Abstract
Carbon-carbon bond cleavage mechanisms play a key role in the selective deconstruction of alkanes and polyolefins. Here, we show that the product distribution, which encompasses carbon range and formation of unsaturated and isomerization products, serves as a distinctive feature that allows the reaction pathways of different catalysts to be classified. Co, Ni, or Ru nanoparticles immobilized on amorphous silica-alumina, Zeo-Y and ZSM-5, were evaluated as catalysts in the deconstruction of n-hexadecane model substrate with hydrogen to delineate between different mechanisms, i.e., monofunctional- (acid site dominated) or bifunctional-hydrocracking (acid site & metal site) versus hydrogenolysis (metal site dominated), established from the product distributions. The ZSM-5-based catalysts were further studied in the depolymerization of polyethylene. Based on these studies, the catalysts are plotted on an activity-mechanism map that functions as an expandable basis to benchmark catalytic activity and to identify optimal catalysts that afford specific product distributions. The systematic approach reported here should facilitate the acceleration of catalyst discovery for polyolefin depolymerization.
Subject terms: Heterogeneous catalysis, Renewable energy, Sustainability
Product distributions have been used to classify the depolymerization pathways of polyolefins catalyzed by silica-alumina-based catalysts to construct an activity-mechanism map as a benchmarking tool to facilitate catalyst discovery.
Introduction
Valorization of end-of-life plastic materials continues to receive significant attention due to emerging environmental concerns and public awareness1–3. Major post-treatments of waste plastics include recycling (~11%), incinerating (~14%), and landfilling (~75%) among the 380 M tons total annually produced4. More sustainable approaches are foreseen with a significantly increased proportion of recycling5. Polyolefins (polyethylene, PE, polypropylene, PP, polystyrene, PS, etc.) comprise more than 50% of the global synthetic polymers produced6, and their transformation to short-medium carbon range chemicals, e.g., gases (C1–4), gasoline (C5–12), diesel (C12–20), or waxes (C18–36), are desired pathways either for direct applications as energy carriers7–9 or as circular feedstocks for the chemical industry10–13. Studies have focused on the chemical conversion of saturated polyolefins, especially PE, given its considerable quantity and typical application in single-use consumer products14,15.
Various approaches to depolymerize polyolefins have been reported. Thermal cracking using solely heat under an oxygen-absent atmosphere takes place at temperatures of 550–600 °C typically, though lower temperatures (~400 °C) can be applied in the presence of a catalyst16,17. For instance, Figueiredo et al. reported the conversion of low-density PE (LDPE) to afford gaseous (C1–4) and liquid (C5–20) hydrocarbons in 46 and 44% yield, respectively, including isomerization and unsaturated products, via monofunctional cracking over ZSM-5 at 400 °C (Table 1, Entry 1)18. Munir et al. achieved near-quantitative conversion of mixed plastics (PE, PP, and Polystyrene, PS) with gaseous and liquid hydrocarbons obtained in 36 and 59% yield, respectively, via monofunctional hydrocracking using Beta zeolite under H2 (20 bar) at 400 °C (Table 1, Entry 2)19. Jumah et al. reported the depolymerization of LDPE to gaseous and liquid hydrocarbons in 44 and 52% yield, respectively, including isomerization products, via bifunctional hydrocracking employing Pt_1 wt%/Beta as catalyst under H2 (20 bar) at 330 °C (Table 1, Entry 3)20. In addition to hydrocracking via silica-alumina materials, tandem catalysis approaches combining precious metal clusters immobilized on other solid acid supports (i.e., not silica-alumina structure) have also been reported. For example, Liu et al. were able to convert LDPE to gaseous and liquid hydrocarbons in 9 and 83% yield, respectively, by applying 1 wt% Pt loaded on WO3/ZrO2 and Zeolite-Y under H2 (30 bar) at 250 °C (Table 1, Entry 4)21. However, the tandem catalysis has not yet been resolved in detail or struck by a consensus as to the silica-alumina-based catalysts and, thus, will not be intensively studied and incorporated in our following studies.
Table 1.
Plastic material depolymerizations via monofunctional-, bifunctional- hydrocracking, tandem catalysis, and hydrogenolysis pathways
| Entry | Sub. | Catalyst | S/C ratio* | Type | Temp. (ºC) | Time (h) | Press. (bar) | Conv. (%) | Yield† (Gas, %) | Yield‡ (Liq., %) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LDPEa | ZSM-5 | 100 | Monofunctional cracking | 400 | 2 | N/A | 90 | 46 | 44 |
| 2 | Mixedb | Beta | 20 | Monofunctional hydrocracking | 400 | 1 | 20 | 95 | 36 | 59 |
| 3 | LDPEc | Pt_1 wt%/Beta | 10 | Bifunctional hydrocracking | 330 | 1 | 20 | 99 | 44 | 52 |
| 4 | LDPEd | Pt/WO3/ZrO2 + Zeo-Y | 10 | Tandem catalysis | 250 | 2 | 30 | 99 | 9 | 83 |
| 5 | LDPEe | Ru/C | 28 | Hydrogenolysis | 225 | 16 | 20 | 99 | 55 | 45 |
| 6 | HDPEf | Ru/C | 2 | Hydrogenolysis | 220 | 1 | 60 | 99 | 12 | 68 |
| 7 | PPg | Ru/TiO2 | 20 | Hydrogenolysis | 250 | 16 | 30 | >94 | 28 | 66 |
*S/C ratio substrate/catalyst weight ratio; Note that the selected carbon range for the reported liquid hydrocarbon yields (wt%) may have slight differences among literature.
†Gaseous products are typically defined as C1–4.
‡Liquid products are typically defined as C5-20.
aPlastic properties/source not provided.
bHDPE (ρ = 0.952 g/cm3, Sigma-Aldrich) + LDPE (ρ = 0.918 g/cm3, Sigma-Aldrich) + PP (Mw = 250,000 g/mol, ρ = 0.9 g/cm3) + PS (Mw = 192,000 g/mol) = (40 + 10 + 30 + 20)wt%.
cLDPE (Mw ~150,000 g/mol).
dLDPE (Mw ~250,000 g/mol, Sigma-Aldrich).
eLDPE (Mw ~4000 g/mol, Sigma-Aldrich).
fHDPE water jug from local source, properties not specified.
gIsotactic PP (Mw ~250,000, Sigma-Aldrich).
Hydrogenolysis of C-C bonds using Ru-based catalysts has also been used for the selective deconstruction of plastics, limiting isomerization and unsaturated products. The formation of methane is considered an important indicator of a hydrogenolysis mechanism with alkylidyne intermediates, as C1 intermediates such as the methenium cation are disfavored in (hydro)cracking mechanisms22. Ru NPs on activated charcoal (Ru/C) has been shown to cleave PE into shortened hydrocarbons under H2 (20–60 bar) at 200–225 °C23,24. Julie et al. reported quantitative conversion of LDPE to gaseous and liquid hydrocarbons in 55 and 45% yield, respectively, using Ru_5wt%/C under H2 (20 bar) at 200 °C (Table 1, Entry 5)23. The same catalyst was shown to transform high-density PE (HDPE) into gaseous and liquid hydrocarbons in 12 and 68% yield, respectively, under H2 (60 bar) at 220 °C in hexane (Table 1, Entry 6)24. Ru NPs immobilized on a reducible solid support such as TiO2 have also been reported to catalyze PP hydrogenolysis (Table 1, Entry 7)25. Pt NPs immobilized on metal oxide (SrTiO3) or fabricated into a mesoporous shell/active site/core structure (mSiO2/Pt/SiO2) have also been used in the hydrogenolysis of PE to afford wax-lubricant range products (C18–40+) under 9–14 bar H2 at temperatures ranging from 250 to 300 °C reacting for 24–96 h26–28.
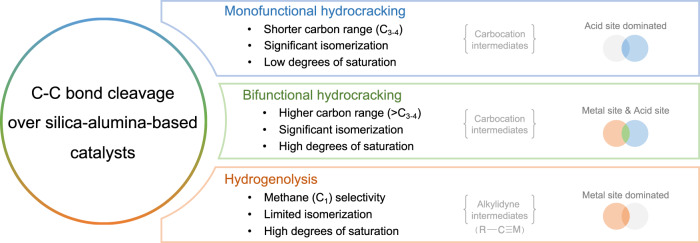
Despite the abundant literature describing catalysts that deconstruct alkanes and polyolefins, systematic comparisons among the catalysts are largely absent and their mechanistic pathways are often overlooked, which hinders further rational catalyst design. To overcome these limitations, we studied the catalytic deconstructions of n-hexadecane (nC16—used as a model substrate and functioning as a benchmark reaction) and polyethylene (PE, Mw ~4000 g/mol) in the presence of hydrogen using various heterogeneous catalysts prepared using an identical synthetic procedure. We use the product distribution, including the carbon range and distinctive features such as degrees of saturation and the formation of isomerization products, were evaluated22, to classify the C-C bond cleavage mechanisms as:
Monofunctional hydrocracking—acid site dominated cleavage with a unimodal distribution comprising C3–4 as major products including significant isomerization and unsaturated products.
Bifunctional hydrocracking—combined acid/metal site cleavage with a unimodal distribution with >C3–4 as major products including isomerization and unsaturated or a bimodal distribution product range in certain cases.
Hydrogenolysis—metal site dominated cleavage with a unimodal distribution with C1 as the major product.
A schematic summarizing the product distributions for each type of mechanism is given in Fig. 1. Furthermore, these studies allowed an activity-mechanism map to be constructed for catalyst benchmarking, which should enable new, superior polyolefin depolymerization catalysts to be developed that provide specific products or distributions.
Fig. 1.
Schematic summary of the product distributions for different C–C bond cleavage mechanisms.
Results
Preparation and characterization of the catalysts
Three different types of silica-alumina were used as catalysts/supports, i.e., amorphous silica-alumina (SiO2-Al2O3), zeolite-Y_hydrogen (Zeo-Y_H, Si/Al = 30:1) with a relatively large pore opening and zeolite socony mobil-5_hydrogen (ZSM-5_H, Si/Al = 23:1) with a relatively small pore size and similar Si/Al ratio29–31. Co, Ni, or Ru nanoparticles (NPs) were immobilized on the three silica-alumina materials (2.5 wt% metal loading) via a solution-based synthetic procedure (see further details in Methods) to give the main library of 12 catalysts, i.e., SiO2-Al2O3, Co/SiO2-Al2O3, Ni/SiO2-Al2O3, Ru/SiO2-Al2O3, Zeo-Y_H, Co/Zeo-Y_H, Ni/Zeo-Y_H, Ru/Zeo-Y_H, ZSM-5_H, Co/ZSM-5_H, Ni/ZSM-5_H, and Ru/ZSM-5_H. Co and Ni NPs were selected as Earth-abundant first-row transition metals with suitable characteristics for C-C bond cleavage32–35. Ru NPs were used as they have been shown to be particularly effective in activating C-C bonds32,36–38. In addition to the main library of 12 catalysts, Zeo-Y_H catalysts with various Si/Al ratios and the corresponding Ni- and Ru-modified catalysts were tested to afford a subset of 6 catalysts, i.e., Zeo-Y_H (Si/Al = 60:1) termed Zeo-Y_H [60], Zeo-Y_H (Si/Al = 80:1) termed Zeo-Y_H [80], Ni/Zeo-Y_H [60], Ni/Zeo-Y_H [80], Ru/Zeo-Y_H [60], and Ru/Zeo-Y_H [80].
All SiO2-Al2O3-, Zeo-Y_H-, and ZSM-5_H-based catalysts were analyzed by powder-XRD, confirming the amorphous nature of SiO2-Al2O3-based catalysts (Supplementary Fig. 1) and the crystalline nature of Zeo-Y_H- and ZSM-5_H-based catalysts (Supplementary Figs. 2, 3). Both the amorphous and crystalline features are well-preserved after NP immobilization to the corresponding unmodified silica-alumina materials. ZSM-5_H-based catalysts were proceeded with further characterization due to their extended applications in our studies. X-ray photoelectron spectroscopy (XPS) analysis of ZSM-5_H confirmed the surface relative atomic concentration to comprise Si (28.6%), Al (2.8%), and O (68.7%) (Supplementary Table 1). Co/ZSM-5_H, Ni/ZSM-5_H, and Ru/ZSM-5_H have surface transition metal concentrations of 9.8, 8.3, and 2.6%, respectively (Supplementary Table 1). Various ratios of metal, metal oxide, and metal hydroxide species were assigned via appropriate fitting methodology specifically for Co, Ni, and Ru at the resting state of all the catalysts (see details in Supplementary Table 2 and Supplementary Figs. 4–6)39,40.
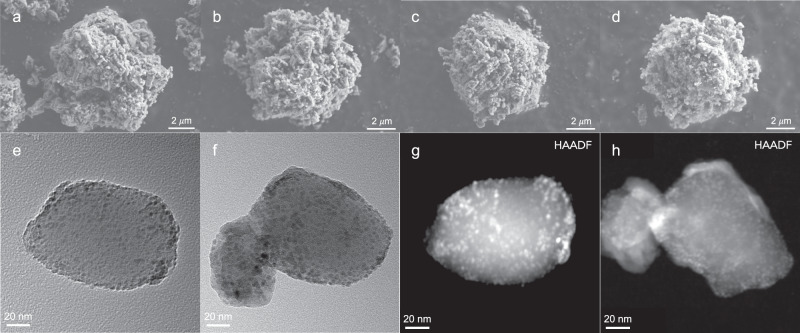
Scanning electron microscopy (SEM) images show that the particle size of all the ZSM-5_H-based catalysts ranges from 6 to 10 μm (Fig. 2a–d) and no significant changes compared to the support material are observed following the immobilization of Co, Ni, or Ru NPs, and the surface morphology is also maintained (cf. Fig. 2a–d with Supplementary Fig. 7). Transmission electron microscopy (TEM) images of ZSM-5_H show a smooth surface with no apparent surface structure (Supplementary Fig. 8). Well-dispersed NPs were observed on the surface of Co/ZSM-5_H and Ni/ZSM-5_H (Fig. 2e, f), and more aggregated NPs were formed on the surface of Ru/ZSM-5_H (Supplementary Fig. 9). High-angle annular dark-field (HAADF) images also reveal the surface fine-particle structures of Co/ZSM-5_H, Ni/ZSM-5_H, and Ru/ZSM-5_H catalysts. The average particle size of the Co, Ni and Ru NPs was estimated as 3.6 ± 0.5, 3.1 ± 0.4, and 5.4 ± 0.8 nm, respectively (see further details in Supplementary Figs. 9–11). Similar particle distributions of Co and Ni NPs ensure that the further reactivity comparisons are mainly based on the types of metal modification instead of acid/metal site intimacy during their working state (hydrogen atmosphere with heat, see below)41. The more aggregated particle distribution, on the other hand, rationalizes the relatively lower Ru surface atomic concentration than Co and Ni via XPS analysis (2.6 vs. 9.8 and 8.3%).
Fig. 2. Electron microscopy images of the ZSM-5_H-based catalysts.
SEM images (scale bar = 2 μm) of an individual a ZSM-5_H, b Co/ZSM-5, c Ni/ZSM-5_H, and d Ru/ZSM-5_H. TEM bright-field images (scale bar = 20 nm) of e Co/ZSM-5_H and f Ni/ZSM-5_H. HAADF images (scale bar = 20 nm) of g Co/ZSM-5_H and h Ni/ZSM-5_H.
Activity studies
In the initial phase of the study n-hexadecane (nC16) was used as a model substrate23 for PE, a saturated aliphatic polymer. Each catalyst was evaluated under sufficient H2 (~1.15 eq.) to quantitatively produce methane, to ensure that selectivity would not be restricted by a lack of hydrogen, and at three temperatures typical of catalytic hydrocracking reactions, i.e., 275, 325, and 375 °C16,17,42,43. The results from the main library catalysts are summarized in Table 2 and Supplementary Figs. 12–15 provide detailed product distributions, and Supplementary Table 3 provides the carbon balance in weight and hydrogen consumption.
Table 2.
n-Hexadecane deconstructions with the 12 catalysts from the main library at 275, 325, and 375 °C
| Entry | Catalyst | Temp. (ºC) | Conv. (%) | C1-4 Yield (%)* | C5-16 Yield (%)* |
|---|---|---|---|---|---|
| 1 | SiO2-Al2O3 | 275 °C | 2.1 ± 0.2 | 0.0 | 2.1 |
| 2 | SiO2-Al2O3 | 325 °C | 3.8 ± 1.0 | 0.1 | 3.7 |
| 3 | SiO2-Al2O3 | 375 °C | 2.1 ± 0.2 | 0.1 | 2.0 |
| 4 | Zeo-Y_H | 275 °C | 4.5 ± 0.3 | 0.3 | 4.2 |
| 5 | Zeo-Y_H | 325 °C | 8.5 ± 0.5 | 0.7 | 7.7 |
| 6 | Zeo-Y_H | 375 °C | 26.7 ± 1.4 | 3.3 | 23.3 |
| 7 | ZSM-5_H | 275 °C | 13.7 ± 2.4 | 3.8 | 10.0 |
| 8 | ZSM-5_H | 325 °C | 91.6 ± 4.4 | 35.5 | 56.2 |
| 9 | ZSM-5_H | 375 °C | 98.0 ± 2.0 | 77.3 | 20.8 |
| 10 | Co/SiO2-Al2O3 | 275 °C | 2.3 ± 0.2 | 0.1 | 2.2 |
| 11 | Co/SiO2-Al2O3 | 325 °C | 2.3 ± 0.3 | 0.0 | 2.2 |
| 12 | Co/SiO2-Al2O3 | 375 °C | 2.4 ± 0.1 | 0.1 | 2.2 |
| 13 | Co/Zeo-Y_H | 275 °C | 1.9 ± 0.4 | 0.0 | 1.9 |
| 14 | Co/Zeo-Y_H | 325 °C | 5.5 ± 1.2 | 0.2 | 5.2 |
| 15 | Co/Zeo-Y_H | 375 °C | 6.1 ± 1.2 | 1.2 | 4.9 |
| 16 | Co/ZSM-5_H | 275 °C | 1.9 ± 0.1 | 0.1 | 1.8 |
| 17 | Co/ZSM-5_H | 325 °C | 5.9 ± 0.1 | 1.2 | 4.7 |
| 18 | Co/ZSM-5_H | 375 °C | 49.0 ± 3.7 | 12.7 | 36.3 |
| 19 | Ni/SiO2-Al2O3 | 275 °C | 2.6 ± 0.6 | 0.1 | 2.6 |
| 20 | Ni/SiO2-Al2O3 | 325 °C | 3.3 ± 1.4 | 0.1 | 3.2 |
| 21 | Ni/SiO2-Al2O3 | 375 °C | 3.8 ± 1.2 | 0.2 | 3.7 |
| 22 | Ni/Zeo-Y_H | 275 °C | 2.1 ± 0.6 | 0.6 | 1.5 |
| 23 | Ni/Zeo-Y_H | 325 °C | 4.4 ± 0.4 | 0.4 | 4.0 |
| 24 | Ni/Zeo-Y_H | 375 °C | 20.9 ± 1.3 | 0.4 | 20.5 |
| 25 | Ni/ZSM-5_H | 275 °C | 2.2 ± 0.2 | 0.3 | 1.9 |
| 26 | Ni/ZSM-5_H | 325 °C | 8.2 ± 1.8 | 0.5 | 7.7 |
| 27 | Ni/ZSM-5_H | 375 °C | 85.6 ± 4.1 | 28.2 | 57.3 |
| 28 | Ru/SiO2-Al2O3 | 275 °C | 95.6 ± 4.0 | 41.5 | 54.2 |
| 29 | Ru/SiO2-Al2O3 | 325 °C | 98.5 ± 0.3 | 75.8 | 22.6 |
| 30 | Ru/SiO2-Al2O3 | 375 °C | 99.8 ± 0.2 | 96.6 | 3.3 |
| 31 | Ru/Zeo-Y_H | 275 °C | 96.0 ± 0.9 | 56.6 | 39.3 |
| 32 | Ru/Zeo-Y_H | 325 °C | 99.6 ± 0.3 | 91.5 | 8.3 |
| 33 | Ru/Zeo-Y_H | 375 °C | 99.0 ± 1.0 | 92.4 | 6.9 |
| 34 | Ru/ZSM-5_H | 275 °C | 99.4 ± 0.6 | 92.8 | 6.6 |
| 35 | Ru/ZSM-5_H | 325 °C | 98.0 ± 2.0 | 99.7 | 0.3 |
| 36 | Ru/ZSM-5_H | 375 °C | 99.8 ± 0.2 | 98.8 | 1.1 |
Reaction conditions: n-hexadecane (1.59 g, 7.0 mmol), catalyst (0.1 g, metal loading = 2.5 wt%), S/C ratio (substrate/catalyst weight ratio) ~16, 45 bar H2, 2 h.
*All yields were calculated as the carbon yield and isomerized C16 (isoC16) are considered as products.
Unmodified silica-alumina catalysts
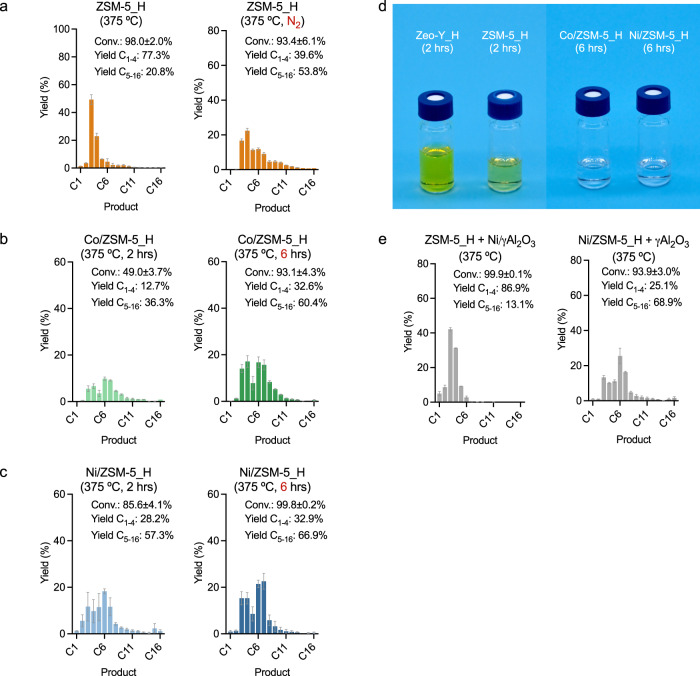
Under the typical conditions used (nC16: ~1.6 g, 7.0 mmol, 112 mmol carbon, catalyst: 0.1 g, H2: 45 bar, reaction time: 2 h), SiO2-Al2O3 showed limited activity even at 375 °C with a maximum conversion of 2.1 ± 0.2% (Table 2, Entries 1–3). With Zeo-Y_H the activity increases over the temperature range employed, accomplishing a conversion of 26.7 ± 1.4% at 375 °C (Table 2, Entries 4–6). ZSM-5_H achieved 13.7 ± 2.4% conversion at 275 °C (Table 2, Entry 7), and near-quantitative conversion (98.0 ± 2.0%) at 375 °C (Table 2, Entries 8, 9). Light hydrocarbon (C1–5) products, with C3 as the major products, were identified in all the reactions (Supplementary Fig. 12). Significant portions of isomerization products were detected, and limited methane was obtained confirming a monofunctional hydrocracking mechanism. Under the reaction conditions employed, the reactivity of the silica-alumina materials follows the order: ZSM-5_H > Zeo-Y_H > SiO2-Al2O. The differences in activity among amorphous SiO2-Al2O3, crystalline Zeo-Y_H, and crystalline ZSM-5_H at different temperatures (Table 2, Entries 1–9) reveal the significance of local confinements and the corresponding topology of a given silica-alumina material towards the activity and selectivity of the catalyst (see below)44,45.
Control experiments were conducted in which the reaction of nC16 was studied under a nitrogen atmosphere in the presence of the unmodified silica-alumina catalysts at 375 °C (Fig. 3a and Supplementary Fig. 16). Interestingly, the nC16 conversion in the presence of ZSM-5_H was reduced under the nitrogen atmosphere compared to the reaction under hydrogen, and a wider unimodal product distribution was obtained (cf. Fig. 3a-left and Supplementary Fig. 16-left with Fig. 3a-right and Supplementary Fig. 16-right). The major products consist of short carbon range (C3–4) hydrocarbons with a limited amount of methane, confirming a monofunctional cracking mechanism under nitrogen. Despite the absence of an external hydrogen source when the reaction is carried out under a nitrogen atmosphere, the hydrogen atoms in the hydrocarbon substrate are able to be transferred, to afford saturated products together with the corresponding unsaturated products resulting from the hydrogen transfer46. The different applied atmospheres do not lead to significantly different C-C bond cleavage pathways. Nevertheless, under a hydrogen atmosphere, the reaction should be classified as hydrocracking due to the influence of the hydrogen on the kinetics and product distribution, i.e. wider vs. narrower unimodal distribution under N2 or H2 atmospheres47. Despite the unmodified silica-alumina catalysts lacking metal sites for efficient hydrogenation/dehydrogenation steps, the hydrogen may affect the concentration and diffusion behavior of surface species48.
Fig. 3. Product distributions and appearances of liquid products after n-hexadecane (nC16) deconstruction.
a nC16 (1.59 g, 7.0 mmol) deconstruction using ZSM-5_H (0.1 g) under hydrogen and under nitrogen, pressure = 45 bar, 2 h. b nC16 (1.59 g, 7.0 mmol) deconstruction using Co/ZSM-5_H (0.1 g, metal loading = 2.5 wt%), 45 bar H2 for 2 and 6 h. c nC16 (1.59 g, 7.0 mmol) deconstruction using Ni/ZSM-5_H (0.1 g, metal loading = 2.5 wt%), 45 bar H2 for 2 and 6 h. d Color of the resulting liquid products from the nC16 deconstructions using Zeo-Y_H, ZSM-5_H, Co/ZSM-5_H, and Ni/ZSM-5_H with 45 bar H2 at 375 °C. e nC16 (1.59 g, 7.0 mmol) deconstruction using Ni/ZSM-5_H (0.1 g, metal loading = 2.5 wt%) + γAl2O3 (0.1 g) and ZSM-5_H (0.1 g) + Ni/γAl2O3 (0.1 g, metal loading = 2.5 wt%), 45 bar H2, 2 h. Error bars = standard deviation. Source data are provided as a Source Data file.
Immobilized Co and Ni NP catalysts
The Co/SiO2-Al2O3 and Ni/SiO2-Al2O3 catalysts only result in low conversions of nC16, i.e., <5%, similar to that observed with the silica-alumina supports alone under the benchmark conditions at 375 °C (Table 2, cf. Entries 1–3 with 10–12 and 19–21). Co/Zeo-Y_H led to a conversion of 6.1 ± 1.2% and Ni/Zeo-Y_H led to a conversion of 20.9 ± 1.3% at 375 °C, ca. 25 and 80% of the activity of Zeo-Y_H alone (Table 2, cf. Entry 6 with 15, 24). Co/ZSM-5_H resulted in a conversion of 49.0 ± 3.7% and Ni/ZSM-5_H resulted in a conversion of 85.6 ± 4.1%, at 375 °C, ca. 50 and 90% of the activity of ZSM-5_H alone (Table 2, cf. Entry 9 with 18, 27).
A bimodal product distribution including isomerized hydrocarbons was obtained with Co/ZSM-5_H and Ni/ZSM-5_H, with both affording C3–4 and C7–8 hydrocarbons as major products (Fig. 3b-left, 3c-left and Supplementary Figs. 13, 14), which contrasts with a unimodal distribution dominated by C3–4 products when ZSM-5_H was applied under the typical conditions (cf. Fig. 3a-left with 3b-left, 3c-left). The change in the product distributions may be appreciated by considering the proportion of liquid to gas ratio of the products (L/G ratio = C5–16 yield/C1–4 yield), which corresponds to 2.9 for Co/ZSM-5_H (conv. = 49.0 ± 3.7%) and 2.0 for Ni/ZSM-5_H (conv. = 85.6 ± 4.1%) compared to 0.3 for ZSM-5_H (conv. = 98.0 ± 2.0%). The bimodal product distribution and higher carbon range C7–8 products obtained using Co/ZSM-5_H and Ni/ZSM-5_H implies a bifunctional pathway. A set of controls with a prolonged reaction time of 6 h in the presence of Co/ZSM-5_H and Ni/ZSM-5-H was conducted to establish whether the bimodal product distribution is due to an insufficient reaction time. Both Co/ZSM-5_H and Ni/ZSM-5_H result in near-quantitative conversion of the nC16 substrate and bimodal product distributions, i.e., a L/G ratio of 1.9 for Co/ZSM-5_H (conv. = 93.1 ± 4.3%) and a L/G ratio of 2.0 for Ni/ZSM-5_H (conv. = 99.8 ± 0.2%), confirming a bifunctional hydrocracking mechanism (cf. Fig. 3a-left with 3b-right, 3c-right). The Co and Ni NPs are unable to cleave C-C bonds efficiently, and therefore the catalytic activity is mainly determined by the acid sites when the reaction is conducted above the onset temperature of the silica-alumina support, evidenced by the proportion of C3 product for the hydrogenolysis of nC16 using Co/ZSM-5_H or Ni/ZSM-5_H (Fig. 3b, c). Hence, Co/ZSM-5_H and Ni/ZSM-5_H are more active than the Zeo-Y_H-based counterparts, i.e., Co/Zeo-Y_H and Ni/Zeo-Y_H, due to the higher activity of the ZSM-5_H support, and despite having identical NP coverages. Such interplay between the immobilized NPs and surface is often referred to as the acid-metal balance and can further affect product selectivity, in these cases not specifically favoring C4 products49,50.
Although the catalysts composed of Co or Ni NPs immobilized on Zeo-Y_H or ZSM-5_ H are less active than the zeolite supports alone (Table 2, cf. Entries 4–9 with 13–18 and 22–27), the extent of unsaturated products (i.e., alkenes, aromatics, etc., determined using 1H NMR spectroscopy) is lower (see further details in Table 3). This difference is because the Co and Ni NPs efficiently catalyze the hydrogenation of unsaturated bonds formed during the cracking process. For example, the degrees of saturation of the resulting liquids using ZSM-5_H under the typical conditions correspond to 87.4, 7.9, and 4.8% of saturated, unsaturated, and aromatic hydrocarbons, respectively (Table 3, Entry 1). In contrast, degrees of saturation shift to 99.4, 0.5, and 0.1% using Co/ZSM-5_H and 97.6, 2.3, and 0.1% with Ni/ZSM-5_H under the same reaction conditions (Table 3, Entries 2, 3). After a reaction time of 6 h, the degree of saturated liquid hydrocarbons increases to 96.2% for Co/ZSM-5_H and 99.9% for Ni/ZSM-5_H (Table 3, Entries 4, 5). Further control experiments using nC16 as a substrate under different hydrogen pressures revealed that the degrees of saturated liquid hydrocarbons increase along the increased pressure (i.e., the amount of H2 increases). When ZSM-5-H is applied as the catalyst, the percentage of saturated products increases from 86.5% under 30 bar of H2 to 88.1% under 60 bar of H2 (corresponding to ~0.80 and ~1.55 eq. required to quantitatively produce methane, respectively, Table 3, Entries 6, 7, and Supplementary Fig. 17). With Ni/ZSM-5_H, 98.6% and 99.9% of saturated products are obtained at 30 and 60 bar of H2, respectively (Table 3, Entries 8,9, and Supplementary Fig. 18). The higher the hydrogen pressure shows the higher the percentage of saturated products, though only to a modest extent. In contrast, the metal modification has a greater influence, significantly increasing the amount of saturated products in the final product distribution. The higher content of saturated products is a distinctive feature of the bifunctional pathway, which reduces potential coking through the suppression of coking precursors such as polyaromatic compounds51–53. Note that as the concentration of unsaturated products increases, the color of the solution also becomes more intensely yellow in color (Fig. 3d).
Table 3.
Degrees of saturation of the liquid products obtained from the deconstruction of n-hexadecane under hydrogen
| Entry | Catalyst | H2 (bar) | Time (h) | Conv. (%) | Saturated (%, δ = 0.25–2.0) | Unsaturated (%, δ = 2.0-6.0) | Aromatic (%, δ = 6.0-8.0) |
|---|---|---|---|---|---|---|---|
| 1 | ZSM-5_H | 45 | 2 | 98.0 ± 2.0 | 87.4 ± 4.3 | 7.9 ± 2.6 | 4.8 ± 1.9 |
| 2 | Co/ZSM-5_H | 45 | 2 | 49.0 ± 3.7 | 99.4 ± 0.5 | 0.5 ± 0.4 | 0.1 ± 0.1 |
| 3 | Ni/ZSM-5_H | 45 | 2 | 85.6 ± 4.1 | 97.6 ± 0.8 | 2.3 ± 0.7 | 0.1 ± 0.1 |
| 4 | Co/ZSM-5_H | 45 | 6 | 93.1 ± 4.3 | 96.2 ± 1.8 | 3.1 ± 1.6 | 0.6 ± 0.2 |
| 5 | Ni/ZSM-5_H | 45 | 6 | 99.8 ± 0.2 | 99.9 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| 6 | ZSM-5_H | 30 | 2 | 99.8 ± 0.1 | 86.5 ± 0.5 | 8.8 ± 0.5 | 4.7 ± 0.1 |
| 7 | ZSM-5_H | 60 | 2 | 99.9 ± 0.1 | 88.1 ± 0.2 | 7.4 ± 0.2 | 4.5 ± 0.1 |
| 8 | Ni/ZSM-5_H | 30 | 2 | 99.5 ± 0.5 | 98.6 ± 0.8 | 1.0 ± 0.7 | 0.4 ± 0.1 |
| 9 | Ni/ZSM-5_H | 60 | 2 | 94.9 ± 2.2 | 99.9 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
Reaction conditions: n-hexadecane (1.59 g, 7.0 mmol), catalyst (0.1 g, metal loading = 2.5 wt%), 375 °C. Note that degrees of saturation are defined by the ratio of proton integrations in 1H NMR spectra to indicate the adjacent carbon-carbon bonds (saturated: δ = 0.25–2.0, unsaturated: δ = 2.0–6.0, and aromatics: δ = 2.0-6.0) given the C-H and C-C bond exclusivity of hydrocarbons.
The proximity of the acid sites and metal sites was investigated by comparing the products obtained using ZSM-5_H + Ni/γAl2O3 (Ni-modified gamma-alumina prepared using identical procedure as Ni/ZSM-5_H) and Ni/ZSM-5_H + γAl2O3 as catalysts54. ZSM-5_H + Ni/γAl2O3 may be considered a low intimacy combination and Ni/ZSM-5_H + γAl2O3 as a high intimacy combination. The low intimacy combination resulted in near-quantitative conversion with a unimodal distribution indicative of a monofunctional pathway typical of ZSM-5_H (cf. Fig. 3a-left with 3e-left), whereas the high intimacy combination led to a conversion of 93.9 ± 0.3% with a bimodal distribution similar to the bifunctional pathway observed for Ni/ZSM-5_H (cf. Fig. 3c-left with 3e-right). These results confirm the importance of acid and metal site proximity in influencing the product distribution as reported elsewhere41,54,55. Note that γAl2O3 shows some activity (conv. = 6.8% ± 0.9) in the transformation of nC16 at 375 °C, explaining the slightly higher activity of the Ni/ZSM-5_H + γAl2O3 combination compared to Ni/ZSM-5_H alone. Moreover, the Co and Ni metal sites appear to provide an alternative route facilitating the dissociation of intermediates away from the acid sites that would otherwise be cleaved further by them to afford higher degrees of saturated products in higher carbon ranges.
Immobilized Ru NP catalysts
The three Ru-modified catalysts all result in the near-quantitative conversion of nC16 with high selectivity to methane, with high conversions even obtained at 275 °C (Table 2, Entries 28–36, and Supplementary Fig. 15). The high yield of methane and trace amount of isomerized products in the C5–12 range (Table 2, Entries 28, 31) indicate that a hydrogenolysis mechanism is dominant23,24,56. Note that evaluating metal NPs dispersed on an inactive support material may provide insights on the onset temperature for C–C bond hydrogenolysis of a given metal. Ru NPs immobilized on carbon (Ru/C) were shown to depolymerize PE via a hydrogenolysis mechanism with an onset temperature around 220 °C, whereas Pt/C, Pd/C, and Rh/C require temperature ≥280 °C23,24. As Ru NPs efficiently catalyze C–C bond hydrogenolysis, the activity and selectivity are dominated by the Ru NPs, especially where the silica-alumina support is less active, e.g., SiO2-Al2O3, and below the onset temperature of the support.
Traces of isomers and chain-end initiated linear alkanes were identified at 325 °C (Supplementary Fig. 19), indicative of hydrocracking attributed to the silica-alumina supports (ZSM-5_H in this case). Therefore, the kinetics of the catalysts for methane production follows the order: Ru/ZSM-5_H (88.6%) > Ru/Zeo-Y_H (76.4%) > Ru/SiO2-Al2O3 (54.5%) at 325 °C. With the exception of Ru, the influence of a metal on the selectivity of the reaction is less predictable, especially when the hydrogenolysis onset temperature is close to the temperature required by the support to initiate hydrocracking. Thus, the overhead of reaction temperature with respect to hydrogenolysis/hydrocracking onset temperature critically influences which mechanism prevails. For instance, ultra-stable Y zeolite and beta zeolite modified with Pt NPs (i.e., ≥280 °C for hydrogenolysis) behave as bifunctional hydrocracking catalysts at around 300 °C20.
Impact of the Si/Al ratio
Zeo-Y_H-based catalysts with different Si/Al ratios (SARs) were also evaluated in the deconstructions of nC16 at 375 °C, see Table 4 and Supplementary Fig. 20 for the full product distributions, and Supplementary Table 4 for carbon balance in weight and hydrogen consumption. Zeo-Y_H [60] (Si/Al = 60:1) and Zeo-Y_H [80] (Si/Al = 80:1) result in conversions of 24.6 ± 1.2% and 14.9 ± 0.6% compared to the 26.7 ± 1.4% for Zeo-Y_H (Si/Al = 30:1) under the standard reaction conditions, i.e., 45 bar H2, 375 °C, 2 h (Table 4, Entries 1–3). As the SAR increases the catalytic activity decreases, which may be associated with their surface acidity, and is in accordance with the literature21. The presence of isomerized and unsaturated products confirm that a monofunctional pathway is in operation (Supplementary Table 5, Entries 2, 3) as would be expected. The differences in activity between the different types of zeolites with comparable SARs (Si/Al = 20–30) are more considerable, i.e., Zeo-Y_H results in a conversion of 26.7 ± 1.4% whereas ZSM-5_H results in a conversion of 98.0 ± 2.0% under the standard reaction conditions (cf. Table 2, Entries 6, 9 with Table 4, Entries 2, 3). Such difference also reveals that the confinements originating from the zeolite topology play a more dominant role than the SAR57. Furthermore, Zeo-Y_H shows selectivity toward higher carbon range products due to the larger pore sizes whereas ZSM-5_H favors the C1–4 hydrocarbons which may be attributed to smaller pores, typically referred as shape selectivity and correlated to the zeolite topology30,58,59.
Table 4.
n-Hexadecane deconstruction with the additional Zeo-Y_H-based catalysts with varying SARs
| Entry | Catalyst | Conv. (%) | C1-4 Yield (%)* | C5-16 Yield (%)* |
|---|---|---|---|---|
| 1 | Zeo-Y_H | 26.7 ± 1.4 | 3.3 | 23.3 |
| 2 | Zeo-Y_H [60] | 24.6 ± 1.2 | 3.8 | 20.8 |
| 3 | Zeo-Y_H [80] | 14.9 ± 0.6 | 2.0 | 12.8 |
| 4 | Ni/Zeo-Y_H | 20.9 ± 1.3 | 0.4 | 20.5 |
| 5 | Ni/Zeo-Y_H [60] | 5.8 ± 0.5 | 0.9 | 4.9 |
| 6 | Ni/Zeo-Y_H [80] | 4.9 ± 0.7 | 0.6 | 4.3 |
| 7 | Ru/Zeo-Y_H | 99.0 ± 1.0 | 92.4 | 6.9 |
| 8 | Ru/Zeo-Y_H [60] | 99.0 ± 1.0 | 98.0 | 2.0 |
| 9 | Ru/Zeo-Y_H [80] | 99.0 ± 1.0 | 97.2 | 2.8 |
Reaction conditions: n-hexadecane (1.59 g, 7.0 mmol), catalyst (0.1 g, metal loading = 2.5 wt%), S/C ratio (substrate/catalyst weight ratio) ~16, 45 bar H2, 375 °C, 2 h.
*All yields were calculated as the carbon yield and isomerized C16 (isoC16) are considered as products.
Ni/Zeo-Y_H [60] and Ni/Zeo-Y_H [80] result in conversions of 5.8 ± 0.5% and 4.9 ± 0.7% of nC16 under 45 bar H2 at 375 °C reacting for 2 hs compared to the conversion of 20.9 ± 1.3% via Ni/Zeo-Y_H (Table 4, Entries 4–6). Similar trends were observed to that of the main library catalysts, i.e., the activity decreases following the immobilization of Ni NPs of Ni/Zeo-Y_H [60] and Ni/Zeo-Y_H [80] (Table 4, cf. Entries 2, 3 with 5, 6). The activity of the catalysts follows the order: Ni/Zeo-Y_H > Ni/Zeo-Y_H [60] > Ni/Zeo-Y_H [80], the same order as the unmodified supports, and confirms that C-C bond cleavage principally depends on the acid sites of the silica-alumina support at 375 °C. High degrees of saturated products obtained using Ni/Zeo-Y_H [60] and Ni/Zeo-Y_H [80] confirm a bifunctional pathway (Supplementary Table 5, Entries 5, 6). Both Ru/Zeo-Y_H [60] and Ru/Zeo-Y_H [80] led to quantitative conversion as observed for Ru/Zeo-Y_H at 375 °C (Table 4, Entries 7–9), with methane as the dominant product, confirming the hydrogenolysis pathway is in operation (Supplementary Fig. 20).
PE depolymerization
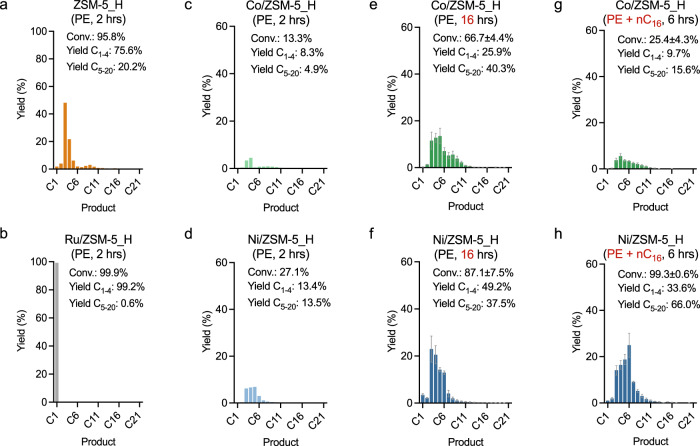
The catalysts employing ZSM-5_H as the support material are the most active and were further used to depolymerize PE (Mw ~4000 g/mol, Sigma-Aldrich). ZSM-5_H results in a conversion of 95.8% (PE: ~1.6 g, ~112 mmol of carbon, catalyst: 0.2 g, H2: 45 bar, reaction time: 2 h, solvent-free) at 375 °C. The product distribution is similar to that obtained with nC16, i.e., with C3–4 hydrocarbons as the major products (Fig. 4a) and unsaturated liquid products, confirming a monofunctional hydrocracking mechanism. Under identical reaction conditions, quantitative depolymerization of PE to methane was observed in the presence of Ru/ZSM-5_H, clearly demonstrating a hydrogenolysis mechanism (Fig. 4b).
Fig. 4. Polyethylene (PE) depolymerization under solvent-free and solvent-assisted conditions.
a PE (1.59 g, ~112 mmol of carbon) depolymerization under solvent-free condition: catalyst (0.2 g, metal loading = 2.5 wt%), 45 bar H2, 375 °C, 2 h via ZSM-5_H, b Ru/ZSM-5_H, c Co/ZSM-5_H, d Ni/ZSM-5_H, e Co/ZSM-5_H, reaction time = 16 h, f Ni/ZSM-5_H, reaction time = 16 h. g PE (0.2 g, ~14 mmol of carbon) depolymerization under the solvent-assisted condition: nC16 (1.4 g, ~98 mmol based on carbon, as a reactive solvent), catalyst (0.1 g, metal loading = 2.5 wt%), 45 bar H2, 375 °C, 6 h via Co/ZSM-5_H and h Ni/ZSM-5_H. Error bars = standard deviation. Source data are provided as a Source Data file.
Low conversions of 13.3 and 27.1% were obtained at 375 °C in the presence of Co/ZSM-5_H and Ni/ZSM-5_H, respectively (Fig. 4c, d). Prolonging the reaction time to 16 h increases the conversion of PE to 66.7 ± 4.4% for Co/ZSM-5_H and 87.1 ± 7.5% for Ni/ZSM-5_H (Fig. 4e, f). With the prolonged reaction time of 16 h, unimodal product distributions were observed with C3–4 hydrocarbons being the major products using Co/ZSM-5_H and Ni/ZSM-5_H under solvent-free conditions. Inherent mass- and heat-transfer limitations of the solvent-free reaction involving a polymeric starting material60–62, could be mitigated by employing a solvent63,64. Hence, nC16 was applied as a reactive solvent (note that the total carbon molar number was maintained, i.e., nC16 + PE = ~1.4 + 0.2 g corresponding to ~112 mmol of carbon, catalyst = 0.1 g). Significant coking was not observed presumably due to the short reaction time and the application of hydrogen (Tables 2, 4), evidenced by the similar curves obtained from thermogravimetric analysis (TGA) of fresh and used catalysts (Supplementary Fig. 21). No apparent differences in TGA curves above 500 °C were observed, indicating a well-closed carbon balance by the gaseous and liquid products due to limited solid residual formation during a reaction. The use of nC16 a solvent is also expected to prevent coke formations and to preserve catalytic performance for the more challenging polymer substrates over longer-term operation65,66, particularly in the cases via highly acidic catalysts67–69. Nonetheless, regeneration processes for coke removal could be applied if required70,71.
Co/ZSM-5_H shows the conversion of 25.4 ± 4.3%, whereas Ni/ZSM-5_H results in a near-quantitative conversion of 99.3 ± 0.6% at 375 °C with a reaction time of 6 h (Fig. 4g, h). No significant differences in the product distribution or selectivity were observed with Co/ZSM-5_H under solvent-free or solvent-assisted depolymerization conditions, presumably due to the general low activity of the applied catalyst (cf. Fig. 4e with 4g). However, the selectivity shifts toward longer hydrocarbons with Ni/ZSM-5_H under solvent-assisted conditions, resulting in C5–6 hydrocarbons as the main products (cf. Fig. 4f with 4h). The intrinsic activity of a given catalyst, therefore, has more significant effects on transfer issues toward selectivity and should be considered a primary factor. Despite the shift toward a higher carbon range when employing the solvent-assisted condition, it is worth noting that the resulting unimodal distribution with C5–6 as the major products after PE depolymerization in the presence of Ni/ZSM-5_H differs from the bimodal distribution obtained for nC16 (cf. Fig. 3c-right with 4h). Co- and Ni-modified catalysts afford predominantly saturated products following the PE depolymerizations, indicating a bifunctional hydrocracking mechanism for PE depolymerization in the presence of Co/ZSM-5_H and Ni/ZSM-5_H under solvent-assisted conditions. These, however, revealed a more sensitive nature of the bifunctional pathway upon transfer issues than the two other pathways. Recent studies also demonstrated that the migration of intermediates between the acid and metal sites affects reactivity as well as selectivity, and rationalizes the sensitive nature of a bifunctional pathway compared to the monofunctional and hydrogenolysis pathways which have more localized active sites53,54.
Activity-mechanism map
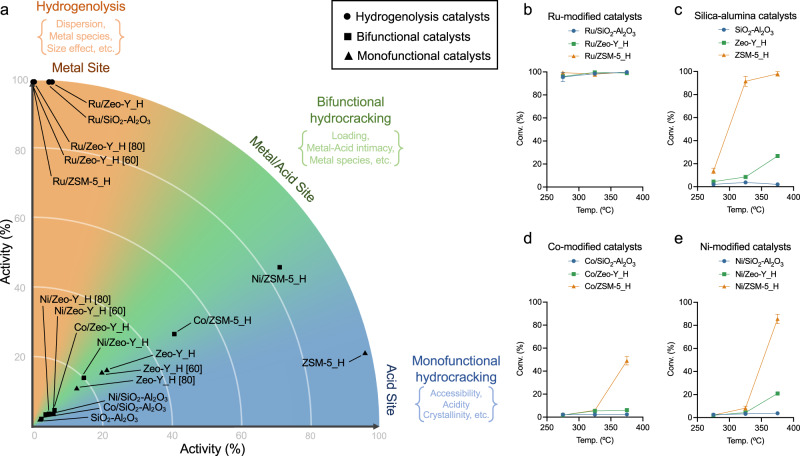
The assignment of the principal mechanism for each catalyst is based on the product distribution, i.e., carbon range, isomerization, and unsaturated products. The catalysts were plotted on an activity-mechanism map in a polar coordinate system (Fig. 5a) for the reactions of nC16 deconstructions under 45 bar H2 at 375 °C with a reaction time of 2 h (i.e., the benchmark conditions). Differences in activity (r) and mechanistic pathway (θ) are indicated by the map (see further details in Methods), allowing rapid benchmarking of new catalysts and enabling the prediction of specific product distributions for depolymerizations.
Fig. 5. Activity-mechanism map and catalytic n-hexadecane (nC16) decompositions via types of metal modifications.
a Activity-mechanism map with the 18 tested catalysts positioned according to their activity and mechanism (note that the activity is denoted by the nC16 conversion at the benchmark condition: 45 bar H2, 375 °C, 2 h). C-C bond cleavage is dominated by hydrogenolysis (orange area), bifunctional hydrocracking (green area), or monofunctional hydrocracking (blue area). b nC16 (1.59 g) deconstructions with 45 bar H2, at 275, 325, and 375 °C, 2 h, via Ru-modified catalysts (0.1 g, metal loading = 2.5 wt%): Ru/SiO2-Al2O3, Ru/Zeo-Y_H, and Ru/ZSM-5_H, c unmodified silica-alumina catalysts (0.1 g): SiO2-Al2O3, Zeo-Y_H, and ZSM-5_H, d Co-modified catalysts (0.1 g, metal loading = 2.5 wt%): Co/SiO2-Al2O3, Co/Zeo-Y_H, and Co/ZSM-5_H, and e Ni-modified catalysts (0.1 g, metal loading = 2.5 wt%): Ni/SiO2-Al2O3, Ni/Zeo-Y_H, and Ni/ZSM-5_H. Error bars = standard deviation. Source data are provided as a Source Data file.
The types of metal modifications strongly influence the principal cleavage mechanism (Fig. 5b–e). All unmodified silica-alumina supports function via a similar mechanism as confirmed by the similar product distributions (cf. Fig. 5a with Supplementary Fig. 12). Immobilization of Co and Ni NPs on the silica-alumina supports switches the mechanism to bifunctional hydrocracking with the Ni-modified catalysts being more active than the Co-analogs (cf. Fig. 5c with 5d, e). The catalysts containing immobilized Ru NPs operate predominantly via hydrogenolysis as methane is the major product (cf. Fig. 5a with Supplementary Fig. 15). The types of silica-alumina support affect the rate of reaction considerably via the acidic and confinement of the support structure but not the principal mechanism (cf. Fig. 5b–e with Supplementary Fig. 22)72–75. The Si/Al ratio influences the catalytic activity but to a lesser degree than the type of metal NPs used and the topology of the silica-alumina support (Supplementary Fig. 23). In contrast, the applied reaction temperature has shown no apparent patterns for predicting activity and prevailing mechanism (Supplementary Fig. 24), unless the onset temperatures of the catalytic components are known.
Discussion
The depolymerization of polyolefins is an attractive way to recycle/reuse the plastic waste in comparison to mechanical recycling to afford high-quality products76–78 and has a greater impact on sustainability than landfilling79,80. Despite the current cost of hydrogen, which is predicted to decrease81,82, the ability to fine-tune the reaction mechanism and hence the product distribution is highly valuable. Moreover, as a direct consequence of the reductive environment, the process is expected to be cleaner and result in less coke formation than alternative methods such as incineration and pyrolysis83–85.
In order to transfer the process from the laboratory scale to the industrial arena, new catalysts with superior activity and selectivity to the current range are required. To this end, we classified depolymerization mechanisms according to the product distribution, which includes the carbon range, isomerization, and degrees of saturation. Based on the study of 18 catalysts, an activity-mechanism map was constructed according to the defined benchmark conditions employing nC16 as a model substrate. The activity-mechanism map serves three major functions. First, it can be used to classify catalysts that afford a specific product distribution. Second, new catalysts can be plotted on the map to allow their activity and mechanism to be gauged relative to the known catalysts. Third, the optimal components, i.e., support, metal type, etc., to provide a specific product distribution can be ascertained from the map. We expect this benchmarking strategy accelerates the development of new catalysts for both plastic-to-fuel and plastic-to-chemical scenarios.
Methods
General
All reactions with nC16 deconstructions and certain reactions with PE depolymerizations were performed at least 3 times, and the statistics of average conversion and standard deviation were calculated. n-Hexadecane and polyethylene (PE, Mw ~4000, Mn ~1700) were purchased from Sigma-Aldrich. n-Dodecane was acquired from Abcr. CoCl2, NiCl2·6H2O, and RuCl3·3H2O were purchased from ChemPur, Abcr, and Precious Metal Online, respectively. All metal salts were stored in desiccators. Amorphous silica-alumina (catalyst support, grade 135), zeolite-Y_hydrogen (Si/Al = 30:1, 780 m2/g; Si/Al = 60:1, 720 m2/g; Si/Al = 80:1, 780 m2/g), and ZSM-5_ammonium (Si/Al = 23:1, 425 m2/g) were obtained from Sigma-Aldrich, Abcr, and Zeolyst, respectively. ZSM-5_ammonium (2.0 g) was calcined at 550 °C under 300 mL/min dry air for 60 min to transform into ZSM-5_hydrogen86. Gamma-alumina (γAl2O3 = 97% min., 185 m2/g) was purchased from Strem.
Typical synthesis of metal-modified catalyst
The support material (1.50 g) and a metal salt (CoCl2, 82.7 mg, 0.64 mmol; NiCl2·6H2O, 151.8 mg, 0.64 mmol; RuCl3·3H2O, 97.1 mg, 0.37 mmol) were placed in a round bottle flask (100 mL) with a magnetic stir bar and then dispersed in deionized (DI) water (36 mL). The suspension was stirred at 500 rpm at 35 °C for 1 h. NaBH4 (70.1 mg, 1.85 mmol for RuCl3·3H2O; 120.8 mg, 3.19 mmol for CoCl2 and NiCl2·6H2O; 5 eq. to the applied metal) was dissolved in DI water (36 mL) and added to the suspension in one portion. The suspension was stirred at 800 rpm for 1 h. The resulting solid was vacuum filtered and washed with DI water (3 × 25 mL). The solid was then dried at 100 °C in an oven for 18 h. Various batches of the catalyst were prepared, characterized, and evaluated, and no significant differences were observed.
Typical reaction of n-hexadecane deconstruction
n-Hexadecane (1.59 g, 7.0 mmol; 112 mmol based on carbon) and the catalyst (0.10 g) were added to a glass vial (20 mL) with a glass magnetic stir bar, and the vial was then placed into an autoclave (75 mL, Parr Instrument). The autoclave was purged three times with H2 and then pressurized to 45 bar and sealed. The pressurized autoclave was placed in a heating block at the desired temperature (275–375 °C) and stirred (350 rpm) for the given reaction time (2–6 h). After the reaction, the autoclave was cooled to room temperature in a water bath. The gaseous products were transferred into a gas-sampling bag (1 L) and injected into a specialized gas-sampling gas chromatography-flame ionization detector (GC-FID) for analysis. For GC-mass spectrometry (GC-MS) analysis of the liquid products, n-dodecane (0.085 g, 0.5 mmol) was added to the vial as an internal standard and diethyl ether was used as the solvent if the resulting liquid was insufficient for GC sample preparation. In certain cases, dichloromethane was used to wash (8 mL × 3) the catalyst, and the catalyst was then vacuum dried overnight before conducting the thermogravimetric analysis (TGA).
Typical reaction of polyethylene depolymerization
PE (1.59 g, Mw ~4000, Sigma-Aldrich; ~112 mmol of carbon) and the catalyst (0.20 g) were added to a glass vial (20 mL) with a glass-coated magnetic stir bar, and the vial was then placed into an autoclave (75 mL, Parr Instrument). The autoclave was purged three times with H2 and then pressurized to 45 bar and sealed. The pressurized autoclave was placed in a heating block at the desired temperature (275–375 °C) and stirred (500 rpm) for the given reaction time (2–16 h). After the reaction, the autoclave was cooled to room temperature in a water bath. The gaseous products were transferred into a gas-sampling bag (1 L) and injected into a specialized gas-sampling gas chromatography-flame ionization detector (GC-FID) for analysis. For GC-mass spectrometry (GC-MS) analysis of the liquid products, n-dodecane (0.085 g, 0.5 mmol) was added to the vial as an internal standard and diethyl ether was used as the solvent if the resulting liquid was insufficient (<0.5 mL) for GC sample preparation.
Instruments
Analysis of the liquid products was performed using an Agilent 7890B Gas Chromatograph coupled with Agilent 7000C MS triple quad detector with He as a carrier gas, Agilent HP-5ms ultra inert capillary column, with a ramping rate of 7.5 °C/min from 40 to 197.5 °C (for samples of nC16 conversion) or from 40 to 295 °C (for samples of PE conversions). Analysis of gaseous products was performed using an Agilent 7890B Gas Chromatograph with N2 as a carrier gas, Agilent PoraPLOT Q capillary column, with a ramping rate of 10 °C/min from 35 to 175 °C. The corresponding retention times of C1–5 hydrocarbons was identified by a reference gas mixture obtained from Carbagas comprising H2, CO, CO2, CH4, C2H4, C2H6, C3H6, C3H8, and nC4H10 in N2. FID area signals were calibrated using reference methane, ethane, propane, and n-butane gas bottle purchased from Sigma-Aldrich.
1H NMR spectra were recorded on a Bruker 400 MHz instrument. Powder X-ray powder diffraction (Powder-XRD) patterns were acquired by Bruker D8 Discover. X-ray photoelectron spectroscopy (XPS) measurements were obtained on a Kratos Axis Supra. Scanning electron microscopy (SEM) images were recorded on a Carl Zeiss Gemini 300 microscope. Transmission electron microscopy (TEM) images and scanning transmission electron microscopy (STEM) mappings were obtained on the FEI Talos microscope and analyzed under 200 keV acceleration energy. Thermogravimetric analysis (TGA) was conducted by Mettler Toledo TGA/DSC 3+ with a ramping rate of 5 °C/min from 35 to 900 °C and a flow rate of 20 mL/min under air.
Data analysis and quantification
Conversion of nC16 and quantification of the C5–16 products was estimated from the GC-FID signal ratio with nC12 as the internal standard with documented approaches of effective carbon number (ECN) calculations for correlations among the resulted hydrocarbon products87. Quantification of the C1–4 fraction was determined from the GC-FID signal ratio compared to signals with 100% reference gas injection.
| 1 |
| 2 |
Conversion of PE was calculated using the following equation and quantification of the products was determined from the GC-FID signal ratio using nC12 as an internal standard:
| 3 |
Activity-mechanism map
The activity-mechanism map is constructed on a two-dimensional polar coordinate system composed of activity as the radius (r) and mechanism as the theta (θ). The value for “activity” is denoted by the nC16 conversion at the selected benchmark conditions (45 bar H2, 375 °C, 2 h as the current case). The benchmark conditions would be further adapted (preferably lower applied temperature) in the case of a new catalyst that outperforms all the tested catalysts by relocating the maximum activity (r = 100%). The value for “mechanism” is denoted by the similarity compared to a perfect mechanism with product selectivity as the major feature:
0º (perfect monofunctional hydrocracking): C3–4 products only.
45º (perfect bifunctional hydrocracking): >C3–4 products only.
90º (perfect hydrolysis): C1 product only.
Supplementary information
Acknowledgements
This work is supported by the EPFL (Switzerland) and created as part of NCCR Catalysis (grant number 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation. We thank prof. Holger Frauenrath, Julian Bleich, Matthieu Wendling, Yann Lavanchy, and Yevhen Hryshunin for technical support.
Source data
Author contributions
W.-T.L., A.v.M., F.D.B., and P.J.D. contributed to the design of the experiments and data analysis. W.-T.L. and J.R.C. performed the experiments and M.D.M. performed the XPS analysis; W.-T.L. and P.J.D. wrote the manuscript, and all the authors discussed, commented on, and proofread the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The data that support the figures and findings of this work are provided in the Supplementary Information and in the Source Data files. Source data are provided with this paper.
Competing interests
W.-T.L., A.v.M., F.D.B., and P.J.D. are founders of a spin-off company. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-32563-y.
References
- 1.Patrício Silva AL, et al. Increased plastic pollution due to COVID-19 pandemic: challenges and recommendations. Chem. Eng. J. 2021;405:126683. doi: 10.1016/j.cej.2020.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae Y, An YJ. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: a review. Environ. Pollut. 2018;240:387–395. doi: 10.1016/j.envpol.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Windsor FM, et al. A catchment-scale perspective of plastic pollution. Glob. Change Biol. 2019;25:1207–1221. doi: 10.1111/gcb.14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017). [DOI] [PMC free article] [PubMed]
- 5.Department of Economic and Social Affairs. The Sustainable Development Goals Report 2020 (United Nations, 2020).
- 6.Ritchie, H. & Roser, M. “Plastic Pollution”. Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/plastic-pollution [Online Resource] (2018).
- 7.Kunwar B, Cheng HN, Chandrashekaran SR, Sharma BK. Plastics to fuel: a review. Renew. Sustain. Energy Rev. 2016;54:421–428. doi: 10.1016/j.rser.2015.10.015. [DOI] [Google Scholar]
- 8.Mohanraj C, Senthilkumar T, Chandrasekar M. A review on conversion techniques of liquid fuel from waste plastic materials. Int. J. Energy Res. 2017;41:1534–1552. doi: 10.1002/er.3720. [DOI] [Google Scholar]
- 9.Wu C, Williams PT. Pyrolysis-gasification of plastics, mixed plastics and real-world plastic waste with and without Ni-Mg-Al catalyst. Fuel. 2010;89:3022–3032. doi: 10.1016/j.fuel.2010.05.032. [DOI] [Google Scholar]
- 10.Gracida-Alvarez UR, Winjobi O, Sacramento-Rivero JC, Shonnard DR. System analyses of high-value chemicals and fuels from a waste high-density polyethylene refinery. Part 1: conceptual design and techno-economic assessment. ACS Sustain. Chem. Eng. 2019;7:18254–18266. doi: 10.1021/acssuschemeng.9b04763. [DOI] [Google Scholar]
- 11.Gracida-Alvarez UR, Winjobi O, Sacramento-Rivero JC, Shonnard DR. System analyses of high-value chemicals and fuels from a waste high-density polyethylene refinery. Part 2: carbon footprint analysis and regional electricity effects. ACS Sustain. Chem. Eng. 2019;7:18267–18278. doi: 10.1021/acssuschemeng.9b04764. [DOI] [Google Scholar]
- 12.Palos R, et al. Waste refinery: the valorization of waste plastics and end-of-life tires in refinery units. A review. Energy Fuels. 2021;35:3529–3557. doi: 10.1021/acs.energyfuels.0c03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez E, et al. Towards waste refinery: co-feeding HDPE pyrolysis waxes with VGO into the catalytic cracking unit. Energy Convers. Manag. 2020;207:112554. doi: 10.1016/j.enconman.2020.112554. [DOI] [Google Scholar]
- 14.Jubinville D, Esmizadeh E, Saikrishnan S, Tzoganakis C, Mekonnen T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustain. Mater. Technol. 2020;25:e00188. [Google Scholar]
- 15.Butler E, Devlin G, McDonnell K. Waste polyolefins to liquid fuels via pyrolysis: Review of commercial state-of-the-art and recent laboratory research. Waste Biomass. Valoriz. 2011;2:227–255. doi: 10.1007/s12649-011-9067-5. [DOI] [Google Scholar]
- 16.Sadrameli SM. Thermal/catalytic cracking of hydrocarbons for the production of olefins: a state-of-the-art review I: thermal cracking review. Fuel. 2015;140:102–115. doi: 10.1016/j.fuel.2014.09.034. [DOI] [Google Scholar]
- 17.Sadrameli SM. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: a state-of-the-art review II: catalytic cracking review. Fuel. 2016;173:285–297. doi: 10.1016/j.fuel.2016.01.047. [DOI] [Google Scholar]
- 18.Figueiredo AL, et al. Catalytic cracking of LDPE over nanocrystalline HZSM-5 zeolite prepared by seed-assisted synthesis from an organic-template-free system. J. Anal. Appl. Pyrolysis. 2016;117:132–140. doi: 10.1016/j.jaap.2015.12.005. [DOI] [Google Scholar]
- 19.Munir D, Amer H, Aslam R, Bououdina M, Usman MR. Composite zeolite beta catalysts for catalytic hydrocracking of plastic waste to liquid fuels. Mater. Renew. Sustain. Energy. 2020;9:1–13. doi: 10.1007/s40243-020-00169-3. [DOI] [Google Scholar]
- 20.Bin Jumah A, Anbumuthu V, Tedstone AA, Garforth AA. Catalyzing the hydrocracking of low density polyethylene. Ind. Eng. Chem. Res. 2019;58:20601–20609. doi: 10.1021/acs.iecr.9b04263. [DOI] [Google Scholar]
- 21.Liu S, Kots PA, Vance BC, Danielson A, Vlachos DG. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021;7:8283–8304. doi: 10.1126/sciadv.abf8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weitkamp J. Catalytic hydrocracking-mechanisms and versatility of the process. ChemCatChem. 2012;4:292–306. doi: 10.1002/cctc.201100315. [DOI] [Google Scholar]
- 23.Rorrer JE, Beckham GT, Román-Leshkov Y. Conversion of polyolefin waste to liquid alkanes with Ru-based catalysts under mild conditions. JACS Au. 2021;1:8–12. doi: 10.1021/jacsau.0c00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia C, et al. Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolysis over Ru catalyst. Chem. Catal. 2021;1:437–455. doi: 10.1016/j.checat.2021.04.002. [DOI] [Google Scholar]
- 25.Kots PA, et al. Polypropylene plastic waste conversion to lubricants over Ru/TiO2Catalysts. ACS Catal. 2021;11:8104–8115. doi: 10.1021/acscatal.1c00874. [DOI] [Google Scholar]
- 26.Wu X, et al. Size-controlled nanoparticles embedded in a mesoporous architecture leading to efficient and selective hydrogenolysis of polyolefins. J. Am. Chem. Soc. 2022;144:5323–5334. doi: 10.1021/jacs.1c11694. [DOI] [PubMed] [Google Scholar]
- 27.Tennakoon A, et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 2020;3:893–901. doi: 10.1038/s41929-020-00519-4. [DOI] [Google Scholar]
- 28.Celik G, et al. Upcycling single-use polyethylene into high-quality liquid products. ACS Cent. Sci. 2019;5:1795–1803. doi: 10.1021/acscentsci.9b00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson DH, Kokotailo GT, Lawton SL, Meier WM. Crystal structure and structure-related properties of ZSM-5. J. Phys. Chem. 1981;85:2238–2243. doi: 10.1021/j150615a020. [DOI] [Google Scholar]
- 30.Den Hollander MA, Wissink M, Makkee M, Moulijn JA. Gasoline conversion: reactivity towards cracking with equilibrated FCC and ZSM-5 catalysts. Appl. Catal. A Gen. 2002;223:85–102. doi: 10.1016/S0926-860X(01)00745-1. [DOI] [Google Scholar]
- 31.Al-Ani A, Freitas C, Zholobenko V. Nanostructured large-pore zeolite: the enhanced accessibility of active sites and its effect on the catalytic performance. Micropor. Mesopor. Mater. 2020;293:109805. doi: 10.1016/j.micromeso.2019.109805. [DOI] [Google Scholar]
- 32.Chen PH, Billett BA, Tsukamoto T, Dong G. Cut and sew transformations via transition-metal-catalyzed carbon-carbon bond activation. ACS Catal. 2017;7:1340–1360. doi: 10.1021/acscatal.6b03210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurahashi T, De Meijere A. C-C bond activation by octacarbonyldicobalt: [3+1] cocyclizations of methylenecyclopropanes with carbon monoxide. Angew. Chem. Int. Ed. 2005;44:7881–7884. doi: 10.1002/anie.200502596. [DOI] [PubMed] [Google Scholar]
- 34.Fan C, Lv XY, Xiao LJ, Xie JH, Zhou QL. Alkenyl exchange of allylamines via nickel(0)-catalyzed C-C bond cleavage. J. Am. Chem. Soc. 2019;141:2889–2893. doi: 10.1021/jacs.8b13251. [DOI] [PubMed] [Google Scholar]
- 35.Su B, Cao ZC, Shi ZJ. Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations. Acc. Chem. Res. 2015;48:886–896. doi: 10.1021/ar500345f. [DOI] [PubMed] [Google Scholar]
- 36.Vom Stein T, et al. Ruthenium-catalyzed C-C bond cleavage in lignin model substrates. Angew. Chem. Int. Ed. 2015;54:5859–5863. doi: 10.1002/anie.201410620. [DOI] [PubMed] [Google Scholar]
- 37.Shangguan J, Olarte MV, Chin YH. Mechanistic insights on C-O and C-C bond activation and hydrogen insertion during acetic acid hydrogenation catalyzed by ruthenium clusters in aqueous medium. J. Catal. 2016;340:107–121. doi: 10.1016/j.jcat.2016.04.024. [DOI] [Google Scholar]
- 38.Ritleng V, Sirlin C, Pfeffer M. Ru-, Rh-, and Pd-catalyzed C-C bond formation involving C-H activation and addition on unsaturated substrates: reactions and mechanistic aspects. Chem. Rev. 2002;102:1731–1769. doi: 10.1021/cr0104330. [DOI] [PubMed] [Google Scholar]
- 39.Biesinger MC, et al. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011;257:2717–2730. doi: 10.1016/j.apsusc.2010.10.051. [DOI] [Google Scholar]
- 40.Morgan DJ. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015;47:1072–1079. doi: 10.1002/sia.5852. [DOI] [Google Scholar]
- 41.Oenema J, et al. Influence of nanoscale intimacy and zeolite micropore size on the performance of bifunctional catalysts for n-heptane hydroisomerization. ACS Catal. 2020;10:14245–14257. doi: 10.1021/acscatal.0c03138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano DP, Aguado J, Escola JM. Catalytic cracking of a polyolefin mixture over different acid solid catalysts. Ind. Eng. Chem. Res. 2000;39:1177–1184. doi: 10.1021/ie9906363. [DOI] [Google Scholar]
- 43.Miskolczi N, Bartha L, Deák G, Jóver B, Kalló D. Thermal and thermo-catalytic degradation of high-density polyethylene waste. J. Anal. Appl. Pyrolysis. 2004;72:235–242. doi: 10.1016/j.jaap.2004.07.002. [DOI] [Google Scholar]
- 44.Leydier F, Chizallet C, Costa D, Raybaud P. Revisiting carbenium chemistry on amorphous silica-alumina: unraveling their milder acidity as compared to zeolites. J. Catal. 2015;325:35–47. doi: 10.1016/j.jcat.2015.02.012. [DOI] [Google Scholar]
- 45.Chai Y, Dai W, Wu G, Guan N, Li L. Confinement in a zeolite and zeolite catalysis. Acc. Chem. Res. 2021;54:2894–2904. doi: 10.1021/acs.accounts.1c00274. [DOI] [PubMed] [Google Scholar]
- 46.Shimada I, Takizawa K, Fukunaga H, Takahashi N, Takatsuka T. Catalytic cracking of polycyclic aromatic hydrocarbons with hydrogen transfer reaction. Fuel. 2015;161:207–214. doi: 10.1016/j.fuel.2015.08.051. [DOI] [Google Scholar]
- 47.Sonnemans J, Mars P. The mechanism of pyridine hydrogenolysis on molybdenum-containing catalysts. III. Cracking, hydrocracking, dehydrogenation and disproportionation of pentylamine. J. Catal. 1974;34:215–229. doi: 10.1016/0021-9517(74)90031-1. [DOI] [Google Scholar]
- 48.Xiong M, Gao Z, Qin Y. Spillover in heterogeneous catalysis: new insights and opportunities. ACS Cent. Sci. 2021;11:3159–3172. [Google Scholar]
- 49.Anaya F, Zhang L, Tan Q, Resasco DE. Tuning the acid-metal balance in Pd/ and Pt/zeolite catalysts for the hydroalkylation of m-cresol. J. Catal. 2015;328:173–185. doi: 10.1016/j.jcat.2015.01.004. [DOI] [Google Scholar]
- 50.Monteiro CAA, Costa D, Zotin JL, Cardoso D. Effect of metal-acid site balance on hydroconversion of decalin over Pt/Beta zeolite bifunctional catalysts. Fuel. 2015;160:71–79. doi: 10.1016/j.fuel.2015.07.054. [DOI] [Google Scholar]
- 51.Cerqueira HS, Magnoux P, Martin D, Guisnet M. Coke formation and coke profiles during the transformation of various reactants at 450 °C over a USHY zeolite. Appl. Catal. A Gen. 2001;208:359–367. doi: 10.1016/S0926-860X(00)00734-1. [DOI] [Google Scholar]
- 52.Bjørgen M, Olsbye U, Kolboe S. Coke precursor formation and zeolite deactivation: mechanistic insights from hexamethylbenzene conversion. J. Catal. 2003;215:30–44. doi: 10.1016/S0021-9517(02)00050-7. [DOI] [Google Scholar]
- 53.Liu B, et al. Microwaves effectively examine the extent and type of coking over acid zeolite catalysts. Nat. Commun. 2017;8:1–7. doi: 10.1038/s41467-016-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendes PSF, Silva JM, Ribeiro MF, Daudin A, Bouchy C. Bifunctional intimacy and its interplay with metal-acid balance in shaped hydroisomerization catalysts. ChemCatChem. 2020;12:4582–4592. doi: 10.1002/cctc.202000624. [DOI] [Google Scholar]
- 55.Zhang Y, et al. Hydroisomerization of n-dodecane over bi-porous Pt-containing bifunctional catalysts: effects of alkene intermediates’ journey distances within the zeolite micropores. Fuel. 2019;236:428–436. doi: 10.1016/j.fuel.2018.09.017. [DOI] [Google Scholar]
- 56.Nakagawa Y, et al. Regioselectivity and reaction mechanism of Ru-catalyzed hydrogenolysis of squalane and model alkanes. ChemSusChem. 2017;10:189–198. doi: 10.1002/cssc.201601204. [DOI] [PubMed] [Google Scholar]
- 57.Alaithan ZA, Mallia G, Harrison NM. Monomolecular cracking of propane: effect of zeolite confinement and acidity. ACS Omega. 2022;7:7531–7540. doi: 10.1021/acsomega.1c05532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smit B, Maesen TLM. Towards a molecular understanding of shape selectivity. Nature. 2008;451:671–678. doi: 10.1038/nature06552. [DOI] [PubMed] [Google Scholar]
- 59.Smit B, Maesen TLM. Molecular simulations of zeolites: adsorption, diffusion, and shape selectivity. Chem. Rev. 2008;108:4125–4184. doi: 10.1021/cr8002642. [DOI] [PubMed] [Google Scholar]
- 60.Nargessi Z, Karimzadeh R. Analysis of heat and mass transfer and parametric sensitivity in an experimental fixed-bed reactor for the catalytic cracking of heavy hydrocarbons based on modeling and experiments. Ind. Eng. Chem. Res. 2021;60:4831–4846. doi: 10.1021/acs.iecr.0c06223. [DOI] [Google Scholar]
- 61.Hoff TC, et al. Decoupling the role of external mass transfer and intracrystalline pore diffusion on the selectivity of HZSM-5 for the catalytic fast pyrolysis of biomass. ACS Sustain. Chem. Eng. 2017;5:8766–8776. doi: 10.1021/acssuschemeng.7b01578. [DOI] [Google Scholar]
- 62.Mukherjee S, Vannice MA. Solvent effects in liquid-phase reactions. I. Activity and selectivity during citral hydrogenation on Pt/SiO2 and evaluation of mass transfer effects. J. Catal. 2006;243:108–130. doi: 10.1016/j.jcat.2006.06.021. [DOI] [Google Scholar]
- 63.Rodríguez E, et al. Co-cracking of high-density polyethylene (HDPE) and vacuum gasoil (VGO) under refinery conditions. Chem. Eng. J. 2020;382:122602. doi: 10.1016/j.cej.2019.122602. [DOI] [Google Scholar]
- 64.Arandes JM, Ereña J, Azkoiti MJ, López-Valerio D, Bilbao J. Valorization by thermal cracking over silica of polyolefins dissolved in LCO. Fuel Process. Technol. 2004;85:125–140. doi: 10.1016/S0378-3820(03)00110-3. [DOI] [Google Scholar]
- 65.Zachariah A, Wang L, Yang S, Prasad V, De Klerk A. Suppression of coke formation during bitumen pyrolysis. Energy Fuels. 2013;27:3061–3070. doi: 10.1021/ef400314m. [DOI] [Google Scholar]
- 66.Wan, H., Chaudhari, R. V. & Subramaniam, B. Catalytic hydroprocessing of p-cresol: Metal, solvent and mass-transfer effects. In Topics in Catalysis. vol. 55 129–139 (Springer, 2012).
- 67.Gobin K, Manos G. Polymer degradation to fuels over microporous catalysts as a novel tertiary plastic recycling method. Polym. Degrad. Stab. 2004;83:267–279. doi: 10.1016/S0141-3910(03)00272-6. [DOI] [Google Scholar]
- 68.Xian X, et al. Acidity tuning of HZSM-5 zeolite by neutralization titration for coke inhibition in supercritical catalytic cracking of n-dodecane. Appl. Catal. A Gen. 2021;623:118278. doi: 10.1016/j.apcata.2021.118278. [DOI] [Google Scholar]
- 69.Cheng K, et al. Impact of the spatial organization of bifunctional metal–zeolite catalysts on the hydroisomerization of light alkanes. Angew. Chem. Int. Ed. 2020;59:3592–3600. doi: 10.1002/anie.201915080. [DOI] [PubMed] [Google Scholar]
- 70.Nakasaka Y, Tago T, Konno H, Okabe A, Masuda T. Kinetic study for burning regeneration of coked MFI-type zeolite and numerical modeling for regeneration process in a fixed-bed reactor. Chem. Eng. J. 2012;207–208:368–376. doi: 10.1016/j.cej.2012.06.138. [DOI] [Google Scholar]
- 71.Kassargy C, et al. Study of the effects of regeneration of USY zeolite on the catalytic cracking of polyethylene. Appl. Catal. B Environ. 2019;244:704–708. doi: 10.1016/j.apcatb.2018.11.093. [DOI] [Google Scholar]
- 72.Pyra K, Tarach KA, Majda D, Góra-Marek K. Desilicated zeolite BEA for the catalytic cracking of LDPE: the interplay between acidic sites’ strength and accessibility. Catal. Sci. Technol. 2019;9:1794–1801. doi: 10.1039/C9CY00326F. [DOI] [Google Scholar]
- 73.Tarach K, et al. Catalytic cracking performance of alkaline-treated zeolite Beta in the terms of acid sites properties and their accessibility. J. Catal. 2014;312:46–57. doi: 10.1016/j.jcat.2014.01.009. [DOI] [Google Scholar]
- 74.Niwa M, et al. Dependence of cracking activity on the Brønsted acidity of y zeolite: DFT study and experimental confirmation. Catal. Sci. Technol. 2013;3:1919–1927. doi: 10.1039/c3cy00195d. [DOI] [Google Scholar]
- 75.Suzuki K, Noda T, Katada N, Niwa M. IRMS-TPD of ammonia: direct and individual measurement of Brønsted acidity in zeolites and its relationship with the catalytic cracking activity. J. Catal. 2007;250:151–160. doi: 10.1016/j.jcat.2007.05.024. [DOI] [Google Scholar]
- 76.Thiounn T, Smith RC. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020;58:1347–1364. doi: 10.1002/pol.20190261. [DOI] [Google Scholar]
- 77.Rahimi AR, Garciá JM. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017;1:1–11. doi: 10.1038/s41570-017-0046. [DOI] [Google Scholar]
- 78.Ragaert K, Delva L, Van Geem K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017;69:24–58. doi: 10.1016/j.wasman.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 79.Das S, et al. Solid waste management: scope and the challenge of sustainability. J. Clean. Prod. 2019;228:658–678. doi: 10.1016/j.jclepro.2019.04.323. [DOI] [Google Scholar]
- 80.Cucchiella F, D’Adamo I, Gastaldi M. Sustainable waste management: waste to energy plant as an alternative to landfill. Energy Convers. Manag. 2017;131:18–31. doi: 10.1016/j.enconman.2016.11.012. [DOI] [Google Scholar]
- 81.Statista. Forecasted breakdown of renewable hydrogen production costs worldwid e from 2020 to 2030. Statistahttps://www.statista.com/statistics/1220812/global-hydrogen-production-cost-forecast-by-scenario/ (2021).
- 82.IEA. The Future of Hydrogen. International Energy Agencyhttps://www.iea.org/reports/the-future-of-hydrogen (2019).
- 83.Yan Q, et al. A comprehensive review on selective catalytic reduction catalysts for NOx emission abatement from municipal solid waste incinerators. Environ. Prog. Sustain. Energy. 2016;35:1061–1069. doi: 10.1002/ep.12328. [DOI] [Google Scholar]
- 84.Tseng HH, Wey MY, Liang YS, Chen KH. Catalytic removal of SO2, NO and HCl from incineration flue gas over activated carbon-supported metal oxides. Carbon N. Y. 2003;41:1079–1085. doi: 10.1016/S0008-6223(03)00017-4. [DOI] [Google Scholar]
- 85.Hart A, Leeke G, Greaves M, Wood J. Down-hole heavy crude oil upgrading by CAPRI: effect of hydrogen and methane gases upon upgrading and coke formation. Fuel. 2014;119:226–235. doi: 10.1016/j.fuel.2013.11.048. [DOI] [Google Scholar]
- 86.Serrano DP, García RA, Linares M, Gil B. Influence of the calcination treatment on the catalytic properties of hierarchical ZSM-5. Catal. Today. 2012;179:91–101. doi: 10.1016/j.cattod.2011.06.029. [DOI] [Google Scholar]
- 87.Scanlon JT, Willis DE. Calculation of flame ionization detector relative response factors using the effective carbon number concept. J. Chromatogr. Sci. 1985;23:333–340. doi: 10.1093/chromsci/23.8.333. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the figures and findings of this work are provided in the Supplementary Information and in the Source Data files. Source data are provided with this paper.