Abstract
Background
People with severe mental illness (SMI) are at higher risk of physical health conditions compared to the general population, however, the impact of specific underlying health conditions on the use of secondary care by people with SMI is unknown. We investigated hospital use in people managed in the community with SMI and five common physical long-term conditions: cardiovascular diseases, COPD, cancers, diabetes and liver disease.
Methods
We performed a systematic review and meta-analysis (Prospero: CRD42020176251) using terms for SMI, physical health conditions and hospitalisation. We included observational studies in adults under the age of 75 with a diagnosis of SMI who were managed in the community and had one of the physical conditions of interest. The primary outcomes were hospital use for all causes, physical health causes and related to the physical condition under study. We performed random-effects meta-analyses, stratified by physical condition.
Results
We identified 5,129 studies, of which 50 were included: focusing on diabetes (n = 21), cardiovascular disease (n = 19), COPD (n = 4), cancer (n = 3), liver disease (n = 1), and multiple physical health conditions (n = 2). The pooled odds ratio (pOR) of any hospital use in patients with diabetes and SMI was 1.28 (95%CI:1.15–1.44) compared to patients with diabetes alone and pooled hazard ratio was 1.19 (95%CI:1.08–1.31). The risk of 30-day readmissions was raised in patients with SMI and diabetes (pOR: 1.18, 95%CI:1.08–1.29), SMI and cardiovascular disease (pOR: 1.27, 95%CI:1.06–1.53) and SMI and COPD (pOR:1.18, 95%CI: 1.14–1.22) compared to patients with those conditions but no SMI.
Conclusion
People with SMI and five physical conditions are at higher risk of hospitalisation compared to people with that physical condition alone. Further research is warranted into the combined effects of SMI and physical conditions on longer-term hospital use to better target interventions aimed at reducing inappropriate hospital use and improving disease management and outcomes.
Introduction
People with severe mental illness (SMI) have more physical health comorbidities [1–5] and poorer prognoses from those comorbidities [6] than the general population. Physical health comorbidities can lead to reduced quality of life [7], worsening mental health [8], and drives excess mortality in people with SMI [9, 10].
Previous systematic reviews have found that people with SMI are at a higher risk of 30-day readmissions compared to those without SMI [11, 12], and that those with SMI and physical health comorbidities are at higher risk of psychiatric admissions compared to those with SMI alone [13].
Studies based on hospital records alone have found that people with SMI use hospitals for physical health more frequently than people without SMI for emergency admissions [14], preventable admissions [15] and all-cause admissions [16]. However, without accounting for underlying physical comorbidities, whether this represents inappropriate use of services is unclear. A recent meta-analysis by Ronaldson et al. [17] found that in studies controlling for physical health comorbidities there were more hospitalisations, ED visits and longer length of stays in people with SMI compared to those without SMI, suggesting the higher service use is not explained by higher prevalence of physical health conditions alone.
The relationship between physical and mental health and the effect on service utilisation is likely complex, dependent on a range of patient and provider factors. Known drivers of hospital utilisation in the general population, such as poor medication adherence, polypharmacy [18] or inappropriate prescribing [19], continuity of care, and patient satisfaction [20–22] may influence hospital utilisation differently depending on the number and type of underlying mental and physical health conditions in a population.
In order to understand the effect of having both a diagnosis of SMI and of physical health conditions on hospital utilisation, we undertook a systematic review and meta-analysis of observational hospital utilisation studies, comparing people with SMI and one of five common physical long-term conditions (LTCs), compared to those with either SMI or LTCs alone. These diseases (cardiovascular diseases, chronic obstructive pulmonary disease (COPD), cancers, diabetes and liver disease) were chosen because of their high burden of disease globally and/or their impact on those with SMI.
Methods
Search strategy
We searched the following sources on 24 March 2020 for publications or grey literature within the remit of the study without date restrictions: PubMED, EmBase, Web of Science, PsychInfo, PsychExtra, Health Management Information Centre. Searches for new publications were performed on 17 December 2020 and 17 March 2022. Searches included terms for severe mental illness, physical health conditions and hospitalisation (S1 Appendix). We performed forward and backward citation searching of relevant studies, reviews and editorials. Where conference abstracts were identified searches for related articles were performed. Conference abstracts were excluded from the final analysis, though those with available data were included in a sensitivity analysis. The study protocol was registered with Prospero: CRD42020176251.
Outcomes
The primary outcomes were planned or unplanned hospital admissions, for either all-causes, all physical health causes, causes specific to the physical LTC under study, or ambulatory care sensitive conditions (ACSC), a list of conditions for which emergency admission is thought to be avoidable [23]. Secondary outcomes were readmissions and attendance at EDs or other acute outpatient care for these causes.
Inclusion and exclusion criteria
We included observational studies of adults under the age of 75, managed in the community, and diagnosed with SMI and at least one of the physical LTCs of interest (cardiovascular diseases, COPD, cancers, diabetes and liver disease). We defined SMI as patients with a diagnosis of either schizophrenia, bipolar disorder or other non-organic long-term psychotic disorders, in line with the Quality Outcomes Framework used by the NHS in England [24]. We therefore excluded studies that included major depression in their definition of SMI, without stratifying results by mental health condition.
We excluded studies without comparator populations, interventional studies, and reviews. We also excluded studies focused solely on children and young people (under 18) or the elderly (over 75 years), or in populations not managed in the community. We excluded studies focused on planned outpatient care, preventative services such as cancer screening where the setting of service provision was unclear and context specific, and studies focused on admissions for specific procedures. Finally, we excluded studies where the outcome was hospitalisation for a specific physical health condition other than the physical LTC of interest.
Data screening and extraction
We collated the results of the literature search using EndNote X9 (Clarivate Analytics, PA, USA) and removed duplicates. The first researcher (NL) screened titles and abstracts against inclusion and exclusion criteria in Microsoft Access, and records obtained in March 2020 (70%) were screened by the second researcher (KD). We resolved disagreements through discussion and calculated the Kappa statistic for inter-rater agreement. We acquired full text articles for all studies identified for inclusion which were screened by the first researcher and a 20% sample was screened by the second researcher. We extracted data from included studies using a standardised form, which was piloted on a sub-set of articles prior to finalisation. This form included variables describing the study focus (exposure, outcome, study population, location); design (methodology, effect measure and size, matching or adjusting variables, follow up time, study period), and publication (publication year).
Statistical analysis
We analysed the data both as a narrative synthesis, and a meta-analysis stratified by physical LTCs. Studies providing adjusted odds ratios (OR) or hazard ratios (HR) were included in the meta-analyses. Pooled OR and HR were calculated on aggregate data and the relationship between SMI and physical health and secondary care utilisation quantified using a random effects meta-analysis, performed in R [25] and R Studio [26]. In-study bias was be assessed using Newcastle-Ottawa scale (NOS) assessment for observational studies. We assessed publication bias by visual scrutiny of funnel plots of effect size against standard error, and where more than ten studies were considered, using an Egger’s test. Study heterogeneity was measured using the I2 statistic [27]. We undertook subgroup analysis to account for SMI diagnosis group and outcome measures. Where differences were found between groups in subgroup analysis, meta-regression was performed to determine the effect of controlling for these groups on heterogeneity. We performed a sensitivity analysis using three-level hierarchical meta-analysis. This method allows for the inclusion of multiple results from single studies, accounting for variance between participants and between studies as in random effects meta-analysis, but also the variance between multiple effect sizes within a study [28].
Results
We identified 5,129 records, of which 3,646 remained after deduplication (Fig 1). Inter-rater agreement of title and abstract screening was 91.4%, with a Kappa statistic of 0.57. Following screening, 50 studies [29–78] were included in the narrative synthesis, published between 2006 and 2022 (Table 1).
Fig 1. PRISMA flow chart.
WoS: Web of Science; HMIC: Health management information consortium.
Table 1. Study description.
| Authors | Pub year | Study design | Exposure | Outcome | Population | Notes | Study period | Follow up | Pop size | Unit of measure | Country | Area | Age | Matched |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies of diabetes and SMI | ||||||||||||||
| Egglefield et al. [29] | 2020 | Cross sectional | Antipsychotic adherence | Preventable diabetes admissions | Medicaid registered patients with diabetes | Unadjusted data provided for patients with schizophrenia | 2012 | 1 year | 191,521 | Person | US | One region | 18–64 | No |
| Helmer et al. [30] | 2020 | Cohort | SMI and other MH conditions | Any, acute and chronic ACSC admissions | Veterans Affairs registered patients with diabetes | 2010 | 1 year | 151,614 | Person | US | National | >66 | No | |
| Stockbridge et al. [31] | 2019 | Cross sectional | Schizophrenia, bipolar disorder, and other MH conditions | Diabetes admissions | Insured patients with diabetes | 2011–2013 | 3 years | 229,039 | Person | US | National | 20–64 | No | |
| Tsai et al. [32] | 2019 | Cohort | Bipolar disorder | Hyperglycaemia admissions | Patients with diabetes | 1999–2013 | Up to 11 years | 30,477 | Person | Taiwan | National | Adults | Yes | |
| Goueslard et al. [33] | 2018 | Cohort | Schizophrenia | Acute diabetes complications long-term readmissions | Patients with type 1 diabetes | 2009–2012 | 3 years | 45,655 | Person | France | National | 15–35 | No | |
| Edwards et al. [34] | 2014 | Cohort | Home-Based Primary Care | ACSC admissions | Veterans Affairs registered patients with diabetes | Psychosis is a covariate | 2006–2010 | Up to 5 years | 56,608 | Person | US | National | >67 | No |
| Druss et al. [35] | 2012 | Cross sectional | Schizophrenia, bipolar disorder, and other MH conditions | ACSC admissions | Medicaid registered patients with diabetes | 2003–2004 | 2 years | 657,628 | Person | US | National | < = 65 | No | |
| Leung et al. [36] | 2011 | Cohort | Schizophrenia, bipolar disorder, and other MH conditions | Diabetes admissions | Medicaid or medicare registered patients with type 2 diabetes | 2005 | 1 year | 106,174 | Person | US | One region | >18 | No | |
| Mai et al. [37] | 2011 | Cohort | Schizophrenia, affective psychosis, other psychoses, and other MH conditions | Diabetes admissions | Patients with diabetes | 1990–2006 | Up to 15.5 years | 43,671 | Person | Australia | One region | >18 | Yes | |
| Cramer et al. [38] | 2010 | Cross sectional | Risk factors and comorbidities | More than one all-cause long-term readmission | Medicaid registered patients with diabetes | Psychosis is one of many risk factors considered | 2005 | 1 year | 695 | Person | US | National | Adults | No |
| Yan et al. [39] | 2019 | Cohort | Risk factors and comorbidities | All-cause admissions | Patients with antipsychotic-treated schizophrenia, bipolar 1 disorder or major depressive disorder | Type 2 diabetes is one of many risk factors considered | 2013–2016 | 1 year | 38,195 | Person | US | Multiple regions | >18 | No |
| Chen et al. [40] | 2012 | Cohort | Outpatient quality of care | All-cause 30-day readmissions | Commercially insured patients with diabetes | Psychosis is a covariate | 2010 | 30 days | 30,139 | Person | US | National | >19 | No |
| Guerrero Fernandez de Alba et al. [41] | 2020 | Cohort | Schizophrenia, and other MH conditions | All-cause and diabetes admissions and ED attendances | Patients with type 2 diabetes | 2012 | 1 year | 63,365 | Person | Spain | One region | >18 | No | |
| Chwastiak et al. [42] | 2014 | Cohort | SMI | All cause 30-day and long-term readmissions | Patients with diabetes | 2010–2011 | 30 days / up to 2 years | 82,060 | Person | US | One region | >18 | No | |
| Becker et al. [43] | 2011 | Cohort | Schizophrenia | Hyperglycaemia or hypoglycaemia admissions or ED attendances | Patients with diabetes | 1996–2006 | 1–10 years | 5,033 | Person | Canada | One region | 18–50 | Yes | |
| Krein et al. [44] | 2006 | Cross sectional | SMI | All-cause admissions | Veterans Affairs registered patients with diabetes | 1997–1998 | 1 year | 36,546 | Person | US | National | Mean 58 | Yes | |
| Kurdyak et al. [45] | 2017 | Cohort | Schizophrenia | Diabetes and all-cause admissions and ED attendances | Patients with diabetes | 2011–2013 | 2 years | 1,131,375 | Person | Canada | One region | 19–105 | No | |
| Shim et al. [46] | 2014 | Cohort | Schizophrenia or diabetes | Diabetes and all-cause ED attendances | Medicaid registered patients with diabetes and/or schizophrenia | 2006–2007 | 2 years | 432,112 | Person | US | Multiple regions | 18–64 | No | |
| Sullivan et al. [47] | 2006 | Cross sectional | Bipolar disorder, and other MH conditions | Admissions in those attending ED for diabetes | Patients with diabetes | 1994–1998 | 4.5 years | 4,275 | Admissions | US | Single site | >18 | No | |
| Wang et al. [78] | 2021 | Cohort | SMI | All cause admissions | Patients with diabetes | 2000–2016 | 6.4 years | 6,383 | Person | UK | England | >18 | Yes | |
| Huang et al. [73] | 2021 | Cohort | Schizophrenia | All cause admissions | Patients with diabetes | 2002–2013 | 11 | 10,604 | Person | Taiwan | National | Not given | Yes | |
| Studies of cardiovascular disease and SMI | ||||||||||||||
| Attar et al. [48] | 2020 | Cohort | Schizophrenia | Major adverse cardiac event long-term readmissions | Patients with acute myocardial infarction | 2000–2018 | 5 years | 286,333 | Person | Sweden | National | >18 | No | |
| Chamberlain et al. [49] | 2017 | Cohort | Multimorbidity | All-cause long-term readmissions | Patients with atrial fibrillation | Schizophrenia is one of many risk factors considered | 2000–2014 | Up to 14 years | 2,860 | Person | US | One region | >18 | No |
| Sayers et al. [50] | 2007 | Cross sectional | Psychosis, bipolar disorders, and other MH conditions | All-cause long-term readmissions | Medicare registered patient with congestive heart failure | 1999 | 1 year | 21,429 | Person | US | National | 65+ | No | |
| Shah et al. [51] | 2018 | Cross sectional | Risk factors and comorbidities | All-cause 30-day readmissions | Patients with non-acute myocardial infarction cardiogenic shock | Psychosis is one of many risk factors considered | 2013–2014 | 30 days | 24,665 | Person | US | Multiple regions | >16 | No |
| Pham et al. [52] | 2019 | Cross sectional | Risk factors and comorbidities | All-cause and heart failure 7- and 30-day unplanned readmissions | Medicare registered patient with heart failure | Psychosis is one of many risk factors considered | 2014 | 30 days | 234,298 | Admissions | US | Multiple regions | >65 | No |
| Chamberlain et al. [53] | 2018 | Cross sectional | Risk factors and comorbidities | Heart failure 30-day readmissions | Patients with heart failure | Psychosis is one of many risk factors considered | 2006–2011 | 30 days | 1,007,807 | Person | US | Multiple regions | Not given | No |
| Shah et al. [54] | 2018 | Cross sectional | Risk factors and comorbidities | All cause 31-day readmissions | Patients with Takotsubo cardiomyopathy | Psychosis is one of many risk factors considered | 2013–2014 | 31 days | 5,997 | Person | US | Multiple regions | >18 | No |
| Shah et al. [55] | 2018 | Cross sectional | Risk factors and comorbidities | All-cause 30-day readmissions | Patients with acute myocardial infarction and cardiogenic shock | Psychosis is one of many risk factors considered | 2013–2014 | 30 days | 26,016 | Person | US | Multiple regions | >16 | No |
| Jorgensen et al. [56] | 2017 | Cohort | Schizophrenia | All-cause 28-day readmissions | Patients with heart failure | 2004–2013 | 28 days | 36,718 | Person | Denmark | National | >18 | No | |
| Ahmedani et al. [57] | 2015 | Cohort | Bipolar disorders, schizophrenia-spectrum disorders, other psychoses, and other MH conditions | All-cause 30-day readmissions | Patients with heart failure or myocardial infarction | 2009–2011 | 30 days | 123,921 | Admissions | US | Multiple regions | >18 | No | |
| Coffey et al. [58] | 2012 | Cross sectional | Risk factors and comorbidities | Congestive heart failure 30-day readmissions | Patients with congestive heart failure | Psychosis is one of many risk factors considered | 2006 | 30 days | Admissions | US | Multiple regions | >18 | No | |
| Lu et al. [59] | 2017 | Cohort | Schizophrenia, bipolar mood disorder, and other MH conditions | Heart failure 30-day and long-term readmissions | African American patients with heart failure | 2010–2013 | 30 days / ave 3.2 years | 611 | Person | US | Single site | >20 | No | |
| Kallio et al. [69] | 2022 | Cohort | Schizophrenia | Stroke and myocardial infarction long term readmissions | Patients with coronary artery disease who underwent coronary artery bypass grafting surgery | 2004–2018 | Up to 10 years | 29,220 | Person | Finland | Multiple sites | Not given | Yes | |
| Fleetwood et al. [71] | 2021 | Cohort | Schizophrenia and bipolar disorder | Stroke and myocardial infarction long term readmissions | Patients hospitalised with myocardial infarction | 1999–2018 | Up to 20 years | 184,134 | Person | UK | Scotland | >18 | No | |
| Ghani et al. [72] | 2021 | Cohort | SMI | All-cause 30-day emergency readmissions | Patients who underwent vascular surgery | 2007–2018 | 30 days | 8,973 | Person | UK | One region | >18 | No | |
| Fleetwood et al. [70] | 2021 | Cohort | Schizophrenia and bipolar disorder | Stroke and myocardial infarction long term readmissions | Patients hospitalised with stroke | 1991–2018 | Up to 28 years | 169,923 | Person | UK | Scotland | >18 | No | |
| Paredes et al. [75] | 2020 | Cohort | SMI | All-cause 30-day readmissions | Medicare registered patients who underwent coronary artery bypass grafting surgery | 2013–2017 | 30 days | 118,837 | Person | US | National | >65 | No | |
| Sreenivasan et al. [76] | 2022 | Cohort | Bipolar disorder and schizophrenia or other psychotic illnesses | All-cause 30-day readmissions | Patients hospitalised with myocardial infarction | 2016–2017 | 30 days | 904,575 | Person | US | National | >18 | No | |
| Andres et al. [77] | 2012 | Cross sectional | Schizophrenia | Long-term readmission for myocardial infarction | Patients hospitalised with myocardial infarction | 2000–2007 | 8 years | 19,016 | Person | Spain | One region | >15 | No | |
| Studies of COPD and SMI | ||||||||||||||
| Buhr et al. [60] | 2019 | Cross sectional | Charlson and Elixhauser indicies | All-cause 30-day readmissions | Patients with COPD | Psychosis included in the Elixhauser index | 2010–2016 | 30 days | 1,622,983 | Admissions | US | National | >40 | No |
| Jorgensen et al. [61] | 2018 | Cohort | Schizophrenia | All-cause 30-day readmissions | Patients with COPD | 2008–2013 | 30 days | 211,868 | Person | Denmark | National | >30 | No | |
| Lau et al. [62] | 2017 | Cross sectional | Risk factors and comorbidities | COPD 30-day readmissions | Patients with COPD | Psychosis is one of many risk factors considered | 2006–2011 | 30 days | 597,502 | Person | US | Multiple regions | >40 | No |
| Singh et al. [63] | 2016 | Cohort | Psychosis, and other MH conditions | All-cause 30-day readmissions | Medicare registered patients with COPD | 2001–2011 | 30 days | 135,498 | Admissions | US | National | >66 | No | |
| Studies of cancer, liver disease or multiple diseases and SMI | ||||||||||||||
| Basta et al. [64] | 2016 | Cohort | Risk factors and comorbidities | Complicated lymphedema long-term readmissions | Women who had undergone breast cancer related mastectomy /lumpectomy | Psychosis is one of many risk factors considered | 2007–2012 | 2 years | 56,075 | Person | US | Multiple regions | >18 | No |
| Kashyap et al. [68] | 2021 | Cohort | Bipolar and psychoses | All-cause 30-day ED attendance | Medicare registered patients with gastrointestinal malignancies in the last 30 days of life | 2004–2014 | 30 days | 110,325 | Person | US | National | >66 | No | |
| Ratcliff et al. [74] | 2021 | Cohort | Bipolar disorder and psychoses | All cause 90-day readmissions | Veterans Affairs registered patients who underwent surgery for colorectal cancer | Not given | 90 days | 50,611 | Person | US | National | Not given | No | |
| Huckans et al. [65] | 2010 | Cohort | Schizophrenia | All-cause readmissions during anti-viral therapy | Veterans Affairs registered patients with hepatitis C | 1998–2006 | During antiviral therapy | 60 | Person | US | Multiple regions | Mean 50 | Yes | |
| Davydow et al. [66] | 2016 | Cohort | SMI | ACSC admissions | General population | Table 1 provides unadjusted effect for patients with underlying cardiovascular disease, diabetes, liver disease and cancer | 1999–2013 | 14 years | 5,945,540 | Person | Denmark | National | >18 | No |
| Guo et al. [67] | 2008 | Cohort | Risk factors and comorbidities | All-cause admissions and ED attendances | Commercially insured patients with bipolar disorder | Diabetes, COPD and heart disease are some of many risk factors considered | 1998–2002 | Up to 5 years | 67,862 | Person | US | Multiple regions | Mean 37.1 | No |
Study characteristics
Most studies were conducted in the United States (US) (n = 33; Table 1). Forty-four studies quantified the risk of admissions, readmissions or ED visits in a patient population (median population size: 53,343; interquartile range (IQR): 23,856–185,981); while in five studies the focus was the number of index admissions which resulted in a readmission (median admissions: 184,898, IQR: 132,604–581,469), and one investigated the admission ratio of 4,275 ED visits. The majority of studies (n = 38) included adults with an age range of 20 to 65 or wider, while seven focused on those over the age of 65. The remaining studies excluded patients under the age of 30 or 40 (n = 3), those over the age of 50 (n = 1) or those over 35 (n = 1). The included studies were heterogeneous in population, exposure, outcome, and effect measure and 27 could be stratified into multiple analyses based on these factors (Table 2). Of the 104 unique analyses, 59 investigated inpatient admissions over at least a year, with a median follow up of five years (IQR: 2–14). A further 27 investigated inpatient admissions limited to a 28 to 31 day period following an index admission (termed 30-day readmissions) and 12 investigated ED visits (median follow up: 2 years, IQR: 2–5 years). Two analyses investigated 7-day readmissions, two investigated 90-day readmissions, one combined inpatient admissions and ED visits over a ten year period, and one calculated the odds of admission in those attending an ED (Table 2). ED use was the only acute outpatient care outcome identified, and we did not identify any studies of planned inpatient admissions.
Table 2. Description of analyses.
| Authors | Year | Baseline condition | Exposure | Utilisation | Utilisation type | NOS score | Adjusted for age and sex | Adjusted for physical comorbidities | Adjusted for prior utilisation | Effect measure | Effect size | 95%CI/p-value | Included in meta-analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The effect of diabetes on hospital utilisation in patients with SMI | |||||||||||||
| Yan et al. [39] | 2019 | Schizophrenia | Diabetes T2 | Inpatient | All cause | 9 | Yes | Yes | Yes | aOR | 1.19 | 1.05–1.36 | NA |
| Shim et al. [46] | 2014 | Schizophrenia | Diabetes T1/T2 | ED | All cause | 4 | No | No | No | OR | 1.46 | 1.41–1.51 | NA |
| Yan et al. [39] | 2019 | Bipolar | Diabetes T2 | Inpatient | All cause | 9 | Yes | Yes | Yes | aOR | 1.23 | 1.13–1.34 | NA |
| Guo et al. [67] | 2008 | Bipolar | Diabetes | Inpatient | All cause | 6 | Yes | Yes | No | aRR | 1.44 | 1.36–1.52 | NA |
| Guo et al. [67] | 2008 | Bipolar | Diabetes | ED | All cause | 6 | Yes | Yes | No | aRR | 1.17 | 1.08–1.25 | NA |
| The effect of cardiovascular disease on hospital utilisation in patients with SMI | |||||||||||||
| Guo et al. [67] | 2008 | Bipolar | Ischemic heart disease | Inpatient | All cause | 6 | Yes | Yes | No | aRR | 1.89 | 1.78–2.02 | NA |
| Guo et al. [67] | 2008 | Bipolar | Ischemic heart disease | ED | All cause | 6 | Yes | Yes | No | aRR | 1.67 | 1.53–1.81 | NA |
| The effect of COPD on hospital utilisation in patients with SMI | |||||||||||||
| Guo et al. [67] | 2008 | Bipolar | COPD | Inpatient | All cause | 6 | Yes | Yes | No | aRR | 1.94 | 1.81–2.06 | NA |
| Guo et al. [67] | 2008 | Bipolar | COPD | ED | All cause | 6 | Yes | Yes | No | aRR | 1.61 | 1.47–1.76 | NA |
| The effect of SMI on hospital utilisation in patients with diabetes | |||||||||||||
| Stockbridge et al. [31] | 2019 | Diabetes T1/T2 | Bipolar | Inpatient | Diabetes | 7 | Yes | Yes | No | aOR | 0.99 | 0.78–1.25 | Yes |
| Druss et al. [35] | 2012 | Diabetes T1/T2 | Bipolar | Inpatient | ACSC | 7 | Yes | Yes | No | aOR | 1.03 | 0.98–1.09 | Yes |
| Leung et al. [36] | 2011 | Diabetes T2 | Bipolar | Inpatient | Diabetes | 7 | Yes | No | Yes | aOR | 1.07 | 0.91–1.26 | Yes |
| Chen et al. [40] | 2012 | Diabetes T1/T2 | Psychosis | 30-day | All cause | 8 | Yes | Yes | Yes | aOR | 1.15 | 1.03–1.29 | Yes |
| Stockbridge et al. [31] | 2019 | Diabetes T1/T2 | Schizophrenia | Inpatient | Diabetes | 7 | Yes | Yes | No | aOR | 1.61 | 1.29–2.01 | Yes |
| Goueslard et al. [33] | 2018 | Diabetes T1 | Schizophrenia | Inpatient | Diabetes | 6 | Yes | Yes | No | aOR | 2.21 | 1.69–2.88 | Yes |
| Druss et al. [35] | 2012 | Diabetes T1/T2 | Schizophrenia | Inpatient | ACSC | 7 | Yes | Yes | No | aOR | 1.26 | 1.21–1.30 | Yes |
| Leung et al. [36] | 2011 | Diabetes T2 | Schizophrenia | Inpatient | Diabetes | 7 | Yes | No | Yes | aOR | 0.75 | 0.63–0.89 | Yes |
| Guerrero Fernandez de Alba et al. [41] | 2020 | Diabetes T2 | Schizophrenia | Inpatient | All cause | 6 | Yes | Yes | No | aOR | 1.40 | 1.18–1.66 | Yes |
| Guerrero Fernandez de Alba et al. [41] | 2020 | Diabetes T2 | Schizophrenia | Inpatient | Diabetes | 6 | Yes | Yes | No | aOR | 1.25 | 0.55–2.82 | Yes |
| Guerrero Fernandez de Alba et al. [41] | 2020 | Diabetes T2 | Schizophrenia | ED | All cause | 6 | Yes | Yes | No | aOR | 1.28 | 1.11–1.47 | Yes |
| Kurdyak et al. [45] | 2017 | Diabetes T1/T2 | Schizophrenia | ED | Diabetes | 6 | Yes | Yes | No | aOR | 1.34 | 1.28–1.41 | Yes |
| Kurdyak et al. [45] | 2017 | Diabetes T1/T2 | Schizophrenia | ED | All causea | 6 | Yes | Yes | No | aOR | 1.72 | 1.68–1.77 | Yes |
| Kurdyak et al. [45] | 2017 | Diabetes T1/T2 | Schizophrenia | Inpatient | Diabetes | 6 | Yes | Yes | No | aOR | 1.36 | 1.28–1.43 | Yes |
| Kurdyak et al. [45] | 2017 | Diabetes T1/T2 | Schizophrenia | Inpatient | All causea | 6 | Yes | Yes | No | aOR | 1.85 | 1.79–1.92 | Yes |
| Helmer et al. [30] | 2020 | Diabetes T1/T2 | SMI | Inpatient | ACSC | 7 | Yes | Yes | No | aOR | 1.00 | 0.94–1.07 | Yes |
| Chwastiak et al. [42] | 2014 | Diabetes T1/T2 | SMI | 30-day | All causea | 8 | Yes | Yes | Yes | aOR | 1.24 | 1.07–1.44 | Yes |
| Wang et al. [78] | 2021 | Diabetes T2 | SMI | Inpatient | All causea | 9 | Yes | Yes | Yes | aOR | 1.36 | 1.13–1.65 | Yes |
| Cramer et al. [38] | 2010 | Diabetes T1/T2 | Psychosis | Inpatient | All cause | 5 | No | Yes | No | aOR | 2.15 | 1.18–3.92 | Yes, but also excluded as does not adjusted for age and sex |
| Helmer et al. [30] | 2020 | Diabetes T1/T2 | SMI | Inpatient | Chronic ACSC | 7 | Yes | Yes | No | aOR | 0.88 | 0.82–0.96 | No: subset of all ACSC |
| Helmer et al. [30] | 2020 | Diabetes T1/T2 | SMI | Inpatient | Acute ACSC | 7 | Yes | Yes | No | aOR | 1.21 | 1.11–1.31 | No: subset of all ACSC |
| Egglefield et al. [29] | 2020 | Diabetes T1/T2 | Schizophrenia | Inpatient | Diabetes | 4 | No | No | No | ORd | 1.69 | 1.54–1.86 | No: unadjusted |
| Krein et al. [44] | 2006 | Diabetes T1/T2 | SMI | Inpatient | All cause | 4 | No | No | No | OR | 2.80 | 2.67–2.94 | No: unadjusted |
| Shim et al. [46] | 2014 | Diabetes T1/T2 | Schizophrenia | ED | Diabetes | 4 | No | No | No | OR | 1.17 | 1.12–1.21 | No: unadjusted |
| Shim et al. [46] | 2014 | Diabetes T1/T2 | Schizophrenia | ED | All causea | 4 | No | No | No | ORd | 1.30 | 1.25–1.34 | No: unadjusted |
| Tsai et al. [32] | 2019 | Diabetes T1/T2 | Bipolar | Inpatient | Diabetes | 8 | Yes | Yes | No | aHR | 1.41 | 1.15–1.71 | Yes |
| Mai et al. [37] | 2011 | Diabetes T1/T2 | Affective psychosis | Inpatient | Diabetes | 8 | Yes | Yes | No | aHRe | 1.22 | 1.15–1.30 | Yes |
| Edwards et al. [34] | 2014 | Diabetes T1/T2 | Psychosis | Inpatient | ACSC | 6 | Yes | Yes | No | aHR | 1.01 | 0.98–1.04 | Yes |
| Mai et al. [37] | 2011 | Diabetes T1/T2 | Other psychosis | Inpatient | Diabetes | 8 | Yes | Yes | No | aHRe | 1.18 | 1.10–1.27 | Yes |
| Mai et al. [37] | 2011 | Diabetes T1/T2 | Schizophrenia | Inpatient | Diabetes | 8 | Yes | Yes | No | aHRe | 1.06 | 0.94–1.20 | Yes |
| Becker et al. [43] | 2011 | Diabetes T1/T2 | Schizophrenia | Inpatient or ED | Diabetes | 8 | Yes | Yes | Yes | aHR | 1.68 | 1.34–2.10 | Yes |
| Chwastiak et al. [42] | 2014 | Diabetes T1/T2 | SMI | Inpatient | All causea | 7 | Yes | Yes | Yes | aHR | 1.14 | 1.05–1.23 | Yes |
| Goueslard et al. [33] | 2018 | Diabetes T1 | Schizophrenia | Inpatient | Diabetes | 6 | Yes | Yes | No | aHR | 2.13 | 1.69–2.69 | Yes, but also excluded as an outlier |
| Stockbridge et al. [31] | 2019 | Diabetes T1/T2 | Bipolar | Inpatient | Diabetes | 7 | Yes | Yes | No | aRR | 1.34 | 0.78–2.31 | No: RR |
| Stockbridge et al. [31] | 2019 | Diabetes T1/T2 | Schizophrenia | Inpatient | Diabetes | 7 | Yes | Yes | No | aRR | 1.41 | 0.94–2.12 | No: RR |
| Huang et al. [73] | 2021 | Diabetes T2 | Schizophrenia | Inpatient | All causea | 7 | No | No | No | Average number of admissions | 1.09 vs 0.92 | p = 0.001 | No: Average utilisation |
| Sullivan et al. [47] | 2006 | Diabetes T1/T2 | SMI | Admission ratio | Diabetes | 6 | Yes | No | No | aOR | 0.77 | 0.45–1.33 | No: Admission ratio |
| The effect of SMI on hospital utilisation in patients with cardiovascular disease | |||||||||||||
| Shah et al. [51] | 2018 | Cardiogenic shock (no AMI) | Psychosis | 30-day | All cause | 8 | Yes | Yes | No | aOR | 0.90 | 0.78–1.05 | Yes |
| Pham et al. [52] | 2019 | Heart failure | Psychosis | 30-day | All cause | 7 | Yes | Yes | No | aOR | 1.11 | 1.04–1.18 | Yes |
| Pham et al. [52] | 2019 | Heart failure | Psychosis | 30-day | Cardiovascular | 7 | Yes | Yes | No | aOR | 1.02 | 0.93–1.13 | Yes |
| Chamberlain et al. [53] | 2018 | Congestive heart failure | Psychosis | 30-day | Cardiovascular | 8 | Yes | Yes | No | aOR | 1.07 | 1.01–1.12 | Yes |
| Chamberlain et al. [53] | 2018 | Congestive heart failure | Psychosis | 30-day | Cardiovascular | 8 | Yes | Yes | No | aOR | 1.08 | 1.00–1.16 | Yes |
| Shah et al. [54] | 2018 | Takotsubo cardiomyopathy | Psychosis | 30-day | All cause | 8 | Yes | Yes | No | aOR | 1.90 | 1.36–2.66 | Yes |
| Shah et al. [55] | 2018 | Cardiogenic shock (with AMI) | Psychosis | 30-day | All cause | 8 | Yes | Yes | No | aOR | 1.14 | 0.97–1.35 | Yes |
| Coffey et al. [58] | 2012 | Congestive heart failure | Psychosis | 30-day | Cardiovascular | 7 | Yes | Yes | No | aOR | 1.16 | p<0.001 | Yes |
| Jorgensen et al. [56] | 2017 | Heart failure | Schizophrenia | 30-day | All causea | 9 | Yes | Yes | No | aOR | 1.77 | 0.79–3.92 | Yes |
| Ghani et al. [72] | 2021 | Vascular surgery | SMI | 30-day | All causec | 6 | Yes | No | Yes | aOR | 2.02 | 1.10–3.70 | Yes |
| Paredes et al. [75] | 2020 | CABG surgery | SMI | 30-day | All cause | 7 | Yes | Yes | No | aORe | 2.28 | 2.10–2.46 | Yes |
| Pham et al. [52] | 2019 | Heart failure | Psychosis | 7-day | All cause | 7 | Yes | Yes | No | aOR | 1.10 | 1.00–1.22 | No: 7-day readmission |
| Pham et al. [52] | 2019 | Heart failure | Psychosis | 7-day | Cardiovascular | 7 | Yes | Yes | No | aOR | 1.04 | 0.87–1.23 | No: 7-day readmission |
| Ahmedani et al. [57] | 2015 | Heart failure | Schizophrenia | 30-day | All cause | 6 | No | No | No | ORd | 1.06 | 0.78–1.44 | No: unadjusted |
| Ahmedani et al. [57] | 2015 | MI | Schizophrenia | 30-day | All cause | 6 | No | No | No | ORd | 1.55 | 0.69–3.45 | No: unadjusted |
| Ahmedani et al. [57] | 2015 | Heart failure | Bipolar | 30-day | All cause | 6 | No | No | No | ORd | 1.25 | 1.05–1.50 | No: unadjusted |
| Ahmedani et al. [57] | 2015 | MI | Bipolar | 30-day | All cause | 6 | No | No | No | ORd | 0.98 | 0.61–1.58 | No: unadjusted |
| Ahmedani et al. [57] | 2015 | Heart failure | Other psychoses | 30-day | All cause | 6 | No | No | No | ORd | 1.70 | 1.40–2.07 | No: unadjusted |
| Andres et al. [77] | 2012 | MI | Schizophrenia | Inpatient | MI | 6 | No | No | No | ORd | 0.83 | 0.25–2.81 | No: unadjusted |
| Sreenivasan et al. [76] | 2022 | MI | Psychosis | 30-day | All cause | 8 | Yes | Yes | No | aHR | 1.56 | 1.43–1.69 | Yes |
| Lu et al. [59] | 2017 | Heart failure | Bipolar | Inpatient | Cardiovascular | 6 | Yes | Yes | No | aHR | 2.08 | 1.05–4.11 | Yes, but also excluded as an outlier |
| Fleetwood et al. [71] | 2021 | MI | Bipolar | Inpatient | MI or stroke | 8 | Yes | No | No | aHR | 1.40 | 1.20–1.62 | Yes |
| Fleetwood et al. [70] | 2021 | Stroke | Bipolar | Inpatient | MI or stroke | 8 | Yes | No | No | aHR | 1.14 | 1.01–1.28 | Yes |
| Sreenivasan et al. [76] | 2022 | MI | Bipolar | 30-day | All cause | 8 | Yes | Yes | No | aHR | 1.32 | 1.19–1.45 | Yes |
| Lu et al. [59] | 2017 | Heart failure | Bipolar | 30-day | Cardiovascular | 7 | Yes | Yes | No | aHR | 3.44 | 1.19–10.00 | Yes, but also excluded as an outlier |
| Attar et al. [48] | 2020 | MI | Schizophrenia | Inpatient | Re-infarction | 8 | Yes | Yes | Yes | aHR | 1.29 | 0.77–2.13 | Yes |
| Chamberlain et al [49] | 2017 | Atrial fibrillation | Schizophrenia | Inpatient | All cause | 7 | Yes | Yes | No | aHR | 1.22 | 0.98–1.52 | Yes |
| Lu et al. [59] | 2017 | Heart failure | Schizophrenia | Inpatient | Cardiovascular | 6 | Yes | Yes | No | aHR | 2.33 | 1.51–3.61 | Yes, but also excluded as an outlier |
| Lu et al. [59] | 2017 | Heart failure | Schizophrenia | 30-day | Cardiovascular | 7 | Yes | Yes | No | aHR | 4.92 | 2.49–9.71 | Yes, but also excluded as an outlier |
| Fleetwood et al. [71] | 2021 | MI | Schizophrenia | Inpatient | MI or stroke | 8 | Yes | No | No | aHR | 1.46 | 1.29–1.65 | Yes |
| Fleetwood et al. [70] | 2021 | Stroke | Schizophrenia | Inpatient | MI or stroke | 8 | Yes | No | No | aHR | 1.21 | 1.10–1.34 | Yes |
| Fleetwood et al. [71] | 2021 | MI | Schizophrenia | Inpatient | MI | 8 | Yes | No | No | aHR | 1.42 | 1.24–1.63 | No: Population included in other outcome |
| Fleetwood et al. [71] | 2021 | MI | Bipolar | Inpatient | MI | 8 | Yes | No | No | aHR | 1.34 | 1.13–1.58 | No: Population included in other outcome |
| Fleetwood et al. [70] | 2021 | Stroke | Schizophrenia | Inpatient | Stroke | 8 | Yes | No | No | aHR | 1.24 | 1.11–1.38 | No: Population included in other outcome |
| Fleetwood et al. [70] | 2021 | Stroke | Bipolar | Inpatient | Stroke | 8 | Yes | No | No | aHR | 1.17 | 1.03–1.32 | No: Population included in other outcome |
| Attar et al. [48] | 2020 | MI | Schizophrenia | Inpatient | Stroke | 8 | Yes | Yes | Yes | aHR | 1.72 | 1.00–2.98 | No: Population included in other outcome |
| Attar et al. [48] | 2020 | MI | Schizophrenia | Inpatient | Heart failure | 8 | Yes | Yes | Yes | aHR | 1.39 | 1.04–1.86 | No: Population included in other outcome |
| Kallio et al. [69] | 2022 | Coronary artery disease and CABG | Schizophrenia | Inpatient | MI | 6 | No | No | No | HR | 1.86 | 1.25–2.78 | No: unadjusted |
| Kallio et al. [69] | 2022 | Coronary artery disease and CABG | Schizophrenia | Inpatient | Stroke | 6 | No | No | No | HR | 0.91 | 0.50–1.66 | No: unadjusted |
| Sayers et al. [50] | 2007 | Heart failure | Psychosis | Inpatient | All cause | 7 | Yes | Yes | No | Predicted increase | 0.30 | p<0.001 | No: predicted increase |
| Sayers et al. [50] | 2007 | Heart failure | Bipolar | Inpatient | All cause | 7 | Yes | Yes | No | Predicted increase | 0.38 | p = 0.001 | No: predicted increase |
| Davydow et al. [66] | 2016 | MI | SMI | Inpatient | ACSC | 5 | No | No | No | RRd | 1.41 | 1.36–1.47 | No: RR |
| Davydow et al. [66] | 2016 | CHF | SMI | Inpatient | ACSC | 5 | No | No | No | RRd | 1.19 | 1.15–1.22 | No: RR |
| Davydow et al. [66] | 2016 | Cerebrovascular disease | SMI | Inpatient | ACSC | 5 | No | No | No | RRd | 1.47 | 1.43–1.52 | No: RR |
| The effect of SMI on hospital utilisation in patients with COPD | |||||||||||||
| Lau et al. [62] | 2017 | COPD | Psychosis | 30-day | COPD | 8 | Yes | Yes | No | aOR | 1.19 | 1.13–1.25 | Yes |
| Lau et al. [62] | 2017 | COPD | Psychosis | 30-day | COPD | 8 | Yes | Yes | No | aOR | 1.16 | 1.08–1.24 | Yes |
| Singh et al. [63] | 2016 | COPD | Psychosis | 30-day | All cause | 6 | Yes | No | No | aOR | 1.18 | 1.10–1.27 | Yes |
| Jorgensen et al. [61] | 2018 | COPD | Schizophrenia | 30-day | All cause | 8 | Yes | Yes | No | aOR | 1.08 | 0.92–1.28 | Yes |
| Buhr et al. [60] | 2019 | COPD | Psychosis | 30-day | All cause | 5 | No | No | No | ORd | 1.27 | 1.25–1.29 | No: unadjusted |
| The effect of SMI on inpatient admissions in liver disease patients | |||||||||||||
| Huckans et al. [65] | 2010 | HCV | Schizophrenia | Inpatient | All causea | 5 | No | No | No | OR | 5.80 | 0.63–53.01 | No: unadjusted |
| Huckans et al. [65] | 2010 | HCV | Schizophrenia | ED | All causea | 5 | No | No | No | OR | 3.27 | 0.77–13.83 | No: unadjusted |
| Davydow et al. [66] | 2016 | Liver disease | SMI | Inpatient | ACSC | 5 | No | No | No | RRd | 1.53 | 1.45–1.61 | No: unadjusted |
| The effect of SMI on inpatient admissions in cancer patients | |||||||||||||
| Davydow et al. [66] | 2016 | Cancer | SMI | Inpatient | ACSC | 5 | No | No | No | RRd | 1.54 | 1.48–1.60 | No: unadjusted |
| Basta et al. [64] | 2016 | Breast cancer related mastectomy/ lumpectomy | Psychosis | Inpatient | Cancer | 8 | Nob | Yes | No | aOR | 2.15 | 1.51–3.06 | No: limited comparison |
| Kashyap et al. [68] | 2021 | Gastrointestinal malignancies | Bipolar | ED | All cause end of life | 8 | Yes | Yes | No | aOR | 1.12 | 1.01–1.24 | No: limited comparison |
| Kashyap et al. [68] | 2021 | Gastrointestinal malignancies | Psychosis | ED | All cause end of life | 8 | Yes | Yes | No | aOR | 0.98 | 0.85–1.12 | No: limited comparison |
| Ratcliff et al. [74] | 2021 | Surgery for colorectal cancer | Bipolar | 90-day | All cause | 6 | No | No | No | ORd | 1.24 | 1.04–1.47 | No: unadjusted |
| Ratcliff et al. [74] | 2021 | Surgery for colorectal cancer | Psychosis | 90-day | All cause | 6 | No | No | No | ORd | 1.25 | 1.03–1.52 | No: unadjusted |
a: Excluded psychiatric hospitalisations
b: Adjusted for age and only included females so scored as if adjusted for age and sex
c: emergency admissions
d: calculated from raw data
e: extracted from figure using ImageJ: https://imagej.nih.gov/ij/; COPD: Chronic Obstructive Pulmonary Disease; ED: Emergency Department; OR: odds ratio; HR: hazard ratio; RR: risk ratio; HCV: hepatitis C virus; SMI: severe mental illness; CABG: coronary artery bypass graft; MI: myocardial infarction; ACSC: ambulatory care sensitive condition.
Study quality and risk of bias
The majority of studies had pre-existing psychiatric illness as a focus (n = 37/50), while 11 considered a broad range of risk factors for hospital admission, of which SMI was one. Two studies included SMI as a covariate for a different exposure of interest. The majority (n = 42) of studies were in unmatched populations and 11 did not provide adjusted effect measures. Ten studies were limited to a single region of a country, and two to single hospitals (S1 Table). Denominator populations were sourced from hospital records in 31 studies, hospital and outpatient or pharmacy records in eleven and primary care records in eight (S1 Table).
Of the 39 studies which provided adjusted effect estimates, 37 controlled for age and gender, one controlled for gender but not age [38] and one controlled for age and was limited to the female population only [64]. Thirty-three studies controlled for physical health comorbidities and eight for prior healthcare utilisation (S1 Table). Almost half the studies (n = 24/50) had a NOS of between 6 and 7 (fair quality), while 19 had a score of 8 or 9 (high quality) and seven had a score of under 6 (poor quality; S1 and S2 Tables). Two studies with multiple analyses had differing NOS for analyses presenting ORs and HRs (S1 and S2 Tables). Funnel plots for all analyses presenting ORs (Egger’s test: p = 0.3733, S1 Fig) and risk ratios (Egger’s test: p = 0.2809, S1 Fig) were not suggestive of publication bias, however the funnel plot for analyses presenting HRs was asymmetrical (Egger’s test: p<0.0001, S1 Fig).
Hospital utilisation in people with SMI, comparing people with or without physical LTCs
Nine analyses from three studies [39, 46, 67] investigated the impact of diabetes (n = 5), cardiovascular disease (n = 2) and COPD (n = 2) on hospitalisation in a patient population with pre-existing schizophrenia (n = 2) or bipolar disorder (n = 7). The outcome was all-cause ED attendances for four studies and all-cause admissions for five. All analyses found a higher risk of hospital utilisation in those with SMI and a physical health condition compared to those with SMI alone (Table 2). The low number and heterogenous study characteristics meant that these studies were deemed unsuitable for meta-analysis.
Hospital utilisation in people with physical LTCs, comparing people with and without SMI
Ninety-five analyses from 48 studies investigated the impact of SMI diagnosis on hospital utilisation in a patient population with diagnoses of diabetes, cardiovascular disease, COPD, liver disease or cancer.
Hospital utilisation in people with diabetes, with and without SMI
Thirty-seven analyses from 20 studies investigated the effect of SMI on hospital utilisation in patients with diabetes. Most analyses included patients diagnosed with either type I or II diabetes mellitus (n = 28; Table 2). Twenty-seven analyses were included in meta-analysis, reasons for exclusions are detailed in Table 2.
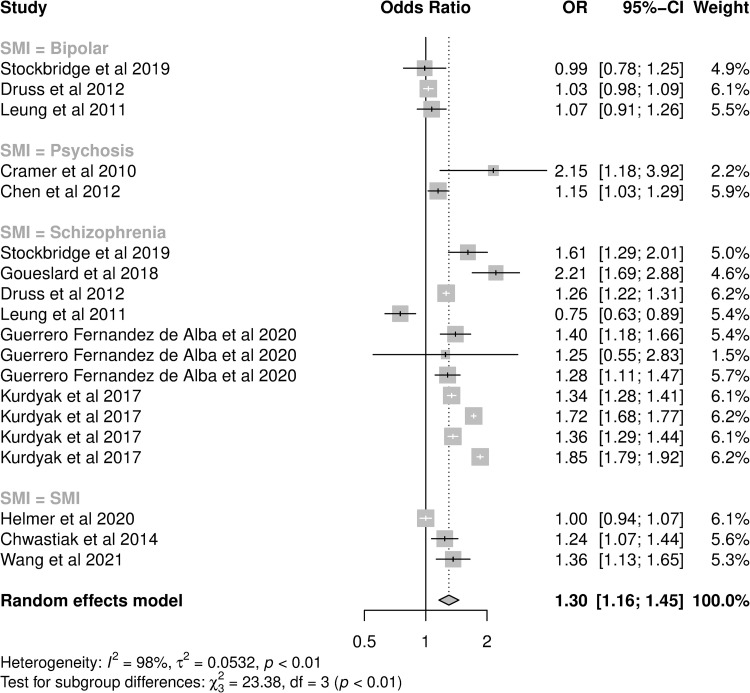
The meta-analysis of adjusted OR included 19 analyses from 14 studies (Fig 2). Schizophrenia was the most frequent exposure (11 analyses) and admissions the most frequent outcome (14 analyses; Table 2). The funnel plot of these analyses did not show asymmetry (Egger’s test: p = 0.0738, S2 Fig). For patients with diabetes, the pooled OR for hospital utilisation in patients with a diagnosis of any SMI was 1.30 (95%CI: 1.16–1.45) compared to those without an SMI diagnosis, however heterogeneity was high (I2 = 97.8%). When one study which did not control for age was removed [38] the pooled odds ratio was 1.28 (95% confidence interval (CI) 1.15–1.44, I2 = 97.9%) In subgroup analysis, the effect size was greater in patients with schizophrenia (OR: 1.42, 95%CI: 1.25–1.60) than patients with other SMI diagnoses, and analyses of all-cause hospitalisations had higher pooled OR (1.43, 95%CI: 1.28–1.60) compared to those reporting ACSC conditions or diabetes-specific hospitalisations (Table 3). Studies performed in the US had a lower pooled OR (1.10, 95%CI: 0.99–1.22) than studies in other countries (Table 3). While the pooled OR for analyses of 30-day readmissions was lower, confidence intervals of all outcome types overlapped (Table 3). Controlling for these variables in meta-regression reduced heterogeneity (I2 = 82.8%).
Fig 2. Forest plot of studies presenting adjusted odds ratios of hospital utilisation in diabetes patients with SMI compared to diabetes patients without SMI.
Table 3. Subgroup analyses of studies of hospital use in people with underlying diabetes, cardiovascular disease and COPD: Comparing those with and without SMI with outliers removed.
| No. of studies | Pooled effect size (95%CI) of hospital use in people with SMI compared to those without | I2 (%) | p-value for between group differences | |||
|---|---|---|---|---|---|---|
| The effect of SMI on hospital use in people with diabetes (OR) | ||||||
| SMI diagnosis | <0.0001 | |||||
| Bipolar disorder | 3 | 1.03 (0.98–1.08) | 0 | |||
| Psychosis | 1 | 1.15 (1.03–1.29) | -- | |||
| Schizophrenia | 11 | 1.42 (1.25–1.60) | 97.7 | |||
| SMI | 3 | 1.17 (0.96–1.44) | 85.9 | |||
| Outcome: service | 0.2015 | |||||
| 30-day readmission | 2 | 1.18 (1.08–1.29) | 0 | |||
| ED attendance | 3 | 1.44 (1.18–1.77) | 97.8 | |||
| Inpatient admissions | 13 | 1.26 (1.08–1.47) | 97.9 | |||
| Outcome: Cause | 0.0225 | |||||
| All-cause | 7 | 1.43 (1.28–1.60) | 94.7 | |||
| Diabetes | 8 | 1.25 (1.08–1.44) | 90.3 | |||
| Ambulatory care sensitive | 3 | 1.09 (0.93–1.28) | 96.6 | |||
| Country of study | <0.0001 | |||||
| US | 9 | 1.10 (0.99–1.22) | 91.6 | |||
| Canada | 4 | 1.55 (1.34–1.80) | 98.2 | |||
| France | 1 | 2.21 (1.69–2.89) | -- | |||
| Spain | 3 | 1.33 (1.19–1.48) | 0 | |||
| UK | 1 | 1.36 (1.13–1.65) | -- | |||
| The effect of SMI on hospital use in people with diabetes (HR) | ||||||
| SMI diagnosis | 0.3654 | |||||
| Bipolar disorder | 2 | 1.27 (1.12–1.44) | 46.2 | |||
| Psychosis | 2 | 1.09 (0.93–1.27) | 93.2 | |||
| Schizophrenia | 2 | 1.32 (0.85–2.07) | 91.8 | |||
| SMI | 1 | 1.14 (1.05–1.23) | -- | |||
| Outcome: Cause | <0.0001 | |||||
| All-cause | 1 | 1.14 (1.05–1.23) | -- | |||
| Diabetes | 5 | 1.25 (1.13–1.37) | 73.5 | |||
| Ambulatory care sensitive | 1 | 1.01 (0.98–1.04) | -- | |||
| Country of study | 0.0016 | |||||
| US | 2 | 1.07 (0.95–1.20) | 87.3 | |||
| Canada | 1 | 1.68 (1.34–2.10) | -- | |||
| Australia | 3 | 1.17 (1.10–1.25) | 46.5 | |||
| Taiwan | 1 | 1.41 (1.16–1.72) | -- | |||
| The effect of SMI on hospital use in people with cardiovascular disease (OR) | ||||||
| SMI diagnosis | <0.0001 | |||||
| Psychosis | 8 | 1.09 (1.02–1.16) | 66.4 | |||
| Schizophrenia | 1 | 1.77 (0.79–3.94) | -- | |||
| SMI | 2 | 2.28 (2.11–2.46) | 0 | |||
| Outcome: Cause | 0.0861 | |||||
| All-cause | 7 | 1.46 (1.03–2.08) | 97.5 | |||
| Cardiovascular disease | 4 | 1.07 (1.04–1.11) | 0 | |||
| Country of study | 0.2259 | |||||
| US | 9 | 1.22 (1.01–1.48) | 97.5 | |||
| Denmark | 1 | 1.77 (0.79–3.94) | -- | |||
| UK | 1 | 2.02 (1.10–3.70) | -- | |||
| The effect of SMI on hospital use in people with cardiovascular disease (HR) | ||||||
| SMI diagnosis | 0.0056 | |||||
| Bipolar | 3 | 1.28 (1.13–1.43) | 62.9 | |||
| Psychosis | 1 | 1.56 (1.44–1.67) | -- | |||
| Schizophrenia | 4 | 1.30 (1.15–1.46) | 47.8 | |||
| Outcome: Service | 0.2218 | |||||
| 30-day readmission | 2 | 1.44 (1.22–1.69) | 53.7 | |||
| Inpatient admissions | 6 | 1.28 (1.17–1.40) | 84.4 | |||
| Outcome: Cause | 0.4218 | |||||
| All-cause | 3 | 1.39 (1.20–1.60) | 77.0 | |||
| Cardiovascular disease | 5 | 1.29 (1.16–1.43) | 62.5 | |||
| Country of study | 0.7365 | |||||
| Sweden | 1 | 1.29 (0.78–2.14) | -- | |||
| UK | 4 | 1.29 (1.15–1.44) | 71.8 | |||
| US | 3 | 1.39 (1.20–1.60) | 77.0 | |||
| The effect of SMI on hospital use in people with COPD (OR) | ||||||
| SMI diagnosis | 0.3059 | |||||
| Psychosis | 3 | 1.18 (1.14–1.22) | 0 | |||
| Schizophrenia | 1 | 1.08 (0.92–1.27) | -- | |||
| Outcome: Cause | 0.7298 | |||||
| All-cause | 2 | 1.16 (1.09–1.24) | 0 | |||
| COPD | 2 | 1.18 (1.13–1.23) | 0 | |||
| Country of study | 0.3059 | |||||
| US | 3 | 1.18 (1.14–1.22) | 0 | |||
| Denmark | 1 | 1.08 (0.92–1.27) | -- | |||
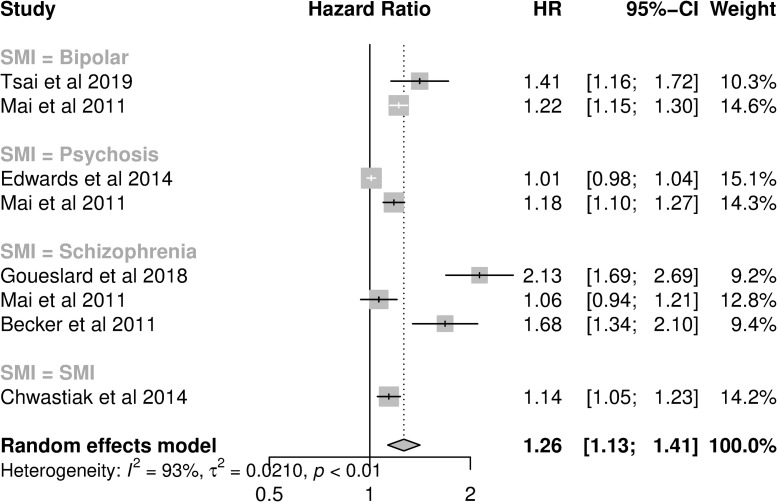
Fewer studies in populations with diabetes assessed HR (eight analyses from six studies, Fig 3). Seven analyses investigated admissions, while one investigated admissions or ED attendance combined (Table 2). The funnel plot identified one outlier, with a large effect size [33] (S3 Fig). When this outlier was removed, the pooled HR was reduced from 1.26 (1.13–1.41; I2 = 92.7%, Fig 3) to 1.19 (95%CI: 1.08–1.31, I2 = 90.6%). In subgroup analysis, analyses of diabetes admissions had a higher pooled HR (1.25; 95%CI: 1.13–1.37) than all-cause or ACSC admissions studies, while analyses performed in the US had a lower pooled HR (1.07; 95%CI: 0.95–1.20) than studies in other countries (Table 3). Pooled HRs were similar across SMI diagnoses. When controlling for country and type of hospital utilisation in meta-regression, the residual heterogeneity was reduced (I2: 46.5%).
Fig 3. Forest plot of studies presenting adjusted hazard ratios of hospital utilisation in diabetes patients with SMI compared to diabetes patients without SMI.
For studies of both hazard ratios and odds ratios based in the US, there was evidence that pooled effect sizes of hospital utilisation in people with SMI were lower in studies of patients registered in Veteran’s Affairs, Medicare or Medicaid, compared to studies of commercially insured people or studies including both state-insured and commercially insured individuals (Table 4).
Table 4. Subgroup analyses of studies of hospital use in the US in people with underlying diabetes: Comparing those with and without SMI.
| No. of studies | Pooled effect size (95%CI) of hospital use in people with SMI compared to those without | I2 (%) | p-value for between group differences | |||
|---|---|---|---|---|---|---|
| The effect of SMI on hospital use in people with diabetes (OR) | ||||||
| Study population | 0.0365 | |||||
| Medicaid/Medicare | 4 | 1.03 (0.86–1.22) | 95.4 | |||
| Veterans’ health | 1 | 1.00 (0.94–1.07) | -- | |||
| Insured | 3 | 1.22 (0.96–1.56) | 80.1 | |||
| Complete | 1 | 1.24 (1.07–1.44) | -- | |||
| The effect of SMI on hospital use in people with diabetes (HR) | ||||||
| Study population | 0.005 | |||||
| Veterans’ health | 1 | 1.01 (0.98–1.04) | ||||
| Insured | 1 | 1.14 (1.05–1.23) | ||||
Hospitalisation use in people with cardiovascular disease, with and without SMI
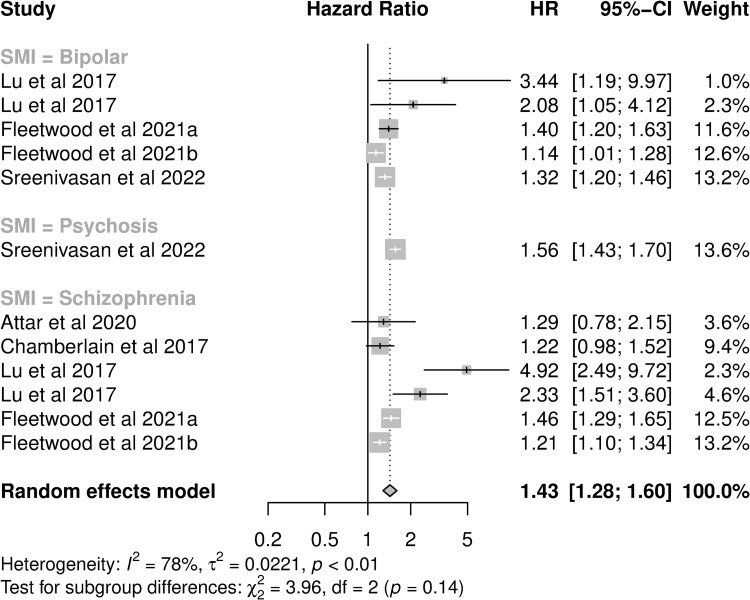
Forty-four analyses from 20 studies were based in populations with underlying cardiovascular disease, the most common of which was heart failure (n = 7, Table 1). Eleven analyses from nine studies providing adjusted ORs for hospital utilisation in people with SMI compared to those without SMI were included in meta-analysis, and twelve analyses from six studies presented adjusted HR. The funnel plot for these analyses did not show asymmetry (meta-analysis of ORs: Egger’s test: p = 0.6751, S4 Fig; meta-analysis of HRs: Egger’s test: p = 0.1535, S5 Fig), and for ORs did not show any outliers.
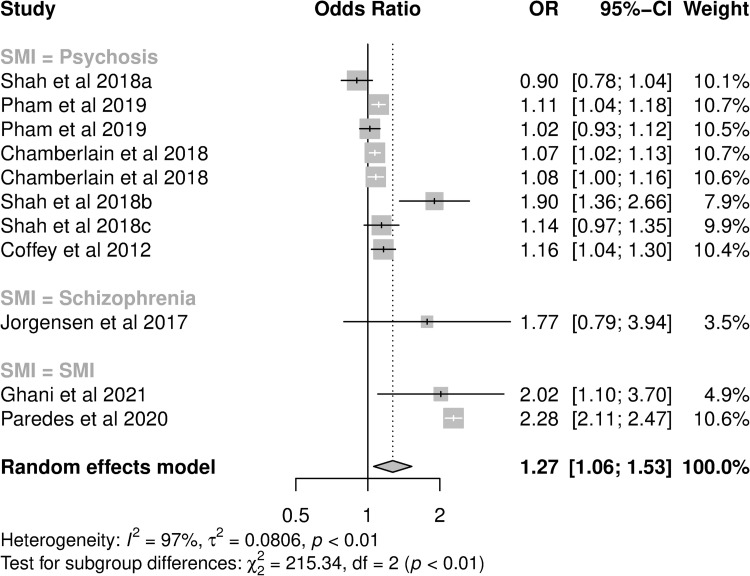
For those presenting ORs, all were 30-day readmission studies, and psychosis was the exposure for eight analyses (Table 2). The pooled OR for hospital utilisation in patients with a diagnosis of any SMI was 1.27 (95%CI: 1.06–1.53; I2: 96.9%, Fig 4). In subgroup analysis, pooled OR were not significantly different between cause of hospitalisation or country of study, but did differ by SMI diagnosis (Table 3). The majority of analyses examined broad risk factors for hospitalisation, while only three focused on SMI specifically. Those with SMI as a focus had greater pooled OR (pOR: 2.27, 95%CI: 2.10–2.46 vs. pOR 1.09, 95%CI: 1.02–1.16). Controlling for these variables in meta-regression reduced heterogeneity (I2 = 61.9%).
Fig 4. Forest plot of studies presenting adjusted odds ratios of hospital utilisation in cardiovascular disease patients with SMI compared to cardiovascular disease without SMI.
For those presenting HRs, the pooled HR for hospital utilisation was 1.43 (95%CI: 1.28–1.60, I2: 78.4%, Fig 5). Most analyses investigated inpatient admissions (8/12) and cardiovascular outcomes (n = 9). One study, contributing four analyses, was identified as an outlier (S5 Fig). This study was a small single-site study of African American patients in the US [59]. Removal of this study from the meta-analysis reduced the pooled HR to 1.33 (95%CI: 1.21–1.46, I2: 74.0%). In subgroup analysis, pooled HRs were not significantly different between cause of hospitalisation, hospitalisation type or country of study (Table 3). However, there were differences by SMI diagnosis, and controlling for this did reduce heterogeneity (I2 = 55.13%).
Fig 5. Forest plot of studies presenting adjusted hazard ratios of hospital utilisation in cardiovascular disease patients with SMI compared to cardiovascular disease without SMI.
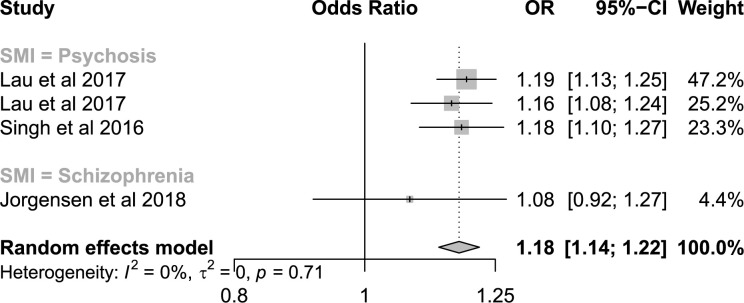
Hospitalisation use in people with COPD, with and without SMI
Five analyses from four studies were in populations with underlying COPD. All five presented ORs for 30-day readmissions in patients with SMI compared to those without SMI, of which four presented adjusted ORs. The funnel plot of these analyses did not show asymmetry of outliers (S6 Fig). The pooled OR for hospital use in patients with a diagnosis of any SMI was 1.18 (95%CI: 1.14–1.22, I2 = 0%, Fig 6). In subgroup analysis, pooled ORs were not significantly different between cause of hospitalisation, country of study or SMI diagnosis (Table 3).
Fig 6. Forest plot of studies presenting adjusted odds ratios of hospital utilisation in COPD patients with SMI compared to COPD patients without SMI.
Hospitalisation use in people with cancer or liver disease, with and without SMI
Two studies were identified which considered SMI as an exposure for hospitalisation in people with and without SMI in populations with underlying liver disease and four in populations with underlying cancer (Table 1). Neither of the liver disease studies presented adjusted effect estimates, and both were low quality for the exposures and outcomes considered in this synthesis (NOS score = 5). Huckans et al. [65] found that people with schizophrenia were more likely to attend EDs and have inpatient admissions during hepatitis C treatment than those without schizophrenia, though due to the small population size (n = 60) confidence intervals were wide and included one. Davydow et al. [66] found higher ACSC admissions for in those with liver disease and SMI compared to those with liver disease without SMI (Table 2).
For cancer, two studies presented adjusted effect measures of hospital utilisation. Basta et al. [64] studied readmissions for lymphedema in the two years after breast cancer diagnosis in women. They found that women with a diagnosis of psychosis were at higher risk of readmission (aOR: 2.15, 95%CI: 1.51–3.06). Kashyap et al. [68] found higher utilisation of emergency departments in the 30 days prior to death in those with gastrointestinal malignancies and SMI compared to those with gastrointestinal malignancies alone. Finally, an unadjusted analysis by Ratcliff et al. [74] found higher risk of 90-day readmissions after surgery for colorectal cancer in those with SMI, while Davydow et al. [66] found higher risk of ACSC admissions in those with cancer and SMI compared to those with cancer alone, in unadjusted analysis (Table 2).
Sensitivity analysis. In sensitivity analysis, re-running the analysis as a three-level hierarchical model did not result in improved model fit, nor substantial change the pooled OR (1.26, 95%CI: 1.10–1.45) or HR (1.23; 95%CI 1.01–1.50) for studies in people with diabetes and SMI, or the pooled OR (1.34, 95%CI: 1.07–1.69) or HR (1.47. 95%CI: 1.16–1.85) for people with cardiovascular disease and SMI. For COPD, the two analyses from one study included in the meta-analysis were from different populations and so sensitivity analysis was not performed. Only three conference abstracts providing adjusted effect measures for hospitalisation were retrieved. The first was a study of risk factors for 30- and 90-day rehospitalisation following radical cystectomy for bladder cancer. The authors found that people with psychosis had an elevated HR for readmission (aHR: 1.82, p<0.05) [79]. The second was a small study of 373 people with diabetes, which found that those with two or more admissions were more likely to have a diagnosis of schizophrenia (aOR: 4.99, p<0.05) than those with only one admission [80]. Finally, a study of all-cause 30-day readmissions in people with acute ischaemic stroke, found those with SMI we at higher risk (aOR: 1.24, 95%CI: 1.20–1.27) [81].
Discussion
This review and meta-analysis demonstrates that people with SMI and one of five physical health conditions have consistently higher hospital utilisation than either people with SMI alone or with physical health conditions alone. This is the first systematic review to consider the impact of having SMI and a specific physical health condition on hospital utilisation, allowing a better understanding of the impact of SMI on hospital use in those with underlying physical illness, and highlighting areas for future research.
We found that in people with underlying cardiovascular disease, COPD or diabetes, people with a diagnosis of SMI had higher hospital use compared to those without SMI. This finding is in line with other systematic reviews or meta-analyses [11–13, 17], which consider the impact of SMI on hospitalisations in the general population, or when controlling for physical health comorbidities. The same appeared to be true for people with cancer and liver disease, though studies presenting adjusted analyses were limited to one study of breast cancer complications [64], and one of end of life emergency department use in people with gastrointestinal malignancies [68]. No studies of liver disease reported adjusted effect measures. Only five studies were identified which considered a population with underlying severe mental illness, with and without physical LTCs. In these studies, the addition of physical LTC increased the risk of hospital utilisation.
In populations with underlying diabetes, cardiovascular disease and COPD, people with SMI were at higher risk of 30-day readmissions compared to those without SMI, and the pooled OR were similar for 30-day readmission in these populations. This suggests that over this short timeframe, the risk of readmission does not differ substantially by underlying physical disease. While the effect size of having SMI was relatively small for all three diseases, any increased risk of hospital admission represents a major burden given the underlying high rate of admissions for these diseases in the general population [82, 83].
A strength of focusing on studies in populations with underlying physical LTC, is that it provides further evidence that the higher emergency hospitalisation in people with SMI is not due to higher prevalence of that LTC in the SMI population. It also allows the investigation of the impact of hospitalisations for the underlying LTC, compared to all-cause hospitalisations. In 30-day readmission studies of both COPD and cardiovascular disease we found little difference between studies of all-cause or cause-specific hospitalisations, suggesting that 30-day readmissions for the index condition are likely driving the difference between those with and without a diagnosis of SMI. The consistently higher risk in those with SMI, may indicate systematic differences in management and treatment of physical health conditions in people with SMI, such as lower adherence to medication, reduced access or attendance at planned outpatient care [84] and less guideline-recommended treatment [56, 61, 85–87], as well as more complex medication regimens and medical histories.
For studies examining hospital admissions for populations with underlying diabetes, we found that while patients with SMI had higher pooled OR of diabetes-specific admissions than those without SMI, the greatest difference was in all-cause admissions. This was also true in studies investigating both all-cause and diabetes admissions in the same study [41, 45]. These findings suggest that while a higher risk of diabetes admissions and sub-optimal management and treatment of diabetes [35, 37, 45] account for some of the higher hospital use in people with SMI, there are other factors involved. A study of patients with underlying diabetes found high rates of all-cause hospitalisations in people with SMI, even once acute psychiatric admissions were excluded from the outcome [45], suggesting that higher rates of multimorbidity, and therefore higher general physical health admissions, as well as higher risk of trauma and infectious disease hospitalisations [16], may be adding to the burden of hospitalisations in these patients. While we did not find the same in the subgroup analysis of diabetes studies presenting hazard ratios, only one study investigated all-cause admissions and the total number of available studies was small, limiting interpretation.
We also found evidence that specific populations may have elevated risk of hospital use. We found a high risk of hospitalisation in people with SMI in studies examining the effect of SMI on readmissions during hepatitis C treatment [65], on cardiovascular hospital use in African American patients with heart failure [59], on diabetes readmissions in patients under the age of 35 with type I diabetes [33], and following breast cancer surgery [64]. For diabetes, patients with schizophrenia appeared to be at higher risk of hospitalisation compared to other SMI diagnoses in studies presenting adjusted odds ratios. This has been reported elsewhere [17], and is in line with other studies that have found people with schizophrenia suffer more ill-health, greater all-cause mortality and poorer physical health and treatment outcomes than people with other SMI diagnoses [9, 35, 37, 88, 89]. However, for studies of people with underlying diabetes or cardiovascular disease presenting adjusted hazard ratios, there was little difference between diagnoses of bipolar disorder and schizophrenia. Of the seven studies included in our review which considered schizophrenia alongside other SMI diagnoses, two found patients with schizophrenia were more likely to be hospitalised than other SMI diagnoses [31, 35], one found that those with schizophrenia were less likely to be hospitalised [36], and four found no significant difference [37, 59, 70, 71].
Finally, we found that while still elevated, the risk of readmission in patients with diabetes and SMI was lower in the US compared to other countries. While this finding has been documented before [17], the reason for this is unclear. For these studies, we found differences in effect size based on the healthcare system under investigation, and therefore patients with SMI may face different barriers and drivers to hospital use across payers in the US healthcare system. It is not clear whether this is limited to diabetes management, as the small number of studies in patients with COPD or cardiovascular disease did not permit comparisons by country.
Limitations
Although this review has better described the pattern of hospital utilisation in people with SMI and physical health conditions, there are limitations. Although our search strategy was thorough, we may have missed studies which include SMI as a risk factor for higher healthcare utilization, but which do not include terms for SMI in the title or abstract. These studies are unlikely to have SMI as their main exposure variable and given that SMI is not common in the general population are less likely to provide well powered estimations. We identified 11 studies for which SMI was not the main focus, and while inclusion of these studies provides further evidence, caution is needed as they may be subject to confounding and issues of power [90]. In addition, while our search strategy was thorough, and overall agreement between reviewers was high (91%), the interrater reliability of screened abstracts as measured by the Kappa statistic was moderate (0.57). This is in part due to the large number of studies screened and the rarity of relevant studies [91], but also the complexity of multiple exposures and outcomes. All disagreements were discussed thoroughly to ensure the accuracy of study inclusion.
We found marked heterogeneity in the study results, particularly for studies of diabetes. While definitions of SMI, physical LTCs and outcome measures accounted for some of this, underlying differences in the population and healthcare system, as well as differences in study design are likely major causes of this heterogeneity.
While most studies we identified were of fair or good quality, there were limitations to many of them. Few studies utilised matched cohorts of patients, and most did not evaluate the impact of prior healthcare utilisation, despite this being a known predictor of hospital use in the general population [92]. Furthermore, many studies were performed in the US, which limits the generalisability of results to other healthcare systems. Despite being based in longitudinal populations, under half of studies performed a time-to-event analysis. Where this was performed, very few accounted for multiple hospitalisations or included time-varying covariates. Most studies included only patients who had accessed secondary care, both to define SMI and physical health conditions. Without access to primary care records, these studies exclude those patients who may be managed solely in primary care or attend secondary care very infrequently. These excluded patients may provide important information on protective factors that reduce secondary care use.
Knowledge gaps and future research
There were few studies investigating hospital use in a population of patients with SMI, comparing hospital use in those with or without physical LTC. The underlying heterogeneity of these studies made them unsuitable for meta-analysis. Given that people with SMI are at an higher risk of many physical LTCs, further research is required to identify the drivers of physical health hospitalisations in people with SMI, and subsets of this population at higher risk.
There was also a lack of data regarding hospital use in patients with cancer, and the impact of SMI diagnoses on hospital utilisation. Given the higher risk of mortality following cancer diagnosis in those with SMI, and evidence of sub-optimal cancer screening and late diagnoses [93], it is important to understand hospital utilisation in this population.
Finally, there was a lack of information on the impact of SMI on hospitalisation for liver disease, and on the long-term risk of hospitalisation in patients with COPD or cardiovascular disease. These common diseases represent a huge burden in terms of hospital resource use and ill health in the general population [94]. Given people with SMI may be at higher risk of these diseases [2], receive poorer care [6, 56, 61, 84–87, 89, 95–98] and worse outcomes [6], more research is required into the impact of an SMI diagnosis on hospital utilisation in people with these conditions.
Conclusions
This systematic review and meta-analysis found that patients with SMI and underlying physical health conditions are at a higher risk of hospital use for that condition, and for other causes. Further research is warranted into the effects of different physical health conditions and different SMI diagnoses on hospital use, particularly over longer time periods, and of pathways and drivers of hospitalisation in those with SMI. This will allow targeted interventions aimed at reducing inappropriate hospital use and improving disease management and outcomes in people with SMI.
Supporting information
(DOC)
(DOCX)
*Does control for age and is limited to females.
(DOCX)
*One point. a: Analysis presenting odds ratios; b: analysis presenting hazard ratios c: Analysis of 30-day readmissions; d: analysis of long-term readmissions.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by Public Health England (PhD2019/002 - NL), the Wellcome Trust (211085/Z/18/Z - JFH), the Medical Research Council (MC\PC\17216 - DPJO), University College London Hospitals NIHR Biomedical Research Centre (NL, DPJO, JFH) and the NIHR ARC North Thames Academy (DPJO, JFH). This report is independent research supported by the National Institute for Health Research ARC North Thames. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health Research, Public Health England, or the Department of Health and Social Care
References
- 1.Launders N, Hayes J. F., Price G., Osborn D. P. J. Clustering of physical health multimorbidity in 68,392 people with severe mental illness and matched comparators: a lifetime prevalence analysis of United Kingdom primary care data. PLoS Med. 2022;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health England. Severe mental illness (SMI) and physical health inequalities. 2018.
- 3.Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6(8):675–712. doi: 10.1016/S2215-0366(19)30132-4 [DOI] [PubMed] [Google Scholar]
- 4.Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Serious mental illness and medical comorbidities: Findings from an integrated health care system. J Psychosom Res. 2017;100:35–45. doi: 10.1016/j.jpsychores.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baughman KR, Bonfine N, Dugan SE, Adams R, Gallagher M, Olds RS, et al. Disease Burden Among Individuals with Severe Mental Illness in a Community Setting. Community Ment Health J. 2016;52(4):424–32. doi: 10.1007/s10597-015-9973-2 [DOI] [PubMed] [Google Scholar]
- 6.Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature Mortality Among Adults With Schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–81. doi: 10.1001/jamapsychiatry.2015.1737 [DOI] [PubMed] [Google Scholar]
- 7.Barnes AL, Murphy ME, Fowler CA, Rempfer MV. Health-related quality of life and overall life satisfaction in people with serious mental illness. Schizophr Res Treatment. 2012;2012:245103. doi: 10.1155/2012/245103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filipcic I, Simunovic Filipcic I, Ivezic E, Matic K, Tunjic Vukadinovic N, Vuk Pisk S, et al. Chronic physical illnesses in patients with schizophrenia spectrum disorders are independently associated with higher rates of psychiatric rehospitalization; a cross-sectional study in Croatia. Eur Psychiatry. 2017;43:73–80. doi: 10.1016/j.eurpsy.2017.02.484 [DOI] [PubMed] [Google Scholar]
- 9.Hoang U, Goldacre MJ, Stewart R. Avoidable mortality in people with schizophrenia or bipolar disorder in England. Acta Psychiatr Scand. 2013;127(3):195–201. doi: 10.1111/acps.12045 [DOI] [PubMed] [Google Scholar]
- 10.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–41. doi: 10.1001/jamapsychiatry.2014.2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germack HD, Caron A.,Solomon R.,Hanrahan N. P. Medical-surgical readmissions in patients with co-occurring serious mental illness: A systematic review and meta-analysis. General Hospital Psychiatry. 2018;55:65–71. doi: 10.1016/j.genhosppsych.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 12.Jansen L, van Schijndel M, van Waarde J, van Busschbach J. Health-economic outcomes in hospital patients with medical-psychiatric comorbidity: A systematic review and meta-analysis. PLoS One. 2018;13(3):e0194029. doi: 10.1371/journal.pone.0194029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprah L, Dernovsek M. Z.,Wahlbeck K.,Haaramo P. Psychiatric readmissions and their association with physical comorbidity: A systematic literature review. BMC Psychiatry. 2017;17(1). doi: 10.1186/s12888-016-1172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorning H DA, Blunt I. Focus on: people wth mental ill health and hospital use. The Health Foundation and Nuffield Trust; 2015. [Google Scholar]
- 15.Lin HC, Huang CC, Chen SF, Chen YH. Increased risk of avoidable hospitalization among patients with schizophrenia. Can J Psychiatry. 2011;56(3):171–8. doi: 10.1177/070674371105600307 [DOI] [PubMed] [Google Scholar]
- 16.Jayatilleke N, Hayes R. D.,Chang C. K.,Stewart R. Acute general hospital admissions in people with serious mental illness. Psychol Med. 2018;48(16):2676–83. doi: 10.1017/S0033291718000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronaldson A, Elton L, Jayakumar S, Jieman A, Halvorsrud K, Bhui K. Severe mental illness and health service utilisation for nonpsychiatric medical disorders: A systematic review and meta-analysis. PLoS Med. 2020;17(9):e1003284. doi: 10.1371/journal.pmed.1003284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wimmer BC, Bell JS, Fastbom J, Wiese MD, Johnell K. Medication Regimen Complexity and Number of Medications as Factors Associated With Unplanned Hospitalizations in Older People: A Population-based Cohort Study. J Gerontol A Biol Sci Med Sci. 2016;71(6):831–7. doi: 10.1093/gerona/glv219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobrovitz N, Heneghan C, Onakpoya I, Fletcher B, Collins D, Tompson A, et al. Medications that reduce emergency hospital admissions: an overview of systematic reviews and prioritisation of treatments. BMC Med. 2018;16(1):115. doi: 10.1186/s12916-018-1104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huntley A, Lasserson D, Wye L, Morris R, Checkland K, England H, et al. Which features of primary care affect unscheduled secondary care use? A systematic review. BMJ Open. 2014;4(5):e004746. doi: 10.1136/bmjopen-2013-004746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tammes P, Morris RW, Brangan E, Checkland K, England H, Huntley A, et al. Exploring the relationship between general practice characteristics and attendance at Walk-in Centres, Minor Injuries Units and Emergency Departments in England 2009/10-2012/2013: a longitudinal study. BMC Health Serv Res. 2017;17(1):546. doi: 10.1186/s12913-017-2483-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whittaker W, Anselmi L, Kristensen SR, Lau YS, Bailey S, Bower P, et al. Associations between Extending Access to Primary Care and Emergency Department Visits: A Difference-In-Differences Analysis. PLoS Med. 2016;13(9):e1002113. doi: 10.1371/journal.pmed.1002113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson K, Deeny SR, Steventon A. Ambulatory care-sensitive conditions: their potential uses and limitations. BMJ Qual Saf. 2019;28(6):429–33. doi: 10.1136/bmjqs-2018-008820 [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence. NICE Quality and Outcomes Framework indicator 2022 [Available from: https://www.nice.org.uk/Standards-and-Indicators/QOFIndicators?categories=&page=3.
- 25.R Core Team. A language and environment for statistical computing. 2018.
- 26.RStudio Team. RStudio: Integrated Development for R. 2015.
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Noortgate W, Lopez-Lopez JA, Marin-Martinez F, Sanchez-Meca J. Three-level meta-analysis of dependent effect sizes. Behav Res Methods. 2013;45(2):576–94. doi: 10.3758/s13428-012-0261-6 [DOI] [PubMed] [Google Scholar]
- 29.Egglefield K, Cogan L., Leckman-Westin E., Finnerty M. Antipsychotic Medication Adherence and Diabetes-Related Hospitalizations Among Medicaid Recipients With Diabetes and Schizophrenia. Psychiatr Serv. 2020;71(3):236–42. doi: 10.1176/appi.ps.201800505 [DOI] [PubMed] [Google Scholar]
- 30.Helmer DA, Dwibedi N, Rowneki M, Tseng CL, Fried D, Rose D, et al. Mental Health Conditions and Hospitalizations for Ambulatory Care Sensitive Conditions Among Veterans with Diabetes. Am Health Drug Benefits. 2020;13(2):61–71. [PMC free article] [PubMed] [Google Scholar]
- 31.Stockbridge EL, Chhetri S.,Polcar L. E.,Loethen A. D.,Carney C. P. Behavioral health conditions and potentially preventable diabetes-related hospitalizations in the United States: Findings from a national sample of commercial claims data. PLoS One. 2019;14(2):e0212955. doi: 10.1371/journal.pone.0212955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai CL, Yang YC, Chen SF, Hsu CY, Shen YC. Risk of hyperglycemic crisis episode in diabetic patients with bipolar disorder: A nationwide population-based cohort study. J Affect Disord. 2019;257:281–6. doi: 10.1016/j.jad.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 33.Goueslard K, Petit J. M., Cottenet J., Chauvet-Gelinier J. C., Jollant F., Quantin C. Increased risk of rehospitalization for acute diabetes complications and suicide attempts in patients with type 1 diabetes and comorbid schizophrenia. Diabetes care. 2018;41(11):2316–21. doi: 10.2337/dc18-0657 [DOI] [PubMed] [Google Scholar]
- 34.Edwards STP J.; Simon S. R.; Pizer S. D. Home-based primary care is associated with reduced ambulatory care sensitive hospitalizations in veterans with diabetes. Journal of General Internal Medicine. 2014;29:S109. [Google Scholar]
- 35.Druss BG, Zhao L., Cummings J. R., Shim R. S., Rust G. S., Marcus S. C. Mental comorbidity and quality of diabetes care under medicaid: A 50-state analysis. Medical Care. 2012;50(5):428–33. doi: 10.1097/MLR.0b013e318245a528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung G, Zhang J.,Lin W. C.,Clark R. E. Behavioral disorders and diabetes-related outcomes among Massachusetts Medicare and Medicaid beneficiaries. Psychiatr Serv. 2011;62(6):659–65. doi: 10.1176/ps.62.6.pss6206_0659 [DOI] [PubMed] [Google Scholar]
- 37.Mai Q, Holman C. D. J., Sanfilippo F. M., Emery J. D., Preen D. B. Mental illness related disparities in diabetes prevalence, quality of care and outcomes: A population-based longitudinal study. BMC Medicine. 2011;9 (no pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cramer S, Chapa G., Kotsos T., Jenich H. Assessing multiple hospitalizations for health-plan-managed Medicaid diabetic members. J Healthc Qual. 2010;32(3):7–14. doi: 10.1111/j.1945-1474.2010.00089.x [DOI] [PubMed] [Google Scholar]
- 39.Yan T, Greene M., Chang E., Broder M. S., Touya M., Munday J., et al. Hospitalization risk factors in antipsychotic-Treated schizophrenia, bipolar i disorder or major depressive disorder. Journal of Comparative Effectiveness Research. 2019;8(4):217–27. doi: 10.2217/cer-2018-0090 [DOI] [PubMed] [Google Scholar]
- 40.Chen JY, Ma Q, Chen H, Yermilov I. New bundled world: quality of care and readmission in diabetes patients. J Diabetes Sci Technol. 2012;6(3):563–71. doi: 10.1177/193229681200600311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerrero Fernandez de Alba IG-M A.; Poblador-Plou B.; Gimeno-Feliu L. A.;Ioakeim-Skoufa I.; Rojo-Martinez G.; Forjaz M. J.; Prados-Torres A. Association between mental health comorbidity and health outcomes in type 2 diabetes mellitus patients. Scientific reports. 2020;10(1):19583. doi: 10.1038/s41598-020-76546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chwastiak LA, Davydow D. S., McKibbin C. L., Schur E., Burley M., McDonell M. G., et al. The Effect of Serious Mental Illness on the Risk of Rehospitalization Among Patients With Diabetes. Psychosomatics. 2014;55(2):134–43. doi: 10.1016/j.psym.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker T, Hux J. Risk of acute complications of diabetes among people with schizophrenia in Ontario, Canada. Diabetes Care. 2011;34(2):398–402. doi: 10.2337/dc10-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krein SL, Bingham C. R., McCarthy J. F., Mitchinson A., Payes J., Valenstein M. Diabetes treatment among VA patients with comorbid serious mental illness. Psychiatr Serv. 2006;57(7):1016–21. doi: 10.1176/ps.2006.57.7.1016 [DOI] [PubMed] [Google Scholar]
- 45.Kurdyak P, Vigod S., Duchen R., Jacob B., Stukel T., Kiran T. Diabetes quality of care and outcomes: Comparison of individuals with and without schizophrenia. Gen Hosp Psychiatry. 2017;46:7–13. doi: 10.1016/j.genhosppsych.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 46.Shim RS, Druss B. G., Zhang S., Kim G., Oderinde A., Shoyinka S., et al. Emergency department utilization among Medicaid beneficiaries with schizophrenia and diabetes: The consequences of increasing medical complexity. Schizophr Res. 2014;152(2–3):490–7. doi: 10.1016/j.schres.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan G, Han X., Moore S., Kotrla K. Disparities in hospitalization for diabetes among persons with and without co-occurring mental disorders. Psychiatr Serv. 2006;57(8):1126–31. doi: 10.1176/ps.2006.57.8.1126 [DOI] [PubMed] [Google Scholar]
- 48.Attar RW A.; Koul S.; Eggert S.; Polcwiartek C.; Jernberg T.; Erlinge D.; et al. Higher risk of major adverse cardiac events after acute myocardial infarction in patients with schizophrenia. Open Heart. 2020;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chamberlain AM, Alonso A., Gersh B. J., Manemann S. M., Killian J. M., Weston S. A., et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: A population-based study. American Heart Journal. 2017;185:74–84. doi: 10.1016/j.ahj.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sayers SL, Hanrahan N, Kutney A, Clarke SP, Reis BF, Riegel B. Psychiatric comorbidity and greater hospitalization risk, longer length of stay, and higher hospitalization costs in older adults with heart failure. J Am Geriatr Soc. 2007;55(10):1585–91. doi: 10.1111/j.1532-5415.2007.01368.x [DOI] [PubMed] [Google Scholar]
- 51.Shah M, Patel B, Tripathi B, Agarwal M, Patnaik S, Ram P, et al. Hospital mortality and thirty day readmission among patients with non-acute myocardial infarction related cardiogenic shock. Int J Cardiol. 2018;270:60–7. doi: 10.1016/j.ijcard.2018.06.036 [DOI] [PubMed] [Google Scholar]
- 52.Pham PNX H.; Sarayani A.; Chen M.; Brown J. D. Risk Factors Associated With 7-Versus 30-Day Readmission Among Patients With Heart Failure Using the Nationwide Readmission Database. Medical Care. 2019;57(1):1–7. doi: 10.1097/MLR.0000000000001006 [DOI] [PubMed] [Google Scholar]
- 53.Chamberlain RS, Sond J, Mahendraraj K, Lau CS, Siracuse BL. Determining 30-day readmission risk for heart failure patients: the Readmission After Heart Failure scale. Int J Gen Med. 2018;11:127–41. doi: 10.2147/IJGM.S150676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah MR, P.; Lo K. B. U.; Sirinvaravong N.; Patel B.; Tripathi B.; Patil S.; et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clinical Cardiology. 2018;41(7):916–23. doi: 10.1002/clc.22974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah M, Patil S, Patel B, Agarwal M, Davila CD, Garg L, et al. Causes and Predictors of 30-Day Readmission in Patients With Acute Myocardial Infarction and Cardiogenic Shock. Circ Heart Fail. 2018;11(4):e004310. doi: 10.1161/CIRCHEARTFAILURE.117.004310 [DOI] [PubMed] [Google Scholar]
- 56.Jorgensen M, Mainz J., Egstrup K., Johnsen S. P. Quality of Care and Outcomes of Heart Failure Among Patients With Schizophrenia in Denmark. Am J Cardiol. 2017;120(6):980–5. doi: 10.1016/j.amjcard.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 57.Ahmedani BK, Solberg L. I., Copeland L. A., Fang-Hollingsworth Y., Stewart C., Hu J., et al. Psychiatric comorbidity and 30-day readmissions after hospitalization for heart failure, AMI, and pneumonia. Psychiatr Serv. 2015;66(2):134–40. doi: 10.1176/appi.ps.201300518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coffey RMM A.; Barrett M.; Andrews R. M.; Mutter R.; Moy E. Congestive Heart Failure: Who Is Likely to Be Readmitted? Med Care Res Rev. 2012;69(5):602–16. doi: 10.1177/1077558712448467 [DOI] [PubMed] [Google Scholar]
- 59.Lu MLR, De Venecia T. A., Goyal A., Rodriguez Ziccardi M., Kanjanahattakij N., Shah M. K., et al. Psychiatric conditions as predictors of rehospitalization among African American patients hospitalized with heart failure. Clin Cardiol. 2017;40(11):1020–5. doi: 10.1002/clc.22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buhr RGJ N. J.; Kominski G. F.; Dubinett S. M.; Ong M. K.; Mangione C. M. Comorbidity and thirty-day hospital readmission odds in chronic obstructive pulmonary disease: a comparison of the Charlson and Elixhauser comorbidity indices. BMC health services research. 2019;19(1):701. doi: 10.1186/s12913-019-4549-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jorgensen M, Mainz J., Lange P., Johnsen S. P. Quality of care and clinical outcomes of chronic obstructive pulmonary disease in patients with schizophrenia. A Danish nationwide study. International Journal for Quality in Health Care. 2018;30(5):351–7. doi: 10.1093/intqhc/mzy014 [DOI] [PubMed] [Google Scholar]
- 62.Lau CSMS B. L.; Chamberlain R. S. Readmission after COPD exacerbation scale: Determining 30-day readmission risk for COPD patients. International Journal of COPD. 2017;12:1891–902. doi: 10.2147/COPD.S136768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh G, Zhang W., Kuo Y. F., Sharma G. Association of Psychological Disorders With 30-Day Readmission Rates in Patients With COPD. Chest. 2016;149(4):905–15. doi: 10.1378/chest.15-0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basta MN, Fox JP, Kanchwala SK, Wu LC, Serletti JM, Kovach SJ, et al. Complicated breast cancer-related lymphedema: evaluating health care resource utilization and associated costs of management. Am J Surg. 2016;211(1):133–41. doi: 10.1016/j.amjsurg.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 65.Huckans M, Mitchell A., Ruimy S., Loftis J., Hauser P. Antiviral therapy completion and response rates among hepatitis c patients with and without schizophrenia. Schizophrenia Bulletin. 2010;36(1):165–72. doi: 10.1093/schbul/sbn065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davydow DS, Ribe A. R., Pedersen H. S., Fenger-Gron M., Cerimele J. M., Vedsted P., et al. Serious Mental Illness and Risk for Hospitalizations and Rehospitalizations for Ambulatory Care-sensitive Conditions in Denmark A Nationwide Population-based Cohort Study. Medical Care. 2016;54(1):90–7. doi: 10.1097/MLR.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 67.Guo JJ, Keck PE Jr., Li H, Jang R, Kelton CM. Treatment costs and health care utilization for patients with bipolar disorder in a large managed care population. Value Health. 2008;11(3):416–23. doi: 10.1111/j.1524-4733.2007.00287.x [DOI] [PubMed] [Google Scholar]
- 68.Kashyap MH, Chang J. P., Pollom D. T., L E. Impact of mental illness on end-of-life emergency department use in elderly patients with gastrointestinal malignancies. Cancer Med. 2021;10(6):2035–44. doi: 10.1002/cam4.3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kallio MK, Malmberg J., Gunn M., Rautava J., Korhonen P., Kytö P., V. Impaired long-term outcomes of patients with schizophrenia spectrum disorder after coronary artery bypass surgery: nationwide case-control study. BJPsych Open. 2022;8(2):e48. doi: 10.1192/bjo.2022.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleetwood K, Smith Sarah H., Mercer Daniel J., Licence Stewart W., Sudlow Kirsty, Jackson Cathie L. M., et al. Association of severe mental illness with stroke outcomes and process-of-care quality indicators: Nationwide cohort study. The British Journal of Psychiatry. 2021(Pagination). [DOI] [PubMed] [Google Scholar]
- 71.Fleetwood KW, Smith S. H., Mercer D. J., Licence S. W., Sudlow K., Jackson C. L. M., A C. Severe mental illness and mortality and coronary revascularisation following a myocardial infarction: a retrospective cohort study. BMC Med. 2021;19(1):67. doi: 10.1186/s12916-021-01937-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghani MK, Pritchard S., Harris M., Weerakkody M., Stewart R., Perera R., G. Vascular surgery receipt and outcomes for people with serious mental illnesses: Retrospective cohort study using a large mental healthcare database in South London. J Psychosom Res. 2021;147:110511. doi: 10.1016/j.jpsychores.2021.110511 [DOI] [PubMed] [Google Scholar]
- 73.Huang CJL T. L. Huang Y. T. Hsieh H. M. Chang C. C. Chu C. C. Wei C. W. Weng S. F. Healthcare burden and factors of type 2 diabetes mellitus with Schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2021. [DOI] [PubMed] [Google Scholar]
- 74.Ratcliff CGM N. N. Sansgiry S. Dindo L. Cully J. A. Impact of psychiatric diagnoses and treatment on postoperative outcomes among patients undergoing surgery for colorectal cancer. Psychiatr Serv. 2021;72(4):391–8. doi: 10.1176/appi.ps.201900559 [DOI] [PubMed] [Google Scholar]
- 75.Paredes AZ, Hyer JM, Diaz A, Tsilimigras DI, Pawlik TM. The Impact of Mental Illness on Postoperative Outcomes Among Medicare Beneficiaries: A Missed Opportunity to Help Surgical Patients? Ann Surg. 2020;272(3):419–25. doi: 10.1097/SLA.0000000000004118 [DOI] [PubMed] [Google Scholar]
- 76.Sreenivasan J, Kaul R, Khan MS, Malik A, Usman MS, Michos ED. Mental health disorders and readmissions following acute myocardial infarction in the United States. Sci Rep. 2022;12(1):3327. doi: 10.1038/s41598-022-07234-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andres E, Garcia-Campayo J, Magan P, Barredo E, Cordero A, Leon M, et al. Psychiatric morbidity as a risk factor for hospital readmission for acute myocardial infarction: an 8-year follow-up study in Spain. Int J Psychiatry Med. 2012;44(1):63–75. doi: 10.2190/PM.44.1.e [DOI] [PubMed] [Google Scholar]
- 78.Wang HI, Han L, Jacobs R, Doran T, Holt RIG, Prady SL, et al. Healthcare resource use and costs for people with type 2 diabetes mellitus with and without severe mental illness in England: longitudinal matched-cohort study using the Clinical Practice Research Datalink. Br J Psychiatry. 2021:1–8. [DOI] [PubMed] [Google Scholar]
- 79.Nepple KO P.; Strope S.; Sandhu G.; Kallogjeri D.; Kibel A. Hospital readmission after radical cystectomy for bladder cancer: Results of a population-based analysis. Journal of Urology. 2012;187(4):e646–e7. [Google Scholar]
- 80.Duquette EK A.; Wang N. E.; Shearer E. Social determinants of health associated with multiple emergency department visits in patients with diabetes. Academic Emergency Medicine. 2020;27 (Supplement 1):S134. [Google Scholar]
- 81.Salih YW, H. Keeney, B. Leyenaar, J. Robbins, N. M. Hospital readmission rates among acute ischemic stroke survivors with severe mental illness. Neurology Conference: 73rd Annual Meeting of the American Academy of Neurology, AAN. 2021;96(15 SUPPL 1).
- 82.Agency for Healthcare Research and Quality. HCUP Fast Stats. Healthcare Cost and Utilization Project (HCUP) Rockville, MD2021 [Available from: www.hcup-us.ahrq.gov/faststats/national/inpatientcommondiagnoses.jsp. [PubMed]
- 83.NHS Digital. Hospital Admitted Patient Care and Adult Critical Care Activity 2019 [Available from: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2018-19.
- 84.Kurdyak P, Vigod S.,Calzavara A.,Wodchis W. P. High mortality and low access to care following incident acute myocardial infarction in individuals with schizophrenia. Schizophr Res. 2012;142(1–3):52–7. doi: 10.1016/j.schres.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 85.Kisely S, Campbell LA, Wang Y. Treatment of ischaemic heart disease and stroke in individuals with psychosis under universal healthcare. Br J Psychiatry. 2009;195(6):545–50. doi: 10.1192/bjp.bp.109.067082 [DOI] [PubMed] [Google Scholar]
- 86.Chang WC, Chan J. K. N., Wong C. S. M., Hai J. S. H., Or P. C. F., Chen E. Y. H. Mortality, Revascularization, and Cardioprotective Pharmacotherapy After Acute Coronary Syndrome in Patients With Psychotic Disorders: A Population-Based Cohort Study. Schizophr Bull. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McGinty EE, Blasco-Colmenares E., Zhang Y., Dosreis S. C., Ford D. E., Steinwachs D. M., et al. Post-myocardial-infarction quality of care among disabled Medicaid beneficiaries with and without serious mental illness. Gen Hosp Psychiatry. 2012;34(5):493–9. doi: 10.1016/j.genhosppsych.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heiberg IH, Jacobsen BK, Balteskard L, Bramness JG, Naess O, Ystrom E, et al. Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatr Scand. 2019;139(6):558–71. doi: 10.1111/acps.13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schulman-Marcus J, Goyal P., Swaminathan R. V., Feldman D. N., Wong S. C., Singh H. S., et al. Comparison of Trends in Incidence, Revascularization, and In-Hospital Mortality in ST-Elevation Myocardial Infarction in Patients With Versus Without Severe Mental Illness. Am J Cardiol. 2016;117(9):1405–10. doi: 10.1016/j.amjcard.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 90.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–8. doi: 10.1093/aje/kws412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–3. [PubMed] [Google Scholar]
- 92.Wallace E, Stuart E, Vaughan N, Bennett K, Fahey T, Smith SM. Risk prediction models to predict emergency hospital admission in community-dwelling adults: a systematic review. Med Care. 2014;52(8):751–65. doi: 10.1097/MLR.0000000000000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solmi M, Firth J, Miola A, Fornaro M, Frison E, Fusar-Poli P, et al. Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta-analysis including 4 717 839 people. Lancet Psychiatry. 2020;7(1):52–63. doi: 10.1016/S2215-0366(19)30414-6 [DOI] [PubMed] [Google Scholar]
- 94.GBD 2019 collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laursen TM, Munk-Olsen T., Agerbo E., Gasse C., Mortensen P. B. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Archives of General Psychiatry. 2009;66(7):713–20. doi: 10.1001/archgenpsychiatry.2009.61 [DOI] [PubMed] [Google Scholar]
- 96.Murugiah K, Kumar G., Deshmukh A., Sachdeva R., Mehta J. Schizophrenia and use of revascularization procedures after acute myocardial infarction. Journal of the American College of Cardiology. 2012;59(13):E1898. [Google Scholar]
- 97.Shao M, Zhuo C., Gao X., Chen C., Xu Y., Tian H., et al. Reduced rate of revascularization in schizophrenic patients with acute myocardial infarction: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109870. doi: 10.1016/j.pnpbp.2020.109870 [DOI] [PubMed] [Google Scholar]
- 98.Druss BG, Bradford D. W., Rosenheck R. A., Radford M. J., Krumholz H. M Mental disorders and use of cardiovascular procedures after myocardial infarction. Jama. 2000;283(4):506–11. doi: 10.1001/jama.283.4.506 [DOI] [PubMed] [Google Scholar]