Abstract
Determine efficacy and adverse events (AEs) of hydroxyurea (HU) in mast cell activation syndrome (MCAS) patients who were refractory to standard medical therapy. An electronic chart review was performed to find MCAS patients who received HU in a MCAS medical practice. Diagnosis of MCAS was established on the basis of mast cell (MC) activation symptoms in ≥ 5 systems plus ≥ 1 abnormal MC mediators and/or ≥ 20 MC/high power field on duodenal biopsies. Medicines not providing significant clinical improvement prior to HU were tabulated. The following symptoms were evaluated by patients on a 0–10 scale prior to and at the study conclusion: bone pain, abdominal pain, diarrhea, bloating, and nausea. Safety labs were obtained on a regular basis. Twenty out of three hundred ten (8.4%) MCAS patients received HU. Patients included 22 females, average age 42.4 years. Dysautonomia was present in 60%. An average of 10.6 (SD 1.7, range 8–13) medications were used prior to adding HU to various concomitant medications. Average dose of HU was 634 mg. In 20 patients who continued therapy for ≥ 2 months, there was statistically significant reduction of bone pain, abdominal pain, diarrhea, bloating, and nausea. Fourteen patients noted prolonged success with therapy. Six patients stopped HU within 6 weeks owing to AEs. Four patients treated ≥ 2 months had AEs and 2 led to HU cessation. All AEs were reversible. Refractory MCAS patients showed clear significant improvement in bone pain and gastrointestinal symptoms on HU. Systematic monitoring was effective in preventing the occurrence of severe HU-induced adverse events.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00210-022-02282-8.
Keywords: Mast cell activation syndrome, Treatment, Hydroxyurea, Efficacy, Toxicity
Introduction
Mast cell activation syndrome (MCAS) is the most common variant of mast cell activation disease (MCAD) and causes chronic inflammatory and allergic symptoms and syndromes (Hamilton 2018; Afrin et al. 2017). In a German study, the prevalence of MCAD was 17% (Molderings et al. 2013). The pathologic behavior of mast cells (MCs) in MCAS is due to uncontrolled activation and release of mediators by aberrant MCs leading to harmful local and distant effects (Frieri 2018; Ravanbakhsh and Kesavan 2019). This may be due to mutations of the mast cell (MC) regulatory genes (Molderings et al. 2007; Molderings 2015). MCAS, in its wide variety of clinical presentations, features inappropriate MC activation with relatively modest MC proliferation (in contrast to systemic mastocytosis (SM), the rare variant of MCAD).
Establishing the diagnosis of MCAS is paramount. Although being highly prevalent, this syndrome is generally not considered in the differential diagnosis of a multisystemic disorder (Afrin et al. 2016). Diagnosis may be difficult to prove but is important because the disease is treatable (Afrin et al. 2020). In a study of 413 MCAS patients, symptoms that were present in 50% or more patients were, in descending frequency, fatigue, abdominal pain, altered bowel habits, muscle pain, pre-syncope or syncope, headaches, itching, urticaria, nausea, chills, edema, eye irritation, dyspnea, and heartburn (Afrin et al. 2017). Unrecognized and untreated MCAS may account for refractory gastrointestinal (GI) symptoms which is often attributed to functional GI disorders including irritable bowel syndrome (Hsieh 2018; Weinstock et al. 2021). Among the common often severe symptoms affecting all body systems, migratory bone pain is a particularly severe clinical phenomenon (Afrin 2013). In the differential diagnosis of migratory bone pain, MCAS is not considered a potential etiology (Mantyh 2019). In light of the migratory nature of bone pain in MCAS, it is possible that release of mediators locally or distant leads to inflammation of the periosteal nerves leading to pain (Mach et al. 2002). Bone pain in MCAS patients responds poorly to narcotics, non-narcotic analgesics, antidepressants, and anticonvulsants (Afrin 2013).
There are no FDA-approved or EU-approved medications specifically for MCAS, treatment can be difficult in a sizable number of patients, and, thus, alternative medical therapy is important to investigate. Hydroxyurea (HU) is an oral ribonucleotide reductase inhibitor with antimetabolic and antineoplastic properties (Musiałek and Rybaczek 2021). It inhibits ribonucleotide reductase, a ubiquitous intracellular enzyme that converts ribonucleotides to deoxyribonucleotides, which are required for DNA synthesis and repair. First used 60 years ago for chronic myeloproliferative neoplasms, HU is now standard of care in reducing the severity of sickle cell disease (SCD) (McGann and Ware 2015). In this context, it is of interest that in animal models and human subjects with SCD, medications that reduce MC activation decreased bone pain (Vincent et al. 2013, 2016). HU also inhibits replication of human immunodeficiency virus-1 (HIV-1) and has been used in therapy of cyanotic congenital heart disease (Lori and Lisziewicz 2000; Reiss et al. 2007). A theoretic explanation for effectiveness of HU in these diseases include reducing MC activity independent of anti-proliferative effects. HU could decrease activity and ability of GI mucosal MCs that capture HIV-1 and mediate trans-infection of CD4 + T cells (Jiang et al. 2015). Similarly, HU could reduce the activity of the increased numbers of chymase-containing MCs in lung tissue of congenital heart disease patients and, thereby, decrease severity of these pulmonary and cardiac diseases (Hamada et al. 1999).
HU has also been used in the treatment of systemic MC activation disease (Afrin 2013, 2014). It achieved modest benefit in SM with an associated hematological neoplasm (Lim et al. 2009). Five MCAS patients treated with HU had marked reduction in diffuse body and bone pain (Afrin 2013). In that study, cytopenia needed cessation of the drug in one patient. Reactions to excipients in three patients required trials of an alternative formulation which were then successful.
The aim of the present retrospective analysis is to address the effectiveness and toxicity of HU in MCAS patients who are refractory to standard medical treatment options.
Methods
The study was reviewed by the Sterling Investigational Review Board (IRB) in Atlanta, Georgia. The Sterling IRB Chairperson decided that the study (ID #9768) was exempt from full IRB review pursuant to the terms of the U.S. Department of Health and Human Service’s Policy for Protection of Human Research Subjects at 45 C.F.R. §46.104(d). The IRB found that the exemption category 2 applied. All patients signed an informed consent form that described all risks of HU and allowed collection and reporting of clinical data.
One of the investigators (LBW) is an internist and gastroenterologist specializing in MCAS patients. An electronic chart review search of his patient data was performed to find MCAS patients who were prescribed HU. These patients had failed to show any significant clinical response to step 1, 2, 3, and/or 4 MC medicines plus elimination dietary trials as per recent guidelines (Weinstock et al. 2021). A variety of other medications reported to be effective in MCAS were employed including benzodiazepines, tricyclic antidepressants, imatinib, and low-dose naltrexone (Molderings et al. 2016; Weinstock et al. 2018; Weinstock and Blasingame 2020).
The diagnosis of MCAS was established on the basis of the diagnostic Consensus-2 criteria (Afrin et al. 2020), in particular on the constellation of complaints attributable to pathologically increased MC activity in ≥ 5 systems plus either ≥ 1 minor criteria, i.e., abnormal MC mediators and/or ≥ 20 MC/high power field on duodenal biopsies. The standardized validated mast cell mediator release syndrome (MCMRS) checklist was used to determine the number of MC symptoms and systems for each patient (Appendix 1). The total score considers the points for the number of symptoms, abnormal MC mediators, and abnormal MC count on biopsy (Molderings et al. 2013; Weinstock et al. 2021). At a score of ≥ 14 points, the presence of a MCMRS has a probability of 95%. We also used a MCMRS score for symptoms alone. Prior medicines that did not give significant clinical improvement and concomitant medications were tabulated. The following symptoms were evaluated by a 0 to 10 scale prior to HU therapy: bone pain, abdominal pain, diarrhea, bloating, and nausea. The baseline symptoms were derived from the intake MCMRS checklist administered to all new complex patients seen in the clinic. These symptoms were determined at a second point in time at the date of study commencement in November 2021. HU was prescribed either as the generic form at 500 mg daily or as branded Droxia (Bristol-Meyers Squibb Company, Princeton, NJ) 600 mg daily Droxia directions were 200 mg daily and increase the dose by 200 mg every three days until 600 mg was reached. The choice was dependent on insurance coverage. Dose escalation beyond 500 or 600 mg was considered on a case-by-case basis for non-responders.
Safety lab monitoring included a baseline complete blood count (CBC) and complete metabolic profile (CMP). The CBC was obtained weekly for the first month, biweekly for the second month, and then monthly if stable. The CMP was performed monthly. Patients were encouraged to contact the clinician for any adverse events and were seen in the clinic at 3-month intervals. Statistical tests for symptom score changes were performed using R software and statistical significance was defined as p < 0.05. Repeated measure t-tests were used to decide whether symptoms significantly improved after treatment with HU.
Results
Of 310 MCAS patients, 26 (8.5%) were prescribed HU. There were 22 females and 4 males with an average age of 42.4 years. In the twenty-six patients, the mean MCMRS symptom score was 24. Co-morbid syndromes included postural orthostatic tachycardia syndrome (POTS) in 61.5% and hypermobile Ehlers-Danlos syndrome in 34.6%. An average of 10.6 (SD 1.7, range 8–13) medications were prescribed prior to adding HU to an average of 6.0 concomitant medications. The duration of HU therapy was < 2 months in 6 patients and an average of 15 months (SD 12, range 2–42) in the other 20 patients. Table 1 summarizes the clinical characteristics, medication history, and adverse events.
Table 1.
Clinical characteristics, medicine history, and adverse events for twenty-six MCAS patients treated with hydroxyurea (HU)
| Females, N (%) | 22 (84.6%) |
| Males, N (%) | 4 (15.4%) |
| Age, mean (SD) | 42.4 (14.3) |
| MCMRS score, mean (SD)a | 24.2 (4.2) |
| Percentage of patients with abnormal mast cell mediator level(s) | 70% |
| Percentage of patients with positive biopsyb | 95% |
| Postural orthostatic tachycardia syndrome, N (%) | 16 (61.5%) |
| Hypermobile Ehlers-Danlos syndrome, N (%) | 9 (34.6%) |
| Number of medications used prior to starting HU, mean (SD, range) | 10.6 (1.7, 8–13) |
| Number concomitant medications taken with HU, mean (SD, range) | 6.0 (2.3, 1–9) |
| HU dose, mean (SD) | 634.6 (135.5) |
| Duration of HU therapy, mo, mean (SD) | 9.9 (11.1) |
| Adverse events, N (%) | 10 (38.5%) |
| Cessation due to adverse events, N (%)c | 8 (30.8%) |
aThe mast cell mediator release syndrome (MCMRS) score was calculated by tabulating a standardized validated checklist. A score of ≥ 14 is highly suggestive of a MCMRS. bThe immunohistochemistry CD117 stain was performed in duodenal biopsies in 20 patients. In the patient with a normal biopsy, the diagnostic minor criterion of an increased mediator release was positive. cAdverse events leading to cessation of therapy in 8 patients included: mild changes in the leukocyte count (1), creatinine (2), and liver enzymes (1) in 4 patients; and worsening of chronic symptoms of poor healing in 1 patient; and a variety of symptoms including bone pain, nausea, headaches, and fatigue in 3 patients
Medications used prior to HU are delineated in Table 2. Initial therapy usually was a combination of histamine-1 and -2 receptor antagonists, quercetin, vitamins C and D, and low-dose naltrexone. Then, one or more of the following drugs were added or substituted by another one: cromolyn, oral ketotifen, montelukast, aspirin, tricyclic antidepressants, and benzodiazepines. Invariably blockade of both histamine receptor types were continued throughout the course of therapy. Omalizumab was used concomitantly in eight patients—it often improved urticaria, but did not affect other MCAS symptoms. Other advanced medications that did not help prior to starting HU included zileuton (in seven patients), imatinib (in two patients), and intravenous immune globulin (in two patients). Short courses of budesonide or prednisone were prescribed to five patients.
Table 2.
Medications that failed to provide significant improvement in those twenty mast cell activation syndrome patients who for that reason were treated with hydroxyurea for ≥ 2 months
| Medication | N | % |
|---|---|---|
| Histamine H1 receptor antagonist | 20 | 100 |
| Histamine H2 receptor antagonist | 19 | 95 |
| Cromolyn | 19 | 95 |
| Vitamin C | 19 | 95 |
| Montelukast | 18 | 90 |
| Low-dose naltrexone | 17 | 85 |
| Quercetin | 16 | 80 |
| Vitamin D | 15 | 75 |
| Benzodiazepine | 12 | 60 |
| Ketotifen | 11 | 55 |
| Aceytsalicylic acid | 9 | 45 |
| Omalizumab | 8 | 40 |
| Zileuton | 7 | 35 |
| Norepinephrine/5-hydroxytryptamine enhancers | 6 | 30 |
| Glucocorticoid receptor agonist a | 5 | 25 |
| Imatinib | 2 | 10 |
| Intravenous immunoglobulinb | 2 | 10 |
| Pentosan polysufate | 1 | 1 |
aGlucocorticoid receptor agonist used included budesonide and short courses of prednisone
bIntravenous immunoglobulin was used for severe concomitant dysautonomia
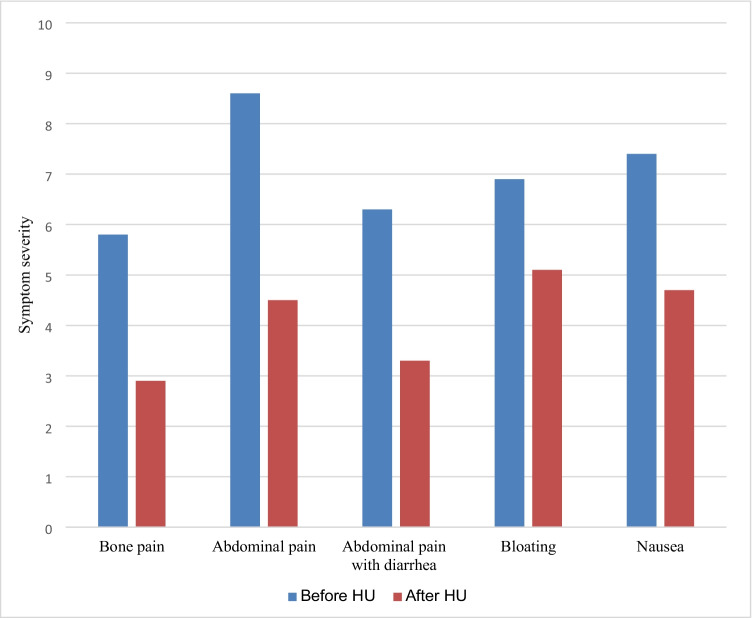
The average dose of HU was 634 mg (SD 135, range 500–1000) which was administered for 9.9 months (SD 11.1, range 0.5–42.0) in all twenty-six patients (Table 1). The analysis for the 20 patients who took HU ≥ 2 months showed overall statistically significant benefit in all of the symptom domains (Table 3, Fig. 1). Fourteen of these twenty patients (70%) had significant benefit and were considered to be “long-term users”: 32.6 months (SD 11.8, range 4–42). One patient needed a change in formulation due to hives from an excipient. Five patients took HU ≥ 2 months but did not achieve significant clinical response and one of patient had worsening symptoms. One patient had an excellent response for 12 months but stopped HU owing to a self-limited increase in creatinine. To decide whether there was a difference in response to HU as determined by the presence of bone pain, we separated the patient group by presence or absence of bone pain (N = 15 vs. N = 5) and compared symptom response of abdominal pain, diarrhea, bloat, and nausea between both groups. Improvement scores were not statistically different between those with and without bone pain.
Table 3.
Symptoms rated on a scale of 0 to 10 before and after hydroxyurea (HU) treatment in twenty MCAS patients who took HU ≥ 2 months
| Symptom | Mean (SD) severity before HU | Mean (SD) severity after HU | t(19) = | p value |
|---|---|---|---|---|
| Bone pain | 5.8 (4.0) | 2.9 (3.4) | 3.0 | 0.0066 |
| Abdominal pain | 8.6 (1.8) | 4.5 (2.7) | 6.0 | < 0.0001 |
| Diarrhea | 6.3 (3.9) | 3.3 (3.6) | 4.2 | 0.0005 |
| Bloating | 6.9 (3.4) | 5.1 (3.1) | 3.3 | 0.0040 |
| Nausea | 7.4 (3.3) | 4.7 (3.2) | 3.9 | 0.0008 |
Abbreviations: SD, standard deviation; t(19), t tests with 19 degrees of freedom
Fig. 1.
Outcome of twenty MCAS patients treated with hydroxyurea (HU) ≥ 2 months (mean 15 ± 12; range 2–42). The differences in the symptom severity before and after hydroxyurea treatment were at least significant at < 0.01, t(19), t tests with 19 degrees of freedom
Self-limited adverse events led to cessation of therapy within 2 months in six patients. Mild changes in the leukocyte count, creatinine, and/or liver enzymes were detected in three patients. There was worsening of poor healing of skin wounds in one patient who suffered from IgG deficiency; bone pain in one patient; and nausea/headaches/fatigue in one patient. Two of the twenty other patients had self-limited adverse events that led to cessation of HU: 1) one patient who had complaints of fatigue, edema, and dizziness and subsequently improved with imatinib and (2) one patient with transient increased creatinine. Two long-term patients had self-limited adverse events but were able to continue HU therapy: (1) one patient had a transient increased liver enzyme (γGT) due to intake of high dose acetaminophen during a COVID-19 infection and (2) one patient with mild leukopenia resolved by dose reduction. One patient had urticaria in response to taking multiple medicines. She had hives with two forms of hydroxyurea but found a third that did not have a dye that she reacted to.
Discussion
In the present retrospective study, we addressed the effectiveness and toxicity of HU in MCAS patients who were refractory to conventional treatment options. Twenty-six patients did not respond to dietary changes and an average of 10.6 medicines and 6.0 concomitant medicines before adding HU to the regimen. The prevalence of postural orthostatic tachycardia and hypermobile Ehlers-Danlos syndromes were higher in this cohort than in other case series of MCAS patients (Afrin et al. 2017; Weinstock and Blasingame 2020). These MCAS patients are considered to be more difficult to treat and there is an overlap of symptoms in the three syndromes (Weinstock et al. 2021). Overall, 14/20 (70%) patients who took HU ≥ 2 months experienced significant clinical benefit from HU and continued it long-term (average of 32.6 months).
AEs were reversible with drug cessation or decreasing the dose. Laboratory changes were all mild and included leukopenia (1), neutropenia (1), liver enzyme changes (2), and increased creatinine (2). The safety laboratory plan employed was effective in detecting the above problems before clinical symptoms arose. This is important because when using HU long-term the possibility of developing severe adverse effects must be kept in mind. HU may cause severe myelosuppression (Agrawal et al. 2014). Therefore, it should not be prescribed if the bone marrow function is depressed. HU has been used primarily for the treatment of myeloproliferative diseases, which has an inherent risk of transforming to acute myeloid leukemia. There has been a longstanding concern that HU itself carries a leukemia risk, but large studies have shown that the risk of secondary malignancy is absent (Szikriszt et al. 2016; Santoro et al. 2017). In patients with essential thrombocythemia treated with HU, the rate of secondary malignancy was 7% and, hence, not different from those patients without any therapy (5%) (Santoro et al. 2017). Concerns about the secondary malignancy risk from HU, originally thought to be fairly high, have been diminishing over the decades, to the point where long-term HU use is now standard of care in certain subpopulations of polycythemia vera patients and in many sickle cell patients. In fact, there are many studies now demonstrating modest to no increased risk for malignancies from long-term HU use (e.g., Rodriguez et al. 2018; Tolu et al. 2020; Liggett et al. 2022). Even among the studies suggesting an increased risk of “skin cancers” in long-term HU users, it is critical to note that the observed excess of cutaneous cancers were non-melanomatous cancers, with far different prognosis than melanomas, the development of which long-term HU use does not seem to drive (Soutou et al. 2020). Nevertheless, patients should use sun protection with UV blocking cream. Liver and renal toxicity was observed in one study (Lisziewicz et al. 2003). HU pulmonary toxicity is a rare complication (Sandhu et al. 2000). There may be potential risk to a fetus and mothers who are breast feeding should not take HU (Stevens 1999). In men with SCD, a risk of reduced fertility after HU therapy has been reported (DeBaun 2014).
The main limitations of the study are the retrospective and open label design. Another issue is that symptom assessments were conducted only at the date of entry into the clinic and at the time of data collection at the end of the study. The patients who stopped taking HU earlier than the end of data collection completed the symptom questionnaire in a retrospective manner. The possibility of recall bias cannot be excluded in this study. Finally, after step-1 therapy failed to be effective, the subsequent replacements and concomitant medicines were not prescribed in a regimented manner so that such medicine changes could have contributed to the HU-induced improvement.
In conclusion, refractory MCAS patients showed clear statistically significant improvement of bone pain and gastrointestinal symptoms by HU. Systematic monitoring was effective in detecting minor laboratory changes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
L. W. conceived of the study, performed the research, helped analyze data, and drafted the manuscript. J. B. helped perform the research, analyze data, and reviewed the chart. J. B. reviewed and contributed to the manuscript. G. M. helped analyze the data. G. M. critically reviewed and contributed to the manuscript. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The raw data are given in the supplementary file ESM_2-xlsx.
Declarations
Ethics approval
The study was reviewed by the Sterling Investigational Review Board (IRB) in Atlanta, Georgia. The Sterling IRB Chairperson decided that the study (ID #9768) was exempt from full IRB review pursuant to the terms of the U.S. Department of Health and Human Service’s Policy for Protection of Human Research Subjects at 45 C.F.R. §46.104(d). The IRB found that the exemption Category 2 applied.
Consent to participate and consent to publish
All patients signed an informed consent form that described all risks of HU and allowed collection and reporting of their clinical data.
Competing interests
Dr. Weinstock is an uncompensated, volunteer medical advisor to the startup company MC Sciences, Ltd. Dr. Weinstock is a compensated medical advisor to Statera BioPharma. Mrs. Brook has no disclosures. Dr. Molderings is the co-founder and chief medical officer of the startup company MC Sciences, Ltd.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Leonard B. Weinstock, Email: lw@gidoctor.net
Jill B. Brook, Email: jillbrook@msn.com
Gerhard J. Molderings, Email: molderings@uni-bonn.de
References
- Afrin LB. Utility of hydroxyurea in mast cell activation syndrome. Exp Hematol Oncol. 2013;2:28. doi: 10.1186/2162-3619-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrin LB. Mast cell activation syndrome as a significant comorbidity in sickle cell disease. Am J Med Sci. 2014;348:460–464. doi: 10.1097/MAJ.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrin LB, Butterfield JH, Raithel M, Molderings GJ. Often seen, rarely recognized: mast cell activation disease–a guide to diagnosis and therapeutic options. Ann Med. 2016;48:190–201. doi: 10.3109/07853890.2016.1161231. [DOI] [PubMed] [Google Scholar]
- Afrin LB, Self S, Menk J, Lazarchick J. Characterization of mast cell activation syndrome. Am J Med Sci. 2017;353:207–215. doi: 10.1016/j.amjms.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrin LB, Ackerley MB, Bluestein LS, et al. Diagnosis of mast cell activation syndrome: a global “consensus-2”. Diagnosis (berl) 2020;8:137–152. doi: 10.1515/dx-2020-0005. [DOI] [PubMed] [Google Scholar]
- Agrawal RK, Patel RK, Shah V, Nainiwal L, Trivedi B. Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus. 2014;30:91–96. doi: 10.1007/s12288-013-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR. Hydroxyurea therapy contributes to infertility in adult men with sickle cell disease: a review. Expert Rev Hematol. 2014;7:767–773. doi: 10.1586/17474086.2014.959922. [DOI] [PubMed] [Google Scholar]
- Frieri M. Mast cell activation syndrome. Clin Rev Allergy Immunol. 2018;54:353–365. doi: 10.1007/s12016-015-8487-6. [DOI] [PubMed] [Google Scholar]
- Hamada H, Terai M, Kimura H, Hirano K, Oana S, Niimi H. Increased expression of mast cell chymase in the lungs of patients with congenital heart disease associated with early pulmonary vascular disease. Am J Respir Critic Care Med. 1999;160:1303–1308. doi: 10.1164/ajrccm.160.4.9810058. [DOI] [PubMed] [Google Scholar]
- Hamilton MJ. Nonclonal mast cell activation syndrome: a growing body of evidence. Immunol Allergy Clin North Am. 2018;38:469–481. doi: 10.1016/j.iac.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh FH. Gastrointestinal involvement in mast cell activation disorders. Immunol Allergy Clin North Am. 2018;38(429–441):10. doi: 10.1016/j.iac.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Jiang AP, Jiang JF, Wei JF, Guo MG, Qin Y, Guo QQ, Ma L, Liu BC, Wang X, Veazey RS, Ding YB, Wang JH. Human mucosal mast cells capture HIV-1 and mediate viral trans-infection of CD4+ T-cells. J Virol. 2015;90:2928–2937. doi: 10.1128/JVI.03008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett LA, Cato LD, Weinstock JS, Zhang Y, Nouraie SM, Gladwin MT, Garrett ME, Ashley-Koch A, Telen MJ, Custer B, Kelly S, Dinardo CL, Sabino EC, Loureiro P, Carneiro-Proietti AB, Maximo C, NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium. Reiner AP, Abecasis GR, Williams DA, Natarajan P, Bick AG, Sankaran VG. Clonal hematopoiesis in sickle cell disease. J Clin Invest. 2022;132:e156060. doi: 10.1172/JCI156060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisziewicz J, Foli A, Wainberg M, Lori F. Hydroxyurea in the treatment of HIV infection: clinical efficacy and safety concerns. Drug Saf. 2003;26:605–624. doi: 10.2165/00002018-200326090-00002. [DOI] [PubMed] [Google Scholar]
- Lim KH, Pardanani A, Butterfield JH, Li CY, Tefferi A. Cytoreductive therapy in 108 adults with systemic mastocytosis: outcome analysis and response prediction during treatment with interferon-alpha, hydroxyurea, imatinib mesylate or 2-chlorodeoxyadenosine. Am J Hematol. 2009;84:790–794. doi: 10.1002/ajh.21561. [DOI] [PubMed] [Google Scholar]
- Lori F, Lisziewicz J. Rationale for the use of hydroxyurea as an anti-human immunodeficiency virus drug. Clin Infect Dis. 2000;30(Suppl 2):S193–S197. doi: 10.1086/313851. [DOI] [PubMed] [Google Scholar]
- Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O'Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Mechanisms that drive bone pain across the lifespan. Brit J Clin Pharmacol. 2019;85:1103–1113. doi: 10.1111/bcp.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert Opin Drug Safety. 2015;14:1749–1758. doi: 10.1517/14740338.2015.1088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molderings GJ, Kolck UW, Scheurlen C, Kolck UW, Scheurlen C, Brüss M, Homann J, Von Kügelgen I. Multiple novel alterations in Kit tyrosine kinase in patients with gastrointestinally pronounced systemic mast cell activation disorder. Scand J Gastroenterol. 2007;42:1045–1053. doi: 10.1080/00365520701245744. [DOI] [PubMed] [Google Scholar]
- Molderings GJ, Haenisch B, Bogdanow M, Fimmers R, Nöthen MM. Familial occurrence of systemic mast cell activation disease. PLoS One. 2013;8(9):e76241. doi: 10.1371/journal.pone.0076241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molderings GJ. The genetic basis of mast cell activation disease - looking through a glass darkly. Crit Rev Oncol Hematol. 2015;93:75–89. doi: 10.1016/j.critrevonc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Molderings GJ, Haenisch B, Brettner S, Homann J, Menzen M, Dumoulin FL, Panse J, Butterfield J, Afrin LB. Pharmacological treatment options for mast cell activation disease. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:671–694. doi: 10.1007/s00210-016-1247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiałek MW, Rybaczek D. Hydroxyurea-the good, the bad and the ugly. Genes. 2021;12:1096. doi: 10.3390/genes12071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanbakhsh N, Kesavan A. The role of mast cells in pediatric GI disease. Clin Rev Allergy Immunol. 2019;32:338–345. doi: 10.20524/aog.2019.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss UM, Bensimhon P, Zimmerman SA, Ware RE. Hydroxyurea therapy for management of secondary erythrocytosis in cyanotic congenital heart disease. Am J Hematol. 2007;82:740–743. doi: 10.1002/ajh.20925. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Duez P, Dedeken L, Cotton F, Ferster A. Hydroxyurea (hydroxycarbamide) genotoxicity in pediatric patients with sickle cell disease. Pediatr Blood Cancer. 2018;65:e27022. doi: 10.1002/pbc.27022. [DOI] [PubMed] [Google Scholar]
- Sandhu HS, Barnes PJ, Hernandez P. Hydroxyurea-induced hypersensitivity pneumonitis: a case report and literature review. Can Respir J. 2000;7:491–495. doi: 10.1155/2000/297045. [DOI] [PubMed] [Google Scholar]
- Santoro C, Sperduti I, Latagliata R, Baldacci E, Anaclerico B, Avvisati G, Breccia M, Buccisano F, Cedrone M, Cimino G, De Gregoris C, De Muro M, Di Veroli A, Leonetti Crescenzi S, Montanaro M, Montefusco E, Porrini R, Rago A, Spadea A, Spirito F, Villivà N, Andriani A, Alimena G, Mazzucconi MG. Role of treatment on the development of secondary malignancies in patients with essential thrombocythemia. Cancer Med. 2017;6:1233–1239. doi: 10.1002/cam4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutou B, Senet P, Lionnet F, Habibi A, Aractingi S. Sickle cell disease induces resistance to cutaneous carcinogenesis. Orphanet J Rare Dis. 2020;6(15):66. doi: 10.1186/s13023-020-1341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikriszt B, Póti Á, Pipek O, Krzystanek M, Kanu N, Molnár J, Ribli D, Szeltner Z, Tusnády GE, Csabai I, Szallasi Z, Swanton C, Szüts D. A comprehensive survey of the mutagenic impact of common cancer cytotoxics. Genome Biol. 2016;9(17):99. doi: 10.1186/s13059-016-0963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MR. Hydroxyurea: an overview. J Biolog Reg Homeostatic Agents. 1999;13:172–175. [PubMed] [Google Scholar]
- Tolu SS, Wang K, Yan Z, Zhang S, Roberts K, Crouch AS, Sebastian G, Chaitowitz M, Fornari ED, Schwechter EM, Uehlinger J, Manwani D, Minniti CP, Bouhassira EE. Characterization of hematopoiesis in sickle cell disease by prospective isolation of stem and progenitor cells. Cells. 2020;9:2159. doi: 10.3390/cells9102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent L, Vang D, Nguyen J, Benson B, Lei J, Gupta K. Cannabinoid receptor-specific mechanisms to alleviate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation. Haematologica. 2016;101:566–577. doi: 10.3324/haematol.2015.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock LB, Brook JB, Myers TL, Goodman B (2018) Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin, and antibiotic treatment. BMJ Case Rep bcr2017221405. 10.1136/bcr-2017-221405 [DOI] [PMC free article] [PubMed]
- Weinstock LB, Blasingame K (2020) Low dose naltrexone and gut health. In Elsegood L (ed) The LDN Book, vol 2. Chelsea Green Publishing, Vermont, pp 35–54
- Weinstock LB, Pace LA, Rezaie A, Afrin LB, Molderings GJ. Mast cell activation syndrome: a primer for the gastroenterologist. Dig Dis Sci. 2021;66:965–982. doi: 10.1007/s10620-020-06264-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data are given in the supplementary file ESM_2-xlsx.