Abstract
Extracellular vesicles have emerged as prominent regulators of the immune response during tumor progression. EVs contain a diverse repertoire of molecular cargo that plays a critical role in immunomodulation. Here, we identify the role of EVs as mediators of communication between cancer and immune cells. This expanded role of EVs may shed light on the mechanisms behind tumor progression and provide translational diagnostic and prognostic tools for immunologists.
Cells communicate via contact-dependent and contact-independent interactions through the secretion of chemokines, cytokines, growth factors, and associated cell receptors. Over the past 30 years, the secretion of extracellular vesicles (EVs) has also emerged as a major mechanism for cell-cell and cell-environment interactions. These 30–5000 nm lipid membrane-bound vesicles contain functional proteins, nucleic acids, lipids, and other bioactive molecules that can modulate the behavior of recipient cells1.
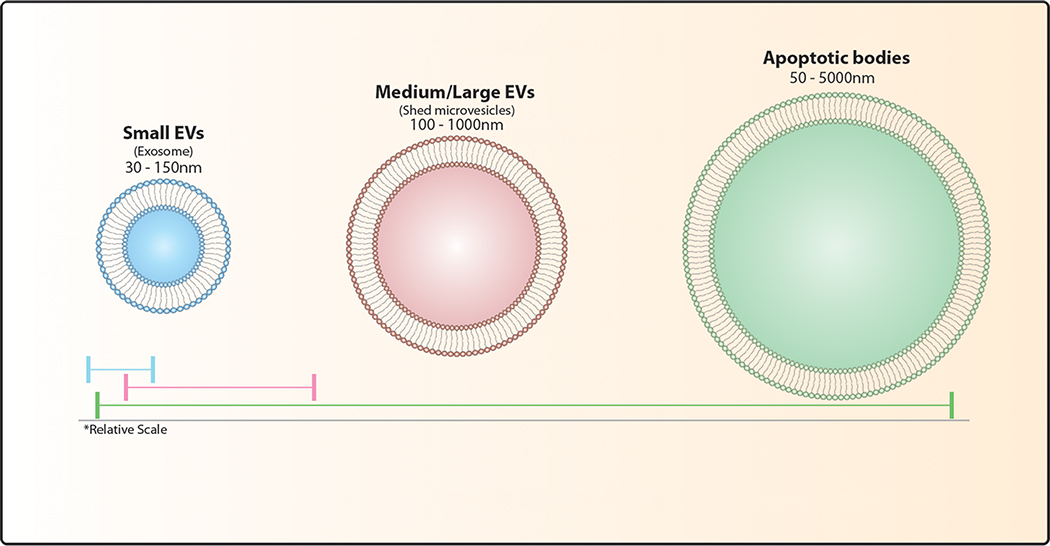
EVs are generally split into three subtypes based on their mechanism of biogenesis – exosomes, shed microvesicles, and apoptotic bodies (Box 1). EV biogenesis and secretion have been well reviewed elsewhere2,3,4. In brief, exosomes are formed via the inward budding of the cell plasma membrane and the subsequent formation of multi-vesicular bodies. Conversely, shed microvesicles - also referred to as ectosomes - are produced via the outward budding of the plasma membrane. Apoptotic bodies are released during cellular apoptosis. Due to the difficulty of isolating EV subtypes, they are often labeled according to size as small, medium, and large EVs (Box 1). Following the International Society for Extracellular Vesicles (ISEV) nomenclature5, we will use the term EV for all lipid bilayer particles secreted by cells, specifying size where appropriate. While some proteins may be shared within an EV subtype, vesicular cargo is highly dependent on the type and state of the donor cell6. This diversity in cargo allows EVs to modulate several important cellular processes, including cell differentiation, blood coagulation, and angiogenesis in tissue homeostasis and development7. Studies have demonstrated the key role that EVs play in tumor progression and antitumor immune responses8. Here, we review the role of EVs as mediators of communication between cancer and immune cells, as well as the potential clinical uses of EVs that arise from these interactions.
Box 1. Subtypes of extracellular vesicles.
Classically defined by size, no specific molecular markers exist to distinguish EV subtypes. While a number of molecules tend to be enriched in EV fractions; many traditional markers (CD9, CD63, CD81, TSG101, Alix, Flotillin-1, HSC70, Actin, MHC I and MHC II) co-isolate with multiple EV subtypes leading to confusion and misinterpretation in the literature. Special consideration should be directed towards extensive characterization of EV isolations prior to publication.
The role of extracellular vesicles in the immune cascade
Immune-derived EVs carry a variety of cargo that function in immune activation and suppression (Fig. 1a). The role of EVs in an immune response was first described in 1996 (Ref.9). This study observed that small EVs released by B cells not only carried major histocompatibility complex (MHC) class II molecules, but also elicited immune responses in T cells9. Soon after, a second study10 described the ability of dendritic cell (DC) small EVs to induce tumor suppression in vivo. In 2002, another study elaborated on this work to describe a mechanism for indirect T cell stimulation by DC small EVs11. These discoveries established EVs as a major mechanism of cellular communication and provided a basis for immune EV research over the past two decades.
Figure 1: Heterogeneous cargo of EVs.

Secreted from tumor and immune cells alike, EVs contain molecular cargo that facilitates immune stimulatory and suppressive interactions. Trafficking transmembrane and cytosolic proteins, RNAs, and DNAs, EVs regulate autoimmune and disease responses. A) EVs secreted by immune cells carry cargo that directly controls peripheral immune function by either promoting a regulatory response or conditioning surrounding cells, reducing inflammation. B) Tumor-derived EVs express surface and cytosolic markers from their cellular progenitor, which in turn induce phenotypic changes in recipient cells.
DCs are antigen presenting cells (APCs) that orchestrate the immune response against a specific antigen. DC EVs have become a focus of the field due to their ability to stimulate antitumor immune responses. DC EVs harbor MHC I and MHC II molecules, tetraspanins, adhesion molecules, heat shock proteins, and costimulatory molecules that confer their immunomodulatory capabilities12–19. It is debated, however, whether EVs can carry out these functions via direct interaction with target cells18,20,21, or if the presence of DCs is necessary11,22–24. Interestingly, in vitro modeling of primary mouse DCs might set a new example for the immunomodulatory capabilities of EVs. Shown to transfer tumor antigen loaded vesicles via tight synaptic connections, DCs highlight a potential downstream mechanism for EV antigen presentation25.. Further in vitro modeling involving priming of DCs via antigen laden EVs might reveal internalized EV cargo is also shared between APCs during this synaptic transfer.
Whether their effects are direct or indirect, APC-derived EVs, and specifically DC EVs, play a major role in antigen presentation and immune activation26–28. EVs have the remarkable ability to transfer pre-formed functional peptide-MHC complexes from APCs to recipient cells29,30. Amongst the MHC II molecules present on DC EVs, HLA-DQ enables DC EVs to promote T cell proliferation23,31,32. DC small EVs induce maturation and differentiation in several cell types, including immature DCs and monocytes33,34. DC EVs also stimulate interferon-gamma (IFN-γ) production by naive CD4+ T cells and induce differentiation into T helper 1, T helper 2, and regulatory T (Treg) cells23,35–38. Interestingly, the molecular cargo of DC EVs varies based on the presence of surrounding cells. Co-culture assays using primary DCs derived from human monocytes reveal that in the presence of bystander T cells, DCs secrete small EVs enriched in microRNAs (miRNAs) miR-30b, miR-146a, and miR-15539. These miRNAs carried by DC EVs are functionally active in promoting further CD8+ T cell activation39.
While T cells generally function to carry out immune responses, their EVs have also emerged as important immunomodulators. Small EVs from activated T cells are enriched in nucleic acids with a variety of functions. Found to contain mitochondrial DNA (mtDNA), small T cell EVs prime DCs to more efficiently respond to future attacks by a familiar antigen40. Acting through the cGAS-STING cytosolic DNA-sensing pathway, DNA bound to the surface of small T cell-derived EVs confers an increased resistance to infection in recipient DCs40. Activated T cells secrete EVs containing specific tRNA fragments, which would otherwise function to inhibit T cell activation41. Secretomic analysis of cell culture-derived T cell EVs revealed systemic encapsulation of immunomodulatory cytokines21. EV-associated cytokine encapsulation by T cells further stabilize the functional ability of these cytokines in comparison to their free counterparts21. This stabilizing effect of EVs has been exploited in drug delivery applications (described in detail below).
While the differences among EV subtypes are well accepted, reports suggest that heterogeneity within EV subtypes remains largely unaccounted for23,35,42,43. The maturity of donor DCs as well as variation in EV enrichment profiles affect naive T cell development23,35,42. Small EVs and EVs secreted by mature DCs preferentially induce T helper 1 activation, while larger EVs induce T helper 2 differentiation23. Similarly, T cell-derived EVs bearing different markers – namely CD47, CD63, and MHC I – are enriched in different sets of RNAs43. These developments have shed light on why EV research often produces sometimes conflicting results and provide more evidence for why it is typically inaccurate to attribute any effect to a single subpopulation of EVs.
Autoimmune suppression via extracellular vesicles
The primary function of Tregs, is to prevent autoimmunity by promoting self-tolerance of the immune system44. Tregs employ EVs to suppress the activity of other immune cells44. Treg-derived EVs contain several miRNAs and miRNA precursors45–50. Uptake of EVs containing miR-142–3p and miR-150–5p by DCs induces a decrease in pro-inflammatory cytokine IL-6 expression and an increase in immunosuppressive cytokine IL-10 expression45. These miRNAs interfere with antigen processing and presentation in DCs51, thus inhibiting immune activation (Fig. 2).
Figure 2: Mechanisms of EV immune regulation.
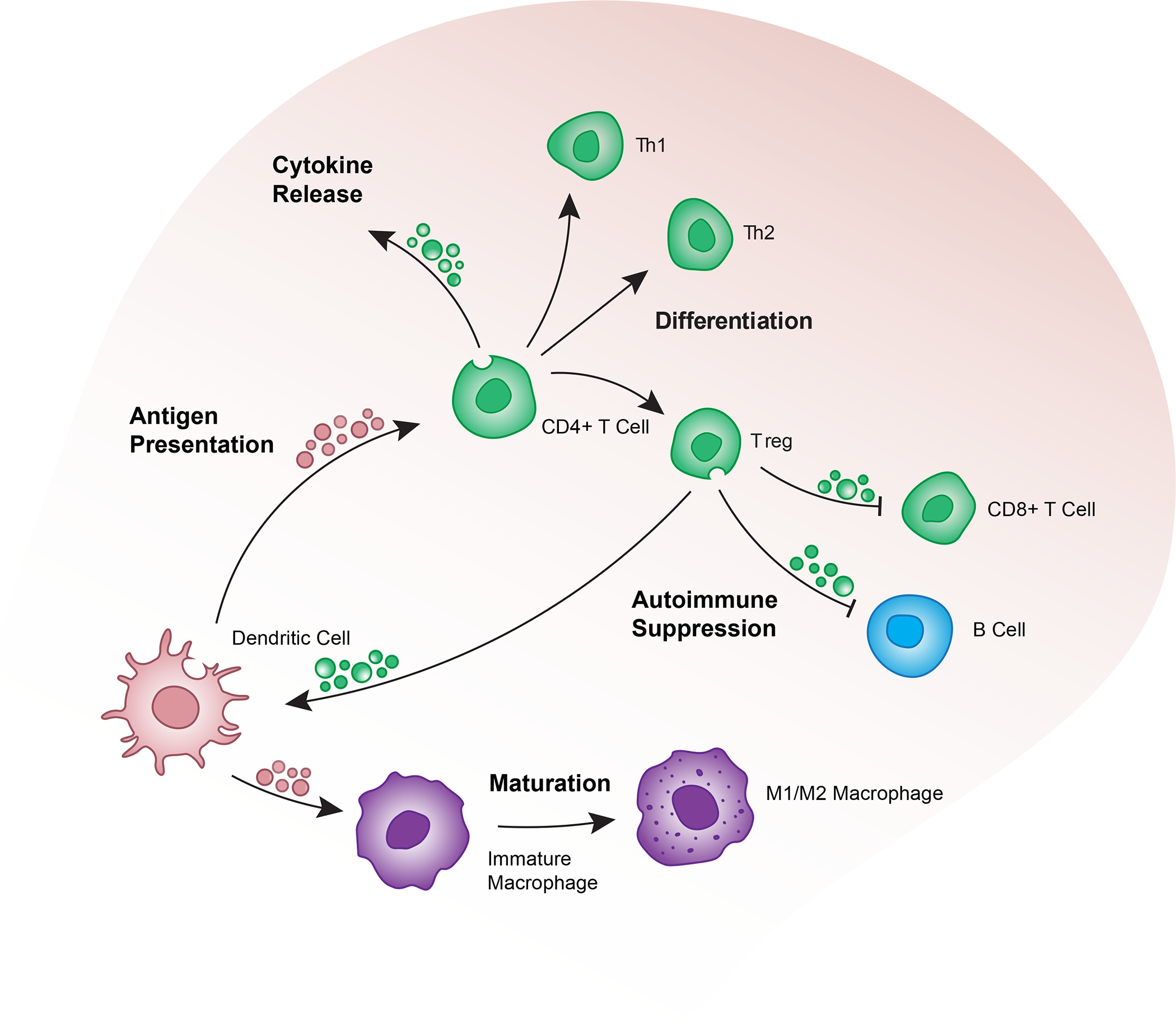
EVs released by immune cells constantly balance immune regulatory and autoimmune responses of surrounding cells in a highly interwoven network of immune responses. EVs promote cell maturation, polarization, and differentiation in T cells and mononuclear cells. Encapsulation and stabilization of EV-associated cytokines further extend the functional capacity of immune EVs by regulating cytokine release and downstream proliferation.
Tregs also target other T cells as a method of immune regulation. Treg-derived EVs suppress the proliferation of T cells via EV-mediated transfer of miRNAs and miRNA precursors46–50. Treg-derived EVs are enriched in miR-146a-5p, which targets and suppresses signal transducer and activator of transcription 1 (STAT1) and interleukin-1 receptor-associated kinase-like 2 (IRAK2) in recipient CD4+ T cells to inhibit proliferation49. The miRNA precursor Let-7d is carried by Treg-derived EVs and transferred to the target cell, where it appears to inhibit Cox-2 and decrease IFN-γ expression48. A specialized subset of CD4+CD25− Tregs release EVs found to induce a Treg phenotype in naïve CD4+ T cells (Fig. 2). These EVs containing miR-9, miR-330, miR-503, and inducible nitric oxide synthase (iNOS) mRNA suppress proliferation and induce an increase in IL-10 secretion in recipient T cells47.
In addition to directly influencing immune cell functions, Treg-derived EVs exert secondary immune suppression effects by transferring proteins to recipient cells. On their surface, Treg-derived EVs display both the Ebi3 and p35 subunits of IL-35 in association with tetraspanin CD8152. Cells that uptake these EVs exogenously express IL-35 on their surface. IL-35 interacts with the cell’s own IL-35 receptors (IL-35R) to induce cell exhaustion via expression of the inhibitory receptors programmed cell death protein 1 (PD-1), T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), and lymphocyte-activation gene 3 (LAG3)53. Other lymphocytes may also interact with IL-35 on the surface of these recipient cells, causing secondary suppression of the neighboring cells. This effect has been observed in CD4+ T cells, CD8+ T cells, and B cells52 (Fig. 2).
Antigen-specific immunosuppression is exhibited by EVs derived from APCs54,55. APC-derived EVs express MHC II molecules and Fas ligand (FasL), which allow them to induce apoptosis in T cells56. Since this mechanism of immunosuppression is antigen-specific, the host must be immunized against the antigen for the effect to occur. Similarly, overactivated T cells undergoing activation-induced cell death secrete EVs expressing FasL, which suppresses the immune response57.
The role of cancer-derived extracellular vesicles in immune evasion
A hallmark of cancer is the ability of tumor cells to evade detection by an individual’s immune system58. Colloquially termed “immune escape”, successful immune evasion utilizes a variety of mechanisms to stifle both adaptive and innate immune responses (Fig. 3)8. Immune cells experience a wide variety of inhibitory interactions from cancer cells, both via direct physical contact and through endogenous soluble factors58–61. As a significant driver of these interactions, cancer EVs can exhibit both immune evasion62–65 and immunogenic properties (Fig. 2b)66.
Figure 3: Cancer EVs directly regulate tumor progression.
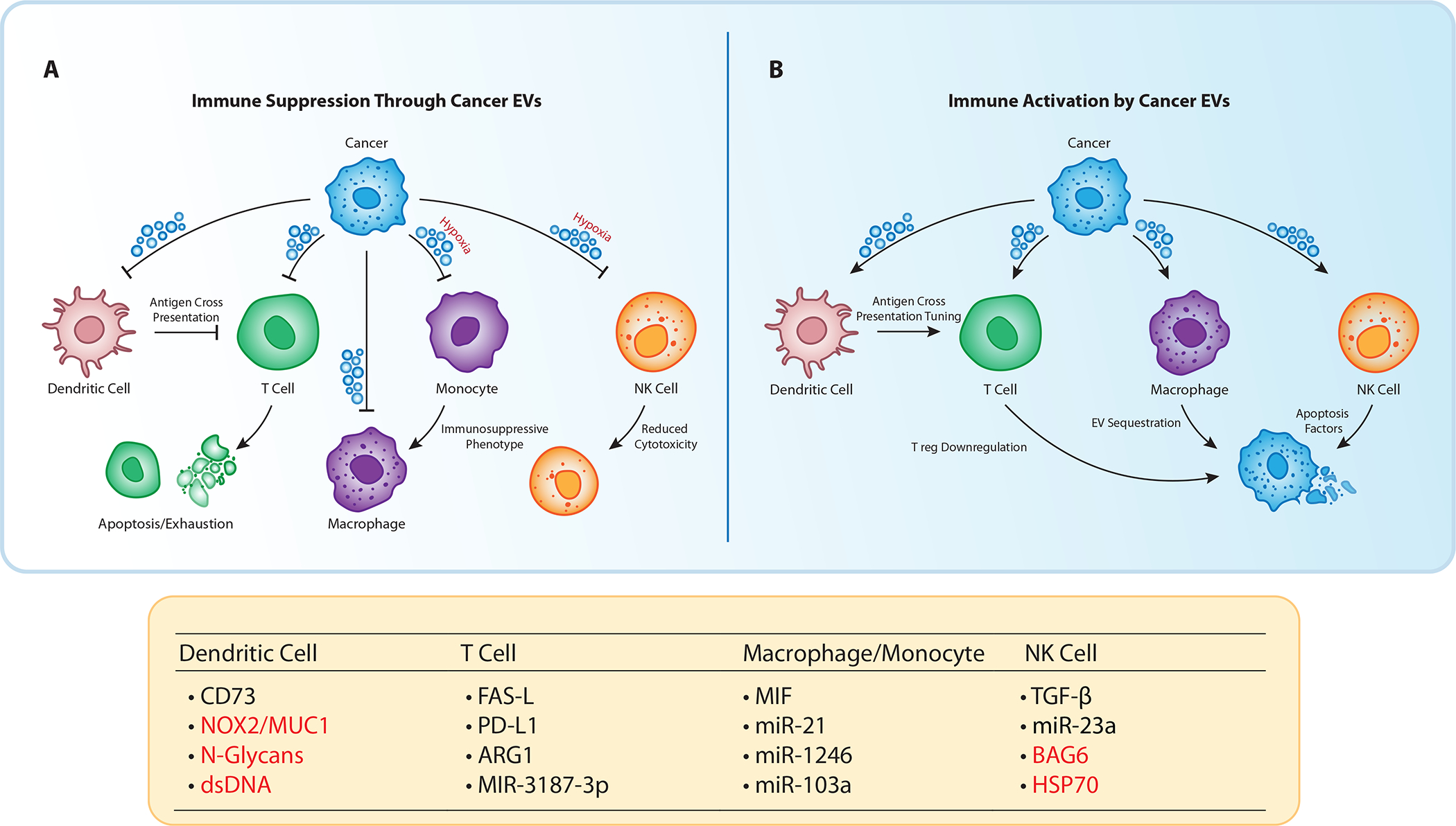
A) EVs released by cancer cells promote tumor progression through the suppression of adaptive and innate immune cells. Impeding effective antigen cross presentation in dendritic cells, EVs also contribute to T cell dysfunction through check point inhibition. Additionally, cancer EVs polarize mononuclear cells towards an immunosuppressive phenotype, while reducing cytotoxicity in NK cells. Less restricted by physical constraints, cancer EVs demonstrate far reaching immunosuppressive effects on circulatory and distal immune cells. B) Cancer EVs function as vessels for tumor recognition. During a functional immune response, EVs deliver foreign antigens to dendritic cells indirectly stimulating cytotoxic T cells. While macrophages actively sequester cancer EVs from circulation promoting cancer cell recognition. Molecules highlighted in red stimulate immune activation; molecules highlighted in black describe immunosuppressive cargo.
Early work on metastatic melanoma revealed that upregulation of the protein tyrosine kinase MET (MET) in small EVs permanently re-educates circulating bone marrow progenitors into pro-tumorigenic mediators of pre-metastatic niche formation in vivo67. Similarly, small EVs from pancreatic cancer cells, which contain a high amount of macrophage migration inhibitory factor (MIF), induce transforming growth factor beta (TGF-β) release by Kupffer cells to remodel the extracellular matrix (ECM) in the liver68. More recent work has shown that cancer EVs carry an abundance of functional mRNA, non-coding RNA, and proteins thought to be crucial for interacting with both the innate and adaptive immune responses (Fig. 3)69–71.
Highlighting the diverse role for cancer cell EVs, RNA sequencing of small chronic lymphocytic leukemia EVs revealed the non-coding Y RNA hY4 increased programmed death-ligand 1 (PD-L1) protein expression in circulating monocytes via toll-like receptor (TLR) 7 signaling72. PD-1/PD-L1 interactions regulate T cell receptor (TCR) activation to prevent autoimmune response73. Like its tumor cell progenitor, PD-L1 on small EVs isolated from glioblastoma and metastatic melanomas demonstrate the ability to directly activate the PD-1/PD-L1 immune checkpoint69,74 (Fig. 3). Small EVs isolated from metastatic melanoma feature identical PD-L1 membrane topology69 and, as such, functionally bind PD-169,74. A syngeneic mouse model of melanoma further revealed that PD-L1+ EVs prevent the proliferation of PD-1+ CD8+ T cells, and consequently the number of tumor infiltrating lymphocytes69. Expressed in a concentration-dependent manner, PD-L1+ EVs are upregulated by soluble IFN-γ and correlate to overall tumor burden69,74. The identification of EVs as vessels for PD-L1 has emerged as a prominent subclass of EV-mediated immune suppression. Exosomal PD-L1 in immune evasion has been well reviewed elsewhere75.
In addition to functional membrane-bound PD-L1, small EVs can carry miRNAs and enzymatically active Arginase-1 (ARG1) which both directly impact T cell activation, proliferation, and cytokine release via T cell receptor (TCR) downregulation70,76. Transfer of miR-498 in vitro directly impacts the release of tumor necrosis factor (TNF) release in CD8+ T cells in metastatic melanoma76. Meanwhile, miR-3187–3p can suppress CD45 membrane expression and consequent TCR activation in melanoma (Fig. 3)76. ARG1, found in ovarian carcinoma EVs, has emerged as a metabolic mechanism for T cell dysfunction. A catalyst for the urea cycle, ARG1-mediated depletion of L-arginine suppresses T cell immune response in vitro by down-regulating TCR complex component CD3ζ77. and by causing cell cycle arrest in the G1 phase via RICTOR in the mTORC2 complex78,79. Isolated from patient plasma and ascites, ARG1+ ovarian cancer EVs actively suppress T cell proliferation, both directly and via DC antigen cross-presentation70. ARG1+ cancer EVs have potentially far-reaching effects in T cell inhibition, as ARG1+ macrophages have been observed to actively arrest T cell proliferation in the absence of TCR signaling78.
There are also indirect routes for EV-mediated suppression of T cells. Since CD8+ T cells primarily undergo activation and proliferation via APC upregulation of MHC I, indirect activation via APCs presents an alternative avenue for EV-mediated suppression11. shRNA knockdowns of G-protein Rab27a in an in vitro irradiated prostate cancer model revealed a predominantly immunosuppressive role for EVs during antigen cross presentation80. Inducing a non-native CD73+ phenotype to DCs, EVs derived from irradiated prostate cancer promote an adenosine mediated suppression of CD8+ T cell activation via impaired DC antigen presentation80.
While EVs and their contents can drastically affect immune responses, it is important to understand where those signals originate. A significant body of evidence suggests external factors unique to the tumor microenvironment regulate the content and quantity of cancer EVs81–84. Consequently, environmental stresses are often reflected by EV protein and RNA expression82. Hypoxia, an intrinsic property of desmoplastic and late-stage cancers, promotes increased secretion of EVs often rich in immunosuppressive proteins and miRNAs83,85–87. A syngeneic mouse model of macrophage infiltration revealed that hypoxic small EVs isolated from B16F0 melanoma cells promote an anti-inflammatory M2-like phenotype in infiltrating macrophage cells83. Furthermore, small EV transfer of miR-103a and Let-7a upregulated under hypoxic conditions promote M2-like polarization in lung and melanoma cancers81,83, the latter working through the downregulation of an insulin-AKT-mTOR signaling pathway83. Meanwhile, microvesicular miR-23a and TGF-β carried by hypoxia-induced EVs has implications for natural killer (NK) cell suppression via downregulation of CD107a and NKG2D in vitro (Fig. 3) 87. Although chemical and physical cues may regulate EV content, the mechanism through which EVs systematically disseminate in tissue may also have implications for the downstream immunoregulatory capabilities of EVs.
Strikingly, EVs may acquire innate diffusive capacities within the tumor microenvironment. Physically restricted by a biocompatible nano-porous matrix, labeled EVs from mouse mesenchymal stromal cells were observed to readily diffuse through an otherwise spatially confined environment84. Indicative of mechanosensing properties, changes in the matrix stiffness and stress served to enhanced EV transport through the nano-porous matrix. This enhanced diffusion is also accompanied by physical deformation of the EV itself84. Mediated by the transport of water through aquaporins, AQP1 depletion increased the Young’s modulus (i.e. stiffness) of EVs and reduced diffusivity within the porous matrix84. Interestingly, whether and how the physical properties of the tumor microenvironment can affect EV production, cargo, and function remain unexplored. Further research into the impact of biomechanical cues – including stromal matrix stiffness88, topological cues89,90, fluid shear stresses91,92 – on EV content and function could also elucidate why many pre-clinical immune therapies based on EVs fail to return clinical benefits.
The role of extracellular vesicles in the antitumor immune response
While cancer EVs can mediate tumor progression, they also play an immunogenic role in the body’s response to cancer93–103. Cancer EVs carry tumor-associated antigens (TAAs), damage-associated molecular patterns (DAMPs), and other cargo that are taken up by immune cells and leveraged to mount an antitumor response93,94,96–101 (Fig. 1). Cancer EVs deliver TAAs and peptide-MHC complexes (pMHC) for antigen presentation and tumor-specific T cell stimulation93,95,98,100,103. More recently, lymphoma and melanoma EVs have been found to affect antigen processing machinery in DCs. Carrying mucin 1 (MUC1), NADPH oxidase 2 (NOX2), and reactive oxygen species (ROS), cancer EVs may alkalinize the DC phagosomal compartment via ROS production, making antigen processing within the DC more efficient93,103. Cancer EVs may also carry DNA fragments, including GAPDH gene fragments and mtDNA, which induce maturation in DCs via the cGAS-STING pathway96,100,102. However, the presence of dsDNA in small EVs has been disputed. Shown to co-isolate with small EVs, dsDNA may not be trafficked by small EVs at all, but instead released primarily and independently through a similar endosomal mechanism104.
Cancer EVs can also exert immune-stimulating effects directly on effector cells105. Melanoma EVs can increase IFN-γ secretion, promote proliferation, and increase the tumor-cytotoxic activity of NK cells106. Displaying an activating NKp30 ligand BAG6 on their surfaces, melanoma EVs can trigger an antitumor response in NK cells upon binding106. Furthermore, pancreatic and colon carcinoma EVs carrying heat shock protein 70 (Hsp70) stimulate NK cells to secrete granzyme B, an apoptosis factor in vitro107. In the long term, however, this effect is reversed with tumor EVs promoting a decrease in cytotoxic activity108. The ability of cancer EVs to directly stimulate effector cells is dependent upon the presence of adhesion and costimulatory molecules on their surfaces, in addition to the maturation status of the target cell109. The mechanisms by which cancer EVs stimulate effector cells require further study.
EVs contribute to the immune response in several other ways. For example, glioma EVs reduce the presence of Tregs at the tumor site, attenuating inherent immune suppression110. EVs from non-small lung cancer activate mast cells in vitro and increase TNF and CCL2 production111. Immune cell-derived EVs can also directly carry out antitumor functions112–115. CD8+ T cell EVs are cytotoxic and can directly kill tumor cells113,114. Furthermore, CD8+ T cell EVs deplete mesenchymal tumor cells via the transfer of cytotoxic miR-298 to prevent invasion and metastasis113,114. DC EVs from hepatocellular carcinoma patients reduce Tregs at the tumor site115. Macrophages have also been found to suppress tumor immune evasion by taking up melanoma EVs to prevent their tumor-promoting interactions with B cells112.
Several studies have noted discrepancies in function between soluble factors and their vesicular counterparts. Tumor antigens seem to be more efficiently taken up by immune cells when associated with EVs rather than in soluble form93,95,100. Additionally, vesicular Hsp70 can contribute to radiotherapy resistance in tumors116, while immunization with non-vesicular Hsp70-peptide complex is associated with more positive effects117,118. These findings cast doubt on whether functions are always correctly attributed to EVs instead of soluble factors. This is especially concerning since it is difficult to segregate soluble factors from EV preparations104. While ideal single EV subtype models and validation techniques remain largely elusive, effects attributed to EV populations should always be extensively validated through multiple isolation and analysis techniques35.
Clinical applications of extracellular vesicles in immunotherapy, drug delivery, and prognostics
The immunotherapeutic potential of EVs was first demonstrated in 1998 (Ref. 10). The properties (i.e., antigen presentation and costimulatory molecules) that made EVs a functional communication pathway have since been widely explored, leading to the inception of a new class of EV-centered cancer immunotherapies. After more than two decades of probing EV function in immune-cancer interactions, the field has turned its focus to the engineering and targeting of EVs for cancer therapy and diagnosis. As more immunological and oncogenic characteristics of EVs come to light, new therapeutic targets and mechanisms will become available (Fig. 4).
Figure 4: Potential clinical translations for EVs.

EVs have emerged as powerful tools for cancer treatment. Synthetic and re-appropriated natural EVs carry bioactive molecules that enhance the immunological roles of EVs, indicate disease prognosis, or facilitate drug delivery to the tumor site. These capacities are the focus of several ongoing clinical trials.
The involvement of EVs in cancer-immune cell crosstalk presents many opportunities for cancer immunotherapy. Proposed therapies use natural and engineered EVs to enhance the existing immune response to cancer, or to inhibit their immunosuppressive functions119,120. Currently, there are no approved EV-based immunotherapies. However, a considerable number of clinical trials have started over the past five years.
EVs are ideal candidates for drug development and delivery due to their biocompatibility, stability, targeting capabilities, and scalability. EVs are enriched in adhesion and signaling molecules, which allow them to hone to target cells and stimulate uptake121. The presence of transmembrane CD47 permits EVs to avoid immune rejection via CD47-SIRPα “don’t eat me’ signaling122. This immune escape mechanism contributes to the extended circulation time of EVs in comparison to free drug or cell-based therapies, which are more susceptible to immune clearance. In addition to extended half-life, EVs demonstrate greater cellular targeting and uptake than free drug delivery123,124.
A major advantage of EVs over cell-based therapies is their amenability to specialized and scaled-up production. Several platforms are being developed for scaled-immunoprecipitation (IP) production and purification of EVs125,126. Recently described methods even allow for rapid, automated harvest and surface modification of EVs on a microfluidic device127. For internal cargo modification, several methods have been established, including sonication, electroporation, and passive diffusion128. Furthermore, EVs are better suited to long term storage than cells, experiencing limited loss of function129,130. Cytokine release syndrome (CRS), however, remains a safety concern for the use of EVs in cancer immunotherapy. CRS is a potentially fatal reaction to immunotherapies that target T cells131. While it has not been investigated or observed in EV therapies, CRS should be taken into account during the development of EV-based cancer immunotherapies.
The presence of TAAs on cancer EVs makes DCs a major target for cancer EV-based immunotherapy132–138. Treatment with EVs from colorectal carcinoma, glioblastoma, myeloid leukemia, renal carcinoma, melanoma, tongue carcinoma, and lung cancer has demonstrated increased T cell activation, proliferation, tumor infiltration, and tumor cytotoxicity with simultaneous decrease in immunosuppression and tumor growth132–139. Proposed treatments collect, modify, and return tumor EVs to improve in vivo targeting of DCs and enhance the immune response132,134–138,140. Melanoma EVs have been engineered to present immunostimulatory CpG-DNA for preferential uptake by DCs136,140. Upregulation of IL-12135 or the addition of miR-155132, pH-sensitive fusogenic GALA peptide137, or DAMPs138 to cancer EVs enhances antigen presentation and immune activation by DCs. Myeloid leukemia EVs have also been used to condition DCs ex vivo prior to DC vaccination to improve efficacy of treatment134 (Fig. 4).
Other EV-based therapies inhibiting tumor progression involve EV-mediated immunosuppression strategies that preserve their immunostimulatory functions94,133,135,141. Depletion of suppressive factors such as Siglec-9 ligands133 or TGF-β94,135,141 in glioblastoma, colon carcinoma, and leukemia EVs resulted in increased uptake by DCs and an enhanced antitumor immune response. Subjecting colon cancer cells to heat stress produces EVs enriched in Hsp70 that stimulates IL-6 production in DCs, increases T helper 17 polarization, and decreases Treg polarization leading to an enhanced immune response142. Similarly, irradiated hepatoma cells secrete EVs enriched in TAAs, like CDCP1, and DAMPs, including Hsp70 and Hsp9098. Another potential target to inhibit tumor EV-mediated immune suppression is myeloid-derived suppressor cells (MDSCs)143. Hsp70 on breast, lung, and ovarian cancer EVs was found to bind TLR2 on MDSCs, stimulating EV-mediated suppression of T cells and NK cells. Addition of the peptide aptamer A8 blocks TLR2 binding and may prevent immune suppression by MDSCs143.
Immune cell-derived EVs also have potential for cancer immunotherapy. DC EVs, in particular, have garnered attention for their ability to stimulate tumor-specific immune responses144–146. DC EVs loaded with IFN-γ enhance this effect, increasing secretion of IFN-γ and TNF by NK cells147. It was hypothesized that the addition of melanoma antigen recognized by T cells 1 (MART1) to these IFN-γ-enriched EVs might stimulate a tumor-specific response in NK cells, but the effect was small in humans147. Other DC EV modifications aim to enhance their immune stimulating capabilities. Adding TAAs – such as melanoma-associated antigen 3 (MAGE-A3), MART1, glycoprotein 100 (gp100), or HPV16 E7 – to the surfaces of DC EVs increases uptake by monocytes and promotes T cell proliferation, stimulation, and cytotoxicity via DCs127,148. Increasing alpha-fetoprotein (AFP) expression in DC EVs also enhances tumor immunity. AFP promotes enrichment of MHC I, MHC II, and costimulatory molecules on DC EVs, which induces a change in the tumor microenvironment from immunoinhibitory to immunostimulatory by increasing T cell tumor infiltration and reducing the presence of Tregs149. Similarly, adding ovalbumin, lipopolysaccharides (LPS), and IFN-γ to DC EVs promotes the conversion of immunosuppressive M2 macrophages into immunostimulatory M1 macrophages, promotes antigen presentation in DCs, and directly activates T cells150.
EVs from NK cells and macrophages have also been investigated for immunotherapy. NK cell EVs contain FasL and TNF, making them cytotoxic to cancer cells151,152. Vesicles generated from NK cell membranes also exhibit these properties151. Modifications to enhance tumor attack include priming the NK cells with IL-15. This treatment increases TNF-related apoptosis-inducing ligand (TRAIL), and expression of the activatory receptors NKp46, and NKp30 on NK cell EVs, leading to more efficient tumor targeting and cytotoxicity153. Macrophage EVs have been employed for antigen presentation to DCs. The addition of hyaluronic acid (HA), 3-(diethylamino)propylamine (DEAP), monophosphoryl lipid A (MPLA), and MUC1 trigger uptake by DCs and the release of TAA in the endocytic compartment for improved antigen presentation and T cell activation154.
Clinical trials for extracellular vesicle-based cancer diagnostics and therapy
Early clinical trials investigating the use of EVs in cancer immunotherapy showed little more than that EVs are safe for human use144–146. This has not deterred further attempts, however, as our knowledge of EV function continues to expand. Current clinical studies aim to determine the effects of immunotherapy, among other treatments, on vesicular cargo, including PD-L1 and miRNA expression profiles155–160. Other clinical research is investigating the role of EVs in therapeutic resistance159,161. EVs are hypothesized to contribute to therapeutic resistance via enrichment of immunotherapeutic targets, like CD20 and PD-L1. A possible solution, currently undergoing Early Feasibility Phase I clinical trials, is to deplete circulating EVs via a proprietary hemopurifier device 162, neutralizing their immunosuppressive and therapeutic resistance effects. Another Phase I clinical trial is investigating the immune modulation and anticancer effects of curcumin-loaded plant EVs 163. An upcoming Phase 2 clinical trial investigating the use of an antisense oligodeoxynucleotide drug (IMV-001) against insulin-like growth factor type I has shown dependence on TAA-bearing tumor EVs to stimulate antitumor immunity. The IMV-001 drug released from an implanted bio-diffusion chamber for the treatment of malignant gliomas, induces apoptotic cell death in surrounding tumor cells. IMV-001 is hypothesized to work together with these TAA-bearing tumor EVs released from apoptotic cells to stimulate antitumor immunity in the surrounding tissue164. The results of this treatment strategy have been positive thus far and emphasize the downstream potential for TAA loaded EVs to promote immune response165–167.
Engineered and synthetic vesicles are gaining popularity for application in drug delivery due to their stability and homing capabilities. For example, oncolytic virus is an established cancer treatment, but due to rapid immune clearance, it must be administered locally168. Encapsulation of the virus in cancer EVs enables systemic delivery and tumor site homing for the targeting of cancer metastases168. Macrophage EVs have likewise been used to deliver the antitumor drug doxorubicin to the tumor site in vivo154. DNA vaccinations may even be targeted to EVs to enhance immunogenicity. An expression plasmid encoding antigen-fused CD63 was able to deliver the antigen onto EVs in vivo, eliciting a stronger antitumor response in a syngeneic lymphoma model 169.
EVs derived from the human HEK293 cell line are another common platform for cancer therapy. HEK293 EVs have been modified with signal regulatory protein alpha (SIRPα) to block CD47 at the tumor site, resulting in increased phagocytosis of tumor cells by macrophages and increased CD8+ T cell tumor infiltration120. HEK293 EVs can also be loaded with PH20 hyaluronidase to break down high molecular weight HA in the tumor microenvironment. The resulting oligo-HA induce DC maturation via TLR4 activation and elicits a more potent antitumor response170. Vesicles made from HEK293 cell membrane expressing PD-1 were used to block tumor PD-L1 and deliver indoleamine 2,3-dioxygenase-1 (IDO) inhibitor to the tumor microenvironment, resulting in reduced Treg presence and improved antitumor response171. Efforts to decrease liver uptake of HEK293 EVs, thus increasing circulation time and tumor site accumulation, use dextran sulfate to block scavenger receptor class A (SR-A) on the EVs, optimizing their performance125.
Efforts to engineer synthetic vesicles attempt to recapitulate the tumor-targeting and immune-stimulating properties of EVs, via the repurposing of cell membranes, for instance. Leukocyte membranes have been harvested and extruded into vesicles for the coating of synthetic microcapsules172. Melanoma cell membranes were similarly used to generate poly(ethylene glycol) (PEG)ylated nanovesicles capable of inducing an antitumor immune response119. Synthetic multivalent antibodies retargeted exosomes (SMART-Exos) are modified with tumor antigen and CD3 to induce antigen-specific immune responses173,174.
EV isolation remains bound by labor-intensive purification techniques. Techniques such as immunoprecipitation (IP) and size exclusion chromatography (SEC) supplement the ad hoc-standard ultracentrifugation in a laboratory setting. However, extensive sample preparation and incubation steps negate the practical benefits of IP and SEC techniques clinically. While advancements in microfluidic “chip” isolations promise to bridge this laboratory-clinic divide, it reflects a time-untested methodology175.
The quickest route to clinical practicality of EVs is as prognostic biomarkers. Currently limited by low throughput of isolation techniques, patient-derived EVs isolated from bodily fluids – whole blood, plasma, urine, ascites, etc. – and tissue biopsy have the potential to be a critical prognostic tool for immunologists. In addition to catapulting EVs into mainstream clinical use, high throughput EV isolation methods for liquid and solid biopsies might further reveal systematic vs local EV function. The focus of over 100 completed and ongoing clinical trials, the EV prognostic field is rapidly expanding. EVs from patient samples could possibly indicate diseases progression, as well as response to therapy, allowing for more personalized treatment.
Characterization of patient samples has, however, correlated several immune-related vesicular biomarkers with poor prognosis176,177. Vesicular annexin II is involved in the activation of proinflammatory signaling in macrophages leading to breast cancer metastasis177. This role and the correlation of vesicular annexin II with breast cancer progression suggest it may serve as a biomarker for breast cancer prognosis (Fig. 4). Similarly, elevated serum levels of vesicular miR-200b and miR-200c have been correlated with the spread of epithelial ovarian cancer to the lymph nodes (FIGO stage III-IV)176.
In addition to predicting and measuring disease progression, EVs may indicate patient response to cancer treatment178,179. In general, patients with long-term clinical remission of acute myeloid leukemia, as well as patients tested after induction of chemotherapy, have lower vesicular expression levels of TGF-β than those tested at diagnosis178. Another study in anaplastic astrocytoma patients found that decreases in vesicular IL-8 and TGF-β correspond to an increase in immune activity after antitumor vaccination179. The relationship between vesicular TGF-β and cancer progression is likely due to its role in NK cell suppression178. TGF-β may, therefore, serve as a prognostic biomarker for patient response to chemotherapy.
EVs may emerge as powerful indicators of immunotherapeutic resistance as well69,180–182. The monoclonal antibody drug trastuzumab is used to treat HER2+ breast cancer, but not all patients respond to the treatment. HER2 and epithelial cell adhesion molecule (EpCAM) present on breast cancer EVs have been found to sequester therapeutic antibodies and interfere with tumor targeting (Fig. 4)180,181. Meanwhile circulating vesicular PD-L1 has proved to be a predictor of clinical response to the landmark immunotherapy pembrolizumab (anti-PD-1)69,182. Thus, the presence of vesicular tumor antigens may serve as indicators of immunotherapeutic resistance.
Conclusion
Burdened by low yield and labor-intensive isolation techniques, the purity, quality, and characterization of EVs remain paramount for both pre-clinical and clinical research. Pre-clinical studies have revealed a diverse repertoire of EV functions, particularly with regards to cell maturation, antigen presentation, and immune suppression. Essential to both the antitumor and immune regulatory responses, the emergence of EVs as immune checkpoint vessels continue to be an exciting avenue for clinical translation. Moreover, the contribution of the physical properties of the extracellular matrix (e.g. matrix stiffness and porosity) to EV secretion, cargo, and consequent immune suppression remain largely unexplored. A holistic approach to immune suppression beginning with environment origin, immune cell migration, and consequent adaptive immunity may elucidate why and when EVs are able to suppress immune response.
Currently secondary in clinical applications, significant progress has been made in the past five years towards the use of EVs in cancer immunotherapy. Their safety, targeting capabilities, and clinical practicality make EVs an attractive candidate for drug development and delivery. Tumor and immune cell EVs have shown significant effects on cancer and the antitumor immune response in disease models. The challenge remains, however, to harness these abilities to produce a meaningful benefit in patients. For the time being, EVs are better suited to diagnostic and prognostic use than treatment. Future work should focus on developing EVs as adjuvant therapies to combat the side effects and drawbacks of established treatments.
Table 1:
Ongoing and completed clinical trials involving EVs at www.ClinicalTrials.gov
| Clinical Trial | Identifier |
|---|---|
| Prediction of Immunotherapeutic Effect of Advanced Non-small Cell Lung Cancer | NCT04427475 |
| Clinical Research for the Consistency Analysis of PD-L1 in Cancer Tissue and Plasma Exosome (RadImm01) | NCT02890849 |
| Clinical Research for the Consistency Analysis of PD-L1 in Lung Cancer Tissue and Plasma Exosome Before and After Radiotherapy (RadImm02) | NCT02869685 |
| Anaplastic Thyroid Cancer and Follicular Thyroid Cancer-derived Exosomal Analysis Via Treatment of Lovastatin and Vildagliptin and Pilot Prognostic Study Via Urine Exosomal Biological Markers in Thyroid Cancer Patients | NCT02862470 |
| Study of Molecular Mechanisms Implicated in the Pathogenesis of Melanoma. Role of Exosomes (EXOSOMES) | NCT02310451 |
| Study of Exosomes in Monitoring Patients With Sarcoma (EXOSARC) (EXOSARC) | NCT03800121 |
| Exosomes and Immunotherapy in Non-Hodgkin B-cell Lymphomas (ExoReBLy) | NCT03985696 |
| Hemopurifier Plus Pembrolizumab in Head and Neck Cancer | NCT04453046 |
| Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue | NCT01294072 |
| Pilot Immunotherapy Trial for Recurrent Malignant Gliomas | NCT01550523 |
| Antisense102: Pilot Immunotherapy for Newly Diagnosed Malignant Glioma | NCT02507583 |
Acknowledgments
This work was supported through grants from the National Institutes of Health (T32GM008764), the National Cancer Institute (U54CA143868), and the National Institute on Aging (U01AG060903) to DW. Figures designed with BioRender.com.
References
- 1.Yáñez-mó M et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo M, Raposo G & Théry C Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Kalra H, Drummen GPC & Mathivanan S Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 17, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raposo G & Stoorvogel W Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Théry C et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niel G. Van, Angelo GD & Raposo G Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Xu R et al. Extracellular vesicles in cancer — implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 15, 617–638 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Becker A et al. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 30, 836–848 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raposo G et al. B Lymphocytes Secrete Antigen-presenting Vesicles. J. Exp. Med. 183, 1161–1172 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, A. S. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4, 594–600 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Théry C et al. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 3, 1156–1162 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Mallegol J et al. T84-Intestinal Epithelial Exosomes Bear MHC Class II/Peptide Complexes Potentiating Antigen Presentation by Dendritic Cells. Gastroenterology 132, 1866–1876 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Admyre C et al. Exosomes with major histocompatibility complex class II and costimulatory molecules are present in human BAL fluid. Eur. Respir. J. 22, 578–583 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M & Minami M MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol. Lett. 89, 125–131 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Clayton A et al. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods 247, 163–174 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Lynch S et al. Novel MHC Class I Structures on Exosomes. J. Immunol. 183, 1884–1891 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Bobrie A, Colombo M, Raposo G & Théry C Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 12, 1659–1668 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Hwang I, Shen X & Sprent J Direct stimulation of naïve T cells by membrane vesicles from antigen-presenting cells: Distinct roles for CD54 and B7 molecules. Proc. Natl. Acad. Sci. U. S. A. 100, 6670–6675 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander M et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Cell Biol. 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Admyre C, Johansson SM, Paulie S & Gabrielsson S Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur. J. Immunol. 36, 1772–1781 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald W et al. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qazi KR, Gehrmann U, Jordo ED, Karlsson MCI & Gabrielsson S Antigen-loaded exosomes alone induce Th1-type memory through a B cell – dependent mechanism. Immunobiology 113, 2673–2683 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Tkach M et al. Qualitative differences in T‐cell activation by dendritic cell‐derived extracellular vesicle subtypes. EMBO J. 36, 3012–3028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent-Schneider H Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int. Immunol. 14, 713–722 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Ruhland MK et al. Visualizing Synaptic Transfer of Tumor Antigens among Dendritic Cells. Cancer Cell 37, 786–799.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Jiménez VD et al. Extracellular Vesicles Released from Mycobacterium tuberculosis-Infected Neutrophils Promote Macrophage Autophagy and Decrease Intracellular Mycobacterial Survival. Front. Immunol. 9, 272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danesh A et al. Granulocyte-derived extracellular vesicles activate monocytes and are associated with mortality in intensive care unit patients. Front. Immunol. 9, 956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y et al. Macrophages transfer antigens to dendritic cells by releasing exosomes containing dead-cell-associated antigens partially through a ceramide-dependent pathway to enhance CD4 +T-cell responses. Immunology 149, 157–171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker PD et al. B lymphocytes contribute to indirect pathway T cell sensitisation via acquisition of extracellular vesicles. Am. J. Transplant. (2020) doi: 10.1111/ajt.16088. [DOI] [PubMed] [Google Scholar]
- 30.Zeng F & Morelli AE Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Seminars in Immunopathology vol. 40 477–490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L et al. Exosomes from Thymic Stromal Lymphopoietin-Activated Dendritic Cells Promote Th2 Differentiation through the OX40 Ligand. Pathobiology 86, 111–117 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Kremer AN et al. Natural T-cell ligands that are created by genetic variants can be transferred between cells by extracellular vesicles. Eur. J. Immunol. 48, 1621–1631 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei G et al. Dendritic cells derived exosomes migration to spleen and induction of inflammation are regulated by CCR7. Sci. Rep. 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schierer S et al. Extracellular vesicles from mature dendritic cells (DC) differentiate monocytes into immature DC. Life Sci. Alliance 1, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowal J et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U. S. A. 113, E968–E977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui X et al. Thyrocyte-derived exosome-targeted dendritic cells stimulate strong CD4+ T lymphocyte responses. Mol. Cell. Endocrinol. 506, 110756 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Cai Z et al. Immunosuppressive exosomes from TGF-$β$1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Research vol. 22 607–610 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M et al. Inhibition of MicroRNA <em>let-7i</em> Depresses Maturation and Functional State of Dendritic Cells in Response to Lipopolysaccharide Stimulation via Targeting Suppressor of Cytokine Signaling 1. J. Immunol. 187, 1674 LP–1683 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Lindenbergh MFS et al. Bystander T-cells support clonal T-cell activation by controlling the release of dendritic cell-derived immune-stimulatory extracellular vesicles. Front. Immunol. 10, 448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torralba D et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 9, 2658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiou N et al. Selective Export into Extracellular Vesicles and Function of tRNA Fragments during T Cell Activation. Cell Rep. 25, 3356–3370.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segura E, Amigorena S & The C Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells, Mol. Dis. 35, 89–93 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Kaur S et al. CD63, MHC class 1, and CD47 identify subsets of extracellular vesicles containing distinct populations of noncoding RNAs. Sci. Rep. 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakaguchi S, Sakaguchi N, Asano M, Itoh M & Toda M Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor a-Chains (CD25). J. Immunol. 155, 1151–1165 (1995). [PubMed] [Google Scholar]
- 45.Tung SL et al. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci. Rep. 8, 6065 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tung SL et al. Regulatory T Cell Extracellular Vesicles Modify T-Effector Cell Cytokine Production and Protect Against Human Skin Allograft Damage. Front. Cell Dev. Biol. 8, 317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aiello S et al. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci. Rep. 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okoye IS et al. MicroRNA-Containing T-Regulatory-Cell-Derived Exosomes Suppress Pathogenic T Helper 1 Cells. Immunity 41, 89–103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torri A et al. Extracellular MicroRNA signature of human helper T cell subsets in health and autoimmunity. J. Biol. Chem. 292, 2903–2915 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L et al. Exosomes Derived From T Regulatory Cells Suppress CD8 + Cytotoxic T Lymphocyte Proliferation and Prolong Liver Allograft Survival. Med. Sci. Monit. 25, 4877–4884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naqvi AR, Fordham JB, Ganesh B & Nares S MiR-24, miR-30b and miR-142–3p interfere with antigen processing and presentation by primary macrophages and dendritic cells. Sci. Rep. 6, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan JA et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 30, 1039–1051.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turnis ME et al. Interleukin-35 Limits Anti-Tumor Immunity. Immunity 44, 316–329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SH, Bianco NR, Shufesky WJ, Morelli AE & Robbins PD MHC Class II + Exosomes in Plasma Suppress Inflammation in an Antigen-Specific and Fas Ligand/Fas-Dependent Manner. J. Immunol. 179, 2235–2241 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Kim SH et al. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 13, 289–300 (2006). [DOI] [PubMed] [Google Scholar]
- 56.O Reilly LA et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 461, 659–663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monleón I et al. Differential Secretion of Fas Ligand- or APO2 Ligand/TNF-Related Apoptosis-Inducing Ligand-Carrying Microvesicles During Activation-Induced Death of Human T Cells. J. Immunol. 167, 6736–6744 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Hanahan D & Weinberg RA Hallmarks of Cancer: The Next Generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Hiratsuka S, Watanabe A, Aburatani H & Maru Y Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Oskarsson T et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kano A Tumor cell secretion of soluble factor(s) for specific immunosuppression. Sci. Rep. 5, 8913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoshino A et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller L, Mitsuhashi M, Simms P, Gooding WE & Whiteside TL Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci. Rep. 6, 20254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clayton A & Tabi Z Exosomes and the MICA-NKG2D system in cancer. Blood Cells, Mol. Dis. 34, 206–213 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Capello M et al. Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat. Commun. 10, 254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahaweni NM, Kaijen-Lambers MEH, Dekkers J, Aerts JGJV & Hegmans JPJJ Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J. Extracell. Vesicles 2, 22492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peinado H et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costa-Silva B et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen G et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Czystowska-Kuzmicz M et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 10, 3000 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ricklefs FL et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, eaar2766 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haderk F et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci. Immunol. 2, 28 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Karwacz K et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8 + T cells. EMBO Mol. Med. 3, 581–592 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ricklefs FL et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, eaar2766 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daassi D, Mahoney KM & Freeman GJ The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 20, 209–215 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Vignard V et al. MicroRNAs in tumor exosomes drive immune escape in melanoma. Cancer Immunol. Res. 8, 255–267 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez PC et al. Regulation of T Cell Receptor CD3ζ Chain Expression byl-Arginine. J. Biol. Chem. 277, 21123–21129 (2002). [DOI] [PubMed] [Google Scholar]
- 78.Van De Velde LA et al. T Cells encountering myeloid cells rogrammed for amino acid-dependent Immunosuppression Use Rictor/mTORC2 Protein for proliferative checkpoint decisions. J. Biol. Chem. 292, 15–30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez PC, Quiceno DG & Ochoa AC l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109, 1568–1573 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salimu J et al. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J. Extracell. Vesicles 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsu YL et al. Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN. Mol. Ther. 26, 568–581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Jong OG et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 1, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park JE et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene 38, 5158–5173 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Lenzini S, Bargi R, Chung G & Shin J-W Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 15, 217–223 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilkes DM, Semenza GL & Wirtz D Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer 14, 430–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diaz B & Yuen A The impact of hypoxia in pancreatic cancer invasion and metastasis. Hypoxia 2, 91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berchem G et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim D-H & Wirtz D Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials 48, 161–172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fraley SI, Feng Y, Giri A, Longmore GD & Wirtz D Dimensional and temporal controls of three-dimensional cell migration by zyxin and binding partners. Nat. Commun. 3, 719 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khatau SB et al. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. 106, 19017 LP–19022 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wirtz D, Konstantopoulos K & Searson PC The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Follain G et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat. Rev. Cancer 20, 107–124 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Dionisi M et al. Tumor-Derived Microvesicles Enhance Cross-Processing Ability of Clinical Grade Dendritic Cells. Front. Immunol. 9, 2481 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang F, Wan J, Hu W & Hao S Enhancement of Anti-Leukemia Immunity by Leukemia-Derived Exosomes Via Downregulation of TGF-β1 Expression. Cell. Physiol. Biochem. 44, 240–254 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Rughetti A et al. Microvesicle cargo of tumor-associated MUC1 to dendritic cells allows cross-presentation and specific carbohydrate processing. Cancer Immunol. Res. 2, 177–186 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Kitai Y et al. DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING-Dependent Pathway and Reinforce Antitumor Immunity. J. Immunol. 198, 1649–1659 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Kremer AN et al. Natural T-cell ligands that are created by genetic variants can be transferred between cells by extracellular vesicles. Eur. J. Immunol. 48, 1621–1631 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin W et al. Radiation-induced small extracellular vesicles as ‘carriages’ promote tumor antigen release and trigger antitumor immunity. Theranostics 10, 4871–4884 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Squadrito ML, Cianciaruso C, Hansen SK & De Palma M EVIR: Chimeric receptors that enhance dendritic cell cross-dressing with tumor antigens. Nat. Methods 15, 183–186 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol. Res. 3, 196–205 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Caruso Bavisotto C et al. Immunomorphological Pattern of Molecular Chaperones in Normal and Pathological Thyroid Tissues and Circulating Exosomes: Potential Use in Clinics. Int. J. Mol. Sci. 20, 4496 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diamond JM et al. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol. Res. 6, 910–920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma J et al. Mechanisms by Which Dendritic Cells Present Tumor Microparticle Antigens to CD8+ T cells. Cancer Immunol. Res. 6, 1057–1069 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Jeppesen DK et al. Reassessment of Exosome Composition. Cell 177, 428–445.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menay F et al. Exosomes Isolated from Ascites of T-Cell Lymphoma-Bearing Mice Expressing Surface CD24 and HSP-90 Induce a Tumor-Specific Immune Response. Front. Immunol. 8, 286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Daßler-Plenker J et al. RIG-I activation induces the release of extracellular vesicles with antitumor activity. Oncoimmunology 5, e1219827 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gastpar R et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 65, 5238–5247 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Q et al. Bifacial effects of engineering tumour cell-derived exosomes on human natural killer cells. Exp. Cell Res. 363, 141–150 (2018). [DOI] [PubMed] [Google Scholar]
- 109.Greening DW, Gopal SK, Xu R, Simpson RJ & Chen W Exosomes and their roles in immune regulation and cancer. Seminars in Cell and Developmental Biology vol. 40 72–81 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Scholl JN et al. Characterization and antiproliferative activity of glioma-derived extracellular vesicles. Nanomedicine 15, 1001–1018 (2020). [DOI] [PubMed] [Google Scholar]
- 111.Salamon P, Mekori YA & Shefler I Lung cancer-derived extracellular vesicles: a possible mediator of mast cell activation in the tumor microenvironment. Cancer Immunol. Immunother. 69, 373–381 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pucci F et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science (80-. ). 352, 242–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seo N et al. Activated CD8+ T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat. Commun. 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peters PJ et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 173, 1099–1109 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu Z et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J. Hepatol. 67, 739–748 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Ostheimer C, Gunther S, Bache M, Vordermark D & Multhoff G Dynamics of heat shock protein 70 serum levels as a predictor of clinical response in non-small-cell lung cancer and correlation with the hypoxia-related marker osteopontin. Front. Immunol. 8, 1305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gao Y et al. Enhancing the treatment effect on melanoma by heat shock protein 70-peptide complexes purified from human melanoma cell lines. Oncol. Rep. 36, 1243–1250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blachere BNE et al. Heat Shock Protein – Peptide Complexes, Reconstituted In. J. Exp. Med. 186, 1315–22 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ochyl LJ et al. PEGylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials 182, 157–166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koh E et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 121, 121–129 (2017). [DOI] [PubMed] [Google Scholar]
- 121.Hoshino A et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kamerkar S et al. Therapeutic targeting of oncogenic KRAS in pancreatic cancer by engineered exosomes. Nature vol. 546 498–503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rana S, Yue S, Stadel D & Zöller M Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 44, 1574–1584 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Sun H et al. A multifunctional liposomal nanoplatform co-delivering hydrophobic and hydrophilic doxorubicin for complete eradication of xenografted tumors. Nanoscale 11, 17759–17772 (2019). [DOI] [PubMed] [Google Scholar]
- 125.Watson DC et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 105, 195–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Whitford W & Guterstam P Exosome manufacturing status. Future Med. Chem. 11, 1225–1236 (2019). [DOI] [PubMed] [Google Scholar]
- 127.Zhao Z, McGill J, Gamero-Kubota P & He M Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab Chip 19, 1877–1886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luan X et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica vol. 38 754–763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou H et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 69, 1471–1476 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jeyaram A & Jay SM Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS Journal vol. 20 1–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shimabukuro-Vornhagen A et al. Cytokine release syndrome. J. Immunother. cancer 6, 56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asadirad A et al. Phenotypical and functional evaluation of dendritic cells after exosomal delivery of miRNA-155. Life Sci. 219, 152–162 (2019). [DOI] [PubMed] [Google Scholar]
- 133.Dusoswa SA et al. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J. Extracell. Vesicles 8, 1648995 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gu X, Erb U, Büchler MW & Zöller M Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int. J. Cancer 136, E74–E84 (2015). [DOI] [PubMed] [Google Scholar]
- 135.Rossowska J et al. Antitumor Potential of Extracellular Vesicles Released by Genetically Modified Murine Colon Carcinoma Cells With Overexpression of Interleukin-12 and shRNA for TGF-β1. Front. Immunol. 10, 211 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matsumoto A, Takahashi Y, Ariizumi R, Nishikawa M & Takakura Y Development of DNA-anchored assembly of small extracellular vesicle for efficient antigen delivery to antigen presenting cells. Biomaterials 225, 119518 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Morishita M, Takahashi Y, Nishikawa M, Ariizumi R & Takakura Y Enhanced Class i Tumor Antigen Presentation via Cytosolic Delivery of Exosomal Cargos by Tumor-Cell-Derived Exosomes Displaying a pH-Sensitive Fusogenic Peptide. Mol. Pharm. 14, 4079–4086 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Zuo B et al. Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat. Commun. 11, 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Al-Samadi A et al. Crosstalk between tongue carcinoma cells, extracellular vesicles, and immune cells in in vitro and in vivo models. Oncotarget 8, 60123–60134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morishita M, Takahashi Y, Matsumoto A, Nishikawa M & Takakura Y Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 111, 55–65 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Huang F et al. TGF-β1-silenced leukemia cell-derived exosomes target dendritic cells to induce potent anti-leukemic immunity in a mouse model. Cancer Immunol. Immunother. 66, 1321–1331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo D et al. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology 154, 132–143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gobbo J et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes with a HSP70 Peptide Aptamer. J. Natl. Cancer Inst. 108, 1–11 (2016). [DOI] [PubMed] [Google Scholar]
- 144.Bell BM, Kirk ID, Hiltbrunner S, Gabrielsson S & Bultema JJ Designer exosomes as next-generation cancer immunotherapy. Nanomedicine: Nanotechnology, Biology, and Medicine vol. 12 163–169 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Pitt JM et al. Dendritic cell-derived exosomes for cancer therapy. Journal of Clinical Investigation vol. 126 1224–1232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tran TH, Mattheolabakis G, Aldawsari H & Amiji M Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 160, 46–58 (2015). [DOI] [PubMed] [Google Scholar]
- 147.Besse B et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5, e1071008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen S et al. Poly(I:C) enhanced anti-cervical cancer immunities induced by dendritic cells-derived exosomes. Int. J. Biol. Macromol. 113, 1182–1187 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Lu Z et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J. Hepatol. 67, 739–748 (2017). [DOI] [PubMed] [Google Scholar]
- 150.Matsumoto A, Asuka M, Takahashi Y & Takakura Y Antitumor immunity by small extracellular vesicles collected from activated dendritic cells through effective induction of cellular and humoral immune responses. Biomaterials 252, 120112 (2020). [DOI] [PubMed] [Google Scholar]
- 151.Zhu L et al. Novel alternatives to extracellular vesicle-based immunotherapy–exosome mimetics derived from natural killer cells. Artif. Cells, Nanomedicine Biotechnol. 46, S166–S179 (2018). [DOI] [PubMed] [Google Scholar]
- 152.Zhu L et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics 7, 2732–2745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu L et al. Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials 190–191, 38–50 (2019). [DOI] [PubMed] [Google Scholar]
- 154.Lee H, Park H, Noh GJ & Lee ES pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 202, 323–333 (2018). [DOI] [PubMed] [Google Scholar]
- 155.Prediction of Immunotherapeutic Effect of Advanced Non-small Cell Lung Cancer - NCT04427475.
- 156.Clinical Research for the Consistency Analysis of PD-L1 in Cancer Tissue and Plasma Exosome - NCT02890849. https://clinicaltrials.gov/show/NCT02890849.
- 157.Clinical Research for the Consistency Analysis of PD-L1 in Lung Cancer Tissue and Plasma Exosome Before and After Radiotherapy - NCT02869685. https://clinicaltrials.gov/show/NCT02869685.
- 158.Anaplastic Thyroid Cancer and Follicular Thyroid Cancer-derived Exosomal Analysis Via Treatment of Lovastatin and Vildagliptin and Pilot Prognostic Study Via Urine Exosomal Biological Markers in Thyroid Cancer Patients - NCT02862470. https://clinicaltrials.gov/show/NCT02862470.
- 159.Study of Molecular Mechanisms Implicated in the Pathogenesis of Melanoma. Role of Exosomes - NCT02310451. https://clinicaltrials.gov/show/NCT02310451%0A.
- 160.Study of Exosomes in Monitoring Patients With Sarcoma (EXOSARC) - NCT03800121. https://clinicaltrials.gov/show/NCT03800121%0A.
- 161.Exosomes and Immunotherapy in Non-Hodgkin B-cell Lymphomas - NCT03985696. https://clinicaltrials.gov/show/NCT03985696.
- 162.Hemopurifier Plus Pembrolizumab in Head and Neck Cancer - NCT04453046. https://clinicaltrials.gov/show/NCT04453046.
- 163.Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue - NCT01294072. https://clinicaltrials.gov/show/NCT01294072.
- 164.Pilot Immunotherapy Trial for Recurrent Malignant Gliomas - NCT01550523. https://clinicaltrials.gov/show/NCT01550523%0A.
- 165.Hackett DW Glioblastoma Vaccine Shows Positive Phase Ib Study Results. Precision Vaccinations https://www.precisionvaccinations.com/imvax-novel-igv-001-autologous-tumor-cell-vaccine-delivering-multi-pronged-response-against-tumor (2019).
- 166.Andrews DW et al. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J. Clin. Oncol. 19, 2189–2200 (2001). [DOI] [PubMed] [Google Scholar]
- 167.Antisense102: Pilot Immunotherapy for Newly Diagnosed Malignant Glioma. https://clinicaltrials.gov/ct2/show/NCT02507583.
- 168.Garofalo M et al. Systemic administration and targeted delivery of immunogenic oncolytic adenovirus encapsulated in extracellular vesicles for cancer therapies. Viruses 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kanuma T et al. CD63-Mediated Antigen Delivery into Extracellular Vesicles via DNA Vaccination Results in Robust CD8 + T Cell Responses. J. Immunol. 198, 4707–4715 (2017). [DOI] [PubMed] [Google Scholar]
- 170.Hong Y et al. Degradation of tumour stromal hyaluronan by small extracellular vesicle-PH20 stimulates CD103+ dendritic cells and in combination with PD-L1 blockade boosts anti-tumour immunity. J. Extracell. Vesicles 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang X et al. PD-1 Blockade Cellular Vesicles for Cancer Immunotherapy. Adv. Mater. 30, 1707112 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Gao C, Wu Z, Lin Z, Lin X & He Q Polymeric capsule-cushioned leukocyte cell membrane vesicles as a biomimetic delivery platform. Nanoscale 8, 3548–3554 (2016). [DOI] [PubMed] [Google Scholar]
- 173.Cheng Q, Shi X & Zhang Y Reprogramming exosomes for immunotherapy. in Methods in Molecular Biology vol. 2097 197–209 (Humana Press Inc., 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheng Q et al. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 140, 16413–16417 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kang Y-T, Hadlock T, Jolly S & Nagrath S Extracellular vesicles on demand (EVOD) chip for screening and quantification of cancer-associated extracellular vesicles. Biosens. Bioelectron. 168, 112535 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Meng X et al. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Adv. Exp. Med. Biol. 924, 3–8 (2016). [DOI] [PubMed] [Google Scholar]
- 177.Maji S et al. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol. Cancer Res. 15, 93–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hong CS, Muller L, Whiteside TL & Boyiadzis M Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front. Immunol. 5, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Muller L et al. Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ciravolo V et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J. Cell. Physiol. 227, 658–667 (2012). [DOI] [PubMed] [Google Scholar]
- 181.Battke C et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol. Immunother. 60, 639–648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cordonnier M et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J. Extracell. Vesicles 9, 1710899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]