Abstract
Background
Acute kidney injury (AKI) is a well-recognized complication of coronavirus disease 2019 (COVID-19). The short and long-term outcomes of patients who develop AKI have not been well characterized.
Methods
In this multicenter retrospective cohort study, we describe the clinical characteristics and outcomes of critically ill adults with severe COVID-19 and AKI. Patient-level variables were extracted from the electronic medical record. Using nadir-to-peak serum creatinine, AKI was defined using the KDIGO definition. Multivariable logistic regression analyses examined factors associated with development of moderate-to-severe (stage 2–3) AKI, severe (stage-3) AKI, and the composite of renal replacement therapy (RRT) or in-hospital death.
Results
Among 459 critically ill adults with COVID-19, 371 (80.1%) developed AKI, with 179 (37.9%) developing stage-3 AKI. Male gender, black and Asian/Native American race, lower baseline estimated glomerular filtration rate (eGFR), higher body mass index (BMI), and higher Acute Physiology and Chronic Health Evaluation (APACHE) IV score were more prevalent among patients with severe AKI, as were systemic markers of inflammation. On multivariable analysis, male gender, black and Asian/Native American race, higher APACHE IV score, lower baseline eGFR, and higher BMI (mainly the highest BMI stratum ≥35 kg/m<sup>2</sup>) were independently associated with higher stages of AKI severity. Male gender, lower baseline eGFR, and higher APACHE IV score were also independently associated with the composite of RRT or in-hospital death. Moderate-to-severe AKI and severe AKI were independently associated with in-hospital death, and there was a significant interaction between BMI and moderate-to-severe AKI for the outcome of in-hospital death. Among 83 (18.1%) patients who required RRT, 27 (32.5%) survived, and 12 (44.4%) remained dialysis-dependent at discharge. At 3 and 6 months, 5 (41.7%) and 4 (33.3%) remained dialysis-dependent, respectively.
Conclusions
AKI is common in critically ill adults with COVID-19. Several patient-level risk factors are associated with higher stages of AKI severity. BMI might be an effect modifier of AKI severity for in-hospital death. Among AKI survivors, there is a high rate of short- and long-term dialysis dependence.
Keywords: Critical illness, Body mass index, Coronavirus disease 2019, Acute kidney injury, Severe acute respiratory coronavirus 2
Introduction
Since the first case of severe acute respiratory coronavirus 2 (SARS-CoV-2) and the resulting acute respiratory illness was reported in Wuhan, China [1] in December 2019, coronavirus disease 2019 (COVID-19) has become a worldwide pandemic, with more than 200 million cases reported as of August 1, 2021 [2]. Massachusetts experienced its first case of COVID-19 on February 28, 2020, with the first surge occurring during the months of April and May 2020 [3]. COVID-19 is recognized as a wide clinical spectrum of disease, ranging from an asymptomatic or mild upper respiratory tract viral infection to severe multiple organ failure. Acute kidney injury (AKI) is a common complication, with reports indicating an incidence rate of 20% among hospitalized patients with COVID-19 and greater than 50% among the critically ill [4]. Renal replacement therapy (RRT) is required in one third of patients with COVID-19 and severe AKI [4, 5]. A 3-fold increased risk of death has been reported in patients with COVID-19 and severe AKI [5]. Little is known regarding risk factors for AKI in critically ill patients with COVID-19 and long-term recovery of kidney function among those with AKI who survive hospitalization.
The aim of our study was to describe the clinical characteristics of critically ill patients with severe COVID-19 and AKI, factors associated with the development and severity of AKI, and the composite outcome of RRT or in-hospital death, AKI severity, and in-hospital death, and the long-term outcomes of patients with AKI discharged with an ongoing dialysis requirement.
Methods
Study Design
This was a multicenter, retrospective cohort study of critically ill adults with a confirmed diagnosis of COVID-19 hospitalized in the intensive care units (ICUs) at a large integrated health care system (Steward Health Care, Dallas, TX, USA) during the first wave of the pandemic in the state of Massachusetts. The study was approved by the Institutional Review Board and the requirement for informed consent was waived due to the retrospective nature of the study (IRB No. EX071). The Massachusetts region of the Steward Health Care System operates nine hospitals in eastern Massachusetts with a total of 125 ICU beds and a robust centralized ICU telemedicine program to provide proactive support to bedside clinicians.
Data Source and Study Population
The primary source for data collection was the hospital electronic health record (MEDITECH Inc., Westwood, MA, USA) and the tele-ICU eCare Manager database (Philips North America, Cambridge, MA, USA), which is the ICU telemedicine information management system that collects data on key patient-related indicators. For our study, we identified all adults (age ≥18 years old) with COVID-19, as defined by a positive nucleic acid amplification test (NAAT) for SARS-CoV-2 from a nasopharyngeal swab, hospitalized in the ICU between March 01, 2020 and July 31, 2020. Since our focus was on patients with AKI, we excluded patients with end-stage kidney failure receiving dialysis at the time of hospitalization.
Data Collection and Definitions of Variables
The data collection for the cohort included demographic and clinical variables (age, gender, race/ethnicity, and body mass index [BMI]) and coexisting conditions (hypertension, diabetes mellitus, coronary artery disease, cerebrovascular disease, heart failure, chronic lung disease [asthma and chronic obstructive pulmonary disease], and obstructive sleep apnea). The Acute Physiology and Chronic Health Evaluation (APACHE) IV score was generated from the tele-ICU eCare Manager database and recorded at the time of ICU admission. Kidney-related laboratory variables of interest included serum creatinine (baseline [up to 12 months prior to hospitalization], at ICU admission, nadir, peak and discharge value) and the urinalysis at the time of AKI diagnosis or nephrology consultation, with a focus on the presence of blood and protein on the dipstick. Additional selected laboratory variables obtained at the time of admission to the ICU included systemic markers of inflammation or tissue injury (procalcitonin, C-reactive protein [CRP], lactate dehydrogenase [LDH], and ferritin), liver function tests (serum aspartate aminotransferase [AST], alanine transaminase, and total bilirubin), and measures of coagulation and fibrinolysis (prothrombin time [PT]/international normalized ratio, partial thromboplastin time, fibrinogen, and D-dimer).
The baseline estimated glomerular filtration rate (eGFR) was calculated using the CKD-Epi equation [6]. Chronic kidney disease (CKD) was defined based on a baseline or nadir eGFR of less than 60 mL/min/1.73 m2. Obesity was defined as BMI ≥30 kg/m2 and morbid obesity as BMI ≥40 kg/m2.
The definition and staging of AKI were in accordance with the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guideline for AKI [7]. In brief, AKI was defined by an absolute increase of 0.3 mg/dL or greater or a relative 1.5-fold increase or greater in the baseline (or nadir) to peak serum creatinine change during the index hospitalization. The following stages of AKI were assessed: stage 1 (increase in serum creatinine by ≥0.3 mg/dL or 1.5- to 2-fold increase in serum creatinine); stage 2 (>2- to 3-fold increase in serum creatinine); and stage 3 (>3-fold increase in serum creatinine, or serum creatinine ≥4.0 mg/dL with an acute increase of >0.5 mg/dL, or dialysis requirement). Dialysis-requirement was defined as worsening AKI necessitating initiation of RRT (intermittent hemodialysis or continuous veno-venous hemodialysis).
Outcomes of Interest
The main outcomes of interest were moderate-to-severe AKI as defined by stage 2 or 3 AKI, and severe AKI was defined as stage 3 AKI or dialysis requirement. Additional outcomes of interest included the composite outcome of dialysis requirement or in-hospital death, in-hospital death, and among patients discharged alive with ongoing dialysis requirement, dialysis independence at 3- and 6-month post-discharge.
Statistical Analyses
Continuous variables are summarized as mean (standard deviation) or median (25th, 75th percentiles) and categorical variables as frequency counts (percentages). We compared baseline continuous variables between AKI stages using analysis of variance (ANOVA) or the Kruskal-Wallis one-way ANOVA, and χ2 test for categorical variables and reported trend p values. We repeated the same analyses using BMI categories as strata.
Multivariable logistic regression analyses were performed to examine factors that were associated with the development of moderate-to-severe AKI, severe AKI, and the composite outcome of dialysis requirement or in-hospital death. The main predictor variables of interest were age, sex, race, baseline eGFR, BMI, and APACHE IV score.
Multivariable logistic regression analyses were also performed to examine the association of moderate-to-severe AKI and severe AKI with in-hospital death after adjustment for age, sex, race, baseline eGFR, BMI, and APACHE IV score. We also tested for an interaction between BMI and AKI severity for the outcome of in-hospital death. We hypothesized that BMI is an effective modifier of AKI severity for mortality. The results of the logistic regression analyses are displayed as odds ratios with a 95% confidence interval. Among patients with dialysis-requiring AKI who survived the index hospitalization, a description of their clinical outcomes was conducted at 3 months and 6 months.
The proportion of missing data for all covariates is shown in online supplementary Table S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000524657). To minimize the loss of power when fitting multivariable models and assuming data were missing at random, we used multiple imputation with chained equations to create 20 multiple complete datasets. The imputation model included all covariates in Table 1, including the outcomes of AKI stages and in-hospital death.
Table 1.
Clinical characteristics of critically ill patients with COVID-19 stratified by AKI and its stages of severity
| Total (n = 459) | No AKI 88 (19.2%) | Stage-1 AKI 103 (22.4%) | Stage-2 AKI 94 (20.5%) | Stage-3 AKI 174 (37.9%) | Trend p value | |
|---|---|---|---|---|---|---|
| Age, years | 66.8 (14.6) | 64.6 (14.8) | 68.4 (15.1) | 67.0 (17.0) | 67.0 (12.6) | 0.6673 |
| Male gender | 293 (63.8) | 43 (48.9) | 64 (62.1) | 59 (62.8) | 127 (73.0) | 0.0002 |
| Race | ||||||
| White | 235 (52.1) | 59 (67.1) | 56 (54.9) | 47 (51.1) | 73 (43.2) | |
| Black/AA | 115 (25.5) | 9 (10.2) | 31 (30.4) | 21 (22.8) | 54 (32.0) | 0.0007 |
| Hispanic | 64 (14.2) | 16 (18.2) | 9 (8.8) | 16 (17.4) | 23 (13.6) | |
| Asian/Native American | 37 (8.2) | 4 (4.6) | 6 (5.9) | 8 (8.7) | 19 (11.2) | |
| Continuous BMI, kg/m2 | 30.3 (7.5) | 30.1 (7.1) | 29.2 (7.7) | 30.3 (7.4) | 31.0 (7.5) | 0.0871 |
| Categorical BMI, kg/m2 | ||||||
| <25 | 115 (25.1) | 27 (30.7) | 34 (33.0) | 20 (21.5) | 34 (19.5) | |
| 25 to <30 | 132 (28.8) | 23 (26.1) | 24 (23.3) | 30 (32.3) | 55 (31.6) | 0.0443 |
| 30 to <35 | 110 (24.0) | 21 (23.9) | 27 (26.2) | 21 (22.6) | 41 (23.6) | |
| ≥35 | 101 (22.1) | 17 (19.3) | 18 (17.5) | 22 (23.7) | 44 (25.3) | |
| APACHE IV score | 74.3 (28.0) | 60.4 (23.6) | 69.2 (25.7) | 73.7 (28.9) | 84.6 (27.1) | <0.0001 |
| Coexisting conditions | ||||||
| Hypertension | 296 (64.5) | 53 (60.2) | 68 (66.0) | 55 (58.5) | 120 (69.0) | 0.244 |
| Hyperlipidemia | 193 (42.1) | 40 (45.5) | 47 (45.6) | 41 (43.6) | 65 (37.4) | 0.1415 |
| Coronary artery disease | 56 (12.2) | 13 (14.8) | 14 (13.6) | 7 (7.5) | 22 (12.6) | 0.5192 |
| Heart failure | 61 (13.3) | 14 (15.9) | 11 (10.7) | 12 (12.8) | 24 (13.8) | 0.9003 |
| Atrial fibrillation | 58 (12.6) | 10 (11.4) | 15 (14.6) | 12 (12.8) | 21 (12.1) | 0.9286 |
| Type-2 diabetes mellitus | 181 (39.4) | 36 (40.9) | 39 (37.9) | 34 (36.2) | 72 (41.4) | 0.8414 |
| COPD | 56 (12.2) | 15 (17.1) | 18 (17.5) | 9 (9.6) | 14 (8.1) | 0.0085 |
| Asthma | 51 (11.1) | 17 (19.3) | 8 (7.8) | 8 (8.5) | 18 (10.3) | 0.1106 |
| Obstructive sleep apnea | 26 (5.7) | 8 (9.1) | 6 (5.8) | 1 (1.1) | 11 (6.3) | 0.3742 |
| Stroke | 48 (10.5) | 5 (5.7) | 14 (13.6) | 8 (8.5) | 21 (12.1) | 0.289 |
| DVT/PE | 32 (7.0) | 8 (9.1) | 6 (5.8) | 7 (7.5) | 11 (6.3) | 0.5569 |
| Dementia | 63 (13.7) | 12 (13.6) | 12 (11.7) | 16 (17.0) | 23 (13.2) | 0.8675 |
| Chronic kidney disease | 120 (26.6) | 14 (15.9) | 27 (26.5) | 19 (20.7) | 60 (35.5) | 0.002 |
| Kidney related variables | ||||||
| Baseline serum creatinine, mg/dL | 1.02 (0.71) | 0.86 (0.41) | 0.94 (0.35) | 0.81 (0.31) | 1.27 (1.01) | <0.0001 |
| Peak serum creatinine, mg/dL | 2.93 (2.41) | 0.98 (0.42) | 1.50 (0.54) | 1.95 (0.80) | 5.30 (2.33) | <0.0001 |
| Baseline eGFR, mL/min/1.73 m2 | 81.4 (29.9) | 87.0 (26.2) | 82.5 (28.1) | 90.1 (28.8) | 73.2 (31.4) | 0.0004 |
| Urine dipstick blood | ||||||
| Negative | 152 (40.1) | 34 (55.7) | 38 (45.8) | 36 (43.9) | 44 (28.8) | |
| 1 + | 85 (22.4) | 15 (24.6) | 16 (19.3) | 17 (20.7) | 37 (24.2) | 0.0005 |
| 2 + | 90 (23.8) | 10 (16.4) | 17 (20.5) | 17 (20.7) | 46 (30.1) | |
| 3 + | 52 (13.7) | 2 (3.3) | 12 (14.5) | 12 (14.6) | 26 (17.0) | |
| Urine dipstick protein | ||||||
| Negative | 98 (25.7) | 24 (39.3) | 25 (30.5) | 30 (36.6) | 19 (12.2) | |
| Trace | 10 (2.6) | 2 (3.3) | 4 (4.9) | 2 (2.4) | 2 (1.3) | |
| 1 + | 113 (29.7) | 20 (32.8) | 17 (20.7) | 24 (29.3) | 52 (33.3) | <0.0001 |
| 2 + | 147 (38.6) | 14 (23.0) | 34 (41.5) | 26 (31.7) | 73 (46.8) | |
| 3 + | 13 (3.4) | 1 (1.6) | 2 (2.4) | 0 (0.0) | 10 (6.4) | |
| Selected laboratory variables | ||||||
| Procalcitonin, ng/mL | 0.28 (0.12, 0.67) | 0.19 (0.10, 0.37) | 0.21 (0.11, 0.47) | 0.31 (0.13, 0.68) | 0.41 (0.18, 0.98) | <0.0001 |
| CRP, mg/dL | 13.9 (7.4, 22.9) | 11.6 (6.2, 20.7) | 10.7 (6.9, 20.0) | 12.4 (7.4, 21.5) | 17.2 (8.3, 24.8) | 0.0063 |
| LDH, U/L | 430 (307, 598) | 375 (295, 477) | 420 (274, 563) | 395 (2,840, 576) | 506 (355, 651) | <0.0001 |
| Ferritin, ng/mL | 764 (338, 1,431) | 558 (185, 1,237) | 660 (313, 1,306) | 701 (281, 1,772) | 935 (488, 1,480) | 0.0039 |
| D-dimer, ng/mL | 1.9 (1.0, 3.6) | 1.5 (0.8, 3.5) | 2.0 (1.0, 5.6) | 1.7 (1.0, 3.2) | 2.2 (1.1, 4.0) | 0.1054 |
| PT, sec | 15.4 (4.9) | 14.7 (3.4) | 15.1 (3.0) | 15.9 (5.6) | 15.7 (5.8) | 0.1703 |
| INR | 1.3 (0.6) | 1.2 (0.4) | 1.2 (0.3) | 1.3 (0.7) | 1.3 (0.7) | 0.3991 |
| aPTT, sec | 36.0 (31.1, 42.2) | 33.9 (29.7, 39.2) | 35.6 (30.4, 42.2) | 35.8 (31.5, 43.5) | 37.6 (32.1, 43.3) | 0.0109 |
| Fibrinogen, mg/dL | 681.9 (222.3) | 621.0 (197.2) | 657.9 (202.0) | 648.3 (236.9) | 736.7 (224.0) | <0.0001 |
| Total bilirubin, mg/dL | 0.5 (0.3, 0.7) | 0.5 (0.3, 0.6) | 0.4 (0.3, 0.7) | 0.5 (0.3, 0.7) | 0.5 (0.3, 0.8) | 0.0488 |
| AST, IU/L | 45 (30, 74) | 34 (24, 56) | 39 (30, 67) | 43 (29, 81) | 56 (37, 84) | <0.0001 |
| ALT, IU/L | 29 (17, 54) | 26 (16, 48) | 25 (15, 54) | 26 (15, 56) | 33 (20, 53) | 0.0095 |
| Alkaline phosphatase, IU/L | 77 (60, 103) | 81 (64, 108) | 80 (61, 98) | 70 (59, 93) | 77 (58, 105) | 0.5289 |
Data summarized as mean (SD) or median (25th, 75th percentiles) for continuous variables or n (%) for categorical variables. BMI, body mass index; APACHE, acute physiology and chronic health evaluation; COPD, chronic obstructive pulmonary disease; DVT/PE, deep vein thrombosis/pulmonary embolism; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; PT, prothrombin time; INR, international normalized ratio; aPTT, activated partial thromboplastin time; AST, aspartate transaminase; ALT, and alanine transaminase.
All analyses were performed using SAS Enterprise Guide (Version 7.14, Cary, NC, USA) and R language (version 4.1.0, R Foundation for Statistical Computing, Vienna, Austria). Differences were considered statistically significant at a p value of less than 0.05.
Results
Clinical Characteristics of Critically Ill Patients with COVID-19 by Stages of AKI
After excluding 20 patients with ESRD on maintenance dialysis, the final analytical cohort included 459 critically ill patients with COVID-19. Table 1 displays the characteristics and outcomes of the cohort stratified by AKI and its stages of severity. In brief, 371 (80.1%) patients developed AKI, with 179 (37.9%) developing stage-3 AKI. Male gender, non-white race, highest strata of BMI (>35 kg/m2), COPD, higher APACHE IV score, and lower baseline eGFR were represented to a greater extent among patients with higher stages of AKI severity. Similarly, procalcitonin, ferritin, CRP, fibrinogen, AST, alanine transaminase, and LDH levels were higher among patients with moderate and severe (stages 2 and 3, respectively) AKI.
Factors Associated with Development of Moderate-to-Severe (Stage 2/3), Severe (Stage-3) AKI, and the Composite of RRT or In-Hospital Death in Critically Ill Patients with COVID-19
The results of the logistic regression analyses examining the factors associated with development of moderate-to-severe AKI (stages 2 and 3), severe AKI (stage 3), and the composite outcome of RRT or in-hospital death are displayed in Tables 2, 3 and 4, with a focus on age, sex, race, baseline eGFR, BMI, and APACHE IV score.
Table 2.
Association of age, sex, race, eGFR, BMI, and APACHE IV score with moderate-to-severe (stage 2 and 3) AKI
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Age (per 10-year ↑) | 1.02 (0.90, 1.16) | 0.97 (0.81, 1.16) |
| Baseline eGFR (per 5 mL/min/1.73 m2 ↑) | 0.97 (0.94, 1.00) | 0.99 (0.95, 1.03) |
| Body mass index (vs. < 25 kg/m2) | ||
| ≥35 kg/m2 | 2.28 (1.31, 3.99) | 3.46 (1.81, 6.60) |
| 30 to <35 kg/m2 | 1.39 (0.83, 2.34) | 1.89 (1.06, 3.38) |
| 25 to <30 kg/m2 | 2.06 (1.23, 3.43) | 2.31 (1.32, 4.05) |
| Male gender (vs. female gender) | 1.79 (1.20, 2.63) | 1.92 (1.27, 2.94) |
| Race (vs. white) | ||
| Black | 1.84 (1.16, 2.91) | 1.79 (1.08, 2.96) |
| Hispanic | 1.52 (0.86, 2.66) | 1.56 (0.83, 2.92) |
| Asian/Native American | 2.62 (1.22, 5.66) | 3.10 (1.37, 7.03) |
| APACHE IV score (per 20-point ↑) | 1.49 (1.28, 1.75) | 1.54 (1.28, 1.84) |
N = 459; Events = 268; models are based on multiple imputation with chained equations to create 20 multiple complete datasets. eGFR, estimated glomerular filtration rate; BMI, body mass index; APACHE, acute physiology and chronic health evaluation; OR, odds ratio; CI, confidence interval.
Table 3.
Association of age, sex, race, eGFR, and BMI with severe (stage 3) AKI
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Age (per 10-year ↑) | 1.01 (0.89, 1.15) | 0.80 (0.66, 0.96) |
| Baseline eGFR (per 5 mL/min/1.73 m2 ↑) | 0.92 (0.89, 0.96) | 0.91 (0.87, 0.95) |
| Body mass index (vs. < 25 kg/m2) | ||
| ≥35 kg/m2 | 1.95 (1.11, 3.41) | 2.23 (1.14, 4.38) |
| 30 to <35 kg/m2 | 1.31 (0.76, 2.28) | 1.57 (0.84, 2.95) |
| 25 to <30 kg/m2 | 1.66 (0.98, 2.82) | 1.71 (0.95, 3.09) |
| Male gender (vs. female gender) | 1.92 (1.28, 2.94) | 2.17 (1.37, 3.45) |
| Race (vs. white) | ||
| Black | 2.02 (1.28, 3.18) | 2.22 (1.33, 3.72) |
| Hispanic | 1.25 (0.70, 2.24) | 1.21 (0.63, 2.34) |
| Asian/Native American | 2.41 (1.20, 4.86) | 3.05 (1.41, 6.60) |
| APACHE IV score (per 20-point ↑) | 1.52 (1.30, 1.77) | 1.48 (1.24, 1.76) |
N = 459; Events = 174; models are based on multiple imputation with chained equations to create 20 multiple complete datasets. eGFR, estimated glomerular filtration rate; BMI, body mass index; APACHE, acute physiology and chronic health evaluation; OR, odds ratio; CI, confidence interval.
Table 4.
Association of age, sex, race, eGFR and BMI with renal replacement therapy or in-hospital death
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Age (per 10-year ↑) | 1.41 (1.23, 1.62) | 1.01 (0.82, 1.23) |
| Baseline eGFR (per 5 mL/min/1.73 m2 ↑) | 0.85 (0.81, 0.88) | 0.86 (0.82, 0.91) |
| Body mass index (vs. < 25 kg/m2) | ||
| ≥35 kg/m2 | 1.02 (0.59, 1.76) | 1.01 (0.50, 2.03) |
| 30 to <35 kg/m2 | 1.06 (0.63, 1.80) | 1.43 (0.75, 2.74) |
| 25 to <30 kg/m2 | 0.79 (0.48, 1.31) | 0.70 (0.38, 1.28) |
| Male gender (vs. female gender) | 1.61 (1.09, 2.33) | 2.17 (1.33, 3.45) |
| Race (vs. white) | ||
| Black | 0.97 (0.62, 1.53) | 1.22 (0.70, 2.12) |
| Hispanic | 0.90 (0.51, 1.56) | 1.26 (0.62, 2.56) |
| Asian/Native American | 0.80 (0.40, 1.60) | 0.78 (0.33, 1.83) |
| APACHE IV score (per 20-point ↑) | 2.03 (1.67, 2.46) | 1.78 (1.45, 2.18) |
N = 459; Events = 258; models are based on multiple imputation with chained equations to create 20 multiple complete datasets. eGFR, estimated glomerular filtration rate; BMI, body mass index; APACHE, acute physiology and chronic health evaluation; OR, odds ratio; CI, confidence interval.
In brief, on univariate analysis, male gender, black and Asian/Native American race, lower baseline eGFR, higher BMI (mainly the highest stratum of ≥35 kg/m2), and higher APACHE IV score were associated with moderate-to-severe or severe AKI and the composite outcome of RRT or in-hospital death (Tables 2, 3, 4). In addition, older age and several comorbidities, including hypertension, coronary artery disease, chronic kidney disease, CVA, and dementia, were associated with RRT or in-hospital death (Table 4 and online suppl. Table S2). Several laboratory variables, including procalcitonin, CRP, LDH, serum ferritin, activated partial thromboplastin time, fibrinogen, and AST were associated with moderate-to-severe or severe AKI and the composite outcome of RRT or in-hospital death (online suppl. Table S2).
On multivariable analysis, male gender, black and Asian/Native-American race, higher BMI, higher APACHE IV score, and lower baseline eGFR were associated with severity of AKI. The highest BMI stratum (≥35 kg/m2) conferred an adjusted odds ratio of 3.46 (95% CI: 1.81, 6.60) and 2.23 (95% CI: 1.14, 4.38) for stage 2/3 AKI and stage 3 AKI, respectively, relative to the BMI stratum <25 kg/m2. Male gender, lower baseline eGFR, and higher APACHE IV score were the only variables that were independently associated with the composite of RRT or in-hospital death.
Association of AKI Severity and In-Hospital Death in Critically Ill Patients with COVID-19
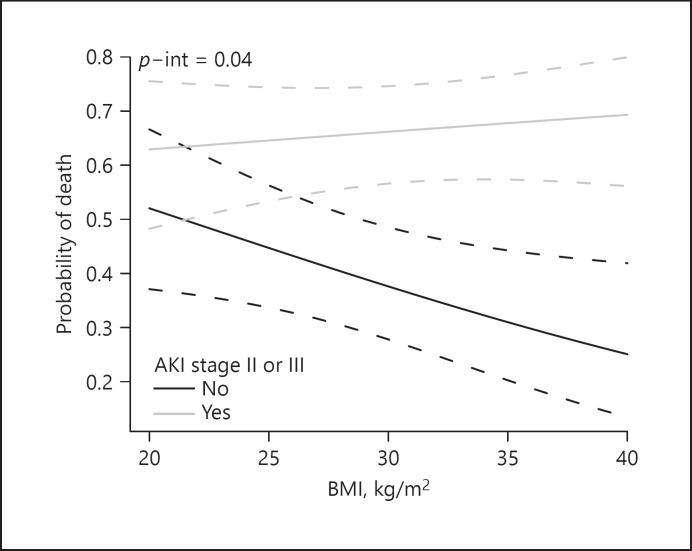
We next examined the association of AKI severity with in-hospital death. After adjustment for age, sex, race, baseline eGFR, BMI, and APACHE IV score, moderate-to-severe AKI and severe AKI were independently associated with in-hospital death, with an adjusted odds ratio of 3.18 (95% CI: 2.00, 5.06) and 3.68 (95% CI: 2.27, 5.97), respectively. We also found a significant interaction between moderate-to-severe AKI and BMI for the outcome of in-hospital death, whereby the independent association of moderate-to-severe AKI with probability of in-hospital death increased among patients with higher BMI (p = 0.04; Fig. 1), the interaction with severe AKI did not reach significance (p = 0.05).
Fig. 1.
Interaction between BMI and moderate-to-severe (stage 2 and 3) AKI for the outcome of in-hospital death (p = 0.04 for interaction).
Long-Term Outcomes of Dialysis-Dependent Critically Ill Patients with COVID-19 and AKI
Among the 83 (18.1%) critically patients with COVID-19 and AKI who required RRT, 27 (32.5%) survived, and 12 (44.4%) remained dialysis-dependent at time of hospital discharge. During the 6-months follow-up period, 1 (8.3%) of the 12-patients died. At 3 months and 6 months, 5 (45.5%) and 4 (36.4%) of the 11 patients remained dialysis dependent, respectively.
Discussion
In this retrospective cohort study, we provide a detailed description of 459 critically ill patients presenting with COVID-19 requiring ICU level of care, with 80.1% developing AKI and 18% requiring RRT. Similar results were seen in a retrospective study performed in an ICU of a large center in the UK where 76.4% of critically ill patients with COVID-19 developed AKI and 31.9% required RRT [8]. Male gender, black and Asian/Native American race, higher BMI, lower baseline eGFR, and higher APAHCE IV score were associated with severity of AKI, as well as systemic markers of inflammation and tissue injury including higher CRP, fibrinogen, ferritin, LDH, and AST.
The cause of AKI in patients with SARS-CoV-2 is multifactorial and likely involves inflammatory and immune responses, endothelial injury, along with activation of coagulation pathways and the renin-angiotensin system [9]. The hypothesis of direct SARS-CoV-2 infection with renal tropism of the virus has been proposed but remains controversial. Indeed, early in the pandemic, in a study on the quantification of SARS-COV-2 viral load in autopsy tissue samples from 22 patients with COVID-19, 17 (77%) showed tropism for the kidneys [10]. Organotropism of the virus beyond the lungs involving the kidneys may be facilitated by angiotensin converting enzyme-2 receptors expressed on both the proximal tubular epithelial cells and podocytes. However, two additional postmortem kidney autopsy studies of 54 patients with COVID-19 and AKI revealed no histological evidence of direct viral infection on histology or through in situ hybridization [11, 12]. While the variable detection of SARS-CoV-2 RNA and live virus in the kidneys may support the hypothesis of organotropism of the virus beyond the lungs, conditions more common in critically ill patients, including hypoxia, hemodynamic instability, use of nephrotoxic agents, and sepsis likely account for how SARS-CoV-2 infection indirectly causes AKI.
Acute tubular injury is the most common histologic diagnosis found on postmortem kidney biopsies from patients with COVID-19 and AKI, which might suggest reversibility of the injury [12, 13]. In our study, of the 83 (18.1%) patients with severe AKI who required RRT, 27 (32.5%) survived, and 12 (44.4%) remained dialysis-dependent at discharge. At 3 months and 6 months, 45.5% and 36.4% of these patients remained dialysis-dependent, respectively. Our findings are consistent with a previous large multicenter cohort study of critically ill adults with COVID-19, where among those who survived hospitalization, 34% were still RRT-dependent at discharge, and 18% remained RRT dependent 60 days after ICU admission [14]. Similarly, in a large cohort of 5,216 hospitalized US veterans with COVID-19, AKI developed in 1,655 (32%) patients with 201 (12%) of these patients requiring RRT; 47% of those who developed AKI did not recover to baseline serum creatinine by discharge and 12 patients (20%) continued RRT post discharge [15]. Pulmonary fibrosis post-COVID-19 has been reported [16], raising concerns of the likelihood of COVID-19 associated kidney fibrosis after AKI recovery and the potential for progression to CKD. Further studies are required to determine the long-term outcome in these patients.
In our cohort, we observed an association between high BMI and severity of AKI as well as a significant interaction between BMI and moderate-to-severe AKI for the outcome of in-hospital death highlighting the potential role of obesity as an effect modifier. Although the association of obesity with CKD has been well described [17], there is paucity of data on its association with AKI and its severity in critically ill patients. The role of obesity and its influence on mortality in the general population has been well established. However, over the past several years, a growing body of literature has also highlighted the paradox where overweight and mild to moderately obese individuals appear to have a better prognosis than their lean counterparts with respect to certain chronic diseases such as cardiovascular disease [18], end-stage kidney disease [19], and respiratory diseases, including COPD [20] and pulmonary embolism [21]. Nonetheless, despite this obesity paradox, multiple recent studies have shown a striking association between BMI and risk for death among patients with COVID-19. In one study, obese individuals were twice as likely to be hospitalized for COVID-19 compared to those who were non-obese and had a 48% higher risk for mortality [22]. A meta-analysis of 61 studies recently demonstrated that obesity (defined as BMI >30 kg/m2) was associated with an increased risk of severe disease and death from COVID-19 [23, 24]. It is well known that patients with high BMI are at greater risk of respiratory dysfunction, thus making them more prone to severe complications of COVID-19 and resultant high mortality [25]. Obesity also places patients with COVID-19 at a higher risk of acute respiratory distress syndrome [26]. The increased risk of disease severity has been attributed to higher rates of metabolic and cardiovascular complications [27] as well as a heightened systemic inflammatory response syndrome induced by numerous host inflammatory mediators, including interleukin-1, interleukin-6, interleukin-17, and tumor necrosis factor alpha [28]. The heightened inflammatory response observed in obesity is explained in part by alterations in adipokines, including an increase in leptin and a decrease in adiponectin [29]. Leptin, a proinflammatory adipokine, predominantly produced by adipose tissue, promotes the production of proinflammatory cytokines interleukin-1, interleukin-6, and tumor necrosis factor alpha [30]. As a result, persons with a higher BMI and excess ectopic fat and adipose tissue have the potential to experience an excessive COVID-19-induced cytokine release syndrome, contributing to higher morbidity. In addition, the physical attributes of an obese person with COVID-19 that might play a role in disease severity include increased waist circumference and body mass resulting in diminished forced vital capacity and diaphragm contractility, making it more difficult to implement supportive therapies such as intubation and prone positioning [31].
Male gender, black and Asian/Native American race, and higher APACHE IV score were associated with development of severe AKI. Black race has previously been shown to be associated with a higher risk of AKI, including severe AKI [32]. In our study, male gender was associated with a higher risk of severe AKI. This observation is consistent with other studies that found an association between male gender and higher risk of AKI [32]. Moreover, higher APACHE IV score, a measure of severity of illness, was associated with AKI severity, likely reflecting hemodynamic instability, hypotension, and sepsis, resulting in organ dysfunction, including higher stages of AKI.
Greater elevations of systemic markers of inflammation and tissue injury, including procalcitonin, CRP, ferritin, fibrinogen, AST, and LDH, were also associated with development of severe AKI. Unlike other studies, we did not find a significant association between hypertension and diabetes and severe AKI.
Our study has several strengths. We analyzed a large, diverse, critically ill adult population with severe COVID-19 and an array of comorbid conditions admitted to an integrated health care system with multiple acute care facilities, including several community hospitals and a tertiary care center. We used a robust ICU dataset to reliably extract the variables of interest, a well-validated consensus definition of AKI, and a composite endpoint to account for competing risks. There are several important limitations to consider. The study involved critically ill patients with COVID-19 and excluded hospitalized patients who did not require ICU-level of care. This explains the very high rate of AKI in our cohort of critically ill patients. Our findings may not be generalizable to other health care systems or regions within the USA during the surge of COVID-19. Furthermore, our definition of AKI may have underestimated severity of AKI as not all patients had a baseline serum creatinine level, which may have been lower than the nadir value observed during the hospitalization, thus resulting in smaller nadir-to-peak serum creatinine differences. As with all cohort studies, other residual known and unknown confounders that were not accounted for in our analyses may have also affected our effect estimates. Finally, our study was conducted during the first wave of the pandemic, and the evolving therapies for COVID-19 may have affected disease severity and rates of AKI. Indeed, it was not until early 2021 that the RECOVERY trial demonstrated that in patients hospitalized with COVID-19, the use of dexamethasone results in lower 28-day mortality among those receiving any respiratory support, and the number patients requiring RRT was lower in the dexamethasone group compared to the usual care group [33].
In conclusion, we observed a high rate of AKI and RRT requirement in our cohort study of critically ill adults with COVID-19 requiring ICU-level of care. Several patient characteristics, including male gender, black/Asian/Native American race, higher BMI, higher APACHE IV score, lower baseline eGFR, and higher systemic markers of inflammation and tissue injury (including procalcitonin, CRP, ferritin, fibrinogen, AST and LDH) were associated with development of AKI. BMI was independently associated with severity of AKI and among patients with moderate-and-severe AKI, was an effect modifier for in-hospital mortality. Among AKI survivors, we also found higher rates of dialysis-dependence at hospital discharge and at 6-months of follow-up. Future studies are needed to validate our findings and further investigate the relationship between BMI and risk of COVID-19-associated AKI and mortality, and the long-term risk of CKD.
Statement of Ethics
The study was approved by the Institutional Review Board (No. IRB#00797) and requirement for informed consent was waived due to retrospective nature of the study.
Conflict of Interest Statement
The authors declare that they have no relevant financial interests pertaining to this study.
Funding Sources
The authors report no external funding source for this study. This project was supported in part by the National Center for Research Resources Grant No. UL1 RR025752 and the National Center for Advancing Translational Sciences, National Institutes of Health, Grant No. UL1 TR000073 and UL1 TR001064. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Author Contributions
B.L. Jaber and V.S. Balakrishnan contributed to the development of the concept and design of the study; A.H. Moraco, J. Dewald, C. El Mouhayyar; H. Tighiouart, B.L. Jaber, V.S. Balakrishnan, C. El Mouhayyar, and J. Dewald contributed to data analysis and interpretation; H. Tighiouart performed the statistical analysis; B.L. Jaber, C. El Mouhayyar, A.H. Moraco, and J. Dewald drafted the manuscript; supervision and mentorship was contributed by V.S. Balakrishnan and B.L. Jaber. All the authors approved the final version of the manuscript. V.S. Balakrishnan takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data Availability Statement
All data is available upon request.
Acknowledgments
The authors would like to acknowledge Alexandra Cawood, Nikolay Korchemny, and Marcel Robles for their contribution to data acquisition.
Christopher El Mouhayyar and Jonathan Dewald equally contributed to the manuscript.
References
- 1.World Health Organization . WHO novel coronavirus: China [Internet] World Health Organization; 2020. [cited 2021 May 18]. Available from: http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ [Google Scholar]
- 2.COVID-19 map [Internet] Johns Hopkins Coronavirus Resour Cent; 2021. [cited 2021 May 18]. Available from: https://coronavirus.jhu.edu/map.html. [Google Scholar]
- 3.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 Mar;382((10)):929–36. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadim MK, Forni LG, Mehta RL, Connor MJ, Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020 Dec;16((12)):747–64. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med. 2020 Oct;17((10)):e1003406. doi: 10.1371/journal.pmed.1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010 Apr;55((4)):622–7. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KDIGO . Acute kidney injury (AKI): KDIGO [Internet] 2020. [cited 2021 Aug 7]. Available from: https://kdigo.org/guidelines/acute-kidney-injury/ [Google Scholar]
- 8.Lumlertgul N, Pirondini L, Cooney E, Kok W, Gregson J, Camporota L, et al. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021 Dec;11((1)):1–11. doi: 10.1186/s13613-021-00914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021 Nov;17((11)):751–64. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet Lond Engl. 2020 Aug;396((10251)):597–8. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golmai P, Larsen CP, DeVita MV, Wahl SJ, Weins A, Rennke HG, et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020 Sep;31((9)):1944–7. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020 Sep;31((9)):2158–67. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020 Sep;31((9)):1948–58. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI treated with renal replacement therapy in critically Ill patients with COVID-19. J Am Soc Nephrol. 2021 Jan;32((1)):161–76. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16((1)):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Casa GD, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020 Aug;8((8)):750–2. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ting SM, Nair H, Ching I, Taheri S, Dasgupta I. Overweight, obesity and chronic kidney disease. Nephron Clin Pract. 2009;112((3)):c121–7. doi: 10.1159/000214206. [DOI] [PubMed] [Google Scholar]
- 18.Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol. 2018 Sep;72((13)):1506–31. doi: 10.1016/j.jacc.2018.08.1037. [DOI] [PubMed] [Google Scholar]
- 19.Naderi N, Kleine C-E, Park C, Hsiung J-T, Soohoo M, Tantisattamo E, et al. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog Cardiovasc Dis. 2018 Jul;61((2)):168–81. doi: 10.1016/j.pcad.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chittal P, Babu AS, Lavie CJ. Obesity paradox: does fat alter outcomes in chronic obstructive pulmonary disease? COPD. 2015 Jan;12((1)):14–8. doi: 10.3109/15412555.2014.915934. [DOI] [PubMed] [Google Scholar]
- 21.Keller K, Hobohm L, Münzel T, Ostad MA, Espinola-Klein C, Lavie CJ, et al. Survival benefit of obese patients with pulmonary embolism. Mayo Clin Proc. 2019 Oct;94((10)):1960–73. doi: 10.1016/j.mayocp.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 22.Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21((11)):e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho JSY, Fernando DI, Chan MY, Sia CH. Obesity in COVID-19: a systematic review and meta-analysis. Ann Acad Med Singap. 2020 Dec;49((12)):996–1008. [PubMed] [Google Scholar]
- 24.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020 Nov;173((10)):773–81. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021 Feb;49((1)):15–28. doi: 10.1007/s15010-020-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010 Jan;65((1)):44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization . Obesity and overweight [Internet] 2021. [cited 2021 Sep 7]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 28.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006 Dec;444((7121)):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 29.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008 Nov;93((11 Suppl 1)):S64–73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conde J, Scotece M, Gómez R, Gómez-Reino JJ, Lago F, Gualillo O. At the crossroad between immunity and metabolism: focus on leptin. Expert Rev Clin Immunol. 2010 Sep;6((5)):801–8. doi: 10.1586/eci.10.48. [DOI] [PubMed] [Google Scholar]
- 31.Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020 Jul;142((1)):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 32.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020 Sep;31((9)):2145–57. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021 Feb;384((8)):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available upon request.