Abstract
Background
Concerns about vaccination increased among patients with multiple sclerosis (MS) regarding side effects, efficacy, and disease exacerbation. Recently there were reports of MS relapses after the COVID-19 vaccination, which emerged the safety concerns. Therefore, we aimed to perform a systematic review of case reports and case series studies to investigate the MS relapses after COVID-19 vaccination with most details.
Methods
We systematically searched three databases, including PubMed, Scopus, and Web of Science, in February 2022. Case reports and case series which reported relapse after COVID-19 vaccination in MS patients were eligible to include in our study.
Results
Seven studies were included in our systematic review after the abstract and full-text screening with a total of 29 cases. The mean duration between COVID-19 vaccination and relapse appearance was 9.48 ± 7.29 days. Among patients, 22 cases experienced relapse after their first dosage of the COVID-19 vaccine, one after the second dose, and five after the booster dose. The type of vaccine was unknown for one patient. The most common symptoms of relapses were sensory deficits (paresthesia, numbness, dysesthesia, and hypoesthesia) and weakness.
Conclusion
Overall, the COVID-19 vaccination may trigger relapses in some MS patients, but as the infection itself can stimulate relapse, the benefit of vaccination outweighs its risk in this population, and mass vaccination against COVID-19, especially in MS patients, should be continued and encouraged.
Keywords: Multiple sclerosis, COVID-19 vaccination, Relapse, Exacerbation
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for coronavirus infection, was declared a worldwide pandemic by the World Health Organization (WHO) in March 2020 [1]. Vaccination against COVID-19 is the primary long-term strategy to stop this pandemic globally [2]; thus, worldwide initiatives were done to develop vaccines against this pandemic which has claimed over 6 million lives and affected over 500 million people as of April 2022 [3]. Patients with comorbidities, especially autoimmune diseases, have been considered at higher risk to develop a more severe form of the disease [4]. A systematic review and meta-analysis in 2021 showed the pooled prevalence of suspected covid-19 in patients with multiple sclerosis (MS) was 4%, hospitalization was 10%, and death in hospitalized patients was 4% [5].
MS is the most prevalent disabling permanently neurological disease among young adults and is associated with high socioeconomic cost and diminished quality of life [6]. Infectious diseases are the leading cause of death and a common cause of comorbidity among patients with MS and may cause the exacerbation of MS symptoms; thus, vaccination in patients with MS should be purposed as a general policy to decrease the risk of infections [6].
Concerns about vaccination increased among health care providers and patients with MS regarding side effects, efficacy, and disease exacerbation [7]. Neurological manifestations are rare complications of COVID-19 infection and vaccination [8]. Among neurological manifestations, autoimmune disorders which affect the nervous system are rare (<0.1%) after COVID-19 vaccination [9]. In another study, after the first dose of Pfizer BioNTech and AstraZeneca vaccines, the most common complications were Guillain–Barré syndrome and Bell’s palsy [10]. Recently there were reports of MS relapses after the COVID-19 vaccination, which emerged safety concerns [11], [12], [13]. A study by Fragoso et al. revealed that patients with no evidence of MS activity and no change in their medications developed a new relapse with new lesions on magnetic resonance imaging (MRI) along with increased disability following their first dose of AstraZeneca vaccine for COVID-19 [14]. Therefore, we aimed to perform a systematic review of case reports and case series studies to investigate the MS relapses after COVID-19 vaccination with most details.
2. Methods
This study was conducted following preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline [15].
2.1. Search strategy
We systematically searched three databases, including PubMed, Scopus, and Web of Science, in February 2022. Our search strategy included the following terms: (Multiple sclerosis) AND (COVID-19 OR SARS-COV-2 OR corona virus OR Coronavirus Disease OR 2019-nCoV Disease) AND (Vaccination OR Vaccine OR immunization).
2.2. Eligibility criteria
All case reports and case series which reported relapse after COVID-19 vaccination in MS patients were eligible to include in our study. The non-English article, studies with other vaccination for another virus, review papers, and other types of original studies (cohorts, case-control, and clinical trials) were excluded.
2.3. Study selection
Two independent reviewers (F.N, K.K) screened the title and abstracts and excluded irrelevant studies. Then the same reviewers checked the full text of the remaining articles to evaluate their eligibility to include in our study.
2.4. Data extraction
The same investigators (F.N, K.K) extracted the following information based on a predesigned datasheet: Study the demographic, type of MS, age, sex, MS duration, clinical presentation before relapse, MRI findings, type of COVID-19 vaccination, vaccine dosage, the interval between relapse and vaccination, the clinical presentation of relapse, treatments, and outcomes.
2.5. Quality assessments
The quality of included studies was assessed using the Joanna Briggs Institute Critical Appraisal tools for Case Reports independently by two reviewers (F.N, K.K) [16]. The answer to the questions was based on “Yes” or “No” and the score ranged from 0 to 8.
3. Results
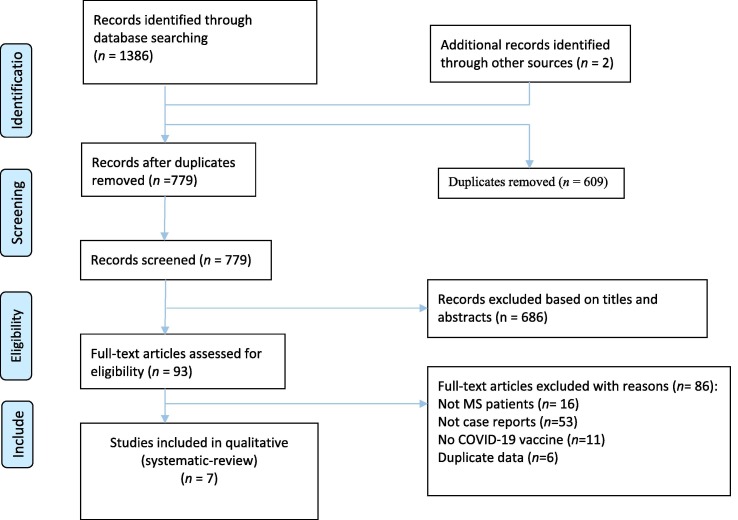
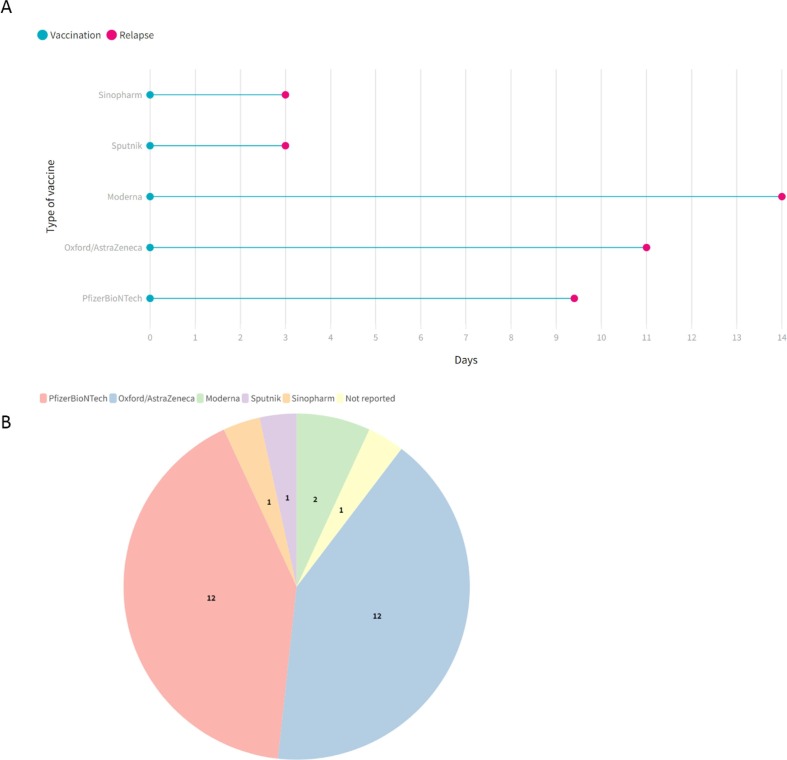
Our initial search yielded 1386 studies (Fig. 1 ). After duplicate removing, 779 papers were screened. At this step, 686 articles were excluded via title and abstract evaluation, and the remaining studies underwent full-text review. Finally, seven studies entered our systematic review [11], [12], [13], [14], [17], [18], [19]. The demographical and clinical characteristics of included studies are detailed in Table 1 . A total of 29 cases with a mean age of 43.2 ± 11.5 and a range [22–66] were included in our study. 68% of the patients were female, and eight cases were reported as RRMS. The mean duration between COVID-19 vaccination and relapse appearance was 9.48 ± 7.29 days (Fig. 2 ). Twelve patients received Oxford/AstraZeneca, twelve received PfizerBioNTech, two Moderna, and each one received Sputnik and Sinopharm (Fig. 2). Among patients, 22 cases experienced relapse after their first dosage of the COVID-19 vaccine, one after the second dose, and five after the booster dose. The type of vaccine was unknown for one patient. The most common symptoms of relapses were sensory deficits (n = 14) (paresthesia, numbness, dysesthesia, and hypoesthesia) and weakness (n = 6). After relapse, most of the patients received glucocorticoids, and 13 patients recovered, five partially recovered, and five patients not yet recovered until the end of the study follow-up (see Fig. 3 ).
Fig. 1.
PRISMA flow diagram depicting the flow of information through the different phases of a systematic review.
Table 1.
Demographical and clinical findings of the included studies.
| Study | Country | Type of MS | Age | Sex | MS disease duration | Clinical presentation of MS before relapse | DMTs | MRI Findings | Type COVID-19 vaccine | Vaccine dosage | Time interval between vaccination and relapse | Relapse clinical presentations | Treatments | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lagosz et al. 2022 | Poland | NR | 64 | M | NR | NR | NR | A hypodense lesion in the left frontal-parietal area | NR | NR | 1 day | Feeling numbness, worsened mobility in the arms and fatigue | Glucocorticoids | Recovered |
| Kataria et al. 2022 | USA | NR | 57 | F | 6 years | NR | Interferon-beta | Multiple confluent and distinct hyperintense white matter enhancing lesions in both hemispheres on T2-weighted and diffusion-weighted images. Spine MRI was normal. | BNT162b2/PfizerBioNTech | 2nd | 18 days | Fatigue, involuntary eye movements, numbness, tingling, stiffness in her left upper and lower limbs | Intravenous methylprednisolone and physiotherapy , baclofen | Recovered |
| Etemadifar et al. 2021 | Iran | RRMS | 34 | F | 13 years | optic neuritis and bilateral lower limb paresthesia / paraparesis | Interferon-beta 1a. | Several new periventricular, juxtacortical and brainstem lesions on T2 | Sputnik | 1st | 3 days | Severe right hemiplegia and ataxia | Oral methylprednisolone for 3 weeks | Recovered |
| Ahadi et al. 2021 | Iran | RRMS | 42 | F | 20 years | optic neuritis/ hemiparesthesia and monoparesis/ paraparesis | Interferon-beta 1b. | Showed numeral periventricular, anterior medullary white matter hyper-intensities | Sinopharm | 1st | 2 days | Progressive paraparesis without paresthesia | Intravenous methylprednisolone | Recovered |
| Maniscalco et al. 2021 | Italy | NR | 31 | F | 5 years | Tinnitus and dizziness | Fingolimod | Three new voluminous enhancing lesions | BNT162b2/PfizerBioNTech | 1st | 48 hours | Paraesthesia and weakness in her left arm and limbs | Intravenous methylprednisolone | Recovered |
| Fragoso et al. 2021 | Brazil | RRMS | 22 | F | 5 years | NR | Fingolimod | Non-Gd tumefactive lesion | Oxford/AstraZeneca | 1st | 7 days | Facial paralysis, hemiparesis, ataxia | Pulsotherapy methylprednisolone | Not yet recovered |
| RRMS | 32 | F | 2 years | NR | Dimethyl fumarate | New Gd + lesions in the left eye | Oxford/AstraZeneca | 1st | 10 days | Loss of vision and papillitis in the left eye | Pulsotherapy methylprednisolone Immunoglobulin | Partial recoved | ||
| SPMS | 35 | M | 3 years | NR | Natalizumab | High lesion load , new lesions | Oxford/AstraZeneca | 1st | 7 days | Worsening of disability, could not walk, severe weakness of both legs | Oral prednisone | Not yet recovered | ||
| RRMS | 30 | F | 1 year | NR | Natalizumab | New Gd + lesions | Oxford/AstraZeneca | 1st | 25 days | Right hemiparesis | Pulsotherapy methylprednisolone | Recovered | ||
| RRMS | 42 | F | 3 years | NR | Fingolimod | New Gd+ lesions in spinal cord, T2 level | Oxford/AstraZeneca | 1st | 15 days | Rapidly progressive weakness in both arms , grade III at its worst | Pulsotherapy methylprednisolone | Recovered | ||
| RRMS | 35 | M | 4 years | NR | Teriflunomide | New Gd+ lesions in brainstem | Oxford/AstraZeneca | 1st | 20 days | Incoordination of right arm and hand | Pulsotherapy methylprednisolone | Not yet recovered | ||
| PPMS | 51 | M | 2 years | NR | NR | New Gd+ lesions in cervical cord | Oxford/AstraZeneca | 1st | 25 days | Hypoesthesia in both arms | No treatment | Not yet recovered | ||
| RRMS | 32 | F | 6 years | NR | Glatiramer acetate | New Gd+ lesions+ new lesions | Oxford/AstraZeneca | 1st | 7 days | Motor and sensitive deficits in right leg and foot | Pulsotherapy methylprednisolone | Not yet recovered | ||
| Nistri et al. 2021 |
Italy |
NR | 48 | F | New diagnosis | visual acuity deficit from right eye | NR | Enhancing lesion in the corpus callosum, multiple white matter unenhanced lesions and lesions in the occipital lobe were detected | Oxford/AstraZeneca | 1 st | 8 days | Visual acuity deficit from right eye | High dose of intravenous methylprednisolone | Recovered |
| NR | 45 | M | 9 years | NR | Ocrelizumab | Two new lesions in the temporal gyri and a new spinal cord lesion at T3 level | Oxford/AstraZeneca | 1st | 3 weeks | Dysesthesia in both legs | Steroids | NR | ||
| NR | 54 | F | 28 years | NR | NR | One enhancing lesion in the spinal cord | Oxford/AstraZeneca | 1 st | 3 days | Developed hypoesthesia below the T6 level | Intravenous methylprednisolone | Recovered | ||
| NR | 66 | F | New diagnosis | visual disturbance and postural instability on the right limbs | NR | Multiple white matter lesions, four of them enhancing in the left paratrigonal and periventricular white matter | Oxford/AstraZeneca | 1 st | 1 week | Visual disturbance and postural instability on the right limbs | Intravenous methylprednisolone | Partial recovered | ||
| NR | 42 | F | 2 years | progressive weakness on the right side of body | Ocrelizumab | Enhancing brain lesion in the right corona radiata | Moderna | 1 st | 2 weeks | Slight weakness of the left upper limb | NR | NR | ||
| NR | 57 | M | 20 yeas | NR | NR | Enhancing pontine lesion | Moderna | booster | 2 weeks | Severe motor deficit in both legs | Intravenous methylprednisolone | Partial recovered | ||
| NR | 49 | F | 8 years | NR | Dimethyl fumarate | A periventricular lesion and a spinal lesion at C3 level, both enhancing | BNT162b2/PfizerBioNTech | 1 st | 5 days | Numbness on the left hand and left side of her head | Intravenous methylprednisolone | Recovered | ||
| NR | 39 | M | 7 years | hypoesthesia on left side | Dimethyl fumarate | Three new lesions, two of which were enhancing in the left parietal lobe and in the periventricular white matter | BNT162b2/PfizerBioNTech | 1st | 10 days | Paresthesia on left leg | Oral steroids | Partial recoverd | ||
| NR | 39 | F | New diagnosis | NR | NR | A new enhancing lesion in the mesencephalon | BNT162b2/PfizerBioNTech | 1st | 3 days | Dysesthesia on her right hand and foot | Intravenous methylprednisolone | Recovered | ||
| NR | 60 | F | 23 years | NR | Dimethyl fumarate | One enhancing brain lesion in the left periventricular white matter | BNT162b2/PfizerBioNTech | 1st | 2 days | Fatigue and numbness in both legs | NR | NR | ||
| NR | 30 | F | 3 years | optic neuritis | Cladribine | Two enhancing brain lesions, one in the right corona radiata and one with conspicuous oedema in the left centrum semiovale | BNT162b2/PfizerBioNTech | booster | 20 days | Language disturbance | NR | NR | ||
| NR | 58 | F | 21 years | NR | NR | A new area with ring enhancement in the white matter of the left frontal lobe | BNT162b2/PfizerBioNTech | 1st | 3 days | Headache, balance disturbance, urinary incontinence, difficulties in walking and dysphagia | Intravenous methylprednisolone | Recovered | ||
| NR | 34 | F | 3 months | numbness and hyposthenia on her right hand | NR | Three brain enhancing lesion (one right posterior paraventricular and two in the left periventricular white matter) and a new unenhanced lesion on spinal cord | BNT162b2/PfizerBioNTech | booster | 4 days | Neck pain and hypoesthesia on right arm | NR | NR | ||
| NR | 35 | F | 16 years | NR | Dimethyl fumarate | Three enhancing lesions in the left temporal lobe and left centrum semiovale | BNT162b2/PfizerBioNTech | booster | 1 day | Paresthesia on the left side of body | NR | NR | ||
| NR | 54 | M | 18 years | NR | Teriflunomide | Two ring-enhancing lesions located in the left periventricular white matter | bNT162b2/PfizerBioNTech | 1st | 1 week | Right hemiparesis | Intravenous methylprednisolone | Recovered | ||
| NR | 37 | M | 2 years | NR | Dimethyl fumarate | A new tumefactive contrast-enhancing lesion in the left fronto-parietal white matter | BNT162b2/PfizerBioNTech | booster | 11 days | Weakness on right limbs | Intravenous methylprednisolone | Partial recovered |
Abbreviations: NR, Not Reported, RRMS, relapsing remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis; PPMS, primary progressive multiple sclerosis, DMTs, disease modyfing therapies.
Fig. 2.
The mean duration between vaccination and relapse based on type of vaccine (A), and type of COVID-19 vaccine used among cases (B).
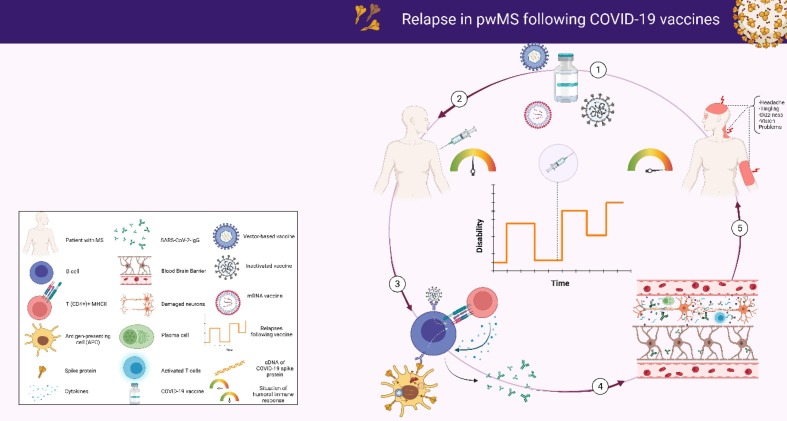
Fig. 3.
Mechanism of relapse in patients with MS following COVID-19 vaccination, by BioRender.
The result of the quality assessment using JBI criteria revealed that six studies scored more than 7, and only one study scored 5 (Table 2 ). The mean JBI score for all included studies was 7.28.
Table 2.
The Joanna Briggs Institute Critical Appraisal tools for Case Reports.
| Lagosz et al. 2022 | Kataria et al. 2022 | Etemadifar et al. 2021 | Ahadi et al. 2021 | Maniscalco et al. 2021 | Fragoso et al. 2021 | Nistri et al. 2021 | |
|---|---|---|---|---|---|---|---|
| Were patient’s demographic characteristics clearly described? | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the patient’s history clearly described and presented as a timeline? | No | No | Yes | Yes | Yes | Yes | No |
| Was the current clinical condition of the patient on presentation clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were diagnostic tests or assessment methods and the results clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the intervention(s) or treatment procedure(s) clearly described? | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the post-intervention clinical condition clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were adverse events (harms) or unanticipated events identified and described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Does the case report provide takeaway lessons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total rank | 5 | 7 | 8 | 8 | 8 | 8 | 7 |
4. Discussion
Although mass vaccination against COVID-19 is the preferred way of controlling the disease, concerns around the long-term safety of these vaccines have remained unclear, in particular in patients with underlying comorbidities [20]. Autoimmune disorders comprise a group of these comorbidities for which vaccination may trigger undesired responses. Post-vaccination relapses in neurological autoimmune disorders such as MS and Guillen Barre have been previously reported with HBV, Influenza, polio, and tetanus vaccines [21], [22]. Available COVID-19 vaccines are no exception, and reports of Bell's palsy, transverse myelitis, Guillen Barre syndrome and MS relapses have emerged [23].
To the current time point, there is no contraindication for COVID-19 vaccination in MS patients except for living attenuated vaccines in patients under immunosuppressive or immunomodulatory regimens [24]. In addition, no vaccine is favored for MS patients [14]. However, reports of relapses after either first or booster doses of COVID-19 vaccines indicate an association between disease pathophysiology and vaccination. In an interval ranging from one to 25 days after vaccination, a portion of MS patients manifested neurological symptoms, with the most common ones being paralysis, visual loss, weakness, and motor deficits. The extent of immune response in MS patients depends on both individual genetic susceptibility, and the type of vaccine used [13], [25].
Cross-reactivity and bystander activation are well-established theories justifying autoimmunity after vaccination. Depending on the vaccine type, one of these mechanisms may be more relevant. In the case of Pfizer, which is an RNA virus coding for spike proteins in lipid membrane without any adjuvant, cross-reactivity may explain the situation as the COVID-19 spike protein antibody is structurally similar to myelin basic protein [25]. Besides, the interaction between spike proteins and Angiotensin-Converting Enzyme 2 (ACE2) receptors located in the Blood-Brain Barrier (BBB) and spinal neurons have been reported in several in vivo studies [26]. This is true for Coronavirus itself, as it can cross BBB either with transcytosis using ACE2 receptors or reach brain parenchyma via the olfactory bulb [14]. However, autoimmunity after the AstraZeneca vaccine is less likely to happen because of this cross-reactivity. AstraZeneca has an adjuvant (MF59) that has clearly been shown to induce inflammation by secretion of cytokines, including IL-6, IL-8, chemokine CCL-2, CCL-3, and CCL-4 [27]. The adjuvant can activate the Toll-Like Receptor (TLR) that per se prompts nuclear factor kappa B (NF-κB) phosphorylation. NF-κB is a transcription factor of up to 1500 inflammatory genes, including cytokines and chemokines. These molecules supply T and B cells with adequate stimuli to recognize their specific antigen and initiate clonal activation. In MS patients, these inflammatory molecules can interfere with control over self-reacting clones and activate unrelated lymphocytes, something that is called bystander activation [28], [29]. In this way, clonal expansion occurs, and the disease relapses.
Despite the fact that these relapses were temporally associated with vaccine administration, with current studies, it is impossible to disentangle post-vaccination relapses from the relapses that would have manifested regardless of COVID-19 vaccination [13]. In a study on 555 MS patients, 2.1% of patients receiving the first dose and 1.6% with the second dose experienced relapses; however, no difference in the relapse rate was highlighted when the results were compared to previous years [30]. This study was limited to a short follow-up period and therefore its results should be interpreted with caution. More studies are warranted to show a causal association.
Overall, the COVID-19 vaccination may trigger relapses in some MS patients but as the infection itself can stimulate relapse, the benefit of vaccination outweighs its risk in this population, and mass vaccination against COVID-19 especially in MS patients should be continued and encouraged [31]. In the meanwhile, most of the relapsed cases were fully recovered after receiving methylprednisolone showing the relapse can be controlled without consequences [11], [13], [14], [17], [18].
Funding
We do not have any financial support for this study.
Ethical approval
Since the data in this paper were obtained from the PPMI database (ppmi.loni.usc.edu), it does not include any research involving human or animal subjects.
Availability of data and material
The datasets analyzed during the current study are available upon request with no restriction.
Consent for publication
This manuscript has been approved for publication by all authors.
Author contributions
All the authors listed in the manuscript have participated actively in preparing the final version of this case report.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hassaniazad M., Farshidi H., Gharibzadeh A., Bazram A., Khalili E., Noormandi A., et al. Efficacy and safety of favipiravir plus interferon-beta versus lopinavir/ritonavir plus interferon-beta in moderately ill patients with COVID-19: A randomized clinical trial. J Med Virol. 2022;94(7):3184–3191. doi: 10.1002/jmv.27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y., Rodgers W.J., Middleton R.M., Baheerathan A., Tuite-Dalton K.A., Ford D.V., et al. Willingness to receive a COVID-19 vaccine in people with multiple sclerosis – UK MS Register survey. Multiple Scler Related Disorders. 2021;55:103175. doi: 10.1016/j.msard.2021.103175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moghadasi A.N., Mirmosayyeb O., Barzegar M., Sahraian M.A., Ghajarzadeh M. The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): a systematic review and meta-analysis. Neurol Sci. 2021;42(8):3093–3099. doi: 10.1007/s10072-021-05373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persson R., Lee S., Ulcickas Yood M., Wagner C.M., Minton N., Niemcryk S., et al. Infections in patients diagnosed with multiple sclerosis: a multi-database study. Mult Scler Relat Disord. 2020;41:101982. doi: 10.1016/j.msard.2020.101982. [DOI] [PubMed] [Google Scholar]
- 7.Yazdani A., Mirmosayyeb O., Ghaffary E.M., Hashemi M.S., Ghajarzadeh M. COVID-19 vaccines and patients with multiple sclerosis: willingness, unwillingness and hesitancy: a systematic review and meta-analysis. Neurol Sci. 2022;43(7):4085–4094. doi: 10.1007/s10072-022-06051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patone M., Handunnetthi L., Saatci D., Pan J., Katikireddi S.V., Razvi S., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etemadifar M., Sigari A.A., Sedaghat N., Salari M., Nouri H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum Vaccin Immunother. 2021;17(10):3481–3483. doi: 10.1080/21645515.2021.1928463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataria H., Hart C.G., Alizadeh A., Cossoy M., Kaushik D.K., Bernstein C.N., et al. Neuregulin-1 beta 1 is implicated in pathogenesis of multiple sclerosis. Brain. 2021;144(1):162–185. doi: 10.1093/brain/awaa385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nistri R., Barbuti E., Rinaldi V., Tufano L., Pozzilli V., Ianniello A., et al. Case report: multiple sclerosis relapses after vaccination against SARS-CoV2: a series of clinical cases. Front Neurol. 2021;12:765954. doi: 10.3389/fneur.2021.765954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fragoso Y.D., Gomes S., Gonçalves M.V.M., Mendes Junior E., Oliveira B.E.S., Rocha C.F., et al. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult Scler Relat Disord. 2022;57:103321. doi: 10.1016/j.msard.2021.103321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed]
- 16.M P. Moola S MZ, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis P. JBI Manual for Evidence Synthesis. JBI Manual for Evidence Synthesis. 2020.
- 17.Łagosz P., Biegus J., Gruszka E., Zymliński R. The surprising course of multiple sclerosis relapse in a patient after SARS-CoV-2 vaccination. Kardiol Pol. 2022;80(2):237–238. doi: 10.33963/KP.a2022.0005. [DOI] [PubMed] [Google Scholar]
- 18.Maniscalco G.T., Manzo V., Di Battista M.E., Salvatore S., Moreggia O., Scavone C., et al. Severe multiple sclerosis relapse after COVID-19 vaccination: a case report. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seyed Ahadi M., Ghadiri F., Ahraian M.A., Naser Moghadasi A. Acute attack in a patient with multiple sclerosis 2 days after COVID vaccination: a case report. Acta Neurol Belg. 2021 doi: 10.1007/s13760-021-01775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knoll M.D., Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Confavreux C., Suissa S., Saddier P., Bourdès V., Vukusic S. Vaccinations and the risk of relapse in multiple sclerosis. N Engl J Med. 2001;344(5):319–326. doi: 10.1056/NEJM200102013440501. [DOI] [PubMed] [Google Scholar]
- 22.Geier M.R., Geier D.A., Zahalsky A.C. Influenza vaccination and Guillain Barre syndrome. Clin Immunol. 2003;107(2):116–121. doi: 10.1016/s1521-6616(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Raventós B, Roel E, Pistillo A, Martinez-Hernandez E, Delmestri A, et al. Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis. BMJ. 2022;376:e068373. [DOI] [PMC free article] [PubMed]
- 24.Dema M., Eixarch H., Villar L.M., Montalban X., Espejo C. Immunosenescence in multiple sclerosis: the identification of new therapeutic targets. Autoimmun Rev. 2021;20(9):102893. doi: 10.1016/j.autrev.2021.102893. [DOI] [PubMed] [Google Scholar]
- 25.Langer-Gould A., Qian L., Tartof S.Y., Brara S.M., Jacobsen S.J., Beaber B.E., et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014;71(12):1506. doi: 10.1001/jamaneurol.2014.2633. [DOI] [PubMed] [Google Scholar]
- 26.Farez M.F., Correale J., Armstrong M.J., Rae-Grant A., Gloss D., Donley D., et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology. 2019;93(13):584–594. doi: 10.1212/WNL.0000000000008157. [DOI] [PubMed] [Google Scholar]
- 27.Ko E.J., Kang S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum Vaccin Immunother. 2018;14(12):3041–3045. doi: 10.1080/21645515.2018.1495301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaral M.P., Branco L.M., Strasser A., Dixit V.M., Bortoluci K.R. Paradise revealed III: why so many ways to die? Apoptosis, necroptosis, pyroptosis, and beyond. Cell Death Differ. 2020;27(5):1740–1742. doi: 10.1038/s41418-020-0526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 30.Achiron A., Dolev M., Menascu S., Zohar D.-N., Dreyer-Alster S., Miron S., et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diem L., Friedli C., Chan A., Salmen A., Hoepner R. Vaccine hesitancy in patients with multiple sclerosis: preparing for the SARS-CoV-2 vaccination challenge. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e991. doi: 10.1212/NXI.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available upon request with no restriction.