Summary
Cable bacteria (CB) are non-isolated filamentous bacteria in the family of Desulfobulbaceae, known for fostering centimeter-long electron transfer in sediments with pronounced redox zonation. This protocol details steps to extract CB filaments from cultured natural sediment, inoculate autoclaved sediment with extracted filaments, and subsequently evaluate the growth and enrichment of CB. We also describe the approaches for collecting suitable sediment, preparing autoclaved sediment, and manufacturing glass needles and hooks for the extraction of CB.
Subject areas: Cell culture, Microbiology, Environmental sciences
Graphical abstract
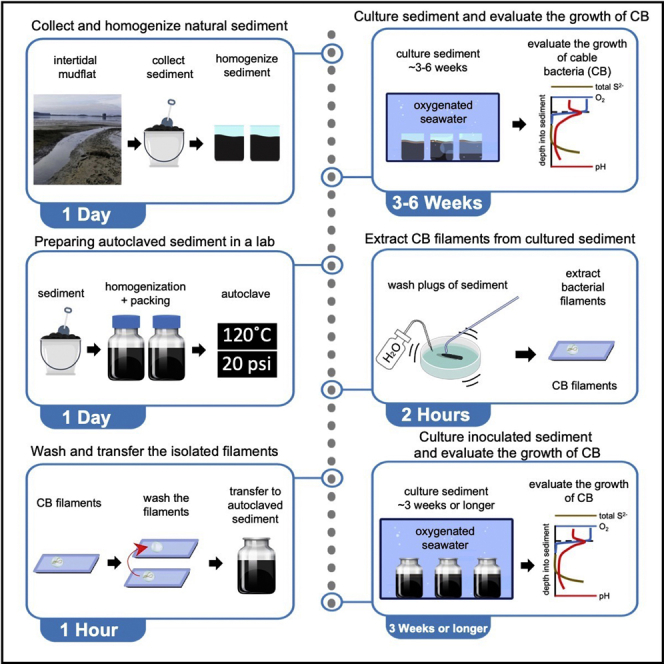
Highlights
-
•
Prepare autoclaved sediment as an enrichment medium for cable bacteria
-
•
Manufacture glass needles and hooks as tools to extract cable bacteria
-
•
Video demonstration of cable bacteria extraction and autoclaved sediment inoculation
-
•
Recover prolific cable bacteria biomass grown in autoclaved sediment
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Cable bacteria (CB) are non-isolated filamentous bacteria in the family of Desulfobulbaceae, known for fostering centimeter-long electron transfer in sediments with pronounced redox zonation. This protocol details steps to extract CB filaments from cultured natural sediment, inoculate autoclaved sediment with extracted filaments, and subsequently evaluate the growth and enrichment of CB. We also describe the approaches for collecting suitable sediment, preparing autoclaved sediment, and manufacturing glass needles and hooks for the extraction of CB.
Before you begin
Cable bacteria (CB) are as of yet non-isolated taxa within the family of Desulfobulbaceae, known for establishing centimeter-long filamentous networks in the uppermost layers of sediments with redox zonation (Pfeffer et al., 2012). The long distances spanned by individual filaments allow CB to access energy harvested from sulfide oxidation (½ H2S + 2H2O → ½ SO42- + 4e- + 5H+) within sulfidic zones and oxygen reduction (O2 + 4e- + 4H+ → 2H2O) within oxic zones (Kjeldsen et al., 2019; Pfeffer et al., 2012). To transfer electrons between the reactants of the two half-reactions and to acquire metabolic energy, CB construct electrically conductive fibers within their periplasmic envelope that extend longitudinally along filaments (Boschker et al., 2021; Cornelissen et al., 2018; Meysman et al., 2019). The periplasmic fibers and the associated structures passing charge onto and off the fibers are being investigated for a host of biomaterial applications, while the phylogeny and lifestyle behavior of CB are of fundamental interest in microbial ecology and environmental applications such as remediation of contaminants. In this protocol, we detail a simple approach utilizing autoclaved natural sediment as a medium to enrich and obtain CB biomass for easier study.
The first step we follow to enrich CB in autoclaved sediment is to cultivate CB in natural sediment from which CB filaments have been observed. The electrically connected but separated half-reactions thus create (or permit) a suboxic zone where no oxygen or sulfide is detected. The natural presence of CB and the geochemical signature of their associated suboxic zone has been observed in various surficial sediments from diverse habitats, including intertidal mudflats, mangroves, rivers, salt marshes, and certain bioturbated sediments exhibiting stable biogenic structures (Aller et al., 2019; Burdorf et al., 2016; Dam et al., 2021; Li et al., 2020a, 2020b; Malkin et al., 2014, 2017; Malkin and Meysman, 2015; Risgaard-Petersen et al., 2015; Trojan et al., 2016). Prior research has not only provided a list of locations where CB can be found (Table 1), but it suggests that the ideal sedimentary matrix to enrich CB should contain 1) dissolved hydrogen sulfide in pore waters below the sediment-water interface, 2) persistent dissolved oxygen in the overlying seawater, and 3) a stably stratified zonation of redox layers. Evidence from geographic DNA databases has continued to show the existence of CB in a multitude of settings. However, care must be taken when collecting from a local site. Estuaries are the most common access point for fresh culturing material and can exhibit a range of conditions. In addition to these being potentially dangerous places to collect samples, local conservation efforts may be in place to protect existing or restore historic, mudflats and adjacent habitats. Even if the volume of culturing mud collected is small, be sure to determine the impact of the collection. CB would appear to thrive in human-disturbed settings, and we recommend focusing on collections that are purely for biomaterial work in already disturbed, high sedimentation environments such as harbors. Bulk collection of material from conservation sites, seagrass meadows, and protected mudflats should be avoided.
Table 1.
Locations listed in previous studies where cable bacteria can be found
| Sediment type | Approximate salinity (PSU) | Location | Coordinates | Reference |
|---|---|---|---|---|
| Marine or Brackish | ||||
| Harbor sediments | 20 | Aarhus Harbor Denmark | 56.14°N, 10.22°E | (Nielsen et al., 2010; Pfeffer et al., 2012) |
| Bay sediment | 30 | Aarhus Bay Denmark | 56.10°N, 10.46°E | (Nielsen et al., 2010; Pfeffer et al., 2012) |
| Unvegetated intertidal sediment | 28 | Rattekaai Salt Marsh, the Netherlands | 51.44°N, 4.17°E | (Malkin et al., 2014; Malkin and Meysman, 2015) |
| Muddy subtidal sediment | N/A | Station 130 in the Belgian Coastal Zone | 51.27°N, 2.91°E | (Malkin et al., 2014) |
| Muddy sediment in a seasonally stratified coastal basin. | 30 | Marine Lake Grevelingen, the Netherlands | 51.75°N, 3.89°E | (Malkin et al., 2014; Seitaj et al., 2015) |
| Muddy intertidal mudflat sediment | 29 | Sally’s bend, Yaquina Bay, Oregon, USA | 44.63°N, 124.01°W | (Li et al., 2020a) |
| Subtidal deposits from downstream of a commercial oyster farm | 29 | Yaquina Bay, Oregon, USA | 44.58°N, 123.99°W | (Li et al., 2020a) |
| Carbonate muddy bay sediment | 25 | Florida Bay, Florida, USA | 25.12°N, 80.82°W | (Yin et al., 2021) |
| Muddy sediment within the bivalve reef | 29 | Northeast shore of the barrier island of Texel, Wadden Sea, The Netherlands | 53.15°N, 4.90°E | (Malkin et al., 2017) |
| Muddy sediment bioturbated by the parchment worm | 26–31 | Great Peconic Bay, Long Island, New York, USA | 40.95°N, 72.49°W | (Aller et al., 2019) |
| Muddy intertidal mudflat sediment bioturbated by mud shrimps | 29 | Idaho flat, Yaquina Bay, Oregon, USA | 44.62°N, 124.04°W | (Li et al., 2020b) |
| Tidal sediment covered by a biofilm dominated by diatoms | 28 | Oosterschelde tidal inlet, the Netherlands | 51.44°N, 4.17°E | (Malkin et al., 2014; Malkin and Meysman, 2015) |
| Seagrass sediment | 30–32 | The Swan River estuary, Australia | 32.03°S, 115.76°E | (Martin et al., 2019) |
| Mangrove sediment | 30–32 | Western Port and Anderson Inlet, Australia | 38.633°S, 145.183°E and 38.229°S, 145.309°E | (Burdorf et al., 2016) |
| Freshwater or groundwater | ||||
| Surface sediment of a lowland, hard-water stream | 0 | Giber Å, Eastern Jutland, Denmark | 56.07°N, 10.17°E | (Risgaard-Petersen et al., 2015; Scholz et al., 2019) |
| Freshwater ponds sediment | 0 | Vennelystparken, Aarhus, Denmark | 56.16°N, 10.21°E | (Kjeldsen et al., 2019) |
| Groundwater aquifer sediment contaminated by tar oil | 0 | Düsseldorf-Flingern, Germany | 51.22°N, 6.82°E | (Müller et al., 2016, 2020) |
Harvest sediment that contains cable bacteria from an accessible location
Timing: 1 day
-
1.
Identify a safely accessible location to harvest marine sediment away from protected habitats or highly polluted areas.
-
2.
Collect the suboxic to the sulfidic zone of the sediment (usually between 0–30 cm depth) using a shovel or other digging tools.
Note: The geochemical patterns created by the CB in porewater profiles can be measured onsite using microelectrodes before selecting a collection depth for the sediment. Details about how to perform onsite microelectrode profiling can be found in Malkin et al. (2014).
-
3.
Put the sediment in a large container (e.g., a large bucket with a lid), gathering only as much as needed.
-
4.
If possible, collect some overlying water to measure the salinity by using a conductivity-based salinity meter or a refractometer.
-
5.
If enough overlying water is present, collect several liters or retrieve water from a nearby location with similar salinity in a different container.
-
6.
Bring sediment and seawater to a laboratory.
-
7.
Store the sediment and seawater at a refrigerated temperature (5°C or lower) or process the sediment right away as described in the next step.
CRITICAL: Prior to sampling, be sure to determine appropriate personal protective equipment for the collection location, such as boots and waders in cold environments. When sampling tidal locations, check tide tables prior to collection and be aware of your surroundings. Do not sample alone.
Alternatives: Sediment can be collected by other retrieval tools such as push cores, sediment grabs, and traditional digging equipment.
Homogenize sediment in a laboratory
Timing: 1 day
-
8.
Sieve the stored sediment through a 0.5 mm mesh screen to remove any large fauna, shells, rocks, and debris, capturing sieved material into a clean receptacle.
-
9.
Homogenize the sieved sediment by physical mixing.
Note: Oxygen exposure can be reduced during the homogenization process by introducing a tube that flows nitrogen gas into the sediment.
-
10.
Pack the homogenized sediment into containers with a large opening (e.g., a core liner or a wide-mouth glass bottle).
CRITICAL: The homogenization of sulfidic sediment may release hydrogen sulfide, a toxic chemical in its gaseous form. We highly recommend the use of personal protection equipment such as a sulfide-specific face respirator or conducting the homogenization process under a ventilation hood or in a very well-ventilated open space.
Alternatives: A kitchen strainer with a similar mesh size can replace the use of a mesh screen. Nitrogen gas can be replaced by other inert gases such as helium and argon.
Culture natural sediment and evaluate the growth of cable bacteria
Timing: 3–6 weeks
-
11.
Place the containers into an aquarium filled with autoclaved seawater and keep the seawater aerated by using a commercially available aquarium air pump and bubbling stone.
-
12.
Evaluate the growth and presence of CB by measuring the vertical chemical profiles of oxygen, sulfide, and pH.
Note: The vertical chemical profiles of oxygen, sulfide, and pH that are characteristic of CB can be measured by microelectrodes. More details about performing microelectrode profiling in a lab can be found in Malkin et al. (2014) and Li et al., 2020a, Li et al., 2020b.
Alternatives: Artificial seawater can also be used as the culture media in commercially available aquarium tanks. The presence of CB can also be verified by using microscopic techniques (e.g., scanning electronic microscopy and transmission electron microscopy) to identify their unique morphological features namely the longitudinal ridges and cartwheel cell-cell junctions (Li et al., 2020b; Pfeffer et al., 2012), molecular techniques (e.g., fluorescent in situ hybridization) (Li et al., 2020b; Malkin et al., 2014, 2017), and sequencing techniques (e.g., maker gene analysis and metagenomics) (Geelhoed et al., 2020; Kjeldsen et al., 2019; Li et al., 2020b; Trojan et al., 2016). The growth and density of CB can be evaluated using counts of stained filaments and quantitative polymerase chain reaction methods (Aller et al., 2019; Geelhoed et al., 2020).
Prepare thin glass hooks and needles for extracting CB filaments
Timing: 1 h
-
13.
Hold the two ends of a glass capillary tube and put the middle of the tube over the flame of a Bunsen burner.
-
14.
Rotate the tube slowly to heat evenly.
-
15.
When the middle of the glass tube becomes soft, quickly move the tube away from the flame and pull the two ends evenly to make a thin tapered mid-section. Troubleshooting.
-
16.
Separate the glass tube into two pipettes by melting at the middle of the newly pulled section and shorten the thin end to a desired length.
-
17.
Use heat from outside a lighter flame to bend the thin tip of each glass pipette needle into the shape of a hook.
CRITICAL: Wear gloves, do not touch hot glass, and be watchful to avoid burns (Methods video S1).
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Other | ||
| 5 Gallon White Plastic Bucket & Lid | Amazon | https://www.amazon.com/dp/B075X4SPSG/ref=cm_sw_em_r_mt_dp_VRDRT98F03K0PSCWR5Q9 |
| Heavy Duty Carbon Steel Garden Hand Shovel | Amazon | https://www.amazon.com/dp/B079P8HBY1/ref=cm_sw_em_r_mt_dp_TBCZYCT64JN2FYCHQM0N |
| Water and slip resistant Boots | VWR International LLC | Cat# 76104-862 |
| Fisherbrand™ U.S. Standard Brass Sieves with Steel Cloth (0.5 mm) | Fisher Scientific LLC | Cat# 04-881-19 |
| Stainless Steel Mesh Strainer | Amazon | https://www.amazon.com/dp/B007TUQF9O/ref=cm_sw_em_r_mt_dp_WWK4NAXVPA8JXS8166VS |
| Compressed nitrogen gas | Airgas | Part# NI 230LT22 |
| Core tubes | McMaster-Carr | https://www.mcmaster.com/polycarbonate-tube/high-pressure-hard-plastic-tubing-for-air-and-water/ |
| Core tube caps | Caplugs | https://www.caplugs.com/sleeve-caps-sc |
| Regular-Mouth Glass Mason Jars, 8-Ounce | Amazon | https://www.amazon.com/dp/B096WBXF9R/ref=cm_sw_em_r_mt_dp_A98C3JFEEYEEV7NC1CVK |
| 10-gallon aquarium | Aqueon | https://www.aqueon.com/products/aquariums/standard-glass-rectangle-aquariums |
| AQUANEAT Aquarium Air Pump 300GPH | Amazon | https://www.amazon.com/dp/B088THVBZF/ref=cm_sw_em_r_mt_dp_X5BV4775MT61X2WPPGXH?_encoding=UTF8&psc=1 |
| Standard Bunsen Burners | VWR International LLC | Cat# 470148-926 |
| Capillary Melting Point Tubes, 0.20 mm Wall Thickness | VWR International LLC | Cat# 80061-548 |
| Media/Storage Bottles with GL Screw Caps, 1 L, With Cap | VWR International LLC | Cat# 10754-820 |
| Media/Storage Bottles with GL Screw Caps, 100 mL, With Cap | VWR International LLC | Cat# 10754-814 |
| Syringe 1 cc | VWR International LLC | Cat# 100500-888 |
| VWR® Petri Dishes, Glass | VWR International LLC | Cat# 75845-544 |
| VWR VistaVision™ Microscope Slides, Plain | VWR International LLC | Cat# 16004-422 |
| Fisherbrand™ Nichrome Inoculating Needles with Handles | Fisher Scientific LLC | Cat# 14-956-101 |
Step-by-step method details
Prepare autoclaved sediment and seawater in a laboratory
Timing: 1 day
The following procedure describes the steps required to prepare autoclaved sediment from aliquots of the sediment previously harvested.
-
1.
As was done for incubations, sieve the stored sediment through a 0.5 mm mesh screen to remove large fauna, rocks, and debris.
-
2.
Homogenize the sieved sediment by physical mixing. Troubleshooting.
Note: Oxygen exposure can be reduced during the homogenization process by introducing a tube that flows nitrogen gas into the sediment.
-
3.
Fill 100 mL autoclave-safe glass bottles with homogenized sediment to about ¾ of the volume.
-
4.
Fill 1000 mL autoclave-safe bottles with seawater to about ¾ of the volume.
Note: Sterilized artificial seawater can be used as well.
-
5.
Autoclave the glass bottles at 120°C for 1–2 h.
-
6.
Allow the autoclaved sediment and seawater to cool down to about 25°C before use.
Note: Autoclaved sediment can also be stored and sealed at about 25°C until inoculation. The containers remain sealed and are stored in a cool place, for up to two weeks in this study.
CRITICAL: The homogenization of sulfidic sediment and mishandling of iron sulfide may release hydrogen sulfide, a highly toxic chemical in its gaseous form. We again recommend the use of personal protective equipment such as a sulfide-specific face respirator when in enclosed spaces, and the homogenization process should be conducted under a ventilation hood or in an open well-ventilated space. The autoclaved sediment and seawater should be prepared at least one day to two weeks ahead of the following steps.
Alternatives: Nitrogen gas can be replaced by other inert gases such as helium and argon.
Extract cable bacteria filaments from the cultured natural sediment
Timing: 2 h
The following procedure describes the steps required to isolate CB filaments from the cultured natural sediment matrix.
-
7.
Take sub-cores (sediment plugs approximately 0.5 cm in diameter and 1 cm long) from the cultured sediment by using cut-off 1 mL syringes (Figure 1).
-
8.Reduce the sediment volume around the sampled mass of CB filaments by gentle washing Troubleshooting.
-
a.Place the extracted plugs of sediment in a glass Petri dish.
-
b.Add seawater or artificial seawater to the Petri dish.
-
c.Gently shake the Petri dish to allow the sediment particles to separate from the CB filaments.
-
d.Remove sediment-laden water from the Petri dish.
-
e.Repeat b–d until the filamentous structure of CB can be observed, and the amount of adhering sediment grains can no longer be reduced (Methods video S2).
-
a.
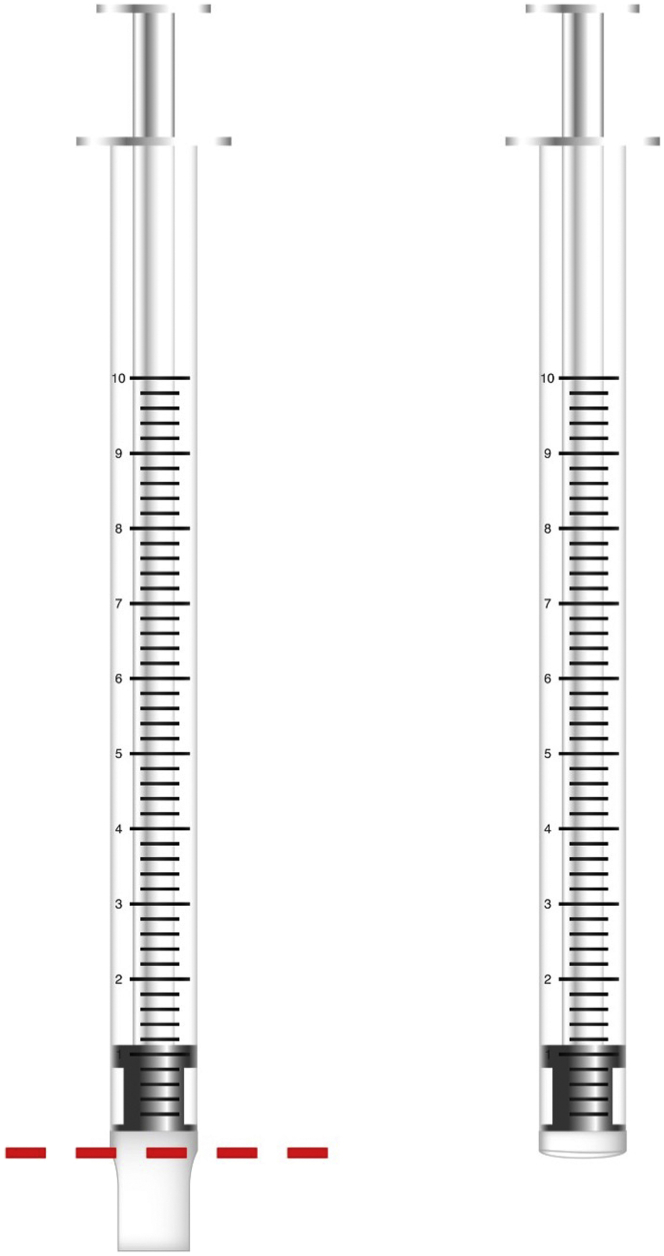
Figure 1.
Graphical demonstration of the preparation of a cut-off syringe
Left: cutting the tip of the 1cc syringe to allow the proper intake of a sediment plug (the red line indicates where to make the cut); right: the finished look of the cut-off syringe.
Note: Deionized water can be used in this step as well.
-
9.Use the fabricated glass hook to pick CB filaments from the remainder of the washed sediment plug(s). Troubleshooting.
-
a.Push the hook gently into a suboxic zone of the remaining washed sample, which can be identified by its grayish color.
-
b.Swirl the hook gently several times (3–7).
-
c.Slowly pull the hook away from the sample and look for filamentous structures of CB connecting (extending between) the hook and the remainder of the sediment.
-
d.Collect the filaments by pulling the hook apart from the sediment (Methods video S2).
-
a.
Note: After being pulled out from the sediment, a mass of CB will shrink onto the hook and appear like a “glob”.
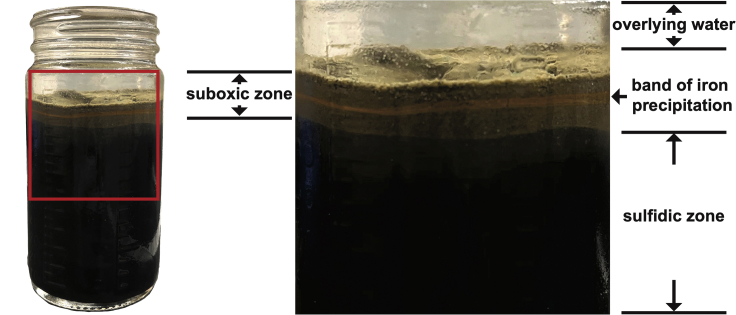
CRITICAL: Samples should be taken from cultured sediments which have developed a pronounced suboxic zone evident by color changes from black to gray and the presence of a band of iron precipitation at the subsurface (Figure 2).
Figure 2.
The stratified zonation of topmost sediment inhabited by cable bacteria
Wash and transfer the isolated filaments into the autoclaved sediment
Timing: 1 h
The following procedure describes the steps followed to further wash the isolated CB filaments and transfer the filaments into the autoclaved sediment.
-
10.Transfer the isolated CB filaments to a droplet of the washing solution placed on a microscopic slide (Methods video S3).
-
a.Use a clean glass needle to scrape the biomass off the glass hook.
-
b.Resuspend the biomass in the droplet of the washing solution.
-
a.
Note: Washing solution can be autoclaved seawater, artificial seawater, phosphate-buffered saline (PBS) solution, or DI water.
-
11.
If necessary, repeat step 10 until the biomass appears free of sediment particles.
-
12.
Use another clean glass needle to pick up the washed biomass and transfer the biomass into the surface layer of the autoclaved sediment (Methods video S3).
-
13.
Increase the probability of transferred CB survival by performing multiple transfers into the surface layer of the autoclaved sediment.
Optional: A sterilized inoculation needle can be used to transfer the CB biomass.
Culture inoculated sediment and evaluate the growth of cable bacteria
Timing: 3 weeks or longer
The following procedure describes the steps required to culture inoculated sediment and to evaluate the growth of cable bacteria.
-
14.
Slowly submerge the inoculated sediment into an aerated aquarium filled with an autoclaved medium.
-
15.
Culture the inoculated sediment. Troubleshooting.
-
16.
Evaluate the growth and presence of CB by measuring the vertical chemical profiles of oxygen, sulfide, and pH.
Note: The vertical chemical profiles of oxygen, sulfide, and pH that are characteristic of CB can be measured by the microelectrodes. More details about performing microelectrode profiling in a lab can be found in Malkin et al. (2014) and Li et al., 2020a, Li et al., 2020b.
Alternatives: Artificial seawater can also be used as the culture media in commercially available aquarium tanks. The presence of CB can also be verified by using microscopic techniques (e.g., scanning electronic microscopy and transmission electron microscopy) to identify their unique morphological features namely the longitudinal ridges and cartwheel cell-cell junctions (Li et al., 2020b; Pfeffer et al., 2012), molecular techniques (e.g., fluorescent in situ hybridization) (Li et al., 2020b; Malkin et al., 2014, 2017), and sequencing techniques (e.g., maker gene analysis and metagenomics) (Geelhoed et al., 2020; Kjeldsen et al., 2019; Li et al., 2020b; Trojan et al., 2016). The growth and density of CB can be evaluated using counts of stained filaments and quantitative polymerase chain reaction methods (Aller et al., 2019; Geelhoed et al., 2020).
Expected outcomes
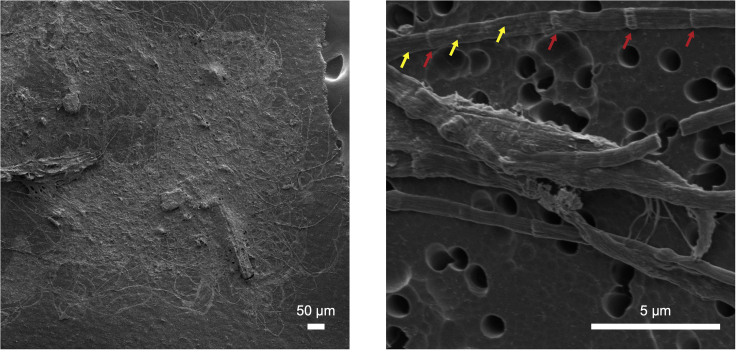
Electrogenic sulfur oxidation catalyzed by CB will significantly alter porewater chemistry, and these changes can be utilized as indicators of successful inoculation (Malkin et al., 2014; Nielsen and Risgaard-Petersen, 2015; Pfeffer et al., 2012). The signature of spatially separated and yet electrically connected sulfide oxidation/oxygen reduction is a pH minimum at depth and a pH maximum near the surface of the sediment column. The development of the two pH extremes and a suboxic zone can be measured by chemical profiling using microelectrodes (see Unisense microelectrode products and studies mentioned above) as early as 2 weeks after inoculation. Secondly, at the pH minimum, buried minerals such as iron are being redissolved and will diffuse towards the sediment-water interface (Otte et al., 2018b; Rao et al., 2016; Seitaj et al., 2015; Sulu-Gambari et al., 2016a, 2016b). At the pH maximum, iron is subsequently oxidized and reprecipitated in the presence of oxygen. Over time, starting from weeks 4–5, the precipitated minerals can be visualized as a distinctive band of orange color at the subsurface (Figure 2), suggesting that the enrichment culture is mature. Finally, established microscopic methods mentioned in the previous sections can be applied to examine the filamentous biomass of CB (Figures 3 and 4). The longitudinal ridges and cartwheel cell-cell junctions of the filaments can be identified by scanning electron microscopy (Figure 4). The purity and the phylogeny of the cultured CB can be assessed by molecular and sequencing techniques (e.g., marker gene analysis and metagenomics) (Geelhoed et al., 2020; Kjeldsen et al., 2019; Trojan et al., 2016).
Figure 3.
Light microscope images of the cable bacteria filaments enriched in autoclaved intertidal sediments originally collected from Yaquina Bay, OR
Figure 4.
Images of the enriched cable bacteria filaments captured by a scanning electron microscope (Helios 650 Ultra Resolution Dual Beam)
Left: a bundle of the extracted filaments; right: tangled cable bacteria filaments within the bundle exhibiting the typical morphological features (indicated on the topmost filament, yellow arrows: the longitudinal ridges, and red arrows: the cartwheel-like cell-cell junctions).
Limitations
Natural sediment is a complex matrix that contains various microorganisms, some of which may evade the sterilization of an autoclave, or leave their DNA behind (Otte et al., 2018a). The biomass of CB used as inoculum is almost certain to contain other microorganisms. The method in the present study is therefore designed to ensure the enrichment of CB and its dominant growth in culture, not to achieve true isolation of CB.
Troubleshooting
Problem 1
Unable to make a thin tapered mid-section when pulling the heated glass tube. (Related to step 15 in “prepare thin glass hooks and needles for extracting CB filaments”).
Potential solution
Different types of glass tubes may have different softening temperatures. However, it is critical to quickly move the tube away from the flame and pull when the middle of the glass tube becomes soft.
Problem 2
Low to no sulfide in sediment after autoclave. (Related to step 2).
Potential solution
The continuously boiling during autoclaving will likely diminish the concentration of total sulfides in the sediment. To ensure sustainable growth of CB, iron sulfide serving as an additional sulfide source can be added to the sediment (1:100 weight to weight) during homogenization. Alternatively, other sulfide sources such as sodium sulfide can be supplied to the sediment after the autoclave process.
Problem 3
Unable to extract filaments from the reduced volume sub-core from natural cultured sediments. (Related to steps 7 and 8).
Potential solution
There are various possible reasons for this problem. Firstly, the growth of CB in the topmost layer of cultured sediment is often heterogeneous. Therefore, some spots may contain little to no CB biomass. Taking another sub-core in a different location can be a potential solution. Secondly, when in less abundance, CB filaments are easily broken and overlooked. Use gentle force for washing and a thin glass hook with a long neck for extracting.
Problem 4
A suboxic zone does not appear to develop during the first few weeks of culture in autoclaved sediment. (Related to step 15).
Potential solution
Keep inoculating the sediment with freshly extracted CB biomass to enhance the survival and growth of CB. Additionally, burying the CB biomass in the topmost layer of the autoclaved sediment (∼1–2 mm depth) may also enhance the survival rate.
Problem 5
A limited amount of CB biomass is found in the inoculated autoclaved sediment. (Related to step 15).
Potential solution
Possible reasons are that the conditions required for CB biomass to grow have not been met. When preparing the autoclaved sediment and inoculum, ensure that 1) the sediment contains sulfide minerals as a substrate for the CB and 2) the filaments extracted from the cultured natural sediment are CB. If the sediments do not contain enough sulfide, iron sulfide can be supplied to the sediment before autoclave. The identity of the inoculum can be verified by using microscopic or phylogenetic analyses. Additionally, ensure the oxygen concentration is sufficient in the overlying water.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Cheng Li (Cheng.Li@oregonstate.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This research was funded through grants N00014-17-1-2599 and N00014-21-1-2251 from the Office of Naval Research to C.E.R. and C.L. The authors would like to thank Philip Ley and Filip Meysman from the University of Antwerp for communicating skills of light microscope imaging. We thank Teresa Sawyer and Pete Eschbach, directors of the Electron Microscopy Facility at Oregon State University for assistance with the SEM imaging. C.L. would like to thank his mother, Ying Wang (1957–2021), a wonderful chemistry teacher who taught C.L. the importance of demonstration as a teaching strategy.
Author contributions
Grants supporting this study were awarded to C.E.R. and C.L. C.L. and C.E.R. conceived the study. C.L. developed the protocols of sediment culture. P.C. provided essential support in method development. C.L. wrote the manuscript, with major editing contributed by C.E.R. and P.C.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101604.
Data and code availability
This study did not generate datasets/code.
References
- Aller R.C., Aller J.J., Zhu Q., Heilbrun C., Klingensmith I., Kaushik A. Worm tubes as conduits for the electrogenic microbial grid in marine sediments. Sci. Adv. 2019;5:eaaw3651. doi: 10.1126/sciadv.aaw3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschker H.T.S., Cook P.L.M., Polerecky L., Eachambadi R.T., Lozano H., Hidalgo-Martinez S., Khalenkow D., Spampinato V., Claes N., Kundu P., et al. Efficient long-range conduction in cable bacteria through nickel protein wires. Nat. Commun. 2021;12:3996. doi: 10.1038/s41467-021-24312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdorf L.D.W., Hidalgo-Martinez S., Cook P.L.M., Meysman F.J.R. Long-distance electron transport by cable bacteria in mangrove sediments. Mar. Ecol. Prog. Ser. 2016;545:1–8. [Google Scholar]
- Cornelissen R., Bøggild A., Eachambadi R.T., Koning R.I., Kremer A., Hidalgo-Martinez S., Zetsche E.-M., Damgaard L.R., Bonné R., Drijkoningen J., et al. The cell envelope structure of cable bacteria. Front. Microbiol. 2018;9:3044. doi: 10.3389/fmicb.2018.03044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam A., Marshall I.P.G., Risgaard-Petersen N., Burdorf L.D.W., Marzocchi U. Effect of salinity on cable bacteria species composition and diversity. Environ. Microbiol. 2021 doi: 10.1111/1462-2920.15484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelhoed J.S., van de Velde S.J., Meysman F.J.R. Quantification of cable bacteria in marine sediments via qPCR. Front. Microbiol. 2020;11:1506. doi: 10.3389/fmicb.2020.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen K.U., Schreiber L., Thorup C.A., Boesen T., Bjerg J.T., Yang T., Dueholm M.S., Larsen S., Risgaard-Petersen N., Nierychlo M., et al. On the evolution and physiology of cable bacteria. Proc. Natl. Acad. Sci. USA. 2019;116:19116–19125. doi: 10.1073/pnas.1903514116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Reimers C.E., Alleau Y. Inducing the attachment of cable bacteria on oxidizing electrodes. Biogeosciences. 2020;17:597–607. doi: 10.5194/bg-17-597-2020. [DOI] [Google Scholar]
- Li C., Reimers C.E., Chapman J.W. Microbiome analyses and presence of cable bacteria in the burrow sediment of Upogebia pugettensis. Mar. Ecol. Prog. Ser. 2020;648:79–94. doi: 10.3354/meps13421. [DOI] [Google Scholar]
- Malkin S.Y., Meysman F.J.R. Rapid redox signal transmission by “cable bacteria” beneath a photosynthetic biofilm. Appl. Environ. Microbiol. 2015;81:948–956. doi: 10.1128/aem.02682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S.Y., Rao A.M., Seitaj D., Vasquez-Cardenas D., Zetsche E.-M., Hidalgo-Martinez S., Boschker H.T., Meysman F.J.R. Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor. ISME J. 2014;8:1843–1854. doi: 10.1038/ismej.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S.Y., Seitaj D., Burdorf L.D.W., Nieuwhof S., Hidalgo-Martinez S., Tramper A., Geeraert N., Stigter H.D., Meysman F.J.R. Electrogenic sulfur oxidation by cable bacteria in bivalve reef sediments. Front. Mar. Sci. 2017;4:28. doi: 10.3389/fmars.2017.00028. [DOI] [Google Scholar]
- Martin B.C., Bougoure J., Ryan M.H., Bennett W.W., Colmer T.D., Joyce N.K., Olsen Y.S., Kendrick G.A. Oxygen loss from seagrass roots coincides with colonisation of sulphide-oxidising cable bacteria and reduces sulphide stress. ISME J. 2019;13:707–719. doi: 10.1038/s41396-018-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meysman F.J.R., Cornelissen R., Trashin S., Bonne R., Martinez S.H., van der Veen J., Blom C.J., Karman C., Hou J.-L., Eachambadi R.T., et al. A highly conductive fibre network enables centimetre-scale electron transport in multicellular cable bacteria. Nat. Commun. 2019;10:4120. doi: 10.1038/s41467-019-12115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H., Bosch J., Griebler C., Damgaard L.R., Nielsen L.P., Lueders T., Meckenstock R.U. Long-distance electron transfer by cable bacteria in aquifer sediments. ISME J. 2016;10:2010–2019. doi: 10.1038/ismej.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H., Marozava S., Probst A.J., Meckenstock R.U. Groundwater cable bacteria conserve energy by sulfur disproportionation. ISME J. 2020;14:623–634. doi: 10.1038/s41396-019-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L., Risgaard-Petersen N., Fossing H., Christensen P., Sayama M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature. 2010;463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- Nielsen L.P., Risgaard-Petersen N. Rethinking sediment biogeochemistry after the discovery of electric currents. Ann. Rev. Mar. Sci. 2015;7:425–442. doi: 10.1146/annurev-marine-010814-015708. [DOI] [PubMed] [Google Scholar]
- Otte J.M., Blackwell N., Soos V., Rughöft S., Maisch M., Kappler A., Kleindienst S., Schmidt C. Sterilization impacts on marine sediment---Are we able to inactivate microorganisms in environmental samples? FEMS Microbiol. Ecol. 2018;94 doi: 10.1093/femsec/fiy189. [DOI] [PubMed] [Google Scholar]
- Otte J.M., Harter J., Laufer K., Blackwell N., Straub D., Kappler A., Kleindienst S. The distribution of active iron-cycling bacteria in marine and freshwater sediments is decoupled from geochemical gradients. Environ. Microbiol. 2018;20:2483–2499. doi: 10.1111/1462-2920.14260. [DOI] [PubMed] [Google Scholar]
- Pfeffer C., Larsen S., Song J., Dong M., Besenbacher F., Meyer R.L., Kjeldsen K.U., Schreiber L., Gorby Y.A., El-Naggar M.Y., et al. Filamentous bacteria transport electrons over centimetre distances. Nature. 2012;491:218–221. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- Rao A.M.F., Malkin S.Y., Hidalgo-Martinez S., Meysman F.J.R. The impact of electrogenic sulfide oxidation on elemental cycling and solute fluxes in coastal sediment. Geochem. Cosmochim. Acta. 2016;172:265–286. doi: 10.1016/j.gca.2015.09.014. [DOI] [Google Scholar]
- Risgaard-Petersen N., Kristiansen M., Frederiksen R.B., Dittmer A., Bjerg J., Trojan D., Schreiber L., Damgaard L., Schramm A., Nielsen L. Cable bacteria in freshwater sediments. Appl. Environ. Microbiol. 2015;81:6003–6011. doi: 10.1128/aem.01064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz V.V., Müller H., Koren K., Nielsen L.P., Meckenstock R.U. The rhizosphere of aquatic plants is a habitat for cable bacteria. FEMS Microbiol. Ecol. 2019;95:fiz062. doi: 10.1093/femsec/fiz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitaj D., Schauer R., Sulu-Gambari F., Hidalgo-Martinez S., Malkin S.Y., Burdorf L.D.W., Slomp C.P., Meysman F.J.R. Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins. Proc. Natl. Acad. Sci. USA. 2015;112:13278–13283. doi: 10.1073/pnas.1510152112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulu-Gambari F., Seitaj D., Behrends T., Banerjee D., Meysman F.J.R., Slomp C.P. Impact of cable bacteria on sedimentary iron and manganese dynamics in a seasonally-hypoxic marine basin. Geochem. Cosmochim. Acta. 2016;192:49–69. doi: 10.1016/j.gca.2016.07.028. [DOI] [Google Scholar]
- Sulu-Gambari F., Seitaj D., Meysman F.J.R., Schauer R., Polerecky L., Slomp C.P. Cable bacteria control iron–phosphorus dynamics in sediments of a coastal hypoxic basin. Environ. Sci. Technol. 2016;50:1227–1233. doi: 10.1021/acs.est.5b04369. [DOI] [PubMed] [Google Scholar]
- Trojan D., Schreiber L., Bjerg J.T., Bggild A., Yang T., Kjeldsen K.U., Schramm A. A taxonomic framework for cable bacteria and proposal of the candidate genera Electrothrix and Electronema. Syst. Appl. Microbiol. 2016;39:297–306. doi: 10.1016/j.syapm.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Aller R.C., Zhu Q., Aller J.Y. The dynamics of cable bacteria colonization in surface sediments: a 2D view. Sci. Rep. 2021;11:7167. doi: 10.1038/s41598-021-86365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets/code.