Abstract
PL promoters direct the transcription of the duplicated cbb operons from the facultative chemoautotroph Ralstonia eutropha H16. The operons encode most enzymes of the Calvin-Benson-Bassham carbon reduction cycle required for CO2 assimilation. Their transcription depends on the activator protein CbbR. Structure-function relationships in the cloned chromosomal promoter region were analyzed by site-directed mutagenesis. PL was altered in its presumed hexameric −35 and/or −10 box or in the spacer region between the boxes to achieve a greater or lesser resemblance to the structure of the ς70 consensus promoter of Escherichia coli. PL::lacZ transcriptional fusions of various promoter variants were assayed in transconjugant strains of R. eutropha as well as in corresponding cbbR deletion mutants. Mutations increasing the similarity of the −35 and/or −10 box to the consensus sequence stimulated PL activity to various extents, whereas mutations deviating from the consensus decreased the activity. The length of the spacer region also proved to be critical. The conversion of the boxes, either individually or simultaneously, into the consensus sequences resulted in a highly active PL. All improved PL mutants, however, retained the activation under inducing or derepressing growth conditions, although the full-consensus promoter was nearly constitutive. They were also activated in the cbbR mutants. The activity of the overlapping, divergently oriented cbbR promoter was less affected by the mutations. The half- and full-consensus PL mutants were comparably active in E. coli. Two major conclusions were drawn from the results: (i) the location and function of PL were verified, and (ii) indirect evidence was obtained for the involvement of another regulator(s), besides CbbR, in the transcriptional control of the R. eutropha cbb operons.
Autotrophy denotes the ability of an organism to gain the majority of its cell carbon by the assimilation of CO2. The Calvin-Benson-Bassham carbon reduction cycle (2) is the quantitatively predominating route of CO2 fixation among autotrophs (28). Ralstonia eutropha (Alcaligenes eutrophus) is an aerobic, facultatively chemoautotrophic β-proteobacterium, which fixes CO2 via the Calvin cycle, by using either hydrogen or formate as an energy source for litho- or organoautotrophic growth, respectively (5). Most genes of Calvin cycle enzymes in R. eutropha, including those of ribulose-1,5-bisphosphate carboxylase oxygenase (RubisCO)—the actual CO2-fixing enzyme of the cycle—are encoded within a chromosomal operon (cbbc operon) of about 15 kbp. Strain H16 harbors a second, highly homologous, and identically organized copy of the operon (cbbp operon) located on the megaplasmid pHG1 (20). The cbbp operon lacks, however, the 3′-terminal cbbBc gene present in the cbbc operon (4), whereas the large subunit gene cbbL of RubisCO forms the 5′ ends of both operons. Although the organizations and sizes of cbb operons vary considerably among autotrophic bacteria, they also show some common features (11, 20, 33).
The cbbc operon of R. eutropha H16 is separated by 167 bp from the divergently oriented regulatory gene cbbR, whose product is required for transcription activation of the duplicate cbb operons. CbbR belongs to the LysR family of transcriptional regulator proteins (32). A deficient cbbR gene (cbbR′) is situated exactly in the position corresponding to the cbbp operon on pHG1 (37). The intergenic segments between cbbR or cbbR′ and cbbLc or cbbLp constitute the control regions of the operons, as they contain the operators and promoters of the system (19, 21). CbbR has been shown to bind preferentially between positions −29 and −74 relative to the transcriptional start point of the cbbc operon (19). Consistent with metabolic economy, the heterotrophic growth of the organism on most organic substrates completely represses the transcription of the cbb operons, to avoid wasting of energy that would be caused by CO2 fixation under these conditions. Partial derepression occurs during growth on a few substrates, such as fructose and gluconate, but only autotrophic growth results in the full derepression or induction of the operons (6, 10, 21). The transcriptional control is postulated to involve the transduction of a still unknown metabolic signal sensed by CbbR, which modulates the activity of the cbbc and cbbp operon promoter PL (20). No significant subpromoter activity was detected within the operons (31). Structural motifs tentatively assigned to PL resemble those of ς70-dependent promoters of Escherichia coli (12), and this applies also, although in a less pronounced way, to the presumed cbbR and cbbR′ promoter PR. The divergent promoters PL and PR are arranged in a back-to-back configuration, according to Beck and Warren (3), with overlapping −35 boxes (21).
As a prerequisite to understanding the regulatory mechanism acting upon the cbb operons of R. eutropha H16, critical structural properties of PL must be known. Therefore, a mutational analysis of chromosomal PL was performed that aimed at defining sequence elements important for the promoter activity. The activities of altered PL promoters were determined not only in the homologous host R. eutropha but also in E. coli and in newly constructed cbbR deletion mutants of R. eutropha. The results verified the location and function of the promoter and provided a first hint that, in addition to CbbR, another regulator(s) might participate in the transcriptional control of the cbb operons in R. eutropha.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are shown in Table 1. Strains of R. eutropha were grown in a nutrient broth or mineral medium (MM) at 30°C as described previously (36). MM was routinely supplemented with 0.2% (wt/vol) organic substrate for either heterotrophic or organoautotrophic growth on formate. Mixotrophic cultures were initially grown on 0.1% (wt/vol) fructose until reaching an optical density at 436 nm of 1 to 2, before 0.2% (wt/vol) formate was added and the incubation was continued for another 4 h. Lithoautotrophic cultures were gassed with a mixture of H2, CO2, and O2 (8:1:1 [vol/vol/vol]). E. coli was propagated in Luria-Bertani medium at 37 or 30°C. If required, the media contained ampicillin (50 μg/ml), kanamycin (50 μg/ml for E. coli, 450 μg/ml in MM for R. eutropha, or 120 μg/ml in nutrient broth), or tetracycline (15 μg/ml for E. coli or 20 μg/ml for R. eutropha) as selective antibiotics.
TABLE 1.
List of bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| R. eutropha | ||
| H16 | Cfx Hox Fox, pHG1; wild type | ATCC 17699 |
| HF210 | Smr Cfx Hox− Fox, pHG1−; derivative of strain HF39, a Smr mutant of strain H16 | 17 |
| HB14 | Cfx− Hox Fox, pHG1; cbbRΔ; derivative of strain H16 | This study |
| HB15 | Smr Cfx− Hox− Fox, pHG1−; cbbRΔ; derivative of strain HF210 | This study |
| E. coli | ||
| XL1-Blue | Tcr; recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 lac(F′[proAB lacIq lacZΔM15 Tn10]) | 8 |
| JW1 | ara Δ(lac-proAB) rpsL thi Φ80(lacZΔM15) F′(lacIq lacZΔM15 proAB+) | 16 |
| S17-1 | Smr Tprmod+res thi pro recA; integrated RP4 (Tc::Mu-Km::Tn7) | 34 |
| Plasmids | ||
| pUC18/19 | AprlacPOZ′ | 38 |
| pBluescript KS | AprlacPOZ′ | Stratagene |
| pBBR1MCS-3 | TcrlacPOZ′ | 18 |
| pBH2241b | pUC19::224-bp BglII-HinfI fragment containing the intergenic region between cbbR and cbbLc | 21 |
| pAEC1200 | pUC18::1.8-kb HindIII-SalI fragment containing cbbR | This study |
| pAEC1200Δ | pUC18::0.9-kb HindIII-SalI fragment, derivative of pAEC1200 with deleted 0.9-kb BglII-SmaI segment in cbbR | This study |
| pLO1 | Kmr; sacB RP4-oriT ColE1-oriT | 24 |
| pNHG1 | Kmr Tcr; pLO1::1.48-kb DdeI-VspI fragment from pBBR1MCS-3 | This study |
| pNHG110 | pNHG1::0.9-kb HindIII-SalI fragment from pAEC1200Δ | This study |
| pUW7 | Tcr; pVK101::1,031-bp DdeI fragment containing cbbR | 37 |
| pBK | Tcr; double operon fusion plasmid with divergently oriented lacZ (β-galactosidase) and gusA (β-glucuronidase) as reporter genes | 21 |
| pBK2241c | pBK::236-bp XbaI-PstI fragment from pBH2241 | 21 |
| pBKM1 through pBKM14 | pBK::236-bp XbaI-PstI fragment with different mutations in PL (Table 2) | This study |
Apr, ampicillin resistance; Smr, streptomycin resistance; Tcr, tetracycline resistance; Tpr, trimethoprim resistance; Cfx, ability for autotrophic CO2 fixation; Hox, ability for H2 oxidation; Fox, ability for formate oxidation; pHG1, megaplasmid.
In pBH2241 cbbc operon promoter PL is oriented divergently, relative to lacZ.
In pBK2241 and pBKM1 through pBKM14, PL is oriented colinearly, relative to lacZ.
Manipulation and sequencing of DNA.
Standard procedures (1, 29) were employed to isolate genomic and plasmid DNA from bacteria, to transform plasmid DNA into E. coli, and for general DNA handling. Restriction endonucleases and DNA-modifying enzymes were used under the reaction conditions recommended by the manufacturers. DNA probes to be applied in Southern hybridizations were nonradioactively labelled with peroxidase by means of a special reaction system (ECL direct labelling and detection kit; Amersham, Brunswick, Germany). For the hybridizations, DNA fragments were separated by agarose gel electrophoresis and transferred onto a nylon membrane (Biodyne B; Pall, Dreieich, Germany) by vacuum blotting. DNA sequences were determined by the dideoxy chain termination method (30) and PCR cycle sequencing (SequiTherm cycle sequencing kit; Biozym, Hessisch Oldendorf, Germany) with 35S- or fluorescence-labelled oligonucleotide primers. Plasmids were conjugally transferred from E. coli S17-1 to strains of R. eutropha by biparental mating (35).
Generation of mutations in PL and construction of transcriptional fusions.
Oligonucleotide-directed mutagenesis carried out in a two-stage PCR (9) was employed to introduce mutations into the cbbc operon promoter PL. The specifically designed oligonucleotides M1 through M14 (24-mers; not shown) contained the desired mutations close to their centers. Together with a reverse primer (24-mer) they served individually to perform the first amplification (30 cycles at 95°C for 30 s, 59°C for 30 s, and 72°C for 60 s) of a segment (121 to 147 bp) of the 224-bp BglII-HinfI DNA fragment inserted in pBH2241. The reaction mixtures (100 μl) included 200 ng of pBH2241 as a template, a 2 μM concentration of mutagenesis primer, a 2 μM concentration of reverse primer, 2 mM concentrations of dATP, dGTP, dCTP, and dTTP, 25 mM MgCl2, and 2 U of Tfl DNA polymerase in a buffer system formulated by the supplier of the polymerase (Biozym) and were placed in a thermal cycler (model PTC-100; MJ Research, Watertown, Mass.). The mutagenized PCR product was purified by electrophoresis in low-melting-point agarose. It was used as the primer in a second PCR (30 cycles at 95°C for 30 s, 50°C for 60 s, and 72°C for 60 s) together with a universal primer (24-mer) to accomplish the amplification of the complete BglII-HinfI fragment. If insufficient amounts of the product (330 bp) were obtained in the second PCR, it was reamplified in a third PCR (the same conditions as in the first PCR) by means of the universal and reverse primers. The product of the final PCR was digested with either EcoRI and HindIII or XbaI and PstI prior to being cloned into correspondingly cleaved pUC19 or pBluescript KS, respectively. DNA sequencing of the respective plasmids pM1 through pM14 (not listed) confirmed the specific mutations. Finally, the inserts of these plasmids were excised with XbaI and PstI and recloned into correspondingly digested pBK. The resulting plasmids, pBKM1 through pBKM14, carried the cbbc::lacZ transcriptional fusions with differently modified PL promoters.
Construction of suicide plasmid pNHG1.
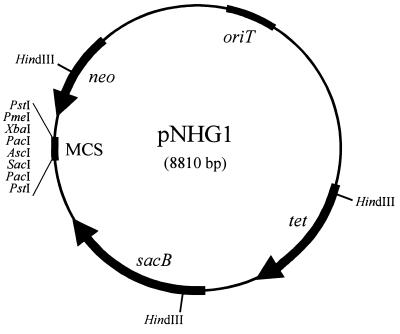
The suicide vector pLO1 (7,322 bp [23]) has been designed as a tool for gene replacement mutagenesis in gram-negative bacteria, especially in R. eutropha (24). However, because of the inherently low sensitivities of R. eutropha H16 and particularly of strain HF210 to kanamycin, an additional, easily selectable marker in these strains was incorporated into pLO1. For this purpose, the tetracycline resistance gene tet was excised from pBBR1MCS-3 as a 1,484-bp DdeI-VspI DNA fragment. Plasmid pLO1 was partially digested with BamHI, and the recessed 3′ ends of the linearized plasmid and of the DdeI-VspI fragment were filled in by treatment with the Klenow fragment of DNA polymerase I. The ligation of the two DNA fragments resulted eventually in the isolation of the new suicide vector pNHG1 (Fig. 1).
FIG. 1.
Map of suicide vector pNHG1. The plasmid encompasses 8,810 bp and was constructed by inserting a fragment carrying the tetracycline resistance gene tet from pBBR1MCS-3 into pLO1. Only unique cleavage sites of restriction endonucleases are indicated within the multiple cloning site (MCS). neo, the gene encoding kanamycin resistance; oriT, the origin of transfer replication; sacB, the gene encoding levansucrase.
Construction of cbbR deletion mutants.
To construct isogenic cbbR deletion mutants of R. eutropha H16 and HF210, a 1.8-kb HindIII-SalI fragment containing cbbR was first cloned into pUC18, yielding pAEC1200. The plasmid was linearized with BglII and further digested with HindIII after filling in the recessed 3′ ends of the BglII site by treatment with Klenow fragment. In a separate reaction pAEC1200 was cut with HindIII and SmaI. The resulting 3.2-kb HindIII-BglII and 0.4-kb HindIII-SmaI fragments, respectively, were ligated to produce pAEC1200Δ, which lacked the internal 891 bp of cbbR (total length of the gene, 954 bp). To reclone the 0.9-kb HindIII-SalI fragment, pAEC1200Δ was digested with HindIII, treated with Klenow fragment to fill in the 3′ ends of the site, and cleaved with XbaI. The 0.9-kb fragment carrying cbbRΔ was finally ligated to pNHG1 digested with XbaI and PmeI, generating pNHG110.
An allelic exchange of cbbRΔ for cbbR was achieved by two consecutively selected recombination events. Single recombinants (heterogenotes) of H16 and HF210 were characterized by simultaneously acquired tetracycline and kanamycin resistance after the conjugal transfer of pNHG1 from E. coli S17-1. Double recombinants (homogenotes), which gained sucrose resistance and concomitantly lost both antibiotic resistances, were obtained from heterogenotes as described by Lenz et al. (24).
Enzyme assays.
The activity of RubisCO in R. eutropha was determined in a radiometric, whole-cell assay based on the fixation of 14CO2 as described previously (22). A colorimetric assay was used for β-galactosidase (25), as well as a fluorimetric assay for β-glucuronidase (14), employing crude cell extracts prepared from R. eutropha or E. coli. One unit of activity represents the amount of enzyme catalyzing the formation of 1 μmol of product per min. Cultures of the strains were grown to an optical density at 436 nm of 2 to 3. The cells were harvested, resuspended in the appropriate buffer, and disrupted by sonication. Cell extracts were obtained after centrifugation at 14,000 × g for 20 min to remove unbroken cells and cell debris. Protein concentrations in the extracts were estimated by the method of Bradford (7).
RESULTS
Mutational modification of PL.
The proposed ς70-type PL promoters of the two cbb operons of R. eutropha H16 are believed to have the following structure on the nontemplate DNA strand: [−35] TTTACC-N17-TATACC [−10] (Table 2). The resemblance of PL to the canonical ς70-dependent promoter of E. coli (12) is evident for the hexameric −35 and −10 boxes as well as the length (17 bp) of the spacer region. In order to study the functional significance of the PL substructures, various mutations were introduced into the chromosomal wild-type promoter cloned within plasmid pBH2241. Specifically designed oligonucleotides were used to direct the PCR-based mutageneses. Fourteen different mutant PL promoters were produced that carried single or multiple sequence alterations (Table 2). The −10 and −35 boxes of mutants M1 and M6, respectively, matched the corresponding elements of the E. coli consensus promoter. Mutant M9, which was generated from M1, contains the consensus sequence in both regions. The spacer mutants included clones with either a single nucleotide deletion (M12), an insertion (M13), or a double substitution (M14). PL::lacZ transcriptional fusions were constructed for each mutant (pM1 through pM14) to enable the subsequent determination of the different promoter activities in R. eutropha as well as in E. coli.
TABLE 2.
Sequences of mutant PL promoters of the cbbc operon from R. eutropha H16
| Promoter | DNA sequence of PLa |
|---|---|
| −35 —spacer region— −10 | |
| Wild type | cbbR-TTTACCTTATGTGGGTGGGCTTATATCTT-cbbLc |
| M1 | TTTACC -N17- TATAATb |
| M2 | TTTACC -N17- TATCAT |
| M3 | TTTACC -N17- TATATT |
| M4 | TTTACC -N17- CCTCTT |
| M5 | TTGACC -N17- TATCTT |
| M6 | TTGACA -N17- TATCTT |
| M7 | TTTACA -N17- TATCTT |
| M8 | CCTACC -N17- TATCTT |
| M9 | TTGACA -N17- TATAAT |
| M10 | CTGACA -N17- TATAAT |
| M11 | TTTACA -N17- TACCTT |
| M12 | TTTACCTTATGTGGG-GGGCTTATATCTT |
| M13 | TTTACCTTATGTGGGTGGGGCTTATATCTT |
| M14 | TTTACCTTATCAGGGTGGGCTTATATCTT |
| ς70-dependent consensus of E. coli | TTGACA -N17- TATAAT |
The −35 and −10 boxes of wild-type PL are boxed; N17 indicates an unmutated spacer region in PL and an unspecified spacer sequence in the E. coli consensus promoter.
Mutated, deleted (M12), and inserted (M13) bases are in boldface type.
Isolation and phenotype of cbbR deletion mutants.
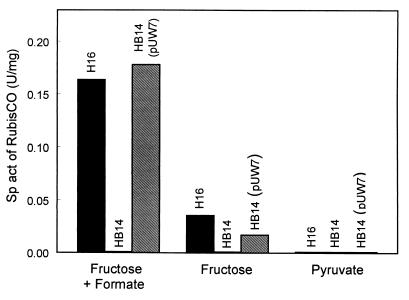
Because the cbb promoter activities were intended to be determined in both the wild-type and a CbbR-free background, cbbR null mutants of R. eutropha wild-type H16 and pHG1-cured HF210 were constructed by gene replacement mutagenesis. The derived mutants, HB14 and HB15, respectively, carried an 891-bp in-frame deletion within cbbR, as verified by the sequencing of corresponding PCR-generated fragments and by Southern hybridizations (data not shown). The potential product of cbbRΔ is a small protein of 20 amino acid residues that lacks 297 residues, including the helix-turn-helix structural motif in the NH2-terminal domain, of the authentic CbbR. A cbbRΔ-derived product with binding capacity to the cbb operator region could therefore not be formed in the mutants. Both strains concurrently lost the ability to grow autotrophically and to derepress or induce the cbb operons. They regained these properties by in trans complementation with cbbR present on plasmid pUW7. The transconjugant HB14(pUW7) showed RubisCO activities similar to that of wild-type H16 after mixotrophic growth on fructose plus formate or heterotrophic growth on fructose, whereas no activity was detected in cells grown on pyruvate (Fig. 2). These data corroborated the function of CbbR as an activator of the cbb operons and the suitability of the mutants for the subsequent promoter assays.
FIG. 2.
Activities of RubisCO in R. eutropha H16, HB14, and HB14(pUW7) grown on fructose plus formate, fructose, or pyruvate. The enzyme activity was determined by means of a whole-cell assay. Sp act, specific activity.
Activities of mutant PL in R. eutropha.
The β-galactosidase reporter activities originating from the various mutant PL::lacZ fusions were first determined in transconjugants of wild-type R. eutropha H16 grown under lithoautotrophic (H2-CO2) or heterotrophic (fructose or pyruvate) conditions. Confidence in the significance of the individual data on promoter activities was ensured by obtaining them from at least three independent cultures. Promoters with a closer match of the −10 (M1, M2, and M3) or −35 (M5, M6, and M7) box of PL to those of the ς70 consensus promoter of E. coli showed increased activity relative to that of the wild-type promoter present in pBK2241 (Table 3). The increase depended on the position and number of base substitutions but occurred independently of the growth substrate. This was particularly evident for mutants M1 (−10 consensus), M6 (−35 consensus), and M9 (full consensus), whose activities in autotrophic cells reached a level about twofold that of the wild type. More significant, however, was the drastic increase in heterotrophic cells, even after growth on the normally strongly repressing pyruvate. The basal activity of PL M9 in pyruvate-grown cells was actually higher than that of the wild-type promoter under autotrophic conditions. At least M9 can thus be considered to be nearly constitutive. Nevertheless, all improved variants of PL still exhibited the principal regulatory pattern of the wild-type promoter characterized by derepression or induction in autotrophically or fructose-grown cells. Diminishing the resemblance of either the −10 (M4) or −35 (M8) box to the consensus by replacing the conserved distal bases TA or TT with CC resulted in a nearly total loss of promoter activity. The substitution of the distal T of the −35 box in the full-consensus M9 converted the constitutive into a repressible promoter (M10). Replacing one consensus base in the −10 box of wild-type PL and simultaneously introducing a 1-base match in the −35 box gave a promoter (M11) which was slightly more active than the wild type and also retained the activation. In contrast, the deletion of one base within the spacer region (N17→N16) largely inactivated PL (M12), whereas the insertion of one base (N17→N18) caused only a partial loss of activity (M13). An exchange of two adjacent bases (GT→TA) within the spacer had no significant effect on the promoter (M14).
TABLE 3.
Activities of mutant PL promoters determined by PL::lacZ transcriptional fusions in transconjugants of R. eutropha H16
| Transconjugant | Sp act of β-galactosidase (mU/mg of protein)a grown on:
|
||
|---|---|---|---|
| H2-CO2 | Fructose | Pyruvate | |
| pBKb | 4 | 3 | 3 |
| pBK2241c | 930 | 100 | 6 |
| pBKM1d | 1,590 | 1,200 | 90 |
| pBKM2 | 1,120 | 560 | 16 |
| pBKM3 | 930 | 360 | 8 |
| pBKM4 | 5 | 5 | 6 |
| pBKM5 | 1,770 | 300 | 16 |
| pBKM6 | 1,940 | 1,440 | 90 |
| pBKM7 | 1,580 | 240 | 12 |
| pBKM8 | 15 | 7 | 5 |
| pBKM9 | 2,200 | 1,610 | 1,270 |
| pBKM10 | 1,300 | 320 | 24 |
| pBKM11 | 1,580 | 120 | 6 |
| pBKM12 | 40 | 9 | 6 |
| pBKM13 | 320 | 70 | 6 |
| pBKM14 | 1,140 | 100 | 6 |
The activity values represent mean values obtained from at least three independent determinations. They varied up to ±5 mU/mg in the low-level activity range (3 to 40 mU/mg), up to ±20 mU/mg in the intermediate range (70 to 360 mU/mg), and up to ±100 mU/mg in the high range (930 to 2,200 mU/mg).
Transconjugant H16(pBK) served as a background reference.
pBK2241 contains wild-type PL fused to lacZ and PR fused to gusA.
pBKM1 through pBKM14 contain mutant PL M1 through M14, respectively.
The activities of M1, M6, and M9 were also monitored in the cbbRΔ mutants HB14 and HB15, to evaluate the effect of the CbbR activator protein on these most active promoters. HB15 was included in these studies to examine the possible influence of plasmid pHG1 on the activity of the promoters. As expected, wild-type PL was inactive in the CbbR-less background of the HB14 and HB15 transconjugants (Table 4). The modified PL promoters, however, were active in the mutants, although at levels reduced by one-quarter to one-third compared to those of the parent strains H16 and HF210, respectively, when grown on pyruvate. Since the transconjugants of both mutants displayed a similar regulatory pattern, the presence of pHG1 did not principally affect the activities of these altered promoters. The activities appeared to be largely independent of CbbR yet were enhanced 1.3- to 3.2-fold during mixotrophic growth. Therefore, a basal activation or derepression mechanism is proposed to affect PL even in the absence of CbbR.
TABLE 4.
Activities of mutant PL promoters determined by PL::lacZ transcriptional fusions in transconjugants of R. eutropha
| Transconjugant | Sp act of β-galactosidase (mU/mg of protein)a for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Fructose + formate
|
Pyruvate
|
|||||||
| H16 | HB14 | HF210 | HB15 | H16 | HB14 | HF210 | HB15 | |
| pBKb | 4 | 3 | 3 | 2 | 3 | 3 | 3 | 4 |
| pBK2241c | 780 | 6 | 490 | 5 | 6 | 4 | 6 | 2 |
| pBKM1d | 1,980 | 160 | 1,100 | 130 | 90 | 60 | 60 | 40 |
| pBKM6 | 1,570 | 170 | 1,590 | 70 | 90 | 60 | 40 | 30 |
| pBKM9 | 1,800 | 1,150 | 1,630 | 790 | 1,270 | 860 | 730 | 480 |
The activity values represent mean values obtained from at least three independent determinations. They varied up to ±5 mU/mg in the low-level activity range (2 to 40 mU/mg), up to ±10 mU/mg in the intermediate range (60 to 170 mU/mg), and up to ±100 mU/mg in the high range (480 to 1,980 mU/mg).
Transconjugant H16(pBK) served as a background reference.
pBK2241 contains wild-type PL fused to lacZ and PR fused to gusA.
pBKM1, pBKM6, and pBKM9 contain PL M1, M6, and M9, respectively.
Activities of mutant PL promoters in E. coli.
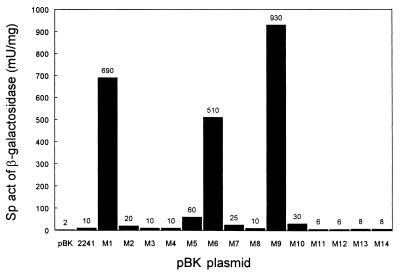
Because the rationale to mutagenize PL of R. eutropha was based on the consensus structure of an E. coli ς70 promoter, the activities of the various PL::lacZ fusions were also determined in E. coli transformants. Wild-type PL showed very low but reproducible activity in the foreign host as did most of the mutant promoters (Fig. 3). A significant increase was observed with mutant M5, but only M1, M6, and M9 yielded high PL activities. The full-consensus mutant M9 was most active and reached the same level as the activated wild-type PL in autotrophically grown R. eutropha. These data basically supported the findings made on the activities of mutant PL promoters in the authentic host.
FIG. 3.
Activities of mutant PL promoters determined by PL::lacZ fusions (pBKM1 through pBKM14) in transformants of E. coli JW1 grown in Luria-Bertani medium. The strains harboring pBK and pBK2241 served as background and wild-type references, respectively. The specific activity (sp act) values represent mean values obtained from at least three independent determinations. They varied up to ±5 mU/mg in the low-level activity range (2 to 60 mU/mg) and up to ±30 mU/mg in the high range (510 to 930 mU/mg).
PR activities associated with mutant PL.
The presumed cbbR promoter PRa active in autotrophically growing R. eutropha overlaps PL. Its −35 box is located within the spacer region of the cbb operon promoter (21). Mutations in PL were thus thought to potentially influence PR activity. The β-glucuronidase reporter activities of the PR::gusA fusions were determined for PL mutants M1, M6, and M9 in transconjugants of R. eutropha H16 and HF210 as well as of the corresponding cbbRΔ mutants HB14 and HB15 grown under heterotrophic or mixotrophic conditions. The previously observed, relatively low PR activity in pyruvate-grown cells (19, 21) was confirmed for the wild-type PL region and for mutants M6 and M9 (Table 5). Surprisingly, mutant M1 showed a strongly enhanced activity in all four transconjugants. Determinations with the remaining PL variants revealed no significant influence of the mutations on PR activity (data not shown). Growth on fructose plus formate led to significantly higher PR activities, although the increase was less pronounced in HF210 and HB15 (Table 5). This putative activation or derepression of PR, which apparently occurs in the wild type as well as in a CbbR-free background, parallels the findings obtained for PL. PR activities were not detected in transformants of E. coli.
TABLE 5.
PR promoter activities associated with the mutant PL promoter M1, M6, or M9 and determined by PR::gusA transcriptional fusions in transconjugants of R. eutropha
| Transconjugant | Sp act of β-glucuronidase (μU/mg of protein)a for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Fructose + formate
|
Pyruvate
|
|||||||
| H16 | HB14 | HF210 | HB15 | H16 | HB14 | HF210 | HB15 | |
| pBKb | 4 | 4 | 6 | 4 | 6 | 9 | 3 | 9 |
| pBK2241c | 480 | 2,990 | 40 | 50 | 18 | 240 | 30 | 8 |
| pBKM1d | 2,750 | 3,430 | 7,020 | 7,170 | 910 | 900 | 990 | 990 |
| pBKM6 | 160 | 290 | 70 | 120 | 6 | 10 | 35 | 12 |
| pBKM9 | 150 | 390 | 80 | 170 | 20 | 10 | 50 | 17 |
The activity values represent mean values obtained from at least three independent determinations. They varied up to ±5 μU/mg in the low-level activity range (3 to 40 μU/mg), up to ±50 μU/mg in the intermediate range (50 to 480 μU/mg), and up to ±300 μU/mg in the high range (900 to 7,170 μU/mg).
Transconjugant H16(pBK) served as a background reference.
pBK2241 contains wild-type PL fused to lacZ and PR fused to gusA.
pBKM1, pBKM6, and pBKM9 contain PL M1, M6, and M9, respectively, fused to lacZ and PR fused to gusA.
DISCUSSION
cbb promoter activities in a wild-type background.
The present work reports on a detailed mutational study of the cbb operon promoter of R. eutropha. Its intention was to verify the proposed location of PL and identify structural attributes which are of importance for the activity and regulation of the promoter. Our previous supposition of PL as a ς70-type promoter located at the predicted position within the cbb control region of R. eutropha H16 was confirmed by the data. The resemblance of PL to the E. coli ς70 consensus promoter is supported by the low but significant level of expression of the RubisCO genes cbbLS in the heterologous host (13). In order to investigate structure-function relationships of PL, alterations were introduced into the presumed promoter elements to either increase or diminish their similarity to the consensus promoter. Mutations that strongly reduced or abolished the activity of PL were due to changes deviating from the consensus nucleotides within the −10 or −35 box. The modification of the length of the spacer region between the boxes by a 1-bp deletion or insertion also resulted in drastic decreases of activated promoter activity, corroborating both the structure and location of PL. The optimal length of the spacer (N17) rather than its sequence is a critical parameter because the exchange of two base pairs in PL M14 had no effect on the promoter. This finding is consistent with the equivalent activities of the chromosomal and pHG1-borne PL promoters of R. eutropha H16, which differ only in the two spacer bases (GG versus TA) located directly downstream of the mutated bases in M14 (21).
Mutations resulting in a closer match of the −35 and/or −10 box to the consensus stimulated PL activity. A partial derepression was observed with the half-consensus promoters M1 and M6, and the full-consensus promoter M9 exhibited a strong derepression even in pyruvate-grown cells. Although the basal activity of PL increased dramatically upon closer matching to the consensus, the principal property of activation during autotrophic growth or growth on fructose was retained by all improved promoter mutants. The position and identity of PL are further confirmed by comparing the cbb control regions of various chemo- and photoautotrophic bacteria. Particularly the TTTAC pentamer in the −35 box of the potential ς70-type cbb operon promoters seems to be conserved among the organisms (11, 20, 33). In accordance with this suggestion, the substitution of the C for the distal T in the −35 box of the full-consensus mutant promoter M9 caused a severe decrease of the high-level basal activity, again without affecting the activation of mutant M10. It is of interest that the exchange of this strongly conserved base also resulted in a drastic drop of PL activity in the heterologous host, E. coli. The results suggest that both the −35 and the −10 boxes are required for the activity of the promoter. Moreover, a promoter structure resembling or matching that of the E. coli ς70 consensus appears also to be favorable for activity in R. eutropha, although additional promoters from the latter organism need to be characterized to verify this conclusion. The hierarchies of base pair preferences found for the different positions of the boxes in E. coli (26) are predicted to be similar in R. eutropha. In accordance with these considerations, only PL mutants M1, M6, and especially M9 displayed high-level activities in E. coli that approached those observed in the homologous host and corresponded to that of the autotrophically activated wild-type promoter. Whether PL and its mutants are also activated in the heterologous host in the presence of CbbR is an open question relating to the overall mechanism of the activation process.
The mutations introduced into PL were not necessarily expected to affect the activity of the partially overlapping PR promoter as they changed only sequence segments not part of the presumed −35 and −10 boxes of PR. In fact, all PL mutants except M1 did not show large differences in PR activities between R. eutropha H16 and HF210. Why the activity of M1 was vastly enhanced remains unclear. A shift of the PL transcriptional start point as a conceivable cause of affecting the transcription from PR was not observed with M1, M6, and M9 (data not shown). The mutations in the −10 box of PL might have created an alternative PR promoter structure. However, the low-level PR activity of M9, which also contains these mutations, is not consistent with this possibility, unless the additional alterations present in the −35 box neutralized the stimulatory effect of the mutations in the −10 box on PR activity. The observed increase of PR activities under growth conditions inducing or derepressing PL confirms earlier results (19, 21). Its explanation must await the detailed elucidation of the cbb gene regulation in R. eutropha.
cbb promoter activities in a CbbR-free background.
A CbbR-deficient mutant, strain HB13, of R. eutropha has already been isolated (37). In this mutant cbbR is disrupted by a Tn5 insertion within the 3′ third of the gene (21), potentially permitting the production of a truncated CbbR protein, which might still be able to bind to the operator in the cbb control region. Therefore, to provide a noninterfering CbbR-free background for the cbb promoter analysis, the cbbRΔ mutant strains HB14 and HB15 were constructed.
The comparison of the PL activities in the transconjugants of the parent strains H16 and HF210 and of the corresponding mutants allows several conclusions to be drawn: (i) CbbR is absolutely required for the transcriptional activation of the cbb operon from wild-type PL; (ii) activator CbbR is also required for the high-level activation of the most-improved PL mutants, M1, M6, and M9, under both inducing and repressing growth conditions; and (iii) the general activation feature of the promoter, very intriguingly, was not lost in the absence of CbbR, as indicated by the weak but significant induction (1.3- to 3.2-fold) of M1, M6, and M9 in HB14 or HB15 grown under mixotrophic conditions. Because of its high, almost constitutive activity, M9 exhibited only low-level inducibility, even in the parent strains (1.3- to 2.2-fold), in which M1 and M6 were induced considerably more strongly (18- to 36-fold). For unknown reasons all three PL mutants showed an approximately twofold higher activity level in HB14 than in HB15. It is tempting to speculate that this difference is a reflection of a positive regulatory influence of megaplasmid pHG1 on the activation of PL.
In contrast to the PL activities of the tested fusions, the PR activities were slightly enhanced in HB14 and HB15 compared to those of their parent strains (1.3- to 6.2-fold), when the transconjugants were grown under PL-inducing conditions. However, there was no principal derepression of PR due to the lack of CbbR. Considering the observed negative autoregulation of cbbR transcription (19), such derepression of PR would be expected in the absence of CbbR, unless an additional regulatory protein(s) binds to the cbb control region.
The mutational increase of the transcriptional competence of PL resulted in the release of promoters M1, M6, and M9 from complete dependency on CbbR. In these PL variants the normally essential need for CbbR in the activation of the wild-type promoter seems to be largely compensated for by an improved promoter structure. However, CbbR alone is not thought to be sufficient for the activation of the wild-types, as well as results of promoter. Our experimental concept of the cbbR deletion mutants functional studies of the modified promoters in the CbbR-free background, allowed us to obtain an indirect hint at the existence of one or more additional cbb regulatory proteins, which are proposed to act in concert with CbbR in the transcriptional control of wild-type PL and which might be activators or repressors. The occurrence of an additional cbb regulator(s) in R. eutropha would correspond to the postulated participation of the RegB-RegA two-component signal transduction system, besides CbbR, in the regulation of the cbb operons in the phototroph Rhodobacter sphaeroides (15, 27). A facultative autotroph does most likely require transcriptional control of its cbb operon(s) by more than one regulatory component to adjust to the different modes of energy and carbon metabolism when switching between autotrophic and heterotrophic growth or vice versa. In view of these considerations, the genetic control of autotrophic CO2 fixation should be part of an integrated regulatory network (15, 21, 33).
ACKNOWLEDGMENTS
We thank Gertrud Stahlhut for her expert technical assistance.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft, Bonn, Germany.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates; 1987. [Google Scholar]
- 2.Bassham J A, Calvin M. The path of carbon in photosynthesis. Englewood Cliffs, N.J: Prentice-Hall; 1957. [Google Scholar]
- 3.Beck C F, Warren R A J. Divergent promoters, a common form of gene organization. Microbiol Rev. 1988;52:318–326. doi: 10.1128/mr.52.3.318-326.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bömmer D, Schäferjohann J, Bowien B. Identification of cbbBc as an additional distal gene of the chromosomal cbb CO2 fixation operon from Ralstonia eutropha. Arch Microbiol. 1996;166:245–251. doi: 10.1007/s002030050380. [DOI] [PubMed] [Google Scholar]
- 5.Bowien B, Schlegel H G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- 6.Bowien B, Windhövel U, Yoo J-G, Bednarski R, Kusian B. Genetics of CO2 fixation in the chemoautotroph Alcaligenes eutrophus. FEMS Microbiol Rev. 1990;87:445–450. [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Bullock W O, Fernandez J M, Short J M. XL1-Blue, a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques. 1987;5:376–378. [Google Scholar]
- 9.Chen B, Przybyla A E. An efficient site-directed mutagenesis method based on PCR. BioTechniques. 1994;17:657–659. [PubMed] [Google Scholar]
- 10.Friedrich C G, Friedrich B, Bowien B. Formation of enzymes of autotrophic metabolism during heterotrophic growth of Alcaligenes eutrophus. J Gen Microbiol. 1981;122:69–78. [Google Scholar]
- 11.Gibson J L, Tabita F R. The molecular regulation of the reductive pentose phosphate pathway in proteobacteria and cyanobacteria. Arch Microbiol. 1996;166:141–150. doi: 10.1007/s002030050369. [DOI] [PubMed] [Google Scholar]
- 12.Harley C B, Reynolds R P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987;15:2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husemann M, Klintworth R, Büttcher V, Salnikow J, Weissenborn C, Bowien B. Chromosomally and plasmid-encoded gene clusters for CO2 fixation (cfx genes) in Alcaligenes eutrophus. Mol Gen Genet. 1988;214:112–120. [Google Scholar]
- 14.Jefferson R A, Burgess S M, Hirsh D. β-Glucuronidase from Escherichia coli as gene fusion marker. Proc Natl Acad Sci USA. 1986;83:8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi H M, Tabita F R. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc Natl Acad Sci USA. 1996;93:14515–14520. doi: 10.1073/pnas.93.25.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolmar H, Friedrich K, Pschorr J, Fritz H J. Hybrids of circular DNA single strands as intermediates in DNA cloning, sequence analysis and directed mutagenesis. Technique. 1990;2:237–245. [Google Scholar]
- 17.Kortlüke C, Horstmann K, Schwartz E, Rhode M, Binsack R, Friedrich B. A gene complex coding for membrane-bound hydrogenase of Alcaligenes eutrophus. J Bacteriol. 1992;174:6277–6289. doi: 10.1128/jb.174.19.6277-6289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop R M, Peterson K M. Four new derivatives of the broad-host-range vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 19.Kusian B, Bowien B. Operator binding of the CbbR protein, which activates the duplicate cbb CO2 assimilation operons of Alcaligenes eutrophus. J Bacteriol. 1995;177:6568–6574. doi: 10.1128/jb.177.22.6568-6574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusian B, Bowien B. Organization and regulation of cbb CO2 assimilation genes in autotrophic bacteria. FEMS Microbiol Rev. 1997;21:135–155. doi: 10.1111/j.1574-6976.1997.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 21.Kusian B, Bednarski R, Husemann M, Bowien B. Characterization of the duplicate ribulose-1,5-bisphosphate carboxylase genes and cbb promoters of Alcaligenes eutrophus. J Bacteriol. 1995;177:4442–4450. doi: 10.1128/jb.177.15.4442-4450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leadbeater L, Siebert K, Schobert P, Bowien B. Relationship between activities and protein levels of ribulosebisphosphate carboxylase and phosphoribulokinase in Alcaligenes eutrophus. FEMS Microbiol Lett. 1982;14:263–266. [Google Scholar]
- 23.Lenz, O., and B. Friedrich. Personal communication.
- 24.Lenz O, Schwartz E, Dernedde J, Eitinger M, Friedrich B. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J Bacteriol. 1994;176:4385–4393. doi: 10.1128/jb.176.14.4385-4393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller J H. Assay of β-galactosidase. In: Platt T, Müller-Hill B, Miller J H, editors. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 319–353. [Google Scholar]
- 26.Moyle H, Waldburger C, Susskind M M. Hierarchies of base pair preferences in the P22 ant promoter. J Bacteriol. 1991;173:1944–1950. doi: 10.1128/jb.173.6.1944-1950.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Y, Tabita F R. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J Bacteriol. 1996;178:12–18. doi: 10.1128/jb.178.1.12-18.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raven J A. The role of autotrophs in global CO2 cycling. In: Lidstrom M E, Tabita F R, editors. Microbial growth on C1 compounds. Dordrecht, The Netherlands: Kluwer; 1996. pp. 351–358. [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schäferjohann J, Bednarski R, Bowien B. Regulation of CO2 assimilation in Ralstonia eutropha: premature transcription termination within the cbb operon. J Bacteriol. 1996;178:6714–6719. doi: 10.1128/jb.178.23.6714-6719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 33.Shively J M, van Keulen G, Meijer W G. Something from almost nothing: carbon dioxide fixation in chemoautotrophs. Annu Rev Microbiol. 1998;52:191–230. doi: 10.1146/annurev.micro.52.1.191. [DOI] [PubMed] [Google Scholar]
- 34.Simon R, Priefer U, Pühler A. A broad-host-range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 35.Srivastava S, Urban M, Friedrich B. Mutagenesis of Alcaligenes eutrophus by insertion of the drug-resistance transposon Tn5. Arch Microbiol. 1982;131:203–207. doi: 10.1007/BF00405879. [DOI] [PubMed] [Google Scholar]
- 36.Windhövel U, Bowien B. On the operon structure of the cfx gene clusters in Alcaligenes eutrophus. Arch Microbiol. 1990;154:85–91. doi: 10.1007/BF00249183. [DOI] [PubMed] [Google Scholar]
- 37.Windhövel U, Bowien B. Identification of cfxR, an activator gene of autotrophic CO2 fixation in Alcaligenes eutrophus. Mol Microbiol. 1991;5:2695–2705. doi: 10.1111/j.1365-2958.1991.tb01978.x. [DOI] [PubMed] [Google Scholar]
- 38.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]