ABSTRACT
Our understanding of the causes and natural course of restless legs syndrome (RLS) is incomplete. The lack of objective diagnostic biomarkers remains a challenge for clinical research and for the development of valid animal models. As a task force of preclinical and clinical scientists, we have previously defined face validity parameters for rodent models of RLS. In this article, we establish new guidelines for the construct validity of RLS rodent models. To do so, we first determined and agreed on the risk, and triggering factors and pathophysiological mechanisms that influence RLS expressivity. We then selected 20 items considered to have sufficient support in the literature, which we grouped by sex and genetic factors, iron-related mechanisms, electrophysiological mechanisms, dopaminergic mechanisms, exposure to medications active in the central nervous system, and others. These factors and biological mechanisms were then translated into rodent bioequivalents deemed to be most appropriate for a rodent model of RLS. We also identified parameters by which to assess and quantify these bioequivalents. Investigating these factors, both individually and in combination, will help to identify their specific roles in the expression of rodent RLS-like phenotypes, which should provide significant translational implications for the diagnosis and treatment of RLS.
KEY WORDS: Restless legs syndrome, Rodent models, Construct validity, Guidelines
Summary: We summarize the consensus guidelines on the construct validity of rodent models of restless legs syndrome, which have been recently established by a task force of the International Restless Legs Syndrome Study Group.
Introduction
Restless legs syndrome (RLS), also known as Willis-Ekbom disease, is a common sensorimotor disorder with a prominent circadian pattern. According to the RLS Epidemiology, Symptoms and Treatment (REST) study, about 5% of US and European adults reported experiencing RLS symptoms at least weekly (Allen et al., 2005). RLS is characterized by a rest-induced, movement responsive, mostly nocturnal, urge to move the legs or akathisia, which in RLS is typically associated with uncomfortable or unpleasant sensations in the legs (Allen et al., 2014). Sleep disruption is the primary factor producing most of the morbidity (Kushida et al., 2004), but RLS patients usually do not report sleepiness during daytime, despite their reduced total sleep time (Allen et al., 2010).
RLS diagnosis, therefore, relies upon subjective clinical features or symptoms (Box 1; Allen et al., 2014). Objective clinical features or signs, such as periodic limb movements (PLM) during sleep (PLMS), are only included as features supportive of diagnosis (Box 1; Allen et al., 2014). The fact that an RLS diagnosis mostly depends on subjective clinical features is a significant challenge for preclinical research. Addressing this issue is particularly challenging when developing animal models of RLS with face validity, that is, animal models that closely reproduce the clinical features of human RLS. The challenge of defining parameters for rodent models of RLS with face validity was recently addressed by a task force convened by the International Restless Legs Syndrome Study Group (IRLSSG) (Salminen et al., 2021).
Box 1. International RLS Study Group (IRLSSG) consensus diagnostic criteria for RLS.
Essential diagnostic criteria:
The urge to move the legs usually but not always accompanied by, or felt to be caused by, uncomfortable and unpleasant sensations in the legs.
The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity, such as lying down or sitting.
The urge to move the legs and any accompanying unpleasant sensations are partially or totally relieved by movement, such as walking or stretching.
The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are worse in the evening or night than during the day.
The occurrence of the above features is not solely accounted for as symptoms primary to another medical or behavioral condition (e.g. myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping).
Clinical features supporting the diagnosis of RLS:
Periodic limb movements (PLM); presence of PLM during sleep or wakefulness (PLMS or PLMW, respectively) at a rate or intensity that is greater than expected for age or medical and medication status.
Dopaminergic treatment response; reduction in symptoms at least initially upon treatment with low doses of dopaminergic agonists.
Family history of RLS among first-degree relatives.
Lack of profound daytime sleepiness.
In addition to face validity, a valid translational rodent model should also have construct validity. Construct validity considers how well the mechanisms used to induce the clinical features of a (neuropsychiatric) disorder in an animal model reflect its currently understood pathophysiology (Salminen et al., 2021). Doing so requires an in-depth understanding of the biological basis of a human disorder. However, an additional challenge for RLS preclinical research is the lack of consensus about its main risk and triggering factors, and pathophysiological mechanisms. These challenges hinder reaching an agreement about the construct validity of RLS animal models.
The IRLSSG task force that established guidelines for rodent models of RLS with face validity reconvened to establish guidelines for construct validity. The starting point was to determine the most accepted risk and triggering factors, and the pathophysiological mechanisms of RLS. Selected factors and biological mechanisms were then evaluated for their possible translation in rodent models of RLS. Recommendations were then presented to assess and quantify these parameters and to identify potential approaches to induce or reproduce them in rodents.
Methods used to determine RLS models with construct validity
Following the compilation of the IRLSSG consensus guidelines on rodent models of RLS (Salminen et al., 2021), the IRLSSG Executive Committee approved a motion in October 2020 that the previously appointed animal models task force continue its work and assess the construct validity of potential RLS models. Twelve of the original 14 members undertook this work. As previously reported, a modified Delphi method (Salminen et al., 2021) was used to reach a consensus and to ensure anonymity. Following an initial telephone conference during which all participants approved the aims and the general methodology of the task force, three phases of guideline development were then conducted through e-mail correspondence, with the assistance of a facilitator.
Phase one
This phase involved the development of a list of potential risk or triggering factors and pathophysiological mechanisms of RLS in humans, based on the existing literature. In the first round, each task force member submitted a list of objective clinical findings, including but not restricted to genetic, analytical, imaging and pathological findings, that they posited to be associated with RLS. To facilitate the identification of missing items, the list was categorized before undergoing a second round of suggestions to give each task force member the opportunity to add any potentially overlooked items. To promote discussion, members were permitted to write counterpoints to any items that they believed should be removed and rebuttals to any of the counterpoints that had been made. Each task force member then voted anonymously for the inclusion or exclusion of each item on the list. Items receiving ≤50% of votes were excluded. The remaining items were categorized into one of three categories: risk factors, trigger factors and pathophysiological mechanisms, and ranked according to the percentage of positive votes received.
Phase two
In the second phase, the leading committee (A.S., S.C., S.F.) translated, where possible, each item into factors relevant to rodent models. These translations were circulated among the task force for discussion.
Phase three
Using the list of translated items, the task force developed actionable guidelines. In the first round of this phase, the leading committee proposed methods to measure each of the previously identified factors in a rodent model, as well as methods that could be used to induce or reproduce each feature in rodents, as specifically as possible. Modifications were made following a review by the task force, and a final version was unanimously accepted. After approval of the written report by all task force members, the recommendations were forwarded to the IRLSSG Executive Committee for review and endorsement.
RLS risk factors, triggering factors, and pathophysiological mechanisms
The task force suggested a total of 51 options for the final list of RLS risk factors, triggering factors and pathophysiological mechanisms; of these, 31 were excluded by consensus, resulting in a final list of 20 items, which the task force considered to have sufficient support in the literature. These items were classified into six groups, which we discuss below.
Genetic and sex-related factors
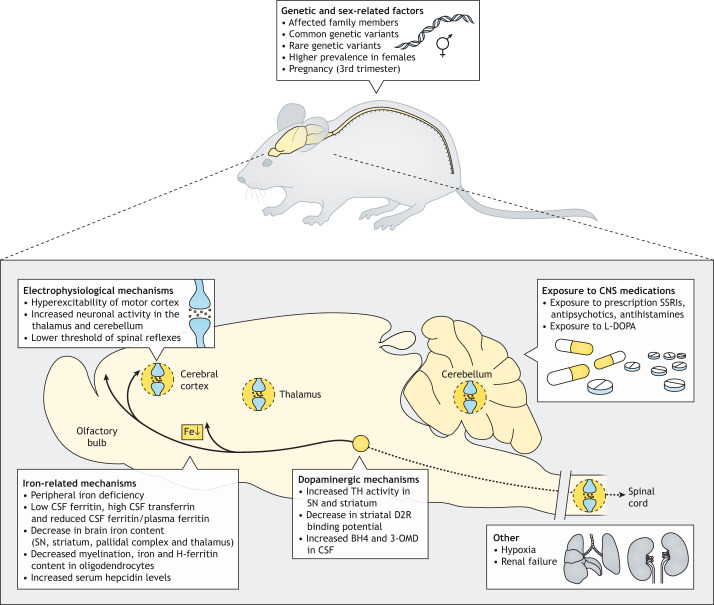
Genetic and sex-related factors include affected family members, common genetic variants, rare genetic variants, higher prevalence of RLS in females, and pregnancy (Fig. 1A).
Fig. 1.
RLS risk and triggering factors and pathophysiological mechanisms. Schematics of selected risk factors, triggering factors and pathophysiological mechanisms in RLS, which should be implemented or reproduced in rodents to provide animal models of RLS with construct validity. The schematic at the bottom depicts a rodent brain, with anterior to the left and dorsal uppermost, and the caudal section of the spinal cord. It shows factors, such as hyperexcitability in the frontal cortex, thalamus, cerebellum and spinal cord, involvement of the ascending dopaminergic system and possible additional involvement of the descending dopaminergic system, hypoxia (lungs) and renal insufficiency (kidneys). Abbreviations: BH4, tetrahydrobiopterin; CNS, central nervous system; CSF, cerebrospinal fluid; D2R, dopamine D2 receptor; Fe, iron; 3-OMD, 3-ortho-methyl-DOPA; SN, substantia nigra; SSRIs, selective serotonin reuptake inhibitors; TH, tyrosine hydroxylase.
RLS is a complex genetic disorder; its heritability is determined by a combination of genetic risk variants and non-genetic environmental factors (Schormair and Winkelmann, 2011). Family and twin studies have estimated that the heritability of RLS is 50–60% (Schormair and Winkelmann, 2011). The genetic risk factors of RLS can be divided into common and rare, according to the frequency of the respective risk allele (Schormair and Winkelmann, 2011). Common genetic variants associated with human disease can be identified in genome-wide association studies (GWAS) and can influence disease progression with low penetrance by affecting the expression patterns of target genes, for example, when localized in regulatory elements. Two recent GWAS meta-analyses confirmed a total of 23 RLS risk variants in 22 genomic risk loci, including the first-reported RLS risk loci near MEIS1, BTBD9 and PTPRD (Schormair et al., 2017; Didriksen et al., 2020). However, caution should be applied when interpreting the effects of common variants, as the affected gene might not be the gene that is nearest to the associated genetic locus (Mumbach et al., 2017). Rare variants, detected by targeted sequencing or by whole-exome or whole-genome sequencing, are often located in the coding regions of the genome (Schormair and Winkelmann, 2011). These variants may have a direct effect on the amino acid composition and function of the encoded protein.
RLS has a higher prevalence among women than men (Manconi et al., 2012). Hormonal differences between men and women are unlikely to account for this difference (Ghorayeb et al., 2008) but might, instead, be partly due to pregnancy (Pantaleo et al., 2010), as a high prevalence of RLS occurs during the third trimester of pregnancy (Darvishi et al., 2020). Furthermore, even though RLS regularly resolves upon delivery, women who develop RLS symptoms during pregnancy are, compared to nulliparous women, up to four times more susceptible to developing RLS later in life (Cesnik et al., 2010; Earley et al., 2014). Therefore, pregnancy is a triggering factor for RLS, whereas previous pregnancies are a risk factor for the increased susceptibility to developing RLS again in the future.
Iron-related mechanisms
Iron-related factors for RLS include: peripheral iron deficiency (PID); low levels of cerebrospinal fluid (CSF) ferritin, high levels of CSF transferrin and a reduced CSF ferritin-to-plasma ferritin ratio; decrease in brain iron content, particularly in the substantia nigra (SN), striatum, pallidal complex and thalamus; decrease in iron content and decrease in H-ferritin, predominantly in oligodendrocytes; decreased myelination; and increased levels of serum hepcidin (Fig. 1B).
Iron-related findings for RLS can be grouped into CSF/plasma, post-mortem and imaging findings, which provide significant evidence that brain iron deficiency (BID) is often involved in the pathophysiology of RLS (Earley et al., 2014; Ferré et al., 2019). PID, in contrast, constitutes a risk or triggering factor, enabling the development of BID in a vulnerable individual. The prevalence of RLS in patients with anemia secondary to iron deficiency is as high as 30% (Allen et al., 2013a), i.e. six times higher than the prevalence of RLS in the general population (Allen et al., 2005).
Nevertheless, many RLS patients do not show systemic iron deficiency but rather appear to have a specific iron deficiency in the brain, substantiated by relatively lower CSF ferritin, higher CSF transferrin and reduced CSF ferritin/plasma ferritin levels compared to those of controls (Earley et al., 2000; Mizuno et al., 2005), as well as decreased iron content of the SN, striatum, pallidal complex and thalamus (Allen et al., 2001; Godau et al., 2007, 2008; Rizzo et al., 2013; Li et al., 2016). Although less frequently, negative findings have also been reported (Knake et al., 2009; Margariti et al., 2012).
In post-mortem tissue, this specific iron deficiency in the brain appears as decreased levels of iron and H-ferritin, predominantly in oligodendrocytes (Connor et al., 2003, 2011a), the brain cells with the highest iron and ferritin content, and which synthesize myelin (Todorich et al., 2009). Decreased myelination in RLS might, thus, arise from an iron-dependent impairment of oligodendrocyte function (Connor et al., 2011b). Hepcidin is a central regulatory molecule of systemic iron homeostasis, which is secreted by the liver and decreases systemic iron levels (Hentze et al., 2010). Increased serum hepcidin levels without a concomitant decrease in plasma iron concentrations have been reported in RLS patients (Dauvilliers et al., 2018; Chenini et al., 2020) and imply the existence of a not-yet-understood dysregulation of systemic iron metabolism in RLS. However, one recent report does not differentiate between plasma hepcidin levels in RLS patients and in controls from a large population of blood donors (Dowsett et al., 2021).
Electrophysiological mechanisms
Electrophysiological RLS-related factors include: hyperexcitability of the motor cortex, increased neuronal activity in the thalamus and cerebellum, and lower threshold of spinal reflexes (Fig. 1C).
The most-important evidence for hyperexcitability of the motor cortex in RLS patients stems from transcranial magnetic stimulation (TMS) studies, which indicate increased excitability in the motor cortex with reduced intracortical inhibition (Tergau et al., 1999; Quatrale et al., 2003; Scalise et al., 2004; Lanza et al., 2015; Magalhães et al., 2019). Most of these studies conclude that the pyramidal tract is intact in RLS patients, and that altered cortical excitability depends on cortical and subcortical mechanisms. Importantly, a TMS study reported a predominant increase in excitability within the part of the primary motor cortex representing the leg (Salas et al., 2018).
Functional magnetic resonance imaging (fMRI) has repeatedly shown significantly increased activation of the thalamus and cerebellum in RLS patients (Bucher et al., 1997; Margariti et al., 2012; Zhuo et al., 2017). Neuronal overactivity in the thalamus of RLS patients is also supported by results obtained by using proton magnetic resonance spectroscopy (MRS), which showed an increased Glx signal, i.e. the combined signal of glutamine and glutamate, in RLS patients compared to that in controls (Allen et al., 2013b). The increased excitability of the motor cortex, and increased neuronal activity of the thalamus and cerebellum observed in studies using TMS, fMRI and MRS were independent of the presence of RLS symptoms. Decreased concentrations of N-acetylaspartate without neuronal loss, indicative of neuronal dysfunction, have been specifically detected in the medial thalamus of RLS patients (Rizzo et al., 2012). This thalamic area includes the midline–intralaminar nuclei, which project to the cortex and striatum (Groenewegen and Berendse, 1994), and which are directly connected to the dentate nucleus of the cerebellum (Bostan and Strick, 2018).
RLS is nearly always associated with PLM, which, together with spinal reflexes, are in large part mediated by neural circuits in the spinal cord (Telles et al., 2011). A functional role for the spinal circuits in the emergence of RLS symptoms is supported by data that have identified altered spinal cord flexor withdrawal reflexes in RLS, where spinal reflex excitability is often increased in the evening during both wakefulness and sleep (Dafkin et al., 2017, 2018; Bara-Jimenez et al., 2000; Aksu and Bara-Jimenez, 2002; Abdulhadi et al., 2021). Additional evidence comes from findings that PLM can occur independently of descending control after spinal cord injury (Telles et al., 2011) and can improve after treatment with dopaminergic agonists (Salminen et al., 2013).
Dopaminergic mechanisms
Dopaminergic RLS-related factors include: increased levels of tetrahydrobiopterin (BH4) and 3-ortho-methyl-DOPA (3-OMD) in the CSF, increased activity of tyrosine hydroxylase (TH) in the SN and striatum, and decreased binding potential of striatal dopamine D2 receptor (D2R) (Fig. 1D).
CSF, post-mortem, and imaging findings indicate that increased dopamine synthesis and release occurs in the brains of RLS patients. Increased BH4 and 3-OMD levels in the CSF of RLS patients has been reported in several studies (Earley et al., 2001, 2006; Allen et al., 2009). Increased expression of active (phosphorylated) TH in the SN (pars compacta) and striata of RLS patients has been reported in a study where the same results were demonstrated in rats with diet-induced BID (Connor et al., 2009), strongly suggesting a connection between BID and the increase in dopamine synthesis and release. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) studies have assessed the status of D2R in the striata of patients with RLS, with most but not all studies showing a decrease in D2R ligand binding (reviewed in Earley et al., 2014). A more-recent combined PET/SPECT study showed evidence for a decrease in the D2R binding potential being mostly dependent on increased synaptic dopamine (Earley et al., 2013), which is compatible with increased dopamine synthesis and release. This apparent ‘cerebral hyperdopaminergic state’ associated with RLS seems counterintuitive, given the effective therapeutic response to low doses of dopaminergic agonists.
However, this apparent ‘dopaminergic paradox’ can be explained by their effect on presynaptic D2-like receptors (D2R, D3R or D4R subtypes) (Ferré et al., 2021). D2-like receptors that localize to dopaminergic cells (autoreceptors) exert a strong inhibitory control on their neuronal activity and mediate the decreased locomotor activity in rodents that is induced by low doses of non-selective D2-like receptors (Maj et al., 1997; Chernoloz et al., 2009; Li et al., 2010). Results obtained in rats with diet-induced BID also indicate a significant role for presynaptic D2-like receptors in RLS that localize to cortico-striatal glutamatergic terminals (Yepes et al., 2017). Finally, the spinal cord, which receives descending dopaminergic innervation, represents another plausible localization of D2-like receptors involved in the therapeutic effects of dopaminergic agonists for RLS (Thorpe et al., 2011).
Exposure to CNS-activating medications
Exposure to prescribed selective serotonin reuptake inhibitors (SSRIs), antipsychotics or antihistamines as well as exposure to L-DOPA is linked to RLS (Fig. 1E).
RLS can develop owing to exposure to several CNS-active medications, such as SSRIs (Yang et al., 2005; Rottach et al., 2008), that increase the extracellular levels of serotonin, and can secondarily exert excitatory effects on the dopaminergic system (Howell and Cunningham, 2015). Antipsychotic treatment has also been associated with RLS (Patatanian and Claborn, 2018) but is more commonly found together with akathisia, i.e. the inability to remain still, but without the unpleasant sensations in the legs and the circadian component of RLS. This has been suggested to depend on a blockade of presynaptic dopamine receptors in the ventral striatum (Ferré et al., 2021). Treatment with antihistamines, particularly those that cross the blood-brain-barrier and bind to the H1-receptor subtype, can markedly worsen RLS symptoms (Mackie and Winkelman, 2015). This outcome may also be related to the ability of antihistamines to interact with the dopaminergic system (Oleson et al., 2012; Flik et al., 2015).
Levodopa (L-DOPA) and several D2-like receptor agonists are, initially, very effective therapeutic agents for RLS. They do not trigger RLS but lead to a worsening of symptoms over time, a phenomenon known as ‘augmentation’ (Allen and Earley, 1996; Allen et al., 2011).
Other factors
Other factors linked to RLS include hypoxia and renal failure (Fig. 1F). Peripheral hypoxia, as measured in the legs during the symptomatic period, is more frequent in patients with RLS and correlates with symptom severity (Salminen et al., 2014). Markers of hypoxia are also overexpressed in the skeletal muscles of RLS patients (Wåhlin-Larsson et al., 2009). Association of RLS with systemic hypoxia is supported by its association with prolonged exposure to high altitudes (Gupta et al., 2017) and with its recently described moderate, but significant, increased association with chronic obstructive pulmonary disease (Thi Truong et al., 2021). In addition, the activation of hypoxic pathways has been demonstrated in the SN and brain microvasculature of RLS patients (Patton et al., 2011).
The prevalence of RLS among patients suffering from renal insufficiency may be ≤30% (Lin et al., 2019), significantly higher than in the general population. In some cases, RLS resolves after a successful kidney transplant (Capelli et al., 2019), supporting the causal relationship between the two conditions. Therefore, renal failure may be classified as a triggering factor for RLS.
Translation of risk factors, triggering factors and pathophysiological mechanisms into rodent models
Next, the task force attempted to translate the selected items into rodent models and proposed guidelines on how to both measure and induce or reproduce these factors in rodent models.
Genetics and sex-related factors
Affected family members
As noted in our previous guidelines (Salminen et al., 2021), rodents of an inbred colony are genetically identical to each other and share an identical environment. Therefore, observation and comparison of rodent families would not be as applicable as they are when using clinical cohorts. Instead, the focus of rodent studies should be on the specific genetic and environmental causes that influence the heritability of RLS.
Common genetic variants
Common genetic variants can often not be translated directly to rodents owing to the incomplete conservation of non-coding regions of the genome between species, but humans may share more genomic commonalities with other species than previously thought (Leypold and Speicher, 2021). RLS research should focus on the effect that a specific variant has on gene expression. When the expression of a target gene is downregulated by the risk allele of the variant, heterozygous or homozygous knockout (KO) models of the target gene may be considered. This approach has been used extensively in RLS animal models (DeAndrade et al., 2012; Drgonova et al., 2015; Salminen et al., 2017). Gene silencing methods, such as RNA interference, may also be considered in both in vivo and in vitro models. When an RLS risk variant is associated with the upregulation of a target gene, overexpression approaches should be used. If tissue-specific effects on gene expression are known to be associated with the variant, conditional KO systems specific to the organs or cell types of interest should be considered. The most common tool used to generate tissue-specific genetic modifications in rodents is the Cre-LoxP system (Tsien, 2016). This approach may also be used when a hypothesis concerning a disease-relevant tissue has been established. Conditional genetic modifications using the Cre-LoxP system have already been used in RLS animal models (Lyu et al., 2019a, 2020a). If the non-coding variant is located within a highly conserved regulatory region that is also identified in the rodent genome, the genetic manipulation of those regulatory elements may be considered.
Rare genetic variants
Rare genetic variants can, in some cases, be directly translated to rodent models. When sufficient inter-species conservation of the target region is present, an individual variant may be introduced into the rodent genome by using gene-editing technology, such as CRISPR-Cas9. When an exaggerated effect of the variant is warranted, a larger part of the genetic sequence or an entire domain of the affected protein could be altered. However, such approaches may introduce unwanted effects on protein folding and would need to be well validated. If modification of gene expression is preferred, similar approaches to those outlined above for common genetic variants can be used.
Higher prevalence in females
As recommended in our previous guidelines (Salminen et al., 2021), both sexes should be used in RLS animal experiments because of the many sex-related biological differences. That sex-related differences may be driven by previous pregnancies, as in humans, should also be considered.
Pregnancy (third trimester)
The biological differences between human and rodent pregnancies are significant, ranging from the typical number of offspring per pregnancy to the timing of delivery relative to the development of the offspring. Although technically possible, monitoring rodents during pregnancy can be challenging for practical and ethical reasons. The time of pregnancy onset should be determined by using a vaginal plug. If possible, the litter size should be recorded and controlled for by data analysis. Pregnant rodents can be observed during the second half of their pregnancy. However, this may not correspond biologically to the third trimester of human pregnancy and, therefore, the interpretation of such results is challenging. One study has investigated the motor behavior of pregnant rats as a potential model for RLS (Mariano et al., 2014).
Iron-related mechanisms
Peripheral iron deficiency
PID may be indirectly assessed by measuring hemoglobin levels. However, the analysis of serum ferritin more accurately evaluates non-anemic PID (Quiroz et al., 2016; Lo Martire et al., 2018; Zhu et al., 2020). An iron-deficient diet can induce PID without concomitant BID in the adult rodent, which is – unlike weaning animals − very resistant to BID (see section below).
Low CSF ferritin, high CSF transferrin and reduced CSF ferritin-to-plasma ferritin levels
In human RLS patients, these measures are used as indirect markers of general BID and may be analyzed in rodents. However, rodent models allow for more-direct measurements of BID (see below). Currently, in the most-used animal model of BID, i.e. the diet-induced BID model, rodents are exposed to a strict iron-deficient diet during the post-weaning period, a time when they are specifically sensitive to developing BID (Earley et al., 2014). However, this approach is associated with PID, which could determine or modify the experimental results. Inbred strain recombination has provided mouse strains with a predominant BID versus PID upon an iron-deficient diet during the post-weaning period, such as females of the BXD S40 mouse strain (Jellen et al., 2009, 2012; Allen et al., 2020). The BXD S40 female mouse might, therefore, represent a better model for RLS with regards to BID, since it could provide clues about the mechanisms involved in the specific dysregulation of iron levels within the brain during RLS, as well as identify the loci that cause the BXD 40 strain to exhibit such dysregulation, thereby helping to identify orthologous loci influencing RLS in human patients. Mice with non-anemic BID and PID also have been obtained by using a less-severe but longer-lasting iron-deficient diet during the post-weaning period (Quiroz et al., 2016).
Decrease in brain iron content
Decreased iron levels, particularly in the SN, striatum, pallidal complex and thalamus, can be directly determined by measuring the iron content, or the density or expression of the transferrin receptor, which is specifically upregulated upon chronic cellular iron deficiency (Lok and Loh, 1998; Han et al., 2003; Gulyani et al., 2009). A strong inverse correlation between iron content and density or mRNA expression of the transferrin receptor protein has been demonstrated in the rat brain, indicating that these factors can be used as indirect measures of BID in rodents (Han et al., 2003). Iron content can be measured histochemically by Perls Prussian Blue staining or by mass spectrometry, and transferrin receptor density or expression can be measured by immunohistochemistry, western blotting, quantitative PCR (qPCR) or RNA in situ hybridization, e.g. RNAscope (Connor et al., 2011a; Quiroz et al., 2016; Han et al., 2003; Gulyani et al., 2009). The SN (pars reticulata), striatum, pallidal complex and thalamus are brain areas with the highest iron content, and appear to experience a more-profound decrease in iron content than other brain areas upon BID in RLS (Allen et al., 2001; Godau et al., 2007, 2008; Rizzo et al., 2013; Li et al., 2016). Therefore, a more-profound decrease in brain iron content in these areas should also be expected in the BID rodent. The specific role of iron deficiency within each distinct brain area in the pathophysiology of RLS symptomatology could be evaluated by local infusion of iron chelators, such as deferiprone. This approach has been recently used to study axonal iron transport between different areas of the mouse brain (Wang et al., 2019). Genome editing tools could also be used to induce a decrease of intracellular iron content in specific cells, such as neurons or glia, and in brain areas, by using Cre-LoxP-mediated recombination techniques, e.g. by conditionally knocking down the expression of transferrin receptors.
Decreased myelination, and reduced iron and H-ferritin content in oligodendrocytes
Iron content can be measured histochemically by Perls Prussian Blue staining, and markers of H-ferritin and oligodendrocytes by immunohistochemical techniques (Connor et al., 2011a; Wan et al., 2020). H-ferritin from isolated crude myelin fractions extracted from brain tissue can also be analyzed in western blotting (Connor et al., 2011b). Oligodendrocytes are the producers of myelin in the CNS and are the cells with the highest iron content in the CNS (Todorich et al., 2009). They also seem to experience a more-profound decrease in iron content than other cell types upon BID in RLS (see above). Their specific role in the pathophysiology of RLS symptomatology could be evaluated in transgenic rodents by using selective and conditional KO of H-ferritin in oligodendrocytes. Such a conditional KO has recently been published and shows severe oligodendrocyte dysfunction and hypomyelination, particularly when the selective genetic blockade of H-ferritin expression occurs during the post-weaning period (Wan et al., 2020).
Increased serum hepcidin levels
Serum hepcidin levels can be directly measured in rodents. Transgenic mice that overexpress hepcidin in their hepatocytes have been recently generated (Zhang et al., 2018). These animals show a significant increase in serum hepcidin levels and could be used to study a possible pathogenetic mechanism of increased hepcidin.
Electrophysiological mechanisms
Hyperexcitability of the motor cortex
In vivo techniques that track neuronal activation in rodents include manganese-enhanced MRI, an approach that has already been used to study cortical hyperexcitability in a genetic rodent model of RLS (Lyu et al., 2020a); [18F]-fluorine-labeled 2-fluoro-2-deoxy-D-glucose PET imaging of metabolic activity (Cai et al., 2019); and the use of miniaturized fluorescence microscopes (miniscopes) or fiber photometry, both of which measure fluctuations of calcium ions (Ca2+) (Girven and Sparta, 2017; Shiromani et al., 2021). Optogenetic stimulation combined with in vivo microdialysis/voltammetry/fiber photometry can be used to determine an increased sensitivity of motor cortical pyramidal cells to the release of glutamate by their nerve terminals in the striatum or spinal cord (Yepes et al., 2017). In vitro techniques could be used to measure the neuronal properties at both the somatodendritic and nerve-terminal level. These techniques include electrophysiological analysis of ex vivo cortico-striatal slices, and measurement of intrinsic excitability and spontaneous firing activity, as well as electrically or optogenetically induced pre- and postsynaptic events (i.e. the probability of neurotransmitter release and excitatory postsynaptic currents) (Lyu et al., 2020a). The hyperexcitability of the motor cortex could be induced by using Cre-LoxP recombination techniques to promote the selectively targeted expression of ion channel subunits within the motor cortex that can alter neuronal excitability. This approach could generate, for example, a gain-of-function sodium channel in glutamatergic neurons or a loss-of-function potassium channel in GABAergic neurons. This strategy has been used in global knock-in mice – without a targeted expression to specific neuronal populations – to obtain genetic rodent models of epilepsy (Oyrer et al., 2018). As a more-translational method, experimental repetitive TMS (rTMS) was recently introduced in awake rats to promote the focal stimulation of the motor cortex (Cermak et al., 2020), and has been used clinically to modify cortical excitability (Lanza et al., 2018). Optogenetics and ultrasound techniques are alternative methods that could also be used in vivo to increase neuronal excitability of the motor cortex through repetitive stimulation or other stimulation techniques (Magno et al., 2019; Qiu et al., 2021).
Increased neuronal activity in the thalamus and cerebellum
The imaging and miniscope approaches discussed above are used to measure neuronal activation in the motor cortex but could also be used to measure neuronal activation in thalamic and cerebellar areas. The same in vivo techniques, such as PET imaging, miniscope, fiber photometry, optogenetics, in vivo microdialysis, as well as in vitro techniques, such as brain slice electrophysiology, could be applied to the midline and intralaminar thalamic nuclei and their glutamatergic outputs to the striatum and cortex (Groenewegen and Berendse, 1994), and their glutamatergic input from the cerebellar dentate nucleus (Bostan and Strick, 2018). Similarly, the same approaches suggested to induce motor cortex hyperexcitability – excluding rTMS, which cannot target deep and discrete brain areas – could also be used to induce increased neuronal activity in the thalamic and cerebellar nuclei.
Lower threshold of spinal reflexes
Thresholds of spinal cord reflexes can be readily assessed in rodents both in vivo and in vitro. In vivo, this can involve the assessment of thermal withdrawal latencies in awake animals by using a Hargreaves or a tail-flick assay (Brewer et al., 2014; Rodgers et al., 2019). Sensory stimulation can also be performed in anesthetized animals to identify the responses of specific neuronal populations (Douglass and Carstens, 1997). In addition to thermal withdrawal reflexes, behavioral assessments can test for changes in mechanoreceptor sensitivity using von Frey filaments (Christensen et al., 1996). Another approach would be to implant intrathecal minipumps to deliver a drug with a high spatial resolution to specific parts of the spinal cord (Dableh et al., 2009). Surgical interventions, such as spinal transection, can be performed to evaluate the role of the sensorimotor circuitry in vivo without the influence of a descending modulatory control (Kisucká et al., 2015). In addition, interventional approaches enable the study of the correlation of spinal with supraspinal mechanisms (Watanabe et al., 2015). Spinal cords can also be readily isolated in vitro and placed in a dish with artificial cerebrospinal fluid, where sensory input can be electrically stimulated and the elicited motor outputs recorded (Brewer et al., 2014; Machacek et al., 2001; Clemens and Hochman, 2004; Keeler et al., 2012; Johnson and Clemens, 2021).
Dopaminergic mechanisms
Increased TH activity in the SN and striatum
Changes in TH activity are usually measured by immunohistochemistry or western blotting to determine the phosphorylated fraction of total TH in the corresponding brain areas (Connor et al., 2009). A transgenic mouse with tissue-specific overexpression of TH in catecholaminergic neurons has already been described and studied extensively (Kaneda et al., 1991; Kiuchi et al., 1993; Nakahara et al., 1993). However, unexpectedly, the significantly higher expression and activity of TH in this animal model were not accompanied by a significant increase in the basal striatal extracellular concentration of dopamine. This finding was attributed to compensatory adaptations. Therefore, to study the possible contribution of increased TH activity to the development of an RLS-like phenotype, it would be desirable to develop a conditional transgenic rodent that overexpresses TH during the postnatal or adult period in order to decrease the likelihood of compensatory developmental adaptations.
Decrease in striatal dopamine D2R binding potential
The density or expression of striatal D2R in rodents can be measured via several commonly used techniques, such as western blotting, immunohistochemistry, qPCR or in situ hybridization. The density and affinity of striatal D2R can be measured in radioligand-binding experiments. In vivo methods can also be used to measure striatal extracellular dopamine concentration, including microdialysis, cyclic voltammetry and fiber photometry. Although there are several pharmacological ways to increase striatal dopamine, one translationally sound mechanism would be to secondarily induce this by increasing dopamine synthesis (see above).
Increased BH4 and 3-OMD in the CSF
BH4 and 3-OMD are markers of increased L-DOPA synthesis in the brain, and can directly be analyzed in rodents. An increase in these markers might be secondary to an increase in TH activity, since 3-OMD is a byproduct of L-DOPA and BH4 is a TH co-factor. However, whether an increase in 3-OMD or BH4 in the brain is directly involved in RLS symptomatology has yet to be studied. An increase in brain BH4 can be produced by administering its natural precursor sepiapterin, as recently reported in humans (Smith et al., 2019).
Exposure to CNS-active medications
Exposure to prescription SSRIs, antipsychotics or antihistamines
As already discussed in our previous guidelines (Salminen et al., 2021), SSRIs or other drugs can be applied by oral, systemic, intracranial or intrathecal administration, either acutely – to probe for possible fast effects – or continuously – by using slow-release miniature osmotic pumps. Intrathecal administration would distinguish between spinal and supra-spinal mechanisms.
Exposure to L-DOPA
To reproduce augmentation in a rodent model of RLS, L-DOPA could be administered repeatedly or continuously via mini pumps, systemically or centrally, for prolonged periods that have yet to be established.
Hypoxia and renal failure
Hypoxia
Hypoxia in rodents can be achieved by using different gas mixtures in individually ventilated cages. This can be done in the animal's home cage or in a separate chamber by ventilating the cage with a hypoxic gas mixture of ≤15% oxygen (O2) partial pressure. Mild hypoxia has previously been tested in a putative RLS animal model and did not elicit RLS-like signs in mice, either alone or in combination with non-anemic PID (Lo Martire et al., 2018).
Renal failure
Renal failure can be modeled in rodents by using three different strategies, the first of which is to a use a pre-existing genetic model of renal failure. Some of these models were developed to study primary podocyte-specific genetic focal segmental glomerulosclerosis (Matsusaka et al., 2005), HIV-associated nephropathy (Dickie et al., 1991) and Alport syndrome (Hanifa et al., 2019). The second strategy involves surgically induced renal failure by using sub-total nephrectomy (Hanifa et al., 2019), radiation necropathy (Robbins et al., 1995) or unilateral ureteral obstruction (Chevalier et al., 2009). The third strategy is to use spontaneous models of renal failure, which include the Buffalo/Mna rat (Nakamura et al., 1986), the Munich Wistar Frömter (MWF/Fub) rat (Fassi et al., 1998) and spontaneously hypertensive rat strains, such as the Wistar-derived one (Zhou and Frohlich, 2007).
Other existing RLS rodent models
In addition to the constructs discussed above, several other approaches have been used to model RLS in rodents. We briefly mention these below and highlight why they have not been selected as having sufficient construct validity by this task force.
The A11 dopaminergic cell cluster lesion and other lesion models
The main mesencephalic ascending dopaminergic cell systems originate in the SN (pars compacta), the ventral tegmental area and the retrorubral field, which are located dorsally and caudally to the SN, and correspond to the originally described A9, A10 and A8 dopaminergic cell clusters. Additionally, there are other diencephalic dopaminergic cell clusters. In particular, the A11 hypothalamic cluster is the origin of descending spinal dopaminergic innervation (Clemens et al., 2006; Qu et al., 2006). The A11 model of RLS is based on the hypothesis that decreased dopamine function in the spinal cord mimics RLS-like phenotypes (Ondo et al., 2000; Clemens et al., 2006; Zhao et al., 2007; Lopes et al., 2012). However, an autopsy study of the brains of RLS patients found no evidence of degeneration of or biochemical changes, such as TH levels, to the A11 area of their brains (Earley et al., 2009). Nevertheless, a reduction in spinal monoaminergic innervation with age has been reported in rats (Ranson et al., 2003) and might contribute to the age-related increased prevalence of PLM. Furthermore, a significant role for spinal cord dopamine in RLS is supported by clinical evidence of therapeutic improvement in PLM, in patients with spinal cord injury treated with a dopamine agonist (Earley et al., 2001; Kumru et al., 2015). In addition to the A11 hypothalamic cluster, targeted lesions of the corticospinal and rubrospinal tracts and their afferent sources have also been reported to induce RLS-like movements (Guo et al., 2017).
Opioid receptor knockout models
Genetic inactivation of the µ-opioid receptors in mice has recently been shown to reproduce some RLS-like behavioral phenotypes (Lyu et al., 2019b). However, despite the therapeutic effects of opioids, only one pilot study has shown some alterations in the levels of enkephalins and dynorphins in the thalamus and SN regions of RLS patient brains (Walters et al., 2009). Furthermore, opioid receptors are as yet not genetically associated with RLS. Therefore, the task force concluded that the validity of opioid system modifications as a construct for RLS was not sufficiently supported by the currently available evidence.
Conclusions
These guidelines for the construct validity of RLS animal models can be used to evaluate previously published research and to generate novel RLS rodent models. The translation of the discussed RLS risk and triggering factors and pathophysiological mechanisms to rodent models requires researchers to identify the most appropriate methodologies to induce or replicate these factors in animals. Genetically modified rodents have already been studied individually for their ability to recapitulate several RLS pathogenetic mechanisms and RLS-like behavioral phenotypes (DeAndrade et al., 2012; Drgonova et al., 2015; Salminen et al., 2017, 2019; Lyu et al., 2019a, 2020a). In addition, PID during the post-weaning period – either alone or in combination with hypoxia or pregnancy – has been used to reproduce the RLS-like behavioral phenotype in rodents (Mariano et al., 2014; Lo Martire et al., 2018; Lai et al., 2017). Other potential methods, such as renal failure and CNS-active medications, remain to be studied in the context of RLS. In the future, these factors should be studied in compound study designs, where, for example, a genetic model is exposed to PID or renal failure. Based on the cumulative effects of different risk factors in humans (Trenkwalder et al., 2016), this would be expected to strengthen the construct validity of the rodent model of RLS and may ultimately lead to a better interpretation and a more widely accepted recognition of the mechanisms underlying RLS.
The analysis and study of the different putative pathophysiological mechanisms in isolation are also encouraged. This approach could causally link these mechanisms to a specific RLS-like behavioral phenotype and investigate their impact and role in the chain of pathophysiological events. The appearance of a specific pathophysiological mechanism upon the manipulation or induction of an RLS risk or triggering factor or pathophysiological mechanism would strengthen our understanding of the disease's progression. Successful examples of this approach include the use of dietary-induced BID in rodents, which recapitulates several dopaminergic and electrophysiological RLS features (Earley et al., 2014; Connor et al., 2009; Ferré et al., 2021) and the global or conditional cortical KO of the Btbd9 gene, whose protein product enhances motor cortico-striatal transmission in mice (Lyu et al., 2020a).
Rodent models established by using the constructs discussed here should also be investigated for their face validity following our guidelines for RLS-like behavior. Investigating RLS-linked factors separately and in different combinations will help to elucidate which specific risks, triggering factors and pathophysiological mechanisms lead to which RLS-like behavioral phenotypes. These guidelines do not promote the exclusive use of rodents, since other animal models, such as invertebrates (Box 2, Invertebrate animal models of RLS) and zebrafish (Spieler et al., 2014), and in vitro models (Gulyani et al., 2009) are also contributing to our understanding of RLS; these are, however, beyond the purview of the task force. Finally, these guidelines are based on the present understanding of RLS pathophysiology and should be revised in the future, as and when new data and approaches become available.
Box 2. Invertebrate animal models of RLS.
Several single-nucleotide polymorphisms (SNPs) associated with restless leg syndrome (RLS) have been investigated in Caenorhabditis elegans and Drosophila melanogaster. While these organisms cannot recapitulate the complexity of human RLS, they might provide important clues about the role of genetic risk factors in the clinical phenotype of RLS. C. elegans has several orthologs of known RLS genetic risk factors, including MEIS1, BTBD9 and PTPRD (unc-62, hpo-9 and ptp-3, respectively) (Chen et al., 2019). It has eight identifiable dopaminergic neurons as well as orthologs of all the genes involved in dopamine synthesis, packaging, reuptake and metabolization (Jayanthi et al., 1998; Hobert, 2013). It also expresses several behavioral paradigms to evaluate dopaminergic function (Chen et al., 2019). The dop-1 and dop-3 genes encode for C. elegans dopamine receptors. HPO-9 and DOP-1 proteins function similarly in egg-laying and locomotor behaviors, and HPO-9 deficiency leads to increased transcription of dop-3 (Lyu et al., 2020a). Both unc-62 and hpo-9 (Chen et al., 2022) are expressed in dopaminergic neurons, and suppression of unc-62 impairs dopaminergic neuron terminal differentiation and dopamine pathway gene expression (Lyu et al., 2020b; Jimeno-Martín et al., 2022). Furthermore, interference with unc-62 expression increases expression of ferritin, indicating a possible connection between the MEIS1 gene and iron metabolism in humans (Catoire et al., 2011).
D. melanogaster has a highly regulated rest/activity cycle of ∼24 h and has been extensively used to investigate sleep disorders (Donelson and Sanyal, 2015). It has a single BTBD9 ortholog (locus tag CG1826), the genetic deletion of which mildly shortens lifespan and produces RLS-like phenotypes, including increased locomotor activity, significant sleep fragmentation and responsiveness to dopamine agonists, such as pramipexole (Freeman et al., 2012).
Acknowledgements
We acknowledge Anne-Marie Williams for her editorial assistance, Allan O'Bryan (IRLSSG) for logistic support, and the IRLSSG Executive Committee for their input and continued support. Richard Allen, at the center of many advances in RLS research over the last 25 years, contributed greatly to this publication but sadly passed away on 12/09/2020. All the authors are honored to have been able to work alongside him.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The IRLSSG funded the research reported in this manuscript. The corresponding author is an NIH Federal employee, funded by the intramural funds of the National Institute on Drug Abuse.
References
- Abdulhadi, I. G., Al-Mahdawi, A. M. and Hamdan, F. B. (2021). Electrophysiological findings in patients with restless legs syndrome. Sleep Med. 87, 151-157. 10.1016/j.sleep.2021.09.012 [DOI] [PubMed] [Google Scholar]
- Aksu, M. and Bara-Jimenez, W. (2002). State dependent excitability changes of spinal flexor reflex in patients with restless legs syndrome secondary to chronic renal failure. Sleep Med. 3, 427-430. 10.1016/S1389-9457(02)00073-4 [DOI] [PubMed] [Google Scholar]
- Allen, R. P. and Earley, C. J. (1996). Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep 19, 205-213. 10.1093/sleep/19.3.205 [DOI] [PubMed] [Google Scholar]
- Allen, R. P., Barker, P. B., Wehrl, F. W., Song, H. K. and Earley, C. J. (2001). MRI measurement of brain iron in patients with restless legs syndrome. Neurology 56, 263-265. 10.1212/WNL.56.2.263 [DOI] [PubMed] [Google Scholar]
- Allen, R. P., Walters, A. S., Montplaisir, J., Hening, W., Myers, A., Bell, T. J. and Ferini-Strambi, L. (2005). Restless legs syndrome prevalence and impact: REST general population study. Arch. Int. Med. 165, 1286-1292. 10.1001/archinte.165.11.1286 [DOI] [PubMed] [Google Scholar]
- Allen, R. P., Connor, J. R., Hyland, K. and Earley, C. J. (2009). Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 10, 123-128. 10.1016/j.sleep.2007.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, R. P., Stillman, P. and Myers, A. J. (2010). Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med. 11, 31-37. 10.1016/j.sleep.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Allen, R. P., Ondo, W. G., Ball, E., Calloway, M. O., Manjunath, R., Higbie, R. L., Lee, M. R. and Nisbet, P. A. (2011). Restless legs syndrome (RLS) augmentation associated with dopamine agonist and levodopa usage in a community sample. Sleep Med. 12, 431-439. 10.1016/j.sleep.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Allen, R. P., Auerbach, S., Bahrain, H., Auerbach, M. and Earley, C. J. (2013a). The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am. J. Hematol. 88, 261-264. 10.1002/ajh.23397 [DOI] [PubMed] [Google Scholar]
- Allen, R. P., Barker, P. B., Horská, A. and Earley, C. J. (2013b). Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology 80, 2028-2034. 10.1212/WNL.0b013e318294b3f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, R. P., Picchietti, D. L., Garcia-Borreguero, D., Ondo, W. G., Walters, A. S., Winkelman, J. W., Zucconi, M., Ferri, R., Trenkwalder, C., Lee, H. B.et al. (2014). Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 30, 860-873. 10.1016/j.sleep.2014.03.025 [DOI] [PubMed] [Google Scholar]
- Allen, R. P., Earley, C. J., Jones, B. C. and Unger, E. L. (2020). Iron-deficiency and dopaminergic treatment effects on RLS-Like behaviors of an animal model with the brain iron deficiency pattern of the restless legs syndrome. Sleep Med. 71, 141-148. 10.1016/j.sleep.2020.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara-Jimenez, W., Aksu, M., Graham, B., Sato, S. and Hallett, M. (2000). Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology 54, 1609-1616. 10.1212/WNL.54.8.1609 [DOI] [PubMed] [Google Scholar]
- Bostan, A. C. and Strick, P. L. (2018). The basal ganglia and the cerebellum: nodes in an integrated network. Nat. Rev. Neurosci. 19, 338-350. 10.1038/s41583-018-0002-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, K. L., Baran, C. A., Whitfield, B. R., Jensen, A. M. and Clemens, S. (2014). Dopamine D3 receptor dysfunction prevents anti-nociceptive effects of morphine in the spinal cord. Front. Neural Circuits 8, 62. 10.3389/fncir.2014.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher, S. F., Seelos, K. C., Oertel, W. H., Reiser, M. and Trenkwalder, C. (1997). Cerebral generators involved in the pathogenesis of the restless legs syndrome. Ann. Neurol. 41, 639-645. 10.1002/ana.410410513 [DOI] [PubMed] [Google Scholar]
- Cai, N. S., Quiroz, C., Bonaventura, J., Bonifazi, A., Cole, T. O., Purks, J., Billing, A. S., Massey, E., Wagner, M., Wish, E. D.et al. (2019). Opioid-galanin receptor heteromers mediate the dopaminergic effects of opioids. J. Clin. Invest. 129, 2730-2744. 10.1172/JCI126912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli, I., Pizza, F., Ruggeri, M., Gasperoni, L., Carretta, E., Donati, G., Cianciolo, G., Plazzi, G. and La Manna, G. (2019). Time evolution of restless legs syndrome in haemodialysis patients. Clin. Kidney J. 14, 341-347. 10.1093/ckj/sfz148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoire, H., Dion, P. A., Xiong, L., Amari, M., Gaudet, R., Girard, S. L., Noreau, A., Gaspar, C., Turecki, G., Montplaisir, J. Y.et al. (2011). Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann. Neurol. 70, 170-175. 10.1002/ana.22435 [DOI] [PubMed] [Google Scholar]
- Cermak, S., Meng, Q., Peng, K., Baldwin, S., Mejías-Aponte, C. A., Yang, Y. and Lu, H. (2020). Focal transcranial magnetic stimulation in awake rats: Enhanced glucose uptake in deep cortical layers. J. Neurosci. Methods 339, 108709. 10.1016/j.jneumeth.2020.108709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesnik, E., Casetta, I., Turri, M., Govoni, V., Granieri, E., Strambi, L. F. and Manconi, M. (2010). Transient RLS during pregnancy is a risk factor for the chronic idiopathic form. Neurology 75, 2117-2120. 10.1212/WNL.0b013e318200d779 [DOI] [PubMed] [Google Scholar]
- Chen, P., Ijomone, O. M., Lee, K. H. and Aschner, M. (2019). Caenorhabditis elegans and its applicability to studies on restless legs syndrome. Adv. Pharmacol. 84, 147-174. 10.1016/bs.apha.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P., Cheng, H., Zheng, F., Li, S., Bornhorst, J., Yang, B., Lee, K. H., Ke, T., Li, Y., Schwerdtle, T.et al. (2022). BTBD9 attenuates manganese induced oxidative stress and neurotoxicity by regulating insulin growth factor signaling pathway. Hum. Mol. Genet 31, 2207-2222. 10.1093/hmg/ddac025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenini, S., Delaby, C., Rassu, A. L., Barateau, L., Vialaret, J., Hirtz, C., Dupuy, A. M., Lehmann, S., Jaussent, I. and Dauvilliers, Y. (2020). Hepcidin and ferritin levels in restless legs syndrome: a case-control study. Sci. Rep. 10, 11914. 10.1038/s41598-020-68851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoloz, O., El Mansari, M. and Blier, P. (2009). Sustained administration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology 34, 651-661. 10.1038/npp.2008.114 [DOI] [PubMed] [Google Scholar]
- Chevalier, R. L., Forbes, M. S. and Thornhill, B. A. (2009). Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75, 1145-1152. 10.1038/ki.2009.86 [DOI] [PubMed] [Google Scholar]
- Christensen, M. D., Everhart, A. W., Pickelman, J. T. and Hulsebosch, C. E. (1996). Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain 68, 97-107. 10.1016/S0304-3959(96)03224-1 [DOI] [PubMed] [Google Scholar]
- Clemens, S. and Hochman, S. (2004). Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J. Neurosci. 24, 11337-11345. 10.1523/JNEUROSCI.3698-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, S., Rye, D. and Hochman, S. (2006). Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology 67, 125-130. 10.1212/01.wnl.0000223316.53428.c9 [DOI] [PubMed] [Google Scholar]
- Connor, J. R., Boyer, P. J., Menzies, S. L., Dellinger, B., Allen, R. P., Ondo, W. G. and Earley, C. J. (2003). Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 61, 304-309. 10.1212/01.WNL.0000078887.16593.12 [DOI] [PubMed] [Google Scholar]
- Connor, J. R., Wang, X. S., Allen, R. P., Beard, J. L., Wiesinger, J. A., Felt, B. T. and Earley, C. J. (2009). Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 132, 2403-2412. 10.1093/brain/awp125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor, J. R., Ponnuru, P., Wang, X. S., Patton, S. M., Allen, R. P. and Earley, C. J. (2011a). Profile of altered brain iron acquisition in restless legs syndrome. Brain 134, 959-968. 10.1093/brain/awr012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor, J. R., Ponnuru, P., Lee, B. Y., Podskalny, G. D., Alam, S., Allen, R. P., Earley, C. J. and Yang, Q. X. (2011b). Postmortem and imaging based analyses reveal CNS decreased myelination in restless legs syndrome. Sleep Med. 12, 614-619. 10.1016/j.sleep.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dableh, L. J., Yashpal, K. and Henry, J. L. (2009). Physiological evidence of a postsynaptic inhibition of the tail flick reflex by a cannabinoid receptor agonist. Eur. J. Pharmacol. 602, 36-40. 10.1016/j.ejphar.2008.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafkin, C., Green, A., Olivier, B., McKinon, W. and Kerr, S. (2017). Plantar reflex excitability is increased in the evening in restless legs syndrome patients. Neurosc. Lett. 660, 74-78. 10.1016/j.neulet.2017.09.027 [DOI] [PubMed] [Google Scholar]
- Dafkin, C., Green, A., Olivier, B., Mckinon, W. and Kerr, S. (2018). Circadian variation of flexor withdrawal and crossed extensor reflexes in patients with restless legs syndrome. J. Sleep Res. 27, e12645. 10.1111/jsr.12645 [DOI] [PubMed] [Google Scholar]
- Darvishi, N., Daneshkhah, A., Khaledi-Paveh, B., Vaisi-Raygani, A., Mohammadi, M., Salari, N., Darvishi, F., Abdi, A. and Jalali, R. (2020). The prevalence of Restless Legs Syndrome/Willis-ekbom disease (RLS/WED) in the third trimester of pregnancy: a systematic review. BMC Neurol. 20, 132. 10.1186/s12883-020-01709-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers, Y., Chenini, S., Vialaret, J., Delaby, C., Guiraud, L., Gabelle, A., Lopez, R., Hirtz, C., Jaussent, I. and Lehmann, S. (2018). Association between serum hepcidin level and restless legs syndrome. Mov. Disord. 33, 618-627. 10.1002/mds.27287 [DOI] [PubMed] [Google Scholar]
- DeAndrade, M. P., Johnson, R. L., Jr, Unger, E. L., Zhang, L., van Groen, T., Gamble, K. L. and Li, Y. (2012). Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in Btbd9 mutant mice. Hum. Mol. Genet. 21, 3984-3992. 10.1093/hmg/dds221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie, P., Felser, J., Eckhaus, M., Bryant, J., Silver, J., Marinos, N. and Notkins, A. L. (1991). HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology 185, 109-119. 10.1016/0042-6822(91)90759-5 [DOI] [PubMed] [Google Scholar]
- Didriksen, M., Nawaz, M. S., Dowsett, J., Bell, S., Erikstrup, C., Pedersen, O. B., Sørensen, E., Jennum, P. J., Burgdorf, K. S., Burchell, B.et al. (2020). Large genome-wide association study identifies three novel risk variants for restless legs syndrome. Commun. Biol. 3, 703. 10.1038/s42003-020-01430-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson, N. C. and Sanyal, S. (2015). Use of Drosophila in the investigation of sleep disorders. Exp. Neurol. 274, 72-79. 10.1016/j.expneurol.2015.06.024 [DOI] [PubMed] [Google Scholar]
- Douglass, D. K. and Carstens, E. (1997). Responses of rat sacral spinal neurons to mechanical and noxious thermal stimulation of the tail. J. Neurophysiol. 77, 611-620. 10.1152/jn.1997.77.2.611 [DOI] [PubMed] [Google Scholar]
- Dowsett, J., Didriksen, M., Larsen, M. H., Burgdorf, K. S., Thørner, L. W., Sørensen, E., Erikstrup, C., Pedersen, O. B., Ostrowski, S. R. and Ullum, H. (2021). No association between plasma hepcidin levels and restless legs syndrome - results from the Danish Blood Donor Study. Sleep Med. 88, 68-73. 10.1016/j.sleep.2021.10.008 [DOI] [PubMed] [Google Scholar]
- Drgonova, J., Walther, D., Wang, K. J., Hartstein, G. L., Lochte, B., Troncoso, J., Uetani, N., Iwakura, Y. and Uhl, G. R. (2015). Mouse model for protein tyrosine phosphatase D (PTPRD) Associations with restless leg syndrome or willis-ekbom disease and addiction: reduced expression alters locomotion, sleep behaviors and cocaine-conditioned place preference. Mol. Med. 21, 717-725. 10.2119/molmed.2015.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley, C. J., Connor, J. R., Beard, J. L., Malecki, E. A., Epstein, D. K. and Allen, R. P. (2000). Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology 54, 1698-1700. 10.1212/WNL.54.8.1698 [DOI] [PubMed] [Google Scholar]
- Earley, C. J., Hyland, K. and Allen, R. P. (2001). CSF dopamine, serotonin, and biopterin metabolites in patients with restless legs syndrome. Mov. Disord. 16, 144-149. [DOI] [PubMed] [Google Scholar]
- Earley, C. J., Hyland, K. and Allen, R. P. (2006). Circadian changes in CSF dopaminergic measures in restless legs syndrome. Sleep Med. 7, 263-268. 10.1016/j.sleep.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Earley, C. J., Allen, R. P., Connor, J. R., Ferrucci, L. and Troncoso, J. (2009). The dopaminergic neurons of the A11 system in RLS autopsy brains appear normal. Sleep Med. 10, 1155-1157. 10.1016/j.sleep.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley, C. J., Kuwabara, H., Wong, D. F., Gamaldo, C., Salas, R. E., Brašić, J. R., Ravert, H. T., Dannals, R. F. and Allen, R. P. (2013). Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep 36, 51-57. 10.5665/sleep.2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley, C. J., Connor, J., Garcia-Borreguero, D., Jenner, P., Winkelman, J., Zee, P. C. and Allen, R. (2014). Altered brain iron homeostasis and dopaminergic function in Restless Legs Syndrome (Willis-Ekbom Disease). Sleep Med. 15, 1288-1301. 10.1016/j.sleep.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Fassi, A., Sangalli, F., Maffi, R., Colombi, F., Mohamed, E. I., Brenner, B. M., Remuzzi, G. and Remuzzi, A. (1998). Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J. Am. Sci. Nephrol. 9, 1399-1406. 10.1681/ASN.V981399 [DOI] [PubMed] [Google Scholar]
- Ferré, S., García-Borreguero, D., Allen, R. P. and Earley, C. J. (2019). New insights into the neurobiology of restless legs syndrome. Neuroscientist 25, 113-125. 10.1177/1073858418791763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré, S., Guitart, X., Quiroz, C., Rea, W., García-Malo, C., Garcia-Borreguero, D., Allen, R. P. and Earley, C. J. (2021). Akathisia and restless legs syndrome: solving the dopaminergic paradox. Sleep Med. Clin. 16, 249-267. 10.1016/j.jsmc.2021.02.012 [DOI] [PubMed] [Google Scholar]
- Flik, G., Folgering, J. H., Cremers, T. I., Westerink, B. H. and Dremencov, E. (2015). Interaction between brain histamine and serotonin, norepinephrine, and dopamine systems: In Vivo microdialysis and electrophysiology study. J. Mol. Neurosci. 56, 320-328. 10.1007/s12031-015-0536-3 [DOI] [PubMed] [Google Scholar]
- Freeman, A., Pranski, E., Miller, R. D., Radmard, S., Bernhard, D., Jinnah, H. A., Betarbet, R., Rye, D. B. and Sanyal, S. (2012). Sleep fragmentation and motor restlessness in a Drosophila model of Restless Legs Syndrome. Curr. Biol. 22, 1142-1148. 10.1016/j.cub.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorayeb, I., Bioulac, B., Scribans, C. and Tison, F. (2008). Perceived severity of restless legs syndrome across the female life cycle. Sleep Med. 9, 799-802. 10.1016/j.sleep.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Girven, K. S. and Sparta, D. R. (2017). Probing deep brain circuitry: new advances in In Vivo calcium measurement strategies. ACS Chem. Neurosci. 8, 243-251. 10.1021/acschemneuro.6b00307 [DOI] [PubMed] [Google Scholar]
- Godau, J., Schweitzer, K. J., Liepelt, I., Gerloff, C. and Berg, D. (2007). Substantia nigra hypoechogenicity: definition and findings in restless legs syndrome. Mov. Disord. 22, 187-192. 10.1002/mds.21230 [DOI] [PubMed] [Google Scholar]
- Godau, J., Klose, U., Di Santo, A., Schweitzer, K. and Berg, D. (2008). Multiregional brain iron deficiency in restless legs syndrome. Mov. Disord. 23, 1184-1187. 10.1002/mds.22070 [DOI] [PubMed] [Google Scholar]
- Groenewegen, H. J. and Berendse, H. W. (1994). The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 17, 52-57. 10.1016/0166-2236(94)90074-4 [DOI] [PubMed] [Google Scholar]
- Gulyani, S., Earley, C. J., Camandola, S., Maudsley, S., Ferré, S., Mughal, M. R., Martin, B., Cheng, A., Gleichmann, M., Jones, B. C.et al. (2009). Diminished iron concentrations increase adenosine A(2A) receptor levels in mouse striatum and cultured human neuroblastoma cells. Exp. Neurol. 215, 236-242. 10.1016/j.expneurol.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C. N., Yang, W. J., Zhan, S. Q., Yang, X. F., Chen, M. C., Fuller, P. M. and Lu, J. (2017). Targeted disruption of supraspinal motor circuitry reveals a distributed network underlying Restless Legs Syndrome (RLS)-like movements in the rat. Sci. Rep. 7, 9905. 10.1038/s41598-017-10284-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, R., Ulfberg, J., Allen, R. P. and Goel, D. (2017). High prevalence of restless legs syndrome/Willis Ekbom Disease (RLS/WED) among people living at high altitude in the Indian Himalaya. Sleep Med. 35, 7-11. 10.1016/j.sleep.2017.02.031 [DOI] [PubMed] [Google Scholar]
- Han, J., Day, J. R., Connor, J. R. and Beard, J. L. (2003). Gene expression of transferrin and transferrin receptor in brains of control vs. iron-deficient rats. Nutr. Neurosci. 6, 1-10. [PubMed] [Google Scholar]
- Hanifa, M. A., Skott, M., Maltesen, R. G., Rasmussen, B. S., Nielsen, S., Frøkiær, J., Ring, T. and Wimmer, R. (2019). Tissue, urine and blood metabolite signatures of chronic kidney disease in the 5/6 nephrectomy rat model. Metabolomics 15, 112. 10.1007/s11306-019-1569-3 [DOI] [PubMed] [Google Scholar]
- Hentze, M. W., Muckenthaler, M. U., Galy, B. and Camaschella, C. (2010). Two to tango: regulation of Mammalian iron metabolism. Cell 142, 24-38. 10.1016/j.cell.2010.06.028 [DOI] [PubMed] [Google Scholar]
- Hobert, O. (2013). The neuronal genome of Caenorhabditis elegans. WormBook 1-106. 10.1895/wormbook.1.161.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, L. L. and Cunningham, K. A. (2015). Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol. Rev. 67, 176-197. 10.1124/pr.114.009514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi, L. D., Apparsundaram, S., Malone, M. D., Ward, E., Miller, D. M., Eppler, M. and Blakely, R. D. (1998). The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol. Pharmacol. 54, 601-609. [PubMed] [Google Scholar]
- Jellen, L. C., Beard, J. L. and Jones, B. C. (2009). Systems genetics analysis of iron regulation in the brain. Biochimie 91, 1255-1259. 10.1016/j.biochi.2009.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellen, L. C., Unger, E. L., Lu, L., Williams, R. W., Rousseau, S., Wang, X., Earley, C. J., Allen, R. P., Miles, M. F. and Jones, B. C. (2012). Systems genetic analysis of the effects of iron deficiency in mouse brain. Neurogenetics 13, 147-157. 10.1007/s10048-012-0321-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno-Martín, A., Sousa, E., Brocal-Ruiz, R., Daroqui, N., Maicas, M. and Flames, N. (2022). Joint actions of diverse transcription factor families establish neuron-type identities and promote enhancer selectivity. Genome Res. 32, 459-473. 10.1101/gr.275623.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, T. L. and Clemens, S. (2021). Differential dopamine modulation of spinal reflex amplitudes is associated with the presence or absence of the autonomic nervous system. Neurosci. Lett. 742, 135514. 10.1016/j.neulet.2020.135514 [DOI] [PubMed] [Google Scholar]
- Kaneda, N., Sasaoka, T., Kobayashi, K., Kiuchi, K., Nagatsu, I., Kurosawa, Y., Fujita, K., Yokoyama, M., Nomura, T. and Katsuki, M. (1991). Tissue-specific and high-level expression of the human tyrosine hydroxylase gene in transgenic mice. Neuron 6, 583-594. 10.1016/0896-6273(91)90061-4 [DOI] [PubMed] [Google Scholar]
- Keeler, B. E., Baran, C. A., Brewer, K. L. and Clemens, S. (2012). Increased excitability of spinal pain reflexes and altered frequency-dependent modulation in the dopamine D3-receptor knockout mouse. Exp. Neurol. 238, 273-283. 10.1016/j.expneurol.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Kisucká, A., Hricová, Ľ., Pavel, J., Strosznajder, J. B., Chalimoniuk, M., Langfort, J., Gálik, J., Maršala, M., Radoňak, J. and Lukáčová, N. (2015). Baclofen or nNOS inhibitor affect molecular and behavioral alterations evoked by traumatic spinal cord injury in rat spinal cord. Spine J. 15, 1366-1378. 10.1016/j.spinee.2014.08.013 [DOI] [PubMed] [Google Scholar]
- Kiuchi, K., Kiuchi, K., Kaneda, N., Sasaoka, T., Hidaka, H. and Nagatsu, T. (1993). Regulatory mechanism of dopamine biosynthesis in the striatum of transgenic mice carrying human tyrosine hydroxylase gene. Neurosci. Lett. 151, 55-58. 10.1016/0304-3940(93)90044-L [DOI] [PubMed] [Google Scholar]
- Knake, S., Heverhagen, J. T., Menzler, K., Keil, B., Oertel, W. H. and Stiasny-Kolster, K. (2009). Normal regional brain iron concentration in restless legs syndrome measured by MRI. Nature Sci. Sleep 2, 19-22. 10.2147/NSS.S7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru, H., Vidal, J., Benito, J., Barrio, M., Portell, E., Valles, M., Flores, C. and Santamaria, J. (2015). Restless leg syndrome in patients with spinal cord injury. Parkinsonism Relat. Disord. 21, 1461-1464. 10.1016/j.parkreldis.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Kushida, C. A., Allen, R. P. and Atkinson, M. J. (2004). Modeling the causal relationships between symptoms associated with restless legs syndrome and the patient-reported impact of RLS. Sleep Med. 5, 485-488. 10.1016/j.sleep.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Lai, Y. Y., Cheng, Y. H., Hsieh, K. C., Nguyen, D., Chew, K. T., Ramanathan, L. and Siegel, J. M. (2017). Motor hyperactivity of the iron-deficient rat - an animal model of restless legs syndrome. Mov. Disord. 32, 1687-1693. 10.1002/mds.27133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, G., Cantone, M., Lanuzza, B., Pennisi, M., Bella, R., Pennisi, G. and Ferri, R. (2015). Distinctive patterns of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome, restless legs syndrome, insomnia, and sleep deprivation. Sleep Med. Rev. 19, 39-50. 10.1016/j.smrv.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Lanza, G., Cantone, M., Aricò, D., Lanuzza, B., Cosentino, F., Paci, D., Papotto, M., Pennisi, M., Bella, R., Pennisi, G.et al. (2018). Clinical and electrophysiological impact of repetitive low-frequency transcranial magnetic stimulation on the sensory-motor network in patients with restless legs syndrome. Ther. Adv. Neurol. Disord. 11, 1756286418759973. 10.1177/1756286418759973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold, N. A. and Speicher, M. R. (2021). Evolutionary conservation in noncoding genomic regions. Trends Genet. 37, 903-918. 10.1016/j.tig.2021.06.007 [DOI] [PubMed] [Google Scholar]
- Li, S. M., Collins, G. T., Paul, N. M., Grundt, P., Newman, A. H., Xu, M., Grandy, D. K., Woods, J. H. and Katz, J. L. (2010). Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behav. Pharmacol. 21, 171-181. 10.1097/FBP.0b013e32833a5c68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Allen, R. P., Earley, C. J., Liu, H., Cruz, T. E., Edden, R., Barker, P. B. and van Zijl, P. (2016). Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med. 22, 75-82. 10.1016/j.sleep.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. W., Zhang, J. F., Qiu, M. Y., Ni, L. Y., Yu, H. L., Kuo, S. H., Ondo, W. G., Yu, Q. and Wu, Y. C. (2019). Restless legs syndrome in end stage renal disease patients undergoing hemodialysis. BMC Neurol. 19, 47. 10.1186/s12883-019-1265-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Martire, V., Alvente, S., Bastianini, S., Berteotti, C., Valli, A., Manconi, M., Zoccoli, G. and Silvani, A. (2018). Sleep and Tibialis anterior muscle activity in mice with mild hypoxia and iron deficiency: implications for the restless legs syndrome. Front. Physiol. 9, 1818. 10.3389/fphys.2018.01818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok, C. N. and Loh, T. T. (1998). Regulation of transferrin function and expression: review and update. Biol. Signals Recept. 7, 157-178. 10.1159/000014542 [DOI] [PubMed] [Google Scholar]
- Lopes, C., Esteves, A. M., Frussa-Filho, R., Tufik, S. and de Mello, M. T. (2012). Evaluation of periodic limb movements in a putative animal model of restless leg syndrome. Mov. Disord. 27, 413-420. 10.1002/mds.24058 [DOI] [PubMed] [Google Scholar]
- Lyu, S., Xing, H., DeAndrade, M. P., Liu, Y., Perez, P. D., Yokoi, F., Febo, M., Walters, A. S. and Li, Y. (2019a). The role of BTBD9 in striatum and restless legs syndrome. eNeuro 6, ENEURO.0277-19.2019. 10.1523/ENEURO.0277-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, S., DeAndrade, M. P., Mueller, S., Oksche, A., Walters, A. S. and Li, Y. (2019b). Hyperactivity, dopaminergic abnormalities, iron deficiency and anemia in an in vivo opioid receptors knockout mouse: Implications for the restless legs syndrome. Behav. Brain. Res. 374, 112123. 10.1016/j.bbr.2019.112123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, S., Xing, H., DeAndrade, M. P., Perez, P. D., Zhang, K., Liu, Y., Yokoi, F., Febo, M. and Li, Y. (2020a). The role of BTBD9 in the cerebral cortex and the pathogenesis of restless legs syndrome. Exp. Neurol. 323, 113111. 10.1016/j.expneurol.2019.113111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, S., Xing, H., Liu, Y., Girdhar, P., Zhang, K., Yokoi, F., Xiao, R. and Li, Y. (2020b). Deficiency of Meis1, a transcriptional regulator, in mice and worms: Neurochemical and behavioral characterizations with implications in the restless legs syndrome. J. Neurochem. 155, 522-537. 10.1111/jnc.15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek, D. W., Garraway, S. M., Shay, B. L. and Hochman, S. (2001). Serotonin 5-HT(2) receptor activation induces a long-lasting amplification of spinal reflex actions in the rat. J. Physiol. 537, 201-207. 10.1111/j.1469-7793.2001.0201k.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie, S. and Winkelman, J. W. (2015). Long-term treatment of restless legs syndrome (RLS): an approach to management of worsening symptoms, loss of efficacy, and augmentation. CNS Drugs 29, 351-357. 10.1007/s40263-015-0250-2 [DOI] [PubMed] [Google Scholar]
- Magalhães, S. C., Queiroz de Paiva, J. P., Kaelin-Lang, A., Sterr, A., Eckeli, A. L., Winkler, A. M., Fernandes do Prado, G., Amaro, E., Jr. and Conforto, A. B. (2019). Short-interval intracortical inhibition is decreased in restless legs syndrome across a range of severity. Sleep Med. 62, 34-42. 10.1016/j.sleep.2019.03.021 [DOI] [PubMed] [Google Scholar]
- Magno, L., Tenza-Ferrer, H., Collodetti, M., Aguiar, M., Rodrigues, A., da Silva, R. S., Silva, J., Nicolau, N. F., Rosa, D., Birbrair, A.et al. (2019). Optogenetic stimulation of the M2 cortex reverts motor dysfunction in a mouse model of Parkinson's disease. J. Neurosci. 39, 3234-3248. 10.1523/JNEUROSCI.2277-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj, J., Rogóz, Z., Skuza, G. and Kołodziejczyk, K. (1997). The behavioural effects of pramipexole, a novel dopamine receptor agonist. Eur. J. Pharmacol. 324, 31-37. 10.1016/S0014-2999(97)00066-6 [DOI] [PubMed] [Google Scholar]
- Manconi, M., Ulfberg, J., Berger, K., Ghorayeb, I., Wesström, J., Fulda, S., Allen, R. P. and Pollmächer, T. (2012). When gender matters: restless legs syndrome. Report of the “RLS and woman” workshop endorsed by the European RLS Study Group. Sleep Med. Rev, 16, 297-307. [DOI] [PubMed] [Google Scholar]
- Margariti, P. N., Astrakas, L. G., Tsouli, S. G., Hadjigeorgiou, G. M., Konitsiotis, S. and Argyropoulou, M. I. (2012). Investigation of unmedicated early onset restless legs syndrome by voxel-based morphometry, T2 relaxometry, and functional MR imaging during the night-time hours. AJNR. Am. J. Neuroradiol. 33, 667-672. 10.3174/ajnr.A2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano, M. O., Esteves, A. M., Frank, M. K., Caperuto, L. C., Manconi, M., Tufik, S. and De Mello, M. T. (2014). Changes in motor behavior during pregnancy in rats: the basis for a possible animal model of restless legs syndrome. Rev. Bras. Ginecol. Obstet. 36, 436-441. 10.1590/SO100-720320140005105 [DOI] [PubMed] [Google Scholar]
- Matsusaka, T., Xin, J., Niwa, S., Kobayashi, K., Akatsuka, A., Hashizume, H., Wang, Q. C., Pastan, I., Fogo, A. B. and Ichikawa, I. (2005). Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J. Am Soc. Nephrol. 16, 1013-1023. 10.1681/ASN.2004080720 [DOI] [PubMed] [Google Scholar]
- Mizuno, S., Mihara, T., Miyaoka, T., Inagaki, T. and Horiguchi, J. (2005). CSF iron, ferritin and transferrin levels in restless legs syndrome. J. Sleep Res. 14, 43-47. 10.1111/j.1365-2869.2004.00403.x [DOI] [PubMed] [Google Scholar]
- Mumbach, M. R., Satpathy, A. T., Boyle, E. A., Dai, C., Gowen, B. G., Cho, S. W., Nguyen, M. L., Rubin, A. J., Granja, J. M., Kazane, K. R.et al. (2017). Enhancer connectome in primary human cells identifies target genes of disease-associated DNA elements. Nat. Genet. 49, 1602-1612. 10.1038/ng.3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara, D., Hashiguti, H., Kaneda, N., Sasaoka, T. and Nagatsu, T. (1993). Normalization of tyrosine hydroxylase activity in vivo in the striatum of transgenic mice carrying human tyrosine hydroxylase gene: a microdialysis study. Neurosci. Lett. 158, 44-46. 10.1016/0304-3940(93)90608-N [DOI] [PubMed] [Google Scholar]
- Nakamura, T., Oite, T., Shimizu, F., Matsuyama, M., Kazama, T., Koda, Y. and Arakawa, M. (1986). Sclerotic lesions in the glomeruli of Buffalo/Mna rats. Nephron 43, 50-55. 10.1159/000183718 [DOI] [PubMed] [Google Scholar]
- Oleson, E. B., Ferris, M. J., España, R. A., Harp, J. and Jones, S. R. (2012). Effects of the histamine H1 receptor antagonist and benztropine analog diphenylpyraline on dopamine uptake, locomotion and reward. Eur. J. Pharmacol. 683, 161-165. 10.1016/j.ejphar.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondo, W. G., He, Y., Rajasekaran, S. and Le, W. D. (2000). Clinical correlates of 6-hydroxydopamine injections into A11 dopaminergic neurons in rats: a possible model for restless legs syndrome. Mov. Disord. 15, 154-158. [DOI] [PubMed] [Google Scholar]
- Oyrer, J., Maljevic, S., Scheffer, I. E., Berkovic, S. F., Petrou, S. and Reid, C. A. (2018). Ion channels in genetic epilepsy: from genes and mechanisms to disease-targeted therapies. Pharmacol. Rev. 70, 142-173. 10.1124/pr.117.014456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo, N. P., Hening, W. A., Allen, R. P. and Earley, C. J. (2010). Pregnancy accounts for most of the gender difference in prevalence of familial RLS. Sleep Med. 11, 310-313. 10.1016/j.sleep.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patatanian, E. and Claborn, M. K. (2018). Drug-induced restless legs syndrome. Ann. Pharmacother. 52, 662-672. 10.1177/1060028018760296 [DOI] [PubMed] [Google Scholar]
- Patton, S. M., Ponnuru, P., Snyder, A. M., Podskalny, G. D. and Connor, J. R. (2011). Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur. J. Neurol. 18, 1329-1335. 10.1111/j.1468-1331.2011.03397.x [DOI] [PubMed] [Google Scholar]
- Qiu, Z., Kala, S., Guo, J., Xian, Q., Zhu, J., Zhu, T., Hou, X., Wong, K. F., Yang, M., Wang, H.et al. (2021). Targeted neurostimulation in mouse brains with non-invasive ultrasound. Cell Rep. 34, 108595. 10.1016/j.celrep.2020.108595 [DOI] [PubMed] [Google Scholar]
- Qu, S., Ondo, W. G., Zhang, X., Xie, W. J., Pan, T. H. and Le, W. D. (2006). Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp. Brain Res. 168, 152-156. 10.1007/s00221-005-0075-1 [DOI] [PubMed] [Google Scholar]
- Quatrale, R., Manconi, M., Gastaldo, E., Eleopra, R., Tugnoli, V., Tola, M. R. and Granieri, E. (2003). Neurophysiological study of corticomotor pathways in restless legs syndrome. Clin. Neurophysiol. 114, 1638-1645. 10.1016/S1388-2457(03)00137-8 [DOI] [PubMed] [Google Scholar]
- Quiroz, C., Gulyani, S., Ruiqian, W., Bonaventura, J., Cutler, R., Pearson, V., Allen, R. P., Earley, C. J., Mattson, M. P. and Ferré, S. (2016). Adenosine receptors as markers of brain iron deficiency: implications for restless legs syndrome. Neuropharmacology 111, 160-168. 10.1016/j.neuropharm.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson, R. N., Dodds, A. L., Smith, M. J., Santer, R. M. and Watson, A. H. (2003). Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 972, 149-158. 10.1016/S0006-8993(03)02521-6 [DOI] [PubMed] [Google Scholar]
- Rizzo, G., Manners, D., Vetrugno, R., Tonon, C., Malucelli, E., Plazzi, G., Marconi, S., Pizza, F., Testa, C., Provini, F.et al. (2012). Combined brain voxel-based morphometry and diffusion tensor imaging study in idiopathic restless legs syndrome patients. Eur. J. Neurol. 19, 1045-1049. 10.1111/j.1468-1331.2011.03604.x [DOI] [PubMed] [Google Scholar]
- Rizzo, G., Manners, D., Testa, C., Tonon, C., Vetrugno, R., Marconi, S., Plazzi, G., Pizza, F., Provini, F., Malucelli, E.et al. (2013). Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov. Disord. 28, 1886-1890. 10.1002/mds.25576 [DOI] [PubMed] [Google Scholar]
- Robbins, M. E., Bonsib, S. M., Soranson, J. A., Wilson, G. D., Ikeda, A., Rezvani, M., Golding, S. J., Whitehouse, E. and Hopewell, J. W. (1995). Radiation-induced changes in glomerular and tubular cell kinetics and morphology following irradiation of a single kidney in the pig. Int. J. Radiat. Oncol. Biol. Phys. 32, 1071-1081. 10.1016/0360-3016(95)00548-D [DOI] [PubMed] [Google Scholar]
- Rodgers, H. M., Yow, J., Evans, E., Clemens, S. and Brewer, K. L. (2019). Dopamine D1 and D3 receptor modulators restore morphine analgesia and prevent opioid preference in a model of neuropathic pain. Neuroscience 406, 376-388. 10.1016/j.neuroscience.2019.03.034 [DOI] [PubMed] [Google Scholar]
- Rottach, K. G., Schaner, B. M., Kirch, M. H., Zivotofsky, A. Z., Teufel, L. M., Gallwitz, T. and Messer, T. (2008). Restless legs syndrome as side effect of second generation antidepressants. J. Pshichiatr. Res. 43, 70-75. 10.1016/j.jpsychires.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Salas, R., Kalloo, A., Earley, C. J., Celnik, P., Cruz, T. E., Foster, K., Cantarero, G. and Allen, R. P. (2018). Connecting clinical aspects to corticomotor excitability in restless legs syndrome: a TMS study. Sleep Med. 49, 105-112. 10.1016/j.sleep.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Salminen, A. V., Manconi, M., Rimpilä, V., Luoto, T. M., Koskinen, E., Ferri, R., Ohman, J. and Polo, O. (2013). Disconnection between periodic leg movements and cortical arousals in spinal cord injury. J. Clin. Sleep Med. 9, 1207-1209. 10.5664/jcsm.3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, A. V., Rimpilä, V. and Polo, O. (2014). Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease). Neurology 82, 1856-1861. 10.1212/WNL.0000000000000454 [DOI] [PubMed] [Google Scholar]
- Salminen, A. V., Garrett, L., Schormair, B., Rozman, J., Giesert, F., Niedermeier, K. M., Becker, L., Rathkolb, B., Rácz, I., German Mouse Clinic Consortium et al. (2017). Meis1: effects on motor phenotypes and the sensorimotor system in mice. Dis. Model. Mech. 10, 981-991. 10.1242/dmm.030080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, A. V., Lam, D. D. and Winkelmann, J. (2019). Role of MEIS1 in restless legs syndrome: From GWAS to functional studies in mice. Adv. Pharmacol. 84, 175-184. 10.1016/bs.apha.2019.03.003 [DOI] [PubMed] [Google Scholar]
- Salminen, A. V., Silvani, A., Allen, R. P., Clemens, S., Garcia-Borreguero, D., Ghorayeb, I., Ferré, S., Li, Y., Ondo, W., Picchietti, D. L., et al. (2021). Consensus guidelines on rodent models of restless legs syndrome. Mov. Disord 36, 558-569. 10.1002/mds.28401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalise, A., Cadore, I. P. and Gigli, G. L. (2004). Motor cortex excitability in restless legs syndrome. Sleep Med. 5, 393-396. 10.1016/j.sleep.2004.01.011 [DOI] [PubMed] [Google Scholar]
- Schormair, B. and Winkelmann, J. (2011). Genetics of restless legs syndrome: mendelian, complex, and everything in between. Sleep Med. Clin. 6, 203-215. 10.1016/j.jsmc.2011.04.006 [DOI] [Google Scholar]
- Schormair, B., Zhao, C., Bell, S., Tilch, E., Salminen, A. V., Pütz, B., Dauvilliers, Y., Stefani, A., Högl, B., Poewe, W.et al. (2017). Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Lancet. Neurol. 16, 898-907. 10.1016/S1474-4422(17)30327-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromani, P. J., Blanco-Centurion, C. and Vidal-Ortiz, A. (2021). Mapping network activity in sleep. Front. Neurosci. 15, 646468. 10.3389/fnins.2021.646468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N., Longo, N., Levert, K., Hyland, K. and Blau, N. (2019). Exploratory study of the effect of one week of orally administered CNSA-001 (sepiapterin) on CNS levels of tetrahydrobiopterin, dihydrobiopterin and monoamine neurotransmitter metabolites in healthy volunteers. Mol. Genet. Metab. Rep. 21, 100500. 10.1016/j.ymgmr.2019.100500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieler, D., Kaffe, M., Knauf, F., Bessa, J., Tena, J. J., Giesert, F., Schormair, B., Tilch, E., Lee, H., Horsch, M.et al. (2014). Restless legs syndrome-associated intronic common variant in Meis1 alters enhancer function in the developing telencephalon. Genome Res. 24, 592-603. 10.1101/gr.166751.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telles, S. C., Alves, R. C. and Chadi, G. (2011). Periodic limb movements during sleep and restless legs syndrome in patients with ASIA A spinal cord injury. J. Neurol. Sci. 303, 119-123. 10.1016/j.jns.2010.12.019 [DOI] [PubMed] [Google Scholar]
- Tergau, F., Wischer, S. and Paulus, W. (1999). Motor system excitability in patients with restless legs syndrome. Neurology 52, 1060-1063. 10.1212/WNL.52.5.1060 [DOI] [PubMed] [Google Scholar]
- Thi Truong, B. E., Sung, F. C., Lin, C. L., Hang, L. W., Teng, Y. K. and Tzeng, Y. L. (2021). A follow-up study on restless legs syndrome in chronic obstructive pulmonary disease population. Sleep Med. 80, 9-15. 10.1016/j.sleep.2021.01.016 [DOI] [PubMed] [Google Scholar]
- Thorpe, A. J., Clair, A., Hochman, S. and Clemens, S. (2011). Possible sites of therapeutic action in restless legs syndrome: focus on dopamine and α2δ ligands. Eur. Neurol. 66, 18-29. 10.1159/000328431 [DOI] [PubMed] [Google Scholar]
- Todorich, B., Pasquini, J. M., Garcia, C. I., Paez, P. M. and Connor, J. R. (2009). Oligodendrocytes and myelination: the role of iron. Glia 57, 467-478. 10.1002/glia.20784 [DOI] [PubMed] [Google Scholar]
- Trenkwalder, C., Allen, R., Högl, B., Paulus, W. and Winkelmann, J. (2016). Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology 86, 1336-1343. 10.1212/WNL.0000000000002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien, J. Z. (2016). Cre-Lox Neurogenetics: 20 Years of versatile applications in brain research and counting…. Front. Genet. 7, 19. 10.3389/fgene.2016.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wåhlin-Larsson, B., Ulfberg, J., Aulin, K. P. and Kadi, F. (2009). The expression of vascular endothelial growth factor in skeletal muscle of patients with sleep disorders. Muscle Nerve 40, 556-561. 10.1002/mus.21357 [DOI] [PubMed] [Google Scholar]
- Walters, A. S., Ondo, W. G., Zhu, W. and Le, W. (2009). Does the endogenous opiate system play a role in the Restless Legs Syndrome? A pilot post-mortem study. J. Neurol.Sci. 279, 62-65. [DOI] [PubMed] [Google Scholar]
- Wan, R., Cheli, V. T., Santiago-González, D. A., Rosenblum, S. L., Wan, Q. and Paez, P. M. (2020). Impaired postnatal myelination in a conditional knockout mouse for the ferritin heavy chain in oligodendroglial cells. J. Neurosci. 40, 7609-7624. 10.1523/JNEUROSCI.1281-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Zeng, Y. N., Yang, P., Jin, L. Q., Xiong, W. C., Zhu, M. Z., Zhang, J. Z., He, X. and Zhu, X. H. (2019). Axonal iron transport in the brain modulates anxiety-related behaviors. Nat. Chem. Biol. 15, 1214-1222. 10.1038/s41589-019-0371-x [DOI] [PubMed] [Google Scholar]
- Watanabe, T., Sasaki, M., Komagata, S., Tsukano, H., Hishida, R., Kohno, T., Baba, H. and Shibuki, K. (2015). Spinal mechanisms underlying potentiation of hindpaw responses observed after transient hindpaw ischemia in mice. Sci. Rep. 5, 11191. 10.1038/srep11191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C., White, D. P. and Winkelman, J. W. (2005). Antidepressants and periodic leg movements of sleep. Biol. Psychiatry. 58, 510-514. 10.1016/j.biopsych.2005.04.022 [DOI] [PubMed] [Google Scholar]
- Yepes, G., Guitart, X., Rea, W., Newman, A. H., Allen, R. P., Earley, C. J., Quiroz, C. and Ferré, S. (2017). Targeting hypersensitive corticostriatal terminals in restless legs syndrome. Ann. Neurol. 82, 951-960. 10.1002/ana.25104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., Wang, S., Wang, L., Shan, B. C., Zhang, H., Yang, F., Zhou, Z. Q., Wang, X., Yuan, Y. and Xu, Y. J. (2018). Hepcidin is an endogenous protective factor for osteoporosis by reducing iron levels. J. Mol. Endocrinol. 60, 297-306. [DOI] [PubMed] [Google Scholar]
- Zhao, H., Zhu, W., Pan, T., Xie, W., Zhang, A., Ondo, W. G. and Le, W. (2007). Spinal cord dopamine receptor expression and function in mice with 6-OHDA lesion of the A11 nucleus and dietary iron deprivation. J. Neurosci. Res. 85, 1065-1076. 10.1002/jnr.21207 [DOI] [PubMed] [Google Scholar]
- Zhou, X. and Frohlich, E. D. (2007). Analogy of cardiac and renal complications in essential hypertension and aged SHR or L-NAME/SHR. Med. Chem. 3, 61-65. 10.2174/157340607779317634 [DOI] [PubMed] [Google Scholar]
- Zhu, X. Y., Wu, T. T., Wang, H. M., Li, X., Ni, L. Y., Chen, T. J., Qiu, M. Y., Shen, J., Liu, T., Ondo, W. G.et al. (2020). Correlates of nonanemic iron deficiency in restless legs syndrome. Front. Neurol. 11, 298. 10.3389/fneur.2020.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, Y., Wu, Y., Xu, Y., Lu, L., Li, T., Wang, X. and Li, K. (2017). Combined resting state functional magnetic resonance imaging and diffusion tensor imaging study in patients with idiopathic restless legs syndrome. Sleep Med. 38, 96-103. 10.1016/j.sleep.2017.06.033 [DOI] [PubMed] [Google Scholar]