Abstract
Acutely, pain serves to protect us from potentially harmful stimuli, however damage to the somatosensory system can cause maladaptive changes in neurons leading to chronic pain. Although acute pain is fairly well controlled, chronic pain remains difficult to treat. Chronic pain is primarily a neuropathic condition, but studies examining the mechanisms underlying chronic pain are now looking beyond afferent nerve lesions and exploring new receptor targets, immune cells, and the role of the autonomic nervous system in contributing chronic pain conditions. The studies outlined in this review reveal how chronic pain is not only confined to alterations in the nervous system and presents findings on new treatment targets and for this debilitating disease.
Keywords: chronic pain, DRG neurons, glia, immune cells, peripheral mechanisms
Introduction
Pain is defined by The International Association for the Study of Pain (IASP) [1] as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.” Most studies consider pain in an acute/chronic dichotomy. Chronic pain is defined as pain that persists or reoccurs for more than three months and causes immense suffering [2]. Chronic pain affects an estimated 20% of people worldwide and account for 15%–20% of physician visits [3]. Recently, the corona virus disease 2019 (COVID-19) pandemic has led to an increase in pain reports, as one common symptom of COVID-19 is pain. It is likely that due to both virus and stress triggers the prevalence of chronic pain has increased [4]. When felt acutely, pain evokes an innate nociceptive response that aids the body in avoiding further harm. While this response may be evolutionarily useful, numerous conditions and diseases can lead to the generation of pathological chronic pain. Pathological pain conditions are generally categorized into (1) inflammatory pain following tissue injury, (2) cancer pain, (3) neuropathic pain following nerve, spinal cord or brain injury, and (4) drug-induced pain. Pain can elicit a characteristic behavioral response in humans [5, 6] as well as laboratory animals. Pain assays generally measure two categories of behaviors: spontaneous pain and evoked pain. Spontaneous pain can be measured using the grimace scale, burrowing assays, gait analysis, weight bearing and automated behavioral analysis without stimuli [7]. Evoked pain assays measure the subjects’ responses to external stimuli and quantify two pain states, hyperalgesia and allodynia. Hyperalgesia refers to increased response to noxious mechanical and thermal stimuli, where allodynia refers to nociceptive responses to innocuous stimuli. Numerous animal models have been developed to measure pathological pain conditions. In Table 1, we summarize the common pain conditions and their corresponding animal models.
Table 1:
Common pain conditions in human and corresponding animal models.
| Chronic pain category | Human disease | Animal models |
|---|---|---|
| Neuropathic pain | Neuroma | Axotomy (complete sciatic nerve transection) |
| Causalgia; neuropathies; chronic widespread pain (CWP); complex regional pain syndrome (CRPS); temporomandibular disorder (TMD); peripheral nerve injured by trauma | Chronic constriction injury (CCI); partial sciatic nerve ligation (PSL); spinal nerve ligation (SNL); cuffing-induced sciatic nerve injury (PNI); spared nerve injury (SNI) | |
| Trigeminal neuralgia | Compression of trigeminal ganglion; CCI to infra-orbital nerve | |
| Low back pain | Nucleus pulposus (NP) applied to the lumbar dorsal root ganglia (DRG) and/or adjacent nerve roots (NP) model; chronic compression of the DRG model (CCD); L5 DRG is inflamed by zymosan in incomplete Freund’s adjuvant | |
| Postoperative pain | Incision model | |
| Diabetes-induced neuropathy | Streptozotocin (STZ) -induced neuropathy model | |
| Post-herpetic neuralgia | Varicella zoster virus-induced neuropathy | |
| Inflammatory pain | Peripheral nerve inflammation | Cutaneous and subcutaneous models: complete Freund’s adjuvant (CFA); formalin model; carrageenan model |
| Inflammatory bowel disease (IBD) | Dextran sodium sulfate (DSS); 2,4,6-trinitrobenzene sulfonic acid (TNBS) | |
| Arthritis | Joint inflammation models: CFA; carrageenan; Collagen-induced arthritis (CIA); Collagen-antibody-induced arthritis | |
| Fibromyalgia symptoms (FMS) | Intraperitoneal injection with IgG fibromyalgia patients | |
| Cancer pain | Tumor mestastases to the skeleton | Bone cancer pain models: femur, calcaneus, tibial with inoculation of cancerous cells into respective bones |
| Melanoma skin cancer | Injection of melanoma cells in plantar region of hind paw | |
| Drug-induced pain | Cancer patients after drug treatment | Anti-cancer agents (vincristine, cisplatin, oxaliplatin, paclitaxel) models |
| Anti-HIV drugs-induced neuropathy | Anti-HIV agents (2,3-dideoxycytidine, didanosine) |
Significant conceptual and technological advances in the field of pain and somatosensory physiology in the past several decades have led us to understand pain as a unique sensory modality within the somatosensory system (along with the other senses, such as of proprioception, itch, touch and temperature) [8]. Somatosensory information including pain is detected by primary sensory neurons whose cell bodies are located in the dorsal root ganglion (DRG). The peripheral branches of DRG neurons extend into the skin and internal organs where they detect external stimuli via various mechanical, chemical and thermal receptors. This information is transmitted to the spinal cord and eventually the brain.
It is generally understood that neuronal plasticity in the peripheral nervous system (PNS) and central nervous system (CNS) is crucial in the transition from acute to chronic pain. Yet the initiation of chronic pain is also influenced by other non-neuronal cell types, including epithelial, immune and glial cells.
In this review, we summarize our current understanding of the non-neuronal and neural mechanisms of chronic pain. We will focus on peripheral tissues, where chronic pain originates, and ask the question from a neuron-centric standpoint: what types of neurons sense pain? What receptors do these neurons utilize to detect what types of stimuli? Which molecules in the periphery activate or modulate pain sensory neurons and how do these signals affect action potential initiation and transmission in pain sensory neurons?
Pain sensory neurons
Sensory information first triggers receptors found on peripheral afferents which then transmit these signals through nerves to the spinal cord and onto the brain. The cell bodies of these peripheral nerves reside in the DRG and trigeminal ganglia (TG). DRG sensory neurons display a wide range of characteristics and properties that help to sort and transmit various types of signals. DRG neurons can be classified by cell body size, degree of myelination and axon caliber, electrophysiological properties including conduction velocities and gene expression profiles. There are four widely accepted categories: (1) myelinated Aα-type, large diameter cell bodies, fast conduction velocities, thickly proprioceptive neurons; (2) myelinated Aβ-type, large diameter cell bodies, fast conduction velocities, low-threshold mechanoreceptors (LTMRs) that mediate touch; (3) lightly myelinated Aδ-type, medium sized, intermediate conduction velocities, nociceptive neurons and (4) unmyelinated C-type, small diameter, slowest conduction velocities, thermoreceptors, low-threshold mechanoreceptors, pruriceptors and nociceptors. Electrophysiological recordings revealed that most pain neurons belong to C-type, Aδ-type neurons and a small population are Aβ-type neurons [9].
The gene-expression profiles of DRG neurons are extremely useful in assigning physiological functions of neuron types: receptor expression is a good predictor of ligand specificity, while ion channels and transmitter synthesis/transport machineries indicate the mode of conductance and synaptic transmission in the spinal cord. Recently, different groups have performed transcriptome profiling of DRG neurons at the single-cell and population level using next generation RNA sequencing (RNA-seq) technologies [10], [11], [12], [13], [14], [15], [16]. Despite minor difference on the specificity and expression of certain genes, the RNA-seq analyses are generally in agreement, and the assignment of neuronal subtypes is consistent with traditional criteria based on cell body size and electrophysiological properties. Based on principal component analysis of single-cell transcriptomes, DRG neurons in mouse were generally categorized by 11 subgroups: neurofilament containing (NF) 1–3 subtypes represent myelinated Aβ-type LTMRs which transmit tactile information; NF4 and 5 are proprioceptors which sense the position and movement of the body; tyrosine hydroxylase containing (TH) are unmyelinated C-type LTMRs that mediate mechanical pain and pleasant touch; peptidergic nociceptors (PEP1) are unmyelinated peptidergic thermosensitive nociceptors that co-express genes encoding neuropeptides, such as calcitonin gene-related peptide (CGRP) and substance P; PEP2 are lightly myelinated peptidergic Aδ nociceptors that express CGRP and the neurofilament heavy chain protein (NEFH); non-peptidergic nociceptors (NP)1 correspond to Mas1-related G protein-coupled receptor (Mrgpr)D+ neurons, non-peptidergic unmyelinated polymodal nociceptors that respond to noxious mechanical, thermal stimuli and pruritic stimuli.
The neuronal types relevant to pain have been carefully reviewed recently [17]. Consistent with classical electrophysiological characterizations, small diameter unmyelinated Aδ and C fibers (including PEP1, 2 and NP) are primary sensory neurons of pain. It is worth noting that these categories are based on transcriptomic profiling of healthy animals. In chronic pain conditions, the gene expression profiles of nearly all neuronal types undergo change [18, 19].
In the skin, where peripheral branches of nociceptive neurons are best characterized, the axons of terminal branches extend into both the dermis and epidermis, receiving a wide variety of stimuli via cell surface receptors (see Figure 1). We will briefly review the causes of chronic pain in the context of the affected fields innervated by DRG neurons and go over the major categories of known chronic pain mediators, their cellular sources, their receptors on the neuronal membrane and the signal transduction pathways that affect the firing of an action potential by which sensory information is transmitted to the CNS.
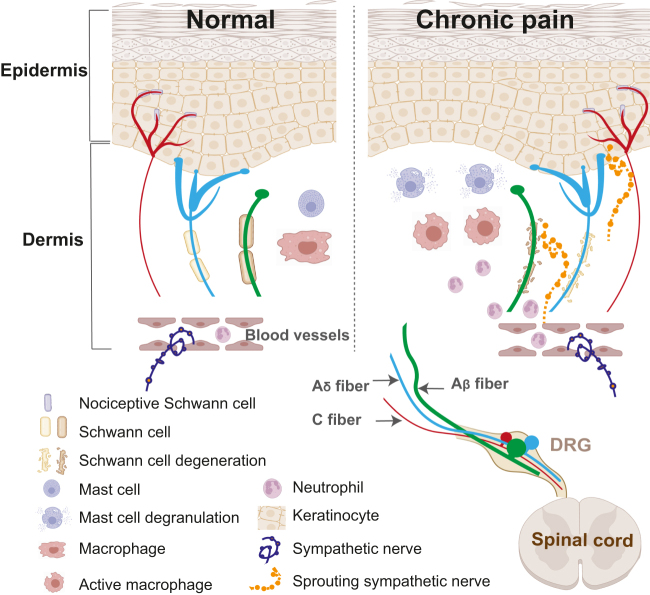
Figure 1:
Major cell types involved in pain in the peripheral tissue. Pain sensory information is primarily received by small diameter Aδ and C type sensory neurons. The peripheral axons of sensory neurons form elaborate terminal branches that extend into both the dermis and epidermis, receiving a wide variety of stimuli via cell surface receptors. Under physiological conditions, the microenvironment is in dynamic balance. In chronic pain condition, keratinocytes, mast cells and macrophages are activated and release mediators that stimulate or sensitize nociceptive neurons. Circulating immune cells such as neutrophils, are recruited from the blood stream. Schwann cells that normally ensheath the axon bundles degenerate. In addition, sympathetic nerves, which normally do not innerve the dermis, sprout into the upper dermis and epidermis and directly interact with the sensory system. DRG, dorsal root ganglia.
Peripheral terminals of DRG neurons innervate many types of tissues, each of which contain a multitude of receptors for various mediators. These organs and tissues have certain histological structures, including blood and lymphatic vessels, where a variety of immune cells reside. Decades of study has found that immune cell infiltration and release of inflammatory mediators at sites of neuronal trauma contribute to chronic pain. Since the focus of this review is on neuronal mechanisms of chronic pain, we will focus our discussion on immune cells and inflammatory mediators with well-established neuronal receptors that directly affect nociceptor activation. And we will discuss the peripheral nerve terminal activation (see Figure 1) and cell body activation in DRG (see Figure 2). We will briefly review the causes of chronic pain in the context of the affected fields innervated by DRG neurons and go over the major categories of known chronic pain mediators, their cellular sources, their receptors on the neuronal membrane and the signal transduction pathways that affect the afferent firing.
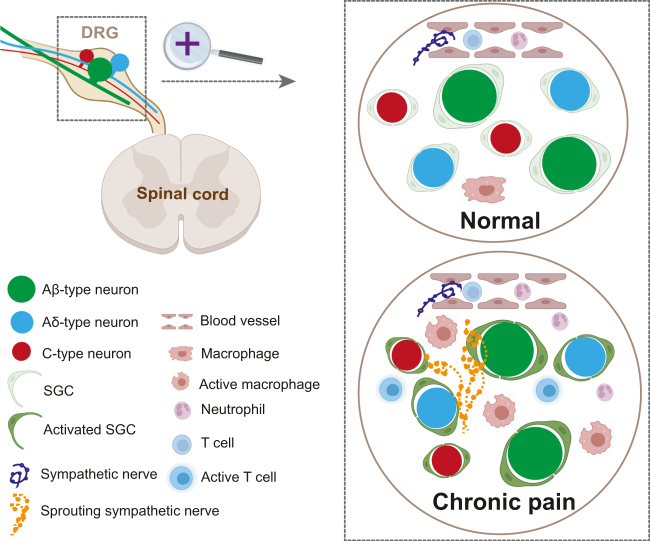
Figure 2:
Major cell types involved in pain in the DRG. Under physiological condition, DRG neurons are isolated from each other by SGCs and the neurons, glial cells and a small number of tissue resident immune cells form a microenvironment in dynamic balance. In chronic pain, macrophages are activated and release a milieu of inflammatory factors, and some blood-borne immune cells including neutrophils and T cells are recruited into the DRG. Sympathetic nerves, which usually terminate at the blood vessels, sprout into the DRG and form synaptic connection with DRG neurons, resulting in ectopic sensory activation. DRG, dorsal root ganglion; SGC, satellite glia cell.
Below, we review the major non-neuronal cell types and the mediators they release that activate primary nociceptors during pain.
Peripheral nerve terminal activation
Role of epithelial cells
Here, we collectively refer to keratinocytes in the skin and epithelial cell types in the colon and other internal organs as epithelial cells [20]. Keratinocytes are the primary cells of skin. The peripheral terminals of DRG neurons extend to Keratinocytes. When epithelial cells are injured, they secrete various neuroactive mediators that contribute to pain. These include adenosine 5′-triphosphate (ATP), nerve growth factor (NGF), interleukin (IL)-6, IL-1b, tumor necrosis factor α (TNF-α), and endothelin-1. We discuss some of these important mediators and their neuronal receptors in detail.
Administration of ATP to the skin evokes pain behavior both in human [21, 22] and animals [23]. Innocuous and noxious touch induce release of ATP from keratinocytes, which directly acts on ionotropic purinergic receptor (P2X4) ion channel receptors on the sensory neurons [24]. Other diseases such as post-herpetic neuralgia and complex regional pain syndrome are accompanied by an increase in epidermal ATP release which can lead to excessive activation of P2X receptors on sensory neurons and may elicit pain [25]. ATP can also act on purinergic receptor (P2Y) G-protein-coupled receptor (GPCR)-coupled receptors on sensory neurons [26]. The underlying excitation mechanisms of the P2Y receptor could be induced thru sensitization of the transient receptor potential cation channel subfamily V1 (TRPV1) [27] and modulation of other ion channels such as potassium channels (Kv7) [28] and mechanotransduction channels [29].
NGF was discovered originally as a neuron survival factor during development. NGF binds to its high-affinity receptor, tropomyosin receptor kinase (Trk) A expressed on nociceptor neurons [30]. NGF/TrkA signaling can sensitize TRPV1 [31] and alter the expression of genes including those encoding Nav1.8 Na+ channels (Scn10a) [32, 33], acid-sensing ion channel 3(Accn3) [34], TRPV1receptors (Trpv1) [35], brain derived neurotrophic factor (BDNF)(Bdnf) [36] and substance P(Tac1) [37].
IL-6 is a family of neuropoietic cytokines. Intraplantar injection of IL-6 induces mechanical hyperalgesia in a dose dependent manner [38]. The effect of IL-6 on pain neurons may be direct, as IL-6 can elicit rapid calcium transients in around 33% of cultured DRG neurons [39]. IL-6 has can also lead to neuronal activation via the soluble IL-6 receptor together with gp130 to promote phosphorylation of TRPV1 through activation of protein kinase C (PKC)-δ via Grb2-associated binder-like protein (Gab)1/2/phosphatidylinositol 3-kinase (PI3K) [40]. IL-6/soluble IL-6 receptor/gp130 signaling can potentiate the heat-evoked CGRP release [41].
IL-1b and tumor necrosis factor α (TNF-α) are pro-inflammatory cytokines. Intraplantar injection of IL-1b or TNF-α can elicit both mechanical and thermal hyperalgesia [38, 42]. TNF-α binds to its receptor-TNFR1 and potentiate tetrodotoxin (TTX)-resistant Na+ channels through the activation of p38 mitogen-activated protein kinase (MAPK) pathway [43, 44]. Similarly, IL-1b binds to IL-1R receptor to potentiate TTX-resistant Na+ channels via the activation of the p38 MAPK pathway as well [45]. In addition, IL-1b can also induce pain neurons releasing SP via the cyclooxygenase-2 system [46].
Endothelin-1 is a 21-amino-acid peptide known to have vasoconstrictive properties [47]. Subcutaneous plantar injections with endothelin-1 were shown to produce pain behavior through activation of endothelin (ET)-A receptors [48, 49]. Taken together, these findings illustrate the diversity of epithelial–neuronal pain pathways.
Role of immune cells
There are various types of immune cells that contribute to chronic pain including mast cell, neutrophils, activated macrophages, and T lymphocytes. Here, we provide an overview of immune cells and how they modulate peripheral sensory nerves.
As a first responder of the immune system, mast cells are distributed widely in the body and found anatomically to coexist with sensory nerves. Mast cells are characterized by their numerous granules that contain a larger number of bioactive substances. Upon activation, mast cells undergo degranulation releasing the content of their cytosolic granules. These granules contain include histamine, proteases, serotonin, cytokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-1,3,4,5,6 and TNF-α, and chemokines such as CC chemokine ligand (CCL)1, CCL2, CCL3, CCL4 [50, 51]. Those mediators can target their specific receptors on sensory nerve terminals leading to peripheral afferent sensitization and inflammatory pain [52], [53], [54]. Mast cell degranulation also regulates nociceptive sensation via histaminergic and non-histaminergic pathways. Our lab found that activation of the mast cell specific receptor Mas-related G-protein-coupled receptor (MrgprB2), as well as its human homolog MRGPRX2, preferentially leads to tryptase release over histamine, leading to itch that was distinct from classical IgE-FcεRI histaminergic itch [55]. Moreover, our group demonstrated that MrgprB2 receptor can be activated directly by the neuropeptide substance P [56]. This result may explain why blockade of the canonical substance P receptor, neurokinin (NK)1, was found to be insufficient to prevent substance P induced pain. Furthermore, our lab identified that MrgprB2 as a receptor specific to connective tissue mast cells, not on DRG neurons, and proceeded to demonstrate that mice lacking the MrgprB2 receptor had reductions in inflammatory hypersensitivity after injury [57, 58]. This effect was induced by release of multiple pro-inflammatory cytokines and chemokines which facilitate immune cells infiltration and concomitant peripheral afferent sensitization.
Neutrophils are the earliest recruited innate immune cell, infiltrating tissues from the blood in response to tissue injury. Although inflammatory mediators released by neutrophils such as interleukins, prostaglandin E2 (PGE2) and TNF-α, can sensitize the nociceptive neurons [59], knowledge regarding the crosstalk between neutrophils and nociceptive never terminals is still limited. Interestingly, studies have shown that inhibition of neutrophil accumulation in the periphery can decrease pain after inflammation [60], [61], [62].
Activated macrophages can modulate pain dependent on their class, one of which is the pro-inflammatory M1-like phenotype, while the other anti-inflammatory M2 phenotypes [63]. Here, we focus on their pro-inflammatory function. Like other types of immune cells, activated macrophages can release many inflammatory mediators such as IL1, NGF, and TNF-α sensitizing the nerve. Angiotensin II type 2 receptor (AT2R) antagonists have been shown to reduce pain behavior in rodents with different pain models [64], [65], [66]. A recent study reported that activation of AT2R in macrophages found at the site of injury, but not in DRG neurons, triggers production of reactive oxygen/nitrogen species which can activate nociceptors via activation of the cell damage/pain-sensing ion channel TRPA1 [67]. In addition, the complement system component C5a can induce thermal and mechanical hyperalgesia depending on activated macrophages in the skin, involving the release of NGF [68, 69]. NGF binding to its receptor on the terminals of nociceptors sensitizes TRPV1 receptors on nociceptors, eliciting hyperalgesia. Phagocytosis is a key function of macrophage in the resolution of inflammation. Activation of putative peptidergic G protein-coupled receptor (GPR) [37] expressed on macrophage by neuroprotectin D1 (NPD1) was found to be involved in phagocytosis and phenotypic switching from proinflammatory to anti-inflammatory signaling [70].
In summary, increasing evidence suggests that immune cell play a major role in many chronic pain conditions. Peripherally targeting immune cells and their mediators may offer novel therapeutic potential.
The role of terminal Schwann cells
Neurons in both the peripheral and central nervous systems are sheathed by glial cells. Glia provides structural and nutritional support for neurons and are critical insulators for the conduction of electrical signal along the sometimes meters long axons. In the peripheral nervous system axons of DRG neurons are ensheathed by Schwann cells [71].
Schwann cells are classified by two types: the myelinating Schwann cells wrapping A fibers including Aβ and Aδ in a 1:1 ratio to produce a myelin sheath, and the non-myelinating Schwann cell encompassing C fibers, in what is called a Remak bundle [72, 73]. Under physiological conditions, both types of Schwann cells can support, trophically nourish and insulate axons. After nerve injury, Schwann cells can detect and respond to the injury rapidly. Schwann cells undergo phenotypic change, proliferating and interacting with axons to produce a large number of glia mediators such as ATP, growth factors (NGF, BDNF, and neurotrophin-3 and 4), erythropoietin (Epo), cytokines (TNF- α, IL-1 and IL-6) and chemokines (CCL1 and COX2) [74]. Some glia mediators such as TNFα, IL-1α, and IL-1β released by Schwann cells can recruit macrophages, which can indirectly affect the condition of axon excitability [75]. Along with signaling to neurons, some mediators released by Schwann cells also act on Schwann cells themselves in an autocrine manner. For example, NGF can target both on Schwann cells and axons. This trophic factor can act on its’ high-affinity tropomyosin-related kinase A receptor (TrkA) on the axons of DRG neurons, as well as its’ low-affinity nerve growth factor receptor (LNGFR) on Schwann cells [76, 77].
In response to nerve injury, Schwann cells receive signals that reduce their cell death and prevent axon degeneration, thereby ameliorating chronic pain states. These include the activation of Epo receptors (EpoR) by Epo [78], low density lipoprotein (LDL) receptor-related protein (LRP1) by LDL [79], [80], [81], NMDA receptors (NMDA-R) [82] and γ-aminobutyric acid type B (GABA-B) receptors [83], [84], [85] by glutamate.
Conversely, the activation of P2X7receptors by ATP [86, 87], Toll-like receptor 2 (TLR2) by damage-associated molecular patterns (DAMPs) [88], Lysophosphatidic acid 1(LPA1) receptors by LPA [89], [90], [91], and TRPA1 receptors [92] signaling in Schwann cells can exacerbate axonal degeneration and worsen chronic pain.
ErbB signaling can be pro- or anti-pain depending ligand activation. Deficiency of Neuregulin/ErbB–mediated ErbB phosphorylation signaling in myelinating Schwann cells leads to hypomyelination and hypersensitivity to mechanical stimuli [93], [94], [95]. Leprosy induces neuropathic pain in both patients and animal models [96, 97]. M. leprae was shown to directly bind to and activate ErbB2 and Erk1/2 and induce demyelination [98]. This demyelination may lead to sensory alterations and chronic pain.
Schwann cell degeneration can also affect neighboring axons. Transection of the fourth and fifth lumbar ventral root (L4/5 ventral rhizotomy) model, in which Wallerian degeneration is restricted only to myelinated efferent fibers, sparing unmyelinated axons, triggers mitosis of Remak Schwann cells (but not myelinating Schwann cells) surrounding uninjured C-fibers [99]. Thus, the abnormal activities of Schwann cells can have broad impacts beyond the axons they immediately ensheath.
It has long been thought that nociceptive neurons exist as free nerve endings in the skin free of glial ensheathment and are directly activated by noxious stimuli. However, a specialized cutaneous glial cell type termed “nociceptive Schwann cell” was recently characterized, forming a mesh-like organ with nociceptive fibers in the epidermal-dermal border [100, 101]. Selective activation of these cells caused activation of nociceptive neurons and initiated pain. Ablation of nociceptive Schwann cells or nociceptive nerves can lead to the other’s retraction, resulting in neuropathic pain-like hypersensitivity including mechanical, cold, and heat hyperalgesia [102]. These terminal glial cells, similar to their axon-wrapping counterparts, play key roles in the initiation and transmission of pain signaling.
Overall, the nociceptive sensory neurons are ensheathed by glial cells in their terminal branches, on their axons, and around their cell bodies in the DRG. These glial cells regulate the extracellular environment under both healthy or chronic pain conditions. Activation of these cells is both necessary and sufficient for enhanced nociceptive sensory via the release of myriad mediators.
The role of sympathetic nerve sprouting
The sympathetic nervous system consists of two populations of neurons, preganglionic and postganglionic sympathetic neurons. Preganglionic neurons reside in the spinal cord and extend their axons to connect with postganglionic neurons that lie in paravertebral ganglia chains along or in prevertebral ganglia. Postganglionic neurons project efferents to innervate peripheral tissue such as blood vessels, exocrine glands and organs [103, 104]. Normally, in healthy conditions, sympathetic postganglionic nerves have no communication with DRG sensory neurons in the periphery. The sympathetic nervous system (SNS) is involved in many protective reflexes related to pain, but not in the generation of pain [105]. However, under some chronic pain conditions, the sympathetic nervous system is thought to generate, maintain and exacerbate pain [106], [107], [108], [109], [110]. Local sympathetic blockade or lesion is commonly used to treat certain pain conditions including complex regional pain syndrome, phantom limb pain, postherpetic neuralgia, ischemic pain, cancer pain, skeletal pain and arthritis pain [111, 112]. Consistently intracutaneous injection with norepinephrine, one of the neurotransmitters released from sympathetic postganglionic nerve, can evoke or worsen pain both pain patients and animals [113], [114], [115], [116]. This suggests that peripheral sympathetic efferents may release neurotransmitters, such as norepinephrine, to activate receptors on nearby cells. Indeed, under some pathological conditions, sympathetic postganglionic nerves can sprout into peripheral tissue and DRG. We will discuss sympathetic sprouting in the peripheral tissue in this section, and discuss ectopic sympathetic innervation of the DRG in the next part.
Normally, sympathetic nerves innervate the lower dermis and hypodermis and do not intersect with the nociceptive sensory neurons that occupy the upper dermis and the epidermis. However, sympathetic nerve sprouting was found in the upper dermis of the lower lip, an area normally absent of sympathetic innervation, after mental nerve transection or chronic constriction injury (CCI) of the mental nerve [117, 118]. Sympathetic nerves were also found to sprout into the upper dermis (in some cases, these sympathetic fibers were observed to penetrate the epidermis) of the plantar skin after CCI of the sciatic nerve [119], subcutaneous injection with complete Freund’s adjuvant (CFA) into the hindpaw [120] and Cuff and spared nerve injury (SNI) [121]. Similarly, sprouting of sympathetic nerve fibers with accompanying increased norepinephrine was detected in experimental sciatic nerve neuromas after sciatic nerve section and avulsion of the distal stumps [122]. The Schwann cells which enclosed myelinated sensory fibers and sympathetic nerve sprouts within the same basal lamina was also identified in the acute neuroma [123]. In addition, the neuroma-like structures which consist of exuberant sprouting sympathetic and CGRP+, NF200+ sensory nerves form in the periosteum in development of the bone cancer pain [124]. Similar neuroma-like structures formed by robust sprouting of sympathetic and sensory nerves was observed in the synovium and periosteum in painful arthritic knee joints of geriatric mice [125], as well as near the fracture site in mouse models of bone pain [126]. Those finding indicate that the abnormal sprout sympathetic nerve can cross-talk with pain sensory neuron terminals at the site of periphery and influence the pain signal.
To date, few studies which have directly assessed the mechanisms or what specifically triggers sympathetic sprouting in peripheral tissues. Using next gen RNA-Seq analysis, one study showed significant increases in expression and enrichment of the nerve growth factor receptor, TrkA (Ntrk1), in sympathetic ganglia compared too sensory ganglia [127]. NGF and its high-affinity TrkA receptor may play an important role in guiding the sprouting of sympathetic nerves. It has been demonstrated that collateral sprouting of sympathetic axons is dependent on the availability of NGF [128]. The potential sources of NGF may include nonneuronal cells such as macrophages and Schwann cells as increased NGF expression have been observed in these cell types after nerve injury [129].
Clinically, blocking sympathetic activation using stellate ganglion block, lumbar sympathetic block, celiac plexus block, superior hypogastric block, and ganglion Impar block can alleviate chronic pain in about 50% of complex regional pain syndrome (CRPS) patients. These patients are thus identified as suffering from sympathetically maintained pain (SMP) [130, 131]. The exact pathological mechanism of SMP remains unclear. Intradermal injection with physiologically relevant doses of norepinephrine in patients with SMP evokes more severe pain in the SMP-affected region of patients than in the contralateral unaffected region, and in control subjects [115]. Similarly, in animal models, norepinephrine injected cutaneous can revive pain during temporary pain relief via sympathetic blockade [132]. These observations indicate cutaneous adrenergic receptors/adrenergic hypersensitivity might be involved in the mechanisms of SMP [133]. The adrenergic receptor is a class of G protein-coupled receptors that consist of α-adrenoceptors including α1- and α2- adrenoceptors, and β-adrenoceptors including β1-, β2- and β3- adrenoceptors. Enhanced expression of α1-adrenoceptors (α1-ARs) on isolectin B4 (IB4)+ and NF200+ afferent fibers was found in injured sciatic nerve, dermal nerve bundles, and the papillary dermis in rats after peripheral nerve injury [134]. Elevated α1-AR expression was observed on nerve fibers in the skin of patients suffering from complex regional pain syndrome [134]. In the rat CCI model, α1-AR expression was increased in the epidermis and on all types of dermal nerve fibers including CGRP+, IB4+, and NF200+ fibers in plantar, but not dorsal paw skin [135] and α2-adrenergic receptors were found to be involved in the interaction between sprouted sympathetic nerves and sensory terminals. The location of α2- receptors is under debate. Some studies indicate that these receptors are located on the terminals of postganglionic sympathetic neuron (PGSN) [136, 137], whereas others have shown them to be absent on sympathetic nerves [138]. The expression and location of β- adrenoceptors on peripheral nerves after nerve injury have not been comprehensively reported either.
With regards to chronic pain only a few studies have examined parasympathetic nervous system sprouting. Parasympathetic fiber sprouting into the upper dermis following sensory denervation of the rat lower lip skin was observed after transections of the mental nerve [139] and chronic constriction injury (CCI) of the mental nerve [140]. In the chronic cyclpphosphanmide-induced cystitis rat model, both CGRP+ sensory nerves and parasympathetic nerve sprouting was observed in the mucosa of the rat urinary bladder [141].
In summary, though the autonomic and sensory systems generally do not overlap under healthy conditions, sympathetic nerves have been found to ectopically sprout under various chronic pain conditions and can directly activate/potentiate/sensitize nociceptive neural terminals. These findings suggest that peripheral parasympathetic sprouting could be an important pathway underlying chronic pain.
DRG activation
Immune cells and DRG
In addition to peripheral nerve terminals, modulation of sensory neuron cell bodies can occur in the ganglia following peripheral nerve injury and inflammation. The proliferation and recruitment of a wide range of immune cells such as macrophages [142, 143], neutrophils [144], T cells [145, 146] can influence DRG microenvironment and sensitize the excitability of DRG neurons. A comprehensive single-cell transcriptional profiling of DRG cells after peripheral injury (sciatic nerve crush) and central injury (dorsal root crush and spinal cord injury) showed that all injuries increased the proportion of a cells share features of immune cells while also increasing in macrophage markers [147]. In addition, a recent study demonstrated a critical role for DRG macrophages, but not those at the nerve injury site, in both the initiation and maintenance of neuropathic pain induced mechanical hypersensitivity in both male and female mice [148]. Interestingly the signaling pathways underlying the interaction between immune cells and afferents appears to be the same to what is found in immune cell and DRG cell body interactions. Thus, targeting these pathways may offer up broad treatments for chronic pain pathologies.
Satellite glial cells and DRG
Satellite glial cells (SGCs) closely envelop cell bodies in DRGs and TGs. In the tightly packed ganglion, neurons are usually surrounded by SGCs and are completely isolated from each other. Small clusters of two or three neurons, however, are sometimes enclosed within a common SGCs’ envelope, separated only by a thin layer of extracellular space or a thin SGC sheet [149, 150]. This cluster organization is high in neonates but decreases progressively with age [151]. Notably, the interval of extracellular space between the SGC sheath and the wrapped neuronal surface has a very constant distance, which is about 20 nm, similar to the distance found in a synaptic cleft. The structure allows for close interactions between SGCs and neurons [152] and indicates that SGCs have an important role in the function of the DRG neurons under healthy or disease conditions. Recent studies demonstrate that SGCs in DRG are activated in chronic pain conditions. In this section, we present studies illustrating how SGCs are activated and contribute to chronic pain.
Nerve damage induces prominent changes in SGCs, even when the lesion site is distal to the DRG. It was found that early blockade of injured primary sensory afferents via local DRG perfusion with TTX reduces glial cell activation in both SNI and spinal nerve ligation (SNL) rats [153]. One important messenger between DRG neurons and SGCs is ATP. Indeed, it is a major messenger utilized reciprocally in neuron- SGC communication. Both cell types can release ATP, and both express P2Rs to detect ATP signal. There are various types of P2Rs expressed on DRG/TG on both rodents and human, and under pathological condition, the expression of P2Rs is upregulated [154], [155], [156]. Bradykinin can sensitize P2Rs on the SGC in response to ATP [157], and ATP released by DRG neurons can act on P2Rs in SGCs and activate SGCs [158], [159], [160]. The other of important mediator is nitric oxide (NO). NO synthesized and released by DRG neurons after peripheral inflammation diffuse into SGCs, and upregulates cyclic GMP (cGMP) production in SCGs and activated SGCs [161], [162], [163]. Recently, studies observed that CGRP receptor components calcitonin receptor-like receptor (CLR) and receptor activity modifying protein (RAMP) 1 are expressed in SCGs in the TG in both animals and human [164, 165]. This observation suggests that this CGRP receptor signaling might be involved in DRG neuron- SGC communication.
Activated SGCs exhibit increased glial fibrillary acidic protein (GFAP) expression, proliferation, increased response to ATP and nitric oxide, which generate positive feedback. Meanwhile, the permeability of K+ channel which is the main channel affecting the depolarization of SGCs is reduced and receptors such as P2Rs and gap junctions are upregulated. There is no expression of voltage-dependent Na+ or Ca2+ channels in SGCs [166]. The dominant K+ channel in SGCs is Kir4.1. Inhibition or silencing of Kir4.1 can increase ATP release which can excite DRG neurons and induce pain behavior [167], [168], [169].
Components of gap junctions are abundantly expressed in both DRG neurons and SGCs. These include connexins (Cx) and Pannexins (Panx). Connexins are the most abundant, including Cx26, Cx30, Cx32, Cx36, Cx37, Cx43, Cx45 and Cx46 in neurons and SGCs in DRG and TG. Under myriad pathological pain conditions including nerve section, inflammation, chemotherapy-induced neuropathy, the expression of Connexins and coupling by gap junctions is increased [166, 170], [171], [172], [173]. The gap junction could couple between SGCs, between neuron and SGC, or between neurons [170, 173, 174]. Neurons isolated by SGCs do not interact with each other under normal conditions. However, it was observed that in mice with chronic pain, depolarization of DRG neurons can evoke electrical activities in adjacent DRG neurons. This phenomenon was termed cross depolarization. The SGCs have a key role in cross depolarization mediated via ATP-P2R signaling [175, 176] and by gap junctions [177]. Gap junctions can be identified by injecting a dye that can cross gap junctions and examining whether the dye enters nearby cells, or by electrophysiological recording from dissociated DRG and TG neurons in vitro. Recently, we developed a new imaging technique which allows for simultaneous monitoring of over 1,600 neurons per DRG in anesthetized mouse. Using this tool, we found that increased electrical activity in one neuron can activate adjacent neurons which was named coupled activation in nerve injury and inflammation (see Figure 3) [178]. Coupled activation resulted from gap junction strengthening via upregulation of Cx43 in SGCs. Coupled activation and mechanical hyperalgesia were reduced by both local and systemic application of a gap junction blocker and also in Cx43 SGCs conditional knockout mice. Though the underlying mechanism of coupled activation is not very clear, the transmission from neuron to SGC to neuron is feasible, with neurons and SGCs forming heterotypic gap junctions. Thus, SGCs are a major contributor to coupled activation and the resultant mechanical and thermal hypersensitivity [179]. Panx is homologues of gap junction proteins. However, it forms membrane channels which mediate the release of ATP instead of forming gap junctions [180, 181]. DRG expression of Panx1 increased in both neuron and SGCs after nerve injury or inflammation [182, 183]. Panx1 deletion in GFAP-positive glia cells in DRG totally abolished hypersensitivity, whereas deletion of neuronal Panx1 reduced baseline sensitivity and the duration of hypersensitivity [184]. This effect indicates that the dominant role of SGCs is ATP release.
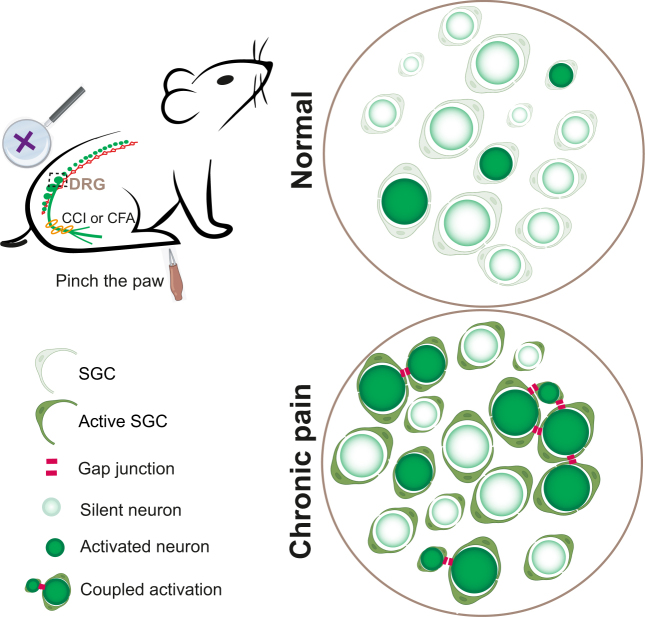
Figure 3:
Coupled activation of adjacent DRG neurons in DRG induced by peripheral stimuli after tissue injury. In healthy DRG, individual neurons are usually isolated from each other by SGCs and do not fire together. In neuropathy-induced chronic pain, however, multiple neurons become connected by gap junctions either directly or via SGCs. This form of ectopic DRG activity is associated with evoked pain. DRG, dorsal root ganglia; CCI, chronic constriction injury; CFA, complete Freund’s adjuvant; SGC, satellite glial cell.
The activated SGCs can release various factors such as nitric oxide, and cytokines IL-1β, IL-6, TNF via p38MAPK or Erk phosphorylation pathway [185], [186], [187], [188], [189]. ATP can act on its’ canonical receptor found on both SGCs and DRG neurons. Cytokines can act on each of their receptor on neurons and increase their excitability. Here, we will not discuss those cytokines and its’ receptor signaling in DRG neurons repeatedly.
Recently, one study observed that injected IgG can primarily bind to SGC in the mice injected with fibromyalgia syndrome (FMS) patient IgG [190]. FMS is a chronic pain condition characterized by widespread pain, with 10–30% of patients were diagnosed with autoimmune rheumatological conditions. Despite these predilections of autoimmunity, the injected FMS IgG does not induce cytokine production or systemic inflammation in mice. Yet the injected mice developed pain symptoms similar to those seen in FMS patients. The IgG only primarily binds to SGC and increases signs of SGC activity and sensitizes the DRG neuron. In addition, FMS IgG is restricted to the DRG, not in the brain and spinal cord. These results suggest that pronociceptive actions of FMS IgG were driven by peripheral mechanisms.
Overall, the past decade of studies has revealed that SGCs changed under chronic pain situation. Understanding these abnormal characteristics and interactions of SGCs with sensory neurons could provide a way that might be utilized therapeutically in the prevention and treatment of pain.
Sympathetic nerve sprouting into DRGs
Sympathetic sprouting into DRG after nerve injury was has been reported in rodents [191, 192] and chronic pain patients since the 1990s [193]. The early studies focused on the functions of sprouted sympathetic nerves forming “baskets” around DRG neurons [194]. Later studies revealed that sprouted sympathetic nerves consisted of both basket-like structures and excess fibers [195, 196]. Electron microscopy revealed that sympathetic postganglionic nerves, identified by tyrosine hydroxylase (TH), were unmyelinated fibers, with some ending in growth cones [197]. Many vesicle-containing axonal enlargements (synaptic varicosities) of sympathetic fibers were found to be among sensory fibers within the same Remak bundle, as well as located within the interstitial space of the DRG, and some were even found to be enclosed within the satellite cell capsule surrounding the DRG soma [197]. These findings indicated that axo-somatic interactions between sprouting sympathetic nerves and DRG sensory neuron were mediated via “en passant” type sympathetic varicosities, not traditional synapses.
The discovery of sympathetic sprouting into DRG provided a possible anatomical basis for clinical syndromes of SMP. Several studies showed that the activities of DRG sensory neurons can be modulated by direct sympathetic stimulation [198], and the activation of DRG neurons and evoked pain behaviors including mechanical and thermal hyperalgesia was attenuated by sympathectomy or sympathetic blockade in different nerve injury animal models [195, 199], [200], [201]. One study showed that delayed and restricted to cold allodynia-like behavior: SNI-related cold scores were lower in the SNI rat with sympathectomy compared to the SNI rat [202]. However, the functional relevance of sympathetic sprouting to neuropathic pain has been questioned because the degree of sympathetic dependence of mechanical and cold allodynia was not correlated to the extent of sympathetic fiber sprouting [203]. Furthermore, sympathetic sprouting was found in rats who did not have neuropathic pain and the sprouting seemed to be temporally delayed in comparison to the onset of mechanical and cold allodynia after distal nerve injury [202]. Interestingly, sympathetic nerve sprouting has been found to co-localize with spontaneously active individual neuron in certain pain models, indicating potential involvement of ectopic sympathetic activation in spontaneous, but not evoked pain [198, 204], [205], [206]. Recently, we directly demonstrated the necessity and sufficiency of DRG sympathetic sprouting in neuropathic mediated spontaneous pain using in vivo imaging and chemogenetic approaches [207]. Intriguingly, in vivo whole DRG imaging revealed that sprouting sympathetic nerves following peripheral nerve injury triggered a previously uncharacterized form of abnormal spontaneous activity in DRG neurons (see Figure 4). This distinct form of spontaneous activity was seen as clusters of adjacent DRG neurons firing synchronously and sporadically, a phenomenon we termed “cluster firing”. Importantly, the level of cluster firing directly correlated with spontaneous pain behaviors. Cluster firing occurred in areas of DRGs that colocalize with sympathetic nerve fiber sprouting. Using specific chemogenetic and pharmacological manipulations, we showed that the activity of sprouting sympathetic nerves is both necessary and sufficient for DRG cluster firing and spontaneous pain [207, 208]. Further, we identified norepinephrine (NE) as a key neurotransmitter in this pathway by visualizing NE activity using a genetically encoded NE sensor and by pharmacological blockade of α- and β- adrenergic receptors. These findings provide direct evidence that sympathetic nerves can evoke populations of DRG neurons in vivo, leading spontaneous pain during neuropathy.
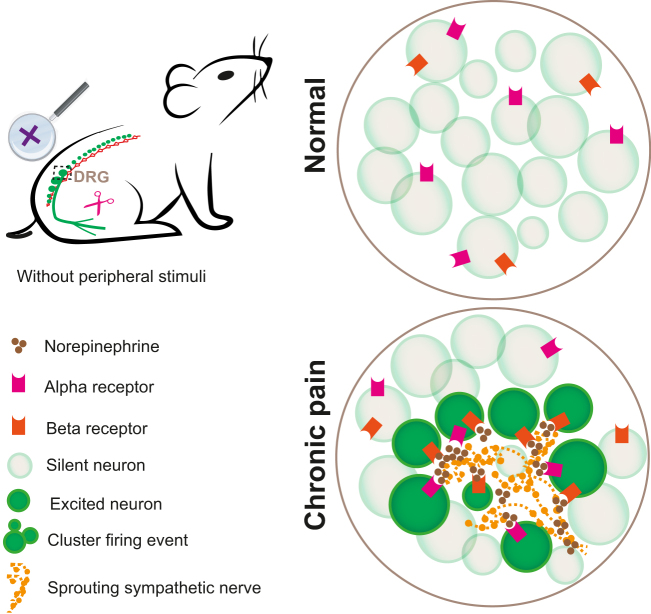
Figure 4:
DRG cluster firing induced by ectopic sympathetic sprouting in neuropathic pain. Under normal conditions, there is no crosstalk between sympathetic nerve and the cell bodies of DRG neurons. Under pathological conditions, such as peripheral nerve injury, sympathetic fibers sprout into the DRG, release norepinephrine that activate adrenergic receptors on DRG neurons, causing large clusters of neurons to fire simultaneously. This phenomenon is closely associated with spontaneous pain without any external stimuli. DRG, dorsal root ganglia.
Next, we will discuss what kinds of DRG neurons are involved in interactions with sympathetic nerve sprouting. Using histochemical staining, a wide range of DRG cell body sizes were observed to be wrapped by sympathetic nerve sprouting. This wrapping took on two forms, a basket formation, while the other was characterized by excess sympathetic fiber encapsulation [2, 20]. Both basket formation and excess fibers can affect excitability of nearby individual DRG neurons [12, 21]. In addition, using electrophysiological recordings, both myelinated A and unmyelinated C afferents were observed to respond to sympathetic stimulation in consequence to transection and ligation of the sciatic nerve [200]. Interestingly, the cluster firing contained all sizes of DRG neurons and the cluster firing sites colocalized with sites of sympathetic nerve sprouting [207]. Norepinephrine was identified as the key neurotransmitter released from sprouting sympathetic nerves, but there is no consensus on the sensitivity to norepinephrine of DRG neurons after nerve injury. NE increased excitability of all sizes of DRG cell bodies when measured using whole-cell patch-clamp recording [209], [210], [211]. All studies of the effect of NE on DRG neurons were limited to in vitro dissociated DRG neurons. Under in vivo conditions, we found that the DRG neurons of SNI mice with cluster firing show more hypersensitivity to NE, compared to DRG neurons of naïve mice or SNI mice without cluster firing at 21–28 day after SNI. The neurons with hypersensitivity to NE contain all neuron sizes, with most neuron clusters responding to 500 μmol/L NE.
Local blockade of α or β receptors at the level of the DRG reduced spontaneous cluster firing initiated by sympathetic nerve sprouting due to peripheral nerve injury. We detected the expression of α1, α2, and β receptor mRNA in the DRG in the SNI model, consistent with our pharmacological results [207]. There are many studies that are consistent with the idea that both alpha and beta receptors may be expressed, although unfortunately there are some disagreements in the literature about the exact subtypes present. Analysis of expression in particular subtypes of neurons in uninjured mice found that α1, α2, and β subtypes are expressed in some types of both myelinated and nociceptor categories [15]. In mice, there was relatively high expression of both α and aβ subtypes of adrenoreceptor in the DRG as well as some additional subtypes at 10–20-fold lower levels; these were maintained in the SNI model (day 30) [212]. A recent study of gene expression in subtypes of mouse DRG sensory neurons over the course of various pain models confirms the presence of several types of α and β subtypes in both myelinated and nociceptive subtypes; however, the absolute expression levels were not available in this study [213]. In that study the model most similar to SNI that was studied was sciatic nerve transection (for which expression changes were modest), and the latest time point given was seven days. However, in general, the literature supports the presence of both α and β subtypes in multiple types of mouse sensory neurons. More specifically, extensive literature shows that rodent DRG sensory neurons can be excited by norepinephrine, especially after nerve injury, and although there are some discrepancies about which particular receptor subtype is involved, there are studies showing that α1 receptors [214], [215], [216], α2 receptors [211, 217], [218], [219], [220], [221] and β [222, 223] receptors are expressed in DRG. Most of those studies relied on pharmacological approaches, with both local or systemic administration of the specific agonists or antagonists of each adrenergic receptor subtype. Notably, adrenergic receptors have also found to be expressed in immune cells and keratinocytes. Even on the sympathetic nerve terminal, there is data supporting expression of α2 receptors. Activation of this subtype was found to be inhibitory rather than excitatory similar to activation of α2 receptors on DRG cell bodies as referenced above.
There are currently two proposed sources of sympathetic neuron sprouting depending on the different injury models. After CCI, sympathetic nerves that innervate blood vessels and dura (probably intact) sprouted into DRG, but after SNL, sympathetic nerves (probably axotomized) invaded from the injured spinal nerve [201]. There is a myriad of molecular factors involved in sympathetic DRG sprouting, such as NGF [201, 224, 225], neurotrophin-3 (NT3) [226], interleukines [201], or Leukemia inhibitor factor (LIF) [194, 227]. The precise molecular mechanism by which sympathetic nerves invade the DRG during neuropathic pain remains yet to be elucidated.
The sympathetic nerve sprouting can affect not only DRG neurons, but also glia and immune cells. For this review we focused discussion on the intraganglion sympathetic-sensory interactions.
The potential for therapeutic targets
Although therapeutic options for chronic pain exist, chronic pain still remains poorly controlled and undermanaged. Originally contraindicated for chronic pain, opioid use for chronic pain treatment began to increase in the 1990s. Although opioids can be modestly effective, their adverse effects and risks of abuse that accompanies long term use has motivated efforts to find other therapeutic targets for pain. Clinical translation of novel basic science targets found in both the CNS and PNS have emerged in recent years. Sensitization of peripheral sensory neurons leads to chronic pain, thus factors which can reduce this hyperexcitability of sensory neurons can be thought of as potential clinical therapeutic targets. Various receptors and ion channels expressing on pain sensory neurons can affect the excitabilities of neurons directly. Ion channels such as TRPV1 [228] or TRPA1 [229], GPCRs such as CGRP receptors [230], as well as voltage gated sodium [231, 232] or potassium channels [233, 234] have undergone or are undergoing clinical trials for the treatment of chronic pain. Furthermore, the elements which influence the trafficking and expression of these receptors and ion channels could lead to further clinical translation. Additionally, non-neuronal elements mentioned in this review could lead to further translational targets, these include neurotrophin signaling [235, 236], chemokine signaling [237] and peripheral glia cells [238, 239]. Finally, the promise of precision medicine may soon come to fruition, as more research has illuminated the specific genetic loci of individuals who may be more susceptible to various chronic pain conditions.
Conclusion and remarks
An unbiased single-cell transcriptional profiling of DRG cells demonstrated distinct molecular changes in non-neuronal cells in response to peripheral nerve injury and dorsal root injury. In Schwann cells, macrophage and SGC gene expression changes were the largest after peripheral injury, but also occurred after dorsal root crush and spinal cord injury [147]. This suggests that non-neuronal cells play a key role in peripheral sensitization.
Multiple mediators released by non-neuronal cell types affect the functions of nociceptive neurons during chronic pain. Advances in our understanding of these molecular mechanisms provide ample opportunity for the development of novel pharmacological treatments for pain syndromes. Although this review took a neuron-centric view and discussed factors and cell types that directly affect sensory neuron function, complex interactions between the epithelial, immune and glial cell types are also important in neuropathic pain.
Detailed cellular and behavioral analyses reveal that the triggers for evoked pain differ significantly from those of spontaneous pain, suggesting future research and clinical treatments should consider differentiating between the two. Several clinical trials targeting mediators such as NGF- the TrkA for chronic pain syndromes have reached Phase II and III, and for osteoarthritis Phase III are currently underway.
Several key questions remain to be answered about the pathogenesis of chronic pain. First, the precise pathology of how acute pain transitions to a chronic phase remains unclear. Second, the neuro-immune interactions causing persistent immune cell activity without proper resolution remain incomplete. Third, the molecular factors that trigger sympathetic sprouting in both the periphery and DRG are yet to be uncovered. Finally, the COVID-19 pandemic has many features that could potentially increase the prevalence of chronic pain; however, the mechanisms of COVID-related pain remain unclear. It is worthwhile to determine if chronic pain is observed in COVID-19 animal model.
In the future cell type specific genetic manipulations, gene therapy, targeted pharmacological interventions hold the promise to eventually resolve these puzzles and hopefully lead to new and effective chronic pain treatments.
Footnotes
Research funding: The work was supported by the Howard Hughes Medical Institute.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
Contributor Information
Qin Zheng, Email: qzheng9@jh.edu.
Xinzhong Dong, Email: xdong2@jhmi.edu.
References
- 1.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–82. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160:53–9. doi: 10.1097/j.pain.0000000000001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–7. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clauw DJ, Häuser W, Cohen SP, Fitzcharles MA. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. 2020;161:1694–7. doi: 10.1097/j.pain.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 6.McCahon S, Strong J, Sharry R, Cramond T. Self-report and pain behavior among patients with chronic pain. Clin J Pain. 2005;21:223–31. doi: 10.1097/00002508-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017;10:284. doi: 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donkelaar HJT. Clinical neuroanatomy: brain circuitry and its disorders. 2nd ed. Berlin: Springer; 2020. [Google Scholar]
- 9.Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev. 2004;46:131–45. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife. 2014;3:e04660. doi: 10.7554/eLife.04660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupari J, Usoskin D, Parisien M, Lou D, Hu Y, Fatt M, et al. Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat Commun. 2021;12:1510. doi: 10.1038/s41467-021-21725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emery EC, Ernfors P. In: The Oxford handbook of the neurobiology of pain. Wood JN, editor. London: Oxford University Press; 2018. Dorsal root ganglion neuron types and their functional specialization. [Google Scholar]
- 13.Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 2016;26:83–102. doi: 10.1038/cr.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD. The emergence of transcriptional identity in somatosensory neurons. Nature. 2020;577:392–8. doi: 10.1038/s41586-019-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–53. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 16.Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.e22. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Q. A functional subdivision within the somatosensory system and its implications for pain research. Neuron. 2022;110:749–69. doi: 10.1016/j.neuron.2021.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu G, Huang K, Hu Y, Du G, Xue Z, Zhu X, et al. Single-cell RNA-seq reveals distinct injury responses in different types of DRG sensory neurons. Sci Rep. 2016;6:31851. doi: 10.1038/srep31851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Wang S, Chen Y, Wu D, Hu X, Lu Y, et al. Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain. Cell Res. 2021;31:904–18. doi: 10.1038/s41422-021-00479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Najjar SA, Davis BM, Albers KM. Epithelial-neuronal communication in the colon: implications for visceral pain. Trends Neurosci. 2020;43:170–81. doi: 10.1016/j.tins.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coutts AA, Jorizzo JL, Eady RA, Greaves MW, Burnstock G. Adenosine triphosphate-evoked vascular changes in human skin: mechanism of action. Eur J Pharmacol. 1981;76:391–401. doi: 10.1016/0014-2999(81)90110-2. [DOI] [PubMed] [Google Scholar]
- 22.Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–77. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- 23.Bland-Ward PA, Humphrey PP. Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br J Pharmacol. 1997;122:365–71. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, et al. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. Elife. 2018;7:e31684. doi: 10.7554/eLife.31684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao P, Barr TP, Hou Q, Dib-Hajj SD, Black JA, Albrecht PJ, et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain. 2008;139:90–105. doi: 10.1016/j.pain.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Gerevich Z, Illes P. P2Y receptors and pain transmission. Purinergic Signal. 2004;1:3–10. doi: 10.1007/s11302-004-4740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yousuf A, Klinger F, Schicker K, Boehm S. Nucleotides control the excitability of sensory neurons via two P2Y receptors and a bifurcated signaling cascade. Pain. 2011;152:1899–908. doi: 10.1016/j.pain.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lechner SG, Lewin GR. Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J Physiol. 2009;587:3493–503. doi: 10.1113/jphysiol.2009.175059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, et al. trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci. 2005;25:4868–78. doi: 10.1523/jneurosci.0249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–62. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 32.Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo. J Neurophysiol. 1998;79:2668–76. doi: 10.1152/jn.1998.79.5.2668. [DOI] [PubMed] [Google Scholar]
- 33.Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12:3077–80. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- 34.Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2003;278:48907–13. doi: 10.1074/jbc.m309468200. [DOI] [PubMed] [Google Scholar]
- 35.Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001;89:181–6. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- 36.Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. 1996;7:134–42. doi: 10.1006/mcne.1996.0010. [DOI] [PubMed] [Google Scholar]
- 37.Marlin MC, Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol. 2015;314:239–57. doi: 10.1016/bs.ircmb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–4. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segond von Banchet G, Kiehl M, Schaible H. Acute and long-term effects of IL-6 on cultured dorsal root ganglion neurones from adult rat. J Neurochem. 2015;94:238–48. doi: 10.1111/j.1471-4159.2005.03185.x. [DOI] [PubMed] [Google Scholar]
- 40.Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, et al. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci. 2009;29:13473–83. doi: 10.1523/jneurosci.1822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96:57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 42.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1β as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 43.Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998;151:138–42. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- 44.Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-α. J Neurosci. 2006;26:246–55. doi: 10.1523/jneurosci.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1β sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/jneurosci.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, et al. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999;73:2206–13. [PubMed] [Google Scholar]
- 47.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- 48.Gokin AP, Fareed MU, Pan HL, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21:5358–66. doi: 10.1523/jneurosci.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–61. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 50.Galli SJ, Gordon JR, Wershil BK. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3:865–72. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 51.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 52.White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005;4:834–44. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–48. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aich A, Afrin LB, Gupta K. Mast cell-mediated mechanisms of nociception. Int J Mol Sci. 2015;16:29069–92. doi: 10.3390/ijms161226151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of mast-cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity. 2019;50:1163–71.e5. doi: 10.1016/j.immuni.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–41. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navratilova E, Porreca F. Substance P and inflammatory pain: getting it wrong and right simultaneously. Neuron. 2019;101:353–5. doi: 10.1016/j.neuron.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 58.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron. 2019;101:412–20.e3. doi: 10.1016/j.neuron.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanashiro A, Hiroki CH, da Fonseca DM, Birbrair A, Ferreira RG, Bassi GS, et al. The role of neutrophils in neuro-immune modulation. Pharmacol Res. 2020;151:104580. doi: 10.1016/j.phrs.2019.104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farquhar-Smith WP, Rice AS. A novel neuroimmune mechanism in cannabinoid-mediated attenuation of nerve growth factor–induced hyperalgesia. Anesthesiology. 2003;99:1391–401. doi: 10.1097/00000542-200312000-00024. [DOI] [PubMed] [Google Scholar]
- 61.Chou TC, Chang LP, Li CY, Wong CS, Yang SP. The antiinflammatory and analgesic effects of baicalin in carrageenan-evoked thermal hyperalgesia. Anesth Analg. 2003;97:1724–9. doi: 10.1213/01.ane.0000087066.71572.3f. [DOI] [PubMed] [Google Scholar]
- 62.Bennett G, Al-Rashed S, Hoult JRS, Brain SD. Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain. 1998;77:315–22. doi: 10.1016/s0304-3959(98)00114-6. [DOI] [PubMed] [Google Scholar]
- 63.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakrabarty A, Liao Z, Smith PG. Angiotensin II receptor type 2 activation is required for cutaneous sensory hyperinnervation and hypersensitivity in a rat hind paw model of inflammatory pain. J Pain. 2013;14:1053–65. doi: 10.1016/j.jpain.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muralidharan A, Wyse BD, Smith MT. Analgesic efficacy and mode of action of a selective small molecule angiotensin II type 2 receptor antagonist in a rat model of prostate cancer-induced bone pain. Pain Med. 2014;15:93–110. doi: 10.1111/pme.12258. [DOI] [PubMed] [Google Scholar]
- 66.Smith MT, Anand P, Rice ASC. Selective small molecule angiotensin II type 2 receptor antagonists for neuropathic pain: preclinical and clinical studies. Pain. 2016;157(1 Suppl):S33–41. doi: 10.1097/j.pain.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 67.Shepherd AJ, Copits BA, Mickle AD, Karlsson P, Kadunganattil S, Haroutounian S, et al. Angiotensin II triggers peripheral macrophage-to-sensory neuron redox crosstalk to elicit pain. J Neurosci. 2018;38:7032–57. doi: 10.1523/jneurosci.3542-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shutov LP, Warwick CA, Shi X, Gnanasekaran A, Shepherd AJ, Mohapatra DP, et al. The complement system component C5a produces thermal hyperalgesia via macrophage-to-nociceptor signaling that requires NGF and TRPV1. J Neurosci. 2016;36:5055–70. doi: 10.1523/jneurosci.3249-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warwick CA, Shutov LP, Shepherd AJ, Mohapatra DP, Usachev YM. Mechanisms underlying mechanical sensitization induced by complement C5a: the roles of macrophages, TRPV1 and CGRP receptors. Pain. 2019;160:702. doi: 10.1097/j.pain.0000000000001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bang S, Xie Y, Zhang Z, Wang Z, Xu Z, Ji R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest. 2018;128:3568–82. doi: 10.1172/jci99888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 72.Murinson BB, Griffin JW. C-fiber structure varies with location in peripheral nerve. J Neuropathol Exp Neurol. 2004;63:246–54. doi: 10.1093/jnen/63.3.246. [DOI] [PubMed] [Google Scholar]
- 73.Salzer JL. Schwann cell myelination. Cold Spring Harbor Perspect Biol. 2015;7:a020529. doi: 10.1101/cshperspect.a020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–76. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 75.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-α, interleukin-1α, and interleukin-1β. J Neurosci. 2002;22:3052–60. doi: 10.1523/jneurosci.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto M, Sobue G, Li M, Arakawa Y, Mitsuma T, Kimata K. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and low-affinity nerve growth factor receptor (LNGFR) mRNA levels in cultured rat Schwann cells; differential time-and dose-dependent regulation by cAMP. Neurosci Lett. 1993;152:37–40. doi: 10.1016/0304-3940(93)90477-3. [DOI] [PubMed] [Google Scholar]
- 77.Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci USA. 1994;91:2795–9. doi: 10.1073/pnas.91.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X, Gonias SL, Campana WM. Schwann cells express erythropoietin receptor and represent a major target for Epo in peripheral nerve injury. Glia. 2005;51:254–65. doi: 10.1002/glia.20202. [DOI] [PubMed] [Google Scholar]
- 79.Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci. 2006;26:11197–207. doi: 10.1523/jneurosci.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mantuano E, Henry K, Yamauchi T, Hiramatsu N, Yamauchi K, Orita S, et al. The unfolded protein response is a major mechanism by which LRP1 regulates Schwann cell survival after injury. J Neurosci. 2011;31:13376–85. doi: 10.1523/jneurosci.2850-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orita S, Henry K, Mantuano E, Yamauchi K, de Corato A, Ishikawa T, et al. Schwann cell LRP1 regulates remak bundle ultrastructure and axonal interactions to prevent neuropathic pain. J Neurosci. 2013;33:5590–602. doi: 10.1523/jneurosci.3342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brifault C, Romero H, Van-Enoo A, Pizzo D, Azmoon P, Kwon H, et al. Deletion of the gene encoding the NMDA receptor GluN1 subunit in Schwann cells causes ultrastructural changes in remak bundles and hypersensitivity in pain processing. J Neurosci. 2020;40:9121–36. doi: 10.1523/jneurosci.0663-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Procacci P, Ballabio M, Castelnovo LF, Mantovani CM, Magnaghi V. GABA-B receptors in the PNS have a role in Schwann cells differentiation? Front Cell Neurosci. 2013;6:68. doi: 10.3389/fncel.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Magnaghi V, Ballabio M, Cavarretta IT, Froestl W, Lambert JJ, Zucchi I, et al. GABAB receptors in Schwann cells influence proliferation and myelin protein expression. Eur J Neurosci. 2004;19:2641–9. doi: 10.1111/j.0953-816x.2004.03368.x. [DOI] [PubMed] [Google Scholar]
- 85.Magnaghi V, Castelnovo LF, Faroni A, Cavalli E, Caffino L, Colciago A, et al. Nerve regenerative effects of GABA-B ligands in a model of neuropathic pain. Biomed Res Int. 2014;2014:368678. doi: 10.1155/2014/368678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faroni A, Rothwell SW, Grolla A, Terenghi G, Magnaghi V, Verkhratsky A. Differentiation of adipose-derived stem cells into Schwann cell phenotype induces expression of P2X receptors that control cell death. Cell Death Dis. 2013;4:e743. doi: 10.1038/cddis.2013.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faroni A, Smith RJP, Procacci P, Castelnovo LF, Puccianti E, Reid AJ, et al. Purinergic signaling mediated by P2X7 receptors controls myelination in sciatic nerves. J Neurosci Res. 2014;92:1259–69. doi: 10.1002/jnr.23417. [DOI] [PubMed] [Google Scholar]
- 88.Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, et al. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27:12565–76. doi: 10.1523/jneurosci.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weiner JA, Fukushima N, Contos JJA, Scherer SS, Chun J. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci. 2001;21:7069–78. doi: 10.1523/jneurosci.21-18-07069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2014;10:712–8. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- 91.Poplawski G, Ishikawa T, Brifault C, Lee‐Kubli C, Regestam R, Henry KW, et al. Schwann cells regulate sensory neuron gene expression before and after peripheral nerve injury. Glia. 2018;66:1577–90. doi: 10.1002/glia.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Logu F, Nassini R, Materazzi S, Carvalho Gonçalves M, Nosi D, Rossi Degl’Innocenti D, et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun. 2017;8:1887. doi: 10.1038/s41467-017-01739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, et al. Neuregulin 1–erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–86. doi: 10.1523/jneurosci.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–3. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 95.Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, et al. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6:1186–93. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 96.Shetty VP. Animal model to study the mechanism of nerve damage in leprosy; a preliminary report. Int J Lepr. 1993;61:70–5. [PubMed] [Google Scholar]
- 97.Reis FJ, Gomes MK, Saadi L, Gosling AP, Cunha AJ. Chronic pain in leprosy: new aspects to be considered. Pain Manag. 2013;3:201–10. doi: 10.2217/pmt.13.12. [DOI] [PubMed] [Google Scholar]
- 98.Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12:961–6. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- 99.Murinson BB, Archer DR, Li Y, Griffin JW. Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J Neurosci. 2005;25:1179–87. doi: 10.1523/jneurosci.1372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doan RA, Monk KR. Glia in the skin activate pain responses. Science. 2019;365:641–2. doi: 10.1126/science.aay6144. [DOI] [PubMed] [Google Scholar]
- 101.Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang M, Usoskin D, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365:695–9. doi: 10.1126/science.aax6452. [DOI] [PubMed] [Google Scholar]
- 102.Rinwa P, Calvo-Enrique L, Zhang MD, Nyengaard JR, Karlsson P, Ernfors P. Demise of nociceptive Schwann cells causes nerve retraction and pain hyperalgesia. Pain. 2021;162:1816–27. doi: 10.1097/j.pain.0000000000002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scott-Solomon E, Boehm E, Kuruvilla R. The sympathetic nervous system in development and disease. Nat Rev Neurosci. 2021;22:685–702. doi: 10.1038/s41583-021-00523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morgan RF, Reisman NR, Wilgis EF. Anatomic localization of sympathetic nerves in the hand. J Hand Surg Am. 1983;8:283–8. doi: 10.1016/s0363-5023(83)80161-0. [DOI] [PubMed] [Google Scholar]
- 105.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–89. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 106.Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127–33. doi: 10.1016/0304-3959(95)00110-e. [DOI] [PubMed] [Google Scholar]
- 107.Borchers AT, Gershwin ME. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2004;13:242–65. doi: 10.1016/j.autrev.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 108.Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci. 2014;39:508–19. doi: 10.1111/ejn.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines. Pain Med. 2013;14:180–229. doi: 10.1111/pme.12033. [DOI] [PubMed] [Google Scholar]
- 110.Drummond PD. Sensory–autonomic interactions in health and disease. Handb Clin Neurol. 2013;117:309–19. doi: 10.1016/b978-0-444-53491-0.00024-9. [DOI] [PubMed] [Google Scholar]
- 111.Benzon HT. Essentials of pain medicine. 3rd ed. Philadalphia: Elsevier Saunders; 2011. [Google Scholar]
- 112.Longo G, Osikowicz M, Ribeiro-da-Silva A. Sympathetic fiber sprouting in inflamed joints and adjacent skin contributes to pain-related behavior in arthritis. J Neurosci. 2013;33:10066–74. doi: 10.1523/jneurosci.5784-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zotterman Y. Sensory functions of the skin in primates. Oxford: Pergamon; 1976. [Google Scholar]
- 114.Choi B, Rowbotham MC. Effect of adrenergic receptor activation on post-herpetic neuralgia pain and sensory disturbances. Pain. 1997;69:55–63. doi: 10.1016/s0304-3959(96)03245-9. [DOI] [PubMed] [Google Scholar]
- 115.Ali Z, Raja SN, Wesselmann U, Fuchs PN, Meyer RA, Campbell JN. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain. 2000;88:161–8. doi: 10.1016/s0304-3959(00)00327-4. [DOI] [PubMed] [Google Scholar]
- 116.Baik E, Chung JM, Chung K. Peripheral norepinephrine exacerbates neuritis-induced hyperalgesia. J Pain. 2003;4:212–21. doi: 10.1016/s1526-5900(03)00617-5. [DOI] [PubMed] [Google Scholar]
- 117.Ruocco I, Cuello AC, Ribeiro‐Da‐Silva A. Peripheral nerve injury leads to the establishment of a novel pattern of sympathetic fibre innervation in the rat skin. J Comp Neurol. 2000;422:287–96. doi: 10.1002/(sici)1096-9861(20000626)422:2<287::aid-cne9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 118.Grelik C, Bennett GJ, Ribeiro‐da‐Silva A. Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. Eur J Neurosci. 2005;21:2475–87. doi: 10.1111/j.1460-9568.2005.04089.x. [DOI] [PubMed] [Google Scholar]
- 119.Yen LD, Bennett GJ, Ribeiro‐da‐Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol. 2006;495:679–90. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]
- 120.Almarestani L, Longo G, Ribeiro-da-Silva A. Autonomic fiber sprouting in the skin in chronic inflammation. Mol Pain. 2008;4:56. doi: 10.1186/1744-8069-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nascimento FP, Magnussen C, Yousefpour N, Ribeiro-da-Silva A. Sympathetic fibre sprouting in the skin contributes to pain-related behaviour in spared nerve injury and cuff models of neuropathic pain. Mol Pain. 2015;11:59. doi: 10.1186/s12990-015-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Small JR, Scadding JW, Landon DN. A fluorescence study of changes in noradrenergic sympathetic fibres in experimental peripheral nerve neuromas. J Neurol Sci. 1990;100:98–107. doi: 10.1016/0022-510x(90)90019-j. [DOI] [PubMed] [Google Scholar]
- 123.Small JR, Scadding JW, Landon DN. Ultrastructural localization of sympathetic axons in experimental rat sciatic nerve neuromas. J Neurocytol. 1996;25:573–82. doi: 10.1007/bf02284825. [DOI] [PubMed] [Google Scholar]
- 124.Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–98. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther. 2012;14:R101. doi: 10.1186/ar3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chartier SR, Thompson ML, Longo G, Fealk MN, Majuta LA, Mantyh PW. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain. 2014;155:2323–36. doi: 10.1016/j.pain.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sapio MR, Vazquez FA, Loydpierson AJ, Maric D, Kim JJ, LaPaglia DM, et al. Comparative analysis of dorsal root, nodose and sympathetic ganglia for the development of new analgesics. Front Neurosci. 2020;14:615362. doi: 10.3389/fnins.2020.615362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gloster A, Diamond J. Sympathetic nerves in adult rats regenerate normally and restore pilomotor function during an anti-NGF treatment that prevents their collateral sprouting. J Comp Neurol. 1992;326:363–74. doi: 10.1002/cne.903260305. [DOI] [PubMed] [Google Scholar]
- 129.Mearow KM, Kril Y, Diamond J. Increased NGF mRNA expression in denervated rat skin. Neuroreport. 1993;4:351–4. doi: 10.1097/00001756-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 130.Gibbs GF, Drummond PD, Finch PM, Phillips JK. Unravelling the pathophysiology of complex regional pain syndrome: focus on sympathetically maintained pain. Clin Exp Pharmacol Physiol. 2008;35:717–24. doi: 10.1111/j.1440-1681.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- 131.Gunduz OH, Kenis-Coskun O. Ganglion blocks as a treatment of pain: current perspectives. J Pain Res. 2017;10:2815–26. doi: 10.2147/jpr.s134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xie J, Yoon YW, Yom SS, Chung JM. Norepinephrine rekindles mechanical allodynia in sympathectomized neuropathic rat. Analgesia. 1995;1:107–13. doi: 10.3727/107156995819564310. [DOI] [Google Scholar]
- 133.Torebjörk E, Wahren L, Wallin G, Hallin R, Koltzenburg M. Noradrenaline-evoked pain in neuralgia. Pain. 1995;63:11–20. doi: 10.1016/0304-3959(95)00140-n. [DOI] [PubMed] [Google Scholar]
- 134.Drummond PD, Drummond ES, Dawson LF, Mitchell V, Finch PM, Vaughan CW, et al. Upregulation of α1-adrenoceptors on cutaneous nerve fibres after partial sciatic nerve ligation and in complex regional pain syndrome type II. Pain. 2014;155:606–16. doi: 10.1016/j.pain.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 135.Drummond ES, Dawson LF, Finch PM, Bennett GJ, Drummond PD. Increased expression of cutaneous α1-adrenoceptors after chronic constriction injury in rats. J Pain. 2014;15:188–96. doi: 10.1016/j.jpain.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 136.Levine JD, Taiwo YO, Collins SD, Tam JK. Noradrenaline hyperalgesia is mediated through interaction with sympathetic postgahglionic neurone terminals rather than activation of primary afferent nociceptors. Nature. 1986;323:158–60. doi: 10.1038/323158a0. [DOI] [PubMed] [Google Scholar]
- 137.Kingery WS, Guo TZ, Davies FM, Limbird L, Maze M. The α2A adrenoceptor and the sympathetic postganglionic neuron contribute to the development of neuropathic heat hyperalgesia in mice. Pain. 2000;85:345–58. doi: 10.1016/s0304-3959(99)00286-9. [DOI] [PubMed] [Google Scholar]
- 138.Sato J, Suzuki S, Tamura R, Kumazawa T. Norepinephrine excitation of cutaneous nociceptors in adjuvant-induced inflamed rats does not depend on sympathetic neurons. Neurosci Lett. 1994;177:135–8. doi: 10.1016/0304-3940(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 139.Ramien M, Ruocco I, Cuello AC, St-Louis M, Ribeiro-Da-Silva A. Parasympathetic nerve fibers invade the upper dermis following sensory denervation of the rat lower lip skin. J Comp Neurol. 2004;469:83–95. doi: 10.1002/cne.10998. [DOI] [PubMed] [Google Scholar]
- 140.Taylor AMW, Ribeiro-da-Silva A. GDNF levels in the lower lip skin in a rat model of trigeminal neuropathic pain: implications for nonpeptidergic fiber reinnervation and parasympathetic sprouting. Pain. 2011;152:1502–10. doi: 10.1016/j.pain.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 141.Dickson A, Avelino A, Cruz F, Ribeiro-da-Silva A. Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience. 2006;139:671–85. doi: 10.1016/j.neuroscience.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 142.Chen O, Donnelly CR, Ji RR. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol. 2020;62:17–25. doi: 10.1016/j.conb.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang ZZ, Li D, Liu CC, Cui Y, Zhu HQ, Zhang WW, et al. CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun. 2014;40:155–65. doi: 10.1016/j.bbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 144.Morin N, Owolabi SA, Harty MW, Papa EF, Tracy TF, Shaw SK, et al. Neutrophils invade lumbar dorsal root ganglia after chronic constriction injury of the sciatic nerve. J Neuroimmunol. 2007;184:164–71. doi: 10.1016/j.jneuroim.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 145.Vicuña L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med. 2015;21:518–23. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, et al. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci. 2016;36:11074–83. doi: 10.1523/jneurosci.3708-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Avraham O, Feng R, Ewan EE, Rustenhoven J, Zhao G, Cavalli V. Profiling sensory neuron microenvironment after peripheral and central axon injury reveals key pathways for neural repair. Elife. 2021;10:e68457. doi: 10.7554/eLife.68457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun. 2020;11:264. doi: 10.1038/s41467-019-13839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hanani M, Spray DC. Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci. 2020;21:485–98. doi: 10.1038/s41583-020-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pannese E, Ledda M, Arcidiacono G, Rigamonti L. Clusters of nerve cell bodies enclosed within a common connective tissue envelope in the spinal ganglia of the lizard and rat. Cell Tissue Res. 1991;264:209–14. doi: 10.1007/bf00313957. [DOI] [PubMed] [Google Scholar]
- 151.Pannese E, Procacci P, Ledda M, Conte V. The percentage of nerve cell bodies arranged in clusters decreases with age in the spinal ganglia of adult rabbits. Anat Embryol (Berl) 1993;187:331–4. doi: 10.1007/BF00185890. [DOI] [PubMed] [Google Scholar]
- 152.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev. 2005;48:457–76. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 2009;160:847–57. doi: 10.1016/j.neuroscience.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Magni G, Riccio D, Ceruti S. Tackling chronic pain and inflammation through the purinergic system. Curr Med Chem. 2018;25:3830–65. doi: 10.2174/0929867324666170710110630. [DOI] [PubMed] [Google Scholar]
- 155.Kushnir R, Cherkas PS, Hanani M. Peripheral inflammation upregulates P2X receptor expression in satellite glial cells of mouse trigeminal ganglia: a calcium imaging study. Neuropharmacology. 2011;61:739–46. doi: 10.1016/j.neuropharm.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 156.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–96. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 157.Ceruti S, Fumagalli M, Villa G, Verderio C, Abbracchio MP. Purinoceptor-mediated calcium signaling in primary neuron-glia trigeminal cultures. Cell Calcium. 2008;43:576–90. doi: 10.1016/j.ceca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 158.Zhang X, Chen Y, Wang C, Huang LYM. Neuronal somatic ATP release triggers neuron–satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA. 2007;104:9864–9. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–7. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- 160.Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33:784–92. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 161.Morris R, Southam E, Braid DJ, Garthwaite J. Nitric oxide may act as a messenger between dorsal root ganglion neurones and their satellite cells. Neurosci Lett. 2002;137:29–32. doi: 10.1016/0304-3940(92)90290-n. [DOI] [PubMed] [Google Scholar]
- 162.Thippeswamy T, Morris R. The roles of nitric oxide in dorsal root ganglion neurons. Ann N Y Acad Sci. 2002;962:103–10. doi: 10.1111/j.1749-6632.2002.tb04060.x. [DOI] [PubMed] [Google Scholar]
- 163.Belzer V, Hanani M. Nitric oxide as a messenger between neurons and satellite glial cells in dorsal root ganglia. Glia. 2019;67:1296–307. doi: 10.1002/glia.23603. [DOI] [PubMed] [Google Scholar]
- 164.Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–96. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 165.Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015;1600:93–109. doi: 10.1016/j.brainres.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 166.Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110:290–8. doi: 10.1016/j.pain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 167.Vit JP, Ohara PT, Bhargava A, Kelley K, Jasmin L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J Neurosci. 2008;28:4161–71. doi: 10.1523/jneurosci.5053-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tang X, Schmidt TM, Perez-Leighton CE, Kofuji P. Inwardly rectifying potassium channel Kir4.1 is responsible for the native inward potassium conductance of satellite glial cells in sensory ganglia. Neuroscience. 2010;166:397–407. doi: 10.1016/j.neuroscience.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Takeda M, Takahashi M, Nasu M, Matsumoto S. Peripheral inflammation suppresses inward rectifying potassium currents of satellite glial cells in the trigeminal ganglia. Pain. 2011;152:2147–56. doi: 10.1016/j.pain.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 170.Pannese E, Ledda M, Cherkas PS, Huang TY, Hanani M. Satellite cell reactions to axon injury of sensory ganglion neurons: increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anat Embryol (Berl) 2003;206:337–47. doi: 10.1007/s00429-002-0301-6. [DOI] [PubMed] [Google Scholar]
- 171.Huang TY, Belzer V, Hanani M. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Eur J Pain. 2010;14:49.e1–11. doi: 10.1016/j.ejpain.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 172.Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21:592–8. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 173.Ledda M, Blum E, de Palo S, Hanani M. Augmentation in gap junction-mediated cell coupling in dorsal root ganglia following sciatic nerve neuritis in the mouse. Neuroscience. 2009;164:1538–45. doi: 10.1016/j.neuroscience.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 174.Spray DC, Iglesias R, Shraer N, Suadicani SO, Belzer V, Hanstein R, et al. Gap junction mediated signaling between satellite glia and neurons in trigeminal ganglia. Glia. 2019;67:791–801. doi: 10.1002/glia.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hanani M. Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: implications for chronic pain. Brain Res. 2012;1487:183–91. doi: 10.1016/j.brainres.2012.03.070. [DOI] [PubMed] [Google Scholar]
- 176.Carvalho GB, Mulpuri Y, Damasio A, Spigelman I. A role for the P2Y1 receptor in nonsynaptic cross-depolarization in the rat dorsal root ganglia. Neuroscience. 2019;423:98–108. doi: 10.1016/j.neuroscience.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 177.Spray DC, Hanani M. Gap junctions, pannexins and pain. Neurosci Lett. 2019;695:46–52. doi: 10.1016/j.neulet.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, et al. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron. 2016;91:1085–96. doi: 10.1016/j.neuron.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Seal RP. Illuminating the gap: neuronal cross-talk within sensory ganglia and persistent pain. Neuron. 2016;91:950–1. doi: 10.1016/j.neuron.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 180.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, et al. Pannexin channels are not gap junction hemichannels. Channels. 2011;5:193–7. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dahl G, Qiu F, Wang J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology. 2013;75:583–93. doi: 10.1016/j.neuropharm.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hanstein R, Zhao J, Gulinello M, Hanani M, Spray DC. Transient inflammation causes chronic tactile hypersensitivity: the new role of the P2X7R-pannexin1 complex in pain. Glia. 2011;59:S105. [Google Scholar]
- 183.Zhang Y, Laumet G, Chen S, Hittelman WN, Pan H. Pannexin-1 up-regulation in the dorsal root ganglion contributes to neuropathic pain development. J Biol Chem. 2015;290:14647–55. doi: 10.1074/jbc.m115.650218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hanstein R, Hanani M, Scemes E, Spray DC. Glial pannexin1 contributes to tactile hypersensitivity in a mouse model of orofacial pain. Sci Rep. 2016;6:38266. doi: 10.1038/srep38266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ji R, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(1 Suppl):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang Y, Song N, Liu F, Lin J, Liu M, Huang C, et al. Activation of the RAS/B-RAF-MEK-ERK pathway in satellite glial cells contributes to substance p-mediated orofacial pain. Eur J Neurosci. 2020;51:2205–18. doi: 10.1111/ejn.14619. [DOI] [PubMed] [Google Scholar]
- 187.Lin J, Liu F, Zhang Y, Song N, Liu M, Fang X, et al. P2Y14 receptor is functionally expressed in satellite glial cells and mediates interleukin-1β and chemokine CCL2 secretion. J Cell Physiol. 2019;234:21199–210. doi: 10.1002/jcp.28726. [DOI] [PubMed] [Google Scholar]
- 188.Qin G, Gui B, Xie J, Chen L, Chen L, Cui Z, et al. Tetrandrine alleviates nociception in a rat model of migraine via suppressing S100B and p-ERK activation in satellite glial cells of the trigeminal ganglia. J Mol Neurosci. 2018;64:29–38. doi: 10.1007/s12031-017-0999-5. [DOI] [PubMed] [Google Scholar]
- 189.Song J, Ying Y, Wang W, Liu X, Xu X, Wei X, et al. The role of P2X7R/ERK signaling in dorsal root ganglia satellite glial cells in the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR) Brain Behav Immun. 2018;69:180–9. doi: 10.1016/j.bbi.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 190.Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, et al. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest. 2021;131:e144201. doi: 10.1172/JCI144201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McLachlan EM, Jänig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–6. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- 192.Chung K, Kim HJ, Na HS, Park MJ, Chung JM. Abnormalities of sympathetic innervation in the area of an injured peripheral nerve in a rat model of neuropathic pain. Neurosci Lett. 1993;162:85–8. doi: 10.1016/0304-3940(93)90566-4. [DOI] [PubMed] [Google Scholar]
- 193.Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, et al. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1999;28:743–61. doi: 10.1023/a:1007090105840. [DOI] [PubMed] [Google Scholar]
- 194.Ramer MS, Thompson SWN, McMahon SB. Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain. 1999;Suppl 6:S111–20. doi: 10.1016/s0304-3959(99)00144-x. [DOI] [PubMed] [Google Scholar]
- 195.Xie W, Strong JA, Zhang J. Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models. Pain. 2020;161:1925–36. doi: 10.1097/j.pain.0000000000001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ramer MS, French GD, Bisby MA. Wallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRG. Pain. 1997;72:71–8. doi: 10.1016/s0304-3959(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 197.Chung K, Yoon YW, Chung JM. Sprouting sympathetic fibers form synaptic varicosities in the dorsal root ganglion of the rat with neuropathic injury. Brain Res. 1997;751:275–80. doi: 10.1016/s0006-8993(96)01408-4. [DOI] [PubMed] [Google Scholar]
- 198.Devor M, Jänig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- 199.Iwase T, Takebayashi T, Tanimoto K, Terashima Y, Miyakawa T, Kobayashi T, et al. Sympathectomy attenuates excitability of dorsal root ganglion neurons and pain behaviour in a lumbar radiculopathy model. Bone Joint Res. 2012;1:198–204. doi: 10.1302/2046-3758.19.2000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Michaelis M, Devor M, Jänig W. Sympathetic modulation of activity in rat dorsal root ganglion neurons changes over time following peripheral nerve injury. J Neurophysiol. 1996;76:753–63. doi: 10.1152/jn.1996.76.2.753. [DOI] [PubMed] [Google Scholar]
- 201.Ramer MS, Bisby MA. Differences in sympathetic innervation of mouse DRG following proximal or distal nerve lesions. Exp Neurol. 1998;152:197–207. doi: 10.1006/exnr.1998.6855. [DOI] [PubMed] [Google Scholar]
- 202.Pertin M, Allchorne AJ, Beggah AT, Woolf CJ, Decosterd I. Delayed sympathetic dependence in the spared nerve injury (SNI) model of neuropathic pain. Mol Pain. 2007;3:21. doi: 10.1186/1744-8069-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim HJ, Na HS, Sung B, Hong SK. Amount of sympathetic sprouting in the dorsal root ganglia is not correlated to the level of sympathetic dependence of neuropathic pain in a rat model. Neurosci Lett. 1998;245:21–4. doi: 10.1016/s0304-3940(98)00167-0. [DOI] [PubMed] [Google Scholar]
- 204.Xie W, Strong JA, Li H, Zhang J. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol. 2007;97:492–502. doi: 10.1152/jn.00899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang J, Strong JA. Recent evidence for activity-dependent initiation of sympathetic sprouting and neuropathic pain. Sheng Li Xue Bao. 2008;60:617–27. [PubMed] [Google Scholar]
- 206.Xie W, Strong JA, Zhang J. Increased excitability and spontaneous activity of rat sensory neurons following in vitro stimulation of sympathetic fiber sprouts in the isolated dorsal root ganglion. Pain. 2010;151:447–59. doi: 10.1016/j.pain.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zheng Q, Xie W, Lückemeyer DD, Lay M, Wang X, Dong X, et al. Synchronized cluster firing, a distinct form of sensory neuron activation, drives spontaneous pain. Neuron. 2022;110:209–20.e6. doi: 10.1016/j.neuron.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Geron M, Tassou A, Scherrer G. Sympathetic yet painful: autonomic innervation drives cluster firing of somatosensory neurons. Neuron. 2022;110:175–7. doi: 10.1016/j.neuron.2021.12.025. [DOI] [PubMed] [Google Scholar]
- 209.Petersen M, Zhang J, Zhang J, LaMotte RH. Abnormal spontaneous activity and responses to norepinephrine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain. 1996;67:391–7. doi: 10.1016/0304-3959(96)03146-6. [DOI] [PubMed] [Google Scholar]
- 210.Abdulla FA, Smith PA. Ectopic α2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons. J Neurosci. 1997;17:1633–41. doi: 10.1523/jneurosci.17-05-01633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ji Y, Shi W, Yang J, Ma B, Jin T, Cao B, et al. Effect of sympathetic sprouting on the excitability of dorsal root ganglion neurons and afferents in a rat model of neuropathic pain. Biochem Biophys Res Commun. 2022;587:49–57. doi: 10.1016/j.bbrc.2021.11.096. [DOI] [PubMed] [Google Scholar]
- 212.Uttam S, Wong C, Amorim IS, Jafarnejad SM, Tansley SN, Yang J, et al. Translational profiling of dorsal root ganglia and spinal cord in a mouse model of neuropathic pain. Neurobiol Pain. 2018;4:35–44. doi: 10.1016/j.ynpai.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Renthal W, Tochitsky I, Yang L, Cheng Y, Li E, Kawaguchi R, et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron. 2020;108:128–44.e9. doi: 10.1016/j.neuron.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xie J, Lee YH, Wang C, Chung JM, Chung K. Differential expression of alpha1-adrenoceptor subtype mRNAs in the dorsal root ganglion after spinal nerve ligation. Mol Brain Res. 2001;93:164–72. doi: 10.1016/s0169-328x(01)00201-7. [DOI] [PubMed] [Google Scholar]
- 215.Maruo K, Yamamoto H, Yamamoto S, Nagata T, Fujikawa H, Kanno T, et al. Modulation of P2X receptors via adrenergic pathways in rat dorsal root ganglion neurons after sciatic nerve injury. Pain. 2006;120:106–12. doi: 10.1016/j.pain.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 216.Zhang Q, Tan Y. Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience. 2011;193:72–9. doi: 10.1016/j.neuroscience.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 217.Chen Y, Michaelis M, Janig W, Devor M. Adrenoreceptor subtype mediating sympathetic-sensory coupling in injured sensory neurons. J Neurophysiol. 1996;76:3721–30. doi: 10.1152/jn.1996.76.6.3721. [DOI] [PubMed] [Google Scholar]
- 218.Leem JW, Gwak YS, Nam TS, Paik KS. Involvement of α2-adrenoceptors in mediating sympathetic excitation of injured dorsal root ganglion neurons in rats with spinal nerve ligation. Neurosci Lett. 1997;234:39–42. doi: 10.1016/s0304-3940(97)00658-7. [DOI] [PubMed] [Google Scholar]
- 219.Shi TS, Winzer-Serhan U, Leslie F, Hökfelt T. Distribution and regulation of α2-adrenoceptors in rat dorsal root ganglia. Pain. 2000;84:319–30. doi: 10.1016/s0304-3959(99)00224-9. [DOI] [PubMed] [Google Scholar]
- 220.Ogon I, Takebayashi T, Iwase T, Emori M, Tanimoto K, Miyakawa T, et al. Sympathectomy and sympathetic blockade reduce pain behavior via alpha-2 adrenoceptor of the dorsal root ganglion neurons in a lumbar radiculopathy model. Spine (Phila Pa 1976) 2015;40:E1269–75. doi: 10.1097/brs.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 221.Ogon I, Takebayashi T, Miyakawa T, Iwase T, Tanimoto K, Terashima Y, et al. Attenuation of pain behaviour by local administration of alpha-2 adrenoceptor antagonists to dorsal root ganglia in a rat radiculopathy model. Eur J Pain. 2016;20:790–9. doi: 10.1002/ejp.804. [DOI] [PubMed] [Google Scholar]
- 222.Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci. 2006;24:527–34. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- 223.Pelegrini-da-Silva A, Oliveira MCG, Parada CA, Tambeli CH. Nerve growth factor acts with the β2-adrenoceptor to induce spontaneous nociceptive behavior during temporomandibular joint inflammatory hyperalgesia. Life Sci. 2008;83:780–5. doi: 10.1016/j.lfs.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 224.Ramer M, Bisby M. Reduced sympathetic sprouting occurs in dorsal root ganglia after axotomy in mice lacking low-affinity neurotrophin receptor. Neurosci Lett. 1997;228:9–12. doi: 10.1016/s0304-3940(97)00356-x. [DOI] [PubMed] [Google Scholar]
- 225.Jones MG, Munson JB, Thompson SWN. A role for nerve growth factor in sympathetic sprouting in rat dorsal root ganglia. Pain. 1999;79:21–9. doi: 10.1016/s0304-3959(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 226.Zhou XF, Deng YS, Chie E, Xue Q, Zhong JH, McLachlan EM, et al. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11:1711–22. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]
- 227.Thompson SW, Majithia AA. Leukemia inhibitory factor induces sympathetic sprouting in intact dorsal root ganglia in the adult rat in vivo. J Physiol. 1998;506:809–16. doi: 10.1111/j.1469-7793.1998.809bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Iftinca M, Defaye M, Altier C. TRPV1-targeted drugs in development for human pain conditions. Drugs. 2021;81:7–27. doi: 10.1007/s40265-020-01429-2. [DOI] [PubMed] [Google Scholar]
- 229.de Araujo DSM, Nassini R, Geppetti P, De Logu F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets. 2020;24:997–1008. doi: 10.1080/14728222.2020.1815191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338–50. doi: 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 231.Goodwin G, McMahon SB. The physiological function of different voltage-gated sodium channels in pain. Nat Rev Neurosci. 2021;22:263–74. doi: 10.1038/s41583-021-00444-w. [DOI] [PubMed] [Google Scholar]
- 232.Ma RSY, Kayani K, Whyte-Oshodi D, Whyte-Oshodi A, Nachiappan N, Gnanarajah S, et al. Voltage gated sodium channels as therapeutic targets for chronic pain. J Pain Res. 2019;12:2709–22. doi: 10.2147/jpr.s207610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Abd-Elsayed A, Jackson M, Gu SL, Fiala K, Gu J. Neuropathic pain and Kv7 voltage-gated potassium channels: the potential role of Kv7 activators in the treatment of neuropathic pain. Mol Pain. 2019;15:1744806919864256. doi: 10.1177/1744806919864256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stevens EB, Stephens GJ. Recent advances in targeting ion channels to treat chronic pain. Br J Pharmacol. 2018;175:2133–7. doi: 10.1111/bph.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hirose M, Kuroda Y, Murata E. NGF/TrkA signaling as a therapeutic target for pain. Pain Pract. 2016;16:175–82. doi: 10.1111/papr.12342. [DOI] [PubMed] [Google Scholar]
- 236.Kashyap MP, Roberts C, Waseem M, Tyagi P. Drug targets in neurotrophin signaling in the central and peripheral nervous system. Mol Neurobiol. 2018;55:6939–55. doi: 10.1007/s12035-018-0885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jiang B, Liu T, Gao Y. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther. 2020;212:107581. doi: 10.1016/j.pharmthera.2020.107581. [DOI] [PubMed] [Google Scholar]
- 238.Gazerani P. Satellite glial cells in pain research: a targeted viewpoint of potential and future directions. Front Pain Res (Lausanne) 2021;2:646068. doi: 10.3389/fpain.2021.646068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Reed CB, Feltri ML, Wilson ER. Peripheral glia diversity. J Anat. 2021;15:10. doi: 10.1111/joa.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]