Abstract
Background
Immunological markers and related signaling molecules in the blood are altered in schizophrenia mouse models, in acutely relapsed patients with schizophrenia, and in persons at a clinically high risk for subsequently developing psychosis, highlighting their potential as prognostic and theranostic biomarkers. Therefore, we herein aimed to identify novel potential biomarkers in the serum that are associated with purinergic signaling.
Methods
To our knowledge, this is the first study to assess the correlations among the levels of human serum adenine nucleotides (ATP, ADP), adenosine, P2X7 receptor, and disease activity in patients hospitalized due to an acute relapse of schizophrenia (n = 53) and healthy controls (n = 47). In addition, to validate these findings using a reverse translational approach, we examined the same parameters in an acute phencyclidine-induced schizophrenia mouse model.
Results
We found consistently elevated levels of ATP, ADP, interleukin (IL)-6, and IL-10 in both schizophrenia groups compared with the controls. The levels of adenosine, IL-1β, IL-12, and C-reactive protein were also increased in the human patient samples. Moreover, ATP and ADP were significantly positively correlated with the Positive and Negative Symptom Scale item “lack of judgment and insight”; IL-1β, IL-12, and tumour necrosis factor alpha were significantly positively correlated with “tension” and “depression”; and “disorientation” and “poor attention” were correlated significantly with IL-6 and IL-8.
Conclusions
Our study suggests the promising potential of blood purines and inflammatory markers as future prognostic tools.
Keywords: Biomarkers, neuroinflammation, purinergic signaling, P2X7 receptor, schizophrenia
Significance Statement.
In the present study, we report for the first-time, to our knowledge, elevated serum purine levels in schizophrenic patients and the association between serum adenine nucleotides (ATP, ADP) and intensity of clinical symptoms in patients hospitalized due to an acute phase of schizophrenia. Furthermore, to validate these findings with a reverse translational approach, we analyzed the identical factors in an acute phencyclidine-induced schizophrenia mouse model. We found consistently elevated levels of ATP, ADP, IL-6, and IL-10 in both schizophrenia groups compared with the controls. Our study suggests the promising potential of blood purines and inflammatory markers as future prognostic tools.
Introduction
Schizophrenia is a complex neurodevelopmental disorder that affects approximately 0.5%–1% of the global population and is 1 of the top 10 global causes of disability (Marder and Cannon, 2019). This chronic, debilitating psychiatric disorder is characterized by positive symptoms, negative symptoms, and cognitive deficits, which often persist despite treatment (Bitter et al., 2019; Marder and Cannon, 2019). Like most mental disorders, schizophrenia appears to be caused by a combination of genetic, environmental, and social risk factors (Byrne et al., 2004; van Os et al., 2010; Varese et al., 2012; Stilo and Murray, 2019) linked to each other by epigenetic mechanisms regulating gene expression levels and molecular pathways (van de Leemput et al., 2016; Javidfar et al., 2017). Although the pathophysiology of schizophrenia is still not completely understood, there is a growing awareness that dysregulation of immune system components is fundamentally linked to the disorder (Carter et al., 2014; Çakici et al., 2019).
Robust evidence collected over the past few years suggests that peripheral and central inflammation play important roles in neuropsychiatric diseases, leading to the hypothesis that mental health is modulated by systemic inflammatory tone (Bhattacharya, 2018; Leighton et al., 2018). Although the elevated levels of blood cytokines in schizophrenia patients have been confirmed by meta-analyses (Miller et al., 2011; Goldsmith et al., 2016; Çakici et al., 2019; Reale et al., 2021), whether this elevated inflammatory tone is the cause, mediator, or consequence of the disorder remains unknown. Likewise, it is not completely clear whether this systemic increase in inflammatory tone can serve as a biomarker of acute relapse, prognosis, or treatment response.
The purinergic signaling system consists of enzymes transporters and receptors responsible for the synthesis, release, action, and inactivation of extracellular ATP and its breakdown product, adenosine (Ado). Purinergic signaling has already been implicated in schizophrenia, although most of the studies have examined the involvement of Ado pointing to a general hypoadenosinergic state in the brain in schizophrenia and imbalance between A1 and A2A Ado receptors (for further references, see Cheffer et al., 2018). As for P2 receptor–mediated signaling, the role of both P2Y and P2X receptors has been raised (Krügel, 2016; Cheffer et al., 2018; Huang et al., 2019; Andrejew et al., 2020). Recently, increased ADP levels in cerebrospinal fluid and a dysregulation of the expression of different P2X, P2Y, and Ado receptors was found in the social isolation model of schizophrenia (Andrejew et al., 2021).
The P2X7 receptor (P2X7R) is an ATP-sensitive ligand gated nonselective cation channel (Sperlágh and Illes, 2014) and one of the most widely studied P2 receptors in brain diseases (Jimenez-Mateos et al., 2019) due to its prominent role in promoting neuroinflammation in the central nervous system (CNS).
In healthy tissues, the concentration of extracellular ATP is low at the nanomolar range (Falzoni et al., 2013)—its concentration in human blood is normally approximately 20–100 nM (Forrester, 1972); nevertheless, under stress and cellular damage, the ATP concentration increases considerably. At high concentrations, extracellular ATP acts as a danger-/damage-associated molecular pattern and activates the P2X7R (Di Virgilio et al., 2018); this induces K+ efflux, which is needed for efficient NOD-like receptor protein 3 inflammasome activation. The NOD-like receptor protein 3 inflammasome triggers the activation of caspase-1, which causes the maturation of interleukin (IL)-1β and IL-18 and, consequently, increases proinflammatory cytokine release (Jo et al., 2016; He et al., 2017; Bhattacharya and Jones, 2018).
We recently demonstrated that both pharmacological and genetic blockade of P2X7Rs could alleviate schizophrenia-like behavior using a phencyclidine (PCP)-induced mouse model of schizophrenia, suggesting that these receptors are endogenously activated in this model (Koványi et al., 2016; Calovi et al., 2020). Another indication for the role of P2X7R in psychoses is that antipsychotic drugs such as prochlorperazine and trifluoperazine alleviate schizophrenia-like behavioral alterations in both animals and humans, allosterically and negatively modulating the function of P2X7Rs (Hempel et al., 2013). Thus, the antipsychotic-induced inhibition of P2X7R may contribute to therapeutic efficacy (Cheffer et al., 2018). As for genetic studies, although researchers in an early study could not find an association between the P2RX7 gene variants and schizophrenia (Hansen et al., 2008), a more recent genome-wide environment-interaction study found that P2RX7 plays a role in vulnerability to develop psychotic symptoms when using cannabis (Boks et al., 2020).
However, to fully understand how P2X7R contributes to disease pathology and to translate the findings to the development of more efficient P2X7R-based therapies, the regulatory mechanisms underlying the pathological expression and function of P2X7R in diseases (Jimenez-Mateos et al., 2019) must be defined. Therefore, we hypothesized that increased purine levels and the consequentially elevated systemic inflammatory tone are hallmarks of the acute episodes of chronic schizophrenia. During these inflammatory “flares,” which are characterized by increased purine levels acting as danger-/damage-associated molecular patterns, purinergic mechanisms might regulate the accompanying inflammatory response and, potentially and indirectly, the symptoms of the acute psychotic episode.
Therefore, we aimed to identify potential serum biomarkers that are associated with purinergic signaling in patients hospitalized due to an acute relapse of schizophrenia. Furthermore, we examined the correlations among the levels of purines, P2X7R, and disease activity in schizophrenia. In addition, to validate these findings using a reverse translational approach, we examined the same parameters in an acute PCP-induced schizophrenia mouse model representing the acute phase of the disorder.
METHODS
Human Study
Participants
—Fifty-three patients with a diagnosis of schizophrenia and 47 healthy volunteers with no psychiatric history participated in the study. We calculated the sample sizes of the control and schizophrenic groups using G*Power 3.1.9.7 software with the following parameters: Student’s t test: a priori: compute required sample size; power: 0.9; α error probability: 0.05; effect size: 0.3; and total sample size: 109. The included patients were recruited from 3 Hungarian sites (Department of Psychiatry and Psychotherapy, Semmelweis University; Department of Psychiatry and Psychiatric Rehabilitation, Jahn Ferenc Hospital; and Department of Psychiatry and Psychiatric Rehabilitation, Saint John Hospital), and the selection of the control group was performed by opportunity sampling. The enrollment and assessment of the participants took place between October 2018 and October 2020. The initial evaluation included demographic details and history of the disorder.
The study was carried out in accordance with the Declaration of Helsinki and approved by the Scientific and Research Ethical Committee of the Hungarian Medical Research Council (permission number: 947-5/2018/EÜIG). Prior to participation in the study, the procedures were explained in detail, and written informed consent was obtained from all participants.
Eligible patients were 18–65 years old, diagnosed with any subtype of schizophrenia according to International Classification of Diseases (ICD)-10, hospitalized due to an acute phase, and enrolled a maximum 3 days after admission. Acute psychotic exacerbation was defined by a total Positive and Negative Syndrome Scale (PANSS) score of 70 or higher, based on the findings of Leucht et al. (2005), with a slight alteration. Patients with a history of substance abuse or dependence within 2 years of the testing, mental retardation at any stages (mild to severe), schizoaffective disorder, drug-induced psychosis, or a significant general medical condition were excluded. In all cases, where psychoactive drug use was suspected—based on the at-the-time status or the clinical history of the patient—a urinary test was prescribed by the patient’s doctor to ensure the state of abstinence. Patients with minor abnormalities in laboratory values were not excluded only if their treating physician considered them as having a severe somatic condition/s (“significant general medical condition”). The exclusion criteria for the control group included a history of any ICD-10 psychiatric diagnosis, including substance abuse or dependence (within the past 2 years).
All patients were being treated with antipsychotic medication at the time of the study. Forty of the patients with schizophrenia were taking second-generation antipsychotics, 1 was taking a first-generation antipsychotic, and 11 were taking both types of antipsychotics. The duration of illness was defined as the number of years between the time of the examination and the first recorded hospitalization. The presence and severity of positive, negative, and general symptoms were evaluated by the PANSS (Kay et al., 1987), and comorbid depressive symptoms were assessed using the Montgomery-Åsberg Rating Scale (MADRS) (Montgomery, 1979).
Both the patient group and the control group included more males than females, and the education levels (P < .0001) and mean ages (P = .0348) of the participants significantly differed between the groups. Details of the patients’ demographic and clinical information, including their CRP levels, were obtained from their medical records and are presented in detail in Tables 1 and 2.
Table 1.
Descriptive Statistics of the Study Sample
| Patient group | Control group | P value | |||
|---|---|---|---|---|---|
| Gender | n | % | n | % | Chi-square |
| Male | 34 | 64.2 | 27 | 57.4 | .4927 |
| Female | 19 | 35.8 | 20 | 42.6 | .4927 |
| Mann-Whitney U-test | |||||
| Mean age | 39.7 | 35 | .0348 | ||
| Mean duration of education, y | 12 | 17.4 | <.0001 | ||
| Marital status, % | Chi-square | ||||
| Single | 71.7 | 34 | .0002 | ||
| Married | 13.2 | 31.9 | .0242 | ||
| Long-term RS | 9.4 | 25.5 | .0324 | ||
| Divorced | 5.7 | 8.5 | .5772 | ||
| Psychiatric history, % | |||||
| Sch. in close family | 15.1 | 2.1 | .0237 | ||
| Sch. among distant rel. | 1.9 | 0 | .3439 | ||
| Other in whole family | 30.2 | 2.1 | .0002 | ||
| Negative | 52.8 | 95.7 | <.0001 | ||
| Mean duration of illness, y | 13.6 | — | — | ||
| Alcohol use, % | |||||
| Never | 43.4 | 19.1 | .0095 | ||
| Rare | 28.3 | 21.3 | .4181 | ||
| Occasional | 17 | 46.8 | .0013 | ||
| Regular | 11.3 | 12.8 | .8243 | ||
| Drug use, % | |||||
| Never tried | 81.1 | 91.5 | .1363 | ||
| Rare | 1.9 | 4.3 | .4883 | ||
| Occasional | 1.9 | 2.1 | .9316 | ||
| Regular | 0 | 0 | — | ||
| Occasional in the past | 15.1 | 2.1 | .0237 | ||
| Smoking, % | |||||
| Never | 39.6 | 66 | .0085 | ||
| Rare | 5.7 | 2.1 | .3682 | ||
| Occasional | 1.9 | 10.6 | .0659 | ||
| Regular | 52.8 | 21.3 | .0012 | ||
| Suicid attempt, % | |||||
| 0 | 64.2 | 100 | <.0001 | ||
| 1–2 | 22.7 | — | .0005 | ||
| 3–5 | 11.3 | — | .0174 | ||
| >5 | 1.9 | — | .3439 | ||
| Somatic diseases (PP) | |||||
| DZ of the musculoskeletal system and connective tissue | 1 | — | .3439 | ||
| DZ of the circulatory system | 10 | 1 | .0076 | ||
| DZ of the respiratory system | 1 | 2 | .4883 | ||
| DZ of the genitourinary system | 2 | 1 | .6301 | ||
| DZ of the digestive system | 3 | 2 | .7476 | ||
| Endocrine, nutritional, and metabolic DZ | 6 | 2 | .1937 | ||
| Certain infectious and parasitic DZ | 1 | — | .3439 | ||
| DZ of the nervous system | 1 | .2859 | .3439 | ||
Abbreviations: DZ, disease; n, sample size; PP, per person; RS, relationship; Sch., schizophrenia; y, in years.
Table 2.
Descriptive Statistics of Patient Sample (PANSS mean + SD, Antipsychotic and Other Treatment (PP))
| PANSS Total | 93.2 + 9.0 | |
| PANSS Positive | 23.2 + 4.7 | |
| PANSS Negative | 22.2 + 5.4 | |
| PANSS General | 47.9 + 4.9 | |
| MADRS | 10.5 + 7.3 | |
| Antipsychotic medication | PP | Chlorpromazine equivalent dose Mean + SD |
| Risperidone | 19 | 466.7 + 137.2 |
| Clozapine | 16 | 312.5 + 157.9 |
| Olanzapine | 14 | 300 + 110.9 |
| Haloperidol | 12 | 200 + 57.74 |
| Aripiprazole | 9 | 225 + 144.2 |
| Quetiapin | 3 | 558.3 + 500.8 |
| Cariprazine | 2 | N/A |
| Other medication | ||
| Benzodiazepines | 37 | |
| Antidepressants | 1 | |
| Antiepileptic drugs | 5 | |
| Lithium | 2 | |
| Other drugs for somatic diseases (Calcium channel blockers, beta blockers, ACE inhibitors, diuretics, proton pump inhibitors, ASA, statins, biguanides, prostaglandin analogs, fatty acid oxidation inhibitors, centrally acting muscle relaxants, anticoagulants) |
25 |
Abbreviations: ACE, angiotensin converting enzyme; ASA: acetylsalicylic acid (aspirin); MADRS, Montgomery Asberg Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; PP, per person; SD: standard deviation.
Sample Collection and Preparation
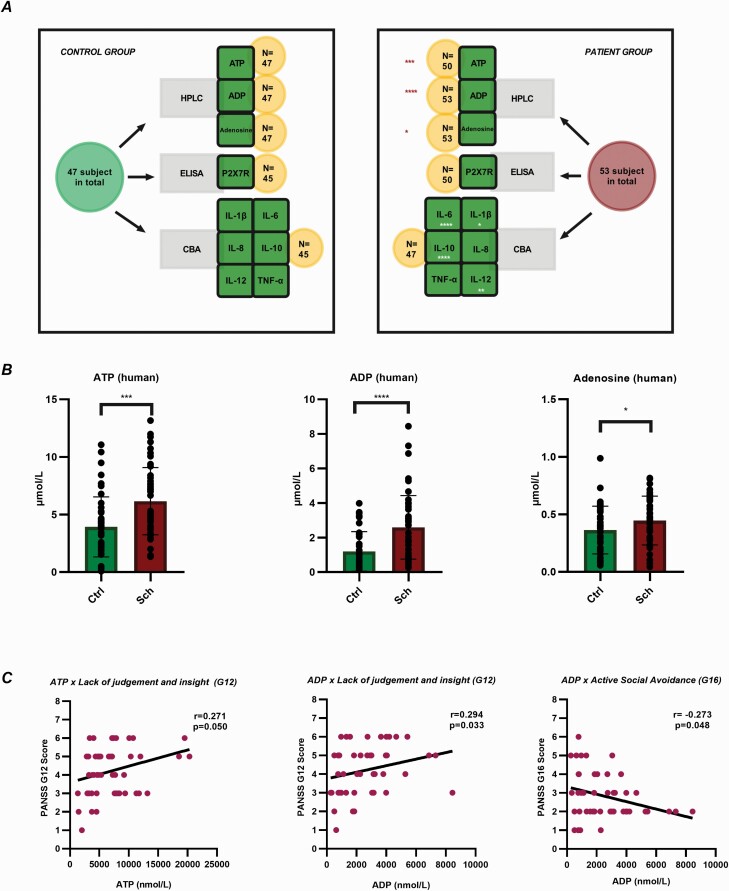
—We obtained blood samples for nucleotide (ATP and ADP), Ado, P2X7R, and cytokine (IL-1β, IL-6, IL-8, IL-10, IL-12, tumour necrosis factor alpha [TNF-α]) measurements, as shown in Figure 1A. To quantify the serum concentrations of soluble P2X7Rs, cytokines, and purines, 3.5 mL of venous blood was collected by a nurse from all participants between 9 and 12 am in a 3.5-mL serum separator tube (VACUETTE TUBE CAT Serum Clot Activator). The tubes were incubated at room temperature for 2 hours to allow the blood to clot. On clotting, the serum was then collected by centrifugation at 20°C for 15 minutes at 1000 × g before being aliquoted (200 µL) into protein low-binding tubes (Eppendorf, Hamburg, Germany) and stored at −80°C until further analysis.
Figure 1.
Study design and purine levels in blood serum and their associations with psychopathology. (A) Design of the human study. CBA, cytometric bead array; ELISA, enzyme-linked immunosorbent assay; HPLC, high-performance liquid chromatography. (B) Significantly increased serum levels of adenine nucleotides and adenosine in humans with schizophrenia. Ctrl, control group; Sch, schizophrenia. The data were analyzed by the Mann-Whitney U-test. Sch: n = 53, Ctrl: n = 47. ATP: P = .0002; ADP: P < .0001; Ado: P = .0417. The mean, SEM, and SD are shown in supplementary Table 1. (C) Scatter plots of correlated purine nucleotides and PANSS scores. Significant correlations were identified between “lack of judgment and insight” and ADP and ATP as well as between “active social avoidance” and ADP. Correlations were calculated by Spearman’s rho. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; N, sample size; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation; SEM, standard error of mean.
Animal Study
Laboratory Animals
—Drug- and test-naïve C57/BL6 male wild-type mice (80–90 days old) were housed in a light- (12 hours on, 12 hours off) and temperature-controlled (23°C+/- 2°C) room room in individual plastic cages (15 × 45 × 20 cm) with ad libitum access to food and water. The experimental procedures were approved by the local Animal Care Committee of the Institute of Experimental Medicine (Budapest, Hungary, ref. no. PE/EA/297–1/2021) and were conducted in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Design
—We herein utilized the acute PCP-induced protocol used and validated in our previous studies (Koványi et al., 2016; Calovi et al., 2020). For the animal study, G*Power 3.1.9.7 software was used to calculate the sample sizes for 4 different treatment groups: PCP+saline (SAL), PCP+risperidone (RIS), SAL + SAL, and SAL + RIS with the following parameters: ANOVA fixed effects, omnibus, 1-way: a priori: compute required sample size; power: 0.9; α error probability: 0.05; effect size: 0.5; and total sample size: 64 (n = 20, 14, 20, and 8 mice/group, respectively). PCP (2 mg/kg) and its vehicle (10 mL/kg) were injected i.p. at an equal volume (100 μL). RIS (Sigma-Aldrich, Budapest, Hungary) was dissolved in normal saline and administered as a single i.p. treatment at a dose of 0.3 mg/kg 30 minutes before administration of the PCP/SAL regimen. The doses of PCP (Koványi et al., 2016; Calovi et al., 2020) and RIS (Chadman, 2011) were chosen based on previous literature data. Forty-five minutes after the PCP/SAL treatment, schizophrenia-like behavioral phenotypes were evaluated using the open-field test. The behavioral tests were carried out between 8:30 am and 1:00 pm by trained observers who were blinded to the test groups.
Open-Field Test
—The open-field test was carried out in a circular open field, where 2 unfamiliar mice receiving the same pharmacological treatment were placed at the opposite sides of the chamber and then allowed to explore freely. We previously published the details of this method (Koványi et al., 2016; Calovi et al., 2020). PCP-induced stereotypical behavior was scored manually according to the protocol described by Nabeshima et al. (1987) and Sams-Dodd (1995), with some modifications. Rapid and sudden changes in the path, rotating around itself forming infinite circles and head backs, as well as head twitching while motionless, were considered stereotypical behaviors.
Preparation of Blood for Immunological Assays
—Five minutes after the behavioral assessments, blood was collected from the caudal vena cava under deep isoflurane (Hungaropharma, Budapest, Hungary) anesthesia. To prevent blood clotting, 10 mL/kg heparin (Teva Pharmaceutical Industries, Budapest, Hungary) was administered i.p. 2 minutes before the mouse was killed. The heparin-treated blood was placed in ice immediately after collection and then gently centrifuged at 4°C for 10 minutes at 2000 rpm. The plasma samples were recentrifuged to remove platelets and remaining cells (4°C, 5 min, 5000 rpm) and then stored at −80°C until the assay.
Analysis of Purines and Inflammatory Markers in Serum and Plasma
High-Performance Liquid Chromatography (HPLC)
—The concentrations of nucleotides (ATP and ADP) and Ado in human serum and mouse plasma samples were determined using a previously described HPLC method (Baranyi et al., 2006). Briefly, a human/mouse serum aliquot (100 µL) stored at −80°C in 4 M perchloric acid solution (10 µL) containing 10 µM theophylline served as the internal standard. HPLC separation was performed on a Shimadzu LC-20 AD Analytical System using UV (Agilent 1100 VW set at 253 nm) detection. A phenyl-hexyl packed (7.5 × 2.1 mm) column was used for online sample enrichment, and separation was completed by coupling to the analytical C-18 (150 × 2.1 mm) column. Concentrations were calculated using a 2-point standard addition calibration and an internal standard method. From the volume of the accurately measured sample mixture and the area of the internal standard peak, the total analytical recovery value was calculated (R = 63.27 ± 2.10%; n = 100). Nucleotide concentrations are expressed as µmol/L.
Enzyme-Linked Immunosorbent Assay and Cytometric Bead Array
—Soluble P2X7R in serum/plasma was measured by enzyme-linked immunosorbent assay (human: Cusabio, Houston, TX, USA; mice: MyBiosource, San Diego, CA, USA) according to the manufacturer’s protocol. Samples were measured in duplicate, and absorbance was read at 450 nm using a Cytation 5 Cell Imaging Multi-Mode Reader (BioTek, Winooski, VT, USA).
Cytokines (IL-1β, IL-6, IL-8/IL-17, IL-10, IL-12, and TNF-α) were assessed in human serum using a Human Inflammatory Cytokine CBA kit (BD Biosciences, San Jose, CA, USA) and in mouse plasma by Cytometric Bead Array Flex Sets (BD Biosciences) according to the manufacturer’s protocol. Multiplex bead array technology was used to simultaneously detect multiple cytokine proteins. Samples were measured in duplicate on a BD FACSVerse flow cytometer, and the results were analyzed with FCAP Array software (Soft Flow, Pécs, Hungary). Cytokine levels are expressed as pg/mL.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism v. 8.0.2 (GraphPad Software, San Diego, CA, USA). The ROUT method was used to identify outliers (Q = 1%), and these data were omitted from further analysis. Most of the immunological data were significantly skewed and analyzed using nonparametric tests. Specifically, pairwise Mann–Whitney U-tests were used to compare 2 groups. Differences among multiple groups were analyzed with 2-way ANOVA followed by Tukey’s post hoc test. Correlations between variables were assessed using Spearman’s rho. Significance was accepted at P < .05, and data are expressed as the mean ± SD. Receiver operator characteristic (ROC) analyses were performed to assess the diagnostic abilities of the measured parameters, and the Youden index was used to determine the optimal cutoff point.
RESULTS
Purine Levels and Their Association With Psychopathology
Examination of the serum adenine nucleotide and Ado levels in the human patient and control groups revealed significantly increased levels of ATP (6.161 ± 2.919 nmol/mL vs 3.931 ± 2.608 nmol/mL; P = .0002), ADP (2.593 ± 1.840 nmol/mL vs 1.204 ± 1.147 nmol/mL; P < .0001), and Ado (0.446 ± 0.212 nmol/mL vs 0.3643.6 ± 0.208 nmol/L; P = .0417) in schizophrenic patients compared with controls (Figure 1B).
In terms of clinical symptomatology, no correlations were found between total scores on the PANSS positive, negative, and general scales and purine levels. For the single items, exploratory analysis of the correlations with symptomatology identified a significant inverse relationship between the item “active social avoidance” and ADP (r = −0.273, P = .048). “Lack of judgment and insight” was shown to exhibit a significant positive relationship with both ATP and ADP (r = 0.271, P = .050; r = 0.294, P = .033) (Figure 1C). No significant correlations were found between the purine levels and total scores on the MADRS.
Inflammatory Markers and Their Association With Psychopathology
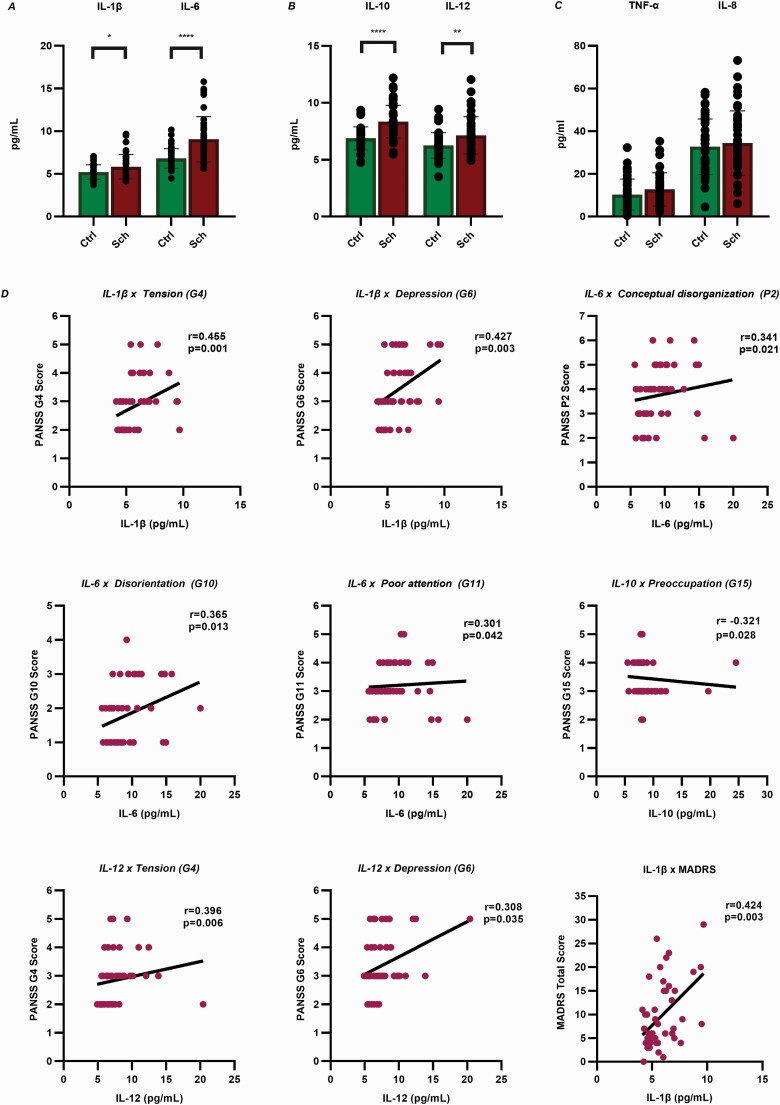
IL-1β, IL-6, IL-10, and IL-12 levels were significantly increased in the sera of schizophrenic patients compared with controls, whereas the levels of IL-8 and TNF-α did not significantly differ between the 2 groups (Figure 2A–C).
Figure 2.
Cytokine levels and their associations with psychopathology. (A-B) Mann-Whitney U-tests revealed significantly elevated levels of IL-1β, IL-6, IL-10, and IL-12 in the sera of schizophrenic patients compared with controls. IL-1β: P = .0418, IL-6: P < .0001, IL-10: P < .0001, IL-12: P = .0085. (C) The levels of IL-8 and TNF-α did not significantly differ between the 2 groups. IL-8: P = .5612, TNF-α: P = .0782. The mean, SEM, and SD are shown in supplementary Table 1. (D) Spearman’s correlation analyses revealed significant positive associations of the PANSS items “tension” and “depression” with IL-1β, IL-12, and TNF-α, whereas “disorientation” and “poor attention” were positively correlated with IL-6 and IL-8. A significant positive correlation was identified between “conceptual disorganization” and IL-6. IL-10 exhibited a significant inverse relationship with the item “preoccupation.” IL-1β was also positively correlated with the total score on the Montgomery-Åsberg Depression Rating Scale. Only the correlations of the significantly increased cytokines are presented here, and the correlations of IL-8 and TNF-α are shown in supplementary Figure 2. Abbreviations: IL, interleukin; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation; SEM, standard error of mean; TNF-α, tumour necrosis factor alpha.
We found no significant correlations between the levels of cytokines and overall positive, negative, and general PANSS scale scores. However, more correlations were found between cytokine levels and some PANSS items. “Tension” and “depression” were correlated with IL-1β (r = 0.455, P = .001; r = 0.427, P = .003), IL-12 (r = 0.396, P = .006; r = 0.308, P = .035), and TNF-α (r = 0.389, P = .008; r = 0.360, P = .015), whereas “disorientation” and “poor attention” were correlated with IL-6 (r = 0.365, P = .013; r = 0.301, P = .042) and IL-8 (r = 0.384, P = .006; r = 0.328, P = .021). A significant inverse relationship was found between “preoccupation” and IL-10 (r = −0.321, P = .028). Furthermore, a significant positive correlation was identified between “conceptual disorganization” and IL-6 (r = 0.341, P = .021). We found a positive correlation between IL-1β and the total MADRS scores (r = 0.424, P = .003) in patients with schizophrenia (Figure 2D).
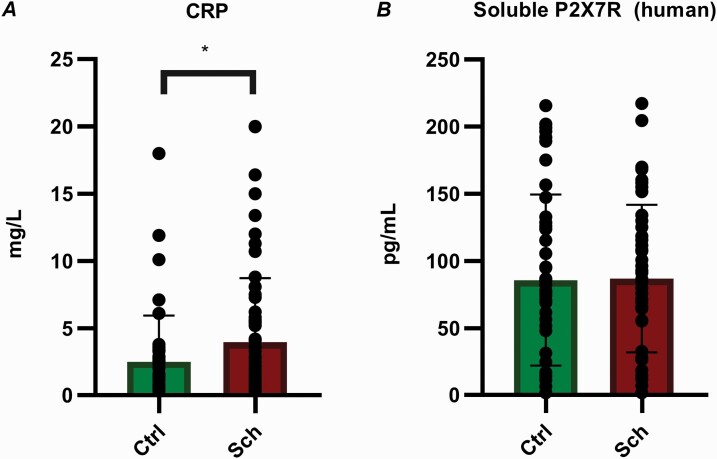
The CRP levels were also more markedly elevated in the sera of patients with schizophrenia than in the sera of the controls (Figure 3A).
Figure 3.
CRP and P2X7R concentrations in human serum. (A) The CRP levels in the patient samples were significantly higher than those in the healthy control group. Sch: n = 50, Ctrl: n = 47. P = .0444 (B) The P2X7R concentrations did not significantly differ between the 2 groups. Sch: n = 50, Ctrl: n = 45. P = .6601. Abbreviations: Ctrl, control group; CRP, C-reactive protein; P2X7R, P2X7 receptor; Sch, schizophrenia. The data were analyzed by the Mann-Whitney U-test.
Soluble P2X7R Concentration in the Serum
The P2X7R levels did not significantly differ between the patients and controls. In healthy control participants, the sP2X7R concentration ranged from 1.795 to 215.6 pg/mL (85.68 ± 63.88), whereas this value ranged from 1.795 to 217.2 pg/mL (86.82 ± 54.89) in the schizophrenic patients (Figure 3B).
Plasma Levels of Purines, Cytokines, and P2X7R in the PCP-Induced Mouse Model of Acute Schizophrenia
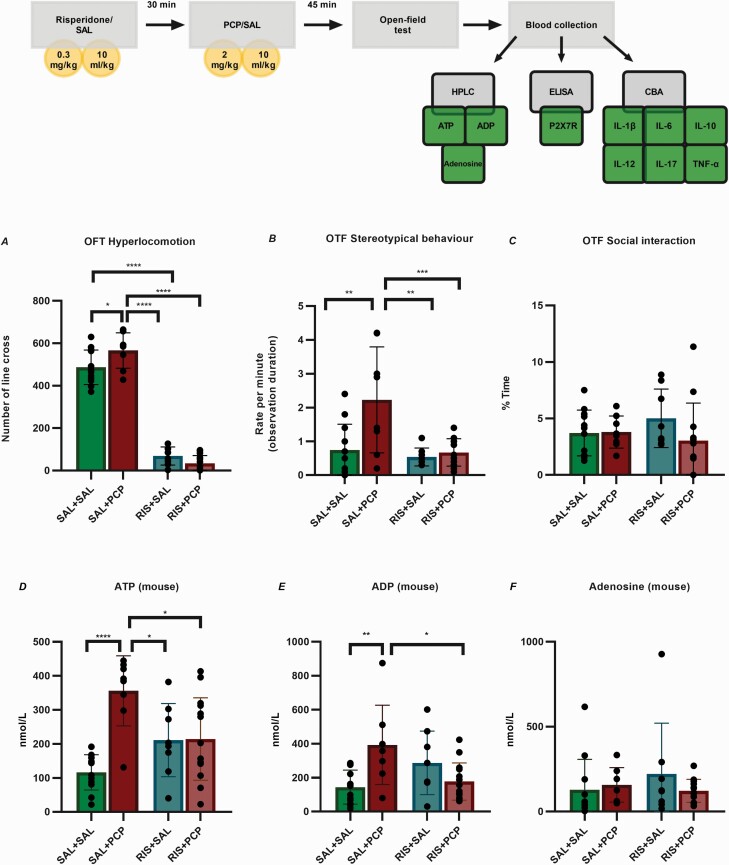
PCP treatment significantly increased the basal locomotor activity measured and the stereotypical behaviors, although it did not affect the social interactions. The RIS regimen by itself significantly decreased both the basal locomotor activity and stereotypical behaviors, whereas it had no effect on the time spent on social interactions. Moreover, RIS treatment completely ameliorated the effect of PCP on behavioral alterations (Figure 4A–C).
Figure 4.
Study design, behavioral validation of the phencyclidine (PCP)-induced acute mouse model of schizophrenia, and purine levels in mouse plasma. (A-B) PCP treatment significantly increased the basal locomotor activity and stereotypical behaviors, whereas risperidone (RIS) pretreatment significantly decreased both phenomena. (C) Neither PCP nor RIS treatment affected social interactions. (D) The level of ATP in the saline (SAL)+PCP group was significantly higher than those in all the other treatment groups. The ATP levels in PCP-treated mice were significantly lower than those in mice receiving RIS. (E) The levels of ADP in the SAL+PCP group were significantly increased compared with those in the SAL+SAL and RIS+PCP groups. (F) The levels of Ado did not significantly differ between the groups. RIS alone did not affect the extracellular purine levels. SAL+SAL N = 12; SAL+PCP, n = 8; RIS+SAL N = 8; RIS+PCP, n = 14. The data were analyzed by 2-way ANOVA followed by Tukey’s post hoc test. P values and statistical details are provided in supplementary Table 2. Abbreviations: Ado, adenosine; ADP, adenosine diphosphate; ANOVA, analysis of variance; ATP, adenosine triphosphate; CBA, cytometric bead array; ELISA, enzyme-linked immunosorbent assay; HPLC, high-performance liquid chromatography; IL, interleukin; OFT, open-field test.
Analysis of the purine levels in plasma samples revealed significant differences between the SAL+SAL and SAL+PCP groups regarding ATP (116.2 ± 51.93 nmol/mL vs 355.9 ± 102.8 nmol/mL; P < .0001) and ADP (144.1 ± 100.4 nmol/mL vs 392.8 ± 233.8 nmol/mL; P = .0055) but not Ado. The ATP and ADP levels were significantly lower in the RIS+SAL and RIS+PCP groups than in the SAL+PCP group. RIS alone did not affect the extracellular purine levels (Figure 4D–F).
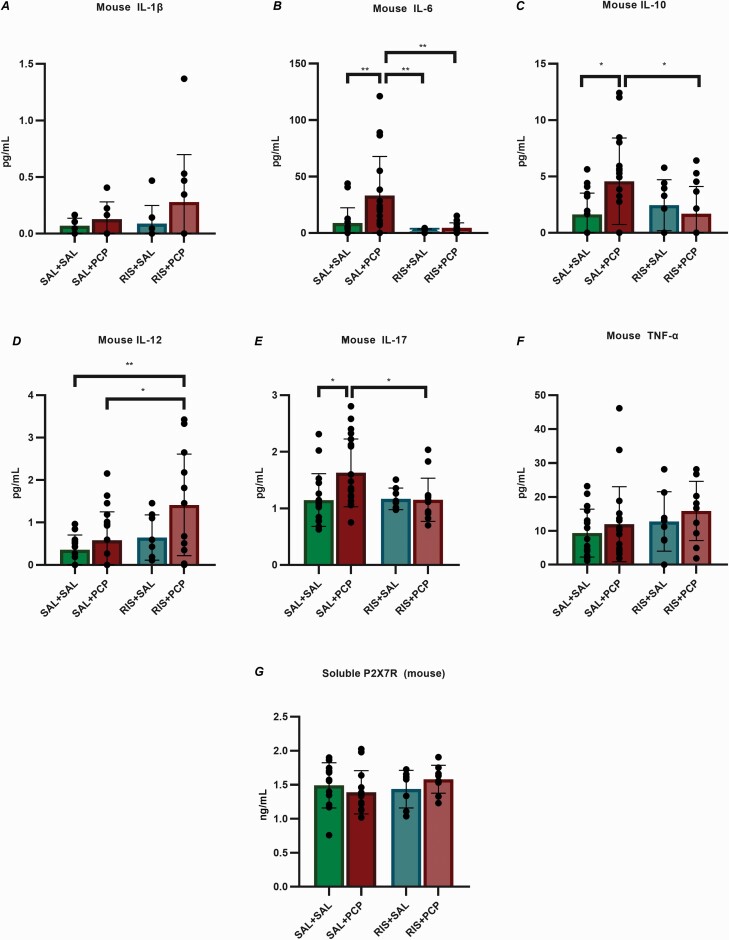
In our rodent model, 6 cytokines were examined: IL-1β, IL-6, IL-10, IL-12, and TNF-α. Since mice do not express the IL-8 gene, IL-17 was measured instead. Significantly increased levels of IL-6, IL-10, and IL-17 were found in the plasma of mice in the SAL+PCP group compared with the SAL+SAL group mice, whereas RIS pretreatment (RIS+PCP) normalized the levels of all 3 elevated cytokines. No significant differences in the IL-1β and TNF-α plasma levels were observed between the SAL+PCP and SAL+SAL groups (Figure 5A–F).
Figure 5.
Cytokine and P2X7R levels in mouse plasma. (A,F) The levels of IL-1β and TNF-α did not significantly differ among the groups. (B,C,E) The plasma levels of IL-6, IL-10, and IL-17 were significantly increased in saline (SAL) + phencyclidine (PCP)-treated mice compared with SAL+SAL control mice. Risperidone (RIS) pretreatment (RIS+PCP) normalized the levels of all 3 of these cytokines. (D) The levels of IL-12 were significantly increased in the RIS+PCP group compared with the SAL+PCP and SAL+SAL groups. (G) The P2X7R concentrations did not significantly differ among the 4 groups. SAL+SAL: n=12; SAL+PCP: n=8; RIS+SAL: n=8; RIS+PCP: n=14. The data were analyzed by 2-way ANOVA followed by Tukey’s post hoc test. P values and statistical details are provided in supplementary Table 3. Abbreviations: ANOVA, analysis of variance; IL, interleukin; P2X7R, P2X7 receptor; TNF-α, tumour necrosis factor alpha.
Similar to the human study, the plasma levels of soluble P2X7R did not significantly differ between the 2 groups, and the overall detection range was 0.76–2.02 ng/mL (Figure 5G).
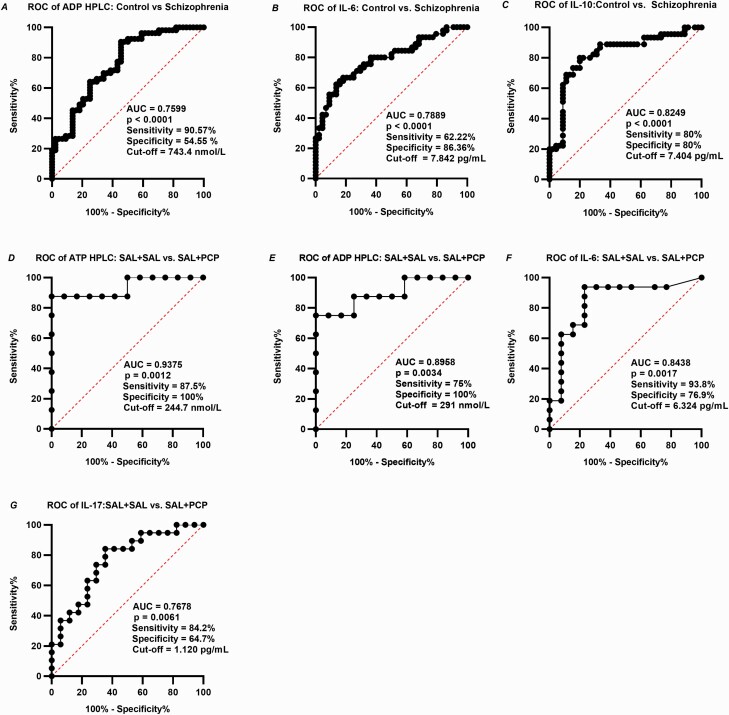
ROC Analyses
ROC analyses were performed to assess the capabilities of the measured blood parameters to serve as diagnostic, prognostic, and stratification markers of patients with schizophrenia. The blood purine and cytokine levels were successfully differentiated between the controls and schizophrenic patients (Figure 6A–C) and between control and PCP-treated mice (Figure 6D–G), with adequate sensitivity and specificity.
Figure 6.
Receiver operator characteristic (ROC) curves of human ADP, IL-6, IL-10 (A-C) and mouse ATP, ADP, IL-6 ,and IL-17 (D-G). ROC analysis presents that blood purine and cytokine concentrations have Y% sensitivity and X% specificity to diagnose schizophrenia at a certain cutoff point. The cutoff point was determined based on the Youden Index. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; AUC, area under the curve; IL, interleukin.
Discussion
The principal novel objective of the present study was to measure the blood purine levels in human schizophrenic patients and in a PCP-induced mouse model of schizophrenia for the first time, to our knowledge, to identify novel purinergic/inflammatory biomarkers. The basal levels of ATP, ADP, and Ado were in the micromolar range in humans and in the high nanomolar range in mice, which is consistent with previous reports (Fisher et al., 2019; Martin et al., 2019; Beamer et al., 2021). The purinergic compounds were purely sera-derived residual platelets, and cellular elements (in case of the plasma samples) were completely removed during the centrifugation procedure. The origin of purines in the serum could be any circulating cell types of the blood stream, including platelets, as well as endothelial cells, because ATP and its metabolites are present in all living cells (Burnstock, 2017). For instance, it is known that inflammatory stimuli, such as bacterial lipopolysaccharides, release purines to the extracellular space from macrophages (Sperlagh et al., 1998). Therefore, it is reasonable to assume that purines are released from circulating monocytes, macrophages, but other sources cannot be excluded either. The levels of ATP and ADP were significantly higher in acutely hospitalized schizophrenic patients and in mice following acute PCP treatment than in the corresponding controls. In addition, the levels of Ado, the breakdown product of ATP, were also found to be elevated in human patient samples but not in mice. This disparity might be explained by the differential kinetics of Ado between the 2 species (Joolharzadeh and St Hilaire, 2019). One of the most notable novel findings of our study is the potential relationship between blood purine levels and disease activity. We revealed that both ATP and ADP were significantly positively correlated with the PANSS item “lack of judgment and insight.” Lack of judgment and insight is an important feature of schizophrenia because it is known to be associated with functioning over time (Lincoln et al., 2007). A significant inverse relationship was found between ADP and the item “active social avoidance.”
We observed markedly increased IL-6, IL-10, and IL-12 levels in both schizophrenic patients and PCP-treated mice. The activation of both pro- and anti-inflammatory networks is in accordance with previous results (Drexhage et al., 2011). Additionally, the serum level of IL-1β was increased in only the schizophrenic patient group, whereas the level of IL-17 was increased in only mice. However, both the human and mouse levels of TNF-α or IL-8 did not differ from those in the corresponding controls. These results are not only in line with previous studies on elevated inflammatory cytokine levels in schizophrenia (Miller et al., 2011; Goldsmith et al., 2016; Dahan et al., 2018) but also further support the roles of IL-1β, IL-6, IL-10, and IL-12. Similar to our mouse model, the IL-6 level in both mice and drug-naïve patients with first-episode psychosis was reduced after RIS treatment (Song et al., 2014; da Silva Araújo et al., 2017). Furthermore, in agreement with previous studies (Orsolini et al., 2018; North et al., 2021), the level of CRP, an acute-phase marker of inflammation, was higher in patients with schizophrenia than in healthy controls.
There are conflicting results in the literature concerning the association of cytokine levels and disease activity (Miller et al., 2011; Dimitrov et al., 2013). Therefore, we examined not only the levels of these proinflammatory markers but also their relationships with psychopathology. Despite no significant correlations between overall positive, negative, and general PANSS scores and IL levels, coherent patterns emerged for the individual items. The PANSS items “tension” and “depression” were correlated with IL-1β, IL-12, and TNF-α, whereas “disorientation” and “poor attention” were correlated with IL-6 and IL-8. In addition, a significant positive correlation was identified between “conceptual disorganization” and IL-6. Moreover, the first 2 items are part of the MADRS, in which we found a positive correlation of the overall score with IL-1β. Previous studies on healthy volunteers and nonhuman primates have also reported inflammatory markers that are associated with anhedonia-like behaviors and reward alterations (Capuron et al., 2012; Harrison, 2017; Mehta et al., 2020). On examining individuals at a clinically high risk for psychosis, Goldsmith et al. found that the baseline TNF level predicted the development of negative symptoms independent of the baseline depression or depression trajectory over time (Goldsmith et al., 2019). Notably, IL-10 exhibited a significant inverse relationship with the item “preoccupation,” which is in line with the previous finding of a negative correlation between IL-10 and the intensity of schizophrenia symptoms (Dahan et al., 2018).
The interrelationships between inflammatory markers, P2X7Rs, and schizophrenia-related behaviors have been intensively studied in the last few years (Koványi et al., 2016; Calovi et al., 2020). To our knowledge, the present study is the first to measure soluble P2X7R concentrations in human and mouse samples in the context of a psychiatric disorder. Despite that we found no differences in the concentrations of soluble P2X7R between the groups, previous studies found significantly higher levels in subjects suffering from serious somatic diseases than in the healthy controls (Martínez-García et al., 2019; Conte et al., 2021; Meng et al., 2021). Nevertheless, evidence exists that P2X7R stimulation promotes behavioral sensitization and that P2X7R inhibition prevents or alleviates psychotic-like motor activation in experimental studies, which might be related to the engagement of central P2X7Rs (Koványi et al., 2016; Krügel, 2016; Calovi et al., 2020).
As previously discussed, the circulating levels of immunological markers and related signaling molecules are consistently found to be altered in schizophrenia. This observation appears in not only mouse models (Zhu et al., 2014; Giridharan et al., 2020), unmedicated first-episode psychosis patients (Steiner et al., 2020), and acutely relapsed patients (Upthegrove and Khandaker, 2020) but also in persons at a clinically high risk for subsequently developing psychosis (Khoury and Nasrallah, 2018; Goldsmith et al., 2019). Furthermore, elevated levels of IL-6 in childhood, measured years before the onset of psychosis, are associated with psychotic symptoms in early adulthood (Khandaker et al., 2014). Therefore, these biomarkers could feasibly be used as diagnostic, prognostic, and theranostic biomarkers in the future. Although clinical interviews remain the gold standard for clinical diagnoses, clinicians routinely use a combination of approaches, including brain scans and various psychological tests; thus, it is not clear what additional information a blood test could provide (Weickert et al., 2013). Hence, the greatest promise in biomarker development might lie not in diagnosis but in the predictions of prognosis and/or treatment responses (Pinto et al., 2017). The ability to identify and predict who will become ill before or during the prodromal stages would be extremely beneficial for patients. Early diagnosis of schizophrenia would shorten the duration of untreated psychosis, which is associated with better disease outcomes, and prevent the use of unwarranted treatments and stigmas due to an incorrect diagnosis (Weickert et al., 2013; Chan et al., 2015).
The earlier onset and higher prevalence of somatic comorbidities in schizophrenia—compared with those in various control groups—is well documented in the literature (Mitchell and Malone, 2006; Bitter et al., 2017); among these, cardiac and cerebrovascular diseases (Newcomer and Hennekens, 2007; Laursen et al., 2012), hypertension (Leonard et al., 2012), and metabolic syndrome (Holt et al., 2005) are the most common. We observed the same in this study, because the incidence rates of these somatic diseases were significantly higher among patients than among the controls. Furthermore, we observed significantly increased levels of CRP and IL-6 when patients had cardiovascular disease and higher levels of IL-8 when they had endocrine-metabolic disease (supplementary Figure 1). Several studies have highlighted the potential contributions of common underlying inflammatory processes to metabolic/cardiovascular abnormalities as well as to psychiatric diseases (Leonard et al., 2012; Lasić et al., 2014). However, as clearly shown in our mouse model, the inflammation in question was present in the absence of any somatic diseases, and it indicates the same pattern as seen in our human sample.
Because biomarker research is mainly based on the investigation of peripheral tissues, particularly when focusing on molecular markers, it is important to consider the impacts of peripheral purines and inflammatory markers on events taking place in the CNS. A possible route via which these markers can cross the blood-brain barrier (BBB) is transfer by extracellular vesicles. The most well-characterized form of extracellular vesicles are exosomes, which are able to cross the BBB while their contents, such as proteins, lipids, and nucleic acids, remain active (Saeedi et al., 2019; Jin et al., 2021). In a meta-analysis, Wang and Miller (2018) reported that cytokine alterations in the CSF were concordant with those in the peripheral blood, particularly in patients with schizophrenia (Wang and Miller, 2018).
In summary, the neuroinflammatory process is largely emergent in CNS diseases. Recent studies have outlined that the genetic deletion or pharmacological inhibition of P2X7R signaling reduces schizophrenia-like behavioral alterations (Hempel et al., 2013; Koványi et al., 2016). Thus, in recent years, various classes of small-molecule and drug-like P2X7R inhibitors have been developed that are capable of crossing the BBB and display high degrees of target engagement in the CNS (Chrovian et al., 2016; Thawkar and Kaur, 2019).
Our study has several limitations. Our sample size is relatively small, mainly due to the availability of patients who were willing to undergo an additional venipuncture right after their hospitalization. Consequently, the current sample includes only a small fraction of admitted patients. Furthermore, various drugs were administered for the treatment of comorbid somatic diseases. It is well-known that some of these drugs may have an effect on the level of inflammatory markers (Ohtsuka et al., 2001; Komoda et al., 2010; Vasigar and Batmanabane, 2013; Kortekaas et al., 2014); however, as we applied a reverse translational model, which shows very similar results in both sides of the study, we might suppose the effect of these drugs on inflammation is not so significant in this case. The present study measured the above-mentioned parameters at only 1 instant in time; longitudinal, follow-up studies would be necessary to examine purine and cytokine alterations across the different phases of the disease. Our data are based only on biological samples from outside of the brain; however, to further strengthen and validate these findings, investigation of post-mortem brains of people with the diagnosis of schizophrenia would be substantial. Future studies should further elucidate the role of P2X7R in the pathomechanism of schizophrenia, because P2X7R antagonists are potential drug targets in a variety of CNS diseases.
In conclusion, although the soluble P2X7R levels did not differ between the patient and control samples, the elevated serum levels of purines indicate widespread activation of various purine receptors that are expressed on circulating immune cells and involved in the regulation of the inflammatory response. Our study also offers evidence that the detection of blood purines and inflammatory markers has substantial potential as a future prognostic tool capable of diagnosing schizophrenia at an early stage and guiding treatment choices. Thus, these biomarkers could improve patient quality of life and decrease both the enormous burden of this illness and health care costs worldwide.
Supplementary Material
Acknowledgments
The authors are grateful for the colleagues at the 3 participating psychiatric wards (Department of Psychiatry and Psychotherapy, Semmelweis University; Department of Psychiatry and Psychiatric Rehabilitation, Jahn Ferenc Hospital; Saint John Hospital); for the colleagues of the Department of Otorhinolaryngology and Laboratory Medicine, Semmelweis University for helping with the blood collection; and for Springer Nature Author Services for the language editing.
This work was supported by the Hungarian Research and Development Fund (grant no. 131629), the Hungarian Brain Research Program (2017-1.2.1-NKP-2017-00002 awarded to B.S. [laboratory studies] and to I.B. [clinical study]), and the European Union’s Horizon 2020 Research and Innovation Program Marie Skłodowska Curie grant (no. 766124).
Interest Statement: None.
Contributor Information
Zsüliet Kristóf, Laboratory of Molecular Pharmacology, Institute of Experimental Medicine, Budapest, Hungary; Doctoral School of Mental Health Sciences, Semmelweis University, Budapest, Hungary.
Mária Baranyi, Laboratory of Molecular Pharmacology, Institute of Experimental Medicine, Budapest, Hungary.
Pál Tod, Laboratory of Molecular Pharmacology, Institute of Experimental Medicine, Budapest, Hungary.
Paula Mut-Arbona, Laboratory of Molecular Pharmacology, Institute of Experimental Medicine, Budapest, Hungary; János Szentágothai Neuroscience Doctoral School, Semmelweis University, Budapest, Hungary.
Kornél Demeter, Behavior Unit, Institute of Experimental Medicine, Budapest, Hungary.
István Bitter, Doctoral School of Mental Health Sciences, Semmelweis University, Budapest, Hungary; Department of Psychiatry and Psychotherapy, Semmelweis University, Budapest, Hungary.
Beáta Sperlágh, Laboratory of Molecular Pharmacology, Institute of Experimental Medicine, Budapest, Hungary; János Szentágothai Neuroscience Doctoral School, Semmelweis University, Budapest, Hungary.
References
- Andrejew R, Oliveira-Giacomelli Á, Ribeiro DE, Glaser T, Arnaud-Sampaio VF, Lameu C, Ulrich H (2020) The P2X7 receptor: central hub of brain diseases. Front Mol Neurosci 13:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejew R, Paim M, Moritz CEJ, Carreño F, Rates SMK, Elisabetsky E, Souza DO, de Almeida RF, Battastini AMO (2021) Post-weaning social isolation impairs purinergic signaling in rat brain. Neurochem Int 148:105111. [DOI] [PubMed] [Google Scholar]
- Baranyi M, Milusheva E, Vizi ES, Sperlágh B (2006) Chromatographic analysis of dopamine metabolism in a Parkinsonian model. J Chromatogr A 1120:13–20. [DOI] [PubMed] [Google Scholar]
- Beamer E, Lacey A, Alves M, Conte G, Tian F, de Diego-Garcia L, Khalil M, Rosenow F, Delanty N, Dale N, El-Naggar H, Henshall DC, Engel T (2021) Elevated blood purine levels as a biomarker of seizures and epilepsy. Epilepsia 62:817–828. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A (2018) Recent advances in CNS P2X7 physiology and pharmacology: focus on neuropsychiatric disorders. Front Pharmacol 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Jones DNC (2018) Emerging role of the P2X7-NLRP3-IL1β pathway in mood disorders. Psychoneuroendocrinology 98:95–100. [DOI] [PubMed] [Google Scholar]
- Bitter I, Czobor P, Borsi A, Fehér L, Nagy BZ, Bacskai M, Rakonczai P, Hegyi R, Németh T, Varga P, Gimesi-Országh J, Fadgyas-Freyler P, Sermon J, Takács P (2017) Mortality and the relationship of somatic comorbidities to mortality in schizophrenia. A nationwide matched-cohort study. Eur Psychiatry 45:97–103. [DOI] [PubMed] [Google Scholar]
- Bitter I, Lieberman JA, Gaudoux F, Sokoloff P, Groc M, Chavda R, Delsol C, Barthe L, Brunner V, Fabre C, Fagard M, Montagne A, Tonner F (2019) Randomized, double-blind, placebo-controlled study of F17464, a preferential D 3 antagonist, in the treatment of acute exacerbation of schizophrenia. Neuropsychopharmacology 44:1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks MP, He Y, Schubart CD, Gastel WV, Elkrief L, Huguet G, Eijk KV, Vinkers CH, Kahn RS, Paus T, Conrod P, Hol EM, de Witte LD (2020) Cannabinoids and psychotic symptoms: a potential role for a genetic variant in the P2X purinoceptor 7 (P2RX7) gene. Brain Behav Immun 88:573–581. [DOI] [PubMed] [Google Scholar]
- Burnstock G (2017) Purinergic signaling in the cardiovascular system. Circ Res 120:207–228. [DOI] [PubMed] [Google Scholar]
- Byrne M, Agerbo E, Eaton WW, Mortensen PB (2004) Parental socio-economic status and risk of first admission with schizophrenia- a Danish national register-based study. Soc Psychiatry Psychiatr Epidemiol 39:87–96. [DOI] [PubMed] [Google Scholar]
- Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC (2019) An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med 49:2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calovi S, Mut-Arbona P, Tod P, Iring A, Nicke A, Mato S, Vizi ES, ønnesen J T, Sperlagh B (2020) P2X7 receptor-dependent layer-specific changes in neuron-microglia reactivity in the prefrontal cortex of a phencyclidine induced mouse model of schizophrenia. Front Mol Neurosci 13:566251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH (2012) Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Bullmore ET, Harrison P (2014) Is there a flame in the brain in psychosis? Biol Psychiatry 75:258–259. [DOI] [PubMed] [Google Scholar]
- Chadman KK (2011) Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav 97:586–594. [DOI] [PubMed] [Google Scholar]
- Chan MK et al. (2015) Development of a blood-based molecular biomarker test for identification of schizophrenia before disease onset. Transl Psychiatry 5:e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheffer A, Castillo ARG, Corrêa-Velloso J, Gonçalves MCB, Naaldijk Y, Nascimento IC, Burnstock G, Ulrich H (2018) Purinergic system in psychiatric diseases. Mol Psychiatry 23:94–106. [DOI] [PubMed] [Google Scholar]
- Chrovian CC, Soyode-Johnson A, Ao H, Bacani GM, Carruthers NI, Lord B, Nguyen L, Rech JC, Wang Q, Bhattacharya A, Letavic MA (2016) Novel phenyl-substituted 5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine P2X7 antagonists with robust target engagement in rat brain. ACS Chem Neurosci 7:490–497. [DOI] [PubMed] [Google Scholar]
- Conte G, Menéndez-Méndez A, Bauer S, El-Naggar H, Alves M, Nicke A, Delanty N, Rosenow F, Henshall DC, Engel T (2021) Circulating P2X7 receptor signaling components as diagnostic biomarkers for temporal lobe epilepsy. Cells 10:2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Araújo T, Maia Chaves Filho AJ, Monte AS, Isabelle de Góis Queiroz A, Cordeiro RC, de Jesus Souza Machado M, de Freitas Lima R, Freitas de Lucena D, Maes M, Macêdo D (2017) Reversal of schizophrenia-like symptoms and immune alterations in mice by immunomodulatory drugs. J Psychiatr Res 84:49–58. [DOI] [PubMed] [Google Scholar]
- Dahan S, Bragazzi NL, Yogev A, Bar-Gad M, Barak V, Amital H, Amital D (2018) The relationship between serum cytokine levels and degree of psychosis in patients with schizophrenia. Psychiatry Res 268:467–472. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Schmalzing G, Markwardt F (2018) The elusive P2X7 macropore. Trends Cell Biol 28:392–404. [DOI] [PubMed] [Google Scholar]
- Dimitrov DH, Lee S, Yantis J, Valdez C, Paredes RM, Braida N, Velligan D, Walss-Bass C (2013) Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: potential role for IL-17 pathway. Schizophr Res 151:29–35. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJ, Drexhage HA (2011) An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol 14:746–755. [DOI] [PubMed] [Google Scholar]
- Falzoni S, Donvito G, Di Virgilio F (2013) Detecting adenosine triphosphate in the pericellular space. Interface Focus 3:20120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher O, Benson RA, Tian F, Dale NE, Imray CH (2019) Purine nucleoside use as surrogate markers of cerebral ischaemia during local and general anaesthetic carotid endarterectomy. SAGE Open Med 7:2050312119865120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T (1972) An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol 224:611–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giridharan VV, Scaini G, Colpo GD, Doifode T, Pinjari OF, Teixeira AL, Petronilho F, Macêdo D, Quevedo J, Barichello T (2020) Clozapine prevents poly (I:C) induced inflammation by modulating NLRP3 pathway in microglial cells. Cells 9:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Miller AH, Addington J, Bearden C, Cadenhead K, Cannon T, Cornblatt B, Mathalon D, McGlashan T, Seidman L, Tsuang M, Wood s SW, Walker EF, Perkins DO (2019) Association of baseline inflammatory markers and the development of negative symptoms in individuals at clinical high risk for psychosis. Brain Behav Immun 76:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T, Jakobsen KD, Fenger M, Nielsen J, Krane K, Fink-Jensen A, Lublin H, Ullum H, Timm S, Wang AG, Jørgensen NR, Werge T (2008) Variation in the purinergic P2RX(7) receptor gene and schizophrenia. Schizophr Res 104:146–152. [DOI] [PubMed] [Google Scholar]
- Harrison NA (2017) Brain structures implicated in inflammation-associated depression. Curr Top Behav Neurosci 31:221–248. [DOI] [PubMed] [Google Scholar]
- He Y, Taylor N, Fourgeaud L, Bhattacharya A (2017) The role of microglial P2X7: modulation of cell death and cytokine release. J Neuroinflammation 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel C, Nörenberg W, Sobottka H, Urban N, Nicke A, Fischer W, Schaefer M (2013) The phenothiazine-class antipsychotic drugs prochlorperazine and trifluoperazine are potent allosteric modulators of the human P2X7 receptor. Neuropharmacology 75:365–379. [DOI] [PubMed] [Google Scholar]
- Holt RI, Bushe C, Citrome L (2005) Diabetes and schizophrenia 2005: are we any closer to understanding the link? J Psychopharmacol 19:56–65. [DOI] [PubMed] [Google Scholar]
- Huang L, Otrokocsi L, Sperlágh B (2019) Role of P2 receptors in normal brain development and in neurodevelopmental psychiatric disorders. Brain Res Bull 151:55–64. [DOI] [PubMed] [Google Scholar]
- Javidfar B, Park R, Kassim BS, Bicks LK, Akbarian S (2017) The epigenomics of schizophrenia, in the mouse. Am J Med Genet B Neuropsychiatr Genet 174:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Smith J, Nicke A, Engel T (2019) Regulation of P2X7 receptor expression and function in the brain. Brain Res Bull 151:153–163. [DOI] [PubMed] [Google Scholar]
- Jin T, Gu J, Li Z, Xu Z, Gui Y (2021) Recent advances on extracellular vesicles in central nervous system diseases. Clin Interv Aging 16:257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo EK, Kim JK, Shin DM, Sasakawa C (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joolharzadeh P, St Hilaire C (2019) CD73 (Cluster of Differentiation 73) and the differences between mice and humans. Arterioscler Thromb Vasc Biol 39:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB (2014) Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury R, Nasrallah HA (2018) Inflammatory biomarkers in individuals at clinical high risk for psychosis (CHR-P): state or trait? Schizophr Res 199:31–38. [DOI] [PubMed] [Google Scholar]
- Komoda H, Inoue T, Node K (2010) Anti-inflammatory properties of azelnidipine, a dihydropyridine-based calcium channel blocker. Clin Exp Hypertens 32:121–128. [DOI] [PubMed] [Google Scholar]
- Kortekaas KE, Meijer CA, Hinnen JW, Dalman RL, Xu B, Hamming JF, Lindeman JH (2014) ACE inhibitors potently reduce vascular inflammation, results of an open proof-of-concept study in the abdominal aortic aneurysm. PLoS One 9:e111952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koványi B, Csölle C, Calovi S, Hanuska A, Kató E, Köles L, Bhattacharya A, Haller J, Sperlágh B (2016) The role of P2X7 receptors in a rodent PCP-induced schizophrenia model. Sci Rep 6:36680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel U (2016) Purinergic receptors in psychiatric disorders. Neuropharmacology 104:212–225. [DOI] [PubMed] [Google Scholar]
- Lasić D, Bevanda M, ošnjak N B, Uglešić B, Glavina T, Franić T (2014) Metabolic syndrome and inflammation markers in patients with schizophrenia and recurrent depressive disorder. Psychiatr Danub 26:214–219. [PubMed] [Google Scholar]
- Laursen TM, Munk-Olsen T, Vestergaard M (2012) Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry 25:83–88. [DOI] [PubMed] [Google Scholar]
- Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J (2018) Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry 23:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, Schwarz M, Myint AM (2012) The metabolic syndrome in schizophrenia: is inflammation a contributing cause? J Psychopharmacol 26:33–41. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR (2005) What does the PANSS mean? Schizophr Res 79:231–238. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Lüllmann E, Rief W (2007) Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr Bull 33:1324–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Cannon TD (2019) Schizophrenia. N Engl J Med 381:1753–1761. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Dale N, Imray CHE, Roffe C, Smith CJ, Tian F, Price CI (2019) The association between early neurological deterioration and whole blood purine concentration during acute stroke. Biomark Res 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García JJ et al. (2019) P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat Commun 10:2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V, Felger JC (2020) Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc Cogn Affect Neurosci 15:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Chen P, Guo X, Li X, Wu Y, Liu W, Jiang F, Liu H, Wang L (2021) Correlations between serum P2X7, vitamin A, 25-hydroxy vitamin D, and mycoplasma pneumoniae pneumonia. J Clin Lab Anal 35:e23760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Malone D (2006) Physical health and schizophrenia. Curr Opin Psychiatry 19:432–437. [DOI] [PubMed] [Google Scholar]
- Montgomery SM (1979) Depressive symptoms in acute schizophrenia. Prog Neuropsychopharmacol 3:429–433. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Ishikawa K, Yamaguchi K, Furukawa H, Kameyama T (1987) Phencyclidine-induced head-weaving observed in mice after ritanserin treatment. Eur J Pharmacol 139:171–178. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Hennekens CH (2007) Severe mental illness and risk of cardiovascular disease. JAMA 298:1794–1796. [DOI] [PubMed] [Google Scholar]
- North HF, Bruggemann J, Cropley V, Swaminathan V, Sundram S, Lenroot R, Pereira AM, Zalesky A, Bousman C, Pantelis C, Weickert TW, Shannon Weickert C (2021) Increased peripheral inflammation in schizophrenia is associated with worse cognitive performance and related cortical thickness reductions. Eur Arch Psychiatry Clin Neurosci 271:595–607. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Hamada M, Hiasa G, Sasaki O, Suzuki M, Hara Y, Shigematsu Y, Hiwada K (2001) Effect of beta-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol 37:412–417. [DOI] [PubMed] [Google Scholar]
- Orsolini L, Sarchione F, Vellante F, Fornaro M, Matarazzo I, Martinotti G, Valchera A, Di Nicola M, Carano A, Di Giannantonio M, Perna G, Olivieri L, De Berardis D (2018) Protein-C reactive as biomarker predictor of schizophrenia phases of illness? A systematic review. Curr Neuropharmacol 16:583–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JV, Moulin TC, Amaral OB (2017) On the transdiagnostic nature of peripheral biomarkers in major psychiatric disorders: a systematic review. Neurosci Biobehav Rev 83:97–108. [DOI] [PubMed] [Google Scholar]
- Reale M, Costantini E, Greig NH (2021) Cytokine imbalance in schizophrenia. from research to clinic: potential implications for treatment. Front Psychiatry 12:536257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi S, Israel S, Nagy C, Turecki G (2019) The emerging role of exosomes in mental disorders. Transl Psychiatry 9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams-Dodd F (1995) Automation of the social interaction test by a video-tracking system: behavioural effects of repeated phencyclidine treatment. J Neurosci Methods 59:157–167. [DOI] [PubMed] [Google Scholar]
- Song X, Fan X, Li X, Zhang W, Gao J, Zhao J, Harrington A, Ziedonis D, Lv L (2014) Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naïve, first-episode schizophrenia. Psychopharmacology 231:319–325. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Hasko G, Nemeth Z, Vizi ES (1998) ATP released by lipopolysaccharide increases nitric oxide production in RAW 264.7 macrophages via P2Z/P2X7 receptors. Neurochem Int 33:209–215. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Illes P (2014) P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci 35:537–547. [DOI] [PubMed] [Google Scholar]
- Steiner J, Frodl T, Schiltz K, Dobrowolny H, Jacobs R, Fernandes BS, Guest PC, Meyer-Lotz G, Borucki K, Bahn S, Bogerts B, Falkai P, Bernstein HG (2020) Innate immune cells and C-reactive protein in acute first-episode psychosis and schizophrenia: relationship to psychopathology and treatment. Schizophr Bull 46:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilo SA, Murray RM (2019) Non-genetic factors in schizophrenia. Curr Psychiatry Rep 21:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thawkar BS, Kaur G (2019) Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J Neuroimmunol 326:62–74. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Khandaker GM (2020) Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Curr Top Behav Neurosci 44:49–66. [DOI] [PubMed] [Google Scholar]
- van de Leemput J, Hess JL, Glatt SJ, Tsuang MT (2016) Genetics of schizophrenia: historical insights and prevailing evidence. Adv Genet 96:99–141. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP (2010) The environment and schizophrenia. Nature 468:203–212. [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP (2012) Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull 38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasigar P, Batmanabane M (2013) Anti-inflammatoryactivity of calciumchannel blocker lercanidipine hydrochloride. J Pharmacol Pharmacother 4:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AK, Miller BJ (2018) Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull 44:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Weickert TW, Pillai A, Buckley PF (2013) Biomarkers in schizophrenia: a brief conceptual consideration. Dis Markers 35:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wang H, Shi R, Zhang R, Wang J, Kong L, Sun Y, He J, Kong J, Wang JF, Li XM (2014) Chronic phencyclidine induces inflammatory responses and activates GSK3β in mice. Neurochem Res 39:2385–2393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.