Abstract
It has been shown in multiple experimental and biological investigations that kaempferol, an edible flavonoid generated from plants, may be used as an anti-cancer drug and has been shown to have anti-cancer properties. Many signaling pathways are altered in cancer cells, resulting in cell growth inhibition and death in various tumor types. Cancer is a multifaceted illness coordinated by multiple external and internal mechanisms. Natural extracts with the fewest side effects have piqued the attention of researchers in recent years, attempting to create cancer medicines based on them. An extensive array of natural product-derived anti-cancer agents have been examined to find a successful method. Numerous fruits and vegetables have high levels of naturally occurring flavonoid kaempferol, and its pharmacological and biological effects have been studied extensively. Certain forms of cancer are sensitive to kaempferol-mediated anti-cancer activity, although complete research is needed. We have endeavored to concentrate our review on controlling carcinogenic pathways by kaempferol in different malignancies. Aside from its extraordinary ability to modify cell processes, we have also discussed how kaempferol has the potential to be an effective therapy for numerous tumors.
Keywords: Anti-cancer, Natural compounds, Kaempferol, Mechanisms, Signaling pathways
Introduction
Cancer is an irregularity in the proliferation of various cells in different body tissues and is referred to as a group of diseases with an unmanageable growth and abnormal mechanism of cell division [1]. At the same time, there is always a balance between cell division, death, and differentiation [2]. The exact reasons for cancer are still unknown. Still, genetic and environmental factors such as ionizing radiations, viral infections, chemical and toxic substances, or excessive sunlight that disrupt cell function can cause abnormalities in the cell nucleus [3, 4]. The body's defense immune response is insufficient to manage the condition [2].
Biology, environment, lifestyle, and healthcare organization are central to preventing cancer and other chronic diseases. However, all of these issues are limited by environmental factors such as economic context, agricultural dimensions, nutrition, pollution, social condition, and education of individuals that can make healthy behavioral choices impossible [5–7].
More than 200 types of cancer are known. Many factors can lead to abnormal cell growth and eventually cancer, but what is important is early detection of cancer, which raises hopes for the effectiveness of the treatment [8–12]. It is not uncommon to utilize a mix of the most prevalent cancer therapies (such as surgery or chemotherapy) to treat the disease, depending on the patient's natural state and the kind of cancer [13–15]. Due to the adverse effects and drug resistances of mentioned treatments, natural compounds have been considered in the prevention and treatment of cancers and also reducing the chemo-radiotherapies side effects [15–17]. The use of natural compounds in treating various diseases has been prevalent since ancient times. About 80% of people worldwide use these compounds and are widely dependent on them [18]. These structures target the tumor cells by regulating cell death pathways, including apoptosis and autophagy [19].
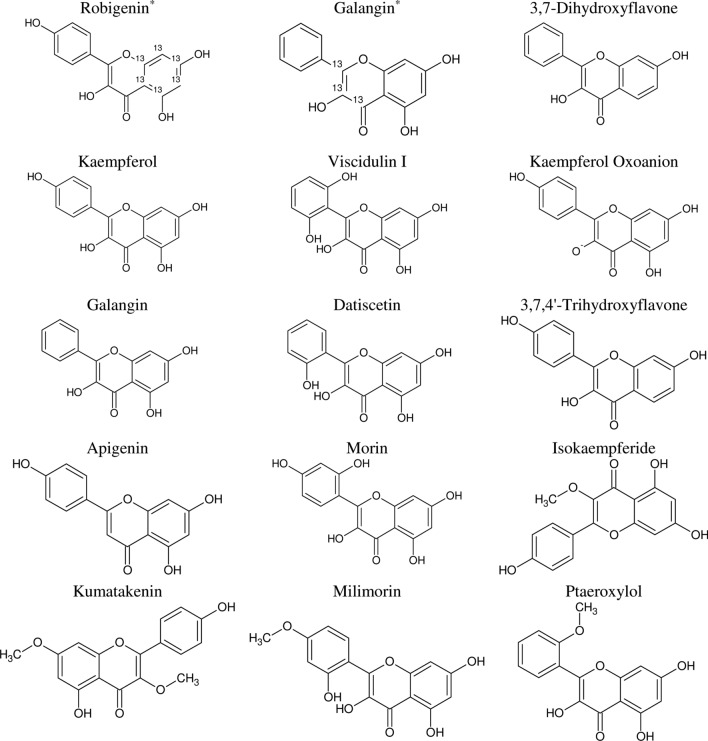
One of the most common flavonoid compounds in plants is a tetrahydroxyflavone from the flavonol family known as kaempferol (i.e., 3,4′,5,7-tetrahydroxyflavone), as shown in Fig. 1, along with its potential analogous structures. It is found in many plants, particularly edible or medicinally important [20]. Previous epidemiological research shows kaempferol has been linked to various cardiovascular, cancer, inflammatory, neurodegenerative, and obese diseases [21]. Researchers have well-described kaempferol's chemopreventive and therapeutic activities in previous studies [15, 22]. In this research, we will conduct a comprehensive investigation and discussion to determine how kaempferol may be used to prevent and treat different cancer types.
Fig. 1.
Kaempferol's chemical structure and its analogous compounds using the Tanimoto similarity index of 97% in PubChem database (*[13C6]-Robigenin and Galangin-13C3 have Carbon 13 (.13C))
Pharmacokinetics of kaempferol
The biological and pharmacological effects of flavonoid structures are related to their original characteristic mechanisms of action and the activity of their metabolites due to their widespread and rapid biotransformation [23, 24]. Due to the variety of the binding substituents, most natural flavonoid structures, such as kaempferol, are commonly presented and consumed as glycoside forms [20, 25]. Although the high polarity of the attached glycosyl groups to the aglycon reduces their absorption, previous studies have revealed that flavonoid glycosides absorption without hydrolysis is feasible [26]. The lipophilic nature of this structure leads to passive and facilitated diffusion absorption; however, scientific research demonstrated that it might also be absorbed as an active transport process [27, 28]. Conjugation enzymes metabolize kaempferol to glucuronides and sulfoconjugates in the small intestine. It can also be hydrolyzed by colon microflora and converted to simple phenolics (e.g., 4-hydroxyphenyl acetic acid, phloroglucinol, and 4-methyl phenol) to absorb or exert in feces.
Moreover, the absorbed part of kaempferol is metabolized into glucuronides and sulfo-conjugates in the liver. Finally, the conjugated forms of kaempferol create phenolic chemicals in the colon, and kaempferol and its glycoside derivatives are eliminated in the urine [20, 28]. Earlier studies demonstrated that 3- glucuronide conjugate of kaempferol was the main detected component in plasma and urine. Likewise, the urine excretion of consumed kaemferol was approximately 1.9% to 2.5%. [29, 30]. Following oral ingestion of kaempferol, many human investigations have shown that the concentration of the compound in plasma is confined to nanomolar levels. For example, in a 2002 study by Radtke, the plasma concentration of kaempferol was reported to be 10.7 nM, while the intake amount was 4.7 mg/day [31]. Another research found that ingesting 27 mg of kaempferol in the form of daily tea resulted in a plasma concentration of 52 nM (15 g/L), which was attained by consuming 52 nM (15 g/L) of kaempferol [30]. A plasma concentration of 57.86 nM of kaempferol was measured in a dietary intervention study on 92 healthy students (mean age: 24.16 years; mean BMI: 21.31 kg/m2) [32].
Anti-cancer activities of kaempferol in literature cancer types
A thorough review of the Scopus database was carried out on different types of cancer using “kaempferol” as their treatments, which will be described as follows.
Bladder cancer
According to the GLOBOCAN latest report, bladder cancer considers 3% of diagnoses of global cancer and mainly occurs in developed countries [33].
Urinary tract carcinoma is the most frequent urinary system cancer [34]; Xie et al. demonstrated kaempferol's preventative and therapeutic benefits from human bladder cancer with EJ cells. They reported significant anti-proliferative and anti-migration activities of this natural flavonol. The apoptotic mechanism activated by kaempferol is connected to the PTEN tumor suppressor gene through the PI3K/Akt signaling pathway [35]. In the other study, the assessment of MTT assay on two different bladder cancer cell lines (5637 and T24 cells) demonstrated that kaempferol increased cell cytotoxicity. Still, the analysis of flow cytometry, DNA ladder, and TUNEL assays resulted in significant kaempferol-induced apoptosis and cell cycle arrest. In vivo investigations (subcutaneous xenografted mouse models) supported these in vitro findings, with kaempferol-treated animals showing less cancer formation, whereas apoptotic markers are elevated. The research revealed that kaempferol might suppress cell growth by significantly downregulating the c-Met/p38 signaling pathway [36]. Kaemferol altered DNA methylation by creating 103 gene-specific differential DNA methylation locations (dDMPs), as was reported in a cell line research (using 5637 and T24 cells) and a 31-day in vivo investigation in nude mice with bladder cancer distress. The study concluded that kaempferol could promote the DNMT3B degradation through the ubiquitin–proteasome pathway and introduced as a novel DNMT3B inhibitor [37]. Wu and colleagues discussed the radical scavenging activity of kaempferol, its function in stimulating antioxidant enzymes, controlling ROS formation, and the anti-hemolytic effects of lipid peroxidation. Their study showed that kaempferol was a potent inhibitor in bladder cancer with high safety on normal cells. Besides, it suppressed the phosphorylation of AKT, CyclinD1, CDK4, Bid, Mcl-1, and Bcl-xL, while boosting the expression of p-BRCA1, ATM, p53, p21, and p38, as well as Bax and Bid. It also promoted apoptosis and S phase arrest in EJ bladder cancer cells while decreasing proliferation [34].
Bone cancer
Primary bone cancers are the most severe type of bone cancers, consisting of about 0.2% of all worldwide malignancies derived from primitive mesenchymal cells involving several subtypes of osteosarcoma chondrosarcoma Ewing sarcoma [38]. Previous research on osteosarcoma cells showed that kaempferol might increase the expression of Box protein and decrease Bcl-2 protein, decrease mitochondrial membrane potential and increase caspase-3, -7, and -9 activities in the U-2 OS cell line. A rise in AIF protein levels was also observed, indicating that apoptosis was induced through a caspase-independent mitochondrial mechanism. The endoplasmic reticulum stress pathways are the other mechanism induced by kaempferol in the human osteosarcoma cell line. In a study, the in vivo evaluation confirmed the antitumor effects of this bioflavonoid in mice models [39]. In another research, kaempferol inhibited cell metastasis in U-2 OS cells by inhibiting various signaling pathways (e.g., ERK, AP-1, JNK, and p38) [40]. When osteoblasts stopped producing osteoclastogenic cytokines and osteoclast precursor cells stop differentiating, kaempferol had an anti-osteoclastogenic impact on bone. It is due to antagonizing the TNF receptor family action on bone cells [41].
Breast cancer
Female breast cancer accounts for 11.7% of all cases and 6.9% of cancer deaths globally [42]. There are several studies on kaempferol's effects and possible mechanisms on breast cancer. In 2004, Hung published an article that showed the significant efficacy of kaempferol on the impairment of the estrogen receptor-α and blocking the estradiol-induced cell proliferation, while estrogen receptor (ER)-negative breast cancer cells didn't show growth resistance toward the kaempferol [43]. In some in vitro and in vivo studies, kaempferol is a phytoestrogen that inhibits triclosan and exhibits estrogen's effects on the growth of breast cancer cells [44]. Cyclin D1, cyclin E, and cathepsin D expressions were upregulated in cells treated with triclosan, whereas p21 and Bax expressions were downregulated, and these gene expressions were blocked by kaempferol. This compound also inhibited 17β-estradiol and triclosan-induced expression of pIRS-1, pAkt, and pMEK1/2 [44]. Similar results to in vitro studies on tumor growth inhibition were observed in the in vivo xenografted mouse model [44]. In addition to ER-agonist, this phytoconstituent was known as AHR-antagonist (aryl hydrocarbon receptor), which was related to the inhibition activity on transcription of AHR in ER-α-negative breast cancer cell lines independent of ER-α expression [45]. Generally, the biphasic responses (via ER-dependent and ER-independent pathways) of kaempferol on.
Estrogen receptors were reported, which explained the role of this compound in regulating the body’s estrogenic activity and its valuable potency in inhibiting estrogen imbalance diseases [46]. As an ERK activation inducer, kaempferol may also activate MEK1 and ELK1 (a substrate of the ERK). Another study has demonstrated that they effectively reduce breast cancer cell viability [47]. The suggested chemoprotective mechanism of kaempferol can result in toxicity and proliferation arrest due to the downregulation of glucose transporter 1 (GLUT1) gene expression and inhibition of cellular glucose uptake in cancer cell lines. Furthermore, this flavonol structure could inhibit the cancer cell's monocarboxylate transporter (MCT1)-mediated lactate reuptake and destroy [48]. Downregulation of the matrix metalloproteinase-9 (MMP) expression and activity was the other process in breast cancer invasion treated with kaempferol using the MDA-MB-231 cell line [49]. This component inhibited AP-1, PMA-induced MMP-9 production, PKC-δ translocation, and MAPK signaling [49]. Furthermore, treating MCF-7 cells with kaempferol influenced the aryl hydrocarbon receptor in inhibiting CYP1A1 transcription, and CAT transcription was likewise suppressed by kaempferol. Moreover, kaempferol totally reduced the induction of 2,3,7,8-tetrachlorodibenzo-p-dioxin in band shift [50].
Cancer stem cell markers, such as Oct-4, Nanog, ABCB1, and ALDH1A1, were significantly reduced in MCF-7 cells treated with kaempferol and docetaxel. This study reported kaempferol as a more effective anti-cancer agent than docetaxel [51]. Kaempferol was a suppressor of primary tumor growth and lung cancer metastases in a mouse breast tumor model [52]. It reduced the expression of H3-cit (citrullinated histone H3) as a neutrophil extracellular traps biomarker (NETs), involved in NADPH/ROS-NETs signaling, and reduced tumor metastasis by inhibiting the ROS-PAD4 pathway [52]. Kaempferol could also suppress the proliferation of triple-negative breast cancer, contribute to the G2/M arrest induction, induce apoptosis and DNA damage, increase the expression of γ-H2AX and cleave the caspase-9, caspase-3, and p-ATM in comparison with the control group [53].
According to previous studies, various isolated kaempferol derivatives from the plant species, such as prenylated kaempferol and kaempferol-3-O-rhamnoside, also have noticeable inhibitory effects on breast cancer cells [54, 55]. Activating the caspase cascade signaling system could suppress breast cancer cells (MCF-7) growth and promote the apoptotic process in breast cancer cells [55]. Moreover, according to a recent study, a conjugate form of 1-deoxynojirimycin and kaempferol linked by an undecane chain revealed significant lipophilicity, anti-proliferative, and anti-α-glucosidase activities. The new derivative showed downregulation of the expression of COX-2, inhibited migration, decreased intracellular ROS and calcium cation levels, decreased Bcl-2 expression, and increased Bax expression in MCF-7 cells [56].
The other factors influencing the kaempferol mechanism of action in breast cancer are its pharmaceutical formulation and particle size, considered in two previous studies. The gold nanoparticles (AuNPs) and nanostructured lipid carriers (NLCs) of kaempferol are suggested as powerful drug delivery techniques in the management of breast cancer cells through the antioxidant, anti-proliferative, and antiangiogenic properties [57, 58].
Cervical cancer
Cervical cancer was the fourth most often diagnosed and the fourth major cause of cancer mortality in women globally in 2020, with over 604,000 new cases and 342,000 fatalities [42]. Kaempferol impacted the glucose uptake and inhibited mitochondrial respiration in HeLa cells (derived from cervical cancer cells). In addition, HeLa cells activated autophagy as a survival response to this bioflavonoid. This study revealed that autophagy was related to early activation of the AMPK/mTOR-mediated pathway [59]. Apoptosis and cell cycle arrest was observed in HeLa cells when Kaempferol-7-O-b-D-glucoside, an extraction of Smilax china L. rhizome, was tested. This glycoside also reduced NF-κB nuclear translocation, increased Bax protein, and decreased Bcl-2, indicating a mitochondrial pathway in apoptosis [60].
Colon cancer
The GLOBOCAN 2020 research showed that rates of colon cancer are much higher in transitioned countries than in transitional countries. However, there is no significant difference in mortality rates because of the greater mortality in transitioning nations [42]. A previous study on the kaempferol effects on three colon cancer cell lines (MSU-2, HCT116, and KNC) with diverse expressed gap junction genes and function profiles concluded the possibility of anti-cancer properties with different functions [61]. The results of evaluations on KNC cells (it is expressed in connexin43 mRNA but lacked in gap junctional intercellular communication (GJIC) and Cx43 expression) indicated the ability of this flavonol structure on the induction effect of alkaline phosphatase (ALPase), reduction on Stat3, restoration of connexin43 protein (Cx43) phosphorylation, and functional GJIC. The Jak/Stat3 signaling pathway suppression was suggested as the important mechanism in the cells mentioned above. According to the results of this experiment among three different cell lines (MSU-2, HCT116, and KNC), kaempferol is considered a cytostatic component for only KNC cells [61]. According to Lee et al. studies, kaempferol made a series of molecular changes in the growth inhibition and HT-29 colon cancer cell line apoptotic function. In addition to a decrease in Bcl-xL and intact Bid, as well as an increase in Bik, Bad mitochondrial proteins, and the activation and cleavage of caspase-9, apoptosis function was caused by changes in the Bcl-2 family protein levels and localization (such as the reduction of Bcl-xL and intact Bid, as well as an increase in the Bik and mitochondrial Bad proteins).
Furthermore, the activation of caspase-8, the cleavage of caspase-3 and Bid, and the decrease of phospho-Akt and Akt activity by kaempferol led to Bad mitochondrial translocation [62]. Based on Budisan et al.'s studies, cell viability impairment, cell proliferation inhibition, apoptosis induction and autophagy, modifications in coding, and noncoding expression of genes have occurred in RKO and HCT-116 colon cancer Cell Lines treated with kaempferol [63]. Also, fewer cells in the G1 phase, but more in the G2 phase, were seen after kaempferol treatment [63].
The activation of death receptor 5 (DR5) by kaempferol increased the sensitivity of colon cancer cells to TRAIL-induced apoptosis in studies including medication combinations. In contrast, combining these two components didn't have significant cytotoxic effects on normal cells. Subsequently, the variety of kaempferol and TRAIL was introduced as a beneficial approach to cancer therapies [64]. Similar outcomes were observed in the kaempferol and 5-fluorouracil combination in HcT-8 and HcT-116 cell lines. The results indicated the synergistic effects of this bioflavonoid and 5-fluorouracil on cell viability, proliferation reduction, and apoptosis induction [65]. The molecular evaluation showed the upregulation of the Bax expression levels, downregulation of Bcl-2 and TS expression levels, and inhibition of the PI3K/Akt pathway [65]. A recent study reported that the combination of fluoxetine and kaempferol improved several biological properties such as the antioxidant, anti-inflammatory, and anti-proliferative effects with developed apoptotic activity and could exhibit a more potent chemopreventive effect against 2-dimethylhydrazine-induced preneoplastic lesion in rat colon than using fluoxetine alone [66]. Eventually, a recent experiment demonstrated that the combination of doxorubicin (DOX) and kaempferol was more efficient in induction of apoptosis and cytotoxic effect against HT-29 colon cancer cell line in comparison with each drug alone; also combined drug nanoformulations (polyethylene glycolated gold nanoparticles of doxorubicin-kaempferol) revealed a critical decreasing in the volume of colon tumor on the in vivo model without any severe side effects [67].
Endometrial cancer
Endometrial cancer is the sixth most frequent malignancy in women and one of the most common malignancies in gynecology [68]. Nulliparity and metabolic abnormalities such as obesity have been linked to an increased incidence of this condition, which is one of the estrogen-dependent diseases [69]. Kaempferol inhibited the viability of Ishikawa and HEC 265 cells in previous research (estrogen receptor-positive, human endometrial adenocarcinoma cell line). At concentrations as low as 80 µg per milliliter, kaempferol inhibits the development of Ishikawa and HEC-265 cells. It induced the G2/M phase and suppressed the G1 phase. According to the results, this bioflavonoid structure led to apoptotic cell death in Ishikawa and HEC-265 cancer cells by decreasing endoplasmic reticulum (ER), survivin, and Bcl-2 levels and increasing p53 and PARP expression [69]. HEC108 cell line and growth of HEC180 were unaffected by kaempferol in the same research [69]. Using the human endometrial cancer cell line MFE-280 as a model, researchers found that inhibiting mTOR/PI3K/Akt signaling led to triggering apoptosis and halting cell cycle progression with substantial anti-cancer effects [70].
Gastric cancer
There are around 1.1 million new cases of this disease each year, and 768,793 deaths from it are reported in the GLOBOCAN database, making it one of the most common cancers and the fourth-leading cause of death globally. Men have double the values of women [42]. There are several studies on the effects of kaempferol on different gastric cancer cells. In 2010, kaempferol revealed dosage and time-dependent inhibitory effects on MGC-803 cells, causing apoptosis and G2/M phase arrest [71]. Song et al. found that kaempferol promoted apoptosis and G2/M phase cell cycle arrest in gastric cancer cell lines, including MKN-28 and SGC-7901. Still, it had no impact on standard gastric epithelial cell lines, as previously reported (GSE-1) [72]. This investigation discovered that the xenograft tumor development was suppressed without any evident impact on the subjects' body weight, liver, or spleen. The G2/M cell cycle proteins, cyclin B1, Cdk1, and Cdc25C were reduced in kaempferol-treated cells, whereas Bcl-2 was reduced, and cleaved PARP, caspase-3, and -9 were enhanced. The expression of p-Akt, p-ERK, and COX-2 was reduced in MKN-28 and SGC-7901 cell lines [72]. The Korean researchers investigated kaempferol-mediated stomach cancer therapy's molecular mechanisms and biological activities in 2018. This bioflavonoid enhanced LC3-I to LC3-II transition, decreased p62 production, and activated autophagy and cell death through IRE1–JNK1-mediated Bcl-2–Beclin-1 interaction [73]. The outcomes also suggested HDAC/G9a pathway as another mechanism of kaempferol-mediated epigenetic changes for autophagy and cell death [73].
Leukemia
Leukemia ranks 15th and 11th in terms of the prevalence of common world cancer and the leading cause of death worldwide, with approximately 500,000 and 300,000 cases reported for incidence and death rates, respectively, in 2020 [42]. Proliferation and maturation of hematopoietic myeloid cells are actively addressed in acute myeloid leukemia (AML) [74]. In addition to a poor prognosis and an undesirable treatment outcome, AML has several undesirable traits, such as higher recurrence rates and resistance to anti-cancer drugs [75, 76]. Kaempferol and quercetin might be used to more effectively inhibit the growth and viability of human leukemia THP-1 cells by targeting genes associated with survival [74]. Some studies say that kaempferol is hazardous for Jurkat T cells because it stops them from going through the G2/M phase and makes them die [77]. When kaempferol is administered to human HCT116 colon cancer cells, it stimulates the ataxia-telangiectasia mutated-p53 pathway, resulting in cell death [78]. However, when kaempferitrin enters HeLa cells, it causes apoptosis and has anti-cancer effects. Apoptosis was produced in promyelocytic leukemia cells by raising the Bax/Bcl-2 ratio and inhibiting MDR when kaempferol was administered [79]. Multi-drug resistance, apoptosis (PI3K and AKT), and differentiation (PML-RAR and HDAC1) were found to be upregulated in the study [79]. When the PI3K/Akt signaling pathways are inhibited, the growth of human leukemia cell lines (i.e., K562 and U937) is slowed, and their viability is reduced [74]. Tumor necrosis factor-related apoptosis-inducing ligand-resistant cells such as MOLT-4 have gained resistance to it via various mechanisms. Kaempferol enhanced the number of sub-G1 cells in the promyelocytic leukemia cells and triggered apoptosis. Also, it inhibited the production of FGF-8 and VEGFB, likely due to the reduction of survival-related signaling pathways [74]. According to real-time PCR, human leukemia K562 and U937 cell growth and viability are reduced when kaempferol inhibits PI3K/Akt signaling pathways [74, 79].
Liver cancer
It is estimated that the world's most common primary cancer diagnosis and the third leading cause of cancer-related death will both be liver cancers in 2020 [42]. Additionally, there were more than 900,000 new cases and 800,000 deaths, with males having 2–3 times the prevalence and fatality rates [42]. A review of the previous research has shown that kaempferol affects liver cancer cells by affecting various molecular mechanisms and pathways in cancer progression. For example, Mylonis et al. revealed the influential role of kaempferol in inhibiting the viability of hepatoma (Huh-7) cancer cells, HIF-1, and MAPK in hypoxic conditions [80]. According to an in vivo study, kaempferol could modulate the levels of nucleic acids, membrane-bound ATPase (Na+/K+ ATPase, Mg2+ ATPase, and Ca2+ ATPase), mitochondrial TCA cycle, and carbohydrate metabolizing enzymes (hexokinase, phosphogluco-isomerase, aldolase, glucose-6-phosphatase, and fructose-1,6-diphosphatase) in aflatoxin B1 (AFB1) induced hepatocellular carcinoma. It was introduced as a potent anti-carcinogenic factor [81]. In another study, kaempferol acted as an anti-inflammatory agent. Its ability was proven to abolish the downregulatory action of TNF-α on liver-X-receptor alpha (LXR-α) in a dose-dependent manner [82].
Moreover, autophagy induction via upregulation of AMPK, downregulation of AKT and mTOR signaling pathways, and the induction of G2/M arrest through CDK1/cyclin B downregulation were observed in kaempferol-treated SK-HEP-1 cancer cells [83]. The assessment of different concentrations of kaempferol on ABCA1, mRNA expression levels in TNF-α stimulated HepG2 cell line demonstrated that although in the TNF-α-simulated inflammatory condition, kaempferol couldn't reduce the expression of ABCA1 rising back to untreated levels; high dose of this compound could lonely upregulated the ABCA1 mRNA expression considerably, which showed a positive relevance between ABCA1 and kaempferol. It was suggested that this bioflavonoid could be used as a suitable preventative agent in Tangier disease [84]. One of the main mechanisms proposed by Guo et al. in their in vitro investigation of HepG2 cells was the ER stress CHOP pathway, which increased the production of protein and mRNA of ER stress indicators [85]. The flavonoid structure was shown to promote apoptosis in hepatocytes in the rat model of hepatocellular carcinoma through the mitochondrial-dependent pathway by targeting upstream activities such as the enhancement of ROS formation, MMP downregulation, and the increasing of the caspase-3 activity in the cytosol [86]. Several pieces of literature described the protective effects of kaempferol on the liver against various oxidative stresses [87, 88]. By activating mitochondria and inhibiting the signaling pathways of PI3K/mTOR/MMP, kaempferol limits liver cancer cell proliferation and migration. And results showed that kaempferol didn't have any significant toxicity on normal cells [89]. This report also demonstrated a synergistic influence and more significant inhibitive effects of proliferation, migration, and invasion on liver cancer in combined treatment with doxorubicin and kaempferol. It was suggested that this bioflavonoid could interrupt cancer cell survival signaling pathways and may compensate for the shortcomings of monotherapies. Furthermore, the multi-targeting activity of kaempferol can reduce drug resistance during treatment [89].
Following Shakya et al.'s report, kaempferol protected the liver against alcohol- and thermally oxidized polyunsaturated fatty acid-induced oxidative stress [88]. Moreover, a recent study anticipated that kaempferol and kaempferide (kaempferol 4′-methyl ether) possessed significant effects on Nonalcoholic fatty liver disease (NAFLD) prevention and treatment, which might cause some necroinflammatory responses such as nonalcoholic steatohepatitis and consequently cancer [87, 90]. The induced molecular mechanisms of these two flavonol structures were reduced lipogenesis-related protein expression (e.g., SREBP1, FAS, and SCD-1). Moreover, they have decreased the expression of PPARγ, C/EBPβ, and two adipogenic transcription factors, HO-1 and Nrf2 [87]. Moreover, the molecular docking approach presented that the binding of kaempferol and kaempferid to SCD-1 could introduce an essential regulator in lipid metabolism [87]. Along with in vitro and in vivo experiments, in silico evaluations also confirmed the anti-liver cancer potential of kaempferol. The well-binding affinities to the receptor FKBP12-rapamycin binding domain and AKT serine/threonine-protein kinase concluded the improved efficacy in inhibiting mTORC1 compared with everolimus as a standard drug [91].
The studies above and several previous data show that kaempferol's different synthetic and natural derivatives also presented significant cancer-fighting effects against hepatocarcinoma cells [54, 92].
Lung cancer
In 2020, greater and less than 2.0 million new cases and fatalities were due to lung cancer, respectively [42]. Cancer experts think that non-small cell lung cancer (NSCLC) is responsible for more than 11% of the worldwide cancer burden and more than 18% of all cancer fatalities [93]. The authors found that kaempferol significantly decreased in vivo lung metastasis using a realistic and uncomplicated B16F10 melanoma metastatic model. These inhibitory effects decreased MMP-9 synthesis and activity by reducing the MAPK and PKC signaling pathways. A549 cells die due to kaempferol's ability to inhibit the PI3K/AKT and ERK pathways. According to the results [94, 95], kaempferol suppressed A549 cells via activating the apoptosis pathway. A different test in NSCLC cells uses Nrf2 reporter luciferase as a biomarker (A549 and NCIH460). Nrf2 siRNA decreased NQO1 protein levels in A549 and NCIH460 cells [96]. In comparison to normal lung epithelial cells, NSCLC cells (A549 and NCIH460) express more significant amounts of Nrf2, as well as its downstream proteins NQO1 and HO1 (L132) [97]. At zero and 24 h following treatment with K-AuNCs, the researchers evaluated if kaempferol-conjugated AuNCs showed equivalent anti-migratory effects on A549 lung cancer cells. Kaempferol, toxic to lung cancer cells but not HK-2 human kidney normal cells, can be efficiently conjugated to AuNCs. It has been shown that K-AuNCs govern the development of A549 cells [98]. Pretreatment of A549 cells with kaempferol significantly decreased the toxicity of TGF-1. Through dephosphorylation of Smad3 at the Thr179 site in A549 lung cancer cells, kaempferol inhibits the EMT, migration, and MMP-2 activation induced by TGF-1 in these cancer cells. Kaempferol's ability to inhibit cancer cell development has been linked to estrogen-related receptors (ERRs) and its activity [99]. Finally, the fact that A549 lung cancer cells were resistant to TGF-1-induced EMT, migration, and activation of MMP-2 suggests that kaempferol's antimetastatic effect may target essential components of numerous signaling pathways implicated in tumor metastasis. In addition, in A549 cells, kaempferol-induced apoptosis seems to need MEK-MAPK activation, which acts before caspase-7. For the A549 lung cancer cell line, kaempferol is toxic to the cells. Kaempferol enhances the MEK/MAPK, affects the Bcl-2 proteins, and inhibits the phosphorylation of Akt1 to activate apoptosis [100]. By cleaving PARP and caspase-7, the MEK1/2 inhibitor prevents kaempferol-induced apoptosis. A549 cell death requires MEK-MAPK activation, which occurs before caspase activation, according to the literature findings [94].
Nervous system cancer
According to the 2020 GLOBOCAN report, brain and nervous system cancer comprised more than 308,000 and 251,000 new cases and deaths, respectively [42]. It has been shown that the combination of kaempferol and TRAIL might be an essential strategy for treating glioma by suppressing survivin protein degradation [101]. Apoptosis was reduced when survivin was overexpressed in the cell. Kaempferol was also responsible for downregulating phosphorylated Akt and reducing the quantity of survivin protein to stop survivin [101]. It was discovered in this study that TRAIL and kaempferol were able to imitate caspase-8 cleavage while also having an anti-apoptotic effect that was not inhibited by survivin, which has been demonstrated in earlier research not to affect caspase-8 activation. As well as this, the apoptosis inhibitory Bcl-2, Bcl-xL, and Mcl-1 family members were all reduced in concentration by kaempferol [101]. Kaempferol was also shown to elicit caspase-dependent pathways, including the downregulation of XIAP and survivin, which ERK and Akt regulate in the other investigation on A172 human glioma cells [102]. Kaempferol was also shown to have neuroprotective properties against 4-hydroxynonenal-induced apoptosis in PC12 cells. This natural flavonoid prevented the 4-hydroxynonenal-induced apoptosis, linked to nuclear condensation, Bcl-2 downregulation, and proapoptotic caspase-3 activation. Additionally, it prevented phosphorylation of c-JNK in response to 4-hydroxynonenal, which was bound to p47phox, and inhibited NADPH oxidase activity in response to 4-hydroxynonenal (NOX). Results indicated that kaempferol might be a preventative agent against NADPH oxidase-mediated neurodegenerative disorders [103].
In 2018, an investigation on IMR32 and Neuro2A cells (human and mouse neuroblastoma cell lines), showed this flavonol structure induced neuroblastoma differentiation, decreased cell viability, promoted apoptosis, modulated the IRE1α (inositol-requiring enzyme one alpha) expression, and activated the IRE1α endoribonuclease [104].
Ovarian cancer
As mentioned in the GLOBOCAN report, ovarian cancer has been considered a severe women malignancy, with 313,959 new cases in 2020 worldwide [42]. Based on the previous studies on OVCAR-3 (mutant p53), wild-type p53 of A2780/CP70 cells, anti-angiogenesis, and the significant inhibitory effects of kaempferol on VEGF (Vascular endothelial growth factor) gene expression via both Akt/HIF and ESRRA pathways were described [105]. The other study confirmed tumor anti-angiogenesis effects of kaempferol and added the induction effects of this flavonoid compound on G2/M cell cycle arrest through the Chk2/Cdc25C/Cdc2 and Chk2/p21/Cdc2 pathways in A2780/CP70 ovarian cancer cells [106]. The researchers described that kaempferol could increase the DR5 and Fas expression level and stimulate the extrinsic apoptosis pathway through the death receptors/FADD/Caspase-8 and Chk2 was not a responsible factor for the apoptosis induction and upregulation of p53 by kaempferol [106]. Kaempferol also displayed anti-proliferative properties on several human ovarian cancer cells (Caov-3, TOV-112D, SKOV-3, and OVACAR-3) by triggering autophagy and apoptosis G0/G1 cell cycle arrest and inhibition of MEK/ERK and STAT3 pathways [107]. In a recent study on A2780 cells, kaempferol effects increased cell apoptosis and decreased viability and proliferation. Furthermore, rising GRP78, PERK, ATF6, IRE-1, LC3II, beclin 1, and caspase-4 levels stimulated the cytotoxic endoplasmic reticulum/ autophagy mechanism, which was related to enhancing intracellular calcium cation levels. Cancer cells were made more sensitive to cisplatin by kaempferol, which did so via lowering the protein p-Akt in the cells [108]. Various studies have suggested using kaempferol combined with cisplatin to control the drug resistance problem in ovarian cancer [106, 108]. Earlier studies have also shown the synergistic effects of kaempferol and cisplatin inhibiting ABCC6 and cMyc gene transcription in ovarian cancer cells [109]. It should be noted that nano-formulations of kaempferol had a more influential role in reducing the survival of ovarian cancer cells than kaempferol alone [110].
Pancreatic cancer
Affecting both men and women throughout the world, pancreatic cancer has a bleak outlook, making it one of the most dangerous and lethal tumors to develop [42]. Scientific research on three human pancreatic cancer cell lines (Miapaca-2, Panc-1, and SNU-213 cells) presented the anti-cancer effect of kaempferol via viability reduction and apoptosis increasing in a dose-depend manner [111]. This study introduced kaempferol as an anti-viable, antioxidant, anti-migration agent with no toxicity in low doses. It could mediate the anti-cancer activities against pancreatic cancer by blocking EGFR-related Src, ERK1/2, and AKT signaling pathways. Two glucoside derivatives of kaempferol (kaempferol-3-O-glucoside and kaempferol-4’-O-glucoside) didn't show any anti-cancer effects in the mentioned study [111].
Moreover, a recent study showed that kaempferol could successfully suppress pancreatic cancer in vivo and in vitro models. Akt/mTOR signaling activation might diminish the TGM2 mRNA and protein levels and ROS generation. Raising the expression of tissues transglutaminase is the cause of downregulation of ROS production, inhibition of related signaling pathways, and poor prognosis in pancreatic ductal adenocarcinoma [112].
Prostate cancer
Prostate cancer is anticipated to be the second most common cancer in men and the fifth leading cause of cancer deaths globally by 2020, with an estimated 1.5 million new cases and 0.4 million fatalities [42]. As mentioned above, even though the inhibition of cancer cell growth and apoptosis induction were the main mechanisms of kaempferol, promoting the body's immunity also plays an essential role in fighting against various types of cancer. As an example, an investigation of the effects of kaempferol on the production of granulocyte–macrophage colony-stimulating factor (GM-CSF) in prostate cancer cells (PC-3) demonstrated the increasing of GM-CSF release by PC-3 kaempferol -treated cells without any disturbance on the mRNA levels could improve the activation of the antigen-presenting dendritic cells and the host immune system and consequently could develop the tumoricidal immune response. This natural flavonol stimulated the production of GM-CSF by activating the PLC, PKC, and MEK1/2 cascade [22]. The other literature study represented the apoptosis stimulating role of kaempferol in a dose-depend manner in the presence of dihydrotestosterone on LNCaP cells (androgen-sensitive human prostate adenocarcinoma cells). Moreover, it could inhibit the activities of dihydrotestosterone-induced androgen receptors and decrease the downstream targets of androgen receptors (e.g., PSA, TMPRSS2, and TMEPA1) and PSA protein levels, and also inhibit androgen receptor protein expression and nuclear accumulation. Eventually, kaempferol could suppress the vasculogenic mimicry formation and.
invasive potency in a dose-dependent manner [113]. Other research has shown that it may have an anti-proliferative impact depending on how much kaempferol-3-O rhamnoside is applied to LNCaP cells. For this reason, in LNCaP cells, it caused an increase in the expression of the enzymes caspase-8, -9, -3, and ADP-ribose polymerase[114].
Skin cancer
Skin cancer, which develops when skin cells grow abnormally and unchecked, is one kind of cancer that may spread throughout the body. This malignancy is divided into two main groups keratinocyte cancers known as non-melanoma and melanoma [115]. According to the last GLOBOCAN data, over one million new cases with 63,731 death of non-melanoma (excluding basal cell carcinoma) and 324,635 new cases with 57,043 death of melanoma were reported in 2020 [42]. Based on the previous study of kaempferol-treated HaCaT cells, the results demonstrated 147 transcripts (including 18 upregulation and 129 downregulation) in cDNA microarray analysis that caused significant expression changes [116]. The results showed the role of kaempferol on the simulation of PPAR transcriptional activity in the HaCaT cell line, which transiently transfected with the PPRE-tk-Luc reporter gene, and suggested that its action mode could be mediated by PPAR pathways [116].
Moreover, recently published literature reported Prunus cerasus L. extract (sour cherry) as a source of essential polyphenols such as kaempferol, quercetin, and chlorogenic acid during apoptotic cell death of HaCaT cell line exposed to airborne particles with a diameter less than 10 µm. Cell viability decreased, reactive oxygen species were inhibited, and apoptosis-related gene expression (e.g., Bax, Bcl-2, and caspase-3) was inhibited due to the activation of transcription factor NF-κB [117]. The other experiment revealed that kaempferol significantly inhibited EGF-induced neoplastic transformation in JB6 P1 mouse epidermal cells. In addition, it could suppress AP-1 and NF-κB activation and PI3K/Akt signaling pathways, and the binding of kaempferol to PI3K inhibited UVB-induced activities [118]. Moreover, this natural compound targeted RSK2 and MSK1 to suppress and prevent UV radiation-induced skin cancer [119]. Kaempferol suppressed cell migration and apoptosis in the presence of melanoma malignancy, as well as downregulating the levels of mTOR, phosphorylated (p) mTOR, PI3K, and Akt proteins [120].
Others
We summarized their information in this section because of fewer published experimental data for kaempferol effects on the other types of cancer cells. A study performed on the effects of kaempferol on head and neck squamous cell carcinomas revealed that this compound could inhibit the rate of oxygen consumption and reduce the content of intracellular ATP in tumor cells, which could induce anti-proliferative activities and tumor cells apoptosis and also could inhibit the capacity of migration. Furthermore, the results showed that combining this bioflavonoid and cisplatin could improve cisplatin resistance limitation in mentioned cancer cells [121].
On esophageal squamous cell carcinoma, kaempferol showed significant inhibitory effects on tumor cell proliferation and clone creation in an experiment for colony formation and cell colony formation. Kaempferol induced the G0/G1 phase arrest, changes in protein expression involved in cell cycle regulation, and inhibits tumor glycolysis in tumor cells. The epidermal growth factor receptor (EGFR) signaling pathway was reported as the possible mechanism for described function [122].
Apoptosis was activated, and cell cycle arrest was seen in 786-O and 769-P cells treated with kaempferol discovered by Song et al. They found that this bioflavonoid inhibited the EGFR/p38 MAPK signaling pathways, increased p21, decreased cyclin B1, stimulated PARP cleavages, induced apoptosis, and inhibited cell proliferation in human renal cell carcinoma (RCC) cells [123].
Kaempferol also reduced MMP-9 production by inhibiting NF-κB and AP-1 activation in the HT1080 (human fibrosarcoma) cell line, which was associated with a substantial decrease of IκBα and JNK phosphorylation that was driven by phorbol-12-myristate-13-acetate [124]. These results demonstrated the chemopreventive effects of kaempferol in controlling the inflammation risk, tumor invasion, and metastasis involving MMP-9 [124].
Also, in vitro and in vivo studies revealed that kaempferol suppressed cholangiocarcinoma development and metastasis [125]. According to the in vitro experiments, this natural polyphenol inhibited HCCC-9810 and QBC939 cells' proliferation and colony formation while inhibiting their migration and invasion capabilities. It was found that kaempferol decreased Bcl-2 expression and elevated Bax, Fas, cleaved-caspase-3, -8, -9, and PARP expressions. Furthermore, the phosphorylated AKT, TIMP2, and MMP2 were downregulated by kaempferol treatment [125]. The lung metastasis model saw fewer metastatic foci and lighter mice [125]. Moreover, decreasing the amount of Ki-67-positive cells was reported as a kaempferol effect [125].
According to previous research on retinoblastoma SO-RB50 cells, kaempferol affected the ERR-α. The Wnt/ β-catenin signaling pathway stopped cells from making more copies of themselves and spreading. This report demonstrated the occurrence of G2/M arrest and apoptosis in the SO-RB50 cell line [126].
The extrinsic mechanism of apoptosis was used to show that the kaempferol derivative known as 3-O-b-isorhamninoside, isolated from Rhamnus alaternus L. leaves, caused apoptosis in human lymphoblastoid cells. The obtained results represented that the induced apoptosis of the mentioned glycoside of kaempferol was related to the stimulation of caspase-3 and PARP cleavage [127].
A previous in vitro study indicated that kaempferol and its glycoside (kaempferol.
7-O-rhamnoside) inhibited PD-1/PD-L1 interaction, closely related to the T cell functions impairment and escape cancer cells from the immune surveillance [128]. In addition, decreasing the ERR-regulated target gene expression, such as reducing TFAM, Mfn2, Mn-SOD, and Cu/Zn-SOD gene expression and antagonizing the EER-α and -γ were related to the kaempferol effects on inhibiting mitochondrial biogenesis and cancer cells development [129].
The combined action of kaempferol with other chemicals
Previous research suggests that coordinating flavonoids with transition metal ions may increase the anti-cancer activity of flavonoids. According to the results of an MTT test, kaempferol-Zn was in a better position than free kaempferol to inhibit the growth of cancer cells and reduce their viability. These findings provided sufficient evidence to support the hypothesis that kaempferol-Zn has the potential to trigger apoptosis in EC9706 cells. Consequently, the mitochondria were further damaged by kaempferol-Zn, which led to cell death [129]. Colon cancer cells were treated with a PEGylated AuNPs-DOX@Kaempferol drug delivery system. This system relied on chemically modified PEGylated AuNPs and electrostatic hydrogen-bonding interactions. As the pH became more acidic, the DOX and kaempferol molecules were freed from their critical interactions, and medications could also be released at intracellular locations. It resulted in a more significant antitumor effect and promoted death in colon cancer cells [130].
An investigation presented quercetin and kaempferol's cytotoxic action on HCT-116 cells; some green foods and fruits might contain equivalent amounts of both flavonoids. HCT-116 cells were prevented from entering the G2/M phase of the cell cycle by quercetin and kaempferol's chemopreventive effects, which resulted in a loss of viability in the cells when the two compounds were combined [131]. Chloroquine or sodium phenylbutyrate was added to kaempferol-treated ovarian cancer cells to reverse the effects of kaempferol on ovarian cancer cells (ER stress inhibitor). According to the results of this research, using kaempferol, either alone or in conjunction with cisplatin, may be an effective way to reduce ovarian cell tumorigenesis [132]. PANC-1 and BxPC-3 were inhibited most effectively when kaempferol and erlotinib were used together. An IHC staining confirmed the in vitro results that kaempferol and erlotinib further decreased the expressions of p-EGFR and AKT, as well as p-ERK1/2 and Bax when used together [133]. A critical factor in nanoparticle anti-cancer efficacy is particle size since nanoparticles enter cancer cell walls through increased permeability and retention (EPR). Compared to kaempferol on its own, treating cancer cells with nanoparticles loaded with kaempferol increased early apoptosis by between 0.1 and 6%. The cytotoxicity of paclitaxel was increased when combined with kaempferol-loaded NLCs against MDA-MB-468 murine breast cancer cells [134].
According to a recent study, NANOG, SOX2, MDR1, and CD44 are all induced pluripotent stem cell markers that may be reduced by kaempferol alone or in conjunction with Verapamil. Cancer cells’ survival and acquisition may be slowed by pharmaceutical combinations that interfere with the CD44–NANOG–MDR1 feedback loop activation network, according to a new study [135]. The kaempferol could be effectively conjugated to the AuNCs. The resultant conjugation was selectively harmful to the lung cancer cells while remaining non-toxic to the normal human kidney cells (i.e., HK-2 cell line). The clonogenic experiment provided conclusive evidence that K-AuNCs were responsible for controlling the growth of A549 cells. The findings offer credence to the natural flavonoid kaempferol's potential as an anti-cancer agent in conjunction with AuNCs [98]. The effects of kaempferol are amplified through the downregulation of cMyc, resulting in ovarian cancer cells being more likely to undergo apoptosis due to cisplatin treatment. It was shown that combining kaempferol and cisplatin might synergistically diminish ovarian cancer cells' vitality. The reduction in cell viability was achieved by blocking the gene transcription of ABCC6 and cMyc [136]. The research presents the findings of kaempferol-cisplatin-doxorubicin combo investigations. The results demonstrated a substantial synergistic therapeutic benefit when doxorubicin or cisplatin was used in conjunction with kaempferol in HCT-15 and MDA-MB-231 cell lines [137].
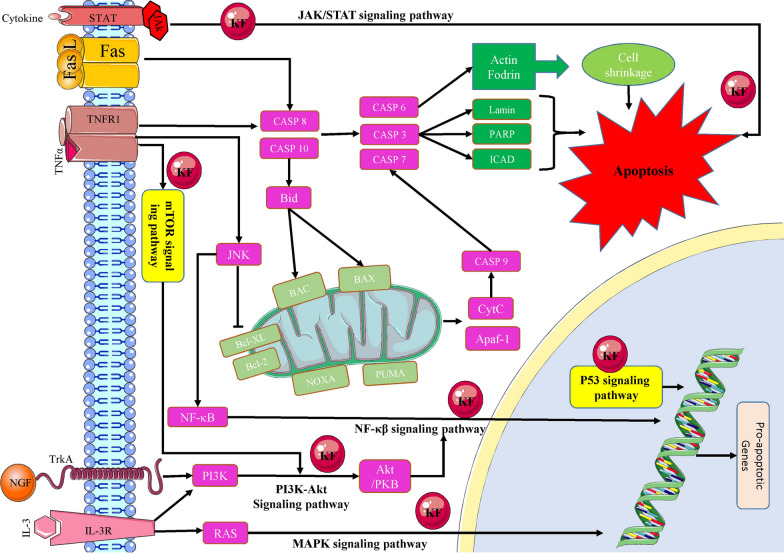
As a result, the regulation of complex biological illnesses may be improved by using medication combinations that co-act with one another. In addition to controlling these potential pharmacological actions, kaempferol has also been found to induce other organ protective benefits, as listed in Table 1. Activation of apoptosis and mitochondrial-related pathways and processes has been shown in some studies to be the mechanism by which kaempferol kills cancer cells (Fig. 2).
Table 1.
Characteristics of twenty one types of cancers in terms of treatment, study type, cell line, sample, dosage, treatment period, and mechanisms and possible involved signaling pathways
| Cancer types | Treatment | Study | Cell Line | Sample | Dosage | Treatment Period | Mechanisms/Signaling Pathway | Ref |
|---|---|---|---|---|---|---|---|---|
| Bladder cancer | Kaempferol | In vitro | EJ | – | 20–160 µM | 24 and 48 h | Activation of Caspase-3 | [35] |
| Kaempferol |
In vitro In vivo |
5637 and T24 | 6 to 8-week old athymic BALB/cnu/nu male mice (subcutaneous xenografted mouse models) |
In vitro: 50–150 µM In vivo: 50–150 mg/kg |
48 h Intraperitoneal injection, daily, for four weeks |
c-Met/p38 signaling pathway | [36] | |
| Kaempferol |
In vitro In vivo |
5637 and T24 | 5-week-old BALB/c nude mice (nude mice bearing tumor xenografts) |
In vitro: 40 µM In vivo: 150 mg/kg |
24 and 48 h Intraperitoneal injection, daily, 31 days |
Ubiquitin–proteasome pathway | [37] | |
| Kaempferol | In vitro | EJ | – | 20–80 µM | 48 h | Activating p53 signal pathway | [34] | |
| Bone cancer | Kaempferol |
In vitro In vivo |
U-2 OS, HOB, 143B cells | BALB/cnu/nu mice (8 weeks old) |
In vitro: 25–200 µM In vivo: 25 or 50 mg/kg |
24 h Oral administration, daily, 40 days |
Endoplasmic reticulum stress pathway and mitochondrial signaling pathway | [39] |
| Kaempferol | In vitro | U-2 OS | – | 25–100 µM | 48 h | Blocking MAPKs and AP-1 signaling pathways | [40] | |
| Breast cancer | Kaempferol | In vitro | ER-positive (MCF-7, T47D, and ZR-75) and ER-negative (MDA231) | – | 17.5–70 µM | 24 and 48 h | Reduction in the expression of both IRS-1 and cyclin D1 | [43] |
| Kaempferol | In vitro | T47D, BT549 and MDA-MB-231 | – | 10 µM | 24 h | Inhibition of AHR-dependent transcription | [45] | |
| Kaempferol | In vitro | MCF-7, T47D and MDA-MB-231 cells | – | 20–100 µM | 48 h | ERK signaling pathway | [47] | |
| Kaempferol | In vitro | MCF-7 | – | 10–100 µM | 24 h | Inhibition of MCT1-mediated lactate reuptake | [48] | |
| Kaempferol |
In vitro In vivo |
MDA-MB-231 | 6 to 8 weeks old male C57BL/6 mice |
In vitro: 10–40 µmol/L In vivo: 50, 100, and 200 mg/kg |
24 h Intragastric administration, daily, 21 days |
Inhibition of MAPK signaling pathway | [49] | |
| Kaempferol |
In vitro In vivo |
MCF-7 | Female BALB/c nu/nu mice, 6-week old |
In vitro: 50–100 µmol/L In vivo: 100 mg/kg |
96 h Subcutaneous Injection 2 or 3 times a week for six weeks |
ER and IGF-1R signaling pathway | [44] | |
| Kaempferol | In vitro | MCF-7 | – | 5–700 µM | 48 h | Downregulation of oct4, nanog, abcb1 and aldh1a1 | [51] | |
| Kaempferol |
In vitro In vivo |
4T1 | 7 to 8 weeks female BALB/C mice |
In vitro: 25 µM /L In vivo 40, 80, and 160 mg/kg |
24 h Oral gavage, daily, four weeks |
Inhibition of ROS-PAD4 pathway | [52] | |
| Kaempferol | In vitro | BT474 and MDA-MB-231 cells | – | 50 µM/L | 72 h | Induction of DNA Damage, Promotion of apoptosis | [53] | |
| Kaempferol-loaded nlcs and paclitaxel | In vitro | MDA-MB-468 cells | – | 1–8 nmol paclitaxel and 10–160 μmol/l kaempferol | 24 and 48 h | PI3K/Akt pathway and apoptosis pathway | [134] | |
| Kaempferol-verapamil |
In vitro Ex-vivo |
BCSC and MDA-MB-231 cells | 34 tumor samples of patients | 104.8 µM kaempferol and 5 µM verapamil | 48 h | Chemoresistance pathways | [135] | |
| Kaempferol-doxorubicin and cisplatin | In vitro | MDA-MB-231 cells | – | 32, 18 and 9 µg/ml | 24 h |
signaling pathways such as apoptosis and angiogenesis |
[136] | |
| Cervical cancer | Kaempferol | In vitro | HeLa | – | 100 and 200 µM | 48 h |
AMP-activated protein kinase-dependent autophagy |
[59] |
| Cholangio carcinoma | Kaempferol |
In vitro In vivo |
HCCC9810 and QBC939 | Male BALB/c athymic nude mice (4 weeks old) |
In vitro: 30- 150 µM In vivo: 20 mg/kg |
24, 48, 72 h Intraperitoneal injection, daily, Three weeks |
Inhibition of PI3K/AKT pathway, apoptosis | [125] |
| Colon cancer | Kaempferol | In vitro | MSU2, HCT116, KNC | – | 2.5, 5, 10, and 20 µM | 24 h | Suppression of Jak/Stat3 signaling pathway | [61] |
| Kaempferol | In vitro | HT29, SW480 | – | 20- 60 µM /L | 24 and 48 h | Apoptosis and reduction of Akt activity | [62] | |
| Kaempferol | In vitro | RKO and HCT116 | – | 0.1–1000 µM | 48 h | Upregulation of MMP28 and downregulation of NTRK3 | [63] | |
| Kaempferol-TRAIL | In vitro | SW480 | – | 10–40 µM | 24 h | Apoptosis | [64] | |
|
Kaempferol- 5‑fluorouracil |
In vitro | HcT8, HcT116 | – | 100 µM | 24 h | PI3K/Akt signaling pathway | [138] | |
| Kaempferol-fluoxetine | In vivo | - | Adult male Sprague–Dawley rats | 200 mg/kg | Oral administration, daily, 6 weeks | Decreasing ROS production, inhibition of lipid peroxidation | [66] | |
| Pegylated aunps-DOX@Kaempferol | In vitro | HT-29 cell line | – | 50–100 nm | 48 h | Intrinsic pathways | [130] | |
| Kaempferol-quercetin | In vitro | HT-116 cell line | – |
1Q:1 K, 2Q:1 K, and 1Q:2 K |
48 h | p53-caspase-3 pathway and pro-apoptotic Bcl-2 family members PUMA and Bax | [131] | |
| Kaempferol-doxorubicin and cisplatin | In vitro | HCT-15 cells | – | 60, 30 and 15 µg/ml | 24 h |
signaling pathways such as apoptosis and angiogenesis |
[136] | |
| Endometrial | Kaempferol | In vitro | Ishikawa, HEC-265, HEC108, and HEC180 cells | – | 36 µM, 72 µM | 48 h | Suppression of the ER-α and the anti-apoptotic proteins | [69] |
| Kaempferol | In vitro | MFE280 | – | 0.5–20 µM | 24 h | Inhibition of mTOR/PI3K/Akt signaling pathway | [139] | |
| Esophageal carcinoma | Kaempferol |
In vitro In vivo |
KYSE150 and Eca109 cells |
Five-week-old female Balb/c athymic nude mice |
In vitro: 30–60 μM In vivo: 100 mg/kg |
24, 48, 72 h Intraperitoneal injection, every three days, 25 days |
Inhibition of EGFR signaling pathway | [122] |
| Kaempferol-zinc (II) complex | In vitro | EC9706 cells | – | 6, 18, 30 µg/mL | 24 h | Cellular signaling pathway | [129] | |
| Fibrosarcoma | Kaempferol | In vitro | HT1080 | – | 10–100 μM | 24 h | Blocking NF-κB | [124] |
| Gastric cancer | Kaempferol |
In vitro In vivo |
MKN28 and SGC7901 | 4-week-old athymic mice |
In vitro: 25–200 μM In vivo: 20 mg/kg |
24, 48, 72 h Intraperitoneal injection, daily, 3 weeks |
Downregulation of G2/M cell cycle-associated proteins |
[72] |
| Kaempferol | In vitro |
AGS, SNU216, NCI-N87, SNU638, and MKN74 |
– | 25–100 μM | 8, 16, and 24 h | Induction of autophagic cell | [73] | |
| Head and neck | Kaempferol | In vitro | Cal27, HEp2, and DOK | – | 70–280 μM | 48 and 72 h | Induction apoptosis and inhibition of the capacity of migration | [121] |
| Leukemia | Kaempferol | In vitro | Human monocytic cell line THP1 | – | 40 µM | 12, 24, 48, and 72 h | Significant decrease in Bcl-xL expression | [74] |
| Kaempferol | In vitro | CCRF-CEM (CCL1199) and Jurkat E61 (TIB152) | – | 10 µg/mL | 4, 12, 24, 48, 72, and 96 h | Production and expression of IL-2 cytokine | [140] | |
| Kaempferol | In vitro | Human promyelocytic leukemia cell lines, HL60 and NB4 | – | 12.5–100 μM | 24, 48, and 72 h | Decreased viability of leukemia cells | [79] | |
| Kaempferol | In vitro | Jurkat/Neo cells and Jurkat/Bcl-2 cells | – | 25–75 μM | 36 h | Bak and PUMA upregulation | [77] | |
| Kaempferol | In vitro | MOLT4 cell | – | 95 μM | 12, 24, and 48 h | Induced apoptosis | [141] | |
| Liver cancer | Kaempferol | In vitro | Huh7 | – | 0–100 μM | 24 and 48 h | Inhibition of HIF-1 and MAPK | [80] |
| Kaempferol | In vivo | – | Male wistar albino rats | 100 mg/kg | Oral administration, daily, 28 days | Mitochondrial TCA cycle | [81] | |
| Kaempferol | In vitro | HepG2 | – | 1–20 μM | 24 h | Suppression of the downregulatory action of TNF-α | [82] | |
| Kaempferol | In vitro | SK-HEP-1 | – | 25–100 μM | 24 h | Autophagy induction via AMPK, AKT, and mTOR signaling pathways | [83] | |
| Kaempferol | In vitro | HepG2 | – | 5–100 μM | 3, 6, 12, and 24 h | Activation of ER stress-CHOP pathway | [85] | |
| Kaempferol | In vitro |
Hepatocytes (obtained from the rat liver of hepatocellular carcinoma) |
Male Sprague–Dawley rats | 2.5–100 μM | 24 and 48 h | Release of cytochrome c via ROS generation before cytotoxicity ensued | [86] | |
| Kaempferol | In vitro |
Huh7, Huh1, HepG2, HepG2.2.15, SK-Hep-1, PLC/PRF/5, HLE, HLF, and Hep3B |
– | 40 μM | 24, 48, and 72 h | Activating of mitochondrial signaling pathways and inhibition of the PI3K/mTOR/MMP signaling pathway | [89] | |
| Kaempferol | In vitro | HepG2 | – | 1–20 μM | 24 h | Upregulated the ABCA1 mRNA expression | [84] | |
| Lung cancer | Kaempferol | In vitro | A549 and NCIH460 | – | 1–50 μM | 24, 48, and 72 h | Downregulation of a unique inhibitor of the NF-κB pathway | [96] |
| Kaempferol | In vitro | A549 | – | 5–20 μM | 24 and 48 h | ERK1/2 signaling pathway | [129] | |
| Kaempferol | In vitro | A549 | – |
17.5–70 μM 14–112 μM |
24 and 48 h | PI3K/AKT and ERK pathways in cell apoptosis | [95, 94] | |
| Kaempferol | In vitro | A549 | – | 10- 50 μM | 24 and 48 h | Activation of MEK-MAPK signaling pathway | [94, 100] | |
| Nervous system cancer | Kaempferol | In vitro | U87 and U251 cells | – | 50–200 μM | 48 h | Apoptosis | [101] |
| Kaempferol | In vitro | A172 | – | 50–200 μM | 6, 12, 24, 36, 48 h | Induction of cell death | [102] | |
| Kaempferol | In vitro | PC12 | – | 5 and 10 μM | 24 h | Inhibiting activation of the NOX-JNK signaling pathway | [103] | |
| Kaempferol | In vitro | IMR32 and Neuro2A | – | 50 μM | 48, 72, 96 h | Induction of IRE1a -XBP1 pathway | [104] | |
| Ovarian cancer | Kaempferol |
In vitro In vivo |
OVCAR3 and A2780/CP70 cells | Chick embryo chorioallantoic membrane (CAM) |
In vitro: 10–80 μM In vivo: 20-µM |
24 h Single-dose, five days incubation |
Downregulation of HIF-1α; repression of AKT phosphorylation | [105] |
| Kaempferol | In vitro | A2780/CP70 | – | 30–50 μM | 48 h | Caspase-8 pathway | [106] | |
| Kaempferol | In vitro | Caov3, TOV-112D, SKOV3, OVACAR3 | – | 12.5–50 μM | 24 h | Upregulation of apoptotic proteins and STAT3 signaling pathways | [107] | |
| Kaempferol | In vitro | A2780 | – | 40 µmol/l | 24 h | Activation autophagy mechanism | [108] | |
| Kaempferol-cisplatin | In vitro | A2780 | – |
40 µmol/l kaempferol with cisplatin (0, 2, 4, 6, 8, 10, 15, 20 µmol/l) |
24 h | PI3K/Akt signalling pathway | [132] | |
| Kaempferol-cisplatin | In vitro | OVCAR-3 | – | 0–80 μM Cisplatin with other chemicals of 0–20 μM | 24 h | Apoptosis caused by down regulation of cMyc | [98] | |
| Pancreatic cancer | Kaempferol | In vitro | Miapaca2, Panc1, and SNU213 cells | – | 0.005–200 μM | 72 h | Inhibition of EGFR and AKT pathways | [111] |
| Kaempferol |
In vitro In vivo |
PANC1, Miapaca2 cells | Tumor Xenograft models (male BALB/c mice) |
In vitro: 2.5–1000 μmol/L In vivo: 25–100 mg/kg |
48 h Gavage for seven days |
Induction of ROS-dependent apoptosis via Akt/mTOR signaling |
[112] | |
| Kaempferol- erlotinib |
In vitro In vivo |
PANC-1 and BxPC-3 cell lines | In vivo: Four-week-old female mice | Various dosages |
In vitro: 72 h In vivo: 4 weeks |
PI3K/AKT signaling pathway and epidermal growth factor receptor | [133] | |
| Prostate cancer | Kaempferol | In vitro | PC3 | – | 10 μM | 18 h | Activation of PLC, PKC, and MEK1/2 cascade | [22] |
| Kaempferol | In vito | LNCaP, PC3 | – | 5- 15 μM | 24, 48, 96, and 144 h | Inhibition of cell proliferation via androgen-dependent pathway | [113] | |
| Renal cancer | Kaempferol | In vitro | 786O and 769P | – | In vitro: 50- 150 μM | 24, 48, 72 h | Inhibition of MAPK signaling pathways, | [123] |
| Retinoblastoma | Kaempferol |
In vitro In vivo |
SO-RB50 |
25 human tissue samples (included five normal, 11 normal pediatric retinas, and 14 retinoblastomas) |
10–30 μM | 24 h | Suppression of Wnt/β-catenin signaling pathway | [126] |
| Skin cancer | Kaempferol | In vitro | HaCaT | – | 1–10 μM | 24 h | PPAR pathways | [116] |
| Kaempferol | In vitro | JB6 P + mouse epidermal cell line | – | 10–40 μM | 24 h | Inhibition of PI3K activity | [118] | |
| Kaempferol |
In vitro In vivo |
A431, A431 sh-RSK2, A431 sh-MSK1, or A431 sh-RSK2/ sh-MSK1, NIH3T3 |
Female SKH-1 hairless mice |
In vitro: 0–50 μM/L In vivo: 0.5 or 1 mg |
2 h Topical application, 12 weeks |
Suppresses RSK2 and MSK1 kinase activities | [119] | |
| Kaempferol | In vitro | HaCaT | – | 200 µg/mL of Prunus cerasus | 24 h | Blocking of the mitochondrial pathway of apoptosis | [117] | |
| Kaempferol | In vitro | A375 | – | 10–80 μM | 24, 48, and 72 h | Induction of apoptosis and downregulation of mTOR/PI3K/AKT pathway | [120] |
The treatments include kaempferol as a single agent or being combined with erlotinib, cisplatin, zinc (II), doxorubicin, TRAIL, 5‑fluorouracil, fluoxetine, PEGylated AuNPs-DOX, quercetin, paclitaxel, verapamil
Fig. 2.
The activation tumour cell apoptosis using kaempferol as a therapeutics agent through diverse range of signaling pathways and mitochondrial mechanisms (KF: kaempferol)
Conclusion
Kaempferol is a bioflavonoid compound involved in reducing the incidence of hormone-related cancers. Oncogene-induced apoptosis and growth inhibition are the primary mechanisms of this compound; however, increasing the host's immunological response is also considered an important mechanism. When present in more significant quantities, kaempferol is associated with an anti-cancer action; yet, when present in lower concentrations, it is associated with a pro-cancer activity. Kaempferol may have a role in activating apoptosis in many different kinds of cancer cells. If this is the case, kaempferol may have an anti-cancer effect via intrinsic and extrinsic apoptotic pathways. Because of its propensity to limit cell proliferation, kaempferol is engaged in different processes. These activities include activation of JAK/STAT3, PI3K/AKT, and NF-κB, as well as interference with TNF-induced activation of MAPK. Considering the interaction induced by various elements such as gender and immunological conditions is necessary. In vitro and animal studies have been done to determine precisely what function kaempferol plays, but more research is needed to figure out the mechanism(s) by which it works in humans.
In summary, based on the literature that has been published so far, the use of kaempferol in combination therapy is successful. However, there is still a lack of sufficient scientific data and regulatory approval from the appropriate authorities. Kaempferol has been shown to have chemotherapeutic potential; however, further data and studies are needed to confirm kaempferol's status as a potentially effective chemotherapeutic drug. Preclinical evidence from human trials must also be obtained before using kaempferol as a chemotherapy drug. And if additional research is done on kaempferol's tumor growth-inhibiting capacity, it might be a valuable therapeutic drug in the future.
Acknowledgements
The authors would like to thank the Research Office of Tabriz University of Medical Sciences for providing support under the support scheme (Grant No. 69284)
Abbreviations
- ABCA1
ATP-binding cassette transporter
- ABCC6
ATP-binding cassette subfamily C member 6
- AFB1
Aflatoxin B1
- AIF
Apoptosis-inducing factor
- AKT
Ak strain transforming
- ALPase
Alkaline phosphatase
- AhR
Aryl hydrocarbon receptor
- AML
Myeloid leukemia
- AMPK
Adenosine monophosphate-activated protein kinase
- ATF6
Activating transcription factor 6
- ATM
Ataxia telangiectasia mutated
- AP-1
Activator protein 1
- ATP
Adenosine tri-phosphate
- BAX
Bcl-2-associated X protein
- Bcl2
B-cell lymphoma 2
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- H2AX
H2A histone family member X
- HDAC
Histone deacetylase
- HIF
Hypoxia-Inducible Factor
- HO-1
Heme oxygenase-1
- IκBα
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- IRE
Inositol-requiring kinase
- IRS
Insulin receptor substrate
- JAK
Janus kinase
- JNK
C-Jun N-terminal kinase
- K-AuNCs
Kaempferol-conjugated gold nanoparticles
- LC3
Microtubule-associated protein 1A/1B-light chain 3
- PERK
Protein kinase RNA- like endoplasmic reticulum kinase
- PCR
Polymerase chain reaction
- PLC
Phospholipase C
- PPAR
Peroxisome proliferator-activated receptor
- PTEN
Phosphatase and tensin homolog
- PI3K/Akt
Phosphatidylinositol 3-kinase/protein kinase B
- PML/RARα
Promyelocytic leukemia/retinoic acid receptor-α
- TNF
Tumor necrosis factor
- Bcl-xL
B-cell lymphoma-extra large
- Bid
A BH3 domain-only death agonist protein
- BMI
Body Mass Index
- BRCA
Breast cancer type
- CAT
Chloramphenicol acetyltransferase
- C/EBPβ
CCAAT/enhancer-binding protein β
- Cx43
Connexin43
- Caspase
Cysteine-aspartic proteases, cysteine aspartases
- CHOP
CCAAT-enhancer-binding protein homologous protein
- c-Met
Mesenchymal-epithelial transition factor
- CYP1A1
Cytochrome P450, family 1, subfamily A, polypeptide 1
- CDK
Cyclin-dependent kinase
- Cdc25C
A cyclin of the specific phosphatase family that activates the cyclin B1/CDK1 complex
- Chk2
The CHEK2 gene encodes for checkpoint kinase 2
- COX
Cyclooxygenase
- MAPK
Mitogen-activated protein kinase
- MCF-7
Michigan Cancer Foundation 7
- MDR
Multiple drug resistance
- MEK
MAPK/ERK kinase
- Mfn2
Mitofusin-2
- Mcl-1
Myeloid cell leukemia 1
- MMP
Matrix metalloproteinase
- MSK
Mitogen and stress-activated kinase
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- mTOR
Mammalian target of rapamycin
- NADPH
Reduced nicotinamide adenine dinucleotide phosphate
- NAFLD
Nonalcoholic fatty liver disease
- PSA
Prostate-specific antigen
- RSK
Ribosomal S6 kinase
- ROS
Reactive oxygen species
- SCD-1
Stearoyl-CoA Desaturase-1
- SOD
Superoxide dismutase
- SREBP1
Sterol Regulatory Element-binding Protein-1
- Stat3
Signal transducer and activator of transcription 3
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- dDMPs
Deoxydinucleoside Monophosphates
- DNMT3B
DNA methyltransferase 3B
- EGFR
Epidermal growth factor receptor
- ELK1
ETS Transcription factor ELK1
- EMT
Epithelial-mesenchymal transition
- ERK
Extracellular signal-regulated kinase
- ER-negative
Estrogen receptor-negative
- ERR-α
Estrogen-related receptor α
- ESRRA
Estrogen-related receptor alpha
- FADD
FAS-associated death domain protein
- FKBP12
12-KDa FK506-binding protein
- FGF-8
Fibroblast growth factor 8
- GRP78
Glucose Regulated Protein 78,000
- GJIC
Gap junction intercellular communication
- GLOBOCAN
Global Cancer Incidence, Mortality, and Prevalence
- NF-κB
Nuclear factor-kappaB
- NOX
ADPH oxidase
- NQO1
NAD(P)H quinone oxidoreductase 1
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PAD4
Protein arginine deiminase 4
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- pAkt
Phosphorylated Protein kinase B
- Phox
Phagocyte oxidase
- PMA
Phorbol myristate acetate
- PKC
Protein kinase C
- PARP
Poly (ADP-ribose) polymerase
- TCA
Tricarboxylic acid cycle
- TFAM
Mitochondrial transcription factor A
- TGF
Transforming growth factor
- TGM2
The transglutaminase 2 gene
- TIMP2
Tissue inhibitor of metalloproteinases 2
- TMEPA1
Transmembrane prostate androgen protein
- TMPRSS2
Transmembrane Serine Protease 2
- XIAP
X-linked inhibitor of apoptosis protein
- RCC
Renal cell carcinoma
Author contributions
BS and SA—conceptualization; EA, BS, and SA—data curation; EA and SA—formal analysis; EA and BS—investigation; EA, BS, and SA—methodology; BS—project administration; BS and SA—supervision; EA, BS, and SA—roles/writing—original draft; EA, BS, and SA—writing—review and editing. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors contributed equally and Joint first authors
Contributor Information
Babak Sokouti, Email: b.sokouti@gmail.com, Email: sokoutib@tbzmed.ac.ir.
Solmaz Asnaashari, Email: asnaasharisolmaz@gmail.com.
References
- 1.Koval S, Murane W, Sasser A, Seiner O, Troth J, Courtine J, Messay B, Jiang A, Guerrero C, Kodali A. Cancer and its treatments. Dartmouth Undergrad J Sci. 2020;1(Summer):172–187. [Google Scholar]
- 2.Cooper G. The cell: a molecular approach. Sunderland: SinauerAssociates; 2000. [Google Scholar]
- 3.Migliore L, Coppedè F. Genetic and environmental factors in cancer and neurodegenerative diseases. Mutat Res. 2002;512(2–3):135–153. doi: 10.1016/S1383-5742(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Yadav AR, Mohite SK. Cancer-a silent killer: an overview. Asian J Pharm Res. 2020;10(3):213–216. doi: 10.5958/2231-5691.2020.00036.2. [DOI] [Google Scholar]
- 5.Martin-Moreno JM, Ruiz-Segovia N, Diaz-Rubio E. Behavioural and structural interventions in cancer prevention: towards the 2030 SDG horizon. Mol Oncol. 2021;15(3):801–808. doi: 10.1002/1878-0261.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y-B, Pan X-F, Chen J, Cao A, Zhang Y-G, Xia L, Wang J, Li H, Liu G, Pan A. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer. 2020;122(7):1085–1093. doi: 10.1038/s41416-020-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Key TJ, Bradbury KE, Perez-Cornago A, Sinha R, Tsilidis KK, Tsugane S. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ. 2020 doi: 10.1136/bmj.m511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amjad E, Asnaashari S, Sokouti B, Dastmalchi S. Systems biology comprehensive analysis on breast cancer for identification of key gene modules and genes associated with TNM-based clinical stages. Sci Rep. 2020;10(1):10816. doi: 10.1038/s41598-020-67643-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amjad E, Asnaashari S, Sokouti B, Dastmalchi S. Impact of gene biomarker discovery tools based on protein-protein interaction and machine learning on performance of artificial intelligence models in predicting clinical stages of breast cancer. Interdiscip Sci Comput Life Sci. 2020;12(4):476–486. doi: 10.1007/s12539-020-00390-8. [DOI] [PubMed] [Google Scholar]
- 10.Saidu R, Morhason-Bello I. Same-day test and treat for early detection and treatment of cervical cancer in LMICs. Lancet Glob Health. S2214109X22003163. [DOI] [PubMed]
- 11.Ahmed Md-E, Bhardwaj A. A novel hybrid convolutional neural network approach for the stomach intestinal early detection cancer subtype classification. Comput Intell Neurosci. 2022;1:7325064. doi: 10.1155/2022/7325064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pericleous S, Bhogal RH, Mavroeidis VK. The role of circulating biomarkers in the early detection of recurrent colorectal cancer following resection of liver metastases. Front Biosci-Landmark. 2022;27(6):189. doi: 10.31083/j.fbl2706189. [DOI] [PubMed] [Google Scholar]
- 13.Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, Capella G. Recent advances in cancer therapy: an overview. Curr Pharm Des. 2010;16(1):3–10. doi: 10.2174/138161210789941847. [DOI] [PubMed] [Google Scholar]
- 14.Baskar R, Lee KA, Yeo R, Yeoh K-W. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci. 2017;18(3):656. doi: 10.3390/ijms18030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers (Basel) 2014;6(3):1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amjad E, Sokouti B, Asnaashari S. An investigation of 6-Shogaol effects on MCF7 cell lines through a systems biology approach. Egypt J Med Hum Gene. 2022;23(1):60. doi: 10.1186/s43042-022-00276-y. [DOI] [Google Scholar]
- 18.El-Saadony MT, Zabermawi NM, Zabermawi NM, Burollus MA, Shafi ME, Alagawany M, Yehia N, Askar AM, Alsafy SA, Noreldin AE. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev Int. 2021 doi: 10.1080/87559129.2021.1944183. [DOI] [Google Scholar]
- 19.Braicu C, Zanoaga O, Zimta A-A, Tigu AB, Kilpatrick KL, Bishayee A, Nabavi SM, Berindan-Neagoe I. Natural compounds modulate the crosstalk between apoptosis-and autophagy-regulated signaling pathways: controlling the uncontrolled expansion of tumor cells. Semin Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Calderon-Montano JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11(4):298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 21.Rasouli H, Farzaei MH, Khodarahmi R. Polyphenols and their benefits: a review. Int J Food Prop. 2017;20(sup2):1700–1741. [Google Scholar]
- 22.Bandyopadhyay S, Romero JR, Chattopadhyay N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol Cell Endocrinol. 2008;287(1–2):57–64. doi: 10.1016/j.mce.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Karabin M, Hudcova T, Jelinek L, Dostalek P. Biotransformations and biological activities of hop flavonoids. Biotechnol Adv. 2015;33(6):1063–1090. doi: 10.1016/j.biotechadv.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Barve A, Chen C, Hebbar V, Desiderio J, Saw CLL, Kong AN. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm Drug Disp. 2009;30(7):356–365. doi: 10.1002/bdd.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mierziak J, Kostyn K, Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules. 2014;19(10):16240–16265. doi: 10.3390/molecules191016240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira E, Watson D, Grant M. Metabolism of quercetin and kaempferol by rat hepatocytes and the identification of flavonoid glycosides in human plasma. Xenobiotica. 2002;32(4):279–287. doi: 10.1080/00498250110107886. [DOI] [PubMed] [Google Scholar]
- 27.Crespy V, Morand C, Besson C, Cotelle N, Vézin H, Demigné C, Rémésy C. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am J Physiol Gastroint Liver Physiol. 2003;284(6):980–988. doi: 10.1152/ajpgi.00223.2002. [DOI] [PubMed] [Google Scholar]
- 28.Alam W, Khan H, Shah MA, Cauli O, Saso L. Kaempferol as a dietary anti-inflammatory agent: current therapeutic standing. Molecules. 2020;25(18):4073. doi: 10.3390/molecules25184073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuPont M, Day A, Bennett R, Mellon F, Kroon P. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur J Clin Nutr. 2004;58(6):947–954. doi: 10.1038/sj.ejcn.1601916. [DOI] [PubMed] [Google Scholar]
- 30.De Vries J, Hollman P, Meyboom S, Buysman M, Zock PL, van Staveren WA, Katan MB. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am J Clin Nutr. 1998;68(1):60–65. doi: 10.1093/ajcn/68.1.60. [DOI] [PubMed] [Google Scholar]
- 31.Radtke J, Linseisen J, Wolfram G. Fasting plasma concentrations of selected flavonoids as markers of their ordinary dietary intake. Eur J Nutr. 2002;41(5):203–209. doi: 10.1007/s00394-002-0377-z. [DOI] [PubMed] [Google Scholar]
- 32.Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr. 2010;103(2):249–255. doi: 10.1017/S000711450999170X. [DOI] [PubMed] [Google Scholar]
- 33.Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci. 2020;8(1):15. doi: 10.3390/medsci8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu P, Meng X, Zheng H, Zeng Q, Chen T, Wang W, Zhang X, Su J. Kaempferol attenuates ROS-induced hemolysis and the molecular mechanism of its induction of apoptosis on bladder cancer. Molecules. 2018;23(10):2592. doi: 10.3390/molecules23102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie F, Su M, Qiu W, Zhang M, Guo Z, Su B, Liu J, Li X, Zhou L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int J Mol Sci. 2013;14(11):21215–21226. doi: 10.3390/ijms141121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang Q, Song W, Xu D, Ma Y, Li F, Zeng J, Zhu G, Wang X, Chang LS, He D. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol Carcinog. 2015;54(9):831–840. doi: 10.1002/mc.22154. [DOI] [PubMed] [Google Scholar]
- 37.Qiu W, Lin J, Zhu Y, Zhang J, Zeng L, Su M, Tian Y. Kaempferol modulates DNA methylation and downregulates DNMT3B in bladder cancer. Cell Physiol Biochem. 2017;41(4):1325–1335. doi: 10.1159/000464435. [DOI] [PubMed] [Google Scholar]
- 38.Pullan JE, Lotfollahzadeh S. Primary Bone Cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022 [PubMed]
- 39.Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF, Li KH, Chen PY, Chung JG, Yang JS. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol Nutr Food Res. 2010;54(11):1585–1595. doi: 10.1002/mnfr.201000005. [DOI] [PubMed] [Google Scholar]
- 40.Chen H-J, Lin C-M, Lee C-Y, Shih N-C, Peng S-F, Tsuzuki M, Amagaya S, Huang W-W, Yang J-S. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep. 2013;30(2):925–932. doi: 10.3892/or.2013.2490. [DOI] [PubMed] [Google Scholar]
- 41.Pang JL, Ricupero DA, Huang S, Fatma N, Singh DP, Romero JR, Chattopadhyay N. Differential activity of kaempferol and quercetin in attenuating tumor necrosis factor receptor family signaling in bone cells. Biochem Pharmacol. 2006;71(6):818–826. doi: 10.1016/j.bcp.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 42.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 43.Hung H. Inhibition of estrogen receptor alpha expression and function in MCF-7 cells by kaempferol. J Cell Physiol. 2004;198(2):197–208. doi: 10.1002/jcp.10398. [DOI] [PubMed] [Google Scholar]
- 44.Kim S-H, Hwang K-A, Choi K-C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J Nutr Biochem. 2016;28:70–82. doi: 10.1016/j.jnutbio.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 45.MacPherson L, Matthews J. Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor α expression in human breast cancer cells. Cancer Lett. 2010;299(2):119–129. doi: 10.1016/j.canlet.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh SM, Kim YP, Chung KH. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch Pharmacal Res. 2006;29(5):354–362. doi: 10.1007/BF02968584. [DOI] [PubMed] [Google Scholar]
- 47.Kim B-W, Lee E-R, Min H-M, Jeong H-S, Ahn J-Y, Kim J-H, Choi H-Y, Choi H, Kim EY, Park SP. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol Ther. 2008;7(7):1080–1089. doi: 10.4161/cbt.7.7.6164. [DOI] [PubMed] [Google Scholar]
- 48.Azevedo C, Correia-Branco A, Araújo JR, Guimaraes JT, Keating E, Martel F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr Cancer. 2015;67(3):504–513. doi: 10.1080/01635581.2015.1002625. [DOI] [PubMed] [Google Scholar]
- 49.Li C, Zhao Y, Yang D, Yu Y, Guo H, Zhao Z, Zhang B, Yin X. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem Cell Biol. 2015;93(1):16–27. doi: 10.1139/bcb-2014-0067. [DOI] [PubMed] [Google Scholar]
- 50.Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340(3):715–722. doi: 10.1042/bj3400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soltanian S, Riahirad H, Pabarja A, Karimzadeh MR, Saeidi K. Kaempferol and docetaxel diminish side population and down-regulate some cancer stem cell markers in breast cancer cell line MCF-7. Biocell. 2017;41(2&3):33. doi: 10.32604/biocell.2017.41.033. [DOI] [Google Scholar]
- 52.Zeng J, Xu H, Fan PZ, Xie J, He J, Yu J, Gu X, Zhang CJ. Kaempferol blocks neutrophil extracellular traps formation and reduces tumour metastasis by inhibiting ROS-PAD4 pathway. J Cell Mol Med. 2020;24(13):7590–7599. doi: 10.1111/jcmm.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu L, Xue L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol Res. 2019;27(6):629. doi: 10.3727/096504018X15228018559434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo B, Liao ZX, Xu C, Liu C. Two novel prenylated kaempferol derivatives from fresh bud’s fur of Platanus acerifolia and their anti-proliferative activities. Nat Prod Res. 2016;30(22):2523–2528. doi: 10.1080/14786419.2015.1118632. [DOI] [PubMed] [Google Scholar]
- 55.Diantini A, Subarnas A, Lestari K, Halimah E, Susilawati Y, Supriyatna S, Julaeha E, Achmad TH, Suradji EW, Yamazaki C. Kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol Lett. 2012;3(5):1069–1072. doi: 10.3892/ol.2012.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang R, Zhang Y, Xin X, Huang G, Zhang N, Zeng Q, Tang L, Attaribo T, Lee KS, Jin BR. Dual-targeting antiproliferation hybrids derived from 1-deoxynojirimycin and kaempferol induce MCF-7 Cell apoptosis through the mitochondria-mediated pathway. J Nat Prod. 2021 doi: 10.1021/acs.jnatprod.1c00014. [DOI] [PubMed] [Google Scholar]
- 57.Aghazadeh T, Bakhtiari N, Rad IA, Ramezani F. Formulation of kaempferol in nanostructured lipid carriers (NLCs): a delivery platform to sensitization of MDA-MB468 breast cancer cells to paclitaxel. Biointerface Res Appl Chem. 2021 doi: 10.33263/BRIAC116.1459114601. [DOI] [Google Scholar]
- 58.Raghavan BS, Kondath S, Anantanarayanan R, Rajaram R. Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. Process Biochem. 2015;50(11):1966–1976. doi: 10.1016/j.procbio.2015.08.003. [DOI] [Google Scholar]
- 59.Filomeni G, Desideri E, Cardaci S, Graziani I, Piccirillo S, Rotilio G, Ciriolo MR. Carcinoma cells activate AMP-activated protein kinase-dependent autophagy as survival response to kaempferol-mediated energetic impairment. Autophagy. 2010;6(2):202–216. doi: 10.4161/auto.6.2.10971. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Xu W, Li C, Wu H, Liu Y. Kaempferol-7-Ob-Dglucoside (KG) isolated from Smilax china L. rhizome induces G2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett. 2008;264:229–240. doi: 10.1016/j.canlet.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 61.Nakamura Y, Chang C-C, Mori T, Sato K, Ohtsuki K, Upham BL, Trosko JE. Augmentation of differentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis. 2005;26(3):665–671. doi: 10.1093/carcin/bgi003. [DOI] [PubMed] [Google Scholar]
- 62.Lee HS, Cho HJ, Yu R, Lee KW, Chun HS, Park JHY. Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int J Mol Sci. 2014;15(2):2722–2737. doi: 10.3390/ijms15022722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Budisan L, Gulei D, Jurj A, Braicu C, Zanoaga O, Cojocneanu R, Pop L, Raduly L, Barbat A, Moldovan A. Inhibitory effect of CAPE and kaempferol in colon cancer cell lines—possible implications in new therapeutic strategies. Int J Mol Sci. 2019;20(5):1199. doi: 10.3390/ijms20051199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshida T, Konishi M, Horinaka M, Yasuda T, Goda AE, Taniguchi H, Yano K, Wakada M, Sakai T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem Biophys Res Commun. 2008;375(1):129–133. doi: 10.1016/j.bbrc.2008.07.131. [DOI] [PubMed] [Google Scholar]
- 65.Li Q, Wei L, Lin S, Chen Y, Lin J, Peng J. Synergistic effect of kaempferol and 5-fluorouracil on the growth of colorectal cancer cells by regulating the PI3K/Akt signaling pathway. Mol Med Rep. 2019;20(1):728–734. doi: 10.3892/mmr.2019.10296. [DOI] [PubMed] [Google Scholar]
- 66.Hassan ES, Hassanein NM, Ahmed HMS. Probing the chemoprevention potential of the antidepressant fluoxetine combined with epigallocatechin gallate or kaempferol in rats with induced early stage colon carcinogenesis. J Pharmacol Sci. 2021;145(1):29–41. doi: 10.1016/j.jphs.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Meena D, Vimala K, Kannan S. Combined delivery of DOX and Kaempferol using PEGylated gold nanoparticles to target colon cancer. J Clust Sci. 2021 doi: 10.1007/s10876-020-01961-x. [DOI] [Google Scholar]
- 68.Besso MJ, Montivero L, Lacunza E, Argibay MC, Abba M, Furlong LI, Colas E, Gil-Moreno A, Reventos J, Bello R. Identification of early stage recurrence endometrial cancer biomarkers using bioinformatics tools. Oncol Rep. 2020;44(3):873–886. doi: 10.3892/or.2020.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chuwa AH, Sone K, Oda K, Tanikawa M, Kukita A, Kojima M, Oki S, Fukuda T, Takeuchi M, Miyasaka A. Kaempferol, a natural dietary flavonoid, suppresses 17β-estradiol-induced survivin expression and causes apoptotic cell death in endometrial cancer. Oncol Lett. 2018;16(5):6195–6201. doi: 10.3892/ol.2018.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lei X, Guo J, Wang Y, Cui J, Feng B, Su Y, Zhao H, Yang W, Hu Y. Inhibition of endometrial carcinoma by Kaempferol is interceded through apoptosis induction, G2/M phase cell cycle arrest, suppression of cell invasion and upregulation of m-TOR/PI3K signalling pathway. J BUON Off J Balk Union Oncol. 2019;24:1555–1561. [PubMed] [Google Scholar]
- 71.Du BY, Zhou Y, Tan YH, Wu YY, Liu JJ, Liu XJ. Effect of kaempferol in inhibiting proliferation and inducing apoptosis of human gastric cancer cell line MGC-803. 肿瘤. 2010;6.
- 72.Song H, Bao J, Wei Y, Chen Y, Mao X, Li J, Yang Z, Xue Y. Kaempferol inhibits gastric cancer tumor growth: an in vitro and in vivo study. Oncol Rep. 2015;33(2):868–874. doi: 10.3892/or.2014.3662. [DOI] [PubMed] [Google Scholar]
- 73.Kim TW, Lee SY, Kim M, Cheon C, Ko S-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9(9):1–14. doi: 10.1038/s41419-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamid Reza Ghaderi J, Sedigheh S, Abbas Zakeri B. Flavonoids kaempferol (KAE) and quercetine (QUE) inhibited proliferation of human leukemia THP-1 cells by up regulation of pro-apoptotic protein Bax and caspase 3/8 expression and down regulation of anti-apoptotic proteins Bcl-2, Bcl-xl and Mcl-1 expression. Ann Cancer Res Ther. 2021;29(1):41–46. doi: 10.4993/acrt.29.41. [DOI] [Google Scholar]
- 75.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 76.Estey E, Karp JE, Emadi A, Othus M, Gale RP. Recent drug approvals for newly diagnosed acute myeloid leukemia: gifts or a Trojan horse? Leukemia. 2020;34(3):671–681. doi: 10.1038/s41375-019-0704-5. [DOI] [PubMed] [Google Scholar]
- 77.Kim KY, Jang WY, Lee JY, Jun do Y, Ko JY, Yun YH, Kim YH. Kaempferol activates G2-checkpoint of the cell cycle resulting in G2-arrest and mitochondria-dependent apoptosis in human acute leukemia jurkat T cells. J Microbiol Biotechnol. 2016;26(2):287–294. doi: 10.4014/jmb.1511.11054. [DOI] [PubMed] [Google Scholar]
- 78.Li W, Du B, Wang T, Wang S, Zhang J. Kaempferol induces apoptosis in human HCT116 colon cancer cells via the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement of p53 upregulated modulator of apoptosis. Chem Biol Interact. 2009;177(2):121–127. doi: 10.1016/j.cbi.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 79.Moradzadeh M, Tabarraei A, Sadeghnia HR, Ghorbani A, Mohamadkhani A, Erfanian S, Sahebkar A. Kaempferol increases apoptosis in human acute promyelocytic leukemia cells and inhibits multidrug resistance genes. J Cell Biochem. 2018;119(2):2288–2297. doi: 10.1002/jcb.26391. [DOI] [PubMed] [Google Scholar]
- 80.Mylonis I, Lakka A, Tsakalof A, Simos G. The dietary flavonoid kaempferol effectively inhibits HIF-1 activity and hepatoma cancer cell viability under hypoxic conditions. BBRC. 2010;398(1):74–78. doi: 10.1016/j.bbrc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 81.Langeswaran K, Revathy R, Kumar SG, Vijayaprakash S, Balasubramanian MP. Kaempferol ameliorates aflatoxin B1 (AFB1) induced hepatocellular carcinoma through modifying metabolizing enzymes, membrane bound ATPases and mitochondrial TCA cycle enzymes. Asian Pac J Trop Biomed. 2012;2(3):S1653–S1659. doi: 10.1016/S2221-1691(12)60471-7. [DOI] [Google Scholar]
- 82.Ee YY, Hoong CC. Downregulation in the mRNA expression of nuclear hormone receptor liver-X-receptor alpha (LXR-α) by TNF-α is abolished by the antioxidant kaempferol, but not ascorbic acid, in human hepatocarcinoma HepG2 cells. Asian Biomed. 2012;6(4):585–589. [Google Scholar]
- 83.Huang W-W, Tsai S-C, Peng S-F, Lin M-W, Chiang J-H, Chiu Y-J, Fushiya S, Tseng MT, Yang J-S. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int J Oncol. 2013;42(6):2069–2077. doi: 10.3892/ijo.2013.1909. [DOI] [PubMed] [Google Scholar]
- 84.Cheah H-Y, Wong Y-Y, Wong H-K, Lim W-S, Chew C-H. Nicotinic acid, lauric acid and kaempferol abolish ATP-binding cassette transporter subfamily A member 1 (ABCA1) down-regulation by TNF-α in hepatocarcinoma HepG2 cell line. Biomed Res. 2014;25(3):419–425. [Google Scholar]
- 85.Guo H, Ren F, Zhang L, Zhang X, Yang R, Xie B, Li Z, Hu Z, Duan Z, Zhang J. Kaempferol induces apoptosis in HepG2 cells via activation of the endoplasmic reticulum stress pathway. Mol Med Rep. 2016;13(3):2791–2800. doi: 10.3892/mmr.2016.4845. [DOI] [PubMed] [Google Scholar]
- 86.Seydi E, Salimi A, Rasekh HR, Mohsenifar Z, Pourahmad J. Selective cytotoxicity of luteolin and kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: involvement of ROS-mediated mitochondrial targeting. Nutr Cancer. 2018;70(4):594–604. doi: 10.1080/01635581.2018.1460679. [DOI] [PubMed] [Google Scholar]
- 87.Tie F, Ding J, Hu N, Dong Q, Chen Z, Wang H. Kaempferol and Kaempferide Attenuate Oleic Acid-Induced Lipid Accumulation and Oxidative Stress in HepG2 Cells. Int J Mol Sci. 2021;22(16):8847. doi: 10.3390/ijms22168847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shakya G, Manjini S, Hoda M, Rajagopalan R. Hepatoprotective role of kaempferol during alcohol-and ΔPUFA-induced oxidative stress. J Basic Clin Physiol Pharmacol. 2014;25(1):73–79. doi: 10.1515/jbcpp-2013-0051. [DOI] [PubMed] [Google Scholar]
- 89.Yang G, Xing J, Aikemu B, Sun J, Zheng M. Kaempferol exhibits a synergistic effect with doxorubicin to inhibit proliferation, migration, and invasion of liver cancer. Oncol Rep. 2021;45(4):1–10. doi: 10.3892/or.2021.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Machado MV, Diehl AM. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology. 2016;150(8):1769–1777. doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Siniprasad P, Nair B, Balasubramaniam V, Sadanandan P, Namboori PK, Nath LR. Evaluation of kaempferol as AKT dependent mTOR regulator via targeting FKBP-12 in hepatocellular carcinoma: an in silico approach. Lett Drug Des Discov. 2020;17(11):1401–1408. doi: 10.2174/1570180817999200623115703. [DOI] [Google Scholar]
- 92.Wang Q, Huang Y, Zhang J-S, Yang X-B. Synthesis, characterization, DNA interaction, and antitumor activities of La (III) complex with Schiff base ligand derived from kaempferol and diethylenetriamine. Bioinorg Chem Appl. 2014;2014:9. doi: 10.1155/2014/354138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen TT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, Nguyen TH, Lee JJ, Tan Y, Ong CS, et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol. 2003;197(1):110–121. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- 95.Kuo WT, Tsai YC, Wu HC, Ho YJ, Chen YS, Yao CH, Yao CH. Radiosensitization of non-small cell lung cancer by kaempferol. Oncol Rep. 2015;34(5):2351–2356. doi: 10.3892/or.2015.4204. [DOI] [PubMed] [Google Scholar]
- 96.Fouzder C, Mukhuty A, Kundu R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch Biochem Biophys. 2021;697:108700. doi: 10.1016/j.abb.2020.108700. [DOI] [PubMed] [Google Scholar]
- 97.Fouzder C, Mukhuty A, Mukherjee S, Malick C, Kundu R. Trigonelline inhibits Nrf2 via EGFR signalling pathway and augments efficacy of Cisplatin and Etoposide in NSCLC cells. Toxicol In Vitro. 2021;70:105038. doi: 10.1016/j.tiv.2020.105038. [DOI] [PubMed] [Google Scholar]
- 98.Govindaraju S, Roshini A, Lee MH, Yun K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int J Nanomed. 2019;14:5147–5157. doi: 10.2147/IJN.S209773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang H, Gao M, Wang J. Kaempferol inhibits cancer cell growth by antagonizing estrogen-related receptor α and γ activities. Cell Biol Int. 2013;37(11):1190–1196. doi: 10.1002/cbin.10152. [DOI] [PubMed] [Google Scholar]
- 100.Jo E, Park SJ, Choi YS, Jeon WK, Kim BC. Kaempferol suppresses transforming growth factor-β1-induced epithelial-to-mesenchymal transition and migration of A549 lung cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3 at Threonine-179. Neoplasia. 2015;17(7):525–537. doi: 10.1016/j.neo.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siegelin MD, Reuss DE, Habel A, Herold-Mende C, Von Deimling A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol Cancer Ther. 2008;7(11):3566–3574. doi: 10.1158/1535-7163.MCT-08-0236. [DOI] [PubMed] [Google Scholar]
- 102.Jeong JC, Kim MS, Kim TH, Kim YK. Kaempferol induces cell death through ERK and Akt-dependent down-regulation of XIAP and survivin in human glioma cells. Neurochem Res. 2009;34(5):991–1001. doi: 10.1007/s11064-008-9868-5. [DOI] [PubMed] [Google Scholar]
- 103.Jang YJ, Kim J, Shim J, Kim J, Byun S, Oak M-H, Lee KW, Lee HJ. Kaempferol attenuates 4-hydroxynonenal-induced apoptosis in PC12 cells by directly inhibiting NADPH oxidase. J Pharmacol Exp Ther. 2011;337(3):747–754. doi: 10.1124/jpet.110.176925. [DOI] [PubMed] [Google Scholar]
- 104.Abdullah A, Talwar P, d'Hellencourt CL, Ravanan P. IRE 1α is critical for Kaempferol-induced neuroblastoma differentiation. FEBS J. 2019;286(7):1375–1392. doi: 10.1111/febs.14776. [DOI] [PubMed] [Google Scholar]
- 105.Luo H, Rankin GO, Liu L, Daddysman MK, Jiang B-H, Chen YC. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr Cancer. 2009;61(4):554–563. doi: 10.1080/01635580802666281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao Y, Yin J, Rankin GO, Chen YC. Kaempferol induces G2/M cell cycle arrest via checkpoint kinase 2 and promotes apoptosis via death receptors in human ovarian carcinoma A2780/CP70 cells. Molecules. 2018;23(5):1095. doi: 10.3390/molecules23051095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang S, Si L, Jia Y, Jian W, Yu Q, Wang M, Lin R. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. J BUON. 2019;24(3):975–981. [PubMed] [Google Scholar]
- 108.El-Kott A, Shati A, Al-Kahtani M, Alharbi S. Kaempferol induces cell death in A2780 ovarian cancer cells and increases their sensitivity to cisplatin by activation of cytotoxic endoplasmic reticulum-mediated autophagy and inhibition of protein kinase B. Folia Biol. 2020;66:36–46. [PubMed] [Google Scholar]
- 109.Luo H, Daddysman MK, Rankin GO, Jiang B-H, Chen YC. Kaempferol enhances cisplatin's effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc. Cancer Cell Int. 2010;10(1):1–9. doi: 10.1186/1475-2867-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo H, Jiang B, Li B, Li Z, Jiang B-H, Chen YC. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int J Nanomed. 2012;7:3951. doi: 10.2147/IJN.S33670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee J, Kim JH. Kaempferol inhibits pancreatic cancer cell growth and migration through the blockade of EGFR-related pathway in vitro. PLoS ONE. 2016;11(5):e0155264. doi: 10.1371/journal.pone.0155264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang F, Wang L, Qu C, Chen L, Geng Y, Cheng C, Yu S, Wang D, Yang L, Meng Z. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer. 2021;21(1):1–11. doi: 10.1186/s12885-020-07763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Da J, Xu M, Wang Y, Li W, Lu M, Wang Z. Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer. Anal Cell Pathol. 2019;2019:10. doi: 10.1155/2019/1907698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Halimah E, Diantini A, Destiani DP, Pradipta IS, Sastramihardja HS, Lestari K, Subarnas A, Abdulah R, Koyama H. Induction of caspase cascade pathway by kaempferol-3-O-rhamnoside in LNCaP prostate cancer cell lines. Biomed Rep. 2015;3(1):115–117. doi: 10.3892/br.2014.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cullen JK, Simmons JL, Parsons PG, Boyle GM. Topical treatments for skin cancer. Adv Drug Del Rev. 2020;153:54–64. doi: 10.1016/j.addr.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 116.Kang BY, Kim S, Lee K-H, Lee YS, Hong I, Lee M-O, Min D, Chang I, Hwang JS, Park JS. Transcriptional profiling in human HaCaT keratinocytes in response to kaempferol and identification of potential transcription factors for regulating differential gene expression. Exp Mol Med. 2008;40(2):208–219. doi: 10.3858/emm.2008.40.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim D-W, Jung D-H, Sung J, Min IS, Lee S-J. Tart cherry extract containing chlorogenic acid, quercetin, and kaempferol inhibits the mitochondrial apoptotic cell death elicited by airborne PM10 in human epidermal keratinocytes. Antioxidants. 2021;10(3):443. doi: 10.3390/antiox10030443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee KM, Lee DE, Seo SK, Hwang MK, Heo Y-S, Lee KW, Lee HJ. Phosphatidylinositol 3-kinase, a novel target molecule for the inhibitory effects of kaempferol on neoplastic cell transformation. Carcinogenesis. 2010;31(8):1338–1343. doi: 10.1093/carcin/bgq102. [DOI] [PubMed] [Google Scholar]
- 119.Yao K, Chen H, Liu K, Langfald A, Yang G, Zhang Y, Yu DH, Kim MO, Lee M-H, Li H. Kaempferol targets RSK2 and MSK1 to suppress UV radiation-induced skin cancer. Cancer Prev Res. 2014;7(9):958–967. doi: 10.1158/1940-6207.CAPR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang J, Xiao P, Sun J, Guo L. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J BUON. 2018;23:218–223. [PubMed] [Google Scholar]
- 121.Catalán M, Rodríguez C, Olmedo I, Carrasco-Rojas J, Rojas D, Molina-Berríos A, Díaz-Dosque M, Jara JA. Cell Biology and Translational Medicine. Cham: Springer; 2020. Kaempferol induces cell death and sensitizes human head and neck squamous cell carcinoma cell lines to cisplatin. [DOI] [PubMed] [Google Scholar]
- 122.Yao S, Wang X, Li C, Zhao T, Jin H, Fang W. Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumor Biol. 2016;37(8):10247–10256. doi: 10.1007/s13277-016-4912-6. [DOI] [PubMed] [Google Scholar]
- 123.Song W, Dang Q, Xu D, Chen Y, Zhu G, Wu K, Zeng J, Long Q, Wang X, He D. Kaempferol induces cell cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38 signaling. Oncol Rep. 2014;31(3):1350–1356. doi: 10.3892/or.2014.2965. [DOI] [PubMed] [Google Scholar]
- 124.Choi YJ, Lee YH, Lee S-T. Galangin and kaempferol suppress phorbol-12-myristate-13-acetate-induced matrix metalloproteinase-9 expression in human fibrosarcoma HT-1080 cells. Mol Cells. 2015;38(2):151. doi: 10.14348/molcells.2015.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qin Y, Cui W, Yang X, Tong B. Kaempferol inhibits the growth and metastasis of cholangiocarcinoma in vitro and in vivo. Acta Biochim Biophys Sin. 2016;48(3):238–245. doi: 10.1093/abbs/gmv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qin B, Liu J, Liu S, Li B, Ren J. Kaempferol targets estrogen-related receptor alpha and inhibits cell proliferation and invasion in retinoblastoma via Wnt/beta-catenin signaling pathway. Int J Clin Exp Med. 2016;9(11):21415–21423. [Google Scholar]
- 127.Bhouri W, Bouhlel I, Boubaker J, Kilani S, Ghedira K, Ghedira LC. Induction of apoptosis in human lymphoblastoid cells by kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside from Rhamnus alaternus L. (Rhamnaceae) Cell Prolif. 2011;44(3):283–290. doi: 10.1111/j.1365-2184.2011.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim JH, Kim YS, Choi J-G, Li W, Lee EJ, Park J-W, Song J, Chung H-S. Kaempferol and its glycoside, kaempferol 7-O-rhamnoside, inhibit PD-1/PD-L1 interaction in vitro. Int J Mol Sci. 2020;21(9):3239. doi: 10.3390/ijms21093239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tu LY, Pi J, Jin H, Cai JY, Deng SP. Synthesis, characterization and anticancer activity of kaempferol-zinc(II) complex. Bioorg Med Chem Lett. 2016;26(11):2730–2734. doi: 10.1016/j.bmcl.2016.03.091. [DOI] [PubMed] [Google Scholar]
- 130.Meena D, Vimala K, Kannan S. Combined delivery of DOX and Kaempferol using PEGylated gold nanoparticles to target colon cancer. JCS. 2022;33(1):173–187. [Google Scholar]
- 131.Jaramillo Carmona SM, Lopez S, Abia R, Rodriguez-Arcos R, Jiménez Araujo A, Guillén Bejarano R, Muriana FJ. Combination of quercetin and kaempferol enhances in vitro cytotoxicity on human colon cancer (HCT-116) cells. Rec Nat Prod. 2014;8:262–271. [Google Scholar]
- 132.El-Kott AF, Shati AA, Al-Kahtani MA, Alharbi SA. Kaempferol induces cell death in A2780 ovarian cancer cells and increases their sensitivity to cisplatin by activation of cytotoxic endoplasmic reticulum-mediated autophagy and inhibition of protein kinase B. Folia Biol (Praha) 2020;66(1):36–46. [PubMed] [Google Scholar]
- 133.Zhang Z, Guo Y, Chen M, Chen F, Liu B, Shen C. Kaempferol potentiates the sensitivity of pancreatic cancer cells to erlotinib via inhibition of the PI3K/AKT signaling pathway and epidermal growth factor receptor. Inflammopharmacology. 2021;29(5):1587–1601. doi: 10.1007/s10787-021-00848-1. [DOI] [PubMed] [Google Scholar]
- 134.Aghazadeh T, Bakhtiari N, Rad IA, Ramezani F. Formulation of kaempferol in nanostructured lipid carriers (NLCs): a delivery platform to sensitization of MDA-MB468 breast cancer cells to paclitaxel. Biointerface Res Appl Chem. 2021;11(6):14591–14601. doi: 10.33263/BRIAC116.1459114601. [DOI] [Google Scholar]
- 135.Nandi SK, Roychowdhury T, Chattopadhyay S, Basu S, Chatterjee K, Choudhury P, Banerjee N, Saha P, Mukhopadhyay S, Mukhopadhyay A, et al. Deregulation of the CD44-NANOG-MDR1 associated chemoresistance pathways of breast cancer stem cells potentiates the anti-cancer effect of Kaempferol in synergism with Verapamil. Toxicol Appl Pharmacol. 2022;437:115887. doi: 10.1016/j.taap.2022.115887. [DOI] [PubMed] [Google Scholar]
- 136.Luo H, Daddysman MK, Rankin GO, Jiang BH, Chen YC. Kaempferol enhances cisplatin's effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc. Cancer Cell Int. 2010;10:16. doi: 10.1186/1475-2867-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kalyani C, Narasu M, Devi Y. Synergistic growth inhibitory effect of flavonol-kaempferol and conventional chemotherapeutic drugs on cancer cells. Int J Pharm Pharm Sci. 2017;9:123. doi: 10.22159/ijpps.2017v9i2.16021. [DOI] [Google Scholar]
- 138.Hamzah NHC, Mohammed A, Sirajudeen K, Asari MA, Hamzah Z, Shaik IK. Keladi candik (Alocasia longiloba Miq.) petiole extracts promote wound healing in a full thickness excision wound model in rats. Asian Pac J Trop Biomed. 2019;9(4):140. doi: 10.4103/2221-1691.256727. [DOI] [Google Scholar]
- 139.Lei X, Guo J, Wang Y, Cui J, Feng B, Su Y, Zhao H, Yang W, Hu Y. Inhibition of endometrial carcinoma by Kaempferol is interceded through apoptosis induction, G2/M phase cell cycle arrest, suppression of cell invasion and upregulation of m-TOR/PI3K signalling pathway. J Balkan Union Oncol. 2019;24:1555–1561. [PubMed] [Google Scholar]
- 140.Asai K, Moriwaki S, Maeda-Yamamoto M. Kaempferol, a tea flavonol, effect on interleukin-2 signal transduction of human T cell leukemia. Jpn Agric Res Quart. 2005;39(3):175–179. doi: 10.6090/jarq.39.175. [DOI] [Google Scholar]
- 141.Hassanzadeh A, Naimi A, Hagh MF, Saraei R, Marofi F, Solali S. Kaempferol improves TRAIL-mediated apoptosis in leukemia MOLT-4 Cells by the inhibition of anti-apoptotic proteins and promotion of death receptors expression. Anticancer Agents Med Chem. 2019;19(15):1835–1845. doi: 10.2174/1871520619666190731155859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.