Abstract
Type 2 diabetes mellitus (T2DM) and Alzheimer's disease (AD) commonly co‐occur. T2DM increases the risk for AD by approximately twofold. Animal models provide one means of interrogating the relationship of T2DM to AD and investigating brain insulin resistance in the pathophysiology of AD. Animal models show that persistent hyperglycaemia results in chronic low‐grade inflammation that may contribute to the development of neuroinflammation and accelerate the pathobiology of AD. Epidemiological studies suggest that patients with T2DM who received treatment with specific anti‐diabetic agents have a decreased risk for the occurrence of AD and all‐cause dementia. Agents such as metformin ameliorate T2DM and may have other important systemic effects that lower the risk of AD. Glucagon‐like peptide 1 (GLP‐1) agonists have been associated with a decreased risk for AD in patients with T2DM. Both insulin and non‐insulin anti‐diabetic treatments have been evaluated for the treatment of AD in clinical trials. In most cases, patients included in the trials have clinical features of AD but do not have T2DM. Many of the trials were conducted prior to the use of diagnostic biomarkers for AD. Trials have had a wide range of durations and population sizes. Many of the agents used to treat T2DM do not cross the blood brain barrier, and the effects are posited to occur via lowering of peripheral hyperglycaemia and reduction of peripheral and central inflammation. Clinical trials of anti‐diabetic agents to treat AD are ongoing and will provide insight into the therapeutic utility of these agents.
Keywords: Alzheimer's disease, dapagliflozin, diabetes, empagliflozin, GLP‐1 agonist, insulin, liraglutide, metformin, mouse model, pioglitazone, rosiglitazone, semaglutide
T2DM and AD are linked in several important ways. T2DM is a risk factor for AD. T2DM is characterized by chronic low‐grade inflammation, and there is evidence linking peripheral inflammation to central inflammation. Hyperglycaemia in animal models produces AD‐related changes. Neurons in AD have insulin resistance. Agents that lower blood sugar and peripheral inflammation may have beneficial effects on AD. Clinical trials of insulin and other anti‐diabetic agents have not shown benefit in patients with AD; additional trials are on‐going.
Abbreviations and acronyms
- AD
Alzheimer's disease
- ADAS‐cog
Alzheimer's Disease Assessment Scale – cognitive subscale
- ADAS‐Exec
AD Assessment Scale – Executive version
- ADCS‐ADL
Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale
- AGE
advanced glycation end‐products
- AKT
protein kinase B
- AMP
adenosine monophosphate
- AMPA
α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid
- AMPK
AMP‐activated protein kinase
- APOE
apolipoprotein E
- APP
amyloid precursor protein
- Aβ
amyloid beta
- BACE
β‐site APP‐cleaving enzyme
- BBB
blood–brain barrier
- BDNF
brain‐derived neurotrophic factor
- CaMKII
calcium/calmodulin‐dependent protein kinase II
- cdk5
cyclin‐dependent kinase 5
- CDR‐SB
Clinical Dementia Rating ‐ Sum of Boxes
- CIBIC
Clinician's Interview‐Based Impression of Change with Caregiver Input
- CNS
central nervous system
- CRP
C‐reactive protein
- CSF
cerebrospinal fluid
- DM
diabetes mellitus
- DNA
deoxyribonucleic acid
- EGFP
enhanced green fluorescence protein
- ELAD
evaluating liraglutide in Alzheimer's disease
- EV
extracellular vesicles
- EVOKE
A Research Study Investigating Semaglutide in People with Early Alzheimer's Disease
- FABP
fatty acid‐binding protein
- fAD
familial Alzheimer's disease
- FDG
fluorodeoxyglucose
- GABA
γ‐aminobutyric acid
- GIP
glucose‐dependent insulinotropic polypeptide
- GLP
glucagon‐like peptide
- GSK‐3β
glycogen synthase kinase‐3β
- GWAS
genome‐wide association studies
- HDL
high‐density lipoprotein
- HFD
high‐fat diet
- HgbA1c
haemoglobin‐A1c
- HLA
human leukocyte antigen
- ICV
intracerebroventricular
- IDE
insulin‐degrading enzyme
- IFG
insulin‐like growth factor
- IL
interleukin
- INF‐gamma
interferon gamma
- IR
insulin receptor
- IRS
insulin receptor substrate
- JNK
c‐Jun N‐terminal kinase
- LOAD
late onset Alzheimer's disease
- LPS
lipopolysaccharide
- LTD
long‐term depression
- LTP
long‐term potentiation
- MAPK
mitogen‐activated protein kinase
- MCI
mild cognitive impairment
- MMSE
Mini‐Mental State examination
- MRI
magnetic resonance imaging
- MWM
Morris water maze
- NFT
neurofibrillary tangles
- NGS
next‐generation sequencing
- NIA
National Institute on Aging
- NMDA
N‐methyl‐d‐aspartate
- NO
nitric oxide
- PET
positron emission tomography
- PGE2
prostaglandin E2
- PI3K
phosphatidylinositol 3‐kinase
- PI3K
phosphoinositide 3 kinase
- PI3K/AKT
phosphatidylinositol 3‐kinase/protein kinase B
- PKA
protein kinase A
- PPARγ
peroxisome proliferator‐activated receptor γ
- PPY
pancreatic polypeptide
- PS1
presenilin 1
- PS2
presenilin 2
- RAGE
receptor advanced glycation end‐products
- ROS
reactive oxygen species
- SRT
selective reminding test
- STZ
streptozotocin
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- Th1
T helper cells
- TLR
toll‐like receptor
- TNF
tumour necrosis factor
- TZD
thiazolidinediones
- WT
wild type
- XR
extended release
1. INTRODUCTION
Alzheimer's disease (AD) is a neurodegenerative disease that is characterized by progressive synaptic and neuronal loss, learning and memory deficits, and cognitive, functional, and behavioural decline (Scheltens et al., 2021). AD is the most common form of dementia, accounting for 60%–80% of all cases (Alzheimer's Association, 2021). Approximately 6.2 million Americans and nearly 50 million individuals worldwide have AD dementia (Alzheimer's Association, 2021); AD is the sixth leading cause of death in the United States (Alzheimer's Association, 2021). Age is the greatest risk factor for developing AD, although AD is not a normal part of aging (Alzheimer's Association, 2021). Several other factors confer increased risk including apolipoprotein E ε‐4 (APOE4) genotype, stroke and vascular risk factors, head trauma, diabetes mellitus (DM), and obesity (Barnes & Yaffe, 2011). Type 2 diabetes mellitus (T2DM) can confer a 1.5‐fold to fourfold increase in lifetime risk for AD (Lu et al., 2009; Mehla et al., 2014). Furthermore, approximately 80% of individuals with AD have insulin resistance or abnormal fasting glucose levels (Janson et al., 2004). Over 25% of the US population over the age of 65 have T2DM, and aging of the population plays a large role in both the epidemic of T2DM and the increased prevalence of AD (Kirkman et al., 2012). Understanding the mechanistic relationships of T2DM and AD may lead to candidate treatments that control T2DM and ameliorate the risk of progression to AD. Some of these agents may treat AD independent of the occurrence of T2DM.
The pathological hallmarks of AD include amyloid‐beta (Aβ) protein plaques composed of fibrillar Aβ (Hardy & Higgins, 1992; Hyman et al., 2012), neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein (Grundke‐Iqbal et al., 1986; Serrano‐Pozo et al., 2011), and chronic neuroinflammation, or a sustained immune response in the brain, that promotes and accelerates both Aβ and tau pathologies (Heneka et al., 2015; Kinney et al., 2018; Millington et al., 2014).
Here, we review the foundational science linking T2DM and AD, animal models used to explore the relationship of the two disorders, and past and current clinical trials of diabetes therapies tested for the treatment of AD.
1.1. Foundational science links between T2DM and AD
Many types of evidence link T2DM and AD. T2DM affects over 200 million people worldwide and is defined as a sustained state of hyperglycaemia due to dysfunction of insulin receptor (IR) signalling (insulin resistance) despite elevated levels of insulin (Ahmed, 2012). Several studies have shown that insulin resistance is a risk factor for AD and may aggravate the pathology of AD (Boles et al., 2017; de Felice, 2013; Ferreira et al., 2018; Talbot et al., 2012a). Altered insulin signalling disrupts brain function, as insulin in the brain promotes neurite growth, synaptic plasticity, and development and maintenance of excitatory synapses (Gu et al., 2014; Liu et al., 2014; Taouis & Torres‐Aleman, 2019; Zhao, Siu, et al., 2019). Insulin administration via oral, nasal or intracerebroventricular (ICV) injections decreases AD pathology in animal models of T2DM with AD‐like changes (Adzovic et al., 2015; Avgerinos et al., 2018; Cummings et al., 2020; Freiherr et al., 2013; Morris & Burns, 2012; Steinmetz et al., 2016).
Metabolic syndrome is composed of insulin resistance, obesity, hypertension, cardiovascular disease and dyslipidaemia which increase the risk of AD beyond the risk conferred by T2DM (Hildreth et al., 2012; Verdile et al., 2015). Obesity, T2DM and AD have overlapping biological pathologies including insulin resistance, oxidative stress, mitochondrial dysfunction and inflammation (Pugazhenthi et al., 2017).
Insulin interacts with tau pathology via the activation of protein kinases involved in tau phosphorylation. Activation of kinases including protein kinase A (PKA), calcium/calmodulin‐dependent protein kinase II (CaMKII), glycogen synthase kinase‐3β (GSK‐3β) and cyclin‐dependent kinase 5 (cdk5) leads to the hyperphosphorylation of tau and aggregation of tau proteins comprising NFTs (Dolan & Johnson, 2010; Duka et al., 2013; Engin & Engin, 2021; Wang et al., 2007). GSK‐3β is a serine‐threonine kinase that is consistently upregulated in AD brains (Blalock et al., 2004; Hooper et al., 2008; Leclerc et al., 2001; Lovestone et al., 1994; Munoz‐Montano et al., 1997). GSK‐3β can contribute to development of NFTs (Beurel et al., 2015; Hanger et al., 1992; Mandelkow et al., 1992) and Aβ plaque formation (Beurel et al., 2015; Hurtado et al., 2012; Phiel et al., 2003). In a reciprocal relationship, Aβ aggregation appears to promote tau hyperphosphorylation via activation of GSK‐3β (Reddy, 2013).
Animal models are used to explore the mechanistic relationships of T2DM and AD. Streptozotocin (STZ), for example, when given in staggered and low‐dose injections, destroys pancreatic β cells mimicking late‐stage T2DM pancreatic exhaustion (McEvoy et al., 1984; Murtishaw et al., 2018; Reed et al., 2000; Srinivasan et al., 2005; Zhang et al., 2008). Treated animals have sustained hyperglycaemia without any overt illness (Murtishaw et al., 2018); they exhibit learning and memory deficits, increased tau phosphorylation and increased neuroinflammation similar to the pathological changes observed in AD (Murtishaw et al., 2018).
Signalling pathways link T2DM to AD. Fractalkine (CX3CL1) is a chemokine constitutively and specifically released by neurons to regulate microglia involved in neuroinflammation through the fractalkine receptor (CX3CR1) located on central nervous system (CNS) microglia (Chamera et al., 2020). CX3CL1/CX3CR1 signalling interacts with insulin regulation in the brain by modulating microglial function and hippocampal synaptic plasticity (Paolicelli et al., 2014; Sheridan et al., 2014). A tau transgenic (Tg) mouse model (hTau mice) lacking CX3CR1 exhibited enhanced tau phosphorylation and aggregation associated with microglial activation, as well as behavioural impairments (Bhaskar et al., 2010; Bolos et al., 2017).
Genetic factors suggest a link between T2DM and AD. APOE‐4 is a gene encoding a protein that is involved in lipid and cholesterol binding and transport and is the best‐known genetic risk factor for AD (Corder et al., 1993; Kim et al., 2009; Strittmatter et al., 1993). Two copies of the ε4 allele of the gene can confer up to a 15‐fold increase in developing AD (Altmann et al., 2014; Farrer et al., 1997; Payami et al., 1994). APOE‐4 has been shown to increase hyperinsulinaemia in T2DM amplifying the risk for AD in APOE‐4 carriers with T2DM (Luchsinger et al., 2004; Peila et al., 2002).
1.2. Insights from animal models of AD and T2DM
Most of the work on AD‐related mechanisms as well as the testing of candidate therapeutics prior to clinical trials is carried out in animal models that mimic aspects of AD; this has largely relied on the use of genetic mouse models that express mutations associated with familial AD (fAD). These models present some of the core pathological features of AD but do not exhibit the entire range of AD pathology.
Animal models remain indispensable in research due to their biological similarity to humans, allowing researchers to investigate disease mechanisms and progression under controlled conditions. They continue to advance both the scientific and medical fields and provide the scientific basis for the creation of novel therapeutics. Approximately 60% of all preclinical research is conducted in rodents due to their anatomical, physiological, genetic and overall biological similarity to humans (Bryda, 2013). They allow researchers to target and alter specific genes, translational pathways and protein interactions that provide insights into mechanisms and facilitate testing potential therapeutics. Several model‐based approaches have been utilized to investigate mechanisms and features of AD and T2DM both independently and together.
1.3. Common AD mouse models/mechanisms and use in metabolic and diabetes investigations
There is no animal model that exactly mimics AD pathogenesis and progression. Wild‐type (WT) mice do not normally develop AD‐type pathology; hence, human genes consistent with fAD (e.g., amyloid precursor protein [APP], presenilin 1 [PS1] and presenilin 2 [PS2]) are commonly incorporated into mouse genomes to induce AD pathology in Tg mouse models (Balu et al., 2019). The AD pathology of fAD and sporadic late onset AD (LOAD) are morphologically similar. It is common in a laboratory setting to utilize several fAD genetic mutations to create AD animal models. There are currently over 200 animal models of AD tracked by Alzforum (https://www.alzforum.org/research-models/alzheimers-disease), which include models facilitating the pathogenesis of Aβ plaques, NFTs, gliosis, synaptic loss, neuronal loss, changes in long‐term potentiation and long‐term depression (i.e., LTP/LTD, respectively), and cognitive impairment. The APP/PS1 mouse, for example, is arguably the most widely used animal model to investigate Aβ pathology progression and other features of AD. The APP/PS1 model contains mutations in APP (Swedish) and PSEN1 (L166P) genes, under the Thy1 promotor, and express a threefold increase in APP production compared with endogenous murine APP. APP is the precursor protein for Aβ, and these mice develop Aβ plaques in the cortex and hippocampus at approximately 3–4 months of age (Radde et al., 2006), followed by reactive gliosis and proinflammatory cytokine release (Lee et al., 2010; Radde et al., 2006), synaptic loss (Bittner et al., 2012), neuronal loss in the dentate gyrus (Rupp et al., 2011), hippocampal LTP/LTD impairments (Gengler et al., 2010) and cognitive impairment (Webster et al., 2013), all consistent with AD. APP/PS1 mice are often utilized for AD‐related studies because they closely mimic the progression of Aβ in AD patients.
Studies of the relationship between AD and T2DM often rely on manipulations that alter diet, blood glucose, and overall metabolic functions produced artificially in animal models of AD.
1.4. Insulin resistance and AD
It has traditionally been proposed that reduction of brain metabolism occurs after neuronal atrophy and loss in the course of AD (Bokde et al., 2001). However, accumulating evidence indicates that hypometabolism, potentially because of metabolic dysfunction (e.g., insulin resistance observed in T2DM), may occur prior to brain atrophy (Kyrtata et al., 2021). Animal models are utilized to investigate the mechanisms by which this may occur. In mouse models, long‐term feeding with a high‐fat diet (HFD) can cause hyperinsulinaemia, cardiovascular disease, obesity and both peripheral and central insulin resistance (Buettner et al., 2007; Wali et al., 2020). The HFD and obesity have been shown to increase neuroinflammation (Spagnuolo et al., 2015), neurodegeneration (Mazon et al., 2017; Pugazhenthi et al., 2017) and cognitive decline (Balasubramanian et al., 2021; Nguyen et al., 2014) that are consistent with AD. In clinical populations, most, but not all, patients with T2DM are overweight or obese, leading to insulin resistance. Insulin resistance and hyperglycaemia can induce hyperphosphorylated tau protein accumulation and lead to NFTs (Silva et al., 2019). The exact effects of diet‐induced insulin resistance on AD pathology and cognition remain controversial due to differing diet compositions, incubation times, cohort sex and rodent strains used in the experiments. The strength of these approaches is that they reproduce the milieu of changes observed with T2DM; however, they provide limited insight in to specific mechanisms linking T2DM and AD.
Genetic approaches to producing insulin resistance include the ob/ob mouse that is widely utilized to induce obesity and facilitating examination of diet‐induced insulin resistance. This rodent model, in a C57BL/6J background, is unable to produce leptin—a hormone responsible for inhibiting hunger—via altering the leptin gene (i.e., ob or Lep), leading to increased appetite and obesity (Ingalls et al., 1950; Small et al., 2017). These animals exhibit hyperinsulinaemia, mild hyperglycaemia and insulin resistance (Coleman, 1978; Small et al., 2017). Crossing the ob/ob mouse model with APP/PS1 mice (i.e., APP/PS1 + ob/ob) leads to models that exhibit significant increases in Aβ plaque load in the hippocampus and prefrontal cortex; two brain regions affected early in AD (S. Zhang et al., 2017). APP/PS1 + ob/ob mice evidence more abundant hyperphosphorylated tau, neuroinflammation (i.e., activated astrocytes and microglia) and synaptic loss and have more severe learning and memory deficits compared to APP/PS1 mice (Zhang et al., 2017).
Over the last 15 years, considerable attention has been devoted to understanding the sustained inflammatory response seen in the brain in AD. Chronic inflammation and insulin resistance are observed in both T2DM and AD (O'Brien et al., 2017; Parimisetty et al., 2016; Vinuesa et al., 2021). Systemic inflammation may precede the development of insulin resistance in insulin‐sensitive tissue (Klöting & Blüher, 2014; Parimisetty et al., 2016). In addition to peripheral and central inflammation in both T2DM and AD, there is evidence of inflammation‐related changes in the blood–brain barrier (BBB) in both conditions. The BBB is a neurovascular unit that limits peripheral toxins, immune cells and pathogens from entering and damaging the brain (van Dyken & Lacoste, 2018). Chronic peripheral and inflammation induced by DM and obesity can cause BBB breakdown and permeability to infiltrating macrophages, leading to exacerbation of the immune response in the brain, disruption of glial and neuronal cell integrity, impaired hormonal function, increased insulin insensitivity and impaired cognition (van Dyken & Lacoste, 2018). The invasion of leukocytes releases proinflammatory cytokines that exacerbate AD pathology including neuronal damage and death (Kinney et al., 2018; Varatharaj & Galea, 2017). In animal models of AD, chronic neuroinflammation can be seen before Aβ accumulation in the hippocampus (Beauquis et al., 2013; Heneka et al., 2015). Several glial signalling cascades implicated in regulation of immune responses are altered in AD and T2DM. For example, Toll‐like receptor 4 (TLR4) and triggering receptor expressed on myeloid cells 2 (TREM2) are expressed on microglia that regulate inflammation in the brain (Zhou et al., 2019). Activation of TLR4 produces large increases of proinflammatory cytokines such as tumour necrosis factor‐α (TNF‐α), nitric oxide (NO), prostaglandin E2 (PGE2) and interleukin‐1β (IL‐1β), which can promote and exacerbate both T2DM and AD pathology (Murtishaw et al., 2016; Zhao, Bi, et al., 2019). Lipopolysaccharide (LPS), an immunostimulatory component of gram‐negative bacteria, and a ligand for TLR‐4, induces inflammation in the brain (Zhao, Bi, et al., 2019). LPS‐induced CNS inflammation in 3xTg‐AD mice showed significant increase of tau hyperphosphorylation via cdk5 (Kitazawa et al., 2005). LPS‐induced inflammation following ICV injection of STZ induces IR insensitivity in the brain, increased tau phosphorylation in the hippocampus and learning and memory deficits in male Sprague–Dawley rats assessed in the Morris mater maze (MWM) (Murtishaw et al., 2016). These studies suggest that chronic inflammation as a result for T2DM, may be one mechanism by which T2DM confers increased risk for developing LOAD.
Additional signalling cascades that regulate both neuronal and glial function are altered in T2DM and AD. c‐Jun N‐terminal kinases (JNKs) are members of the mitogen‐activated protein kinase (MAPK) family and are involved in cellular stress responses; their activity has been linked to T2DM. JNK is among the most investigated molecules in obesity‐induced insulin resistance (Pal et al., 2016). JNK has three genetic isoforms, MAPK8 coding for JNK1, MAPK9 coding for JNK2 and MAPK10 coding for JNK3 (Yarza et al., 2016). JNK3 has been implicated in the development of AD (Yarza et al., 2016). It is highly expressed and chronically active in brain tissue and cerebrospinal fluid (CSF) in AD patients and has been implicated in the cognitive deficits consistent with AD (Gourmaud et al., 2015). Several downstream cascades that arise from JNK signalling are linked to cell survival and pathologic features of AD. JNK can directly induce insulin resistance through a phosphorylated IR substrate (IRS) 1, inhibiting insulin cascades and potentially increasing the risk for AD (Sabio et al., 2008). ICV injection of Aβ oligomers can activate IRS‐1pSer and JNK in the hippocampus of cynomolgus monkeys (Bomfim et al., 2012). Administration of exendin‐4—an anti‐diabetic agent—to Tg mice, decreased IRS‐1pSer and activated JNK, resulting in amelioration of behavioural and cognitive deficits (Bomfim et al., 2012). Mice given HFD to induce obesity exhibit JNK activation. Genetic manipulations leading to depletion of JNK prevent obesity by decreasing adiposity and ameliorating insulin sensitivity and IR signalling (Hirosumi et al., 2002). Genetic‐based depletion of JNK3 in fAD mice leads to a dramatic reduction of Aβ42 peptide and Aβ plaque load and improved cognition (Yoon et al., 2012). Aβ42 can indirectly activate JNK signalling, resulting in neuroinflammation and neurodegeneration (Yoon et al., 2012). JNK modulates NFT formation via phosphorylating tau (Lagalwar et al., 2006; Yarza et al., 2016). JNK‐related signalling represents a specific conserved pathway that intersects with obesity, insulin resistance and cell survival in AD and T2DM.
Taken together, insulin resistance may not directly cause AD; however, insulin resistance can increase the risk of developing AD by exacerbating AD pathology (e.g., Aβ, tau and chronic neuroinflammation) and, in turn, promote further insulin resistance—a feed‐forward loop (Wei et al., 2021). Therapeutics targeting insulin resistance may be useful to ameliorate this mechanism and reduce the risk of AD and the exacerbation of AD in those with existing pathological changes.
1.5. Hyperglycaemia and AD
Hyperglycaemia is a major characteristic of T2DM and has a high prevalence in aged populations. Twenty‐five percent of the US population over the age of 65 have T2DM (as defined by hyperglycaemia), and another ~88 million in the United States exhibit pre‐DM hyperglycaemia (elevated blood glucose but not yet meeting T2DM criteria; Centers for Disease Control and Prevention, 2020). In clinical populations, disruption of glucose homeostasis expedites the progression from mild cognitive impairment (MCI) to AD (Morris et al., 2014), suggesting that dysregulation of glucose metabolism may play a causal role AD pathogenesis (Macauley et al., 2015).
Hyperglycaemia can be modelled in in rodents with STZ, a diabetogenic drug that is toxic to insulin‐producing pancreatic β cells via the alkylation of β‐cell DNA, thus impairing insulin secretion and inducing chronic hyperglycaemia (Murtishaw et al., 2018). This approach has been useful in studying T1DM; however, the severity of the beta cell loss does not mimic T2DM seen in aging populations. To better approximate T2DM, investigators have developed staggered and/or low‐dose injections of STZ to effectively induce a progressive long‐term hyperglycaemia state in an otherwise healthy animal (Murtishaw et al., 2018). Investigations have demonstrated that staggered administration of STZ results in a sustained hyperglycaemic state with induction of learning and memory deficits, increased tau phosphorylation and increased neuroinflammation in mice consistent with AD pathological changes (Murtishaw et al., 2018). Investigations employing central STZ administered via a single ICV injection revealed exacerbation of Aβ accumulation in APP/PS1 mice (Kelliny et al., 2021). The central STZ administration is hypothesized to induce IR resistance centrally, potentially mimicking an important aspect of AD. Peripheral STZ and the resulting hyperglycaemia have been shown to increase APP protein expression in APP/PS1 mice, thus promoting Aβ generation (Yang et al., 2013) and directly exacerbate Aβ and tau pathologies (Arnold et al., 2018; Ferreira et al., 2018; Murtishaw et al., 2018; Yang et al., 2013). Acute hyperglycaemia in APP/PS1 mice can increase Aβ production in the hippocampal interstitial fluid, and the effect is exacerbated with increased age (Macauley et al., 2015). Pdx1+/− mice (a chronic hyperglycaemia mouse model) crossed with an APP/PS1 mouse exhibited increased tau phosphorylation, increased synaptic loss in the hippocampus, increased microglial and astrocyte activation, glucose intolerance and Aβ plaque formation (Guo et al., 2016). These mice exhibited increased advanced glycation end‐products (AGEs) followed by the activation of its receptor (RAGE), which is thought to contribute to impairments in Aβ degradation and Aβ generation (Guo et al., 2016). AGEs can induce synaptic and neuronal death via increased APP processing (i.e., β‐site APP‐cleaving enzyme [BACE] and PS1) and reactive oxygen species (ROS) generation (Ko et al., 2015). Elevated glucose levels can facilitate the formation of Aβ‐42 oligomers (the more toxic form of Aβ) (Kedia et al., 2017). It is hypothesized that glucotoxicity via chronic hyperglycaemia can induce neuronal structural and functional alterations, haemorrhagic interruption of cerebral blood vessels and increased Aβ accumulation. Glucotoxicity can result in cell injury to hepatocytes and insulin‐producing pancreatic β cells via mitochondrial oxidative stress and mitochondrial dysfunction (Lee, Lee, et al., 2011; Mota et al., 2016). Oxidative stress can increase the activity of BACE and gamma secretase, enzymes directly involved in the cleavage of APP and the generation of Aβ (Cheignon et al., 2017; Y. Zhao & Zhao, 2013).
Chronic hyperglycaemia induces hyperphosphorylation of tau via several kinases (Murtishaw et al., 2018; Wang et al., 2007). Activation of PKA, CaMKII, GSK‐3β and cdk5 in T2DM leads to the hyperphosphorylation of tau, aggregation of tau proteins and formation of NFTs seen in AD (Dolan & Johnson, 2010; Duka et al., 2013; Engin & Engin, 2021; Wang et al., 2007). Aβ accumulation appears to promote tau hyperphosphorylation via activation of GSK‐3β (Reddy, 2013). Chronic hyperglycaemia can induce tau modification via tau cleavage, both in vitro and in vivo (Kim et al., 2013). Thus, kinase alterations seen in T2DM can contribute to AD pathogenesis. The investigations of hyperglycaemia provide mechanistic evidence for how T2DM confers increased risk for developing AD.
1.6. Genetic relationships of T2DM and AD
1.6.1. Apolipoprotein E
APOE is a protein that is involved in lipid and cholesterol binding and transport and is the most common genetic risk factor for AD (Corder et al., 1993; Kim et al., 2009; Strittmatter et al., 1993). In humans, there are three APOE alleles (i.e., E2, E3 and E4) that produce apo‐E2, apo‐E3 and apo‐E4 proteins. Approximately ~60% of AD patients have an APOE4 genotype (Rebeck et al., 1993). Having two copies of the ε2 allele is the strongest genetic protective factor for LOAD, whereas two copies of the ε4 variant can confer up to a 15‐fold increase risk in developing LOAD (Altmann et al., 2014; Farrer et al., 1997; Payami et al., 1994; Riedel et al., 2016; Serrano‐Pozo et al., 2021). Mice express only one form of APOE, and the amino acid homology between mouse and human APOE is 70% (Rajavashisth et al., 1985). APOE reporter mice with enhanced green fluorescence protein (EGFP) insertion reveal that microglia and astrocytes constitutively express APOE, and neurons synthesize APOE under stress conditions (Xu et al., 2006). Despite the increased risk in APOE‐4 human carriers, Tg fAD mice do not closely mimic the effects of human APOE isoforms (Balu et al., 2019), and a majority of Tg fAD mouse models use murine APOE instead of human APOE; this presents a limitation when investigating Aβ accumulation, synaptic integrity and neuroinflammation seen in AD (Balu et al., 2019). Given the large role that APOE plays in AD pathophysiology, several animal models of both DM and AD with APOE modifications have been investigated.
Although the APOE 4 gene significantly increases the likelihood of developing AD, many individuals who are APOE4 carriers do not develop the disease; it is hypothesized that APOE4 interacts with several factors, including obesity, that increase AD risk (Moser & Pike, 2017). For example, APOE4 has been shown to increase hyperinsulinaemia and T2DM, contributing to the risk of AD (Luchsinger et al., 2004; Peila et al., 2002). Diet‐induced obesity (i.e., western diet) in 5xfAD/human APOE‐ε4+/+ mice exhibit a significant increase in amyloid deposits, Aβ burden, and reactive gliosis compared to 5xfAD/human APOE‐E3+/+; suggesting that there is an interaction between obesity and APOE in increasing AD pathogenesis (Moser & Pike, 2017). Mice with human APOE4 with HFD‐induced insulin resistance replicate diabetic‐related states such as increased glucose and insulin resistance and decreased insulin secretion (Koren‐Iton et al., 2020). When mice with human APOE3 are fed a HFD, they show similar results as APOE4 mice, suggesting that diabetic modifications play an important role in the pathological effects of APOE (Koren‐Iton et al., 2020).
1.6.2. Non‐APOE risk genes for AD and DM
Genome‐wide association studies (GWAS), next‐generation sequencing (NGS) and other technological advances point to several additional genetic loci, rare genetic variants and mutations that have a role in LOAD (Giri et al., 2016). These techniques have helped identify genetic influences that vary from low risk (e.g., CR1, CD33, CD2AP, etc.), to medium risk (e.g., ADAM12, PLD3, ABCA7, etc.), high risk (e.g., APOE, TREM2 and SORL1, etc.) and causal (e.g., APP, PS1 and PS2) associations with AD. Over 280 autosomal dominant variants have been observed in AD (Aguilar et al., 2019; Cruts et al., 2012; Yamazaki et al., 2016). The impact of genes influencing JNK and GSK‐3β discussed above suggest overlapping changes relevant to T2DM and AD.
Similarly, there are numerous candidate genes implicated in DM that may have direct linkage to AD pathogenesis. Risk genes that are strongly associated with T1DM include human leukocyte antigen (HLA) HLA‐DR3‐DQ2 or HLA‐DR4‐DQ8 haplotypes (Pociot & Lernmark, 2016). Over 50 genes, 58 genomic regions and more than 100 single nucleotide polymorphisms (SNPs) are associated with T1DM (Paschou et al., 2018; Pociot & Lernmark, 2016). Individuals with T1DM, especially the elderly, have an increased risk of developing AD (Lacy et al., 2018).
Genes strongly associated with T2DM include TCF7L2 (involved in insulin secretion and glucose production), ABBC8 (regulates insulin), CAPN10 (involved in insulin sensitivity and secretion), GLUT2 (transports glucose into pancreatic β cells), GCGR (involved in glucagon regulation) (Naseri et al., 2020) and others (Naseri et al., 2020; Park, 2011). These genes are involved in the overall production and regulation of glucose and insulin and in how glucose is sensed in the body; dysfunction of these processes can lead to T2DM and in parallel, increased risk for AD.
Some of these genes are known to link T2DM and AD. For example, the TCF7L2 gene is strongly associated with T2DM (Grant et al., 2006), and increased TCF7L2 mRNA has been observed in AD brains (Blom et al., 2010). ABCA1 regulates cholesterol efflux and is involved in high‐density lipoprotein (HDL) formation (Fitz et al., 2012). APP/PS1 mice with APOE4 insertion and ABCA1−/+ exhibited memory impairments and increased Aβ deposition (Fitz et al., 2012). Consistent with human studies, the CAPN10 gene plays an important role in DM in mice (Cheverud et al., 2010), and it may indirectly increase the risk of AD by contributing to T2DM.
1.7. Animal model treatment‐related insights
1.7.1. Insulin
Insulin is a hormone produced primarily by pancreatic β cells (Rorsman & Ashcroft, 2018), although it has been shown that neurons can produce insulin (Blázquez et al., 2014; Gray et al., 2014). Insulin mRNA is found in brain regions relevant to AD such as the hippocampus (i.e., CA1 and CA3) (Devaskar et al., 1994). Elevated glucose levels initiate the synthesis and release of insulin via pancreatic β cells and promote cellular glucose uptake for energy generation. Downstream insulin signalling pathways include their role in protein transcription and synthesis, regulation of apoptosis and modulation of lipid synthesis in the brain (Arnold et al., 2018). Insulin plays a large role in brain development, neuronal health, and brain ageing. Both insulin and insulin‐like growth factor (IFG‐1) can modulate the proliferation, differentiation and survival of neural stem cells (Spinelli et al., 2019).
IRs are highly expressed on various cell types in the brain (i.e., neurons, microglia, oligodendrocytes, etc.) and across several brain regions including the hypothalamus, olfactory bulb, cerebellum, striatum, cortex and hippocampus (Arnold et al., 2018). IRs are highly localized in both pre‐ and post‐synaptic areas, playing an important role in neuroplasticity (Abbott et al., 1999; Bockmann et al., 2002; Mielke et al., 2006; Werther et al., 1989).
Insulin in the brain can promote neurite growth, regulates the expression and localization of γ‐aminobutyric acid (GABA), N‐methyl‐d‐aspartate (NMDA) and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors, and is involved in synaptic plasticity (i.e., LTP and LTD) in the hippocampus (van der Heide et al., 2005). Insulin helps maintain and promote excitatory synapses (Chiu et al., 2008) and dendritic spines (Lee, Huang, & Hsu, 2011) and can promote neuronal health and survival by inhibiting apoptosis (Arnold et al., 2018; Kim & Han, 2005). Neurons do not depend on insulin‐dependent GLUT‐4 receptors for glucose uptake as required for glucose uptake in peripheral cells. Instead, insulin‐independent receptors (e.g., GLUT‐3) are expressed on neurons and—indirectly activated via NMDA receptors—facilitate neuronal glucose uptake (Talbot et al., 2012b; Uemura & Greenlee, 2006).
Insulin and IRs decrease with normal ageing and in AD. The binding of insulin to IRs is directly associated with the production of insulin‐degrading enzyme (IDE). IDE is a key enzyme involved in the degradation of Aβ (Farris et al., 2003). IR activation suppresses the activation of GSK3β, a kinase involved in the phosphorylation of tau (Murtishaw et al., 2018). These mechanisms may explain why insulin treatment rescued learning and memory deficits in rodents (Adzovic et al., 2015; Steinmetz et al., 2016). Six weeks of treatment with intranasal insulin rescued brain insulin signalling dysfunction, ameliorated cognitive impairments, inhibited JNK activation, increased neurogenesis and reduced Aβ accumulation and plaques in 4.5‐month‐old APP/PS1 mice (Mao et al., 2016). Additional data, from an amyloid and tau mouse model of AD (3x‐Tg‐fAD mice) treated with intranasal insulin for 2 months, demonstrated improved memory (in novel object recognition task and MWM), reduced depression‐like behaviour (via tail suspension and forced swim tests) and decreased hyperphosphorylated tau, Aβ oligomers and 3‐nitrotyrosine in the frontal cortex and hippocampus (Barone et al., 2019). Additional data showed that ICV administration of insulin significantly decreased inflammatory markers in the hippocampus and improved spatial memory performance (Adzovic et al., 2015). It remains to be determined if the benefits of insulin administration are impacted when IR insensitivity is present in AD, aging or T2DM.
Soluble Aβ oligomers can impair insulin signalling via downregulation of IRs on neurons (Zhao et al., 2008). Elevated brain insulin initiates increased levels of IDE. Studies with mouse models of both T2DM and AD demonstrate that activating peroxisome proliferator‐activated receptor γ (PPARγ) and the adenosine monophosphate (AMP)‐activated protein kinase (AMPK) pathways significantly increase IDE leading to decreased Aβ levels and rescue of recognition and learning memory deficits (Li et al., 2018).
1.7.2. Metformin
Metformin is the most utilized medication to treat T2DM as it decreases hepatic glucose production, decreases intestinal glucose absorption and increases insulin sensitivity. The effects of metformin in AD animal models, however, have not been entirely consistent across experiments. Studies suggest that metformin may have beneficial effects in age‐related diseases including AD (Rotermund et al., 2018). For example, mice with STZ‐induced hyperglycaemia given metformin showed reduce levels of phosphorylated tau and Aβ plaque burden in the hippocampus, decreased phosphorylated GSK‐3β in the cortex and improved learning and memory (Oliveira et al., 2021). In another study, administration of metformin led to a reduction of Aβ levels, improved learning and memory (i.e., MWM and Y‐Maze), enhanced mRNA expression of genes involved in synaptic plasticity (i.e., brain‐derived neurotrophic factor [BDNF]), deceased oxidative stress (i.e., malondialdehyde and superoxide dismutase), reduced inflammation (i.e. IL‐1β and IL‐6) and increased IDE protein levels in APP/PS1 mice (Lu et al., 2020). Long‐term metformin treatment in HFD aged C57BL/6J mice found that treatment with metformin prevented spatial learning and memory deficits (Allard et al., 2016). HFD mice given metformin showed enhanced glucose tolerance, as well as decreased oxidative stress and inflammation (Lennox et al., 2014). Administration of metformin to HFD rats led to reduced peripheral insulin resistance, decreased brain and plasma markers of oxidative stress, improved mitochondrial function and prevention of learning and memory impairments (Pintana et al., 2012). Senescence‐accelerated mouse‐prone 8 (SAMP8)—an AD mouse model—injected with metformin for 8 weeks exhibited improved learning and memory and decreased accumulation of APPc99 and hyperphosphorylated tau (Farr et al., 2019). Chronic metformin treatment rescued spine density, LTP and spatial memory in APP/PS1 mice via the suppression of cdk5 (Wang et al., 2020). Metformin also improves cognition of aged mice by promoting cerebrovascular integrity, enhanced glycolysis in blood and neurogenesis (Zhu et al., 2020).
However, not all studies have found beneficial responses to metformin in animal models of AD. Db/db mice injected with metformin did not exhibit improved spatial learning and memory (Li et al., 2012). One study found that metformin treatment in P301S mice—a tauopathy mouse model—showed reduced hyperphosphorylated tau but simultaneously exhibited increased tau aggregation (Barini et al., 2016). Similarly, short‐term metformin treatment reduced hyperphosphorylated tau but simultaneously promoted expression and processing of APP and BACE‐1 (Kickstein et al., 2010; Picone et al., 2015). The inconsistency observed in studies of the effects of metformin on AD‐related changes may be due to differing diet compositions as well as mechanistic differences in the various animal models.
1.7.3. PPAR‐gamma agonists
Peroxisome proliferation‐activated receptors (PPARs) have a role in cellular functions relevant to T2DM and AD. PPARs are a type of ligand‐inducible nuclear hormone receptor superfamily that are regulated by steroids and lipid metabolites (Heneka et al., 2011; Nicolakakis & Hamel, 2010). PPARs are involved in lipid storage, adipocyte differentiation and glucose homeostasis in all organs, including the brain (Heneka et al., 2011; Nicolakakis & Hamel, 2010). Three isoforms of PPRAs exist (α, γ and β/δ), each encoded by different genes (Nicolakakis & Hamel, 2010). PPARγ has a role in insulin sensitizing effects of the PPARγ agonists thiazolidinediones, a class of oral anti‐diabetic agents (Lehmann et al., 1995; Nicolakakis & Hamel, 2010). PPARγ has relevance to both T2DM and AD, as PPARγ can regulate obesity, diabetes and neuroinflammation (de Carvalho et al., 2021). In animal models, PPARγ agonists have been observed to reduce microglial and astrocytic activation in the hippocampus and cortex, decrease BACE‐1 mRNA and protein levels and reduce Aβ deposits in the hippocampus and cortex (Heneka et al., 2005). PPARγ plays an important role in mitochondrial function and biogenesis, fatty acid storage, energy metabolism and antioxidant defence (Rodríguez‐Pascau et al., 2021). More recently, bis(ethylmaltolato)‐oxidovanadium (IV) (BEOV), a vanadium compound, was shown to be involved in DM and AD via PPARγ activity (He et al., 2021). Administration of BEOV in APP/PS1 mice significantly decreased levels of TNF‐α, IL‐6, IL‐1β, NO synthase and cyclooxygenase‐2 in hippocampus of APP/PS1 mice (He et al., 2021). Furthermore, these effects were observed in BV2 microglia cell cultures with Aβ (He et al., 2021). BEOV reduced Aβ levels and improved learning and memory in APP/PS1 mice (He et al., 2021).
There are several endogenous and synthetic agonists with a spectrum of affinity for PPARγ. Natural PPARγ agonists with high affinity include linoleic acid (9‐ and 13‐HODE), prostaglandin 15‐Deoxi‐Delta(12,14)‐prostaglandin J(2) (15d‐PGJ(2)) (Cocca et al., 2009; Khan et al., 2019), as well as gamolenic acid, eicosapentaenic acid, polyunsaturated fatty acid metabolites and others (Khan et al., 2019). Synthetic PPARγ agonists primarily include thiazolidinediones (e.g., pioglitazone [Actos]: troglitazone [Rezulin], ciglitazone and rosiglitazone [Avandia]) (Khan et al., 2019), as well as ibuprofen, indomethacin, flurbiprofen and others (Khan et al., 2019). APP/PS1 mice administered pioglitazone showed reduced microglia and astrocyte activation and reduced Aβ plaques in the hippocampus (Mandrekar‐Colucci et al., 2012). Pioglitazone increased microglial Aβ phagocytosis in the hippocampus in an AD mouse model (Yamanaka et al., 2012). Pioglitazone was shown to increase levels of APOE‐related genes, decrease proinflammatory genes and decrease Aβ levels in the hippocampus of an AD mouse model (Skerrett et al., 2015). Pioglitazone reduced tau phosphorylation at multiple sites in the cortex and CA1 region of the hippocampus and improved cognition in 3xTg mice (Adler et al., 2014). Similarly, administration of rosiglitazone reduced astrocytic and microglial activation, Aβ oligomers and aggregates, and spatial memory impairments in APP/PS1 mice (Toledo & Inestrosa, 2010). More recently, telmisartan, an antagonist for angiotensin receptor II type 1, commonly used for hypertension treatment, was shown to activate PPARγ with anti‐inflammatory and anti‐apoptotic effects (Khan et al., 2019). Telmisartan improved memory deficits seen in AD mouse models generated by ICV injection of STZ (Singh et al., 2013) and ICV injection of Aβ (Khan et al., 2019; Shindo et al., 2012; Tsukuda et al., 2009).
1.7.4. GLP‐1 agonists
Glucagon‐like peptide 1 (GLP‐1) is a hormone that is released by the gut, is involved in the gut/brain axis, protects insulin‐producing pancreatic β cells and assists in insulin secretion (Cabou & Burcelin, 2011). GLP‐1 can directly modulate neurotransmitter release, is involved in LTP and protects synapses involved in LTP from Aβ oligomer‐induced damage (Gault & Hölscher, 2008). GLP‐1 reduces oxidative stress, is involved in autophagy regulation and exhibits anti‐inflammatory protective functions (e.g., anti‐inflammatory signalling) in the CNS (Li et al., 2009). GLP‐1 signal transduction is mediated by the GLP‐1 receptor (GLP‐1R), a G‐protein‐coupled receptor (Grieco et al., 2019). GLP‐1R can activate the phosphatidylinositol 3‐kinase/protein kinase B (PI3K/AKT) pathway leading to protection against apoptosis and inhibition of pro‐inflammatory cytokines (Farilla et al., 2003; Grieco et al., 2019; Tramutola et al., 2017; Yang et al., 2018).
GLP‐1R agonist agents (e.g., exenatide, lixisenatide, liraglutide, semaglutide, etc.) lower glucose levels and reduce cognitive deficits observed in T2DM (Aroda, 2018; Gomez‐Peralta & Abreu, 2019; Grieco et al., 2019). Administration of exenatide to rats reduced neuroinflammation (i.e., TNF‐α) (Solmaz et al., 2015) and rescued LTP from Aβ‐induced compromise of hippocampus function (Wang et al., 2016, p. 4).
Administration of lixisenatide reduced NFTs, Aβ plaques and chronic neuroinflammation in the hippocampus of APP/PS1/tau female mice (Cai et al., 2018). The effects of lixisenatide are via the PI3K/AKT/GSK‐3β signalling pathway and can prevent spatial memory and synaptic insults that are induced by Aβ oligomers (Cai et al., 2014). Peripheral administration of lixisenatide in mice on HFDs showed improvement of recognition memory, increased numbers of immature neurons in the dentate gyrus and upregulation of hippocampal expression of neurotrophic tyrosine kinase receptor type 2 (NTRK2) and mammalian target of rapamycin (mTOR) involved in modulating synaptic plasticity and LTP (Lennox et al., 2014).
Administration of liraglutide to mice with STZ‐induced hyperglycaemia resulted in improved learning and memory and reduced neuronal death in the hippocampus (Palleria et al., 2017). Treatment with liraglutide before administration of STZ led to neuroprotective effects against STZ‐related hippocampal neuronal death and cognitive impairments associated with the AMPK/mTOR signalling pathway (Kong et al., 2018). Liraglutide prevented tau hyperphosphorylation in the hippocampus of db/db mice via activating insulin signalling pathways and suppressed GSK‐3β activation (Ma et al., 2015). Liraglutide has been reported to reduce tau hyperphosphorylation, prevent learning and memory impairments, and alleviate the structural changes of pyramidal neurons in the hippocampus of mice with ICV injection of Aβ (Qi et al., 2016)
A novel dual GLP‐1 and glucose‐dependent insulinotropic polypeptide (GIP) combination (i.e., DA‐JC4) prevented hyperphosphorylation of tau, reduced chronic neuroinflammation, decreased apoptotic signalling and improved IR sensitivity in ICV‐injected STZ rats (Shi et al., 2017). A GLP‐1/gastric inhibitory polypeptide dual agonist (DA5‐CH) reduced Aβ plaque load and phosphorylated tau protein and improved learning and memory in APP/PS1 mice (Grieco et al., 2019). A triple receptor agonist that activated GIP‐1, GIP and glucagon receptors simultaneously significantly reduced Aβ accumulation, neuroinflammation (e.g., activated astrocytes and microglia) and mitochondrial oxidative stress in the hippocampus and cortex of APP/PS1 mice; the animals exhibited improved learning and memory (Tai et al., 2018).
1.7.5. Commentary on non‐clinical and animal model observations linking T2DM and AD
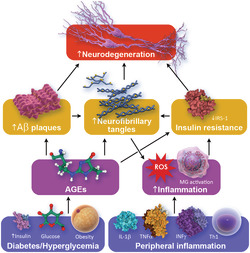
The non‐clinical studies reviewed provide an overview of the complex relationship between AD and T2DM. There is shared biology at the level of insulin resistance, IDE function, vascular effects, inflammation and genetic influences. Animal models have been informative regarding the adverse influences of T2DM features (e.g., hyperglycaemia and insulin resistance) on AD pathology, either by inducing AD‐like changes or exacerbating AD pathology in AD models. Animal studies provide substantial insight into the mechanistic effects of anti‐diabetes medications and how they affect both T2DM and AD‐related pathology and behaviour. Figure 1 shows the complex interaction of T2DM and AD biological mechanisms.
FIGURE 1.
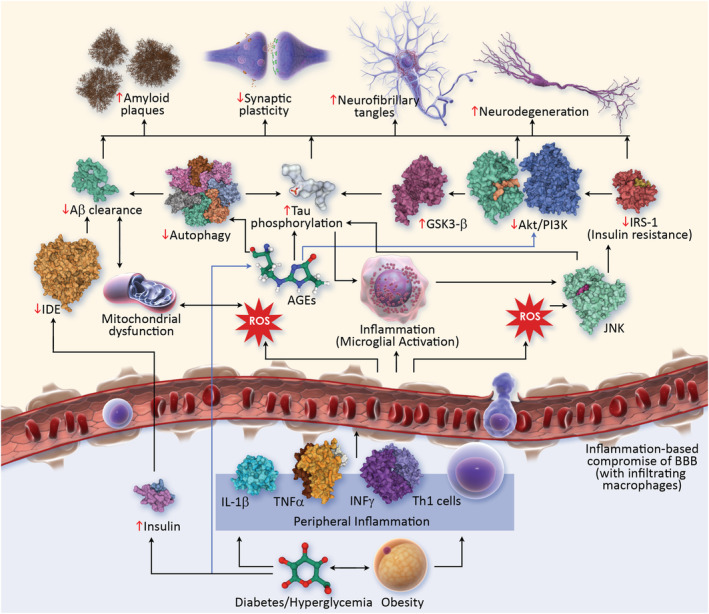
Pathophysiological links between T2DM and AD (AD, Alzheimer's disease; AGEs, advanced glycation end products; Akt, protein kinase B; BBB, blood–brain barrier; IDE, insulin degrading enzyme; IL1‐B, interleukin 1 B; INF, interferon‐gamma; IRS1, insulin receptor substrate 1; GSK3B, glycogen synthase kinase‐3B; JNK, c‐Jun N‐terminal kinase; PI3K, phosphoinositide 3 kinase; ROS, reactive oxygen species; T2DM, type 2 diabetes mellitus; Th1, T helper cells 1; TNF‐α, tumour necrosis factor‐α)
1.8. Clinical trials of anti‐diabetic drugs for the treatment of AD
The many links between diabetes and AD suggest that therapies for T2DM might be beneficial in the treatment of AD. We performed a review of clinicaltrials.gov, a comprehensive registry of clinical trials identifying all T2DM therapies in trials for AD. We identified 10 trials assessing the impact of insulin in AD and 20 AD trials involving 10 non‐insulin anti‐diabetic agents conducted since 2006. Among the insulin trials: eight were completed, one was terminated, and one was withdrawn. Among the non‐insulin anti‐diabetic agents, seven trials are on‐going, eight trials have been completed, and five trials were terminated. Table 1 provides the details of the insulin trials including the trial phase, intended number of participants, Mini‐Mental Status (MMSE) score range of the eligible participants, treatment duration in the trial and clinical and biomarker outcomes included in the trial. Table 2 provides the same information for the non‐insulin anti‐diabetic agents. Classes of agents represented in the trials include insulin, biguanides (e.g., metformin and metformin‐extended release [XR]), thiazolidinediones (e.g., pioglitazone and rosiglitazone), sodium‐glucose co‐transporter 2 (SGLT2) inhibitors (e.g., dapagliflozin and empagliflozin) and GLP‐1 analogs (e.g., exenatide, liraglutide and semaglutide).
TABLE 1.
Characteristics of trials of insulin for the treatment of Alzheimer's disease
| Trial number | Agent | Date registered | Trial status | Trial phase | Enrolment | MMSE range | Treatment duration | Primary clinical outcomes | Outcome biomarkers |
|---|---|---|---|---|---|---|---|---|---|
| NCT00581867 | Insulin aspart | 12/19/07 | Completed |
Phase 1 Phase 2 |
31 | Single dose | fMRI measure of hippocampal activation | ||
| NCT02503501 | Insulin glulisine | 7/14/15 | Terminated | Phase 2 | 49 | MoCA 18–27 | 26 weeks |
ADAS‐cog13 CDR |
CSF Aβ 42 CSF tau CSF phosphotau cerebral glucose metabolism via FDG PET |
| NCT01145482 | Insulin aspart | 6/15/10 | Completed | Not applicable | 12 | >15 | 2 doses | Cerebral glutamate concentration | |
| NCT02462161 | Insulin aspart | 6/1/15 | Completed | Phase 1 | 24 | 12 weeks |
ADAS‐cog MCI scores |
CSF Aβ CSF tau Plasma Aβ Plasma tau Cortical thickness in AD‐vulnerable regions Inflammatory markers |
|
| NCT01636596 | Insulin lispro | 7/6/12 | Withdrawn | Not applicable | 0 | ≥15 | 26 weeks |
MMSE CDT |
Cerebral glucose metabolism via FDG PET Basal metabolism |
| NCT01436045 | Insulin glulisine | 9/16/11 | Completed | Phase 2 | 12 | 18–26 | Single dose |
Trails B ‐ seconds Trails B ‐ errors Cognitive performance via RBANS |
|
| NCT01767909 | Insulin regular (Humulin R) | 1/10/13 | Completed |
Phase 2 Phase 3 |
240 | ≥20 | 26 weeks | ADAS‐cog12 |
CSF Aβ CSF tau Degree of hippocampal/entorhinal atrophy |
| NCT01595646 | Insulin detemir | 5/8/12 | Completed | Phase 2 | 37 | 16 weeks | Verbal memory composite (delayed story recall and Buschke selective reminding test) |
CSF Aβ CSF tau CSF TTau‐P181/Aβ 42 ratio Plasma Aβ Plasma tau Cerebral blood flow via MRI OGTT |
|
| NCT01547169 | Insulin detemir | 2/7/12 | Completed | Phase 2 | 60 | 3 weeks | Verbal memory composite (immediate + delayed story recall and immediate + delayed list recall) |
Plasma Aβ Plasma tau OGTT |
|
| NCT00438568 | Insulin regular (Novolin R) | 7/21/2007 | Completed | Phase 2 | 173 | 16 weeks | Changes in cognition |
CSF Aβ Plasma Aβ Cerebral glucose metabolism via PET |
Abbreviations: Aβ, amyloid beta; ADAS‐cog, Alzheimer's Disease Assessment Scale ‐ Cognitive Subscale; CSF, cerebrospinal fluid; CT, computed tomography; FDG PET, fluorodeoxyglucose positron emission tomography; GDS, Global Deterioration Scale; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Exam; MRI, magnetic resonance imaging; MoCA, Montreal Cognitive Assessment; OGTT, Oral Glucose Tolerance Test; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
TABLE 2.
Characteristics of trials of non‐insulin anti‐diabetic agents tested for the treatment of Alzheimer's disease
| Trial number | Agent | Date registered | Trial status | Trial phase | Enrolment | MMSE range | Treatment duration | Primary clinical outcomes | Outcome biomarkers |
|---|---|---|---|---|---|---|---|---|---|
| NCT04098666 | Metformin XR | 9/19/19 | Recruiting |
Phase 2 Phase 3 |
370 | ≥20 | 104 weeks | FCSRT | Brain Aβ SUVR, brain tau SUVR, plasma Aβ |
| NCT01965756 | Metformin | 10/16/13 | Completed | Phase 2 | 20 | >21 | 16 weeks | ADAS‐cog | CSF Aβ, Total tau, phosphorylated tau concentration |
| NCT00620191 | Metformin | 2/7/08 | Completed | Phase 2 | 80 | ≥20 | 52 weeks | ADAS‐cog, Total recall score in SRT | Plasma Aβ‐42 |
| NCT00982202 | Pioglitazone | 9/22/09 | Completed | Phase 2 | 25 | 12–26 | 78 weeks | ADAS‐cog, CDR‐SB, CIBIC‐plus | Laboratory abnormalities |
| NCT01931566 | Pioglitazone | 8/26/13 | Terminated | Phase 3 | 3,494 | ≥25 | Up to 260 weeks | Time to diagnosis of MCI‐AD | |
| NCT02284906 | Pioglitazone | 11/4/14 | Terminated | Phase 3 | 40 | ≥25 | 104 weeks | Change from extension study baseline in composite score of cognitive test battery | |
| NCT04251182 | T3D‐959 | 1/24/20 | Recruiting | Phase 2 | 256 | 14–26 | 24 weeks | ADAS‐cog11, ADCS‐CGIC | Plasma Aβ 42/40 ratio |
| NCT00428090 | Rosiglitazone XR | 1/25/07 | Completed | Phase 3 | 862 | 10–23 | 24 weeks | ADAS‐cog, CIBIC+ global functioning Total score | HgbA1c, body weight |
| NCT00348309 | Rosiglitazone XR | 6/30/06 | Completed | Phase 3 | 1,496 | 10–26 | 52 weeks | ADAS‐cog, CDR‐SB | HgbA1c |
| NCT00490568 | Rosiglitazone XR | 6/21/07 | Terminated | Phase 2 | 1,461 | 10–26 | 76 weeks | AEs | HgbA1c, body weight |
| NCT00348140 | Rosiglitazone XR | 6/30/06 | Completed | Phase 3 | 1,468 | 10–26 | 54 weeks | ADAS‐cog, CDR‐SB | HgbA1c, body weight |
| NCT00550420 | Rosiglitazone XR | 10/25/07 | Terminated | Phase 3 | 331 | 10–26 | 52 weeks | AEs | HgbA1c, body weight |
| NCT01374438 | MSDC‐0160 (Mitoglitazone) | 6/14/11 | Completed | Phase 2 | 29 | ≥20 | 12 weeks | Cerebral glucose metabolic rate | High molecular weight adiponectin |
| NCT03801642 | Dapagliflozin | 12/21/18 | Recruiting |
Phase 1 Phase 2 |
48 | 15–26 | 12 weeks | Cerebral N‐acetylaspartate | Cerebral glucose metabolism via FDG PET, HgbA1c, Plasm β‐hydroxybutyrate, activated AKT levels, mTOR phosphorylation, platelet cytochrome oxidase activity, MCP‐1, Eotaxin‐1, TNF‐α, CRP |
| NCT03852901 | Empagliflozin | 2/22/19 | Recruiting | Phase 1 | 100 | 2 weeks | Ketone bodies | Ketone bodies | |
| NCT01255163 | Exenatide | 12/4/10 | Terminated | Phase 2 | 57 | >20 | 78 weeks | Incidence of nausea | CSF total tau, CSF p181‐tau, CSF Aβ‐42, BMI |
| NCT01843075 | Liraglutide | 4/24/13 | Active, not recruiting | Phase 2 | 204 | ≥20 | 52 weeks | Cerebral glucose metabolic rate | Cortical amyloid, tau deposition, microglial activation |
| NCT01469351 | Liraglutide | 11/1/11 | Completed | Not applicable | 34 | ≥18 | 26 weeks | Intracerebral amyloid deposition via PIB PET scan | Intracerebral amyloid deposition, glucose uptake in CNS by FDG PET |
| NCT04777396 | Semaglutide | 2/26/21 | Recruiting | Phase 3 | 1,840 | ≥22 | 173 weeks | CDR‐SB | High sensitivity CRP level |
| NCT04777409 | Semaglutide | 2/26/21 | Recruiting | Phase 3 | 1,840 | ≥22 | 173 weeks | CDR‐SB |
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; ADAS‐cog, Alzheimer's Disease Assessment Scale ‐ Cognitive Subscale; ADCS, Alzheimer's Disease Cooperative Study; ADFACS, Alzheimer's Disease Functional Assessment of Change Scale; ADL, Activities of Daily Living; ADLMCI, Activities of Daily Living Scale for Mild Cognitive Impairment; AEs, adverse events; BMI, body mass index; CDR‐SB, Clinical Dementia Rating Scale Sum of Boxes; CGIC, Clinical Global Impressions of Change; CIBIC‐Plus, Clinician's Interview‐Based Impression of Change Plus Caregiver Input; CRP, C‐reactive protein; CSF, cerebrospinal fluid; FCSRT, Free and Cued Selective Reminding Test; FDG PET, fluorodeoxyglucose positron emission tomography; HgbA1c, haemoglobin‐A1c; MCI, mild cognitive impairment; MCP‐1, monocyte chemotactic protein 1; MMSE, Mini‐Mental State Exam; mTOR, mammalian target of rapamycin; SRT, spaced retrieval training; SUVR, standardized uptake value ratio; TNF, tumour necrosis factor; XR, extended release.
1.8.1. Insulin
In most insulin trials investigating effects on memory or AD, insulin is delivered by intranasal administration to avoid hypoglycemia (Craft et al., 2012a). A trial of memory effects 15 minutes after intranasal insulin in normal controls or patients with mild‐moderate AD dementia or MCI (N = 61) showed improvement in those without the APOE‐4 genotype (Reger et al., 2006). A 21‐day randomized controlled study (N = 25) using intranasal administration of insulin found improved memory, attention and function (Reger et al., 2008). In a study comparing two doses of insulin with placebo, Craft and colleagues showed an improvement in memory in the low‐dose group (Craft et al., 2012a). There were no effects of insulin on CSF amyloid levels and modest effects on stabilizing decline of hypometabolism on fluorodeoxyglucose (FDG) and positron emission tomography (PET) among participants treated for 4 months (N = 104) (Craft et al., 2012a). A 4‐month study (N = 36) comparing regular insulin, long‐acting insulin, and placebo found improved memory and change in the Aβ42/p‐tau ratio in the CSF in those receiving regular insulin (Craft et al., 2017). In a larger 12‐month study (N = 289), no drug‐placebo differences were observed on the AD Assessment Scale – cognitive subscale (ADAS‐cog) or CSF markers of AD (Craft et al., 2020). Treatment of participants with mild to moderate AD given intranasal insulin for 2 days following a course of high‐dose Vitamin D demonstrated improved memory on some assessments and not others (Stein et al., 2011). A blinded cross‐over study of rapid‐acting insulin (i.e., glulisin insulin) in patients with the APOE4 genotype reported no benefit for memory function (Rosenbloom et al., 2014).
No insulin trials have been rigorously conducted using contemporary standards of diagnosis (e.g., biological confirmation of the diagnosis of AD). Many of the trials have involved relatively small numbers of patients. Some of the trials have shown measurable effects on recent memory; few have shown effects on other cognitive functions, global ratings or functional assessments. Overall, trials of insulin for the treatment of AD do not comprise a body of evidence in favour of this intervention in patients with symptomatic AD.
1.8.2. Biguanides (metformin, metformin‐XR)
Metformin was assessed for its cognitive benefits in a population of patients with MCI and obesity. Eighty participants were randomized to metformin or placebo. Few subjects (10%) tolerated the intended dose of 1,000 mg twice daily. Primary outcomes were the Selective Reminding Test (SRT) and the ADAS‐cog. At trial end, there was a significant drug‐placebo difference in favour of metformin on the SRT; other measures including FDG PET showed not drug‐placebo difference (Luchsinger et al., 2004). In another trial, 20 non‐diabetic subjects with MCI or mild AD dementia were randomized to active treatment or placebo in an 8‐week cross‐over study. The metformin group exhibited significantly improved executive function and a trend towards improved memory and learning; no drug‐placebo difference was seen on other cognitive assessments or on measures of cerebral blood flow (Koenig et al., 2017). Given its pleiotropic effects, metformin has been proposed as an anti‐aging and multi‐organ disease modifying intervention; trials have been designed in which cognition would be included among several age‐related outcomes (Justice et al., 2018).
Metformin has not been extensively assessed in clinical trials. Trials for which results are available offer modest support for further testing in AD. Improved diagnostic specificity, larger sample sizes, and longer durations of treatment are required to thoroughly test metformin for possible efficacy in AD.
1.8.3. Thiazolidinediones (pioglitazone, rosiglitazone)
Thiazolidinedione PPARγ agonists exert anti‐diabetic effects, lowering peripheral insulin and increasing insulin sensitivity. In a randomized unblinded study of 32 participants with MCI or mild to moderate AD dementia treated for 6 months with pioglitazone, those on active treatment had significantly improved scores on the ADAS‐cog and a test of logical memory. No drug‐placebo difference was observed on the MMSE (Hanyu et al., 2009). In a 6‐month randomized unblinded study of 42 patients with mild AD dementia and T2DM, those on active treatment exhibited improved cognition and increased blood flow in the parietal lobes not seen in those assigned to placebo. A decrease in fasting peripheral insulin levels suggested improved insulin sensitivity in those on pioglitazone (Sato et al., 2011). A randomized, double‐blind 18‐month trial of 25 non‐diabetic participants with AD dementia showed pioglitazone to be safe. No cognitive benefits were observed (Geldmacher et al., 2011).
Pioglitazone has been assessed in a Phase 3 AD prevention trial (the TOMMORROW trial) intended to determine whether treatment would delay time to progression to MCI due to AD. Eligible participants were stratified based a combination of TOMM40 rs 10524523 genotype, APOE genotype and age, with high‐risk individuals receiving low‐dose pioglitazone or placebo and low‐risk individuals receiving placebo. A sample size of 2,346 participants was estimated to be required to assess the intended outcome (Burns et al., 2019). During the study, the sample size was reduced and the trial shortened. No drug‐placebo difference was observed in time to progression to MCI due to AD at the time of trial termination.
Rosiglitazone is a PPARγ agonist assessed for possible efficacy in the treatment of AD. A 6‐month Phase 2 proof‐of‐concept study with 30 participants randomized to rosiglitazone or placebo demonstrated significant improvement in delayed recall and selective attention in the active treatment group (Watson et al., 2005). A 6‐month Phase 2 randomized controlled trial including 511 participants assessed cognitive and global outcomes for 2, 4 and 8 mg of rosiglitazone compared with placebo. No drug‐placebo difference was observed on the primary outcomes for any dose. An exploratory analysis of APOE4 non‐carriers showed significant benefit on the ADAS‐cog for those on active treatment (Risner et al., 2006). A follow‐up 24‐week Phase 3 study comparing rosiglitazone 2 mg, rosiglitazone XR 8 mg, donepezil and placebo (N = 693) found no drug‐placebo difference on cognitive or global measures in the total population or the APOE4 non‐carriers. Participants in the donepezil arm of the study had no drug‐placebo difference on the ADAS‐cog raising questions about the trial conduct. The participants receiving donepezil exhibited a significant benefit of treatment as measured on the Clinician's Interview‐Based Impression of Change with Caregiver Input (CIBIC+) (Gold et al., 2010). In a 12‐month randomized controlled trial of rosiglitazone XR compared with placebo using FDG PET as the primary outcome, participants on active treatment showed a non‐significant increase in glucose metabolism in the first month of treatment. No drug‐placebo differences were observed in decline in glucose metabolism, rate of cerebral atrophy, or cognitive measures (Tzimopoulou et al., 2010). Two Phase 3 48‐week randomized controlled trials including nearly 3,000 patients found no drug‐placebo difference on cognitive or global measures in the total study population, APOE4 non‐carriers or all patients except APOE4 homozygotes (Harrington et al., 2011). A follow‐up study of these trials showed that a predefined 6‐protein metabolic and inflammatory biomarker panel (i.e., IL‐6, IL‐10, C‐reactive protein [CRP], TNF‐α, heart‐type fatty acid‐binding protein 3 [FABP‐3] and pancreatic polypeptide [PPY]) correctly identified a treatment response among those receiving rosiglitazone with 98% accuracy (O'Bryant et al., 2021).
These large studies of rosiglitazone in mild‐moderate AD dementia demonstrate no treatment benefit. The post hoc analysis of responders with a characteristic biomarker profile leaves open the possibility that within the population, there is a subgroup of rosiglitazone‐responsive individuals.
1.8.4. SGLT2 inhibitors (dapagliflozin, empagliflozin)
SGLT2 inhibitors reduce blood glucose levels by inhibiting glucose reabsorption by the kidney, inducing glucosuria. They reduce fasting and postprandial blood glucose levels, body weight and blood pressure. SGLT2 inhibitors reduce mTOR kinase activity that may contribute to lysosomal and mitochondrial dysfunction in AD. mTOR activity is associated with BBB endothelial cell dysfunction, tau hyperphosphorylation and Aβ plaque formation (Esterline et al., 2020). The first trials of SGLT2 inhibitors in AD have been inaugurated.
1.8.5. Incretin mimetics/GLP‐1 analogs (exenatide, liraglutide, semaglutide)
The National Institute on Aging (NIA) with support from AstraZeneca, conducted an 8‐month, double‐blind, randomized, placebo‐controlled Phase 2 clinical trial to assess the safety and tolerability of exenatide in early AD. Eighteen participants with high probability of AD completed the entire study prior to its early termination. Exenatide was shown to be safe and well‐tolerated in this population. Among outcomes assessed, there were no differences compared with placebo for clinical and cognitive measures, magnetic resonance imaging (MRI) assessments of cortical thickness and volume, or biomarkers in CSF, or plasma. There was a reduction in Aβ42 in neuronal extracellular vesicles. The investigators note that the study was underpowered due to early termination, and firm conclusions regarding efficacy cannot be drawn (Mullins et al., 2019).
In a 26‐week, randomized, placebo‐controlled, double‐blind trial conducted in Demark and including 24 non‐diabetic participants with AD, those treated with liraglutide were noted to have no difference in amyloid deposition or cognition compared with those on placebo. The researchers note that in patients with long‐standing AD, the 26 weeks of liraglutide treatment had prevented the expected decline of regional cerebral glucose measure on FDG PET (Gejl et al., 2016).
Liraglutide's effect on cerebral glucose metabolism and cognitive function was assessed in a 12‐month, multicentre, randomized, double‐blind, placebo‐controlled, Phase 2 trial (Evaluating Liraglutide in AD [ELAD] study). Two hundred and four participants diagnosed with probable AD were enrolled (Femminella et al., 2019). No drug‐placebo differences were observed in the primary outcome of cerebral glucose metabolism or in the secondary outcomes of Clinical Dementia Rating ‐ Sum of Boxes (CDR‐SB) score and AD Cooperative Study – Activities of Daily Living (ADCS‐ADL) scale. An improvement was noted in the AD Assessment Scale – Executive version (ADAS‐Exec), and there was reduced loss of temporal lobe and total brain grey matter volume in participants receiving liraglutide (Alzforum, 2021).
Novo Nordisk A/S is currently recruiting for two trials investigating semaglutide in participants with early AD, the EVOKE and EVOKE+ trials. Participant diagnosis is confirmed by amyloid PET or CSF amyloid measures consistent with AD. The primary outcome is drug‐placebo difference in change in cognition from baseline using the CDR‐SB score (ClinicalTrials.gov, 2021). A secondary outcome is time to progression to AD dementia in those with MCI at trial baseline. Participants of these trials have amyloid PET at baseline to confirm the diagnosis of AD. EVOKE+ allows participants with subcortical cerebrovascular disease to enter the study, the EVOKE trial does not allow such participants.
1.8.6. Trial commentary
Among the insulin trials, two were Phase 1 trials, seven were Phase 2 trials, and one was a Phase 3 trial (two trials did not have identified phases). The trials of non‐insulin anti‐diabetic agents included two Phase 1 trials, nine Phase 2 trials and nine Phase 3 trials (one trial had no declared phase). The non‐insulin agents in Phase 3 included metformin (one trial), pioglitazone (two trials), rosiglitazone (four trials) and semaglutide (two trials).
Some insulin trials showed clinical benefit in exploratory trials (Claxton et al., 2015; Craft et al., 2012b, 2017). Very acute trials with intravenous insulin suggested improvement on memory measures (Craft et al., 2003; Watson et al., 2009). These positive outcomes were not replicated in larger longer term studies (e.g., 6‐ to 12‐month duration). All the completed studies on the use of non‐insulin T2DM treatments for AD have been negative; seven of the 20 are currently ongoing. A trial with liraglutide showed a numerical but not significant stabilization of brain metabolism on FDGF PET contrasting with metabolic decline in the placebo group (Gejl et al., 2016). The observation supports the suggestion that GLP‐1 agonists have CNS effects despite not crossing the BBB.
Of the 20 non‐insulin trials of agents used to treat T2DM, only four confirmed the diagnosis of AD with amyloid biomarkers. Of the 10 insulin trials, none had confirmatory amyloid biomarkers assessed. Studies show that of patients recruited for trials of early AD (MCI due to AD and mild AD dementia) and exhibiting AD clinical features, 40% are excluded due to negative amyloid scans (Sevigny et al., 2016). Similarly, in a trial of mild‐moderate AD, 30% of patients with the mild AD phenotype and 15% of these diagnosed with moderate AD had negative amyloid imaging (Degenhardt et al., 2016). These observations suggest that most of the trials of insulin and of non‐insulin T2DM therapies not using diagnostic biomarkers likely have included 15%–40% of participants who lack the biology of AD; the canonical biology of AD—Aβ plaque formation—is absent in these amyloid negative participants. Non‐amyloid bearing participants do not have AD and exhibit slower decline, compromising the ability to observe treatment‐placebo differences (Ballard et al., 2019). Inclusion of non‐AD patients may have negatively affected the outcomes of these trials.
There has been only one AD prevention trial using an anti‐diabetic agent—an assessment of pioglitazone in cognitively normal individuals at increased genetic risk for AD (Burns et al., 2019). This study was terminated before the planned outcomes could be determined. Based on the increased risk for AD observed in epidemiologic studies of T2DM and the animal model studies demonstrating the ability to induce aspects of AD pathology with hyperglycaemia, additional long duration prevention trials are warranted.
Ten of the non‐insulin trials include individuals with MMSE scores of 20 or above consistent with early AD; the other trials include patients with mild‐moderate AD dementia. Entry criteria for the insulin trials often did not specify an MMSE range; of those providing this information, most of the trials included patients with mild or moderate AD dementia. No patients with severe dementia are included in any of the trials.
None of the insulin trials and two of the non‐insulin trials required participants to have risk factors for T2DM. In both trials including T2DM risks, AD with concomitant obesity was the identified trial population.
The primary outcomes of the trials commonly included the ADAS‐cog, CDR‐SB or measures of memory and delayed recall. Cerebral glucose metabolism measured by FDG PET, ketone bodies or adverse events were primary outcomes in some trials. Diabetes‐related biomarkers including haemoglobin‐A1c (HgbA1c) and CRP were included in eight of 20 non‐insulin trials and none of the insulin trials.
Trial duration and number of participants vary greatly among these studies. Insulin trials recruited between 12 and 240 participants and were from a few hours for single dose studies to 24 weeks in duration. Non‐insulin trials vary between short observation periods of 2 weeks (empagliflozin) to studies intended to have up to 5 years of exposure (pioglitazone). Non‐insulin trial populations have varied between 20 and 1,840 trial participants among completed or ongoing trials. The size and the duration of the trials are determined by the specific question being asked and the anticipated effect size of the therapy. Industry‐sponsored trials are typically larger and longer than academic or government‐sponsored trials. Short‐term trials can detect improvement above baseline and the possible cognitive enhancing properties of T2DM agents; they can assess short‐term effects on biomarkers that may predict benefits with longer term treatment. Trials of 12‐month duration or longer are required to collect clinical and biomarker data showing slowing of cognitive decline supportive of disease modification (Cummings, 2019; Cummings et al., 2018).
2. DISCUSSION
Many avenues of information link T2DM with the AD continuum. Animal models of T2DM with hyperglycaemia exhibit AD‐type pathological changes in the brain including tau hyperphosphorylation, tau aggregates and neuroinflammation (Engin & Engin, 2021; Murtishaw et al., 2018). Double and triple Tg mouse models of AD exhibit insulin resistance and energy dyshomeostasis similar to that observed T2DM patients (Velazquez et al., 2017). Amyloid pathology is more severe in several of the studies using AD model animals with concomitant physiological changes of T2DM. In humans, T2DM increases the risk for AD. T2DM is associated with obesity, and obesity is a risk for AD. Epidemiologic observations suggest that treatment of T2DM with some types of agents including rosiglitazone and GLP‐1 agonists is associated with a diminished risk for AD compared with those treated with other agents (Akimoto et al., 2020). The biology of AD includes insulin resistance similar to that seen in peripheral tissues of T2DM patients (Talbot et al., 2012b).
Peripheral inflammation stimulates neuroinflammation through transfer of inflammatory exosomes to the brain from the periphery and entry of peripheral inflammatory cells through a compromised BBB (Li et al., 2018; Pugazhenthi et al., 2017; Ransohoff, 2016; Zlokovic, 2008); both diabetes and obesity exhibit chronic low‐grade inflammation in association with insulin resistance (Osborn & Olefsky, 2012). Some classes of T2DM therapies—metformin and GLP‐1 agonists—reduce peripheral inflammation and this may contribute to both their anti‐diabetic properties and the reduction in AD incidence reported with their use (Lee & Jun, 2016; Saisho, 2015). GLP‐1 agonists may facilitate insulin entry in the brain, reduce insulin resistance, decrease neuroinflammation and restore neurogenesis in the absence of elevated peripheral inflammation suggesting that they may be useful in the treatment of non‐diabetic AD patients (Bae & Song, 2017; Cai et al., 2018).
Questions to be resolved in applying T2DM agents to AD are raised by non‐clinical and clinical observations. Animal models of the relationship of T2DM to AD most often involve STZ‐induced hyperglycaemia and investigation of the ensuing AD‐like pathological changes observed in the brain (Murtishaw et al., 2018). This is a strong model of the cognitive and pathological changes occurring in diabetes, diabetes as a risk factor for AD and brain changes in pre‐diabetes. It is less suited to the study of AD in the absence of T2DM. Most AD patients do not have T2DM, peripheral insulin resistance or hyperglycaemia, although an undetermined number may have pre‐diabetes. Among the studies reporting an increased incidence of AD in patients with T2DM, few had AD diagnostic confirmation with amyloid biomarkers, and—given the common occurrence of cerebrovascular disease in T2DM—the dementia syndromes observed may be AD, vascular dementia or mixed dementia (Ahtiluoto et al., 2010; R. Yang et al., 2019). The reductions in dementia incidence observed with treatment with T2DM agents typically occurred in samples observed for many years in registries or in long diabetes trials; this may translate into challenges showing reductions in dementia with shorter term trials and in patients without predisposing T2DM. Agents that reduce dementia incidence in T2DM cohorts do not necessarily exert effects on AD biology once the disease is expressed in the brain or may have limited effects that are difficult to demonstrate in trials. Some agents used for the treatment of T2DM are of high molecular weight and may pass the BBB in only limited amounts. More investigation of the central versus peripheral effects of these agents is needed to optimize effects on AD. Novel GLP‐1/GIP mimetics have shown neuroprotective effects in animal models of neurodegenerative disease and may warrant testing in next generation clinical trials (Holscher, 2018; Zhang et al., 2020).
An unresolved issue central to clinical trial design is whether to anticipate cognitive improvement as observed in short‐term (e.g., 6 months) cognitive enhancer‐type trials or to conduct disease‐modifying‐type trials that are longer and designed to determine if there is slowing of clinical decline (e.g., 12–24 months). Biomarkers relevant to cognitive enhancer‐type trials might include electroencephalography, FDG PET, of functional MRI. Biomarkers relevant to trials designed to show disease modification are those reflective of AD biology including measures of amyloid, tau, inflammation, and neurodegeneration. Anti‐diabetic agents may improve neuronal function producing cognitive enhancement; they may enhance neuronal survival leading to slowing of disease progression; or they may do both. Defining the expected response to these treatments is critical to designing trials most likely to capture a treatment effect. Trials in preclinical populations at‐risk for AD with DM or pre‐diabetes are another avenue of study of these agents.
New data on the relationship of T2DM and AD are accruing rapidly, and more insight into unresolved issues is anticipated. The identification of discrete mechanisms/signalling cascades that link T2DM and AD is needed to differentiate interventions for diabetic patients at risk for developing AD versus treatments targeting the core AD biology that overlaps with T2DM. If therapeutic benefits of agents used to treat T2DM can be extended to AD based on the many links between these two disorders, development of novel therapeutic regimens for patients with AD will be facilitated.
CONFLICT OF INTEREST
JC has provided consultation to AB Science, Acadia, Alkahest, AlphaCognition, ALZPath, Annovis, AriBio, Artery, Avanir, Biogen, Cassava, Cerevel, Clinilabs, Cortexyme, Diadem, EIP Pharma, Eisai, GatehouseBio, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lexeo, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Otsuka, PharmacotrophiX, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, Unlearn AI, Vaxxinity, VigilNeuro, Zai Laboratories pharmaceutical, assessment and investment companies. AO, JC and JK have no conflicts of interest.
AUTHOR CONTRIBUTIONS
C.D. and D.E. verified the analytical methods. B.C. encouraged A.B. to investigate (a specific aspect) and supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15619.
ACKNOWLEDGEMENTS
JC is supported by National Institute of General Medical Sciences grant P20GM109025; National Institute of Neurological Disorders and Stroke grant U01NS093334; National Institute on Aging grant R01AG053798; P20AG068053; R35AG71476; the Alzheimer's Disease Drug Discovery Foundation (ADDF); and the Joy Chambers‐Grundy Endowment. AO and JC have no acknowledgements. JK is supported by NIH grant R56AG062762
Cummings, J. , Ortiz, A. , Castellino, J. , & Kinney, J. (2022). Diabetes: Risk factor and translational therapeutic implications for Alzheimer's disease. European Journal of Neuroscience, 56(9), 5727–5757. 10.1111/ejn.15619
Edited by: Srikant Rangaraju
Funding information National Institute of Neurological Disorders and Stroke, Grant/Award Number: U01NS093334; NIH, Grant/Award Number: R56AG062762; Joy Chambers‐Grundy Endowment; Alzheimer's Disease Drug Discovery Foundation (ADDF); National Institute on Aging, Grant/Award Numbers: P20AG068053, R01AG053798, R35AG71476; National Institute of General Medical Sciences, Grant/Award Number: P20GM109025
DATA AVAILABILITY STATEMENT
All new data in this article are derived from clinicaltrials.gov and are freely publicly available. Our analyses as applied to the data are described in the methods section and can be reproduced. A web‐portal to enhance access to clinical trial data is under construction and will be available by contacting the lead author or by searching Alzheimer's Clinical Trial Innovation (ACTION) Initiative.
REFERENCES
- Abbott, M. A. , Wells, D. G. , & Fallon, J. R. (1999). The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(17), 7300–7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, B. L. , Yarchoan, M. , Hwang, H. M. , Louneva, N. , Blair, J. A. , Palm, R. , Smith, M. A. , Lee, H. , Arnold, S. E. , & Casadesus, G. (2014). Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiology of Aging, 35(4), 793–801. 10.1016/j.neurobiolaging.2013.10.076 [DOI] [PubMed] [Google Scholar]
- Adzovic, L. , Lynn, A. E. , D'Angelo, H. M. , Crockett, A. M. , Kaercher, R. M. , Royer, S. E. , Hopp, S. C. , & Wenk, G. L. (2015). Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. Journal of Neuroinflammation, 12, 63–63. 10.1186/s12974-015-0282-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar, L. R. , Acosta‐Uribe, J. , Giraldo, M. M. , Moreno, S. , Baena, A. , Alzate, D. , Cuastumal, R. , Aguillón, D. , Madrigal, L. , Saldarriaga, A. , Navarro, A. , Garcia, G. P. , Aguirre‐Acevedo, D. C. , Geier, E. G. , Cochran, J. N. , Quiroz, Y. T. , Myers, R. M. , Yokoyama, J. S. , Kosik, K. S. , & Francisco, L. R. (2019). Genetic origin of a large family with a novel psen1 mutation (ILE416THR). Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 15(5), 709–719. 10.1016/j.jalz.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, S. (2012). Diabetes: An old disease, a new insight. Springer Science+Business Media. [Google Scholar]
- Ahtiluoto, S. , Polvikoski, T. , Peltonen, M. , Solomon, A. , Tuomilehto, J. , Winblad, B. , Sulkava, R. , & Kivipelto, M. (2010). Diabetes, Alzheimer disease, and vascular dementia: A population‐based neuropathologic study. Neurology, 75(13), 1195–1202. 10.1212/WNL.0b013e3181f4d7f8 [DOI] [PubMed] [Google Scholar]
- Akimoto, H. , Negishi, A. , Oshima, S. , Wakiyama, H. , Okita, M. , Horii, N. , Inoue, N. , Ohshima, S. , & Kobayashi, D. (2020). Antidiabetic drugs for the risk of Alzheimer disease in patients with type 2 DM using FAERS. American Journal of Alzheimer's Disease & Other Dementias, 35, 1533317519899546. 10.1177/1533317519899546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard, J. S. , Perez, E. J. , Fukui, K. , Carpenter, P. , Ingram, D. K. , & de Cabo, R. (2016). Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behavioural Brain Research, 301, 1–9. 10.1016/j.bbr.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann, A. , Tian, L. , Henderson, V. W. , & Greicius, M. D. (2014). Sex modifies the APOE‐related risk of developing Alzheimer's disease. Annals of Neurology, 75(4), 563–573. 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzforum (2021). Therapeutics. Liraglutide. https://www.alzforum.org/therapeutics/liraglutide
- Alzheimer's Association . (2021). 2021 Alzheimer's disease facts and figures. Alzheimer's & Dement, 17(3), 327–406. [DOI] [PubMed] [Google Scholar]
- Arnold, S. E. , Arvanitakis, Z. , Macauley‐Rambach, S. L. , Koenig, A. M. , Wang, H.‐Y. , Ahima, R. S. , Craft, S. , Gandy, S. , Buettner, C. , Stoeckel, L. E. , Holtzman, D. M. , & Nathan, D. M. (2018). Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nature Reviews Neurology, 14(3), 168–181. 10.1038/nrneurol.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroda, V. R. (2018). A review of GLP‐1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes, Obesity & Metabolism, 20(Suppl 1), 22–33. 10.1111/dom.13162 [DOI] [PubMed] [Google Scholar]
- Association, A. D . (2021). Classification and diagnosis of diabetes: Standards of medical Care in Diabetes—2021. Diabetes Care, 44(Supplement 1), S15–S33. 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- Avgerinos, K. I. , Kalaitzidis, G. , Malli, A. , Kalaitzoglou, D. , Myserlis, P. G. , & Lioutas, V.‐A. (2018). Intranasal insulin in Alzheimer's dementia or mild cognitive impairment: A systematic review. Journal of Neurology, 265(7), 1497–1510. 10.1007/s00415-018-8768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C.S., Song J (2017). The Role of Glucagon‐Like Peptide 1 (GLP1) in Type 3 Diabetes: GLP‐1 Controls Insulin Resistance, Neuroinflammation and Neurogenesis in the Brain. International Journal of Molecular Sciences, 18, (11), 2493. 10.3390/ijms18112493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, P. , Kiss, T. , Tarantini, S. , Nyúl‐Tóth, Á. , Ahire, C. , Yabluchanskiy, A. , Csipo, T. , Lipecz, A. , Tabak, A. , Institoris, A. , Csiszar, A. , & Ungvari, Z. (2021). Obesity‐induced cognitive impairment in older adults: A microvascular perspective. American Journal of Physiology‐Heart and Circulatory Physiology, 320(2), H740–H761. 10.1152/ajpheart.00736.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard, C. , Atri, A. , Boneva, N. , Cummings, J. L. , Frolich, L. , Molinuevo, J. L. , Tariot, P. N. , & Raket, L. L. (2019). Enrichment factors for clinical trials in mild‐to‐moderate Alzheimer's disease. Alzheimers Dement (N Y), 5, 164–174. 10.1016/j.trci.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu, D. , Karstens, A. J. , Loukenas, E. , Maldonado Weng, J. , York, J. M. , Valencia‐Olvera, A. C. , & LaDu, M. J. (2019). The role of APOE in transgenic mouse models of AD. Neuroscience Letters, 707, 134285. 10.1016/j.neulet.2019.134285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barini, E. , Antico, O. , Zhao, Y. , Asta, F. , Tucci, V. , Catelani, T. , Marotta, R. , Xu, H. , & Gasparini, L. (2016). Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Molecular Neurodegeneration, 11(1), 16. 10.1186/s13024-016-0082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, D. E. , & Yaffe, K. (2011). The projected impact of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurology, 10(9), 819–828. 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone, E. , Tramutola, A. , Triani, F. , Calcagnini, S. , di Domenico, F. , Ripoli, C. , Gaetani, S. , Grassi, C. , Butterfield, D. A. , Cassano, T. , & Perluigi, M. (2019). Biliverdin reductase—A mediates the beneficial effects of intranasal insulin in Alzheimer disease. Molecular Neurobiology, 56(4), 2922–2943. 10.1007/s12035-018-1231-5 [DOI] [PubMed] [Google Scholar]
- Beauquis, J. , Pavía, P. , Pomilio, C. , Vinuesa, A. , Podlutskaya, N. , Galvan, V. , & Saravia, F. (2013). Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer's disease. Experimental Neurology, 239, 28–37. 10.1016/j.expneurol.2012.09.009 [DOI] [PubMed] [Google Scholar]
- Beurel, E. , Grieco, S. F. , & Jope, R. S. (2015). Glycogen synthase kinase‐3 (GSK3): Regulation, actions, and diseases. Pharmacology & Therapeutics, 148, 114–131. 10.1016/j.pharmthera.2014.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar, K. , Konerth, M. , Kokiko‐Cochran, O. N. , Cardona, A. , Ransohoff, R. M. , & Lamb, B. T. (2010). Regulation of tau pathology by the microglial fractalkine receptor. Neuron, 68(1), 19–31. 10.1016/j.neuron.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner, T. , Burgold, S. , Dorostkar, M. M. , Fuhrmann, M. , Wegenast‐Braun, B. M. , Schmidt, B. , Kretzschmar, H. , & Herms, J. (2012). Amyloid plaque formation precedes dendritic spine loss. Acta Neuropathologica, 124(6), 797–807. 10.1007/s00401-012-1047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock, E. M. , Geddes, J. W. , Chen, K. C. , Porter, N. M. , Markesbery, W. R. , & Landfield, P. W. (2004). Incipient Alzheimer's disease: Microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proceedings of the National Academy of Sciences of the United States of America, 101(7), 2173–2178. 10.1073/pnas.0308512100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez, E. , Velázquez, E. , Hurtado‐Carneiro, V. , & Ruiz‐Albusac, J. M. (2014). Insulin in the brain: Its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Frontiers in Endocrinology, 5, 161. 10.3389/fendo.2014.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom, E. S. , Wang, Y. , Skoglund, L. , Hansson, A. C. , Ubaldi, M. , Lourdusamy, A. , Sommer, W. H. , Mielke, M. , Hyman, B. T. , Heilig, M. , Lannfelt, L. , Nilsson, L. N. G. , & Ingelsson, M. (2010). Increased mRNA levels of TCF7L2 and MYC of the Wnt pathway in Tg‐ArcSwe mice and Alzheimer's disease brain. International Journal of Alzheimer's Disease, 2011, 936580. 10.4061/2011/936580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmann, J. , Kreutz, M. R. , Gundelfinger, E. D. , & Böckers, T. M. (2002). ProSAP/shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. Journal of Neurochemistry, 83(4), 1013–1017. 10.1046/j.1471-4159.2002.01204.x [DOI] [PubMed] [Google Scholar]
- Bokde, A. L. , Pietrini, P. , Ibáñez, V. , Furey, M. L. , Alexander, G. E. , Graff‐Radford, N. R. , Rapoport, S. I. , Schapiro, M. B. , & Horwitz, B. (2001). The effect of brain atrophy on cerebral hypometabolism in the visual variant of Alzheimer disease. Archives of Neurology, 58(3), 480–486. 10.1001/archneur.58.3.480 [DOI] [PubMed] [Google Scholar]
- Boles, A. , Kandimalla, R. , & Reddy, P. H. (2017). Dynamics of diabetes and obesity: Epidemiological perspective. Oxidative Stress and Mitochondrial Quality in Diabetes/Obesity and Critical Illness Spectrum of Diseases, 1863(5), 1026–1036. 10.1016/j.bbadis.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos, M. , Llorens‐Martin, M. , Perea, J. R. , Jurado‐Arjona, J. , Rabano, A. , Hernandez, F. , & Avila, J. (2017). Absence of CX3CR1 impairs the internalization of tau by microglia. Molecular Neurodegeneration, 12(1), 59. 10.1186/s13024-017-0200-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomfim, T. R. , Forny‐Germano, L. , Sathler, L. B. , Brito‐Moreira, J. , Houzel, J.‐C. , Decker, H. , Silverman, M. A. , Kazi, H. , Melo, H. M. , McClean, P. L. , Holscher, C. , Arnold, S. E. , Talbot, K. , Klein, W. L. , Munoz, D. P. , Ferreira, S. T. , & de Felice, F. G. (2012). An anti‐diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease–associated Aβ oligomers. The Journal of Clinical Investigation, 122(4), 1339–1353. 10.1172/JCI57256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryda, E. C. (2013). The mighty mouse: The impact of rodents on advances in biomedical research. Missouri Medicine, 110(3), 207–211. [PMC free article] [PubMed] [Google Scholar]
- Buettner, R. , Schölmerich, J. , & Bollheimer, L. C. (2007). High‐fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity, 15(4), 798–808. 10.1038/oby.2007.608 [DOI] [PubMed] [Google Scholar]
- Burns, D. K. , Chiang, C. , Welsh‐Bohmer, K. A. , Brannan, S. K. , Culp, M. , O'Neil, J. , Runyan, G. , Harrigan, P. , Plassman, B. L. , Lutz, M. , Lai, E. , Haneline, S. , Yarnall, D. , Yarbrough, D. , Metz, C. , Ponduru, S. , Sundseth, S. , & Saunders, A. M. (2019). The TOMMORROW study: Design of an Alzheimer's disease delay‐of‐onset clinical trial. Alzheimers Dement (N Y), 5, 661–670. 10.1016/j.trci.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabou, C. , & Burcelin, R. (2011). GLP‐1, the gut‐brain, and brain‐periphery axes. The Review of Diabetic Studies: RDS, 8(3), 418–431. 10.1900/RDS.2011.8.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, H.‐Y. , Hölscher, C. , Yue, X.‐H. , Zhang, S.‐X. , Wang, X.‐H. , Qiao, F. , Yang, W. , & Qi, J.‐S. (2014). Lixisenatide rescues spatial memory and synaptic plasticity from amyloid β protein‐induced impairments in rats. Neuroscience, 277, 6–13. 10.1016/j.neuroscience.2014.02.022 [DOI] [PubMed] [Google Scholar]
- Cai, H.‐Y. , Yang, J.‐T. , Wang, Z.‐J. , Zhang, J. , Yang, W. , Wu, M.‐N. , & Qi, J.‐S. (2018). Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer's disease. Biochemical and Biophysical Research Communications, 495(1), 1034–1040. 10.1016/j.bbrc.2017.11.114 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2020). National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020.
- Chamera, K. , Trojan, E. , Szuster‐Głuszczak, M. , & Basta‐Kaim, A. (2020). The potential role of dysfunctions in neuron‐microglia communication in the pathogenesis of brain disorders. Current Neuropharmacology, 18(5), 408–430. 10.2174/1570159X17666191113101629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheignon, C. , Tomas, M. , Bonnefont‐Rousselot, D. , Faller, P. , Hureau, C. , & Collin, F. (2017). Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biology, 14, 450–464. 10.1016/j.redox.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheverud, J. M. , Fawcett, G. L. , Jarvis, J. P. , Norgard, E. A. , Pavlicev, M. , Pletscher, L. S. , Polonsky, K. S. , Ye, H. , Bell, G. I. , & Semenkovich, C. F. (2010). Calpain‐10 is a component of the obesity‐related quantitative trait locus Adip1. Journal of Lipid Research, 51(5), 907–913. 10.1194/jlr.M900128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, S.‐L. , Chen, C.‐M. , & Cline, H. T. (2008). Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron, 58(5), 708–719. 10.1016/j.neuron.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton, A. , Baker, L. D. , Hanson, A. , Trittschuh, E. H. , Cholerton, B. , Morgan, A. , Callaghan, M. , Arbuckle, M. , Behl, C. , & Craft, S. (2015). Long‐acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early‐stage Alzheimer's disease dementia. Journal of Alzheimer's Disease, 44(3), 897–906. 10.3233/JAD-141791 [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov . (2021). A research study investigating semaglutide in people with early Alzheimer's disease (EVOKE). National Institutes of Health. https://www.clinicaltrials.gov/ct2/show/NCT04777396
- Cocca, C. , Dorado, J. , Calvo, E. , López, J. A. , Santos, A. , & Perez‐Castillo, A. (2009). 15‐Deoxi‐delta(12,14)‐prostaglandin J2 is a tubulin‐binding agent that destabilizes microtubules and induces mitotic arrest. Biochemical Pharmacology, 78(10), 1330–1339. 10.1016/j.bcp.2009.06.100 [DOI] [PubMed] [Google Scholar]
- Coleman, D. L. (1978). Obese and diabetes: Two mutant genes causing diabetes‐obesity syndromes in mice. Diabetologia, 14(3), 141–148. 10.1007/BF00429772 [DOI] [PubMed] [Google Scholar]
- Corder, E. H. , Saunders, A. M. , Strittmatter, W. J. , Schmechel, D. E. , Gaskell, P. C. , Small, G. W. , Roses, A. D. , Haines, J. L. , & Pericak‐Vance, M. A. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science, 261(5123), 921–923. [DOI] [PubMed] [Google Scholar]
- Craft, S. , Asthana, S. , Cook, D. G. , Baker, L. D. , Cherrier, M. , Purganan, K. , Purganan, K. , Wait, C. , Petrova, A. , Latendresse, S. , Watson, G. S. , Newcomer, J. W. , Schellenberg, G. D. , & Krohn, A. J. (2003). Insulin dose‐response effects on memory and plasma amyloid precursor protein in Alzheimer's disease: Interactions with apolipoprotein E genotype. Psychoneuroendocrinology, 28(6), 809–822. 10.1016/s0306-4530(02)00087-2 [DOI] [PubMed] [Google Scholar]
- Craft, S. , Baker, L. D. , Montine, T. J. , Minoshima, S. , Watson, G. S. , Claxton, A. , Arbuckle, M. , Callaghan, M. , Tsai, E. , Plymate, S. R. , Green, P. S. , Leverenz, J. , Cross, D. , & Gerton, B. (2012a). Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Archives of Neurology, 69(1), 29–38. 10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft, S. , Baker, L. D. , Montine, T. J. , Minoshima, S. , Watson, G. S. , Claxton, A. , Arbuckle, M. , Callaghan, M. , Tsai, E. , Plymate, S. R. , Green, P. S. , Leverenz, J. , Cross, D. , & Gerton, B. (2012b). Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Archives of Neurology, 69(1), 29–38. 10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft, S. , Claxton, A. , Baker, L. D. , Hanson, A. J. , Cholerton, B. , Trittschuh, E. H. , Dahl, D. , Caulder, E. , Neth, B. , Montine, T. J. , Jung, Y. , Maldjian, J. , Whitlow, C. , & Friedman, S. (2017). Effects of regular and long‐acting insulin on cognition and Alzheimer's disease biomarkers: A pilot clinical trial. Journal of Alzheimer's Disease, 57(4), 1325–1334. 10.3233/JAD-161256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft, S. , Raman, R. , Chow, T. W. , Rafii, M. S. , Sun, C. K. , Rissman, R. A. , Donohue, M. C. , Brewer, J. B. , Jenkins, C. , Harless, K. , Gessert, D. , & Aisen, P. S. (2020). Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: A randomized clinical trial. JAMA Neurology, 77(9), 1099–1109. 10.1001/jamaneurol.2020.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts, M. , Theuns, J. , & van Broeckhoven, C. (2012). Locus‐specific mutation databases for neurodegenerative brain diseases. Human Mutation, 33(9), 1340–1344. 10.1002/humu.22117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, J. (2019). The role of biomarkers in Alzheimer's disease drug development. Advances in Experimental Medicine and Biology, 1118, 29–61. 10.1007/978-3-030-05542-4_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, J. , Lee, G. , Ritter, A. , Sabbagh, M. , & Zhong, K. (2020). Alzheimer's disease drug development pipeline: 2020. Alzheimer's & Dementia: Translational Research & Clinical Interventions, 6(1), e12050. 10.1002/trc2.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, J. , Ritter, A. , & Zhong, K. (2018). Clinical trials for disease‐modifying therapies in Alzheimer's disease: A primer, lessons learned, and a blueprint for the future. Journal of Alzheimer's Disease, 64(s1), S3–S22. 10.3233/JAD-179901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho, M. V. , Gonçalves‐de‐Albuquerque, C. F. , & Silva, A. R. (2021). PPAR gamma: From definition to molecular targets and therapy of lung diseases. International Journal of Molecular Sciences, 22(2), 805. 10.3390/ijms22020805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felice, F. G. (2013). Alzheimer's disease and insulin resistance: Translating basic science into clinical applications. The Journal of Clinical Investigation, 123(2), 531–539. 10.1172/JCI64595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt, E. K. , Witte, M. M. , Case, M. G. , Yu, P. , Henley, D. B. , Hochstetler, H. M. , D'Souza, D. N. , & Trzepacz, P. T. (2016). Florbetapir F18 PET amyloid neuroimaging and characteristics in patients with mild and moderate Alzheimer dementia. Psychosomatics, 57(2), 208–216. 10.1016/j.psym.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Devaskar, S. U. , Giddings, S. J. , Rajakumar, P. A. , Carnaghi, L. R. , Menon, R. K. , & Zahm, D. S. (1994). Insulin gene expression and insulin synthesis in mammalian neuronal cells. Journal of Biological Chemistry, 269(11), 8445–8454. 10.1016/S0021-9258(17)37214-9 [DOI] [PubMed] [Google Scholar]
- Dolan, P. J. , & Johnson, G. V. W. (2010). The role of tau kinases in Alzheimer's disease. Current Opinion in Drug Discovery & Development, 13(5), 595–603. PubMed. [PMC free article] [PubMed] [Google Scholar]
- Duka, V. , Lee, J.‐H. , Credle, J. , Wills, J. , Oaks, A. , Smolinsky, C. , Shah, K. , Mash, D. C. , Masliah, E. , & Sidhu, A. (2013). Identification of the sites of tau hyperphosphorylation and activation of tau kinases in synucleinopathies and Alzheimer's diseases. PLoS ONE, 8(9), e75025. 10.1371/journal.pone.0075025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin, A. B. , & Engin, A. (2021). Alzheimer's disease and protein kinases. In Engin A. B. & Engin A. (Eds.), Protein kinase‐mediated decisions between life and death (pp. 285–321). Springer International Publishing. 10.1007/978-3-030-49844-3_11 [DOI] [Google Scholar]
- Esterline, R. , Oscarsson, J. , & Burns, J. (2020). A role for sodium glucose cotransporter 2 inhibitors (SGLT2is) in the treatment of Alzheimer's disease? International Review of Neurobiology, 155, 113–140. 10.1016/bs.irn.2020.03.018 [DOI] [PubMed] [Google Scholar]
- Farilla, L. , Bulotta, A. , Hirshberg, B. , Li Calzi, S. , Khoury, N. , Noushmehr, H. , Bertolotto, C. , di Mario, U. , Harlan, D. M. , & Perfetti, R. (2003). Glucagon‐like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology, 144(12), 5149–5158. 10.1210/en.2003-0323 [DOI] [PubMed] [Google Scholar]
- Farr, S. A. , Roesler, E. , Niehoff, M. L. , Roby, D. A. , McKee, A. , & Morley, J. E. (2019). Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer's disease. Journal of Alzheimer's Disease, 68(4), 1699–1710. 10.3233/JAD-181240 [DOI] [PubMed] [Google Scholar]
- Farrer, L. A. , Cupples, L. A. , Haines, J. L. , Hyman, B. , Kukull, W. A. , Mayeux, R. , Myers, R. H. , Pericak‐Vance, M. A. , Risch, N. , & van Duijn, C. M. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta‐analysis. JAMA, 278(16), 1349–1356. 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- Farris, W. , Mansourian, S. , Chang, Y. , Lindsley, L. , Eckman, E. A. , Frosch, M. P. , Eckman, C. B. , Tanzi, R. E. , Selkoe, D. J. , & Guénette, S. (2003). Insulin‐degrading enzyme regulates the levels of insulin, amyloid β‐protein, and the β‐amyloid precursor protein intracellular domain in vivo. Proceedings of the National Academy of Sciences of the United States of America, 100(7), 4162–4167. 10.1073/pnas.0230450100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminella, G. D. , Frangou, E. , Love, S. B. , Busza, G. , Holmes, C. , Ritchie, C. , Lawrence, R. , McFarlane, B. , Tadros, G. , Ridha, B. H. , Bannister, C. , Walker, Z. , Archer, H. , Coulthard, E. , Underwood, B. R. , Prasanna, A. , Koranteng, P. , Karim, S. , Junaid, K. , … Edison, P. (2019). Evaluating the effects of the novel GLP‐1 analogue liraglutide in Alzheimer's disease: Study protocol for a randomised controlled trial (ELAD study). Trials, 20(1), 191. 10.1186/s13063-019-3259-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, L. S. S. , Fernandes, C. S. , Vieira, M. N. N. , & de Felice, F. G. (2018). Insulin resistance in Alzheimer's disease. Frontiers in Neuroscience, 12, 830–830. 10.3389/fnins.2018.00830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz, N. F. , Cronican, A. A. , Saleem, M. , Fauq, A. H. , Chapman, R. , Lefterov, I. , & Koldamova, R. (2012). Abca1 deficiency affects Alzheimer's disease‐like phenotype in human ApoE4 but not in ApoE3‐targeted replacement mice. The Journal of Neuroscience, 32(38), 13125–13136. 10.1523/JNEUROSCI.1937-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiherr, J. , Hallschmid, M. , Frey, W. H. 2nd , Brünner, Y. F. , Chapman, C. D. , Hölscher, C. , Craft, S. , de Felice, F. G. , & Benedict, C. (2013). Intranasal insulin as a treatment for Alzheimer's disease: A review of basic research and clinical evidence. CNS Drugs, 27(7), 505–514. 10.1007/s40263-013-0076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault, V. A. , & Hölscher, C. (2008). GLP‐1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta‐amyloid. European Journal of Pharmacology, 587(1–3), 112–117. 10.1016/j.ejphar.2008.03.025 [DOI] [PubMed] [Google Scholar]
- Gejl, M. , Gjedde, A. , Egefjord, L. , Moller, A. , Hansen, S. B. , Vang, K. , Rodell, A. , Braendgaard, H. , Gottrup, H. , Schacht, A. , Moller, N. , Brock, B. , & Rungby, J. (2016). In Alzheimer's disease, 6‐month treatment with GLP‐1 analog prevents decline of brain glucose metabolism: Randomized, placebo‐controlled, double‐blind clinical trial. Frontiers in Aging Neuroscience, 8(24), 108. 10.3389/fnagi.2016.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher, D. S. , Fritsch, T. , McClendon, M. J. , & Landreth, G. (2011). A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Archives of Neurology, 68(1), 45–50. 10.1001/archneurol.2010.229 [DOI] [PubMed] [Google Scholar]
- Gengler, S. , Hamilton, A. , & Hölscher, C. (2010). Synaptic plasticity in the hippocampus of a APP/PS1 mouse model of Alzheimer's disease is impaired in old but not young mice. PLoS ONE, 5(3), e9764. 10.1371/journal.pone.0009764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri, M. , Zhang, M. , & Lü, Y. (2016). Genes associated with Alzheimer's disease: An overview and current status. Clinical Interventions in Aging, 11, 665–681. 10.2147/CIA.S105769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, M. , Alderton, C. , Zvartau‐Hind, M. , Egginton, S. , Saunders, A. M. , Irizarry, M. , Craft, S. , Landreth, G. , Linnamagi, U. , & Sawchak, S. (2010). Rosiglitazone monotherapy in mild‐to‐moderate Alzheimer's disease: Results from a randomized, double‐blind, placebo‐controlled phase III study. Dementia and Geriatric Cognitive Disorders, 30(2), 131–146. 10.1159/000318845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Peralta, F. , & Abreu, C. (2019). Profile of semaglutide in the management of type 2 diabetes: Design, development, and place in therapy. Drug Design, Development and Therapy, 13, 731–738. 10.2147/DDDT.S165372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourmaud, S. , Paquet, C. , Dumurgier, J. , Pace, C. , Bouras, C. , Gray, F. , Laplanche, J.‐L. , Meurs, E. F. , Mouton‐Liger, F. , & Hugon, J. (2015). Increased levels of cerebrospinal fluid JNK3 associated with amyloid pathology: Links to cognitive decline. Journal of Psychiatry & Neuroscience: JPN, 40(3), 151–161. 10.1503/jpn.140062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, S. F. A. , Thorleifsson, G. , Reynisdottir, I. , Benediktsson, R. , Manolescu, A. , Sainz, J. , Helgason, A. , Stefansson, H. , Emilsson, V. , Helgadottir, A. , Styrkarsdottir, U. , Magnusson, K. P. , Walters, G. B. , Palsdottir, E. , Jonsdottir, T. , Gudmundsdottir, T. , Gylfason, A. , Saemundsdottir, J. , Wilensky, R. L. , … Stefansson, K. (2006). Variant of transcription factor 7‐like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nature Genetics, 38(3), 320–323. 10.1038/ng1732 [DOI] [PubMed] [Google Scholar]
- Gray, S. M. , Meijer, R. I. , & Barrett, E. J. (2014). Insulin regulates brain function, but how does it get there? Diabetes, 63(12), 3992–3997. 10.2337/db14-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco, M. , Giorgi, A. , Gentile, M. C. , d'Erme, M. , Morano, S. , Maras, B. , & Filardi, T. (2019). Glucagon‐like peptide‐1: A focus on neurodegenerative diseases. Frontiers in Neuroscience, 13(18), 1112. 10.3389/fnins.2019.01112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke‐Iqbal, I. , Iqbal, K. , Quinlan, M. , Tung, Y. C. , Zaidi, M. S. , & Wisniewski, H. M. (1986). Microtubule‐associated protein tau. A component of Alzheimer paired helical filaments. The Journal of Biological Chemistry, 261(13), 6084–6089. [PubMed] [Google Scholar]
- Gu, T. , Zhao, T. , & Hewes, R. S. (2014). Insulin signaling regulates neurite growth during metamorphic neuronal remodeling. Biology Open, 3(1), 81–93. 10.1242/bio.20136437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C. , Zhang, S. , Li, J.‐Y. , Ding, C. , Yang, Z.‐H. , Chai, R. , Wang, X. , & Wang, Z.‐Y. (2016). Chronic hyperglycemia induced via the heterozygous knockout of Pdx1 worsens neuropathological lesion in an Alzheimer mouse model. Scientific Reports, 6(1), 29396. 10.1038/srep29396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger, D. P. , Hughes, K. , Woodgett, J. R. , Brion, J.‐P. , & Anderton, B. H. (1992). Glycogen synthase kinase‐3 induces Alzheimer's disease‐like phosphorylation of tau: Generation of paired helical filament epitopes and neuronal localisation of the kinase. Neuroscience Letters, 147(1), 58–62. 10.1016/0304-3940(92)90774-2 [DOI] [PubMed] [Google Scholar]
- Hanyu, H. , Sato, T. , Kiuchi, A. , Sakurai, H. , & Iwamoto, T. (2009). Pioglitazone improved cognition in a pilot study on patients with Alzheimer's disease and mild cognitive impairment with diabetes mellitus. Journal of the American Geriatrics Society, 57(1), 177–179. 10.1111/j.1532-5415.2009.02067.x [DOI] [PubMed] [Google Scholar]
- Hardy, J. A. , & Higgins, G. A. (1992). Alzheimer's disease: The amyloid cascade hypothesis. Science, 256(5054), 184–185. [DOI] [PubMed] [Google Scholar]
- Harrington, C. , Sawchak, S. , Chiang, C. , Davies, J. , Donovan, C. , Saunders, A. M. , Irizarry, M. , Jeter, B. , Zvartau‐Hind, M. , van Dyck, C. H. , & Gold, M. (2011). Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild‐to‐moderate Alzheimer's disease: Two phase 3 studies. Current Alzheimer Research, 8(5), 592–606. 10.2174/156720511796391935 [DOI] [PubMed] [Google Scholar]
- He, Z. , You, G. , Liu, Q. , & Li, N. (2021). Alzheimer's disease and diabetes mellitus in comparison: The therapeutic efficacy of the vanadium compound. International Journal of Molecular Sciences, 22(21), 11931. 10.3390/ijms222111931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , Carson, M. J. , el Khoury, J. , Landreth, G. E. , Brosseron, F. , Feinstein, D. L. , Jacobs, A. H. , Wyss‐Coray, T. , Vitorica, J. , Ransohoff, R. M. , Herrup, K. , Frautschy, S. A. , Finsen, B. , Brown, G. C. , Verkhratsky, A. , Yamanaka, K. , Koistinaho, J. , Latz, E. , Halle, A. , … Kummer, M. P. (2015). Neuroinflammation in Alzheimer's disease. The Lancet. Neurology, 14(4), 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , Reyes‐Irisarri, E. , Hüll, M. , & Kummer, M. P. (2011). Impact and therapeutic potential of PPARs in Alzheimer's disease. Current Neuropharmacology, 9(4), 643–650. 10.2174/157015911798376325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , Sastre, M. , Dumitrescu‐Ozimek, L. , Hanke, A. , Dewachter, I. , Kuiperi, C. , O'Banion, K. , Klockgether, T. , van Leuven, F. , & Landreth, G. E. (2005). Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1‐42 levels in APPV717I transgenic mice. Brain: A Journal of Neurology, 128(Pt 6), 1442–1453. 10.1093/brain/awh452 [DOI] [PubMed] [Google Scholar]
- Hildreth, K. L. , van Pelt, R. E. , & Schwartz, R. S. (2012). Obesity, insulin resistance, and Alzheimer's disease. Obesity (Silver Spring, Md.), 20(8), 1549–1557. 10.1038/oby.2012.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi, J. , Tuncman, G. , Chang, L. , Görgün, C. Z. , Uysal, K. T. , Maeda, K. , Karin, M. , & Hotamisligil, G. S. (2002). A central role for JNK in obesity and insulin resistance. Nature, 420(6913), 333–336. 10.1038/nature01137 [DOI] [PubMed] [Google Scholar]
- Holscher, C. (2018). Novel dual GLP‐1/GIP receptor agonists show neuroprotective effects in Alzheimer's and Parkinson's disease models. Neuropharmacology, 136(Pt B), 251–259. 10.1016/j.neuropharm.2018.01.040 [DOI] [PubMed] [Google Scholar]
- Hooper, C. , Killick, R. , & Lovestone, S. (2008). The GSK3 hypothesis of Alzheimer's disease. Journal of Neurochemistry, 104(6), 1433–1439. 10.1111/j.1471-4159.2007.05194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado, D. E. , Molina‐Porcel, L. , Carroll, J. C. , MacDonald, C. , Aboagye, A. K. , Trojanowski, J. Q. , & Lee, V. M.‐Y. (2012). Selectively silencing GSK‐3 isoforms reduces plaques and tangles in mouse models of Alzheimer's disease. The Journal of Neuroscience, 32(21), 7392–7402. 10.1523/JNEUROSCI.0889-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, B. T. , Phelps, C. H. , Beach, T. G. , Bigio, E. H. , Cairns, N. J. , Carrillo, M. C. , Dickson, D. W. , Duyckaerts, C. , Frosch, M. P. , Masliah, E. , Mirra, S. S. , Nelson, P. T. , Schneider, J. A. , Thal, D. R. , Thies, B. , Trojanowski, J. Q. , Vinters, H. V. , & Montine, T. J. (2012). National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's & Dementia, 8(1), 1–13. 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalls, A. M. , Dickie, M. M. , & Snell, G. D. (1950). Obese: A new mutation in the house mouse*. Journal of Heredity, 41(12), 317–318. 10.1093/oxfordjournals.jhered.a106073 [DOI] [PubMed] [Google Scholar]
- Janson, J. , Laedtke, T. , Parisi, J. E. , O'Brien, P. , Petersen, R. C. , & Butler, P. C. (2004). Increased risk of type 2 diabetes in Alzheimer disease. Diabetes, 53(2), 474–481. 10.2337/diabetes.53.2.474 [DOI] [PubMed] [Google Scholar]
- Justice, J. N. , Niedernhofer, L. , Robbins, P. D. , Aroda, V. R. , Espeland, M. A. , Kritchevsky, S. B. , Kuchel, G. A. , & Barzilai, N. (2018). Development of clinical trials to extend healthy lifespan. Cardiovasc Endocrinol Metab, 7(4), 80–83. 10.1097/XCE.0000000000000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia, N. , Almisry, M. , & Bieschke, J. (2017). Glucose directs amyloid‐beta into membrane‐active oligomers. Physical Chemistry Chemical Physics: PCCP, 19(27), 18036–18046. 10.1039/c7cp02849k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliny, S. , Lin, L. , Deng, I. , Xiong, J. , Zhou, F. , Al‐Hawwas, M. , Bobrovskaya, L. , & Zhou, X.‐F. (2021). A new approach to model sporadic Alzheimer's disease by intracerebroventricular streptozotocin injection in APP/PS1 mice. Molecular Neurobiology, 58(8), 3692–3711. 10.1007/s12035-021-02338-5 [DOI] [PubMed] [Google Scholar]
- Khan, M. A. , Alam, Q. , Haque, A. , Ashafaq, M. , Khan, M. J. , Ashraf, G. M. , & Ahmad, M. (2019). Current progress on peroxisome proliferator‐activated receptor gamma agonist as an emerging therapeutic approach for the treatment of Alzheimer's disease: An update. Current Neuropharmacology, 17(3), 232–246. 10.2174/1570159X16666180828100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickstein, E. , Krauss, S. , Thornhill, P. , Rutschow, D. , Zeller, R. , Sharkey, J. , Williamson, R. , Fuchs, M. , Köhler, A. , Glossmann, H. , & Schweiger, R. (2010). Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21830–21835. 10.1073/pnas.0912793107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. , Backus, C. , Oh, S. , & Feldman, E. L. (2013). Hyperglycemia‐induced tau cleavage in vitro and in vivo: A possible link between diabetes and Alzheimer's disease. Journal of Alzheimer's Disease: JAD, 34(3), 727–739. 10.3233/JAD-121669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Basak, J. M. , & Holtzman, D. M. (2009). The role of apolipoprotein E in Alzheimer's disease. Neuron, 63(3), 287–303. 10.1016/j.neuron.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.‐J. , & Han, Y. (2005). Insulin inhibits AMPA‐induced neuronal damage via stimulation of protein kinase B (Akt). Journal of Neural Transmission (Vienna, Austria: 1996), 112(2), 179–191. 10.1007/s00702-004-0163-6 [DOI] [PubMed] [Google Scholar]
- Kinney, J. W. , Bemiller, S. M. , Murtishaw, A. S. , Leisgang, A. M. , Salazar, A. M. , & Lamb, B. T. (2018). Inflammation as a central mechanism in Alzheimer's disease. Alzheimer's & Dementia: Translational Research & Clinical Interventions, 4, 575–590. 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman, M. S. , Briscoe, V. J. , Clark, N. , Florez, H. , Haas, L. B. , Halter, J. B. , Huang, E. S. , Korytkowski, M. T. , Munshi, M. N. , Odegard, P. S. , Pratley, R. E. , & Swift, C. S. (2012). Diabetes in older adults. Diabetes Care, 35(12), 2650–2664. 10.2337/dc12-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa, M. , Oddo, S. , Yamasaki, T. R. , Green, K. N. , & LaFerla, F. M. (2005). Lipopolysaccharide‐induced inflammation exacerbates tau pathology by a cyclin‐dependent kinase 5‐mediated pathway in a transgenic model of Alzheimer's disease. The Journal of Neuroscience, 25(39), 8843–8853. 10.1523/JNEUROSCI.2868-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöting, N. , & Blüher, M. (2014). Adipocyte dysfunction, inflammation and metabolic syndrome. Reviews in Endocrine & Metabolic Disorders, 15(4), 277–287. 10.1007/s11154-014-9301-0 [DOI] [PubMed] [Google Scholar]
- Ko, S.‐Y. , Ko, H.‐A. , Chu, K.‐H. , Shieh, T.‐M. , Chi, T.‐C. , Chen, H.‐I. , Chang, W.‐C. , & Chang, S.‐S. (2015). The possible mechanism of advanced glycation end products (AGEs) for Alzheimer's disease. PLoS ONE, 10(11), e0143345. 10.1371/journal.pone.0143345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, A. M. , Mechanic‐Hamilton, D. , Xie, S. X. , Combs, M. F. , Cappola, A. R. , Xie, L. , Detre, J. A. , Wolk, D. A. , & Arnold, S. E. (2017). Effects of the insulin sensitizer metformin in Alzheimer disease: Pilot data from a randomized placebo‐controlled crossover study. Alzheimer Disease and Associated Disorders, 31(2), 107–113. 10.1097/WAD.0000000000000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, F.‐J. , Wu, J.‐H. , Sun, S.‐Y. , Ma, L.‐L. , & Zhou, J.‐Q. (2018). Liraglutide ameliorates cognitive decline by promoting autophagy via the AMP‐activated protein kinase/mammalian target of rapamycin pathway in a streptozotocin‐induced mouse model of diabetes. Neuropharmacology, 131, 316–325. 10.1016/j.neuropharm.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Koren‐Iton, A. , Salomon‐Zimri, S. , Smolar, A. , Shavit‐Stein, E. , Dori, A. , Chapman, J. , & Michaelson, D. M. (2020). Central and peripheral mechanisms in ApoE4‐driven diabetic pathology. International Journal of Molecular Sciences, 21(4), 1289. 10.3390/ijms21041289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrtata, N. , Emsley, H. C. A. , Sparasci, O. , Parkes, L. M. , & Dickie, B. R. (2021). A systematic review of glucose transport alterations in Alzheimer's disease. Frontiers in Neuroscience, 15, 626636. 10.3389/fnins.2021.626636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy, M. E. , Gilsanz, P. , Karter, A. J. , Quesenberry, C. P. , Pletcher, M. J. , & Whitmer, R. A. (2018). Long‐term glycemic control and dementia risk in type 1 diabetes. Diabetes Care, 41(11), 2339–2345. 10.2337/dc18-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagalwar, S. , Guillozet‐Bongaarts, A. L. , Berry, R. W. , & Binder, L. I. (2006). Formation of phospho‐SAPK/JNK granules in the hippocampus is an early event in Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 65(5), 455–464. 10.1097/01.jnen.0000229236.98124.d8 [DOI] [PubMed] [Google Scholar]
- Leclerc, S. , Garnier, M. , Hoessel, R. , Marko, D. , Bibb, J. A. , Snyder, G. L. , Greengard, P. , Biernat, J. , Wu, Y.‐Z. , Mandelkow, E.‐M. , Eisenbrand, G. , & Meijer, L. (2001). Indirubins inhibit glycogen synthase kinase‐3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease: A property common to most cyclin‐dependent kinase inhibitors?*210. Journal of Biological Chemistry, 276(1), 251–260. 10.1074/jbc.M002466200 [DOI] [PubMed] [Google Scholar]
- Lee, C.‐C. , Huang, C.‐C. , & Hsu, K.‐S. (2011). Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology, 61(4), 867–879. 10.1016/j.neuropharm.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Lee, E.‐M. , Lee, Y.‐E. , Lee, E. , Ryu, G. R. , Ko, S.‐H. , Moon, S.‐D. , Song, K.‐H. , & Ahn, Y.‐B. (2011). Protective effect of heme oxygenase‐1 on high glucose‐induced pancreatic β‐cell injury. Diabetes & Metabolism Journal, 35(5), 469–479. 10.4093/dmj.2011.35.5.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Varvel, N. H. , Konerth, M. E. , Xu, G. , Cardona, A. E. , Ransohoff, R. M. , & Lamb, B. T. (2010). CX3CR1 deficiency alters microglial activation and reduces beta‐amyloid deposition in two Alzheimer's disease mouse models. The American Journal of Pathology, 177(5), 2549–2562. 10.2353/ajpath.2010.100265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. S. , & Jun, H. S. (2016). Anti‐inflammatory effects of GLP‐1‐based therapies beyond glucose control. Mediators of Inflammation, 2016, 3094642. 10.1155/2016/3094642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, J. M. , Moore, L. B. , Smith‐Oliver, T. A. , Wilkison, W. O. , Willson, T. M. , & Kliewer, S. A. (1995). An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator‐activated receptor γ (PPARγ). Journal of Biological Chemistry, 270(22), 12953–12956. 10.1074/jbc.270.22.12953 [DOI] [PubMed] [Google Scholar]
- Lennox, R. , Porter, D. W. , Flatt, P. R. , Holscher, C. , Irwin, N. , & Gault, V. A. (2014). Comparison of the independent and combined effects of sub‐chronic therapy with metformin and a stable GLP‐1 receptor agonist on cognitive function, hippocampal synaptic plasticity and metabolic control in high‐fat fed mice. Neuropharmacology, 86, 22–30. 10.1016/j.neuropharm.2014.06.026 [DOI] [PubMed] [Google Scholar]
- Li, H. , Wu, J. , Zhu, L. , Sha, L. , Yang, S. , Wei, J. , Ji, L. , Tang, X. , Mao, K. , Cao, L. , Wei, N. , Xie, W. , & Yang, Z. (2018). Insulin degrading enzyme contributes to the pathology in a mixed model of type 2 diabetes and Alzheimer's disease: Possible mechanisms of IDE in T2D and AD. Bioscience Reports, 38(1), BSR20170862. 10.1042/BSR20170862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Deng, J. , Sheng, W. , & Zuo, Z. (2012). Metformin attenuates Alzheimer's disease‐like neuropathology in obese, leptin‐resistant mice. Pharmacology, Biochemistry, and Behavior, 101(4), 564–574. 10.1016/j.pbb.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Perry, T. , Kindy, M. S. , Harvey, B. K. , Tweedie, D. , Holloway, H. W. , Powers, K. , Shen, H. , Egan, J. M. , Sambamurti, K. , Brossi, A. , Lahiri, D. K. , Mattson, M. P. , Hoffer, B. J. , Wang, Y. , & Greig, N. H. (2009). GLP‐1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and parkinsonism. Proceedings of the National Academy of Sciences of the United States of America, 106(4), 1285–1290. 10.1073/pnas.0806720106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Spéder, P. , & Brand, A. H. (2014). Control of brain development and homeostasis by local and systemic insulin signalling. Diabetes, Obesity and Metabolism, 16(S1), 16–20. 10.1111/dom.12337 [DOI] [PubMed] [Google Scholar]
- Lovestone, S. , Reynolds, C. H. , Latimer, D. , Davis, D. R. , Anderton, B. H. , Gallo, J.‐M. , Hanger, D. , Mulot, S. , Marquardt, B. , Stabel, S. , Woodgett, J. R. , & Miller, C. C. J. (1994). Alzheimer's disease‐like phosphorylation of the microtubule‐associated protein tau by glycogen synthase kinase‐3 in transfected mammalian cells. Current Biology, 4(12), 1077–1086. 10.1016/S0960-9822(00)00246-3 [DOI] [PubMed] [Google Scholar]
- Lu, F. P. , Lin, K. P. , & Kuo, H. K. (2009). Diabetes and the risk of multi‐system aging phenotypes: A systematic review and meta‐analysis. PLoS ONE, 4(1), e4144. 10.1371/journal.pone.0004144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, X.‐Y. , Huang, S. , Chen, Q.‐B. , Zhang, D. , Li, W. , Ao, R. , Ao, R. , Leung, F. C.‐Y. , Zhang, Z. , Huang, J. , Tang, Y. , & Zhang, S.‐J. (2020). Metformin ameliorates Aβ pathology by insulin‐degrading enzyme in a transgenic mouse model of Alzheimer's disease. Oxidative Medicine and Cellular Longevity, 2020, 2315106. 10.1155/2020/2315106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger, J. A. , Tang, M.‐X. , Shea, S. , & Mayeux, R. (2004). Hyperinsulinemia and risk of Alzheimer disease. Neurology, 63(7), 1187–92. 10.1212/01.WNL.0000140292.04932.87 [DOI] [PubMed] [Google Scholar]
- Ma, D.‐L. , Chen, F.‐Q. , Xu, W.‐J. , Yue, W.‐Z. , Yuan, G. , & Yang, Y. (2015). Early intervention with glucagon‐like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. Journal of Neurochemistry, 135(2), 301–308. 10.1111/jnc.13248 [DOI] [PubMed] [Google Scholar]
- Macauley, S. L. , Stanley, M. , Caesar, E. E. , Yamada, S. A. , Raichle, M. E. , Perez, R. , Mahan, T. E. , Sutphen, C. L. , & Holtzman, D. M. (2015). Hyperglycemia modulates extracellular amyloid‐β concentrations and neuronal activity in vivo. The Journal of Clinical Investigation, 125(6), 2463–2467. 10.1172/JCI79742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow, E.‐M. , Drewes, G. , Biernat, J. , Gustke, N. , Lint, J. V. , Vandenheede, J. R. , & Mandelkow, E. (1992). Glycogen synthase kinase‐3 and the Alzheimer‐like state of microtubule‐associated protein tau. FEBS Letters, 314(3), 315–321. 10.1016/0014-5793(92)81496-9 [DOI] [PubMed] [Google Scholar]
- Mandrekar‐Colucci, S. , Karlo, J. C. , & Landreth, G. E. (2012). Mechanisms underlying the rapid peroxisome proliferator‐activated receptor‐γ‐mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer's disease. The Journal of Neuroscience, 32(30), 10117–10128. 10.1523/JNEUROSCI.5268-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Y. , Guo, Z. , Zheng, T. , Jiang, Y. , Yan, Y. , Yin, X. , Chen, Y. , & Zhang, B. (2016). Intranasal insulin alleviates cognitive deficits and amyloid pathology in young adult APPswe/PS1dE9 mice. Aging Cell, 15(5), 893–902. 10.1111/acel.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazon, J. N. , de Mello, A. H. , Ferreira, G. K. , & Rezin, G. T. (2017). The impact of obesity on neurodegenerative diseases. Life Sciences, 182, 22–28. 10.1016/j.lfs.2017.06.002 [DOI] [PubMed] [Google Scholar]
- McEvoy, R. C. , Andersson, J. , Sandler, S. , & Hellerstrom, C. (1984). Multiple low‐dose streptozotocin‐induced diabetes in the mouse. Evidence for stimulation of a cytotoxic cellular immune response against an insulin‐producing beta cell line. Journal of Clinical Investigation, 74(3), 715–722. 10.1172/JCI111487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla, J. , Chauhan, B. C. , & Chauhan, N. B. (2014). Experimental induction of type 2 diabetes in aging‐accelerated mice triggered Alzheimer‐like pathology and memory deficits. Journal of Alzheimer's Disease: JAD, 39(1), 145–162. 10.3233/JAD-131238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, J. G. , Taghibiglou, C. , & Wang, Y. T. (2006). Endogenous insulin signaling protects cultured neurons from oxygen‐glucose deprivation‐induced cell death. Neuroscience, 143(1), 165–173. 10.1016/j.neuroscience.2006.07.055 [DOI] [PubMed] [Google Scholar]
- Millington, C. , Sonego, S. , Karunaweera, N. , Rangel, A. , Aldrich‐Wright, J. R. , Campbell, I. L. , Gyengesi, E. , & Münch, G. (2014). Chronic neuroinflammation in Alzheimer's disease: New perspectives on animal models and promising candidate drugs. BioMed Research International, 2014, e309129. 10.1155/2014/309129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. K. , & Burns, J. M. (2012). Insulin: An emerging treatment for Alzheimer's disease dementia? Current Neurology and Neuroscience Reports, 12(5), 520–527. 10.1007/s11910-012-0297-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. K. , Vidoni, E. D. , Honea, R. A. , Burns, J. M. , & Initiative, A.'s. D. N. (2014). Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiology of Aging, 35(3), 585–589. 10.1016/j.neurobiolaging.2013.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, V. A. , & Pike, C. J. (2017). Obesity accelerates Alzheimer‐related pathology in APOE4 but not APOE3 mice. ENeuro, 4(3), ENEURO.0077–17. 10.1523/ENEURO.0077-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota, M. , Banini, B. A. , Cazanave, S. C. , & Sanyal, A. J. (2016). Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism: Clinical and Experimental, 65(8), 1049–1061. 10.1016/j.metabol.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, R. J. , Mustapic, M. , Chia, C. W. , Carlson, O. , Gulyani, S. , Tran, J. , Li, Y. , Mattson, M. P. , Resnick, S. , Egan, J. M. , Greig, N. H. , & Kapogiannis, D. (2019). A pilot study of exenatide actions in Alzheimer's disease. Current Alzheimer Research, 16(8), 741–752. 10.2174/1567205016666190913155950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz‐Montano, J. R. , Moreno, F. J. , Avila, J. , & Diaz‐Nido, J. (1997). Lithium inhibits Alzheimer's disease‐like tau protein phosphorylation in neurons. FEBS Letters, 411(2–3), 183–188. 10.1016/s0014-5793(97)00688-1 [DOI] [PubMed] [Google Scholar]
- Murtishaw, A. S. , Heaney, C. F. , Bolton, M. M. , Belmonte, K. C. D. , Langhardt, M. A. , & Kinney, J. W. (2018). Intermittent streptozotocin administration induces behavioral and pathological features relevant to Alzheimer's disease and vascular dementia. Neuropharmacology, 137, 164–177. 10.1016/j.neuropharm.2018.04.021 [DOI] [PubMed] [Google Scholar]
- Murtishaw, A. S. , Heaney, C. F. , Bolton, M. M. , Sabbagh, J. J. , Langhardt, M. A. , & Kinney, J. W. (2016). Effect of acute lipopolysaccharide‐induced inflammation in intracerebroventricular‐streptozotocin injected rats. Neuropharmacology, 101, 110–122. 10.1016/j.neuropharm.2015.08.044 [DOI] [PubMed] [Google Scholar]
- Naseri, R. , Navabi, S. J. , Samimi, Z. , Mishra, A. P. , Nigam, M. , Chandra, H. , & Farzaei, M. H. (2020). Targeting glycoproteins as a therapeutic strategy for diabetes mellitus and its complications. DARU Journal of Pharmaceutical Sciences, 28(1), 333–358. 10.1007/s40199-020-00327-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, J. C. D. , Killcross, A. S. , & Jenkins, T. A. (2014). Obesity and cognitive decline: Role of inflammation and vascular changes. Frontiers in Neuroscience, 8, 375–375. 10.3389/fnins.2014.00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis, N. , & Hamel, E. (2010). The nuclear receptor PPARγ: As a therapeutic target for cerebrovascular and brain dysfunction in Alzheimer's disease. Frontiers in Aging Neuroscience, 2, 21. 10.3389/fnagi.2010.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, P. D. , Hinder, L. M. , Callaghan, B. C. , & Feldman, E. L. (2017). Neurological consequences of obesity. The Lancet Neurology, 16(6), 465–477. 10.1016/S1474-4422(17)30084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryant, S. E. , Zhang, F. , Petersen, M. , Johnson, L. , Hall, J. , & Rissman, R. A. (2021). A precision medicine approach to treating Alzheimer's disease using rosiglitazone therapy: A biomarker analysis of the REFLECT trials. Journal of Alzheimer's Disease, 81(2), 557–568. 10.3233/JAD-201610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, W. H. , Braga, C. F. , Lós, D. B. , Araújo, S. M. R. , França, M. R. , Duarte‐Silva, E. , Rodrigues, G. B. , Rocha, S. W. S. , & Peixoto, C. A. (2021). Metformin prevents p‐tau and amyloid plaque deposition and memory impairment in diabetic mice. Experimental Brain Research.. 239(9), 2821–2839. 10.1007/s00221-021-06176-8 [DOI] [PubMed] [Google Scholar]
- Osborn, O. , & Olefsky, J. M. (2012). The cellular and signaling networks linking the immune system and metabolism in disease. Nature Medicine, 18(3), 363–374. 10.1038/nm.2627 [DOI] [PubMed] [Google Scholar]
- Pal, M. , Febbraio, M. A. , & Lancaster, G. I. (2016). The roles of c‐Jun NH2‐terminal kinases (JNKs) in obesity and insulin resistance. The Journal of Physiology, 594(2), 267–279. 10.1113/JP271457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleria, C. , Leo, A. , Andreozzi, F. , Citraro, R. , Iannone, M. , Spiga, R. , Sesti, G. , Constanti, A. , de Sarro, G. , Arturi, F. , & Russo, E. (2017). Liraglutide prevents cognitive decline in a rat model of streptozotocin‐induced diabetes independently from its peripheral metabolic effects. Behavioural Brain Research, 321, 157–169. 10.1016/j.bbr.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Paolicelli, R. C. , Bisht, K. , & Tremblay, M. E. (2014). Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Frontiers in Cellular Neuroscience, 8, 129. 10.3389/fncel.2014.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parimisetty, A. , Dorsemans, A.‐C. , Awada, R. , Ravanan, P. , Diotel, N. , & Lefebvre d'Hellencourt, C. (2016). Secret talk between adipose tissue and central nervous system via secreted factors—An emerging frontier in the neurodegenerative research. Journal of Neuroinflammation, 13(1), 67. 10.1186/s12974-016-0530-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, K. S. (2011). The search for genetic risk factors of type 2 diabetes mellitus. Diabetes & Metabolism Journal, 35(1), 12–22. 10.4093/dmj.2011.35.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschou, S. A. , Papadopoulou‐Marketou, N. , Chrousos, G. P. , & Kanaka‐Gantenbein, C. (2018). On type 1 diabetes mellitus pathogenesis. Endocrine Connections, 7(1), R38–R46. 10.1530/EC-17-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payami, H. , Montee, K. R. , Kaye, J. A. , Bird, T. D. , Yu, C.‐E. , Wijsman, E. M. , & Schellenberg, G. D. (1994). Alzheimer's disease, apolipoprotein E4, and gender. Jama, 271(17), 1316–1317. 10.1001/jama.1994.03510410028015 [DOI] [PubMed] [Google Scholar]
- Peila, R. , Rodriguez, B. L. , & Launer, L. J. (2002). Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu‐Asia aging study. Diabetes, 51(4), 1256–1262. 10.2337/diabetes.51.4.1256 [DOI] [PubMed] [Google Scholar]
- Phiel, C. J. , Wilson, C. A. , Lee, V. M. , & Klein, P. S. (2003). GSK‐3alpha regulates production of Alzheimer's disease amyloid‐beta peptides. Nature, 423(6938), 435–439. 10.1038/nature01640 [DOI] [PubMed] [Google Scholar]
- Picone, P. , Nuzzo, D. , Caruana, L. , Messina, E. , Barera, A. , Vasto, S. , & di Carlo, M. (2015). Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF‐κB activation: Use of insulin to attenuate metformin's effect. Biochimica et Biophysica Acta (BBA) – Molecular. Cell Research, 1853(5), 1046–1059. 10.1016/j.bbamcr.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Pintana, H. , Apaijai, N. , Pratchayasakul, W. , Chattipakorn, N. , & Chattipakorn, S. C. (2012). Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sciences, 91(11), 409–414. 10.1016/j.lfs.2012.08.017 [DOI] [PubMed] [Google Scholar]
- Pociot, F. , & Lernmark, Å. (2016). Genetic risk factors for type 1 diabetes. The Lancet, 387(10035), 2331–2339. 10.1016/S0140-6736(16)30582-7 [DOI] [PubMed] [Google Scholar]
- Pugazhenthi, S. , Qin, L. , & Reddy, P. H. (2017). Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Oxidative Stress and Mitochondrial Quality in Diabetes/Obesity and Critical Illness Spectrum of Diseases, 1863(5), 1037–1045. 10.1016/j.bbadis.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, L. , Ke, L. , Liu, X. , Liao, L. , Ke, S. , Liu, X. , Wang, Y. , Lin, X. , Zhou, Y. , Wu, L. , Chen, Z. , & Liu, L. (2016). Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase‐3β pathway in an amyloid β protein induced alzheimer disease mouse model. European Journal of Pharmacology, 783, 23–32. 10.1016/j.ejphar.2016.04.052 [DOI] [PubMed] [Google Scholar]
- Radde, R. , Bolmont, T. , Kaeser, S. A. , Coomaraswamy, J. , Lindau, D. , Stoltze, L. , Calhoun, M. E. , Jäggi, F. , Wolburg, H. , Gengler, S. , Haass, C. , Ghetti, B. , Czech, C. , Hölscher, C. , Mathews, P. M. , & Jucker, M. (2006). Abeta42‐driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Reports, 7(9), 940–946. 10.1038/sj.embor.7400784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth, T. B. , Kaptein, J. S. , Reue, K. L. , & Lusis, A. J. (1985). Evolution of apolipoprotein E: Mouse sequence and evidence for an 11‐nucleotide ancestral unit. Proceedings of the National Academy of Sciences, 82(23), 8085–8089. 10.1073/pnas.82.23.8085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff, R. M. (2016). How neuroinflammation contributes to neurodegeneration. Science, 353(6301), 777–783. 10.1126/science.aag2590 [DOI] [PubMed] [Google Scholar]
- Rebeck, G. W. , Reiter, J. S. , Strickland, D. K. , & Hyman, B. T. (1993). Apolipoprotein E in sporadic Alzheimer's disease: Allelic variation and receptor interactions. Neuron, 11(4), 575–580. 10.1016/0896-6273(93)90070-8 [DOI] [PubMed] [Google Scholar]
- Reddy, P. H. (2013). Amyloid beta‐induced glycogen synthase kinase 3β phosphorylated VDAC1 in Alzheimer's disease: Implications for synaptic dysfunction and neuronal damage. Biochimica et Biophysica Acta, 1832(12), 1913–1921. 10.1016/j.bbadis.2013.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, M. J. , Meszaros, K. , Entes, L. J. , Claypool, M. D. , Pinkett, J. G. , Gadbois, T. M. , & Reaven, G. M. (2000). A new rat model of type 2 diabetes: The fat‐fed, streptozotocin‐treated rat. Metabolism, 49(11), 1390–1394. 10.1053/meta.2000.17721 [DOI] [PubMed] [Google Scholar]
- Reger, M. A. , Watson, G. S. , Frey, W. H. 2nd , Baker, L. D. , Cholerton, B. , Keeling, M. L. , Belongia, D. A. , Fishel, M. A. , Plymate, S. R. , Schellenberg, G. D. , Cherrier, M. M. , & Craft, S. (2006). Effects of intranasal insulin on cognition in memory‐impaired older adults: Modulation by APOE genotype. Neurobiology of Aging, 27(3), 451–458. 10.1016/j.neurobiolaging.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Reger, M. A. , Watson, G. S. , Green, P. S. , Wilkinson, C. W. , Baker, L. D. , Cholerton, B. , Fishel, M. A. , Plymate, S. R. , Breitner, J. C. , DeGroodt, W. , Mehta, P. , & Craft, S. (2008). Intranasal insulin improves cognition and modulates beta‐amyloid in early AD. Neurology, 70(6), 440–448. 10.1212/01.WNL.0000265401.62434.36 [DOI] [PubMed] [Google Scholar]
- Riedel, B. C. , Thompson, P. M. , & Brinton, R. D. (2016). Age, APOE and sex: Triad of risk of Alzheimer's disease. The Journal of Steroid Biochemistry and Molecular Biology, 160, 134–147. 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner, M. E. , Saunders, A. M. , Altman, J. F. , Ormandy, G. C. , Craft, S. , Foley, I. M. , Zvartau‐Hind, M. E. , Hosford, D. A. , Roses, A. D. , & Rosiglitazone in Alzheimer's Disease Study Group . (2006). Efficacy of rosiglitazone in a genetically defined population with mild‐to‐moderate Alzheimer's disease. The Pharmacogenomics Journal, 6(4), 246–254. 10.1038/sj.tpj.6500369 [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Pascau, L. , Britti, E. , Calap‐Quintana, P. , Dong, Y. N. , Vergara, C. , Delaspre, F. , Medina‐Carbonero, M. , Tamarit, J. , Pallardó, F. V. , Gonzalez‐Cabo, P. , Ros, J. , Lynch, D. R. , Martinell, M. , & Pizcueta, P. (2021). PPAR gamma agonist leriglitazone improves frataxin‐loss impairments in cellular and animal models of Friedreich ataxia. Neurobiology of Disease, 148, 105162. 10.1016/j.nbd.2020.105162 [DOI] [PubMed] [Google Scholar]
- Rorsman, P. , & Ashcroft, F. M. (2018). Pancreatic β‐cell electrical activity and insulin secretion: Of mice and men. Physiological Reviews, 98(1), 117–214. 10.1152/physrev.00008.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom, M. H. , Barclay, T. R. , Pyle, M. , Owens, B. L. , Cagan, A. B. , Anderson, C. P. , Frey, W. H. 2nd , & Hanson, L. R. (2014). A single‐dose pilot trial of intranasal rapid‐acting insulin in apolipoprotein E4 carriers with mild‐moderate Alzheimer's disease. CNS Drugs, 28(12), 1185–1189. 10.1007/s40263-014-0214-y [DOI] [PubMed] [Google Scholar]
- Rotermund, C. , Machetanz, G. , & Fitzgerald, J. C. (2018). The therapeutic potential of metformin in neurodegenerative diseases. Frontiers in Endocrinology, 9, 400. 10.3389/fendo.2018.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp, N. J. , Wegenast‐Braun, B. M. , Radde, R. , Calhoun, M. E. , & Jucker, M. (2011). Early onset amyloid lesions lead to severe neuritic abnormalities and local, but not global neuron loss in APPPS1 transgenic mice. Neurobiology of Aging, 32(12), 2324.e1–2324.e6. 10.1016/j.neurobiolaging.2010.08.014 [DOI] [PubMed] [Google Scholar]
- Sabio, G. , Das, M. , Mora, A. , Zhang, Z. , Jun, J. Y. , Ko, H. J. , Barrett, T. , Kim, J. K. , & Davis, R. J. (2008). A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science (New York, N.Y.), 322(5907), 1539–1543. 10.1126/science.1160794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisho, Y. (2015). Metformin and inflammation: Its potential beyond glucose‐lowering effect. Endocrine, Metabolic & Immune Disorders Drug Targets, 15(3), 196–205. 10.2174/1871530315666150316124019 [DOI] [PubMed] [Google Scholar]
- Sato, T. , Hanyu, H. , Hirao, K. , Kanetaka, H. , Sakurai, H. , & Iwamoto, T. (2011). Efficacy of PPAR‐gamma agonist pioglitazone in mild Alzheimer disease. Neurobiology of Aging, 32(9), 1626–1633. 10.1016/j.neurobiolaging.2009.10.009 [DOI] [PubMed] [Google Scholar]
- Scheltens, P. , de Strooper, B. , Kivipelto, M. , Holstege, H. , Chételat, G. , Teunissen, C. E. , Cummings, J. , & van der Flier, W. M. (2021). Alzheimer's disease. Lancet (London, England), 397(10284), 1577–1590. 10.1016/S0140-6736(20)32205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano‐Pozo, A. , Das, S. , & Hyman, B. T. (2021). APOE and Alzheimer's disease: Advances in genetics, pathophysiology, and therapeutic approaches. The Lancet. Neurology, 20(1), 68–80. 10.1016/S1474-4422(20)30412-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano‐Pozo, A. , Frosch, M. P. , Masliah, E. , & Hyman, B. T. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 1(1), a006189. 10.1101/cshperspect.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny, J. , Suhy, J. , Chiao, P. , Chen, T. , Klein, G. , Purcell, D. , Oh, J. , Verma, A. , Sampat, M. , & Barakos, J. (2016). Amyloid PET screening for enrichment of early‐stage Alzheimer disease clinical trials: Experience in a phase 1b clinical trial. Alzheimer Disease and Associated Disorders, 30(1), 1–7. 10.1097/WAD.0000000000000144 [DOI] [PubMed] [Google Scholar]
- Sheridan, G. K. , Wdowicz, A. , Pickering, M. , Watters, O. , Halley, P. , O'Sullivan, N. C. , Mooney, C. , O'Connell, D. J. , O'Connor, J. J. , & Murphy, K. J. (2014). CX3CL1 is up‐regulated in the rat hippocampus during memory‐associated synaptic plasticity. Frontiers in Cellular Neuroscience, 8, 233. 10.3389/fncel.2014.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. , Zhang, Z. , Li, L. , & Hölscher, C. (2017). A novel dual GLP‐1/GIP receptor agonist alleviates cognitive decline by re‐sensitizing insulin signaling in the Alzheimer icv. STZ rat model. Behavioural Brain Research, 327, 65–74. 10.1016/j.bbr.2017.03.032 [DOI] [PubMed] [Google Scholar]
- Shindo, T. , Takasaki, K. , Uchida, K. , Onimura, R. , Kubota, K. , Uchida, N. , Irie, K. , Katsurabayashi, S. , Mishima, K. , Nishimura, R. , Fujiwara, M. , & Iwasaki, K. (2012). Ameliorative effects of telmisartan on the inflammatory response and impaired spatial memory in a rat model of Alzheimer's disease incorporating additional cerebrovascular disease factors. Biological & Pharmaceutical Bulletin, 35(12), 2141–2147. 10.1248/bpb.b12-00387 [DOI] [PubMed] [Google Scholar]
- Silva, M. V. F. , Loures, C. M. G. , Alves, L. C. V. , de Souza, L. C. , Borges, K. B. G. , & das Carvalho, M. G. (2019). Alzheimer's disease: Risk factors and potentially protective measures. Journal of Biomedical Science, 26(1), 33. 10.1186/s12929-019-0524-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, B. , Sharma, B. , Jaggi, A. S. , & Singh, N. (2013). Attenuating effect of lisinopril and telmisartan in intracerebroventricular streptozotocin induced experimental dementia of Alzheimer's disease type: Possible involvement of PPAR‐γ agonistic property. Journal of the Renin‐Angiotensin‐Aldosterone System: JRAAS, 14(2), 124–136. 10.1177/1470320312459977 [DOI] [PubMed] [Google Scholar]
- Skerrett, R. , Pellegrino, M. P. , Casali, B. T. , Taraboanta, L. , & Landreth, G. E. (2015). Combined liver X receptor/peroxisome proliferator‐activated receptor γ agonist treatment reduces amyloid β levels and improves behavior in amyloid precursor protein/presenilin 1 mice. The Journal of Biological Chemistry, 290(35), 21591–21602. 10.1074/jbc.M115.652008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, L. , Brandon, A. E. , Turner, N. , & Cooney, G. J. (2017). Modeling insulin resistance in rodents by alterations in diet: What have high‐fat and high‐calorie diets revealed? American Journal of Physiology‐Endocrinology and Metabolism, 314(3), E251–E265. 10.1152/ajpendo.00337.2017 [DOI] [PubMed] [Google Scholar]
- Solmaz, V. , Çınar, B. P. , Yiğittürk, G. , Çavuşoğlu, T. , Taşkıran, D. , & Erbaş, O. (2015). Exenatide reduces TNF‐α expression and improves hippocampal neuron numbers and memory in streptozotocin treated rats. European Journal of Pharmacology, 765, 482–487. 10.1016/j.ejphar.2015.09.024 [DOI] [PubMed] [Google Scholar]
- Spagnuolo, M. S. , Mollica, M. P. , Maresca, B. , Cavaliere, G. , Cefaliello, C. , Trinchese, G. , Scudiero, R. , Crispino, M. , & Cigliano, L. (2015). High fat diet and inflammation—Modulation of haptoglobin level in rat brain. Frontiers in Cellular Neuroscience, 9, 479–479. 10.3389/fncel.2015.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli, M. , Fusco, S. , & Grassi, C. (2019). Brain insulin resistance and hippocampal plasticity: Mechanisms and biomarkers of cognitive decline. Frontiers in Neuroscience, 13, 788. 10.3389/fnins.2019.00788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, K. , Viswanad, B. , Asrat, L. , Kaul, C. L. , & Ramarao, P. (2005). Combination of high‐fat diet‐fed and low‐dose streptozotocin‐treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacological Research, 52(4), 313–320. 10.1016/j.phrs.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Stein, M. S. , Scherer, S. C. , Ladd, K. S. , & Harrison, L. C. (2011). A randomized controlled trial of high‐dose vitamin D2 followed by intranasal insulin in Alzheimer's disease. Journal of Alzheimer's Disease, 26(3), 477–484. 10.3233/JAD-2011-110149 [DOI] [PubMed] [Google Scholar]
- Steinmetz, A. B. , Johnson, S. A. , Iannitelli, D. E. , Pollonini, G. , & Alberini, C. M. (2016). Insulin‐like growth factor 2 rescues aging‐related memory loss in rats. Neurobiology of Aging, 44, 9–21. 10.1016/j.neurobiolaging.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter, W. J. , Saunders, A. M. , Schmechel, D. , Pericak‐Vance, M. , Enghild, J. , Salvesen, G. S. , & Roses, A. D. (1993). Apolipoprotein E: High‐avidity binding to beta‐amyloid and increased frequency of type 4 allele in late‐onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 90(5), 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, J. , Liu, W. , Li, Y. , Li, L. , & Hölscher, C. (2018). Neuroprotective effects of a triple GLP‐1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer's disease. Brain Research, 1678, 64–74. 10.1016/j.brainres.2017.10.012 [DOI] [PubMed] [Google Scholar]
- Talbot, K. , Wang, H.‐Y. , Kazi, H. , Han, L.‐Y. , Bakshi, K. P. , Stucky, A. , Fuino, R. L. , Kawaguchi, K. R. , Samoyedny, A. J. , Wilson, R. S. , Arvanitakis, Z. , Schneider, J. A. , Wolf, B. A. , Bennett, D. A. , Trojanowski, J. Q. , & Arnold, S. E. (2012a). Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF‐1 resistance, IRS‐1 dysregulation, and cognitive decline. The Journal of Clinical Investigation, 122(4), 1316–1338. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot, K. , Wang, H.‐Y. , Kazi, H. , Han, L.‐Y. , Bakshi, K. P. , Stucky, A. , Fuino, R. L. , Kawaguchi, K. R. , Samoyedny, A. J. , Wilson, R. S. , Arvanitakis, Z. , Schneider, J. A. , Wolf, B. A. , Bennett, D. A. , Trojanowski, J. Q. , & Arnold, S. E. (2012b). Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF‐1 resistance, IRS‐1 dysregulation, and cognitive decline. The Journal of Clinical Investigation, 122(4), 1316–1338. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taouis, M. , & Torres‐Aleman, I. (2019). Editorial: Insulin and the brain. Frontiers in Endocrinology, 10, 299. 10.3389/fendo.2019.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo, E. M. , & Inestrosa, N. C. (2010). Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer's disease. Molecular Psychiatry, 15(3), 272–285. 10.1038/mp.2009.72 [DOI] [PubMed] [Google Scholar]
- Tramutola, A. , Arena, A. , Cini, C. , Butterfield, D. A. , & Barone, E. (2017). Modulation of GLP‐1 signaling as a novel therapeutic approach in the treatment of Alzheimer's disease pathology. Expert Review of Neurotherapeutics, 17(1), 59–75. 10.1080/14737175.2017.1246183 [DOI] [PubMed] [Google Scholar]
- Tsukuda, K. , Mogi, M. , Iwanami, J. , Min, L.‐J. , Sakata, A. , Jing, F. , Iwai, M. , & Horiuchi, M. (2009). Cognitive deficit in amyloid‐beta‐injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator‐activated receptor‐gamma activation. Hypertension, 54(4), 782–787. 10.1161/HYPERTENSIONAHA.109.136879 [DOI] [PubMed] [Google Scholar]
- Tzimopoulou, S. , Cunningham, V. J. , Nichols, T. E. , Searle, G. , Bird, N. P. , Mistry, P. , Dixon, I. J. , Hallett, W. A. , Whitcher, B. , Brown, A. P. , Zvartau‐Hind, M. , Lotay, N. , Lai, R. Y. , Castiglia, M. , Jeter, B. , Matthews, J. C. , Chen, K. , Bandy, D. , Reiman, E. M. , … Matthews, P. M. (2010). A multi‐center randomized proof‐of‐concept clinical trial applying [(1)(8)F]FDG‐PET for evaluation of metabolic therapy with rosiglitazone XR in mild to moderate Alzheimer's disease. Journal of Alzheimer's Disease, 22(4), 1241–1256. 10.3233/JAD-2010-100939 [DOI] [PubMed] [Google Scholar]
- Uemura, E. , & Greenlee, H. W. (2006). Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Experimental Neurology, 198(1), 48–53. 10.1016/j.expneurol.2005.10.035 [DOI] [PubMed] [Google Scholar]
- van der Heide, L. P. , Kamal, A. , Artola, A. , Gispen, W. H. , & Ramakers, G. M. J. (2005). Insulin modulates hippocampal activity‐dependent synaptic plasticity in a N‐methyl‐d‐aspartate receptor and phosphatidyl‐inositol‐3‐kinase‐dependent manner. Journal of Neurochemistry, 94(4), 1158–1166. 10.1111/j.1471-4159.2005.03269.x [DOI] [PubMed] [Google Scholar]
- van Dyken, P. , & Lacoste, B. (2018). Impact of metabolic syndrome on neuroinflammation and the blood–brain barrier. Frontiers in Neuroscience, 12, 930. 10.3389/fnins.2018.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj, A. , & Galea, I. (2017). The blood‐brain barrier in systemic inflammation. Brain, Behavior, and Immunity, 60, 1–12. 10.1016/j.bbi.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Velazquez, R. , Tran, A. , Ishimwe, E. , Denner, L. , Dave, N. , Oddo, S. , & Dineley, K. T. (2017). Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer's disease. Neurobiology of Aging, 58, 1–13. 10.1016/j.neurobiolaging.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdile, G. , Keane, K. N. , Cruzat, V. F. , Medic, S. , Sabale, M. , Rowles, J. , Wijesekara, N. , Martins, R. N. , Fraser, P. E. , & Newsholme, P. (2015). Inflammation and oxidative stress: The molecular connectivity between insulin resistance, obesity, and Alzheimer's disease. Mediators of Inflammation, 2015, 105828. 10.1155/2015/105828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa, A. , Pomilio, C. , Gregosa, A. , Bentivegna, M. , Presa, J. , Bellotto, M. , Saravia, F. , & Beauquis, J. (2021). Inflammation and insulin resistance as risk factors and potential therapeutic targets for Alzheimer's disease. Frontiers in Neuroscience, 15, 653651. 10.3389/fnins.2021.653651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali, J. A. , Jarzebska, N. , Raubenheimer, D. , Simpson, S. J. , Rodionov, R. N. , & O'Sullivan, J. F. (2020). Cardio‐metabolic effects of high‐fat diets and their underlying mechanisms—A narrative review. Nutrients, 12(5), 1505. 10.3390/nu12051505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Grundke‐Iqbal, I. , & Iqbal, K. (2007). Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. In European Journal of Neuroscience, 25(1), 59–68. Premier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Wang, L. , Jiang, R. , Xu, Y. , Zhao, X. , & Li, Y. (2016). Exendin‐4 antagonizes Aβ1‐42‐induced attenuation of spatial learning and memory ability. Experimental and Therapeutic Medicine, 12(5), 2885–2892. 10.3892/etm.2016.3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhao, J. , Guo, F.‐L. , Gao, X. , Xie, X. , Liu, S. , Yang, X. , Yang, X. , Zhang, L. , Ye, Y. , Fan, L. , & Wang, J. (2020). Metformin ameliorates synaptic defects in a mouse model of AD by inhibiting Cdk5 activity. Frontiers in Cellular Neuroscience, 14, 170. 10.3389/fncel.2020.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, G. S. , Baker, L. D. , Cholerton, B. A. , Rhoads, K. W. , Merriam, G. R. , Schellenberg, G. D. , Asthana, S. , Cherrier, M. , & Craft, S. (2009). Effects of insulin and octreotide on memory and growth hormone in Alzheimer's disease. Journal of Alzheimer's Disease, 18(3), 595–602. 10.3233/JAD-2009-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, G. S. , Cholerton, B. A. , Reger, M. A. , Baker, L. D. , Plymate, S. R. , Asthana, S. , Fishel, M. A. , Kulstad, J. J. , Green, P. S. , Cook, D. G. , Kahn, S. E. , Keeling, M. L. , & Craft, S. (2005). Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: A preliminary study. The American Journal of Geriatric Psychiatry, 13(11), 950–958. 10.1176/appi.ajgp.13.11.950 [DOI] [PubMed] [Google Scholar]
- Webster, S. J. , Bachstetter, A. D. , & van Eldik, L. J. (2013). Comprehensive behavioral characterization of an APP/PS‐1 double knock‐in mouse model of Alzheimer's disease. Alzheimer's Research & Therapy, 5(3), 28. 10.1186/alzrt182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z. , Koya, J. , & Reznik, S. E. (2021). Insulin resistance exacerbates Alzheimer disease via multiple mechanisms. Frontiers in Neuroscience, 15, 687157. 10.3389/fnins.2021.687157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werther, G. A. , Hogg, A. , Oldfield, B. J. , McKinley, M. J. , Figdor, R. , & Mendelsohn, F. A. (1989). Localization and characterization of insulin‐like growth factor‐i receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry* A distinct distribution from insulin receptors. Journal of Neuroendocrinology, 1(5), 369–377. 10.1111/j.1365-2826.1989.tb00131.x [DOI] [PubMed] [Google Scholar]
- Xu, Q. , Bernardo, A. , Walker, D. , Kanegawa, T. , Mahley, R. W. , & Huang, Y. (2006). Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(19), 4985–4994. 10.1523/JNEUROSCI.5476-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka, M. , Ishikawa, T. , Griep, A. , Axt, D. , Kummer, M. P. , & Heneka, M. T. (2012). PPARγ/RXRα‐induced and CD36‐mediated microglial amyloid‐β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. The Journal of Neuroscience, 32(48), 17321–17331. 10.1523/JNEUROSCI.1569-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, Y. , Painter, M. M. , Bu, G. , & Kanekiyo, T. (2016). Apolipoprotein E as a therapeutic target in Alzheimer's disease: A review of basic research and clinical evidence. CNS Drugs, 30(9), 773–789. 10.1007/s40263-016-0361-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R. , Pedersen, N. L. , Bao, C. , Xu, W. , Xu, H. , Song, R. , Qi, X. , & Xu, W. (2019). Type 2 diabetes in midlife and risk of cerebrovascular disease in late life: A prospective nested case‐control study in a nationwide Swedish twin cohort. Diabetologia, 62(8), 1403–1411. 10.1007/s00125-019-4892-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Fang, H. , Xu, G. , Zhen, Y. , Zhang, Y. , Tian, J. , Zhang, D. , Zhang, G. , & Xu, J. (2018). Liraglutide improves cognitive impairment via the AMPK and PI3K/Akt signaling pathways in type 2 diabetic rats. Molecular Medicine Reports, 18(2), 2449–2457. 10.3892/mmr.2018.9180 [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Wu, Y. , Zhang, S. , & Song, W. (2013). High glucose promotes Aβ production by inhibiting APP degradation. PLoS ONE, 8(7), e69824. 10.1371/journal.pone.0069824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarza, R. , Vela, S. , Solas, M. , & Ramirez, M. J. (2016). C‐Jun N‐terminal kinase (JNK) signaling as a therapeutic target for Alzheimer's disease. Frontiers in Pharmacology, 6, 321. 10.3389/fphar.2015.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, S. O. , Park, D. J. , Ryu, J. C. , Ozer, H. G. , Tep, C. , Shin, Y. J. , Lim, T. H. , Pastorino, L. , Kunwar, A. J. , Walton, J. C. , Nagahara, A. H. , Lu, K. P. , Nelson, R. J. , Tuszynski, M. H. , & Huang, K. (2012). JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron, 75(5), 824–837. 10.1016/j.neuron.2012.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, L. , Li, Y. , Li, L. , Melchiorsen, J. U. , Rosenkilde, M. , & Holscher, C. (2020). The novel dual GLP‐1/GIP receptor agonist DA‐CH5 is superior to single GLP‐1 receptor agonists in the MPTP model of Parkinson's disease. Journal of Parkinson's Disease, 10(2), 523–542. 10.3233/JPD-191768 [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Lv, X. Y. , Li, J. , Xu, Z. G. , & Chen, L. (2008). The characterization of high‐fat diet and multiple low‐dose streptozotocin induced type 2 diabetes rat model. Experimental Diabetes Research, 2008, 704045. 10.1155/2008/704045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Chai, R. , Yang, Y.‐Y. , Guo, S.‐Q. , Wang, S. , Guo, T. , Xu, S.‐F. , Zhang, Y.‐H. , Wang, Z.‐Y. , & Guo, C. (2017). Chronic diabetic states worsen Alzheimer neuropathology and cognitive deficits accompanying disruption of calcium signaling in leptin‐deficient APP/PS1 mice. Oncotarget, 8(27), 43617–43634. 10.18632/oncotarget.17116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, F. , Siu, J. J. , Huang, W. , Askwith, C. , & Cao, L. (2019). Insulin modulates excitatory synaptic transmission and synaptic plasticity in the mouse hippocampus. Neuroscience, 411, 237–254. 10.1016/j.neuroscience.2019.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Bi, W. , Xiao, S. , Lan, X. , Cheng, X. , Zhang, J. , Lu, D. , Wei, W. , Wang, Y. , Li, H. , Fu, Y. , & Zhu, L. (2019). Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Scientific Reports, 9(1), 5790. 10.1038/s41598-019-42286-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, W.‐Q. , de Felice, F. G. , Fernandez, S. , Chen, H. , Lambert, M. P. , Quon, M. J. , Krafft, G. A. , & Klein, W. L. (2008). Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 22(1), 246–260. 10.1096/fj.06-7703com [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , & Zhao, B. (2013). Oxidative stress and the pathogenesis of Alzheimer's disease. Oxidative Medicine and Cellular Longevity, 2013, e316523. 10.1155/2013/316523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Yu, W. , Zhang, M. , Tian, X. , Li, Y. , & Lü, Y. (2019). Imbalance of microglial TLR4/TREM2 in LPS‐treated APP/PS1 transgenic mice: A potential link between Alzheimer's disease and systemic inflammation. Neurochemical Research, 44(5), 1138–1151. 10.1007/s11064-019-02748-x [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Shen, J. , Feng, S. , Huang, C. , Liu, Z. , Sun, Y. E. , & Liu, H. (2020). Metformin improves cognition of aged mice by promoting cerebral angiogenesis and neurogenesis. Aging, 12(18), 17845–17862. 10.18632/aging.103693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic, B. V. (2008). The blood‐brain barrier in health and chronic neurodegenerative disorders. Neuron, 57(2), 178–201. 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All new data in this article are derived from clinicaltrials.gov and are freely publicly available. Our analyses as applied to the data are described in the methods section and can be reproduced. A web‐portal to enhance access to clinical trial data is under construction and will be available by contacting the lead author or by searching Alzheimer's Clinical Trial Innovation (ACTION) Initiative.


