Abstract
Background:
There is evidence for endothelial dysfunction in youth living with perinatally acquired HIV (YLPHIV). However little data exist on its mechanisms.
Methods:
YLPHIV and age-matched HIV-uninfected (HIV-) youth enrolled in the Cape Town Adolescent Antiretroviral Cohort (CTAAC) in South Africa between 9–14 years of age were included. YLPHIV were on antiretroviral therapy (ART)> 6 months with viral load <400 copies/mL at baseline and 24 months. Serum biomarkers of systemic inflammation, monocyte activation, intestinal integrity, and oxidized LDL-cholesterol were measured at baseline and after 24 months. Endothelial function was measured at 24 months using reactive hyperemic index (RHI); endothelial dysfunction was defined as RHI<1.35. Spearman correlation coefficient and quantile regression were used to examine associations between RHI and different biomarkers.
Results:
We included 266 YLPHIV and 69 HIV- participants. At baseline, median (Q1, Q3) age was 12 (11, 13) years and 53% were females. YLPHIV had poorer endothelial function compared to HIV- youth (RHI=1.36 vs 1.52, p<0.01). At baseline and 24 months, YLPHIV had higher markers of monocyte activation (sCD14), gut barrier dysfunction (intestinal fatty acid binding protein, IFAB-P) and oxidized LDL cholesterol (p≤0.04) compared to HIV- youth. Among YLPHIV, sCD14 remained associated with endothelial dysfunction after adjusting for age, sex, Tanner stage, and ART duration (β:−0.05, p=0.01).
Conclusion:
Despite viral suppression, South African YLPHIV have poor endothelial function and persistent evidence of monocyte activation and gut barrier dysfunction compared to HIV-youth. The long-term clinical significance of gut integrity and monocyte activation needs to be further assessed in YLPHIV.
Keywords: Pediatric HIV, gut integrity, inflammation, translocation, monocyte activation, vascular function
Introduction
Adolescents and young adults represent a growing share of people living with HIV worldwide. As cardiovascular disease (CVD) is now a leading cause of death in adults living with HIV [1–3], the evaluation of CVD risk in youth who are facing a lifetime of exposure to both HIV and ART is becoming a high research priority.
Changes in the endothelium are one of the earliest alterations of the vessel wall which occur prior to atherosclerosis[4]. Peripheral Arterial Tonometry (endoPAT) measures vascular function in an automated and non-invasive manner by assessing vascular morphology and function through finger pulse volume and infrared light transmission plethysmography[5]. We reported previously that youth living with perinatally-acquired HIV (YLPHIV) both in the US[6] and in South Africa[7] have poorer endothelial function compared to that of uninfected controls as measured by reactive hyperemic index (RHI) using endoPAT.
The association between chronic inflammation and CVD is well documented in adults living with HIV [8–16]. The etiologies for vascular dysfunction in YLPHIV are likely multifactorial, however the specific role of systemic inflammation, immune activation, and intestinal integrity on the vascular process in the setting of few traditional CVD risk factors in this population is unclear. In this study, our objectives were to determine whether markers of inflammation, immune activation and gut integrity were different between YLPHIV and HIV- South African youth; second, to investigate the relationship between these markers and measures of endothelial function as measured by endoPAT and third to explore independent predictors of endothelial dysfunction.
Methods
Study design
The Cape Town Adolescent Antiretroviral Cohort (CTAAC) is an ongoing prospective cohort in South Africa which enrolled YLPHIV aged 9–14 years on ART for >6 months as well as age, sex, and ethnicity-matched HIV-uninfected youth between October 2013 and March 2015 as previously described[17, 18]. Parental written consent was obtained and adolescent assent provided. Children were recruited from primary care and hospital-based clinics in Cape Town, South Africa. The study was approved by the Faculty of Health Sciences, University of Cape Town Human Research Ethics Committee. For this substudy, only participants with plasma samples available at enrollment and after 24 months were included, and among YLPHIV, only those with an HIV-1 RNA PCR of <400 copies/mL at both timepoints and through the 2-year observation period were included.
Study evaluation
At baseline and 24 months, participants were fasting for > 8 hours and blood draws were obtained for real time measurements of lipid profiles, insulin resistance, high sensitivity C-reactive protein (hsCRP) and CD4 cell count. The derived homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as described [19]. Blood was processed and serum stored. Frozen, never previously thawed serum was shipped to University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA. The samples were used for measurement of soluble and cellular markers of monocyte immune activation, markers of systemic inflammation, gut integrity and oxidized lipids.
Inflammation, soluble immune activation and gut markers
Measurements were performed by the Dahms Clinical Research Unit Core Laboratory [Dr McComsey, laboratory Director]. In this analysis, we selected biomarkers based on prior data in HIV suggesting their association with CVD, metabolic complications, or overall mortality. Intestinal fatty acid binding protein (I-FABP) is considered a marker of enterocyte damage and has been associated with increased mortality[20, 21]. Soluble CD14 (sCD14) is a soluble marker of monocyte activation and microbial translocation and is associated with mortality and progression of atherosclerosis[26]. Oxidized lipids (oxidized LDL) is the form of LDL that has undergone oxidative changes and has been shown to contribute to endothelial cell activation[22]. The remaining biomarkers [soluble CD163 (sCD163), interleukin-6 (IL-6), and soluble tumor necrosis alpha receptor II (sTNFRII)] correlate with cardiometabolic complications, or drive other hallmarks of immune dysregulation in HIV.
I-FABP, sCD14, sCD163, sTNFRII, hsCRP, IL-6 (R &D Systems, Minneapolis, Minnesota, USA) and oxidized LDL (Mercodia, Winston Salem, North Carolina, USA) were measured by ELISA. The intra-assay variability ranged between 4–8% and inter-assay variability was less than 11% for all markers. Laboratory personnel were blinded to group assignments.
Endothelial function
Endothelial function was measured using peripheral arterial tonometry as previously described[6, 23]. Briefly, the device (Itamar Medical Ltd., Caesarea, Israel) consists of two finger probes. The probe allows the application of a constant and evenly distributed near-diastolic counter pressure within the entire probe [24, 25]. The recordings was performed with the patient in a seated position with both hands at the same level. After a period of stabilization, the blood pressure cuff on the study arm was inflated to 60 mm Hg above systolic pressure for 5 min. The cuff was deflated to induce reactive hyperemia and assess PAT. A reactive hyperemia index (RHI) was generated and is the post to pre -occlusion PAT signal ratio in the occluded side normalized to the control side and corrected for baseline vascular tone. An index of >1.35 is considered normal with <1.35 being abnormal [25].. Augmentation index (AI) is calculated from PAT pulses recorded at the baseline period and the result is further normalized to heart rate of 75 beats per minute (AI 75). Lower AI values reflect better arterial elasticity.
Statistical analyses
Demographics, clinical indices and HIV-related factors are presented by group at baseline. Median and interquartile range (IQR) are reported for continuous variables and frequency and percent for nominal variables. Changes from baseline to 24 months in clinical variables and biomarkers were determined. All baseline variables were compared between groups using unpaired t-tests or Wilcoxon Rank Sum tests as warranted by distribution for continuous variables and by Chi-Square tests, Fisher’s Exact tests, or Pearson Exact Chi-Square tests as appropriate for categorical variables. Within-group changes of biomarkers were tested using paired t-tests or Wilcoxon Signed Rank tests as appropriate for the distribution. RHI was log transformed to more closely approximate a normal distribution.
Spearman correlation analysis was utilized to assess the relationships between baseline and 24 months clinical variables with biomarkers at both time points and endothelial dysfunction at 24 months. Next, quantile regression was employed to answer what baseline and 24 month biomarkers as well as change in biomarkers are independently associated in YLPHIV with endothelial dysfunction at 24 months as the outcome, after adjusting for confounders. All statistical tests were two-sided and considered significant with p<0.05. Analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
Results
Baseline Demographics
Overall, 352 CTAAC participants had stored serum available and were included in the present analysis. Demographic information and baseline characteristics of the participants are displayed in Table 1. Median age (Q1, Q3) was 12 years (10.57, 13.19), 53% were females and 30% were in Tanner stages ≥3. YLPHIV had higher waist-to-hip ratio, homeostatic assessment of insulin resistance (HOMA) and worse lipid profiles (p≤ 0.04). No participants had cardiovascular disease or were taking lipid-lowering agents.
Table 1:
Characteristics of Participants at Enrollment
| YLPHIV N=283 | HIV- N=69 | p value | |
|---|---|---|---|
|
| |||
| Female (%) | 147 (51.9) | 39 (56.5) | 0.583 |
| Tanner (=>3 ,%) | 80 (28.7) | 25 (36.8) | 0.248 |
| Age (years) | 11.89 [10.65, 13.09] | 12.01 [10.24, 13.42] | 0.863 |
| Current smoking (%) | 2 (0.7) | 0 (0) | 0.659 |
| CD4 cell count (cells/mm3) | 744.00 [603.00, 951.00] | ---- | |
| CD4 % | 31.07 [26.72, 35.29] | ---- | |
| Viral load (copies/mL) | 39.00 [39.00, 39.00] | --- | |
| ART regimen | |||
| NRTI: | |||
| AZT | 44 (15%) | ||
| ABC | 19 (19%) | ||
| D4T | 177 (62%) | ||
| 3TC | 277 (98%) | --- | |
| NNRTI: | |||
| EFV | 170 (61%) | ||
| NVP | 23 (8%) | ||
| PI (LPV/r) | 84 (30%) | ||
| INSTI (RAL) | 1 (0.3%) | ||
| ART duration (years) | 8.18 [5.42, 9.58] | --- | |
| BMI (kg/m2) | 17.18 [16.07, 19.12] | 18.59 [16.65, 20.93] | 0.004 |
| Waist hip ratio | 0.87 [0.82, 0.95] | 0.81 [0.79, 0.84] | <0.001 |
| Systolic blood pressure (mm Hg) | 105.00 [99.50, 112.00] | 106.00 [100.00, 116.00] | 0.097 |
| Diastolic blood pressure (mm Hg) | 67.00 [62.00, 73.00] | 69.00 [64.00, 75.00] | 0.144 |
| Total cholesterol (mmol/L) | 4.10 [3.70, 4.70] | 3.80 [3.40, 4.30] | <0.001 |
| LDL (mmol/L) | 2.20 [1.80, 2.60] | 2.00 [1.60, 2.32] | 0.015 |
| HDL (mmol/L) | 1.50 [1.30, 1.80] | 1.45 [1.20, 1.73] | 0.275 |
| Triglycerides (mmol/L) | 0.90 [0.70, 1.20] | 0.60 [0.50, 0.80] | <0.001 |
| HOMA IR | 1.96 [1.13, 3.14] | 1.24 [0.19, 2.52] | 0.042 |
Median [Interquartile Range]. Bold values represent p<0.05
3TC: lamivudine, ART: antiretroviral therapy, ABC: abacavir, AZT: zidovudine, BMI: body mass index, D4T: stavudine, EFV: efavirenz, HDL: high density lipids, HOMA IR: homeostatic assessment of insulin resistance, INSTI: integrase strand transfer inhibitor, LDL: low density lipids, LPV,r: lopinavir boosted ritonavir, NRTI: nucleotide reverse transcriptase inhibitor, NNRTI: non nucleotide reverse transcriptase inhibitor, NVP: nevirapine, PI: protease inhibitor, RAL: raltegravir, YLPHIV: youth living with perinatally acquired HIV
YLPHIV had a median CD4 cell count of 744 cells/mm3 (603, 951), a median viral load of 39 copies/mL and median ART duration of 8 years (5.42, 9.58). The majority of participants were on efavirenz (EFV,61%) and the remaining on lopinavir/ritonavir (LPV/r, 30%) with a lamivudine (98%) or stavudine (62%) backbone.
Characteristics at the 24-month visit
Characteristics for the 24 months visit are shown in Table 2. YLPHIV continued to have higher CVD risk factors with higher waist-to-hip ratio and higher LDL and triglycerides (p≤0.002). Within each group, over 24 months, the only significant changes were in the waist to hip ratio and BMI. The waist to hip ratio did not change in YLPHIV (p=0.12), but increased by a median of 0.02 (−0.02, 0.05) in uninfected participants (p=0.01) over 24 months. BMI increased by 1.52 (0.57, 2.43, p<0.01) kg/m2 in YLPHIV and by 1.35 (0.61, 2.33, p<0.01) in uninfected participants over 24 months.
Table 2:
Characteristics of Participants at 24 months of Follow-Up
| YLPHIV N=283 | HIV- N=69 | p value | |
|---|---|---|---|
|
| |||
| Female (%) | 147 (51.9) | 39 (56.5) | 0.583 |
| Tanner (=>3,%) | 188 (68.9) | 50 (72.5) | 0.561 |
| Age | 13.95 [12.68, 15.23] | 14.04 [12.29, 15.34] | 0.765 |
| Current Smoking (%) | 6 (2%) | 2 (3%) | 0.791 |
| CD4 cell count (cells/mm3) | 727.00 [576.00, 905.50] | ||
| CD4 % | 32.39 [27.97, 36.86] | ||
| Viral load (copies/mL) | 39.00 [39.00, 39.00] | ||
| ART regimen | |||
| NRTI: | |||
| AZT | 42 (15%) | ||
| ABC | 214 (76%) | ||
| D4T | 22 (8%) | ||
| 3TC | 267 (94%) | ||
| NNRTI: | |||
| EFV | 176 (62%) | ||
| NVP | 5 (2%) | ||
| PI (LPV/r) | 96 (34%) | ||
| INSTI (RAL) | 2 (0.7%) | ||
| BMI (kg/m2) | 18.82 [17.33, 21.14] | 20.34 [17.88, 22.30] | 0.016 |
| Waist hip ratio | 0.87 [0.83, 0.91] | 0.84 [0.80, 0.89] | 0.002 |
| Systolic blood pressure (mm Hg) | 104.00 [98.00, 111.00] | 107.00 [103.00, 114.00] | 0.015 |
| Diastolic blood pressure (mm Hg) | 67.00 [61.00, 71.00] | 69.00 [64.00, 73.00] | 0.069 |
| Total cholesterol (mmol/L) | 4.10 [3.61, 4.78] | 3.53 [3.17, 4.05] | <0.001 |
| LDL (mmol/L) | 2.18 [1.77, 2.65] | 1.87 [1.58, 2.29] | 0.002 |
| HDL (mmol/L) | 1.47 [1.24, 1.73] | 1.36 [1.14, 1.67] | 0.078 |
| Triglycerides (mmol/L) | 0.91 [0.68, 1.23] | 0.59 [0.50, 0.76] | <0.001 |
Median [Interquartile Range]
Bold values represent p<0.05
BMI: body mass index, HDL: high density lipids, LDL: low density lipids, YLPHIV: youth living with perinatally acquired HIV
In YLPHIV, CD4% increased significantly by a median of 1.4 % (−1.72, 4.07, p=0.013). The median viral load remained at 39 copies/mL. Most YLPHIV (76%) had a switch in ART regimen, with the majority being a switch from a stavudine backbone to abacavir.
Changes in Biomarkers
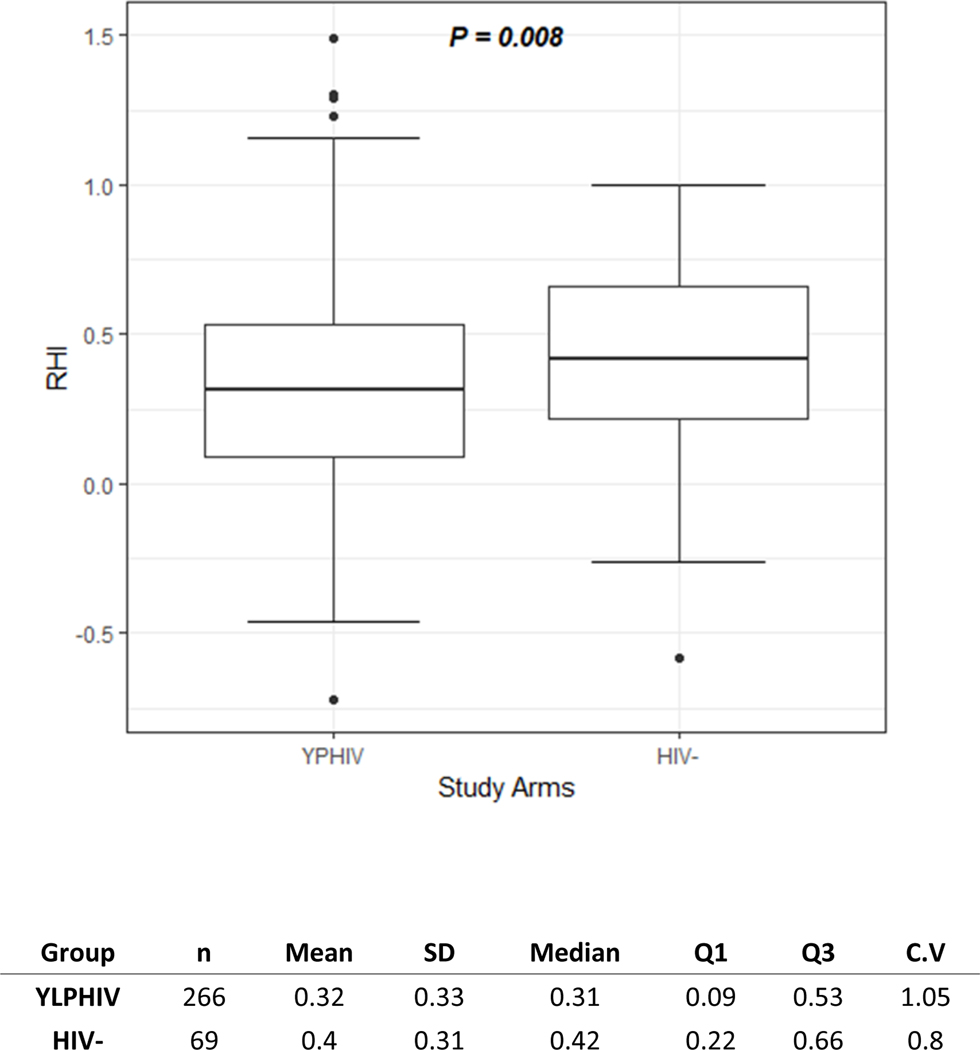
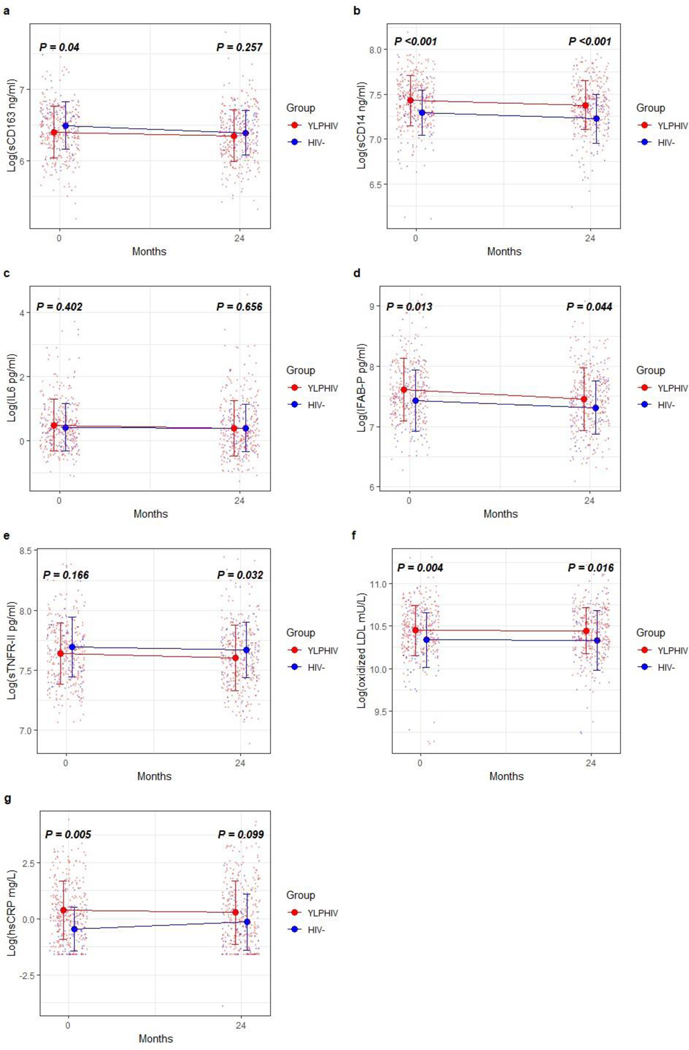
As illustrated in Figure 2, at baseline, hsCRP, sCD14, I-FABP and oxidized LDL were higher in YLPHIV compared to HIV- participants (p≤0.013). All biomarkers, except for hsCRP, remained higher in YLPHIV compared to HIV- participants at 24 months (p≤0.044, Figure 1).
Fig. 2.
EndoPAT. Box plot of ln reactive hyperemic index in youth living with perinatally acquired HIV and HIV. The box represents the interquartile range, whiskers represent the range. Wilcoxon test used for comparison.
Fig. 1.
Changes in biomarkers between the groups at baseline and 24 months. Graph showing median biomarkers with whiskers representing interquartile range, and the colored small dots are all values (a: soluble CD163, b: soluble CD14, c: IL-6, d: intestinal fatty acid binding protein, e: soluble tumor necrosis factor receptor a II, f: oxidized LDL, g: high sensitivity C-reactive protein) in youth living with perinatally acquired HIV and HIV at enrollment and 24 months. Biomarkers were log transformed. Wilcoxon test used, P values represent differences between youth living with perinatally acquired HIV and HIV at each time point. hsCRP, high sensitivity C-reactive protein; IFAB-P, intestinal fatty acid binding protein; IL-6, interleukin 6; sCD14 and 163, soluble CD14 and 163; sTNFRII, soluble tumor necrosis factor receptor a II.
Within groups, sCD14, and I-FABP decreased significantly within YLPHIV over 24 months (p≤0.011, Table 3). There was not significant change within the HIV- arm for any of the biomarkers.
Table 3:
Changes in Biomarkers over 24 months within each groups
| YLPHIV Median [IQR] | P | HIV- Median [IQR] | P | |
|---|---|---|---|---|
|
| ||||
| sCD163 (ng/mL) | −29.00 [−113.50, 44.50] | 0.058 | −82.00 [−126.00, 5.00] | 0.065 |
| sCD14 (ng/mL) | −84.00 [−323.00, 138.00] | 0.011 | −86.00 [−253.00, 61.00] | 0.107 |
| Il6 (pg/mL) | −0.12 [−0.88, 0.58] | 0.062 | −0.10 [−0.64, 0.78] | 0.871 |
| IFAB-P (pg/mL) | −182.00 [−943.50, 447.00] | 0.004 | −190.00 [−795.75, 245.25] | 0.286 |
| sTNF-RII (pg/mL) | −84.00 [−318.00, 198.00] | 0.07 | −51.00 [−319.50, 253.75] | 0.747 |
| oxLDL (mU/L) | 344.50 [−4257.25, 4277.25] | 0.712 | −138.50 [−2424.00, 3786.25] | 0.911 |
| hsCRP (mg/L) | −0.12 [−1.60, 1.27] | 0.184 | 0.07 [−0.35, 0.83] | 0.098 |
Median [Interquartile Range]
Bold values represent p<0.05
high sensitivity C reactive protein, IL6: interleukin 6, hsCRP:, IFAB-P: intestinal fatty acid binding protein, oxLDL: oxidized LDL, sCD14 and 163: soluble CD 14 and 163, sTNFR-II: soluble tumor necrosis factor receptor α II
The increase in CD4 % over 24 months in YLPHIV correlated with decreases in sCD163, IL6, sTNFRII, and oxidized LDL (r=0.15–0.22, p<0.01).
The results of the analyses including all participants did not differ from the sensitivity analyses performed including only participants with endoPAT at 24 months (n=335); therefore only the former data are presented.
EndoPAT measures
Of the participants with plasma available at both time points, 335 also had endoPAT measures at 24 months with no differences in the demographic or HIV characteristics. YLPHIV had worse endothelial function as measured by a lower RHI than uninfected participants (p=<0.01, Figure 2). 43% of YLPHIV vs 30% of uninfected participants (p=0.21) had endothelial dysfunction (RHI<1.35). Compared to participants with higher RHI, those with endothelial dysfunction were overall more likely to be younger males with lower BMI, have higher waist-to-hip ratio and higher triglyceride levels (p≤0.01, data not shown). Among YLPHIV, there was no difference in RHI for the participants on ABC, EFV or LPV/r (p≤0.69).
Baseline Predictors of endoPAT at 24 months
In univariate analyses, higher baseline IFAB-P weakly correlated with worse endothelial dysfunction at 24 months (ρ=0.17, p=0.04). None of the other biomarkers nor variables traditionally associated with CVD risk factors including sex, CD4 cell count, HOMA-IR, blood pressure, BMI, waist hip ratio correlated with endothelial dysfunction (p≤0.78, data not shown).
At 24 months, only higher sCD14 at that time point correlated with endothelial dysfunction (ρ=0.23, p=0.007). Change in each biomarker did not correlate with endothelial dysfunction.
In multivariable analyses, in YLPHIV, after adjusting for confounder including waist hip ratio, age, sex, tanner stage, viral load and ART duration, only higher sCD14 at 24 months remained associated with endothelial dysfunction (β=−0.046, 95% CI −0.081, −0.010, p-0.013).
Discussion
These findings provide novel information on the prevalence and pathogenesis of cardiometabolic complications of YLPHIV in Africa, for which there are limited data. We found that YLPHIV virologically suppressed on ART have persistent monocyte activation and gut barrier dysfunction which may contribute to vascular dysfunction seen in this population. This extends our prior observation of increased carotid intima thickness measurements in youth with HIV and the potential role of intestinal barrier dysfunction in the etiology of vascular disease in this population [26, 27].
Despite viral suppression and long term ART, South African YLPHIV have residual systemic inflammation and monocyte activation consistent with findings in adults living with HIV[28] and other pediatric cohorts[29–32]. Several biomarkers (sCD14 and IFAB-P) decreased significantly after 24 months in YLPHIV, however sCD14, IFAB-P and oxidized LDL remained significantly elevated compared to the uninfected participants. In addition, oxidized LDL did not significantly change after 24 months. Oxidized LDL is a marker of oxidative stress and in uninfected adults has been associated with obesity[33], insulin resistance[34] and cardiovascular disease[35] and in HIV has been associated with markers of immune activation[36]. Increased circulating levels of LDL in YLPHIV may be related to multiple factors including HIV disease and ART. The ongoing inflammation in this population may promote pro-inflammatory alterations of lipid particles[37]. Oxidized LDL in turn may play a role in the persistent monocyte activation seen in YLPHIV[37] supporting the bi-directional relationship between inflammation and lipid alteration. IFAB-P, a marker of gut structural damage, has been associated with higher mortality[20] and alteration in body composition specifically fat accumulation in HIV[38]. IFAB-P levels were found to increase in ART-naïve adults, 96 weeks post ART[38], suggesting that despite ART, gut integrity is not completely restored.
CD14 is expressed on monocytes/macrophages, serves as a co-receptor for toll-like receptor and facilitates cellular responses to lipopolysaccharides, the major component of the outer membrane of gram-negative bacteria[39]. The soluble form of CD14 (sCD14) is produced by the liver as an acute phase reactant and can also be shed by activated monocytes as a marker of lipopolysaccharide activation[40] Studies in children, youth and adults living with HIV randomized to different ART regimens reported that sCD14 levels remain persistently elevated despite ART and viral suppression consistent with our findings [29, 41–44]. Studies in adults suggest that sCD14 decreased only with integrase strand transfer inhibitor (INSTI) use but not with protease inhibitor or NNRTI based regimens[42–44]. One of the mechanisms hypothesized is a higher concentration of INSTI in the enterocytes leading to better control over bacterial translocation[43]. We found that both IFAB-P, a marker of enterocyte necrosis or inflammation, and sCD14, significantly decreased in YLPHIV, however remained elevated over 24 months compared to uninfected participants. This further supports that despite viral suppression and immunologic improvement (CD4% significantly improved over 24 months in YLPHIV), there likely remains a cycle of ongoing bacterial translocation, monocyte activation and disruption in gut integrity. that does not improve with early viral suppression. Future studies will be needed as more children and youth are initiated and switched to INSTI-based regimen as this drug class is now the preferred first line on the World Health Organization HIV treatment guidelines for adults and children[45].
Chronic intestinal mucosal damage, bacterial translocation, inflammation and monocyte activation can have detrimental health consequences and may play a role in cardiometabolic complications in YLPHIV. To our knowledge, only one study has investigated endothelial function as measured by endoPAT and its relationship with inflammatory biomarkers in Southern African adults living with HIV[46]. Bestawros et al. found that persistent systemic inflammation as measured by hsCRP and sTNFRI was associated with impaired endothelial function over 12 weeks in undernourished HIV-infected adults starting ART[46]. We found that both IFAB-P, a marker of enterocyte necrosis or inflammation, and sCD14, a marker of monocyte activation and an indirect measure of microbial translocation, correlated with endothelial dysfunction in YLPHIV. After adjusting for known confounders, only high levels of sCD14 remained associated with worse endothelial function.
In the general population and in HIV, sCD14 has been associated with vascular disease, a surrogate marker for increased risk of future clinical cardiovascular events in older adults and overall mortality [40, 47–49]. This is the first published study to demonstrate a relationship between sCD14 and endothelial dysfunction in YLPHIV in the setting of lack (or few) traditional CVD risk factors. Our previous reports from youth in the US did not identify a relationship between sCD14 and RHI[6]. We hypothesize that this is largely in part due to the smaller sample size of YLPHIV in the US study but may also be secondary to ongoing microbial translocation and altered intestinal integrity which may be different between US and South African youth, who are exposed to a high burden of infectious diseases. Although future larger studies are warranted, our findings of the association of sCD14 with worsening endothelial function in YLPHIV suggests a common pathway in HIV in which activation of the immune system by microbial products may accelerate the progression of co-morbidities even at a young age.
This analysis leverages a well-defined cohort with an age, sex- and ethnicity-matched control group enrolled in parallel in the same country. There are several limitations in our study, we only had serum samples available which restricted the assays we conducted and were unable to further assess the functional integrity of the gut and quantify measure microbial translocation, however, we were able to assess biomarkers of these in a longitudinal manner over 24 months. In addition, were limited by having only a single measure of endothelial function. We cannot prove causal relationships or exclude the possibility of residual confounding. Our study focused on black youth in South Africa and therefore our findings cannot be generalized to different populations, however the vast majority of the world’s population of children/youth with perinatally acquired HIV reside in sub-Saharan Africa[50].
In conclusion, our study showed that YLPHIV in South Africa have evidence of monocyte activation and intestinal damage which persist over time despite viral suppression with ART. In addition, YLPHIV show evidence of subclinical vascular disease. The extent of monocyte activation may play a role in endothelial dysfunction in this population. These findings further highlight the potential early CVD risk factors in YLPHIV as they are advancing into adulthood and the mechanisms underlying this risk in the absence of traditional risk factors emphasizing the need for further investigation.
Acknowledgements
The authors would like to thank the patients who participated in this research.
Lab support was provided by UH CRC and Case Western CTSC.
Source of support:
CTAAC was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD074051) and the South African Medical Research Council (SAMRC). Additional support include NICHD K23HD088295 (SDF), UH CRC and Case CTSC.
Footnotes
Competing interests
GAM served as a consultant for Merck, Gilead, Viiv, and Theratechnologies, and has received research funding from Roche, Astellas, Tetraphase, and BMS. All other authors had no conflict of interest.
References
- 1.Paula AA, Schechter M, Tuboi SH, Faulhaber JC, Luz PM, Veloso VG, et al. Continuous increase of cardiovascular diseases, diabetes, and non-HIV related cancers as causes of death in HIV-infected individuals in Brazil: an analysis of nationwide data. PLoS One 2014,9:e94636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep 2013,10:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsue PY, Waters DD. Time to Recognize HIV Infection as a Major Cardiovascular Risk Factor. Circulation 2018,138:1113–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poredos P. Endothelial dysfunction in the pathogenesis of atherosclerosis. Int Angiol 2002,21:109–116. [PubMed] [Google Scholar]
- 5.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 2003,146:168–174. [DOI] [PubMed] [Google Scholar]
- 6.Dirajlal-Fargo S, Sattar A, Kulkarni M, Bowman E, Funderburg N, McComsey GA. HIV-positive youth who are perinatally infected have impaired endothelial function. Aids 2017,31:1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahtab S, Zar HJ, Ntusi NAB, Joubert S, Asafu-Agyei NAA, Luff NJ, et al. Endothelial dysfunction in South African youth living with perinatally acquired HIV on antiretroviral therapy. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000,342:836–843. [DOI] [PubMed] [Google Scholar]
- 9.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011,204:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA 2012,308:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008,5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ. Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation 1994,90:2236–2240. [DOI] [PubMed] [Google Scholar]
- 13.Empana JP, Canoui-Poitrine F, Luc G, Juhan-Vague I, Morange P, Arveiler D, et al. Contribution of novel biomarkers to incident stable angina and acute coronary syndrome: the PRIME Study. Eur Heart J 2008,29:1966–1974. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010,24:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansoor A, Althoff K, Gange S, Anastos K, Dehovitz J, Minkoff H, et al. Elevated NT-pro-BNP levels are associated with comorbidities among HIV-infected women. AIDS Res Hum Retroviruses 2009,25:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duprez DA, Neuhaus J, Tracy R, Kuller LH, Deeks SG, Orkin C, et al. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS 2011,25:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Githinji LN, Gray DM, Hlengwa S, Machemedze T, Zar HJ. Longitudinal changes in Spirometry in perinatally HIV-infected South African adolescents on antiretroviral therapy. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Githinji LN, Gray DM, Hlengwa S, Myer L, Zar HJ. Lung Function in South African Adolescents Infected Perinatally with HIV and Treated Long-Term with Antiretroviral Therapy. Ann Am Thorac Soc 2017,14:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985,28:412–419. [DOI] [PubMed] [Google Scholar]
- 20.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014,210:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 2003,36:529–535. [DOI] [PubMed] [Google Scholar]
- 22.Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, et al. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J Clin Invest 1995,96:2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sana Mahtab HZ, Susan J. Joubert, Nana Akua Asafu-Agyei, Norme J. Luff, Nomawethu Jele, Liesl Zülke, Landon Myer, Jennifer Jao. ENDOTHELIAL DYSFUNCTION IN SOUTH AFRICAN YOUTH WITH PERINATALLY ACQUIRED HIV ON ART. In: Conference on Retroviruses and Opportunistic INfections. Boston MA; 2019. [Google Scholar]
- 24.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol 2003,41:1761–1768. [DOI] [PubMed] [Google Scholar]
- 25.Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr., Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004,44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 26.Dirajlal-Fargo S, Albar Z, Bowman E, Labbato D, Sattar A, Karungi C, et al. Subclinical Vascular Disease in Children with HIV in Uganda is Associated with Intestinal Barrier Dysfunction. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McComsey GA, O’Riordan M, Hazen SL, El-Bejjani D, Bhatt S, Brennan ML, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. Aids 2007,21:921–927. [DOI] [PubMed] [Google Scholar]
- 28.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids 2015,29:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirajlal-Fargo S, Musiime V, Cook A, Mirembe G, Kenny J, Jiang Y, et al. Insulin Resistance and Markers of Inflammation in HIV-infected Ugandan Children in the CHAPAS-3 Trial. Pediatr Infect Dis J 2017,36:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Augustemak de Lima LR, Petroski EL, Moreno YMF, Silva DAS, Trindade E, Carvalho AP, et al. Dyslipidemia, chronic inflammation, and subclinical atherosclerosis in children and adolescents infected with HIV: The PositHIVe Health Study. PLoS One 2018,13:e0190785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams JC, Zhang X, Karki M, Chi YY, Wallet SM, Rudy BJ, et al. Soluble CD14, CD163, and CD27 biomarkers distinguish ART-suppressed youth living with HIV from healthy controls. J Leukoc Biol 2018,103:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckard AR, Rosebush JC, Lee ST, O’Riordan MA, Habib JG, Daniels JE, et al. Increased Immune Activation and Exhaustion in HIV-infected Youth. Pediatr Infect Dis J 2016,35:e370–e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab 2005,90:6454–6459. [DOI] [PubMed] [Google Scholar]
- 34.Ho RC, Davy K, Davy B, Melby CL. Whole-body insulin sensitivity, low-density lipoprotein (LDL) particle size, and oxidized LDL in overweight, nondiabetic men. Metabolism 2002,51:1478–1483. [DOI] [PubMed] [Google Scholar]
- 35.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998,98:1487–1494. [DOI] [PubMed] [Google Scholar]
- 36.Hileman CO, Turner R, Funderburg NT, Semba RD, McComsey GA. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. Aids 2016,30:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zidar DA, Juchnowski S, Ferrari B, Clagett B, Pilch-Cooper HA, Rose S, et al. Oxidized LDL Levels Are Increased in HIV Infection and May Drive Monocyte Activation. J Acquir Immune Defic Syndr 2015,69:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Kamari V, Moser C, Hileman CO, Currier JS, Brown TT, Johnston L, et al. Lower Pretreatment Gut Integrity Is Independently Associated With Fat Gain on Antiretroviral Therapy. Clin Infect Dis 2019,68:1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res 2005,11:225–229. [DOI] [PubMed] [Google Scholar]
- 40.Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol 2013,33:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudy BJ, Kapogiannis BG, Worrell C, Squires K, Bethel J, Li S, et al. Immune Reconstitution but Persistent Activation After 48 Weeks of Antiretroviral Therapy in Youth With Pre-Therapy CD4 >350 in ATN 061. J Acquir Immune Defic Syndr 2015,69:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012,206:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, et al. Differential Reduction in Monocyte Activation and Vascular Inflammation With Integrase Inhibitor-Based Initial Antiretroviral Therapy Among HIV-Infected Individuals. J Infect Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, et al. Changes in Inflammation and Immune Activation with Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Organization TWH. Update of REcommendations on First-and Second-Line Antiretroviral Regimens. In; 2019. [Google Scholar]
- 46.Bestawros M, Chidumayo T, Blevins M, Canipe A, Bala J, Kelly P, et al. Increased systemic inflammation is associated with cardiac and vascular dysfunction over the first 12 weeks of antiretroviral therapy among undernourished, HIV-infected adults in Southern Africa. J AIDS Clin Res 2015,6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martinez JS, et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One 2012,7:e46073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. Aids 2014,28:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011,203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.UNAIDS. UNAIDS report 2019. 2019.