Abstract
Stroke has a high incidence and disability rate, and rehabilitation is an effective means to reduce the disability rate of patients. To systematize rehabilitation assessment, which is the foundation for rehabilitation therapy, we summarize the assessment methods commonly used in research and clinical applications, including the various types of stroke rehabilitation scales and their applicability, and related biomedical detection technologies, including surface electromyography (sEMG), motion analysis systems, transcranial magnetic stimulation (TMS), magnetic resonance imaging (MRI), and combinations of different techniques. We also introduce some assessment techniques that are still in the experimental phase, such as the prospective application of artificial intelligence (AI) with optical correlation tomography (OCT) in stroke rehabilitation. This review provides a useful bibliography for the assessment of not only the severity of stroke injury, but also the therapeutic effects of stroke rehabilitation, and establishes a solid base for the future development of stroke rehabilitation skills.
Keywords: Stroke, Rehabilitation assessment, Stroke assessment scales, Detection technology, Artificial intelligence
1. Introduction
Stroke refers to a series of diseases caused by cerebral vascular obstruction or rupture. It has a high incidence and disability rate (Liu et al., 2021), and its severity is related to patient age (Lorenzi et al., 2018), gender, race (Tsao et al., 2022), location, and genetics (Biscetti et al., 2016). Stroke rehabilitation is currently an effective means of reducing the disability rate (Stinear et al., 2020). Rehabilitation is complex and some important aspects need to be considered. Rehabilitation assessments based on the functional level, degree of impairment, and recovery of patients after a stroke form an important scientific basis for determining rehabilitation goals and formulating rehabilitation treatment programs. Stroke-related assessment focuses on the structure of the brain injury and recovery (Boyd et al., 2017), and on neurological function rather than simple examination of structural abnormalities of the nervous system. This is because the complexity of the human nervous system determines that patients with similar clinical manifestations often have different neurological performance at a functional level. Structural abnormalities of the nervous system due to stroke can be used as a basis for disease diagnosis, but are difficult to use for the evaluation of functional impairment and rehabilitation, according to the International Classification of Functioning, Disability and Health (ICF) issued by the World Health Organization (WHO, 2001). This classification is an essential tool to identify and measure efficiency and effectiveness of rehabilitation (Marotta et al., 2020).
Considering the importance and complexity of the brain, the difficulty of detection, and the operability of practice, assessment scales based on behavioral performance assessment are currently used clinically to assess the degree of neurological impairment and the level of rehabilitation of patients (Kasner, 2006). In addition, the rapid development of science and technology has introduced a large number of efficient and convenient biomedical detection methods to the medical field. Therefore, to obtain reliable and valid biomarkers for stroke rehabilitation, several tests including surface electromyography (sEMG) (Goen and Tiwari, 2013), motion analysis systems (Schwarz et al., 2019), transcranial magnetic stimulation (TMS) (Eldaief et al., 2022), and magnetic resonance imaging (MRI) (Imura et al., 2021) have been used in recent years for research and clinical applications in stroke rehabilitation assessment. Stroke rehabilitation biomarkers refer to disease state indicators that can be used to reflect potential molecular or cellular processes in the body that are difficult to measure directly in clinical practice, and can be used to predict a patient's recovery status and response to treatment (Bernhardt et al., 2016).
Artificial intelligence (AI) has developed rapidly in recent years and led to many breakthroughs in the field of medicine (Topol, 2019; Feng et al., 2021). A growing number of research teams are now attempting to integrate machine learning and deep learning into the rehabilitation assessment and treatment decision-making process of stroke diseases (Mouridsen et al., 2020). The related work is in its infancy and there are still many issues to be addressed before it can be routinely applied in the clinic. Previous techniques relied on indirect assessments of a patient's injury and recovery status. In contrast, optical coherence tomography (OCT) allows visualization of changes in blood flow, reperfusion or regeneration of diseased vessels (Ma et al., 2018), as well as damage and recovery of neural tissue (Baran et al., 2015). By combining the image information obtained by OCT with AI, specific aspects of recovery during stroke rehabilitation can be monitored in real time, providing guidance for the rehabilitation process. However, research on OCT in cerebrovascular disease is still in the early experimental stages.
Previous relevant literature reviews have covered a relatively narrow technical approach, making it difficult to provide a more comprehensive introduction to the field. In this review, various testing techniques and methods that can be used for the assessment of neurological impairment and rehabilitation of stroke patients will be reviewed, and the advantages, disadvantages, and development prospects of the different techniques will be elaborated.
2. Assessment scales for stroke
Different rehabilitation centers use various types of stroke assessment scales to assess the neurological status and degree of impairment of patients according to the actual assessable conditions in the immediate state, to obtain a comprehensive understanding of the functional status of patients before and after treatment, and to analyze and evaluate the effectiveness of rehabilitation treatment. There are many different types of scales, including motor function, sensory function, cognitive function, daily performance, and life skill scales. Based on the ICF, some clinical experts have summarized the common scales into three levels: body structure and function, activity function, and participate function.
2.1. Assessment of body structure and function
The assessment of the body structure and function of a stroke patient focuses mainly on the neurological damage to structure and function, and can be broadly divided into two categories: comprehensive assessment and specialized assessment.
2.1.1. Comprehensive assessment
A comprehensive scale is used clinically to provide an initial assessment of overall neurological deficits and to assess the patient's recovery by monitoring changes in these deficits (Table 1). Different scales have different focuses and efficacy. For example, Stroke Impairment Assessment Set (SIAS) is considered suitable for stroke patients during the recovery period, European Stroke Scale (ESS) is used mainly for stroke patients with a damaged middle cerebral artery, National Institute of Health Stroke Scale (NIHSS) is designed for patients with cerebral infarction, and Canadian Neurological Scale (CNS) is used to assess stroke patients with clear consciousness or somnolence. The examination focuses on the clinical behavioral performance of stroke patients, and includes the assessment of a number of important functions of neural control such as consciousness, facial movement (whether facial palsy is present), speech, vision, upper and lower limb, hand or foot movements, and walking gait.
Table 1.
Overview of assessment scales for stroke
| Classification based on ICF | Content of evaluation | Commonly used scales | References |
|---|---|---|---|
| Body structure and function | Overall neurological impairment | National Institute of Health Stroke Scale (NIHSS) | Goldstein et al., 1989 |
| Canadian Neurological Scale (CNS) | Côté et al., 1989 | ||
| European Stroke Scale (ESS) | Hantson et al., 1994 | ||
| Scandinavian Stroke Scale (SSS) | Lindenstrøm et al., 1991 | ||
| Stroke Impairment Assessment Set (SIAS) | Zhou, 2002 | ||
| Chinese Stroke Scale (CSS) | Qi, 2005 | ||
| Sensory function | |||
| External sensory | Semmes-Weinstein monofilament method | Weinstein, 1993 | |
| Two-point discrimination test | Takara, 1971 | ||
| Weinstein enhanced sensory test | Weinstein, 1993 | ||
| Internal sensory | Threshold to Detection of Passive Motion (TTDPM) | Han et al., 2016 | |
| Joint Position Reproduction (JPR) | Steinberg et al., 2019 | ||
| Active Movement Extent Discrimination Assessment (AMEDA) | Waddington et al., 2014 | ||
| Motor function | |||
| Muscle tone and spasticity | Ashworth spasticity scale | Platz et al., 2005 | |
| Joint mobility | Beighton | Schlager et al., 2018 | |
| Contompasis | Schlager et al., 2018 | ||
| Hospital del Mar criteria (HdM) | Bevilacqua et al., 2019 | ||
| Balanced walking | Berg Balance Scale | Blum and Korner-Bitensky, 2008 | |
| Arthroscopic organ | Frenchay Dysarthria Assessment (FDA) | Enderby, 1980 | |
| Injury of general motor function | Brunnstrom Hemiplegia Scale | Shah, 1984 | |
| Fugl-Meyer Scale | Fugl-Meyer et al., 1975 | ||
| (Simplified) Fugl-Meyer Motor Function Scale | Gladstone et al., 2002 | ||
| Stroke Rehabilitation Assessment of Movement (STREAM) | Ahmed et al., 2003 | ||
| Chedoke-McMaster Stroke Assessment | Gowland et al., 1993 | ||
| Lindmark Motor Function Assessment | Lindmark and Hamrin, 1988 | ||
| Rivermead Mobility Index | Ekinci et al., 2021 | ||
| Cognitive function | |||
| Overall cognitive impairment | Mini-Mental State Examination (MMSE) | Folstein et al., 1983 | |
| Stroke Unit Mental Status Examination (SUMSE) | Hajek et al., 1989 | ||
| Neurobehavioral Cognitive Status Examination (NCSE) | Osmon et al., 1992 | ||
| Montreal Cognitive Assessment Scale (MoCA) | Nasreddine et al., 2005 | ||
| Halstead-Reitan Neuropsychological Test Battery (HRNB) | Reitan, 1955 | ||
| Loewenstein Occupational Therapy Cognitive Assessment (LOTCA) | Katz et al., 1989 | ||
| Memory | Auditory Verbal Learning Test (AVLT) | Guo et al., 2007 | |
| Attention | Digit Span Test (DST) | Ostrosky-Solís and Lozano, 2006 | |
| Executive force | Shape Trail Test (STT) | Zhao et al., 2013 | |
| Stroop Color and Word Test (SCWT) | Scarpina and Tagini, 2017 | ||
| Language features | Verbal Fluency Test (VF) | Diesfeldt, 1983 | |
| Boston Naming Test (BNT) | Wang et al., 2019 | ||
| Spatial visual function | Clock Drawing Test (CDT) | Royall et al., 1998 | |
| Activity function | Basic activities of daily living (ADL) | ||
| Simple and practicable | Katz index of independence in ADL (Katz ADL Index) | Katz et al., 1970 | |
| Modified Rankin Scale (MRS) | Sulter et al., 1999 | ||
| Athletic activities | Modified Barthel Index (MBI) | Shah et al., 1989 | |
| Physical Self-Maintenance Scale (PSMS) | Lawton and Brody, 1969 | ||
| Cognition and speech | Functional Independence Measure (FIM) | Linacre et al., 1994 | |
| Participation function | Instrumental ability in ADL | Katz index of instrumental ADL based on ADL index (Katz IADL Index) | Katz, 1983 |
| Functional Autonomy Measurement System (SMAF) | Hebert et al., 1988 | ||
| Assessment of Motor and Process Skills (AMPS) | Park et al., 1994 | ||
| Frenchay Activity Index (FAI) | Holbrook and Skilbeck, 1983 | ||
| Functional Activity Questionnaire (FAQ) | Pfeffer et al., 1982 | ||
| Quality of life | |||
| Psychological assessment | Ferrans & Powers Quality of Life Index (QLI-Generic Version) | Ferrans and Powers, 1985 | |
| 36-Item Short-Form Survey (SF-36) | Ware and Sherbourne, 1992 | ||
| Observation and questionnaire | Ferrans & Powers Quality of Life Index (QLI-Stroke Version) | Jaracz and Kozubski, 2003 | |
| Stroke-Specific Quality of Life Scale (SS-QOL) | Williams et al., 1999 |
ICF: International Classification of Functioning, Disability and Health.
2.1.2. Specialized assessments
Specialized assessments provide a more detailed and comprehensive assessment of specific neurological functions, of which sensory, motor, and cognitive functions are the most important.
Depending on the source of the stimulus, sensory functions can be divided into two categories: external and internal. External sensory function involves mainly the perception of stimuli, and includes the familiar visual, auditory, olfactory, taste, and skin senses. The
skin sensation, also called shallow sensation, is often assessed using the Semmes-Weinstein monofilament method (Table 1). Internal sensory function refers to the contraction of the body's muscles or internal organ changes perceived by the deep receptors of the body, including kinesthesia, balance, position, and body sensation, and so is also called deep sensation. The methods for assessing deep sensation include the Threshold to Detection of Passive Motion (TTDPM) (Table 1).
Conventional motor function assessment items include muscle strength, muscle tone, spasticity, and joint mobility, and different sub-items have their own corresponding assessment scales. However, sensation is a prerequisite for movement, and even if there is no significant decrease in muscle strength, sensory dysfunction can have a significant impact on the patient's motor function. Motor function has a significant impact on the patient's quality of life later in life and is often used as an important indicator to assess whether the patient benefits from the intervention (Kim and Winstein, 2017). These functions also have their own assessment scales (Table 1).
Cognitive function is another important specific assessment module, and deficits are assessed mostly using neuropsychological scales used in the assessment of cognitive impairment. The overall cognitive status of the patient can be assessed on the basis of scales such as the Mini-Mental State Examination (MMSE) (Folstein et al., 1983). Similarly, there are corresponding scales for the assessment of specific cognitive functions such as memory, attention, execution, language, and spatial vision (Table 1).
2.2. Assessment of activity function
From an individual perspective, activity refers to the integrated mobilization of the body's functions to accomplish an action or task, and the types, forms, and content of activities vary significantly. However, activities of daily living (ADL) are basic activities that are necessary for people to maintain their daily lives. They are common across ethnic and cultural groups, and can be used for quantitative assessment. The ability to accomplish ADL better reflects the overall activity level of the individual, and is considered the core basis for the evaluation of rehabilitation outcomes (Veerbeek et al., 2011). According to the content of activities, ADL can be roughly divided into basic ADL (BADL) and instrumental ADL (IADL). BADL includes self-care and movement. Specific activities include eating, dressing, using the toilet, getting in and out of bed, taking a bath, and using transportation. The IADL includes faculty work, communication, and social cognitive aspects, with major activities such as cooking, cleaning, shopping, watching TV, making phone calls, financial management, and socializing.
The assessment of active function is based on body structure and function, and the main assessment is the ADL ability of patients. Currently, BADL-related rating scales commonly used clinically include the Modified Barthel Index (MBI) (Table 1), the Katz ADL Index, and Modified Rankin Scale (MRS). These are simple and easy to implement and widely used, but lack items to detect subtle changes in function (Hartigan, 2007). MBI and Physical Self-Maintenance Scale (PSMS) are more specific, but examine mainly the motor aspects of activity content without addressing the level of cognitive function. Functional Independence Measure (FIM) is more sensitive than MBI because it adds cognitive and verbal content based on sports. IADL is more advanced than BADL, the tasks required to complete are more complex, and the degree of social participation is stronger, so it is usually used to evaluate the next level-participation function.
2.3. Assessment of participation function
Participation function is the highest level, reflecting the patient's "participation and participation limitations" in family life, interpersonal communication, study, work, and social life. The assessment of participation functions includes mainly two aspects: IADL ability and quality of life. The assessment period is usually during the follow-up period after rehabilitation treatment, so the assessment involves many non-medical factors, making it difficult and limited in accuracy.
The common IADL scales are based mainly on the BADL scale and are more comprehensive and three-dimensional. For example, Katz (1983) has developed the Katz index of instrumental ADL based on the ADL index (Table 1). Quality of life is an important aspect of this level of assessment, defined as satisfaction with personally important areas of life (Ferrans and Powers, 1992). Therefore, assessment results are strongly influenced by patients' subjectivity.
2.4. Assessment scales: summary and prospects
After decades of development and revision, stroke-related rehabilitation assessment scales have been greatly enhanced, covering a wide range of neurological domains and various stages of rehabilitation treatment. However, the shortcomings of scale-based stroke rehabilitation assessments are obvious. Firstly, the scales are mostly manually determined and completed, and therefore easily influenced by subjective factors of subjects and examiners. The assessments of activity and participation levels are also influenced by non-medical factors, which make it difficult to ensure the consistency, stability, and objectivity of the results. Secondly, a single scale or a single set of scales cannot meet all clinical needs, while the combined use of multiple scales has not resulted in a recognized and perfect system. The operation process requires high professionalism and tests the experience level of the examiners. The assessment items inevitably cross and repeat, the assessment process is time-consuming and laborious, and the assessment results are difficult to quantify, unify, and standardize.
With the development of computer and Internet technology, rehabilitation assessment scales are increasingly becoming electronic. Computer-based or mobile scale software can greatly reduce the time, material, and labor costs of assessment, expand the use of assessment scenarios and time space, and to some extent standardize the assessment process. However, the use of software programs optimizes only the material carrier of the scale and does not change the disadvantages of excessive human involvement, so objectivity or accuracy cannot be effectively improved.
More importantly, the data of quantitative scales, whether in paper or electronic form, originate mainly from observations of patients or simple task activities, so the information obtained is relatively macroscopic and extrinsic. With the development of biotechnology and medical instruments, current biomedical testing technology obtains physiological information from more dimensions and more objectively from the patient's body or specific body parts. Stroke rehabilitation assessment studies based on related technologies have emerged with the aim of obtaining more accurate and efficient neurobiological markers.
3. Biomedical detection technologies
The main biomedical testing techniques used for rehabilitation assessment of stroke patients are sEMG, motion analysis systems, TMS, and MRI. This section focuses on these four main tools and mentions other techniques that have been used (Table 2).
Table 2.
Overview of biomedical technologies for rehabilitation assessments of stroke
| Measurement technique | Advantages | Disadvantages | Application fields | References |
|---|---|---|---|---|
| sEMG | Non-invasive, safe, convenient, and objective; detects the surface muscle activity of various parts of the body | Susceptible to external and nearby muscle interference; limited to the superficial major muscle group; unable to get signals from deep muscles | Upper limb | Kallenberg and Hermens, 2009 |
| Hand | Hu et al., 2015; Vinstrup et al., 2018 | |||
| Lower limb | Rozanski et al., 2020 | |||
| Back | Li et al., 2014 | |||
| Abdomen | Yoon and You, 2017 | |||
| Swallowing | Park et al., 2019 | |||
| External anal sphincter | Dias et al., 2018, 2019 | |||
| Motion analysis systems | Non-invasive, safe, and objective; a variety of sensors to obtain upper and lower limb motion parameters; be used for real-time rehabilitation training | High requirements on-site and for equipment; multi-parameter fusion analysis is complicated to practice | Upper limb movement | Bosecker et al., 2010; van Dokkum et al., 2014; Schwarz et al., 2019 |
| Lower limb movement | Kawamura et al., 2007; Rosa et al., 2014 | |||
| Feedback of rehabilitation training effect | Caliandro et al., 2020 | |||
| Assisted robot adaptive correction | Androwis et al., 2018 | |||
| TMS | Painless and non-invasive; independent control of neural activity in specific brain regions; neuroelectrophysiological examination in central nervous system | The interpretability of the results is poor; the effect of functional recombination of brain regions cannot be eliminated | Motor | McDonnell and Stinear, 2017 |
| Somatosensory | Hwang et al., 2016 | |||
| Swallowing | Gallas et al., 2007; Barritt and Smithard, 2009 | |||
| Sensory function represented by vision | Sack and Linden, 2003 | |||
| MRI | ||||
| Diffusion MRI | Non-invasive, safe, and objective; reflects the integrity of subcortical white matter fiber bundles | No neurological function assessment is performed; part of the mode scanning time is too long; in the field of rehabilitation, it is mainly used for outcome prediction, but cannot be accurately assessed | DWI: prediction of ischemic outcome | Yoo et al., 2010 |
| DTI: significant correlation between related parameters and motor function scores | Parsons et al., 2010 | |||
| DTT: 3D visualization of cortical fiber bundle injury | Lindenberg et al., 2010 | |||
| DKI QSI: reflects the change in microstructure of tissue | Yamada et al., 2013; Rudrapatna et al., 2014 | |||
| fMRI | Non-invasive, safe, and objective; obtains the functional activity of the whole brain | Image accuracy is limited; long scan and task time; the results are often unconvincing | rs-MRI: motor and cognitive functional brain area assessment; longitudinal analysis reflects dynamic changes in patients' motor function | Park et al., 2011; Golestani et al., 2013; Dacosta-Aguayo et al., 2014; Chen et al., 2019 |
| ts-MRI: motor, sensory, and cognitive function assessment, and neurofeedback for efficacy assessment | Calvert et al., 2000; Mintzopoulos et al., 2009; Kim and Winstein, 2017;Wang et al., 2018 | |||
| Combination | ||||
| TMS+fMRI, TMS+EEG, TMS+MEG, TMS+MRS, TMS+NIRS, TMS+PET | Narrows the area of the brain to be examined to make it more accurate; reduces the impact of functional restructuring; improves the accuracy of functional assessment | EEG and NIRS have low resolution and accuracy; MEG and PET equipments have low penetration rates and are expensive; the interpretability of the results needs to be improved | Evaluation of motor and cognitive function and the mechanism of brain recovery and restructuring | Sack and Linden, 2003; Hamzei et al., 2006; Auriat et al., 2015; Kim and Winstein, 2017; Pellicciari et al., 2018 |
| fMRI+EEG | Not only the activity and functional connection of brain regions, but also the activation sequence and the subordinate relationship between brain regions can be understood | Neural connectivity network analysis and functional rehabilitation assessment | Lioi et al., 2020 | |
| TMS+structural imaging | Neurological function and structure tests can predict the level of motor recovery in stroke patients | Assessment of motor functions and neural pathway integrity | Stinear et al., 2012; Auriat et al., 2015 | |
| EMG+NIRS | The anterior nerve activity and terminal muscle activity of the nerve conduction pathway are detected at the same time | Co-detection of muscle electrophysiological signals and brain hemodynamics in motor function | Scano et al., 2019; Caliandro et al., 2020 |
DWI: diffusion-weighted imaging; DTI: diffusion-tensor imaging; DTT: diffusion tensor tractography; DKI: diffuse kurtosis imaging; QSI: q-space imaging; rs-MRI: rest-state MRI; ts-MRI: task-state MRI; EEG: electroencephalogram; MEG: magnetoencephalogram; MRI: magnetic resonance imaging; fMRI: functional MRI; MRS: magnetic resonance spectroscopy; NIRS: near infrared spectroscopy; PET: positron emission tomography; sEMG: surface electromyography; TMS: transcranial magnetic stimulation.
3.1. Surface electromyography
sEMG is a non-invasive technique for assessing neuromuscular functional status during exercise. Electrophysiological time series of signals generated during contraction of the muscle under test are acquired from the skin surface through the receiving electrodes of the sEMG. It has the advantages of being non-invasive, safe, convenient, and objective (Zheng et al., 2007). The assessment of neuromuscular function impairment and its rehabilitation efficacy in stroke patients is currently an important issue in basic research and clinical application of rehabilitation medicine. The sEMG has gradually gained attention in this field in recent years because of these technical advantages.
The sEMG detects superficial muscle groups in patients. It is often integrated into a motion analysis system to evaluate the muscle activity of the upper and lower limbs during movement (Li et al., 2015). The small and flexible signal receiver end of the sEMG device has shown unique value in some specific body sites. For example, neuromuscular dysfunction of the external anal sphincter will lead to fecal incontinence, which is difficult to identify by conventional detection means due to the specific site of onset. The sEMG signals of the external anal sphincter were acquired using a specially designed high-density sEMG collector to clarify the area of onset in patients (Dias et al., 2018) to improve the accuracy of drug injection, and for the study of the pathogenesis and causative factors of this disease (Dias et al., 2019).
However, the limitations of this technique are that the signal acquisition is susceptible to interference, the acquisition area is limited to large muscle groups on the surface of the body, and the signal of deeper muscles or specific muscles cannot be obtained. A needle electrode EMG inserted directly into the muscle through the skin can alleviate these problems to some extent, but as an invasive detection method, its clinical use requires more caution.
3.2. Motion analysis systems
Traditional motion analysis systems consist of high-definition video cameras and positioning markers used to record somatic movements and calculate motion trajectories to obtain relevant kinematic parameters for quantitative assessment of a subject's motor functions and movement characteristics. Such parameters are often used in the field of rehabilitation medicine and sports medicine, where the analysis of lower limb walking or running movements is called gait analysis.
With the introduction of different sensors and signal acquisition devices, the functions of motion analysis systems are continuously expanded, optimized, and improved. Taking the gait analysis system as an example, the relevant system now contains a three-dimensional (3D) dynamic capture system, 3D force measurement platform and plantar pressure measurement instrument, sEMG, accelerometer, gyroscope, and goniometer. The whole system can accurately capture the 3D dynamic coordinates of the human body's marker points during the travel process. Supporting analysis software is used to reconstruct and analyze the 3D model based on the collected multiple data to obtain the relevant gait parameters. Gait parameters include mainly kinematic, kinetic, electromyographic activity, and energy metabolism parameters (Tao et al., 2012). An upper limb motion analysis system has similar sensors and acquisition capabilities (Carnevale et al., 2019).
Motion analysis systems are widely used in the field of stroke rehabilitation assessment. The idea of most studies is mainly to assign rehabilitation patients to complete targeted tasks, obtain motor parameters by analyzing data recorded during exercise, analyze correlations between candidate parameters and motor function scale scores, and select significant characteristic parameters for rehabilitation prediction of motor function. The correlation test task is also a means of motor function rehabilitation. The real-time objective result feedback from the test system can improve the training effect during the rehabilitation training of patients (Caliandro et al., 2020). For some patients who have lost independent limb motor function due to a stroke, the system can transmit the acquired patient motor parameters to an assistive robot for adaptive training and correction of the patient's limbs (Androwis et al., 2018).
3.3. Transcranial magnetic stimulation
TMS is based on the principle of Faraday electromagnetic induction, in which pulsed magnetic field signals are applied to the cerebral cortex through the scalp and skull without attenuation. Induced currents are generated in the tissues of the area of action to stimulate neuronal excitation in the area. This in turn affects the neuroelectrical activity and intracerebral metabolism, triggering a series of physiological and biochemical responses (Eldaief et al., 2022). This technique has the advantage of being painless and non-invasive, and is now widely used in the functional assessment and adjunctive treatment of psychiatric disorders and various neurological disorders including stroke (Johansson, 2011).
Neurophysiological examinations based on TMS, such as motor evoked potential (MEP), somatosensory evoked potential (SEP), and silent period (SP) of cortical magnetic stimulation, are used in the rehabilitation assessment of neurological functions. The neurophysiological examinations can be used to analyze damage to the central neuromotor and sensory conduction pathways in patients after a stroke(Wang and Wang, 2016). Compared to technologies that passively receive neural activity signals, TMS has the advantage of being able to manipulate neural activity in specific areas of the brain on its own, thus providing a clearer picture of the relationship between brain activity and behavior. However, the biggest problem with this technique is that the examination is often performed with the default assumption that specific brain regions always correspond to the motor-sensory function of the specified somatic area before, during, and after the disease and during the recovery phase. However, neurons in the damaged and adjacent areas of the brain will undergo reorganization during the recovery phase to compensate for the loss of the original function of the necrotic area. Repeated examination of neural activity in the designated brain area using TMS technology alone may not be able to determine the recovery of neural function in a timely and effective manner, and it is not practical to blindly try various candidates for functional reorganization of brain areas (Sack and Linden, 2003).
3.4. Magnetic resonance imaging
MRI uses the principle of nuclear magnetic resonance to obtain structural information inside the human body by applying a gradient magnetic field. Energy attenuation varies according to the different structures encountered inside a material. Over the decades, MRI has evolved into various modalities such as diffusion MRI and functional MRI (fMRI). Its application in stroke has also extended from disease diagnosis to rehabilitation assessment (Bernhardt et al., 2016). The associated biological markers have been extended from simple parameters of the lesion volume area to more complex imaging-based structural and functional indices (Kim and Winstein, 2017).
Research work on diffusion MRI in stroke rehabilitation has been emerging (Jang, 2011; Ma et al., 2014). Diffusion MRI techniques with different modalities have different characteristics, and all have corresponding applications and advances in neurological rehabilitation studies of stroke. Diffusion-weighted imaging (DWI) has higher sensitivity and specificity than other examinations, and is an important method for diagnosing acute cerebral ischemia in clinical practice(Kim et al., 2014). High signal area volume of DWI lesion and quantitative index of apparent dispersion coefficient (ADC) analysis also contribute to the prediction of ischemic infarct area outcome (Yoo et al., 2010). Diffusion-tensor imaging (DTI) provides a more precise interpretation of the anisotropy of voxel water molecule dispersion. The parameters correlate significantly with motor function scores after a stroke, but the scan time is too long (Parsons et al., 2010). Diffusion tensor tractography (DTT) can reflect the damage to cortical fiber tracts more visually and accurately (Lindenberg et al., 2010). Diffuse kurtosis imaging (DKI) and q-space imaging (QSI) are based on non-Gaussian diffusion imaging. They take into account the diffusion phenomenon of the non-Gaussian distribution of water molecules due to internal heterogeneity of brain tissues, and reflect the microstructural changes of tissues more realistically.
fMRI is used mainly for analyzing functional activity and functional connectivity of the human brain nervous system. According to the way it is used, MRI can be divided into rest-state MRI (rs-MRI) and task-state MRI (ts-MRI). rs-MRI usually reflects the spontaneous neural activity of the brain while remaining awake. Studies related to stroke have shown that in motor (Park et al., 2011; Chen et al., 2019) and cognitive (Dacosta-Aguayo et al., 2014) functional brain regions, rs-MRI signals significantly correlate with corresponding clinical representations. Longitudinal functional connectivity analysis can reflect changes in patients' motor function with disease progression, suggesting the potential of this method for rehabilitation assessment and condition detection (Golestani et al., 2013). Compared to other neurological methods, ts-MRI can assess not only conventional motor and sensory functions by combining different task modalities (Kim and Winstein, 2017), but also some cognitive functions. The relevant signals can be used as neurofeedback to assess the methods and thus optimize the adjustment of rehabilitation programs (Wang et al., 2018). However, the image accuracy of fMRI is limited by the balance between temporal and spatial resolution at acquisition, and it is difficult to construct a convincing causal link between activated brain regions and corresponding neurological functions identified by fMRI.
3.5. Technology portfolio and other approaches
Each of these techniques has its own unique strengths and weaknesses, so researchers have skillfully combined the different techniques to maximize the accuracy of a patient's neurological assessment.
TMS provides independent and controlled stimulation of neuronal excitation in specific brain regions, whereas fMRI provides relatively timely and comprehensive access to whole-brain neural activity in the brain during external stimulation or when the patient is performing a specific task. Therefore, both techniques are often used in combination to improve the assessment of neurological recovery of the brain and to provide insight into functional reorganization after a stroke (Kim and Winstein, 2017). In addition to fMRI, other detection techniques to obtain neural activity in the brain include electroencephalogram (EEG), magnetoencephalogram (MEG), magnetic resonance spectroscopy (MRS), near-infrared spectroscopy (NIRS), and positron emission tomography (PET). All of these techniques have their own advantages and have been used either alone or in combination with TMS for rehabilitation assessment studies of stroke(Pellicciari et al., 2018).
Two different functional imaging techniques can also be combined so that not only the activity and functional connectivity of brain areas in patients during the rehabilitation phase can be studied, but also the order of activation and subordination of brain areas based on EEG in the temporal dimension. This provides a new perspective for the design and evaluation of future rehabilitation programs (Lioi et al., 2020).
TMS can be combined not only with functional imaging techniques, but also with structural imaging techniques such as DWI to enhance the prediction of motor rehabilitation levels in stroke patients (Stinear et al., 2012; Auriat et al., 2015). Researchers have also combined electromyography, which detects muscle activity at the end of nerve conduction pathways, and NIRS imaging, which detects nerve activity at the front end. This is used to assess muscle characteristics from a joint electrical and hemodynamic perspective in clinical practice. Related studies have also shown potential for future development (de Carlo et al., 2015; Caliandro et al., 2020).
3.6. Summary and prospects
The biomedical testing technologies available for stroke rehabilitation assessment have different strengths and weaknesses, and although the combination of technologies can compensate for their limitations, little research has been translated into routine clinical practice. Because of the large number of neurological functions to be assessed and the available technical options, it is a major challenge to use the appropriate testing technique for a specific function (Bernhardt et al., 2016). Also, the lack of in-depth, large-scale clinical studies makes it difficult to obtain uniform reference evaluation criteria (Kim and Winstein, 2017; Schwarz et al., 2019).
4. New technologies
In addition to the rehabilitation scales and testing techniques already used in clinical research and practice, there are many other techniques that are gradually being applied to the field of stroke rehabilitation assessment, although they are currently in their experimental infancy. The following are two important technologies that hold great promise.
4.1. Artificial intelligence
In recent years, with the development of AI centered on machine learning and deep learning, advances have been made in various aspects of biomedical sciences (Topol, 2019). Some research teams are trying to incorporate AI into the rehabilitation assessment process of stroke diseases. The current applications of AI in this field can be broadly classified into two main categories.
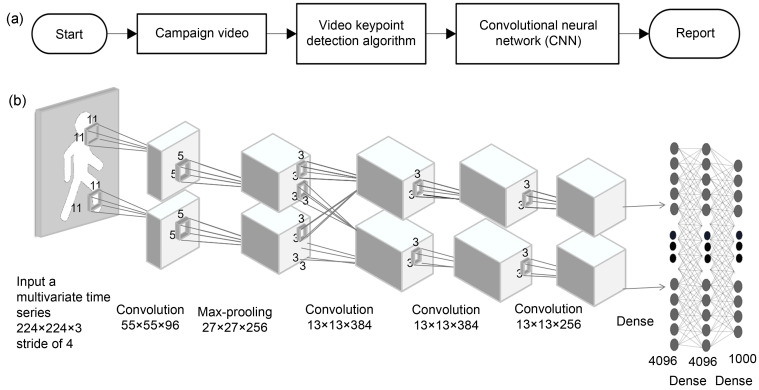
One is the "empowerment" of existing testing methods by incorporating algorithms into the analysis process to improve the accuracy of assessment results. Taking gait analysis as an example, the traditional analysis process is to extract gait parameters based on the original data collected, and select the characteristic parameters with significant differences through statistical tests to assess the patient's walking function (Fig. 1). In contrast, machine learning methods replace the traditional statistical analysis using all candidate gait parameters for feature selection and model construction, thus enabling prediction to discriminate different stages of walking, and identify abnormal gait and severity (Joshi et al., 2013; Wei et al., 2020). Deep learning goes further to replace the original link of gait parameter feature extraction by automatically extracting deep-level features from the raw data species through deep convolutional networks for subsequent classification prediction (Nazmi et al., 2019; Kidziński et al., 2020). In addition, since deep learning networks emerged in the field of machine vision, the main medical application at present is the migration of relevant algorithms to medical images. In stroke rehabilitation assessment, it is possible to predict the injury and rehabilitation level of patients in the chronic phase based on MRI in the acute phase (Mouridsen et al., 2020).
Fig. 1. Running logic of deep learning in gait analysis. (a) Overall flow chart of deep learning applied to gait analysis; (b) Schematic diagram of convolutional neural network architecture.
The second new technological approach is to develop new functions "from scratch" to replace the old methods. In the past, human assessment of the facial muscle and speech function of stroke patients was based mainly on scales, which inevitably introduced many subjective factors causing inaccurate and unstable results. The rise of AI inspired our researchers to analyze facial images and speech information of stroke patients directly through face recognition (Adjabi et al., 2020) and speech recognition (Gupta et al., 2018). These technologies are used to determine the status of facial muscle and speech impairment of patients, and to assess patients' rehabilitation based on an intelligent decision-aid system (Lee et al., 2020). This idea skips the original manual scale assessment stage and replaces some of the assessment scale functions, thus effectively reducing the interference of human factors and greatly improving the objectivity and accuracy of the assessment results.
These methods require a large amount of patient data and physician labeling information, as well as the establishment of an expert knowledge base for the decision-making system, from initial experimental studies to clinical applications. The whole process requires multi-disciplinary cooperation, and the workload is huge.
4.2. Optical coherence tomography
The new optical imaging represented by OCT is considered another important medical imaging technology after MRI, with its powerful non-invasive 3D imaging modality with high temporal and spatial resolution (Aumann et al., 2019). One of the OCT techniques used to extract and visualize information about the structure and distribution of the microvascular network is called OCT angiography (OCTA) (de Carlo et al., 2015). The optical attenuation coefficient (OAC) map of deep biological tissues can also be obtained from raw OCT imaging according to the Lambert-Beer law (Vermeer et al., 2014).
OCT imaging in cerebrovascular disease is currently in its initial experimental stage (Ma et al., 2018; Choi et al., 2019; Yang et al., 2019). Its application in the field of stroke is due mainly to its unique advantages: it is one of the few techniques capable of directly observing diseased vessels and their side branches over time in a living animal model, while being able to capture blood perfusion (Choi et al., 2014) and blood flow velocity (Wang and An, 2009), as well as the optical properties of perfused tissue (Baran et al., 2015). It also enables clear observation, for example, of specially stained subcortical nerve cells (Srinivasan et al., 2012). The image data obtained by OCT can be analyzed by various algorithms, such as those allowing segmentation of blood vessels, to calculate the cerebral vascular perfusion density (Ma et al., 2018). This can be used as an effective indicator to evaluate post-ischemic injury. OCT images also reveal the relationship between neural activity and blood flow (Liu et al., 2016).
In the foreseeable future, sophisticated OCT analysis based on animal models will help not only to understand the mechanisms of recovery after stroke, but also to combine the image data obtained with AI to facilitate a comprehensive assessment of the stroke rehabilitation process, providing many references and guidelines for clinicians to evaluate rehabilitation (Oh et al., 2022). However, this technology has been used in the field of stroke for only a few years and there are still many issues that need to be addressed if it is to be applied in clinical practice. OCT and its derived images are rich in biological information, but the current analysis of such images is relatively simple and superficial, unable to identify distinguish blood vessel types, and requires the addition of more intelligent algorithms. In addition, many researchers are still improving OCT image systems from cells, tissues, and organs to mice, rats, primates, and humans (Wang et al., 2017).
5. Conclusions
Stroke rehabilitation assessment is an important topic in the field of rehabilitation medicine. Although many well-established assessment scales have been widely used in clinical practice, their consistency, stability, and objectivity are difficult to guarantee. Various biomedical testing techniques based on different principles can collect more objective information on human neurological functions and activities, but the lack of large and effective prospective clinical studies and unified and complete guidelines for their use makes it difficult to popularize the application of these techniques in clinical practice. There are two other trends in the development of assessment methods: one is to continue to develop more testing technologies to better understand the recovery mechanism of the brain after stroke and to conduct more comprehensive rehabilitation assessment; the other is to gradually replace the old manual discrimination methods by algorithms based on AI and other computer technologies to make the assessment results more objective, accurate, and standardized. However, these technologies, which are in their infancy, still have a long way to go before they can be used in clinical research and therapy.
Acknowledgments
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY21H170002), the Graduate Scientific Research Foundation of Hangzhou Dianzi University (No. CXJJ2020047), and the National Natural Science Foundation of China (No. 31401008).
Author contributions
Kezhou LIU developed a framework for research directions. Mengjie YIN refined the complete structure of the article and completed the final manuscript. Zhengting CAI completed the preliminary research and participated in the original draft preparation. All authors have read and agreed the final manuscript.
Compliance with ethics guidelines
Kezhou LIU, Mengjie YIN, and Zhengting CAI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Adjabi I, Ouahabi A, Benzaoui A, et al. , 2020. Past, present, and future of face recognition: a review. Electronics, 9(8): 1188. 10.3390/electronics9081188 [DOI] [Google Scholar]
- Ahmed S, Mayo NE, Higgins J, et al. , 2003. The Stroke Rehabilitation Assessment of Movement (STREAM): a comparison with other measures used to evaluate effects of stroke and rehabilitation. Phys Ther, 83(7): 617-630. 10.1093/ptj/83.7.617 [DOI] [PubMed] [Google Scholar]
- Androwis GJ, Pilkar R, Ramanujam A, et al. , 2018. Electromyography assessment during gait in a robotic exoskeleton for acute stroke. Front Neurol, 9: 630. 10.3389/fneur.2018.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumann S, Donner S, Fischer J, et al. , 2019. Optical coherence tomography (OCT): principle and technical realization. In: Bille JF (Ed. ), High Resolution Imaging in Microscopy and Ophthalmology. Springer, Cham, p.59-85. 10.1007/978-3-030-16638-0_3 [DOI] [PubMed] [Google Scholar]
- Auriat AM, Neva JL, Peters S, et al. , 2015. A review of transcranial magnetic stimulation and multimodal neuroimaging to characterize post-stroke neuroplasticity. Front Neurol, 6: 226. 10.3389/fneur.2015.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran U, Li YD, Wang RK, 2015. In vivo tissue injury mapping using optical coherence tomography based methods. Appl Opt, 54(21): 6448-6453. 10.1364/AO.54.006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barritt AW, Smithard DG, 2009. Role of cerebral cortex plasticity in the recovery of swallowing function following dysphagic stroke. Dysphagia, 24(1): 83-90. 10.1007/s00455-008-9162-3 [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Borschmann K, Boyd L, et al. , 2016. Moving rehabilitation research forward: developing consensus statements for rehabilitation and recovery research. Int J Stroke, 11(4): 454-458. 10.1177/1747493016643851 [DOI] [PubMed] [Google Scholar]
- Bevilacqua DE, Maillard S, Ferrari J, 2019. Measuring joint hypermobility using the Hospital Del Mar criteria—a reliability analysis using secondary data analysis. Arch Rheum Arthritis Res, 1(1): 1-6. 10.33552/arar.2019.01.000502 [DOI] [Google Scholar]
- Biscetti F, Giovannini S, Straface G, et al. , 2016. RANK/RANKL/OPG pathway: genetic association with history of ischemic stroke in Italian population. Eur Rev Med Pharmacol Sci, 20(21): 4574-4580. [PubMed] [Google Scholar]
- Blum L, Korner-Bitensky N, 2008. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther, 88(5): 559-566. 10.2522/ptj.20070205 [DOI] [PubMed] [Google Scholar]
- Bosecker C, Dipietro L, Volpe B, et al. , 2010. Kinematic robot-based evaluation scales and clinical counterparts to measure upper limb motor performance in patients with chronic stroke. Neurorehabil Neural Repair, 24(1): 62-69. 10.1177/1545968309343214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Hayward KS, Ward NS, et al. , 2017. Biomarkers of stroke recovery: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair, 31(10-11): 864-876. 10.1177/1545968317732680 [DOI] [PubMed] [Google Scholar]
- Caliandro P, Molteni F, Simbolotti C, et al. , 2020. Exoskeleton-assisted gait in chronic stroke: an EMG and functional near-infrared spectroscopy study of muscle activation patterns and prefrontal cortex activity. Clin Neurophysiol, 131(8): 1775-1781. 10.1016/j.clinph.2020.04.158 [DOI] [PubMed] [Google Scholar]
- Calvert GA, Brammer MJ, Morris RG, et al. , 2000. Using fMRI to study recovery from acquired dysphasia. Brain Lang, 71(3): 391-399. 10.1006/brln.1999.2272 [DOI] [PubMed] [Google Scholar]
- Carnevale A, Longo UG, Schena E, et al. , 2019. Wearable systems for shoulder kinematics assessment: a systematic review. BMC Musculoskelet Disord, 20: 546. 10.1186/s12891-019-2930-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sun DL, Shi YH, et al. , 2019. Dynamic alterations in spontaneous neural activity in multiple brain networks in subacute stroke patients: a resting-state fMRI study. Front Neurosci, 12: 994. 10.3389/fnins.2018.00994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WJ, Reif R, Yousefi S, et al. , 2014. Improved microcirculation imaging of human skin in vivo using optical microangiography with a correlation mapping mask. J Biomed Opt, 19(3): 036010. 10.1117/1.jbo.19.3.036010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WJ, Li YD, Wang RK, 2019. Monitoring acute stroke progression: multi-parametric OCT imaging of cortical perfusion, flow, and tissue scattering in a mouse model of permanent focal ischemia. IEEE Trans Med Imaging, 38(6): 1427-1437. 10.1109/TMI.2019.2895779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté R, Battista RN, Wolfson C, et al. , 1989. The Canadian Neurological Scale validation and reliability assessment. Neurology, 39(5): 638-643. 10.1212/wnl.39.5.638 [DOI] [PubMed] [Google Scholar]
- Dacosta-Aguayo R, Graña M, Savio A, et al. , 2014. Prognostic value of changes in resting-state functional connectivity patterns in cognitive recovery after stroke: a 3T fMRI pilot study. Hum Brain Mapp, 35(8): 3819-3831. 10.1002/hbm.22439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carlo TE, Romano A, Waheed NK, et al. , 2015. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous, 1: 5. 10.1186/s40942-015-0005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias N, Li XH, Zhang C, et al. , 2018. Innervation asymmetry of the external anal sphincter in aging characterized from high-density intra-rectal surface EMG recordings. Neurourol Urodyn, 37(8): 2544-2550. 10.1002/nau.23809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias N, Zhang C, Li XH, et al. , 2019. Neural control properties of the external anal sphincter in young and elderly women. Neurourol Urodyn, 38(7): 1828-1833. 10.1002/nau.24108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesfeldt HFA, 1983. Verbal fluency tests and their significance for psychogeriatric practice. Tijdschr Gerontol Geriatr, 14: 49-59. [PubMed] [Google Scholar]
- Ekinci Y, Yaşaroğlu OF, Düger T, 2021. Content comparison of four commonly used amputee mobility assessment scales in the literature by linking to the International Classification of Functioning, Disability, and Health. Prosthet Orthot Int, 45(6): 544-552. 10.1097/PXR.0000000000000052 [DOI] [PubMed] [Google Scholar]
- Eldaief MC, Dickerson BC, Camprodon JA, 2022. Transcranial magnetic stimulation for the neurological patient: scientific principles and applications. Semin Neurol, 42(2): 149-157. 10.1055/s-0041-1742265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderby P, 1980. Frenchay Dysarthria Assessment. Int J Lang Commun Disord, 15(3): 165-173. 10.3109/13682828009112541 [DOI] [Google Scholar]
- Feng L, Zhou D, Luo C, et al. , 2021. Clinically applicable artificial intelligence algorithm for the diagnosis, evaluation, and monitoring of acute retinal necrosis. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(6): 504-511. 10.1631/jzus.B2000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans CE, Powers MJ, 1985. Quality of life index: development and psychometric properties. Adv Nurs Sci, 8(1): 15-24. 10.1097/00012272-198510000-00005 [DOI] [PubMed] [Google Scholar]
- Ferrans CE, Powers MJ, 1992. Psychometric assessment of the quality of life index. Res Nurs Health, 15(1): 29-38. 10.1002/nur.4770150106 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, Helzer JE, 1983. The Mini-Mental State Examination. Arch Gen Psychiatry, 40(7): 812. 10.1001/archpsyc.1983.01790060110016 [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jääskö L, Leyman I, et al. , 1975. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med, 7(1): 13-31. [PubMed] [Google Scholar]
- Gallas S, Moirot P, Debono G, et al. , 2007. Mylohyoid motor-evoked potentials relate to swallowing function after chronic stroke dysphagia. Neurogastroenterol Motil, 19(6): 453-458. 10.1111/j.1365-2982.2006.00892.x [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, Black SE, 2002. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair, 16(3): 232-240. 10.1177/154596802401105171 [DOI] [PubMed] [Google Scholar]
- Goen A, Tiwari DC, 2013. Review of surface electromyogram signals: its analysis and applications. World Acad Sci Eng Technol Int J Electr Comput Eng, 7(11): 936-943. 10.5281/zenodo.1089094 [DOI] [Google Scholar]
- Goldstein LB, Bertels C, Davis JN, 1989. Interrater reliability of the NIH stroke scale. Arch Neurol, 46(6): 660-662. 10.1001/archneur.1989.00520420080026 [DOI] [PubMed] [Google Scholar]
- Golestani AM, Tymchuk S, Demchuk A, et al. , 2013. Longitudinal evaluation of resting-state fMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair, 27(2): 153-163. 10.1177/1545968312457827 [DOI] [PubMed] [Google Scholar]
- Gowland C, Stratford P, Ward M, et al. , 1993. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke, 24(1): 58-63. 10.1161/01.str.24.1.58 [DOI] [PubMed] [Google Scholar]
- Guo QH, Sun YM, Yu PM, et al. , 2007. Norm of auditory verbal learning test in the normal aged in China community. Chin J Clin Psychol, 15(2): 132-134, 141 (in Chinese). 10.3969/j.issn.1005-3611.2007.02.007 [DOI] [Google Scholar]
- Gupta D, Bansal P, Choudhary K, 2018. The state of the art of feature extraction techniques in speech recognition. In: Agrawal SS, Devi A, Wason R, (Eds.), Speech and Language Processing for Human-Machine Communications. Springer, Singapore, p.195-207. 10.1007/978-981-10-6626-9_22 [DOI] [Google Scholar]
- Hajek VE, Rutman DL, Scher H, 1989. Brief assessment of cognitive impairment in patients with stroke. Arch Phys Med Rehabil, 70(2): 114-117. [PubMed] [Google Scholar]
- Hamzei F, Liepert J, Dettmers C, et al. , 2006. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. NeuroImage, 31(2): 710-720. 10.1016/j.neuroimage.2005.12.035 [DOI] [PubMed] [Google Scholar]
- Han J, Waddington G, Adams R, et al. , 2016. Assessing proprioception: a critical review of methods. J Sport Health Sci, 5(1): 80-90. 10.1016/j.jshs.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantson L, de Weerdt W, de Keyser J, et al. , 1994. The European Stroke Scale. Stroke, 25(11): 2215-2219. 10.1161/01.str.25.11.2215 [DOI] [PubMed] [Google Scholar]
- Hartigan I, 2007. A comparative review of the Katz ADL and the Barthel Index in assessing the activities of daily living of older people. Int J Older People Nurs, 2(3): 204-212. 10.1111/j.1748-3743.2007.00074.x [DOI] [PubMed] [Google Scholar]
- Hebert R, Carrier R, Bilodeau A, 1988. The Functional Autonomy Measurement System (SMAF): description and validation of an instrument for the measurement of handicaps. Age Ageing, 17(5): 293-302. 10.1093/ageing/17.5.293 [DOI] [PubMed] [Google Scholar]
- Holbrook M, Skilbeck CE, 1983. An activities index for use with stroke patients. Age Ageing, 12(2): 166-170. 10.1093/ageing/12.2.166 [DOI] [PubMed] [Google Scholar]
- Hu XG, Suresh AK, Rymer WZ, et al. , 2015. Assessing altered motor unit recruitment patterns in paretic muscles of stroke survivors using surface electromyography. J Neural Eng, 12(6): 066001. 10.1088/1741-2560/12/6/066001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang P, Sohn MK, Kim CS, et al. , 2016. Tibial somatosensory evoked potential can prognosticate for ambulatory function in subacute hemiplegic stroke. J Clin Neurosci, 26: 122-125. 10.1016/j.jocn.2015.05.070 [DOI] [PubMed] [Google Scholar]
- Imura T, Mitsutake T, Iwamoto Y, et al. , 2021. A systematic review of the usefulness of magnetic resonance imaging in predicting the gait ability of stroke patients. Sci Rep, 11: 14338. 10.1038/s41598-021-93717-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SH, 2011. A review of diffusion tensor imaging studies on motor recovery mechanisms in stroke patients. NeuroRehabilitation, 28(4): 345-352. 10.3233/NRE-2011-0662 [DOI] [PubMed] [Google Scholar]
- Jaracz K, Kozubski W, 2003. Quality of life in stroke patients. Acta Neurol Scand, 107(5): 324-329. 10.1034/j.1600-0404.2003.02078.x [DOI] [PubMed] [Google Scholar]
- Johansson BB, 2011. Current trends in stroke rehabilitation. A review with focus on brain plasticity. Acta Neurol Scand, 123(3): 147-159. 10.1111/j.1600-0404.2010.01417.x [DOI] [PubMed] [Google Scholar]
- Joshi CD, Lahiri U, Thakor NV, 2013. Classification of gait phases from lower limb EMG: application to exoskeleton orthosis. Proceedings of 2013 IEEE Point-of-Care Healthcare Technologies (PHT), p.228-231. 10.1109/PHT.2013.6461326 [DOI] [Google Scholar]
- Kallenberg LAC, Hermens HJ, 2009. Motor unit properties of biceps brachii in chronic stroke patients assessed with high-density surface EMG. Muscle Nerve, 39(2): 177-185. 10.1002/mus.21090 [DOI] [PubMed] [Google Scholar]
- Kasner SE, 2006. Clinical interpretation and use of stroke scales. Lancet Neurol, 5(7): 603-612. 10.1016/s1474-4422(06)70495-1 [DOI] [PubMed] [Google Scholar]
- Katz N, Itzkovich M, Averbuch S, et al. , 1989. Loewenstein Occupational Therapy Cognitive Assessment (LOTCA) battery for brain-injured patients: reliability and validity. Am J Occup Ther, 43(3): 184-192. 10.5014/ajot.43.3.184 [DOI] [PubMed] [Google Scholar]
- Katz S, 1983. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc, 31(12): 721-727. 10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed] [Google Scholar]
- Katz S, Downs TD, Cash HR, et al. , 1970. Progress in development of the index of ADL. Gerontologist, 10(1): 20-30. 10.1093/geront/10.1_part_1.20 [DOI] [PubMed] [Google Scholar]
- Kawamura CM, de Morais Filho MC, Barreto MM, et al. , 2007. Comparison between visual and three-dimensional gait analysis in patients with spastic diplegic cerebral palsy. Gait Posture, 25(1): 18-24. 10.1016/j.gaitpost.2005.12.005 [DOI] [PubMed] [Google Scholar]
- KidzińskiŁ, Yang B, Hicks JL, et al. , 2020. Deep neural networks enable quantitative movement analysis using single-camera videos. Nat Commun, 11: 4054. 10.1038/s41467-020-17807-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Winstein C, 2017. Can neurological biomarkers of brain impairment be used to predict poststroke motor recovery? A systematic review. Neurorehabil Neural Repair, 31(1): 3-24. 10.1177/1545968316662708 [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kang HG, Kim HJ, et al. , 2014. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke, 16(3): 131-145. 10.5853/jos.2014.16.3.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Brody EM, 1969. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist, 9(3): 179-186. [PubMed] [Google Scholar]
- Lee MH, Siewiorek DP, Smailagic A, et al. , 2020. Opportunities of a machine learning-based decision support system for stroke rehabilitation assessment. arXiv: 2002. 12261v2. 10.48550/arXiv.2002.12261 [DOI] [Google Scholar]
- Li F, An BC, Zheng JJ, 2015. Evaluating hand neural-muscle function after stroke with surface electromyography (review). Chin J Rehabil Theory Pract, 21(3): 280-283 (in Chinese). 10.3969/j.issn.1006-9771.2015.03.009 [DOI] [Google Scholar]
- Li XY, Shin H, Zhou P, et al. , 2014. Power spectral analysis of surface electromyography (EMG) at matched contraction levels of the first dorsal interosseous muscle in stroke survivors. Clin Neurophysiol, 125(5): 988-994. 10.1016/j.clinph.2013.09.044 [DOI] [PubMed] [Google Scholar]
- Linacre JM, Heinemann AW, Wright BD, et al. , 1994. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil, 75(2): 127-132. 10.1016/0003-9993(94)90384-0 [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, et al. , 2010. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology, 74(4): 280-287. 10.1212/WNL.0b013e3181ccc6d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenstrøm E, Boysen G, Christiansen LW, et al. , 1991. Reliability of Scandinavian Neurological Stroke Scale. Cerebrovasc Dis, 1(2): 103-107. 10.1159/000108825 [DOI] [Google Scholar]
- Lindmark B, Hamrin E, 1988. Evaluation of functional capacity after stroke as a basis for active intervention. Presentation of a modified chart for motor capacity assessment and its reliability. Scand J Rehabil Med, 20(3): 103-109. [PubMed] [Google Scholar]
- Lioi G, Butet S, Fleury M, et al. , 2020. A multi-target motor imagery training using bimodal EEG-fMRI neurofeedback: a pilot study in chronic stroke patients. Front Hum Neurosci, 14: 37. 10.3389/fnhum.2020.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chen S, Zhang H, et al. , 2021. Bioinformatic analysis for potential biological processes and key targets of heart failure-related stroke. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(9): 718-732. 10.1631/jzus.B2000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang Y, Zhao YQ, et al. , 2016. Measurement of cerebral blood flow rate and its relationship with brain function using optical coherence tomography. Proceedings of SPIE 9707, Dynamics and Fluctuations in Biomedical Photonics XIII, p.208-213. 10.1117/12.2214091 [DOI] [Google Scholar]
- Lorenzi M, Bonassi S, Lorenzi T, et al. , 2018. A review of telomere length in sarcopenia and frailty. Biogerontology, 19(3-4): 209-221. 10.1007/s10522-018-9749-5 [DOI] [PubMed] [Google Scholar]
- Ma CC, Liu AJ, Li ZZ, et al. , 2014. Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. J Clin Neurosci, 21(8): 1388-1392. 10.1016/j.jocn.2013.11.032 [DOI] [PubMed] [Google Scholar]
- Ma ZH, Ding N, Yu Y, et al. , 2018. Quantification of cerebral vascular perfusion density via optical coherence tomography based on locally adaptive regional growth. Appl Opt, 57(35): 10117-10124. 10.1364/AO.57.010117 [DOI] [PubMed] [Google Scholar]
- Marotta N, Ammendolia A, Marinaro C, et al. , 2020. International classification of functioning, disability and health (ICF) and correlation between disability and finance assets in chronic stroke patients. Acta Biomed, 91(3): e2020064. 10.23750/abm.v91i3.8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Stinear CM, 2017. TMS measures of motor cortex function after stroke: a meta-analysis. Brain Stimul, 10(4): 721-734. 10.1016/j.brs.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Mintzopoulos D, Astrakas LG, Khanicheh A, et al. , 2009. Connectivity alterations assessed by combining fMRI and MR-compatible hand robots in chronic stroke. NeuroImage, 47(Suppl 2): T90-T97. 10.1016/j.neuroimage.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouridsen K, Thurner P, Zaharchuk G, 2020. Artificial intelligence applications in stroke. Stroke, 51(8): 2573-2579. 10.1161/STROKEAHA.119.027479 [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. , 2005. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 53(4): 695-699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nazmi N, Rahman MAA, Yamamoto SI, et al. , 2019. Walking gait event detection based on electromyography signals using artificial neural network. Biomed Signal Process Control, 47: 334-343. 10.1016/j.bspc.2018.08.030 [DOI] [Google Scholar]
- Oh SS, Kim Y, Lee YB, et al. , 2022. Optical modalities for research, diagnosis, and treatment of stroke and the consequent brain injuries. Appl Sci, 12(4): 1891. 10.3390/app12041891 [DOI] [Google Scholar]
- Osmon DC, Smet IC, Winegarden B, et al. , 1992. Neurobehavioral Cognitive Status Examination: its use with unilateral stroke patients in a rehabilitation setting. Arch Phys Med Rehabil, 73(5): 414-418. [PubMed] [Google Scholar]
- Ostrosky-Solís F, Lozano A, 2006. Digit span: effect of education and culture. Int J Psychol, 41(5): 333-341. 10.1080/00207590500345724 [DOI] [Google Scholar]
- Park CH, Chang WH, Ohn SH, et al. , 2011. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke, 42(5): 1357-1362. 10.1161/STROKEAHA.110.596155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Hwang NK, Kim HH, et al. , 2019. Effect of neuromuscular electrical stimulation combined with effortful swallowing using electromyographic biofeedback on oropharyngeal swallowing function in stroke patients with dysphagia: a pilot study. Medicine (Baltimore), 98(44): e17702. 10.1097/MD.0000000000017702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Fisher AG, Velozo CA, 1994. Using the Assessment of Motor and Process Skills to compare occupational performance between clinic and home settings. Am J Occup Ther, 48(8): 697-709. 10.5014/ajot.48.8.697 [DOI] [PubMed] [Google Scholar]
- Parsons MW, Christensen S, McElduff P, et al. , 2010. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab, 30(6): 1214-1225. 10.1038/jcbfm.2010.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari MC, Bonnì S, Ponzo V, et al. , 2018. Dynamic reorganization of TMS-evoked activity in subcortical stroke patients. Neuroimage, 175: 365-378. 10.1016/j.neuroimage.2018.04.011 [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, et al. , 1982. Measurement of functional activities in older adults in the community. J Gerontol, 37(3): 323-329. 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- Platz T, Eickhof C, Nuyens G, et al. , 2005. Clinical scales for the assessment of spasticity, associated phenomena, and function: a systematic review of the literature. Disabil Rehabil, 27(1-2): 7-18. 10.1080/09638280400014634 [DOI] [PubMed] [Google Scholar]
- Qi R, 2005. Introduction of common stroke efficacy evaluation methods. Chin Acupunct Maribustion, 25(4): 263-264 (in Chinese). [Google Scholar]
- Reitan RM, 1955. Investigation of the validity of Halstead’s measures of biological intelligence. AMA Arch Neurol Psych, 73(1): 28-35. 10.1001/archneurpsyc.1955.02330070030005 [DOI] [PubMed] [Google Scholar]
- Rosa MCN, Marques A, Demain S, et al. , 2014. Lower limb co-contraction during walking in subjects with stroke: a systematic review. J Electromyogr Kinesiol, 24(1): 1-10. 10.1016/j.jelekin.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Royall DR, Cordes JA, Polk M, 1998. CLOX: an executive clock drawing task. J Neurol Neurosurg Psych, 64(5): 588-594. 10.1136/jnnp.64.5.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski GM, Huntley AH, Crosby LD, et al. , 2020. Lower limb muscle activity underlying temporal gait asymmetry post-stroke. Clin Neurophysiol, 131(8): 1848-1858. 10.1016/j.clinph.2020.04.171 [DOI] [PubMed] [Google Scholar]
- Rudrapatna SU, Wieloch T, Beirup K, et al. , 2014. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. NeuroImage, 97: 363-373. 10.1016/j.neuroimage.2014.04.013 [DOI] [PubMed] [Google Scholar]
- Sack AT, Linden DEJ, 2003. Combining transcranial magnetic stimulation and functional imaging in cognitive brain research: possibilities and limitations. Brain Res Rev, 43(1): 41-56. 10.1016/s0165-0173(03)00191-7 [DOI] [PubMed] [Google Scholar]
- Scano A, Zanoletti M, Pirovano I, et al. , 2019. NIRS-EMG for clinical applications: a systematic review. Appl Sci, 9(15): 2952. 10.3390/app9152952 [DOI] [Google Scholar]
- Scarpina F, Tagini S, 2017. The Stroop Color and Word Test. Front Psychol, 8: 557. 10.3389/fpsyg.2017.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlager A, Ahlqvist K, Rasmussen-Barr E, et al. , 2018. Inter- and intra-rater reliability for measurement of range of motion in joints included in three hypermobility assessment methods. BMC Musculoskelet Disord, 19: 376. 10.1186/s12891-018-2290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Kanzler CM, Lambercy O, et al. , 2019. Systematic review on kinematic assessments of upper limb movements after stroke. Stroke, 50(3): 718-727. 10.1161/STROKEAHA.118.023531 [DOI] [PubMed] [Google Scholar]
- Shah S, Vanclay F, Cooper B, 1989. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol, 42(8): 703-709. 10.1016/0895-4356(89)90065-6 [DOI] [PubMed] [Google Scholar]
- Shah SK, 1984. Reliability of the original Brunnstrom recovery scale following hemiplegia. Aust Occup Ther J, 31(4): 144-151. 10.1111/j.1440-1630.1984.tb01473.x [DOI] [Google Scholar]
- Srinivasan VJ, Radhakrishnan H, Jiang JY, et al. , 2012. Optical coherence microscopy for deep tissue imaging of the cerebral cortex with intrinsic contrast. Opt Express, 20(3): 2220-2239. 10.1364/OE.20.002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg N, Adams R, Ayalon M, et al. , 2019. Recent ankle injury, sport participation level, and tests of proprioception. J Sport Rehabil, 28(8): 824-830. 10.1123/jsr.2018-0164 [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Petoe M, et al. , 2012. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain, 135(Pt 8): 2527-2535. 10.1093/brain/aws146 [DOI] [PubMed] [Google Scholar]
- Stinear CM, Lang CE, Zeiler S, et al. , 2020. Advances and challenges in stroke rehabilitation. Lancet Neurol, 19(4): 348-360. 10.1016/s1474-4422(19)30415-6 [DOI] [PubMed] [Google Scholar]
- Sulter G, Steen C, de Keyser J, 1999. Use of the Barthel Index and Modified Rankin Scale in acute stroke trials. Stroke, 30(8): 1538-1541. 10.1161/01.str.30.8.1538 [DOI] [PubMed] [Google Scholar]
- Takara K, 1971. Two-point discrimination on various type of skin graft to hand and foot. Kumamoto Igakkai Zasshi, 45(1): 94-121. [PubMed] [Google Scholar]
- Tao WJ, Liu T, Zheng RC, et al. , 2012. Gait analysis using wearable sensors. Sensors (Basel), 12(2): 2255-2283. 10.3390/s120202255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol EJ, 2019. High-performance medicine: the convergence of human and artificial intelligence. Nat Med, 25(1): 44-56. 10.1038/s41591-018-0300-7 [DOI] [PubMed] [Google Scholar]
- Tsao CW, Aday AW, Almarzooq ZI, et al. , 2022. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation, 145(8): e153-e639. 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- van Dokkum L, Hauret I, Mottet D, et al. , 2014. The contribution of kinematics in the assessment of upper limb motor recovery early after stroke. Neurorehabil Neural Repair, 28(1): 4-12. 10.1177/1545968313498514 [DOI] [PubMed] [Google Scholar]
- Veerbeek JM, Kwakkel G, van Wegen EEH, et al. , 2011. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke, 42(5): 1482-1488. 10.1161/STROKEAHA.110.604090 [DOI] [PubMed] [Google Scholar]
- Vermeer KA, Mo J, Weda JJA, et al. , 2014. Depth-resolved model-based reconstruction of attenuation coefficients in optical coherence tomography. Biomed Opt Express, 5(1): 322-337. 10.1364/BOE.5.000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinstrup J, Calatayud J, Jakobsen MD, et al. , 2018. Hand strengthening exercises in chronic stroke patients: dose-response evaluation using electromyography. J Hand Ther, 31(1): 111-121. 10.1016/j.jht.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Waddington G, Adams R, Han J, et al. , 2014. A new method for measuring dynamic proprioception. J Sci Med Sport, 18(Suppl 1): e141. 10.1016/j.jsams.2014.11.141 [DOI] [Google Scholar]
- Wang C, Meng B, Chen JP, 2019. Applicability of Boston naming test for assessment of postoperative language dysfunction. Zhejiang Med, 41(16): 1742-1745 (in Chinese). 10.12056/j.issn.1006-2785.2019.41.16.2018-2203 [DOI] [Google Scholar]
- Wang H, Magnain C, Sakadžić S, et al. , 2017. Characterizing the optical properties of human brain tissue with high numerical aperture optical coherence tomography. Biomed Opt Express, 8(12): 5617-5636. 10.1364/BOE.8.005617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang HX, 2016. Advance in neuro-electrophysiological techniques in functional evaluation after stroke (review). Chin J Rehabil Theory, 22(12): 1404-1407 (in Chinese). 10.3969/j.issn.1006-9771.2016.12.008 [DOI] [Google Scholar]
- Wang RK, An L, 2009. Doppler optical micro-angiography for volumetric imaging of vascular perfusion in vivo . Opt Express, 17(11): 8926-8940. 10.1364/oe.17.008926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TL, Mantini D, Gillebert CR, 2018. The potential of real-time fMRI neurofeedback for stroke rehabilitation: a systematic review. Cortex, 107: 148-165. 10.1016/j.cortex.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD, 1992. The MOS 36-ltem short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care, 30(6): 473-483. 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- Wei PN, Zhang JH, Wei PP, et al. , 2020. Different sEMG and EEG features analysis for gait phase recognition. Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, p.1002-1006. 10.1109/EMBC44109.2020.9175655 [DOI] [PubMed] [Google Scholar]
- Weinstein S, 1993. Fifty years of somatosensory research. J Hand Ther, 6(1): 11-22. 10.1016/s0894-1130(12)80176-1 [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) , 2001. International Classification of Functioning, Disability and Health (ICF). World Health Organization, Geneva. [Google Scholar]
- Williams LS, Weinberger M, Harris LE, et al. , 1999. Development of a stroke-specific quality of life scale. Stroke, 30(7): 1362-1369. 10.1161/01.str.30.7.1362 [DOI] [PubMed] [Google Scholar]
- Yamada K, Sakai K, Akazawa K, et al. , 2013. Detection of early neuronal damage in CADASIL patients by q-space MR imaging. Neuroradiology, 55(3): 283-290. 10.1007/s00234-012-1105-x [DOI] [PubMed] [Google Scholar]
- Yang SS, Liu KZ, Ding HJ, et al. , 2019. Longitudinal in vivo intrinsic optical imaging of cortical blood perfusion and tissue damage in focal photothrombosis stroke model. J Cereb Blood Flow Metab, 39(7): 1381-1393. 10.1177/0271678X18762636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AJ, Barak ER, Copen WA, et al. , 2010. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with national institutes of health stroke scale score improves the prediction of acute stroke outcome. Stroke, 41(8): 1728-1735. 10.1161/STROKEAHA.110.582874 [DOI] [PubMed] [Google Scholar]
- Yoon HS, You JSH, 2017. Reflex-mediated dynamic neuromuscular stabilization in stroke patients: EMG processing and ultrasound imaging. Technol Health Care, 25(S1): 99-106. 10.3233/THC-171311 [DOI] [PubMed] [Google Scholar]
- Zhao QH, Guo QH, Li F, et al. , 2013. The Shape Trail Test: application of a new variant of the trail making test. PLoS ONE, 8(2): e57333. 10.1371/journal.pone.0057333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JJ, Hu YH, Yu ZW, 2007. Application of surface electromyography in the estimate of neural-muscle function (review). Chin J Rehabil Theory Pract, 13(8): 741-742 (in Chinese). 10.3969/j.issn.1006-9771.2007.08.016. [DOI] [Google Scholar]
- Zhou N, 2002. A new approach to stroke assessment: SIAS. Foreign Med Sci (Sec Phys Med Relakieitation), 22(1): 1-4 (in Chinese). 10.3870/j.issn.1001-117X.2002.01.001 [DOI] [Google Scholar]