Abstract
Background
Covaxin/BBV152 is one of the most widely used vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and one of the few vaccines used extensively in low- and middle-income countries (LMIC).
Methods
We investigated the effect of Covaxin on the SARS-CoV-2 specific IgG and IgA and neutralizing antibody (NAb) levels at baseline (M0) and at Months 1 (M1), 2 (M2), 3 (M3), 4 (M4), 6 (M6) and 12 (M12) following vaccination in healthcare workers. In addition, we also examined the NAb levels against variant lineages of B.1.617.2 (Delta, India), B.1.617.2.1 (Delta Plus, India), B.1.351 (Beta, SA), B.1.1.7 (Alpha, UK) and B.1.1.529 (Omicron).
Results
Covaxin induces enhanced SARS-CoV-2 binding antibodies of IgG and IgA responses against both spike (S) and nucleocapsid (N) antigens at M1, M2, M3, M4, M6 and M12 in comparison with M0. Our data also reveal that NAb levels against the ancestral strain (Wuhan, wild type) are elevated and sustained at M1, M2, M3, M4, M6 and M12 in comparison with M0 and against variant lineages of B.1.617.2 (Delta, India), B.1.617.2.1 (Delta Plus, India), B.1.351 (Beta, SA) and B.1.1.7 (Alpha, UK) are elevated at M3, M6 and M12 in comparison with M0. However, NAb levels against B.1.1.529 (Omicron) was consistently below the limit of detection except at M12.
Conclusion
Thus, Covaxin induces an enhanced humoral immune response, with persistence till at least 12 months post-vaccination against most SARS-CoV-2 variants.
Keywords: COVID-19, SARS-CoV2, binding antibodies, neutralizing antibodies, B cells, vaccination, variants of concern
Introduction
The first coronavirus disease of 2019 (COVID-19) case was reported in Wuhan, China 20191 and the first case in India was reported on 30th January 2020. COVID-19 spread among Indian states happened primarily due to international travel.2 The efficacy of Covaxin/BBV152, a whole-virion inactivated vaccine has been shown previously.3–6 It has been demonstrated that neutralizing antibody (NAb) responses remained elevated in all the participants at 3 months after the vaccination series in clinical trials.7 A phase 3 clinical trial of Covaxin/BBV152 revealed that this vaccine is highly effective with an efficacy of 93% against severe symptomatic COVID-19 disease and with an efficacy of 66% against symptomatic COVID-19.8 Vaccination was well tolerated with no safety concerns observed in this interim analysis. India is considered as the vaccine industrial hub of the world by contributing 60% to the global vaccine supply.9 This country has the capability to manufacture well over 3 billion COVID-19 vaccine doses annually.41 India also has the ability to manufacture low-cost COVID-19 vaccines, which will benefit low-income countries that cannot afford expensive vaccines.10 Covaxin has been approved for use in 13 countries, all of which are low- and middle-income countries (LMIC; https://covid19.trackvaccines.org). Thus far, over 333 million doses of the Covaxin have been administered in India alone. Most of the humoral immune responses reported in the literature are focused on mRNA vaccines11–13 and very little data on the antibody responses to whole-virion inactivated vaccines are available.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is known for the emergence of variants that could be more contagious, cause severe disease, or escape natural or vaccine mediated protective immunity.14 So far, several variants of concern (VOC) have been recognized. The Delta variant (B.1.617.2) and its close relative (Delta Plus, B.1.617.2.1)15 were first reported in India and are associated with enhanced transmissibility and augmented virulence.16 Studies have also reported that the UK variant (Alpha, B.1.1.7) has an augmented risk of transmission 17and mortality.17,18 Similarly, the South African variant (Beta, B.1.351) is associated with increased risk of transmission.19 Finally, the Omicron variant (B.1.1.529), first identified in southern Africa, is characterized by 30 or more changes in the spike protein.20,21 It has also been reported that heterologous prime-boost of ChAdOx1 vaccine, Covishield followed by BBV152, Covaxin resulted elevated levels of binding antibody and neutralizing antibodies against VOC.22 The duration and kinetics of binding and neutralizing antibodies to SARS-CoV-2 VOC has been to shown to wane over time with mRNA vaccines. To determine this property of Covaxin, we estimated the NAb response against the ancestral strain (Wuhan) and variant lineages of B.1.617.2 (Delta, India), B.1.617.2.1 (Delta Plus, India), B.1.351 (Beta, SA), B.1.1.7 (Alpha, UK) and B.1.1.529 (Omicron) in Covaxin vaccinated individuals.
Materials and Methods
Study procedure
The study recruited healthcare workers working in the ICMR research institutes of National Institute for Research in Tuberculosis, Chennai, and National Institute of Epidemiology, who received BBV152/Covaxin (Manufactured by Bharat Biotech, Hyderabad in collaboration with the Indian Council of Medical Research, India) at vaccination centres in Chennai, India from February to May 2021. The inclusion criteria are all adult participants of >18 years of age, vaccinated with COVID-19 vaccine Covaxin and willing to provide written informed consent. The participants were ineligible if they were vaccinated with other COVID-19 vaccines and not willing to provide written informed consent. During the follow-up, participants were excluded if they turned SARS-CoV2 PCR positive during the study. The first dose was administered at baseline or M0 and the second dose was administered 28 ± 2 days later or M1. Blood was drawn at Day 0 (baseline, before vaccination) (M0), Day 28 ± 2 days post-first dose (M1), Day 56 ± 2 days post-first dose (M2), Day 86 ± 2 days post-first dose (M3), Day 116 ± 2 days post-first dose (M4), Day 176 ± 2 days post-first dose (M6) and Day 340–370 days post-first dose (M12).
Antibody assays
Serological testing for antibodies targeting the viral nucleocapsid protein IgG (N) and antibodies targeting the viral Spike protein IgG (S) was performed using YHLO iFlash 1800 Chemiluminescence Immunoassay Analyzer using iFlash-SARS-CoV-2 IgG (N), iFlash-SARS-CoV-2 IgG (S).
The cut-off value for SARS-CoV-2 IgG, according to the manufacturer, IgG concentrations ≥10.00 AU/ml was considered as positive and <10.00 AU/ml was considered as non-reactive. IgA was measured using the Human SARS-CoV-2 Spike protein S1 IgA and Human novel coronavirus nucleoprotein IgA antibody using ELISA platform according to manufacturer’s (MyBioSource) instructions. Plasma samples were used to measure the circulating neutralizing antibodies levels using SARS-CoV2 Surrogate Virus Neutralization Test Kit according to manufacturer’s (GenScript) instructions. The cut-off value ≥20% was considered as positive and <20% was considered as non-reactive for SARS-CoV2 NAb detection.
Statistical analysis
Covaxin vaccinated group at Day 0 (M0), Month 1 (M1), Month 2 (M2), Month 3 (M3), Month 4 (M4), Month 6 (M6) and Month (M12) groups were analysed using Kruskal–Wallis test 1-way analysis of variance (ANOVA) Multiple comparisons (Geometric mean with 95% CI). Multiple comparisons were corrected using the Holm’s correction method. Differences across time points were analysed using paired t-tests. All the analyses were performed using Graph-Pad PRISM Version 9.0.
Ethics statement
The study was approved by the Ethics Committee of ICMR-NIRT (NIRT-IEC No: 2021007). Informed written consent was received from all study individuals.
Results
Study population
We enrolled n = 115 individuals (Table 1) with the median age of 35. We had 71 males and 44 females, the median BMI was 25.3 Kg m2 among which 12 individuals had a history of contact with COVID-19 positive case. We had 46 individuals with co-morbidities including Type 2 diabetes (n = 15) or hypertension (n = 15) or obesity (n = 39). The demographics of the vaccinated individuals—both the whole study population and the enriched co-morbidities group are shown in Table I.
Table 1.
Demographics of the study population
| Study demographics | Whole study population | Comorbidity |
| Total number of participants | 115 | 46 |
| Age in years, median (range) | 35 (23–60) | 42 (38–60) |
| Gender (male/female) | 71/44 | 26/20 |
| BP (systolic), median | 120 | 125 |
| BP (diastolic), median | 80 | 80 |
| BMI Kg/m2 | 25.3 | 28.2 |
| Contact with COVID-19 Case | 12 (10.5%) | 3 (6.5%) |
| Diabetes mellitus | 15 (13.1%) | 15 (32%) |
| Hypertension | 15 (13.1%) | 15 (32%) |
Covaxin induced enhanced IgG and IgA responses persist till 12 months post-vaccination
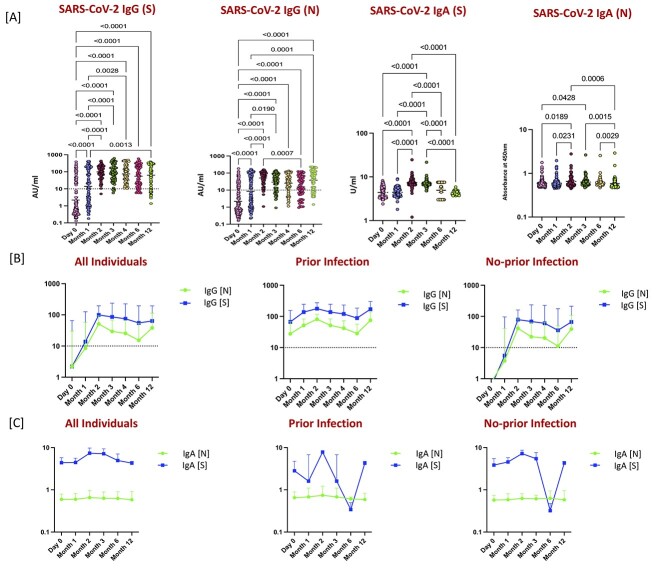
To examine the durability of humoral immunity following two doses of Covaxin, we measured the levels of IgG and IgA (against S and N antigens) at M0, M1, M2, M3, M4, M6 and M12 following the first dose. As shown in Figure 1A, before the first dose (M0), a small proportion of participants had positive IgG (S) and IgG (N) (33 [29%] and 31 [27%]), indicating prior infection. A substantial increase in detectable concentrations of IgG (S) (55 [51%] of 108) and IgG (N) (52 [48%] of 108) was noted at M1. Two months after the first dose and 1 month of the second (M2), a rapid and marked increase in antibody detection and titers was noted, IgG (S) (88 [98%] of 90) and IgG (N) (85 [95%] of 90) antibodies were detected in most participants with a >10-fold increase in titers compared with that at M1. Next, 3 months after the first dose and 2 months of the second (M3), IgG (S) (74 [93%] of 80) and IgG (N) (63 [79%] of 80) antibodies were detected in most participants with M3 levels being significantly higher than M0 and M1. In addition, we assessed the Ab levels at 4 months after the first dose and 3 months after the second (M4) IgG (S) (57 [99%] of 58) and IgG (N) (45 [78%] of 58) antibodies were detected in most participants at M4. At 6 months after the first dose and 5 months after the second (M6), IgG (S) (48 [85%] of 57) and IgG (N) (34 [60%] of 57) antibodies were detected. Finally, 12 months after the first dose and 11 months after the second (M12), IgG (S) (49 [79%] of 62) and IgG (N) (48 [77%] of 62) antibodies were detected. The antibody levels of IgA (S) and IgA (N) levels were shown to have no significant increase at M1, whereas IgA (S) and IgA (N) levels also exhibited a significant increase at M2 in comparison with M0 and M1. In addition, IgA (S) and IgA (N) levels were also significantly higher at M3 compared with M0 and/or M1. Finally, at M6 and M12, IgA (S) levels were significantly lower in comparison with M2 and M3 but IgA (N) levels remained significantly elevated.
Figure 1.
Quantification of antibodies following whole-virion inactivated BBV152 (Covaxin) vaccination. (A) The plasma levels of SARS-CoV2 IgG (S), IgG (N), IgA (S) and IgA (N) Abs were measured at Day 0 (baseline, before vaccination) (M0) [n = 115], Day 28 ± 2 days post-first dose (M1) [n = 108], Day 56 ± 2 days post-first dose (M2) [n = 90], Day 86 ± 2 days post-first dose (M3) [n = 80], Day 116 ± 2 days post-first dose (M4) [n = 58], Day 176 ± 2 days post-first dose (M6) [n = 57] and Day 340–370 days post-first dose (M12) [n = 62] of vaccination. Box plots display the median values with the interquartile range (lower and upper hinge). The data are also represented as scatter plots with each circle representing a single individual. P values were calculated using the linear mixed effect analysis using multiple comparisons. (B) SARS-CoV2 binding antibodies of IgG [S] and IgG [N] at M0, M1, M2, M3, M4, M6 and M12 following the first dose in all individuals and in those with prior infection and with no prior infection. Dotted line indicates the limit of detection (>10). (C) SARS-CoV2 binding antibodies of IgA [S] and IgA [N] at M0, M1, M2, M3, M6 and M12 following the first dose in all individuals and in those with prior infection and with no prior infection.
To examine the kinetics of humoral immunity against SARS-CoV-2 binding antibodies, we measured the SARS-CoV-2 IgG [S] and SARS-CoV-2 IgG [N] at M0, M1, M2, M3, M4, M6 and M12 following the first dose. As shown in Figure 1B, we first measured the patterns of SARS-CoV-2 IgG [S] and SARS-CoV-2 IgG [N] activity over time. SARS-CoV-2 IgG antibodies peaked 2 months after first dose (Month 2), with moderate decline over time through M6 in individuals with and without prior SARS-CoV-2 infection and among which SARS-CoV-2 IgG [S] were relatively higher antibody levels compared with SARS-CoV-2 IgG [N]. Next, we also measured the patterns of SARS-CoV-2 IgA [S] and SARS-CoV-2 IgA [N] activity over time. SARS-CoV-2 IgA [S] antibodies peaked 2 months after first dose (Month 2), with moderate decline over time through M6 in individuals with and without prior SARS-CoV-2 infection. However, there was no significant change in the SARS-CoV-2 IgA [N] antibody levels over time. All respective P values are shown in the Supplementary Table 1, Supplementary data are available at JTM online.
Finally, there was a substantial geometric mean fold increase in the SARS-CoV-2 binding antibodies of IgG (N) with 4-, 24-, 14-, 12-, 7- and 18-fold increase at M1, M2, M3, M4, M6 and M12 compared with the first vaccine dose (Day 0) and SARS-CoV-2 binding antibodies of IgG (S) with 6-, 45-, 39-, 34-, 25- and 29-fold increase at M1, M2, M3, M4, M6 and M12 compared with the first vaccine dose (Day 0; Figure 2B).
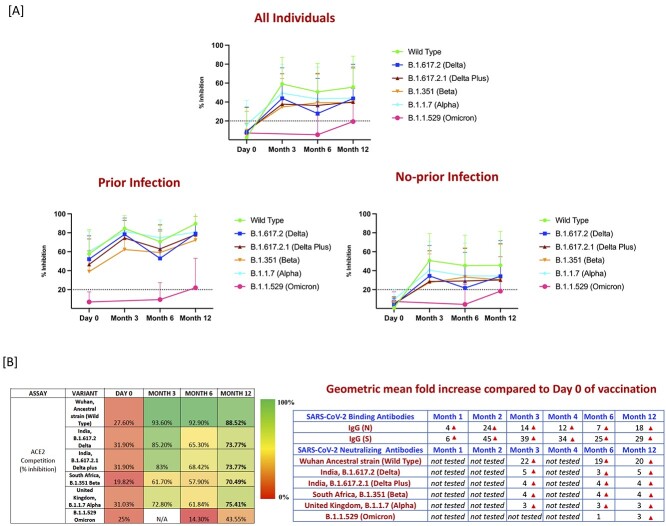
Figure 2.
Binding and neutralizing antibodies persist for 12 months after the second dose of the Covaxin/BBV152 (A) Surrogate Virus Neutralization for wild type and variant lineages of B.1.617.2 (Delta) B.1.617.2.1 (Delta Plus, India) B.1.351 (Beta, SA), B.1.1.7 (Alpha, UK) and B.1.1.529 (Omicron) at M0, M3, M6 and M12 following the first dose in all individuals and in those with prior infection and with no prior infection. Dotted line indicates the limit of detection (>20). (B) Values are the percentage of sera (at each time point) for which antibodies were detected for each variant and fold change increase of SARS-CoV-2 binding and neutralizing antibodies.
Neutralizing antibodies against SARS-CoV-2 viral variants persist for 12 months after the second dose of the Covaxin
We measured the NAb levels against wild type (ancestral strain [Wuhan]) and the variant lineages of B.1.617.2 (Delta), B.1.617.2.1 (Delta Plus, India), B.1.351 (Beta, SA) B.1.1.7 (Alpha, UK) and B.1.1.529 (Omicron) at M0, M3, M6 and M12 following the first dose. As shown in Figure 2A, we first measured the patterns of NAb activity over time. Neutralization measurement against all variants peaked 3 months after first dose (Month 3), with moderate decline over time through M6, among which wild type NAb were relatively higher compared with other variants. However, at M12 there was a mild increase in the NAb to both wild type and to other variants. B.1.1.529 (Omicron) was not detected in the many of the individuals even during at the M12. Furthermore, we assessed the NAb in individuals with and without prior SARS-CoV-2 infection, among which individuals with prior SARS-CoV-2 infection had only minimal decline over time in NAb against wild type and other variants through M6, although there was a moderate increase at M12 in wild type and other variants. However, individuals without prior SARS-CoV-2 infection also had a decline over time in wild type and other variants comparatively till M12. Overall, the individuals without prior SARS-CoV-2 infection had significantly greater decline of antibodies at M6 and M12 in comparison with individuals with prior SARS-CoV-2 infection.
Next, we wanted to evaluate the percentage of individuals in whom the NAb to SARS-CoV-2 variants were detected for each variant. As shown in Figure 2B, at M0 27, 31, 31, 19, 31 and 25% of individuals exhibited NAb against wild type, B.1.617.2, B.1.617.2.1, B.1.351, B.1.1.7 and B.1.1.529. Next, 3 months after the first dose and 2 months of the second (M3), there was a substantial increase in the percentage of individuals with 93, 84 and 83% exhibiting NAb against wild type, B.1.617.2 and B.1.617.2.1 variants, respectively. In contrast, only ~61 and 72% of individuals exhibited NAb against B.1.351 and B.1.1.7 variants, respectively. Then at 6 months after the first dose and 5 months after the second dose (M6), the percentage of individuals exhibiting NAb was 65, 68, 57, 61 and 14% against B.1.617.2, B.1.617.2.1, B.1.351, B.1.1.7 and B.1.1.529 variants, respectively. However, for the wild type ancestral strain (Wuhan), NAb levels were persistently stable with 92% of individuals exhibiting reactivity. Finally, at M12, 88, 73, 73, 70, 75 and 43% of individuals exhibited NAb against wild type, B.1.617.2, B.1.617.2.1, B.1.351, B.1.1.7 and B.1.1.529 variants, respectively. Thus, there was a moderate increase in the NAb percentage in individuals to both wild type and other variants at M12. All respective P values are shown in the Supplementary Table 2, Supplementary data are available at JTM online.
In addition, there was a considerable geometric mean fold increase in the SARS-CoV-2 neutralizing antibodies against wild type and other VOC. As shown the Figure 2B wild type ancestral strain with 22-, 19- and 20-fold increase, B.1.617.2 with 5-, 3- and 5-fold increase, B.1.617.2.1 with 4-, 4- and 4-fold increase, B.1.351 with 4-, 4- and 4-fold increase, B.1.1.7 with 3-, 3- and 3-fold increase at M3, M6 and M12, compared with the first vaccine dose (Day 0). However, for the B.1.1.529 (Omicron) there was no fold increase at M6 but there was a 3-fold increase at M12 compared with the first vaccine dose (Day 0).
Discussion
Interim results from Covaxin efficacy trials have shown 78% efficacy after 2 doses. After the first dose, 65% of participants had anti-spike antibodies, which rose to 98% at Day 14 following the second dose.3,7,23 Similarly, 48% had NAbs after the first dose, which rose to 97% at Day 14 following the second dose. However, the breadth and depth of the antibody response as well as its persistence has not been well detailed.
We, therefore, attempted to examine the antibody responses in terms of isotypes (IgG and IgA), function (NAb activity) and kinetics (M0, M1, M2, M3, M4 M6 and M12). Our data reveal the following salient features of humoral immunity following Covaxin administration. First, a single dose of Covaxin induces significantly elevated IgG but not IgA responses to spike and nucleocapsid. Second, the second dose of the vaccine results in a major increase in the levels of IgG and IgA as demonstrated at the levels at M2. Third, enhanced IgA antibody induction against both spike and nucleocapsid appears to require both doses of the vaccine. Fourth, the second dose of the vaccine elicits elevated antibody production in comparison with not only pre-vaccinated values but also in comparison with post-first dose vaccination, indicating a truly booster effect of the vaccine on humoral immunity. In addition, the vaccine elicited humoral immunity is significantly elevated at 3-, 6- and 12-month post-vaccination in comparison with pre-vaccination levels.
The correlates of protective immunity to SARS-CoV-2 are not completely understood. However, the general consensus is that both B cell and T cell responses play an important role in protection against infection and disease.24–27 In terms of B cell responses, antibody mediated responses include both binding and NAb effects as well as non-neutralizing functions.28,29 In this study, we focused on only binding and NAb functions in terms of humoral immunity following Covaxin. Our data clearly indicate that in over 50% of the participants who had prior SARS-CoV-2 infection, positive IgG and NAb responses were detected indicating that a single dose of the vaccine is sufficient to induce robust humoral responses in these individuals. Interestingly, IgG responses against spike proteins continue to be present in 98% of vaccinees at M2 and 93% of vaccinees at M3, 99% of vaccinees at M4, 80% of vaccinees at M6 and 79% of vaccines at M12, whereas IgG against nucleocapsid is present in 95% of vaccinees at M2, 78% of vaccinees at M3, 79% of vaccinees at M4, 59% of vaccinees at M6 and 77% of vaccines at M12. As most of the studies thus far suggest that IgG antibodies targeting the spike proteins are the major component of immunity to SARS-CoV-2,13 our data suggest the presence of robust anti-COVID immune responses following Covaxin administration. Similarly, NAbs are detectable in 93% of individuals at M2, 94% at M3, 100% at M4 and 92% at M6 and 89% at M12. Since NAbs are considered to be the most accurate correlate of vaccine-induced protective immunity,13 our data also reveal stable Covaxin induced immunity that lasts at least 12 months in vaccinated individuals. Little is known about the ability of COVID vaccines to induce IgA immunity. Previous studies with endemic coronaviruses indicate that nasal IgA levels correlate with protective immunity against these infections.30,31 Although we have not examined mucosal IgA, our data on serum IgA levels clearly indicate enhanced levels of IgA responses against both spike and nucleocapsid antigens engendered by Covaxin.
As the virus is well-known to frequently mutate, the emergence of VOC including B.1.617.2 (Delta), B.1.617.2.1 (Delta Plus) B.1.351 (Beta) and B.1.1.7 (Alpha) has been associated with an increased risk of transmission and mortality.14,16–19 Omicron variant is a latest among the profoundly mutated SARS-CoV-2 variants known as B.1.1.529, and was designated as a VOC by the World Health Organization on 26 November 2021.32,33 Reports from various groups suggest that VOC display a genetic variation related with enhanced transmissibility, more serious illness (e.g. increased hospitalizations or deaths), considerable drop of neutralization by antibodies produced during natural infection or vaccination, these in turn having a major impact on diagnostics, treatments or vaccines.34 Recent findings have revealed that NAb responses were significantly increased after third dose (booster) dose of Covaxin against the homologous B.1 (19 fold), Delta (16 fold), Beta (14 fold) and Omicron (18 fold) VOC.35 In addition, other factors might also play an important role in disease transmission like changes in the serial interval and incubation period before and after transmission peaks.36 Few published studies have reported that more vaccine effectiveness studies are entirely required to fully appreciate the effectiveness of BBV152 against these VOC.37 Our data suggest that the original vaccination series of two doses is sufficient to induce long lasting antibody responses against most VOC. One of the main aims of this study is to assess the vaccine-induced immune response and most particularly to shed light on the still provocative question of how competently antibodies can bind and neutralize SARS-CoV-2 VOC. Our findings suggest that neutralization potency of Covaxin elicited Abs is enhanced against lineage variants of B.1.617.2 (Delta), B.1.617.2.1 (Delta Plus), B.1.351 (Beta) and B.1.1.7 (Alpha) indicating good humoral response in ~60–80% at M3, >60% at 6-month post-vaccination, and > 70% of individuals at month 12 post-vaccination. These findings reinforce the conclusions of the clinical studies that demonstrated protection against severe disease by Covaxin against Delta, Delta AY.1, B.1.617.3 variants and Alpha.38,39 However, in the case of B.1.1.529 (Omicron), there was no detectable response in all individuals at M6 and modest response in 43% of individuals at month 12 post-vaccination, most of which likely was due to exposure to infection, rather than any vaccine mediated effects, in corroboration with other studies.40
Our study suffers from few limitations including the small sample size, and the inclusion of a homogenous group of only healthcare workers. Our study also did not examine functional effect of T cell responses against VOC. Finally, our study did not directly assess efficacy of the vaccine against SARS-CoV-2. Nevertheless, our study provides a major insight into the depth and breadth of the humoral response induced by Covaxin and is one of the first studies to examine the kinetics of antibody induction by this vaccine. This study also highlights the significance of a complete assessment of humoral immune responses to VOC. Finally, our study findings conclude that Covaxin produces a robust humoral immune response, with persistence till at least 12 months post-vaccination against most SARS-CoV-2 variants.
Authors’ contributions
S.B., C.P. and N.P.K designed the study; N.P.K. and A.N. conducted experiments; N.P.K., K.R.U.D., L.E.H., G.K. and A.N. acquired data; N.P.K., A.N. analysed data; S.B., C.P., K.R.U.D. and L.E.H. contributed reagents and also revised subsequent drafts of the manuscript; C.P., V.V.B., M.M. and S.S. responsible for the enrolment of the participants and also contributed to acquisition and interpretation of clinical data; S.B., N.P.K. wrote the manuscript. All authors read and approved the final manuscript.
Data and Materials Availability
All the reported data are available within the manuscript.
Supplementary Material
Acknowledgments
We thank the staff members of the Department of Immunology, ICER department and department of Clinical Research for the timely help. We thank the data entry operators Mr Jaiganesh and Mr Vigneshwaran. We greatly thank all the study participants.
Contributor Information
Nathella Pavan Kumar, Department of Immunology, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, India.
V V Banurekha, Department of Clinical Research, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, India.
C P Girish Kumar, Laboratory Division, ICMR-National Institute of Epidemiology, Chennai 600077, India.
Arul Nancy, International Centre for Excellence in Research, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, India.
Chandrasekaran Padmapriyadarsini, Department of Clinical Research, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, India.
Sakila Shankar, Department of Clinical Research, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, India.
Luke Elizabeth Hanna, Department of Virology and Biotechnology, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, India.
Manoj Murhekar, Epidemiology and Biostatistics Division, ICMR-National Institute of Epidemiology, Chennai 600077, India.
K R Uma Devi, Department of Immunology, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, India.
Subash Babu, International Centre for Excellence in Research, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, India.
Funding
This work was supported by the Indian Council of Medical Research (ICMR) and partially also supported by NIRT-ICER programe. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of Interest
All other authors declare no competing interests.
References
- 1. Chan JF, Yuan S, Kok KH et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azad S, Devi S. Tracking the spread of covid-19 in india via social networks in the early phase of the pandemic. J Travel Med 2020; 27:taaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganneru B, Jogdand H, Daram VK et al. Th1 skewed immune response of whole virion inactivated sars cov 2 vaccine and its safety evaluation. iScience 2021; 24:102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yadav PD, Ella R, Kumar S et al. Immunogenicity and protective efficacy of inactivated sars-cov-2 vaccine candidate, bbv152 in rhesus macaques. Nat Commun 2021; 12:1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Philbin VJ, Dowling DJ, Gallington LC et al. Imidazoquinoline toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol 2012; 130:195–204 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shukla NM, Salunke DB, Balakrishna R et al. Potent adjuvanticity of a pure tlr7-agonistic imidazoquinoline dendrimer. PLoS One 2012; 7:e43612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ella R, Reddy S, Jogdand H et al. Safety and immunogenicity of an inactivated sars-cov-2 vaccine, bbv152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis 2021; 21:950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ella R, Reddy S, Blackwelder W et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated sars-cov-2 vaccine (bbv152): Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet 2021; 398:2173–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharun K, Dhama K. Covid-19 vaccine diplomacy and equitable access to vaccines amid ongoing pandemic. Arch Med Res 2021; 52:761–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chakraborty C, Agoramoorthy G. India's cost-effective covid-19 vaccine development initiatives. Vaccine 2020; 38:7883–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang C, Maruggi G, Shan H, Li J. Advances in mrna vaccines for infectious diseases. Front Immunol 2019; 10:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Achiron A, Mandel M, Dreyer-Alster S et al. Humoral immune response to covid-19 mrna vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021; 14:17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against covid-19 in humans. Nat Rev Immunol 2021; 21:475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo CH, Morris CP, Sachithanandham J et al. Infection with the sars-cov-2 delta variant is associated with higher infectious virus loads compared to the alpha variant in both unvaccinated and vaccinated individuals Clin Infect Dis. 2021; 18:ciab986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richi FT, Alam S, Ahmed F, Al-Hossain A. The outbreak of delta plus variant: The notorious and novel strain of sars-cov-2. Clin Epidemiol Glob. Health 2022; 14:100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hendaus MA, Jomha FA. Delta variant of covid-19: a simple explanation. Qatar Med J 2021; 2021:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies NG, Abbott S, Barnard RC et al. Estimated transmissibility and impact of sars-cov-2 lineage b.1.1.7 in england. Science 2021; 372:eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NG Davies, CI Jarvis, CC-W Group, et al. Increased mortality in community-tested cases of sars-cov-2 lineage b.1.1.7. Nature 2021; 593:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tegally H, Wilkinson E, Giovanetti M et al. Detection of a sars-cov-2 variant of concern in south africa. Nature 2021; 592:438–43. [DOI] [PubMed] [Google Scholar]
- 20. He X, Hong W, Pan X et al. Sars-cov-2 omicron variant: characteristics and prevention. MedComm 2020; 2021; 2:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolter N, Jassat W, Walaza S et al. Early assessment of the clinical severity of the sars-cov-2 omicron variant in south africa: a data linkage study. Lancet 2022; 399:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kant R, Dwivedi G, Zaman K et al. Immunogenicity and safety of a heterologous prime-boost covid-19 vaccine schedule: Chadox1 vaccine covishield followed by bbv152 covaxin. J Travel Med 2021; 28:taab166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ella R, Vadrevu KM, Jogdand H et al. Safety and immunogenicity of an inactivated sars-cov-2 vaccine, bbv152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis 2021; 21:637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shrotri M, van Schalkwyk MCI, Post N et al. T cell response to sars-cov-2 infection in humans: a systematic review. PLoS One 2021; 16:e0245532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathew D, Giles JR, Baxter AE et al. Deep immune profiling of covid-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020; 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le Bert N, Tan AT, Kunasegaran K et al. Sars-cov-2-specific t cell immunity in cases of covid-19 and sars, and uninfected controls. Nature 2020; 584:457–62. [DOI] [PubMed] [Google Scholar]
- 27. Paces J, Strizova Z, Smrz D, Cerny J. Covid-19 and the immune system. Physiol Res 2020; 69:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang AT, Garcia-Carreras B, Hitchings MDT et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun 2020; 11:4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cox RJ, Brokstad KA. Not just antibodies: B cells and t cells mediate immunity to covid-19. Nat Rev Immunol 2020; 20:581–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect 1990; 105:435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Callow KA. Effect of specific humoral immunity and some non-specific factors on resistance of volunteers to respiratory coronavirus infection. J Hyg (Lond) 1985; 95:173–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karim SSA, Karim QA. Omicron sars-cov-2 variant: a new chapter in the covid-19 pandemic. Lancet 2021; 398:2126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Araf Y, Akter F, Tang YD et al. Omicron variant of sars-cov-2: genomics, transmissibility, and responses to current covid-19 vaccines. J Med Virol 2022; 94:1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeyaullah M, AlShahrani AM, Muzammil K et al. Covid-19 and sars-cov-2 variants: current challenges and health concern. Front Genet 2021; 12:693916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deshpande GR, Yadav PD, Abraham P et al. Booster dose of the inactivated covid-19 vaccine bbv152 (covaxin) enhances the neutralizing antibody response against alpha, beta, delta and omicron variants of concern. J Travel Med 2022; 29:taac039. [DOI] [PubMed] [Google Scholar]
- 36. Du Z, Liu C, Wang L et al. Shorter serial intervals and incubation periods in sars-cov-2 variants than the sars-cov-2 ancestral strain. J Travel Med 2022; taac052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yadav PD, Sapkal GN, Ella R et al. Neutralization of beta and delta variant with sera of covid-19 recovered cases and vaccinees of inactivated covid-19 vaccine bbv152/covaxin. J Travel Med 2021; 28:taab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yadav PD, Sahay RR, Sapkal G et al. Comparable neutralization of sars-cov-2 delta ay.1 and delta with individuals sera vaccinated with bbv152. J Travel Med 2021; 28:taab154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sapkal GN, Yadav PD, Ella R et al. Inactivated covid-19 vaccine bbv152/covaxin effectively neutralizes recently emerged b.1.1.7 variant of sars-cov-2. J Travel Med 2021; 28:taab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y, Rocklov J. The effective reproductive number of the omicron variant of sars-cov-2 is several times relative to delta. J Travel Med 2022; 29:taac037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puranwala A. Covid-19 vaccine update—The Covid-19 vaccination race has just begun; India aptly placed. 3 December 2020, date last accessed. https://images.assettype. com/bloombergquint/2020–12/c8d891f5-e8b7-4ae3-acbd-e6697bcf 70a4/Anand_Rathi_Covid_19_Vaccine_Update.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.