Abstract
Purpose
The objective of this study was to investigate the molecular characteristics and potential resistance mechanisms of linezolid-resistant (LZR) Staphylococcus capitis isolates from a tertiary hospital in China.
Methods
S. capitis isolates were obtained from clinical patient specimens; three of the isolates came from blood cultures and one from the hydrothorax. The agar dilution and E-test methods were used to identify antibiotic resistance. The chloramphenicol-florfenicol resistance (cfr) gene carrier status of the strains was determined by PCR. Whole-genome sequencing (WGS) was used to identify point mutations and L3, L4, and L22 mutations and to study the genetic environment of the cfr gene and the relationships between strains.
Results
The 4 isolates obtained in this study were all linezolid-resistant Staphylococcus strains. A similar of susceptibility profile pattern was observed in all four S. capitis strains, each of which exhibited a multidrug-resistant phenotype. A potentially novel mutation, C2128T, was identified, and the cfr genes of S. capitis strains were all positive. Additionally, the same mutations (C2128T and G2600T) were identified in all 23S rRNA sequences of the isolates, whereas mutations were lacking in the L3, L4, and L22 ribosomal proteins. The genetic environments surrounding cfr were identical in all four isolates. A schematic diagram of the phylogenetic tree showed that they were closely related to AYP1020, CR01, and TW2795, and a total of seven drug resistance genes were identified in these strains.
Conclusions
The study indicated that the resistance of the Staphylococcus capitis strains to linezolid was caused by multiple mechanisms, and a potential novel mutation, C2128T, that may have an impact on bacterial resistance was identified.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-022-02616-9.
Keywords: Linezolid resistance, C2128T mutation, Coagulase-negative Staphylococcus, Chloramphenicol-florfenicol resistance, 23S rRNA gene
Introduction
Staphylococcus capitis, a coagulase-negative staphylococcus (CoNS) bacterium, is a normal component of the human skin tissue microbiota that can cause diseases of the body under certain conditions, including skin infections, bloodstream infections, and even sepsis [1, 2]. In recent years, due to the wide application of a wide range of broad-spectrum antibacterial drugs, immunosuppressants, and chemotherapy drugs in the clinic and the widespread implementation of various invasive surgeries, the bacterial harvest rate has increased each year, and drug resistance has continuously risen. At present, the phenomenon of bacterial drug resistance is increasing at an alarming rate [3]. Linezolid was the first oral oxazolidinone antibiotic approved by the U.S. Food and Drug Administration (FDA) for clinical applications in 2000 [4]. This antibiotic is active against a broad spectrum of gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae, and vancomycin-resistant Enterococci (VRE) [5]. Linezolid inhibits bacterial protein production by binding to rRNA on the 50S ribosomal subunit [6]. Linezolid is primarily used to block the development of the initiation complex during protein synthesis; it can inhibit the formation of the initiation complex but does not affect the elongation or termination of the peptide chain during protein synthesis [7].
The incidence of linezolid resistance is gradually increasing with the widespread use of linezolid. There have been many previous reports of linezolid-resistant (LZR) S. capitis infections. According to previous studies, the best-defined mechanism of change in LZR is a mutation of the 23S rRNA gene target and acquisition of the chloramphenicol-florfenicol resistance (cfr) gene [8–10]. 23S rRNA is a component of the bacterial ribosome 50S subunit, and 23S rRNA mutations can arise during exposure to linezolid; hence, the drug stops working, eventually leading to drug resistance. Gene mutation sites also vary among different strains [11, 12]. G2447U and G2576U gene mutations are the most common in Staphylococcus [10, 13]. Cfr, which was first discovered in plasmids of Staphylococcus isolates from German cows, encodes a methyltransferase that can modify 23S rRNA [14]. The cfr gene can mediate the methylation at large subunit sites of ribosomes, causing linezolid resistance [15, 16].
In addition, LZR S. aureus was reported in the first year of the clinical use of linezolid in China [17]. Since then, LZR Staphylococcus isolates have been successively documented in the United States [18], Mexico [19], Japan [20], Spain [21], and Italy [22]. Therefore, there is an urgent need to elucidate the resistance mechanism of LZR in China. This study was conducted to investigate the clinical features of LZR S. capitis found in clinical infections, to further explore its molecular characteristics and mechanisms of resistance and provide a clinical treatment for the prevention of LZR S. capitis.
Materials and methods
Bacterial isolates and patients
From 2018 to 2019, the study of 4 linezolid-resistant bacterial strains isolated from patients and associated data was authorized by the ethics committee of the Fourth Affiliated Hospital of Harbin Medical University, China. Before their inclusion in the current study, all patients signed a written informed consent form. Four Staphylococcus capitis isolates resistant to linezolid were identified in the study, all of which were included in further analyses. The strains were first isolated and identified using the VITEK 2 COMPACT system (VITEK 2 Compact, Biomerieux, France) and then confirmed by 16S sequencing. The isolates were recovered from patients with chronic obstructive pulmonary disease (COPD), acute coronary syndrome, rectal cancer, and a male pelvic abscess. Clinical data of the patients harbouring each isolate, including their age, sex, prior exposure to linezolid, and clinical outcome, were obtained retrospectively. The data were kept anonymous.
Antimicrobial susceptibility testing
The tested antimicrobial agents include oxacillin, penicillin, gentamicin, ciprofloxacin, levofloxacin, moxifloxacin, erythromycin, clindamycin, quinupristin/dalfopristin, vancomycin, tetracycline, tigecycline, rifampicin, trimethoprim/sulfamethoxazole, and linezolid, as recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines [23]. The minimal inhibitory concentration (MIC) (VITEK 2 Compact, Biomerieux, France) of every antibiotic was calculated via the agar dilution MIC method, and the results were interpreted according to the CLSI. The MIC of linezolid was also calculated with the E-test (Biomerieux, France) according to the instructions provided by the manufacturer. S. aureus ATCC 25923 and ATCC 29213 were tested concurrently for quality control (both showed a linezolid MIC of ≤1 μg/ml, Laboratory Department of the Fourth Affiliated Hospital of Harbin Medical University, China).
PCR identification of cfr
PCR was used to determine the existence of cfr in S. capitis. The colonies harvested from agar plates were incubated for 5 minutes in 500 μl of H2O at a temperature of 100 °C. Following 2 minutes of centrifugation, 1 μl of the resultant supernatant was used as a template for PCR conducted using two cfr-specific primers (forward primer: 5′-GAAGCTCTAGCCAACCGTCA-3′, reverse primer: 5′-TCTACCTGCCCTTCGTTTGC-3′, amplicon size: 458 base pairs (bp), overall gene fragment size: 1050 bp, GenBank reference sequence: AM408573). The amplification conditions were as follows: 5 minutes at a temperature of 94 °C; 30 cycles of 94 °C for 30 seconds, 55 °C for 30 seconds, and 72 °C for 1 minute; and a final extension step of 72 °C for 7 minutes. The PCR products were imaged by using a visible light transilluminator (Bioteke, Beijing, China).
Whole-genome sequencing (WGS)
Products of bacterial genomic DNA were sequenced on the Illumina HiSeq (Illumina, America) and PacBio RS (Pacific Biosciences, America) platforms after they were extracted and purified by using a purification kit (TaKaRa, Dalian, China). On the basis of the 16S rRNA nucleotide sequence, a phylogenetic analysis was performed. The obtained 16S and 23S genome sequences are stored in GenBank (accession numbers SUB11152030 and SUB11152217, respectively). The four strains’ representative 16S rRNA nucleotide sequences were compared against the sequences of other Staphylococcus strains stored in GenBank (Supplementary materials). The evolutionary history was inferred using the neighbour-joining method [24]. The optimal tree with a sum of branch length = 0.10605004 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to the branches [25]. The tree was drawn to scale with branch lengths in the same units as the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method and are presented in units of the number of base differences per site. The analysis involved 27 nucleotide sequences. The included codon positions were 1st + 2nd + 3rd + Noncoding. All positions with less than 50% site coverage were eliminated. That was, fewer than 50% of alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 1496 positions in the final dataset. Phylogenetic inferences were made using the neighbour-joining method within MEGA software (version 7.0.26) [26].
Genetic environment of the cfr gene
The genetic context of the cfr gene was determined by WGS. The reference sequences of S. capitis pXWZ (GenBank reference sequence MT096435), S. aureus pSR01 (GenBank reference sequence CP048644), S. capitis pcfr-XZ03 (GenBank reference sequence CP077712), and S. xylosus pSX01 (GenBank reference sequence KP890694) were downloaded from the National Center of Biotechnology Information (NCBI). Resistance genes were obtained from the Comprehensive Antibiotic Research Database (CARD) (https://card.mcmaster.ca/). The polished assembly report and annotation information of the obtained genomic sequences of the 4 strains are shown in supplementary tables (Supplementary Tables 1 and 2).
Results
Characteristics of linezolid-resistant S. capitis
The 4 isolates obtained in this study were all linezolid-resistant Staphylococcus strains. Three strains of linezolid-resistant S. capitis were recovered from blood samples, and one strain was recovered from a pleural effusion sample. Table 1 summarizes the demographic and clinical characteristics of the patients. All patients had received linezolid for an average of 11.5 days leading up to the isolation of linezolid-resistant S. capitis. A similar susceptibility profile pattern was observed in the four S. capitis strains. All of the isolates exhibited a multidrug-resistant phenotype (resistant to three or more different antibiotics). All isolates were resistant to linezolid (MIC 256 mg/L), penicillin, oxacillin, ciprofloxacin, gentamicin, erythromycin, moxifloxacin, and levofloxacin. Their resistance to clindamycin and quinupristin/dalfopristin was variable (Table 2).
Table 1.
Clinical information of patients with linezolid-resistant Staphylococcus capitis isolates
| Isolate | Age (years)/Gender | Underlying diseases | Date of isolation (dd/mm/yy) | Source | Department | Prior linezolid therapy | Time to isolation of LZR S. capitis (days) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 701 | 68/M | COPD | 04/08/2018 | Blood | ICU | Yes | 13 | Survived |
| 703 | 53/M | Acute coronary syndrome | 03/09/2018 | Blood | Cardiology | Yes | 12 | Died |
| 708 | 52/M | Rectal cancer | 11/10/2018 | Blood | ICU | Yes | 8 | Survived |
| 709 | 60/M | Male pelvic abscess | 10/07/2019 | Hydrothorax | ICU | Yes | 13 | Survived |
ICU Intensive care unit, M Male, COPD Chronic obstructive pulmonary disease, LZR S. capitis Linezolid-resistant Staphylococcus capitis
Table 2.
Antibiotic susceptibilities and linezolid resistance mechanisms of linezolid-resistant Staphylococcus capitis isolates
| Isolate | MIC (mg/L) | Genetic resistance markers | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LZD | P | OXA | CN | CIP | LEV | MXF | E | DA | QD | VA | TE | TGC | RA | SXT | cfr | 23S rRNA | L3 | L4 | L22 | |
| 701 | ≥256 | ≥0.5 | ≥4 | ≥16 | ≥8 | 4 | 2 | ≥8 | 2 | 1 | ≤0.5 | 1 | 0.25 | ≤0.5 | 20 | + | C2128, G2600T | – | – | – |
| 703 | ≥256 | ≥0.5 | ≥4 | ≥16 | ≥8 | ≥8 | 2 | ≥8 | ≥8 | 2 | ≤0.5 | 2 | 0.5 | ≤0.5 | 20 | + | C2128T, G2600T | – | – | – |
| 708 | ≥256 | ≥0.5 | ≥4 | ≥16 | ≥8 | 4 | 2 | ≥8 | ≥8 | 2 | ≤0.5 | 2 | 0.5 | ≤0.5 | 20 | + | C2128T, G2600T | – | – | – |
| 709 | ≥256 | ≥0.5 | ≥4 | ≥16 | ≥8 | 4 | 2 | ≥8 | ≥8 | 2 | ≤0.5 | 2 | 0.5 | ≤0.5 | 20 | + | C2128T, G2600T | – | – | – |
MIC Minimum inhibitory concentration, LZD Linezolid, P Penicillin, OXA Oxacillin, CN Gentamicin, CIP Ciprofloxacin, LEV levofloxacin, MXF Moxifloxacin, E Erythromycin, DA Clindamycin, QD Quinupristin/dalfopristin, VA Vancomycin, TE Tetracycline, TGC Tigecycline, RA Rifampicin, SXT Trimethoprim/sulfamethoxazole (19 mg/L /1 mg/L)
Resistance genes and mutations
The PCR screening results were positive for the cfr gene in all isolates, which was further confirmed by sequencing. Additionally, an investigation of the 23S rRNA sequences revealed identical mutations (C2128T and G2600T) in all isolates but no alterations in ribosomal proteins L3, L4, and L22. A potentially novel mutation, C2128T, was identified in all four linezolid-resistant S. capitis isolates (GenBank accession number SUB11152217).
Genetic environment of the cfr gene in the plasmids
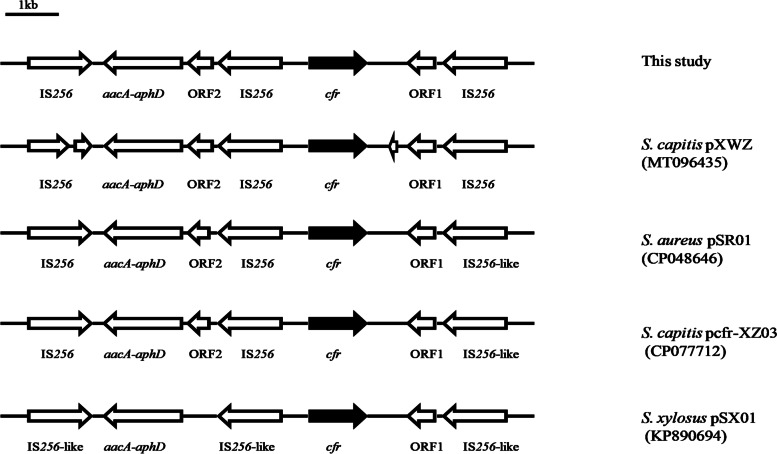
The genetic environment surrounding cfr was similar in all four isolates (Fig. 1). A complete Tn4001-like transposon was identified upstream of cfr in the plasmid. The aminoglycoside resistance gene pair aacA-aphD carried by Tn4001 was flanked by two IS256 elements containing transposase genes. Another copy of the IS256-associated transposase gene was discovered downstream of cfr; this copy was oriented towards the cfr gene and identical in sequence to the right (cfr-proximal) transposase gene of the Tn4001-like transposon. This transposase gene was followed by a short open reading frame 1 (ORF1) encoding a protein with similarity to a transcriptional regulator of a helix-turn-helix (HTH) protein. The genetic environment surrounding the cfr gene exhibited striking similarity to those of previously reported plasmids, including pcfr-XZ03 (100% coverage, 100% identity, CP077712) and pXWZ (100% coverage, 100% identity, MT096435) from S. capitis strains, pSR01 (100% coverage, 99.98% identity, CP048646) from S. aureus strains, and pSX01 (99% coverage, 99.97% identity, KP890694) from an S. xylosus strain. These findings are consistent with the spread of cfr-positive sequences among different species and genera via various health care facilities.
Fig. 1.
Schematic diagram of the genetic environment of the cfr gene in this study. The arrows represent the positions and direction of the elements. The cfr gene is shown in black
Genetic relationship with other strains and drug resistance genes
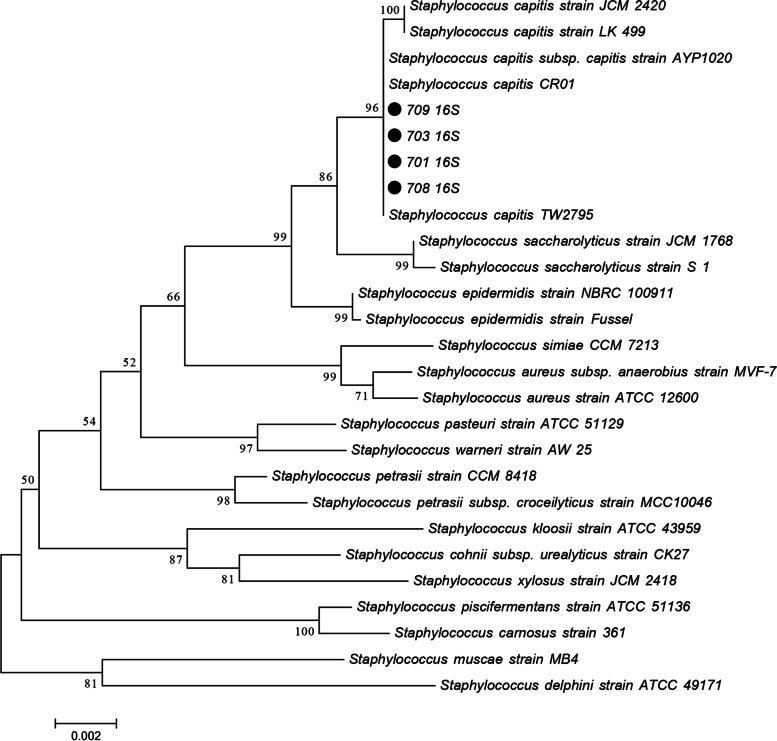
The 16S rRNA genes of the four linezolid-resistant strains were identical to the sequences of the corresponding region (GenBank accession number SUB11152030), and they were more closely related to AYP1020, CR01, and TW2795 than to other strains (Fig. 2). The comparison with the CARD revealed that the resistance genes carried by the four linezolid-resistant strains of S. capitis were identical to those shown in Table 3. There were a total of seven drug resistance genes, among which cfr, blaZ, qacA, and aac(6′)-aph(2″) were located on the plasmids.
Fig. 2.
Schematic diagram of the phylogenetic tree of the four strains. The strains of the research group have been marked with emphasis. The strains and their corresponding GenBank accession numbers for 16S rRNA genes are shown following the organism names. Numbers at the branching nodes are percentages of bootstrap values based on 500 replications. Bootstrap values greater than 50% are shown at the branch points. The scale bar represents 0.002 substitutions per nucleotide position
Table 3.
Drug resistance genes from WGS
| Isolates | Aminoglycoside | Beta-lactam | Macrolide | Oxazolidinone | Phenicol | Disinfectant |
|---|---|---|---|---|---|---|
| 701 | AadD;aac(6′)-aph(2″); aadD; ant(9)-Ia | mecA;blaZ | ermA;cfr | cfr | cfr | qacA |
| 703 | AadD;aac(6′)-aph(2″); aadD; ant(9)-Ia | mecA;blaZ | ermA;cfr | cfr | cfr | qacA |
| 708 | AadD;aac(6′)-aph(2″); aadD; ant(9)-Ia | mecA;blaZ | ermA;cfr | cfr | cfr | qacA |
| 709 | AadD;aac(6′)-aph(2″); aadD; ant(9)-Ia | mecA;blaZ | ermA;cfr | cfr | cfr | qacA |
WGS Whole-genome sequencing
Discussion
Health care settings have seen a notable increase in the isolation of drug-resistant strains since the extensive use of linezolid was initiated. In the current study, we determined the incidence of the cfr gene and mutations of C2128T and G2600T in 23S rRNA that existed in linezolid-resistant S. capitis isolates recovered from 4 patients from a tertiary hospital in China. In general, cfr methyltransferase mediates resistance to antibiotics targeting the 50S ribosome subunit, including florfenicol, lincosamide, oxazolidinone, pleuromutilin, and streptomycin [10]. Thus, cfr-carrying staphylococci exhibit a multidrug-resistant phenotype that is consistent with the resistance profile of the isolates obtained in this study. A novel mutation was identified in the V region of the 23S rRNA, located near the linezolid binding site. Notably, the cfr gene was shown to be carried by plasmids, making it amenable to horizontal transmission across staphylococci. Previously, it was shown that the cfr gene plays a critical role in mediating linezolid resistance. The isolates expressing the cfr gene exhibited significantly higher levels of linezolid resistance than the isolates without the cfr gene [9]. The observation of a high prevalence of cfr in our drug-resistant strains raises the possibility of horizontal gene transfer, which is a reminder of the need for a proactive approach to infection control.
In addition, previous studies have reported that His 146 and Gly155Arg in L3 from a research lab strain of S. aureus and a mutation in the conserved region (63KPWRQKGTGRAR74) of the L4 protein are all related to the cross-resistance of S. pneumoniae, S. aureus, and C. perfringens to linezolid [27–29]. However, the L3, L4, and L22 ribosomal proteins of the four linezolid-resistant isolates studied in our investigation did not exhibit similar mutations. Furthermore, a unique mutation, C2128T, was identified in the 23S rRNA gene; this mutation was distinct from the typical changes in the central loop of 23S rRNA domain V observed in most linezolid-resistant strains, such as Enterococcus and Staphylococcus. To the best of our knowledge, resistance to linezolid has been attributed to G2528U, G2576U, and G2505A (Enterococcus strains); G2447U, G2576U and G2603T (Staphylococcus strains); and C2534T, G2447T, G2576T, T2504A, C2109T and G2474T (Coagulase-negative Staphylococci strains) [13, 30, 31]. Notably, there was an absence of the most prevalent mutation, G2576T, in the V region of the 23S rRNA gene in all four linezolid-resistant isolates, whereas the G2600T mutation was identified in all isolates. Additionally, investigations have discovered a correlation between the prevalence of linezolid resistance and the number of mutations in a copy of the 23S rRNA-encoding gene [32]. The close genetic relatedness of the clones examined in this study to the CR01 and AYP1020 genomes was surprising, as both CR01 and AYP1020are fully susceptible strains. To date, no explanation has been suggested for this unexpected clustering. Both lineages may represent preresistance lineages of linezolid clones, but there is presently no epidemiological evidence to substantiate this hypothesis [33].
Since 3 of the strains of LZR S. capitis included in this study were recovered from blood samples, it is important for clinicians to perform blood cultures to confirm the presence of bloodstream infections. The timely detection of linezolid-resistant staphylococci in the early stage is of great significance for ensuring optimal antibiotic treatment and limiting the emergence of multidrug-resistant bacteria. A previous report showed that the mean isolation time of linezolid-resistant coagulase-negative staphylococci (LRCoNS) strains was 11.0 ± 8.0 days in patients following linezolid treatment, but resistant strains were obtained in a few cases because of cross-infection [13]. This may provide more substantial proof of the theory that the use of linezolid is a potential independent risk factor for the development of LRCoNS strains [34]. This has been shown by the finding that all patients carrying linezolid-resistant S. capitis had taken linezolid medication for an average of 11.5 days prior to the harvesting of the linezolid-resistant S. capitis in our investigation. Taking into consideration that the same drug-resistant clones were recovered from these patients and the time periods during which the strains were harvested, we hypothesize that this could have been due to clonal propagation between strains 701, 703, and 708 [35]. While the incidence of LZR staphylococci remains low, factors such as prolonged hospitalization, numerous interventions, and irrational antibiotic use may hasten the emergence and spread of LZR staphylococci. The discreet use of linezolid and monitoring of staphylococcal resistance are necessary for therapeutic efficacy.
Conclusion
In conclusion, we analysed mutations in the 23S rRNA of 4 LZR S. capitis strains, together with isolated quality control strains and sensitive strains such as AYP1020 and CR01, to identify the potentially novel mutation C2128T, which may have an impact on linezolid resistance. This work provides new ideas for improving the rational use of antibacterial drugs and the prevention and control of hospital infections in the future and minimizing the emergence of multidrug resistance.
Supplementary Information
Additional file 2: Supplementary Table 1. Polished Assembly Report of 4 strains. Supplementary Table 2. Annotation Information of 4 strains.
Acknowledgements
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Authors’ contributions
Xiao Han: Data analysis and writing original draft preparation; Guiling Zou: Conceptualization and Data curation; Jiaren Liu, Chun Yang, and Xuefei Du: Project administration and Supervision; Guoyu Chen, Zhe Sun, Xinyu Zhang, Yu Sun, and Wanying Zhang: Investigation, Resources, Validation; Xiaofeng Jiang: Writing – review and editing. All authors contributed to the study conception and design and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant 81171657).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the GenBank repository, https://cipotato.org/genebankcip/.
Declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the ethics committee of the Fourth Affiliated Hospital of Harbin Medical University. The informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not Applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Natsis NE, Cohen PR. Coagulase-negative staphylococcus skin and soft tissue infections. Am J Clin Dermatol. 2018;19(5):671–677. doi: 10.1007/s40257-018-0362-9. [DOI] [PubMed] [Google Scholar]
- 2.Tchana-Sato V, Defraigne JO. Staphylococcus capitis causing infective endocarditis: not so uncommon. Infection. 2020;48(6):975–977. doi: 10.1007/s15010-020-01514-4. [DOI] [PubMed] [Google Scholar]
- 3.Roca I, Akova M, Baquero F, et al. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach KL, Brickner SJ, Noe MC, et al. Linezolid, the first oxazolidinone antibacterial agent. Ann N Y Acad Sci. 2011;1222:49–54. doi: 10.1111/j.1749-6632.2011.05962.x. [DOI] [PubMed] [Google Scholar]
- 5.Brickner SJ, Barbachyn MR, Hutchinson DK, et al. Linezolid (ZYVOX), the first member of a completely new class of antibacterial agents for treatment of serious gram-positive infections. J Med Chem. 2008;51(7):1981–1990. doi: 10.1021/jm800038g. [DOI] [PubMed] [Google Scholar]
- 6.Batts DH. Linezolid--a new option for treating gram-positive infections. Oncology (Williston Park, NY) 2000;14(8 Suppl 6):23–29. [PubMed] [Google Scholar]
- 7.Bobkova EV, Yan YP, Jordan DB, et al. Catalytic properties of mutant 23 S ribosomes resistant to oxazolidinones. J Biol Chem. 2003;278(11):9802–9807. doi: 10.1074/jbc.M209249200. [DOI] [PubMed] [Google Scholar]
- 8.Cai JC, Hu YY, Zhang R, et al. Linezolid-resistant clinical isolates of meticillin-resistant coagulase-negative staphylococci and Enterococcus faecium from China. J Med Microbiol. 2012;61(Pt 11):1568–1573. doi: 10.1099/jmm.0.043729-0. [DOI] [PubMed] [Google Scholar]
- 9.Yang XJ, Chen Y, Yang Q, et al. Emergence of cfr-harbouring coagulase-negative staphylococci among patients receiving linezolid therapy in two hospitals in China. J Med Microbiol. 2013;62(Pt 6):845–850. doi: 10.1099/jmm.0.051003-0. [DOI] [PubMed] [Google Scholar]
- 10.Long KS, Vester B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother. 2012;56(2):603–612. doi: 10.1128/AAC.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz S, Werckenthin C, Kehrenberg C. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob Agents Chemother. 2000;44(9):2530–2533. doi: 10.1128/AAC.44.9.2530-2533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee JE, De BK, Levett PN, et al. Use of 16S rRNA gene sequencing for rapid confirmatory identification of Brucella isolates. J Clin Microbiol. 2004;42(8):3649–3654. doi: 10.1128/JCM.42.8.3649-3654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu B, Kelesidis T, Tsiodras S, et al. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother. 2013;68(1):4–11. doi: 10.1093/jac/dks354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefani S, Bongiorno D, Mongelli G, et al. Linezolid resistance in staphylococci. Pharmaceuticals (Basel) 2010;3(7):1988–2006. doi: 10.3390/ph3071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu BG, Yuan XL, He DD, et al. Research progress on the oxazolidinone drug linezolid resistance. Eur Rev Med Pharmacol Sci. 2020;24(18):9274–9281. doi: 10.26355/eurrev_202009_23009. [DOI] [PubMed] [Google Scholar]
- 16.Sadowy E. Linezolid resistance genes and genetic elements enhancing their dissemination in enterococci and streptococci. Plasmid. 2018;99:89–98. doi: 10.1016/j.plasmid.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Jones RN, Fritsche TR, Sader HS, et al. LEADER surveillance program results for 2006: an activity and spectrum analysis of linezolid using clinical isolates from the United States (50 medical centers) Diagn Microbiol Infect Dis. 2007;59(3):309–317. doi: 10.1016/j.diagmicrobio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Mendes RE, Deshpande LM, Castanheira M, et al. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob Agents Chemother. 2008;52(6):2244–2246. doi: 10.1128/AAC.00231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendes RE, Deshpande L, Rodriguez-Noriega E, et al. First report of staphylococcal clinical isolates in Mexico with linezolid resistance caused by cfr: evidence of in vivo cfr mobilization. J Clin Microbiol. 2010;48(8):3041–3043. doi: 10.1128/JCM.00880-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda-Dantsuji Y, Hanaki H, Sakai F, et al. Linezolid-resistant Staphylococcus aureus isolated from 2006 through 2008 at six hospitals in Japan. J Infect Chemother. 2011;17(1):45–51. doi: 10.1007/s10156-010-0085-1. [DOI] [PubMed] [Google Scholar]
- 21.Seral C, Sáenz Y, Algarate S, et al. Nosocomial outbreak of methicillin- and linezolid-resistant Staphylococcus epidermidis associated with catheter-related infections in intensive care unit patients. Int J Med Microbiol. 2011;301(4):354–358. doi: 10.1016/j.ijmm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Mendes RE, Deshpande LM, Farrell DJ, et al. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J Antimicrob Chemother. 2010;65(11):2329–2335. doi: 10.1093/jac/dkq331. [DOI] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing; 23rd informational supplement M100-S23. Wayne: CLSI; 2013. [Google Scholar]
- 24.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 25.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hölzel CS, Harms KS, Schwaiger K, et al. Resistance to linezolid in a porcine Clostridium perfringens strain carrying a mutation in the rplD gene encoding the ribosomal protein L4. Antimicrob Agents Chemother. 2010;54(3):1351–1353. doi: 10.1128/AAC.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locke JB, Hilgers M, Shaw KJ. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700) Antimicrob Agents Chemother. 2009;53(12):5265–5274. doi: 10.1128/AAC.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolter N, Smith AM, Farrell DJ, et al. Novel mechanism of resistance to oxazolidinones, macrolides, and chloramphenicol in ribosomal protein L4 of the pneumococcus. Antimicrob Agents Chemother. 2005;49(8):3554–3557. doi: 10.1128/AAC.49.8.3554-3557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamon RC, Iorio NL, Cavalcante FS, et al. Linezolid-resistant Staphylococcus haemolyticus and Staphylococcus hominis: single and double mutations at the domain V of 23S rRNA among isolates from a Rio de Janeiro hospital. Diagn Microbiol Infect Dis. 2014;80(4):307–310. doi: 10.1016/j.diagmicrobio.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Maarouf L, Omar H, El-Nakeeb M, et al. Prevalence and mechanisms of linezolid resistance among staphylococcal clinical isolates from Egypt. Eur J Clin Microbiol Infect Dis. 2021;40(4):815–823. doi: 10.1007/s10096-020-04045-w. [DOI] [PubMed] [Google Scholar]
- 32.Meka VG, Pillai SK, Sakoulas G, et al. Linezolid resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA. J Infect Dis. 2004;190(2):311–317. doi: 10.1086/421471. [DOI] [PubMed] [Google Scholar]
- 33.Cameron DR, Jiang JH, Hassan KA, et al. Insights on virulence from the complete genome of Staphylococcus capitis. Front Microbiol. 2015;6:980. doi: 10.3389/fmicb.2015.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potoski BA, Adams J, Clarke L, et al. Epidemiological profile of linezolid-resistant coagulase-negative staphylococci. Clin Infect Dis. 2006;43(2):165–171. doi: 10.1086/505114. [DOI] [PubMed] [Google Scholar]
- 35.Mulanovich VE, Huband MD, McCurdy SP, et al. Emergence of linezolid-resistant coagulase-negative Staphylococcus in a cancer centre linked to increased linezolid utilization. J Antimicrob Chemother. 2010;65(9):2001–2004. doi: 10.1093/jac/dkq238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Supplementary Table 1. Polished Assembly Report of 4 strains. Supplementary Table 2. Annotation Information of 4 strains.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the GenBank repository, https://cipotato.org/genebankcip/.