Abstract
Pseudomonas syringae pv. syringae B728a, a causal agent of bacterial brown spot on snap beans, swarms with a characteristic dendritic pattern on semisolid (0.4%) agar plates. Filamentation of swarming cells of B728a was not observed. Mutations in either the gacS (formerly lemA) or gacA gene of B728a eliminate the ability of this P. syringae isolate to swarm without obvious effects on bacterial motility. Three field isolates showed a similar dependence on gacS for swarming. Since gacS and gacA mutants are known to be deficient in N-acyl-l-homoserine lactone (acyl-HSL) production, a mutant was constructed by disruption of the ahlI gene of B728a. This mutant did not make any acyl-HSL detectable by the Agrobacterium traG::lacZ reporter system, yet was unaffected in its ability to swarm. Other phenotypes of gacS and gacA mutations were similarly unaffected in the ahlI mutant.
The histidine sensor kinase gene gacS (formerly designated lemA) and the response regulator gene gacA compose a two-component regulatory pair that is widely distributed in genera of gram-negative bacteria as taxonomically diverse as Erwinia (7), Pseudomonas (17, 21, 23, 25), and Vibrio (26). Originally described as affecting lesion formation on beans by Pseudomonas syringae (14, 23, 25) and cyanide and antibiotic production in Pseudomonas fluorescens, respectively (17), gacS and gacA have since been implicated in regulating the expression of a wide variety of systems, including pigment production (9), motility (9), protease (15, 24), pectate lyase (18), cellulase (7), siderophores (28), and homoserine lactone (16, 21, 27), as well as an impressive list of toxins and antibiotics (3, 15, 17, 26). A unifying theme appears to run through the products of gacS and gacA regulation, in that nearly all of the affected end products are extracellular and these tend to actively modify the environment surrounding the bacterial cell. As such, it is not terribly surprising that along with these more specific phenotypes, mutations in gacS and gacA often have profound effects on microbe-host interactions, including those that result in pathogenicity (9, 18, 25). In P. syringae pv. syringae B728a, a causal agent of bacterial brown spot on beans (Phaseolus vulgaris), gacS and gacA mutants are deficient in the production of syringomycin, N-acyl-l-homoserine lactone (acyl-HSL), extracellular protease, and necrotic lesions (16). The ability of these mutants to persist in a field setting is also greatly reduced, although the bacteria appear to grow nearly as well as the wild type in planta (13). In this report, we add conditional swarming to the list of gacS gacA phenotypes in B728a.
When inoculated to a central point on a 0.4% agar plate at 28°C, wild-type B728a begins to grow as a colony with a morphology not unlike that seen at more usual agar concentrations (i.e., 1.5%). After this colony achieves a diameter of 2 to 4 mm (12 to 18 h), irregular blebbing begins to appear at the margins. Generally, one of the blebs elongates into a fluid tendril that moves directly away from the central colony. Other tendrils develop both from this initial stream and from the central colony, eventually creating a characteristic dendritic pattern of branching liquid streams. The nature of the liquid produced is unknown, but its presence parallels the slime production seen with many swarming bacteria (10). Movement across the agar surface is rapid, with streams reaching the limits of the plate within approximately 36 h and resulting in a messy confluent growth across most of the plate area within 72 h. Swarming is not medium dependent, in that it occurred more or less identically on King’s B, Luria, and SWM (see legend to Fig. 1) 0.4% agar (Difco) plates. It should be noted that swarming by wild-type B728a is somewhat variable, with usually minor variations in both the time course and the total amount of swarming per plate occurring on a plate-by-plate basis.
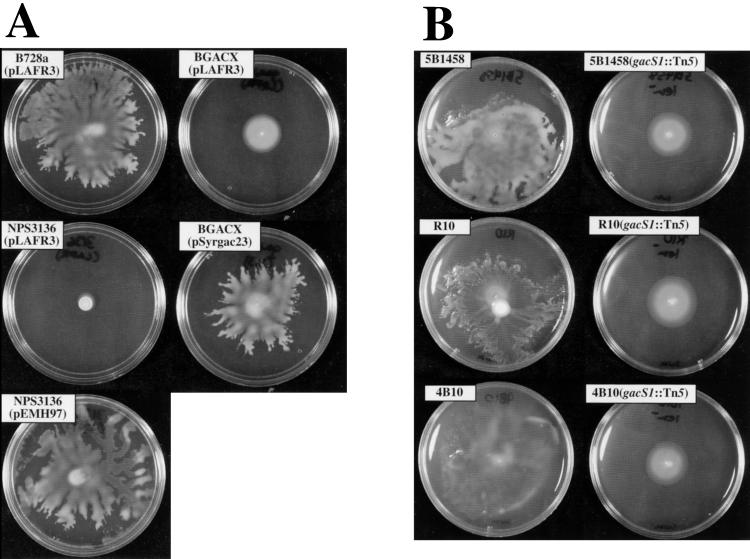
FIG. 1.
(A) Swarming ability of B728a and mutant derivatives. Cells were inoculated with an applicator stick to the center of an SWM plate (5 g of peptone, 3 g of yeast extract, and 4 g of granulated agar per liter; all medium components are from Difco) supplemented with 5 μg of tetracycline per ml to provide plasmid selection. Results were obtained after approximately 48 h of incubation at 28°C. NPS3136 (25) and BGACX (23) are gacS and gacA insertion mutants, respectively. The ability to swarm is restored to the appropriate mutant by plasmid-borne copies of gacS (i.e., pEMH97) (11) and gacA (i.e., pSyrgac23) (20), but not by the plasmid vector pLAFR3. The surface colony sizes of NPS3136(pLAFR3) and BGACX(pLAFR3) are about the same, with the larger apparent diameter of BGACX(pLAFR3) resulting from growth inside the agar matrix (see text). SWM medium in all swarming figures in this report was also supplemented with 1% potato infusion broth; this was done for consistency with other work in our laboratory and has no effect on swarming. (B) Swarming ability of field isolates and gacS mutant derivatives (19). Strains were inoculated on SWM medium (described above [B]) without tetracycline. Pictured are results after approximately 48 h of incubation at 28°C. Most of the visible growth of the gacS mutants represents subsurface spreading within the agar matrix, with the lighter spot at the center being the surface colony.
As shown in Fig. 1A, mutants in either the gacS or gacA gene of B728a are completely defective for this swarming phenotype. The liquid blebbing that signals the initiation of the swarming event never occurs, and the growing central colony continues to grow as a colony, often becoming mucoid after a period of several days. Spreading under the agar surface is usually apparent to various degrees, presumably representing bacterial motility in the liquid channels of the agar matrix. This subsurface spreading also occurs with the wild type, although the surface swarming phenotype makes it somewhat difficult to see. Swimming motility in gacS and gacA mutants from low-agar medium plates appears identical to that of wild-type B728a by microscopy. Provision of the wild-type gacS or gacA gene on a plasmid to gacS and gacA mutants, respectively, completely restores the swarming phenotype (Fig. 1A). Cross-complementation of a gacS mutation by gacA on a plasmid or of gacA by gacS on a plasmid was not observed, confirming that copy number suppression of the swarming-minus phenotype of the sensor mutation by the response regulator or the response regulator mutation by the sensor does not occur. Mutations in salA (16), a regulator downstream in the gac regulon that is involved in syringomycin expression and pathogenicity, had no effect on the ability of B728a to swarm (data not shown).
Previous work had demonstrated that gacS (as lemA) was as important for the development of brown spot symptoms by a set of recent field isolates as it was for B728a (22). Three of these isolates were checked both for the ability to swarm and for the involvement of gacS in the swarming process. All of the field strains tested swarmed on the low-agar medium in a manner very similar to that of B728a (Fig. 1B); all of the gacS mutants derived from the same isolates had lost the ability to swarm (Fig. 1B). These results indicate that conditional swarming is a trait that is often associated with P. syringae pv. syringae and that in this background, the trait is regulated by the gacS gacA regulon. Interestingly, two well-studied laboratory strains of P. syringae pv. syringae that we also tested, B3A and 310D (4), did not swarm under the same conditions. It is unknown whether the two strains are representative of a subset of wild-type P. syringae pv. syringae that naturally lack the ability to swarm or whether the trait has been lost during storage of the laboratory strain.
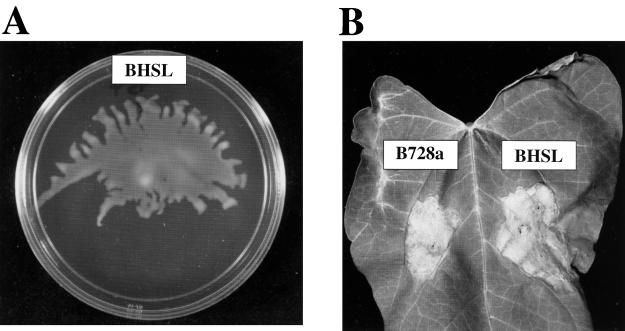
The single predominant N-acyl-l-homoserine lactone produced by B728a has been shown to cochromatograph with N-(3-oxo-hexanoyl)-l-HSL standard by using the traG::lacZ reporter system from Agrobacterium (2). As mentioned above, acyl-HSL production is clearly deficient in gacS and gacA mutants of B728a (16). Homoserine lactones have previously been found to be important to the swarming response in at least one other bacterial species (5), and so it seemed probable that the lack of swarming in our sensor-response regulator mutants was simply due to downstream effects of the defects in quorum sensing. The likelihood of this possibility was reinforced by the finding that ethyl acetate extracts of B728a culture supernatants appeared to weakly restore the initiation of swarming in gacS and gacA mutants when applied to the site of inoculation (19). We decided to approach this question genetically by constructing a mutation in the B728a biosynthetic gene for acyl-HSL. We used the previously published plasmid pAKC954 (4) to replace the wild-type ahlI gene in the B728a chromosome with a copy containing a MudI1734 transposon insertion in the ahlI gene from P. syringae pv. syringae B3A by recombinational exchange. This gene disruption was confirmed by Southern hybridization. The resulting mutant (named BHSL) was tested for acyl-HSL production by bioassay with the Agrobacterium reporter system (20). The bioassay indicated that BHSL was completely deficient for acyl-HSL production; this is in contrast to the gacS and gacA regulatory mutants that had always made smaller but readily detectable amounts of acyl-HSL in this assay, presumably by virtue of genetic leakiness at the biosynthetic locus (data not shown). Acyl-HSL production was restored to the mutant by providing a copy of the wild-type ahlI gene on a plasmid (pAKC953) (4). Surprisingly, this ahlI mutant of B728a swarmed as well as wild-type B728a (Fig. 2A). Indeed, BHSL was indistinguishable from the wild type for the production of extracellular protease and syringomycin (data not shown) and lesion formation (Fig. 2B), indicating that none of these traits are further manifestations of the homoserine lactone deficiency of gacS and gacA mutants. No phenotypes other than the complete loss of detectable homoserine lactone production, except possibly a small difference in colony morphology, have been observed so far for the ahlI mutation in BHSL.
FIG. 2.
Swarming ability of strain BHSL. The mutant was inoculated on an SWM plate as described above (Fig. 1A). The results shown were obtained after approximately 48 h at 28°C. (B) Foliar symptoms produced by the infiltration of the strains indicated into primary leaves of snap bean cultivar “Bush Blue Lake 274.” Bacterial cultures were adjusted to 106 CFU/ml in sterile water and infiltrated as previously described (25). The leaf was digitally imaged 4 days postinoculation.
Conditional swarming has only in recent years been recognized as a more general trait of gram-negative bacteria, with species like Escherichia coli and Salmonella typhimurium now known to be competent for mass mobilization across the surface of a rich medium plate with a lowered agar concentration (11). The swarming process appears to be complex, with a variety of genes in a variety of organisms having been implicated in the swarming event (1, 5, 8, 10). Differentiated cell elongation has been described as nearly universal for swarmer cells, with the cell filaments usually appearing to be multinucleate and hyperflagellated (10). While P. syringae B728a swarming behavior seems similar to that of other swarming bacteria described in the literature, swarmer cells from the leading edge of the B728a swarm did not exhibit this filamentous phenotype. Wild-type B728a and the gacS and gacA mutants of B728a appeared identical to themselves and to each other by microscopy, regardless of whether the sample source was a low-agar swarm plate or a 1.5% agar plate that did not support the swarming phenotype. This does not rule out the presence of such differentiated swarmer cells, but does indicate that if they are present, they represent a very small proportion of the total cell population, even at the swarm’s leading edge. In some cases, hyperflagellation has been made integral to the definition of “authentic” swarming (10). Thus, until more is understood about the mechanisms underlying the phenotype that we have described in P. syringae, the term “swarming” should be considered used here in its broad sense as described previously (12).
Our results indicate that in B728a, swarming occurs within the framework of the Gac regulon, although the mechanism by which the gac genes exert their control remains to be elucidated. While the previously characterized acyl-HSL from B728a does not appear to play a major role in swarming, this does not rule out the possibility of another acyl-HSL that is undetected by the Agrobacterium system or a nonlactone signal molecule (6) being involved. In a similar fashion, it seems possible that biosurfactants might play some role in the swarming process (19), with the effects of gacS and gacA on swarming potentially resulting from their regulation of surfactant production.
Acknowledgments
We thank Laura Hogan for helpful comments on the manuscript.
This work was supported by NSF grant MCB-9419023 to D. K. Willis.
REFERENCES
- 1.Burkart M, Toguchi A, Harshey R M. The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:2568–2573. doi: 10.1073/pnas.95.5.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha C, Gao P, Chen Y-C, Shaw P D, Farrand S K. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 3.Corbell N, Loper J E. A global regulator of secondary metabolite production in Pseudomonas fluorescens Pf-5. J Bacteriol. 1995;177:6230–6236. doi: 10.1128/jb.177.21.6230-6236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumenyo C K, Mukerejee A, Chun W, Chatterjee A K. Genetic and physiological evidence for the production of N-acyl homoserine lactones by Pseudomonas syringae pv. syringae and other plant pathogenic Pseudomonas species. Eur J Plant Pathol. 1998;104:569–582. [Google Scholar]
- 5.Eberl L, Winson M K, Sternberg C, Stewart G S, Christiansen G, Chhabra S R, Bycroft B, Williams P, Molin S, Givskov M. Involvement of N-acyl-L-homoserine lactone autoinducers in controlling the multicellular behavior of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 6.Flavier A B, Clough S J, Schell M A, Denny T P. Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol Microbiol. 1997;26:251–259. doi: 10.1046/j.1365-2958.1997.5661945.x. [DOI] [PubMed] [Google Scholar]
- 7.Frederick R D, Chiu J L, Bennetzen J L, Handa A K. Identification of a pathogenicity locus, rpfA, in Erwinia carotovora subsp. carotovora that encodes a two-component sensor-regulator protein. Mol Plant-Microbe Interact. 1997;10:407–415. doi: 10.1094/MPMI.1997.10.3.407. [DOI] [PubMed] [Google Scholar]
- 8.Givskov M, Östling J, Eberl L, Lindum P W, Christensen A B, Christiansen G, Molin S, Kjelleberg S. Two separate regulatory systems participate in control of swarming motility of Serratia liquefaciens MG1. J Bacteriol. 1998;180:742–745. doi: 10.1128/jb.180.3.742-745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grewal S I S, Han B, Johnstone K. Identification and characterization of a locus which regulates multiple functions in Pseudomonas tolaasii, the cause of brown blotch disease of Agaricus bisporus. J Bacteriol. 1995;177:4658–4668. doi: 10.1128/jb.177.16.4658-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harshey R M. Bees aren’t the only ones: swarming in gram-negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 11.Harshey R M, Matsuyama T. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc Natl Acad Sci USA. 1994;91:8631–8635. doi: 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano S S, Ostertag E M, Savage S A, Baker L S, Willis D K, Upper C D. Contribution of the regulatory gene lemA to field fitness of Pseudomonas syringae pv. syringae. Appl Environ Microbiol. 1997;63:4304–4312. doi: 10.1128/aem.63.11.4304-4312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hrabak E M, Willis D K. The lemA gene required for pathogenicity of Pseudomonas syringae pv. syringae on bean is a member of a family of two-component regulators. J Bacteriol. 1992;174:3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hrabak E M, Willis D K. Involvement of the lemA gene in production of syringomycin and protease by Pseudomonas syringae pv. syringae. Mol Plant-Microbe Interact. 1993;6:368–375. [Google Scholar]
- 16.Kitten T, Kinscherf T G, McEvoy J L, Willis D K. A newly-identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol Microbiol. 1998;28:917–930. doi: 10.1046/j.1365-2958.1998.00842.x. [DOI] [PubMed] [Google Scholar]
- 17.Laville J, Voisard C, Keel C, Maurhofer M, Défago G, Haas D. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA. 1992;89:1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao C-H, McCallus D, Fett W. Molecular characterization of two gene loci required for production of the key pathogenicity factor pectate lyase in Pseudomonas viridiflava. Mol Plant-Microbe Interact. 1994;7:391–400. doi: 10.1094/mpmi-7-0391. [DOI] [PubMed] [Google Scholar]
- 19.Matsuyama T, Bhasin A, Harshey R M. Mutational analysis of flagellum-independent surface spreading of Serratia marcescens 274 on a low-agar medium. J Bacteriol. 1995;177:987–991. doi: 10.1128/jb.177.4.987-991.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piper K R, von Bodman S B, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 21.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 22.Rich J J, Hirano S S, Willis D K. Pathovar-specific requirement for the Pseudomonas syringae lemA gene in disease lesion formation. Appl Environ Microbiol. 1992;58:1440–1446. doi: 10.1128/aem.58.5.1440-1446.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rich J J, Kinscherf T G, Kitten T, Willis D K. Genetic evidence that the gacA gene encodes the cognate response regulator for the lemA sensor in Pseudomonas syringae. J Bacteriol. 1994;176:7468–7475. doi: 10.1128/jb.176.24.7468-7475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacherer P, Défago G, Haas D. Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHAO. FEMS Microbiol Lett. 1994;116:155–160. doi: 10.1111/j.1574-6968.1994.tb06694.x. [DOI] [PubMed] [Google Scholar]
- 25.Willis D K, Hrabak E M, Rich J J, Barta T M, Lindow S E, Panopoulos N J. Isolation and characterization of a Pseudomonas syringae pv. syringae mutant deficient in lesion formation on bean. Mol Plant-Microbe Interact. 1990;3:149–156. [Google Scholar]
- 26.Wong S M, Carroll P A, Rahme L G, Ausubel F M, Calderwood S B. Modulation of expression of the ToxR regulon in Vibrio cholerae by a member of the two-component family of response regulators. Infect Immun. 1998;66:5854–5861. doi: 10.1128/iai.66.12.5854-5861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood D W, Pierson L S., III The phzI gene of Pseudomonas aureofaciens 30-84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene. 1996;168:49–53. doi: 10.1016/0378-1119(95)00754-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J P, Normark S. Induction of gene expression in Escherichia coli after pilus-mediated adherence. Science. 1996;273:1234–1236. doi: 10.1126/science.273.5279.1234. [DOI] [PubMed] [Google Scholar]