Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new disease that has led to a worldwide pandemic, resulting in millions of deaths and a high economic burden. Here, we analyze the current status of preventive vaccines authorized by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA). Published clinical trials have shown the effectiveness of mRNA (BNT162b2 and Spikevax), adenovirus vector-based (Ad26.COV2.S and ChAdOx1 nCoV-19), and recombinant protein S (NVX-CoV2373) vaccines to be between 52.9% and 100%. The most-frequent adverse effects include local pain, fatigue, headache, or chills. Serious events are associated with Ad26.COV2.S and ChAdOx1 nCoV-19 vaccines.
Introduction
SARS-CoV-2 was described in late 2019 in Wuhan, China. Since then, a worldwide pandemic has emerged, resulting in millions of deaths and economic damage globally.1 The etiological agent is a new coronavirus of the family Coronaviridae, which includes the subfamilies Letovirinae and Orthocoronavirinae. Additionally, Orthocoronavirinae includes the genera Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. Whereas Alphacoronavirus and Betacoronavirus exclusively infect mammals, causing respiratory infections, most gammacoronaviruses and deltacoronaviruses usually infect birds, although some also infect mammals. The betacoronaviruses most pathogenic to humans are SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV).2 The new SARS-CoV-2 is a nonsegmented positive-sense, single-stranded (ss)RNA virus, specifically of the Betacoronavirus genus.3
The general structure of a coronavirus comprises four proteins: the nucleocapsid (N) protein, which forms the helical capsid that includes the viral genome; the spike (S) protein, which forms the lipid envelope that surrounds the virus in the form of a crown; the envelope (E) protein, and the membrane (M) protein. In particular, the S protein of SARS-CoV-2 has a high affinity for angiotensin-converting enzyme-2 (ACE-2), which binds the virus to the host cell, mediated by the transmembrane serine protease 2.4
The usual clinical features of the disease are highly varied and nonspecific, ranging from mild to moderate symptoms, including fever, cough, fatigue, sputum production, dyspnea, sore throat, diarrhea, and headache. It can progress to severe pneumonia in older patients and those with underlying conditions (e.g. hypertension, chronic obstructive pulmonary disease, diabetes, cardiovascular disease, etc.). The overproduction of proinflammatory cytokines in response to SARS-CoV-2, known as a cytokine storm, increases the risk of vascular permeability, organ failure, and, consequently, septic shock and metabolic acidosis leading to death.5
To reduce infections and mitigate the pandemic, population vaccination has been established as a safe and effective strategy to provide protection and reduce the spread of the disease. In this regard, more than 300 candidate vaccines against COVID-19 have been submitted at different stages of development and more than 100 candidates have reached clinical trials thus far.6
COVID-19 vaccine platforms
The platforms on which vaccine development has been based are: (i) a new generation of nucleic acid mRNA-based vaccines, created with the use of a ssRNA molecule as an intermediate step between the translation of protein-coding DNA and protein production by ribosomes in the cytoplasm7; (ii) viral vector vaccines, which involve the use of a recombinant adeno or pox virus, often attenuated to reduce its pathogenicity, carrying the genetic code of the target virus antigen to stimulate an immune response, and created using recombinant DNA techniques8; (iii) inactivated virus vaccines, which use virus fragments or whole viruses that stimulate immunogenicity, but cannot replicate because of destruction of the genetic material by physical or chemical processes9; and (iv) subunit vaccines, in which viral proteins are injected into the host, without introducing viable particles of the pathogen, and, therefore, do not have the full antigenic complexity of the virus.10
On 1 March, 2022, the EMA licensed five vaccines to prevent COVID-19: Comirnaty (developed by BioNTech and Pfizer); Spikevax, previously called COVID-19 Vaccine Moderna (developed by Moderna Biotech); COVID-19 Vaccine Janssen (developed by Janssen-Cilag International), Vaxzevria, previously called COVID-19 Vaccine AstraZeneca (developed by AstraZeneca); and Nuvaxovid (developed by Novavax).11 In addition, on 4 February, 2020 the FDA granted emergency use authorization (EUA) for Comirnaty, Spikevax, and Janssen vaccines.12 Information about the efficacy, dosing, mechanism of action, and safety of vaccines authorized by these agencies requires continuous updating, which is necessary for public health decision-making (Table 1 ).
Table 1.
Summary of the characteristics, efficacy, and adverse effects of SARS-CoV-2 vaccines from the studies included in the review.
| Vaccine characteristics | Administration | Efficacy | Adverse effects | Refs |
|---|---|---|---|---|
| Comirnaty (BNT162b2) | ||||
| Lipid NP vaccine comprising packaged strands of modified mRNA | Dose regimen: two doses of 0.3 ml (30 μg mRNA), with a 21-day interval between doses A booster dose (third dose) can also be administered at least 6 months after second dose |
91.3–100 % efficacy in populations 12 years and older Increased levels of IgG antibodies and virus neutralization Third dose (15 μg) increased anti-S IgG secretion and virus neutralization Also effective against B.1.1.7, B.1.351, and B.1.617.2 variants |
Events at local level: pain at injection site, erythema, urticaria, or swelling Mild-to-moderate systemic reactions: fatigue, headache, chills, muscle pain Severe adverse effects: lymphadenopathy, severe chest pain, hypertension, allergy, paroxysmal ventricular arrhythmia, shoulder injury, paresthesia in right leg |
[13], [14], [16], [17], [19], [21], [22], [23], [24], [25], [26], [27], [28], [29], [31], [32], [33], [34] |
| Spikevax (mRNA-1273) | ||||
| Nucleoside-modified mRNA vaccine | Dose regimen in persons 12 years and older: two doses of 100 μg (0.5 ml) In children 6–11-year old: two doses of 50 μg (0.25 ml) each |
93.2–94.1 % efficacy After second dose, increase in IgG anti-RBD and anti-S antibodies observed Neutralized wild-type, B.1.1.7, B.1.351, P.1, and B.1.617.2 variants |
Mild adverse events at local level: pain at puncture site, erythema, induration or swelling, itching, and lymphadenopathy Most-frequent mild-to-moderate systemic reactions: headache, fatigue, myalgia, arthralgia. Serious adverse effects (cardiorespiratory arrest, hypersensitivity reaction, thrombotic events, pericarditis, death, and Bell’s palsy) could not be attributed to vaccine because of limited data |
[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48] |
| Janssen (Ad26.COV2.S) | ||||
| Monovalent vaccine comprising adenovirus vector without replicative capacity type 26 human adenovirus | Dose regimen: in persons aged 18 years or older, single dose of 0.5 ml (5 × 1010 viral particles) | 52.9–66.1 % efficacy at 28 days after administration To prevent severe disease, efficacy was 85.4 % at 28 days post-vaccination Effective against B.1.1.7, B.1.617.2, P.1, C.37, and B.1.621 variants Administration of booster dose in patients with blood cancers generated immune response against COVID-19 Number of anti-S IgG antibodies similar in patients aged 18–55 and 65 years and older |
Most-frequent local reaction: pain at site injection Systemic reactions: headache, fatigue, myalgia, nausea, fever Severe reactions: lymphadenopathy, Guillain–Barré syndrome and thrombosis combined with thrombocytopenia or immune thrombocytopenia, anaphylaxis and capillary leak syndrome |
[53], [54], [55], [57], [58], [59], [60] |
| Vaxzevria (ChAdOx1 nCoV-19) | ||||
| Replication-deficient recombinant adenovirus vector-based vaccine | Dose regimen: two doses of 0.5 ml intramuscularly | 74 % efficacy 15 days after second dose Hospitalization and death because of COVID-19 reduced after vaccination Effective against wild-type and B.1 0.35, B.1.1.7, B.1.351, P.1, and P.2 variants |
Most-frequent (mild to moderate) local and systemic effects: injection site pain, headache, decreased appetite, hyperthermia, fatigue, muscle pain Severe adverse events: vaccine-induced immune thrombotic thrombocytopenia, hemorrhage, anaphylaxis, Guillain–Barré syndrome, late inflammatory skin reactions, bleeding episodes, death |
[61], [62], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88] |
| Nuvaxovid (NVX-CoV2373) | ||||
| Recombinant, adjuvanted, full-length, purified protein S subunit vaccine, stabilized in its prefusion conformation | Dose regimen: two doses of 0.5 ml with interdose interval of 3–4 weeks | 90.4–100 % efficacy against moderate-to-severe disease Synergic action of NVX-CoV2373 and influenza virus vaccine showed an efficacy of 89.7 % 86.3 % efficacy against B.1.1.7 variant |
Local and systemic adverse effects: headache, nausea and vomiting, myalgia, arthralgia, injection site tenderness, injection site pain, fatigue, malaise, redness and/or swelling at injection site, fever, chills, pain in extremities, lymphadenopathy, hypertension, rash, erythema, pruritus, urticaria | [89], [90], [93], [94], [95], [96], [97], [98], [99] |
In this review, we analyze the current status of vaccines licensed for EUA by the EMA and the FDA to prevent COVID-19 in terms of their mechanism of action, dosage, efficacy, and adverse effects.
Comirnaty
Composition and mechanism of action
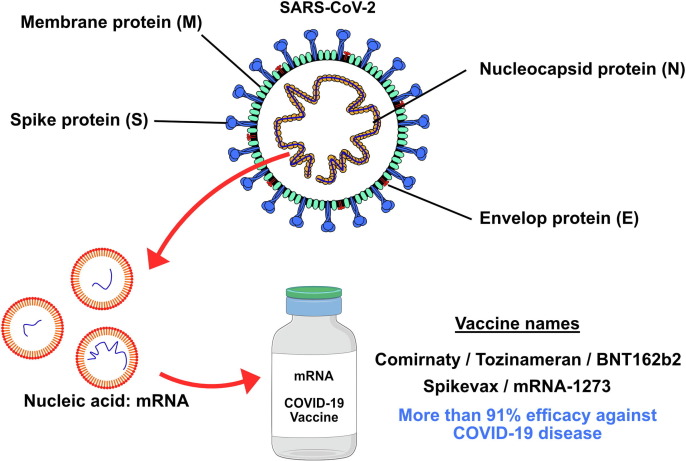
Comirnaty, also known as Tozinameran or BNT162b2, is a vaccine the formulation of which is based on a neutrally charged lipid nanoparticle (NP) with packaged strands of modified mRNA, which is responsible for encoding the full-length transmembrane glycoprotein S of SARS-CoV-2 (Fig. 1 ).[13], [14]
Figure 1.
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) spike (S) mRNA is chemically synthesized and enclosed in lipid nanoparticles.
The NP formulation preserves the RNA, preventing its early degradation and allowing its entry into host cells. Once the genetic material is introduced into the cytoplasm of the cells, it is translated into ribosomes, which synthesize the SARS-CoV-2 S protein. Subsequently, these proteins reach the cell membrane and can mature into the MHC-2 complex of antigen-presenting cells (APCs), such as B cells, macrophages, and dendritic cells (DCs). In this way, these active cells attract T helper (Th) cells, binding their T cell receptor (TCR) to the S protein and triggering the synthesis of interleukins (IL), such as IL-2, IL-4, and IL5. These ILs cause B cells to differentiate into plasma cells, which start to produce numerous antibodies against S proteins. Furthermore, ILs also stimulate Th cells to proliferate into memory T cells, acquiring the ability to neutralize or destroy the virus.15
Dosage and administration
The dose regimen in adults and adolescents ≥ 12-years old comprises two doses of 0.3 ml (30 μg mRNA), with a 21-day interval between doses. A booster dose (third dose) can also be administered at least 6 months after the second dose for persons 18 years of age and older. Moreover, a third dose (0.3 ml) is authorized for persons at least 12-years old who have undergone solid organ transplantation, or who have been diagnosed with immunocompromising conditions at least 28 days after the second dose. In children aged 5–11 years, the vaccination schedule is two 0.2 ml (10 μg) doses 21 days apart. An additional dose can be given to persons 5 years of age or older with a severely weakened immune system at least 28 days after their second dose. Administration of the various doses should be intramuscular, preferably in the deltoid muscle.[16], [17]
Efficacy
In humans, different clinical trials analyzed the effectiveness of various concentrations of vaccine administration (1, 10, 20, and 30 μg) in healthy adults aged 18 years or older who had not been infected with SARS-CoV-2. After administration of two doses at an interval of 21 days, a significant dose-dependent increase in anti-S immunoglobulin (Ig)-G was found, which was enhanced after the second dose. These values were higher in participants aged 65 to 85 years. Likewise, viral neutralization values were also dose-dependently elevated, especially after the second dose of the vaccine and in populations aged 16–55 years.[18], [19], [20] Phase III multicenter clinical trials established an efficacy of 91.3–100 % in the study populations aged 12 years and older 7 days after receiving two doses (30 μg each) of the BNT162b2 vaccine, and of 75 % after receiving the first dose.[21], [22], [23] Recently, the effectiveness of immunization with a third dose of BNT162b2 was evaluated in individuals older than 30 years with no history of SARS-CoV-2 infection. Regardless of the type of vaccine previously applied, 15 μg (half dose) of BTT162b2 increased anti-S IgG as well as virus neutralization compared with the placebo group 28 days after the third dose.24
In relation to the different variants, the BNT162b2 vaccine is effective against the alpha (B.1.1.7), beta (B.1.351), and delta (B.1.617.2) variants.[22], [24], [25]
Different studies of the immunogenicity of the BNT162b2 vaccine in various pathological conditions, such as oncology, hematological problems, autoimmune disorders, and multiple sclerosis, have shown that IgG antibody and virus neutralization titers were higher after vaccine administration. However, the antibody response was significantly lower compared with healthy individuals.[26], [27], [28], [29], [30], [31], [32], [33]
Adverse effects
Reactogenicity in healthy individuals has generated local and systemic effects, most of them mild or moderate, ceasing over the first 2 days after vaccination, and not being present beyond day 14 after the second dose. Events at the local level reported with a higher frequency include pain at the injection site, erythema, urticaria, or swelling.[19], [28], [22], [23] Mild-to-moderate systemic reactions observed have been fatigue, headache, chills, and muscle pain. Others less frequent were fever, diarrhea, abdominal pain, joint pain, loss of appetite, loss of smell, hyperhidrosis, and vomiting; the latter four being unusual.[19], [28], [22], [23] Severe vaccine-associated adverse effects, such as lymphadenopathy, severe chest pain, hypertension, allergy, paroxysmal ventricular arrhythmia, shoulder injury, and paresthesia in the right leg, have been very rare.[21], [22], [34]
Local and systemic adverse events found in patients with cancer, and those with hematological problems, autoimmune disorders, and multiple sclerosis have also been common in healthy patients. By contrast, severe effects, such as impaired liver function, mild neutropenia, mild thrombocytopenia, chronic graft-vs-host disease of the skin, and signs of obliterative bronchiolitis, were more frequent in these participants than in healthy individuals.[27], [31], [35]
Spikevax
Composition and mechanism of action
Spikevax, or mRNA-1273, is a nucleoside-modified mRNA vaccine that encodes a stabilized version of the SARS-CoV-2 S protein, enabling expression of the S antigen in host cells and eliciting an immune response through both T and B cell responses and specific antibody responses against the S antigen, which protects against COVID-19 (Fig. 1).[36], [37]
Dosage and administration
The recommended dosing in persons 12 years of age and older is two doses of 100 μg (0.5 ml). Similarly, in children 6–11-years old, the recommended guideline is two doses of 50 μg (0.25 ml) each. Both regimens should be administered within 28 days between doses and should be administered intramuscularly in the deltoid muscle. In severely immunocompromised patients, a booster dose of 0.5 ml (100 μg) can be administered at least 28 days after the second dose to individuals 12 years of age or older or 0.25 ml (50 μg) to children 6–11 years of age. Similarly, in adults 18 years of age or older, a booster dose of 0.25 ml (50 μg) is indicated at least 3 months after completion of the primary series, comprising another mRNA vaccine or adenoviral vector vaccine.[38], [39]
Efficacy
The efficacy of the vaccine at different doses was tested in Phase I and II clinical trials, in which immunogenicity was observed in healthy patients aged 18 years or older, with no history of COVID-19 infection, and after intramuscular administration of two doses of Spikevax vaccine at concentrations of 50, 100, and 250 μg, with 28 days between doses. A dose-dependent immune response was observed with greater increases in IgG anti-receptor binding domain (RBD) and anti-S antibodies, as well as virus neutralization values, after the second dose.[37], [40], [41], [42] Nonetheless, participants older than 71 years had higher antibody levels.40 In addition, other multicenter Phase III clinical trials reported that efficacies for the prevention of COVID-19 disease after the administration of two doses of 100 μg in healthy individuals aged 18 years or older of 93.2–94.1 %. The results found severe disease prevention levels of 98.2–100 %.[43], [44] Other authors found that serum from patients who received two or three doses of Spikevax neutralized the wild-type (B.1.D614G), B.1.1.7, B.1.351, Gamma (P.1), and B.1.617.2 variants of SARS-CoV-2.[45], [46], [47] In a healthy adolescent population aged 12–17 years, the immune response from administration of two doses of 100 μg each showed an efficacy of 93.3 % 14 days after the second injection.48 Similarly, Stuart et al. 49 found a greater response in participants older than 49 years who received an initial dose of Spikevax and a second heterologous dose of ChAdOx1 or BNT162b2; other than in anti-S IgG antibody values, where there was no difference between the group receiving the homologous (ChAd/ChAd) and heterologous (ChAd/Spikevax) vaccines. Moncunill et al. 50 observed an increase in anti-S and anti-RBD IgA and IgG and neutralization values after the second dose of Spikevax compared with those obtained after the two-dose schedule of BNT162b2 in healthcare professionals with a history of previous SARS-CoV-2 infection. However, no IgM variations were found between the two vaccines.
In addition, in individuals with solid tumors who received chemotherapy, immunotherapy, or both, the administration of two doses of 100 μg of Spikevax showed no difference in efficacy compared with the immunogenicity generated in healthy individuals.51 By contrast, patients with lymphoid malignancies showed lower IgG anti-S antibodies levels compared with healthy patients.32
Adverse effects
Most of the adverse effects of vaccination for all age groups studied were mild to moderate, remitting within the first 10 days after vaccination, and occurred more frequently in those under 65 years of age.43 The most-frequent local reactogenicity associated with vaccine administration was pain at the puncture site. Less-frequently reported symptoms were erythema, induration or swelling, itching, and lymphadenopathy.[37], [40], [41], [43], [44], [48], [52] Systemic effects reported were headache, fatigue, myalgia, and arthralgia in the highest proportion; chills, fever, and nausea or vomiting in the lowest proportion; and dizziness, decreased appetite, muscle spasms, decreased sleep quality, and brain fog in rare cases.[37], [40], [41], [43], [44], [48], [52] Serious events, such as cardiorespiratory arrest, hypersensitivity reaction, thrombotic events, pericarditis, death, or Bell’s palsy, could not be attributed to the vaccine because of limited data.[41], [43], [44]
Ad26.COV2.S: Janssen®
Composition and mechanism of action
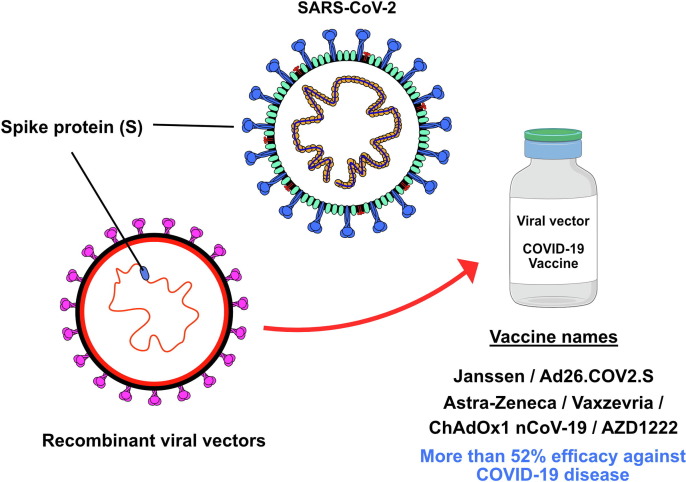
Ad26.COV2.S is a monovalent vaccine comprising an adenovirus vector without replicative capacity type 26 human adenovirus (Ad26). Each 0.5-ml dose contains at least 8.92 log10 infectious units produced by the PER.C6 TetR cell line through recombinant DNA. Its viral vector-based formulation allows delivery of the mRNA to host cells and transient expression of the S protein, stimulating the production of both neutralizing antibodies and other anti-S-specific functional antibodies, contributing to protection against COVID-19 (Fig. 2 ).53
Figure 2.
Viral vector vaccines are created using recombinant DNA techniques and involve the use of an attenuated adenovirus that carries the genetic code for the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) spike (S) protein.
Dosage and administration
Ad26.COV2.S is indicated for use in persons aged 18 years or older. There are currently no data on the safety and efficacy of this drug in the non-adult population. The vaccine is administered as a single dose of 0.5 ml (5 × 1010 viral particles) intramuscularly. A second booster dose of 0.5 ml has been considered, at least 2 months after the first injection. This vaccine could also be used as a heterologous booster in patients who have been vaccinated with other mRNA vaccines, with the same time interval between the first and second dose as indicated above.[54], [55]
Efficacy
The clinical efficacy of this vaccine was initially evaluated in a Phase I clinical trial in 18–55-year-old participants with no history of COVID-19 infection. It was shown that immunogenicity (production of anti-S and anti-RBD IgG antibodies and virus neutralization) was superior after receiving one or two doses of Ad26.COV2.S vaccine versus placebo. By contrast, there was no difference in the immune response produced between individuals who received one or two doses, or between those who received a high (1 × 1011 viral particles of Ad26.COV2.S) or low (5 × 1010 viral particles of Ad26.COV2.S) dose.56
Subsequently, in a Phase I/II clinical trial by Sadoff et al. 57 it was observed that the number of anti-S IgG antibodies was similar in a cohort of patients aged 18–55 and 65 years and older after the use of the high or low dose. In those participants aged 18–55 years, the immune response was higher than in the 65 + age group. In addition, a second dose 56 days after the first dose was found to increase immunogenicity after 14 days.
Other multicenter Phase III clinical trials studied the efficacy of Ad26.COV2.S after one and two doses. Administration of one dose of Ad26.COV2.S (5 × 1010 viral particles) had an efficacy of 56.3–66.9 % 14 days, and of 52.9–66.1 % 28 days after administration. Furthermore, its ability to prevent severe COVID-19 disease was 76.7 % at 14 days, and 85.4 % at 28 days post-vaccination. In addition to this efficacy against the wild-type strain, administration of Ad26.COV2.S showed greater than 69 % effectiveness against the B.1.1.7, B.1.617.2, P.1, C.37 (lambda), and B.1.621 (mu) variants.[58], [59]
Notably, administration of a heterologous booster dose of Ad26.COV2.S in patients with blood cancers, who showed no antibody response after a double-dose of BNT162b2, generated a serologically positive response in 31 % of participants at 28 days. By contrast, although this booster dose increased anti-S antibody levels, the immune response was lower in patients with chronic lymphocytic leukemia or lymphoma.60
Adverse effects
Different clinical trials described the most-frequent local and systemic reactions after the first and second doses of Ad26.COV2.S. These reactions were dose dependent, generally mild to moderate, and more frequent in patients aged 65 years and older. The most-frequent local reaction was pain. In addition, the most common systemic reactions were headache, fatigue, myalgia, nausea, and fever. Other less-frequent local and systemic reactions were reported, including tremors, dizziness, paresthesia, sneezing, oropharyngeal pain, diarrhea, rash, hyperhidrosis, asthenia, malaise, lymphadenopathy, hypersensitivity, urticaria, hypoesthesia, tinnitus, venous thromboembolism, vomiting, Guillain–Barré syndrome and thrombosis combined with thrombocytopenia or immune thrombocytopenia, anaphylaxis, and capillary leak syndrome.[58], [59], [60]
Vaxzevria
Composition and mechanism of action
The vaccine developed by Oxford University in collaboration with AstraZeneca, known as Vaxzevria, ChAdOx1 nCoV-19 or AZD1222, is a replication-deficient recombinant adenovirus vector-based vaccine that induces the expression of SARS-CoV-2 protein S in host cells.[61], [62] Vaccinated individuals generate antibodies against protein S, including those related to RBDs, which have numerous neutralizing epitopes or neutralization antigens (Fig. 2).[63], [64]
Dosage and administration
The vaccine is indicated for persons aged 18 years and older, and the schedule requires two doses of 0.5 ml each, with an interval of 4–12 weeks between doses, preferably administered intramuscularly, in the deltoid muscle.[65], [66]
Efficacy
Preclinical trials in which single or double-dose ChAdOx1 nCoV-19 was administered reported evidence of prevention of SARS-CoV-2 infection in rhesus macaques.67 Moreover, other Phase I/II clinical trials showed the immunogenicity of ChAdOx1 nCoV-19 in healthy individuals aged 18–55 years with no history of SARS-CoV-2 infection, reporting increased levels of anti-S IgG antibody and neutralization against wild-type virus after both one and two doses compared with a control group (Meningococcal ACWY vaccine). Nevertheless, the second dose induced stronger antibody responses.[68], [69], [70]
A multicenter Phase II/III clinical trial showed that administration of a second dose to different age groups produced a positive antibody response to SARS-CoV-2.71 In line with this, a Phase III clinical trial showed how the ChAdOx1 nCoV-19 vaccine is a safe and effective vaccine for the prevention of symptomatic COVID-19. In this study, 74 % efficacy was observed 15 days after the second dose of ChAdOx1 nCoV-19.72 These different clinical trials show that, after the start of vaccination programs in different countries with ChAdOx1 nCoV-19, there was a reduction in hospitalization and death resulting from COVID-19, as well as a decrease in the probability of symptomatic disease and transmission of the virus.73
It has also been shown that the ChAdOx1 nCoV-19 vaccine is effective against variants B.1.1.7, B.1.351, P.1, and P.2 (Zeta).[70], [74], [75]
Adverse effects
The most-frequently reported mild-to-moderate local and systemic effects are injection site pain, headache, decreased appetite, hyperthermia, fatigue, and muscle pain, all appearing within the first 15 days after vaccine administration.[69], [72], [76] Conversely, although the association is unclear, certain serious events have been associated with the administration of the ChAdOx1 nCoV-19 vaccine, such as vaccine-induced immune thrombotic thrombocytopenia, hemorrhage, anaphylaxis,[77], [78], [79], [80], [81] Guillain–Barré syndrome,[82], [83], [84], [85], [86] late inflammatory skin reactions,87 bleeding episodes, and death.88 These severe symptoms caused the temporary suspension of the administration of ChAdOx1 nCoV-19 in different European countries in March 2021.
Nuvaxovid
Composition and mechanism of action
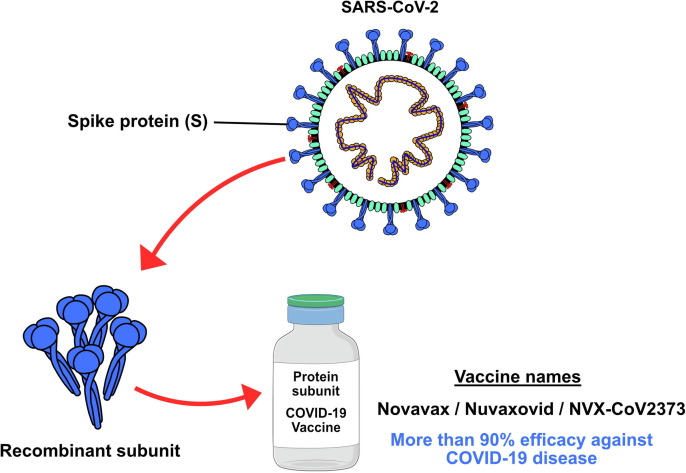
Nuvaxovid (NVX-CoV2373) is a recombinant, adjuvanted, full-length, purified protein S subunit vaccine, stabilized in its prefusion conformation. This protein is responsible for mediating binding to the ACE-2 receptor on host cells. Each dose contains 42.5 μg of A-fraction and 7.5 μg of C-fraction of Matrix-M adjuvant based on a saponin extracted from the bark of the Chilean Quillaja saponaria tree (Fig. 3 ).[89], [90] The components of this vaccine demonstrated clinical efficacy against SARS-CoV-2 by inducing an immune response with high anti-S IgG antibodies that prevent binding of the ACE-2 receptor and achieve protection against the virus.[91], [92], [93] Similarly, a response mediated by CD4+ and CD8+ T cells with a dominant Th1 phenotype and B cells is triggered. Thus, when the virus re-enters the host, the immune system recognizes the S protein, triggering the S cell-mediated immune response.
Figure 3.
Subunit vaccines in which all or part of the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) spike (S) protein is expressed in insect cells without introducing viable particles of the pathogen.
Dosage and administration
The recommended regimen is two doses of 0.5 ml (5 μg of recombinant S protein with 50 μg of Matrix-M adjuvant) administered intramuscularly into the deltoid muscle, with an interdose interval of 3–4 weeks.[94], [95]
Efficacy
The efficacy of this vaccine is being evaluated in several Phase II and III clinical trials. A multicenter, randomized, Phase II clinical trial was conducted in South Africa in subjects aged 18–84 years old who were HIV-positive or stable seropositive and who received two doses of NVX-CoV2373 or placebo 21 days apart. Vaccine efficacy was 49.4 % against mild or moderate COVID-19 disease among all participants (HIV seropositive and seronegative), and 60.1 % against disease prevention in seronegative participants only. In addition, disease efficacy attributed to the B.1.351 variant was 43.0 % for the total population studied, and 51.0 % for the HIV-seronegative population.96
The efficacy of the vaccine in healthy subjects with no history of infection was reported in a multicenter randomized Phase III clinical trial, in which participants received two doses of NVX-CoV2373 separated by 21 days or two placebo injections. The results showed that administration of NVX-CoV2373 prevented mild-to-moderate COVID-19 disease by 90.4 %, with 100 % efficacy against moderate to severe disease.97
A Phase III clinical trial in the UK in individuals aged 18–84 years who received two doses of 5 μg NVX-CoV2373 and a concomitant dose of influenza virus vaccine reported an efficacy of 89.7 %, describing ten cases of virologically confirmed mild, moderate, or severe symptomatic COVID-19 with onset at least 7 days after the second dose in vaccinated patients, and 96 cases in placebo group patients. In terms of the effects described for the different age groups, efficacy was 88.9 % among participants aged 65 years or older, whereas efficacy in the 18–64 age group was 89.8 %.98
According to the virus variant, results showed 86.3 % efficacy against the B.1.1.7 variant. No cases of severe COVID-19 were reported in participants who received NVX-CoV2373 compared with four cases of severe COVID-19 reported in participants who received a placebo.98 Remarkably, among individuals who received concomitant and influenza virus vaccination, no alterations in influenza virus immunization were identified, although a 30 % reduction in antibody responses to NVX-CoV2373 was observed. However, co-administration of both vaccines resulted in increased reactivity compared with a single administration of NVX-CoV2373.99
Adverse effects
Local and systemic adverse effects reported after administration of NVX-CoV2373 vaccine were headache, nausea and vomiting, myalgia, arthralgia, injection site tenderness, injection site pain, fatigue, malaise, redness and/or swelling at the injection site, fever, chills, pain in the extremities, lymphadenopathy, hypertension, rash, erythema, fever, chills, pain in the extremities, lymphadenopathy, hypertension, rash, fatigue, malaise, redness and/or swelling at the injection site, fever, chills, pain in the extremities, lymphadenopathy, hypertension, rash, erythema, pruritus, or urticaria.[89], [93], [96], [97], [99]
Comparative summary by platform
Vaccines the formulation of which is based on modified mRNA encoding the S glycoprotein of SARS-CoV-2, such as BNT162b2 (Comirnaty) and Spikevax (Moderna,) have shown, in different clinical trials, an efficacy of 91.3–100 % (BNT162b2) or 93.2–100 % (Spikevax) to prevent COVID-19 disease in healthy adults aged 18 years or older who had not been infected with SARS-CoV-2. Adenovirus vector-based vaccines, such as Ad26.COV2.S (Janssen) or ChAdOx1 nCoV-19 (Astra-Zeneca), are indicated for use in people aged 18 years or older, and revealed an efficacy of 52.9–66.1 % to prevent SARS-CoV-2 infection and 85.4 % to prevent severe disease at 28 days after administration of Ad26.COV2.S, and 74 % efficacy to prevent COVID-19 disease 15 days after a second dose of ChAdOx1 nCoV-19. NVX-CoV2373 vaccine (Novavax) is a recombinant protein S subunit vaccine indicated for 18 years of age and older. The reported efficacy to prevent mild-to-moderate disease after two doses was 90.4–100 %. A comparative analysis of the different efficacy ranges reported in the literature revealed that mRNA vaccines (BNT162b2 and Spikevax) have a higher percentage of prevention against COVID-19 disease, followed by recombinant protein S subunit vaccines (NVX-CoV2373), and adenovirus vector-based vaccines (Ad26.COV2.S and ChAdOx1 nCoV-19). The vaccine with the lowest preventive effect against SARS-CoV-2 is the ChAdOx1 nCoV-19 vaccine.
Local and systemic adverse effects of the vaccines tested, which have also been equally reported with all the vaccines, include injection site tenderness, pain at the injection site, fatigue, headache, chills, myalgia, or fever. Additionally, certain serious events have been associated with the administration of the Ad26.COV2.S and ChAdOx1 nCoV-19 vaccines, such as vaccine-induced immune thrombotic thrombocytopenia, hemorrhage, Guillain-Barré syndrome, and death.
Concluding remarks
The SARS-CoV-2 pandemic has caused millions of deaths worldwide, resulting from a sanitary and economic burden that has required the development of vaccines in a limited timeframe. Vaccines approved by the EMA and FDA have been developed on different vaccine platforms, such as mRNA-based, viral vector-based, inactivated virus-based, or protein subunit-based vaccines. Based on available evidence, these agency-approved vaccine modalities show a high degree of efficacy (52.9–100 %) and safety, and offer a new paradigm for a future pandemic. However, most of the clinical trials have not been completed and further studies are needed to ensure long-term efficacy and safety.
CRediT authorship contribution statement
Manuel Rueda-Fernández: Conceptualization, Writing – original draft, Writing – review & editing. Lucía Melguizo-Rodríguez: Writing – original draft. Víctor J. Costela-Ruiz: Writing – original draft. Anabel González-Acedo: Writing – original draft. Javier Ramos-Torrecillas: Conceptualization, Writing – review & editing, Supervision. Rebeca Illescas-Montes: Conceptualization, Writing – original draft, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the research group BIO277 (Junta de Andalucía) and the Department of Nursing (University of Granada). Data sets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Akande O.W., Akande T.M. COVID-19 pandemic: a global health burden. Niger Postgrad Med J. 2020;27(3):147–155. doi: 10.4103/npmj.npmj_157_20. [DOI] [PubMed] [Google Scholar]
- 2.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yesudhas D., Srivastava A., Gromiha M.M. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection. 2021;49(2):199–213. doi: 10.1007/s15010-020-01516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Draft Landscape and Tracker of COVID-19 Candidate Vaccines. www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Accessed August 7, 2022].
- 7.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19(8):810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 9.Dong Y., Dai T., Wei Y., Zhang L., Zheng M., Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5:237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pormohammad A., Zarei M., Ghorbani S., Mohammadi M., Razizadeh M.H., Turner D.L., et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines. 2021;9(5):467. doi: 10.3390/vaccines9050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EMA. COVID-19 Vaccines: Authorised. www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised [Accessed August 7, 2022].
- 12.FDA. COVID-19 Vaccines. www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines [Accessed August 7, 2022].
- 13.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. The dawn of mRNA vaccines: the COVID-19 case. J Control Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascellino M.T., Di Timoteo F., De Angelis M., Oliva A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist. 2021;14:3459–3476. doi: 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine [Accessed August 7, 2022].
- 17.EMA. Comirnaty. www.ema.europa.eu/en/medicines/human/EPAR/comirnaty [Accessed August 7, 2022].
- 18.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 19.Walsh E.E., Frenck R.W., Jr, Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei J., Stoesser N., Matthews P.C., Ayoubkhani D., Studley R., Bell I., et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6(9):1140–1149. doi: 10.1038/s41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas S.J., Moreira E.D., Jr, Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frenck R.W., Jr, Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groß R., Zanoni M., Seidel A., Conzelmann C., Gilg A., Krnavek D., et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 2021;75 doi: 10.1016/j.ebiom.2021.103761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iannetta M., Landi D., Cola G., Campogiani L., Malagnino V., Teti E., et al. B- and T-cell responses after SARS-CoV-2 vaccination in patients with multiple sclerosis receiving disease modifying therapies: immunological patterns and clinical implications. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.796482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington P., Doores K.J., Radia D., O'Reilly A., Lam H.P.J., Seow J., et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br J Haematol. 2021;194(6):999–1006. doi: 10.1111/bjh.17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 29.Bergman P., Blennow O., Hansson L., Mielke S., Nowak P., Chen P., et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Vesterbacka J, Aleman S, Nowak P, COVAXID Study Group High seroconversion rate after vaccination with mRNA BNT162b2 vaccine against SARS-CoV-2 among people with HIV - but HIV viremia matters? AIDS. 2022;36(3):479–481. doi: 10.1097/QAD.0000000000003135. [DOI] [PubMed] [Google Scholar]
- 31.Gavriatopoulou M., Terpos E., Ntanasis-Stathopoulos I., Briasoulis A., Gumeni S., Malandrakis P., et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS-CoV-2: a prospective study. Blood Adv. 2021;5(21):4398–4405. doi: 10.1182/bloodadvances.2021005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marasco V., Piciocchi A., Candoni A., Pagano L., Guidetti A., Musto P., et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2022;196(3):548–558. doi: 10.1111/bjh.17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shroff R.T., Chalasani P., Wei R., Pennington D., Quirk G., Schoenle M.V., et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27(11):2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadali R.A.K., Janagama R., Peruru S., Malayala S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pimpinelli F., Marchesi F., Piaggio G., Giannarelli D., Papa E., Falcucci P., et al. Fifth-week immunogenicity and safety of anti-SARS-CoV–2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):81. doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 37.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EMA. Spikevax (previously COVID-19 Vaccine Moderna). www.ema.europa.eu/en/medicines/human/EPAR/spikevaxm [Accessed August 7, 2022].
- 39.FDA. Spikevax and Moderna COVID-19 Vaccine. www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/spikevax-and-moderna-covid-19-vaccine [Accessed August 7, 2022].
- 40.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu L., McPhee R., Huang W., Bennett H., Pajon R., Nestorova B., et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39(20):2791–2799. doi: 10.1016/j.vaccine.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jochum S., Kirste I., Hortsch S., Grunert V.P., Legault H., Eichenlaub U., et al. Clinical utility of Elecsys anti-SARS-CoV-2 S assay in COVID-19 vaccination: an exploratory analysis of the mRNA-1273 Phase 1 trial. Front Immunol. 2022;12 doi: 10.3389/fimmu.2021.798117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El Sahly H.M., Baden L.R., Essink B., Doblecki-Lewis S., Martin J.M., Anderson E.J., et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385(19):1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi A., Koch M., Wu K., Chu L., Ma L., Hill A., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi A., Koch M., Wu K., Dixon G., Oestreicher J., Legault H., et al. Serum neutralizing activity of mRNA-1273 against SARS-CoV-2 variants. J Virol. 2021;95(23):e0131321. doi: 10.1128/JVI.01313-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Stewart-Jones G.B.E., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. 2021;384(15):1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali K., Berman G., Zhou H., Deng W., Faughnan V., Coronado-Voges M., et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385(24):2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stuart A.S.V., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399(10319):36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moncunill G., Aguilar R., Ribes M., Ortega N., Rubio R., Salmerón G., et al. Determinants of early antibody responses to COVID–19 mRNA vaccines in a cohort of exposed and naïve healthcare workers. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oosting S.F., van der Veldt A.A.M., GeurtsvanKessel C.H., Fehrmann R.S.N., van Binnendijk R.S., Dingemans A.C., et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22(12):1681–1691. doi: 10.1016/S1470-2045(21)00574-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadali R.A.K., Janagama R., Peruru S., Gajula V., Madathala R.R., Chennaiahgari N., et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021;93(7):4420–4429. doi: 10.1002/jmv.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forni G., Mantovani A. COVID-19 Commission of Accademia Nazionale dei Lincei, Rome. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.EMA. COVID-19 Vaccine Janssen. www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen [Accessed August 7, 2022].
- 55.FDA. Janssen COVID-19 Vaccine. www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine [Accessed August 7, 2022].
- 56.Stephenson K.E., Le Gars M., Sadoff J., de Groot A.M., Heerwegh D., Truyers C., et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA. 2021;325(15):1–10. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim results of a Phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N Engl J Med. 2022;386(9):847–860. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reimann P., Ulmer H., Mutschlechner B., Benda M., Severgnini L., Volgger A., et al. Efficacy and safety of heterologous booster vaccination with Ad26.COV2.S after BNT162b2 mRNA COVID-19 vaccine in haemato-oncological patients with no antibody response. Br J Haematol. 2022;196(3):577–584. doi: 10.1111/bjh.17982. [DOI] [PubMed] [Google Scholar]
- 61.Ewer K.J., Barrett J.R., Belij-Rammerstorfer S., Sharpe H., Makinson R., Morter R., et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27(2):270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 62.Gelanew T, Mulu A, Abebe M, Bates TA, Wassie L, Teferi M, et al. A single dose ChAdOx1 nCoV-19 vaccine elicits high antibody responses in individuals with prior SARS-CoV-2 infection comparable to that of double dose vaccinated SARS-CoV-2 infection naïve individuals. Res Sq. Published online January 11, 2022: rs.3.rs–1250175. [DOI] [PMC free article] [PubMed]
- 63.Watanabe Y., Mendonça L., Allen E.R., Howe A., Lee M., Allen J.D., et al. Native-like SARS-CoV-2 spike glycoprotein expressed by ChAdOx1 nCoV-19/AZD1222 vaccine. bioRxiv. ACS Cent Sci. 2021;7(4):594–602. doi: 10.1021/acscentsci.1c00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369(6505):806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.EMA. Vaxzevria (previously COVID-19 Vaccine AstraZeneca). www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca [Accessed August 7, 2022].
- 66.Manomaipiboon A., Phumisantiphong U., Maneerit J., Chalearmchai Y., Jirawathin W., Prajongsai A., et al. Immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 with 12-dose vials: an interim analysis. Vaccine. 2022;40(4):587–593. doi: 10.1016/j.vaccine.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586(7830):578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 69.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385(25):2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emary K.R.W., Golubchik T., Aley P.K., Ariani C.V., Angus B., Bibi S., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clemens S.A.C., Folegatti P.M., Emary K.R.W., Weckx L.Y., Ratcliff J., Bibi S., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 lineages circulating in Brazil. Nat Commun. 2021;12:5861. doi: 10.1038/s41467-021-25982-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bosaeed M., Balkhy H.H., Almaziad S., Aljami H.A., Alhatmi H., Alanazi H., et al. Safety and immunogenicity of ChAdOx1 MERS vaccine candidate in healthy Middle Eastern adults (MERS002): an open-label, non-randomised, dose-escalation, phase 1b trial. Lancet Microbe. 2022;3(1):e11–e20. doi: 10.1016/S2666-5247(21)00193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bae S., Lee Y.W., Lim S.Y., Lee J.H., Lim J.S., Lee S., et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36(17):e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Novak N., Tordesillas L., Cabanillas B. Adverse rare events to vaccines for COVID-19: from hypersensitivity reactions to thrombosis and thrombocytopenia. Int Rev Immunol. 2022;41(4):438–447. doi: 10.1080/08830185.2021.1939696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allen C.M., Ramsamy S., Tarr A.W., Tighe P.J., Irving W.L., Tanasescu R., et al. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021;90(2):315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 83.Bagella C.F., Corda D.G., Zara P., Elia A.E., Ruiu E., Sechi E., et al. Chronic inflammatory demyelinating polyneuropathy after ChAdOx1 nCoV-19 vaccination. Vaccines. 2021;9(12):1502. doi: 10.3390/vaccines9121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonifacio G.B., Patel D., Cook S., Purcaru E., Couzins M., Domjan J., et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J Neurol Neurosurg Psychiatry. 2022;93(3):341–342. doi: 10.1136/jnnp-2021-327027. [DOI] [PubMed] [Google Scholar]
- 85.Maramattom B.V., Krishnan P., Paul R., Padmanabhan S., Cherukudal Vishnu Nampoothiri S., Syed A.A., et al. Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann Neurol. 2021;90(2):312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 86.McKean N., Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14(7):e244125. doi: 10.1136/bcr-2021-244125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bogdanov G., Bogdanov I., Kazandjieva J., Tsankov N. Cutaneous adverse effects of the available COVID-19 vaccines. Clin Dermatol. 2021;39(3):523–531. doi: 10.1016/j.clindermatol.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trogstad L., Robertson A.H., Mjaaland S., Magnus P. Association between ChAdOx1 nCoV-19 vaccination and bleeding episodes: Large population-based cohort study. Vaccine. 2021;39(40):5854–5857. doi: 10.1016/j.vaccine.2021.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bengtsson K.L., Song H., Stertman L., Liu Y., Flyer D.C., Massare M.J., et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine. 2016;34(16):1927–1935. doi: 10.1016/j.vaccine.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 91.Gorman MJ, Patel N, Guebre-Xabier M, Zhu A, Atyeo C, Pullen KM, et al. Collaboration between the Fab and Fc contribute to maximal protection against SARS-CoV-2 in nonhuman primates following NVX-CoV2373 subunit vaccine with Matrix-MTM vaccination. bioRxiv. Published online February 5, 2021. http://dx.doi.org/10.1101/2021.02.05.429759.
- 92.Guebre-Xabier M., Patel N., Tian J.H., Zhou B., Maciejewski S., Lam K., et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine. 2020;38(50):7892–7896. doi: 10.1016/j.vaccine.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Formica N., Mallory R., Albert G., Robinson M., Plested J.S., Cho I., et al. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: a phase 2 randomized placebo-controlled trial. PLoS Med. 2021;18(10):e1003769. doi: 10.1371/journal.pmed.1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.EMA. Nuvaxovid. www.ema.europa.eu/en/medicines/human/EPAR/nuvaxovid [Accessed August 7, 2022].
- 95.WHO. WHO recommendation Novavax Inc. COVID-19 vaccine (SARS-CoV-2 rS) - NUVAXOVIDTM | WHO - Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control). https://extranet.who.int/pqweb/vaccines/who-recommendation-novavax-inc-covid-19-vaccine-sars-cov-2-rs-recombinant-adjuvanted [Accessed August 7, 2022].
- 96.Shinde V., Bhikha S., Hoosain Z., Archary M., Bhorat Q., Fairlie L., et al. Efficacy of the NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1899–1909. doi: 10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dunkle L.M., Kotloff K.L., Gay C.L., Áñez G., Adelglass J.M., Barrat Hernández A.Q., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386(6):531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toback S., Galiza E., Cosgrove C., Galloway J., Goodman A.L., Swift P.A., et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):167–179. doi: 10.1016/S2213-2600(21)00409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]