blot Analysis. Following tissue preparation, electrophoresis, transfer and film exposure, optical band densities of pERK1/2 and ERK1/2 were evaluated using NIH ImageJ Software. pERK1/2 levels were normalized to total ERK1/2. A one-way ANOVA showed a significant main effect of treatment on pERK1/2 expression (Fig. 5B; F(4,32) = 4.778; p < .05). Post-hoc analyses revealed that only the synergy group, that received both SKF38393 and PD128907 statistically differed from other groups (p < .05). Rats treated with either SKF38393 or PD128907 individually did not differ from vehicle-treated rats. Interestingly, non-responders (n = 4) treated with SKF38393 + PD128907 had pERK1/2 levels similar to those treated with vehicle and also differed statistically from the synergy group (black bar; Fig. 5B; p < .05).
3.5. Verification of lesion
Severity of lesion was assessed by analyses of DA and DOPAC content in left (lesion) and right (intact) striatal tissue using HPLC. On average, rats displayed an expected significant reduction in DA (Mlesion = 244.38, Mintact = 12,133.86; t55= −9.802, p < .05) and DOPAC (Mlesion = 612.50, Mintact = 5746.66; t55= −11.17, p < .05) content in the lesioned striatum relative to the intact side (97.99% and 93.80% reduction, respectively).
Considering the between-subjects design of Experiment 4, further analysis was needed to check for any differences in lesion severity between groups that might confound results. First, non-responders from Experiment 4 were analyzed. Non-responders (n = 4) did not display depletion significantly different from dyskinetic rats in either DA (Mlesion = 159.80, Mintact = 11,621.86) or DOPAC (Mlesion = 1648.15, Mintact = 10,518.41) and were therefore included in further analysis. A mixed ANOVA revealed a main effect of hemisphere on DA (F(1,32) . 51.025, p < .05) and DOPAC (F(1,32) = 100.259, p < .05) levels with all rats displaying marked deficits in lesion vs. Intact DA (Mlesion = 254.96, Mintact = 13,741.41) and DOPAC (Mlesion = 724.43, Mintact = 9981.47). Importantly, there were no main effects of treatment or treatment by side interactions in either DA or DOPAC, indicating that rats had equal levels of depletion across groups.
4. Discussion
Accumulated evidence clearly supports that chronic L-DOPA treatment initiates aberrant neuroplasticity that significantly alters basal ganglia circuitry. This often produces motor side effects that manifest as dyskinesias. Among these changes, receptor-receptor interactions have been of particular interest, as these have the ability to deviate from canonical cascades and may represent a novel way to interrupt maladaptive signaling (Ferre et al., 2010; Guitart et al., 2014). Although D1R and D3R have been implicated in LID, they have proven to be difficult to manipulate pharmacologically with antagonist treatment, dampening the interest in these receptors as anti-dyskinetic targets (Hsu et al., 2004; Mela et al., 2010; Silverdale et al., 2004) The discovery that chronic L-DOPA treatment may precipitate the expression of a dorsal striatal D1R-D3R heteromer interactions has reinvigorated the relative interest in these receptors as a potentially functional unit. However, until now, the signaling mechanisms by which D1R-D3R stimulation modifies dyskinesia expression and in vivo effects remained speculative. The current study provides evidence of D1R-D3R synergistic interactions both behaviorally and biochemically at the level of striatal pERK1/2 in L-DOPA-primed hemi-parkinsonian rats.
D1R’s role in LID is the most characterized of the DA receptor subtypes. Although it is unclear whether L-DOPA significantly changes D1R expression in the dyskinetic brain (Bordet et al., 1997; Ferre et al., 2014; Guigoni et al., 2005), converging evidence highlights a definite D1R sensitization that drives dyskinetic behaviors. In fact, genetic (Darmopil et al., 2009), pharmacologic (Lindenbach et al., 2016; Taylor et al., 2005), and optogenetic (Hernandez et al., 2017; Perez et al., 2017) strategies have all conclusively demonstrated D1R’s role. On the contrary, the role of D3R in dyskinesia has been particularly difficult to characterize despite clear evidence suggesting involvement. In as early as 1997, Bordet and colleagues noted that the ectopic expression of D3R in the striatum occurs as a result of L-DOPA administration and actively participates in behavioral sensitization (Bordet et al., 1997). Moreover, D3R knockout mice display attenuated LID expression, corresponding with a reduction in LID biomarkers (Solis et al., 2015). To date, the most profound D3R-mediated change occurs at the level of expression. Upregulation is observed in dyskinetic mice (Solis et al., 2015), rats (Bordet et al., 1997; Farre et al., 2015; Visanji et al., 2009), non-human primates (Farre et al., 2015) and PD patients (Payer et al., 2016). Though upregulation was not directly quantified in our study, existing data using similar lesion and priming parameters have consistently demonstrated D3R upregulation in the dorsal striatum in D1R-bearing neurons (Farre et al., 2015; Visanji et al., 2009). Moreover, D3R stimulation with the D3R-preferring agonist PD128907 dose-dependently induced expression of AIMs (Fig. 2B). The lowest dose (0.1 mg/kg) produced dyskinesia with the shortest dyskinetic window and was thus selected for further testing.
Despite the promising data collected in this study relating to D3R, several factors relating to the use of PD128907 should be considered. Some have found that PD128907 induces D3R tolerance at the level of signaling and behavior (Cote and Kuzhikandathil, 2014, 2015). However, in our model we did not find evidence of D3R behavioral tolerance. Rats displayed comparable ALO AIMs whether it was their first exposure to PD128907 (0.1 mg/kg) post-LDOPA priming during experiment 1 or their last exposure to PD128907 (0.1 mg/kg) during experiment 2. Therefore, behavioral D3R responding was stable across the 6-week agonist testing period. Moreover, results did not indicate D3R tolerance at the level of ERK signaling. Others have reported that PD128907 is able to selectively activate ERK1/2 signaling in D3R-positive cells (Cote and Kuzhikandathil, 2014; Guitart et al., 2014), and that this effect is lost in MPTP-treated dyskinetic mice despite D3R upregulation (Cote and Kuzhikandathil, 2015). In experiment 4, L-DOPA-primed animals received a single dose of PD128907 and dorsal striatal tissue collection occurred 30 min later (Fig. 1C). Consistent with experiment 1, rats displayed a sharp increase in AIMs that subsided significantly by the final AIMs rating. Not surprisingly, this decline was also mirrored in levels of pERK1/2, a common biomarker for LID, which were not statistically different from animals treated with vehicle (Fig. 5).
Pharmacological probing of D3R has been notoriously difficult due to its similarity to D2R (Missale et al., 1998). Fortunately, new compounds have been identified that are able to discern D3R from D2R (Cortes et al., 2016). We employed the D3R agonist PD128907. In CHO-K1 cells, PD128907 display 1000-fold selectivity over D2R and 10,000-fold selectivity over the other D2-like receptor, D4R (Akunne et al.,1995). However, others have reported a preference of 50-fold (Newman-Tancredi et al., 1995) or 18-fold (Pugsley et al., 1995). Anatomically, PD128907 displays selective binding in D3Rrich regions such as the Islands of Calleja (Bancroft et al., 1998) and locomotor effects of PD128907 are lost in D3R knockout mice (Pritchard et al., 2003). Therefore, although we cannot absolutely conclude that the dyskinesiogenic effects of PD128907 are D3R-mediated, our results are commensurate with prior D3R-linked effects. Moreover, this assertion is bolstered by prior work demonstrating D1R and D3R cooperativity in LID. Specifically, D3R interactions with D1R in the form of a heteromer have been postulated to play a causal role in the manifestation of LID (Farre et al., 2015; Fiorentini et al., 2008; Guigoni et al., 2005).
In the present study, we demonstrated that co-administration of low doses of PD128907 and SKF38393 potentiated dyskinesia severity and duration beyond the additive effects of each compound alone (Fig. 3A and 5A). This synergism was specific to dyskinesia, as other locomotor behaviors were not significantly modified by SKF38393 + PD128907 co-administration (Fig. 4). That said, it is possible that the lack of synergy in the locomotor activity chambers reflects the nature of the automated data collection system which quantifies infrared beam breaks rather than specific abnormal movements. Synergistic interactions have also been reported as a result of D1R-D3R co-localization at the level of gene expression in the ventral striatum (Ridray et al., 1998) and GABA release in striatonigral terminals (Cruz-Trujillo et al., 2013). Furthermore, changes related to D1R trafficking, affinity, and signaling have all been described in D1R-D3R-expressing cells (Fiorentini et al., 2008; Guigoni et al., 2005; Marcellino et al., 2008). Given that D3R and D1R-D3R heteromers are upregulated in LID, similar effects observed in vitro may be occurring in vivo that potentiate striatal signaling. Namely, D3R stabilizes D1R membrane expression and enhances D1R affinity for DA (Fiorentini et al., 2008; Marcellino et al., 2008). Both of these properties may describe potential mechanisms by which direct pathway signaling in enhanced in LID.
Several downstream effectors have been associated with LID, such as hyperphosphorylation of striatal ERK1/2, protein kinase A, and DARRP-32 (Lindenbach et al., 2016; Pavon et al., 2006; Santini et al., 2007; Westin et al., 2007). Expression of pERK1/2 has been hypothesized to be largely D1R-driven, as D1R antagonists block this effect (Westin et al., 2007). In the current investigation, we found dyskinesiogenic D1R-D3R co-administration produced synergistic induction of pERK1/2 while low doses of individual agonists did not (Fig. 4B). The specificity of the role of pERK1/2 in dyskinesia is further highlighted in the non-responder group which did not display elevated pERK1/2 levels when co-treated with both agonists despite equivalent motor impairment and striatal DA depletion to the responder group (Fig. 5B). This augmentation of striatal pERK1/2 is reminiscent of synergy reported by Guitart et al. (2014) who showed an emergent agonistinduced upregulation of pERK1/2 in D1R-D3R cells. One possible explanation of our effect could be convergence of downstream signaling of D1R and D3R, as stimulation of either can increase pERK1/2 levels (Guitart et al., 2014; Xu et al., 2017). However, it is notable that in vitro, emergent D1R-D3R pERK1/2 signaling is selectively lost following heteromer destabilization via competing peptides (Guitart et al., 2014). This suggests pERK1/2 induction may constitute a biochemical fingerprint for the D1R-D3R heteromer. This signaling may be accomplished via G-proteindependent (Fiorentini et al., 2008) or -independent (Guitart et al., 2014) mechanisms. Further work aimed at dissecting out G-protein-dependent or -independent signaling at the level of cyclic adenosine monophosphate (cAMP) or β-arrestin could help elucidate the ways in which D1R-D3R interactions signal within the dyskinetic brain.
5. Conclusion
Overall, the present study provides strong evidence of striatal D1R-D3R functional cooperativity in vivo. D1R and D3R agonist cotreatment not only synergistically exacerbated dyskinesia but also enhanced downstream signaling commonly associated with LID expression. This effect was found to be dyskinesia-specific, as D1R and D3R agonist co-administration neither modulated general locomotor behaviors nor enhanced pERK1/2 expression in nonresponders. Existing literature suggests that striatal D1R-D3R cooperativity may reflect the formation of heteromers of D1R and D3R homodimers (heterotetramers; Guitart et al., 2014) highlighting a critical need to establish specific compounds or strategies that interfere with or reverse D1R-D3R allosteric interactions. Of broader interest, D1R-D3R interactions have also been implicated in other disorders, further justifying more research explicitly examining D1R-D3R function and dysfunction (Ferre et al., 2010, 2014).
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Melissa Conti for her training and expertise in both surgical techniques and behavioral assays. Additionally, the authors would also like to recognize Sarah Lefkowitz for her assistance in behavioral data collection and Dr. David Werner for his consultation in Western Blot procedures.
Funding
Support for this work came from the Center for Development and Neuroscience (Bishop) and conceptual and experimental contributions of Dr. Ferré were supported by NIDA intramural funds.
Footnotes
Disclosure statement
KL, SM, NC, EN, MD, SF, and CB have no disclosures or conflicts of interest to report.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.neuropharm.2018.06.024.
References
- Ahlskog JE, Muenter MD, 2001. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord 16 (3), 448e458. [DOI] [PubMed] [Google Scholar]
- Akunne HC, Towers P, Ellis GJ, Dijkstra D, Wikstrom H, Heffner TG, et al. , 1995. Characterization of binding of [3H]PD 128907, a selective dopamine D3 receptor agonist ligand, to CHO-K1 cells. Life Sci. 57 (15), 1401e1410. [DOI] [PubMed] [Google Scholar]
- Bancroft GN, Morgan KA, Flietstra RJ, Levant B, 1998. Binding of [3H]PD 128907, a putatively selective ligand for the D3 dopamine receptor, in rat brain: a receptor binding and quantitative autoradiographic study. Neuropsychopharmacology 18 (4), 305e316. 10.1016/S0893-133X(97)00162-0. [DOI] [PubMed] [Google Scholar]
- Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, et al. , 2003. Attenuation of levodopa-induced dyskinesia by normalizing dopamine d3 receptor function. Nat. Med 9 (6), 762e767. 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- Bishop C, Daut GS,Walker PD, 2005. Serotonin 5-HT2A but not 5-HT2C receptor antagonism reduces hyperlocomotor activity induced in dopamine-depleted rats by striatal administration of the D1 agonist SKF 82958. Neuropharmacology 49 (3), 350e358. 10.1016/j.neuropharm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Bishop C, George JA, Buchta W, Goldenberg AA, Mohamed M, Dickinson SO, et al. , 2012. Serotonin transporter inhibition attenuates l-DOPA-induced dyskinesia without compromising l-DOPA efficacy in hemi-parkinsonian rats. Eur. J. Neurosci 36 (6), 2839e2848. 10.1111/j.1460-9568.2012.08202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC, 1997. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc. Natl. Acad. Sci. U. S. A 94 (7), 3363e3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ, 1999. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 88 (2), 617e628. [DOI] [PubMed] [Google Scholar]
- Chiken S, Sato A, Ohta C, Kurokawa M, Arai S, Maeshima J, et al. , 2015. Dopamine D1 receptor-mediated transmission maintains information flow through the cortico-striato-entopeduncular direct pathway to release movements. Cerebr. Cortex 25 (12), 4885e4897. 10.1093/cercor/bhv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MM, Goldenberg AA, Kuberka A, Mohamed M, Eissa S, Lindenbach D, Bishop C, 2016. Effect of tricyclic antidepressants on L-DOPA-induced dyskinesia and motor improvement in hemi-parkinsonian rats. Pharmacol. Biochem. Behav 142, 64e71. 10.1016/j.pbb.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Conti MM, Ostock CY, Lindenbach D, Goldenberg AA, Kampton E, Dell’isola R, et al. , 2014. Effects of prolonged selective serotonin reuptake inhibition on the development and expression of L-DOPA-induced dyskinesia in hemi-parkinsonian rats. Neuropharmacology 77, 1e8. 10.1016/j.neuropharm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes A, Moreno E, Rodriguez-Ruiz M, Canela EI, Casado V, 2016. Targeting the dopamine D3 receptor: an overview of drug design strategies. Expet Opin. Drug Discov 11 (7), 641e664. 10.1080/17460441.2016.1185413. [DOI] [PubMed] [Google Scholar]
- Cote SR, Chitravanshi VC, Bleickardt C, Sapru HN, Kuzhikandathil EV, 2014. Overexpression of the dopamine D3 receptor in the rat dorsal striatum induces dyskinetic behaviors. Behav. Brain Res 263, 46e50. 10.1016/j.bbr.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Cote SR, Kuzhikandathil EV, 2014. In vitro and in vivo characterization of the agonist-dependent D3 dopamine receptor tolerance property. Neuropharmacology 79, 359e367. 10.1016/j.neuropharm.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Cote SR, Kuzhikandathil EV, 2015. Chronic levodopa treatment alters expression and function of dopamine D3 receptor in the MPTP/p mouse model of Parkinson’s disease. Neurosci. Lett 585, 33e37. 10.1016/j.neulet.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Trujillo R, Avalos-Fuentes A, Rangel-Barajas C, Paz-Bermudez F, Sierra A, Escartin-Perez E, et al. , 2013. D3 dopamine receptors interact with dopamine D1 but not D4 receptors in the GABAergic terminals of the SNr of the rat. Neuropharmacology 67, 370e378. 10.1016/j.neuropharm.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Martin AB, De Diego IR, Ares S, Moratalla R, 2009. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol. Psychiatr 66 (6), 603e613. 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA, 2007. Modulation of L-DOPAinduced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav. Brain Res 179 (1), 76e89. 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Farre D, Munoz A, Moreno E, Reyes-Resina I, Canet-Pons J, Dopeso-Reyes IG, et al. , 2015. Stronger dopamine D1 receptor-mediated neurotransmission in dyskinesia. Mol. Neurobiol 52 (3), 1408e1420. 10.1007/s12035-014-8936-x. [DOI] [PubMed] [Google Scholar]
- Fasano S, Bezard E, D’Antoni A, Francardo V, Indrigo M, Qin L, et al. , 2010. Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with L-dopa-induced dyskinesia. Proc. Natl. Acad. Sci. U. S. A 107 (50), 21824e21829. 10.1073/pnas.1012071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ, et al. , 2014. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol. Rev 66 (2), 413e434. 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Lluis C, Lanciego JL, Franco R, 2010. Prime time for G-protein-coupled receptor heteromers as therapeutic targets for CNS disorders: the dopamine D(1)-D(3) receptor heteromer. CNS Neurol. Disord. - Drug Targets 9 (5),596e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C, 2008. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol. Pharmacol 74 (1), 59e69. 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Gomez-Mancilla B, Bedard PJ, 1991. Effect of D1 and D2 agonists and antagonists on dyskinesia produced by L-dopa in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys. J. Pharmacol. Exp. Therapeut 259 (1), 409e413. [PubMed] [Google Scholar]
- Grondin R, Doan VD, Gregoire L, Bedard PJ, 1999. D1 receptor blockade improves L-dopa-induced dyskinesia but worsens parkinsonism in MPTP monkeys. Neurology 52 (4), 771e776. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, et al. , 2005. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Park. Relat. Disord 11 (Suppl. 1), S25eS29. 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E, 2007. Altered D(1) dopamine receptor trafficking in parkinsonian and dyskinetic non-human primates. Neurobiol. Dis 26 (2), 452e463. 10.1016/j.nbd.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sanchez-Soto M, et al. , 2014. Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol. Pharmacol 86 (4), 417e429. 10.1124/mol.114.093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Morris JG, Reid WG, Trafficante R, 2005. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov. Disord 20 (2), 190e199. 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- Hernandez FL, Castela I, Ruiz-DeDiego I, Obeso JA, Moratalla R, 2017. Striatal activation by optogenetics induces dyskinesias in the 6-hydroxydopamine rat model of Parkinson disease. Mov. Disord 32 (4), 530e537. 10.1002/mds.26947. [DOI] [PubMed] [Google Scholar]
- Hsu A, Togasaki DM, Bezard E, Sokoloff P, Langston JW, Di Monte DA, Quik M, 2004. Effect of the D3 dopamine receptor partial agonist BP897 [N-[4-(4-(2-methoxyphenyl)piperazinyl)butyl]-2-naphthamide] on L-3,4-dihydroxyphenylalanine-induced dyskinesias and parkinsonism in squirrel monkeys. J. Pharmacol. Exp. Therapeut 311 (2), 770e777. 10.1124/jpet.104.071142. [DOI] [PubMed] [Google Scholar]
- Lindenbach D, Conti MM, Ostock CY, George JA, Goldenberg AA, Melikhov-Sosin M, et al. , 2016. The role of primary motor cortex (M1) glutamate and GABA signaling in l-dopa-induced dyskinesia in parkinsonian rats. J. Neurosci 36 (38), 9873e9887. 10.1523/JNEUROSCI.1318-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HS, Ohlin KE, Cenci MA, 2009. Differential involvement of D1 and D2 dopamine receptors in L-DOPA-induced angiogenic activity in a rat model of Parkinson’s disease. Neuropsychopharmacology 34 (12), 2477e2488. 10.1038/npp.2009.74. [DOI] [PubMed] [Google Scholar]
- Lindgren HS, Rylander D, Ohlin KE, Lundblad M, Cenci MA, 2007. The “motor complication syndrome” in rats with 6-OHDA lesions treated chronically with L-DOPA: relation to dose and route of administration. Behav. Brain Res 177 (1), 150e159. 10.1016/j.bbr.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, et al. , 2008. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem 283 (38), 26016e26025. 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows SM, Chambers NE, Conti MM, Bossert SC, Tasber C, Sheena E, et al. , 2017. Characterizing the differential roles of striatal 5-HT1A auto- and hetero-receptors in the reduction of l-DOPA-induced dyskinesia. Exp. Neurol 292, 168e178. 10.1016/j.expneurol.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Mela F, Millan MJ, Brocco M, Morari M, 2010. The selective D(3) receptor antagonist, S33084, improves parkinsonian-like motor dysfunction but does not affect L-DOPA-induced dyskinesia in 6-hydroxydopamine hemi-lesioned rats. Neuropharmacology 58 (2), 528e536. 10.1016/j.neuropharm.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG, 1998. Dopamine receptors: from structure to function. Physiol. Rev 78 (1), 189e225. 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Monville C, Torres EM, Dunnett SB, 2005. Validation of the l-dopa-induced dyskinesia in the 6-OHDA model and evaluation of the effects of selective dopamine receptor agonists and antagonists. Brain Res. Bull 68 (1e2), 16e23. 10.1016/j.brainresbull.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Audinot V, Jacques V, Peglion JL, Millan MJ, 1995. [3H](.)S 14297:a novel, selective radioligand at cloned human dopamine D3 receptors. Neuropharmacology 34 (12), 1693e1696. [DOI] [PubMed] [Google Scholar]
- Ostock CY, Hallmark J, Palumbo N, Bhide N, Conti M, George JA, Bishop C, 2015. Modulation of L-DOPA’s antiparkinsonian and dyskinetic effects by alpha2-noradrenergic receptors within the locus coeruleus. Neuropharmacology 95, 215e225. 10.1016/j.neuropharm.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R, 2006. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol. Psychiatr 59 (1), 64e74. 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Payer DE, Guttman M, Kish SJ, Tong J, Adams JR, Rusjan P, et al. , 2016. D3 dopamine receptor-preferring [11C]PHNO PET imaging in Parkinson patients with dyskinesia. Neurology 86 (3), 224e230. 10.1212/WNL.0000000000002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Zhang D, Bordia T, Quik M, 2017. Striatal D1 medium spiny neuron activation induces dyskinesias in parkinsonian mice. Mov. Disord 32 (4), 538e548. 10.1002/mds.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, et al. , 2003. 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology 28 (1), 100e107. 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, et al. , 1995. Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J. Pharmacol. Exp. Therapeut 275 (3), 1355e1366. [PubMed] [Google Scholar]
- Putterman DB, Munhall AC, Kozell LB, Belknap JK, Johnson SW, 2007. Evaluation of levodopa dose and magnitude of dopamine depletion as risk factors for levodopa-induced dyskinesia in a rat model of Parkinson’s disease. J. Pharmacol. Exp. Therapeut 323 (1), 277e284. [DOI] [PubMed] [Google Scholar]
- Ridray S, Griffon N, Mignon V, Souil E, Carboni S, Diaz J, et al. , 1998. Coexpression of dopamine D1 and D3 receptors in islands of Calleja and shell of nucleus accumbens of the rat: opposite and synergistic functional interactions. Eur. J. Neurosci 10 (5), 1676e1686. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, et al. , 2007. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J. Neurosci 27 (26), 6995e7005. 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastianutto I, Maslava N, Hopkins CR, Cenci MA, 2016. Validation of an improved scale for rating l-DOPA-induced dyskinesia in the mouse and effects of specific dopamine receptor antagonists. Neurobiol. Dis 96, 156e170. 10.1016/j.nbd.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Silverdale MA, Nicholson SL, Ravenscroft P, Crossman AR, Millan MJ, Brotchie JM, 2004. Selective blockade of D(3) dopamine receptors enhances the anti-parkinsonian properties of ropinirole and levodopa in the MPTPlesioned primate. Exp. Neurol 188 (1), 128e138. 10.1016/j.expneurol.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Smith Y, Wichmann T, Factor SA, DeLong MR, 2012. Parkinson’s disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology 37 (1), 213e246. 10.1038/npp.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC, 1990. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347 (6289), 146e151. 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Solis O, Garcia-Montes JR, Gonzalez-Granillo A, Xu M, Moratalla R, 2015. Dopamine D3 receptor modulates l-dopa-induced dyskinesia by targeting D1 receptor-mediated striatal signaling. Cerebr. Cortex 27 (1), 435e446. 10.1093/cercor/bhv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Walker PD, 2005. Dopamine D1 and D2 receptor contributions to L-DOPA-induced dyskinesia in the dopamine-depleted rat. Pharmacol. Biochem. Behav 81 (4), 887e893. 10.1016/j.pbb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Visanji NP, Fox SH, Johnston T, Reyes G, Millan MJ, Brotchie JM, 2009. Dopamine D3 receptor stimulation underlies the development of L-DOPAinduced dyskinesia in animal models of Parkinson’s disease. Neurobiol. Dis 35 (2), 184e192. 10.1016/j.nbd.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA, 2007. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol. Psychiatr 62 (7), 800e810. 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang X, Tocker AM, Huang P, Reith ME, Liu-Chen LY, et al. , 2017. Functional characterization of a novel series of biased signaling dopamine D3 receptor agonists. ACS Chem. Neurosci 8 (3), 486e500. 10.1021/acschemneuro.6b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.