Abstract
Transport of water across the plasma membrane is a fundamental process occurring in all living organisms. In bacteria, osmotic movement of water across the cytoplasmic membrane is needed to maintain cellular turgor; however, the molecular mechanisms of this process are poorly defined. Involvement of aquaporin water channels in bacterial water permeability was suggested by the recent discovery of the aquaporin gene, aqpZ, in Escherichia coli. By employing cryoelectron microscopy to compare E. coli cells containing (AqpZ+) and lacking (AqpZ−) aquaporin, we show that the AqpZ water channel rapidly mediates large water fluxes in response to sudden changes in extracellular osmolarity. These findings (i) demonstrate for the first time functional expression of a prokaryotic water channel, (ii) evidence the bidirectional water channel feature of AqpZ, (iii) document a role for AqpZ in bacterial osmoregulation, and (iv) define a suitable model for studying the physiology of prokaryotic water transport.
Plasma membranes exhibit water permeability as a result of diffusion through the lipid bilayer, and some cells are able to transport water at greatly accelerated rates; however, the molecular pathway of this transport remained elusive until discovery of the aquaporins, a large family of water channel proteins (17). Aquaporins have been found in animals and plants (3, 10, 15) as well as in prokaryotes, where the aquaporin gene, aqpZ, was recently identified in wild-type Escherichia coli (4). Several eukaryotic aquaporins have been found to have physiological roles of primary importance, as demonstrated by the phenotypes resulting from naturally occurring mutations and targeted disruptions of related genes (2). In contrast, the role(s) of aquaporin water channels in prokaryotes is still undefined, although osmotically induced movement of water across the cytoplasmic membrane is believed to be one of the first osmoregulatory responses by which bacteria maintain cell turgor within the range needed for growth and survival (7, 19). aqpZ-like genes have been identified in several gram-negative bacterial species, while, surprisingly, canonical aquaporin genes have not been found in the genomes of gram-positive bacteria whose chromosomal DNAs have been sequenced (21). In order to elucidate the role(s) of aquaporins in gram-negative bacteria, we previously illustrated the potential importance of AqpZ by generating a strain of E. coli carrying a null mutation in the aqpZ gene. Compared directly to AqpZ+ parental wild-type bacteria (MM294 Strr), the AqpZ− strain (MM1211) was found to exhibit reduced growth in hypo-osmolar medium, in line with osmotic regulation of the aqpZ gene (5). However, this study did not show direct involvement of AqpZ in osmotically driven transport of water across the cytoplasmic membrane. Here, we have employed cryoelectron microscopy, a technique that preserves cell integrity, to directly evaluate participation of AqpZ in osmotic water flux in E. coli.
MATERIALS AND METHODS
Plasmids and bacterial strains.
The pPD100 plasmid (8) and strain BL21(λDE3) (20) were kindly provided by Erhard Bremer. The pOPAZ plasmid used to overproduce AqpZ was as described previously (6). The aqpZ null E. coli strain MM1211 (AqpZ−) and its parental wild-type strain, MM294 Strr (AqpZ+), as well as plasmid pGC94, were described previously (5, 6).
Osmotic shocks and cryoelectron microscopy.
AqpZ+ and AqpZ− E. coli cells were grown overnight and used to inoculate 30 ml of fresh M9 minimal medium (5). Cultures were grown at 37°C until the exponential phase of growth (optical density at 600 nm OD600 = 0.8). Bacteria were then rapidly pelleted and resuspended in 0.6 ml of M9 medium (osmolality, 240 mosM) at room temperature. A 2.5-μl drop of cell suspension was placed directly on a copper grid coated with a thin carbon film, upon which osmotic challenges were performed. Osmotic up-shocks were induced by rapidly mixing 2.5 μl of a 1.2 M sucrose-M9 solution with the cell suspension (final osmolality, 1,000 mosM). After intervals of up to 90 s, the suspension was briefly blotted with filter paper to a thin film and plunged into liquid ethane held at liquid nitrogen temperature. For osmotic down-shift experiments, bacterial suspensions were equilibrated at 1,000 mosM for 5 min before a 2.5-μl aliquot was placed on a microscope grid and rapidly mixed with an equal volume of 0.3 M sucrose in M9 (final osmolality, 750 mosM) for 15 s. Specimens were examined at −170°C in a Philips CM12 microscope with a Gatan model 626 cryoholder. Micrographs were recorded on Kodak SO 163 film under low-dose conditions and at a nominal magnification of ×6,300. A total of 77 fields was analyzed, corresponding to 150 cells in 10 independent experiments. Cell and cytoplasm volumes were calculated from projection images of E. coli with MACS, an image-processing software (18). In order to avoid cell size variations, results were expressed as ratios of cytoplasmic volume (Vi) to total cell volume (Ve).
RESULTS
Cryoelectron microscopy appearance of unchallenged E. coli cells.
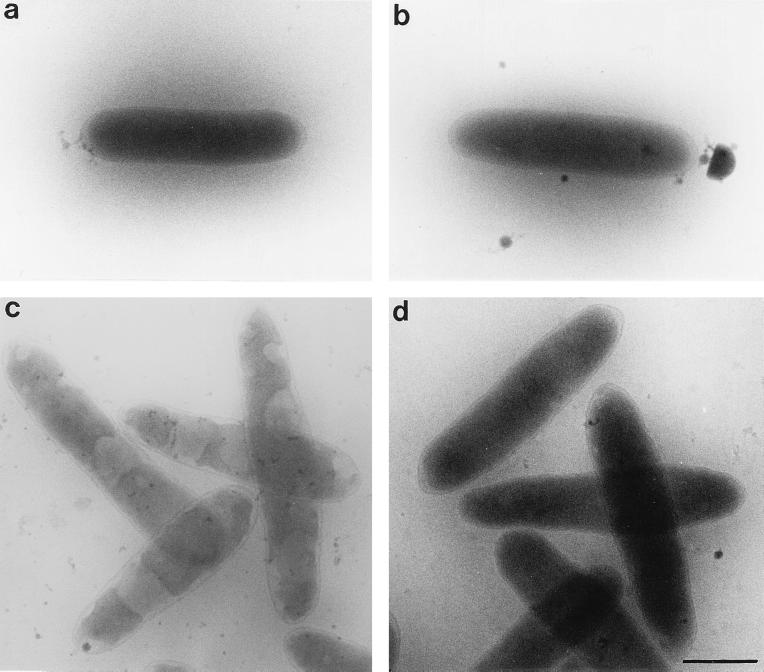
As a first step, the cryoelectron microscopy appearance of osmotically unchallenged (240 mosM) E. coli cells was characterized. The wild-type (MM294 Strr) or knock-out (MM1211) strain was grown to the exponential phase, at which expression of the aqpZ gene is maximal. Under these conditions, AqpZ+ and AqpZ− cell suspensions in vitrified thin layers appear indistinguishable (Fig. 1a and b). Both strains of bacteria are sharply delineated by the bacterial envelope, the periplasmic space is minimal, and no shrinkage of the cytoplasm is apparent.
FIG. 1.
Cryoelectron micrographs of AqpZ+ (MM294 Strr) and AqpZ− (MM1211) E. coli strains before and after osmotic up-shock. In the absence of osmotic challenge, no apparent morphological differences between AqpZ+ (a) and AqpZ− (b) E. coli cells are observed. The outer membrane, the murein layer, and the cytoplasmic membrane adhere to each other by enveloping the cytoplasm, which presents a regular shape. Following osmotic up-shift to 1,000 mosM for 15 s, shrinkage of the cytoplasm is visible in the AqpZ+ strain (c). In contrast, only slight wrinkling of the cell wall is apparent in the AqpZ− strain (d), and contraction of cytoplasmic volume is not detectable. Bar, 1 μm.
Appearance of the osmotically up-shocked aqpZ null mutant.
The AqpZ+ and AqpZ− strains of E. coli were observed by cryoelectron microscopy after challenge with a series of short-term osmotic up-shocks.
Exponentially grown cells were osmotically shocked by addition of an M9 solution containing the nonpermeable solute sucrose to a final osmolarity of 1,000 mosM for 15 s. Under these conditions, the morphology of AqpZ+ cells is strikingly altered (Fig. 1c). The large efflux of water driven by the osmotic gradient causes visible shrinkage of the cytoplasm, leading to separation of the cytoplasmic membrane from the other components of the wall and to consequent formation of plasmolysis spaces. In contrast, under the same experimental conditions, wrinkling of the cell wall occurs in osmotically up-shocked AqpZ− cells but no shrinkage of the cytoplasm is apparent (Fig. 1d).
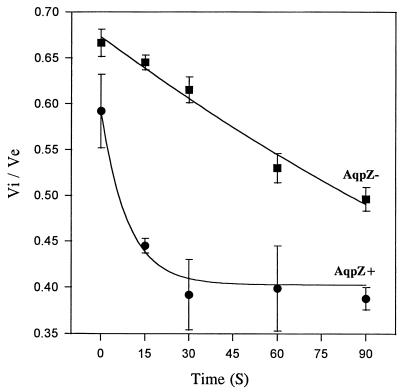
In other experiments, we compared the rates of change of cytoplasmic volume with time. AqpZ+ and AqpZ− cells exhibit markedly different time course responses to osmotic up-shock (Fig. 2). The AqpZ+ strain undergoes a large water efflux within seconds of osmotic up-shock, leading to nearly complete dehydration of the cytoplasm by 15 s. In contrast, the response of the AqpZ− strain is significantly slower, and even after 90 s shrinkage is not completed.
FIG. 2.
Time course of osmotically induced water efflux from AqpZ+ and AqpZ− E. coli strains. Cells were exposed to osmotic up-shocks, and the cytoplasmic (Vi) and total cell (Ve) volumes were measured at specified time intervals. In AqpZ+ cells, efflux of water follows an exponential decay and maximal decrease of cytoplasmic volume is completed within 30 s. AqpZ− cells have a slow response; even after 90 s, shrinkage is not completed.
Appearance of the osmotically up-shocked aqpZ null mutant complemented with a functional aqpZ gene.
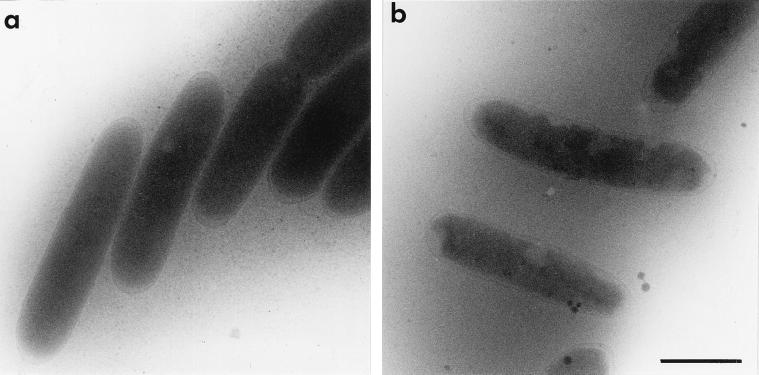
In control experiments, osmotic up-shift of 15 s was induced in AqpZ− E. coli cells transformed with pGC94, a plasmid bearing an intact and functional aqpZ gene. Under these conditions, the aqpZ-complemented null mutant strain shows clear cytoplasm shrinkage, the extent of which is comparable to that of the corresponding AqpZ+ strain (Fig. 3b).
FIG. 3.
Morphological appearance of aqpZ-complemented AqpZ− E. coli cells before and after osmotic up-shock. AqpZ− E. coli cells were transformed with pGC94 (6), a plasmid bearing an intact and functional aqpZ gene, and their morphological appearance was investigated by cryoelectron microscopy. In the absence of osmotic challenge, the morphology of the transformed cells (a) is identical to AqpZ+ and AqpZ− cells under similar conditions. Following osmotic up-shift to 1,000 mosM for 15 s, significant shrinkage of the cytoplasm is visible (b), as in the AqpZ+ strain. Bar, 1 μm.
Appearance of the osmotically down-shocked aqpZ null mutant.
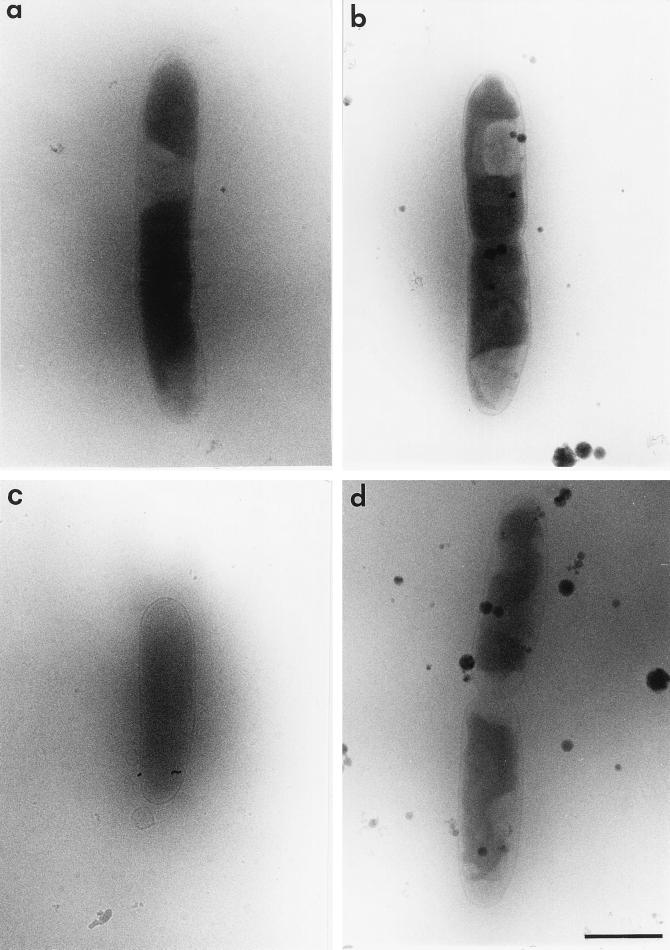
The possibility that AqpZ also mediates osmotic influx of water into E. coli cells was evaluated in experiments comparing AqpZ+ and AqpZ− strains which were previously equilibrated in hyperosmolar medium (1,000 mosM for 5 min) to increase the intracellular osmolarity. The prolonged incubation produces shrinkage of the cytoplasm in both AqpZ+ and AqpZ− cells (Fig. 4a and b). After an osmotic down-shock to 750 mosM for 15 s, AqpZ+ cells clearly undergo rapid and complete rehydration of the cytoplasm (Fig. 4c), whereas cytoplasm shrinkage is still persistent in AqpZ− cells during this interval (Fig. 4d).
FIG. 4.
Cryoelectron micrographs of AqpZ+ and AqpZ− E. coli strains before and after osmotic down-shock. AqpZ+ (a) and AqpZ− (b) cells were equilibrated to 1,000 mosM for 5 min to increase intracellular osmolarity. The cells were then osmotically down-shocked to 750 mosM for 15 s to induce osmotic entry of water. Complete rehydration of the cytoplasm is apparent in the AqpZ+ strain (c), whereas cytoplasm shrinkage is persistent in the AqpZ− strain (d). Bar, 1 μm.
DISCUSSION
Conventional specimen preparation methods for electron microscopy involve steps which are artifact prone. Artifacts are particularly severe with membrane structures and enveloped particles, whose size and material distribution can be dramatically affected by the preparation procedure. To be absolutely sure that any biological specimen is close to its native state, the ideal would be to maintain the initial aqueous environment during observation within the electron microscope. By far, the most successful approach has been development of the method of preparing and observing specimens in a thin layer of vitreous ice (1, 12). Many different types of biological specimens have now been examined in vitreous ice layers, and it has become clear that it is possible to avoid most artifacts. For example, it has been shown that fragile specimens such as liposomes and coated vesicles can be beautifully preserved in vitreous ice (13, 22). In addition, due to the fast freezing necessary for vitrification, structural intermediates can be trapped and visualized. Thus, cryomicroscopy makes it possible to perform time-resolved studies of rapid changes that occur under varying physiological conditions (14).
Abrupt changes in the environmental osmolarity are known to rapidly trigger large fluxes of water across the bacterial envelope; however, the molecular pathways by which this occurs are not clearly defined (7). As shown in this study, lack of cytoplasm shrinkage in AqpZ− E. coli cells submitted to short-term osmotic up-shock indicates that AqpZ is likely responsible for the outwardly directed flux of water triggered by the increase in extracellular osmolarity. This result is strongly supported by additional control experiments in which the ability of the AqpZ− strain to rapidly dehydrate the cytoplasm is restored by transformation of the cells with pGC94 (6), a plasmid bearing an intact and functional aqpZ gene.
Bacteria are small in order to increase their surface-to-volume ratio, with concomitant decreases in problems of transport of solutes and water (11). Thus, diffusional water permeability due to membrane lipids may make a significant contribution to the movement of water across the inner membrane of E. coli. Cytoplasm shrinkage observed with prolonged incubations of the AqpZ− strain (Fig. 4) confirms that low but finite water permeability is an intrinsic feature of membrane lipids (9). This may explain why the AqpZ− E. coli strain does not express a more severe phenotype and why gram-positive bacteria apparently lack aquaporin water channels.
Influx of water into the bacterial cell has also been postulated to be a direct consequence of sudden decreases in extracellular osmolarity (7, 11). Consistent with the anticipated possible role of AqpZ in mediating water influx (5), we show that AqpZ+ cells undergo rapid and complete rehydration of the cytoplasm, whereas AqpZ− cells exhibit little response in this interval. These results, taken with those of osmotic up-shift, indicate that the aqueous pore created by AqpZ permits osmotically driven movement of water in both directions, a property which has also been recently demonstrated for multiple mammalian aquaporins (16).
The data reported in the present work provide the first direct demonstration that the prokaryotic water channel, AqpZ, is functionally expressed in E. coli, where it is able to mediate rapid entry or exit of water in response to abrupt changes in extracellular osmolarity. Although puzzling questions on the physiological necessity of fast water transport in bacteria remain, our findings may provide important insight in better defining the mechanisms of osmoadaptation that make bacteria capable of growth and survival in environments with wide ranges of osmolarity.
ACKNOWLEDGMENTS
We thank Paul Blount and Erhard Bremer for valuable contributions and suggestions, Stefan Hohmann and Carlos Blanco for comments on the manuscript, and Louis Communier and Joëlle Alori for photography. Electron microscopy was performed at the Centre de Microscopie Electronique à Transmission de l’Université de Rennes 1, Rennes, France.
This study was supported by CNRS and Fondation Langlois (to C.D., D.T., J.-P.R., A.F., and J.G.).
REFERENCES
- 1.Adrian M, Dubochet J, Lepault J, McDowall A W. Cryo-electron microscopy of viruses. Nature. 1984;308:32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- 2.Agre P. Aquaporin null phenotypes: the importance of classical physiology. Proc Natl Acad Sci USA. 1998;95:9061–9063. doi: 10.1073/pnas.95.16.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agre P, Bonhivers M, Borgnia M. The aquaporins, blueprints for cellular plumbing systems. J Biol Chem. 1998;273:14659–14662. doi: 10.1074/jbc.273.24.14659. [DOI] [PubMed] [Google Scholar]
- 4.Calamita G, Bishai W R, Preston G M, Guggino W B, Agre P. Molecular cloning and characterization of AqpZ, a water channel from Escherichia coli. J Biol Chem. 1995;270:29063–29066. doi: 10.1074/jbc.270.49.29063. [DOI] [PubMed] [Google Scholar]
- 5.Calamita G, Kempf B, Bonhivers M, Bishai W R, Bremer E, Agre P. Regulation of the Escherichia coli water channel gene aqpZ. Proc Natl Acad Sci USA. 1998;95:3627–3631. doi: 10.1073/pnas.95.7.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calamita G, Kempf B, Rudd K E, Bonhivers M, Kneip S, Bishai W R, Bremer E, Agre P. The aquaporin-Z water channel gene of Escherichia coli: structure, organization and phylogeny. Biol Cell. 1997;89:321–329. [PubMed] [Google Scholar]
- 7.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 8.Dersh P, Fsihi H, Bremer E. Low-copy-number T7 vectors for selective gene expression and efficient protein over production in Escherichia coli. FEMS Microbiol Lett. 1994;123:19–26. doi: 10.1111/j.1574-6968.1994.tb07195.x. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein A. Water movement through lipid bilayers, pores, and plasma membranes: theory and reality. New York, N.Y: Wiley-Interscience; 1987. [Google Scholar]
- 10.Froger A, Tallur B, Thomas D, Delamarche C. Prediction of functional residues in water channels and related proteins. Protein Sci. 1998;7:1458–1468. doi: 10.1002/pro.5560070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch A L. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- 12.Lepault J, Booy F, Dubochet J. Electron microscopy of frozen biological suspensions. J Microsc. 1983;129:89–102. doi: 10.1111/j.1365-2818.1983.tb04163.x. [DOI] [PubMed] [Google Scholar]
- 13.Lepault J, Pattus F, Martin N. Cryo-electron microscopy of artificial biological membranes. Biochim Biophys Acta. 1985;820:315–318. [Google Scholar]
- 14.Lepault J, Erk I, Nicolas G, Ranck J L. Time-resolved cryo-electron microscopy of vitrified muscular components. J Microsc. 1991;161:47–57. doi: 10.1111/j.1365-2818.1991.tb03072.x. [DOI] [PubMed] [Google Scholar]
- 15.Maurel C. Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- 16.Meinild A K, Klaerke D A, Zeuthen T. Bidirectional water fluxes and specificity for small hydrophobic molecules in aquaporin 0-5. J Biol Chem. 1998;273:32446–32451. doi: 10.1074/jbc.273.49.32446. [DOI] [PubMed] [Google Scholar]
- 17.Preston G M, Carroll T P, Guggino W B, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 water channel. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 18.Rolland J P, Bron P, Thomas D. MACS: automatic counting of objects based on shape recognition. Comput Appl Biosci. 1997;5:563–564. doi: 10.1093/bioinformatics/13.5.563. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz H, Koch A L. Phase and electron microscopic observations of osmotically induced wrinkling and the role of endocytotic vesicles in the plasmolysis of the Gram-negative cell wall. Microbiology. 1995;141:3161–3170. doi: 10.1099/13500872-141-12-3161. [DOI] [PubMed] [Google Scholar]
- 20.Studier F W, Moffat B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression. J Mol Biol. 1990;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 21.TIGR Microbial Database. 25 May 1999, revision date. [Online.] The Institute for Genomic Research, Rockville, Md. http://www.tigr.org/tdb/mdb/mdb.html. [31 May 1999, last date accessed.]
- 22.Vigers G P, Crowther R A, Pearse B M. Three-dimensional structure of clathrin cages in ice. EMBO J. 1986;5:529–534. doi: 10.1002/j.1460-2075.1986.tb04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]