Abstract
Since its development about 40 years ago (1981–2021), Morris water maze has turned into a very popular tool for assessing spatial learning and memory. Its many advantages have ensured its pertinence to date. These include its effectiveness in evaluating hippocampal-dependent learning and memory, exemption from motivational differences across diverse experimental manipulations, reliability in various cross-species studies, and adaptability to many experimental conditions with various test protocols. Nonetheless, throughout its establishment, several experimental and analysis loopholes have galvanized researchers to assess ways in which it could be improved and adapted to fill this gap. Therefore, in this review, we briefly summarize these developments since the early years of its establishment through to the most recent advancements in computerized analysis, offering more comprehensive analysis paradigms. In addition, we discuss the adaptability of the Morris water maze across different test versions and analysis paradigms, providing suggestions with regard to the best paradigms for particular experimental conditions. Hence, the proper selection of the experimental protocols, analysis paradigms, and consideration of the assay’s limitations should be carefully considered. Given that appropriate measures are taken, with various adaptations made, the Morris water maze will likely remain a relevant tool to assess the mechanisms of spatial learning and memory.
Keywords: hippocampal-dependent learning and memory, Morris water maze, spatial learning and memory
The Hippocampus and its Role in spatial Learning and memory
The hippocampus has inspired anatomists to study its intricate structure since the first dissections carried out ancient Egyptian that pioneered various neurosurgical techniques [1, 2]. Its physical appearance has been linked to many representations, such as silkworms, the horns of rams, or seahorses. Due to its structural resemblance to various entities, a Danish anatomist, Winslow, proposed the term ram’s horns for the hippocampus in 1732 [3]. During the Renaissance era, French anatomists Garengeot (1742) and Flurant (1752) were captivated by ancient myths, and thus named the curved-shape inner portion of the temporal lobe or the hippocampus the Ammon’s horn, or Cornu Ammonis (CA) in Latin [4]. The name Ammon’s horn or CA reflects the ancient Egyptian mythological god Ammon, whose crown resembles the horns of the ram [5, 6]. In addition, the physical resemblance of this structure to that of seahorse led Arantius (1587), an Italian surgeon and anatomist, to name the structure of the hippocampus, which means seahorse in Latin [2, 3, 7]. In modern history, the involvement of the hippocampus in memory was elucidated by Brenda Milner, a British-Canadian neuropsychologist, and two neurosurgeons, Wilder Penfield and William Scoville [8]. In 1953, Scoville performed bilateral medial temporal lobe resection on a patient, Henry Molaison, better known as HM, who suffered from major epileptic seizures since the age of 16. After the surgery, HM recovered from his seizures, but consequently suffered from a profound memory deficit despite retaining his general knowledge and intelligence [9]. In addition, data on the involvement of the hippocampus in memory is consistent with a clinical study on two other patients, referred to as FC and PB, who suffered from severe amnesia following unilateral medial temporal lobe resection for the treatment of epileptic seizures [8, 10].
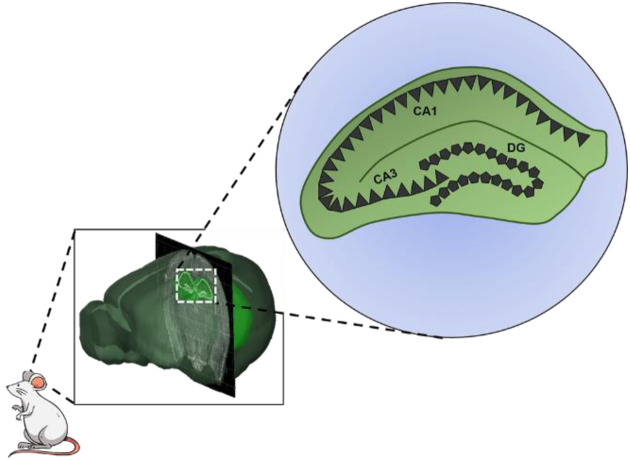
In 1971, the discovery of place cell theory by O’Keefe and Nadel, proposing that neurons in the hippocampus represent points in space of an environment − has represented a fundamental debate in understanding the neural basis of spatial memory [11,12,13,14,15]. According to the theory, place cells (complex spiking pyramidal cells that fire corresponding to the unique location of the animal) have been identified in hippocampal sub-regions, including the CA1 and CA3 (Fig. 1), where collectively they form a neural map corresponding to the environment, thereby pointing to the role of the hippocampus as the brain locus that represents the internal map of one’s spatial environment [11, 12, 16,17,18,19,20]. These exemplary studies have led to the development of biological and experimental inquiries seeking a better understanding of the role of the hippocampus in memory [21].
Fig. 1.
The anatomy of the rodent hippocampus. Place cells (complex spiking pyramidal cells) have been found in the CA3 and CA1 of the hippocampus. Image of the whole brain with the coronal sectioning of the hippocampal structure was created from an open access software (Allen Brain Atlas − Brain Explorer® 2). Pentagonal cells represent granular neurons of dentate gyrus, whereas triangular cells represent pyramidal neurons of CA3 and CA1.
Memory can be defined as the brain function to classify, encode, store and recover the acquired information [22,23,24,25]. Memory can be divided into two main categories which are short- (STM) and long-term memory (LTM) [26, 27]. STM, also known as working memory, is the information that is stored in a limited capacity and in a short period of time − within the range of seconds or minutes. In contrary, LTM can be stored in a larger amount with a longer or unlimited duration of time [28,29,30]. LTM is classified into two major types, which are non-declarative and declarative memory [24, 31]. Non-declarative memory, also known as procedural memory, is defined as the unconscious memory abilities which includes acquiring information about habits as well as motor and cognitive skills [21, 32]. Declarative memory is defined as the conscious recollection of verbal or non-verbal information or materials such as images, words, ideas and sensation [21]. In addition to that, declarative memory is formed on a sequential basis, encompassing acquiring new memory (acquisition), storing the memory (storage), and retrieving the memory (reference) [29]. Endel Tulving proposed further categorization of declarative memory, in which constituted by semantic and episodic components [33, 34]. Semantic memory is related to general knowledge about facts and environment, whereas episodic memory is encoded in a spatiotemporal manner that can be linked with specific experiences, as if one is able to “mentally-travel” back and re-experience the previous events [33, 35, 36]. It is suggested that the hippocampus may provide a platform for experience to be mapped and encoded therefore serve as a neural substrate for episodic memories [36].
Lesioning studies discovered that injury on dorsal structure of the hippocampus led to spatial performance deficits, whereas lesion on the ventral region did not result in those impairments, yet animals were observed exhibiting reduced anxiety-like behaviors, suggesting that the hippocampus manifests dissociative functions across its septotemporal axis [37,38,39,40]. In this context, while dorsal hippocampus is associated with spatial learning and memory, its ventral structure is pivotal for motivational and emotional processing, including depression and anxiety [41,42,43,44,45].
Since the discovery of the hippocampus in spatial learning and memory, various studies addressing this association have been conducted, including in humans [46,47,48,49], primates [50,51,52], and rodents [53, 54]. Given certain limitations in performing navigational studies on human and non-human primates, such as space consumption and insufficient up-to-date advance technology [55], the development of smaller scale experiments with feasible experimental manipulations, including the use of animal-based behavioral assays, is required in order to overcome these limitations. In animal studies, a wide range of experimental evaluations can be used to assess hippocampal-dependent place learning and memory, such as the radial arm maze (RAM), Barnes maze (BM), and Morris water maze (MWM) [18, 24, 29], contextual fear conditioning (CFC) and object location memory (OLM).
Experimental Tools for Assessing Hippocampal-Dependent Learning and Memory
There are various assays for the evaluation of spatial learning and memory. Those include the RAM, BM, MWM, CFC and OLM. Each of them possesses distinct experimental setups and designs in which may offer distinguishing measures for the hippocampal-dependent learning and memory assessment.
Radial arm maze
The radial arm maze (RAM) was developed by Olton and Samuelson in 1976. The RAM was the prevailing test for the evaluation of spatial learning and memory prior to the development of the BM and MWM [18, 56]. The RAM consists of an octagonal central chamber with eight equal-length arms extended from each side of the central chamber [57]. The base of the central chamber is made up of wood, while the wall is made of clear Perspex. The top and side arms are made of see-through clear plastic material to allow animals to identify distal visual cues surrounding the maze. The start of each arm harbors a clear plastic door that controls the animal’s access to the central chamber. Each end of the arm consisted of a cylindrical well in which the food pellet is placed [58]. In terms of the experimental procedure, the RAM consists of pre-training and training sessions to assess working memory [58]. For pre-training, animals are subjected to three sessions of habituation, whereby animals are allowed to freely explore the maze. Throughout habituation sessions, all arm doors are opened, allowing animals to freely explore all arms for 5 min, as the reward food pellets are randomly scattered down in the arms [58]. The standard RAM training protocol is conducted by [59], whereby the animals’ spatial working memory strategy in locating and retrieving the reward pellet from all eight arms, without re-entering the previously visited arm, is monitored [58]. At the start of the trial, reward pellets are placed in all eight arms. The animal is placed in the central chamber, with all arm doors closed. Once the doors are open, the animal chooses to visit a particular arm to retrieve the reward pellet. Once the animal returns to the central chamber, the doors are shut for 10 s. Subsequently, the animal is allowed to choose to visit other arms. The training procedure carries on until all eight arms have been visited, or 10 min have passed [58]. Animal re-entrance into a previously visited arm is denoted as a working memory error, reflecting dysfunctional spatial working memory [60]. Nonetheless, for the assessment of spatial working memory, the RAM assay consists of three phases: the pre-training, training, and retention phases [61]. For the pre-training session, the animal is only subject to a single habituation phase for 10 min [62]. Following the habituation phase, animals are subjected to two consecutive trials, or also known as acquisition trials (consisting of five minutes each trial) for eight consecutive days. During the acquisition trials, only four arms are baited with reward food; these arms are constantly baited throughout the acquisition and retention sessions. Following this, animals are subjected to a series of retention sessions with each session consisting of five minutes time allocation. Different retention sessions are performed at different time points (48, 72, 96, 120, and 144 h after the previous sessions) [62]. Animal entry into the non-baited arms is considered a reference memory error, whereas re-entry into the previously visited arms is considered a working memory error [62]. Nowadays, the number of arms designed in the RAM may vary (from as few as four to as many as 17 arms). The purpose of this modification is to render the task more difficult, such that the method can be improved, especially when assessing working or reference memory [18]. However, there remain several limitations in this maze, where animals may use a serial strategy rather than spatial cues in locating the target arm. In addition, the animal may use the trail of many proximal cues (such as olfactory cues) to identify the visited arms [18, 29, 63]. This makes the maze less sensitive to the measurement of spatial aspects of learning and memory, yet more suitable for decision-making evaluation. In addition, limitations of the previously established behavioral assays, reflecting the conceptualization of the burrow and trail mazes [63], the RAM may not be suitable (provided that if proximal cues such as olfactory cues could not be eliminated) for the study of spatial learning since animals may use a series of choice-point decisions rather than spatial localization to perform the navigational process.
Barnes maze
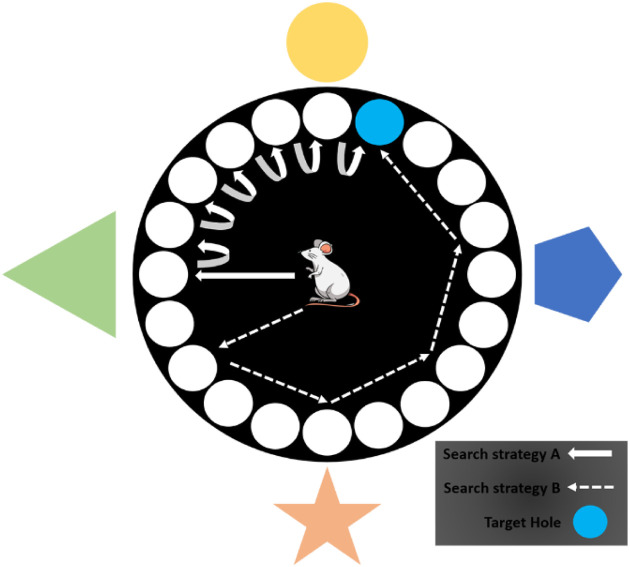
The Barnes maze (BM) is an example of a dry-based test used to assess spatial learning and memory in rodents. The BM was first introduced by Carol Barnes in 1979, a few years after the development of the RAM, to study spatial memory in rats [64, 65]. To date, it has been primarily applied in small rodents, such as mice [65,66,67] and rats [68,69,70]. The maze consists of an elevated circular PVC platform (122 cm diameter) supported by an aluminum support frame containing equally spaced holes. An escape tunnel is constructed underneath one of the holes (target hole), whereas the other holes are empty, without any escape tunnel constructed underneath [65]. Ample visual cues are also fixed in the test room as spatial guides for the animal to locate the target hole (Fig. 2). After sufficient training, the animals acquire the spatial location of the target hole and prefer the hole over the other holes. The dimensions of the equipment vary according to the species used, with smaller dimensions for mice than for rats. For mice, the PVC platform usually contains 40 equally sized holes (5 cm diameter), whereas for rats, 20 equally sized holes (10 cm diameter) are usually used [65, 71,72,73]. An overhead camera is also installed to track and record the animal’s navigational route for analytic purposes [74]. In terms of the protocol, the BM consists of three main phases: the habituation, training, and probe test phases [75]. Habituation is usually conducted 24 h prior to training. During habituation, animals are allowed to freely explore the maze within 60 s and then gently hand-guided to the target hole for two min [76]. In the training phase, animals are usually given two trials (lasting for one to two minutes) per day for five consecutive days. Subsequently, the animal is subjected to the probe trial (with the target hole closed) 24 h after the training phase. Usually, animals are allocated 90, 120, or 180 s throughout the probe trial [69, 72, 76, 77]. However, one potential drawback of the BM is that the lack of aversive stimuli in the maze may result in a slow learning process in animals [65]. This mild aversion may also contribute to less stressful conditions, which decreases the animal’s motivation to search for the escape hole. In addition to greater aversion to the animal, there are various physical motivators incorporated into the test, including shining bright light onto the platform [76, 78], sounding a buzzer to make the animal averse the loud sound [71, 79], and blowing air from above onto the maze [78]. However, the stress that these physical motivators may incur has not yet been assessed [18]. Another disadvantage of BM is the inconsistent spatial ability exhibited between rats and mice; there remains a certain degree of inconsistency between different mouse strains [80]. In comparison to rats, mice in the BM exhibit distinct behaviors, such as a certain degree of hesitation to enter the target hole; instead, they are more likely to explore other holes prior to escape. This behavioral variability may eventually affect the test parameters, including the escape latency, distance traveled, and frequency of hole entrance error [80]. In addition to inter-species variability, inconsistent BM findings associated with different mouse strains include superior spatial learning ability in the C57BL strain compared to 129/SV, BALB/c, and Swiss mouse strains [81]. In another study, C57BL mice exhibited finer spatial learning than CD1 mice [82]. Different spatial learning strategies manifested by different mouse strains may underlie these BM variations, which may highlight the fact that BM only assesses limited aspects of spatial learning.
Fig. 2.
The Barnes Maze. Animal is placed at the centre of the maze platform. Ample visual cues are placed in the test room as spatial guides for navigational purposes. Animal may employ various search strategies to locate the target hole, as illustrated in the figure.
Morris water maze
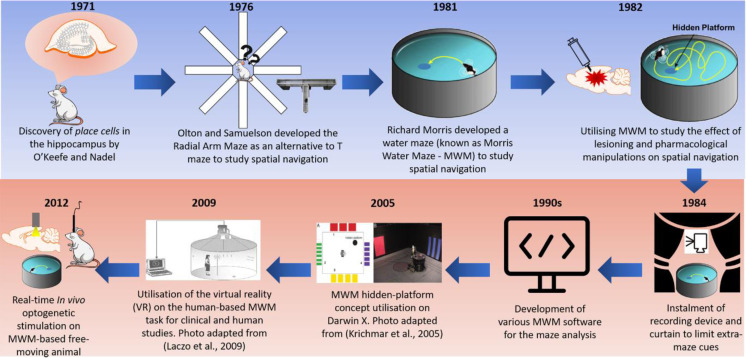
Due to the aforementioned limitations, a novel water-based maze known as the MWM was developed in 1981 by a British neuroscientist, Richard G. M. Morris. Before the development of the water maze, Morris performed various behavioral studies primarily on learning and memory, including assessing the mechanisms of Pavlovian fear-avoidance conditioning in rats [83,84,85], the effect of septum lesioning on fear conditioning [86], and the impact of satiation on non-reward behaviors [87, 88]. Since its development about 40 years ago (Fig. 3), the MWM has become a very popular tool in assessing learning and memory; more than 5,000 publications related to the MWM have been published in PubMed from 1981 to 2013 [18]. More recently, in PubMed, the number exceeded 11,000 publications by 2020, highlighting its wide usage. This may be explained by the numerous advantages of the maze, including its effectiveness in evaluating hippocampal-dependent learning and memory; its exemption to any motivational differences showed by subjects across a wide range of experimental manipulations (lesioning, pharmacological, and genetic studies), which are secondary to the main objective of the task, and its reliability in various cross-species studies including guinea pigs, rats, and mice [49, 89,90,91,92].
Fig. 3.
The discovery of the hippocampal place cells led to the development of Radial Arm Maze (RAM) and Morris Water Maze (MWM). Since then, MWM has been undergoing varieties of improvements and adaptations, including in the computational neuroscience, human and animal studies.
However, MWM was initially developed to study spatial learning in rats [63, 93]. On the other hand, unlike rats, although mice showed different behavioral responses to water maze due to their heterogenous innate characteristics (including swimming behaviors and strategies), many researchers have reported successful and reproducible findings when using mice, despite there are also researches reporting performance inconsistency between rats and mice [93,94,95,96]. There was no study specifically comparing the place learning performance between rats and mice until 1995 such performed by Wishaw. In his study, Wishaw discovered that compared to rats, mice swam well and were able to perform the task, even though their performance was not characterized by proper swimming behavior [97]. The application of rat behavioral assays to mice is due to the early presumption that mice are miniature rats [97]. Innately, rats are semi-aquatic animals that are adaptable to living near water, including in the sewage and drainage system. In contrast, mice are not well adapted to such a habitat, yet prefer inhabiting a drier environment [97, 98]. In terms of swimming phenotypes, rats are good at swimming under obstacles, diving, and staying afloat [99], whereas for mice, despite performing well, they swim vigorously even though sometimes stop and passively stay afloat or even exhibit difficulty to remain afloat [97, 100]. The difference between rats’ and mice’ innate preference for a specific ecological environment may contribute to their performance discrepancies in water-based behavioral assays [97]. For instance, mice exhibit a distinct innate response to water including high anxiety, physical exhaustion, and predisposition to hypothermia [96]. Therefore, to combat this issue, a mouse-specific cognitive assay has been developed known as the Paddling Pool Task (PPT), which is a modification and adaptation from the MWM and BM [96, 100, 101]. With the available modified and robust cognitive assay, the utilization of mice will remain useful due to their experimental advantages, which include availability of diverse genetically modified mouse strains that are useful for investigating neurodevelopmental and neurodegenerative impairments, including learning and memory [93, 95, 102,103,104,105].
Contextual fear conditioning
CFC is an example of associative learning involving an adaptive process which allows animals to learn to anticipate events. In CFC, the dependent measures used is freezing response that takes place following pairing of an unconditioned stimulus (US) such as foot shock or air puff with a conditioned stimulus (CS) such as a particular context (tone) or cue (light). For CFC, animals are allowed to explore the training environment context (context A) for two minutes. After that, animals either receive single or multiple foot shocks prior returning to their respective home cage. Subsequently, animals are subjected to context test, where animals are placed in the similar context A, for five minutes and their freezing times are recorded [106, 107]. Many studies indicate association between the hippocampus and contextual learning are occurred here [108]. There are studies demonstrating that hippocampal lesions result in reduction of freezing behavior during CFC tests [109,110,111]. Nonetheless other studies have reported that lesions on the hippocampus do not disrupt CFC when lesions are made before the task [112, 113]. Plus, a genetic study manipulating CCAAT/enhancer binding protein (C/EBP) gene, a genetic material involved in learning and memory, indicates inconsistent findings between contextual and spatial learning tests. From the study, it is suggested that cellular and molecular events in hippocampal lesions mediating contextual and spatial learning deficits can be further dissociated [114]. Hippocampally-lesioned animals may still be able to learn about the context in the test, suggesting that neocortex, without hippocampal involvement, may mediate this contextual learning [113]. It is suggested that hippocampal lesion may only mediate impairment in acquisition and storage of the contextual representation rather than deficit in the context-stimulus association (contextual conditioning) [108]. One prevalent advantage of the CFC test is its reliability to be conducted on various rat and mouse strains, even with the exhibition of profound motor dysfunctions which significantly confound analysis in other behavioral assays.
Object location memory
Object location memory (OLM) task is also known for its sensitivity to evaluate hippocampal-dependent learning [105]. The experimental apparatus is a white rectangular open field arena with dimension of 30 cm length × 23 cm width × 21.5 cm height for mice [115], or 100 cm length × 100 cm width × 60 cm height for rats [116]. Throughout the test, animals’ behaviors are video recorded. For OLM, the test consists of habituation, training, and retention sessions. Habituation can be conducted for 3–6 consecutive days (3–5 min/day). During the habituation, animals are allowed to freely explore the maze with no object placed in the arena. Subsequently, during the training period, animals are placed in the arena with two similar objects known as familiar object (F). Animals are allowed to explore the objects for 10 min. Following this, animals are subjected to retention test. For the evaluation of STM, retention test is conducted 90 min following training, whereas of LTM, test is conducted 24 h post-training. During retention test, one object is placed at the same location as during the training session, while another similar object is placed at the novel location (somewhere in the middle of the arena). This experimental approach is oppositely different with novel object recognition test (NORT), which is also a widely used behavioral assay for the assessment of LTM formation. While in the OLM test where one object is moved at a novel location without changing its physical novelty, however in the NORT, similar object is replaced with a novel object without manipulating its original location [105]. As for OLM test, animals are allowed to explore the objects within five minutes. This is due to animals’ innate preference for novel object [105, 115, 117, 118]. The OLM test is a cost-effective method for the assessment of LTM mechanisms in rodents. Additionally, its simple protocol only requires two days behavioral testing which does not need extensive and intricate experiment equipment and analyses [119]. However, one concern with OLM task is that motor dysfunctions (due to treatment or surgery manipulations) may profoundly affect animal behaviors during the habituation session. Apart from that, the exhibition of anxiety in normal animals may confound especially LTM discrimination index (which is derived from time difference between exploring novel location and similar location) [105].
Early Years of the Classical MWM: 1980s
In Morris’s early experiments using the novel water maze, he used male rats of the Lister strain; the animals underwent 15 trials for three consecutive days to locate a hidden platform. In one of his experiments, he performed six trials on the first and second days, and another three trials on the third day. To render the platform invisible, the water was made opaque by mixing the water with fresh milk, and the platform was painted silver. From the experiment, he discovered that the rats can rapidly learn to locate a spatially fixed, unseen, and odorless object without the trail of proximal or olfactory cues [63]. Following the task development, Morris et al. conducted several brain lesion studies to investigate their effects on spatial learning and memory using the newly established water maze. In one of the studies, hippocampal lesioning (removal of the dorsal and ventral hippocampi by aspiration) in rats was performed, and the findings revealed a profound and lasting place-navigational impairment in the hippocampally lesioned animals compared to the controls [120]. From this finding, Morris emphasized that the data appeared to support O’Keefe and Nadel’s theory of spatial mapping in the hippocampus [121].
The lesioning study on spatial navigation was further extended by Hagan, Morris and colleagues, whereby the effect of brain catecholamine depletion (disruption of the meso-striatal and meso-limbic dopaminergic innervations) was assessed in the context of the water maze task, since, at that time, there were few studies investigating the effect of brain catecholamine disruption on spatial memory [122]. Therefore, extending the use of the MWM to study the role of brain catecholamines in spatial learning seems promising and reliable. They discovered that the disruption of neo-striatal dopaminergic innervation caused a certain degree of behavioral performance deficit, while damage to the meso-limbic innervations failed to exhibit any performance impairment. However, the performance deficit was not likely to result from impairment in spatial learning, but may instead be due to abnormal swimming paths such as rotation to the left or right and atypical search strategies in the impaired animals. Nonetheless, in their study, they pointed out an important methodological consideration that they did not assess the possible role of central catecholamines in egocentric spatial navigation.
Later in 1984, Morris further improved the MWM procedure by recording and tracking the animals’ swimming path to study the accurate path taken by the rats. In addition, the tracking system was devised with a specific image analyzer to transform the conventional video recording into high-contrast images for edge distinction. Even though Morris emphasized that the main purpose of this methodological improvement was to improvise the labor time for the training procedure [13], from the authors’ viewpoint, the recording device would manage to track the animal’s swimming strategies, which may be useful to address the previously reported consideration, as reflected in the possible presence of an egocentric navigation paradigm in the animal. In addition, this paradigm, which can be referred to as the ability to navigate by internal self-movement cues, can be experimentally assessed by the subject’s route-based integration, which relies on the internal cues on the rate of turns and movements [18]. The latter can only be analyzed with the recording-assisting device and is not possible (or is not reliable) to be performed by manual observation. Furthermore, using the recording device setup, later studies performed computer-based analyses of the maze [74, 75]. In addition to the tracking device, Morris controlled the presence of external cues by setting up curtains around the pool [13, 18].
Schenk and Morris later conducted a series of experiments by lesioning the rat retrohippocampal region, including the lateral and mEC subicular and para-subicular areas using a radiofrequency current. In one of these experiments, they performed pre- and post-operative water maze training on the retrohippocampal-lesioned rats and discovered that rats given preoperative training underwent partial behavioral recovery (in terms of spatial memory); however, the exhibition of residual impairment was still observed in the spatial discrimination task [121]. They also suggested that, from entorhinal cortex lesioning, the declarative aspect of spatial memory could have been impaired; nevertheless, the procedural or habitual aspect could remain intact, eventually contributing to behavioral recovery. Furthermore, they pointed out that, in the water maze, there are four release points and no pathway to the target platform is constrained. In contrast, the simple-plus maze provides a binary choice-point (animals only face a choice between turning left or right) [121], which may highlight a more robust measure of spatial discriminative learning in the MWM by minimizing the possibility of the animals discriminating navigation by chance. Morris and colleagues also extended the use of this maze to the role of the forebrain cholinergic neurons in spatial learning and memory in rats [123]. In this study, the cholinergic neurons were lesioned by depleting choline acetyltransferase (ChAT) using ibotenic acid infusion into the medial septum and diagonal band; they later discovered that cholinergic lesioning in these structures resulted in the impairment of spatial navigation in the water maze task.
Morris et al. conducted a series of pharmacological studies. One of the studies included infusing aminophosphonovaleric acid (AP5), a competitive full NMDA receptor antagonist, into the rat brain to disrupt hippocampal function, revealing a significant place learning impairment in the AP5-infused animals compared to controls, similar to that observed in hippocampally lesioned rats [124]. However, AP5 can induce several sensorimotor disturbances, such as mild ataxia, suprasegmental reflex disruption, and muscle relaxation. Whether these effects could be correlated to the impairment of spatial learning in rats, specifically in the MWM task, remained unknown. In light of this question, Morris later studied and discovered that the AP5-induced sensorimotor disturbances are independent of spatial learning impairment, and concurrently found that MWM pretraining can reduce AP5-induced sensorimotor dysfunction [125]. Morris et al. also performed another pharmacological study on the effect of intraventricular infusion of leupeptin, a calpain inhibitor, on MWM spatial memory [126]. This investigation was performed as a response to a previously reported study in which leupeptin was found to impair the spatial radial maze task [127]. Using MWM, leupeptin was found to induce partial deficits in spatial learning, although the effect was not as profound as that of AP5 [126].
1990s: Advances in MWM Analyses
The MWM has several instrumental characteristics that contribute to its prevalent use, including the lack of required pre-training, high reliability across a wide range of tank configuration and experimental procedures, its applicability to a wide range of species (rats, mice, guinea pigs, and humans), and its specificity for the assessment of hippocampal-dependent spatial and place learning and memory [90]. However, the methodological gap reflecting the imprecise measures of an animal’s performance in the MWM has always been neglected by most researchers [128]. In addition to this, some previous studies also documented less specific criteria linked to well-established and traditional parameters, such as the animal’s path length and escape latency, in measuring the animal’s task performance [93, 129]. In light of this, it is important to identify alternative measures in this maze for assessing the spatial aspect of learning and memory. Therefore, several authors have pointed to other measures, such as swim speed, path directionality, turning preferences, and cumulative distance to the goal, as alternatives to the traditionally established parameters [128,129,130]. However, the manual observation and qualitative analyses of these alternatives may be unreliable and time consuming. Previous investigations addressing these limitations where semi-automated tests using micro-computers were carried out to identify the animal’s turning preference in the context of spatial navigation [131, 132]; however, this method (and other available software at that time) could not offer a more advanced analysis for the detailed features [133]. In an attempt to overcome these limitations, Wolfer and Lipp developed a disk operating system-based (DOS-based) program (TRACK-ANALYZER) for in-depth offline analysis of the MWM, including tortuosity, turning, and directionality preferences of swimming behaviors [133].
2000s: Progress in the Digitalized Version of the MWM
Subsequently, in parallel to the development of plugins and digital software for numerical and graphical analyses, especially for the MWM, Wolfer and colleagues further extended the power and flexibility of the previously developed TRACK-ANALYZER software by designing Wintrack (a more powerful Windows path analysis software, equipped with a drag-and-drop interface option, which can process a variety of data acquired from various tracking systems) [134]. Despite a more powerful and detailed analysis, the evaluation did not distinctly categorize swimming behaviors. Graziano and his team later applied other statistical analyses, known as discriminant analysis (DA), to differentiate distinct swimming behaviors (thigmotaxis, circling, random searching, scanning, self-orienting, approaching, and direct finding) [128]. Considerations of the methodological gap and attempts to overcome those limitations have led to the development of more detailed and advanced computerized analyses, such as those performed in several recent studies on the swimming behavior of animals in the MWM [135,136,137]. Recently, these advances analyses have been extended even further and made available as free open-source software (Pathfinder) accessible by researchers [138]. There have also been several studies using this open-source software for analyzing swimming behavior, such as studying the effect of neurogenesis on spatial alternation [139] and investigating the effect of different animal strains reflecting various genetic models of autism spectrum disorder (ASD) on spatial learning [140].
Different Protocols and Analysis Methods for the Morris Water Maze
One of the main reasons for which MWM has gained high popularity among researchers is its adaptability to many experimental conditions with various test protocols. We will begin with the most basic procedure of the MWM to most clearly highlight and explain its ability to be adapted to different protocols and analytical schemes. Spatial acquisition is the most basic procedure in the MWM [90]. In this procedure, the animal is released from different starting points, and must use distal cues to navigate a direct path to a spatially fixed hidden platform. Animals are commonly given four trials per day with varying numbers of training days in order to train them to locate the platform [18, 90, 141,142,143]. Following these training sessions, animals are subjected to a probe trial, which is usually conducted 24 h after the final acquisition trial to assess memory retrieval. During the probe trial, the platform is removed, and the experimenter may video-record the swimming behaviors and further analyze them using specialized tracking software. There are many different forms of tracking software available for analyses, including San Diego Instruments, Clever Systems, Noldus, Columbus Instruments, Anymaze, Coulbourn Instruments, and Panlab SMART, offering a wide range of analytic criteria.
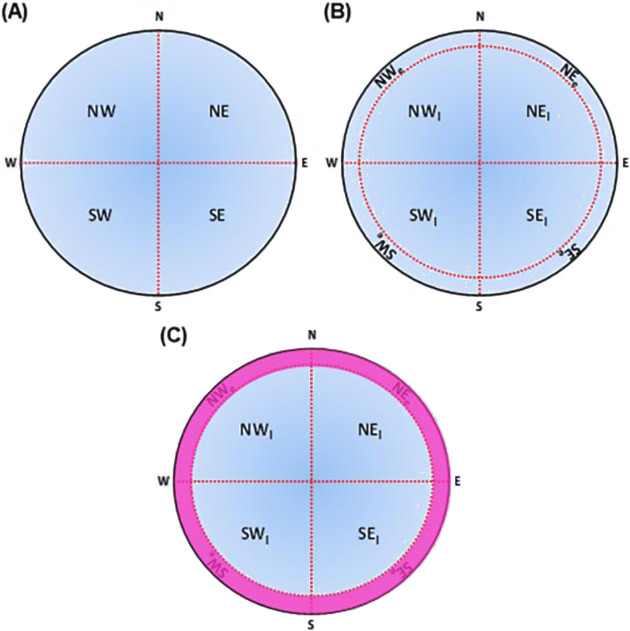
Tracking software allows the experimenter to divide and calibrate the pool arena into multiple zones which can be used to illustrate the quadrants. The pool is evenly divided into four quadrants, each comprising 25% of the total pool area (Fig. 4A) [144, 145]. However, this four-quadrant definition may not be suitable for animal models mimicking brain injury (pharmacological or lesion) that exhibit prolonged thigmotaxis, even after multiple trainings [146,147,148]. Thigmotaxis reflects the animal’s tendency to move along the edge of its environment, or, in the context of the MWM, the animal’s preference to swim along the pool perimeter. Animals that exhibit this behavior may spend most of their time at the pool periphery, including in the external area of the target quadrant. As a result, this may contribute to the overestimation of time spent in the target quadrant, even though the animal shows no sign of searching the platform. Therefore, further dividing the quadrants into several sub-quadrants may reduce data bias (Fig. 4B). In addition, the experimenter may quantitatively assess the animal thigmotactic behaviors that can be measured by path length or time allocation in the peripheral ring (Fig. 4C). Experimentally, thigmotaxis can reflect anxiety and stress in animals, where anxious rodents tend to spend more time along the pool wall [149, 150]. There are many different setups to define the area of the sub-quadrant zones; in [151], the peripheral ring area can be set to 29% of the total pool area, and the remaining 71% is evenly divided into four quadrants, each comprising 17.75% of the total pool area (Fig. 4). Apart from that, Murayama and colleagues [152, 153] virtually dissociated the pool periphery area from the central zone by 36%, leaving 64% area covering the central region. The dissociation of the periphery from the central area provides a reliable tool for the evaluation of thigmotactic behaviors in the MWM. Plus, the utilization of freezing time parameter was used in the study to determine the animals’ depressive-like behavior throughout the water maze test.
Fig. 4.
(A) Four equally sized quadrants in the MWM pool. (B) The quadrants further divided with peripheral ring (each quadrant comprises internal and external area, giving rise to eight sub-quadrants in total). (C) Peripheral ring shaded in pink for thigmotaxis evaluation. N=North; E=East; S=South; W=West.
After calibrating the arena, experimenters can select various criteria for memory evaluation during the training and probe trials. For training, the time latency to locate the hidden platform is one of the most widely used parameters for qualitative evaluation [144, 154]. As training progresses, the time latency to locate the platform decreases, reflecting functional spatial learning and memory throughout the task. However, at the start of the training, animals typically exhibit thigmotaxis [18, 135, 136]. Nonetheless, in subsequent training sessions, they gradually suppress their thigmotactic behaviors in order to actively search for the hidden platform, which can be defined as the escape latency [155, 156]. In addition, swimming distance to the hidden platform can also be used as an applicable measure to evaluate spatial learning, in which decreased swimming distance reflects increased spatial learning and memory [157, 158]. Nonetheless, this parameter remains limited because, in certain navigational strategies, animals may use shorter distances despite their lack of knowledge of where the platform is located [159]. However, there have been attempts to use this parameter to interpret animal swimming strategies across a number of different MWM trials [128, 135]. These strategies can be categorized into three main types: spatial, non-spatial, and thigmotaxis. The spatial and non-spatial types can be further subdivided into several subdivisions, which for the spatial condition is direct finding and self-orienting, whereas for the non-spatial condition is circling, random searching, and scanning [128, 160]. However, this technique remains controversial, especially when used only in the context of a qualitative approach, without any proper quantitative mathematical analyses.
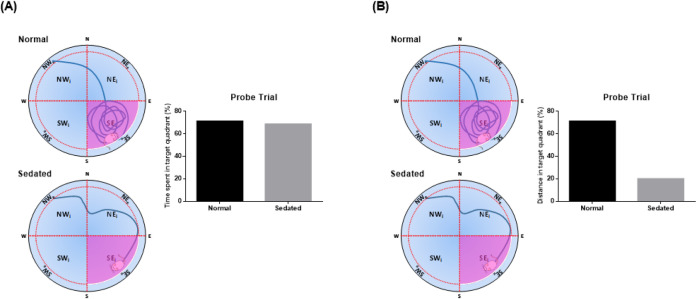
With regard to probe trials, the experimenter can choose several analysis criteria to assess spatial retrieval memory. The criteria include: (i) time spent in the target quadrant in comparison to other quadrants, (ii) distance or path length in the target quadrant, (iii) time latency of first entry to the target quadrant, and (iv) number of platform-site crossovers [90]. For the probe trial, the most frequent parameter used is time spent or distance in the target quadrant compared to the other quadrants [90, 144, 154, 161, 162]. A longer time spent in the target quadrant reflects a superior retrieval memory [162]. However, this measure may not be practical for animals having received sedative drugs shortly before the probe trial. In previous studies, diazepam, which exerts sedative effects, has been shown to increase rat immobility in the forced swimming test [163,164,165]. As for the MWM, sedated animals may, by chance, stay immobile in the target quadrant, resulting in exaggerated time spent in the target quadrant, even though the animal shows neither sign of preferring the quadrant over other quadrants nor searching for the former platform (Fig. 5A). As such, using distance in the target quadrant as the criterion, as performed by [166], who administered diazepam before the MWM trial, may represent a more plausible option to overcome this false positive result (Fig. 5B). Additionally, this parameter is also used in other MWM studies [95, 156, 167, 168], highlighting its relevance for probe trial analysis.
Fig. 5.
Illustrated animal swimming paths and predicted results for normal versus sedated animals during the 60 s-probe trial with different analysis parameters: (A) time spent; (B) distance in the target quadrant. Target quadrant shaded in pink.
In addition, the time latency of the first entry to the target quadrant can also be used in probe trials [169,170,171]. Timić and colleagues studied the effect of midazolam administered immediately before the training sessions or probe trial on different stages of memory in rats [169]. Therefore, to avoid data bias and misanalysis, researchers need to consider appropriate analysis parameters that are suitable for the treatment(s) given to the animals. To date, no study has yet assessed the effect of sedative drugs on animal swimming behavior and immobility in the MWM. However, certain MWM studies have investigated the effect of memory extinction, between adult and aged rats, on animal despair behavior, as reflected by immobility time. Based on these studies, adult rats showed less immobility time compared to aged rats throughout the memory extinction trial, indicating less behavioral despair in adult rats [172, 173]. Using the same parameter, [174] performed a study on behavioral despair between inferior, medial, and superior learner rats during the probe trial. Here, inferior-learners spent more time remained immobile compared to medial and superior learners. Therefore, it may be possible to extend this parameter to study the effect of sedative drugs on animal swimming behavior in the MWM. In addition, the number of platform site crossovers may also be used in the probe trial, whereby more crossovers reflect prominent retrieval memory [175,176,177,178,179]. However, there remain certain limitations linked to using this parameter, potentially leading to crossover undercounting. In addition, crossovers can also be lower than the actual amounts, especially when a large pool diameter is used, or the platform-site size is reduced [90]. Given the limitations, the time spent and distance in the target quadrant and the distance to the target site provide more robust measures than the number of platform crossovers.
Not only adapting to diverse protocols and methods, according to the recent findings utilizing correlation analysis between multiple MWM parameters, Murayama and colleagues have identified association between spatial learning function with neuropsychiatric symptoms. In a combination of cognition and neural cell transplantation approaches, they have recently demonstrated that neural cell transplantation in the hippocampus of the genetically manipulated mouse model of Alzheimer’s disease (AD), [PDGF promoter-driven amyloid precursor protein (PDAPP) transgenic (Tg) mice], improved both spatial learning deficits and neuropsychiatric symptoms such exhibited in the AD, including motivation, anxiety-like behavior, and depression [152]. Furthermore, another work from the same research team, using the same correlation analysis and transplantation method, they discovered that young female PDAPP Tg mice exhibited more severe spatial learning dysfunction accompanied with more prominent neuropsychiatric abnormalities as compared to their young male counterparts. From the findings, they suggest the predisposition of sexual dominance in the AD to heterogenous spatial learning deficits and neuropsychiatric abnormalities, which may be valuable for the AD studies [153]. These two latest works have corroborated that by utilizing multiple MWM analysis parameters simultaneously, those findings can be re-interpretated and extended into correlative studies, linking cognitive processing, or at least spatial learning, with neuropsychiatric symptoms, which may serve as a valuable tool in investigating potential non-cognitive behavioral elements (e.g., abnormal neuropsychiatry) contributing to cognitive impairment.
Limitations and Disadvantages
As with other behavioral assays, such as the RAM and BM, the MWM also has certain experimental limitations which may potentially confound the experiment analysis. One prevalent disadvantage of the MWM is its undulating stress on animals due to the maze aversive condition [18, 93, 180]. The MWM has been shown to increase corticosterone levels, a stress-related hormone, in the rat brain and blood plasma [181, 182]. However, performance due to stress is always reflected as an inverted U-shaped function, whereby either too little or excessive stress can be counterproductive for learning performance [18]. Therefore, the optimal number of trials per day or the number of training days should be considered to avoid excessive or unwanted stress.
Treatments that induce hypoactivity can be dissociated from learning deficits in the MWM [90]. Deficits of learning performance in the MWM are independent of locomotor effects because land-based locomotor reductions did not affect swimming speed [183]. Besides that, the absence of swimming speed differences (a parameter indicating motor abnormality) may verify that abnormal learning performance is exclusively due to spatial impairment, not due to any swimming or movement abnormalities [184].
Besides that, animals’ visual acuity may also confound the MWM analysis. Animals that can directly locate the hidden platform are considered having intact visual function, whereby contributes to their good spatial learning ability [90]. In order to assess and control this non-cognitive factor that potentially affects the spatial performance, the development of cued task are required in the water maze [120, 185]. This test is important so that researchers can identify whether test underperformance is totally due to spatially impaired function or instead due to animals’ non-cognitive impairment such as their inability to visualise the cues. There are evidence suggesting that reduced visual acuity due to eye enucleation in Sprague-Dawley rats and binocularly-deprived Long-Evans rats impaired place learning in the MWM task [185, 186]. Besides that, photoreceptor degeneration in aged rats are also contributable to the MWM spatial underperformance [187, 188]. Hence, to control this non-cognitive factor, respective preliminary or validation cued test can be conducted either before [152, 153] or after spatial assessment [90, 95]. However there is suggestion that conducting cued test before spatial assessment is beneficial for mice, whereas vice versa for rats [18].
Another limitation that may confound the analysis is response variability to water across species. Rats have been suggested to exhibit superior swimming abilities compared to mice [189]. For instance, the C57BL/6J strain performed poorly compared to Long-Evans rats in the swimming-based place learning task as well as in the MWM [97, 190]. Also, the MWM is not conducive to certain mouse strains [90, 189]. In addition, BALB and C3H strains have been shown to perform well in the MWM as compared to C57BL and DBA strains [191]. Therefore, in order to minimize data inaccuracy when using mice, [82] encouraged the use of at least one land-based maze in addition to the MWM. Besides response variation to water, prolonged exposure to water can lead to hypothermia, which can eventually impair spatial learning in rats and mice [192, 193]. Hypothermia can occur due to extensive training schedules, such as in the context of back-to-back trials [18, 182]. Thus, longer intervals between trials, such as those commonly conducted in the MWM task, may address this concern. In addition, maintaining the temperature of the water, such as at ± 25°C, is necessary to prevent hypothermia.
Apart from that, variation in swimming strategies may confound the experiment analysis. For an example, the use of distance paradigm to evaluate the animals’ ability to locate the hidden platform also has its own limitation. This is because in certain navigational strategies, animals may exploit shorter distances despite their lack of knowledge where the platform is located [159]. For an instance, animal can learn about the location of hidden platform that is placed at certain distance from the wall without exploiting visual cues provided. In this context, animal may first swim directly into the pool wall and subsequently along the pool wall (thigmotaxis) in order to efficiently locate the platform that is placed somewhere proximal to the wall [129, 159]. Using this non-spatial strategy, animal may still be able to reach the platform within the allocation time. In addition to that, animals may though exhibit identical path length despite exhibiting dissimilar path trajectory patterns, where comparably, one animal clearly exhibits better knowledge about the platform location compared to another animal. To overcome this issue, several additional indices on the path trajectory including cumulative search error and proximity measure can be respectively used in training and probe trial [129, 159]. Besides, categorizing animals’ swimming strategies into spatial, non-spatial and thigmotaxis, such discussed in the aforementioned subtopic, can further address this confounding factor.
As for MWM flexibility in assessing working memory, where the platform is placed in new positions every day [13], this is not as sensitive as other mazes and has not been widely used as such [18]. Instead, a six- or eight-armed radial arm water maze (RAWM), first conducted by [194], which is a modification and combination of RAM and MWM, has been proven to provide a more robust measure to assess working memory such as in traumatic brain injury or an Alzheimer’s animal model [195,196,197]. However, in comparison to the MWM, it remains more challenging to track the animal’s swimming trajectory in the RAWM because the animal movement is confined to the maze alley, which may be disadvantageous in the context of this modified version. Table 1 summarizes the advantages and disadvantages of RAM, BM, and MWM maze.
Table 1. Advantages and disadvantages of the radial arm maze (RAM), Barnes maze (BM), and Morris water maze (MWM).
| Behavioral assays | Advantages | Disadvantages |
|---|---|---|
| Radial arm maze | • Suitable for spatial working memory assessment. • Less laborious. |
• Possible exploitation of serial strategy. • Selection of choice-point decision rather than spatial strategy due to olfactory cue trails. |
| Barnes maze | • Suitable for spatial learning and memory assessment. • Ability to track individual trajectory of different exploration patterns. |
• Lack of aversive stimuli may reduce animal’s motivation to escape. • Inconsistent escape behaviors between rats and mice. |
| Morris water maze | • Reliable in various cross-species studies including guinea pigs, rats, and mice. • Ability to track various swimming strategies (spatial, non-spatial, and thigmotaxis). • Able to tract non-cognitive factor based on cued task. |
• Possibility of hypothermia and excessive stress due to the extensive and uncontrolled trials. • Not sensitive for spatial working memory assessment. |
| Contextual fear conditioning test | • Reliable for various rat and mouse strains. • Less affectability to motor dysfunction. • Suitable for evaluating associative learning which involves multiple brain regions (hippocampus, amygdala, and neocortex). |
• Assessment on contextual learning does not necessarily represent spatial learning. • Not sensitive for hippocampal-dependent learning. |
| Object location memory test | • Cost-effective. • Simple protocols without integration with intricate experiment instruments and extensive analysis parameters. |
• Motor dysfunction due to treatment or surgical manipulations may confound the test habituation. • Exhibition of anxiety in normal animals can affect the animals’ learning. |
Conclusion
To this date, diverse studies in the behavioral neurosciences have contributed to our understanding of the crucial role of the hippocampus in spatial learning and memory, as well as the underlying mechanisms. In animal studies, MWM has become one of the most frequently used assays to assess hippocampal-dependent learning and memory. This assay possesses diverse test protocols and analysis parameters that are applicable to a wide range of experimental conditions. However, appropriate protocols and parameters should be carefully selected, in addition to considering the limitations of the assay. For example, MWM can be excessively stressful for the animals, yet several measures can be taken to obviate this concern, such as providing an ideal test duration and performing gentle handling on the animals throughout the protocol. Given that proper measures can be taken, MWM can remain a crucial methodology in recapitulating the mechanisms of spatial learning and memory.
Acknowledgments
This review work was funded by the Fundamental Research Grant Scheme (FRGS) 203/PPSP/6171233, sponsored by Ministry of Higher Education, Malaysia.
References
- 1.Fanous AA, Couldwell WT. Transnasal excerebration surgery in ancient Egypt. J Neurosurg. 2012; 116: 743–748. doi: 10.3171/2011.12.JNS11417 [DOI] [PubMed] [Google Scholar]
- 2.Spiers HJ. Hippocampal Formation. 2nd edn, Encyclopedia of Human Behavior: Second Edition. 2nd edn. Elsevier Inc. 2012. [Google Scholar]
- 3.Bir SC, Ambekar S, Kukreja S, Nanda A. Julius Caesar Arantius (Giulio Cesare Aranzi, 1530-1589) and the hippocampus of the human brain: history behind the discovery. J Neurosurg. 2015; 122: 971–975. doi: 10.3171/2014.11.JNS132402 [DOI] [PubMed] [Google Scholar]
- 4.Iniesta I. On the origin of Ammon’s horn. Neurologia. 2014; 29: 490–496(English Edition). doi: 10.1016/j.nrl.2012.03.015 [DOI] [PubMed] [Google Scholar]
- 5.Pearce JMS. Ammon’s horn and the hippocampus. J Neurol Neurosurg Psychiatry. 2001; 71: 351. doi: 10.1136/jnnp.71.3.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duvernoy HM. The human hippocampus: an atlas of applied anatomy. JF Bergmann-Verlag. 2013. [Google Scholar]
- 7.Judaš M, Pletikos M. A note on the sea-horse in the human brain. Transl Neurosci. 2010; 1: 335–337. doi: 10.2478/v10134-010-0041-8 [DOI] [Google Scholar]
- 8.Squire LR. The legacy of patient H.M. for neuroscience. Neuron. 2009; 61: 6–9. doi: 10.1016/j.neuron.2008.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957; 20: 11–21. doi: 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch Neurol Psychiatry. 1958; 79: 475–497. doi: 10.1001/archneurpsyc.1958.02340050003001 [DOI] [PubMed] [Google Scholar]
- 11.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971; 34: 171–175. doi: 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- 12.O’keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press. 1978. [Google Scholar]
- 13.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11: 47–60. doi: 10.1016/0165-0270(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 14.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989; 48: 29–69. doi: 10.3109/00207458909002151 [DOI] [PubMed] [Google Scholar]
- 15.Moser EI, Moser MB, McNaughton BL. Spatial representation in the hippocampal formation: a history. Nat Neurosci. 2017; 20: 1448–1464. doi: 10.1038/nn.4653 [DOI] [PubMed] [Google Scholar]
- 16.O’Keefe J. A review of the hippocampal place cells. Prog Neurobiol. 1979; 13: 419–439. doi: 10.1016/0301-0082(79)90005-4 [DOI] [PubMed] [Google Scholar]
- 17.Bush D, Barry C, Burgess N. What do grid cells contribute to place cell firing? Trends Neurosci. 2014; 37: 136–145. doi: 10.1016/j.tins.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vorhees CV, Williams MT. Assessing spatial learning and memory in rodents. ILAR J. 2014; 55: 310–332. doi: 10.1093/ilar/ilu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moser MB, Rowland DC, Moser EI. Place cells, grid cells, and memory. Cold Spring Harb Perspect Biol. 2015; 7: a021808. doi: 10.1101/cshperspect.a021808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan HM, Wills TJ, Cacucci F. The development of spatial and memory circuits in the rat. Wiley Interdiscip Rev Cogn Sci. 2017; 8: e1424. doi: 10.1002/wcs.1424 [DOI] [PubMed] [Google Scholar]
- 21.Squire LR, Dede AJ. Conscious and unconscious memory systems. Cold Spring Harb Perspect Biol. 2015; 7: a021667. doi: 10.1101/cshperspect.a021667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandel ER, Schwartz JH, Jessell TM, Siegelbaum S, Hudspeth AJ, Mack S., editors. Principles of neural science. New York: McGraw-hill; 2000. Jan. [Google Scholar]
- 23.Okano H, Hirano T, Balaban E. Learning and memory. Proc Natl Acad Sci USA. 2000; 97: 12403–12404. doi: 10.1073/pnas.210381897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul CM, Magda G, Abel S. Spatial memory: Theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res. 2009; 203: 151–164. doi: 10.1016/j.bbr.2009.05.022 [DOI] [PubMed] [Google Scholar]
- 25.Zlotnik G, Vansintjan A. Memory: an extended definition. Front Psychol. 2019; 10: 2523. doi: 10.3389/fpsyg.2019.02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkinson RC, Shiffrin RM. Human memory: a proposed system and its control processes. Psychol Learn Motiv. 1968; 2: 89–195. doi: 10.1016/S0079-7421(08)60422-3 [DOI] [Google Scholar]
- 27.Malmberg KJ, Raaijmakers JGW, Shiffrin RM. 50 years of research sparked by Atkinson and Shiffrin (1968). Mem Cognit. 2019; 47: 561–574. doi: 10.3758/s13421-019-00896-7 [DOI] [PubMed] [Google Scholar]
- 28.Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001; 24: 87–114, discussion 114–185. doi: 10.1017/S0140525X01003922 [DOI] [PubMed] [Google Scholar]
- 29.Sharma S, Rakoczy S, Brown-Borg H. Assessment of spatial memory in mice. Life Sci. 2010; 87: 521–536. doi: 10.1016/j.lfs.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Constantinidis C, Klingberg T. The neuroscience of working memory capacity and training. Nat Rev Neurosci. 2016; 17: 438–449. doi: 10.1038/nrn.2016.43 [DOI] [PubMed] [Google Scholar]
- 31.Squire LR. Mechanisms of memory. Science. 1986; 232: 1612–1619. doi: 10.1126/science.3086978 [DOI] [PubMed] [Google Scholar]
- 32.Squire LR. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J Cogn Neurosci. 1992; 4: 232–243. doi: 10.1162/jocn.1992.4.3.232 [DOI] [PubMed] [Google Scholar]
- 33.Tulving E. 12. Episodic and Semantic Memory. Organization of memory/Eds E. Tulving, W. Donaldson, NY: Academic Press. 1972:381–403. [Google Scholar]
- 34.Greenberg DL, Verfaellie M. Interdependence of episodic and semantic memory: evidence from neuropsychology. J Int Neuropsychol Soc. 2010; 16: 748–753. doi: 10.1017/S1355617710000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002; 53: 1–25. doi: 10.1146/annurev.psych.53.100901.135114 [DOI] [PubMed] [Google Scholar]
- 36.Sugar J, Moser MB. Episodic memory: Neuronal codes for what, where, and when. Hippocampus. 2019; 29: 1190–1205. doi: 10.1002/hipo.23132 [DOI] [PubMed] [Google Scholar]
- 37.Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999; 113: 1170–1188. doi: 10.1037/0735-7044.113.6.1170 [DOI] [PubMed] [Google Scholar]
- 38.Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002; 116: 884–901. doi: 10.1037/0735-7044.116.5.884 [DOI] [PubMed] [Google Scholar]
- 39.Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003; 139: 197–213. doi: 10.1016/S0166-4328(02)00268-1 [DOI] [PubMed] [Google Scholar]
- 40.Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, et al. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999; 113: 1189–1203. doi: 10.1037/0735-7044.113.6.1189 [DOI] [PubMed] [Google Scholar]
- 41.Pothuizen HH, Zhang WN, Jongen-Rêlo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004; 19: 705–712. doi: 10.1111/j.0953-816X.2004.03170.x [DOI] [PubMed] [Google Scholar]
- 42.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010; 65: 7–19. doi: 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014; 15: 181–192. doi: 10.1038/nrn3677 [DOI] [PubMed] [Google Scholar]
- 44.Bagot RC, Parise EM, Peña CJ, Zhang HX, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015; 6: 7062. doi: 10.1038/ncomms8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron. 2018; 97: 670–683.e6. doi: 10.1016/j.neuron.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Commins S, Duffin J, Chaves K, Leahy D, Corcoran K, Caffrey M, et al. NavWell: A simplified virtual-reality platform for spatial navigation and memory experiments. Behav Res Methods. 2020; 52: 1189–1207. doi: 10.3758/s13428-019-01310-5 [DOI] [PubMed] [Google Scholar]
- 47.Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006; 129: 2894–2907. doi: 10.1093/brain/awl286 [DOI] [PubMed] [Google Scholar]
- 48.Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006; 16: 1091–1101. doi: 10.1002/hipo.20233 [DOI] [PubMed] [Google Scholar]
- 49.Zhong JY, Magnusson KR, Swarts ME, Clendinen CA, Reynolds NC, Moffat SD. The application of a rodent-based Morris water maze (MWM) protocol to an investigation of age-related differences in human spatial learning. Behav Neurosci. 2017; 131: 470–482. doi: 10.1037/bne0000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolls ET. Spatial view cells and the representation of place in the primate hippocampus. Hippocampus. 1999; 9: 467–480. doi: [DOI] [PubMed] [Google Scholar]
- 51.Rahman A, Languille S, Lamberty Y, Babiloni C, Perret M, Bordet R, et al. Sleep deprivation impairs spatial retrieval but not spatial learning in the non-human primate grey mouse lemur. PLoS One. 2013; 8: e64493. doi: 10.1371/journal.pone.0064493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huebner F, Fichtel C, Kappeler PM. Linking cognition with fitness in a wild primate: fitness correlates of problem-solving performance and spatial learning ability. Philos Trans R Soc Lond B Biol Sci. 2018; 373: 20170295. doi: 10.1098/rstb.2017.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saffarpour S, Shaabani M, Naghdi N, Farahmandfar M, Janzadeh A, Nasirinezhad F. In vivo evaluation of the hippocampal glutamate, GABA and the BDNF levels associated with spatial memory performance in a rodent model of neuropathic pain. Physiol Behav. 2017; 175: 97–103. doi: 10.1016/j.physbeh.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 54.Soler JE, Robison AJ, Núñez AA, Yan L. Light modulates hippocampal function and spatial learning in a diurnal rodent species: A study using male nile grass rat (Arvicanthis niloticus). Hippocampus. 2018; 28: 189–200. doi: 10.1002/hipo.22822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haley GE, Raber J. Spatial learning and memory in animal models and humans. InAnimal Models of Behavioral Analysis 2011. (pp. 91–109). Humana Press, Totowa, NJ. [Google Scholar]
- 56.Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. J Exp Psychol Anim Behav Process. 1976; 2: 97–116. doi: 10.1037/0097-7403.2.2.97 [DOI] [Google Scholar]
- 57.Preston CJ, Brown KA, Wagner JJ. Cocaine conditioning induces persisting changes in ventral hippocampus synaptic transmission, long-term potentiation, and radial arm maze performance in the mouse. Neuropharmacology. 2019; 150: 27–37. doi: 10.1016/j.neuropharm.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 58.Vann SD. Lesions within the head direction system reduce retrosplenial c-fos expression but do not impair performance on a radial-arm maze task. Behav Brain Res. 2018; 338: 153–158. doi: 10.1016/j.bbr.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978; 139: 295–308. doi: 10.1016/0006-8993(78)90930-7 [DOI] [PubMed] [Google Scholar]
- 60.Gökçek-Saraç Ç, Wesierska M, Jakubowska-Doğru E. Comparison of spatial learning in the partially baited radial-arm maze task between commonly used rat strains: Wistar, Spargue-Dawley, Long-Evans, and outcrossed Wistar/Sprague-Dawley. Learn Behav. 2015; 43: 83–94. doi: 10.3758/s13420-014-0163-9 [DOI] [PubMed] [Google Scholar]
- 61.Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007; 27: 10765–10776. doi: 10.1523/JNEUROSCI.0117-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valladolid-Acebes I, Stucchi P, Cano V, Fernández-Alfonso MS, Merino B, Gil-Ortega M, et al. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol Learn Mem. 2011; 95: 80–85. doi: 10.1016/j.nlm.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 63.Morris RG. Spatial localization does not require the presence of local cues. Learn Motiv. 1981; 12: 239–260. doi: 10.1016/0023-9690(81)90020-5 [DOI] [Google Scholar]
- 64.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979; 93: 74–104. doi: 10.1037/h0077579 [DOI] [PubMed] [Google Scholar]
- 65.Pitts MW. Barnes maze procedure for spatial learning and memory in mice. Bio Protoc. 2018; 8: e2744. doi: 10.21769/BioProtoc.2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Illouz T, Madar R, Clague C, Griffioen KJ, Louzoun Y, Okun E. Unbiased classification of spatial strategies in the Barnes maze. Bioinformatics. 2016; 32: 3314–3320. doi: 10.1093/bioinformatics/btw376 [DOI] [PubMed] [Google Scholar]
- 67.Illouz T, Madar R, Okun E. A modified Barnes maze for an accurate assessment of spatial learning in mice. J Neurosci Methods. 2020; 334: 108579. doi: 10.1016/j.jneumeth.2020.108579 [DOI] [PubMed] [Google Scholar]
- 68.Locklear MN, Kritzer MF. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav. 2014; 66: 298–308. doi: 10.1016/j.yhbeh.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gawel K, Labuz K, Gibula-Bruzda E, Jenda M, Marszalek-Grabska M, Filarowska J, et al. Cholinesterase inhibitors, donepezil and rivastigmine, attenuate spatial memory and cognitive flexibility impairment induced by acute ethanol in the Barnes maze task in rats. Naunyn Schmiedebergs Arch Pharmacol. 2016; 389: 1059–1071. doi: 10.1007/s00210-016-1269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang TY, Gao Z, Liang NC. Sex-Dependent Wheel Running Effects on High Fat Diet Preference, Metabolic Outcomes, and Performance on the Barnes Maze in Rats. Nutrients. 2020; 12: 2721. doi: 10.3390/nu12092721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Leary TP, Brown RE. The effects of apparatus design and test procedure on learning and memory performance of C57BL/6J mice on the Barnes maze. J Neurosci Methods. 2012; 203: 315–324. doi: 10.1016/j.jneumeth.2011.09.027 [DOI] [PubMed] [Google Scholar]
- 72.Morel GR, Andersen T, Pardo J, Zuccolilli GO, Cambiaggi VL, Hereñú CB, et al. Cognitive impairment and morphological changes in the dorsal hippocampus of very old female rats. Neuroscience. 2015; 303: 189–199. doi: 10.1016/j.neuroscience.2015.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibula-Tarlowska E, Kotlinska JH. Kissorphin improves spatial memory and cognitive flexibility impairment induced by ethanol treatment in the Barnes maze task in rats. Behav Pharmacol. 2020; 31: 272–282. doi: 10.1097/FBP.0000000000000557 [DOI] [PubMed] [Google Scholar]
- 74.Vargas-López V, Lamprea MR, Múnera A. Characterizing spatial extinction in an abbreviated version of the Barnes maze. Behav Processes. 2011; 86: 30–38. doi: 10.1016/j.beproc.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 75.Zappa Villar MF, López Hanotte J, Falomir Lockhart E, Trípodi LS, Morel GR, Reggiani PC. Intracerebroventricular streptozotocin induces impaired Barnes maze spatial memory and reduces astrocyte branching in the CA1 and CA3 hippocampal regions. J Neural Transm (Vienna). 2018; 125: 1787–1803. doi: 10.1007/s00702-018-1928-7 [DOI] [PubMed] [Google Scholar]
- 76.Gawel K, Gibula E, Marszalek-Grabska M, Filarowska J, Kotlinska JH. Assessment of spatial learning and memory in the Barnes maze task in rodents-methodological consideration. Naunyn Schmiedebergs Arch Pharmacol. 2019; 392: 1–18. doi: 10.1007/s00210-018-1589-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harloe JP, Thorpe AJ, Lichtman AH. Differential endocannabinoid regulation of extinction in appetitive and aversive Barnes maze tasks. Learn Mem. 2008; 15: 806–809. doi: 10.1101/lm.1113008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stouffer EM, Heisey JL. Latent learning of spatial information is impaired in middle-aged rats. Dev Psychobiol. 2013; 55: 309–315. doi: 10.1002/dev.21021 [DOI] [PubMed] [Google Scholar]
- 79.Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the θ frequency. Cell. 1995; 81: 905–915. doi: 10.1016/0092-8674(95)90010-1 [DOI] [PubMed] [Google Scholar]
- 80.Kennard JA, Woodruff-Pak DS. Age sensitivity of behavioral tests and brain substrates of normal aging in mice. Front Aging Neurosci. 2011; 3: 9. doi: 10.3389/fnagi.2011.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koopmans G, Blokland A, van Nieuwenhuijzen P, Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol Behav. 2003; 79: 683–693. doi: 10.1016/S0031-9384(03)00171-9 [DOI] [PubMed] [Google Scholar]
- 82.Patil SS, Sunyer B, Höger H, Lubec G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res. 2009; 198: 58–68. doi: 10.1016/j.bbr.2008.10.029 [DOI] [PubMed] [Google Scholar]
- 83.Morris RGM. Pavlovian conditioned inhibition of fear during shuttlebox avoidance behavior. Learn Motiv. 1974; 5: 424–447. doi: 10.1016/0023-9690(74)90002-2 [DOI] [Google Scholar]
- 84.Morris RGM. Two independent effects of variation in intertrial interval upon leverpress avoidance learning by rats. Anim Learn Behav. 1974b; 2: 189–192. doi: 10.3758/BF03199174 [DOI] [Google Scholar]
- 85.Morris RGM. Preconditioning of reinforcing properties to an exteroceptive feedback stimulus. Learn Motiv. 1975; 6: 289–298. doi: 10.1016/0023-9690(75)90029-6 [DOI] [Google Scholar]
- 86.Dickinson A, Morris RGM. Conditioned acceleration and free-operant wheel-turn avoidance following septal lesions in rats. Physiol Psychol. 1975; 3: 107–112. doi: 10.3758/BF03337484 [DOI] [Google Scholar]
- 87.Morris RGM, Einon DF, Morgan MJ. Persistent behaviour in extinction after partial deprivation in training. Q J Exp Psychol. 1976; 28: 633–642. doi: 10.1080/14640747608400589 [DOI] [Google Scholar]
- 88.Morgan MJ, Einon D, Morris RGM. Inhibition and isolation rearing in the rat: extinction and satiation. Physiol Behav. 1977; 18: 1–5. doi: 10.1016/0031-9384(77)90084-1 [DOI] [PubMed] [Google Scholar]
- 89.Kallai J, Makany T, Karadi K, Jacobs WJ. Spatial orientation strategies in Morris-type virtual water task for humans. Behav Brain Res. 2005; 159: 187–196. doi: 10.1016/j.bbr.2004.10.015 [DOI] [PubMed] [Google Scholar]
- 90.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006; 1: 848–858. doi: 10.1038/nprot.2006.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008; 28: 5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lewejohann L, Pickel T, Sachser N, Kaiser S. Wild genius - domestic fool? Spatial learning abilities of wild and domestic guinea pigs. Front Zool. 2010; 7: 9. doi: 10.1186/1742-9994-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001; 36: 60–90. doi: 10.1016/S0165-0173(01)00067-4 [DOI] [PubMed] [Google Scholar]
- 94.Baeta-Corral R, Giménez-Llort L. Persistent hyperactivity and distinctive strategy features in the Morris water maze in 3xTg-AD mice at advanced stages of disease. Behav Neurosci. 2015; 129: 129–137. doi: 10.1037/bne0000027 [DOI] [PubMed] [Google Scholar]
- 95.Barnhart CD, Yang D, Lein PJ. Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One. 2015; 10: e0124521. doi: 10.1371/journal.pone.0124521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sankowski R, Huerta TS, Kalra R, Klein TJ, Strohl JJ, Al-Abed Y, et al. Large-scale validation of the paddling pool task in the clockmaze for studying hippocampus-based spatial cognition in mice. Front Behav Neurosci. 2019; 13: 121. doi: 10.3389/fnbeh.2019.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whishaw IQ. A comparison of rats and mice in a swimming pool place task and matching to place task: some surprising differences. Physiol Behav. 1995; 58: 687–693. doi: 10.1016/0031-9384(95)00110-5 [DOI] [PubMed] [Google Scholar]
- 98.Whishaw IQ, Kolb B., editors. The behavior of the laboratory rat: a handbook with tests. Oxford university press; 2004. Sep 2. [Google Scholar]
- 99.Stryjek R, Modlińska K, Pisula W. Species specific behavioural patterns (digging and swimming) and reaction to novel objects in wild type, Wistar, Sprague-Dawley and Brown Norway rats. PLoS One. 2012; 7: e40642. doi: 10.1371/journal.pone.0040642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deacon RM, Rawlins JN. Learning impairments of hippocampal-lesioned mice in a paddling pool. Behav Neurosci. 2002; 116: 472–478. doi: 10.1037/0735-7044.116.3.472 [DOI] [PubMed] [Google Scholar]
- 101.Deacon RM. Shallow water (paddling) variants of water maze tests in mice. J Vis Exp. 2013; 76: 2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008; 57: 809–818. doi: 10.1016/j.neuron.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 103.Jiang YH, Pan Y, Zhu L, Landa L, Yoo J, Spencer C, et al. Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PLoS One. 2010; 5: e12278. doi: 10.1371/journal.pone.0012278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013; 78: 8–27. doi: 10.1016/j.neuron.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vogel-Ciernia A, Wood MA. Examining object location and object recognition memory in mice. Curr Protoc Neurosci. 2014; 69: 31.1–17. doi: 10.1002/0471142301.ns0831s69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learn Mem. 2007; 14: 313–317. doi: 10.1101/lm.430907 [DOI] [PubMed] [Google Scholar]
- 107.Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, et al. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol. 2010; 20: 1336–1344. doi: 10.1016/j.cub.2010.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013; 14: 417–428. doi: 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992; 106: 274–285. doi: 10.1037/0735-7044.106.2.274 [DOI] [PubMed] [Google Scholar]
- 110.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998; 112: 863–874. doi: 10.1037/0735-7044.112.4.863 [DOI] [PubMed] [Google Scholar]
- 111.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001; 11: 8–17. doi: [DOI] [PubMed] [Google Scholar]
- 112.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997; 88: 261–274. doi: 10.1016/S0166-4328(97)00088-0 [DOI] [PubMed] [Google Scholar]
- 113.Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006; 26: 5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sterneck E, Paylor R, Jackson-Lewis V, Libbey M, Przedborski S, Tessarollo L, et al. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein δ. Proc Natl Acad Sci USA. 1998; 95: 10908–10913. doi: 10.1073/pnas.95.18.10908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, et al. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology. 2011; 36: 1545–1556. doi: 10.1038/npp.2011.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty AK. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front Behav Neurosci. 2014; 8: 78. doi: 10.3389/fnbeh.2014.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, et al. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011; 31: 764–774. doi: 10.1523/JNEUROSCI.5052-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vogel-Ciernia A, Matheos DP, Barrett RM, Kramár EA, Azzawi S, Chen Y, et al. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat Neurosci. 2013; 16: 552–561. doi: 10.1038/nn.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Denninger JK, Smith BM, Kirby ED. Novel object recognition and object location behavioral testing in mice on a budget. J Vis Exp. 2018; (141): 10.3791/58593. . doi: 10.3791/58593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982; 297: 681–683. doi: 10.1038/297681a0 [DOI] [PubMed] [Google Scholar]
- 121.Schenk F, Morris RGM. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Exp Brain Res. 1985; 58: 11–28. doi: 10.1007/BF00238949 [DOI] [PubMed] [Google Scholar]
- 122.Hagan JJ, Alpert JE, Morris RGM, Iversen SD. The effects of central catecholamine depletions on spatial learning in rats. Behav Brain Res. 1983; 9: 83–104. doi: 10.1016/0166-4328(83)90015-3 [DOI] [PubMed] [Google Scholar]
- 123.Hagan JJ, Salamone JD, Simpson J, Iversen SD, Morris RGM. Place navigation in rats is impaired by lesions of medial septum and diagonal band but not nucleus basalis magnocellularis. Behav Brain Res. 1988; 27: 9–20. doi: 10.1016/0166-4328(88)90105-2 [DOI] [PubMed] [Google Scholar]
- 124.Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986; 319: 774–776. doi: 10.1038/319774a0 [DOI] [PubMed] [Google Scholar]
- 125.Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989; 9: 3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morris RGM, Hagan JJ, Nadel L, Jensen J, Baudry M, Lynch GS. Spatial learning in the rat: impairment induced by the thiol-proteinase inhibitor, leupeptin, and an analysis of [3H]glutamate receptor binding in relation to learning. Behav Neural Biol. 1987; 47: 333–345. doi: 10.1016/S0163-1047(87)90448-1 [DOI] [PubMed] [Google Scholar]
- 127.Stäubli U, Baudry M, Lynch G. Leupeptin, a thiol proteinase inhibitor, causes a selective impairment of spatial maze performance in rats. Behav Neural Biol. 1984; 40: 58–69. doi: 10.1016/S0163-1047(84)90170-5 [DOI] [PubMed] [Google Scholar]
- 128.Graziano A, Petrosini L, Bartoletti A. Automatic recognition of explorative strategies in the Morris water maze. J Neurosci Methods. 2003; 130: 33–44. doi: 10.1016/S0165-0270(03)00187-0 [DOI] [PubMed] [Google Scholar]
- 129.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993; 107: 618–626. doi: 10.1037/0735-7044.107.4.618 [DOI] [PubMed] [Google Scholar]
- 130.Dalm S, Grootendorst J, de Kloet ER, Oitzl MS. Quantification of swim patterns in the Morris water maze. Behav Res Methods Instrum Comput. 2000; 32: 134–139. doi: 10.3758/BF03200795 [DOI] [PubMed] [Google Scholar]
- 131.West CA, Reid KH, Schurr A. A simple rapid swim test to determine spatial preference in the rat. Physiol Behav. 1986; 36: 393–395. doi: 10.1016/0031-9384(86)90035-1 [DOI] [PubMed] [Google Scholar]
- 132.Denenberg VH, Talgo NW, Waters NS, Kenner GH. A computer-aided procedure for measuring swim rotation. Physiol Behav. 1990; 47: 1023–1025. doi: 10.1016/0031-9384(90)90029-4 [DOI] [PubMed] [Google Scholar]
- 133.Wolfer DP, Lipp HP. A new computer program for detailed off-line analysis of swimming navigation in the Morris water maze. J Neurosci Methods. 1992; 41: 65–74. doi: 10.1016/0165-0270(92)90124-V [DOI] [PubMed] [Google Scholar]
- 134.Wolfer DP, Madani R, Valenti P, Lipp HP. Extended analysis of path data from mutant mice using the public domain software Wintrack. Physiol Behav. 2001; 73: 745–753. doi: 10.1016/S0031-9384(01)00531-5 [DOI] [PubMed] [Google Scholar]
- 135.Gehring TV, Luksys G, Sandi C, Vasilaki E. Detailed classification of swimming paths in the Morris Water Maze: multiple strategies within one trial. Sci Rep. 2015; 5: 14562. doi: 10.1038/srep14562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Higaki A, Mogi M, Iwanami J, Min LJ, Bai HY, Shan BS, et al. Recognition of early stage thigmotaxis in Morris water maze test with convolutional neural network. PLoS One. 2018; 13: e0197003. doi: 10.1371/journal.pone.0197003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vouros A, Gehring TV, Szydlowska K, Janusz A, Tu Z, Croucher M, et al. A generalised framework for detailed classification of swimming paths inside the Morris Water Maze. Sci Rep. 2018; 8: 15089. doi: 10.1038/s41598-018-33456-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cooke MB, O’Leary TP, Harris P, Ma R, Brown RE, Snyder JS. Pathfinder: open source software for analyzing spatial navigation search strategies. F1000 Res. 2019; 8: 1521. doi: 10.12688/f1000research.20352.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu RQ, Cooke M, Seib DR, Zhao J, Snyder JS. Adult neurogenesis promotes efficient, nonspecific search strategies in a spatial alternation water maze task. Behav Brain Res. 2019; 376: 112151. doi: 10.1016/j.bbr.2019.112151 [DOI] [PubMed] [Google Scholar]
- 140.Fertan E, Wong AA, Purdon MK, Weaver ICG, Brown RE. The effect of background strain on the behavioral phenotypes of the MDGA2+/- mouse model of autism spectrum disorder. Genes Brain Behav. 2021; 20: e12696. doi: 10.1111/gbb.12696 [DOI] [PubMed] [Google Scholar]
- 141.Anisman H, McIntyre DC. Conceptual, spatial, and cue learning in the Morris water maze in fast or slow kindling rats: attention deficit comorbidity. J Neurosci. 2002; 22: 7809–7817. doi: 10.1523/JNEUROSCI.22-17-07809.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tamming RJ, Siu JR, Jiang Y, Prado MA, Beier F, Bérubé NG. Mosaic expression of Atrx in the mouse central nervous system causes memory deficits. Dis Model Mech. 2017; 10: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harris RA, Lone A, Lim H, Martinez F, Frame AK, Scholl TJ, et al. Aerobic glycolysis is required for spatial memory acquisition but not memory retrieval in mice. eNeuro. 2019; 6: ENEURO.0389–18.2019. doi: 10.1523/ENEURO.0389-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garthe A, Kempermann G. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci. 2013; 7: 63. doi: 10.3389/fnins.2013.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gong X, Shao Y, Li B, Chen L, Wang C, Chen Y. γ-aminobutyric acid transporter-1 is involved in anxiety-like behaviors and cognitive function in knockout mice. Exp Ther Med. 2015; 10: 653–658. doi: 10.3892/etm.2015.2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, et al. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992; 9: 11–20. doi: 10.1089/neu.1992.9.11 [DOI] [PubMed] [Google Scholar]
- 147.Cain DP, Boon F, Corcoran ME. Thalamic and hippocampal mechanisms in spatial navigation: a dissociation between brain mechanisms for learning how versus learning where to navigate. Behav Brain Res. 2006; 170: 241–256. doi: 10.1016/j.bbr.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 148.Inostroza M, Cid E, Brotons-Mas J, Gal B, Aivar P, Uzcategui YG, et al. Hippocampal-dependent spatial memory in the water maze is preserved in an experimental model of temporal lobe epilepsy in rats. PLoS One. 2011; 6: e22372. doi: 10.1371/journal.pone.0022372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harris AP, D’Eath RB, Healy SD. Environmental enrichment enhances spatial cognition in rats by reducing thigmotaxis (wall hugging) during testing. Anim Behav. 2009; 77: 1459–1464. doi: 10.1016/j.anbehav.2009.02.019 [DOI] [Google Scholar]
- 150.Huang Y, Zhou W, Zhang Y. Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behav Brain Res. 2012; 226: 26–31. doi: 10.1016/j.bbr.2011.08.043 [DOI] [PubMed] [Google Scholar]
- 151.Wagner AK, Brayer SW, Hurwitz M, Niyonkuru C, Zou H, Failla M, et al. Non-spatial pre-training in the water maze as a clinically relevant model for evaluating learning and memory in experimental TBI. Neurobiol Learn Mem. 2013; 106: 71–86. doi: 10.1016/j.nlm.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Murayama MA, Arimitsu N, Shimizu J, Fujiwara N, Takai K, Okada Y, et al. Dementia model mice exhibited improvements of neuropsychiatric symptoms as well as cognitive dysfunction with neural cell transplantation. Exp Anim. 2021; 70: 387–397. doi: 10.1538/expanim.21-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Murayama MA, Arimitsu N, Shimizu J, Fujiwara N, Takai K, Ikeda Y, et al. Female dominance of both spatial cognitive dysfunction and neuropsychiatric symptoms in a mouse model of Alzheimer’s disease. Exp Anim. 2021; 70: 398–405. doi: 10.1538/expanim.21-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maei HR, Zaslavsky K, Wang AH, Yiu AP, Teixeira CM, Josselyn SA, et al. Development and validation of a sensitive entropy-based measure for the water maze. Front Integr Nuerosci. 2009; 3: 33. doi: 10.3389/neuro.07.033.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen CH, Lee PW, Liao HM, Chang PK. Neuroligin 2 R215H mutant mice manifest anxiety, increased prepulse inhibition, and impaired spatial learning and memory. Front Psychiatry. 2017; 8: 257. doi: 10.3389/fpsyt.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li J, Li X, Bi H, Ma K, Li B. Developmental exposure to atrazine impairs spatial memory and downregulates the hippocampal D1 dopamine receptor and cAMP-dependent signaling pathway in rats. Int J Mol Sci. 2018; 19: 2241. doi: 10.3390/ijms19082241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moradpour F, Naghdi N, Fathollahi Y, Javan M, Choopani S, Gharaylou Z. Pre-pubertal castration improves spatial learning during mid-adolescence in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2013; 46: 105–112. doi: 10.1016/j.pnpbp.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 158.Lu CL, Tang S, Meng ZJ, He YY, Song LY, Liu YP, et al. Taurine improves the spatial learning and memory ability impaired by sub-chronic manganese exposure. J Biomed Sci. 2014; 21: 51. doi: 10.1186/1423-0127-21-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tomás Pereira I, Burwell RD. Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci. 2015; 129: 533–539. doi: 10.1037/bne0000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brody DL, Holtzman DM. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp Neurol. 2006; 197: 330–340. doi: 10.1016/j.expneurol.2005.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fukuda MTH, Françolin-Silva AL, Almeida SS. Early postnatal protein malnutrition affects learning and memory in the distal but not in the proximal cue version of the Morris water maze. Behav Brain Res. 2002; 133: 271–277. doi: 10.1016/S0166-4328(02)00010-4 [DOI] [PubMed] [Google Scholar]
- 162.Tucker LB, Velosky AG, McCabe JT. Applications of the Morris water maze in translational traumatic brain injury research. Neurosci Biobehav Rev. 2018; 88: 187–200. doi: 10.1016/j.neubiorev.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 163.Nishimura H, Ida Y, Tsuda A, Tanaka M. Opposite effects of diazepam and β-CCE on immobility and straw-climbing behavior of rats in a modified forced-swim test. Pharmacol Biochem Behav. 1989; 33: 227–231. doi: 10.1016/0091-3057(89)90454-1 [DOI] [PubMed] [Google Scholar]
- 164.Wallace-Boone TL, Newton AE, Wright RN, Lodge NJ, McElroy JF. Behavioral and pharmacological validation of the gerbil forced-swim test: effects of neurokinin-1 receptor antagonists. Neuropsychopharmacology. 2008; 33: 1919–1928. doi: 10.1038/sj.npp.1301586 [DOI] [PubMed] [Google Scholar]
- 165.Anyan J, Amir S. Too depressed to swim or too afraid to stop? A reinterpretation of the forced swim test as a measure of anxiety-like behavior. Neuropsychopharmacology. 2018; 43: 931–933. doi: 10.1038/npp.2017.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McNamara RK, Skelton RW. Diazepam impairs acquisition but not performance in the Morris water maze. Pharmacol Biochem Behav. 1991; 38: 651–658. doi: 10.1016/0091-3057(91)90028-Z [DOI] [PubMed] [Google Scholar]
- 167.Salazar JG, Ribes D, Cabré M, Domingo JL, Sanchez-Santed F, Colomina MT. Amyloid β peptide levels increase in brain of AβPP Swedish mice after exposure to chlorpyrifos. Curr Alzheimer Res. 2011; 8: 732–740. doi: 10.2174/156720511797633197 [DOI] [PubMed] [Google Scholar]
- 168.Karl F, Colaço MBN, Schulte A, Sommer C, Üçeyler N. Affective and cognitive behavior is not altered by chronic constriction injury in B7-H1 deficient and wildtype mice. BMC Neurosci. 2019; 20: 16. doi: 10.1186/s12868-019-0498-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Timić T, Joksimović S, Milić M, Divljaković J, Batinić B, Savić MM. Midazolam impairs acquisition and retrieval, but not consolidation of reference memory in the Morris water maze. Behav Brain Res. 2013; 241: 198–205. doi: 10.1016/j.bbr.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 170.Sanches EF, Arteni N, Nicola F, Aristimunha D, Netto CA. Sexual dimorphism and brain lateralization impact behavioral and histological outcomes following hypoxia-ischemia in P3 and P7 rats. Neuroscience. 2015; 290: 581–593. doi: 10.1016/j.neuroscience.2014.12.074 [DOI] [PubMed] [Google Scholar]
- 171.Singh S, Kumar M, Manda K, Haridas S, Bhatt AN, Saluja D, et al. Chronic dietary administration of 2-deoxy-D-glucose does not compromise neurobehavioral functions at tumor preventive doses in mice. J Behav Brain Sci. 2015; 5: 381–393. doi: 10.4236/jbbs.2015.59037 [DOI] [Google Scholar]
- 172.Schulz D, Topic B, De Souza Silva MA, Huston JP. Extinction-induced immobility in the water maze and its neurochemical concomitants in aged and adult rats: a possible model for depression? Neurobiol Learn Mem. 2004; 82: 128–141. doi: 10.1016/j.nlm.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 173.Schulz D, Huston JP, Buddenberg T, Topic B. “Despair” induced by extinction trials in the water maze: relationship with measures of anxiety in aged and adult rats. Neurobiol Learn Mem. 2007; 87: 309–323. doi: 10.1016/j.nlm.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 174.Doe N, Takahashi T, Kiriyama M. Behavioral despair during a water maze learning task in mice. Exp Anim. 2010; 59: 191–197. doi: 10.1538/expanim.59.191 [DOI] [PubMed] [Google Scholar]
- 175.Haws ME, Kaeser PS, Jarvis DL, Südhof TC, Powell CM. Region-specific deletions of RIM1 reproduce a subset of global RIM1α(-/-) phenotypes. Genes Brain Behav. 2012; 11: 201–213. doi: 10.1111/j.1601-183X.2011.00755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hou XQ, Wu DW, Zhang CX, Yan R, Yang C, Rong CP, et al. Bushen‑Yizhi formula ameliorates cognition deficits and attenuates oxidative stress‑related neuronal apoptosis in scopolamine‑induced senescence in mice. Int J Mol Med. 2014; 34: 429–439. doi: 10.3892/ijmm.2014.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mu S, Wang J, Zhou G, Peng W, He Z, Zhao Z, et al. Transplantation of induced pluripotent stem cells improves functional recovery in Huntington’s disease rat model. PLoS One. 2014; 9: e101185. doi: 10.1371/journal.pone.0101185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bruinsma CF, Savelberg SM, Kool MJ, Jolfaei MA, Van Woerden GM, Baarends WM, et al. An essential role for UBE2A/HR6A in learning and memory and mGLUR-dependent long-term depression. Hum Mol Genet. 2016; 25: 1–8. doi: 10.1093/hmg/ddv436 [DOI] [PubMed] [Google Scholar]
- 179.Konsolaki E, Tsakanikas P, Polissidis AV, Stamatakis A, Skaliora I. Early signs of pathological cognitive aging in mice lacking high-affinity nicotinic receptors. Front Aging Neurosci. 2016; 8: 91. doi: 10.3389/fnagi.2016.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wenk GL. Assessment of spatial memory using the radial arm maze and Morris water maze. Curr Protoc Neurosci. 2004; Chapter 8: 5A. [DOI] [PubMed] [Google Scholar]
- 181.Aguilar-Valles A, Sánchez E, de Gortari P, Balderas I, Ramírez-Amaya V, Bermúdez-Rattoni F, et al. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005; 82: 306–319. doi: 10.1159/000093129 [DOI] [PubMed] [Google Scholar]
- 182.Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009; 198: 247–251. doi: 10.1016/j.bbr.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fitzgerald LW, Dokla CP. Morris water task impairment and hypoactivity following cysteamine-induced reductions of somatostatin-like immunoreactivity. Brain Res. 1989; 505: 246–250. doi: 10.1016/0006-8993(89)91450-9 [DOI] [PubMed] [Google Scholar]
- 184.Xing Y, Qin Y, Jing W, Zhang Y, Wang Y, Guo D, et al. Exposure to Mozart music reduces cognitive impairment in pilocarpine-induced status epilepticus rats. Cogn Neurodyn. 2016; 10: 23–30. doi: 10.1007/s11571-015-9361-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Prusky GT, West PW, Douglas RM. Reduced visual acuity impairs place but not cued learning in the Morris water task. Behav Brain Res. 2000; 116: 135–140. doi: 10.1016/S0166-4328(00)00267-9 [DOI] [PubMed] [Google Scholar]
- 186.Lindner MD, Plone MA, Schallert T, Emerich DF. Blind rats are not profoundly impaired in the reference memory Morris water maze and cannot be clearly discriminated from rats with cognitive deficits in the cued platform task. Brain Res Cogn Brain Res. 1997; 5: 329–333. doi: 10.1016/S0926-6410(97)00006-2 [DOI] [PubMed] [Google Scholar]
- 187.O’Steen WK, Spencer RL, Bare DJ, McEwen BS. Analysis of severe photoreceptor loss and Morris water-maze performance in aged rats. Behav Brain Res. 1995; 68: 151–158. doi: 10.1016/0166-4328(94)00168-F [DOI] [PubMed] [Google Scholar]
- 188.Spencer RL, O’Steen WK, McEwen BS. Water maze performance of aged Sprague-Dawley rats in relation to retinal morphologic measures. Behav Brain Res. 1995; 68: 139–150. doi: 10.1016/0166-4328(94)00167-E [DOI] [PubMed] [Google Scholar]
- 189.Lipp HP, Wolfer DP. Genetically modified mice and cognition. Curr Opin Neurobiol. 1998; 8: 272–280. doi: 10.1016/S0959-4388(98)80151-7 [DOI] [PubMed] [Google Scholar]
- 190.Whishaw IQ, Tomie J. Of mice and mazes: similarities between mice and rats on dry land but not water mazes. Physiol Behav. 1996; 60: 1191–1197. doi: 10.1016/S0031-9384(96)00176-X [DOI] [PubMed] [Google Scholar]
- 191.Upchurch M, Wehner JM. Differences between inbred strains of mice in Morris water maze performance. Behav Genet. 1988; 18: 55–68. doi: 10.1007/BF01067075 [DOI] [PubMed] [Google Scholar]
- 192.Rauch TM, Welch DI, Gallego L. Hypothermia impairs performance in the Morris water maze. Physiol Behav. 1989; 46: 315–320. doi: 10.1016/0031-9384(89)90273-4 [DOI] [PubMed] [Google Scholar]
- 193.Iivonen H, Nurminen L, Harri M, Tanila H, Puoliväli J. Hypothermia in mice tested in Morris water maze. Behav Brain Res. 2003; 141: 207–213. doi: 10.1016/S0166-4328(02)00369-8 [DOI] [PubMed] [Google Scholar]
- 194.Buresová O, Bureš J, Oitzl MS, Zahálka A. Radial maze in the water tank: an aversively motivated spatial working memory task. Physiol Behav. 1985; 34: 1003–1005. doi: 10.1016/0031-9384(85)90028-9 [DOI] [PubMed] [Google Scholar]
- 195.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006; 1: 1671–1679. doi: 10.1038/nprot.2006.275 [DOI] [PubMed] [Google Scholar]
- 196.Penley SC, Gaudet CM, Threlkeld SW. Use of an eight-arm radial water maze to assess working and reference memory following neonatal brain injury. J Vis Exp. 2013; 82: 50940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Macheda T, Roberts KN, Morganti JM, Braun DJ, Bachstetter AD. Optimization and validation of a modified radial-arm water maze protocol using a murine model of mild closed head traumatic brain injury. PLoS One. 2020; 15: e0232862. doi: 10.1371/journal.pone.0232862 [DOI] [PMC free article] [PubMed] [Google Scholar]