Abstract
Many persons with spinal cord injury (SCI) have one or more preventable chronic diseases related to excessive energetic intake and poor eating patterns. Appropriate nutrient consumption relative to need becomes a concern despite authoritative dietary recommendations from around the world. These recommendations were developed for the non-disabled population and do not account for the injury-induced changes in body composition, hypometabolic rate, hormonal dysregulation and nutrition status after SCI. Because evidence-based dietary reference intake values for SCI do not exist, ensuring appropriate consumption of macronutrient and micronutrients for their energy requirements becomes a challenge. In this compressive review, we briefly evaluate aspects of energy balance and appetite control relative to SCI. We report on the evidence regarding energy expenditure, nutrient intake and their relationship after SCI. We compare these data with several established nutritional guidelines from American Heart Association, Australian Dietary Guidelines, Dietary Guidelines for Americans, Institute of Medicine Dietary Reference Intake, Public Health England Government Dietary Recommendations, WHO Healthy Diet and the Paralyzed Veterans of America (PVA) Clinical Practice Guidelines. We also provide practical assessment and nutritional recommendations to facilitate a healthy dietary pattern after SCI. Because of a lack of strong SCI research, there are currently limited dietary recommendations outside of the PVA guidelines that capture the unique nutrient needs after SCI. Future multicentre clinical trials are needed to develop comprehensive, evidence-based dietary reference values specific for persons with SCI across the care continuum that rely on accurate, individual assessment of energy need.
Keywords: Spinal cord injury, Energy expenditure, Energy intake, energetic intake, Macronutrients, Micronutrients, Carbohydrates, Protein, Fat, Alcohol, Fiber, Vitamins, Minerals, Nutrition, Diet
A spinal cord injury (SCI) results from trauma to or disease of the spinal cord, often causing permanent neurological deficits and accelerated morbidity and mortality throughout the lifespan(1,2). Depending on level and completeness of injury, SCI is associated with a range of co-morbidities that can limit functional independence, mobility and nutrient utilisation. These co-morbidities include motor paralysis, sensory loss, neurogenic restrictive and obstructive pulmonary disease, neurogenic bradycardia, neurogenic hypotension, sympathetic dysfunction, neurogenic adaptive myocardial atrophy, coronary artery disease, anabolic deficiency, spasticity, sarcopenia, heterotopic ossification, osteoporosis, upper extremity overuse, neurogenic obesity, cardiometabolic syndrome (CMS; including, dyslipidemia, hypertension and type 2 diabetes mellitus), pressure injuries, sexual dysfunction, and neurogenic bowel and neurogenic bladder(3).
In the acute phase of SCI, spinal shock often occurs in which all motor and sympathetic reflex activity is absent(4). The patient is often mechanically ventilated in the acute phase, such that even muscles of respiration are inactive(5). Basal/resting metabolism plummets as the body sheds unneeded paralysed muscle and bone, and nutrient needs are reduced(6). Once weaned from a ventilator, the individual will at least be able to activate muscles of respiration which may marginally increase total daily energy expenditure (TDEE) as they contract. During this time, the body will continue to lose unused muscle and bone until a homoeostasis is attained with minimal muscle protein reserve and bone mineral content reduced to fracture threshold(5,6). The acute phase may last up to 12 months as the individual completes physical rehabilitation and reintegrates into the community with a new baseline functional level, and subsequent energy and nutrient ‘setpoint’, that falls well below previous baseline levels(5-8). The new setpoint is rarely matched by a similar reduction in energetic intake(9). Overeating relative to energetic need and poor dietary habits (e.g. overeating, consuming sugary drink, etc.) contribute to inadequate nutrition and chronic health problems in the population with SCI(10,11). Therefore, ensuring the appropriate consumption of macronutrients and micronutrients relative to need becomes a challenge despite several dietary recommendations.
Authoritative guidelines provide evidence-based dietary recommendations. These guidelines are used to establish goals in planning healthy diets and lifestyles and provide the public information about nutritional science and a wholesome diet. The US Department of Agricultural (USDA) Dietary Guidelines for Americans (DGA)(12), Public Health England (PHE) Dietary Recommendations(13), Australian Dietary Guidelines (ADG)(14) and Institute of Medicine (IOM) Dietary Reference Intakes(15) are meant for use by healthy populations to meet nutritional needs and maintain an overall healthy diet and lifestyle. Both the WHO Healthy Diet(16) and the American Heart Association (AHA)(17,18) primarily focus on the prevention of obesity and chronic disease and, in the case of the AHA, reducing the risk of cardiovascular disease. Food and estimated average requirements, recommended dietary allowance, adequate intakes and tolerable upper-level intake (definitions are provided in Table 1) recommendations from these organisations are often harmonised. However, they differ in their methodologies, ratings of evidence, geographic location of the population of interest, references and ease of translation. A fundamental shortcoming of the guidelines is their translation to persons with life-changing injuries and/or those who have developed chronic health conditions that require special dietary modifications and considerations, such as those with a SCI. Persons with special needs are typically excluded from consideration when designing these guidelines.
Table 1.
Definition of terms relating to dietary reference intakes
| Adequate intake (AI) | The recommended average daily intake is based on observed or experimentally determined approximations of estimates of nutrient intake by a group(s) of apparently healthy individuals that are assumed to be adequate. AI is used when an RDA cannot be determined. |
| Estimated average requirement (EAR) | The average daily nutrient intake level is estimated to meet the requirement of half the healthy individuals in a particular life stage and sex group. |
| Recommended Dietary Allowance (RDA) | The average daily dietary nutrient intake level sufficient to meet the nutrient requirement of nearly all healthy individuals in a particular life stage and sex group. Developed from EAR. |
| Upper limit (UL) | The highest average daily nutrient intake is expected to pose no adverse health risks to almost all persons in the general population. As intake exceeds the upper limit, the potential for adverse health risks may increase. |
The overall purpose of this narrative review is to (1) critically appraise dietary intake relative to energy needs after SCI and (2) compare the existing literature with authoritative dietary guidelines from several countries that target obesity and cardiometabolic risk reduction. We extend the findings from our published meta-analysis and systematic review(19) that determined greater energetic intake relative to energy expenditure and an imbalance in fibre and micronutrient intake compared with the DGA in chronic SCI. In the present paper, we specifically aim to review both the acute (< 1-year post-injury) and chronic (≥ 1-year post-injury) phases of a SCI, include a wider collection of dietary and energy literature in SCI, critically evaluate energy expenditure and dietary intake assessment methods, incorporate dietary guidelines outside the US, and provide practical assessment and nutritional recommendations to facilitate a healthy dietary pattern after SCI. We also highlight neurogenic obesity and cardiometabolic risk after SCI, explore the potential influence of SCI on the central and peripheral mechanisms regulating energy homoeostasis and provide direction for future research by comparing existing literature on persons with and without SCI.
Neurogenic obesity and cardiometabolic risk after spinal cord injury
The prevalence of neurogenic obesity(7,20) in adults with SCI ranges from 22 % to 97 %, compared with 42 % in the non-disabled population(5,11,21-29). Neurogenic obesity results from the dysfunction of energy metabolism, physical deconditioning(30), a sedentary lifestyle(31), impaired fitness(32), sympathetic nervous system dysfunction(33,34), altered hormonal homoeostasis(5,35-40), changes in satiety(41) and loss of lean body mass after SCI (Table 2)(42-47). The volume of marrow fat increases 36 % following the initial 12 weeks after the injury in part, because increases in fat mass are dissociated from obesity-related mechanical loading(48). Following the SCI, bone loss is prompt(49), with bone mineral density at the knee and hip declining 2 to 4 % every month(49,50) and decreasing up to roughly 20 %(51,52) within the first year of the injury. Precipitous loss of skeletal muscle mass below the level of injury (LOI) is marked by decreased cross-sectional area of up to 48 % as immediate as 6 weeks after the injury(45). Muscle atrophy from 30 to 60 % of total lean body mass has also been reported(53). Significant gains in fat mass occurring 2 to 7 months post-SCI contribute to a pathological cardiometabolic profile observed in the chronic phase of the injury(54).
Table 2.
Factors contributing to neurogenic obesity and cardiometabolic syndrome after spinal cord injury (SCI)
| Neurogenic obesity | Cardiometabolic syndrome |
|---|---|
| • Physical decondition | General health risks |
| • Reduction in lean body mass | •Age |
| • Obligatory sarcopenia | • Family history |
| • Mechanical unloading | • Sex |
| • Blunted anabolic hormones | • Hypertension |
| • Inactivity | • Hypercholesterolemia |
| • Limited range of motion | • Type 2 diabetes |
| • Decreased energy expenditure | • Smoking/tobacco use |
| • Decreased BMR/RMR | Cardiometabolic syndrome risk factors |
| • Altered satiety | • Abdominal obesity/visceral adiposity |
| • Excess energetic intake | • Insulin resistance/type 2 diabetes |
| • Impaired fitness | • Hypertension |
| • Genetic predisposition | •Hypertriacylglycerolaemia |
| •Low HDL-cholesterol | |
| Non-traditional risk factors | |
| •Genetics | |
| •Prothrombic state | |
| •Proatherogenic state | |
| •Malnutrition | |
| •Excess energetic intake | |
| •Chronic, low-grade inflammation | |
| SCI-specific risk factors | |
| •Sympathetic nervous system dysfunction | |
| •Physical deconditioning | |
| •Neurogenic obesity and its causes |
The accumulation of visceral fat is considered the principle mediator in the development of dyslipidemia, insulin resistance, hypertension, arteriosclerosis and CMS in the non-disabled population(55-57). Similar risk factors are used to quantify CMS in persons with SCI (Table 2)(7,58-64). Cirnigliaro et al.(65) reported that, when compared with a non-disabled group, persons with SCI had a 27 % increase in visceral fat volume for every centimetre increase in waist circumference, a marker of central obesity, and a 20 % increase in visceral fat volume for every unit increase in body mass index (BMI). Nash et al.(66) identified that being overweight/obese was significantly associated with CMS diagnosis. In a sample of 477 veterans with SCI, Gater et al.(11) reported that 76·7 % were classified as obese when using an SCI-specific BMI cut-off of 22 kg/m2(67). The authors also reported that 55·1 % had or were undergoing treatment for hypertension; approximately 50 % currently had or were previously diagnosed with type 2 diabetes mellitus; 69·7 % had or were under treatment for HDL-cholesterol < 40 mg/dl; and more than 57 % had CMS using modified International Diabetes Federation criteria(68). More recently, Gater and colleagues(29) studied body composition using the gold standard four-compartment model and CMS in a sample of seventy-two participants with chronic motor complete SCI. The authors identified a mean BMI of 27·3 kg/m2 corresponding to 42 % body fat and CMS was in 59·4 % of the sample(29). These findings demonstrate the high prevalence of neurogenic obesity and cardiometabolic complications after SCI and the need for dietary countermeasures (Table 2).
Central and peripheral mechanisms regulating energy homoeostasis and their implications in spinal cord injury
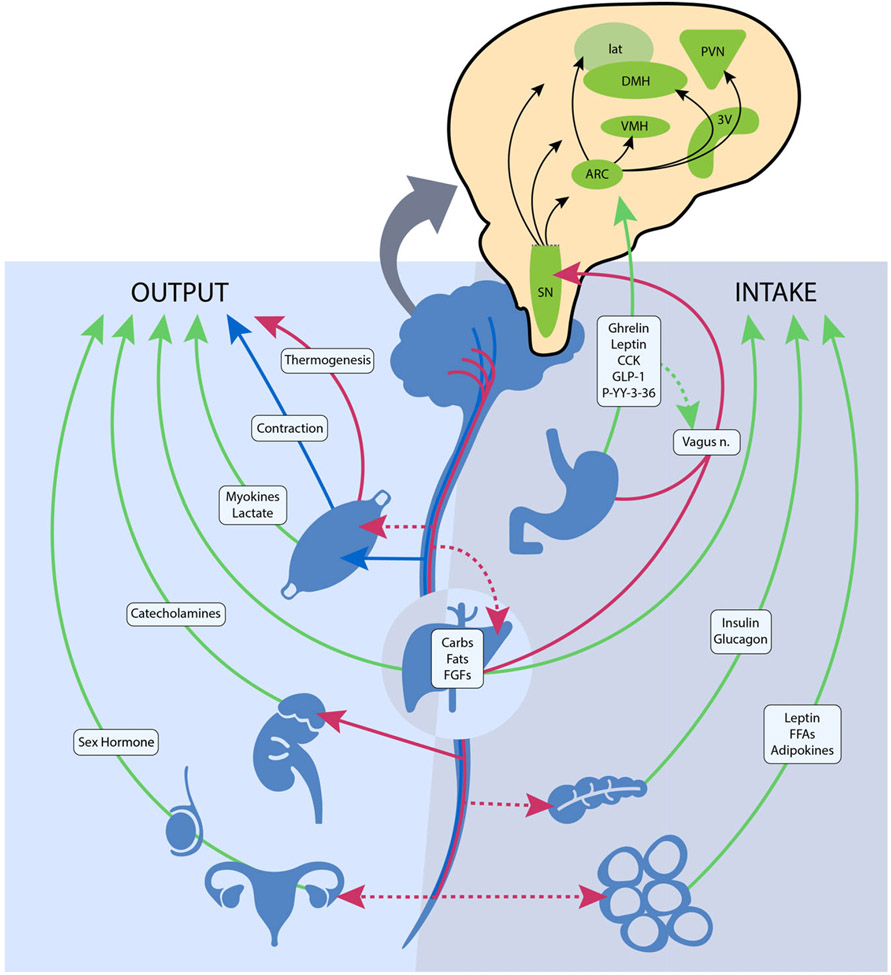
The central nervous system plays a vital role in modulating energy status, and the hypothalamus is the integrating, super-ordinate principal regulator of whole-body energy homoeostasis (Fig. 1). The arcuate nucleus within the hypothalamus plays a critical role in the regulation of feeding and metabolism. It integrates hormonal and nutritional signals from the peripheral circulation, as well as peripheral and central neuronal inputs, to generate a coordinated feedback response. The arcuate nucleus projects to second-order neurons in the paraventricular, dorsomedial, lateral and ventromedial nuclei of the hypothalamus. The second-order neurons further process the received information and project to multiple extrahypothalamic neurocircuits, leading to an integrated response that regulates energy intake and energy expenditure(69). These centres jointly summate influences from various circulating substrates, hormones, neuropeptides and neurotransmitter signals that regulate food intake.
Fig. 1.
The neuroendocrine components involved in the regulation of energy balance relevant to spinal cord injury. Organs and systemic signaling pathways are represented with green lines (circulating hormonal signals), red (voluntary neurological signals) and blue (autonomic neurological signals). The pop-out shows the action of these signals on regions in the hypothalamus and brainstem. Legend: 3V, third ventricle; ARC, arcuate nucleus; Carbs, carbohydrates, CCK, cholecystokinin; DMH, dorsomedial hypothalamic nucleus; FGF, fibroblast growth factors; FFA, free fatty acids; GLP-1, glucagon-like peptide 1; lat, lateral nucleus; n., nerve; PVN, paraventricular nucleus; P-YY-3–36, peptide YY3–36; SN, substantia nigra; and VMH, ventromedial hypothalamic nucleus.  , Humoral;
, Humoral;  , Autonomic;
, Autonomic;  , Voluntary
, Voluntary
Gastrointestinal hormones also have a principal role in regulating central nervous system-dependent energy control. Ghrelin is mainly secreted from the stomach during a fasted state and stimulates body weight gain, adiposity and central feeding centres by activating neurons in the hypothalamus that stimulate food intake(69). Various other hormones, such as peptide YY3–36, cholecystokinin and glucagon-like peptide 1 are secreted from the small intestine upon the ingestion of foodstuff and exert appetite-suppressing effects in various brain regions, such as hypothalamic and brainstem nuclei, and by modulating vagal afferents, the peripheral elements of the brain–gut axis (Fig. 1)(70). Gut peptides provide information on ‘real-time’ food consumption and modify electrical activity of the vagal afferent pathway by attaching to vagal receptors that extend into the digestive tract mucosa. These intestinal-derived signals are sent by the vagus nerve to the nucleus of the solitary tract, with further projection to hypothalamic regions (Fig. 1)(71).
Disruption of the central mechanisms modulating energy metabolism has been previously recognised as the aetiology of obesity in non-disabled persons(69,71). Obesity and cardiometabolic disorders are frequently associated with diminished production or resistance to the production of central and peripheral regulators of energy homoeostasis, including food intake and energy expenditure(72). Naznin et al.(73) and Waise et al.(74) reported that obesity-induced systemic inflammation spreads to the vagus nerve and subsequently the hypothalamus leading to the dysregulation of central and peripheral mechanisms governing satiety, energy regulation and fuel metabolism. With obesity and high-fat, energy-dense diets, increases in the concentration of saturated fatty acids from the periphery cross the blood–brain barrier and induce an inflammatory response on hypothalamic neurons(75). Vinik et al.(76) reported that obesity and type 2 diabetes mellitus-induced neuropathies alter vagal nerve neurotransmission, preventing bidirectional crosstalk between the central nervous system and the gut.
Although supraspinal centres remain intact following SCI, several neurological and endometabolic factors are influenced by the disruption of the central nervous system. With an SCI, compromised afferent and efferent signals from central and peripheral locations lead to dysregulation of the intricate equilibrium of energy metabolism. Physiological cues that are present in persons with SCI and influence appetite, satiety/satiation and energy balance are disrupted, further contributing to an imbalance in energy homoeostasis(41). Besecker et al.(77) have proposed an SCI-induced gastric vagal afferent neuropathy as a cause for homoeostatic dysregulation of energy balance in experimental SCI in rats. The authors hypothesise the disruption of the reflex transmission of chemical feeding-related signals from the gastrointestinal tract to the CNS(77). The central and hypothesised peripheral dysregulation of energy homoeostasis and a deterioration of body composition that results in physical deconditioning produce the ‘perfect storm’ for the onset of neurogenic obesity and cardiometabolic risk in persons with SCI.
Energy expenditure after spinal cord injury
Energy balance reflects a dynamic relationship between energy expenditure and energy intake. TDEE represents the number of energies burned over 24 h and is the sum of basal metabolic rate (BMR), the thermic effect of physical activity (TEPA) and the thermic effect of food digestion (TEF)(78).
In the non-disabled population, TEPA and TEF account for approximately 20 % and 8% of TDEE(78), whereas after SCI they account for about 5 % and 6 %, respectively(34,79). To date, limited research has tested differences in the TEPA and TEF between persons with and without SCI. Monroe et al.(34), measured TEPA using a respiratory chamber. They observed significantly less TEPA in men with SCI compared with men without SCI(34). This finding is likely because movement is restricted to the upper limbs. Consequently, the energy cost of exercise and activities of daily living is significantly lower in SCI compared with a non-disabled person(80). Aksnes et al.(81) and Buchholz et al.(79)did not find differences in TEF between persons with chronic SCI and non-disabled controls, possibly because of a 2-h post-prandial testing window that only captured the parasympathetic-controlled obligatory phase of TEF. Alternatively, Monroe et al.(34) and Asahara and Yamasaki(82) reported significant differences between persons with and without SCI for a 24- and 3-hour test, respectively. The authors of both studies incorporated a longer testing duration, therefore, capturing both the obligatory and the sympathetic and skeletal muscle mass-mediated facultative phases of TEF(82). Both TEF and TEPA present unique assessment challenges attributed to the scarcity of literature. Therefore, TEF and TEPA should remain an activeresearch focus given their influence on energy intake and TDEE.
BMR typically accounts for 60 to 70 % of TDEE in the non-disabled population(83), but in persons with SCI, it accounts for 70 to 80 %(84). Fat-free mass, composed of bone, muscle and organs, contributes the most to BMR, and of fat-free mass, skeletal muscle mass accounts for 85 % of the variance. Attenuation of BMR following SCI originates from a significant reduction in metabolically active tissue(9), sympathetic nervous system dysfunction(85) and altered hormonal milieu(86).
Most SCI literature measures resting metabolic rate (RMR) or resting energy expenditure rather than the more precise measurement of BMR (Table 3). The available literature indicates that the mean measured BMR for persons with SCI ranges from 1022 to 1943 kcal/d, and mean measured RMR ranges from 959 to 2519 kcal/d (Table 3)(9,34,79,81,84,87-120) Buchholz et al.(79) reported resting metabolism was significantly lower in persons with paraplegia (1472 kcal/d) compared with BMI-matched non-disabled controls (1677 kcal/d). In another study by Buchholz et al.(121), authors examined RMR in twenty-seven persons with SCI by injury completeness (complete: 1417 v. incomplete: 1480 kcal/d) and sex (men: 1555 v. women: 1245 kcal/d), only observing significant differences by sex. Gorgey et al.(110) recently reported non-significant differences in BMR by sex (men: 1421 v. women: 1367 kcal/d) and Farkas et al.(9) observed significant differences by LOI (tetraplegia: 1224 and paraplegia: 1517 kcal/d) in motor complete SCI. Collins et al.(80), however, did not report significant differences in RMR by LOI. These differences may stem from population demographics as Farkas et al.(110) examined chronic motor complete SCI, whereas Collins et al.(80) included complete and incomplete SCI. Additionally, Farkas et al.(110) measured BMR and Collins et al.(80) assessed RMR in their respective studies.
Table 3.
BMR/RMR in spinal cord injury literature
| Author, year | Group(s) | n | Sex | Age (years) |
LOI | AIS | TSI/range (years) |
BMR/RMR (kcal/d) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Aksnes et al., 1993 | Group A-meal | 6 | M | 27 | 2 | C6-C7 | Frankel A | 5 | 2 | 1321*,† | |
| Group B-water | 3 | M | 28 | 3 | C6-C7 | Frankel A | 4 | 1 | 1218*,† | ||
| Alexander et al., 1995 | Para-PI | 14 | M | 53 | 3 | Para | 20 | 3 | 1891 | 97* | |
| Para-no PI | 24 | M | 50 | 3 | Para | C/I | 22 | 3 | 1780 | 62* | |
| Aquilani et al., 2001 | All | 10 | M | 42 | 19 | Para | A | ≥ 0·2 | 1469 | 217* | |
| Barco et al., 2014 | All | 11 | M | 32† | C1-C7 | C/I | Acute | 1943**,† | |||
| Bauman et al., 2004 | Twin with SCI | 13 | M/F | 38† | C5-L2 | C/I | 15 | 9 | 1387 | 268** | |
| Bauman et al., 2011 | Testosterone Replacement | 11 | M | 43 | 6 | Para/tetra | C/I | 13 | 10 | 1328 | 262* |
| Control | 11 | M | 35 | 9 | Para/tetra | C/I | 12 | 9 | 1319 | 112* | |
| Bauman et al., 2015 | Testosterone Treatment | 13 | M | 44 | 6 | Para/tetra | A-C | 15 | 10 | 1283 | 246* |
| Control | 11 | M | 35 | 9 | Para/tetra | A-C | 12 | 9 | 1341 | 105* | |
| Broad et al., 2020 | Wheelchair rugby athletes | 14 | M | 31 | 6 | Para/tetra | I | 1735 | 257* | ||
| Buccholz et al., 2003 | Para | 28 | M/F | 34 | 9 | Para | C/I | 11 | 10 | 1465 | 288* |
| Controls | 34 | 29 | 8 | 1677 | 233* | ||||||
| Chun et al., 2017 | All | 50 | M/F | 42 | 11 | Para/tetra | A, B | 12 | 7 | 1284 | 139** |
| Para | 23 | M/F | 42 | 12 | Para | A, B | 11 | 7 | 1250 | 147** | |
| Tetra | 27 | M/F | 42 | 9 | Tetra | A, B | 13 | 8 | 1317 | 124** | |
| Collins et al., 2010 | Tetra | 32 | M/F | 53 | 14 | C5-C8 | A-D | 11 | 12 | 1411 | 315* |
| Para | 34 | M/F | 52 | 12 | T1-L4 | A-D | 16 | 14 | 1433 | 233* | |
| Cox et al., 1985 | All | 45 | M/F | 30† | Para/tetra | 0·18 | 0·04 | 1324**,† | |||
| Farkas et al., 2019 | Tetra | 28 | M/F | 43 | 11 | C4-C8 | A, B | 16 | 11 | 1517 | 398** |
| Para | 13 | M/F | 46 | 10 | T2-L1 | A, B | 13 | 12 | 1224 | 390** | |
| Farkas et al., 2020 | Mid-para | 6 | M/F | 31 | 11 | T6–T8 | A, B | 5 | 6 | 1491 | 241** |
| Low-para | 5 | M | 39 | 11 | T10-L1 | A, B | 10 | 6 | 1693 | 329** | |
| Control | 5 | M/F | 29 | 12 | 1647 | 233** | |||||
| Farkas et al., 2021 | Para | 11 | M/F | 35 | 11 | T5-L1 | A, B | 7 | 6 | 1583 | 289** |
| Controls | 6 | M/F | 29 | 12 | 1647 | 233** | |||||
| Gorgey et al., 2010 | All | 10 | M/F | 33 | 7 | C6-T11 | A, B | 11 | 7 | 1256 | 231* |
| Gorgey et al., 2011 | All | 2 | M | 53† | C4/5, T11 | D | 0·33 | 2 | 1227* | ||
| Gorgey et al., 2012 | Exercise + diet | 5 | M | 36 | 9 | C5-T10 | A, B | 16 | 9 | 1363 | 132* |
| Diet | 4 | M | 33 | 10 | T4-T11 | A, B | 8 | 10 | 1793 | 397* | |
| Gorgey et al., 2015 | All | 16 | M | 38 | 9 | C5-T10 | A, B | 1494 | 34** | ||
| Tetra | 6 | M | 39 | 9 | C5-C7 | A, B | 1411 | 10** | |||
| Para | 10 | M | 38 | 8 | T3–10 | A, B | 1526 | 34** | |||
| Gorgey et al., 2016 | Exercise | 6 | M | 41 | 7 | C5-T10 | A, B | 13 | 9 | 1470 | 173** |
| Control | 5 | M | 35 | 8 | C5-T10 | A, B | 5 | 4 | 1147 | 403** | |
| Gorgey et al., 2018 | Male | 8 | 38 | 9 | 1421 | 503** | |||||
| Female | 8 | 39 | 13 | 1367 | 396** | ||||||
| Gorgey et al., 2019 | Testosterone + exercise | 11 | M | 37 | 12 | C5-T11 | A, B | 10 | 9 | 1443 | 231* |
| Testosterone only | 11 | M | 35 | 8 | C6-T11 | A, B | 7 | 6 | 1519 | 331* | |
| Gorgey & Gater, 2011 | All | 32 | M | 36 | 9 | C5-T11 | A, B | 1431 | 345* | ||
| Tetra | 11 | M | C5-C7 | A, B | 1259 | 204* | |||||
| Para | 25 | M | T4-T11 | A, B | 1483 | 365* | |||||
| Hayes et al., 2002 | All | 11 | M/F | 36 | 8 | Para/tetra | > 3 | 1390 | 245* | ||
| Holmlund et al, 2018 | Tetra-male | 19 | M | 41 | 15 | C5–C8 | A, B | ≥ 0 | 1195 | 207* | |
| Tetra-female | 7 | F | 42 | 12 | C5–C8 | A, B | ≥ 0 | 959 | 140* | ||
| Para-male | 28 | M | 45 | 12 | T7–T12 | A, B | ≥ 0 | 1286 | 223* | ||
| Para-female | 10 | F | 39 | 11 | T7–T12 | A, B | ≥ 0 | 1030 | 206* | ||
| Kearns et al., 1992 | All | 10 | M/F | 32 | 19 | C4-T10 | Frankel A | 0·2† | 1523 | 109* | |
| Kolpek et al., 1989 | All | 7 | M/F | 34 | 5 | C2-T3 | 0–0·05 | 1760 | 288* | ||
| Lee et al., 1985 | Hypometabolic | 6 | M | 44 | 15 | C/I | 16 | 9 | 1588 | 209* | |
| Normometabolic | 5 | M | 41 | 5 | C/I | 19 | 14 | 1757 | 283* | ||
| Hypermetabolic | 6 | M | 48 | 16 | C/I | 19 | 12 | 1786 | 255* | ||
| Liu et al., 1996 | Tetra-PI | 16 | M | 40 | 3 | Tetra | 10 | 2 | 1775 | 296* | |
| Tetra-no PI | 16 | M | 40 | 2 | Tetra | 15 | 3 | 1538 | 264* | ||
| Controls | 16 | M | 43 | 3 | N/A | 1847 | 268* | ||||
| Monroe et al., 1998 | SCI | 10 | M | 36 | 8 | C6-L3 | Frankel A | 9 | 2 | 1756 | 64* |
| Controls | 59 | M | 32 | 7 | 2212 | 317* | |||||
| Nightingale et al., 2017 | All | 33 | 44 | 9 | 1481 | 32* | |||||
| Nightingale & Gorgey, 2018 | All | 30 | M/F | 35 | 11 | C5-L1 | A, B | 35 | 11 | 1499 | 162** |
| Tetra | 9 | 1467 | 178** | ||||||||
| Para | 21 | 1497 | 148** | ||||||||
| Pelly et al., 2017 | All | 7 | M | 31 | 7 | T3-L5 | C/I | 10–15 | 1538 | 139* | |
| Perret & Stoffel-Kurt, 2011 | Acute | 12 | M/F | 28 | 7 | C4-T10 | A, B | 0·4 | 0·3 | 1414 | 327* |
| Chronic | 12 | M/F | 29 | 7 | C5-T12 | A, B | 5 | 2 | 1304 | 232* | |
| Rodriguez et al., 1997 | All | 12 | M/F | 32† | Para/Tetra | C/I | < 1 | 2519 | 693* | ||
| Sedlock & Laventure, 1990 | All | 4 | M | 28 | 2 | T4-L1 | 7 | 3 | 1530 | 330* | |
| Shea et al., 2018 | All | 25 | M/F | 44† | C4-C8 | C/I | 18† | 1414**,† (M)/1104**,† (F) | |||
| Spungen et al., 1993 | All | 12 | M | 42 | 3 | Para | 10 | 2 | 1854 | 70* | |
| Sumrell et al., 2018 | All | 22 | M | 36 | 10 | C5-T11 | A, B | 8 | 8 | 1137 | 280** |
| Para | 14 | M | 35 | 9 | T4-T11 | A, B | 8 | 9 | 1216 | 278** | |
| Tetra | 8 | M | 37 | 12 | C5-C7 | A, B | 8 | 7 | 1022 | 240** | |
| Tanhoffer et al., 2012 | All | 14 | M/F | 40 | 13 | C4-T12 | A, C | 10 | 8 | 1432 | 228* |
| Tanhoffer et al., 2014 | Sedentary group | 8 | M | 39 | 12 | C6-T12 | A, C | 90 | 6 | 1244 | 304* |
| Exercise group | 8 | M | 40 | 15 | C6-T12 | A, C | 90 | 6 | 1200 | 234* | |
| Yilmaz et al., 2007 | AIS A | 22 | M | 32 | 11 | Tetra/para | A | 3† | 1433 | 488* | |
| AIS B | 8 | M | 33 | 11 | Tetra/para | B | 3† | 1170 | 394* | ||
| Tetra | 11 | M | 29 | 10 | Tetra | C | 3† | 1129 | 300* | ||
| Para | 19 | M | 34 | 10 | Para | C | 3† | 1499 | 508* | ||
| Yilmaz et al., 2007 | ≥ T6 | 13 | M | 29 | 9 | Tetra/para | A, B | 3 | 3 | 1407 | 586* |
| ≤ T7 | 7 | M | 37 | 14 | Tetra/para | A, B | 3 | 3 | 1504 | 204* | |
LOI, level of injury; AIS, American Spinal Injury Association Impairment Scale; TSI, time since injury; Para, paraplegia; PI, pressure injuries; C, complete; I, incomplete; Tetra, tetraplegia. Blank spaces indicate data were not provided in the study; data are presented as mean ± standard deviation.
RMR/resting energy expenditure was measured.
Standard deviation not provided.
BMR was measured.
RMR and BMR primarily differ in testing procedures, but both are non-invasively measured with indirect calorimetry using a metabolic cart(78). The participant lies in a supine position in a dark room with minimal movement following at least an 8-h fast for RMR or a 12-h fast for BMR(122). Because RMR is not at a basal state, it is usually higher than BMR for persons with and without SCI as only a short quiescent period is required (10 to 20 min(123)) prior to data acquisition(87). Rather for BMR, the participant is awakened in the morning following an overnight stay, refrains from exercise, caffeine and alcohol for the previous 24 h, is free from emotional stress, and familiar with the apparatus(122,124). Bauman et al.(87) examined BMR and RMR in pairs of monozygotic twins with and without SCI. The authors reported lower basal metabolism (SCI twin: 1387 and non-SCI twin: 1660 kcal/d) in both groups compared with RMR values (SCI twin: 1682 and non-SCI twin: 1854 kcal/d). Additionally, both values were significantly lower in SCI compared with individuals without the injury(87). There is an approximate 20 % difference between BMR and RMR values in persons with SCI compared with an 11% difference in persons without SCI. Considering that resting/basal metabolism are the largest components of TDEE in persons with and without SCI and it is significantly influenced by fat-free mass, several studies have reported that RMR can be used as a strong predictor of energy intake(125-128). When using RMR rather than BMR as a predictor of energetic intake in persons with SCI, dietary need can be overestimated by nearly 400 kcal/d (assuming approximately a 1900 kcal/d diet(19)). These data indicate BMR is a more sensitive indicator of energetic need, and less reliance should be placed on RMR in SCI research. However, TDEE remains superior as it accounts for the multiple components of daily energy expenditure.
According to published studies, during the acute phase of SCI, TDEE ranges from 2030 to 3344 kcal/d (Table 4)(89,93,94,97,101,102,129). In chronic SCI, TDEE is from 1332 to 2834 kcal/d (Table 4)(9,34,94,98-100,121,130). TDEE is reduced in persons with chronic SCI by as much as 54 % in persons with tetraplegia(130) and nearly 20 % in individuals with paraplegia(121). TDEE can be assessed by measuring average daily energy expenditure using direct, or whole body, calorimetry, doubly labelled water, or mechanical ventilation. Of the SCI literature that assessed TDEE, only 33 % measured TDEE. Direct calorimetry measures the amount of heat produced while enclosed within a respiratory chamber and is the gold standard for measuring energy metabolism(78). A test participant is completely enclosed in the chamber where there are no social interactions during the measurements and audiovisual contact with investigators(131). In cross-sectional study designs, the participants spend a minimum of 24 h continuously, and up to a week (or more) in dietary intervention studies(131). This method has several limitations, including the cost of highly specialised equipment, space to house the equipment, confinement of the participant and the need to exclude anything emitting heat other than the research subject. For persons with paralysis, and especially high injury levels, direct calorimetry is unrealistic because of the need for caregiver assistance, power wheelchairs and/or assistive electronic devices. To date, Monroe and colleagues(34) are the only investigators to use direct calorimetry with a respiratory chamber in persons with SCI. The authors demonstrated a TDEE of 1870 kcal/d in chronic complete SCI compared with 2376 kcal in persons without SCI(34), a 24 % difference.
Table 4.
TDEE after SCI
| Author, year | Group(s) | n | Sex | Age (years) |
LOI | AIS | TSI/range (years) |
TDEE (kcal) |
Measured/ predicted |
Measured/prediction method | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | ||||||||
| Buchholz et al., 2003 | Male | 17 | M | 39 | 11 | Para | C/I | 10 | 8 | 2490 | 637 | Predicted | Heart Rate Monitor |
| Female | 10 | F | 32 | 6 | C/I | 16 | 11 | 1870 | 607 | Predicted | Heart Rate Monitor | ||
| Complete | 17 | M/F | 36 | 10 | C | 2072 | 505 | Predicted | Heart Rate Monitor | ||||
| Incomplete | 10 | M/F | 37 | 10 | I | 2582 | 852 | Predicted | Heart Rate Monitor | ||||
| Barco et al., 2014 | All | 11 | M | 32* | C1-C7 | C/I | Acute | 2425–2629 | 434–458 | Measured | Ventilator | ||
| Cox et al., 1985 | All | 45 | M/F | 30* | Tetra/para | C/I | 0·18 | 0·04 | 2030 | 41 | Predicted | BMR × 1·2 (Long Method) | |
| All | 45 | M/F | 30* | 0·18 | 0·04 | 3164 | 61 | Predicted | BMR × 1·75 (Rutten Method) | ||||
| Desneves et al., 2019 | All | 20 | M/F | 43 | 20 | C1-L5 | A-D | 0·05–0·21 | 2354 | 774 | Measured | Doubly labeled water | |
| Farkas et al., 2019 | Tetra | 13 | M/F | 46 | 10 | C4-C8 | A, B | 13 | 12 | 1530. | 640 | Predicted | BMR × 1·2 (Long Method) |
| Para | 28 | 43 | 11 | T2-L1 | 16 | 11 | 1851 | 405 | Predicted | BMR × 1·2 (Long Method) | |||
| Tetra | 13 | 46 | 10 | C4-C8 | 13 | 12 | 1774 | 388 | Predicted | BMR × 1·15 (Farkas Method) | |||
| Para | 28 | 43 | 11 | T2-L1 | 16 | 11 | 1467 | 614 | Predicted | BMR × 1·15 (Farkas Method) | |||
| Farkas et al., 2020 | Mid-para | 6 | M/F | 31 | 11 | T6–T8 | A, B | 5 | 6 | 1712 | 238 | Predicted | BMR × 1·15 (Farkas Method) |
| Low para | 5 | M | 39 | 11 | T10-L1 | A, B | 10 | 6 | 1949 | 456 | Predicted | BMR × 1·15 (Farkas Method) | |
| Mollinger et al., 1985 | High tetra | 14 | M | 35 | 8 | C4-C6 | C | 6 | 5 | 1332 | 112 | Predicted | Measure of Oxygen Consumption |
| Low tetra | 13 | 33 | 6 | C6-C7 | C | 7 | 4 | 2108 | 523 | Predicted | Measure of Oxygen Consumption | ||
| High para | 16 | 33 | 7 | T1-T10 | C | 9 | 5 | 2611 | 620 | Predicted | Measure of Oxygen Consumption | ||
| Low para | 5 | 33 | 9 | T10-L2 | C | 4 | 3 | 2693 | 427 | Predicted | Measure of Oxygen Consumption | ||
| Monroe et al., 1998 | All | 10 | 36 | 8 | C6-L3 | Frankel A | 9 | 2 | 1870 | 73 | Measured | Respiratory chamber | |
| Rodriguez et al., 1997 | All | 12 | M/F | 32* | C3-T12 | C/I | < 1 | 3344 | 431 | Predicted | BMR × 1·2 × 1·6 (Long Method) | ||
| Rowan & Klazemi, 2020 | All | 16 | M/F | 43* | C4-C6 | 0·06 | 2784* | Predicted | 66·5 + (13·7 × weight (kg)) + (5·003 × height (cm)) × (6·755 × age) × 1·2 × 1·1 (Harris-Benedict with Long, Trauma Method) | ||||
| Shea et al., 2018 | All | 25 | M/F | 44* | C4-C8 | C/I | 18* | 1703 | 416 | Predicted | Collins et al., 2010; Ainsworth et al., 1993 Methods | ||
| Tanhoffer et al., 2012 | All | 14 | M/F | 40 | 10 | C4-T12 | A-C | 10 | 8 | 2346 | 595 | Measured | Doubly labeled water |
| All | 14 | 2031 | 362 | Predicted | Heart rate Monitor | ||||||||
| All | 14 | 2728 | 775 | Predicted | Multi-sensor armband | ||||||||
| Tanhoffer et al., 2015 | All | 8 | M/F | 42 | 13 | C6-T12 | A, C | 9 | 6 | 2406 | 552 | Measured | Doubly labeled water |
| All | 8 | M/F | 42 | 13 | C6-T12 | A, C | 9 | 6 | 2834 | 648 | Predicted | Multi-sensor armband | |
| Wouda et al., 2018 | High-intensity interval training | 10 | M/F | 50 | 15 | Tetra/para | D | 0·19 | 0·08 | 2666 | 528 | Predicted | Multi-sensor armband |
| Moderate-intensity training | 10 | 34 | 15 | D | 0·18 | 0·09 | 2736 | 603 | Predicted | Multi-sensor armband | |||
| Control | 10 | 40 | 10 | D | 0.2 | 0·07 | 2437 | 341 | Predicted | Multi-sensor armband | |||
| Wouda et al., 2020 | All | 30 | M/F | 41 | 17 | Tetra/para | D | 0·19* | 2632 | 509 | Predicted | Multi-sensor armband | |
SCI, spinal cord injury; LOI, level of injury; AIS, American Spinal Injury Association Impairment Scale; TSI, time since injury; TDEE, total daily energy expenditure; Para, paraplegia; C, complete; I, incomplete; Tetra, tetraplegia; Blank spaces indicate data were not provided in the study; data are presented as mean ± standard deviation.
Standard deviation are not provided.
Less labour- and time-intensive methods to measure energy expenditure are with doubly labelled water or a metabolic cart during mechanical ventilation. Doubly labelled water is centred around the difference between the apparent turnover rates of the hydrogen and oxygen of body water as a function of carbon dioxide production. The procedure encompasses enriching a research participant with heavy oxygen and heavy hydrogen and then determining the difference in washout kinetics between the isotopes. The oxygen isotope is lost as water and as carbon dioxide due to exchange in the bicarbonate pools. The hydrogen isotope is lost only as water(132). The strength of doubly labelled water is that it is a non-invasive and inconspicuous free-living evaluation of TDEE with no constraint or restriction for the participant. The total number of variables in the equations used to calculate TDEE from doubly labelled water is nine, plus two additional constants, from which the equation for isotope dilution spaces calculation includes five variables and one constant(131), thus, making the technique mathematically complex and prone to miscalculation. Double labelled water is infrequently used (Desneves et al.(94) and Tanhoffer et al.(99,100)) to measure TDEE after SCI. In 2012 and 2015, Tanhoffer et al.(99,100) reported a mean TDEE of 2346 and 2406 kcal/d, respectively, in chronic SCI, while Desneves et al.(94) reported 2354 kcal/d during the acute stage. These observed values far exceed the reported values by Monroe et al.(34) In the acute phase, mechanical ventilators provide a unique option to continuously measure respiratory gases with the addition of a metabolic monitor, similar to indirect calorimetr(133), as published by Barco et al.(93) Because nutritional risk is associated with ventilatory support after SCI and high levels of injury often need respiratory management(134), the use of metabolic monitoring with mechanical ventilators can provide an easy method to determine energetic need during the initial hospitalisation. However, it is likely because of the lack of availability of the specialised equipment, cost and trained personnel required to measure TDEE that most studies rely on predicting TDEE, a method prone to error(78). Of the literature that assessed TDEE, 71 % estimated it through various methods (Table 4). Several of these methods are examined in the next section given their clinical use in defining energetic targets.
Energy (energetic) intake relative to energy expenditure after spinal cord injury
Energy intake reflects energetic gain by the ingestion of foodstuff of different energetic densities. Defining optimal nutrient intake and its management is challenging after SCI because practical guidelines for determining energy requirements for this niche population are limited. Energetic need is dependent on several factors after SCI, including the injury phase(117) and the physical activity/therapy within phase(130), the level(19,89) and completeness(130) of the injury, sex(135), body composition and its post-injury changes(85,103), presence of infection or pressure injuries(120), and frequency and accuracy of reporting on the energetic intake used to assess nutrition(19,84). Consequently, tremendous variability in energetic intake (Table 5) is reported across the literature, even though most studies report it is within or exceeds daily AHA, ADG, DGA and PHE recommendations (Table 6).
Table 5.
Total energetic and macronutrient intake in SCI literature
| Author, year | Group(s) | N | Sex | LOI | AIS | TSI/range (years) |
Energetic intake (kcal/d) |
Protein intake (kcal/d) |
Fat intake (kcal /d) |
Carbohydrate intake (Kcal/d) |
Dietary collection method |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | |||||||
| Abilmona et al., 2018 | All | 22 | M | C5-T11 | A, B | 8 | 8 | 1362 | 500 | 242 | 65 | 534 | 182 | 588 | 267 | SR 5-d dietary recall |
| Allison et al., 2018 | Diet intervention | 12 | M/F | C2-L3 | A-D | 13 | 11 | 1815 | 743 | 290 | 95 | 635 | 313 | 907 | 466 | SR 7-d, 5-d dietary recall |
| Aquilani et al., 2001 | All | 10 | M | Para | A | ≥ 0·2* | 755 + 344 | Weight, type recorded | ||||||||
| Beal et al., 2017 | All | 20 | M | T3-L1 | A, B | 1448 | 484 | 255* | 527* | 667* | SR 3-d dietary recall | |||||
| High vitamin D | 10 | M | T3-L1 | A, B | 19 | 12 | 1683 | 609 | 303* | 606* | 816* | |||||
| Low vitamin D | 10 | M | T3-L1 | A, B | 15 | 12 | 1212 | 358 | 206* | 448* | 519* | |||||
| Chen et al., 2006 | All, baseline | 13 | M/F | Para/tetra | A-D | 18* | 1606 | 672 | Dietary recall | |||||||
| Cox et al., 1985 | All | 45 | M/F | Para/tetra | 0·18 | 0·04 | 1774* | 24-h RD-assessed dietary recall | ||||||||
| Doubelt et al., 2015 | All | 34 | M/F | Para/tetra | C/I | 328 | 144 | FFQ | ||||||||
| Edwards et al., 2008 | All | 15 | M/F | Para/tetra | C/I | ≥ 1* | 2090 | 652 | 320 | 22 | 699 | 54 | 1064 | 83 | SR 3-d dietary recall | |
| Farkas et al., 2019 | Para | 28 | M/F | T2-L1 | A, B | 16 | 11 | 1516 | 548 | 273 | 79 | 523 | 102 | 709 | 114 | SR 3-d dietary recall |
| Tetra | 13 | M/F | C4-C8 | A, B | 13 | 12 | 1619 | 564 | 277 | 81 | 534 | 92 | 762 | 121 | ||
| Gorgey et al., 2012 | Exercise + diet | 5 | M | C5-T10 | A, B | 16 | 9 | 1781 | 228 | 321 | 36 | 623 | 53 | 819 | 89 | 7-d food diaries |
| Diet | 4 | M | T4-T11 | A, B | 8 | 10 | 1731 | 127 | 329 | 69 | 589 | 87 | 814 | 52 | ||
| Gorgey et al., 2015 | All | 16 | M | C5-T10 | A, B | 1350 | 477 | SR 5-d dietary recall | ||||||||
| Gorgey et al., 2019 | Testosterone + exercise | 11 | M | C5-T11 | A, B | 10 | 9 | 1532 | 547 | 291 | 69 | 567 | 107 | 659 | 138 | SR 3-d dietary recall |
| Testosterone only | 11 | M | C6-T11 | A, B | 7 | 6 | 1497 | 127 | 314 | 90 | 539 | 90 | 629 | 120 | ||
| Groah et al., 2009 | Tetra-male | 24 | 2012* | 343* | 733* | 881* | SR 4-d food log | |||||||||
| Para-male | 37 | 2088* | 350* | 744* | 992* | |||||||||||
| Tetra-female | 1 | 2685* | 382* | 945* | 1408* | |||||||||||
| Para-female | 11 | 1662* | 301* | 563* | 805* | |||||||||||
| Iyer et al., 2020 | All | 50 | M/F | Para/tetra | C/I | 0·1–0·4 | 1751 | 294 | 356 | 52 | 486 | 90 | 844 | 180 | SR, RD-assessed 3-d dietary record | |
| Male | 35 | M | Para/tetra | C/I | 1809 | 245 | 364 | 48 | 495 | 90 | 872 | 140 | ||||
| Female | 15 | F | Para/tetra | C/I | 1648 | 372 | 332 | 52 | 459 | 83 | 780 | 240 | ||||
| Kaufman et al., 1985 | Male | 8 | M | C4-L2 | 0·03* | 848 | 414 | 10-d calorie count | ||||||||
| Kearns et al., 1992 | All | 10 | M/F | C4-T10 | Frankel A | 0·2* | 1909 | 43 | RD-interviews, nursing records | |||||||
| Krempien & Barr, 2011 | All | 32 | 2003 | 517 | 352 | 104 | 567 | 189 | 1100 | 304 | SR 3-d food diary | |||||
| Male | 24 | 2028 | 528 | 352 | 88 | 576 | 207 | 1100 | 312 | |||||||
| Female | 8 | 1927 | 510 | 360 | 140 | 522 | 162 | 1088 | 308 | |||||||
| Laven et al., 1989 | All | 29 | M/F | Para/tetra | Frankel A-C | < 0·08* | 1494 | 879 | 232 | 132 | Daily meal tray observation | |||||
| Para | 16 | 1285 | 505 | 212 | 120 | |||||||||||
| Tetra | 13 | 1752 | 1163 | 260 | 148 | |||||||||||
| Lee et al., 1985 | Hypometabolic | 6 | M | C/I | 16 | 9 | 2116 | 415 | 24-h dietary record | |||||||
| Normometabolic | 5 | M | C/I | 19 | 14 | 2152 | 709 | |||||||||
| Hypermetabolic | 6 | M | C/I | 19 | 12 | 2005 | 508 | |||||||||
| Levine et al., 1992 | Male | 24 | C/I | 1682 | 429 | 276 | 83 | 603 | 215 | 816 | 324 | 7-d dietary record | ||||
| Female | 9 | 1282 | 418 | 224 | 86 | 423 | 203 | 664 | 289 | |||||||
| All | 100 | 2601 | 2006 | 401 | 282 | 901 | 785 | 1308 | 1096 | FFQ | ||||||
| Lieberman et al., 2014 | ||||||||||||||||
| Liu et al., 1996 | Tetra-PI | 16 | Tetra | 10 | 2 | 1603 | 604 | Inpatient, Measured from food remaining on hospital tray for 3 d; Outpatient, 3-d dietary record | ||||||||
| Tetra-no PI | 8 | Tetra | 15 | 3 | 1561 | 808 | ||||||||||
| Mollinger et al., 1985 | High tetra | 14 | M | C4-C6 | C | 6 | 5 | 2209 | 894 | Measured food remaining on tray for 3 d, 24-h dietary recall | ||||||
| Low tetra | 13 | M | C6-C7 | C | 7 | 4 | 2213 | 698 | ||||||||
| High para | 16 | M | T1-T10 | C | 9 | 5 | 2384 | 742 | ||||||||
| Low para | 5 | M | T10-L2 | C | 4 | 3 | 2732 | 866 | ||||||||
| Moussavi et al., 2001 | All | 189 | M/F | Para/tetra | A-D | 13 | 10 | 70 | 29 | SR 3-d record | ||||||
| Nightingale et al., 2017 | All | 33 | 1742 | 72 | 306 | 13 | 592 | 30 | 787 | 38 | SR 7-d dietary record, food weighing | |||||
| Peiffer et al., 1981 | Para | 9 | M/F | Para | ≥ 0·3* | 2446 | 251 | 488 | 92 | 24-h dietary recall | ||||||
| Tetra | 9 | M | Tetra | ≥ 0·3* | 1795 | 447 | 288 | 84 | ||||||||
| Perret & Stoffel-Kurt, 2011 | All | 12 | 1775 | 234 | 286 | 32 | 644 | 88 | 775 | 224 | SR 7-d dietary record | |||||
| Rowan & Klazemi, 2020 | All | 16 | M/F | C4-C6 | 0·06* | 2290 | Inpatient, energy content recorded from all sources of feeding (enteral, intravenous) | |||||||||
| Ca only | 36 | M/F | 9 | 8 | 1589 | 709 | 510 | 336 | 886 | 375 | ||||||
| Sabour et al., 2012 | n-3 fatty acid, Ca+ | 39 | M/F | 14 | 26 | 2003 | 658 | 772·2 | 652 | 1001 | 367 | SR 3-d dietary record | ||||
| Sabour et al., 2012 | All | 162 | 2032 | 699 | 746 | 302 | 1077 | 437 | FFQ | |||||||
| Male | 131 | 2078 | 724 | 746 | 284 | 1115 | 462 | |||||||||
| Female | 31 | 1839 | 547 | 748 | 377 | 918 | 260 | |||||||||
| Complete | 48 | 1967 | 726 | 259 | 93 | 704 | 291 | 1042 | 390 | |||||||
| Incomplete | 114 | 2060 | 688 | 257 | 100 | 764 | 307 | 1092 | 456 | |||||||
| Tetra | 94 | 2013 | 681 | 255 | 95 | 735 | 318 | 1071 | 423 | |||||||
| Para | 68 | 2060 | 727 | 261 | 102 | 762 | 281 | 1086 | 459 | |||||||
| Sabour et al., 2016 | Males | 83 | M | Para/tetra | A-D | 12 | 6 | 1826 | 553 | 316 | 96 | 588 | 178 | 922 | 279 | 3-d dietary record/recall |
| Females | 17 | F | Para/tetra | A-D | 15 | 9 | 1413 | 350 | 222 | 53 | 582 | 144 | 619 | 153 | ||
| Sabour et al., 2016 | All | 103 | M/F | Para/tetra | A-D | ≥ 1* | 1756 | 542 | 312 | 100 | 544 | 154 | 900 | 288 | 24-h dietary recall | |
| Sabour et al., 2016 | All | 157 | M/F | Para | C/I | ≥ 1* | 1847 | 589 | 284 | 104 | 675 | 306 | 952 | 332 | 3-d dietary recall | |
| Tomey et al., 2005 | All | 95 | 2265 | 745 | 329 | 127 | 835 | 364 | 1100 | 347 | Modified FFQ to assess 7-d intake | |||||
| Walters et al., 2009 | Male | 63 | 2096 | 420 | 335 | 67 | 629 | 126 | 1090 | 218 | 24-h dietary recall | |||||
| Female | 14 | 1711 | 152 | 291 | 26 | 479 | 43 | 907 | 81 | |||||||
SCI, spinal cord injury; LOI, level of injury; AIS, American Spinal Injury Association Impairment Scale; TSI, time since injury; SR, self-report; Para, paraplegia; Tetra, tetraplegia; C, complete; I, incomplete; PI, pressure injuries; FFQ, food frequency questionnaire; RD, registered dietitian.
Blank spaces indicate data were not provided in the study; data are presented as mean ± standard deviation.
Standard deviation not provided.
Table 6.
Comparison of authoritative, evidence-based non-spinal cord injury dietary guidelines
| American Heart Association(6,7)* |
Australian Dietary Guidelines(8) |
Dietary Guidelines for Americans(1) |
IOM Dietary Reference Intakes(2) |
PHE Government Dietary Recommendations(9,10) |
WHO Healthy Diet(11) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total energy | Female | Male | Female/Male | Female | Male | Female | Male | Female | Male | Female/male |
| Total energy (kcal/d) | 1200–1500 | 1500–1800 | 2108–2259 | 1600–2000 | 2000–3000 | Estimated energy requirement† | Estimated energy requirement‡ | 1840–2000 | 2294–2500 | ≥ 1200 with 500–600/d deficit or in balance with energy expenditure |
| Macronutrients: protein, carbohydrate and fat | ||||||||||
| Protein (kcal) (RDA) | 180–375 | 225–450 | 393–398 | 184 | 224 | Varies based on total energy | 180–186 | 213–222 | Varies based on total energy | |
| Protein (% kcal) (AMDR) | 15 or 25 | 15–25 | 10–35 | 10–35 | 10–35 | 10–35 | 15 | 15 | 15 | |
| Carbohydrates (kcal) (RDA) | 420–975 | 525–1170 | 1088–1108 | 520 | 520 | Varies based on total energy | 980–1068 | 1224–1332 | Varies based on total energy | |
| Carbohydrates (% kcal) (AMDR) | 35, 45, 55 or 65 | 45–65 | 45–65 | 45–65 | 45–65 | 45–65 | 50 | 50 | 55–60 | |
| Dietary fibre (g) | 25–30(12) | 24–26 | 22–28 | 28–34 | 14 (21–38)§ | 30 | 30 | |||
| Total fat (kcal) (AMDR) | 240–600 | 300–720 | 799–820 | 300–700 | 400–1050 | Varies based on total energy | 648–702 | 801–873 | Varies based on total energy | |
| Total fat (% kcal) (DGA) | 20 or 40 | 20–35 | 20–35 | 20–35 | 20–35 | 20–35 | 35 | 35 | ≤ 20–30 | |
| Saturated fat (% kcal) (DGA) | 5–6 | < 10 | < 10 | < 10 | As low as possible | 11 | 11 | < 10 | ||
| MUFA | 8% | 32–34 g | No standards set | No standards set | 27–29 g | 33–36 g | ||||
| n-6 PUFA/linoleic acid (AI) | 2% | 13·4–13·9 g | 11–12 g/d | 14–17 g/d | 5–10% | 5–10% | 13–14 g | 17–18 g | ||
| n-3 PUFA/linolenic acid (AI) | 1·1 g/d | 1·6 g/d | 0·6–1·2% | 0·6–1·2% | ||||||
| Micronutrients: vitamins | ||||||||||
| Choline (mg) (AI) | 425 | 550 | 425 | 550 | ||||||
| Vitamin A (mg/d) (RDA) | 1·2–1·3 | 700 | 900 | 700 | 900 | 600 | 700 | |||
| Vitamin B1/thiamin (mg/d) (RDA) | 1·74–1·83 | 1·1 | 1·2 | 1·1 | 1·2 | 0·7–0·8 | 0·9–1·0 | |||
| Vitamin B2/riboflavin (mg/d) (RDA) | 2·18–2·27 | 1·1 | 1·3 | 1·1 | 1·3 | 1·1 | 1·3 | |||
| Vitamin B3/niacin (mg/d) (RDA) | 45·5–45·9 | 14 | 16 | 14 | 16 | 12·1–13·2 | 15·1–16·5 | |||
| Vitamin B5/pantothenic Acid (mg/d) (AI) | 5 | 5 | ||||||||
| Vitamin B6 (mg/d) (RDA) | 1·3–1·5 | 1·3–17 | 1·3–1·5 | 1·3–1·7 | 1·2 | 1·4 | ||||
| Vitamin B7/biotin (μg/d) (AI) | 30 | 30 | ||||||||
| Vitamin B9/folate (μg/d) (RDA) | 286–299 | 400 | 400 | 400 | 400 | 200 | 200 | |||
| Vitamin B12 (μg/d) (RDA) | 2·4 | 2·4 | 2·4 | 2·4 | 1·5 | 1·5 | ||||
| Vitamin C (mg/d) (RDA) | 130–142 | 75 | 90 | 75 | 90 | 40 | 40 | |||
| Vitamin D (μg/d) | 15 (RDA) | 15 (RDA) | 5–15 (AI) | 5–15 (AI) | 10 | 10 | ||||
| Vitamin E (mg/d) (RDA) | 15 | 15 | 15 | 15 | ||||||
| Vitamin K (μg/d) (AI) | 90 | 120 | 90 | 120 | ||||||
| Micronutrients: minerals | ||||||||||
| Ca (mg/d) | 888–945 | 1000–1200 (RDA) | 1000–1200 (RDA) | 1000–1200 (AI) | 1000–1200 (AI) | 700 (RDA) | 700 (RDA) | |||
| Cr (μg/d) (AI) | 20–25 | 30–35 | ||||||||
| Chloride (mg/d) (AI) | 1800–2300 | 1800–2300 | 2500 | 2500 | ||||||
| Cu (μg/d) (RDA) | 900 | 900 | 900 | 900 | 1·2 | 1·2 | ||||
| Fluoride (mg/d) (AI) | 3 | 4 | ||||||||
| Iodine (μg/d) | 150 | 150 | 140 | 140 | ||||||
| Fe (mg/d) (RDA) | 15·0–15·6 | 8–18 | 8 | 8–18 | 8 | 8·7–14·8 | 8·7 | |||
| Mg (mg/d) (RDA) | 353–366 | 310–320 | 400–420 | 310–320 | 400–420 | 270 | 300 | |||
| Mn (mg/d) (AI) | 1·8 | 2·3 | 1·8 | 2·3 | ||||||
| Mo (μg/d) (RDA) | 45 | 45 | ||||||||
| P (mg/d) (RDA) | 1626–1673 | 700 | 700 | 700 | 700 | 550 | 550 | |||
| K (mg/d) (AI) | 3495–3551 | 4700 | 4700 | 4700 | 4700 | 3500 | 3500 | |||
| Se (μg/d) (RDA) | 55 | 55 | 55 | 55 | 60 | 75 | ||||
| Na (mg/d) (UL) | ≤ 2400 (≤ 1500 is better) | 2300 | 2300 | 2300 | 2300 | 2500 | 2500 | < 5000 | ||
| Zn (mg/d) (RDA) | 12·9–13·3 | 8 | 11 | 8 | 11 | 7 | 9·5 | |||
AMDR, Acceptable Macronutrient Distribution Range; DGA, Dietary Guidelines for American; AI, adequate intake; UL, tolerable upper intake level.
Guidelines were established to prevent CVD and manage overweight and obese adults.
Estimated energy requirement equation for males = 662 – (9·53 × age (years)) + PA × ((15·91 × weight (kg)) + (539·6 × height (m))), where PA is the physical activity coefficient.
Estimated energy requirement equation for females = 354 – (6·91 × age (years)) + PA × ((9·36 × weight (kg)) + (726 × height (m))), where PA is the physical activity coefficient.
Values in parentheses are an example of the total g/d of total fibre calculated from 14 g/1000 kcal multiplied by the median energy intake (kcal/1000 kcal/d) from the Continuing Survey of Food Intakes by Individuals (1994–1996, 1998).
The IOM encourages establishing energetic intake using a sex-specific prediction equation, relying on age, height, weight and physical activity. While less precise than measuring energy requirements, the IOM is the only guideline that makes such a recommendation rather than providing an acceptable macronutrient distribution range. A limitation of IOM’s equation is that it neglects to include resting or basal metabolism as the largest determinant of TDEE, and therefore energetic intake. TDEE can also be estimated using the product of BMR (or RMR) and the common activity correction factor of 1·2(9). Several studies have predicted TDEE using 1·2 and other previously published activity, stress, injury, and/or trauma correction factors to determine energetic intake for persons with SCI(34,89,97,98,101,102,121,129).
In the acute phase of SCI, the literature indicates total energetic intake ranges from 755 to 2290 kcal/d (Table 5)(89,97,119,120,136-138). Over the first 4 weeks of the SCI, this value increases by over 400 kcal/d(119) and likely results from the thermic effect of voluntary respiration (diaphragmatic and intercostal muscle activation) for those weaned from mechanical ventilators. The conversion from the catabolic state to declining energetic needs is not well researched, though RMR has been shown to decrease 10 weeks post-SCI(139). In seminal work by Cox et al.(89), the authors reported that persons in the early rehabilitation phase of the injury require up to 54 % less energy content than would be predicted by most standard formulae. The authors further determined that in the rehabilitation phase, persons with tetraplegia need 22·7 kcal/kg/d and persons with paraplegia need 27·9 kcal/kg/d(89), guidelines that are still widely used today(140). Of note, this calculation published by Cox et al.(89) was developed in fifteen persons with tetraplegia and five with paraplegia and mostly men (86 %) whom typically expend(110,121) and consume(136,141) more than women. The equations also do not account for the serial weight loss and weight regain that occurs during the early phase of the SCI and a drop in energy expenditure that persists through the rehabilitation phases of treatment.
When evaluating the energy intake and expenditure data, persons with acute SCI appear to be in a negative energy balance (TDEE: 2030 to 3344 kcal/d v. energetic intake: 755 to 2290 kcal/d). Distinct from other trauma conditions, persons with acute SCI do not demonstrate hypermetabolism following injury(119,129,142,143). While a negative nitrogen balance does occur, this is obligatory. While the underlying mechanism contributing to a negative nitrogen balance following SCI remains poorly understood(142-145), efforts made to shift the obligatory loss of nitrogen by increasing energetic intake can lead to overfeeding. Confounding this matter is that several studies and registered dietitians use correction factors to increase energetic intake. Kaufman et al.(137) and Barco et al.(93) used an activity factor of 1·1. However, a stress factor of 1·2 to 1·75 is routinely used in in the literature(89,129,137,143). Rodriguez et al.(129) examined the Harris-Benedict equation with an activity factor of 1·2 and an injury factor of 1·6 and identified that the equation overestimated energetic requirements in twelve persons with acute SCI. The same authors reported that establishing nutritional management upon serial indirect calorimetry measurements with higher stress and activity factors result in overfeeding(129). Kearns et al.(119) compared measured RMR with the Harris-Benedict equation in five individuals with tetraplegia and reported that the use of the equation leads to an overfeeding by nearly 70 %. While the time frame of the investigation relative to SCI was not specified, the authors suggested administering 80 % of predicted energetic needs(119).
The 2008 Paralyzed Veterans of America (PVA) Early Acute Management in Adults with Spinal Cord Injury: A Clinical Practice Guideline for Health-Care Professions state ‘Provide appropriate nutrition when resuscitation has been completed and there is no evidence of ongoing (spinal) shock or hypoperfusion’. The PVA recommendations do endorse the determination of energetic requirements for nutritional support using a 30-min energy expenditure measurement by indirect calorimetry. These guidelines for acute SCI do not define or provide a reference for what ‘appropriate nutrition’ entails, and hospitals and inpatient rehabilitation facilities do not use or often have access to metabolic carts, and insurance plans do not cover the cost of indirect calorimetry. Similarly, the Academy of Nutrition and Dietetics (AND) recommends the use of indirect calorimetry during the acute phase of SCI. They state ‘actual energy needs are at least 10 % below predicted needs(140). But the AND recommends in the absence of indirect calorimetry to use the Harris-Benedict formula using admission weight and an injury factor of 1·1 and an activity factor of 1·2(140). This type of prediction method overestimates TDEE and subsequently energetic intake in persons with SCI, thereby leading to overfeeding(129,143). Persons with SCI remain in an obligatory negative nitrogen balance, but providing excess energy content should be avoided. Overfeeding carries unique complications, such as hyperglycaemia, hypercapnia, hypertriacylglycerolaemia, uremia and obesity(8,146).
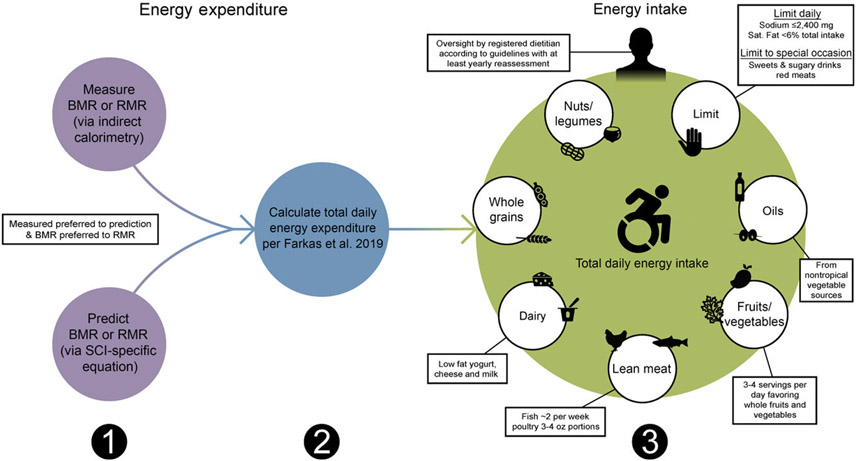
With regard to chronic SCI, the PVA Consortium for Spinal Cord Medicine recently assembled an expert panel to compile the Clinical Practice Guidelines on Identification and Management of Cardiometabolic Risk after SCI (PVA guidelines; Table 7; Fig. 2)(10). The inaugural guidelines recommended when establishing energetic targets, all persons with chronic SCI should undergo an energetic assessment using indirect calorimetry to estimate energy expenditure and assess energy needs(10). Given indirect calorimetry is used to measure resting and basal metabolism, an important consideration is how to determine TDEE. In 2019, Farkas et al.(9) developed a novel SCI-specific correction factor of 1·15 to estimate TDEE from BMR (or RMR) using 2·7 ml of oxygen/kg of body weight/min(80), a MET (metabolic equivalent of task) for SCI. The SCI-specific TDEE prediction equation requires validation against the gold standard respiratory chamber but provides promise. It is a novel method to estimate TDEE, and to accurately determine energetic needs in chronic SCI.
Table 7.
Practical dietary recommendations with example foods to consume and avoid for persons with SCI
| Paralyzed Veterans of American (PVA) Dietary Criteria(5) |
Examples of foods to consume | Examples of foods to avoid |
|---|---|---|
| Fruits | Apples, apricots, avocado, bananas, blueberries, cherries, clementines, cranberries, dates, dried fruit (unsweetened), figs, grapes/raisins, kiwi, mango, melon, nectarines, papaya, pears, pineapples, plums, pomegranates, prunes, raspberries, strawberries and tomatoes. Frozen fruits are a good alternative when fresh fruit is not available. | Fruit cups (with syrup), fruit juice, fruit snacks, jam and jelly. Unsweetened cranberry juice may help reduce excess bacteria in the urinary tract to prevent urinary tract infections. |
| Vegetables | Artichokes, asparagus, beets, broccoli, brussels sprouts, cabbage, carrots, cauliflower, maize, cucumber, eggplant, garlic, green beans, kale, mushrooms, onions, peas, peppers, pickles, potatoes, romaine, spinach, squash, sweet potatoes and turnips. Frozen vegetables are good alternatives when fresh vegetables are not available. | Canned vegetables (high in Na), French fries, ketchup, potato chips, onion rings, relish and sweet potato chips. Avoid high-fat food preparations (e.g. frying/deep frying and use healthier methods such as pan-frying with olive oil, baking, broiling, braising, poaching, steaming and stewing. |
| Poultry | Chicken, duck, eggs, egg whites and turkey | Deep-fried turkey, deviled eggs, fried chicken and fried duck |
| Fish | Cod, halibut, herring, lake trout, mackerel, mahi-mahi, rainbow trout, salmon, sardines, swordfish, tuna (albacore and canned light) and whitefish | Anchovies (cured/canned, high in Na), fried fish (all), fried shellfish (all) and shrimp (high in cholesterol) |
| Low-fat dairy products | Skim/1 % milk, low-fat cheese (e.g. Cheddar, mozzarella, goat, provolone, muenster, feta, swiss, etc.), low-fat cottage cheese and low-fat yogurt | Whole/2 % milk, cream cheese, half and half, creamer, and condensed milk, sour cream, heavy cream, heavy whipping cream and whipped cream |
| Whole grains | Brown rice, buckwheat, millet, oats, quinoa, spelt, wild rice, whole wheat bread and whole-grain pasta | Crackers (all), granola bars (high in sugar), muffins, processed cereals (all) and oatmeal packets with high sugar content and additives, white rice cakes, white bread, white pasta and white rice |
| Legumes | Black beans, black-eyed peas, chickpeas/garbanzo beans, fava (broad) beans, kidney beans, lentils, pinto beans, soyabeans, split peas, tofu and white beans | Baked beans (all) |
| Nuts (and seeds*) | All unsalted: almonds, brazil nuts, cashews, chestnuts, flaxseed, hazelnuts, peanuts, pecans, pumpkin seeds, sesame seeds, sunflower seeds and walnuts | Salted nuts, salted seeds and processed nut butters (e.g. processed peanut butter with extra sugar and additives, hazelnut chocolate spread, etc.) |
| Non-tropical vegetable oils | Rapeseed, maize, olive, peanut, safflower, soyabean and sunflower oils | Coconut, hydrogenated, (full, partial), palm kernel and palm oils. Processed salad dressings and oil-based products (e.g. BBQ sauce, mayonnaise and margarine) |
| Limit† | ||
| Sweets | Dark chocolate (in small quantities), dried fruit (unsweetened) and popcorn (unsalted, no butter) | Cakes, candy, caramel, caramelised popcorn, cookies, croissants, donuts, ice cream, milk chocolate and pastries |
| Sugar-sweetened beverages | Carbonated water (flavoured and unflavoured) and splash of zero (0) calorie liquid water enhancer | Fruit punch, fruit-flavoured beverages, juice and soda (diet and regular) |
| Red meats | Lean cuts with ≤ 5 % fat, trim off fat before cooking‡ and pour off melted fat after cooking, use healthier cooking methods (e.g. bake, broil, stew, grill and roast). Packages for lean cuts will usually say ‘round’, ‘loin’ or ‘sirloin’ | All processed meats: bacon, beef jerky, cold cuts, deli slices, frankfurters, ham, hot dogs, pepperoni, salami and sausages |
| Na intake (≤ 2400 mg)§ | Consult nutrition facts on specific food items | |
| Saturated fat (< 5–6 %) |
The PVA guidelines do not mention seeds; however, the authors are including seeds in the nut category.
Limit to special occasions (i.e., birthdays, weddings, holidays, etc.)
For persons with limited upper extremity function, ask the butcher to trim the fat at the supermarket.
For persons with hypertension, although the authors recommend adopting ≤ 2400 mg of Na for all individuals regardless of hypertension status given the elevated consumption of Na-dense foods reported in the literature.
Fig. 2.
Sequential dietary recommendations for persons with a spinal cord injury (SCI). First, BMR or RMR should be annually measured with indirect calorimetry or estimated using SCI-specific predictions equations (Nightingale and Gorgey(116), Chun et al.(105) or Buchholz et al.(79)) when indirect calorimetry is unavailable. Second, total daily energy expenditure should be estimated as the product of BMR or RMR and 1·15 for persons with SCI using the Farkas et al.(9) equation. Third, a registered dietician should oversee a healthy dietary pattern following the Clinical Practice Guidelines on Identification and Management of Cardiometabolic Risk after SCI and additional recommendations provided in this review(10).
Across the chronic SCI literature, energetic intake ranges from 1212 to 2732 kcal/d (Table 5)(9,34,84,86,92,108,109,115,117,130,135,141,147-157), seemingly appearing to be in energy balance when evaluating TDEE (1332 to 2728 kcal/d). At face value, an energy balance does not appear to be congruent with the reported high rates of obesity(8,9,11,29,60). However, in a recent meta-analysis by Farkas et al.(19), the authors reported in a sample of 606 persons with chronic SCI, a pooled energetic intake of 1876 kcal/d and a pooled RMR of 1492 kcal/d. Estimating a TDEE of 1716 kcal/d (using RMR × 1·15(9)), there is a positive energy balance of over 150 kcal/d. This is further supported by the additional work by Farkas and colleagues(9). The authors reported a greater energetic intake in persons with tetraplegia compared with paraplegia when adjusting energy intake by body weight, thereby accounting for body composition that is significantly different by injury level(9,107,158,159). The authors concluded that their findings may explain why persons with tetraplegia had significantly great percentage body fat relative to paraplegia(9). Collectively, these data provide support that persons with chronic SCI may overconsume relative to their need, and this may contribute to the high rates of neurogenic obesity. However, the findings are subject to how energetic intake was operationalised.
While dietary recalls, dietary diaries/records/logs and food frequency questionnaire (FFQ) have demonstrated a strong agreement amongst themselves, research has identified systematic misreporting errors for all of the self-reported dietary instruments(160). Most of the literature in both acute and chronic SCI describe using dietary assessments without indicating self-report or whether a registered dietitian administered or reviewed the instrument (Table 5). In fact, 40% of the investigators specify the use of self-report dietary assessment techniques, whereas 11 % and 9 % indicated measuring the food consumed off the plate or if a registered dietitian performed the assessment, respectively (the remaining studies provided insufficient detail to clearly determine assessment methods). It is well established that dietary assessment methods underreport true energetic consumption in persons without SCI(160,161), and a similar phenomenon is likely present in the population with SCI(9,84). Moreover, self-report after SCI becomes a challenge, especially with higher levels of injury. Persons with tetraplegia may have difficulty writing down, especially in detail, intake data and may limit, and in some cases omit, what they ate or drank on their assessment instruments. Portion size, food preparation and cooking details may be omitted and are details that can greatly influence the energy content of food. Family members or caregiver(s) may introduce a source of error by recording their food. Accordingly, it is likely that, as with the population without SCI(161), energetic intake is being underreported. Future large-scale studies with more stringent dietary assessment and testing methods are needed to examine the energetic need relative energy expenditure after SCI.
Macronutrient intake
Macronutrients are dietary constituents that provide energy. They include protein, carbohydrates, fats and alcohol. Although alcohol is considered a macronutrient and provides energy, it is not needed for survival.
Protein
Proteins (4 kcal/g) are considered the most abundant macronutrient; they are composed of amino acids, of which nine are essential and cannot be synthesised by the body but must be acquired in the diet. The quality of dietary protein is characterised by the protein’s digestibility and its amino acid profile in relation to requirements as determined by repair, maintenance and growth(12). Several studies report protein ingestion in persons with SCI is within or exceeds recommended daily values for the population without SCI (Tables 5 and 6)(19,108,115,117,135,136,150-154,162-167). Approximately 15 to 19 % of the total daily energy intake came from protein for persons with SCI(108,115,117,147,148,151,164,165). In persons with chronic SCI, Farkas et al.(19) reported consumption of 319 kcal/d of dietary protein surpassed the DGA recommendation of 184 to 224 kcal/d, representing 17 % of their total daily intake, even though fat-free (protein) body mass is markedly reduced. The value reported by Farkas et al.(19) also exceeded the PHE guidelines on protein consumption and was below recommendations by ADG (Table 6). For most individuals with or without SCI, it is not uncommon to meet or exceed total protein recommendations. The sources of dietary protein largely remain unknown. Silveira et al.(150) identified seafood consumption was low in persons with SCI, although Lieberman et al.(164) reported more meat and fish/seafood were consumed compared with non-disabled controls. These conflicting findings may result from the location (Houston, TX(150) v. Charlotte, NC(164)) and/or the race/ethnicity (racially/ethnically diverse(150) v. Black/White(164) of the participants and requires additional research.
Many individuals with SCI are not meeting the recommendations for specific amino acids(12,19). Sabour et al.(154) reported that lysine, leucine, valine and isoleucine were the major constituents of total protein intake in persons with SCI, while arginine, alanine and aspartic acid had the lowest daily intake. Groah et al.(135) demonstrated that amino acid intake after SCI approached, or met, DGA dietary recommendations except for lysine, leucine, threonine, methionine and cysteine. The same authors noted that men with paraplegia consumed a greater amount of every amino acid compared with men with tetraplegia(135). This evidence suggests that while protein consumption remains high among persons with SCI, some may still be missing key essential amino acids that can result in malnutrition and health consequences. Moreover, these essential amino acids are necessary for vital functions such as protein synthesis and tissue repair, which is particularly important in this population group that is prone to pressure injuries.
Pressure injuries after SCI precipitously deplete the limited protein stores as the body attempts to heal the wound, generating a rapid transition to malnutrition. This occurs in the presence of already markedly diminished protein reserves (i.e.. skeletal muscle mass). The AND recommends that in the presence of a pressure injury for persons with SCI, albumin and prealbumin laboratory values should be measured(140). Prealbumin (also known as transthyretin) and albumin have traditionally been utilised as biomarkers of protein nutrition and nutritional status, respectively. A 2012 consensus statement from the AND and the American Society for Parenteral and Enteral Nutrition discouraged the use of prealbumin and albumin as ‘sole’ indicators of undernutrition due to their susceptibility to systemic inflammation(168). In their place, the panel recommended the identification of two or more of the following six characteristics for a malnutrition diagnosis: insufficient energetic intake, weight loss, loss of muscle mass, loss of subcutaneous tissue, localised or generalised fluid accumulation that may sometimes mask weight loss, and diminished functional status as measured by handgrip strength(168). For persons with SCI, lower extremity fluid accumulation is common and due to paralysis, handgrip strength cannot be measured in tetraplegia, thus potentially limiting their utility. The Global Leadership Initiative on Malnutrition recently published guidelines that favoured reinstituting the application of prealbumin as a contributing element to monitor undernutrition in conjunction with C-reactive protein under 15 mg/dl (denoting asymptomatic infection), as prealbumin levels above that are uninterpretable(169-171). This is supported by recent evidence that suggests prealbumin can supplement other markers such as anthropometrics and clinical history to assess and monitor undernutrition(172). In a retrospective chart review of 170 SCI patients with pressure injuries, Lussi et al.(173) reported 15·3% and 34 % of the patients only had pathologic laboratory values of prealbumin and albumin, respectively. Poor protein blood levels, however, were observed in 41 % of the patients(173), suggesting protein blood levels may be a promising measure to assess protein health and pressure injury risk after SCI. Because the use of prealbumin and albumin remain controversial, laboratory examinations, nutritional assessments and anthropometric measures are collectively needed to detect, correct, and treat pressure injuries and protein nutritional deficits after SCI.
Carbohydrate
Carbohydrates (4 kcal/g) are organic compounds in the form of sugars, starches and fibres. The energy source is rich in simple or complex carbohydrates(12). Simple carbohydrates are largely consumed by persons with SCI(136,153), while added sugars surpass consumption by non-disabled individuals and the DGA, PHE, IOM, and ADG recommendations(150). Moreover, simple carbohydrates and added sugars (i.e., processed foods) have a high glycaemic index, meaning consumption of these foods cause a rapid hyperglycemia and insulin release that is difficult to control in persons with SCI and prediabetes and type 2 diabetes mellitus.
Several studies identified that about half of the energy content consumed by persons with SCI were from carbohydrates (Table 5)(108,136,147,150-152,164,165), whereas Nightingale et al.(115) identified 44 % of the daily energy content came from carbohydrates, respectively. Perret and Stoffel-Kurt(117) observed that persons with acute SCI consume a greater percentage of carbohydrates compared with persons with chronic SCI(117). Iyer et al.(136) and Sabour et al.(153) reported higher consumption of carbohydrates in men compared with women with SCI. The latter authors also identified that time since injury, education, and sex were significant predictors for carbohydrate intake in persons with SCI(153). Farkas et al.(19) calculated the average carbohydrate intake for persons with long-standing SCI as 969 kcal/d, a value that exceeds the DGA of 520 kcal/d. This equates to over 50 % of ingested energy content coming from carbohydrates(19). The DGA, IOM and ADG recommend that carbohydrates make up 45 to 65 % of an individual’s total daily energy content when consuming a 2000 kcal/d diet. Although, on average, persons with SCI are consuming less than 2000 kcal/d according to Farkas et al.(19), suggesting 45 to 65 % of the total daily energy content coming from carbohydrates should be reduced for persons with SCI.
Carbohydrate consumption should come from complex carbohydrates with a low glycaemic index, such as whole grains. It is well established that whole grains inherently control blood sugar and increase micronutrients, satiation/satiety and fibre consumption(174). For persons with SCI, data supporting whole-grain consumption are limited. Silveira et al.(150) reported that whole grains made up 15 % of the total grains in the diets of individuals with SCI compared with 19 % in non-disabled controls. Similarly, Lieberman et al.(164) reported significantly lower daily servings of whole grains in SCI compared (1·20) with non-disabled controls (2·44); however, refined grains did not significantly differ (SCI: 5·42 ± 3·45 v. controls: 6·44 ± 6·45), likely to a large variance in the control group. The same authors also showed that 9 % of persons with SCI adhered to consuming ≥ 3 ounces of whole grains compared with 21 % of age- and sex-matched non-disabled controlled(164). The factors contributing to reduced whole-grain consumption in persons with SCI warrant further investigation because of their cardioprotective effects against the risk of heart disease, type 2 diabetes mellitus and obesity, co-morbidities with a high occurrence in the SCI population.
Fat
Fats (9 kcal/g) are a type of lipid and a dense source of energy. Fats are combinations of SFA and unsaturated fats, such as MUFA, PUFA, n-3PUFA, n-6PUFA and trans-fatty acids(12). Fats serve various functions throughout the body, including vitamin transport, organ insulation, maintenance of body temperature, formation of the lipid bilayer of a cell and energy storage.
An abundance of evidence indicates that individuals with SCI ingest amounts of dietary fat that are within or surpass DGA, PHE, ADG and IOM recommendations(9,12,19,117,120,135,136,141,150,151,153-155,162,165,175,176), indicating after carbohydrate consumption, a substantial number of energy content are derived from dietary fat (Table 6). Approximately 34 % to 40 % of daily energy comes from fat in persons with chronic SCI (Table 5)(108,115,147,148,150,151,162,164,165,176) where the upper range is characteristic of a typical US diet(162). In the meta-analysis by Farkas et al.(19), the authors reported that fat intake made up 35 % (663 kcal/d) of the total energetic intake for persons with chronic SCI. The authors noted that fat intake was within the DGA; however, the analysis did not account for the DGA sex- and age-specific ranges for fat intake because of limited power(19). Therefore, these findings should be interpreted with caution under the notion that age and sex were not considered.
High-fat diets often induce greater food intake and weight gain(177), and high saturated fat consumption negatively influences cardiometabolic health and chronic disease risk(178). Intake of saturated fat in persons with SCI is close to the limit or exceeds the recommended daily amount of < 10 to 11 % of total energy content according to DGA, IOM, WHO, PHE and ADG (Table 6)(135,136,147,149,150,153,155,162,165). Tomey et al.(165), Moussavi et al.(176) and Groah et al.(135) reported that saturated fat intake in persons with SCI is higher than the recommended maximum of 10 % of total daily energy content by the USDA’s Food Guide Pyramid, 10 % by the National Cholesterol Education Program and 7 % by the AHA, respectively. The DGA, WHO, PHE and ADG recommend limiting saturated fat to < 10 % to 11 % of daily energetic intake, AHA to 7 %, and IOM to as low as possible (Table 6). The PVA guidelines(10) limit saturated fat to 5 to 6 % of total energetic intake. Persons with SCI exceed recommended values of saturated fat, despite an overall reduced energetic intake(150). The total energy requirements after SCI are less than a non-disabled individual’s, and dietary consumption of saturated fats should mimic the reduced energetic intake, by limiting foods discussed in Table 7.
The DGA, AHA, WHO and ADG recommend replacing saturated fats with unsaturated fatty acids as they provide a cardioprotective effect (Table 6)(12). n-3 and n-6 PUFA are essential in the diet because they cannot be synthesised by humans. Allison et al.(147) reported MUFA consumption did not change following an anti-inflammatory diet, while n-3 and n-6 fatty acids increased and decreased, respectively. Farkas et al.(19) showed greater consumption of MUFA and PUFA in persons with chronic tetraplegia compared with paraplegia; however, this was not a significant finding. Sabour et al.(153) examined dietary fats by injury completeness and noted persons with incomplete injuries consumed more MUFA than those with a complete SCI. According to Silveira et al.(150), MUFA were within healthy ranges, while Groah et al.(135) reported that men and women with paraplegia had lower than the DGA recommended adequate intake of n-6 linoleic acid. According to Sabour et al.(155) linoleic acid consumption exceeded recommended values in persons with SCI. Iyer et al.(136) and Groah et al.(135) also observed that women with SCI exceeded or approached the recommended intake of n-3 linolenic acid, while men with SCI had lower than the recommended intake(135). The latter finding mirrors the results reported by Sabour et al.(155) for men and women with SCI. Silveira and colleagues(150) noted that linoleic and linolenic acids were within normal ranges according to IOM recommendations. These data indicate additional research is needed on MUFA and PUFA and their health-promoting influence after SCI.
Alcohol
Consumed alcohol (7 kcal/g) is known as ethanol and is not a vital macronutrient(12). Ethanol is passively absorbed in the digestive system and metabolised mainly in the liver, although some are also metabolised in the stomach(179). Allison et al.(147) and Nightingale et al.(115) reported that 1·4 % and 3 % of the daily energetic intake came from alcohol in persons with SCI, respectively. Groah and colleagues(135) showed that mean alcohol consumption was overall low (< 10 g/d) among persons with SCI but greater for men than for women with SCI (6·43 v. 2·24 g/d). Another study reported persons with SCI did not consume any alcohol at home(136). Contrary to these data, other studies report high alcohol consumption in persons with SCI(180-183). Study participants with SCI are likely to underreport their true alcohol intake on dietary recalls/logs given the stigma that is often related to alcohol consumption and its effects on body weight and physical and mental health(182). Asking if individuals are current drinkers (i.e. how much they drank in the last month) rather than how much they drink may be a better indicator of alcohol intake in this population.
Fruits and vegetables
Five studies reveal the consumption of fruits and vegetables among individuals with SCI is below the recommended intake according to DGA, ADG, PHE and IOM guidelines(136,150,164,165,184). These data coincide with the reduced consumption of fruits and vegetables in the population without SCI(12-14). Silveria et al.(150) recently revealed that consumption of fruits and vegetables were not only below DGA recommended values, but below the persons without SCI. Lieberman et al.(164) and Tomey(165) demonstrated similar results. Knight et al.(184) observed that fruit and vegetable consumption was greatest among persons with SCI with a high activity level of about 30 min/d compared with lower activity levels. However, the authors did not differentiate between fruit and vegetable intake(184). Interestingly, 80% of the studies that reported fruit and vegetable intake reported vegetable intake was greater than fruit intake(150,164,165). This finding is intriguing given many fruits contain a high amount of simple carbohydrates (i.e. monosaccharides and disaccharides), and as reported by the present review, persons with SCI primarily consume simple carbohydrates v. complex carbohydrates(153).
Fibre
Fibre (about 1·5–2·5 kcal/g) consists of soluble and insoluble complex carbohydrates and lignin that are intrinsic to, and intact in, plants, such as whole grains, vegetables and fruits. Soluble fibre undergoes bacterial degradation in the large intestine to generate volatile free fatty acids that are then absorbed and used as energy(16). Several studies have identified fibre intake in persons with SCI is low independent of sex and injury characteristics(19,117,120,135,147,149,150,152,153,162,165,175) Iyer et al.(136) was the only study to report a high fibre consumption of 30 to 33 g/d, while all other studies report an average intake of 12 to 22 g/d(147,153,162,165,175). Levine et al.(162) showed that dietary fibre intake in men with SCI was a third less than the average intake in the non-disabled population. Farkas et al.(19) quantified fibre intake in the population with chronic SCI as 17 g/d, which is below the recommendations of the DGA, PHE, ADG, IOM and AHA (Table 6). Low intakes of dietary fibre are likely due in part to the low intake of vegetables and fruits, and potentially whole grains.
Conversely, high fibre diets after SCI may cause negative consequences on neurogenic bowel and bladder conditions. Diets high in fibre (> 20 g/d) may instigate unfavourable changes in bowel function and bowel care programmes that do not occur in the non-disabled population(140). Cameron et al.(185) reported that high dietary fibre before a bowel movement does not have the same effect on bowel function in motor complete SCI as in non-disabled individuals. Furthermore, fibre consumption that is too high without commensurate fluid intake can lead to constipation with an already decreased bowel motility(41). The excess fluid intake that is required with high fibre diets may also require additional urethral cathing or lead to bowel/bladder accidents. Consequently, the effects on bowel and bladder care can make high dietary fibre diet recommendations inappropriate for individuals living with SCI(186). Therefore, it is important to develop SCI guidelines on fibre that account for their bowel and bladder programmes (e.g., timing), fluid intake, and their reduced energetic needs relative to an individual without an SCI.
Micronutrients
Micronutrients include vitamins and minerals that are required for cellular communication, water and nutrient transport, the structural integrity of bones, wound healing, and acid–base balance(5). Several micronutrient intakes are within inadequate ranges in persons with SCI according to the recommended guidelines established by the DGA, IOM, PHE and ADG (Table 6)(19,117,135,152,162,164-166). Although others have reported below recommended intake values of vitamins A, B5, B7, C, D and E in individuals with chronic SCI(117,135,152,163,165,175,187), as well as below-recommended intake in the minerals Ca, Mg and K(117,135,152,162,163,175,187). In the meta-analysis by Farkas et al.(19), the authors reported below recommended intakes for vitamins A, B5, B7, B9, D and E, and the minerals K and Ca in persons with chronic SCI according to the DGA report. The authors also found excess intake of vitamins B1, B2, B3, B12 and K, and the minerals Cu, P, Zn and Na according to recommendations(19). Na is one of the most widely studied micronutrients after SCI with an average consumption ranging from 2402 to 4300 mg(117,135,150,152,162,165,166). PVA guidelines recommend Na consumption ≤ 2400 mg/d for all persons with SCI and hypertension. We argue that the high consumption of Na and the prevalence of hypertension in the population(8,11,29) necessitate the need to implement a Na intake ≤ 2400 mg/d for all with SCI.
Three studies have evaluated vitamin and mineral supplementation after SCI. Opperman et al.(188) reported that nutritional supplementation was common in individuals with long-standing SCI, but no common characteristics (e.g., sex, LOI, age, education, etc.) distinguished users from non-users. According to the authors, 71 % of the sample reported using supplements at least once, with approximately 51 % being classified as consistent supplement users at least twice across the three time points assessed in the study. Both Opperman et al.(188) and Walters et al.(152) reported that participants with SCI consumed a micronutrient supplement in the form of Ca, a multivitamin, or vitamin D. The latter authors also observed vitamin C supplementation in their participants(152). Similarly, Wong and colleagues(189) reported that the three most prescribed supplements for persons with SCI were multivitamins, vitamins B and vitamin D at an SCI centre. The same authors noted that micronutrient supplementation was significantly associated with age, nutrition risk and serum albumin concentration(189). Ca and vitamin D supplementation are important for bone health given the high prevalence of osteopenia and osteoporosis in persons with SCI(190). Furthermore, vitamins B and C deficiencies are linked to anaemia and impaired wound healing(191), both of which are reported at high rates after SCI(192,193). Multivitamin and mineral supplementation can correct deficiencies, but if there are no deficiencies, they can potentially be harmful. Persons with SCI should be prescribed vitamin and mineral supplements only if specific deficiencies have been detected or to prevent them (such as vitamin D and Ca for bone density), minimising toxicity risk. Health care professionals should place a greater emphasis on following a healthy dietary pattern (described below) to naturally consume vitamins and minerals rather than relying on supplements that may not completely correct deficiencies and carry some risk (e.g., toxicity).
Dietary recommendations after spinal cord injury
Persons with SCI are instructed to adopt a healthy diet(84,194). But what is a healthy diet for this population? Evidence-based guidelines to ameliorate the risks of obesity and CMS did not exist for the SCI population until recently. The PVA guidelines are the first comprehensive publication to provide data-driven recommendations on healthy eating for individuals with SCI. When considering the PVA guidelines, one should note the small sample sizes in SCI literature relative to non-disabled research in diet and nutritional status. This inherent delimitation of clinical research targeting a niche patient population restricts the depth of conclusions that can be drawn from a truly evidence-based approach. Therefore, the inaugural iteration of the PVA guideline recommendations corresponds to the several current recommendations for identifying and managing CMS in the non-disabled population. However, the guidelines also factor in the alterations in the body composition and the unique endometabolic physiology that accompany SCI.
The PVA guidelines recommend energetic assessment utilising indirect calorimetry to determine energy expenditure and assess energy needs to implement a heart-healthy nutrition plan focusing on vegetables, fruits, poultry, fish, low-fat dairy products, whole grains, legumes, nuts and non-tropical vegetable oils, while limiting sweets, sugar-sweetened beverages and red meats (Table 7; Fig. 2). The PVA report also limits dietary saturated fat to 5 % to 6 % of the total energetic intake and limits daily Na intake to ≤ 2400 mg for individuals with hypertension (Table 7; Fig. 2)(10).
A reduced emphasis should be placed on limiting macronutrients in diets with persons with SCI, but rather focus on providing a healthy dietary pattern (Table 7; Fig. 2). The authors of this review further recommend adopting ≤ 2400 mg of Na for all individuals, regardless of hypertension status given the elevated consumption of Na-dense foods reported in the literature. We also emphasise the importance of lean poultry consisting of a moderate 3 to 4 oz portion, and the consumption of fish two times per week. Vegetables should be consumed between three and four servings per d. They should consist of the five vegetable subgroups (including dark green, red and orange, legumes (beans/peas), starchy, and others). Fruits should favour whole fruits at three least servings per d and significantly limit 100 % fruit juice because of their added/high sugar content and limited fibre content. Emphasis should be placed on low-fat dairy products in the form of milk, yogurt and cheese in small amounts while limiting saturated fat intake below 5–6 %. High-fat, sugary-based sweets and drinks should be replaced with fresh fruit and water, respectively. Flavoured and unflavoured carbonated water and zero energy liquid water enhancers can be used to provide variety and flavour to drinks. Red meat and sweets should be consumed only on special occasions such as birthdays, weddings, holidays, etc. By following the above-referenced dietary patterns, persons with SCI will naturally limit their intake of refined/simple carbohydrates, Na, and saturated fat and increase the consumption of unsaturated fats and fibre. Such dietary patterns will also promote optimal ingestion of micronutrients.
We recommend and recognise the significance of annual dietary assessments (minimally) and nutrition education with a registered dietitian as part of the medical assessment and standard of care for individuals with SCI (Fig. 2). It is our recommendations that in addition to assessing body composition by 3- or 4-compartment modelling(29), registered dietitians should: (1) assess resting metabolism through indirect calorimetry, or, when unavailable, assess RMR/BMR with the Nightingale and Gorgey(116), Chun et al.(105) or Buchholz et al.(79) SCI-specific prediction equation (2) calculate TDEE using the prediction equation and correction factor by Farkas et al.(9) to determine accurate energy needs, (3) encourage adherence to the SCI dietary guidelines detailed above and balanced nutrition (Table 7, Fig. 2) as an overall healthy lifestyle choice, (4) prescribe dietary supplements when specific vitamin and/or mineral deficiencies have been detected or to prevent them when appropriate nutrition/healthy dietary patterns are unsucessful, and (5) explore dietary anomalies specific to SCI, such as avoidance of food groups that may affect bowel/bladder function post-injury. Periodic assessments with the health care team, including a registered dietitian, should be implemented to manage neurogenic obesity, cardiometabolic risk and nutrition status after SCI and allow the individual themselves to take an active role in their overall health.
Future research
Expert advice advocates for pairing diet and physical activity to mitigate neurogenic obesity and reduce the cardiometabolic risk after SCI. However, the interaction of feeding and activity in SCI has only recently begun to be studied and is likely unique in this population. Furthermore, several barriers, such as transportation, overuse injuries, pain, access to facilities, financial restraints, educational knowledge, disability/SCI-specific resources on exercise, and fear of musculoskeletal or integumentary injury, can limit exercise/physical activity engagement. To mitigate barriers to physical exercise and facilitate improvements in overall health, research focused exclusively on dietary intervention may provide a large-scale ‘cure’ to chronic diseases in persons with SCI.
Rather than a nutrient (i.e., low-fat diets) focus, nutrition research is turning its attention to dietary patterns as we consume a whole range of foods and food groups, not just nutrients. Evidence suggests that vegetarian and/or plant-based diets create a negative energy balance and decrease the risk of obesity, diabetes and other chronic health ailments(195,196). Fardet and Boirie(196) examined diet and chronic disease risk from over 300 meta-analyses and systematic reviews published in the last 63 years. The authors reported that plant-based foods were more protective against the risk of developing chronic disease compared with animal-based foods, reinforcing fundamental dietary patterns for good health. Among plant-based foods, whole-grain-based foods had a small edge over fruits and vegetables, while for animal-based foods, dairy products overall were considered neutral on health, and fish was considered protective. Red and processed meats were correlated with elevated chronic disease risk as were sugar-sweetened beverages and highly refined low-fibre grains(196). These data were further supported by evidence published by the AHA that diets higher in plant-based foods and lower in animal-based products were associated with a lower risk of cardiovascular morbidity and mortality(197). Plant-based diets may provide an alternative nutritional plan for cardiometabolic risk factors after SCI and minimise overeating by enhancing satiety relative to their need. Future research should focus on the effects of such diets in the SCI population but also attend to energy balance.
Final considerations
In summary, the loss of metabolically active tissue below the LOI, endometabolic pathophysiology, environmental and physical barriers, and poor dietary choices contribute to the suboptimal dietetics, poor body habitus and cardiometabolic risk in SCI(198). Individuals with SCI meet several evidence-based recommendations published by authoritative guidelines for non-disabled individuals, although they are likely underestimated or overestimated for this population. These guidelines do not account for SCI-induced changes in body composition(199,200), reduced metabolic requirements(19,34,79,87,121), gut dysmotility(41,201) and sympathetic nervous system dysfunction(33,34) and need to be interpreted and used with caution. The PVA guidelines are currently the strongest evidence-based dietary guidelines for the population with SCI and should be followed by persons with SCI and their health care team. Stakeholders and practitioners should have an understanding that the currently limited evidence-based means that guidelines contain might not fully capture the unique nutritional needs of persons with SCI. Therefore, clinical nutritional strategies should also rely on strong inferences from existing studies and use routine monitoring of individual responses to interventions. Because diet-related comorbidities are related to anatomical and physiological changes after SCI, annual nutritional analysis and indirect calorimetry (or, minimally using an SCI-specific BMR/RMR prediction method) with a registered dietitian are encouraged in clinical practice. Future multicentre controlled trials are needed (with less reliance on cross-sectional study design) to collect large data sets on energy expenditure, energy needs and total energetic intake after SCI. These data are needed to help develop comprehensive, evidence-based dietary reference values for nutrients specific for persons with SCI aimed at reducing the secondary complications of the injury.
Acknowledgements
The work is supported by a Postdoctoral Fellowship from the American Association for Anatomy (GJF).
G. J. F. devised the project framework and the conceptual ideas, conducted the literature search, extracted the data, wrote the initial and revised the manuscript, and developed guidelines. D. R. G. assisted with devising the project and revising the manuscript. AS assisted with developing guidelines. G. J. F., A. S. and D. W. M. created the figures. A. S. and D. W. M. assisted with data extraction from the studies and helped to build the tables. E. T., M. S. N. and D. R. G. critically appraised the manuscript. All authors reviewed and approved the final version of the manuscript.
Abbreviations:
- ADG
Australian Dietary Guidelines
- AHA
American Heart Association
- CMS
cardiometabolic syndrome
- DGA
Dietary Guidelines for American
- IOM
Institute of Medicine
- LOI
level of injury
- PHE
Public Health England
- PVA
Paralyzed Veterans of America
- SCI
spinal cord injury
- TEF
thermic effect of food digestion
- TEPA
thermic effect of physical activity
Footnotes
The authors attest that they have no financial or other conflicts of interest.
References
- 1.Cadotte DW & Fehlings MG (2011) Spinal cord injury: a systematic review of current treatment options. Clin Orthop Relat Res 469, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeilig G, Dolev M, Weingarden H, et al. (2000) Long-term morbidity and mortality after spinal cord injury: 50 years of follow-up. Spinal Cord 38, 563–566. [DOI] [PubMed] [Google Scholar]
- 3.Gater DR (2020) Neurogenic bowel and bladder evaluation strategies in spinal cord injury: new directions. J Spinal Cord Med 43, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko HY (2018) Revisit spinal shock: pattern of Reflex evolution during spinal shock. Korean J Neurotrauma 14, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gater DR (2007) Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 18, 333–351. [DOI] [PubMed] [Google Scholar]
- 6.Wing PC (2008) Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med 31, 403–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas GJ & Gater DR (2018) Neurogenic obesity and systemic inflammation following spinal cord injury: a review. J Spinal Cord Med 41, 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash MS, Groah SL, Gater DR, et al. (2019) Identification and management of cardiometabolic risk after spinal cord injury. J Spinal Cord Med 42, 643–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farkas GJ, Gorgey AS, Dolbow DR, et al. (2019) Caloric intake relative to total daily energy expenditure using a spinal cord injury-specific correction factor: an analysis by level of injury. Am J Phys Med Rehabil 98, 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash MS, Groah SL, Gater DR, et al. (2018) Identification and management of cardiometabolic risk after spinal cord injury: clinical practice guideline for health care providers. Top Spinal Cord Inj Rehabil 24, 379–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gater DR, Farkas GJ, Berg AS, et al. (2018) Prevalence of metabolic syndrome in veterans with spinal cord injury. J Spinal Cord Med 42, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services and U.S. Department of Agriculture (2015) 2015–2020 Dietary Guidelines for Americans, 8th ed. Washington, DC: U.S. Department of Health and Human Services and U.S. Department of Agriculture. [Google Scholar]
- 13.Public Health England (2016) Government Dietary Recommendations. London: Public Health England. [Google Scholar]
- 14.National Health and Medical Research Council (2013) Australian Dietary Guidelines. Canberra, Australia: National Health and Medical Research Council [Google Scholar]
- 15.Institute of Medicine of the National Academies (2006) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: Institute of Medicine of the National Academies. [Google Scholar]
- 16.World Health Organization Consultation (2000) Obesity: Preventing and Managing the Global Epidemic. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 17.Jensen MD, Ryan DH, Apovian CM, et al. (2014) 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 63, 2985–3025. [DOI] [PubMed] [Google Scholar]
- 18.Eckel RH, Jakicic JM, Ard JD, et al. (2015) 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 131, E326–E326. [Google Scholar]
- 19.Farkas GJ, Pitot MA, Berg AS, et al. (2019) Nutritional status in chronic spinal cord injury: a systematic review and meta-analysis. Spinal Cord 57, 3–17. [DOI] [PubMed] [Google Scholar]
- 20.Gater DR, Farkas GJ & Tiozzo E (2021) Pathophysiology of neurogenic obesity after spinal cord injury. Top Spinal Cord Inj Rehabil 27, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver FM, Collins EG, Kurichi J, et al. (2007) Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil 86, 22–29. [DOI] [PubMed] [Google Scholar]
- 22.Hales CM, Carroll MD, Fryar CD, et al. (2020) Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS data brief 360, 1–8. [PubMed] [Google Scholar]
- 23.Zhu C, Galea M, Livote E, et al. (2013) A retrospective chart review of heart rate and blood pressure abnormalities in veterans with spinal cord injury. J Spinal Cord Med 36, 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahman K, Nash MS, Lewis JE, et al. (2010) Increased cardiovascular disease risk in Swedish persons with paraplegia: the Stockholm spinal cord injury study. J Rehabil Med 42, 489–492. [DOI] [PubMed] [Google Scholar]
- 25.Rajan S, McNeely MJ, Hammond M, et al. (2010) Association between obesity and diabetes mellitus in veterans with spinal cord injuries and disorders. Am J Phys Med Rehabil 89, 353–361. [DOI] [PubMed] [Google Scholar]
- 26.Liang H, Chen D, Wang Y, et al. (2007) Different risk factor patterns for metabolic syndrome in men with spinal cord injury compared with able-bodied men despite similar prevalence rates. Arch Phys Med Rehabil 88, 1198–1204. [DOI] [PubMed] [Google Scholar]
- 27.Chen YY, Cao Y, Allen V, et al. (2011) Weight Matters: physical and psychosocial well being of persons with spinal cord injury in relation to body mass index. Arch Phys Med Rehabil 92, 391–398. [DOI] [PubMed] [Google Scholar]
- 28.Gupta N, White KT & Sandford PR (2006) Body mass index in spinal cord injury – a retrospective study. Spinal Cord 44, 92–94. [DOI] [PubMed] [Google Scholar]
- 29.Gater DR, Farkas GJ, Dolbow DR, et al. (2021) Body composition and metabolic assessment after aotor complete spinal cord injury: development of a clinically relevant equation to estimate body fat. Top Spinal Cord Inj Rehabil 27, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher JL, McMillan DW & Nash MS (2017) Exercise and health-related risks of physical deconditioning after spinal cord injury. Top Spinal Cord Inj Rehabil 23, 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dearwater SR, Laporte RE, Robertson RJ, et al. (1986) Activity in the spinal cord-injured patient – an epidemiologic analysis of metabolic parameters. Med Sci Sports Exerc 18, 541–544. [PubMed] [Google Scholar]
- 32.Noreau L, Shephard RJ, Simard C, et al. (1993) Relationship of impairment and functional ability to habitual activity and fitness following spinal cord injury. Int J Rehabil Res 16, 265–275. [DOI] [PubMed] [Google Scholar]
- 33.Stjernberg L, Blumberg H & Wallin BG (1986) Sympathetic activity in man after spinal-cord injury – outflow to muscle below the lesion. Brain 109, 695–715. [DOI] [PubMed] [Google Scholar]
- 34.Monroe MB, Tataranni PA, Pratley R, et al. (1998) Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr 68, 1223–1227. [DOI] [PubMed] [Google Scholar]
- 35.Bauman WA, La Fountaine MF & Spungen AM (2014) Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med 37, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbonetti A, Vassallo MRC, Pacca F, et al. (2014) Correlates of low testosterone in men with chronic spinal cord injury. Andrology 2, 721–728. [DOI] [PubMed] [Google Scholar]
- 37.Safarinejad MR (2001) Level of injury and hormone profiles in spinal cord-injured men. Urology 58, 671–676. [DOI] [PubMed] [Google Scholar]
- 38.Durga A, Sepahpanah F, Regozzi M, et al. (2011) Prevalence of testosterone deficiency after spinal cord injury. PMR 3, 929–932. [DOI] [PubMed] [Google Scholar]
- 39.Schopp LH, Clark M, Mazurek MO, et al. (2006) Testosterone levels among men with spinal cord injury admitted to inpatient rehabilitation. Am J Phys Med Rehabil 85, 678–684. [DOI] [PubMed] [Google Scholar]
- 40.Clark MJ, Schopp LH, Mazurek MO, et al. (2008) Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil 87, 758–767. [DOI] [PubMed] [Google Scholar]
- 41.Holmes GM (2012) Upper gastrointestinal dysmotility after spinal cord injury: is diminished vagal sensory processing one culprit? Front Physiol 3, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modlesky CM, Bickel CS, Slade JM, et al. (2004) Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol 96, 561–565. [DOI] [PubMed] [Google Scholar]
- 43.Jones LM, Goulding A & Gerrard DF (1998) DEXA: a practical and accurate tool to demonstrate total and regional bone loss, lean tissue loss and fat mass gain in paraplegia. Spinal Cord 36, 637–640. [DOI] [PubMed] [Google Scholar]
- 44.Nuhlicek DNR, Spurr GB, Barboriak JJ, et al. (1988) Body-composition of patients with spinal-cord injury. Eur J Clin Nutr 42, 765–773. [PubMed] [Google Scholar]
- 45.Castro MJ, Apple DF, Hillegass EA, et al. (1999) Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. J Appl Physiol 80, 373–378. [DOI] [PubMed] [Google Scholar]
- 46.Grimby G, Broberg C, Krotkiewska I, et al. (1976) Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med 8, 37–42. [PubMed] [Google Scholar]
- 47.Gorgey AS & Dudley GA (2007) Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 45, 304–309. [DOI] [PubMed] [Google Scholar]
- 48.Minaire P, Edouard C, Arlot M, et al. (1984) Marrow changes in paraplegic patients. Calcif Tissue Int 36, 338–340. [DOI] [PubMed] [Google Scholar]
- 49.Edwards WB, Schnitzer TJ & Troy KL (2014) Reduction in proximal femoral strength in patients with acute spinal cord injury. J Bone Miner Res 29, 2074–2079. [DOI] [PubMed] [Google Scholar]
- 50.Bieringsorensen F, Bohr HH & Schaadt OP (1990) Longitudinal-study of bone-mineral content in the lumbar spine, the forearm and the lower-extremities after spinal-cord injury. Eur J Clin Investig 20, 330–335. [DOI] [PubMed] [Google Scholar]
- 51.Goenka S, Sethi S, Pandey N, et al. (2018) Effect of early treatment with zoledronic acid on prevention of bone loss in patients with acute spinal cord injury: a randomized controlled trial. Spinal Cord 56, 1207–1211. [DOI] [PubMed] [Google Scholar]
- 52.Bauman WA, Cirnigliaro CM, La Fountaine MF, et al. (2015) Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: an observational cohort study. J Bone Miner Metab 33, 410–421. [DOI] [PubMed] [Google Scholar]
- 53.Qin W, Bauman WA & Cardozo C (2010) Bone and muscle loss after spinal cord injury: organ interactions. Ann NY Acad Sci 1211, 66–84. [DOI] [PubMed] [Google Scholar]
- 54.Groah SL, Nash MS, Ward EA, et al. (2011) Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev 31, 73–80. [DOI] [PubMed] [Google Scholar]
- 55.Balistreri CR, Caruso C, Candore G, et al. (2010) The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm 2010, e802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikeoka D, Mader JK & Pieber TR (2010) Adipose tissue, inflammation and cardiovascular disease. Rev Assoc Med Bras 56, 116–121. [DOI] [PubMed] [Google Scholar]
- 57.Ouchi N, Parker JL, Lugus JJ, et al. (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.da Silva Alves E, de Aquino Lemos V, Ruiz da Silva F, et al. (2013) Low-grade inflammation and spinal cord injury: exercise as therapy? Mediator Inflamm 2013, 971841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farkas GJ, Gorgey AS, Dolbow DR, et al. (2017) The influence of level of spinal cord injury on adipose tissue and its relationship to inflammatory adipokines and cardiometabolic profiles. J Spinal Cord Med 41, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farkas GJ, Gorgey AS, Dolbow DR, et al. (2018) Sex dimorphism in the distribution of adipose tissue and its influence on proinflammatory adipokines and cardiometabolic profiles in motor complete spinal cord injury. J Spinal Cord Med 42, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang T-D, Wang Y-H, Huang T-S, et al. (2007) Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formosan Med Assoc 106, 919–928. [DOI] [PubMed] [Google Scholar]
- 62.Maruyama Y, Mizuguchi M, Yaginuma T, et al. (2008) Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord 46, 494–499. [DOI] [PubMed] [Google Scholar]
- 63.Manns PJ, McCubbin JA & Williams DP (2005) Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil 86, 1176–1181. [DOI] [PubMed] [Google Scholar]
- 64.Morse LR, Stolzmann K, Nguyen HP, et al. (2008) Association between mobility mode and c-reactive protein levels in men with chronic spinal cord injury. Arch Phys Med Rehabil 89, 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cirnigliaro CM, LaFountaine MF, Dengel DR, et al. (2015) Visceral adiposity in persons with chronic spinal cord injury determined by dual energy X-ray absorptiometry. Obesity 23, 1811–1817. [DOI] [PubMed] [Google Scholar]
- 66.Nash MS, Tractenberg RE, Mendez AJ, et al. (2016) Cardiometabolic syndrome in people with spinal cord injury/disease: guideline-derived and nonguideline risk components in a pooled sample. Arch Phys Med Rehabil 97, 1696–1705. [DOI] [PubMed] [Google Scholar]
- 67.Laughton GE, Buchholz AC, Martin Ginis KA, et al. (2009) Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord 47, 757–762. [DOI] [PubMed] [Google Scholar]
- 68.Alberti K, Zimmet P & Shaw J (2006) Metabolic syndrome – a new world-wide definition. A consensus statement from the international diabetes federation. Diabetic Med 23, 469–480. [DOI] [PubMed] [Google Scholar]
- 69.Timper K & Brüning JC (2017) Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech 10, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crespo C, Cachero A, Jiménez L, et al. (2014) Peptides and food intake. Front Endocrinol 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller GD (2019) Appetite regulation: hormones, peptides, and neurotransmitters and their role in obesity. Am J Lifestyle Med 13, 586–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elmquist KWW & Joel K (2012) From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 15, 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naznin F, Toshinai K, Waise TM, et al. (2015) Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J Endocrinol 226, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waise TMZ, Toshinai K, Naznin F, et al. (2015) One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun 464, 1157–1162. [DOI] [PubMed] [Google Scholar]
- 75.Thaler JP, Yi C-X, Schur EA, et al. (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vinik AI, Maser RE, Mitchell BD, et al. (2003) Diabetic autonomic neuropathy. Diabetes Care 26, 1553–1579. [DOI] [PubMed] [Google Scholar]
- 77.Besecker EM, Blanke EN, Deiter GM, et al. (2020) Gastric vagal afferent neuropathy following experimental spinal cord injury. Exp Neurol 323, 113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farkas GJ, Pitot MA & Gater DR (2019) A systematic review of the accuracy of estimated and measured resting metabolic rate in chronic spinal cord injury. Int J Sport Nutr Exerc Metab 29, 548–558. [DOI] [PubMed] [Google Scholar]
- 79.Buchholz AC, McGillivray CF & Pencharz PB (2003) Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr 77, 371–378. [DOI] [PubMed] [Google Scholar]
- 80.Collins EG, Gater D, Kiratli BJ, et al. (2010) Energy cost of physical activities in persons with spinal cord injury. Med Sci Sports Exerc 42, 691–700. [DOI] [PubMed] [Google Scholar]
- 81.Aksnes AK, Brundin T, Hjeltnes N, et al. (1993) Meal-induced rise in resting energy-expenditure in patients with complete cervical spinal-cord lesions. Paraplegia 31, 462–472. [DOI] [PubMed] [Google Scholar]
- 82.Asahara R & Yamasaki M (2016) The thermic response to food intake in persons with thoracic spinal cord injury. J Phys Ther Sci 28, 1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Melanson EL (2017) The effect of exercise on non-exercise physical activity and sedentary behavior in adults. Obes Rev 18, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gorgey AS, Caudill C, Sistrun S, et al. (2015) Frequency of dietary recalls, nutritional assessment, and body composition assessment in men with chronic spinal cord injury. Arch Phys Med Rehabil 96, 1646–1653. [DOI] [PubMed] [Google Scholar]
- 85.Buchholz AC & Pencharz PB (2004) Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care 7, 635–639. [DOI] [PubMed] [Google Scholar]
- 86.Gorgey AS, Khalil RE, Gill R, et al. (2019) Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: an open-label randomized clinical trial. J Neurotrauma 36, 2631–2645. [DOI] [PubMed] [Google Scholar]
- 87.Bauman WA, Spungen AM, Wang J, et al. (2004) The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. J Rehabil Res Dev 41, 1–8. [DOI] [PubMed] [Google Scholar]
- 88.Broad EM, Newsome LJ, Drew DA, et al. (2020) Measured and predicted resting energy expenditure in wheelchair rugby athletes. J Spinal Cord Med 43, 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cox SA, Weiss SM, Posuniak EA, et al. (1985) Energy expenditure after spinal cord injury: an evaluation of stable rehabilitating patients. J Trauma 25, 419–423. [PubMed] [Google Scholar]
- 90.Sedlock DA & Laventure SJ (1990) Body-composition and resting energy-expenditure in long-Term spinal-cord injury. Paraplegia 28, 448–454. [DOI] [PubMed] [Google Scholar]
- 91.Alexander LR, Spungen AM, Liu MH, et al. (1995) Resting metabolic-rate in subjects with paraplegia – the effect of pressure sores. Arch Phys Med Rehabil 76, 819–822. [DOI] [PubMed] [Google Scholar]
- 92.Liu MH, Spungen AM, Fink L, et al. (1996) Increased energy needs in patients with quadriplegia and pressure ulcers. Adv Wound Care 9, 41–45. [PubMed] [Google Scholar]
- 93.Barco KT, Smith RA, Peerless JR, et al. (2002) Energy expenditure assessment and validation after acute spinal cord injury. Nutr Clin Pract 17, 309–313. [DOI] [PubMed] [Google Scholar]
- 94.Desneves KJ, Panisset MG, Rafferty J, et al. (2019) Comparison of estimated energy requirements using predictive equations with total energy expenditure measured by the doubly labelled water method in acute spinal cord injury. Spinal Cord 57, 562–570. [DOI] [PubMed] [Google Scholar]
- 95.Gorgey AS, Poarch H, Harnish C, et al. (2011) Acute effects of locomotor training on neuromuscular and metabolic profile after incomplete spinal cord injury. NeuroRehabilitation 29, 79–83. [DOI] [PubMed] [Google Scholar]
- 96.Holmlund T, Ekblom-Bak E, Franzen E, et al. (2018) Energy expenditure after spinal cord injury in people with motor-complete tetraplegia or motor-complete paraplegia. Spinal Cord 56, 274–283. [DOI] [PubMed] [Google Scholar]
- 97.Rowan C & Kazemi A (2020) An observational study of feeding practice in ventilated patients with spinal cord injury. Clin Nutr Espen 37, 107–113. [DOI] [PubMed] [Google Scholar]
- 98.Shea JR, Shay BL, Leiter J, et al. (2018) Energy expenditure as a function of activity level after spinal cord injury: the need for tetraplegia-specific energy balance guidelines. Front Physiol 9, 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanhoffer RA, Tanhoffer AIP, Raymond J, et al. (2012) Comparison of methods to assess energy expenditure and physical activity in people with spinal cord injury. J Spinal Cord Med 35, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tanhoffer RA, Tanhoffer AIP, Raymond J, et al. (2015) Energy expenditure in individuals with spinal cord injury quantified by doubly labeled water and a multi-sensor armband. J Physical Activity Health 12, 163–170. [DOI] [PubMed] [Google Scholar]
- 101.Wouda MF, Lundgaard E, Becker F, et al. (2018) Effects of moderate- and high-intensity aerobic training program in ambulatory subjects with incomplete spinal cord injury-a randomized controlled trial. Spinal Cord 56, 955–963. [DOI] [PubMed] [Google Scholar]
- 102.Wouda MF, Lundgaard E, Becker F, et al. (2020) Changes in cardiorespiratory fitness and activity levels over the first year after discharge in ambulatory persons with recent incomplete spinal cord injury. Spinal Cord 59, 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yilmaz B, Yasar E, Goktepe S, et al. (2007) Basal metabolic rate and autonomic nervous system dysfunction in men with spinal cord injury. Obesity 15, 2683–2687. [DOI] [PubMed] [Google Scholar]
- 104.Bauman WA, Cirnigliaro CM, La Fountaine MF, et al. (2011) A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res 43, 574–579. [DOI] [PubMed] [Google Scholar]
- 105.Chun SM, Kim HR & Shin HI (2017) Estimating the Basal metabolic rate from fat free mass in individuals with motor complete spinal cord injury. Spinal Cord 55, 844–847. [DOI] [PubMed] [Google Scholar]
- 106.Gorgey AS, Chiodo AE, Zemper ED, et al. (2010) Relationship of spasticity to soft tissue body composition and the metabolic profile in persons with chronic motor complete spinal cord injury. J Spinal Corel Med 33, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gorgey AS & Gater DR (2011) Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab 36, 107–114. [DOI] [PubMed] [Google Scholar]
- 108.Gorgey AS, Mather KJ, Cupp HR, et al. (2012) Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 44, 165–174. [DOI] [PubMed] [Google Scholar]
- 109.Gorgey AS, Martin H, Metz A, et al. (2016) Longitudinal changes in body composition and metabolic profile between exercise clinical trials in men with chronic spinal cord injury. J Spinal Cord Med 39, 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gorgey AS, Farkas GJ, Dolbow DR, et al. (2017) Gender dimorphism in central adiposity may explain metabolic dysfunction after spinal cord injury. PMR 10, 338–348. [DOI] [PubMed] [Google Scholar]
- 111.Hayes M, Chustek M, Wang Z, et al. (2002) DXA: potential for creating a metabolic map of organ-tissue resting energy expenditure components. Obes Res 10, 969–977. [DOI] [PubMed] [Google Scholar]
- 112.Lee BY, Agarwal N, Corcoran L, et al. (1985) Assessment of nutritional and metabolic status of paraplegics. J Rehabil Res Dev 22, 11–17. [DOI] [PubMed] [Google Scholar]
- 113.Spungen AM, Bauman WA, Wang J, et al. (1993) The relationship between total body potassium and resting energy expenditure in individuals with paraplegia. Arch Phys Med Rehabil 74, 965–968. [PubMed] [Google Scholar]
- 114.Yilmaz B, Yasar E, Goktepe AS, et al. (2007) The relationship between basal metabolic rate and femur bone mineral density in men with traumatic spinal cord injury. Arch Phys Med Rehabil 88, 758–761. [DOI] [PubMed] [Google Scholar]
- 115.Nightingale TE, Williams S, Thompson D, et al. (2017) Energy balance components in persons with paraplegia: daily variation and appropriate measurement duration. Int J Behav Nutr Phys Act 14, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nightingale TE & Gorgey AS (2018) Predicting basal metabolic rate in men with motor complete spinal cord injury. Med Sci Sports Exerc 50, 1305–1312. [DOI] [PubMed] [Google Scholar]
- 117.Perret C & Stoffel-Kurt N (2011) Comparison of nutritional intake between individuals with acute and chronic spinal cord injury. J Spinal Cord Med 34, 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pelly FE, Broad EM, Stuart N, et al. (2017) Resting energy expenditure in male athletes with a spinal cord injury. J Spinal Cord Med 41, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kearns PJ, Thompson JD, Werner PC, et al. (1992) Nutritional and metabolic response to acute spinal-cord injury. J Parenter Enter Nutr 16, 11–15. [DOI] [PubMed] [Google Scholar]
- 120.Aquilani R, Boschi F, Contardi A, et al. (2001) Energy expenditure and nutritional adequacy of rehabilitation paraplegics with asymptomatic bacteriuria and pressure sores. Spinal Cord 39, 437–441. [DOI] [PubMed] [Google Scholar]
- 121.Buchholz AC, McGillivray CF & Pencharz PB (2003) Physical activity levels are low in free-living adults with chronic paraplegia. Obes Res 11, 563–570. [DOI] [PubMed] [Google Scholar]
- 122.McMurray RG, Soares J, Caspersen CJ, et al. (2014) Examining variations of resting metabolic rate of adults: a public health perspective. Med Sci Sports Exerc 46, 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Compher C, Frankenfield D, Keim N, et al. (2006) Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 106, 881–903. [DOI] [PubMed] [Google Scholar]
- 124.Henry CJ (2005) Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 8, 1133–1152. [DOI] [PubMed] [Google Scholar]
- 125.Blundell JE, Caudwell P, Gibbons C, et al. (2012) Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br J Nutr 107, 445–449. [DOI] [PubMed] [Google Scholar]
- 126.Blundell JE, Finlayson G, Gibbons C, et al. (2015) The biology of appetite control: do resting metabolic rate and fat-free mass drive energy intake? Physiol Behav 152, 473–478. [DOI] [PubMed] [Google Scholar]
- 127.Caudwell P, Finlayson G, Gibbons C, et al. (2013) Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr 97, 7–14. [DOI] [PubMed] [Google Scholar]
- 128.Hopkins M, Finlayson G, Duarte C, et al. (2016) Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. Int J Obes 40, 312–318. [DOI] [PubMed] [Google Scholar]
- 129.Rodriguez DJ, Benzel EC & Clevenger FW (1997) The metabolic response to spinal cord injury. Spinal Cord 35, 599–604. [DOI] [PubMed] [Google Scholar]
- 130.Mollinger LA, Spurr GB, el Ghatit AZ, et al. (1985) Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil 66, 420–426. [PubMed] [Google Scholar]
- 131.Schutz Y (2018) Respiration chamber calorimetry and doubly labeled water: two complementary aspects of energy expenditure? Eur J Clin Nutr 72, 1310–1313. [DOI] [PubMed] [Google Scholar]
- 132.Westerterp KR (2017) Doubly labelled water assessment of energy expenditure: principle, practice, and promise. Eur J Appl Physiol 117, 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weissman C, Sardar A & Kemper M (1994) An in vitro evaluation of an instrument designed to measure oxygen consumption and carbon dioxide production during mechanical ventilation. Crit Care Med 22, 1995–1200. [PubMed] [Google Scholar]
- 134.Wong S, Derry F, Jamous A, et al. (2012) The prevalence of malnutrition in spinal cord injuries patients: a UK multicentre study. Br J Nutr 108, 918–923. [DOI] [PubMed] [Google Scholar]
- 135.Groah SL, Nash MS, Ljungberg IH, et al. (2009) Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med 32, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iyer P, Beck E & Walton K (2020) Exploring nutrition knowledge and dietary intake of adults with spinal cord injury in specialist rehabilitation. Spinal Cord 58, 1–9. [DOI] [PubMed] [Google Scholar]
- 137.Kaufman HH, Rowlands BJ, Stein DK, et al. (1985) General metabolism in patients with acute paraplegia and quadriplegia. Neurosurgery 16, 309–313. [DOI] [PubMed] [Google Scholar]
- 138.Laven GT, Huang CT, DeVivo MJ, et al. (1989) Nutritional status during the acute stage of spinal cord injury. Arch Phys Med Rehabil 70, 277–282. [PubMed] [Google Scholar]
- 139.Felleiter PKJ, Haeberli Y, Schmid W, et al. (2017) Post-traumatic changes in energy expenditure and body composition in patients with acute spinal cord injury. J Rehabil Med 49, 579–584. [DOI] [PubMed] [Google Scholar]
- 140.Academy of Nutrition and Dietetics (2009) Spinal Cord Injury (SCI) Guidelines, https://andeal.org/topic.cfm?menu=5292&pcat=3487&cat=5448 (accessed August 2020).
- 141.Sabour H, Soltani Z, Latifi S, et al. (2016) Dietary pattern as identified by factorial analysis and its association with lipid profile and fasting plasma glucose among Iranian individuals with spinal cord injury. J Spinal Cord Med 39, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kolpek JH, Ott LG, Record KE, et al. (1989) Comparison of urinary urea nitrogen excretion and measured energy expenditure in spinal cord injury and nonsteroid-treated severe head trauma patients. JPEN J Parenter Enteral Nutr 13, 277–280. [DOI] [PubMed] [Google Scholar]
- 143.Rodriguez DJ, Clevenger FW, Osler TM, et al. (1991) Obligatory negative nitrogen balance following spinal cord injury. J Parenter Enteral Nutr 15, 319–322. [DOI] [PubMed] [Google Scholar]
- 144.Beutler B (1990) The tumor necrosis factors: cachectin and lymphotoxin. Hosp Pract 25, 45–56. [DOI] [PubMed] [Google Scholar]
- 145.Tator CH, van der Jagt RH & Malkin A (1982) The effect of acute spinal cord compression injury on thyroid function in the rat. Surg Neurol 18, 64–68. [DOI] [PubMed] [Google Scholar]
- 146.Todd SR, Gonzalez EA, Turner K, et al. (2008) Update on post-injury nutrition. Curr Opin Crit Care 14, 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Allison DJ, Beaudry KM, Thomas AM, et al. (2018) Changes in nutrient intake and inflammation following an anti-inflammatory diet in spinal cord injury. J Spinal Cord Med 42, 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Abilmona SM & Gorgey AS (2018) Associations of the trunk skeletal musculature and dietary intake to biomarkers of cardiometabolic health after spinal cord injury. Clin Physiol Funct Imaging 38, 949–958. [DOI] [PubMed] [Google Scholar]
- 149.Chen Y, Henson S, Jackson AB, et al. (2006) Obesity intervention in persons with spinal cord injury. Spinal Cord 44, 82–91. [DOI] [PubMed] [Google Scholar]
- 150.Silveira SL, Winter LL, Clark R, et al. (2018) Baseline dietary intake of individuals with spinal cord injury who are overweight or obese. J Acad Nutr Diet 119, 301–309. [DOI] [PubMed] [Google Scholar]
- 151.Edwards LA, Bugaresti JM & Buchholz AC (2008) Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr 87, 600–607. [DOI] [PubMed] [Google Scholar]
- 152.Walters JL, Buchholz AC & Martin Ginis KA (2009) Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord 47, 318–322. [DOI] [PubMed] [Google Scholar]
- 153.Sabour H, Javidan AN, Vafa MR, et al. (2012) Calorie and macronutrients intake in people with spinal cord injuries: an analysis by sex and injury-related variables. Nutrition 28, 143–147. [DOI] [PubMed] [Google Scholar]
- 154.Sabour H, Nazari M, Latifi S, et al. (2016) The relationship between dietary Intakes of amino acids and bone mineral density among individuals with spinal cord injury. Oman Med J 31, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sabour H, Norouzi-Javidan A, Soltani Z, et al. (2016) The correlation between dietary fat intake and blood pressure among people with spinal cord injury. Iran J Neurol 15, 121–127. [PMC free article] [PubMed] [Google Scholar]
- 156.Peiffer SC, Blust P & Leyson JF (1981) Nutritional assessment of the spinal cord injured patient. J Am Diet Assoc 78, 501–505. [PubMed] [Google Scholar]
- 157.Barboriak JJ, Rooney CB, El Ghatit AZ, et al. (1983) Nutrition in spinal cord injury patients. J Am Paraplegia Soc 6, 32–36. [DOI] [PubMed] [Google Scholar]
- 158.Inskip J, Plunet W, Ramer L, et al. (2010) Cardiometabolic risk factors in experimental spinal cord injury. J Neurotrauma 27, 275–285. [DOI] [PubMed] [Google Scholar]
- 159.Spungen AM, Adkins RH, Stewart CA, et al. (2003) Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 95, 2398–2407. [DOI] [PubMed] [Google Scholar]
- 160.Ravelli MN & Schoeller DA (2020) Traditional self-reported dietary instruments are prone to inaccuracies and new approaches are needed. Front Nutr 7, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Subar AF, Freedman LS, Tooze JA, et al. (2015) Addressing current criticism regarding the value of self-report dietary data. J Nutr 145, 2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Levine AM, Nash MS, Green BA, et al. (1992) An examination of dietary intakes and nutritional status of chronic healthy spinal cord injured individuals. Paraplegia 30, 880–889. [DOI] [PubMed] [Google Scholar]
- 163.Doubelt I, de Zepetnek JT, MacDonald MJ, et al. (2015) Influences of nutrition and adiposity on bone mineral density in individuals with chronic spinal cord injury: a cross-sectional, observational study. Bone Rep 2, 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lieberman J, Goff D, Hammond F, et al. (2014) Dietary intake and adherence to the 2010 Dietary Guidelines for Americans among individuals with chronic spinal cord injury: a pilot study. J Spinal Cord Med 37, 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tomey KM, Chen DM, Wang X, et al. (2005) Dietary intake and nutritional status of urban community-dwelling men with paraplegia. Arch Phys Med Rehabil 86, 664–671. [DOI] [PubMed] [Google Scholar]
- 166.Krempien JL & Barr SI (2011) Risk of nutrient inadequacies in elite Canadian athletes with spinal cord injury. Int J Sport Nutr Exerc Metab 21, 417–425. [DOI] [PubMed] [Google Scholar]
- 167.Beal C, Gorgey A, Moore P, et al. (2017) Higher dietary intake of vitamin D may influence total cholesterol and carbohydrate profile independent of body composition in men with chronic spinal cord injury. J Spinal Cord Med 41, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.White JV, Guenter P, Jensen G, et al. (2012) Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics Recommended for the Identification and Documentation of Adult Malnutrition (undernutrition). J Academy Nutr Diet 112, 730–738. [DOI] [PubMed] [Google Scholar]
- 169.Delliere S & Cynober L (2017) Is transthyretin a good marker of nutritional status? Clin Nutr 36, 364–370. [DOI] [PubMed] [Google Scholar]
- 170.Freitas R, Hessel G, Vasques ACJ, et al. (2018) Transthyretin levels: potential biomarker for monitoring nutritional support efficacy and clinical complications risk in patients receiving parenteral nutrition. Clin Nutr Espen 24, 134–139. [DOI] [PubMed] [Google Scholar]
- 171.Keller U (2019) Nutritional laboratory markers in malnutrition. J Clin Med 8, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ranasinghe RN, Biswas M & Vincent RP (2020) Prealbumin: the clinical utility and analytical methodologies. Ann Clin Biochem (In the Press). [DOI] [PubMed] [Google Scholar]
- 173.Lussi C, Frotzler A, Jenny A, et al. (2018) Nutritional blood parameters and nutritional risk screening in patients with spinal cord injury and deep pressure ulcer-a retrospective chart analysis. Spinal Cord 56, 168–175. [DOI] [PubMed] [Google Scholar]
- 174.Della Pepa G, Vetrani C, Vitale M, et al. (2018) Wholegrain intake and risk of type 2 diabetes: evidence from epidemiological and intervention studies. Nutrients 10, 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sabour H, Larijani B, Vafa MR, et al. (2012) The effects of n-3 fatty acids on inflammatory cytokines in osteoporotic spinal cord injured patients: a randomized clinical trial. J Res Med Sci 17, 322–327. [PMC free article] [PubMed] [Google Scholar]
- 176.Moussavi RM, Ribas-Cardus F, Rintala DH, et al. (2001) Dietary and serum lipids in individuals with spinal cord injury living in the community. J Rehabil Res Dev 38, 225–233. [PubMed] [Google Scholar]
- 177.Golay A & Bobbioni E (1997) The role of dietary fat in obesity. Int J Obes Relat Metab Disord 21, S2–S11. [PubMed] [Google Scholar]
- 178.Willett WC, Koplan JP, Nugent R, et al. (2006) Prevention of Chronic Disease by Neans of Diet and Lifestyle Changes. Disease Control Priorities in Developing Countries, 2nd ed. Washington, DC: Oxford University Press. [Google Scholar]
- 179.Cederbaum A (2012) Alcohol metabolism. Clin Liver Dis 16, 667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Garrison A, Clifford K, Gleason SF, et al. (2004) Alcohol use associated with cervical spinal cord injury. J Spinal Cord Med 27, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Frisbie JH & Tun CG (1984) Drinking and spinal cord injury. J Am Paraplegia Soc 7, 71–73. [PubMed] [Google Scholar]
- 182.Tate DG, Forchheimer MB, Krause JS, et al. (2004) Patterns of alcohol and substance use and abuse in persons with spinal cord injury: risk factors and correlates. Arch Phys Med Rehabil 85, 1837–1847. [DOI] [PubMed] [Google Scholar]
- 183.Saunders L & Krause J (2011) Psychological factors affecting alcohol use after spinal cord injury. Spinal Cord 49, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Knight KH, Buchholz AC, Martin Ginis KA, et al. (2011) Leisure-time physical activity and diet quality are not associated in people with chronic spinal cord injury. Spinal Cord 49, 381–385. [DOI] [PubMed] [Google Scholar]
- 185.Cameron KJ, Nyulasi IB, Collier GR, et al. (1996) Assessment of the effect of increased dietary fibre intake on bowel function in patients with spinal cord injury. Spinal Cord 34, 277–283. [DOI] [PubMed] [Google Scholar]
- 186.Sciarra MB, Anna Lucia F, Elisabetta B, et al. (2020) Diet in neurogenic bowel management: a viewpoint on spinal cord injury. World J Gastroenterol 26, 2479–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Javidan AN, Sabour H, Latifi S, et al. (2014) Calcium and vitamin D plasma concentration and nutritional intake status in patients with chronic spinal cord injury: a referral center report. J Res Med Sci 19, 881–884. [PMC free article] [PubMed] [Google Scholar]
- 188.Opperman EA, Buchholz AC, Darlington GA, et al. (2010) Dietary supplement use in the spinal cord injury population. Spinal Cord 48, 60–64. [DOI] [PubMed] [Google Scholar]
- 189.Wong S, Graham A, Green D, et al. (2013) Nutritional supplement usage in patients admitted to a spinal cord injury center. J Spinal Cord Med 36, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McMillan DW, Nash MS, Gater DR, et al. (2021) Neurogenic obesity and skeletal pathology in spinal cord injury. Top Spinal Cord Inj Rehabil 27, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bird JK, Murphy RA, Ciappio ED, et al. (2017) Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients 9, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Frisbie JH (2010) Anemia and hypoalbuminemia of chronic spinal cord injury: prevalence and prognostic significance. Spinal Cord 48, 566–569. [DOI] [PubMed] [Google Scholar]
- 193.Kruger EA, Pires M, Ngann Y, et al. (2013) Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med 36, 572–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Khalil RE, Gorgey AS, Janisko M, et al. (2012) The role of nutrition in health status after spinal cord injury. Aging Dis 4, 14–22. [PMC free article] [PubMed] [Google Scholar]
- 195.Tonstad S, Butler T, Yan R, et al. (2009) Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 32, 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fardet A & Boirie Y (2014) Associations between food and beverage groups and major diet-related chronic diseases: an exhaustive review of pooled/meta-analyses and systematic reviews. Nutr Rev 72, 741–762. [DOI] [PubMed] [Google Scholar]
- 197.Kim H, Caulfield LE, Garcia-Larsen V, et al. (2019) Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J Am Heart Assoc 8, e012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nash MS & Gater DR (2020) Cardiometabolic disease and dysfunction following spinal cord injury: origins and guideline-based countermeasures. Phys Med Rehabil Clin Am 31, 415–436. [DOI] [PubMed] [Google Scholar]
- 199.Gorgey AS, Dolbow DR, Dolbow JD, et al. (2014) Effects of spinal cord injury on body composition and metabolic profile – part I. J Spinal Cord Med 37, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gater DR & Farkas GJ (2016) Alterations in body composition after SCI and the mitigating role of exercise. In The Physiology of Exercise in Spinal Cord injury, pp. 175–198 [Taylor J, editor], Boston, MA: Springer. [Google Scholar]
- 201.Keshavarzian A, Barnes WE, Bruninga K, et al. (1995) Delayed colonic transit in spinal cord-injured patients measured by in-111 amberlite scintigraphy. Am J Gastroenterol 90, 1295–1300. [PubMed] [Google Scholar]