Abstract
The thymus is the primary immune organ responsible for generating self‐tolerant and immunocompetent T cells. However, the thymus gradually involutes during early life resulting in declined naïve T‐cell production, a process known as age‐related thymic involution. Thymic involution has many negative impacts on immune function including reduced pathogen resistance, high autoimmunity incidence, and attenuated tumor immunosurveillance. Age‐related thymic involution leads to a gradual reduction in thymic cellularity and thymic stromal microenvironment disruption, including loss of definite cortical‐medullary junctions, reduction of cortical thymic epithelial cells and medullary thymic epithelial cells, fibroblast expansion, and an increase in perivascular space. The compromised thymic microenvironment in aged individuals substantially disturbs thymocyte development and differentiation. Age‐related thymic involution is regulated by many transcription factors, micro RNAs, growth factors, cytokines, and other factors. In this review, we summarize the current understanding of age‐related thymic involution mechanisms and effects.
Keywords: aging, T cells, thymic epithelial cells, thymic involution, thymus
Age‐related thymic involution has many negative impacts on immune function including reduced pathogen resistance, high autoimmunity incidence, and attenuated tumor immunosurveillance. This review summarizes the current understanding of how age impacts thymic development and function, as well as the mechanisms underlying age‐related thymic involution, particularly TEC transcriptional profile changes during thymic aging.

Abbreviations
- CR
caloric restriction
- cTECs
cortical thymic epithelial cells
- DN
double negative
- DP
double positive
- EMT
epithelial–mesenchymal transition
- ETPs
early T‐lineage progenitors
- FGF21
fibroblast growth factor 21
- GH
growth hormone
- HSCs
hematopoietic stem cells
- LPCs
lymphohematopoietic progenitor cells
- MHCII
major histocompatibility complex class II
- mTECs
medullary thymic epithelial cells
- OSM
oncostatin M
- PPARγ
peroxisome proliferator‐activated receptor gamma
- PVS
perivascular space
- SCF
stem cell factor
- SP
single positive
- TCR
T‐cell receptor
- TECs
thymic epithelial cells
- Tgfbr1
transforming growth factor beta receptor I
- TRECs
T‐cell receptor excision circles
1. INTRODUCTION
Age‐related thymic involution is one of the most ubiquitous changes during immune system senescence. Most vertebrates experience thymic involution, which presents as a decrease in thymic epithelial cells (TECs) and accumulation of adipose tissue within the thymus. Human thymic involution is thought to begin as early as 1 year of age (Hale, 2004; Murray et al., 2003; Palmer et al., 2018). The murine thymus reaches its peak mass at 4 weeks, followed by a gradual diminution (Sutherland et al., 2005). Recently, Wu et al. examined medullary thymic epithelial cell (mTEC) transcriptomes from mice at 2, 6, and 10 weeks of age and showed that age‐related TEC degeneration occurs as early as 6 weeks after birth, as evidenced by reduced cell cycle‐related gene expression and increased inflammatory response‐related gene expression (Wu et al., 2018). Because the thymus involutes as early as 4–6 weeks in mice and 1 year in humans, ages when most other organs do not show any signs of aging, thymic involution may be an evolutionarily conserved process (Chaudhry et al., 2016). Some scientists have speculated that thymic involution has an undiscovered biological purpose and have divided thymic involution into an early phase (growth‐dependent thymic involution) and a late phase (age‐dependent thymic involution) (Aw & Palmer, 2012). The early phase (growth‐dependent thymic involution) may be due to a bioenergetic trade‐off (Boehm & Swann, 2013). In early life, it is essential to maintain high thymic activity and produce broad T‐cell receptor (TCR) diversity to protect against infections (Aw & Palmer, 2012). Once the T‐cell repertoire is established, it may be beneficial for the organism to reduce thymic activity and redistribute energy to other organs. In contrast, the late phase (age‐dependent thymic involution) may be similar to the aging process of other organs (Aw & Palmer, 2012). In this review, we mainly discuss age‐dependent thymic involution.
Thymic involution leads to a decline in new naïve T‐cell production and a collapse in peripheral TCR repertoire, resulting in impaired immune function (Figure 1). Thymic involution is associated with increased susceptibility to many diseases, including cancer, infection, and autoimmunity (Fahy et al., 2019; Goronzy & Weyand, 2003). It is well known that aging is associated with increased incidence of infectious diseases and neoplastic diseases, which is commonly attributed to systematic immunosenescence and a gradual accumulation of genetic mutations (Gavazzi & Krause, 2002; Pawelec, 2017). Recently, using a delicate mathematical model, Palmer et al. showed that age‐related decline in T‐cell production caused by thymic involution is a major risk factor for many cancers and infectious diseases in humans (Palmer et al., 2018). A connection between age‐related thymic demise and autoimmunity has been shown in many studies. For example, Hosaka et al. demonstrated that thymus transplantation could correct autoimmune disease in aging MRL/+ mice that exhibit dramatic thymic involution (Hosaka et al., 1996). In addition, some studies suggest that thymic aging might be involved in rheumatoid arthritis progression in humans (Goronzy & Weyand, 2003). Thus, a more complete understanding of the mechanisms and impacts of age‐related thymic involution will help us to better understand and prevent immunosenescence‐associated diseases during aging. In this review, we summarize the current knowledge on age‐related thymic involution mechanisms and effects.
FIGURE 1.
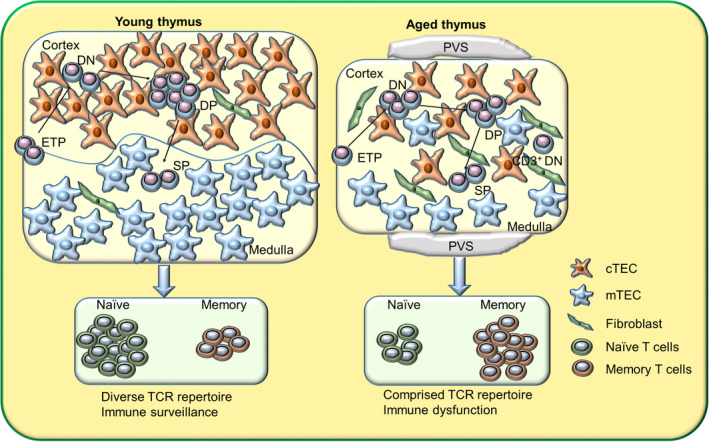
Effects of age on thymic development and function. Age‐related thymic involution leads to a gradual reduction in thymic cellularity and thymic stromal microenvironment disruption, including the loss of definite cortical‐medullary junctions, a reduction in cTECs and mTECs, fibroblast expansion, an increase in perivascular space (PVS), and more. The disrupted thymic stromal microenvironment disturbs thymocyte development causing decreased ETP and DP frequency, increased DN frequency, and abnormal CD3+ DN cell accumulation. The young thymus is able to produce functionally competent T cells expressing a broad TCR repertoire, whereas the aged thymus produces fewer naïve T cells with a restricted TCR repertoire
2. THE IMPACT OF AGE ON THYMIC DEVELOPMENT AND FUNCTION
2.1. The impact of age on thymic stromal cells
Aged‐related thymic involution reduces thymic cellularity in mice by 50% at 16 weeks in comparison with its adult peak at 4 weeks, eventually leading to less than 5% thymic cellularity (Baran‐Gale et al., 2020; Dooley & Liston, 2012; Gray et al., 2006; Sutherland et al., 2005). In humans, thymus size reduction begins as early as 1 year of age, and it continues to decline at a rate of approximately 3% per year until middle age before slowing down to less than 1% per year (George & Ritter, 1996). Morphological analysis has shown that cortical and medullary thymic epithelial region structure becomes increasingly less reticular and less globular with age in mice, and the definite cortical‐medullary junction is also gradually lost with age (Aw et al., 2008; Baran‐Gale et al., 2020) (Figure 1). Furthermore, aging is concomitant with thymic epithelial space contraction and perivascular space (PVS) augmentation in humans (Steinmann et al., 1985) (Figure 1). The aged thymus displays obvious TEC reduction, fibroblast and adipocyte expansion, and senescent cell accumulation (Aw et al., 2008; Gray et al., 2006; Palmer, 2013). Compared with 4 week old mice, TEC cellularity is reduced by about 50% at 16 weeks and over 80% at 50 weeks (Baran‐Gale et al., 2020). In particular, the mTEC population decreases gradually with age, leading to a decline in mTEC/cortical thymic epithelial cell (cTEC) ratio in aged mice (Gray et al., 2006). Although it is widely accepted that TEC number significantly decreases with thymic aging, a recent publication indicated that thymic aging leads to the contraction of cTEC complex cell projections, but has no effect on TEC cell number in mice (Venables et al., 2019). The authors of this article speculated that the use of mechanical/enzymatic methods to isolate TECs in previous studies may have led to a gross underestimate of total TEC number, and they believed that the genetic labeling approach used in their study could overcome this (Venables et al., 2019).
Proliferation of both CD45− non‐TECs stromal cells and TECs decreases dramatically with thymic aging in mice (Gray et al., 2006). Impaired TEC proliferation in aged mice was recently further demonstrated using transcriptome analysis (Cowan et al., 2019; Ki et al., 2014). Thymic aging is accompanied by a decline in TEC marker expression, including EpCAM, keratin, CD205, and Ulex Europaeus Agglutinin 1 (Aw et al., 2008). During thymic aging, the ratio of MHCIIhi TECs to MHCIIlo TECs clearly decreases, reflecting a reduced TEC antigen presentation ability in aged mice (Gray et al., 2006). However, it is notable that some recent studies have shown that the emergence of MHCIIlo TEC subsets during thymic development has other specific roles, such as supporting invariant NKT (iNKT) cell development in the thymus (Kozai et al., 2017; Lucas et al., 2020). Tissue‐restricted antigen expression also diminishes with age, representing a potential mechanism for age‐related increase in autoimmune diseases (Baran‐Gale et al., 2020; A. Griffith et al., 2015; Griffith et al., 2012). Aging also impairs TEC secretion ability, as demonstrated by diminished production of the thymopoietic cytokine IL‐7 in mice (Aspinall & Andrew, 2000; Ortman et al., 2002). IL‐7 administration in older mice and in the rhesus macaque increases thymic output (Aspinall et al., 2007; Pido‐Lopez et al., 2002).
Advances in bulk RNA‐seq and single‐cell RNA‐seq (scRNA‐seq) technology have allowed us to more comprehensively investigate TEC subpopulation changes and transcriptional profile changes during thymic aging. A recent scRNA‐seq study compared TEC subsets in young and old mice. Most mTECs considerably diminished and most cTECs dramatically increased in percentage upon aging, which is consistent with previous reports (Yue et al., 2019). The mTEC progenitor subsets also reduced with age; however, there was a much higher frequency of bipotent TEC progenitors in the aged thymus compared to young mice (Yue et al., 2019). It is worth noting that the mTEC and cTEC subsets in this study were divided roughly based on t‐distributed stochastic neighbor embedding analysis, and the precise nature of these subsets needs to be further elucidated experimentally. A more recent study subdivided TECs from 1, 4, 16, 32, and 52 week old mice into 9 different subtypes using scRNA‐seq analysis. In this study, the authors showed that the proportion of both perinatal cTECs and mature mTECs were significantly reduced with aging, in contrast to the proportion of mature cTECs and intertypical TECs, which increased with aging (Baran‐Gale et al., 2020). By using scRNA‐seq and lineage tracing mouse models, the authors demonstrated that intertypical TECs represent a TEC progenitor state and that aging compromises intertypical TEC differentiation into mature mTECs (Baran‐Gale et al., 2020). They further analyzed the transcriptional signatures of mature cTECs, mature mTECs, and intertypical TECs during aging and found that an inflamm‐aging transcriptional signature was restricted to mature cTECs and mature mTECs, rather than intertypical TECs (Baran‐Gale et al., 2020).
By comparing thymic stromal cell population transcriptomes from 1‐, 3‐, and 6‐month‐old mice, Ki et al. found that the expression of E2F3 transcriptional targets and cell cycle‐associated genes decreased with early thymic aging in cTECs and mTECs (Ki et al., 2014). A similar study showed that the decline in E2F3 transcriptional targets and cell cycle‐associated genes occurs as early as 6 weeks in mice (Wu et al., 2018). E2F3 is a transcription factor that regulates cell proliferation and many cell cycle‐associated genes (Humbert et al., 2000); thus, reduced E2F3 activity results in decreased TEC cell‐cycle progression in aged mice. Cell cycle‐related gene downregulation during thymic aging was further confirmed by another study that showed a decline in myc targets and ribosomal genes with thymic aging in mice (Cowan et al., 2019). By using a FoxN1MycTg mouse model, in which myc is overexpressed in TECs, the authors further demonstrated that myc mainly promotes ribosomal gene expression in TECs, which are distinct from cyclin D1 regulated genes (Cowan et al., 2019). These bulk RNA‐seq and scRNA‐seq results provide an overview of TEC transcriptional and cell subset changes during thymic aging. Some representative cTEC and mTEC transcripts that are downregulated during aging are summarized in Table 1.
TABLE 1.
The expression levels of the representative downregulated genes in mTECs and cTECs during aging
| Cell types | Genes | Average expression level (TPM) | |||
|---|---|---|---|---|---|
| Newborn | Adult | Aged | |||
| mTECs | Cell cycle‐related genes or E2F3 targets | Ccna2 | 75.87 | 63.14 | 41.00 |
| Ccnb1 | 93.8 | 86.26 | 60.38 | ||
| Ccnb2 | 73.15 | 56.97 | 41.63 | ||
| Cdk1 | 96.96 | 78.58 | 57.27 | ||
| Cdkn2d | 38.29 | 34.65 | 19.96 | ||
| Ribosomal genes | Rpl23a | 1224.37 | 1002.92 | 760.76 | |
| Rpl10a | 361.87 | 330.44 | 242.68 | ||
| Rps24 | 601.92 | 545.33 | 344.34 | ||
| Rps29 | 1714.98 | 1339.24 | 919.09 | ||
| Rpl9 | 1313.21 | 1105.58 | 726.28 | ||
| cTECs | Cell cycle‐related genes or E2F3 targets | Ccna2 | 53.09 | 41.22 | 5.92 |
| Ccnb1 | 52.71 | 53.66 | 2.13 | ||
| Ccnb2 | 38.14 | 80.92 | 8.57 | ||
| Cdk1 | 64.35 | 21.59 | 2.75 | ||
| Cdkn2d | 39.73 | 45.84 | 33.06 | ||
| Ribosomal genes | Rpl23a | 1267.49 | 827.83 | 626.10 | |
| Rpl10a | 531.99 | 326.59 | 210.05 | ||
| Rps24 | 728.18 | 398.52 | 180.39 | ||
| Rps29 | 1696.40 | 881.87 | 426.52 | ||
| Rpl9 | 2077.67 | 986.22 | 662.24 | ||
Note: According to the RNA‐seq data of Cowan et al. (2019).
In addition to TECs, aging also affects other stromal cells in the thymus. Thymic aging coincides with adipocyte accumulation around the thymus, and the increase in adipose tissue may inhibit thymic function through adipocytokine secretion (Dixit, 2010). Fibroblast percentage also increases in the aging thymus (Figure 1) in species including mice, humans, and fish, suggesting that this may be a conserved feature (Bertho et al., 1997; Gray et al., 2006; Torroba & Zapata, 2003). Recently, a thymic stromal cell transcriptome analysis revealed that proinflammatory gene expression increased with aging in mouse thymic dendritic cells, which in turn may accelerate thymic aging (Ki et al., 2014). Another interesting study demonstrated that thymic B cell function is also impaired with aging in mice; the authors showed that Aire and Aire‐dependent tissue‐restricted antigen expression decline in aging thymic B cells (Cepeda et al., 2018). Thus, aging impairs many cell subsets in the thymic microenvironment.
2.2. Thymocyte development in the aged thymus
In addition to thymic stromal cells, thymocyte development is also drastically disturbed during thymic aging. Some studies have shown that hematopoietic stem cells (HSCs) of aged mice display an increased bias toward myeloid differentiation concomitant with a diminished lymphoid lineage differentiation ability (Beerman et al., 2010). HSC abnormalities in aged mice may affect the seeding of early T‐lineage progenitors (ETPs) within the thymus. Indeed, ETP frequency declines with aging, and their potential ability to reconstitute the thymus is also reduced (Min et al., 2004, 2005). ETPs from young mice are able to differentiate into all stages of thymocytes when seeded into thymic lobes; in contrast, this differentiation ability is impaired in ETPs from aged mice (Min et al., 2004). However, the effect of aging on HSCs and ETPs is controversial. Zhu et al. established an elegant mouse model in which they transplanted a fetal thymus into the kidney capsule of aged mice, thus providing a young thymic microenvironment for aged lymphohematopoietic progenitor cells (LPCs) (Zhu et al., 2007). Using this model, they demonstrated that the LPCs derived from aged mice and young mice have similar abilities to differentiate into ETPs and subsequent thymocyte subpopulations when transplanted into the young thymic microenvironment, indicating that LPCs do not have a defect synchronized with age‐related thymic involution (Zhu et al., 2007). Another study showed that the ETP defects in aged thymi are mainly due to changes in thymic epithelial architecture, including the poorly defined cortico‐medullary junction and reduced medulla cellularity, rather than ETP‐intrinsic defects (Gui et al., 2007). Thus, although aging may have some effects on HSCs and ETPs, the impairment of ETPs and subsequent thymocyte subpopulations in aged mice can be mainly attributed to thymic microenvironment disruption.
ETPs subsequently differentiate into double negative (DN) (CD4−CD8−) subpopulations that include DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) (Liang, Zhang, Dong, et al., 2021; Luan et al., 2019). The DN subsets subsequently become double positive (DP) (CD4+CD8+) cells that further differentiate into CD4 or CD8 single positive (SP) T cells through the process of positive and negative selection (Germain, 2002). Although both DN and DP population cell numbers are significantly reduced with aging, DN subset frequency increases 2–3 times in aged (24–27 months old) mice compared with young (2–3 months old) mice, whereas the percentage of DP (CD4+CD8+) subpopulations significantly diminishes with age (Thoman, 1995). Among DN subsets, there is a considerable reduction in DN2 and DN3 subset cell numbers with thymic aging in mice (Aspinall, 1997). Additionally, thymic aging is concomitant with the abnormal accumulation of CD3+ DN cells within the thymus (Aw et al., 2009, 2010). Aging also interferes with later stages of thymocyte development. DP and SP thymocytes in aged mice display deregulated CD3 expression, which may lead to attenuated TCR‐dependent stimulation (Aw et al., 2009). Indeed, thymocytes from older mice exhibit an impaired mitogen response ability, which is manifested by a failure to upregulate the activation marker CD69 and proliferate (Aw et al., 2010; Djikic et al., 2014). Consistent with impaired thymocyte differentiation in the aged thymus, T‐cell receptor excision circles (TRECs) within the thymus also significantly decline with aging in mice and humans (Ortman et al., 2002; Palmer et al., 2018). Thus, aging impairs multiple thymocyte developmental stages.
2.3. Thymic involution effects on thymic output
Mature CD4 SP and CD8 SP thymocytes are exported to the periphery where they play a role in immunological surveillance (Liang, Zhang, Zhang, et al., 2021; Zhang et al., 2021). Age‐related thymic involution causes an obvious reduction in the thymic output of naïve T cells and subsequently decreases peripheral T‐cell diversity (Chaudhry et al., 2016; Cowan et al., 2020). Diminished thymic production of naïve T cells leads to homeostatic expansion of existing T cells, resulting in memory T‐cell augmentation (Surh & Sprent, 2000). Although it is well accepted that the thymic output of peripheral naïve T cells progressively declines with aging in mice (den Braber et al., 2012), in humans, the relationship of thymic involution to peripheral naïve T‐cell maintenance is a matter of debate. Many studies using TRECs as a measurement of thymic output demonstrate that peripheral naïve T‐cell thymic output declines with aging in humans (Fagnoni et al., 2000; Ferrando‐Martinez et al., 2011; Mitchell et al., 2010; Naylor et al., 2005). However, Braber et al. showed that adult human peripheral naïve T‐cell pool maintenance occurs almost exclusively through cell proliferation, rather than thymic output (den Braber et al., 2012). Thus, the contribution of thymic output to naïve T‐cell pool maintenance in adults needs further investigation.
Aging also interferes with naïve T‐cell properties and functions (Srinivasan et al., 2021). Naïve T cells from aged mice express elevated levels of senescence markers and display reduced proliferation ability upon antigen stimulation (Akbar & Henson, 2011; Chaudhry et al., 2016). Chemokine receptor expression is also altered in CD4+ T cells of aged mice, exhibiting a deregulation of CCR1, 7, and 8 and CXCR2, 4, and 5, which may impair their migration ability (Mo et al., 2003). The reduced number of naïve T cells together with the disrupted function of naïve T cells during aging leads to impaired immunological surveillance ability in aged organisms.
3. AGE‐RELATED THYMIC INVOLUTION MECHANISMS
3.1. Thymic stromal cell alterations lead to thymic involution
Although the T‐lineage differentiation potential of HSCs and ETPs is partially compromised in aged mice compared with young mice (Min et al., 2004; Zediak et al., 2007), increasing evidence suggests that thymic involution is mainly caused by age‐related thymic stromal cell degeneration, particularly TEC degeneration (Chen et al., 2009; A. V. Griffith et al., 2015; Gui et al., 2012; Zhu et al., 2007). For example, a global transcriptome analysis of thymic stromal cells and lymphocytes revealed that mouse thymic stromal cells, in contrast to lymphocytes, are deficient in catalase (A. V. Griffith et al., 2015). This results in elevated H2O2 levels and stromal cell oxidative damage, which subsequently leads to thymic atrophy. The authors further showed that thymic atrophy could be ameliorated by genetic and biochemical restoration of antioxidant activity (A. V. Griffith et al., 2015). Similar to this study, another publication revealed that the thymi of human Down syndrome patients exhibited premature senescence, and TECs from Down syndrome patients showed increased oxidative stress (Marcovecchio et al., 2021). Using a genome‐wide computational approach, another group showed that age‐associated thymic degeneration is primarily a stromal cell function change (Griffith et al., 2012). Many studies support the pivotal role of thymic stroma in thymic aging. Mackall et al. showed that lethally irradiated older mice exhibit impaired thymopoiesis compared with lethally irradiated young mice after both were injected with young bone marrow (Mackall et al., 1998). A similar experiment showed that intrathymic injection of young ETPs failed to restore normal thymopoiesis in older mice but did so in young mice (Zhu et al., 2007). In contrast, the same study showed that fetal thymi transplants into the kidney capsules of young or old mice had similar thymopoiesis (Zhu et al., 2007). Overall, these findings suggest that the thymic stroma is a key factor in regulating age‐related thymic involution. Likely, the durable identity of the thymus is established by its stromal components because developing thymocytes are only transiently present in the thymus (Petrie & Zuniga‐Pflucker, 2007).
3.2. Molecular regulation of thymic involution
Foxn1 is essential for embryonic thymic organogenesis and TEC maintenance in adults (Zuklys et al., 2016), and emerging evidence suggests that Foxn1 also plays a critical role in preventing age‐related thymic involution (Abramson & Anderson, 2017). Foxn1 expression progressively declines with aging (Figure 2), and Foxn1 overexpression ameliorates age‐related thymic deterioration, indicating that Foxn1 is a pivotal regulator of thymic aging (Bredenkamp et al., 2014; Chen et al., 2009; O'Neill et al., 2016; Rode et al., 2015; Zook et al., 2011). Sun et al. generated a loxP‐floxed‐Foxn1 mouse model carrying a ubiquitous CreERT transgene with a low level of spontaneous activation leading to a gradual loss of Foxn1 expression with age (Sun et al., 2010). By examining this mouse model's phenotype at different ages, Sun et al. demonstrated that gradual Foxn1 loss with age substantially accelerates age‐related thymic involution (Sun et al., 2010). In contrast, Foxn1 overexpression restores most of the changes caused by thymic involution in old mice, including thymic mass enlargement, increased ETP frequency, elevated EpCAM+MHCII+ TEC cell number, and CD4+ and CD8+ naïve compartment expansion in the spleen (Bredenkamp et al., 2014; Zook et al., 2011). Moreover, a recent study showed that engrafting Foxn1‐reprogrammed embryonic fibroblasts could rejuvenate aged thymic architecture and function in both male and female mice (Oh et al., 2020). Collectively, these studies demonstrate a crucial role for Foxn1 in regulating age‐associated thymic degeneration.
FIGURE 2.
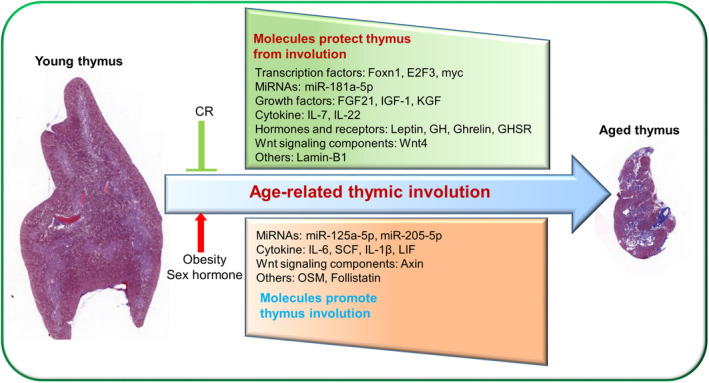
Age‐related thymic involution mechanisms. Both positive and negative regulators of thymic involution have been identified. Positive regulators include Foxn1, E2F3, myc, Wnt4, FGF21, KGF, IL‐7, IL‐22, miR‐181a‐5p, Lamin‐B1, Leptin, GH, IGF‐1, Ghrelin, and GHSR, which exhibit reduced activity with age. Negative regulators include Axin, LIF, OSM, IL‐6, SCF, IL‐1β, miR‐125a‐5p, miR‐205‐5p, and follistatin, which exhibit increased activity with age. In addition, CR can attenuate age‐related thymic involution, while obesity and sex hormones exacerbate age‐related thymic involution
The Wnt signaling pathway attenuates with aging in the thymus (Ferrando‐Martinez et al., 2015; Kvell et al., 2010; Yang, Youm, Sun, et al., 2009) (Figure 2). Ferrando‐Martínez et al. found that the nonadipocytic component of the human thymus expresses higher levels of Wnt pathway inhibitors in the elderly than in the young, thus attenuating the Wnt pathway (Ferrando‐Martinez et al., 2015). Using thymic stromal cell transcriptome analysis, Griffith et al. revealed that Wnt signaling deregulation is the most significant hallmark of thymic degeneration (Griffith et al., 2012). A previous study illustrated that the expression of Axin, a Wnt inhibitor, on mTECs and fibroblasts increases with aging in humans and mice, and Axin knockdown by RNA interference ameliorates age‐related thymic degeneration (Yang, Youm, Sun, et al., 2009). Another study showed that Wnt pathway reduction during aging may involve the epithelial–mesenchymal transition (EMT) process in mice, which we will discuss further below (Kvell et al., 2010).
Growth factors and cytokines also play critical roles in age‐related thymic involution. Prolongevity ketogenic hormone fibroblast growth factor 21 (FGF21) expression gradually declines in the thymus with age, and loss of FGF21 function in middle‐aged mice accelerates age‐dependent thymic deterioration (Figure 2), suggesting that FGF21 expression could protect against age‐related thymic involution (Youm et al., 2016). Leukemia inhibitory factor (LIF), oncostatin M (OSM), IL‐6, and stem cell factor (SCF) expression levels all increase with age in mice (Figure 2), and this elevated expression is associated with thymic involution (Sempowski et al., 2000). Studies have shown that these cytokines may originate from adipocytes or TECs (Dooley & Liston, 2012; Ventevogel & Sempowski, 2013). Another proinflammatory cytokine, IL‐1β, is mainly expressed by macrophages in the thymus (Figure 2), and its increased expression levels also lead to thymic involution (Dixit, 2012; Finn et al., 2018; Guarda et al., 2011). Consistent with this, IL‐1β receptor is primarily expressed in TECs, and ablation of Nlrap3 and Asc, which are required for IL‐1β activation, protect against age‐related thymic demise and immunosenescence in mice (Youm et al., 2012). In contrast, some cytokines and growth factors play positive roles in preventing age‐associated thymic degeneration. Studies have shown that IL‐7, IL‐22, and keratinocyte growth factor administration reverse age‐induced thymic involution in humans and mice (Ventevogel & Sempowski, 2013).
MicroRNAs (miRNAs) have been implicated in the aging process in many organisms, and the role of miRNAs in age‐associated thymic deterioration was recently investigated. Guo et al. compared various miRNA expression levels in TECs from 2‐month‐old and 20‐month‐old mice and identified many differentially expressed miRNAs (Guo et al., 2013). Whole thymus miRNA expression has also been examined during aging. Compared with thymi from 1‐month‐old mice, 50 and 81 miRNAs were differentially expressed in thymi from 10‐month‐old and 19‐month‐old mice, respectively (Ye et al., 2014). Among these differentially expressed miRNAs, miR‐181a‐5p and miR‐125a‐5p, which were downregulated and upregulated during aging, respectively, have been studied further. Guo et al. revealed that miR‐181a‐5p expression decreased in TECs of 10‐ to 19‐month‐old mice compared to 1‐month‐old mice, and miR‐181a‐5p promoted mTEC proliferation by targeting transforming growth factor beta receptor I (Tgfbr1), which exhibits increased expression with aging. This suggests that miR‐181a‐5p could prevent age‐related thymic demise by interfering with TGFβ signaling that could negatively regulate the development of mTECs and promote thymic involution (D. G. Guo et al., 2016; Hauri‐Hohl et al., 2008, 2014; Xu et al., 2018). In contrast to miR‐181a‐5p, Xu et al. revealed that miR‐125a‐5p expression increased in TECs of aged mice compared with TECs of young mice. They found that miR‐125a‐5p suppressed Foxn1 expression, which may underlie its role in promoting age‐related thymic involution (Xu et al., 2018; Xu, Sizova, et al., 2017). A more recent study showed that miR‐205‐5p expression in TECs markedly increased with aging in mice, and miR‐205‐5p promoted age‐associated thymic involution by inhibiting TEC proliferation (Gong et al., 2020). Another study compared differentially expressed miRNAs in the thymi of 10‐month‐old newborn babies and 70‐year‐old humans and showed that 106 miRNAs were significantly changed in elderly thymi (Ferrando‐Martinez et al., 2015). Furthermore, some of the altered miRNAs in this study, such as miR‐25, miR‐134, and miR‐7f, could modulate the Wnt pathway (Ferrando‐Martinez et al., 2015). In addition to age‐related thymic involution, microRNAs also regulate stress‐induced thymic involution. Papadopoulou et al. showed that miR‐29a could prevent pathogen‐associated thymic involution via targeting the IFN‐α receptor in TECs, and Hoover et al. revealed that miR‐205 expression in TECs could maintain thymopoiesis following inflammatory perturbations in mice (Hoover et al., 2016; Papadopoulou et al., 2012). Thus targeting miRNAs may be a potential strategy to rejuvenate age‐induced diminished thymic function (Xu, Zhang, et al., 2017).
Recently, some new thymic involution regulators have been identified. Lamin‐B1 is a cellular architectural protein that has recently been shown to play a critical role in preventing thymic aging in mice (Yue et al., 2019). Yue et al. demonstrated that the increased proinflammatory cytokines produced by thymic myeloid immune cells during aging diminishes Lamin‐B1 expression in TECs and promotes cell senescence, which subsequently induces age‐related thymic involution (Yue et al., 2019) (Figure 2). Other recent studies have shown that imbalances in follistatin, activin A, and BMP4 signaling drive thymic involution in mice (Lepletier et al., 2019), while liver X receptors, a class of nuclear receptors that sense intracellular oxysterols and cholesterol biosynthetic pathway intermediates, may protect against premature thymic involution in mice (Chan et al., 2020). Additionally, results from our lab showed that TEC‐specific deletion of tuberous sclerosis complex 1 (Tsc1), a negative regulator of mTOR activity (Liang, Zhang, Zhang, et al., 2021), also accelerates thymic involution in mice (unpublished data). Interestingly, sirtuin 6 (Sirt6) is a chromatin deacylase that has been implicated as a key factor in aging (Chang et al., 2020); however, our recent publication showed that Sirt6 deficiency in TECs has no obvious effects on thymic aging in mice (Zhang et al., 2021).
3.3. Sex hormones in thymic involution
Steroid hormone levels change dramatically with aging, and steroid hormones play a critical role in promoting age‐related thymic involution (Gui et al., 2012). The role of sex hormones in thymic involution was first reported in 1904 in a study that found that castrated cattle had enlarged thymi (Henderson, 1904). Additionally, the fact that the thymus degenerates most rapidly after puberty, when steroid hormone production reaches its peak, further supports the role of steroid hormones in thymic involution (Abramson & Anderson, 2017). Thymic involution is also more rapid in males than in females (Gui et al., 2012; Hun et al., 2020), implying that androgens may have a more dramatic impact on thymic involution. Although both TECs and thymocytes express androgen receptors (Olsen et al., 2001), androgen‐mediated thymic involution is caused by direct impact on TECs rather than thymocytes because TEC‐specific (but not thymocyte‐specific) androgen receptor deletion leads to androgen‐mediated thymic involution resistant in mice (Olsen et al., 2001). More recently, a comprehensive transcriptome analysis showed that sexual dimorphism significantly affects cTECs (Dumont‐Lagace et al., 2015). cTECs from male mice display low proliferation rates, and androgen‐dependent signaling represses the expression of genes involved in cTEC development and function, such as Foxn1, Dll4, Psmb11, and Ctsl (Dumont‐Lagace et al., 2015). Consistently, another study demonstrated that sex steroid blockade could increase Dll4 expression and its downstream targets on cTECs in mice, which further promotes thymopoiesis by modulating Notch signaling (Velardi et al., 2014). Notably, although castration is an effective way to regenerate the aged thymus, the thymic regrowth induced by castration is transient (Griffith et al., 2012).
Pregnancy also causes thymic involution, mainly mediated by progesterone (Clarke & Kendall, 1989). Studies have shown that progesterone receptor expression in thymic stromal cells is required for thymic involution during pregnancy in mice (Tibbetts et al., 1999). Interestingly, thymic involution during pregnancy may be essential for normal fertility (Tibbetts et al., 1999). A recent study uncovered that RANK expression in TECs promoted sex hormone‐mediated thymic involution and natural regulatory T‐cell development during pregnancy, which is critical for successful pregnancy and prevention of gestational diabetes (Paolino et al., 2021).
3.4. Metabolic regulation of thymic involution
Caloric restriction (CR) has long been known to play a critical role in increasing life span. Recently, CR was also shown to be effective at preventing age‐related thymic involution. Yang et al. showed that CR could inhibit thymic adipogenesis and reduce age‐related thymic involution in mice (Yang, Youm, Vandanmagsar, et al., 2009). Another study conducted on nonhuman primates obtained similar results; the authors showed that long‐term CR effectively improves naïve T‐cell production and preserves T‐cell receptor repertoire diversity (Messaoudi et al., 2006). Thymus transcriptome analysis in short‐term CR mice showed that CR altered catalytic activity and metabolic processes (Omeroglu Ulu et al., 2018). Short‐term CR also altered the expression of leptin, ghrelin, Igf1, and adiponectin, some of which were reported to be associated with age‐related thymic involution (Omeroglu Ulu et al., 2018).
Obesity increases the risk of infections and cancer, which may be partly ascribed to obesity's negative impact on thymic involution. High‐fat diet fed mice display disrupted thymic structure, including a reduced medullary region and an absence of the cortico‐medullary junction (Gulvady et al., 2013). Diet‐induced obesity also leads to thymocyte apoptosis, reduces thymic output, and compromises TCR repertoire diversity in mice (Yang, Youm, Vandanmagsar, et al., 2009). Progressive adiposity in middle‐aged humans also decreases thymic output (Yang, Youm, Vandanmagsar, et al., 2009). Resveratrol, a phytoalexin produced from plants, has been shown to have the potential to inhibit obesity‐induced thymic involution (Gulvady et al., 2013; Wei et al., 2020). Leptin is a potent adipokine that is responsible for sensing a positive energy balance state and reducing food intake (Friedman & Halaas, 1998). Leptin (ob/ob mouse)‐ and leptin receptor (db/db mouse)‐deficient mice display severe obesity that subsequently causes significant thymic involution (Dixit, 2012; Howard et al., 1999). Leptin administration rescues this accelerated thymic involution in ob/ob mice (Howard et al., 1999). Another study showed that leptin receptor is mainly expressed in the medullary region of the thymus (Gruver et al., 2009). Consistent with results in mice, human patients with loss‐of‐function leptin and leptin receptor mutations also display T‐cell functional defects that could be partially reversed by recombinant leptin administration (Farooqi et al., 2002, 2007). Furthermore, naïve CD8 T‐cell maintenance in nonagenarians has been shown to be associated with high leptin levels (Chen et al., 2010).
Growth hormone (GH) and its proximal mediator, IGF‐1, play critical roles in preventing age‐associated thymic involution. Indeed, GH removal by hypophysectomy leads to thymic atrophy in mice and humans (Napolitano et al., 2008; Savino et al., 2002). Circulating GH levels decline with aging, and GH administration partially ameliorates age‐related thymic involution in mice (Taub et al., 2010). Randomized clinical studies in middle‐aged HIV patients showed that GH treatment increases thymic mass and elevates TRECs in peripheral T cells (Napolitano et al., 2008). Similarly, exogenous administration of IGF‐1 enhances thymopoiesis mainly through TEC expansion in mice (Chu et al., 2008). Furthermore, subcutaneous transplantation of GH3 pituitary adenoma cells, which secrete growth hormone, reverses thymic aging in rats (Kelley et al., 1986). A recent clinical trial showed that recombinant human GH administration combined with dehydroepiandrosterone and metformin could promote thymic regeneration and increase protective immunological changes (Fahy et al., 2019). However, GH application in clinical practice needs to be carefully considered due to its significant side effects (Taub et al., 2010). Ghrelin is a stomach hormone that can induce strong GH‐releasing activity through binding to its receptor‐specific 7‐transmembrane GH secretagogue receptor (GHSR) (Sato et al., 2012). Ghrelin and GHSR expression within the thymus decline with progressive age, and administration of both ghrelin and ghrelin‐receptor agonists alleviate age‐associated thymic deterioration in mice and humans (Dixit et al., 2007, 2009; Smith et al., 2007). Consistent with this, genetic studies revealed that ghrelin and GHSR deficiency accelerates age‐associated thymic demise in mice (Youm et al., 2009). Thus, many hormones contribute positively or negatively to thymic involution.
3.5. Adipocyte origin during thymic involution: The EMT process
Adipogenesis is a notable feature of thymic involution (Dixit, 2010). Recently, some progress has been made in understanding how adipocytes are formed during thymic aging. Using genetically modified reporter mice, Youm et al. first reported that TECs can transition to mesenchymal cells through a mechanism called EMT (Youm et al., 2009). These mesenchymal cells are highly plastic and have the potential to differentiate into adipocytes (Mani et al., 2008). Indeed, these mesenchymal cells express pro‐adipogenic genes, which provide a possible adipocyte origin in the thymus (Youm et al., 2009). Some regulators play critical roles in the EMT process. Peroxisome proliferator‐activated receptor gamma (PPARγ), a member of the nuclear receptor superfamily of ligand‐activated transcription factors, is involved in adipocyte development (Tontonoz & Spiegelman, 2008). Thus, it is reasonable to speculate that PPARγ may play a key role in thymic involution. Indeed, an adipocyte‐lineage‐specific constitutively active PPARγ transgene and administration of rosiglitazone, a PPARγ signaling activator, both promote age‐related thymic involution in mice (Youm et al., 2010). In fact, the Ghrl–GHSR interaction and CR both protect against thymic involution by inhibiting EMT and adipogenesis in mice (Yang, Youm, Vandanmagsar, et al., 2009; Youm et al., 2009), and CR‐mediated thymic involution inhibition also involves PPARγ downregulation in mouse thymic stromal cells (Yang, Youm, & Dixit, 2009). Furthermore, decreased Wnt4 and increased LAP2α during thymic aging may promote direct TEC trans‐differentiation into pre‐adipocytes or cause EMT and subsequent pre‐adipocyte differentiation (Kvell et al., 2010). A recent study showed that CD147 deletion from T cells in mice could prevent thymic involution by inhibiting TEC EMT, implying that the interaction between thymocytes and TECs contributes to age‐related thymic involution (Chen et al., 2020). Although some progress has been made, adipocyte origin during thymic aging needs further investigation.
4. CONCLUSIONS
Age‐related thymic involution contributes significantly to immunosenescence. Although some progress has been made in understanding the molecular regulation of thymic involution, the detailed molecular regulation network is still unclear. Comprehensive information about age‐related thymic involution is needed to promote thymic rejuvenation in the elderly. With advances in transcriptome analysis, significant progress has been made in understanding overall thymic stromal cell changes during aging, and the use of scRNA‐seq has revealed comprehensive TEC subset changes. Thymic aging is associated with the downregulation of cell cycle‐related genes and ribosome biogenesis‐related genes in TECs. Recent genetic studies have also identified some new thymic aging regulators, including FGF21, lamin‐B1, liver X receptors, and some miRNAs. With the current understanding of age‐related thymic involution, we can speculate that thymic stromal cells (especially TECs) offer potential targets for thymic rejuvenation in the elderly.
AUTHOR CONTRIBUTIONS
Zhanfeng Liang wrote the manuscript; Xue Dong and Qian Zhang designed figures; Zhaoqi Zhang analyzed the RNA‐seq data; Yong Zhao reviewed the manuscript and supervised the work.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
ACKNOWLEDGMENT
This work was supported by grants from the National Natural Science Foundation for General and Key Programs (31930041, Y.Z., 31800754, Z.L.), the National Key Research and Development Program of China (2017YFA0105002, 2017YFA0104401, and 2017YFA0104402, Y.Z.), and the Knowledge Innovation Program of Chinese Academy of Sciences (XDA16030301, Y.Z.).
Liang, Z. , Dong, X. , Zhang, Z. , Zhang, Q. , & Zhao, Y. (2022). Age‐related thymic involution: Mechanisms and functional impact. Aging Cell, 21, e13671. 10.1111/acel.13671
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abramson, J. , & Anderson, G. (2017). Thymic epithelial cells. Annual Review of Immunology, 35, 85–118. 10.1146/annurev-immunol-051116-052320 [DOI] [PubMed] [Google Scholar]
- Akbar, A. N. , & Henson, S. M. (2011). Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nature Reviews Immunology, 11(4), 289–295. 10.1038/nri2959 [DOI] [PubMed] [Google Scholar]
- Aspinall, R. (1997). Age‐associated thymic atrophy in the mouse due to a deficiency affecting rearrangement of the TCR during intrathymic T cell development. Journal of Immunology, 158(7), 3037–3045. [PubMed] [Google Scholar]
- Aspinall, R. , & Andrew, D. (2000). Thymic atrophy in the mouse is a soluble problem of the thymic environment. Vaccine, 18(16), 1629–1637. 10.1016/S0264-410x(99)00498-3 [DOI] [PubMed] [Google Scholar]
- Aspinall, R. , Pido‐Lopez, J. , Imami, N. , Henson, S. M. , Ngom, P. T. , Morre, M. , Niphuis, H. , Remarque, E. , Rosenwirth, B. , & Heeney, J. L. (2007). Old rhesus macaques treated with interleukin‐7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Research, 10(1), 5–17. 10.1089/rej.2006.9098 [DOI] [PubMed] [Google Scholar]
- Aw, D. , & Palmer, D. B. (2012). It's not all equal: A multiphasic theory of thymic involution. Biogerontology, 13(1), 77–81. 10.1007/s10522-011-9349-0 [DOI] [PubMed] [Google Scholar]
- Aw, D. , Silva, A. B. , Maddick, M. , von Zglinicki, T. , & Palmer, D. B. (2008). Architectural changes in the thymus of aging mice. Aging Cell, 7(2), 158–167. 10.1111/j.1474-9726.2007.00365.x [DOI] [PubMed] [Google Scholar]
- Aw, D. , Silva, A. B. , & Palmer, D. B. (2009). Is thymocyte development functional in the aged? Aging (Albany NY), 1(2), 146–153. 10.18632/aging.100027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw, D. , Silva, A. B. , & Palmer, D. B. (2010). The effect of age on the phenotype and function of developing thymocytes. Journal of Comparative Pathology, 141, S45–S59. 10.1016/j.jcpa.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Baran‐Gale, J. , Morgan, M. D. , Maio, S. , Dhalla, F. , Calvo‐Asensio, I. , Deadman, M. E. , Handel, A. E. , Maynard, A. , Chen, S. , Green, F. , Sit, R. V. , Neff, N. F. , Darmanis, S. , Tan, W. , May, A. P. , Marioni, J. C. , Ponting, C. P. , & Holländer, G. A. (2020). Ageing compromises mouse thymus function and remodels epithelial cell differentiation. eLife, 9, e56221. 10.7554/eLife.56221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman, I. , Maloney, W. J. , Weissmann, I. L. , & Rossi, D. J. (2010). Stem cells and the aging hematopoietic system. Current Opinion in Immunology, 22(4), 500–506. 10.1016/j.coi.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertho, J. M. , Demarquay, C. , Moulian, N. , VanderMeeren, A. , Berrih‐Aknin, S. , & Gourmelon, P. (1997). Phenotypic and immunohistological analyses of the human adult thymus: Evidence for an active thymus during adult life. Cellular Immunology, 179(1), 30–40. 10.1006/cimm.1997.1148 [DOI] [PubMed] [Google Scholar]
- Boehm, T. , & Swann, J. B. (2013). Thymus involution and regeneration: Two sides of the same coin? Nature Reviews Immunology, 13(11), 831–838. 10.1038/nri3534 [DOI] [PubMed] [Google Scholar]
- Bredenkamp, N. , Nowell, C. S. , & Blackburn, C. C. (2014). Regeneration of the aged thymus by a single transcription factor. Development, 141(8), 1627–1637. 10.1242/dev.103614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda, S. , Cantu, C. , Orozco, S. , Xiao, Y. , Brown, Z. , Semwal, M. K. , Venables, T. , Anderson, M. S. , & Griffith, A. V. (2018). Age‐associated decline in thymic B cell expression of Aire and Aire‐dependent self‐antigens. Cell Reports, 22(5), 1276–1287. 10.1016/j.celrep.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, C. T. , Fenn, A. M. , Harder, N. K. , Mindur, J. E. , McAlpine, C. S. , Patel, J. , Valet, C. , Rattik, S. , Iwamoto, Y. , He, S. , Anzai, A. , Kahles, F. , Poller, W. C. , Janssen, H. , Wong, L. P. , Fernandez‐Hernando, C. , Koolbergen, D. R. , van der Laan, A. M. , Yvan‐Charvet, L. , … Swirski, F. K. (2020). Liver X receptors are required for thymic resilience and T cell output. Journal of Experimental Medicine, 217(10), 1–17. 10.1084/jem.20200318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, A. R. , Ferrer, C. M. , & Mostoslavsky, R. (2020). SIRT6, a mammalian Deacylase with multitasking abilities. Physiological Reviews, 100(1), 145–169. 10.1152/physrev.00030.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry, M. S. , Velardi, E. , Dudakov, J. A. , & van den Brink, M. R. (2016). Thymus: The next (re)generation. Immunological Reviews, 271(1), 56–71. 10.1111/imr.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Li, J. , Lim, F. C. , Wu, Q. , Douek, D. C. , Scott, D. K. , & Study, L. H. A. (2010). Maintenance of naive CD8 T cells in nonagenarians by leptin, IGFBP3 and T3. Mechanisms of Ageing and Development, 131(1), 29–37. 10.1016/j.mad.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. Z. , Xiao, S. Y. , & Manley, N. R. (2009). Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage‐sensitive manner. Blood, 113(3), 567–574. 10.1182/blood-2008-05-156265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. , Wang, K. , Feng, Z. , Zhang, M. Y. , Wu, J. , Geng, J. J. , & Chen, Z. N. (2020). CD147 deficiency in T cells prevents thymic involution by inhibiting the EMT process in TECs in the presence of TGF beta. Cellular & Molecular Immunology, 18, 171–181. 10.1038/s41423-019-0353-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, Y. W. , Schmitz, S. , Choudhury, B. , Telford, W. , Kapoor, V. , Garfield, S. , Howe, D. , & Gress, R. E. (2008). Exogenous insulin‐like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood, 112(7), 2836–2846. 10.1182/blood-2008-04-149435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, A. G. , & Kendall, M. D. (1989). Histological‐changes in the thymus during mouse pregnancy. Thymus, 14(1–3), 65–78. [PubMed] [Google Scholar]
- Cowan, J. E. , Malin, J. , Zhao, Y. G. , Seedhom, M. O. , Harly, C. , Ohigashi, I. , Kelly, M. , Takahama, Y. , Yewdell, J. W. , Cam, M. , & Bhandoola, A. (2019). Myc controls a distinct transcriptional program in fetal thymic epithelial cells that determines thymus growth. Nature Communications, 10, 5498. 10.1038/s41467-019-13465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, J. E. , Takahama, Y. , Bhandoola, A. , & Ohigashi, I. (2020). Postnatal involution and counter‐involution of the thymus. Frontiers in Immunology, 11, 897. 10.3389/fimmu.2020.00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Braber, I. , Mugwagwa, T. , Vrisekoop, N. , Westera, L. , Mogling, R. , de Boer, A. B. , Willems, N. , Schrijver, E. H. , Spierenburg, G. , Gaiser, K. , Mul, E. , Otto, S. A. , Ruiter, A. F. , Ackermans, M. T. , Miedema, F. , Borghans, J. A. , de Boer, R. J. , & Tesselaar, K. (2012). Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity, 36(2), 288–297. 10.1016/j.immuni.2012.02.006 [DOI] [PubMed] [Google Scholar]
- Dixit, V. D. (2010). Thymic fatness and approaches to enhance thymopoietic fitness in aging. Current Opinion in Immunology, 22(4), 521–528. 10.1016/j.coi.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit, V. D. (2012). Impact of immune‐metabolic interactions on age‐related thymic demise and T cell senescence. Seminars in Immunology, 24(5), 321–330. 10.1016/j.smim.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Dixit, V. D. , Yang, H. , Cooper‐Jenkins, A. , Giri, B. B. , Patel, K. , & Taub, D. D. (2009). Reduction of T cell‐derived ghrelin enhances proinflammatory cytokine expression: Implications for age‐associated increases in inflammation. Blood, 113(21), 5202–5205. 10.1182/blood-2008-09-181255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit, V. D. , Yang, H. W. , Sun, Y. X. , Weeraratna, A. T. , Youm, Y. H. , Smith, R. G. , & Taub, D. D. (2007). Ghrelin promotes thymopoiesis during aging. Journal of Clinical Investigation, 117(10), 2778–2790. 10.1172/Jci30248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djikic, J. , Nacka‐Aleksic, M. , Pilipovic, I. , Stojic‐Vukanic, Z. , Bufan, B. , Kosec, D. , Dimitrijević, M. , & Leposavic, G. (2014). Age‐associated changes in rat immune system: Lessons learned from experimental autoimmune encephalomyelitis. Experimental Gerontology, 58, 179–197. 10.1016/j.exger.2014.08.005 [DOI] [PubMed] [Google Scholar]
- Dooley, J. , & Liston, A. (2012). Molecular control over thymic involution: From cytokines and microRNA to aging and adipose tissue. European Journal of Immunology, 42(5), 1073–1079. 10.1002/eji.201142305 [DOI] [PubMed] [Google Scholar]
- Dumont‐Lagace, M. , St‐Pierre, C. , & Perreault, C. (2015). Sex hormones have pervasive effects on thymic epithelial cells. Scientific Reports, 5, 12895. 10.1038/srep12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni, F. F. , Vescovini, R. , Passeri, G. , Bologna, G. , Pedrazzoni, M. , Lavagetto, G. , Casti, A. , Franceschi, C. , Passeri, M. , & Sansoni, P. (2000). Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood, 95(9), 2860–2868. [PubMed] [Google Scholar]
- Fahy, G. M. , Brooke, R. T. , Watson, J. P. , Good, Z. , Vasanawala, S. S. , Maecker, H. , Leipold, M. D. , Lin, D. T. S. , Kobor, M. S. , & Horvath, S. (2019). Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell, 18(6), e13028. 10.1111/acel.13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi, I. S. , Matarese, G. , Lord, G. M. , Keogh, J. M. , Lawrence, E. , Agwu, C. , Jebb, S. A. , Perna, F. , Fontana, S. , Lechler, R. I. , AM, D. P. , & O'Rahilly, S. (2002). Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. Journal of Clinical Investigation, 110(8), 1093–1103. 10.1172/Jci200215693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi, I. S. , Wangensteen, T. , Collins, S. , Kimber, W. , Matarese, G. , Keogh, J. M. , Lank, E. , Bottomley, B. , Lopez‐Fernandez, J. , Ferraz‐Amaro, I. , Dattani, M. T. , Ercan, O. , Myhre, A. G. , Retterstol, L. , Stanhope, R. , Edge, J. A. , McKenzie, S. , Lessan, N. , Ghodsi, M. , … O'Rahilly, S. (2007). Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. New England Journal of Medicine, 356, 237–247. 10.1056/NEJMoa063988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando‐Martinez, S. , Ruiz‐Mateos, E. , Dudakov, J. A. , Velardi, E. , Grillari, J. , Kreil, D. P. , Muñoz‐Fernandez, M. Á. , van den Brink, M. R. , & Leal, M. (2015). WNT signaling suppression in the senescent human thymus. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(3), 273–281. 10.1093/gerona/glu030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando‐Martinez, S. , Ruiz‐Mateos, E. , Hernandez, A. , Gutierrez, E. , Rodriguez‐Mendez, M. D. , Ordonez, A. , & Leal, M. (2011). Age‐related deregulation of naive T cell homeostasis in elderly humans. Age, 33(2), 197–207. 10.1007/s11357-010-9170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, J. D. , Smith, A. R. , Patel, M. C. , Shaw, L. , Youniss, M. R. , van Heteren, J. , Dirstine, T. , Ciullo, C. , Lescarbeau, R. , Seitzer, J. , Shah, R. R. , Shah, A. , Ling, D. , Growe, J. , Pink, M. , Rohde, E. , Wood, K. M. , Salomon, W. E. , Harrington, W. F. , … Morrissey, D. V. (2018). A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Reports, 22(9), 2227–2235. 10.1016/j.celrep.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Friedman, J. M. , & Halaas, J. L. (1998). Leptin and the regulation of body weight in mammals. Nature, 395(6704), 763–770. 10.1038/27376 [DOI] [PubMed] [Google Scholar]
- Gavazzi, G. , & Krause, K. H. (2002). Ageing and infection. Lancet Infectious Diseases, 2(11), 659–666. 10.1016/S1473-3099(02)00437-1 [DOI] [PubMed] [Google Scholar]
- George, A. J. T. , & Ritter, M. A. (1996). Thymic involution with ageing: Obsolescence or good housekeeping? Immunology Today, 17(6), 267–272. 10.1016/0167-5699(96)80543-3 [DOI] [PubMed] [Google Scholar]
- Germain, R. N. (2002). T‐cell development and the CD4‐CD8 lineage decision. Nature Reviews Immunology, 2(5), 309–322. 10.1038/nri798 [DOI] [PubMed] [Google Scholar]
- Gong, B. , Wang, X. , Li, B. , Li, Y. , Lu, R. , Zhang, K. , Li, B. , Ma, Y. , & Li, Y. (2020). miR‐205‐5p inhibits thymic epithelial cell proliferation via FA2H‐TFAP2A feedback regulation in age‐associated thymus involution. Molecular Immunology, 122, 173–185. 10.1016/j.molimm.2020.04.011 [DOI] [PubMed] [Google Scholar]
- Goronzy, J. J. , & Weyand, C. M. (2003). T‐cell senescence and contraction of T‐cell repertoire diversity catalysts of autoimmunity and chronic inflammation. Arthritis Research & Therapy, 5(5), 225–234. 10.1186/ar974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, D. H. D. , Seach, N. , Ueno, T. , Milton, M. K. , Liston, A. , Lew, A. M. , Goodnow, C. C. , & Boyd, R. L. (2006). Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood, 108(12), 3777–3785. 10.1182/blood-2006-02-004531 [DOI] [PubMed] [Google Scholar]
- Griffith, A. , Venables, T. , Shi, J. J. , Farr, A. , Van Remmen, H. , Szweda, L. , Fallahi, M. , Rabinovitch, P. , & Petrie, H. (2015). Metabolic damage and premature thymus aging caused by stromal catalase deficiency. Journal of Immunology, 12, 194–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith, A. V. , Fallahi, M. , Venables, T. , & Petrie, H. T. (2012). Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell, 11(1), 169–177. 10.1111/j.1474-9726.2011.00773.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith, A. V. , Venables, T. , Shi, J. J. , Farr, A. , van Remmen, H. , Szweda, L. , Fallahi, M. , Rabinovitch, P. , & Petrie, H. T. (2015). Metabolic damage and premature thymus aging caused by stromal catalase deficiency. Cell Reports, 12(7), 1071–1079. 10.1016/j.celrep.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruver, A. L. , Ventevogel, M. S. , & Sempowski, G. D. (2009). Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia‐induced thymus involution. Journal of Endocrinology, 203(1), 75–85. 10.1677/Joe-09-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda, G. , Zenger, M. , Yazdi, A. S. , Schroder, K. , Ferrero, I. , Menu, P. , Tardivel, A. , Mattmann, C. , & Tschopp, J. (2011). Differential expression of NLRP3 among hematopoietic cells. Journal of Immunology, 186(4), 2529–2534. 10.4049/jimmunol.1002720 [DOI] [PubMed] [Google Scholar]
- Gui, J. G. , Mustachio, L. M. , Su, D. M. , & Craig, R. W. (2012). Thymus size and age‐related thymic involution: Early programming, sexual dimorphism, progenitors and stroma. Aging and Disease, 3(3), 280–290. [PMC free article] [PubMed] [Google Scholar]
- Gui, J. G. , Zhu, X. K. , Dohkan, J. I. , Cheng, L. L. , Barnes, P. F. , & Su, D. M. (2007). The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. International Immunology, 19(10), 1201–1211. 10.1093/intimm/dxm095 [DOI] [PubMed] [Google Scholar]
- Gulvady, A. A. , Ciolino, H. P. , Cabrera, R. M. , & Jolly, C. A. (2013). Resveratrol inhibits the deleterious effects of diet‐induced obesity on thymic function. Journal of Nutritional Biochemistry, 24(9), 1625–1633. 10.1016/j.jnutbio.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Guo, D. G. , Ye, Y. Q. , Qi, J. J. , Zhang, L. H. , Xu, L. F. , Tan, X. T. , Yu, X. , Liu, Q. , Liu, J. , Zhang, Y. , Ma, Y. , & Li, Y. G. (2016). MicroRNA‐181a‐5p enhances cell proliferation in medullary thymic epithelial cells via regulating TGF‐beta signaling. Acta Biochimica et Biophysica Sinica, 48(9), 840–849. 10.1093/abbs/gmw068 [DOI] [PubMed] [Google Scholar]
- Guo, Z. B. , Chi, F. , Song, Y. , Wang, C. S. , Yu, R. X. , Wei, T. L. , Gui, J. , & Zhu, X. K. (2013). Transcriptome analysis of murine thymic epithelial cells reveals age‐associated changes in microRNA expression. International Journal of Molecular Medicine, 32(4), 835–842. 10.3892/ijmm.2013.1471 [DOI] [PubMed] [Google Scholar]
- Hale, L. P. (2004). Histologic and molecular assessment of human thymus. Annals of Diagnostic Pathology, 8(1), 50–60. 10.1016/j.anndiagpath.2003.11.006 [DOI] [PubMed] [Google Scholar]
- Hauri‐Hohl, M. , Zuklys, S. , Hollander, G. A. , & Ziegler, S. F. (2014). A regulatory role for TGF‐beta signaling in the establishment and function of the thymic medulla. Nature Immunology, 15(6), 554–561. 10.1038/ni.2869 [DOI] [PubMed] [Google Scholar]
- Hauri‐Hohl, M. M. , Zuklys, S. , Keller, M. P. , Jeker, L. T. , Barthlott, T. , Moon, A. M. , Roes, J. , & Hollander, G. A. (2008). TGF‐beta signaling in thymic epithelial cells regulates thymic involution and postirradiation reconstitution. Blood, 112(3), 626–634. 10.1182/blood-2007-10-115618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, J. (1904). On the relationship of the thymus to the sexual organs: I. The influence of castration on the thymus. The Journal of Physiology, 31(3–4), 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover, A. R. , Dozmorov, I. , MacLeod, J. , Du, Q. , de la Morena, M. T. , Forbess, J. , Guleserian, K. , Cleaver, O. B. , & van Oers, N. S. C. (2016). MicroRNA‐205 maintains T cell development following stress by regulating Forkhead box N1 and selected chemokines. Journal of Biological Chemistry, 291(44), 23237–23247. 10.1074/jbc.M116.744508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka, N. , Nose, M. , Kyogoku, M. , Nagata, N. , Miyashima, S. , Good, R. A. , & Ikehara, S. (1996). Thymus transplantation, a critical factor for correction of autoimmune disease in aging MRL/+ mice. Proceedings of the National Academy of Sciences of the United States of America, 93(16), 8558–8562. 10.1073/pnas.93.16.8558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, J. K. , Lord, G. M. , Matarese, G. , Vendetti, S. , Ghatei, M. A. , Ritter, M. A. , Lechler, R. I. , & Bloom, S. R. (1999). Leptin protects mice from starvation‐induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. Journal of Clinical Investigation, 104(8), 1051–1059. 10.1172/Jci6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert, P. O. , Verona, R. , Trimarchi, J. M. , Rogers, C. , Dandapani, S. , & Lees, J. A. (2000). E2f3 is critical for normal cellular proliferation. Genes & Development, 14(6), 690–703. [PMC free article] [PubMed] [Google Scholar]
- Hun, M. L. , Wong, K. , Gunawan, J. R. , Alsharif, A. , Quinn, K. , & Chidgey, A. P. (2020). Gender disparity impacts on thymus aging and LHRH receptor antagonist‐induced thymic reconstitution following chemotherapeutic damage. Frontiers in Immunology, 11, 302. 10.3389/fimmu.2020.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, K. W. , Brief, S. , Westly, H. J. , Novakofski, J. , Bechtel, P. J. , Simon, J. , & Walker, E. B. (1986). Gh3 pituitary‐adenoma cells can reverse thymic aging in rats. Proceedings of the National Academy of Sciences of the United States of America, 83(15), 5663–5667. 10.1073/pnas.83.15.5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki, S. , Park, D. , Selden, H. J. , Seita, J. , Chung, H. , Kim, J. , Iyer, V. R. , & Ehrlich, L. I. R. (2014). Global transcriptional profiling reveals distinct functions of thymic stromal subsets and age‐related changes during thymic involution. Cell Reports, 9(1), 402–415. 10.1016/j.celrep.2014.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozai, M. , Kubo, Y. , Katakai, T. , Kondo, H. , Kiyonari, H. , Schaeuble, K. , Luther, S. A. , Ishimaru, N. , Ohigashi, I. , & Takahama, Y. (2017). Essential role of CCL21 in establishment of central self‐tolerance in T cells. Journal of Experimental Medicine, 214(7), 1925–1935. 10.1084/jem.20161864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvell, K. , Varecza, Z. , Bartis, D. , Hesse, S. , Parnell, S. , Anderson, G. , Jenkinson, E. J. , & Pongracz, J. E. (2010). Wnt4 and LAP2alpha as pacemakers of thymic epithelial senescence. PLoS One, 5(5), e10701. 10.1371/journal.pone.0010701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepletier, A. , Hun, M. L. , Hammett, M. V. , Wong, K. , Naeem, H. , Hedger, M. , Loveland, K. , & Chidgey, A. P. (2019). Interplay between follistatin, activin A, and BMP4 signaling regulates postnatal thymic epithelial progenitor cell differentiation during aging. Cell Reports, 27(13), 3887, e3884–3901. 10.1016/j.celrep.2019.05.045 [DOI] [PubMed] [Google Scholar]
- Liang, Z. , Zhang, Q. , Dong, X. , Zhang, Z. , Wang, H. , Zhang, J. , & Zhao, Y. (2021). mTORC2 negatively controls the maturation process of medullary thymic epithelial cells by inhibiting the LTbetaR/RANK‐NF‐kappaB axis. Journal of Cellular Physiology, 236(6), 4725–4737. 10.1002/jcp.30192 [DOI] [PubMed] [Google Scholar]
- Liang, Z. , Zhang, Q. , Zhang, Z. , Sun, L. , Dong, X. , Li, T. , Tan, L. , Xie, X. , Sun, L. , & Zhao, Y. (2021). The development and survival of thymic epithelial cells require TSC1‐dependent negative regulation of mTORC1 activity. Journal of Immunology, 207(8), 2039–2050. 10.4049/jimmunol.2100463 [DOI] [PubMed] [Google Scholar]
- Luan, R. , Liang, Z. F. , Zhang, Q. , Sun, L. G. , & Zhao, Y. (2019). Molecular regulatory networks of thymic epithelial cell differentiation. Differentiation, 107, 42–49. 10.1016/j.diff.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Lucas, B. , White, A. J. , Cosway, E. J. , Parnell, S. M. , James, K. D. , Jones, N. D. , Ohigashi, I. , Takahama, Y. , Jenkinson, W. E. , & Anderson, G. (2020). Diversity in medullary thymic epithelial cells controls the activity and availability of iNKT cells. Nature Communications, 11(1), 2198. 10.1038/s41467-020-16041-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall, C. L. , Punt, J. A. , Morgan, P. , Farr, A. G. , & Gress, R. E. (1998). Thymic function in young/old chimeras: Substantial thymic T cell regenerative capacity despite irreversible age‐associated thymic involution. European Journal of Immunology, 28(6), 1886–1893. [DOI] [PubMed] [Google Scholar]
- Mani, S. A. , Guo, W. , Liao, M. J. , Eaton, E. N. , Ayyanan, A. , Zhou, A. Y. , Brooks, M. , Reinhard, F. , Zhang, C. C. , Shipitsin, M. , Campbell, L. L. , Polyak, K. , Brisken, C. , Yang, J. , & Weinberg, R. A. (2008). The epithelial‐mesenchymal transition generates cells with properties of stem cells. Cell, 133(4), 704–715. 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovecchio, G. E. , Ferrua, F. , Fontana, E. , Beretta, S. , Genua, M. , Bortolomai, I. , Conti, A. , Montin, D. , Cascarano, M. T. , Bergante, S. , D'Oria, V. , Giamberti, A. , Amodio, D. , Cancrini, C. , Carotti, A. , di Micco, R. , Merelli, I. , Bosticardo, M. , & Villa, A. (2021). Premature senescence and increased oxidative stress in the thymus of down syndrome patients. Frontiers in Immunology, 12, 669893. 10.3389/fimmu.2021.669893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi, I. , Warner, J. , Fischer, M. , Park, B. , Hill, B. , Mattison, J. , Lane, M. A. , Roth, G. S. , Ingram, D. K. , Picker, L. J. , Douek, D. C. , Mori, M. , & Nikolich‐Zugich, J. (2006). Delay of T cell senescence by caloric restriction in aged long‐lived nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America, 103(51), 19448–19453. 10.1073/pnas.0606661103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, H. , Montecino‐Rodriguez, E. , & Dorshkind, K. (2004). Reduction in the developmental potential of intrathymic T cell progenitors with age. Journal of Immunology, 173(1), 245–250. 10.4049/jimmunol.173.1.245 [DOI] [PubMed] [Google Scholar]
- Min, H. , Montecino‐Rodriguez, E. , & Dorshkind, K. (2005). Effects of aging on early B‐ and T‐cell development. Immunological Reviews, 205, 7–17. 10.1111/j.0105-2896.2005.00263.x [DOI] [PubMed] [Google Scholar]
- Mitchell, W. A. , Lang, P. O. , & Aspinall, R. (2010). Tracing thymic output in older individuals. Clinical and Experimental Immunology, 161(3), 497–503. 10.1111/j.1365-2249.2010.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo, R. , Chen, J. , Han, Y. , Bueno‐Cannizares, C. , Misek, D. E. , Lescure, P. A. , Hanash, S. , & Yung, R. L. (2003). T cell chemokine receptor expression in aging. Journal of Immunology, 170(2), 895–904. 10.4049/jimmunol.170.2.895 [DOI] [PubMed] [Google Scholar]
- Murray, J. M. , Kaufmann, G. R. , Hodgkin, P. D. , Lewin, S. R. , Kelleher, A. D. , Davenport, M. P. , & Zaunders, J. J. (2003). Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunology and Cell Biology, 81(6), 487–495. 10.1046/j.1440-1711.2003.01191.x [DOI] [PubMed] [Google Scholar]
- Napolitano, L. A. , Schmidt, D. , Gotway, M. B. , Ameli, N. , Filbert, E. L. , Ng, M. M. , Clor, J. L. , Epling, L. , Sinclair, E. , Baum, P. D. , Li, K. , Killian, M. L. , Bacchetti, P. , & McCune, J. M. (2008). Growth hormone enhances thymic function in HIV‐1‐infected adults. Journal of Clinical Investigation, 118(3), 1085–1098. 10.1172/Jci32830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor, K. , Li, G. J. , Vallejo, A. N. , Lee, W. W. , Koetz, K. , Bryl, E. , Witkowski, J. , Fulbright, J. , Weyand, C. M. , & Goronzy, J. J. (2005). The influence of age on T cell generation and TCR diversity. Journal of Immunology, 174(11), 7446–7452. 10.4049/jimmunol.174.11.7446 [DOI] [PubMed] [Google Scholar]
- Oh, J. , Wang, W. , Thomas, R. , & Su, D. M. (2020). Thymic rejuvenation via FOXN1‐reprogrammed embryonic fibroblast (FREF) to counteract age‐related inflammation. JCI Insight, 5, 1–15. 10.1172/jci.insight.140313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, N. J. , Olson, G. , Viselli, S. M. , Gu, X. J. , & Kovacs, W. J. (2001). Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology, 142(3), 1278–1283. 10.1210/en.142.3.1278 [DOI] [PubMed] [Google Scholar]
- Omeroglu Ulu, Z. , Ulu, S. , Dogan, S. , Guvenc Tuna, B. , & Ozdemir Ozgenturk, N. (2018). Transcriptome analysis of the thymus in short‐term calorie‐restricted mice using RNA‐seq. International Journal of Genomics, 2018, 7647980. 10.1155/2018/7647980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, K. E. , Bredenkamp, N. , Tischner, C. , Vaidya, H. J. , Stenhouse, F. H. , Peddie, C. D. , Nowell, C. S. , & Blackburn, C. C. (2016). Foxn1 is dynamically regulated in thymic epithelial cells during embryogenesis and at the onset of thymic involution. PLoS One, 11(3), e0151666. 10.1371/journal.pone.0151666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortman, C. L. , Dittmar, K. A. , Witte, P. L. , & Le, P. T. (2002). Molecular characterization of the mouse involuted thymus: Aberrations in expression of transcription regulators in thymocyte and epithelial compartments. International Immunology, 14(7), 813–822. 10.1093/intimm/dxf042 [DOI] [PubMed] [Google Scholar]
- Palmer, D. B. (2013). The effect of age on thymic function. Frontiers in Immunology, 4, 316. 10.3389/fimmu.2013.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, S. , Albergante, L. , Blackburn, C. C. , & Newman, T. J. (2018). Thymic involution and rising disease incidence with age. Proceedings of the National Academy of Sciences of the United States of America, 115(8), 1883–1888. 10.1073/pnas.1714478115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino, M. , Koglgruber, R. , Cronin, S. J. F. , Uribesalgo, I. , Rauscher, E. , Harreiter, J. , Schuster, M. , Bancher‐Todesca, D. , Pranjic, B. , Novatchkova, M. , Fededa, J. P. , White, A. J. , Sigl, V. , Dekan, S. , Penz, T. , Bock, C. , Kenner, L. , Holländer, G. A. , Anderson, G. , … Penninger, J. M. (2021). RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature, 589(7842), 442–447. 10.1038/s41586-020-03071-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou, A. S. , Dooley, J. , Linterman, M. A. , Pierson, W. , Ucar, O. , Kyewski, B. , Zuklys, S. , Hollander, G. A. , Matthys, P. , Gray, D. H. D. , de Strooper, B. , & Liston, A. (2012). The thymic epithelial microRNA network elevates the threshold for infection‐associated thymic involution via miR‐29a mediated suppression of the IFN‐α receptor. Nature Immunology, 13(2), 181–187. 10.1038/ni.2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec, G. (2017). Immunosenescence and cancer. Biogerontology, 18(4), 717–721. 10.1007/s10522-017-9682-z [DOI] [PubMed] [Google Scholar]
- Petrie, H. T. , & Zuniga‐Pflucker, J. C. (2007). Zoned out: Functional mapping of stromal signaling microenvironments in the thymus. Annual Review of Immunology, 35(25), 649–679. 10.1146/annurev.immunol.23.021704.115715 [DOI] [PubMed] [Google Scholar]
- Pido‐Lopez, J. , Imami, N. , Andrew, D. , & Aspinall, R. (2002). Molecular quantitation of thymic output in mice and the effect of IL‐7. European Journal of Immunology, 32(10), 2827–2836. [DOI] [PubMed] [Google Scholar]
- Rode, I. , Martins, V. C. , Kublbeck, G. , Maltry, N. , Tessmer, C. , & Rodewald, H. R. (2015). Foxn1 protein expression in the developing, aging, and regenerating thymus. Journal of Immunology, 195(12), 5678–5687. 10.4049/jimmunol.1502010 [DOI] [PubMed] [Google Scholar]
- Sato, T. , Nakamura, Y. , Shiimura, Y. , Ohgusu, H. , Kangawa, K. , & Kojima, M. (2012). Structure, regulation and function of ghrelin. Journal of Biochemistry, 151(2), 119–128. 10.1093/jb/mvr134 [DOI] [PubMed] [Google Scholar]
- Savino, W. , Postel‐Vinay, M. C. , Smaniotto, S. , & Dardenne, M. (2002). The thymus gland: A target organ for growth hormone. Scandinavian Journal of Immunology, 55(5), 442–452. 10.1046/j.1365-3083.2002.01077.x [DOI] [PubMed] [Google Scholar]
- Sempowski, G. D. , Hale, L. P. , Sundy, J. S. , Massey, J. M. , Koup, R. A. , Douek, D. C. , Patel, D. D. , & Haynes, B. F. (2000). Leukemia inhibitory factor, Oncostatin M, IL‐6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. The Journal of Immunology, 164(4), 2180–2187. 10.4049/jimmunol.164.4.2180 [DOI] [PubMed] [Google Scholar]
- Smith, R. G. , Sun, Y. X. , Jiang, H. , Albarran‐Zeckler, R. , & Timchenko, N. (2007). Ghrelin receptor (GHS‐R1A) agonists show potential as interventive agents during aging. Molecular Mechanisms and Models of Aging, 1119, 147–164. 10.1196/annals.1404.023 [DOI] [PubMed] [Google Scholar]
- Srinivasan, J. , Lancaster, J. N. , Singarapu, N. , Hale, L. P. , Ehrlich, L. I. R. , & Richie, E. R. (2021). Age‐related changes in thymic central tolerance. Frontiers in Immunology, 12, 676236. 10.3389/fimmu.2021.676236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann, G. G. , Klaus, B. , & Muller‐Hermelink, H. K. (1985). The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scandinavian Journal of Immunology, 22(5), 563–575. 10.1111/j.1365-3083.1985.tb01916.x [DOI] [PubMed] [Google Scholar]
- Sun, L. , Guo, J. , Brown, R. , Amagai, T. , Zhao, Y. , & Su, D. M. (2010). Declining expression of a single epithelial cell‐autonomous gene accelerates age‐related thymic involution. Aging Cell, 9(3), 347–357. 10.1111/j.1474-9726.2010.00559.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh, C. D. , & Sprent, J. (2000). Homeostatic T cell proliferation: How far can T cells be activated to self‐ligands? Journal of Experimental Medicine, 192(4), F9–F14. 10.1084/jem.192.4.F9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, J. S. , Goldberg, G. L. , Hammett, M. V. , Uldrich, A. P. , Berzins, S. P. , Heng, T. S. , Blazar, B. R. , Millar, J. L. , Malin, M. A. , Chidgey, A. P. , & Boyd, R. L. (2005). Activation of thymic regeneration in mice and humans following androgen blockade. Journal of Immunology, 175(4), 2741–2753. 10.4049/jimmunol.175.4.2741 [DOI] [PubMed] [Google Scholar]
- Taub, D. D. , Murphy, W. J. , & Longo, D. L. (2010). Rejuvenation of the aging thymus: Growth hormone‐mediated and ghrelin‐mediated signaling pathways. Current Opinion in Pharmacology, 10(4), 408–424. 10.1016/j.coph.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoman, M. L. (1995). The pattern of T‐Lymphocyte differentiation is altered during thymic involution. Mechanisms of Ageing and Development, 82(2‐3), 155–170. 10.1016/0047-6374(95)01597-S [DOI] [PubMed] [Google Scholar]
- Tibbetts, T. A. , DeMayo, F. , Rich, S. , Conneely, O. M. , & O'Malley, B. W. (1999). Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proceedings of the National Academy of Sciences of the United States of America, 96(21), 12021–12026. 10.1073/pnas.96.21.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz, P. , & Spiegelman, B. M. (2008). Fat and beyond: The diverse biology of PPARgamma. Annual Review of Biochemistry, 77, 289–312. 10.1146/annurev.biochem.77.061307.091829 [DOI] [PubMed] [Google Scholar]
- Torroba, M. , & Zapata, A. G. (2003). Aging of the vertebrate immune system. Microscopy Research and Technique, 62(6), 477–481. 10.1002/jemt.10409 [DOI] [PubMed] [Google Scholar]
- Velardi, E. , Tsai, J. J. , Holland, A. M. , Wertheimer, T. , Yu, V. W. C. , Zakrzewski, J. L. , Tuckett, A. Z. , Singer, N. V. , West, M. L. , Smith, O. M. , Young, L. F. , Kreines, F. M. , Levy, E. R. , Boyd, R. L. , Scadden, D. T. , Dudakov, J. A. , & van den Brink, M. R. M. (2014). Sex steroid blockade enhances thymopoiesis by modulating notch signaling. Journal of Experimental Medicine, 211(12), 2341–2349. 10.1084/jem.20131289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables, T. , Griffith, A. V. , DeAraujo, A. , & Petrie, H. T. (2019). Dynamic changes in epithelial cell morphology control thymic organ size during atrophy and regeneration. Nature Communications, 10(1), 4402. 10.1038/s41467-019-11879-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventevogel, M. S. , & Sempowski, G. D. (2013). Thymic rejuvenation and aging. Current Opinion in Immunology, 25(4), 516–522. 10.1016/j.coi.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, T. T. , Li, M. J. , Guo, L. , Xie, Y. D. , Chen, W. H. , Sun, Y. , Liu, G. H. , Ding, Y. , & Chai, Y. R. (2020). Resveratrol ameliorates thymus senescence changes in D‐galactose induced mice. Microbiology and Immunology, 64(9), 620–629. 10.1111/1348-0421.12833 [DOI] [PubMed] [Google Scholar]
- Wu, H. , Qin, X. , Dai, H. , & Zhang, Y. (2018). Time‐course transcriptome analysis of medullary thymic epithelial cells in the early phase of thymic involution. Molecular Immunology, 99, 87–94. 10.1016/j.molimm.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Xu, M. W. , Gan, T. , Ning, H. T. , & Wang, L. F. (2018). MicroRNA functions in thymic biology: Thymic development and involution. Frontiers in Immunology, 9, 2063. 10.3389/fimmu.2018.02063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. W. , Sizova, O. , Wang, L. F. , & Su, D. M. (2017). A fine‐tune role of Mir‐125a‐5p on Foxn1 during age‐associated changes in the thymus. Aging and Disease, 8(3), 277–286. 10.14336/Ad.2016.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. W. , Zhang, X. L. , Hong, R. Y. , Su, D. M. , & Wang, L. F. (2017). MicroRNAs regulate thymic epithelium in age‐related thymic involution via Down‐ or upregulation of transcription factors. Journal of Immunology Research, 2017 2528957. 10.1155/2017/2528957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , Youm, Y. H. , Sun, Y. X. , Rim, J. S. , Galban, C. J. , Vandanmagsar, B. , & Dixit, V. D. (2009). Axin expression in thymic stromal cells contributes to an age‐related increase in thymic adiposity and is associated with reduced thymopoiesis independently of ghrelin signaling. Journal of Leukocyte Biology, 85(6), 928–938. 10.1189/jlb.1008621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , Youm, Y. H. , Vandanmagsar, B. , Rood, J. , Kumar, K. G. , Butler, A. A. , & Dixit, V. D. (2009). Obesity accelerates thymic aging. Blood, 114(18), 3803–3812. 10.1182/blood-2009-03-213595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. W. , Youm, Y. H. , & Dixit, V. D. (2009). Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age‐related thymic involution. Journal of Immunology, 183(5), 3040–3052. 10.4049/jimmunol.0900562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, Y. Q. , Li, D. T. , Ouyang, D. , Deng, L. , Zhang, Y. , Ma, Y. J. , & Li, Y. G. (2014). MicroRNA expression in the aging mouse thymus. Gene, 547(2), 218–225. 10.1016/j.gene.2014.06.039 [DOI] [PubMed] [Google Scholar]
- Youm, Y. H. , Horvath, T. L. , Mangelsdorf, D. J. , Kliewer, S. A. , & Dixit, V. D. (2016). Prolongevity hormone FGF21 protects against immune senescence by delaying age‐related thymic involution. Proceedings of the National Academy of Sciences of the United States of America, 113(4), 1026–1031. 10.1073/pnas.1514511113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm, Y. H. , Kanneganti, T. D. , Vandanmagsar, B. , Zhu, X. , Ravussin, A. , Adijiang, A. , Owen, J. S. , Thomas, M. J. , Francis, J. , Parks, J. S. , & Dixit, V. D. (2012). The Nlrp3 inflammasome promotes age‐related thymic demise and immunosenescence. Cell Reports, 1(1), 56–68. 10.1016/j.celrep.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm, Y. H. , Yang, H. , Amin, R. , Smith, S. R. , Leff, T. , & Dixit, V. D. (2010). Thiazolidinedione treatment and constitutive‐PPARgamma activation induces ectopic adipogenesis and promotes age‐related thymic involution. Aging Cell, 9(4), 478–489. 10.1111/j.1474-9726.2010.00574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm, Y. H. , Yang, H. W. , Sun, Y. X. , Smith, R. G. , Manley, N. R. , Vandanmagsar, B. , & Dixit, V. D. (2009). Deficient ghrelin receptor‐mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. Journal of Biological Chemistry, 284(11), 7068–7077. 10.1074/jbc.M808302200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, S. , Zheng, X. , & Zheng, Y. (2019). Cell‐type‐specific role of Lamin‐B1 in thymus development and its inflammation‐driven reduction in thymus aging. Aging Cell, 18(4), e12952. 10.1111/acel.12952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zediak, V. P. , Maillard, I. , & Bhandoola, A. (2007). Multiple prethymic defects underlie age‐related loss of T progenitor competence. Blood, 110(4), 1161–1167. 10.1182/blood-2007-01-071605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Liang, Z. , Zhang, J. , Lei, T. , Dong, X. , Su, H. , Chen, Y. , Zhang, Z. , Tan, L. , & Zhao, Y. (2021). Sirt6 regulates the development of medullary thymic epithelial cells and contributes to the establishment of central immune tolerance. Frontiers in Cell and Development Biology, 9, 655552. 10.3389/fcell.2021.655552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Gui, J. G. , Dohkan, J. , Cheng, L. L. , Barnes, P. F. , & Su, D. M. (2007). Lymphohematopoietic progenitors do not have a synchronized defect with age‐related thymic involution. Aging Cell, 6(5), 663–672. 10.1111/j.1474-9726.2007.00325.x [DOI] [PubMed] [Google Scholar]
- Zook, E. C. , Krishack, P. A. , Zhang, S. B. , Zeleznik‐Le, N. J. , Firulli, A. B. , Witte, P. L. , & Le, P. T. (2011). Overexpression of Foxn1 attenuates age‐associated thymic involution and prevents the expansion of peripheral CD4 memory T cells. Blood, 118(22), 5723–5731. 10.1182/blood-2011-03-342097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuklys, S. , Handel, A. , Zhanybekova, S. , Govani, F. , Keller, M. , Maio, S. , Mayer, C. E. , Teh, H. Y. , Hafen, K. , & Hollander, G. A. (2016). Foxn1 regulates key target genes essential for T cell development in postnatal thymic epithelial cells. Nature Immunology, 17(10), 1206–1215. 10.1038/ni.3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


