Abstract
Chronic pain increases the risk of developing anxiety, with limbic areas being likely neurological substrates. Despite high clinical relevance, little is known about the precise behavioral, hormonal, and brain neuroplastic correlates of anxiety in the context of persistent pain. Previous studies have shown that decreased nociceptive thresholds in chronic pain models are paralleled by anxiety-like behavior in rats, but there are conflicting ideas regarding its effects on the stress response and circulating corticosterone levels. Even less is known about the molecular mechanisms through which the brain encodes pain-related anxiety. This study examines how persistent inflammatory pain in a rat model would impact anxiety-like behaviors and corticosterone release, and whether these changes would be reflected in levels of global DNA methylation in brain areas involved in stress regulation. Complete Freund’s adjuvant (CFA) or saline was administered in the right hindpaw of adult male Wistar rats. Behavioral testing included the measurement of nociceptive thresholds (digital anesthesiometer), motor function (open field test), and anxiety-like behaviors (elevated plus maze and the dark-light box test). Corticosterone was measured via radioimmunoassay. Global DNA methylation (enzyme immunoassay) as well as DNMT3a levels (western blotting) were quantified in the amygdala, prefrontal cortex, and ventral hippocampus. CFA administration resulted in persistent reduction in nociceptive threshold in the absence of locomotor abnormalities. Increased anxiety-like behaviors were observed in the elevated plus maze and were accompanied by increased blood corticosterone levels 10 days after pain induction. Global DNA methylation was decreased in the amygdala, with no changes in DNMT3a abundance in any of the regions examined. Persistent inflammatory pain promotes anxiety -like behaviors, HPA axis activation, and epigenetic regulation through DNA methylation in the amygdala. These findings describe a molecular mechanism that links pain and stress in a well-characterized rodent model.
Keywords: Persistent inflammatory pain, stress, anxiety disorders, DNA methylation, corticosterone, amygdala, epigenetics, animal model
Introduction
Chronic pain increases the risk of developing depression and anxiety, 1 and is associated with disorders in affective processes and the involvement of limbic areas both in patients and animal models.2,3 In the central nervous system (CNS), pain processing occurs through a complex organization of ascending and descending neural pathways which often overlap with brain regions involved in emotional behavior, including the prefrontal cortex, amygdala, and hippocampus. 4 These brain regions and their subdivisions are also responsible for the coordination of the stress response. 5 In particular, the amygdala is critically involved both in emotional regulation 6 as well as pain perception.7,8 Chronic pain also affects the hypothalamic-pituitary-adrenal axis (HPA), a neuroendocrine feedback system responsible for the regulation of stress adaptation. The imbalance of the HPA axis is linked to stress-related disorders with increased release of corticosterone leading to alterations in behavior, neurotransmitter systems, and gene expression in the brain. 9 The effects of persistent inflammatory pain on corticosterone release are not well understood.
An attractive mechanism that could explain pain persistence and its co-morbidities is DNA methylation, a covalent reaction at the 5’ position in cytosine-guanine dinucleotides and is often associated with altered gene expression. 10 Indeed, stress-related disorders, anxiodepressive-like behaviors, and chronic pain have been strongly linked to changes in CNS DNA methylation.9,11–14 In a model of peripheral neuropathy, mice displayed anxiety-like behaviors and concomitant reduction of global DNA methylation in the prefrontal cortex and the amygdala. 12 In a mouse model of bone trauma, increased levels of global methylation and decreased levels of hydroxymethylation were observed in the olfactory bulb and were associated with an increased bias towards recalling unpleasant memories. 14 In a rat acute inflammatory pain model induced by complete Freund’s adjuvant (CFA) in the paw, increased phosphorylation of methyl-CpG binding protein 2 was observed in spinal lamina I15,16 and II. 16
The findings above led us to examine whether persistent inflammatory pain would impact anxiety-like behaviors, corticosterone release, and whether these changes would be reflected in levels of global DNA methylation in the brain areas involved in stress regulation. Using a well-characterized rat model of persistent inflammatory pain ,17,18 we hypothesize that reduced nociceptive thresholds are accompanied by increased signs of anxiety, increased levels of circulating cortisol, as well as alterations in the global DNA methylation in the brain.
Methods
Experiments were performed according to the international guidelines for animal use with the approval of the local Animal Care and Use Committee of the University of São Paulo-Brazil, Campus of Ribeirão Preto (Protocol number 2014.1.508.58.1) and Conselho Nacional de Controle de Experimentação Animal - Ministério da Ciência e Tecnologia (Brazil). The number of animals used in each protocol was chosen empirically and all efforts were made to minimize animal suffering.
Animals
Male Wistar adult rats (weighing ∼200 g at arrival) were used. All animals were provided by our local animal vivarium (University of São Paulo, Campus of Ribeirão Preto, Brazil) and kept in Plexiglas wall cages (56 cm × 17 cm × 39 cm, four rats per cage) in a room maintained at 24 ± 1°C, and humidity 60–65% and allowed to acclimate for at least 1 week prior to experiments. Animals were housed in a 12:12-h light/dark cycle with food and water available ad libitum. The experimental rooms for nociceptive and behavioral testing were maintained at the same temperature and humidity as above. The light intensity in the rooms for elevated plus maze, dark-light box, and open field test was ∼50 lux. Visual cues and loud noises were limited for the behavioral and nociceptive testing. All experiments were performed between 09:00 a.m. and 03:00 p.m. to minimize hormonal circadian oscillations. Behavioral experiments in elevated plus maze (EPM), dark-light box (DLB), and open field test (OFT) were documented using a video camera. The ARRIVE guidelines (Animal Research: Reporting of in Vivo Experiments) were followed.
Experimental groups
We randomly assigned each animal to one of the following groups, as described here: Group 1 (n = 8): the rats were tested in the von Frey apparatus to verify the basal latency (BL) of the nociceptive threshold and received in the right hind paw 1 day after BL data. Then, the nociceptive threshold was measured at 3, 7, and 10 days after CFA injection. Right and left hind paws were tested. This group of animals was exposed to the open field test 2 h after the von Frey test on the 10th day after paw injection. Group 2 (n = 8): the rats followed the same timeline as Group 1, however, instead of CFA, 0.9% saline was administered. This served as a control group for the group 1. Group 3 (n = 8): CFA was administered in the right hindpaw and rats underwent EPM 10 days after injection. Group 4 (n = 7): the rats followed the same timeline as group 3, however instead of CFA, 0.9% saline was administered. This served as a control group for the group 3. Group 5 (n = 8): CFA was administered in the right hind paw and underwent DLB 10 days after the injection. Group 6 (n = 8): the rats followed the same timeline as group 5, however instead of CFA, 0.9% saline was administered. This served a control group for the group 5. Group 7 (n = 6): CFA was administered in the right hindpaw, and rats were decapitated 10 days after injection. Immediately following decapitation, the trunk blood was collected and placed into heparinized tubes. Brains were also harvested and stored in −80°C for dissection and subsequent global DNA methylation and DNMT3a abundance analysis. Group 8 (n = 6): the rats followed the same timeline as group 7, however instead of CFA, 0.9% saline was administered. This served as a control group for the group 7.
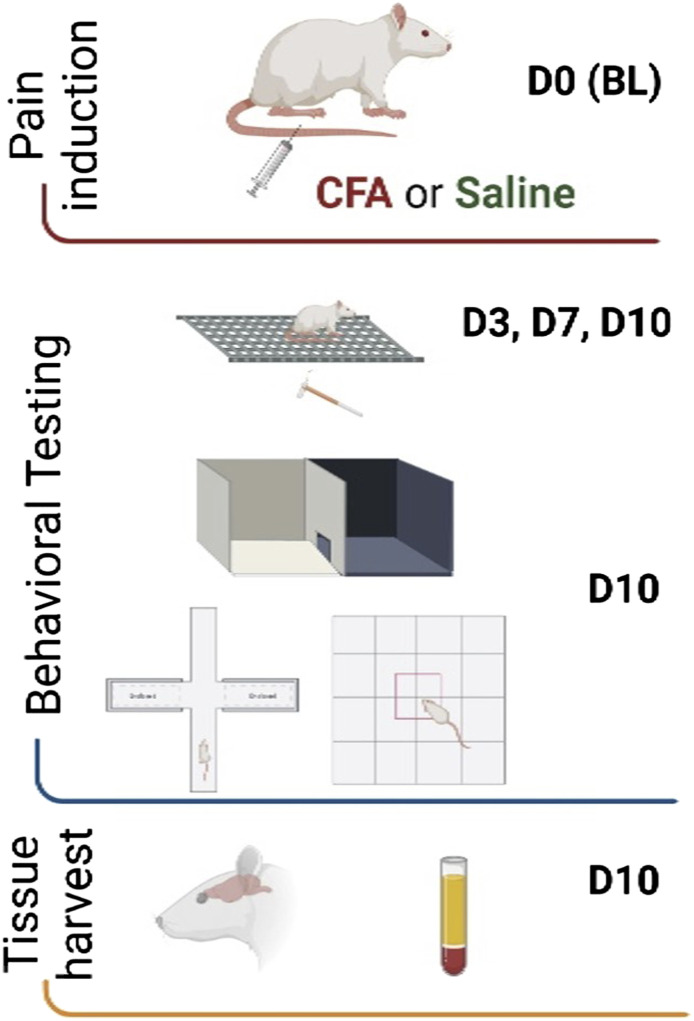
A priori exclusion criteria included animals showing signs of illness and severe discomfort after the intraplantar injections (0 animals were excluded). One animal died due to the ketamine/xylazine anesthesia prior to the intraplantar injection and was therefore excluded from the study. The experimental timeline is depicted in Figure 1.
Figure 1.
Experimental timeline. Image illustrated using Biorender© software.
Induction of persistent pain
A single intraplantar administration of CFA (0.05 mL, 50 μg; Mycobacterium tuberculosis, Sigma-Aldrich #F5881) in the right hind paw surface was performed. All animals were anesthetized with a mixture of ketamine 10%, 100 mg/kg + xylazine 2%, 10 mg/kg intramuscular for the intraplantar CFA or pyrogen-free sterile saline 0.9% injections.
Nociceptive response
The threshold (in grams) of paw withdrawal response was measured. The animals were placed in the experimental box of the apparatus to acclimate for 2 h before the tests and mechanical innocuous stimuli were applied to the hind paws by a digital anesthesiometer (von Frey model, Insight Instruments, Ribeirão Preto, São Paulo, Brazil). After acclimation, a progressive force (0–80 g with a 15-s limit) was applied to the right and left hind paws using a filament (0.5 mm diameter). A baseline (BL) was obtained from all animals prior to the intraplantar saline or CFA injection, and then re-tested at 3, 7, and 10 days after injection. In each experimental day, the mechanical stimulus was applied three times (3–5 min between each stimulus with no fixed interval) to obtain a mean of the force applied.
Locomotor activity
The Open field test (OFT) was used to evaluate locomotor activity. The same sets of animals previously subjected to the von Frey test were also tested in the OFT 2 h after the end of the von Frey test. This was done to determine whether the paw injections would affect locomotion and confound the emotional responses exhibited in the EPM and DLB. Animals were placed in a polyethylene box (60 cm × 60 cm × 60 cm) with the floor divided into 16 equal squares of 15 cm each. Rats could freely ambulate over a 5-min period and the total distance travelled (in cm) was analyzed using the ANY-MAZE software.
Anxiety-like behaviors
Elevated plus-maze
The elevated plus maze (EPM) apparatus was made of wood, had two open arms and two enclosed arms of the same size (50 cm long each), and was raised 50 cm above the floor. Rats were placed in the center of EPM and allowed 5 min of free exploration. The parameters measured were the percentage of time spent in the open arms and number of entries into open and enclosed arms.
Dark-light box
The dark-light box (DLB) apparatus is a box with two equal compartments (50 cm length × 80 cm width × 60 cm height). The dark and white acrylic compartments are connected by a small opening (15 cm height × 10 cm width), which allows for free exploration by the animal. The animals explored the apparatus for 5 min. The number of crossings between compartments and the percentage of time spent in each compartment was measured.
Sample collection and storage
Independent groups of animals were euthanized through decapitation for blood and brain collection 10 days after CFA or saline 0.9% injection. Trunk blood was collected into heparinized tubes and centrifuged (2500 g, 20 min, 4°C). The plasma was extracted and kept at −20°C for the corticosterone assay. Brains were harvested and rapidly frozen in dry ice and maintained in −80°C for later dissection, protein or DNA extraction, DNMT3a quantification, and global DNA methylation analysis.
Corticosterone assay
Radioimmunoassay (RIA) was performed to determine whether CFA-induced persistent inflammatory pain changes circulating corticosterone, a classical marker for stress. Total plasma corticosterone levels were measured following the manufacturer’s instructions for the Corticosterone Antiserum Developed in rabbit (C8784-100TST, Sigma) with modifications. Plasma corticosterone was extracted with ethanol. For each 25 μL of plasma, 500 μL of 100% ethanol was added, vigorously mixed for 15 s, and centrifuged at 2500 g for 15 min at 4°C. Each supernatant was collected and allowed to dry completely. These samples were resuspended in 0.1% gelatin phosphate buffer (pH 7.4) and were maintained at −20° until the analysis. The RIA was performed by incubating the extracted samples or the unlabeled corticosterone with the antiserum and tritiated hormone {[1,2,6,7 – 3H (N)]-corticosterone, Perkin Elmer} at 4°C for 24 h. The separation of free/bound tritiated corticosterone was promoted by the addition of a suspension of dextran-coated charcoal (0.5% activated charcoal, 0.5% dextran in phosphate buffer, pH 7.4) added to each tube, vortexed for 1 min, incubated at 4°C for 15 min, then centrifuged at 2000 g for 15 min at 4°C. The antiserum-bounded 3H-corticosterone was presented at each supernatant, mixed with the scintillation liquid, and the radioactivity of each tube was measured using the beta Trilux counter (Perkin Elmer). A standard curve was set up with known concentrations of unlabeled corticosterone and it was used to estimate the corticosterone levels in the unknown samples. Data were analyzed by Multicalc software. The lower limit for detection for corticosterone was 0.05 ng/mL. All samples were measured in duplicate in the same assay to avoid inter-assay error.
DNA methylation
Genomic DNA extraction and global DNA methylation levels measurement
The following regions were bilaterally microdissected according to stereotaxic coordinates: the amygdala (Bregma −1.92 mm to −3.12), the ventral hippocampus (Bregma −1.92 mm to −1.44 mm), and the prefrontal cortex (Bregma −4.68 mm to −2.76 mm). Genomic DNA from each sample was extracted and purified using SDS/proteinase K. The tissue was placed in 550 μL lysis buffer (NaCl 0.1 m, Tris 50 mm, EDTA 50 mm, final pH 8), 27.5 μL of 20% SDS, and 20 μL of proteinase K (#P2308, Sigma). The tissue was then incubated up to 2 h at 60°C for total tissue digestion. Following the digestion step, 600 μL of 4 m sodium chloride was added to the lysis buffer followed by 15 s of vortexing and centrifuging (14,000 rpm, room temperature, 30 min). An aliquot (1 mL) of the mixture (lysis buffer + NaCl) containing the digested tissue of each sample was placed in another labeled tube and two equal volumes of 100% ethanol were added and kept in −20°C for up to 18 h. The tubes were then centrifuged (14,000 rpm, 4°C, 30 min) the following day and the supernatant was discarded. The DNA was washed (centrifuging 14,000 rpm 4°C, 5 min) three times with 700 μL of 70% ethanol and left at room temperature to allow complete drying. To re-suspend and dilute the DNA, 50 μL of ultrapure molecular grade water was added to the dried tubes. The DNA concentration was quantified by spectrophotometry (Nanodrop 2000c, Thermo Fisher Scientific). This was followed DNA digestion and global DNA methylation quantification. Briefly, the purified DNA was digested with Nuclease P1 (#P2640, Sigma, 2 U/mg of DNA, 4 h, 65°C in acetate buffer 20 mm pH 5.3), alkaline phosphatase enzymes (#N8630, Sigma, 1 U/mg of DNA, 2h, 65°C in Tris-HCl buffer 20 mm pH 7.5), precipitated with 100% ethanol, and NaCl 5m at −20°C for at least 18 h. The samples were then centrifuged at 15,000 g for 15 min. The resulting pellet was re-suspended in ultrapure water and the global levels of DNA methylation were assessed using a DNA Methylation EIA kit (#589324, Cayman Chemicals). The absorbance was measured by spectrophotometer (SpectraMax 190 plate reader, Version 6.2.1, Molecular Devices). Different concentrations of the purified 5-methyl-2’-deoxycytidine (provided by the kit) were used to generate the standard concentration curve. The concentrations (ng/mL) were computed based on the standard curve equation. Each 5-methyl-2’-deoxycytidine concentration was divided by its respective total DNA and expressed as a percentage of the mean of the control (saline group). All samples were measured in the same assay.
DNMT3a protein quantification
Part of the tissue used for genomic DNA extraction was used for this experiment. Tissue was homogenized in RIPA buffer (Sigma-Aldrich # R0278) containing protease inhibitors (10%, dilution 1:10, Sigma-Aldrich) and 0.5% phenylmethanesulfonyl fluoride (PMSF, Sigma-Aldrich). After cold agitation (∼4°C) for 2 h, the samples were centrifuged (3500 rpm, 20 min, 4°C) to collect the supernatant. Following quantification of total protein, 60 μg of protein of each sample was diluted in a 2x Laemmli buffer (1:1; Sigma-Aldrich, S3401). Proteins were separated by electrophoresis (125 V, 90 min) in 12% polyacrylamide gels. A marker of molecular weight (10 kDa–250 kDa; Prism Ultra Protein Ladder, ABCAM ab116028) was used in one well of each gel for assessment of the subsequent transfer. After electrophoresis, the proteins were transferred to a nitrocellulose membrane (0.45 μm pore size; Amersham) in a tank blotting system (125 V, 90 min) semi-immersed in a transfer buffer containing 20% methanol. The membranes were subsequently blocked with 5% BSA for 60 min in slow agitation in room temperature to block non-specific binding. The membranes were incubated overnight in 4°C in a PBS-T BSA 1% buffer (phosphate buffered saline +1% tween 20 + 1% BSA) containing antibodies against DNMT3a (rabbit, 1:1000, H-295 sc-20,703, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin (mouse, 1:1000, C4 sc-47,778, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were then incubated with PBS-T containing BSA 1% and secondary HRP (horseradish peroxidase)-conjugated antibodies (anti-rabbit, 1:10,000; Abcam, ab6721; and anti-mouse 1:10,000; Merck, 12-349) for 2 h in room temperature. A chemiluminescent kit (SuperSignal Chemiluminescent Substrates, Thermo Fisher Scientific) detected the immunolabeled bands in the membranes, and densitometric analysis was made by using the ImageLab 5.2.1 (BioRad) software. The ratio of band optical density of DNMT3 to β-actin (housekeeping protein) was calculated.
Statistical analysis
The time course of mechanical sensitivity was analyzed using a two-way repeated measures ANOVA followed by Tukey’s post hoc test when appropriate. We considered treatment (CFA or vehicle injection) and time (BL, 3rd ,7th, or 10th days after injection) as factors. For the remaining experiments, all statistical analyses were made using the Shapiro-Wilk test for normality followed by Student t test. Welch’s correction was applied when the assumption of equal variances was not met. Results were considered significant is p < .05. All data are reported as mean ± standard error of the mean (SEM) and were analyzed by GraphPad Prism® for Windows, version 7.0 (GraphPad Software, USA).
Results
Intraplantar CFA injection reduces nociceptive thresholds in rats and increases circulating corticosterone levels in the absence of locomotor deficiency
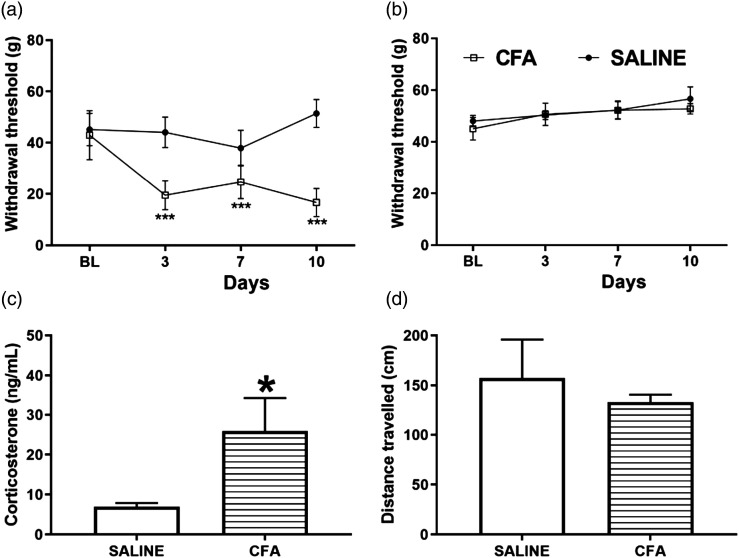
To study the effects of intraplantar CFA injection on the nociceptive response, we tested two groups of animals in the von Frey test before CFA or saline (control) paw injection to establish a baseline; as well as 3,7, and 10 days after the paw injection. Two-way repeated measures ANOVA revealed significant effects of treatment [F (1,14) = 81.95 p < .001], time [F (3,42) = 15.17 p < .001], and treatment × time interaction [F (3,42) = 23.6 p < .001] in the group injected with CFA. Animals that received CFA injection had reduced withdrawal thresholds (in grams) 3, 7, and 10 days after CFA injection in the ipsilateral paw compared to the saline group (two-way repeated measures ANOVA followed by Tukey post-hoc test, p = < .0001; Figure 2(a)). Two-way repeated measures ANOVA did not show evidence of a significant difference in treatment [F (1,14) = 0.4031 p = .5357], time [time F (3,42) = 2.187 p = .1038], or treatment x time interaction [F (3,42) = 0.3551 p = 0.7857] between the groups saline or CFA in the contralateral paw (Figure 2(b)).
Figure 2.
CFA treatment results in reduced mechanical thresholds and increase corticosterone levels in the absence of motor impairment. (a, b): Mechanical thresholds (g) in the von Frey test for the ipsilateral (a) and contralateral (b) hindpaws at baseline (BL) and at 3, 7, and 10 days following the intraplantar administration of 50 μL CFA or 0.9% sterile saline in the right hind paw (Two-way repeated measures ANOVA followed by Tukey’s post-test for multiple comparisons, ***p < .001). (c): Plasma levels of corticosterone assessed by radioimmunoassay at 10 days after CFA or saline administration (Student t test, * p < .05). (d): Total distance travelled in the open field test at 10 days after CFA or saline administration (Student t test). N = 8/group. Results are expressed as mean ± the standard error of the mean (SEM). CFA, Complete Freund’s adjuvant.
We measured circulating total corticosterone levels 10 days after CFA injection in the paw. We found that intraplantar CFA-administered animals showed augmented plasma levels of corticosterone [t (10) = 2.282, p = .0456, Figure 2(c)], compared to the saline control group. To determine if the rats experiencing persistent paw pain would have impaired ambulation, we allowed the animals to freely behave in the OF for 5 min. The OF test is a common tool to assess emotional and locomotor responses in rodents. CFA-injected animals did not differ significantly in the distance of centimeters travelled in the apparatus [t (14) = 0.6209 p = .5446 Figure 2(d)] when compared to the saline control group.
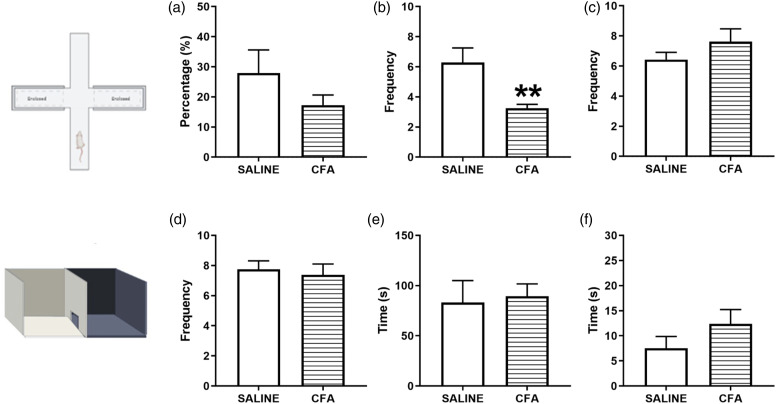
CFA-induced persistent inflammatory pain in rats leads to anxiety-like behaviors
We aimed to investigate whether the established persistent inflammatory pain induced by CFA could promote anxiety-like behaviors in rats. In the EPM test, we quantified the time spent (%) in the open arms and the number of entries into the open and closed arms. CFA-injected rats had a decrease in the number of entries in the open arms [t (13) = 3.228 p = .0066 Figure 3(b)] and there were no differences neither in the entries in the closed arms [t (13) = 1.184 p = .2578 Figure 3(c)] nor time spent (%) in the open arms [t (13) = 1.336 p = .2044 Figure 3(a)] compared to saline group. Decreased frequency of entry and/or duration of time spent in the open arms is an indication of an anxiety-like phenotype. 19 In the DLB, we counted the number of crossings between the dark and white compartments, the time spent in the white compartment, and the time for first latency. We did not observe major differences between CFA and saline groups regarding the number of crossings between compartments [t (14) = 0.4077 p = 0.6897 Figure 3(d)], time spent in the white compartment [t (14) = 0.2473 p = 0.8083 Figure 3(e)), and exhibition of first latency [t (14) = 1.31 p = .2114 Figure 3(f)].
Figure 3.
CFA treatment results in anxiety-like behaviors. (a–c): Rats were tested in the elevated plus maze apparatus 10 days after the intraplantar administration of 50 μL CFA or 0.9% sterile saline in the right hind paw. The following behaviors were measured: (a): Percentage of time spent in the open arms. (b): Frequency of entries in the open arms. (c): Frequency of entries in the closed arms. (d–f): Rats were tested in the dark light box test 10 days after CFA or saline administration. The following behaviors were measured: (d): Number of crossings between the black and white compartments of the apparatus. E: Time spent in the white compartment. (f): Time to exhibit the first latency. N = 7–8/group. Results are expressed as mean ± the standard error of the mean (SEM) and were analyzed by Student t test. **p < .01. Image illustrated using Biorender© software. CFA, Complete Freund’s adjuvant.
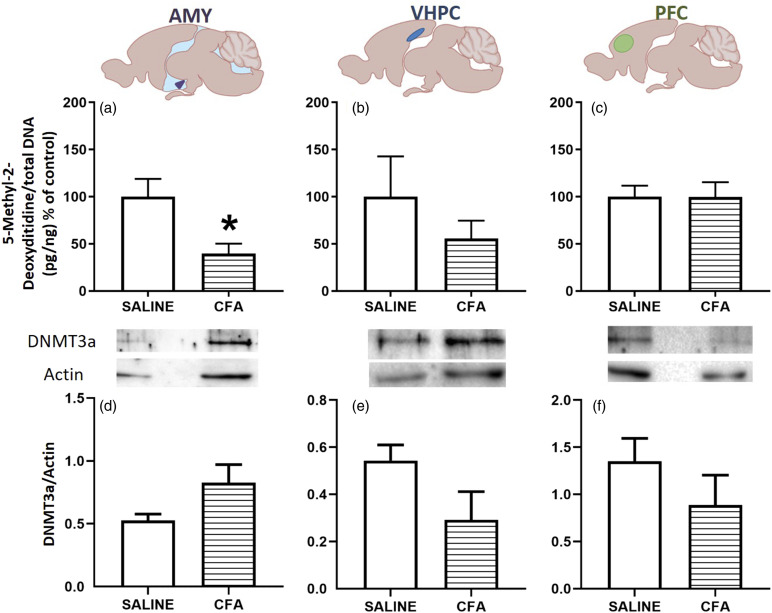
Rats with persistent inflammatory pain show decreased global DNA methylation in the amygdala
After evaluating the behavioral and hormonal effects of persistent pain, we investigated the global DNA methylation levels in the amygdala, ventral hippocampus, and prefrontal cortex. CFA-treated rats showed decreased global DNA methylation in the amygdala when compared to the saline control group [t (10) = 2.777, p = .0195, Figure 4(a)]. There were no significant differences between the CFA and saline control groups in the levels of global DNA methylation in the ventral hippocampus [t (10) = 0.9488, p = .3651, Figure 4(b)] or the prefrontal cortex compared to the saline group [t (10) = 0.0100, p = .9922, Figure 4(c)]. Quantification of the de novo methyltransferase DNMT3a demonstrated no significant differences between the CFA and control group in the amygdala [t (6.3) = 2.01, p = .09, Welch’s correction, Figure 4(d)], ventral hippocampus [t (4) = 1.84, p = 0.14, Figure 4(e)], or prefrontal cortex [t (10) = 1.17, p = .27, Figure 4(f)].
Figure 4.
CFA treatment results in DNA hypomethylation and unchanged DNMT3a levels in the amygdala. (a–c): DNA methylation levels in amygdala (AMY, a), ventral hippocampus (VHPC, b) and prefrontal cortex (PFC, c) 10 days following the intraplantar administration of 50 μL CFA or 0.9% sterile saline in the right hind paw. Each sample result is expressed as 5-methyl-2-deoxycidine/total DNA normalized by the 5-methyl-2-deoxycidine/total DNA of the mean of the control group and expressed as percentage. (d–f): Quantification of DNMT3a levels (relative to actin) in the amygdala (d), ventral hippocampus (e) and prefrontal cortex (f) 10 days after CFA or saline administration. Representative bands can be seen on the upper side. N = 3–6/group. Results are expressed as mean ± the mean standard error (SEM) and were analyzed by Student t test. *p < .05. Image illustrated using Biorender© software. CFA, Complete Freund’s adjuvant.
Discussion
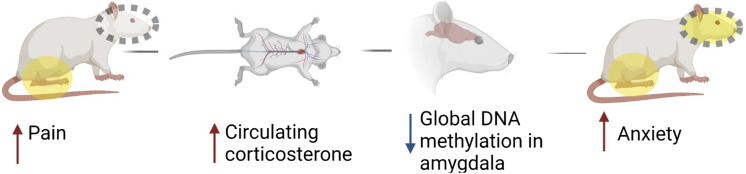
Our data shows that persistent hindpaw inflammation is accompanied by behavioral signs of pain and anxiety, elevated levels of circulating corticosterone, and decreased global DNA methylation in the amygdala (Figure 5).
Figure 5.
Summary of findings. Image illustrated using Biorender© software.
Our behavioral studies supplement the body of prior publications targeting pain and anxiety in preclinical models. For instance, Parent and colleagues showed that hindpaw CFA administration results in a robust anxiety-like response observed in the EPM (reduced percentage of time spent and number of entries in the open arms), OF, and social interaction test 28 days after injection in rats. 18 Similarly, data from our group showed an anxiogenic-like response observed in the EPM 10 days after orofacial CFA injection. 17
There exist conflicting data of how chronic pain and stress hormones (e.g., corticosterone and/or adrenocorticotropic hormone, ACTH) are linked, with reports of increased,20–23 decreased,24–26 or unchanged27–29 blood corticosterone or ACTH levels in subjects with chronic pain. We propose two explanations for such seemingly contradictory findings. First, the discrepancy could be due to the ambiguous nature of the link between pain-related stress and the HPA system. For example, plasma corticosterone or ACTH levels did not change 13 days after mono-neuropathy induction even when the rats were restrained for 120 min compared to sham group. 30 The expression of stress genes (i.e., crf [corticotrophin-releasing factor] and gr [glucocorticoid receptor]) in the central and medial amygdala nuclei in these rats increased, indicating that the chronic pain may change the CRF system independent of the HPA axis. Bomholt and colleagues, 31 using a neuropathic pain model, revealed that rats do not have elevated corticosterone levels at baseline or after physical restriction compared to the sham control, indicating that chronic pain may not directly alter the HPA axis. Second, it is possible that corticosterone levels are less dependent on hyperalgesic state 32 and more dependent on the time course of the painful condition or its specific manifestation. Evidence from human studies shows that the HPA axis may be hyperactive in the early stages of the pain chronicity process and might be hypoactive as the pain persists. 33 For instance, hypercortisolism was shown in regional musculoskeletal pain but not in widespread musculoskeletal pain in women, which makes elevated cortisol an indicator of an intermediate stage in the pain chronicity process. 34 Considering that the CFA animals in our study had elevated blood corticosterone 10 days after the induction of paw inflammation, we speculate that our preclinical model of persistent inflammatory pain could serve to study the hormonal responses to stress and pain chronification processes. A detailed time-course of the effect of the persistent inflammatory pain on the glucocorticoid release and its role in the development of mood-related disorders is therefore needed. It is noteworthy that corticosterone levels alone may not accurately represent HPA axis activation and quantitation of corticosteroid receptor abundance and sensitivity may be required.
Increased levels of stress hormones in the context of chronic pain has been associated with cortical, amygdaloid, and hippocampal plasticity in patients. 35 Similarly, repeated intra-amygdala injections of corticosterone in rats reduced viscerosomatic sensitivity and induced anxiety-like behaviors, an effect that was blocked by CRF receptor 1 antagonist administration. 36 This implies the presence of a plastic molecular mechanism that regulates pain and anxiety in these brain regions. Epigenetic mechanisms, including DNA methylation, have been implicated in pain-related CNS plasticity, both in global12,37 and gene-specific (synaptotagmin 38 and crf 39 ) assays. We observed that CFA-injected animals had reduced global DNA methylation in the amygdala, but not in the prefrontal cortex or the ventral hippocampus. Global hypomethylation in more chronic (6 months) stages of neuropathic pain has been reported in the mouse prefrontal cortex,12,37 thereby emphasizing the sensitivity of these epigenetic modifications both to the time course following injury as well as the type of pain studied. It is noteworthy that while the amygdalar hypomethylation changes in the current study are dramatic, they are often difficult to interpret as they lack cellular context within a complex tissue with high cell heterogeneity. It is unclear whether these changes represent one cell population actively methylating/demethylating protein coding areas of the genome, or an increase of one cell type with a specific methylation profile. As such, further cell specific or single cell platforms will be needed to further interrogate these methylomic landscape changes. No differences in the levels of the de novo methyltransferase, DNMT3a, were observed in any of the regions studied.
We have demonstrated that peripheral CFA administration is associated with the manifestation of both sensory (mechanical sensitization) and affective (stress and anxiety) dimensions of pain and is paralleled by systemic biochemical alterations (increased corticosterone levels) and global DNA hypomethylation in the amygdala. Since only one timepoint was studied, it is not possible to ascertain the precise sequence of these events. However, there is ample evidence of pain leading to amygdalar plasticity and anxiety-like behaviors in preclinical models.40–42 What is less studied is the link between peripheral injuries and brain epigenetic modifications. Previous studies have suggested that epigenetic modifications may predispose individuals to vulnerability to chronic pain and its associated comorbidities 43 while others have noted the involvement of epigenetic mechanisms in the maintenance of pain following the initial pathology or insult. 44 We hypothesize that chronic or persistent pain may lead to DNA methylation changes which, in turn, could initiate (mal)adaptive cellular plasticity underlying comorbidities such as depression and anxiety. A potential candidate gene based on our biochemical data as well as previously published studies is crf. 39 Our future studies will therefore involve crf-specific DNA methylation analysis in our model and its link to the behavioral and hormonal response to persistent inflammatory pain. Furthermore, we will carry out detailed biochemical analyses of DNA methyltransferase localization, abundance, and activity, as well as pharmacological interrogation of DNA methyltransferase function.
Some limitations of the present study should be considered. In this way, our data was collected using male rats only. In the future, it is important to test female subjects since there are well established differences in mechanisms of pain between males and females, 45 including mechanisms of CNS plasticity 46 and epigenetic modifications. 47 While our study does not specify the exact mechanisms by which pain results in global DNA hypomethylation, it is nonetheless an important proof-of-concept finding with potentially high clinical impact and therapeutic relevance. For example, the use of methyl donors (e.g., choline, betaine, folate and vitamin B12 48 ) in the treatment of persistent or chronic pain and its associated behavioral-related disorders could be considered. Our study might therefore guide future treatments for both chronic pain and its sequelae.
Anxiety that is comorbid with chronic pain, in addition to being a clinical problem in itself, can enhance the overall experience of pain and alter the function of commonly-used analgesics, including opiates. 49 It is therefore crucial to study and treat pain in a more integrated manner, considering the entirety of its clinical presentation as well as the underlying peripheral and central mechanisms.
Acknowledgements
We acknowledge Patricia Basile and Flávia Salata for their technical assistance. We thank Dr Sebastian Alvarado for his careful review of the manuscript. We also thank Dr Sâmia Joca for generously providing expert knowledge in addition to laboratory reagents and kits.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES PDSE and Finance Code 001). CRA Leite-Panissi received research fellowship from CNPq (#304215/2016-3 and #309215/2019-1). M Tajerian was funded through the National Institute for Health (1SC2 GM135114-01).
ORCID iDs
Maral Tajerian https://orcid.org/0000-0002-4598-1012
Christie RA Leite-Panissi https://orcid.org/0000-0003-1762-2730
References
- 1.Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, Levinson D, de Girolamo G, Nakane H, Mneimneh Z, Lara C, de Graaf R, Scott KM, Gureje O, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J, Von Korff M. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. Pain 2007; 129: 332–342. DOI: 10.1016/j.pain.2007.01.022 [DOI] [PubMed] [Google Scholar]
- 2.Thompson JM, Neugebauer V. Amygdala plasticity and pain. Pain Res Manag 2017; 2017: 8296501. DOI: 10.1155/2017/8296501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajerian M, Leu D, Zou Y, Sahbaie P, Li W, Khan H, Hsu V, Kingery W, Huang TT, Becerra L, Clark JD. Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology 2014; 121: 852–865. DOI: 10.1097/ALN.0000000000000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy M, Piche M, Chen JI, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci USA 2009; 106: 20900–20905. DOI: 10.1073/pnas.0904706106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci 2008; 1148: 64–73. DOI: 10.1196/annals.1410.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 2015; 517: 284–292. DOI: 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua T, Chen B, Lu D, Sakurai K, Zhao S, Han BX, Kim J, Yin L, Chen Y, Lu J, Wang F. General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nat Neurosci 2020; 23: 854–868. DOI: 10.1038/s41593-020-0632-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, Scherrer G. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 2019; 363: 276–281. DOI: 10.1126/science.aap8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dirven BCJ, Homberg JR, Kozicz T, Henckens M. Epigenetic programming of the neuroendocrine stress response by adult life stress. J Mol Endocrinol 2017; 59: R11–R31. DOI: 10.1530/JME-17-0019 [DOI] [PubMed] [Google Scholar]
- 10.Smrt RD, Zhao X. Epigenetic regulation of neuronal dendrite and dendritic spine development. Front Biol 2010; 5: 304–323. DOI: 10.1007/s11515-010-0650-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hing B, Gardner C, Potash JB. Effects of negative stressors on DNA methylation in the brain: implications for mood and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet 2014; 165B: 541–554. DOI: 10.1002/ajmg.b.32265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajerian M, Alvarado S, Millecamps M, Vachon P, Crosby C, Bushnell MC, Szyf M, Stone LS. Peripheral nerve injury is associated with chronic, reversible changes in global DNA methylation in the mouse prefrontal cortex. PLoS One 2013; 8. e55259. DOI: 10.1371/journal.pone.0055259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denk F, McMahon SB. Chronic pain: emerging evidence for the involvement of epigenetics. Neuron 2012; 73: 435–444. DOI: 10.1016/j.neuron.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tajerian M, Alvarado SG, Clark JD. Differential olfactory bulb methylation and hydroxymethylation are linked to odor location memory bias in injured mice. Mol Pain 2019; 15: 1744806919873475. DOI: 10.1177/1744806919873475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geranton SM, Morenilla-Palao C, Hunt SP. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J Neurosci 2007; 27: 6163–6173. DOI: 10.1523/JNEUROSCI.1306-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geranton SM, Fratto V, Tochiki KK, Hunt SP. Descending serotonergic controls regulate inflammation-induced mechanical sensitivity and methyl-CpG-binding protein 2 phosphorylation in the rat superficial dorsal horn. Mol Pain 2008; 4: 35. DOI: 10.1186/1744-8069-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.do Nascimento GC, Leite-Panissi CR. Time-dependent analysis of nociception and anxiety-like behavior in rats submitted to persistent inflammation of the temporomandibular joint. Physiol Behav 2014; 125: 1–7. DOI: 10.1016/j.physbeh.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 18.Parent AJ, Beaudet N, Beaudry H, Bergeron J, Berube P, Drolet G, Sarret P, Gendron L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res 2012; 229: 160–167. DOI: 10.1016/j.bbr.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007; 2: 322–328. DOI: 10.1038/nprot.2007.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patacchioli FR, Monnazzi P, Simeoni S, De Filippis S, Salvatori E, Coloprisco G, Martelletti P. Salivary cortisol, dehydroepiandrosterone-sulphate (DHEA-S) and testosterone in women with chronic migraine. J Headache Pain 2006; 7: 90–94. DOI: 10.1007/s10194-006-0274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell E, Theorell T, Hasson D, Arnetz B, Saraste H. Stress biomarkers’ associations to pain in the neck, shoulder and back in healthy media workers: 12-month prospective follow-up. Eur Spine J 2008; 17: 393–405. DOI: 10.1007/s00586-007-0554-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sveinsdottir V, Eriksen HR, Ursin H, Hansen AM, Harris A. Cortisol, health, and coping in patients with nonspecific low back pain. Appl Psychophysiol Biofeedback 2016; 41: 9–16. DOI: 10.1007/s10484-015-9300-2 [DOI] [PubMed] [Google Scholar]
- 23.Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tetreault P, Ghantous M, Baria A, Farmer M, Baliki MN, Schnitzer TJ, Apkarian AV. The emotional brain as a predictor and amplifier of chronic pain. J Dent Res 2016; 95: 605–612. DOI: 10.1177/0022034516638027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buryanov A, Kostrub A, Kotiuk V. Endocrine disorders in women with complex regional pain syndrome type I. Eur J Pain 2017; 21: 302–308. DOI: 10.1002/ejp.924 [DOI] [PubMed] [Google Scholar]
- 25.Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Penninx BW, Dekker J. Reduced hypothalamic-pituitary-adrenal axis activity in chronic multi-site musculoskeletal pain: partly masked by depressive and anxiety disorders. BMC Musculoskelet Disord 2014; 15: 227. DOI: 10.1186/1471-2474-15-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahidi B, Sannes T, Laudenslager M, Maluf KS. Cardiovascular responses to an acute psychological stressor are associated with the cortisol awakening response in individuals with chronic neck pain. Physiol Behav 2015; 150: 93–98. DOI: 10.1016/j.physbeh.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meeus M, Van Oosterwijck J, Ickmans K, Baert I, Coppieters I, Roussel N, Struyf F, Pattyn N, Nijs J. Interrelationships between pain processing, cortisol and cognitive performance in chronic whiplash-associated disorders. Clin Rheumatol 2015; 34: 545–553. DOI: 10.1007/s10067-013-2446-5 [DOI] [PubMed] [Google Scholar]
- 28.Nes LS, Carlson CR, Crofford LJ, de Leeuw R, Segerstrom SC. Self-regulatory deficits in fibromyalgia and temporomandibular disorders. Pain 2010; 151: 37–44. DOI: 10.1016/j.pain.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 29.Wingenfeld K, Hellhammer DH, Schmidt I, Wagner D, Meinlschmidt G, Heim C. HPA axis reactivity in chronic pelvic pain: association with depression. J Psychosom Obstet Gynaecol 2009; 30: 282–286. DOI: 10.3109/01674820903254732 [DOI] [PubMed] [Google Scholar]
- 30.Ulrich-Lai YM, Xie W, Meij JT, Dolgas CM, Yu L, Herman JP. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav 2006; 88: 67–76. DOI: 10.1016/j.physbeh.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 31.Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Normal hypothalamo-pituitary-adrenal axis function in a rat model of peripheral neuropathic pain. Brain Res 2005; 1044: 216–226. DOI: 10.1016/j.brainres.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 32.Benedetti M, Merino R, Kusuda R, Ravanelli MI, Cadetti F, dos Santos P, Zanon S, Lucas G. Plasma corticosterone levels in mouse models of pain. Eur J Pain 2012; 16: 803–815. DOI: 10.1002/j.1532-2149.2011.00066.x [DOI] [PubMed] [Google Scholar]
- 33.Nees F, Loffler M, Usai K, Flor H. Hypothalamic-pituitary-adrenal axis feedback sensitivity in different states of back pain. Psychoneuroendocrinology 2019; 101: 60–66. DOI: 10.1016/j.psyneuen.2018.10.026 [DOI] [PubMed] [Google Scholar]
- 34.Riva R, Mork PJ, Westgaard RH, Lundberg U. Comparison of the cortisol awakening response in women with shoulder and neck pain and women with fibromyalgia. Psychoneuroendocrinology 2012; 37: 299–306. DOI: 10.1016/j.psyneuen.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 35.Abdallah CG, Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress 2017; 1: 2470547017704763. DOI: 10.1177/2470547017704763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav Brain Res 2010; 214: 465–469. DOI: 10.1016/j.bbr.2010.05.049 [DOI] [PubMed] [Google Scholar]
- 37.Massart R, Dymov S, Millecamps M, Suderman M, Gregoire S, Koenigs K, Alvarado S, Tajerian M, Stone LS, Szyf M. Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells. Sci Rep 2016; 6: 19615. DOI: 10.1038/srep1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarado S, Tajerian M, Suderman M, Machnes Z, Pierfelice S, Millecamps M, Stone LS, Szyf M. An epigenetic hypothesis for the genomic memory of pain. Front Cell Neurosci 2015; 9: 88. DOI: 10.3389/fncel.2015.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran L, Chaloner A, Sawalha AH, Greenwood Van-Meerveld B. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology 2013; 38: 898–906. DOI: 10.1016/j.psyneuen.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 40.Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 2006; 31: 739–750. DOI: 10.1038/sj.npp.1300858 [DOI] [PubMed] [Google Scholar]
- 41.Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 2007; 3: 13. DOI: 10.1186/1744-8069-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goffer Y, Xu D, Eberle SE, D'Amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci 2013; 33: 19034–19044. DOI: 10.1523/JNEUROSCI.2454-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci 2014; 17: 192–200. DOI: 10.1038/nn.3628 [DOI] [PubMed] [Google Scholar]
- 44.Zhou CH, Zhang MX, Zhou SS, Li H, Gao J, Du L, Yin XX. SIRT1 attenuates neuropathic pain by epigenetic regulation of mGluR1/5 expressions in type 2 diabetic rats. Pain 2017; 158: 130–139. DOI: 10.1097/j.pain.0000000000000739 [DOI] [PubMed] [Google Scholar]
- 45.Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 2020; 21: 353–365. DOI: 10.1038/s41583-020-0310-6 [DOI] [PubMed] [Google Scholar]
- 46.Tajerian M, Sahbaie P, Sun Y, Leu D, Yang HY, Li W, Huang TT, Kingery W, David Clark J. Sex differences in a murine model of complex regional pain syndrome. Neurobiol Learn Mem 2015; 123: 100–109. DOI: 10.1016/j.nlm.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Louwies T, Greenwood-Van Meerveld B. Sex differences in the epigenetic regulation of chronic visceral pain following unpredictable early life stress. Neurogastroenterol Motil 2020; 32: e13751. DOI: 10.1111/nmo.13751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gregoire S, Millecamps M, Naso L, Do Carmo S, Cuello AC, Szyf M, Stone LS. Therapeutic benefits of the methyl donor S-adenosylmethionine on nerve injury-induced mechanical hypersensitivity and cognitive impairment in mice. Pain 2017; 158: 802–810. DOI: 10.1097/j.pain.0000000000000811 [DOI] [PubMed] [Google Scholar]
- 49.Lillywhite A, Woodhams SG, Goncalves SV, Watson DJG, Li L, Burston JJ, Gowler PRW, Canals M, Walsh DA, Hathway GJ, Chapman V. Anxiety enhances pain in a model of osteoarthritis and is associated with altered endogenous opioid function and reduced opioid analgesia. Pain Rep 2021; 6: e956. DOI: 10.1097/PR9.0000000000000956 [DOI] [PMC free article] [PubMed] [Google Scholar]