Abstract
Objective:
Benign adrenal cysts are rare lesions of the adrenal glands. Limited data are available to guide management. We aimed to describe the presentation and outcomes of patients with adrenal cysts.
Design:
Retrospective longitudinal cohort study
Methods:
Consecutive patients with histologically or radiologically confirmed adrenal cysts between 1995–2021 were identified. Pheochromocytomas and malignancy were excluded.
Results:
Benign adrenal cysts were diagnosed in 92 patients (53, 57% women) at a median age of 45 years. Mode of discovery was incidental on imaging in 81 (88%), symptoms of mass effect in 9 (9.8%), and other in 2 (2.2%). Majority (89, 97%) of patients had unilateral cysts (45 right, 44 left) with a median size of 48 mm (range 4–200) at diagnosis. On imaging, most cysts were round/oval (85.4%), homogenous (83.2%) lesions with calcifications (64.0%) and no vascular enhancement (97.7%). During a median follow-up of 65 months (range 7–288), adrenal cysts demonstrated minimal enlargement (median size change 6 mm, median growth rate 2 mm/year). On hormonal evaluation, 10% (5/50 tested) had an abnormal overnight dexamethasone suppression test, and 9.5% (4/42 tested) had an abnormal case detection testing for primary aldosteronism. Patients treated with adrenalectomy (46, 50%) were younger (36.9 vs 50.8 years, P=0.0009) and had more rapidly enlarging cysts (median growth rate 5.5 vs 0.4 mm/year, P=0.0002).
Conclusion:
Benign adrenal cysts are usually incidentally discovered, nonfunctional, homogenous lesions without vascular enhancement that demonstrate minimal growth. Adrenalectomy should be reserved for patients with heterogeneous lesions, abnormal hormonal evaluation, or those with mass effect symptoms.
Keywords: adrenal mass, diagnosis, imaging, Hounsfield unit, computed tomography, adrenalectomy
INTRODUCTION
Benign adrenal cysts are rare lesions of the adrenal glands representing less than 1–2% of all adrenal incidentalomas (1, 2). Based on data from two autopsy studies, the overall prevalence of adrenal cyst is ≈0.06–0.18% (3, 4). Historically, adrenal cysts have been broadly classified on histopathology as endothelial (or simple), pseudocyst (or hemorrhagic), epithelial (or true), and parasitic cysts (5, 6). Limited evidence originating from surgical or histopathology-based series points towards peak incidence between 4th-6th decades of life and a slight female predisposition (7–12). Data on clinical presentation of patients with adrenal cysts derived from surgical series indicate that the majority of patients present with symptoms of mass effect, while most recent imaging-based studies suggest incidental discovery without symptoms (7, 8, 10) Although several studies note benign adrenal cyst to be predominantly unilateral lesions with no side preference, information on other imaging characteristics is limited (7, 8, 12). Furthermore, most of the current evidence on benign adrenal cysts arises from small surgical or pathological cohorts that comprise various other cystic lesions of the adrenal glands, such as cystic pheochromocytoma and adrenocortical carcinoma, or are confined to a single pathological subtype of benign adrenal cyst (7–12). Thus, the clinical presentations, imaging characteristics, natural history and outcomes of benign adrenal cyst remain unclear.
To help address the above knowledge gaps, we conducted a longitudinal study of patients evaluated for benign adrenal cysts at our institution. Our objectives were to: (1) describe the baseline clinical characteristics and outcomes of patients with benign adrenal cyst; (2) determine imaging characteristics and rate of cyst enlargement over time; and (3) identify factors associated with surgical management, and cyst recurrence after surgery.
METHODS
Patient selection and data collection
Mayo Clinic Institutional Review Board approved the study protocol. We used the Adrenal Mass Database to identify all patients with adrenal cysts and other cystic lesions evaluated at Mayo Clinic between January 1st, 1995 and December 31st, 2021. The Adrenal Mass Database includes consecutive patients with adrenal masses evaluated at our institution and was previously utilized in several other studies(13–16). We also used the Mayo Data Explorer, an institutional electronic medical record database to search for imaging, clinical, and histopathology reports for a combination of words: “adrenal” and either “cyst” or “cystic”. Images of every identified cystic adrenal mass generated through this search were reviewed to confirm the results of electronic search. Of 8032 patients in the adrenal database, 21 (0.26%) patients had cystic pheochromocytomas, 7(0.09%) patients had cystic adrenal malignancies (1 with adrenocortical carcinoma, 1 with lymphoma, and 5 patients with adrenal metastases), and 92 (1.2%) had benign adrenal cysts. Patients with a confirmed histopathological diagnosis of pheochromocytomas or malignancy were excluded. Clinical, biochemical, radiological, and surgical information were obtained from the medical record for each patient. Diagnosis of adrenal cyst was made based on histopathology in patients treated with adrenalectomy or based on imaging. Initial cyst size was obtained by measuring the largest diameter on the earliest imaging study. Maximum cyst size was defined as the largest diameter at any time during follow up. In patients with at least two imaging studies at least 6 months apart, the change in size was calculated as the difference between cyst size on the initial and most recent imaging studies. Unenhanced CT attenuation was measured in Hounsfield units (HU) and were obtained in different cystic areas (excluding calcification), and the highest value was recorded. Rim thickness was measured on contrast-enhanced imaging. Subgroup analysis was performed based on initial cyst size (< 50 mm versus ≥ 50 mm), maximum cyst size (< 50 mm versus ≥ 50 mm), cyst growth rate (< 5 mm/year versus > 5 mm/year), and surgical versus conservative management.
Statistical analysis
We presented categorical data as counts and percentages, and continuous data as median and ranges for descriptive characteristics. Association between variables was assessed using the chi square test and Wilcoxon test. Statistical significance was defined as a P-value of < 0.05. We performed statistical analysis using JMP software, version 14.
RESULTS
Demographics and clinical presentation
Of the 8032 patients with adrenal masses evaluated at our institution for the duration of this study, 92 (1.2%) had benign adrenal cysts, diagnosed at a median age of 45.0 years (range, 14.4–83.1). Benign adrenal cysts were discovered incidentally in 81 (88%) patients, during work-up for symptoms of mass effect in 9 (10%) patients, and during cancer staging imaging in 2 (3.3%) patients. Symptoms of mass effect in 9 patients (median cyst size of 99 mm, range 61–200) included abdominal or flank pain in 8 patients and heartburn and loss of appetite in 1 patient (cyst size of 200 mm). Endocrine evaluation was performed in 75 (81.5%) patients at the time of the initial diagnosis or during follow-up (Table 1). In 3 patients with benign adrenal cysts, another adrenal mass was present (adrenocortical adenoma in all).
Table 1.
Clinical presentation, imaging characteristics, management and outcomes of patients with benign adrenal cysts overall and based on initial cyst size. Data are presented as n (%) or as median (range).
| Data available for, n | All patients (n=92) | Initial Cyst Size |
P-value* | ||
|---|---|---|---|---|---|
| <50 mm (n=51) | ≥50 mm (n=41) | ||||
|
| |||||
| Clinical Characteristics | |||||
| Women | 52 (56.5%) | 28 (54.9%) | 24 (58.5%) | 0.8331 | |
| Age at diagnosis, years, median (range) | 45 (14.4–83.1) | 51.2 (14.4–83.1) | 37.1 (15.5–76) | 0.0030 | |
| Race | 0.0316 | ||||
| Caucasian | 80 (87.0%) | 42 (82.4%) | 38 (92.7%) | ||
| African American | 4 (4.5%) | 3 (5.9%) | 1 (2.4%) | ||
| Hispanic | 3 (3.3%) | 2 (3.9%) | 1 (2.4%) | ||
| Native American | 2 (2.2%) | 2 (3.9%) | 0 (0.0%) | ||
| Other or unknown | 3 (3.3%) | 2 (3.9%) | 1 (2.4%) | ||
| Mode of discovery, n/N (%) | 0.0095 | ||||
| Incidental | 81 (88.0%) | 49 (96.0%) | 32 (76.2%) | ||
| Mass effect | 9 (9.8%) | 1 (2.0%) | 8 (19.5%) | ||
| Cancer staging | 2 (2.2%) | 1 (2.0%) | 1 (2.4%) | ||
| Evaluated by endocrinologist | 75 (81.5%) | 39 (75%) | 36 (87.8%) | 0.1877 | |
| Year of diagnosis | 0.0209 | ||||
| < 2012 | 44 (47.8%) | 19 (37.3%) | 25/41 (61.0%) | ||
| ≥ 2012 | 48 (52.2%) | 32 (62.8%) | 16/41 (39.0%) | ||
| Imaging Characteristics | |||||
| Site | 0.3740 | ||||
| Right | 45 (48.9%) | 28 (54.9%) | 17 (41.5%) | ||
| Left | 44 (47.8%) | 22 (43.1%) | 22 (53.7%) | ||
| Bilateral | 3 (3.3%) | 1 (2.0%) | 2 (4.9%) | ||
| Shape | 89 | 0.5959 | |||
| Round/oval | 76 (85.4%) | 42 (84.0%) | 34 (87.2%) | ||
| Lobulated | 13 (14.6%) | 8 (16.0%) | 5 (12.8%) | ||
| Well defined borders | 89 | 87 (97.8%) | 49 (98.0%) | 38 (97.4%) | 0.8553 |
| Homogenous | 89 | 74/89 (83.2%) | 42 (84.0%) | 32 (82.1%) | 0.7975 |
| Vascular enhancement (excluding rim) | 86 | 2 (2.3%) | 2 (4.1%) | 0 (0.0%) | 0.2151 |
| Rim enhancement | 83 | 1.9 (0–71) | 2.1 (0–7) | 1.8 (0–7) | 0.9149 |
| Calcification | 86 | 55 (64.0%) | 26 (55.3%) | 29 (74.4%) | 0.0676 |
| Calcification pattern | |||||
| Peripheral | 49 (89.1%) | 26 (100.0%) | 23 (79.3%) | ||
| Septal | 10 (18.2%) | 5 (19.2%) | 5 (17.2%) | ||
| Scattered | 12 (21.8%) | 6 (23.1%) | 6 (20.7%) | ||
| Unenhanced CT attenuation, HU | 76 | 19 (0–83) | 19.6 (0–83) | 18 (8.9–70) | 0.8014 |
| Cyst growth rate, mm per year | 59 | +2 (−8.9 to 27.4) | +2.3 (−5.9 to 20.7) | +0.22 (−8.9 to 27.4) | 0.3613 |
| Change in cyst size$, mm | 59 | +6 (−60 to 111) | +8.5 (−37.6 to 111) | +3.0 (−60 to 100) | 0.2827 |
| Maximum cyst size, mm | 57.5 (5–200) | 42 (5–159) | 82.8 (51–200) | <0.0001 | |
| Hormonal Assessment | |||||
| Hormonal testing done | 78 (84.8%) | 41 (78.4%) | 37 (90.2%) | 0.1934 | |
| Catecholamine excess | 76 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Cortisol secretory autonomy | 50 | 5 (10%) | 3 (10.7%) | 2 (9.5%) | |
| Aldosterone excess | 42 | 4 (9.5%) | 2 (8.3%) | 2 (11.1%) | |
| Management | |||||
| No surgical intervention | 46 (50.0%) | 29 (56.9%) | 17 (41.5%) | 0.2080 | |
| Laparoscopic adrenalectomy | 35 (38.0%) | 18 (35.3%) | 17 (41.5%) | 1.000 | |
| Open adrenalectomy | 7 (7.6%) | 2 (3.9%) | 5 (12.2%) | ||
| Cystectomy | 4 (4.4%) | 2 (3.9%) | 2 (4.9%) | ||
| Follow-up | |||||
| Imaging follow-up, months | 59 | 65 (7–288) | 71 (12–233) | 46 (7–288) | 0.4206 |
| Clinical follow-up, months | 88 | 58 (1–292) | 79 (1–259) | 50 (1–292) | 0.2800 |
| Appearance of new cyst | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
Abbreviations used: CT = computed tomography; HU = Hounsfield unit
Comparison between initial cyst size < 50 mm and ≥ 50 mm. P value < 0.05 is significant
Difference between cyst size on last and the first imaging studies
Imaging characteristics
On imaging, 89 (96.7%) patients had unilateral cysts (45 on the right and 44 on the left), and 3 (3.3%) presented with bilateral cysts. At the time of initial discovery, the median size was 48 mm (range 5–200), without site differences. Compared to patients with initial cyst size of <50 mm (n=41), patients with initial cyst size of ≥50 mm (n=51), were younger at diagnosis (37.1 vs 51.2 years, P=0.003) and more likely to present with symptoms of mass effect (19.5 vs 2%, P=0.009). No differences in sex, unenhanced CT attenuation, change in size, cyst growth rate, or proportion of surgical intervention were noted between groups (Table 1).
On imaging, benign adrenal cysts were round or oval-shaped in 76 (85.4%) and lobulated (bi- or multilobed) in the remaining 13 (14.6%) patients. Almost all cysts (87, 97.8%) demonstrated well-defined borders with the median rim thickness of 1.9 mm (range, 0–7.1). In 55 (64%) cysts with calcifications, the calcification pattern was peripheral in 49 (89.1%), septate in 10 (18.2%), scattered 12 (21.8%) and a combination of these in 11 (20%) patients. Overall, benign adrenal cysts were homogenous in 74 (83.3%) patients. In 76 patients with available unenhanced CT, the median unenhanced attenuation was 19 HU (range, 0 to 83 HU). On contrast-enhanced CT, most benign adrenal cysts were nonenhancing, with only 2% cysts demonstrating a minor intracystic vascular enhancement (excluding the rim) with an increment of 14.5 HU (Table 1, Figure 1).
Figure 1. Computed tomography (CT) imaging of benign adrenal cysts in selected patients.
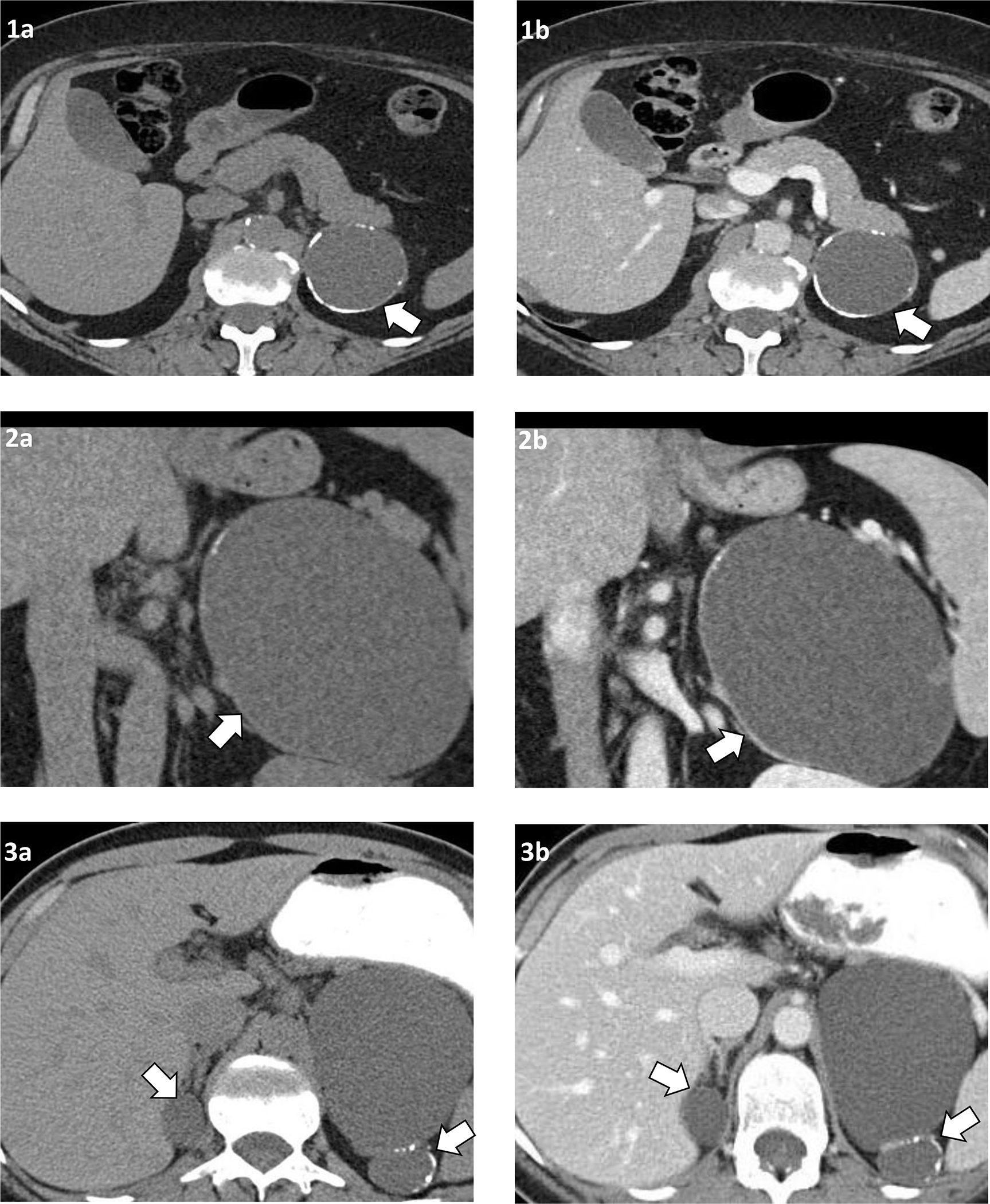
Patient 1: Left adrenal cyst, 60 mm, round shape, egg-shell calcification on cross-sectional unenhanced CT (1a), and after intravenous contrast demonstrating no intracystic enhancement (1b).
Patient 2: Left adrenal cyst, 120 mm, oval shape, peripheral calcification on unenhanced coronal CT (2a), and after intravenous contrast demonstrating partial rim enhancement but no intracystic enhancement (2b).
Patient 3: Bilateral benign adrenal cyst: right adrenal cyst, 26 mm and round shape, and left adrenal cyst, 79 mm and lobulated shape with peripheral and septate calcification. Unenhanced CT image (3a) and after intravenous contrast demonstrating no intracystic enhancement (3b).
Hormonal evaluation
Hormonal work-up in patients included assessment of catecholamine excess in 76 (82.6%) patients, glucocorticoid autonomy in 50 (54.4%) patients, and primary aldosteronism in 42 (45.7%) patients. An overnight 1-mg or 8-mg dexamethasone suppression test was abnormal in 5 patients, 4 of whom had adrenalectomy. Two patients developed postoperative adrenal insufficiency and on histopathology, one was diagnosed with a pseudocyst and the other with an endothelial cyst (Table 2). Case detection testing for primary aldosteronism was abnormal in 4 patients. None had confirmatory testing and 3 underwent adrenalectomy for other reasons (Table 1).
Table 2:
Presentation and outcomes of patients with abnormal preoperative overnight dexamethasone suppression test.
| Patient | Year of evaluation | Post 1-mg DST cortisol (nmol/L) | Baseline ACTH (pmol/L) | Baseline DHEA-S (nmol/L) | 24-hour UFC (nmol/day) | Histopathology | Postoperative adrenal insufficiency |
|---|---|---|---|---|---|---|---|
| 1 | 2003 | 66 | - | - | - | - | - |
| 2 | 2011 | 69 | - | - | - | Pseudocyst (hemorrhagic) | No |
| 3 | 2012 | 85.5 | - | - | 14.1 | Pseudocyst (hemorrhagic) | No |
| 4 | 2017 | 143.5 | 2.9 | 4855.5 | 151.8 | Endothelial cyst | Yes |
| 5* | 2021 | 149 | <1.1 | 184.8 | - | Pseudocyst (hemorrhagic) | Yes |
Abbreviations used: . ACTH = adrenocorticotrophic hormone or corticotropin. DHEA-S = dehydroepiandrosterone sulfate. DST = dexamethasone suppression test; UFC, urinary free cortisol.
Reference range – Baseline ACTH 1.6–13.9 pmol/L. DHEA-S 1793–9214.4 nmol/L. Post DST cortisol ≤50 nmol/L. Urine free cortisol 9.7–127.2nmol/day
Patient on long-term prednisone therapy for rheumatoid arthritis and polymyalgia rheumatica
Intervention, pathology and follow-up
Surgical intervention was performed in 46 (50%) patients and included adrenalectomy in 42 (91%) (35 via laparoscopic and 7 via open approach) and laparoscopic cystectomy in 4 (9%) (Table 1). The primary indication for surgery was concern for an alternate etiology in 19 (41.3%) patients, a large or an enlarging cyst in 17 (37%, median maximum cyst size 102 mm [range, 41–195]), symptoms of mass effect in 5 (10.9%, median maximum cyst size 115 mm [range, 33–200]), patient preference in 3 (6.5%), therapy for ipsilateral hormone excess in 1, and removal as a part of an extensive oncologic surgery in 1. Open adrenalectomy was performed to treat a 180 mm cyst (n=1), due to concern of malignancy (n=1), as a part of oncologic surgery (n=1), and when open adrenalectomy was more frequently utilized in the earlier period of the study (n=4, prior to year 2005). Cyst aspiration was performed in 5 patients, however 3 patients required subsequent adrenalectomy, one patient was later treated with alcohol ablation, with only 1 patient demonstrating no cyst recurrence over 10-year follow-up. Following adrenalectomy, the 30-day surgical complication rate was 4.4% (2 patients), with 1 patient developing postoperative ileus, and another patient who underwent adrenalectomy as a part of multivisceral resection for invasive renal cell carcinoma developed blood loss anemia requiring blood transfusions. When compared to patients managed conservatively (n=46), patients treated with surgery were younger (36.9 vs 50.8 years, P=0.0009), and demonstrated a higher cyst growth rate (5.5 vs 0.4 mm/year, P=0.0002) with a larger increment in size (26 vs 2.25 mm, P=0.0004). Sex, initial cyst size, unenhanced CT attenuation, or presence of symptoms of mass effect were not associated with the decision to pursue surgery (Table 3). The histopathologic subtype in the 46 patients who underwent adrenalectomy included 22 (48%) endothelial cysts, 22 (48%) pseudocysts (including 4 that were associated with adrenocortical adenomas), 1 (2%) epithelial cyst, and 1 (2%) mesothelial cyst. Imaging follow up of at least 6 months was available in 59 patients. Over a median follow-up period of 65 months (range, 6–288), the median cyst growth rate was 2 mm/year (range, −8.9 to 27.4), and the overall median change in size was +6 mm (range, −60 to 111) (Table 1). Patients with cyst growth rate of >5mm/year (n=15) showed a larger median size change (39 vs 2.3 mm, P<0.0001) and were more likely to be treated surgically (87 vs 23%, P<0.0001) compared to patients with growth rate of <5 mm/year (n=44). Sex, age, initial cyst size, unenhanced CT attenuation, or presence of symptoms of mass effect were not associated with cyst growth (Table 4).
Table 3.
Presentation and outcomes of patients based on management. Data are presented as n (%) or as median (range).
| Variable | Management |
P-value | |
|---|---|---|---|
| Conservative | Surgical | ||
|
| |||
| n | 46 | 46 | |
| Age at diagnosis, years | 50.8 (19–83) | 36.9 (14–60) | 0.0009 |
| Women | 24 (52%) | 28 (61%) | 0.5284 |
| Symptoms of mass effect | 2 (4%) | 7 (15%) | 0.1577 |
| Initial cyst size, mm | 45 (12–99) | 50.5 (5–200) | 0.3753 |
| Unenhanced CT attenuation, HU | 18 (0–83) | 19 (0–70) | 0.2892 |
| Cyst growth rate#, mm per year | +0.4 (−9 to 13) | +5.5 (−8 to 27) | 0.0002 |
| Change in cyst size#,$, mm | +2.3 (−60 to 73) | +26 (−30 to 111) | 0.0004 |
Abbreviations used: CT = computed tomography; HU = Hounsfield units
P value < 0.05 is significant
Available for 59 patients with more than 6 months of imaging follow-up
Difference between cyst size on last and the first imaging studies
Table 4.
Presentation and outcomes of patients based on maximum cyst size and growth rate. Data are presented as n (%) or as median (range).
| Variable | Maximum cyst size |
Cyst growth rate |
||||
|---|---|---|---|---|---|---|
| < 50 mm | ≥ 50 mm | P-value | <5mm/year | >5mm/year | P-value | |
|
| ||||||
| n | 38 | 54 | 44 | 15 | ||
| Age at diagnosis, years | 52 (14–81) | 37 (20–76) | 0.0003 | 48 (14–83) | 36 (19–68) | 0.2844 |
| Women | 23 (61%) | 29 (54%) | 0.5309 | 26 (59%) | 7 (47%) | 0.5485 |
| Symptoms of mass effect | 0 (0%) | 9 (17%) | 0.0093 | 3 (7%) | 2 (13%) | 0.5933 |
| Initial cyst size, mm | 35 (5–49) | 61 (20–88) | <0.001 | 48 (11–104) | 46 (17–100) | 0.5833 |
| Unenhanced CT attenuation, HU | 20 (0–83) | 19 (9–70) | 0.7643 | 20 (5–83) | 20 (9–50) | 0.8733 |
| Cyst growth rate#, mm per year | +0.6 (−2.5 to 6) | +3.6 (−9 to 27) | 0.0887 | |||
| Change in cyst size#,$, mm | +3 (−38 to 30) | +12.5 (−60 to 111) | 0.0495 | +2.3 (−60 to 73) | +39 (6 to 111) | <0.0001 |
| Surgical intervention, n (%) | 12 (32%) | 34 (63%) | 0.0056 | 10 (23%) | 13 (87%) | <0.0001 |
Abbreviations used: CT = computed tomography; HU = Hounsfield units
P value < 0.05 is significant
Available for 59 patients with more than 6 months of imaging follow-up
Difference between cyst size on last and the first imaging studies
During follow-up, a total of 54 (58.7%) patients reached a maximum cyst size ≥50 mm. When compared to patients with maximum cyst size of <50 mm (n=38), patients with cyst size ≥50 mm were younger (median age at diagnosis of 36.6 vs 52.4 years, P=0.0003), had larger initial cyst size (61 vs 35 mm, P<0.001), and were more likely to present with symptoms of mass effect (17% vs 0%, P=0.0093) and undergo surgical management (63% vs 31.6%, P=0.006). Though patients with maximum cyst size of ≥50 mm demonstrated a larger overall median increment in size (12.5 vs 3 mm, P=0.0495), no difference in the median cyst growth rate (0.6 vs 3.5 mm per year, P=0.0887) was noted. Maximum cyst size was not associated with sex or unenhanced CT attenuation (Table 4).
During a median clinical follow-up of 58 months (range, 1–292), none of the 88 patients developed new or recurrent cysts. Overall mortality was 4(4.6%), all due to cardiovascular causes. Except for two patients who developed postoperative adrenal insufficiency, no morbidity or mortality related to benign adrenal cysts or intervention was demonstrated (Table 1).
DISCUSSION
In this largest to date longitudinal cohort study of patients with benign adrenal cysts, we characterized clinical presentation, imaging phenotype, and described management and outcomes. We found that most benign adrenal cysts were discovered incidentally and were most commonly unilateral large lesions that did not have enhancement with contrast on CT scan. We further found that larger cysts were more common in younger patients but did not demonstrate an accelerated growth rate when compared to smaller cysts. Patients with benign adrenal cysts had excellent outcomes; however, half were treated with surgery, and only in minority of patients to alleviate symptoms of mass effect.
We found that benign adrenal cysts represented 1.2% of adrenal tumors seen at our institution over the 27-year study period. Similar to previous smaller studies, we found a slight female preponderance (7–10, 12). In our study, patients with benign adrenal cysts were younger at the time of diagnosis than what was previously reported (7–10, 12). Potential explanations for this discrepancy are inclusion of consecutive patients as opposed to only surgically treated patients in previous cohorts, and an earlier discovery of adrenal cysts in our cohort due to more widespread use of imaging over the last decades. Unlike older studies (7, 8), most of the patients in our cohort presented incidentally, and symptoms of mass effect led to cyst discovery in only 10% of patients.
In our study, we were able to examine imaging characteristics in nearly all patients and noted several common findings suggestive of benign adrenal cysts. We found benign adrenal cysts to be predominantly round or oval lesions, with well-defined borders and homogenous appearance. Most benign adrenal cysts were unilateral lesions, with nearly equal left and right distribution. Only 3% of our patients had bilateral cysts, lower than 6–8% prevalence reported by prior studies that included also other cystic lesions (6, 17). The median cyst size in our cohort was 48 mm at the time of discovery and 57.5 mm at the end of the follow-up, with almost 60% achieving a maximum size of 50 mm or more. In prior studies, the average cyst size has varied between 47 and 96 mm, likely due to a higher selection bias (7–12). Information on the imaging phenotype of benign adrenal cysts is limited to small patient cohorts (18–20). In our study we were able to determine the rate of cyst enlargement over time, something that has been described only once before (20). Interestingly, we found no predictors of cyst growth, and the annual cyst growth in larger cysts was similar to smaller cysts.
Notably, we demonstrated that benign adrenal cysts showed no intracystic enhancement with contrast except for two patients with cysts that demonstrated only slight enhancement. Calcification was a relatively common finding in nearly two thirds of patients, predominantly in a peripheral pattern. We examined unenhanced CT attenuation in most of our patients and noted a spectrum of HU measurements (between 0 and 83 HU, with a median of 19 HU). The broad range of unenhanced CT attenuation and heterogenous appearance of some adrenal cysts might have represented the various hematoma stages commonly associated with these lesions. Interestingly, some cysts demonstrated a significant decrease in cyst size by as much as 60 mm during follow-up, a finding also noted by Ricci et al. (20).
Typically, benign adrenal cysts are non-functional lesions (9, 10, 21). Nonetheless, a thorough evaluation for hormone excess should be done, as in other adrenal lesions. Previous studies described rare cases of cystic pheochromocytomas and cystic adrenocortical carcinomas (7–10, 12). No patient in our study had catecholamine excess, however we excluded cystic pheochromocytomas as a part of our study design. Of the 4 patients with a positive case detection testing for primary hyperaldosteronism, none had confirmatory test. In two of the 5 patients with abnormal overnight dexamethasone suppression test, postoperative morning serum cortisol was concerning for adrenal insufficiency, including one patient who had been on prolonged treatment with high-dose steroids for rheumatological conditions. Whether the low postoperative serum cortisol in the one patient was due to preoperative autonomous cortisol secretion causing contralateral gland suppression or exogenous steroids resulting in the hypothalamic-pituitary-adrenal axis suppression is unclear. On histopathological examination, this patient had a hemorrhagic cyst, raising suspicion for a prior adenoma that hemorrhaged or infarcted with subsequent evolution into a cyst containing some remnant cortical tissue.
In most patients, adrenalectomy was performed due to concern for continued enlargement or an alternative etiology and only in a minority to relieve symptoms of mass effect. Younger patients, those with higher cyst growth, and patients with larger cysts were more likely to undergo surgical intervention. Concern for an alternative etiology or malignancy led to adrenalectomy in around 40% of patients, highlighting the value of accurate interpretation of imaging characteristics. Compared to benign adrenal cysts, cystic pheochromocytomas and cystic adrenal malignancies are rarer and more likely to present as heterogeneous lesions composed of a peripherally enhancing solid component of tumor tissue with non-enhancing cystic areas of hemorrhage or necrosis (16, 22, 23). Moreover, almost all cystic pheochromocytomas (16) and 50–60% of adrenocortical carcinomas are hormonally active tumors (24, 25) (Table 5).
Table 5.
Differences between benign adrenal cysts, cystic pheochromocytomas, and cystic adrenal malignancies. Data are presented as n (%) or as median (range).
| Variable | Benign adrenal cyst$ | Cystic pheochromocytoma# | Cystic adrenal malignancy* |
|---|---|---|---|
|
| |||
| n | 92 | 21 | 7 |
| Mode of discovery, % | |||
| Incidental | 90 | 60 | 86 |
| Abdominal mass effect | 10 | 25 | - |
| Symptoms of hormone excess | 0 | 10 | - |
| Genetic screening | 0 | 5 | - |
| Type B symptoms | - | - | 14 |
| Tumor laterality, % | 98% Unilateral | 100% Unilateral | 86% Unilateral |
| Tumor size, cm | 4.8 (0.5–20) | 6.4 (3–19) | 6.7 (3.6–28) |
| Unenhanced CT, HU | 19 (0–83) | ||
| Cystic component | 18 (13–30) | 19 (14–22) | |
| Solid component | 40 (22–50) | 38 (28–44) | |
| Contrast enhancement | Rare | ||
| Cystic component | No | No | |
| Solid component | Yes | Yes | |
| Heterogeneous appearance, % | 10–15 | 85 | 100 |
| Calcifications,% | 64 | 15 | 0 |
| Hormonal | Adrenocortical cancer (n=1): incomplete work up (negative for aldosterone and androgen excess); Adrenal metastases (n=6): not applicable | ||
| Overt hormone excess, % | 0 | 100 | |
| Mild hormone excess, % | 12 | 0 | |
| Hormonally inactive, % | 88 | 0 | |
| Management | Conservative; adrenalectomy if symptoms of mass effect | Adrenalectomy after adequate alpha-blockade | Depending on primary etiology (adrenalectomy, ablation, chemotherapy) |
Abbreviations used: CT = computed tomography; HU = Hounsfield units
Current study
Dogra P, Navin PJ, McKenzie TJ, Foster T, Dy B, Lyden M, Young WF, Jr., Bancos I. Clinical, imaging and biochemical presentation of cystic pheochromocytomas. Clin Endocrinol (Oxf). 2022.
Unpublished data from the Mayo Clinic Adrenal Database
The strengths of our study include the large sample size, the inclusion of consecutive patients irrespective of management, detailed review of imaging characteristics, and the longitudinal follow-up enabling characterization of patient outcomes. Our study has several limitations inherent to the retrospective design with possible underestimation of complications related to cysts and surgery, selection bias, information bias, imaging bias, and referral bias. However, given the rarity of benign adrenal cysts, other study designs may not be feasible. The diagnosis of benign adrenal cysts was not confirmed by pathology in all patients, but the imaging characteristics and growth rate were reassuring of the benign nature. Furthermore, the data on postoperative follow-up for mass effect symptoms and hormonal secretion were scarce, thus, limiting our understanding of response to surgical management. Our study represents experience of a single center and predominantly Caucasian population which may limit generalizability of our observations to a broader population. Lastly, we also acknowledge possible differences in practice over the last 3 decades and different specialties. Decisions on the management of benign adrenal cysts are not standardized and depend on multiple factors, including patient preference.
In conclusion, we describe the clinical and imaging presentation of patients with benign adrenal cysts, both essential to making the initial diagnosis and management decisions. Despite their large size, and continuous cyst enlargement, no adverse outcomes occurred. Symptoms of mass effect did occur in 10% of patients and may be an indication for surgery. While adrenalectomy could be performed without significant morbidity, it is likely not needed in most patients with benign adrenal cysts and should be reserved for patients with hormonal evaluation or imaging characteristics (heterogeneous lesion, thick enhancing rim, or presence of solid component) concerning for an alternate etiology, or in those who develop symptoms of mass effect.
FUNDING:
This research was partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) USA under award K23DK121888 (to I.B). The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health USA.
Footnotes
DECLARATION OF INTEREST: IB reports advisory board participation with Corcept Therapeutics, HRA Pharma, Sparrow Pharmaceutics, Spruce, Recordati Rare Diseases, Adrenas, Lantheus outside the submitted work. Other authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- 1.Ebbehoj A, Li D, Kaur RJ, Zhang C, Singh S, Li T, Atkinson E, Achenbach S, Khosla S, Arlt W, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(11):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichijo T, Ueshiba H, Nawata H, Yanase T. A nationwide survey of adrenal incidentalomas in Japan: the first report of clinical and epidemiological features. Endocr J. 2020;67(2):141–52. [DOI] [PubMed] [Google Scholar]
- 3.Wahl HR. Adrenal cysts. Am J Pathol. 1951;27(758). [Google Scholar]
- 4.Hodges FV, Ellis FR. Cystic lesions of the adrenal glands. AMA Arch Pathol. 1958;66(1):53–8. [PubMed] [Google Scholar]
- 5.Abeshouse GA, Goldstein RB, Abeshouse BS. Adrenal cysts; review of the literature and report of three cases. J Urol. 1959;81(6):711–9. [DOI] [PubMed] [Google Scholar]
- 6.Foster DG. Adrenal cysts. Review of literature and report of case. Arch Surg. 1966;92(1):131–43. [DOI] [PubMed] [Google Scholar]
- 7.Erickson LA, Lloyd RV, Hartman R, Thompson G. Cystic adrenal neoplasms. Cancer. 2004;101(7):1537–44. [DOI] [PubMed] [Google Scholar]
- 8.Chien HP, Chang YS, Hsu PS, Lin JD, Wu YC, Chang HL, Chuang CK, Tsuei KH, Hsueh C. Adrenal cystic lesions: a clinicopathological analysis of 25 cases with proposed histogenesis and review of the literature. Endocr Pathol. 2008;19(4):274–81. [DOI] [PubMed] [Google Scholar]
- 9.Major P, Pedziwiatr M, Matlok M, Ostachowski M, Winiarski M, Rembiasz K, Budzynski A. Cystic adrenal lesions - analysis of indications and results of treatment. Pol Przegl Chir. 2012;84(4):184–9. [DOI] [PubMed] [Google Scholar]
- 10.Cavallaro G, Crocetti D, Paliotta A, De Gori A, Tarallo MR, Letizia C, De Toma G. Cystic adrenal lesions: clinical and surgical management. The experience of a referral centre. Int J Surg. 2015;13:23–6. [DOI] [PubMed] [Google Scholar]
- 11.Koperski L, Pihowicz P, Anysz-Grodzicka A, Gornicka B. Cystic lymphangiomatous lesions of the adrenal gland: A clinicopathological study of 37 cases including previously unreported cysts with papillary endothelial proliferation. Pathol Res Pract. 2019;215(6):152385. [DOI] [PubMed] [Google Scholar]
- 12.Sebastiano C, Zhao X, Deng FM, Das K. Cystic lesions of the adrenal gland: our experience over the last 20 years. Hum Pathol. 2013;44(9):1797–803. [DOI] [PubMed] [Google Scholar]
- 13.Hamidi O, Raman R, Lazik N, Iniguez-Ariza N, McKenzie TJ, Lyden ML, Thompson GB, Dy BM, Young WF, Jr., Bancos I. Clinical course of adrenal myelolipoma: A long-term longitudinal follow-up study. Clin Endocrinol (Oxf). 2020;93(1):11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurtado MD, Cortes T, Natt N, Young WF Jr., Bancos I. Extensive clinical experience: Hypothalamic-pituitary-adrenal axis recovery after adrenalectomy for corticotropin-independent cortisol excess. Clin Endocrinol (Oxf). 2018;89(6):721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao JJ, Baker JE, Rainey WE, Young WF Jr., Bancos I. Concomitant Pheochromocytoma and Primary Aldosteronism: A Case Series and Literature Review. J Endocr Soc. 2021;5(8):bvab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogra P, Navin PJ, McKenzie TJ, Foster T, Dy B, Lyden M, Young WF, Jr., Bancos I. Clinical, imaging and biochemical presentation of cystic pheochromocytomas. Clin Endocrinol (Oxf). 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neri LM, Nance FC. Management of adrenal cysts. Am Surg. 1999;65(2):151–63. [PubMed] [Google Scholar]
- 18.Wang LJ, Wong YC, Chen CJ, Chu SH. Imaging spectrum of adrenal pseudocysts on CT. Eur Radiol. 2003;13(3):531–5. [DOI] [PubMed] [Google Scholar]
- 19.Rowe SP, Bishop JA, Prescott JD, Salvatori R, Fishman EK. CT Appearance of Adrenal Cystic Lymphangioma: Radiologic-Pathologic Correlation. AJR Am J Roentgenol. 2016;206(1):81–5. [DOI] [PubMed] [Google Scholar]
- 20.Ricci Z, Chernyak V, Hsu K, Mazzariol FS, Flusberg M, Oh S, Stein M, Rozenblit A. Adrenal cysts: natural history by long-term imaging follow-up. AJR Am J Roentgenol. 2013;201(5):1009–16. [DOI] [PubMed] [Google Scholar]
- 21.Janevska V, Janevski V, Stankov O, Spasevska L, Kostadinova-Kunovska S, Zhivadinovik J. Non-Tumor Cystic Lesions of the Adrenal Gland. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015;36(3):51–9. [DOI] [PubMed] [Google Scholar]
- 22.Rockall AG, Babar SA, Sohaib SA, Isidori AM, Diaz-Cano S, Monson JP, Grossman AB, Reznek RH. CT and MR imaging of the adrenal glands in ACTH-independent cushing syndrome. Radiographics. 2004;24(2):435–52. [DOI] [PubMed] [Google Scholar]
- 23.Dunnick NR, Heaston D, Halvorsen R, Moore AV, Korobkin M. CT appearance of adrenal cortical carcinoma. J Comput Assist Tomogr. 1982;6(5):978–82. [DOI] [PubMed] [Google Scholar]
- 24.Alaa Sada MA, Bews Katherine A., Thompson Geoffrey B., Young William F. Jr., Bancos Irina, Farley David R., Dy Benzon M., Lyden Melanie L., Habermann Elizabeth B., McKenzie Travis J.. Comparison between functional and non-functional adrenocortical carcinoma. Surgery. 2020;167(1). [DOI] [PubMed] [Google Scholar]
- 25.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]


