Abstract
Background
Considerable attention is currently focused on the potential to switch on brown adipose tissue (BAT), or promote browning of white adipose tissue, to elevate energy expenditure and thereby reduce obesity levels. These processes are already known to be switched on by cold exposure. Yet humans living in colder regions do not show lower levels of obesity. This could be because humans shield themselves from external temperatures, or because the resultant changes in BAT and thermogenesis are offset by elevated food intake, or reductions in other components of expenditure.
Scope of Review
We exposed mice to 11 different ambient temperatures between 5 and 35 °C and characterized their energy balance and body weight/composition. As it got colder mice progressively increased their energy expenditure coincident with changes in thyroid hormone levels and increased BAT activity. Simultaneously, these increases in expenditure were matched by elevated food intake, and body mass remained stable. Nevertheless, within this envelope of unchanged body mass there were significant changes in body composition – with increases in the sizes of the liver and small intestine, presumably to support the greater food intake, and reductions in the level of stored fat – maximally providing about 10% of the total elevated energy demands.
Major Conclusions
Elevating activity of BAT may be a valid strategy to reduce fat storage even if overall body mass is unchanged but if it is mostly offset by elevated food intake, as found here, then the impacts may be small.
Keywords: Brown adipose tissue, Energy expenditure, Body fat, Energy intake, Thermogenesis, Cold
Highlights
-
•
Male and female mice were exposed to 11 different ambient temperatures between 5 and 35 °C.
-
•
As it got colder mice increased both energy expenditure and food intake.
-
•
Increased energy expenditure was coincident with increased THs and BAT activity.
-
•
Stored fat was considerably reduced in colder conditions, providing about 10% of the elevated energy requirements.
-
•
Elevating activity of BAT may be a valid strategy to reduce fat storage.
1. Introduction
Obesity is a global health issue [1,2] because it leads to elevated risks for several non-communicable diseases such as type 2 diabetes, hypertension and cancer [3,4]. Obesity results from an imbalance between energy intake and expenditure [5] the causes of which remain disputed [6,7]. In addition to white adipocytes, the primary role of which is to store triglycerides, Eutherian mammals also have several other types of adipocyte that serve different functions [8]. In particular, brown adipocytes appear to primarily serve to generate heat that can be used for thermoregulation, without the need for shivering – non-shivering thermogenesis (NST). Brown adipocytes generate heat in abundant mitochondria which have the uncoupling protein 1 (UCP1) on their inner membranes. This protein can uncouple the passage of protons across the inner membrane from synthesis of ATP, and this leads to release of their chemi-osmotic potential directly as heat [9]. A third type of adipocyte called beige or brite adipocytes [10,11] seem able to display both white and brown phenotypes under different circumstances [12].
Brown adipose tissue (BAT), is found in large depots in small mammals and neonatal humans [9,13]. It's presence in adult humans was conclusively demonstrated around 10–15 years ago [[14], [15], [16], [17], [18]]. Levels of BAT in adult humans decline as we age [8,16] coincident with a decline in whole body metabolic rate [19] and an increase in adiposity [20]. Moreover, levels of BAT appear to be inversely related to the amount of white adipose tissue (WAT) [18,21,22]. This correlation has been widely interpretated as suggesting activation of BAT might be protective against obesity. However, alternative explanations are possible. For example, higher levels of WAT may reduce thermoregulatory requirements due to its insulative properties [23] and that may reduce the requirement for thermogenesis derived from BAT, and therefore the amount of BAT tissue. Despite these caveats, the potential for obesity treatment by stimulating BAT, or forcing beige adipocytes to adopt their brown phenotype has received enormous attention, particularly in studies of mice [12,22,[24], [25], [26]]. This has been stimulated in part by observations that transplanting BAT but not WAT in mice causes weight loss and can reverse diet induced or genetic obesity [[27], [28], [29]].
When animals are exposed to the cold they increase their energy expenditure to balance the elevated heat loss [30,31]. During acute cold exposure this is mostly generated by shivering but after a period of acclimation this heat requirement is supplied mostly by BAT [32]. This stimulation of BAT by cold is consistent with the fact that levels of BAT in humans, as detected by PET-CT, increase during the temperate winter [17,33], and in relation to colder ambient temperatures [8,[34], [35], [36]]. If these increases in BAT activity protect against obesity, one would predict obesity should be less common in colder areas. Yet within the mainland USA such a relationship is not observed [37]. This absence of an expected relationship may pose an issue for the whole approach of stimulating BAT to treat obesity [38,39]. However, there are several reasons why a relationship between ambient temperature and obesity may not be observed. First, humans may buffer themselves from environmental temperature changes by modulating their external insulation (clothes), and controlling the environments where they spend most of their time, to buffer themselves from the cold outside. This would then decouple their metabolic responses from the apparent environmental exposure, and hence the absence of a relation between cold and obesity may tell us nothing about the effectiveness of switching on brown adipocytes or promoting browning for effects on fat storage. Direct measures of energy expenditure across the USA indeed suggest such decoupling occurs [40]. The fact BAT is stimulated in winter, however, suggests at least some individuals do experience the cold ambient temperature, and elevate their thermoregulatory heat production in response. This may not affect their fat storage for other reasons. For example, the elevated resting energy expenditure may stimulate greater food intake leading to no change in overall energy balance [[41], [42], [43], [44]]. Some evidence suggests such compensatory effects are not complete [45]. Alternatively, increases in BAT derived energy expenditure, when exposed to cold, may be offset by changes in the energy budget at other times, eg reduced physical activity [46]. Either of these effects might complicate a strategy to treat obesity by stimulating BAT/browning. Finally, the reported absence of an effect of cold on obesity levels is based on measures of body mass index (BMI) [37] which is a notoriously poor proxy for fat storage at the individual level [47]. Hence cold may reduce body fat, but if it also stimulated growth of lean tissue (including BAT) there might be no effect on overall BMI, which accounts only for overall mass changes. Contrasting these population level observations, short periods of exposure to mild cold (17 °C) for 2 h per day for 6 weeks did result in an increase in BAT and concomitant decrease in body fat mass by 0.7 kg [48], a difference potentially undetectable in population surveys.
Surprisingly, given the extensive use of the mouse as a model for exploring the mechanisms of stimulating BAT and WAT browning there are relatively few studies that have looked at the detailed energy balance responses of mice across a wide range of ambient temperatures. Here we provide such a study exploring the energy balance and body composition of mice exposed to 11 different ambient temperatures between 5 °C and 35 °C for a period of 2 weeks (roughly equivalent to 1.5 years in a human) in both male and female mice. We show colder ambient temperatures progressively stimulated both energy expenditure and food intake, and hence there was no overall trend in body mass. However, body composition changed, with cold ambient temperature exposure linked to reduced fat and elevated liver and small intestine sizes. Reduced fat contributed about 10% to the elevated energy needs, independent of ambient temperature and sex.
2. Materials and methods
2.1. Animals and experiment protocol
Experimental subjects were the offspring of an outbred breeding colony of Swiss mice maintained at Wenzhou University, Wenzhou, China. Animals were housed individually in plastic cages (Model M1, 29 × 18 × 16 cm, Suzhou Fengshi Experimental Animal Equipment Co., Ltd) with sawdust bedding, and were provided ad libitum with food (standard low fat diet with 11.8% fat: Beijing KeAo Feed Co., Beijing, China) and water. Animals were kept under a 12 h:12 h light:dark cycle (lights on at 08:00 h) at a constant ambient temperature of 21 ± 1 °C prior to ambient temperature manipulations. This experiment was approved by the Wenzhou University Animal Care and Use Committee (WU-ACUC WZU-080), and all experimental procedures complied with guidelines of the WU-ACUC.
2.2. Effects of exposure to a range of ambient temperature from 5 °C to 35 °C
Eighty-eight female and eighty-eight male mice aged 14 weeks were randomly assigned into one of 11 groups: 5, 9, 12, 15, 18, 21, 25, 27.5, 30, 32.5 and 35 °C groups with 8 females and 8 males in each group. All animals were fed a purified low fat diet (D12450B; Research Diets, Inc., New Brunswick, NJ, USA) for one week, and then were transferred into the ambient temperatures in a range from 5 to 35 °C for 2 weeks. We provided the mice with same sawdust bedding (about 30 g) across all 11 temperature groups, and did not provide any other materials or shelters against different temperature. Body mass and food intake were measured before and 14 days of ambient temperature treatment. The food intake was calculated as the difference of the diet mass in the hopper between consecutive two days and averaged over the two weeks.
2.3. Gross energy intake (GEI) and digestibility
The GEI and digestibility were measured over 48 h between day 12–13 of the exposure to different ambient temperature. As described previously [49,50], a known quantity of food was provided, and any uneaten food and orts in bedding material, along with feces from each animal, were collected 48 h later. Food and feces were separated manually after drying at 60 °C to constant mass. The gross energy content of feces was determined using an oxygen bomb calorimeter (C2000 basic 07.278080, IKA, Germany). The GEI, digestive energy intake (DEI), digestibility and gross energy of feces (GEF) were calculated as described previously [49,50].
2.4. Body temperature (Tb)
The Tb was measured between 16:00 and 17:00 on day 14 of the exposure to different ambient temperatures using an encapsulated thermo-sensitive passive transponder (Model GPR + WMQ-30005, diameter 2 mm and length 14 mm; Destron Fearing, South St Paul, USA). Briefly, a transponder was implanted in the abdomen of each mouse according to the instruction of the small device, which allowed Tb data to be collected with a Pocket Reader (Destron Fearing, South St Paul, USA) with minimal disturbance.
2.5. Resting metabolic rate (RMR) and nor-epinephrine stimulated thermogenesis (NeST)
The RMR and NeST were quantified on day 15 of the exposure to different ambient temperature as the rate of oxygen consumption, using an open-flow respirometry system (Model FC-10, Sable Systems, Las Vegas, NV, USA). As described previously [51], air was pumped at a rate of 600–850 mL/min through a cylindrical sealed Perspex chamber. An incubator was used to control the temperature (±0.5 °C) of chambers. Gases leaving the chamber were subsampled at a flow rate of 200 mL/min to an oxygen analyzer (FC-10 oxygen analyzer, Sable Systems). Incurrent air to the chamber (Flowbar-8 Mass Flow Meter System) and excurrent gases were dried using anhydrous silica gel. The data were collected every 10s by an analog-to-digital converter (STD-UI2, Sable Systems). The mouse was transferred into chambers for 1 h for adaptation to the chamber. RMR was measured for 2.5 h at 30 ± 0.5 °C (within the thermoneutral zone of this species) and calculated from the lowest consecutive readings over 10 min.
NeST was determined for another 60min after RMR measurements, which was estimated as the maximal rate of oxygen consumption induced by subcutaneous injection of noradrenaline (norepinephrine; NA) (H31021177, Shanghai Harvest Pharmaceutical Co. Ltd, China) at 25 ± 1 °C. A mass dependent dosage of NA (in mg/kg) was calculated according to the equation: NA = 6.6 Mb−0.458 (where Mb is in g) [52]. NeST was calculated from the highest consecutive readings over 10 min. RMR (mL O2/h) and NeST (mL O2/h) were corrected to standard ambient temperature and air pressure conditions. All measurements were made between 10:00 h and 17:00 h.
2.6. Body composition and body fat depots
Animals were euthanized by decapitation between 9:00 and 11:00 at the end of the experiment, taking circadian rhythmicity into consideration when planning the dissection. Trunk blood was collected, and after coagulating for 2 h, serum was separated from each blood sample by centrifugation (3500 g/min × 10 min) and stored at −80 °C until required for assay. The interscapular BAT, and small intestine without content was removed and weighed (to 1 mg), and immediately frozen using liquid nitrogen and stored at −80 °C until the later measurements. Subcutaneous fat, perirenal fat, mesenteric fat and abdominal fat, and peritesticular fat (for male) were collected and weighed (±1 mg), which were summed as fat depots of the mouse. The liver, heart, spleen, lungs, and kidneys were then removed and weighed (±1 mg). The gastrointestinal tract was removed from each animal, and the stomach, and large intestine and caecum, separated and weighed without their contents (±1 mg). The remaining carcass, including the head and tail, but excluding the reproductive organs, was also weighed.
2.7. The mass, and sucrase, maltase, and aminopeptidase activity of small intestine
As described previously, the activity of several digestive enzymes was measured in whole-tissue homogenates rather than in mucosal samples to avoid underestimation of activity as previously reported [[53], [54], [55], [56]]. We measured the activity of sucrase (EC 3.2.1.48) and maltase (EC 3.2.1.20) using commercial kits (sucrase, A082-2-1; maltase, A082-3-1; Jiancheng Biotech Co. Ltd, Nanjing, China) according to the manufacturer's protocols. The inter- and intra-assay variations were, respectively, 5.0% and 3.1% for sucrase and 5.1% and 3.1% for maltase. Sugar and maltose were used as the substrate for the sucrase and maltase measurements, respectively. The activity of both enzymes was expressed in U/g tissue (1 U was defined as 1 nmol sucrase or maltase hydrolyzed at 37 °C per minute). Aminopeptidase-N activity was measured using methods described previously [53,54,57]. Briefly, l-alanine p-nitroanilide was used as a substrate for the aminopeptidase-N assay (H583, Jiancheng Biotech Co. Ltd, Nanjing, China). Aminopeptidase activity was expressed as U/g tissue. The inter- and intra-assay variations were 7.8% and 5.1%, respectively.
2.8. BAT mass, mitochondria protein and cytochrome c (COX) activity
The BAT was homogenized, and mitochondrial protein was prepared as previously described [58,59]. Mitochondrial protein concentration was determined by the Folin phenol method (P824172, Shanghai Macklin Biochemical Co., Ltd) with BSA as standard [60]. Cytochrome c oxidase (COX) activity in the whole BAT tissue was measured polarographically with cytochrome c as a substrate (C824443, Shanghai Macklin Biochemical Co., Ltd) using oxygen electrode units (Model Oxytherm, Hansatech Instruments Ltd, King's Lynn, Norfolk, UK) [59,61].
2.9. Serum T3 T4, leptin, insulin, and glucose concentrations
As described previously [59], serum T3 and T4 levels were determined by radioimmunoassay using I125 RIA kits (T3, KIP1631, T4, KIP1641, Beijing North Institute of Biological Technology, China). Serum leptin concentration was measured using a Mouse Leptin ELISA Kit (EK297–96, MULTI SCIENCES Biotechnology Co., Ltd, China), according to the instructions. Serum insulin concentration was measured using a commercial Mouse Insulin ELISA Kit (10-1247-01, Mercodia AB, Uppsala Sweden) according to the instructions. The detection limit of serum insulin is ≤ 0.2 ng/mL. Serum glucose was evaluated by the method of glucose oxidase-peroxidase method (GOD-POD) using a commercial Kit (E-BC-K234-S, Elabscience Biotechnology Co., Ltd, China). The detection range of serum glucose is 0.05–30 mmol/L with a sensitivity of 0.05 mmol/L.
2.10. Statistical analysis
Data were analyzed using SPSS 21.0 statistic software. The effect of ambient temperature on body mass, Tb, food intake, GEI, digestibility, RMR, NeST, as well as digestive enzymes activity and serum measures and organs were analyzed using Linear regression models with ambient temperature as a continuous variable. For data where a linear model in relation to temperature was clearly inappropriate (Tb, RMR) we used one-way ANOVA to seek significant temperature effects among the different temperatures. The one-way ANOVA was followed by Tukey's post hoc test where required to examine the differences between the groups. We used Pearson's correlation and linear regression (general linear model, GLM) to model the relationship between food intake, RMR, NeST, fat depot, and serum measures.
3. Results
3.1. Food intake, digestibility, and gut enzyme activities
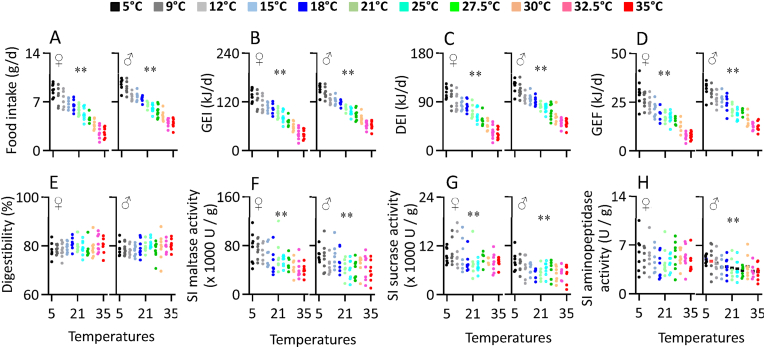
Food intake increased linearly with decreasing ambient temperature in both sexes (female, F1,86 = 421.28, P < 0.001, male, F1,86 = 587.40, P < 0.001, Figure 1A, Table S1). Food intake at 5 °C was 2.5 times greater in females and 1.5 times greater in males compared to intake at 35 °C. Gross energy intake (GEI) also increased linearly with declining ambient temperature (female, F1,86 = 422.33, P < 0.001, male, F1,86 = 587.38, P < 0.001, Figure 1B, Table S1). On average at 35 °C females consumed 39.58 kJ/d (sd = 11.77) and males 61.95 kJ/d (sd = 10.37) while at 5 °C these had increased to 139.10 kJ/d (sd = 15.06) and 152.45 kJ/d (sd = 12.76) respectively, reflecting increases of 251% and 146%. Digested energy intake (DEI) followed a similar pattern with the maximum was observed at 5 °C and the minimum was found at 32.5 °C–35 °C, and a linear change between these ambient temperatures (female, F1,86 = 351.52, P < 0.001, male, F1,86 = 403.81, P < 0.001, Figure 1C, Table S1). Female mice at 5 °C were digesting 77.35 kJ/d more and for males 70.75 kJ/d more than mice at 35 °C. In line with the changed food intake, both females and males produced significantly more fecal energy (GEF) as ambient temperature declined (female, F1,86 = 328.67, P < 0.001, male, F1,86 = 398.12, P < 0.001, Figure 1D, Table S1).
Figure 1.
Food intake (A), gross energy intake, GEI (B), digestive energy intake, DEI (C), gross energy of feces, GEF (D) and digestibility (E), and activity of maltase (F), sucrase (G) and aminopeptidase (H) of the small intestine of Swiss mice exposed to a range of ambient temperatures from 5 to 35 °C. Each point represents a different individual. SI, small intestine; ∗, significant effect of ambient temperature (P < 0.05), ∗∗, P < 0.01.
Despite the very large increase in food intake the digestive efficiency did not differ significantly across the 11 ambient temperature groups (female, F1,86 = 0.92, P = 0.341, male, F1,86 = 2.79, P = 0.099, Figure 1E, Table S1), and averaged 79.45% (sd = 2.83) in females and 79.28% (sd = 2.82) in males. This consistent level of digestive efficiency in the face of a 1.46–2.51 fold increase in energy intake was linked to changes in the activity of major digestive enzymes in the small intestine. Maltase, sucrase and aminopeptidase activities all increased linearly with decreasing ambient temperature in both sexes (Maltase: female, F1,86 = 43.01, P < 0.001, male, F1,86 = 13.52, P < 0.001, Figure 1F; Sucrase: female, F1,86 = 9.63, P = 0.003, male, F1,86 = 17.36, P < 0.001, Figure 1G; Aminopeptidase: male, F1,86 = 14.17, P < 0.001, Figure 1H), except for aminopeptidase activity of females (female, F1,86 = 0.65, P = 0.424, Figure 1H, Table S1).
3.2. Energy expenditure, body temperature (Tb) and BAT activity
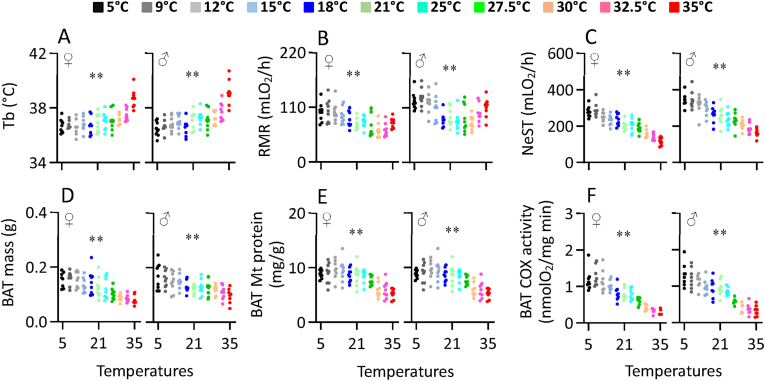
The Tb of both females and males was significantly affected by ambient temperature (females, F10,77 = 7.98, P < 0.001, males, F10,77 = 10.77, P < 0.001, Figure 2A). Levels of Tb were not significantly different between 5 and 27.5 °C, and averaged 36.8 °C (sd = 0.6) in females and 36.9 °C (sd = 0.6) in males, but thereafter increased rapidly. At 35 °C the Tb were 38.7 °C (sd = 0.7) in females and 39.1 °C (sd = 0.9) in males both of which were significantly elevated relative to ambient temperatures below 27.5 °C. The pattern of change in RMR with ambient temperature (females, F10,77 = 5.10, P < 0.001, males, F10,77 = 5.84, P < 0.001, Figure 2B) included a zone beyond which higher and lower ambient temperatures led to significantly increased RMR. In females this zone extended from 27.5 to 32.5 °C. RMR was increased at 35 °C, and increased linearly between 27.5 and 9 °C. There was no difference in RMR between the 5 °C and 9 °C groups. In males the zone where RMR was unchanged extended from 25 to 30 °C. RMR was significantly elevated at 32.5 and 35 °C, and increased linearly below 25 °C–9 °C. As with the females there was no difference in RMR between the 5 °C and 9 °C groups in males. The norepinephrine stimulated thermogenesis (NeST) increased linearly with declining ambient temperature with the maximum observed in the 5 °C and 9 °C groups, and the minimum at 35 °C (females, F1,86 = 247.97, P < 0.001, males, F1,86 = 188.55, P < 0.001, Figure 2C, Table S2). The BAT mass mirrored the change in NeST increasing linearly as ambient temperature declined across the range (female, F1,86 = 79.19, P < 0.001, male, F1,86 = 38.59, P < 0.001, Figure 2D, Table S2). In contrast, BAT mitochondrial protein content increased as ambient temperature declined to 21 °C but thereafter was unchanged at lower ambient temperatures (female, F1,86 = 51.73, P < 0.001, male, F1,86 = 19.96, P < 0.001, Figure 2E, Table S2). In contrast BAT mitochondrial COX activity was increased linearly across the range between 35 and 9 °C (female, F1,86 = 238.71, P < 0.001, male, F1,86 = 222.14, P < 0.001, Figure 2F, Table S2) but was not further increased at 5 °C compared to 9 °C.
Figure 2.
The body temperature, Tb (A), resting metabolic rate, RMR (B) and NE-stimulated thermogenesis, NeST (C), and BAT mass (D), mitochondrial protein content (E) and COX activity (F) of Swiss mice exposed to a range of ambient temperatures from 5 to 35 °C. The X axis in panels A,D,E,F is the ambient temperature at which the animals were housed and measured. For panels B and C, the temperature refers to the housing temperature only. RMR (panel B) was measured at a fixed temperature of 30 °C, and NeST (panel C) was measured at a fixed temperature of 25 °C. Each point represents a different individual. ∗∗, significant effect of ambient temperature (P < 0.01).
3.3. Body mass and composition
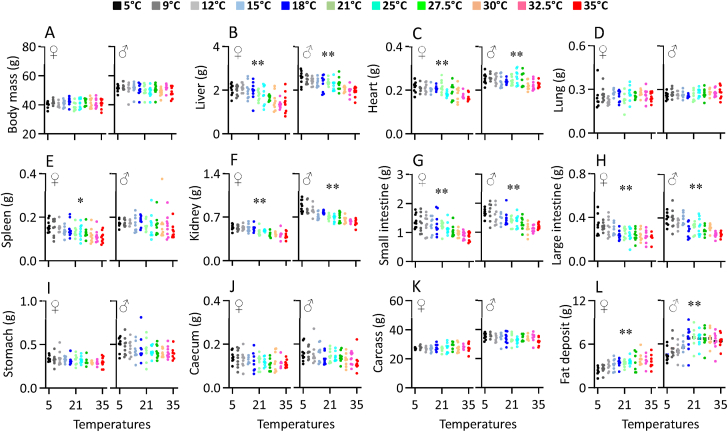
Body mass did not differ significantly over the range of ambient temperatures from 5 to 35 °C (female, F1,86 = 0.28, P = 0.579, male, F1,86 = 4.16, P = 0.054, Figure 3A) and averaged 40.9 g (sd = 2.76) in females and 50.3 g (sd = 3.69) in males. The liver mass of females and males increased as ambient temperatures fell from 35 to 18 °C, but at lower ambient temperatures was unchanged (Figure 3B, Table 1). A similar pattern was observed for the heart mass of the females, but in males heart mass was independent of ambient temperature between 5 and 27.5 °C (Figure 3C, Table 1). Lung mass was independent of ambient temperature in both females and males (Figure 3D, Table 1). The spleen mass significantly increased with decreasing ambient temperature for females but not males (Figure 3E, Table 1). In females the mass of the kidneys showed a similar pattern to the liver, increasing in size between 35 and 18 °C but then remaining unchanged at ambient temperatures below that (Figure 3F, Table 1). In contrast in males the size of the kidneys increased linearly between 35 and 5 °C (Figure 3F, Table 1). The masses of the small and large intestines both increased linearly as it became colder in both sexes (small intestine, Figure 3G; large intestine, Figure 3H, Table 1). In contrast masses of the stomach, caecum and carcass were not significantly associated (p > .05) with temperature (Figure 3I,J and 3K, Table 1). The mass of fat depots of females was independent of ambient temperature between 25 and 35 °C and in this range they averaged 3.66 g (sd = 0.90), but at ambient temperatures below 25 °C they declined and were lowest at 5 °C where they averaged 2.35 g (sd = 0.57). In males the pattern was similar. There was no significant difference between 18 and 35 °C where stored fat averaged 6.67 g (sd = 1.29). Below 18 °C levels of stored fat declined linearly to a minimum at 5 °C where they averaged 4.55 g (sd = 0.99) (Figure 3L, Table 1). Hence at 5 °C females had about 64.2% and males about 68.2% of the body fat they had at 25–35 °C.
Figure 3.
Body mass (A), and the mass liver (B), heart (C), lung (D), spleen (E), kidney (F), stomach (G), small intestine (H), large intestine (I), caecum (J), carcass (K), and fat deposit (L) of Swiss mice exposed to a range of ambient temperatures from 5 to 35 °C. Each point represents a different individual. ∗, significant effect of ambient temperature (P < 0.05), ∗∗, P < 0.01.
Table 1.
The regressions against ambient temperature for each organ.
| Organ | Intercept (g) | Gradient (g) | r2 | F | P |
|---|---|---|---|---|---|
| Females: | |||||
| Body mass (g) | 40.556 ± 0.713 | 0.016471 ± 0.031043 | 0.003 | 0.282 | 0.597 |
| Liver (g) | 2.289 ± 0.088 | −0.024482 ± 0.003838 | 0.322 | 40.842 | 0.000 |
| Heart (g) | 0.229 ± 0.006 | −0.001509 ± 0.000277 | 0.262 | 30.493 | 0.000 |
| Lung (g) | 0.244 ± 0.012 | 0.000533 ± 0.000518 | 0.012 | 1.055 | 0.307 |
| Spleen (g) | 0.168 ± 0.008 | −0.001582 ± 0.000340 | 0.201 | 21.689 | 0.000 |
| Kidney (g) | 0.583 ± 0.013 | −0.004985 ± 0.000578 | 0.464 | 74.327 | 0.000 |
| Stomach (g) | 0.353 ± 0.013 | −0.001719 ± 0.000563 | 0.100 | 9.572 | 0.003 |
| SI (g) | 1.527 ± 0.064 | −0.017703 ± 0.002795 | 0.318 | 40.110 | 0.000 |
| LI (g) | 0.344 ± 0.016 | −0.003796 ± 0.000706 | 0.255 | 29.359 | 0.000 |
| Caecum (g) | 0.142 ± 0.008 | −0.000906 ± 0.000351 | 0.073 | 6.791 | 0.011 |
| Fat depot (g) | 2.387 ± 0.197 | 0.043081 ± 0.008581 | 0.227 | 25.305 | 0.000 |
| Carcass (g) | 26.929 ± 0.571 | 0.028548 ± 0.024865 | 0.015 | 1.318 | 0.254 |
| Males: | |||||
| Body mass (g) | 52.048 ± 0.935 | −0.082984 ± 0.040703 | 0.046 | 4.157 | 0.054 |
| Liver (g) | 2.677 ± 0.065 | −0.019621 ± 0.002840 | 0.350 | 46.337 | 0.000 |
| Heart (g) | 0.263 ± 0.007 | −0.000939 ± 0.000283 | 0.108 | 10.465 | 0.002 |
| Lung (g) | 0.259 ± 0.011 | 0.000525 ± 0.000463 | 0.009 | 0.758 | 0.386 |
| Spleen (g) | 0.181 ± 0.011 | −0.000727 ± 0.000476 | 0.027 | 2.359 | 0.128 |
| Kidney (g) | 0.906 ± 0.018 | −0.008578 ± 0.000767 | 0.591 | 124.302 | 0.000 |
| Stomach (g) | 0.503 ± 0.024 | −0.003136 ± 0.001062 | 0.093 | 8.839 | 0.004 |
| SI (g) | 1.802 ± 0.055 | −0.019141 ± 0.002399 | 0.426 | 63.800 | 0.000 |
| LI (g) | 0.423 ± 0.016 | −0.004890 ± 0.000677 | 0.376 | 51.796 | 0.000 |
| Caecum (g) | 0.169 ± 0.009 | −0.001232 ± 0.000379 | 0.108 | 10.453 | 0.002 |
| Fat depot (g) | 4.750 ± 0.336 | 0.064716 ± 0.014643 | 0.186 | 19.601 | 0.000 |
| Carcass (g) | 35.377 ± 0.720 | −0.067756 ± 0.031358 | 0.051 | 4.669 | 0.034 |
SI, small intestine; LI, large intestine. Data are intercept ± standard deviation or gradient ± standard deviation.
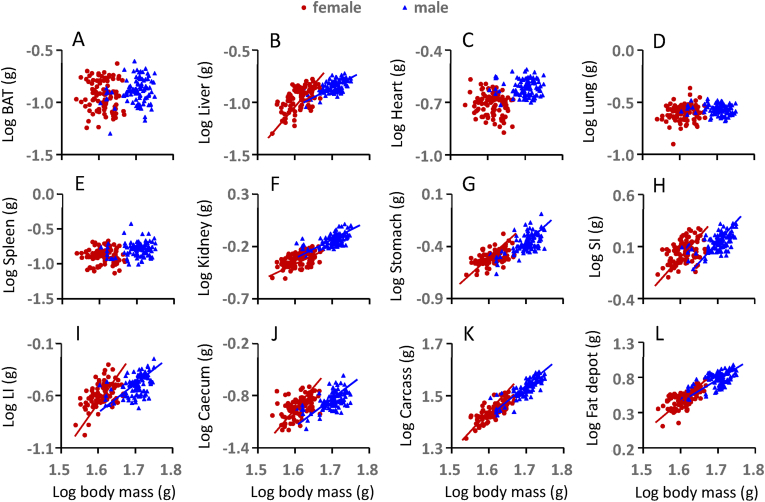
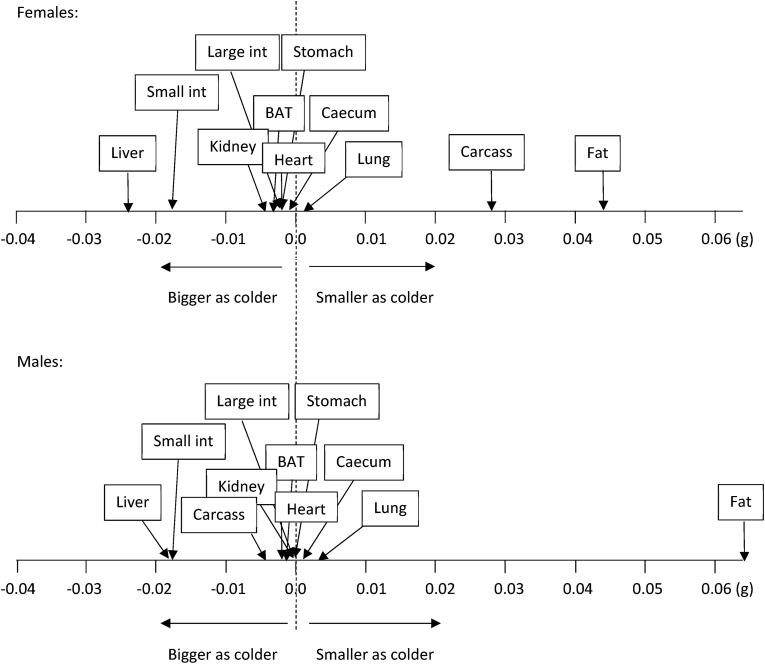
Changes in tissue utilization as ambient temperature changed were explored by plotting the logged size of the different organs in relation to logged body masses (Figure 4, Table S3), and then locating the gradients of these relationships along a utilization axis, with negative values showing tissues that grew larger as it got colder and positive values reflecting tissues that shrunk as it got colder (Figure 5) [62]. This analysis revealed similar body composition remodeling as ambient temperatures fell in both males and females. In particular, the small intestine and liver grew disproportionately larger, while fat stores were the only tissue that became substantially smaller. The overall stability of body mass as ambient temperature changed (Figure 3A) was therefore largely because decreases in white adipose tissue depots were reduced to the same extent that the liver and small intestines were growing.
Figure 4.
The correlations of the log organ weight against the log body weight for all the data across all ambient temperatures. The regression lines were then fitted if the regression was statistically significant (P < 0.05). (A) brown adipose tissue, BAT; (B) liver; (C) heart; (D) lung; (E) spleen; (F) kidney; (G) stomach; (H) small intestine, SI; (I) large intestine, LI; (J) caecum; (K) carcass; and (L) fat deposit. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Figure 5.
Changes in tissue utilization as ambient temperature changed. BAT, brown adipose tissue; Small int, small intestine; Large int, large intestine.
3.4. Serum T3 and T4
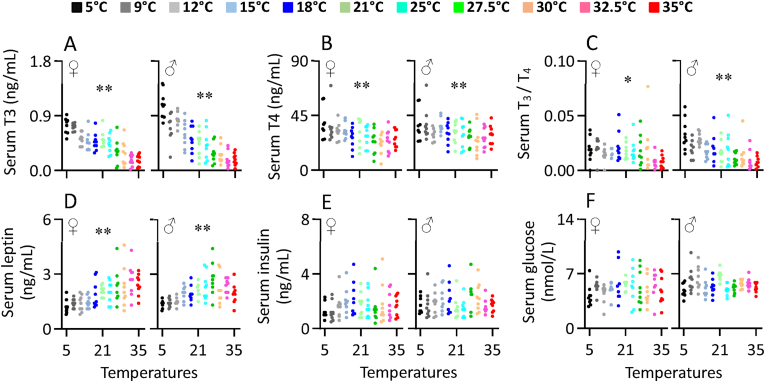
Serum T3 concentration increased linearly with declining ambient temperature over the range form 35 down to 5 °C, in both sexes (females, F1,86 = 122.76, P < 0.001 and males, F1,86 = 143.28, P < 0.001, Figure 6A, Table S4). Serum T3 at 5 °C was increased by 44.6% for females and 147.7% for males compared to that at 21 °C, whereas that at 35 °C decreased by 68.1% for females and 58.2% for males relative to 21 °C. Serum T4 concentration was also significantly increased with decreasing ambient temperature for females (F1,86 = 19.83, P < 0.001) and males (F1,86 = 11.15, P < 0.001, Figure 6B). The ratio of T3 to T4 also increased considerably with decreasing ambient temperature for females (F1,86 = 7.02, P = 0.010) and males (F1,86 = 32.64, P < 0.001, Figure 6C, Table S4).
Figure 6.
The concentration of serum T3 (A), T4 (B) and the ratio of T3 to T4, T3/T4 (C), and the concentration of serum leptin (D), insulin (E) and glucose (F) of Swiss mice exposed to a range of ambient temperatures from 5 to 35 °C. Each point represents a different individual. ∗∗, significant effect of ambient temperature (P < 0.01).
3.5. Serum leptin, insulin and glucose
Serum leptin concentrations as expected mirrored the patterns of change in body fat and were independent of ambient temperature above 21–25 °C, below 21 °C decreased significantly for females (F1,86 = 40.50, P < 0.001) and males (F1,86 = 22.78, P < 0.001, Figure 6D, Table S4). Leptin at 5 °C was decreased by 41.5% and 35.4%, respectively, for females and males compared to that at 21 °C. Serum insulin concentration was not significantly affected by ambient temperature for either females (F1,86 = 0.61, P = 0.436 or males (F1,86 = 0.06, P = 0.811, Figure 6E). Serum glucose level was also not significantly affected by ambient temperature for both females (F1,86 = 0.57, P = 0.453) and males (F1,86 = 0.83, P = 0.366, Figure 6F, Table S4).
3.6. Relationships between food intake, expenditure, and hormone levels
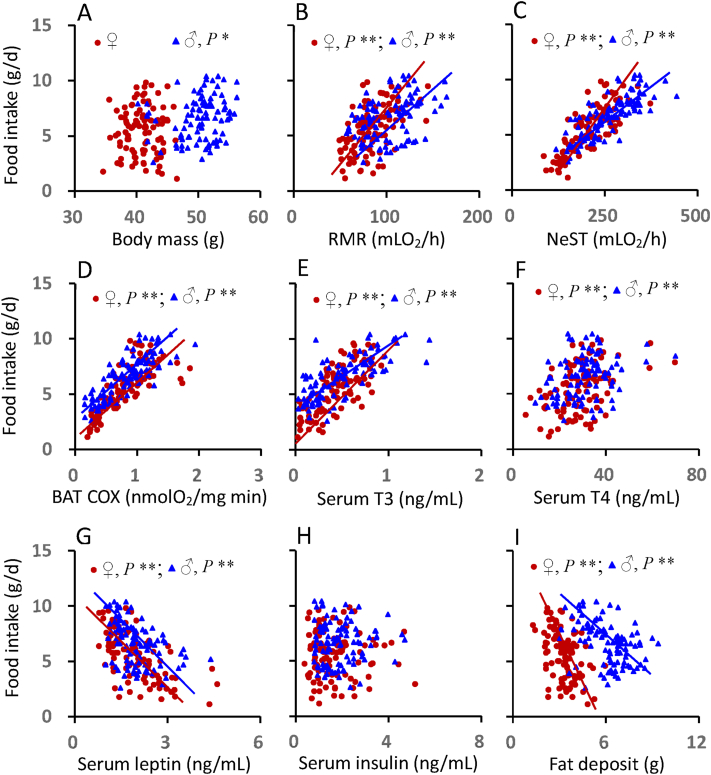
There was significant correlation between food intake and body mass for males, whereas no significant correlation was observed in females (Figure 7A, Table S5). Food intake was positively correlated with RMR, NeST and BAT COX activity for both females and males (Figure 7B,C, 7D, Table S5). Food intake was positively correlated with serum T3 and T4 levels for both sexes (Figure 7E, F, Table S5). Food intake was negatively correlated with serum leptin levels for females and males (Figure 7G, Table S5). There was no significant correlation between food intake and serum insulin levels for either females or males (Figure 7H, Table S5). Because food intake increased as it got colder but fat depots got smaller there was a negative correlation between food intake and fat depot size in both sexes (Figure 7I, Table S5).
Figure 7.
The relationship between food intake and body mass (A), RMR (B), NeST (C), BAT COX activity (D), serum T3 (E) T4 (F), leptin (G), insulin (H) and fat deposit (I) of Swiss mice exposed to a range of ambient temperatures from 5 to 35 °C. Each point represents a different individual. ∗∗, significant correlation (P < 0.01).
Because many of the traits in the univariate relationships are correlated with each other we used multiple regression analysis to identify the most significant factors linked to food intake. In females the most significant regression model included NeST, BAT COX activity, serum T4 and serum leptin levels the best fit regression Food intake = −0.410 + 0.018NeST +0.071BAT.COX +0.038T4 – 0.411Leptin, F1,91 = 73.888, P < 0.0001 explained 76.5% of the variation in food intake between individuals across temperatures. Similarly for males the best fit multiple regression model included NeST and BAT COX activity in addition to circulating insulin levels. The equation y = 0.780 + 0.125BAT.COX +0.012NeST +0.279Insulin, F1,95 = 106.338, P < 0.0001 explained 76.3% of the variation in food intake.
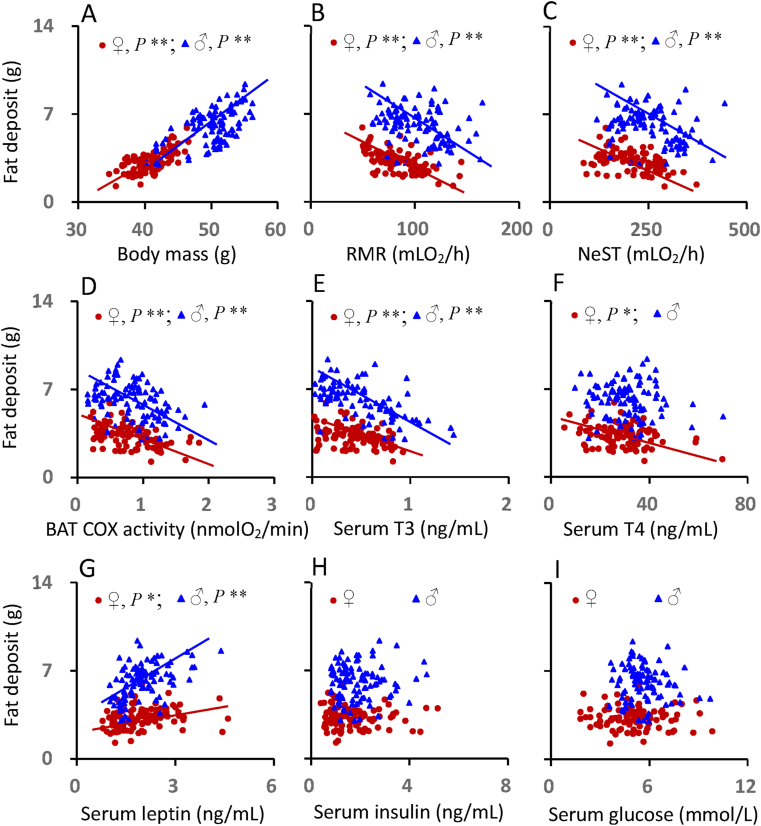
3.7. Relationships of fat depot mass, energy expenditure and hormone levels
The total fat depot was positively correlated with body mass for females and males (Figure 8A, Table S6). There were significantly negative correlations between total fat depot size and RMR, NeST and the BAT COX activity for females and males, indicating that the mice who had higher metabolic thermogenesis had the less fat (Figure 8B,C and 8D, Table S6). The fat depot size was negatively correlated with serum T3 levels for females and males (Figure 8E), whereas the correlations between fat depot and serum T4 was observed in females only (Figure 8F, Table S6). There were positive correlations between the total fat depot and serum leptin levels for both females and males (Figure 8G), whereas no correlations were observed between fat depot and serum insulin (Figure 8H) or serum glucose (Figure 8I, Table S6). In the multivariate analysis the best fit regression model Fat depot size = −2.331 + 0.178 Body mass - 0.012RMR - 0.026BAT COX - 0.117Insulin, F1,91 = 49.898, P < 0.0001 explained 68.7% of the variation in the level of stored fat, while in males the best fit equation was Fat depot size = - 2.830–2.450T3 + 0.202Body mass, F1,96 = 85.046, P < 0.0001; which explained 68.2% of the variation in body fat.
Figure 8.
The relationship between fat deposit and body mass (A), RMR (B), NeST (C), BAT COX activity (D), serum T3 (E) T4 (F), leptin (G), insulin (H) and glucose levels (I) of Swiss mice exposed to a range of ambient temperatures from 5 to 35 °C. Each point represents a different individual. ∗∗, significant correlation (P < 0.01).
4. Discussion
The mice in the present study elevated their food intake, gross energy and digested energy intake in a linear fashion as ambient temperature declined. This is consistent with the ‘Scholander’ thermoregulation curves where the gradient of the relationship reflects the whole body thermal conductance. The food intake was not significantly different between 32.5 and 35 °C suggesting this was the thermoneutral zone (TNZ) bounded by a lower critical ambient temperature of 32.5 °C [30,31,63]. In contrast the body temperature was already significantly elevated at 32.5 °C in females and 30 °C in males, suggesting the actual lower critical temperature was probably slightly lower at around 30 °C. Moreover, constructing the thermoregulation curves based on daily food intake neglected potential shifts in the thermoneutrality between light and dark phases. Measures of RMR at each of the temperatures and in light and dark phases would be necessary to establish the location of the actual lower critical temperature and thermoneutral zone.
These data contribute to the debate regarding the optimal housing ambient temperature for mice to translate studies to humans [[64], [65], [66], [67], [68], [69], [70]] and clearly suggest, given that humans routinely occupy habitats with ambient temperatures about 2–5 °C lower than their LCT [69,70] that this strain of mouse should be ideally maintained around 26–28 °C. The suggestion that mice in general should be housed at 30 °C [65,66,68] is clearly inappropriate (at least for this outbred strain) since for these mice 30 °C is already at the lower critical temperature, an ambient temperature that humans routinely avoid. As we have shown recently, housing this strain at 32.5 °C shortens their lifespan significantly, relative to those kept at 21 °C [71] illustrating there are negative health impacts of keeping rodents at high ambient temperatures.
As ambient temperature fell below the LCT there was a linear increase in RMR to 9 °C but it was not further elevated at 5 °C. The lack of a linear increase in RMR at 5 °C is potentially due to a slightly lower Tb [72]. This pattern is also consistent with metabolic rates measured in non-cold acclimated MF1 mice [73] which reached a summit metabolic response around 0 °C. Core body temperature below LCT was consistent with previous studies of mice and other laboratory rodents [[73], [74], [75]] (early work reviewed in [63]). The increase in metabolism as ambient temperature fell below the LCT was correlated with increased levels of T3, expansion of the BAT mass, increases in BAT mitochondrial protein, BAT COX activity and direct measures of non-shivering thermogenesis. These observations all support extensive previous work showing thermogenesis after about 10 days of cold exposure is provided predominantly by elevated heat production in BAT [26,[76], [77], [78]]. Our work shows that these responses are closely calibrated to the exact levels of cold, and the thermoregulatory requirements to sustain body temperature.
In parallel with the increased metabolic demands the mice elevated their food intake in direct relation to the falling ambient temperature and increased metabolic requirements. In the multivariate model the individual levels of food intake in both males and females were best described by the levels of NeST and BAT COX, along with more minor hormonal effects (T4, Insulin and leptin) that were not consistent across the sexes. We found that serum leptin concentration was significantly decreased in mice exposed to the gradient of decreased ambient temperature, which may partly explain the cold-induced increase in food intake. The main driver of intake therefore appeared to be energy demand. In contrast to these strong changes in intake and expenditure, body mass was constant and independent of ambient temperature. On the face of it these data provide an explanation for why there is no strong relationship between ambient temperature where people live and the local levels of obesity [37], despite at least some individuals in these areas having elevated activity levels of BAT, particularly in winter [79,80]. That is cold may stimulate BAT thermogenesis, but any increase in metabolic rate stimulates an offsetting level of elevated food intake. Other evidence supports such compensatory changes, but suggests the response is incomplete [45].
However, using body mass (or body mass index) as a measure of obesity is extremely crude [[81], [82], [83]] and may miss more subtle effects on body composition changes. That appears to be the case here, because although food intake increased enormously as it got colder this intake was insufficient to meet the elevated thermoregulatory requirements, and increasingly so as it got colder. Consequently, there was a progressive reduction in the level of stored fat as it got colder. At 5 °C the male mice on average lost 2.4 g of fat (maximally about 94 kJ assuming the loss was all lipid) relative to those at 27.5 °C. Over the same ambient temperature range the daily net food assimilation went up by 39 kJ/day. Consequently, over the 2 weeks exposure the white adipose tissue was contributed maximally about 12.1% of the total energy supply. The same calculation at different ambient temperatures for males gives 8.6% at 9 °C, 15.8% at 12 °C, and 11.2% at 15 °C. For females the % values are 7.5% at 5 °C, 7.1% at 9 °C, 6.6% at 12 °C, and 3.9% at 15 °C. The overall average values for males were maximally 11.5% (n = 32 mice below 15 °C) and for females maximally 8.1% (n = 32 mice below 15 °C). Thus the maximal contribution of fat loss to the total elevated requirements appears fairly constant at around 8–12%. A salient question is why is this allocation between fat and food observed? Why, for example, do mice at 12 °C not elevate their intake to the same level as those at 5 °C, thereby covering their entire metabolic demand. The fact they can eat that amount of food at 5 °C shows they are not constrained from achieving that level of intake. Understanding what regulates that allocation may be an important aspect of maximizing the effect of boosting BAT activity.
Because 90% of the elevated energy requirements as it got colder were met by increased food intake the mice had to modify their alimentary tract and associated organs to sustain their digestive efficiency constant at around 80%. Similar changes are observed in mice during lactation in the face of large increases in food intake [[84], [85], [86], [87]]. This included increases in the activity of various digestive enzymes in the small intestine, such as maltase, sucrase and aminopeptidase, combined with expansion in the size of the small intestine and the liver. Progressive expansion in the sizes of the liver and gut as it got colder offset the progressive but opposite change in the size of the adipose tissue depots – leading to the overall stable body mass as ambient temperature fell. The higher RMR in cold-exposed mice may be attributable to the increased weight of liver, and small and large intestine, and kidney, and decreased WAT in these mice.
Our data contrast the observations in rats kept until 12 weeks old at 28 °C and then transferred for 4 weeks to 20 °C [88]. In that study there was a suggested increase in adiposity, the opposite of our observation of decreased adiposity coincident with declining temperatures. However, the sample size in that study was small (two temperature exposure groups with n = 6 per group) and there were no discernible impacts of the temperature manipulation on either food intake or energy expenditure, indicating the exposure did not take the rats outside their thermoneutral zone when ‘cold exposed’. Moreover, while increased adiposity was mentioned in the title and abstract of the paper, the increase in total adipose tissue in the cold was not significant (Figure 2C in ref [88], p = 0.0532).
4.1. Limitations
Although our study makes a comprehensive coverage of the impacts of temperature using 11 different groups the data were generated for only one mouse strain feeding on a single diet. Human diets range from about 10 to 30% fat. And so the diet we used sits at the low end of the spectrum of diets consumed by humans. Higher fat diets may alter the relationships we observed in several different ways. Chief among which is that greater levels of fat might provide elevated insulation and that would be expected to move the positioning of the heat loss curves, and hence the need for greater metabolic rate to balance heat loss. The extent of such effects however depends on the distribution of body fat, and some have suggested that in mice body fat may provide little insulation [89]. This observation however has been disputed [23] and its relevance to humans questioned [90]. Future studies should employ additional strains and additional diets to elucidate the potential diet × strain interactions in the impacts of temperature. Another aspect of activating energy expenditure is whether this will be more effective at preventing the development of obesity than reversing it. This prevention or cure question has generated a vigorous debate with respect to the impacts of physical activity on weight regulation. Without the types of diet by temperature interaction manipulations it is not possible speculate on these effects from the current data. A further limitation of the study is that changes occurring in the 32.5–35 °C or 30–35 °C range are not detected due to only 1–2 points departing from the linear regression lines. Finer resolution sampling of ambient temperatures is needed to examine this.
5. Conclusions
In conclusion, observations that humans living in colder areas do not have reduced rates of obesity (based on BMI), despite some of them having stimulated BAT, do not necessarily negate the potential for stimulation of BAT to effect changes in body fatness. However, if the observations here, that only a small proportion (10%) of the stimulated metabolic rate is matched by reduced fat levels, while most of it is accommodated by increased food intake, proves also to be true in humans (see also ref [91]), then stimulating human BAT by cold may have only modest impacts on obesity levels. Impacts of BAT activity on food intake in humans however are unclear [91,92]. For example, if stimulating BAT increased human total metabolic rate by 10% (approximately 1 MJ/day) [19], but 90% of that was matched by elevated food intake, then the net impact on fat storage might amount to 100 kJ/day – leading to a loss of about 1 kg of body fat over the course of a year (NIH body weight planner simulation tool Body Weight Planner | NIDDK (nih.gov). This may be an optimistic scenario since estimates of the contribution of stimulated BAT to energy expenditure during mild cold stress in humans are much lower [[93], [94], [95], [96]]. However, exposing individuals to 17 °C for 2 h per day did result in 0.7 kg of weight loss after 6 weeks (see also ref [79,80]), suggesting these calculations maybe too pessimistic. Clearly understanding the mechanistic links between stimulated metabolism and stimulated food intake should be a key goal for future work so that the benefits of stimulating BAT are maximised.
Author contributions
Z.J.Z and J.R.S. conceived the project, designed the experiments, performed the data analysis, and wrote the manuscript. M.L. performed the data analysis and contributed first draft of the manuscript. Z.J.Z, R.Y., D.L.H., M.H.B. and J.C. performed majority of the experiments. R.Y. edited the subsequent versions. All authors read and approved the final version of the manuscript.
Data availability statement
All data that support the findings of this study are publicly available https://osf.io/am8nw/
Acknowledgments
This work was partly supported by grants (31670417 and 31870388 to Z.J.Z., and 92057206 to J.R.S) from the National Natural Science Foundation of China and the National Key R&D Program of China (2019YFA0801900 to J.R.S).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101551.
Contributor Information
Zhijun Zhao, Email: zhaozj@wzu.edu.cn.
John R. Speakman, Email: J.Speakman@abdn.ac.uk.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nature Reviews Endocrinology. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.Gregg E.W., Shaw J.E. Global health effects of overweight and obesity. New England Journal of Medicine. 2017;377:80–81. doi: 10.1056/NEJMe1706095. [DOI] [PubMed] [Google Scholar]
- 3.Babu G.R., Murthy G.V.S., Ana Y., Patel P., Deepa R., Neelon S.E.B., et al. Association of obesity with hypertension and type 2 diabetes mellitus in India: a meta-analysis of observational studies. World Journal of Diabetes. 2018;9:40–52. doi: 10.4239/wjd.v9.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg H., Pi-Sunyer F.X. Preventing preventable chronic disease: an essential goal. Progress in Cardiovascular Diseases. 2019;62:303–305. doi: 10.1016/j.pcad.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Hall K.D., Heymsfield S.B., Kemnitz J.W., Klein S., Schoeller D.A., Speakman J.R. Energy balance and its components: implications for body weight regulation. American Journal of Clinical Nutrition. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall K.D., Farooqi I.S., Friedman J.M., Klein S., Loos R.J.F., Mangelsdorf D.J., et al. The energy balance model of obesity: beyond calories in, calories out. American Journal of Clinical Nutrition. 2022;178:1098–1103. doi: 10.1093/ajcn/nqac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speakman J.R., Hall K.D. Carbohydrates, insulin, and obesity. Science. 2021;372:577–578. doi: 10.1126/science.aav0448. [DOI] [PubMed] [Google Scholar]
- 8.Cypess A.M. Reassessing human adipose tissue. New England Journal of Medicine. 2022;386:768–779. doi: 10.1056/NEJMra2032804. [DOI] [PubMed] [Google Scholar]
- 9.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi J., Seale P. Medicine. Beige can be slimming. Science. 2021;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J., Boström P., Sparks L.M., Ye L., Choi J.H., Giang A.H., et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenwald M., Perdikari A., Rülicke T., Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nature Cell Biology. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 13.Dawkins M.J., Scopes J.W. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature. 1965;206:201–202. doi: 10.1038/206201b0. [DOI] [PubMed] [Google Scholar]
- 14.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B., et al. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American Journal of Physiology - Endocrinology And Metabolism. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 16.Pfannenberg C., Werner M.K., Ripkens S., Stef I., Deckert A., Schmadl M., et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D., et al. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 19.Pontzer H., Yamada Y., Sagayama H., Ainslie P.N., Andersen L.F., Anderson L.J., et al. Daily energy expenditure through the human life course. Science. 2021;373:808–812. doi: 10.1126/science.abe5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schautz B., Later W., Heller M., Müller M.J., Bosy-Westphal A. Total and regional relationship between lean and fat mass with increasing adiposity--impact for the diagnosis of sarcopenic obesity. European Journal of Clinical Nutrition. 2012;66:1356–1361. doi: 10.1038/ejcn.2012.138. [DOI] [PubMed] [Google Scholar]
- 21.Betz M.J., Enerbäck S. Human Brown adipose tissue: what we have learned so far. Diabetes. 2015;64:2352–2360. doi: 10.2337/db15-0146. [DOI] [PubMed] [Google Scholar]
- 22.Wang G.X., Zhao X.Y., Lin J.D. The brown fat secretome: metabolic functions beyond thermogenesis. Trends in Endocrinology and Metabolism. 2015;26:231–237. doi: 10.1016/j.tem.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speakman J.R. Obesity and thermoregulation. Handbook of Clinical Neurology. 2018;156:431–443. doi: 10.1016/B978-0-444-63912-7.00026-6. [DOI] [PubMed] [Google Scholar]
- 24.Li G., Zhong L., Han L., Wang Y., Li B., Wang D., et al. Genetic variations in adiponectin levels and dietary patterns on metabolic health among children with normal weight versus obesity: the BCAMS study. International Journal of Obesity. 2022;46:325–332. doi: 10.1038/s41366-021-01004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metabolism. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Li L., Li B., Li M., Speakman J.R. Switching on the furnace: regulation of heat production in brown adipose tissue. Molecular Aspects of Medicine. 2019;68:60–73. doi: 10.1016/j.mam.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Zheng Z., Zhu X., Meng M., Li L., Shen Y., et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Research. 2013;23:851–854. doi: 10.1038/cr.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X., Wang S., You Y., Meng M., Zheng Z., Dong M., et al. Brown adipose tissue transplantation reverses obesity in ob/ob mice. Endocrinology. 2015;156:2461–2469. doi: 10.1210/en.2014-1598. [DOI] [PubMed] [Google Scholar]
- 29.Stanford K.I., Middelbeek R.J., Townsend K.L., An D., Nygaard E.B., Hitchcox K.M., et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. Journal of Clinical Investigation. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholander P.F., Hock R., Walters V., Irving L. Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic rate. Biological Bulletin. 1950;99:259–271. doi: 10.2307/1538742. [DOI] [PubMed] [Google Scholar]
- 31.Scholander P.F., Hock R., Walters V., Johnson F., Irving L. Heat regulation in some arctic and tropical mammals and birds. Biological Bulletin. 1950;99:237–258. doi: 10.2307/1538741. [DOI] [PubMed] [Google Scholar]
- 32.Foster D.O., Frydman M.L. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Canadian Journal of Physiology and Pharmacology. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 33.Au-Yong I.T., Thorn N., Ganatra R., Perkins A.C., Symonds M.E. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–2587. doi: 10.2337/db09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cypess A.M., Chen Y.C., Sze C., Wang K., English J., Chan O., et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proceedings of the National Academy of Sciences of the United U S A. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orava J., Nuutila P., Lidell M.E., Oikonen V., Noponen T., Viljanen T., et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metabolism. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Ouellet V., Labbé S.M., Blondin D.P., Phoenix S., Guérin B., Haman F., et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. Journal of Clinical Investigation. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speakman J.R., Heidari-Bakavoli S. Type 2 diabetes, but not obesity, prevalence is positively associated with ambient temperature. Scientific Reports. 2016;6 doi: 10.1038/srep30409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marlatt K.L., Chen K.Y., Ravussin E. Is activation of human brown adipose tissue a viable target for weight management? American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2018;315:R479–R483. doi: 10.1152/ajpregu.00443.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen K.Y., Brychta R.J., Abdul Sater Z., Cassimatis T.M., Cero C., Fletcher L.A., et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. Journal of Biological Chemistry. 2020;295:1926–1942. doi: 10.1074/jbc.REV119.007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X.Y., Yamada Y., Sagayama S., Ainslie P.N., Blaak E.E., Buchowski M.S., et al. 2022. Human total, basal and activity energy expenditures are independent of ambient environmental temperature. iScience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caudwell P., Finlayson G., Gibbons C., Hopkins M., King N., Näslund E., et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. American Journal of Clinical Nutrition. 2013;97:7–14. doi: 10.3945/ajcn.111.029975. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins M., Finlayson G., Duarte C., Whybrow S., Ritz P., Horgan G.W., et al. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. International Journal of Obesity. 2016;40:312–318. doi: 10.1038/ijo.2015.155. [DOI] [PubMed] [Google Scholar]
- 43.Blundell J.E., Finlayson G., Gibbons C., Caudwell P., Hopkins M. The biology of appetite control: do resting metabolic rate and fat-free mass drive energy intake? Physiology & Behavior. 2015;152:473–478. doi: 10.1016/j.physbeh.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Blundell J.E., Gibbons C., Beaulieu K., Casanova N., Duarte C., Finlayson G., et al. The drive to eat in homo sapiens: energy expenditure drives energy intake. Physiology & Behavior. 2020;219 doi: 10.1016/j.physbeh.2020.112846. [DOI] [PubMed] [Google Scholar]
- 45.Cannon B., Nedergaard J. Thermogenesis challenges the adipostat hypothesis for body-weight control. Proceedings of the Nutrition Society. 2009;68(4):401–407. doi: 10.1017/S0029665109990255. [DOI] [PubMed] [Google Scholar]
- 46.Careau V., Halsey L.G., Pontzer H., Ainslie P.N., Andersen L.F., Anderson L.J., et al. Energy compensation and adiposity in humans. Current Biology. 2021;31:4659–4666. doi: 10.1016/j.cub.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero-Corral A., Somers V.K., Sierra-Johnson J., Thomas R.J., Collazo-Clavell M.L., Korinek J., et al. Accuracy of body mass index in diagnosing obesity in the adult general population. International Journal of Obesity. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., et al. Recruited brown adipose tissue as an antiobesity agent in humans. Journal of Clinical Investigation. 2013;123(8):3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grodzinski W., Wunder B.A. In: Small mammals: their productivity and population dynamics. Golley F.B., Petrusewicz K., Ryszkowski L., editors. Cambridge University Press; Cambridge: 1975. Ecological energetics of small mammals; pp. 173–204. [Google Scholar]
- 50.Zhao Z.J., Hambly C., Shi L.L., Bi Z.Q., Cao J., Speakman J.R. Late lactation in small mammals is a critically sensitive window of vulnerability to elevated ambient temperature. Proceedings of the National Academy of Sciences of the United U S A. 2020;117:24352–24358. doi: 10.1073/pnas.2008974117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Z.J. Energy budget during four successive bouts of lactation in striped hamsters exposed to decreases in ambient temperature. Comparative Biochemistry & Physiology. 2011;160:229–236. doi: 10.1016/j.cbpa.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 52.Heldmaier G. Nonshivering thermogenesis and body size in mammals. Journal of Comparative Physiology. 1971;73:222–248. [Google Scholar]
- 53.Brzęk P., Kohl K., Caviedes-Vidal E., Karasov W.H. Developmental adjustments of house sparrow (Passer domesticus) nestlings to diet composition. Journal of Experimental Biology. 2009;212:1284–1293. doi: 10.1242/jeb.023911. [DOI] [PubMed] [Google Scholar]
- 54.Liu Q.S., Wang D.H. Effects of diet quality on phenotypic flexibility of organ size and digestive function in Mongolian gerbils (Meriones unguiculatus) Journal of Comparative Physiology B. 2007;177:509–518. doi: 10.1007/s00360-007-0149-4. [DOI] [PubMed] [Google Scholar]
- 55.Martínez del Rio C. Dietary, phylogenetic, and ecological correlates of intestinal sucrase and maltase activity in birds. Physiological and Biochemical Zoology. 1990;63:987–1011. [Google Scholar]
- 56.Zhang J.Y., Zhao X.Y., Wen J., Tan S., Zhao Z.J. Plasticity in gastrointestinal morphology and enzyme activity in lactating striped hamsters (Cricetulus barabensis) Journal of Experimental Biology. 2016;219:1327–1336. doi: 10.1242/jeb.138396. [DOI] [PubMed] [Google Scholar]
- 57.Maroux S., Louvard D., Barath J. The aminopeptidase from hog intestinal brush border. Biochimica et Biophysica Acta. 1973;321:282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 58.Wiesinger H., Heldmaier G., Buchberger A. Effect of photoperiod and acclimation temperature on nonshivering thermogenesis and GDP-binding of brown fat mitochondria in the Djungarian hamster Phodopus s. sungorus. Pfluegers Archiv European Journal of Physiology. 1989;413:667–672. doi: 10.1007/BF00581818. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Z.J., Wang D.H. Short photoperiod enhances thermogenic capacity in Brandt's voles. Physiology & Behavior. 2005;85:143–149. doi: 10.1016/j.physbeh.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 60.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 61.Sundin U., Moore G., Nedergaard J., Cannon B. Thermogenin amount and activity in hamster brown fat mitochondria: effect of cold acclimation. American Journal of Physiology. 1978;252:822–832. doi: 10.1152/ajpregu.1987.252.5.R822. [DOI] [PubMed] [Google Scholar]
- 62.Mitchell S.E., Tang Z., Kerbois C., Delville C., Konstantopedos P., Bruel A., et al. The effects of graded levels of calorie restriction: I. impact of short term calorie and protein restriction on body composition in the C57BL/6 mouse. Oncotarget. 2015;6(18):15902–15930. doi: 10.18632/oncotarget.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon C.J. Cambridge University Press; 1993. Temperature regulation in laboratory rodents. [Google Scholar]
- 64.Speakman J.R., Keijer J. Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans. Molecular Metabolism. 2012;2:5–9. doi: 10.1016/j.molmet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon C.J. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiology & Behavior. 2017;179:55–66. doi: 10.1016/j.physbeh.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ganeshan K., Chawla A. Warming the mouse to model human diseases. Nature Reviews Endocrinology. 2017;13:458–465. doi: 10.1038/nrendo.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer A.W., Cannon B., Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Molecular Metabolism. 2018;7:161–170. doi: 10.1016/j.molmet.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reitman M.L. Of mice and men - environmental temperature, body temperature, and treatment of obesity. FEBS Letters. 2018;592:2098–2107. doi: 10.1002/1873-3468.13070. [DOI] [PubMed] [Google Scholar]
- 69.Keijer J., Li M., Speakman J.R. To best mimic human thermal conditions, mice should be housed slightly below thermoneutrality. Molecular Metabolism. 2019;26:4. doi: 10.1016/j.molmet.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keijer J., L,i M ., Speakman J.R. What is the best housing temperature to translate mouse experiments to humans? Molecular Metabolism. 2019;25:168–176. doi: 10.1016/j.molmet.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Z., Cao J., Niu C., Bao M., Xu J., Huo D., et al. Body temperature is a more important modulator of lifespan than metabolic rate in two small mammals. Nature Metabolism. 2022;4:320–326. doi: 10.1038/s42255-022-00545-5. [DOI] [PubMed] [Google Scholar]
- 72.Abreu-Vieira G., Xiao C., Gavrilova O., Reitman M.L. Integration of body temperature into the analysis of energy expenditure in the mouse. Molecular Metabolism. 2015;4(6):461–470. doi: 10.1016/j.molmet.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Speakman J.R. Measuring energy metabolism in the mouse - theoretical, practical, and analytical considerations. Frontiers in Physiology. 2013;4:34. doi: 10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Škop V., Guo J., Liu N., Xiao C., Hall K.D., Gavrilova O., et al. Mouse thermoregulation: introducing the concept of the thermoneutral point. Cell Reports. 2020;31 doi: 10.1016/j.celrep.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Škop V., Xiao C., Liu N., Gavrilova O., Reitman M.L. The effects of housing density on mouse thermal physiology depend on sex and ambient temperature. Molecular Metabolism. 2021;53 doi: 10.1016/j.molmet.2021.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bauwens J.D., Schmuck E.G., Lindholm C.R., Ertel R.L., Mulligan J.D., Hovis I., et al. Cold tolerance, cold-induced hyperphagia, and nonshivering thermogenesis are normal in α₁-AMPK-/- mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;301:R473–R483. doi: 10.1152/ajpregu.00444.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arefanian H., Al-Khairi I., Khalaf N.A., Cherian P., Kavalakatt S., Madhu D., et al. Increased expression level of ANGPTL8 in white adipose tissue under acute and chronic cold treatment. Lipids in Health and Disease. 2021;20:117. doi: 10.1186/s12944-021-01547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ichikawa N., Sasaki H., Lyu Y., Furuhashi S., Watabe A., Imamura M., et al. Cold exposure during the active phase Affects the short-chain fatty acid production of mice in a time-specific manner. Metabolites. 2021;12:20. doi: 10.3390/metabo12010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoneshiro T., Aita S., Matsushita M., Kameya T., Nakada K., Kawai Y., et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity. 2011;19:13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 80.Yoneshiro T., Matsushita M., Nakae S., Kameya T., Sugie H., Tanaka S., et al. Brown adipose tissue is involved in the seasonal variation of cold-induced thermogenesis in humans. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2016;310:R999–R1009. doi: 10.1152/ajpregu.00057.2015. [DOI] [PubMed] [Google Scholar]
- 81.Toplak H., Hoppichler F., Wascher T.C., Schindler K., Ludvik B. Obesity and type 2 diabetes. Wiener Klinische Wochenschrift. 2016;2:S196–S200. doi: 10.1007/s00508-016-0986-9. [DOI] [PubMed] [Google Scholar]
- 82.Toplak H., Leitner D.R., Harreiter J., Hoppichler F., Wascher T.C., Schindler K., et al. Diabesity"-Obesity and type 2 diabetes (Update 2019) Wiener Klinische Wochenschrift. 2019;131:71–76. doi: 10.1007/s00508-018-1418-9. [DOI] [PubMed] [Google Scholar]
- 83.Zakri F.K.A., El-Wahid H.A.A., Sani M., Mahfouz M.S. A body shape index in a small sample of Saudi adults with type 2 diabetes. Journal of Family Medicine and Primary Care. 2019;8:3179–3184. doi: 10.4103/jfmpc.jfmpc_532_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson M.S., Thomson S.C., Speakman J.R. Limits to sustained energy intake. II. Inter-relationships between resting metabolic rate, life-history traits and morphology in Mus musculus. Journal of Experimental Biology. 2001;204(Pt 11):1937–1946. doi: 10.1242/jeb.204.11.1937. [DOI] [PubMed] [Google Scholar]
- 85.Kristan D.M. Effects of intestinal nematodes during lactation: consequences for host morphology, physiology and offspring mass. Journal of Experimental Biology. 2002;205(Pt 24):3955–3965. doi: 10.1242/jeb.205.24.3955. [DOI] [PubMed] [Google Scholar]
- 86.Król E., Johnson M.S., Speakman J.R. Limits to sustained energy intake. VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. Journal of Experimental Biology. 2003;206(Pt 23):4283–4291. doi: 10.1242/jeb.00676. [DOI] [PubMed] [Google Scholar]
- 87.Bao M.H., Chen L.B., Hambly C., Speakman J.R., Zhao Z.J. Exposure to hot temperatures during lactation in Swiss mice stunts offspring growth and decreases future reproductive performance of female offspring. Journal of Experimental Biology. 2020;223(Pt 9):jeb223560. doi: 10.1242/jeb.223560. [DOI] [PubMed] [Google Scholar]
- 88.Aldiss P., Lewis J.E., Lupini I., Bloor I., Chavoshinejad R., Boocock D.J., et al. Cold exposure drives weight gain and adiposity following chronic suppression of brown adipose tissue. International Journal of Molecular Sciences. 2022;23(3):1869. doi: 10.3390/ijms23031869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fischer A.W., Csikasz R.I., von Essen G., Cannon B., Nedergaard J. No insulating effect of obesity. American Journal of Physiology - Endocrinology And Metabolism. 2016;311:E202–E213. doi: 10.1152/ajpendo.00093.2016. [DOI] [PubMed] [Google Scholar]
- 90.Brychta R.J., Huang S., Wang J., Leitner B.P., Hattenbach J.D., Bell S.L., et al. Quantification of the capacity for cold-induced thermogenesis in young men with and without obesity. Journal of Clinical Endocrinology and Metabolism. 2019;104:4865–4878. doi: 10.1210/jc.2019-00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carey A.L., Formosa M.F., Van Every B., Bertovic D., Eikelis N., Lambert G.W., et al. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–155. doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- 92.Sanchez-Delgado G., Acosta F.M., Martinez-Tellez B., Finlayson G., Gibbons C., Labayen I., et al. Brown adipose tissue volume and 18F-fluorodeoxyglucose uptake are not associated with energy intake in young human adults. AmericanJournal of Clinical Nutrition. 2020;111:329–339. doi: 10.1093/ajcn/nqz300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muzik O., Mangner T.J., Leonard W.R., Kumar A., Janisse J., Granneman J.G. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. Journal of Nuclear Medicine. 2013;54:523–531. doi: 10.2967/jnumed.112.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ravussin E., Galgani J.E. The implication of brown adipose tissue for humans. Annual Review of Nutrition. 2011;31:33–47. doi: 10.1146/annurev-nutr-072610-145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen K.Y., Brychta R.J., Linderman J.D., Smith S., Courville A., Dieckmann W., et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. Journal of Clinical Endocrinology and Metabolism. 2013;98:E1218–E1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fernández-Verdejo R., Marlatt K.L., Ravussin E., Galgani J.E. Contribution of brown adipose tissue to human energy metabolism. Molecular Aspects of Medicine. 2019;68:82–89. doi: 10.1016/j.mam.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are publicly available https://osf.io/am8nw/