Abstract
Background
Asthma is a respiratory disease characterised by variable airflow limitation and the presence of respiratory symptoms including wheeze, chest tightness, cough and/or dyspnoea. Exercise training is beneficial for people with asthma; however, the response to conventional models of pulmonary rehabilitation is less clear.
Objectives
To evaluate, in adults with asthma, the effectiveness of pulmonary rehabilitation compared to usual care on exercise performance, asthma control, and quality of life (co‐primary outcomes), incidence of severe asthma exacerbations/hospitalisations, mental health, muscle strength, physical activity levels, inflammatory biomarkers, and adverse events.
Search methods
We identified studies from the Cochrane Airways Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform, from their inception to May 2021, as well as the reference lists of all primary studies and review articles.
Selection criteria
We included randomised controlled trials in which pulmonary rehabilitation was compared to usual care in adults with asthma. Pulmonary rehabilitation must have included a minimum of four weeks (or eight sessions) aerobic training and education or self‐management. Co‐interventions were permitted; however, exercise training alone was not.
Data collection and analysis
Following the use of Cochrane's Screen4Me workflow, two review authors independently screened and selected trials for inclusion, extracted study characteristics and outcome data, and assessed risk of bias using the Cochrane risk of bias tool. We contacted study authors to retrieve missing data. We calculated between‐group effects via mean differences (MD) or standardised mean differences (SMD) using a random‐effects model. We evaluated the certainty of evidence using GRADE methodology.
Main results
We included 10 studies involving 894 participants (range 24 to 412 participants (n = 2 studies involving n > 100, one contributing to meta‐analysis), mean age range 27 to 54 years). We identified one ongoing study and three studies awaiting classification. One study was synthesised narratively, and another involved participants specifically with asthma‐COPD overlap. Most programmes were outpatient‐based, lasting from three to four weeks (inpatient) or eight to 12 weeks (outpatient). Education or self‐management components included breathing retraining and relaxation, nutritional advice and psychological counselling. One programme was specifically tailored for people with severe asthma.
Pulmonary rehabilitation compared to usual care may increase maximal oxygen uptake (VO2 max) after programme completion, but the evidence is very uncertain for data derived using mL/kg/min (MD between groups of 3.63 mL/kg/min, 95% confidence interval (CI) 1.48 to 5.77; 3 studies; n = 129) and uncertain for data derived from % predicted VO2 max (MD 14.88%, 95% CI 9.66 to 20.1%; 2 studies; n = 60). The evidence is very uncertain about the effects of pulmonary rehabilitation compared to usual care on incremental shuttle walk test distance (MD between groups 74.0 metres, 95% CI 26.4 to 121.4; 1 study; n = 30). Pulmonary rehabilitation may have little to no effect on VO2 max at longer‐term follow up (9 to 12 months), but the evidence is very uncertain (MD −0.69 mL/kg/min, 95% CI −4.79 to 3.42; I2 = 49%; 3 studies; n = 66).
Pulmonary rehabilitation likely improves functional exercise capacity as measured by 6‐minute walk distance, with MD between groups after programme completion of 79.8 metres (95% CI 66.5 to 93.1; 5 studies; n = 529; moderate certainty evidence). This magnitude of mean change exceeds the minimally clinically important difference (MCID) threshold for people with chronic respiratory disease. The evidence is very uncertain about the longer‐term effects one year after pulmonary rehabilitation for this outcome (MD 52.29 metres, 95% CI 0.7 to 103.88; 2 studies; n = 42).
Pulmonary rehabilitation may result in a small improvement in asthma control compared to usual care as measured by Asthma Control Questionnaire (ACQ), with an MD between groups of −0.46 (95% CI −0.76 to −0.17; 2 studies; n = 93; low certainty evidence); however, data derived from the Asthma Control Test were very uncertain (MD between groups 3.34, 95% CI −2.32 to 9.01; 2 studies; n = 442). The ACQ finding approximates the MCID of 0.5 points. Pulmonary rehabilitation results in little to no difference in asthma control as measured by ACQ at nine to 12 months follow‐up (MD 0.09, 95% CI −0.35 to 0.53; 2 studies; n = 48; low certainty evidence).
Pulmonary rehabilitation likely results in a large improvement in quality of life as assessed by the St George's Respiratory Questionnaire (SGRQ) total score (MD −18.51, 95% CI −20.77 to −16.25; 2 studies; n = 440; moderate certainty evidence), with this magnitude of change exceeding the MCID. However, pulmonary rehabilitation may have little to no effect on Asthma Quality of Life Questionnaire (AQLQ) total scores, with the evidence being very uncertain (MD 0.87, 95% CI −0.13 to 1.86; 2 studies; n = 442). Longer‐term follow‐up data suggested improvements in quality of life may occur as measured by SGRQ (MD −13.4, 95% CI −15.93 to −10.88; 2 studies; n = 430) but not AQLQ (MD 0.58, 95% CI −0.23 to 1.38; 2 studies; n = 435); however, the evidence is very uncertain.
One study reported no difference between groups in the proportion of participants who experienced an asthma exacerbation during the intervention period. Data from one study suggest adverse events attributable to the intervention are rare.
Overall risk of bias was most commonly impacted by performance bias attributed to a lack of participant blinding to knowledge of the intervention. This is inherently challenging to overcome in rehabilitation studies.
Authors' conclusions
Moderate certainty evidence shows that pulmonary rehabilitation is probably associated with clinically meaningful improvements in functional exercise capacity and quality of life upon programme completion in adults with asthma. The certainty of evidence relating to maximal exercise capacity was very low to low. Pulmonary rehabilitation appears to confer minimal effect on asthma control, although the certainty of evidence is very low to low. Unclear reporting of study methods and small sample sizes limits our certainty in the overall body of evidence, whilst heterogenous study designs and interventions likely contribute to inconsistent findings across clinical outcomes and studies. There remains considerable scope for future research.
Keywords: Adult; Humans; Middle Aged; Asthma; Dyspnea; Dyspnea/rehabilitation; Hospitalization; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/drug therapy; Quality of Life
Plain language summary
What are the benefits of supervised programmes of exercise and education (known as pulmonary rehabilitation) compared with usual care for adults with asthma?
Key messages
‐ We found that people with asthma who take part in supervised programmes of exercise and education (known as pulmonary rehabilitation) are likely to get fitter (can walk further) and have better wellbeing immediately after completing these programmes compared to those who receive usual care. However, we are not certain if these benefits persist up to one year later.
‐ Due to a lack of evidence, the effects of pulmonary rehabilitation on outcomes such as rates of asthma attacks or hospitalisations, anxiety and depression, or physical activity levels is unclear.
‐ Larger, well‐designed studies are needed to better estimate the true benefit of pulmonary rehabilitation for adults with asthma.
What is asthma?
Asthma is a common lung disease where the breathing tubes can become inflamed and narrowed and may produce extra mucus. People with asthma can experience cough, wheezing, chest tightness, and breathlessness, with those most severely affected experiencing difficulty going about their everyday lives.
Asthma cannot be cured, but symptoms can be controlled. Different medications can help keep symptoms under control, whilst physical exercise can also help. However, some people with asthma may find it challenging to undertake comprehensive exercise programmes.
What is pulmonary rehabilitation?
Supervised programmes of exercise and education (called pulmonary rehabilitation) are commonly used for people with chronic lung conditions and help improve breathing, fitness, and wellbeing. These programmes may be based at hospitals, outpatient clinics, or even at home.
Pulmonary rehabilitation is a recommended standard of care for many chronic lung conditions; however, its effects in adults with asthma are less clear.
What did we want to find out?
We wanted to see how pulmonary rehabilitation affects physical fitness, control of asthma symptoms, and wellbeing of adults with asthma compared to usual clinical care involving no pulmonary rehabilitation. We also wanted to learn how it affects the rate of severe asthma attacks/hospitalisations, mental health (anxiety and depression), muscle strength, physical activity levels, and markers of inflammation (in sputum or blood). Finally, we wanted to see whether it is associated with any unwanted effects.
What did we do?
We searched for studies that compared pulmonary rehabilitation to usual care in adults with asthma. Treatment must have lasted at least four weeks (or eight or more sessions) and must have included aerobic exercises (such as walking or cycling) and education or self‐management.
We compared and summarised findings across all eligible studies and rated our confidence in the evidence based on factors such as study methods and size.
What did we find?
‐ We found 10 studies involving 894 adults with asthma.
‐ The studies ranged in size from 24 to 412 people.
‐ Most studies were conducted in Europe.
‐ Where reported, most study participants were female, with the average age ranging from 27 to 54 years.
‐ One study specifically included people with severe forms of asthma. Another study specifically included people who had a condition involving overlapping features of both asthma and chronic obstructive pulmonary disease (COPD).
‐ The way pulmonary rehabilitation was delivered varied across studies. Inpatient programmes lasted 3 to 4 weeks, whilst outpatient programmes lasted 8 to 12 weeks.
‐ The specific nature of exercise or education components amongst the included studies varied widely.
Main results
‐ Pulmonary rehabilitation probably causes a large increase in physical fitness immediately after completion of the programme, resulting in an ability to walk an average of 80 metres further in 6 minutes than in people who receive usual care. There may be little to no effect on physical fitness measured up to one year later.
‐ Pulmonary rehabilitation may result in small improvements in or little to no impact on asthma control immediately after completion of the programme or up to one year later compared to usual care.
‐ Pulmonary rehabilitation probably causes a large improvement in wellbeing as measured by the St George’s Respiratory Disease Questionnaire immediately after completion of the programme. Results may differ slightly according to different quality of life instruments. The effects potentially last up to one year, but results are very uncertain.
‐ Little to no effect on wellbeing was observed after programme completion or up to nine months follow‐up when the Asthma Quality of Life Questionnaire was used.
‐ There was very limited evidence to determine the effect of pulmonary rehabilitation on rates of asthma attacks/hospitalisations, measures of anxiety and depression, limb muscle strength, levels of physical activity, or markers of inflammation in the blood or sputum.
‐ Data from one study suggested pulmonary rehabilitation resulted in no direct unwanted or harmful effects.
Limitations of the evidence
Our confidence in the evidence relating to outcomes such as physical fitness, wellbeing, and asthma control is limited due to concerns regarding unclear methods in some studies, the potential for participants or assessors (or both) to have influenced outcomes due to the awareness of assigned treatments, and the varied ways in which pulmonary rehabilitation was delivered.
The evidence is up‐to‐date to May 2021.
Summary of findings
Summary of findings 1. Pulmonary rehabilitation compared to usual care for adults with asthma.
| Pulmonary rehabilitation compared to usual care for adults with asthma | |||||||
| Patient or population: Adults with asthma Setting: Inpatient hospitals and outpatient centres Intervention: Pulmonary rehabilitation Comparison: Usual care | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with usual care in people with asthma (end‐treatment) | Risk with pulmonary rehabilitation | ||||||
| Exercise performance: peak oxygen uptake (VO2 peak) on cycle ergometer incremental CPET | End‐intervention: range 8 to 12 weeks | Mean peak oxygen uptake (VO2 peak) was 23.7 mL/kg/min. | MD 3.63 mL/kg/min higher (1.48 higher to 5.77 higher); adjusted model data used | ‐ | 129 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Higher value denotes greater peak oxygen uptake (i.e. better) following completion of pulmonary rehabilitation compared to usual care. |
| Follow‐up: range 9 to 12 months | Mean peak oxygen uptake (VO2 peak) was 24.8 mL/kg/min. | MD 0.69 mL/kg/min lower (4.79 lower to 3.42 higher) | ‐ | 66 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 5 | ||
| Exercise performance: % predicted VO2 max on incremental cardiopulmonary exercise test | End‐intervention: mean 3 months | Mean peak oxygen uptake (% predicted VO2 max) was 58.2%. | MD 14.88% higher (9.66 higher to 20.1 higher) | ‐ | 60 (2 RCTs) | ⊕⊕⊝⊝ LOW 6 7 | Higher value denotes greater peak oxygen uptake (i.e. better) following completion of pulmonary rehabilitation compared to usual care. |
| Follow‐up: mean 12 months | Mean change in peak oxygen uptake (% predicted VO2 max) was 1.33%. | MD 10.37% higher (1.6 lower to 22.34 higher) | ‐ | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 8 9 | ||
| Exercise performance: incremental shuttle walk test distance, metres | End‐intervention: mean 12 weeks | Mean incremental shuttle walk test distance was 403 metres. | MD 74.0 metres further (26.4 further to 121.4 further); adjusted model data used | ‐ | 30 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 4 9 | Higher distance denotes greater exercise performance (i.e. better) following completion of pulmonary rehabilitation compared to usual care. |
| Follow‐up: mean 9 months | Mean incremental shuttle walk test distance was 421 metres. | MD 9 metres lower (140.38 lower to 122.38 further) | ‐ | 23 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 4 9 | ||
| Exercise performance: 6‐minute walk test distance, metres | End‐intervention: range 3 to 12 weeks | Mean 6‐minute walk test distance was 483 metres. | MD 79.8 metres further (66.5 further to 93.1 further) | ‐ | 529 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 10 11 | Further distance denotes greater exercise performance (i.e. better) following completion of pulmonary rehabilitation compared to usual care. MCID = 26 to 30 m |
| Follow‐up: mean 12 months | Mean change in 6‐minute walk test distance was −25.5 metres. | MD 52.3 metres further (0.7 further to 103.9 further) | ‐ | 42 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 5 8 12 | ||
| Asthma control: ACQ score | End‐intervention: range 8 to 12 weeks | Mean change in ACQ score ranged from −0.3 to 0.4 points. | MD 0.5 points lower (0.8 lower to 0.2 lower); adjusted model data used | ‐ | 93 (2 RCTs) | ⊕⊕⊝⊝ LOW 4 5 | Lower score denotes better asthma control compared to usual care. MCID = 0.5 points |
| Follow‐up: range 9 to 12 months | Mean ACQ score was 1.4 points. | MD 0.1 points higher (0.4 lower to 0.5 higher) | ‐ | 48 (2 RCTs) | ⊕⊕⊝⊝ LOW 4 5 | ||
| Asthma control: ACT score | End‐intervention: range 3 to 8 weeks | Mean change in ACT score was 2.3 points. | MD 3.3 points higher (2.3 lower to 9.0 higher); adjusted model data used | ‐ | 442 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 13 14 15 | Higher score denotes better asthma control compared to usual care. MCID = 3 points |
| Follow‐up: mean 3 months | Mean ACT score was 15.8 points. | MD 4.6 points higher (3.8 higher to 5.5 higher) | ‐ | 412 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 8 16 | ||
| Quality of life: AQLQ total score | End‐intervention: range 3 to 12 weeks | Mean change in AQLQ total score ranged from −0.1 to 0.3 points. | MD 0.9 points higher (0.1 lower to 1.9 higher); adjusted model data used | ‐ | 442 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 13 14 15 | Higher score denotes better quality of life compared to usual care. MCID = 0.5 points |
| Follow‐up: range 3 to 9 months | Mean change in AQLQ total score ranged from 0 to 0.5 points. | MD 0.6 points higher (0.2 lower to 1.4 higher); adjusted model data used | ‐ | 435 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 15 17 | ||
| Quality of life: SGRQ total score | End‐intervention: range 3 to 6 weeks | Mean change in SGRQ total score was −2.2 points. | MD 18.5 points lower (20.8 lower to 16.3 lower); adjusted model data used | ‐ | 440 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 11 13 | Lower score denotes better quality of life compared to usual care. MCID = 4 points |
| Follow‐up: range 3 to 12 months | Mean change in SGRQ total score ranged from −6 to 1.5 points. | MD 13.4 points lower (15.9 lower to 10.9 lower); adjusted model data used | ‐ | 430 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 8 11 18 19 20 | ||
| Adverse events | ‐ | ‐ | No data reported. | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; CPET: cardiopulmonary exercise test; MCID: minimally clinically important difference threshold; MD: mean difference; RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire; VO2 max: maximal oxygen consumption; VO2 peak: oxygen uptake during peak exercise. | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
1All included studies had at least one domain assessed as at unclear risk of bias. Downgraded one level due to risk of bias. 2The proportion of variability in effect estimates due to true heterogeneity rather than to chance was high. Downgraded one level due to inconsistency. 3Small sample size and wide confidence interval included potential for both small and large treatment effect. Downgraded one level due to imprecision. 4All included studies had at least one domain at high risk of bias and one domain at unclear risk of bias. Downgraded one level due to risk of bias. 5Small sample size limits generalisability of findings and/or wide confidence intervals do not exclude potential for both benefit and little to no effect. Downgraded one level due to imprecision. 6All included studies had at least one domain assessed as at unclear risk of bias. At least one study had multiple domains at unclear risk of bias. Downgraded one level due to risk of bias. 7Small overall sample size reduces our confidence in the accuracy of the observed effect size. Downgraded one level due to imprecision. 8All included studies had more than one domain at high risk of bias. Downgraded two levels due to risk of bias. 9Small sample size of single study limits generalisability of findings and/or wide confidence intervals span potential for both clinically meaningful benefit and a lack of benefit and/or harm. Downgraded two levels due to imprecision. 10All studies had at least one domain at unclear risk of bias, and one study was at high risk of attrition bias. Downgraded two levels due to risk of bias. 11Upgraded one level due to large treatment effect size. 12Point estimates suggest the potential for both benefit and harm with no overlapping of confidence intervals. High statistical heterogeneity. Downgraded one level for inconsistency. 13All included studies had multiple domains at unclear and/or high risk of bias. Downgraded two levels due to risk of bias. 14The proportion of variability in effect estimates due to true heterogeneity rather than to chance was high with no overlap of confidence intervals between studies. Downgraded one level due to inconsistency. 15Wide confidence intervals include potential for both benefit and no benefit and/or harm. Downgraded one level due to imprecision. 16Single study of inpatient rehabilitation limits generalisability of findings. Downgraded one level due to imprecision. 17Effect estimates of included studies include the potential for both benefit and no benefit. Proportion of variance in effect estimates due to true heterogeneity rather than to chance was very high. Downgraded one level due to inconsistency. 18Follow‐up time frames were markedly different between included studies (3 months vs 12 months). Downgraded one level due to indirectness. 19Effect estimates of included studies were markedly contradictory to each other (large benefit vs large harm); proportion of variance in effect estimates due to true heterogeneity rather than to chance was very high. Downgraded two levels due to inconsistency. 20Wide confidence interval margins of effect estimate exceeds minimally important difference threshold. Downgraded one level due to imprecision.
Background
Description of the condition
Asthma is a heterogenous respiratory disease characterised by variable airflow limitation and the presence of respiratory symptoms including wheeze, chest tightness, cough, or dyspnoea. Symptoms may vary over time both in frequency and severity (GINA 2021). Several disease processes (and clusters thereof) exist in asthma, often referred to as asthma 'phenotypes' (e.g. allergic, non‐allergic, adult‐onset, Type 2 high asthma versus Type 2 low asthma). A common consequence of these processes is resultant chronic airway inflammation that causes bronchoconstriction, airway wall thickening, and increased mucous production (GINA 2021). Precise mechanisms explaining the variability in symptoms are challenging to identify; however, intermittent exposure to any number of 'triggers' and the degree of airflow reversibility on spirometry may partly contribute to this phenomenon. Asthma is primarily diagnosed on the basis of clinical presentation and symptom history rather than any individual biomarker, which can lead to under‐ or overdiagnosis. Whilst this poses some challenges to accurately appreciate its global impact, estimates suggest that asthma affects over 300 million people worldwide and imposes a large social and financial burden (GAN 2018). Despite the existence of many established pharmacotherapies to manage asthma, morbidity and mortality remain high: the Global Burden of Disease collaboration estimates that 420,000 people died from asthma in 2016 (FIRS 2017).
Asthma severity is assessed according to the degree of treatment required to manage the condition. Compared to people with mild to moderate asthma, those with more severe disease experience poor symptom control (Reddel 2015), impaired quality of life (Foster 2017; McDonald 2018), increased risk of hospitalisation (Eisner 2000; Poulos 2014), and increased risk of death (Ebmeier 2017). The severe asthma population may therefore represent a specific subgroup in need of high levels of support. Asthma is also associated with several 'extra‐pulmonary' features (i.e. those occurring outside the lungs); evidence confirms that people with asthma are less active than 'healthy' counterparts (Cordova‐Rivera 2018), and higher levels of physical activity is associated with better measures of lung function (Ritz 2010), disease control (Dogra 2011), health status (Lucas 2005), and healthcare use (Dogra 2009). A proportion of adults with asthma, particularly those of older age, may present with clinical features of chronic obstructive pulmonary disease (COPD) (i.e. asthma‐COPD overlap (ACO)), such as significant functional impairment, symptom burden, poor quality of life, comorbidities, and history of respiratory exacerbations. ACO has been defined as the presence of incompletely reversible airflow obstruction on spirometry in addition to clinical features of asthma, and is estimated to occur in approximately 20% of people with asthma or COPD (Gibson 2015). Asthma can therefore be challenging to distinguish from COPD, particularly where shared risk factors may be present. Factors such as older age (e.g. older than 50 years) and significant smoking history (e.g. more than 10 to 20 pack years) are common exclusion criteria from pharmacotherapy trials, which may result in under‐representation of such individuals. This may occur less in studies of rehabilitation. People with asthma are typically encouraged to participate in structured exercise training programmes where possible, and data suggest this to be safe (Cordova‐Rivera 2018a), even when performed at high intensity (da Silva 2016; Toennesen 2018). Regardless, many people struggle to achieve this in an independent or unsupervised environment.
Exercise is also a known trigger for asthma in some individuals. Exercise‐induced bronchoconstriction (EIB) describes acute airway narrowing that is transient and reversible, that occurs as a result of (i.e. during or after) exercise (Aggarwal 2018; Parsons 2013). Its presence is typically confirmed by a minimum of a 10% decline or greater in forced expiratory volume in the first second (FEV1) between pre‐exercise and postexercise (within 30 minutes of completion) spirometry (Crapo 2000). Whilst the precise prevalence of EIB is challenging to identify, it is reported to occur in up to 90% of people with asthma (Weiler 2010), and those with more severe and poorly controlled asthma are considered more likely to exhibit EIB (Weiler 2010). International guidelines indicate that EIB can be effectively managed using strategies such as administration of inhaled short‐acting beta2‐agonist (SABA) medication at least 15 minutes prior to commencing exercise (Parsons 2013). Despite this, the presence of EIB and concerns about the safety of exercise may discourage some people with EIB from participating in exercise programmes or daily physical activity. Strategies to identify and manage EIB may therefore be an important component of exercise training interventions for people with asthma.
Description of the intervention
Current leading international guidelines define pulmonary rehabilitation (PR) as "a comprehensive intervention based on a thorough patient assessment followed by patient‐tailored therapies that include, but are not limited to, exercise training, education, and behaviour change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long‐term adherence to health‐enhancing behaviours" (Spruit 2013). The precise extent to which specific components should be included within PR programmes is not agreed upon, nor is the number of specific components or the way in which they are implemented. The most widely accepted definition of PR for use in scientific research defines the core criteria of PR as "any inpatient, outpatient, community‐based or home‐based rehabilitation programme of at least four weeks' duration that include[s] exercise therapy with or without any form of education and/or psychological support delivered to patients with exercise limitation attributable to [their disease]" (McCarthy 2015). Current literature supports PR as an effective treatment for people with a range of chronic respiratory diseases including COPD, bronchiectasis, interstitial lung disease, and pulmonary hypertension. Adults with asthma exhibit similar dysfunction and respiratory symptoms to many of these conditions, yet their circumstances may also differ substantially. For example, people with asthma may be younger, may have concurrent employment or studying commitments, and may be less physically compromised due to ‘reversibility’ of their airways' disease. It is also challenging to determine whether responses to PR would differ in people with ACO compared to those with clearly defined asthma or COPD (or both).
How the intervention might work
The cornerstone element underpinning many of the observed benefits from PR is exercise training incorporating aerobic/lower limb endurance exercise. The main benefits are considered to be due to adaptations to the peripheral skeletal muscles, including increased capillary proliferation, improved (local) oxygen uptake, improved mitochondrial function, reduced oxidative stress, and a shift in muscle fibre type composition. Other mechanisms contributing to improvements from exercise may include desensitisation to the discomfort of dyspnoea sensations, reductions in anxiety associated with exercise performance, and possible improvements in respiratory mechanics such as reduced dynamic hyperinflation (Osadnik 2019). However, many of these mechanisms are founded upon evidence in people with COPD, with relatively less evidence derived specifically from people with asthma. One novel pathway that appears more relevant to asthma, but that has yet to be fully elucidated, is the possible positive impact of physical exercise upon inflammatory biomarkers.
A randomised controlled trial (RCT) of people with moderate to severe asthma found that aerobic training decreased bronchial hyperresponsiveness and serum pro‐inflammatory cytokines (interleukin‐6, interleukin‐8, monocyte chemoattractant protein‐1) (França‐Pinto 2015). Benefits were also observed in one RCT conducted in obese people with asthma, where the addition of exercise to a programme of weight loss and psychological therapy increased anti‐inflammatory biomarkers and vitamin D levels, and significantly reduced airway and systemic inflammation (fractional concentration of exhaled nitric oxide (FeNO), and serum biomarkers) (Freitas 2017). Improvements in exhaled nitric oxide have even been demonstrated following a single session of moderate‐intensity exercise (30 minutes of treadmill walking) in physically inactive adults with asthma (Scott 2015); this study also suggests that exercise may exert an anti‐inflammatory effect that could be attenuated by interleukin‐1 receptor antagonists.
There may be plausible reason to exert caution in assuming equal physiological responses to exercise between people with COPD and those with asthma. Ventilatory limitations to exercise are less common in people with asthma compared to those with COPD, meaning the contributory roles of physical inactivity and deconditioning to observed exercise intolerance in people with asthma are likely relevant. This may be due to avoidance behaviours associated with a fear of exacerbations due to exercise. Qualitative research supports this notion, indicating that many adolescents with asthma withdraw from exercise as a coping strategy, despite deriving a strong sense of enjoyment from it (Winn 2018). PR programmes also typically involve some form of education or self‐management (or both) or psychological support. Whilst the precise extent and nature of these components can vary markedly between programmes, their incorporation distinguishes PR from isolated 'exercise training' studies. Evidence regarding the role of education and support in people with asthma is scarce; however, it stands to reason that, for a condition that is heavily reliant upon effective self‐management, medication technique and adherence, and timely responses in the event of an acute exacerbation, the inclusion of such components would be considered valuable. This may be particularly relevant for the improvement of disease control, which is a common outcome of importance (somewhat uniquely) for people with asthma. Limited data suggest that people with poorer levels of asthma control may achieve greater gains in asthma control after PR compared to those who commence with better control, thereby potentially lending support to this notion (Sahin 2019).
Why it is important to do this review
International guidelines recommend PR for the management of chronic lung conditions such as COPD (Alison 2017; Bolton 2013; Spruit 2013), bronchiectasis (Alison 2017; Bolton 2013; Spruit 2013), interstitial lung disease (Alison 2017; Spruit 2013), and pulmonary hypertension (Alison 2017; Spruit 2013). Recommendations for people with asthma are less convincing, and referrals for adults with asthma to PR are not a widespread standard of care in clinical practice. American Thoracic Society/European Respiratory Society PR guidelines advocate for the inclusion of adults with 'persistent asthma' in PR (Spruit 2013). British Thoracic Society guidelines advocate that routine referrals for patients with asthma to PR are not recommended (Bolton 2013); however, they do suggest that discussions regarding the benefits of exercise may be appropriate. Asthma was not included in the Australian and New Zealand PR guidelines (Alison 2017), whilst current Global Initiative for Asthma (GINA) guidelines suggest that advice should be provided about pulmonary rehabilitation for those with "COPD or asthma‐COPD overlap" (GINA 2021). It is unclear whether this lack of strong support for PR in people with asthma may reflect an historic predominance of evidence from people with COPD and a need for clearer evidence in people with asthma.
A Cochrane Review of the effects of exercise training specifically for people with asthma demonstrates positive effects on clinically important outcomes such as exercise performance, quality of life, and asthma control (Carson 2013). However, this evidence offers limited applicability to many adults encountered in clinical respiratory medicine practice. For example, participants included within the review had a mean age of approximately 22 years. Distinct differences are also apparent between the nature of some included exercise interventions (e.g. one‐hour outdoor running tracks for children, indoor swimming six days per week) and those typically offered by PR programmes in adult clinical respiratory medicine. The findings of this previous Cochrane Review may therefore only apply to younger people with asthma. The training potential of these younger individuals may differ considerably to adults of older age who typically present with increased chronic health comorbidities (McDonald 2019). For example, those who are younger may prefer, and be capable of, independent exercise training at high intensity or duration (or both) at community‐based gymnasiums or pools rather than group‐based rehabilitation conducted at hospital or healthcare service sites. The former settings also allow flexibility for exercise to be conducted at more convenient times that may fall outside typical daytime, weekday offerings of many PR programmes, thereby potentially impacting training compliance and programme effectiveness. It is also difficult to postulate and identify whether PR may only be suitable for select subgroups (or phenotypes). Little research has been conducted in this area in asthma; however, evidence from other diseases such as COPD suggests that those with more established disability (e.g. moderate to severe disease severity, worse symptom limitation and exercise intolerance) may benefit more than those with milder disease. It is therefore possible that traditional PR models may better suit people who are more limited by their asthma (e.g. older, more severe disease) than those who are not (e.g. younger, athletes).
The structure and delivery of conventional PR programmes may not suit the needs of adults with asthma ideally. Factors such as concurrent employment or personal preferences to avoid training alongside people with severe respiratory disease (e.g. those on long‐term oxygen therapy) may be realistic barriers to attendance. It is not common, or necessarily feasible, to run PR programmes exclusively for people with asthma, hence it is essential to determine whether the 'typical' PR model confers clinically worthwhile benefits for this patient group. If the intervention can demonstrate effectiveness, efforts can subsequently be directed towards the overcoming of disease‐specific barriers such as competing time demands via flexible class scheduling. At present, we cannot confidently advocate that traditional PR models benefit people with asthma, despite its intuitive likely benefit. In order to therefore help clarify the precise role of PR for adults with asthma, it is essential we gain clearer insight into the precise effects of PR in people with asthma.
Objectives
To evaluate, in adults with asthma, the effectiveness of pulmonary rehabilitation compared to usual care on exercise performance, asthma control, and quality of life (co‐primary outcomes), incidence of severe asthma exacerbations/hospitalisations, mental health, muscle strength, physical activity levels, inflammatory biomarkers, and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including those with a cluster design. We included studies reported in full text, those published as an abstract only, and unpublished data. We included randomised cross‐over trials using pre‐cross‐over data only at the end‐intervention time point, despite the known limitations of this approach, due to challenges associated with determining and overcoming adequate 'washout' periods in cross‐over studies involving rehabilitation and/or behavioural intervention components.
Types of participants
We included adults with a primary clinical diagnosis of asthma (defined by international guidelines or according to study author descriptions). A comorbid principal respiratory condition of COPD was allowed due to the known significant overlap between asthma and COPD (ACO). We excluded participants described as having any primary clinical diagnosis other than asthma or COPD/ACO. We did not exclude participants with other comorbidities/characteristics if they were deemed suitable to participate in the rehabilitation intervention within the original study. For studies involving participants of mixed clinical diagnoses, we included the subgroup of data relating specifically to adults with asthma if this was available. If this was unavailable, we only included the data in their entirety if more than 75% of participants were noted as having asthma upon commencement of the intervention.
Types of interventions
We included studies comparing pulmonary rehabilitation to usual care. Pulmonary rehabilitation must have involved a minimum of four weeks (or eight or more sessions) aerobic exercise training (e.g. walking, cycling), including some form of education or self‐management strategy. Pulmonary rehabilitation must have been received as an inpatient or outpatient at a hospital centre, community‐based facility, or home‐based environment (including interventions delivered to the home via tele‐rehabilitation), but the exercise training component must have been supervised by a suitably qualified therapist.
Co‐interventions such as other forms of exercise training (e.g. strength, balance, inspiratory muscle training), breathing techniques (e.g. Buteyko method), dietary supplementation, relaxation, or airway clearance techniques were permitted, as these are commonly integrated within PR programmes. Interventions comprising exercise training modalities alone were not eligible for inclusion. Usual care must not have involved participation in a supervised exercise training programme during the study period, but could comprise no formal intervention (e.g. usual medical or self‐care management, without rehabilitation), delayed‐onset or waitlist‐controlled rehabilitation, or provision of generalised self‐management advice such as educational materials encouraging general physical activity in daily life.
Types of outcome measures
We evaluated the effects of pulmonary rehabilitation on the following outcomes.
Primary outcomes
Exercise performance: this was derived from tests of maximal exercise capacity (e.g. incremental cardiopulmonary exercise test (CPETinc), incremental shuttle walk test (ISWT)) and functional exercise capacity (e.g. 6‐minute walk test (6MWT), constant work rate (CPETcwr), endurance shuttle walk test (ESWT)). The principal metrics of interest for these tests were peak oxygen uptake (VO2 peak) and peak work rate (WRmax) for CPETinc tests; distance in metres for ISWT and 6MWT; and time in seconds for CPETcwr and ESWT. All measures were reported upon completion of the PR intervention and the latest time point up to 12 months after completion of the intervention.
Asthma control (e.g. Asthma Control Questionnaire (ACQ) or Asthma Control Test (ACT)): this was reported upon completion of the PR intervention, and the latest time point up to 12 months after completion of the intervention.
Health‐related quality of life: measured via disease‐specific questionnaires (e.g. Asthma Quality of Life Questionnaire (AQLQ), St George's Respiratory Questionnaire (SGRQ), Asthma Impact Survey, Living with Asthma Questionnaire, Chronic Respiratory Disease Questionnaire (CRQ)) or generic health questionnaires (e.g. 36‐item Short Form Health Survey (SF‐36), Euro‐Qol). We used both total scores and symptom‐specific subdomain scores but reported them separately. We analysed data from disease‐specific and generic instruments separately. We considered disease‐specific quality of life total scores to be the principal analysis of interest. All measures were reported upon completion of the PR intervention, and the latest time point up to 12 months after completion of the intervention.
Secondary outcomes
Severe asthma exacerbations/hospitalisations: measured as the incidence or rate of severe acute asthma exacerbations (episodes requiring oral systemic corticosteroid use) or respiratory‐related hospitalisation, or both (Reddel 2009). Where possible, data from hospitalisations were analysed separately to those of exacerbations. Data were reported using the longest time point available up to 12 months after completion of the intervention.
Mental health: this comprised measures of anxiety and depression (e.g. Hospital Anxiety and Depression Scale (HADS), Beck Depression Inventory, Hamilton Anxiety/Depression Rating Scale). Where available, anxiety data were analysed distinct from depression data. This was assessed upon completion of the PR intervention, and at the longest time point available up to 12 months after completion of the intervention.
Peripheral skeletal muscle force: this included measures of muscle strength (kilograms), power (Newtons) or torque (Newton‐metres). We pooled data from muscle groups of the upper limb together, and data from muscle groups of the lower limb together. Upper limb muscle force data were analysed separately from lower limb muscle force data. This was assessed upon completion of the exercise training intervention, and the longest time point available up to 12 months after completion of the intervention.
Levels of physical activity: this comprised objectively measured outcomes of movement (e.g. steps, time spent in light/moderate/ vigorous activity) but not sedentary behaviour. We did not consider subjective recall methods (e.g. surveys) for inclusion. This was assessed upon completion of the PR intervention, and the longest time point available up to 12 months after intervention completion.
Inflammatory biomarkers: these comprised commonly used markers of airway and systemic inflammation. Examples of airway inflammation may include fractional exhaled nitric oxide (FeNO) and eosinophils (sputum and blood samples). Markers of systemic inflammation may include C‐reactive protein (CRP), white cell count (WCC), and interleukins (e.g. IL‐6). These were assessed upon completion of the PR intervention, and the longest time point available up to 12 months after intervention completion.
Adverse events/side effects: this comprised events related to the PR intervention (e.g. within‐session incidents), such as respiratory‐related hospitalisations, falls and musculoskeletal injuries, as well as incidence of significant exercise‐induced bronchoconstriction (where reported in adequate detail). Mortality was not included within this outcome.
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from searches of the following databases and trial registries:
Cochrane Airways Trials Register (Cochrane Airways 2019), via the Cochrane Register of Studies, all years to 11 May 2021;
Cochrane Central Register of Controlled Trials (CENTRAL), via the Cochrane Register of Studies, all years to 11 May 2021;
MEDLINE (Ovid SP) ALL, 1946 to 11 May 2021;
Embase (Ovid SP), 1974 to 11 May 2021;
PEDro (Physiotherapy Evidence Database), all years to 11 May 2021;
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/), all years to 11 May 2021;
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/), all years to 11 May 2021.
The database search strategies are listed in Appendix 1. These were adapted for use in the other databases. The Cochrane Airways Information Specialist developed the search strategies in collaboration with the review authors; these were peer reviewed by another Cochrane Information Specialist using the Peer Review of Electronic Search Strategies (PRESS) checklist (McGowan 2016).
All databases and trials registries were searched from their inception on 11 May 2021, with no restriction on language or type of publication. Handsearched conference abstracts and grey literature were identified through the Cochrane Airways Trials Register and CENTRAL.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for study information. We searched PubMed for errata or retractions from included studies published in full text, on 21 December 2021.
Data collection and analysis
Selection of studies
We used Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components:
known assessments, a service that matches records in the search results to records that have already been screened in Cochrane Crowd (Cochrane's citizen science platform where the Crowd help to identify and describe health evidence) and labelled as 'RCT' or 'not an RCT';
the RCT classifier, a machine‐learning model that distinguishes RCTs from non‐RCTs; and
Cochrane Crowd, if appropriate (crowd.cochrane.org).
More detailed information about the Screen4Me components can be found in the following publications: Marshall 2018; McDonald 2017; Noel‐Storr 2018; Thomas 2017.
Following this initial assessment, two review authors (CO, CG) independently screened the remaining titles and abstracts of records identified by the search using Covidence software (Covidence), classifying them as 'yes' or 'maybe' (eligible or potentially eligible/unclear) or 'no' (do not retrieve). We retrieved the full‐text study reports of all potentially eligible studies, and two review authors (CO, CG) independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion or via consultation with a third review author (AH) if required. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data that was piloted on one study in the review. One review author (CG) extracted the study characteristic (methods, participants, interventions, outcomes) from the included studies, and another review author (VM) checked these for accuracy. Two review authors (CG, VM) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. Any disagreements were resolved by consensus or by involving a third review author (CO). One review author (CG) transferred data into Review Manager Web (RevMan Web 2020). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (CO) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (CG, AH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Any disagreements were resolved by discussion or by involving another review author (CO). We assessed risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We judged each study as being at low, high, or unclear risk of bias for each domain, and provided a quote from the study report together with a justification for our judgement in the risk of bias table in Characteristics of included studies. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for subjectively and non‐subjectively reported outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and justified any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We planned to analyse dichotomous data as risk ratios (RRs), for ease of interpretation, and continuous data as mean difference (MD) or standardised mean difference (SMD) with 95% confidence intervals (95% CIs). We used SMDs where outcome data were reported via different metrics but were deemed clinically homogenous (e.g. data from different field walking tests or different quality of life instruments); however, they were not used where such outcome data comprised a combination of both endpoint and change data. Where SMDs were to be used for outcome data expressed as change from baseline (principal unit of interest), we planned to employ the standard deviation (SD) of baseline values as the unit of measurement to calculate the SMD and adjust standard errors to take correlation into account, where appropriate data were available. Results from analyses conducted using SMDs were to be transformed back to native metrics of commonly used instruments for ease of interpretation; however, this was not required. Meta‐analyses involving data from rating scales were checked to ensure they were entered with a consistent direction of effect (e.g. lower scores always indicative of improvement).
We undertook meta‐analyses only where this was meaningful, that is if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. We described skewed data narratively (e.g. as medians and interquartile ranges for each group). Where multiple trial arms were reported in a single study, we included only the relevant arms. If two comparisons (e.g. intervention A versus control and intervention B versus control) were combined in the same meta‐analysis, we would either combine the active arms or halve the control group to avoid double‐counting.
If adjusted analyses were available (analysis of variance (ANOVA) or analysis of covariance (ANCOVA)), we used these as a preference in our meta‐analyses. Where both change‐from‐baseline and endpoint scores were available within individual studies for continuous data, we used change‐from‐baseline unless there was reported low correlation between measurements in individuals. If a study reported outcomes at multiple time points, we used the data closest to the primary time point of interest.
We used intention‐to‐treat (ITT) or 'full analysis set' analyses where they were reported (i.e. those where data were imputed for participants who were randomly assigned but did not complete the study) instead of completer or per‐protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we intended to use participants, rather than events, as the unit of analysis (i.e. number of people admitted to hospital, rather than number of admissions per individual). If rate ratios were reported in a study, we would analyse them on this basis. However, no dichotomous data were included in the review.
We only intended to meta‐analyse data from cluster‐RCTs if the available data were adjusted (or could be adjusted) to account for the clustering; however, no such studies were included.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for the affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity amongst the studies in each analysis. If we identified substantial heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis. Whilst we hypothesise that treatment effects could differ on the basis of participants' age, no simple cut‐off is appropriate to examine this via traditional subgroup analysis. We therefore extracted information on mean participant age within the included studies and considered how between‐study heterogeneity may have impacted upon effect estimates. Furthermore, where individual studies presented outcomes stratified by age, we extracted and reported this information.
Assessment of reporting biases
We planned that if we identified more than 10 studies, we would create and examine a funnel plot to explore possible small‐study and publication biases. However, no analysis included more than five studies, thereby precluding the creation of funnel plots.
Data synthesis
We used a random‐effects model for all meta‐analyses and performed a sensitivity analysis with a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Programmes that adopt total training durations lasting ≤ 8 weeks versus those that are > 8 weeks.
Participants characterised by severe asthma versus those characterised by non‐severe asthma (Chung 2014), where identifiable.
We planned to use the following outcomes in subgroup analyses.
Exercise performance (6MWT or ISWT only, considering the predominant use of these tests in clinical practice, measured upon intervention completion).
Asthma control, measured upon intervention completion.
Health‐related quality of life (disease‐specific total scores only, measured upon intervention completion).
We planned to use the formal test for subgroup interactions in Review Manager Web (RevMan Web 2020).
Sensitivity analysis
In order to explore whether the effect of PR on the primary outcomes may be moderated by the inclusion of participants with a mixed ACO diagnosis, we carried out a sensitivity analysis where we removed studies in which more than 50% of participants had ACO (where this was possible to identify).
We also planned to compare the results from the random‐effects model (principal method of analysis) with those using a fixed‐effect model.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the following outcomes: exercise capacity, asthma control, quality of life, and adverse events. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Higgins 2019), and created the table using GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
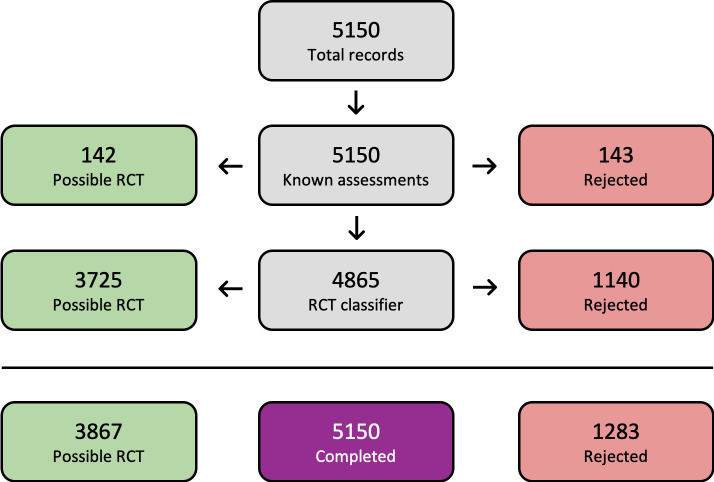
A detailed overview of the study flow is presented in Figure 1. We identified 9446 records from the initial search of the prespecified databases and trial registries, of which 5150 records remained after duplicates were removed. We used Cochrane's Screen4Me workflow (Figure 2) to assess the search results and excluded 1283 records, leaving a total of 3867 records remaining. We excluded 3746 records on the basis of title and abstract, and evaluated 121 records for eligibility via full text, of which 88 were excluded as they did not meet the review criteria. We assessed three records (three studies) as awaiting classification, as limited study characteristics were available from scientific abstracts or clinical trial register information only (Budnevsky 2018; IRCT2014041617299N; NTR4398), and three additional duplicate records were identified. Ten studies (26 records) met the criteria for inclusion in the review, whilst one other study (one record) was identified as ongoing (NCT03630432). We performed searches of PubMed for errata or corrections in December 2021, but identified no amendments.
1.
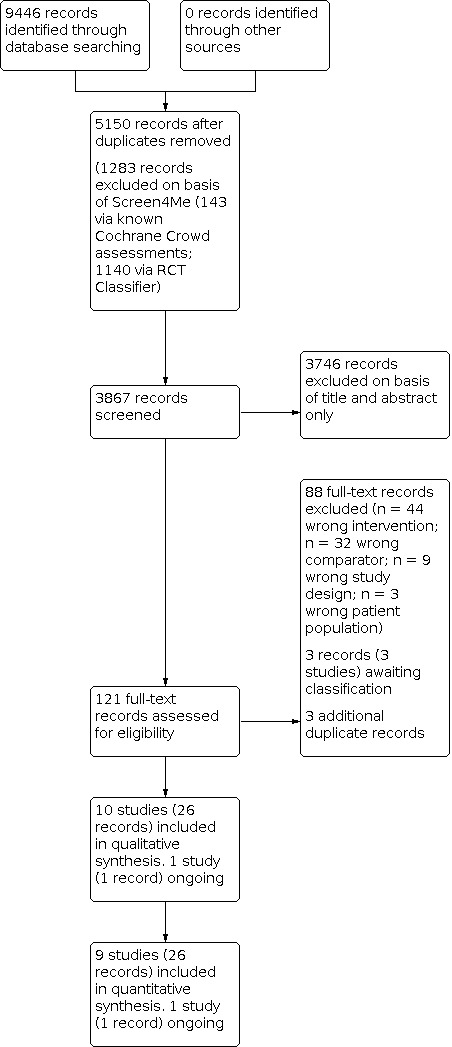
Study flow diagram.
2.
Overview of Cochrane Crowd Known Assessments and Screen4Me workflows for original search.
For study details, see: Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Included studies
Ten studies met the eligibility criteria for this review. The 10 studies were published between 1990 and 2021. Full details can be found in the Characteristics of included studies section. Nine studies were published in full text in peer‐reviewed journals (Cambach 1997; Cochrane 1990; Foglio 2001; Majd 2020; Nathell 2005; Orooj 2020; Schultz 2021; Toennesen 2017; Turk 2020). One study was published in abstract form only (Manzak 2020). The studies were conducted in the Netherlands (Cambach 1997; Turk 2020), the UK (Cochrane 1990; Majd 2020), Italy (Foglio 2001), Sweden (Nathell 2005), Germany (Schultz 2021), Denmark (Toennesen 2017), Turkey (Manzak 2020), and India (Orooj 2020). Nine studies contributed to quantitative synthesis. The study by Nathell 2005 did not report on any of the primary or secondary outcomes of this review and contributed to narrative synthesis only.
Design
All studies included in the review were RCTs. One study was a randomised cross‐over trial that was included but only with consideration given to pre‐cross‐over data involving short‐term (i.e. end‐treatment) outcomes (Cambach 1997). This is described further in Differences between protocol and review. One study was described as a feasibility study (Majd 2020). One study employed a randomised controlled parallel‐group design involving four groups (exercise only, dietary education only, exercise plus dietary education, and control) (Toennesen 2017). We used data from the exercise plus dietary education group (pulmonary rehabilitation) and control groups for analysis. The study by Turk 2020 employed a randomised controlled parallel‐group design involving three groups (pulmonary rehabilitation only, pulmonary rehabilitation plus self‐management support, and control). We used data from the pulmonary rehabilitation‐only and control groups for analysis, as the self‐management co‐intervention involved a novel internet‐based self‐management tool that was applied throughout both the intervention and follow‐up periods. The study by Foglio 2001 randomised participants with a diagnosis of COPD or asthma who had completed pulmonary rehabilitation one year previously to repeat a pulmonary rehabilitation programme or to usual care. One of the study authors provided data relating to asthma participants only at one year.
Participants
The 10 studies involved a total of 894 participants, with sample sizes ranging from 24 to 412 participants. Schultz 2021 was the largest study, with 412 participants; the next‐largest study was Nathell 2005, with 197 participants, although it contributed to the narrative synthesis only. Of the remaining eight studies, the average sample size was 36 participants. The studies by Manzak 2020 and Orooj 2020 did not provide a breakdown of number of participants by gender. Of the eight studies that provided a breakdown of participants by gender, there were 257 male participants and 448 female participants. The mean age of participants ranged from 27 years to 54 years. Five studies did not report on the smoking history of participants (Cambach 1997; Foglio 2001; Majd 2020; Manzak 2020; Turk 2020). The study by Majd 2020 excluded those with a smoking history of ≥ 10 pack years. Participants in the study by Cochrane 1990 were all non‐smokers. Toennesen 2017, Schultz 2021, and Nathell 2005 reported the proportion of current smokers in pulmonary rehabilitation and usual care groups in their respective studies. Asthma diagnostic criteria varied between studies. One study based asthma diagnosis on clinical examination only (Nathell 2005). One study did not state how the diagnosis of asthma was made (Manzak 2020). One study included participants with severe asthma only (Majd 2020). Two studies included mixed participant groups involving people with asthma and people with COPD (Cambach 1997; Foglio 2001). We included the subgroup of data relating specifically to participants with asthma from the study by Cambach 1997. Data for asthma participants only were provided from Foglio 2001 through email correspondence with one of the study authors. One study involved participants with a specific diagnosis of ACO (Orooj 2020). All participants in the study by Foglio 2001 had completed pulmonary rehabilitation one year prior to enrolment; the study examined the outcomes of repeating versus not repeating pulmonary rehabilitation at one year.
Interventions
All studies compared pulmonary rehabilitation to usual care. One study employed a waitlist control group (Schultz 2021). One study was a randomised cross‐over trial, but only data pre‐ and postintervention before cross‐over occurred were extracted for this review (Cambach 1997). Seven studies evaluated pulmonary rehabilitation conducted in the outpatient setting (Cambach 1997; Cochrane 1990; Foglio 2001; Majd 2020; Orooj 2020; Toennesen 2017; Turk 2020). Two studies examined pulmonary rehabilitation conducted in an inpatient setting (Nathell 2005; Schultz 2021). Only one study evaluated a home‐based pulmonary rehabilitation programme (Manzak 2020). The length of pulmonary rehabilitation programmes varied from three to four weeks for inpatient pulmonary rehabilitation programmes, and from eight to 12 weeks for outpatient pulmonary rehabilitation. Only one study specifically stated that there was a run‐in period (Cochrane 1990).
The exercise component of pulmonary rehabilitation included aerobic training (Cambach 1997; Cochrane 1990; Toennesen 2017; Turk 2020), a combination of aerobic and resistance training (Foglio 2001; Majd 2020; Manzak 2020; Orooj 2020; Schultz 2021), and “physical training”, which was not explained further (Nathell 2005). Toennesen 2017 and Turk 2020 employed high‐intensity interval training as the aerobic training modality. All studies included some form of education or self‐management component, such as breathing retraining and relaxation (Cambach 1997), “nutritional advice and psychological counselling when appropriate” (Foglio 2001), coping skill acquisition (Nathell 2005), and a structured self‐management education programme including relaxation techniques, smoking cessation, and nutrition (Orooj 2020). The inpatient pulmonary rehabilitation programme evaluated in Schultz 2021 included respiratory physiotherapy and (if needed) psychosocial support, smoking cessation, and nutritional counselling as well as inspiratory muscle training. In Cochrane 1990, the educational sessions were designed to encourage a greater understanding and to improve self‐management of asthma as well as description of the training programme principles. The education component in both Toennesen 2017 and Turk 2020 consisted of a nutritional intervention: group and individual counselling sessions regarding dietary advice in Toennesen 2017, and psychological group sessions focusing on behavioural modification and motivational strategies in Turk 2020. One study evaluated “asthma tailored pulmonary rehabilitation”, which was based on traditional pulmonary rehabilitation but was targeted exclusively for those with severe asthma (Majd 2020). Half of the education sessions were developed to be more specific to patients with asthma, and the other half were based on motivational consultation and delivered by a health psychologist. In Manzak 2020, participants were provided with a pedometer and exercise diary. A summary of key characteristics of interventions is presented in Table 2.
1. Summary of interventions.
| Study | Participants | Setting | Intervention | Control | Duration (weeks) | Frequency | Follow‐up (weeks) |
| Cambach 1997 | Obstructive lung disease COPD/asthma | OP (physiotherapy practices) Supervised |
Aerobic: cycle ergometer (60 to 75% Wmax), rower and stair walking (> 60% PMHR) x 2 sessions/week. “Recreational activities” at > 60% PMHR x 1 session/week for 45 min Education: breathing retraining, evacuation of mucus, relaxation sessions |
Usual care (before cross‐over occurred) | 12 | 3 OP sessions/week | None |
|
Cochrane 1990 |
Mild‐ moderate asthma | OP (hospital) Supervised |
Aerobic: cycling, jogging, “aerobics” x 30 min. Target 75% PMHR. Audiotape instructions for home use if unable to attend. Education: sessions to improve self‐management and principles of the training programme |
Education sessions to improve self‐management | 12 | 3 OP sessions/week | None |
| Foglio 2001 | COPD and asthma | OP (hospital) Supervised |
Aerobic: cycling up to 30 min at 50% to 70% Wmax resistance: abdominal, UL, LL progressively increasing light weights (300 to 500 g) Education: “patient and family education”, nutritional programme, and psychosocial counselling when appropriate |
Usual care | 8 to 10 | 3 OP sessions/week | 1 year |
| Majd 2020 | Severe asthma | OP (hospital) Supervised |
Aerobic: ground walking (85% VO2 peak), treadmill walking, cycling (60% to 80% peak VO2) x 20 to 30 minutes. Resistance: UL, LL (6 to 12 reps at 80% 1 RM). Education: 12 topics delivered by MDT + individualised session led by health psychologist |
Usual care | 12 | 2 OP sessions/week + HB (minimum 1 session/week) and daily walk encouraged | 9 months |
| Manzak 2020 | Asthma | Home based | Aerobic: regular physical activity such as walking Resistance: strengthening exercises for upper and lower extremities Other: stretching exercises, breathing exercises Education: participants also provided with a pedometer and exercise diary |
Booklets on breathing exercises and physical activity in addition to 1 education session on the course of the disease. Also provided with a pedometer and exercise diary | 8 | Minimum of 3 days per week, with 1 session supervised by a physiotherapist | None |
| Nathell 2005 | Asthma | IP | “Personal physical training programme” Education: delivered by MDT covering “all aspects of asthma” plus coping skill acquisition |
Usual care | 4 | No details provided. | 1, 2, 3 years |
| Orooj 2020 | Asthma/COPD overlap | OP (hospital) Supervised | Aerobic: endurance training on treadmill at 60% to 80% or VO2 peak 5 times per week. Resistance: upper and lower limbs resistance training at 50% to 70% 1 RM 3 times per week. Education: structured self‐management education programme including relaxation techniques, smoking cessation, and nutrition | Usual care | 6 | 5 OP sessions/ week | None |
| Schultz 2021 | Uncontrolled asthma ACT < 20 | IP | Aerobic: outdoor sports and training in water x 5 sessions/week x 45 to 60 min. Resistance: 3 sessions/week x 45 to 60 min. Other: whole body vibration training x 7 sessions/week. Education: asthma, inhalation technique, allergies, trigger avoidance, respiratory physiotherapy |
Usual care (waitlist) | 3 | 5 to 7 sessions per week | 6, 9, 12 months |
| Toennesen 2017 | Asthma ACQ > 1.0 | OP (hospital) Supervised |
Aerobic: HIIT on spinning bikes. Progressively increased to four 5‐minute intervals using 10‐20‐30 concept. Education: 5 group and 1 individual counselling sessions with a dietician |
Usual care | 8 | 3 OP sessions/week | 12 months |
| Turk 2020 | Asthma with ACQ > 0.75 and BMI > 30 | OP (hospital) Supervised |
Aerobic: HIIT body weight exercises (90% VO2 max) x 40 to 60 min. Education: psychological group sessions focusing on behavioural modification and motivational strategies. Nutritional intervention: prescription of a caloric diet 1500 kcal/day |
Usual care “advised to lose weight and to exercise” | 12 | 3 OP sessions/week | 12 months |
ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; BMI: body mass index; COPD: chronic obstructive pulmonary disease. HB: home based; HIIT: high‐intensity interval training; IMT: inspiratory muscle training; IP inpatient; kcal: kilocalories; LL: lower limb; MDT: multidisciplinary team; OP: outpatient; PMHR: predicted maximum heart rate; RM: repetition maximum; UL; upper limb; VO2 max: maximal oxygen uptake; VO2 peak: peak oxygen uptake; Wmax: maximal workload.
Outcomes
One study did not report upon any of the primary or secondary outcomes for this review and contributed to the narrative synthesis only (Nathell 2005). All other studies reported on a measure of exercise capacity, most commonly a cardiopulmonary exercise test (Cambach 1997; Cochrane 1990; Foglio 2001; Majd 2020; Toennesen 2017; Turk 2020). A number of studies also reported on exercise capacity using a functional exercise test, most commonly the 6‐minute walk test (Cambach 1997; Foglio 2001; Manzak 2020; Orooj 2020; Schultz 2021; Turk 2020). Majd 2020 performed the incremental and endurance shuttle walk tests. In Schultz 2021, 6‐minute walk test data were available pre‐ and post‐pulmonary rehabilitation programme for both the intervention and control groups, with end of pulmonary rehabilitation occurring three months before the control group started pulmonary rehabilitation (waitlist‐controlled study). 6‐minute walk test data were available for Foglio 2001 at one‐year follow‐up only and not upon completion of pulmonary rehabilitation. Asthma control was measured in three studies using the ACQ (Majd 2020; Toennesen 2017; Turk 2020), and in two studies using the ACT (Manzak 2020; Schultz 2021). Health‐related quality of life was assessed using the CRQ in two studies (Cambach 1997; Majd 2020); the SGRQ in three studies (Foglio 2001; Manzak 2020; Orooj 2020; Schultz 2021); the AQLQ in three studies (Majd 2020; Schultz 2021; Turk 2020); the Mini Asthma Quality of Life Questionnaire (MiniAQLQ) in one study (Toennesen 2017); and the EQ‐5D in one study (Majd 2020). Data on total AQLQ scores in the study by Majd 2020 were provided through email correspondence with one of the study authors. Data for the longer‐term effects of pulmonary rehabilitation on health‐related quality of life were available from three studies (Foglio 2001; Majd 2020; Schultz 2021). Follow‐up ranged from three months to nine months to one year. Schultz 2021 reported both AQLQ (total and domain scores) and SGRQ (total and domain scores) at three months following rehabilitation. Majd 2020 reported AQLQ (domain scores only; total score obtained through correspondence with authors) and CRQ (domain scores only) at nine‐month follow‐up. Foglio 2001 reported SGRQ total score at one‐year follow‐up.
Exacerbation rate was reported in Turk 2020, where an asthma exacerbation was defined as worsening of symptoms with the need for oral corticosteroids or antibiotics, or both. Foglio 2001 also recorded the number of exacerbations as well as hospitalisations in their mixed COPD/asthma study population, although data for these outcomes for asthma participants only were not available in the published paper or via email correspondence with the authors. Two studies reported mental health outcomes. Majd 2020 reported HADS scores, and Schultz 2021 reported anxiety and depression scores using the Patient Health Questionnaire‐9 (PHQ‐9) and the General Anxiety Disorder‐7 (GAD‐7), respectively. One study assessed peripheral skeletal muscle force (quadriceps maximal voluntary contraction) (Majd 2020). One study reported levels of physical activity as an outcome (Turk 2020). Daily activity such as daily steps and physical activity level was measured with a portable MoveMonitor. Three studies assessed inflammatory biomarkers (markers of airway and systemic inflammation): Majd 2020 (FeNO, sputum eosinophils %, sputum eosinophil count), Toennesen 2017 (FeNO, sputum eosinophils %, sputum neutrophils%, blood eosinophils, serum CRP, and serum IL‐6), and Turk 2020 (FeNO, sputum eosinophils%, sputum neutrophils%, CRP, blood eosinophils, leucocytes). One study measured the incidence of adverse events (Majd 2020), and any adverse events directly or indirectly related to the exercise measurements and training sessions were recorded. Nathell 2005 reported sick leave days, use of steroids, and smoking habits and one, two, and three years following the intervention.
Follow‐up periods ranged from three months, Schultz 2021, to nine months, Majd 2020, to one year, Foglio 2001; Toennesen 2017; Turk 2020, following intervention completion. Participants in Nathell 2005 were followed up at yearly intervals for three years. There was no follow‐up beyond the intervention completion in Cochrane 1990, Manzak 2020, and Orooj 2020. Whilst participants in Cambach 1997 were followed up at six months following intervention completion, group cross‐over occurred at three months. As we only used pre‐cross‐over data, we did not consider follow‐up information in the review.
Excluded studies
We excluded 88 records after full‐text review. The most common reasons for exclusion were that studies did not include an intervention that met our definition of pulmonary rehabilitation (n = 44), and the comparator did not meet our definition of usual care (n = 32). For study details, see Characteristics of excluded studies.
Risk of bias in included studies
We completed risk of bias assessment for all 10 included studies. An overview of risk of bias judgements across studies is provided in Figure 3 and Figure 4.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All studies reported random allocation to study arms. Six studies specified the method of randomisation as computer‐generated random sequences and were judged as at low risk of bias (Majd 2020; Nathell 2005; Orooj 2020; Schultz 2021; Toennesen 2017; Turk 2020). The remaining studies did not provide sufficient information to determine how the sequence was generated. Five studies reported that the allocation sequence was concealed in sufficient detail to permit a judgement of low risk of bias (Majd 2020; Nathell 2005; Schultz 2021; Toennesen 2017; Turk 2020). Four studies used an independent researcher to provide group allocation (Majd 2020; Nathell 2005; Schultz 2021; Turk 2020), and one study used opaque, sealed envelopes (Toennesen 2017). The remaining studies did not provide sufficient information to assess risk of bias for allocation concealment.
Blinding
Blinding of participants was not possible given the nature of the rehabilitation intervention. One study reported that personnel running the rehabilitation programme were unaware of group allocation (Schultz 2021). We rated the risk of bias related to performance bias and detection bias separately for subjectively and non‐subjectively reported outcomes. All studies except for one, Cochrane 1990, included self‐reported outcomes, and were all therefore assessed as having high risk of performance bias for subjectively reported outcomes. We assessed all studies as having high risk of performance bias for non‐subjectively reported outcomes, as they all included a measure of exercise capacity, and it is possible that the assessor could have altered the outcome with more or less encouragement. Two studies reported the use of a blinded outcome assessor and were therefore judged as at low risk of bias for outcome assessment (Majd 2020; Toennesen 2017). In one study (Foglio 2001), it was not clear who collected questionnaire data, although laboratory measurements were blinded. Three studies stated that the outcome assessors were not blinded (Nathell 2005; Schultz 2021; Turk 2020). In the other studies, insufficient data were provided to demonstrate whether outcome assessors were blinded (Cambach 1997; Cochrane 1990; Manzak 2020; Orooj 2020). No studies reported whether data analysts were blinded to group allocation.
Incomplete outcome data
Only two studies reported no dropouts (Cochrane 1990; Orooj 2020). One study available as an abstract only did not report whether dropouts occurred (Manzak 2020). Six studies reported reasons for attrition (Cambach 1997; Foglio 2001; Majd 2020; Schultz 2021; Toennesen 2017; Turk 2020). Nathell 2005, Schultz 2021, and Turk 2020 analysed data for randomised participants according to ITT principles and were therefore judged as at low risk of bias. We assessed two studies as at high risk of bias for this domain. Cambach 1997 reported a significant number of dropouts before and after randomisation. The study population was a mixed COPD/asthma cohort, and it was not clear how many participants with a diagnosis of asthma had dropped out. No ITT analysis was performed. Similarly, there were a high numbers of dropouts and no ITT analysis in Foglio 2001.
Selective reporting
Four studies were prospectively reported on a clinical trial registry (Majd 2020; Schultz 2021; Toennesen 2017; Turk 2020), of which two had a published protocol (Majd 2020; Schultz 2021). Each of these four studies reported results for all outcomes and were judged to be at low risk of bias. Whilst one further study was also prospectively reported on a clinical trial registry (Manzak 2020), it was presented in abstract form only and did not report on the prespecified primary outcome of dyspnoea and the secondary outcome of activities of daily living (London Chest Activity of Daily Living), and was therefore judged to be at unclear risk of bias. It was not possible to determine whether all data were available for five full‐text studies because there was no published protocol, which may reflect the age of some of these studies (Cambach 1997; Cochrane 1990; Foglio 2001; Nathell 2005; Orooj 2020).
Other potential sources of bias
We identified other potential sources of bias in seven studies (Cochrane 1990; Foglio 2001; Manzak 2020; Nathell 2005; Orooj 2020; Toennesen 2017; Turk 2020). In Cochrane 1990, nine of the 26 participants had their treatment altered during the study period, and was therefore rated as having an unclear risk of bias. The participants in the study by Foglio 2001 had already completed pulmonary rehabilitation a year previously, and it was not clear how this would affect the generalisability of the study findings. In Nathell 2005, participants were recruited from a sickness insurance scheme, mainly for manual workers. The diagnosis of asthma was made on clinical examination, and the proportion of current smokers at randomisation was high. One study was available in abstract form only (Manzak 2020), which limited our ability to determine whether there were other potential sources of bias. This study was also retrospectively registered. No data relating to the gender of participants were provided by Orooj 2020. There was a considerable gender imbalance at baseline between intervention and control groups in Toennesen 2017, and it is unclear if this may have affected outcomes. The clinical trial registry record for this study also indicated an original target of 200 participants, which was not achieved. The study by Turk 2020 did not achieve its target sample size.
Effects of interventions
See: Table 1
This review is based on a published protocol (Osadnik 2019a). An overview of the main review findings is provided in Table 1.
Primary outcomes
Exercise performance (tests of maximal exercise capacity, e.g. incremental CPET, ISWT)
Four studies reported that an incremental cardiopulmonary exercise test on a cycle ergometer was performed following pulmonary rehabilitation (Cochrane 1990; Majd 2020; Toennesen 2017; Turk 2020), and one study used the incremental shuttle walk test (Majd 2020). Data were available from two studies in relation to % predicted maximal oxygen uptake (VO2 max) (Cochrane 1990; Turk 2020). The study by Turk 2020 reported change rather than endpoint data. Pooled data from these two studies suggest that pulmonary rehabilitation may result in an increase in % predicted VO2 max immediately following the intervention (mean difference (MD) 14.88%, 95% confidence interval (CI) 9.66 to 20.1; I2 = 0%; 2 studies; n = 60; low certainty evidence; Analysis 1.1). Data were available for three studies in relation to VO2 peak (Cochrane 1990; Majd 2020; Toennesen 2017). Pooled data from these three studies suggest that pulmonary rehabilitation may lead to improvements in VO2 peak immediately following pulmonary rehabilitation, but the evidence is very uncertain (MD 3.63 mL/kg/min, 95% CI 1.48 to 5.77; I2 = 55%; 3 studies; n = 129; very low certainty evidence; Analysis 1.2; Figure 5). Majd 2020 reported a mean change of 74 metres (95% CI 26.4 to 121.4) on the incremental shuttle walk test distance between groups following the intervention in favour of pulmonary rehabilitation (1 study; n = 30).
1.1. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 1: Exercise performance: % predicted VO2 max on incremental cardiopulmonary exercise test at end‐intervention
1.2. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 2: Exercise performance: Peak oxygen uptake (VO2 peak) on incremental cardiopulmonary exercise test at end‐intervention
5.

Analysis 1.2 Peak oxygen uptake (VO2 peak, mL/kg/min) on incremental cardiopulmonary exercise test at end‐intervention
Data regarding the longer‐term effects of pulmonary rehabilitation on maximal exercise capacity were available from four studies (Foglio 2001; Majd 2020; Toennesen 2017; Turk 2020). Data on VO2 peak at follow‐up were reported in three studies, with one study reporting results at nine months (Majd 2020), and the others at one year (Foglio 2001; Toennesen 2017). The evidence is very uncertain for the effect of pulmonary rehabilitation on VO2 peak at follow‐up (MD −0.69 mL/kg/min, 95% CI −4.79 to 3.42; I2 = 49%; 3 studies; n = 66; very low certainty evidence; Analysis 2.1). In Foglio 2001, there was a mean difference in peak work rate (WRmax) on incremental CPET of −13.5 maximal workload (Wmax) (95% CI −34.15 to 7.15) between pulmonary rehabilitation and usual care groups at one‐year follow‐up (1 study; n = 18). There was a mean difference of 10.37% (95% CI −1.6 to 22.34) between pulmonary rehabilitation and usual care groups in % predicted VO2 max at one‐year follow‐up in Turk 2020 (1 study; n = 24). In Majd 2020, there was a mean difference of −9 metres (95% CI −140.38 to 122.38) on the ISWT between groups at follow‐up.
2.1. Analysis.

Comparison 2: Pulmonary rehabilitation vs usual care for adults with asthma (follow‐up), Outcome 1: Exercise performance: Peak oxygen uptake (VO2 peak) on incremental cardiopulmonary exercise test at follow‐up
Exercise performance (tests of functional exercise capacity, e.g. 6MWT, constant work rate CPET, ESWT)
Six studies reported that exercise capacity was measured using the 6‐minute walk test. Data at end intervention were available from five of these studies (Cambach 1997; Manzak 2020; Orooj 2020; Schultz 2021; Turk 2020), with the study by Foglio 2001 reporting data one year after pulmonary rehabilitation completion. Cambach 1997 and Turk 2020 reported change from baseline rather than endpoint data. Pooled data from these five studies suggest that pulmonary rehabilitation likely results in a large improvement in 6‐minute walk distance immediately following the intervention (MD 79.79 metres, 95% CI 66.47 to 93.11; I2 = 0%; 5 studies; n = 529; moderate certainty evidence; Analysis 1.3; Figure 6). One study also measured constant work rate (time in seconds) at end intervention on cardiopulmonary exercise test on a cycle ergometer and reported a mean difference of 425 seconds (95% CI 299.08 to 570.92) between groups following the intervention in favour of pulmonary rehabilitation (1 study; n = 39) (Cambach 1997). Three studies involved interventions lasting ≤ 8 weeks (Manzak 2020; Orooj 2020; Schultz 2021), and two studies involved interventions lasting > 8 weeks (Cambach 1997; Turk 2020). Subgroup analysis revealed no difference in 6‐minute walk distance between subgroups on the basis of programme duration (test for subgroup differences: Chi2 = 3.56; df = 1; P = 0.20; I2 = 38.1%; Analysis 1.4). No subgroup analysis was possible on the basis of disease severity as all included studies related to this outcome involved people who did not have severe asthma.
1.3. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 3: Exercise performance: 6‐minute walk test distance at end‐intervention
6.

Analysis 1.4 Exercise performance: 6‐minute walk test distance at end‐intervention.
Schultz 2018 is endpoint data. Cambach 1997 and Turk 2017 are change from baseline data.
1.4. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 4: Exercise performance: 6‐minute walk test distance at end‐intervention (Subgroup: duration of pulmonary rehabilitation)
We were able to pool data on the longer‐term effects of pulmonary rehabilitation on functional exercise capacity (6MWT) from two studies, with both studies reporting results at one year (Foglio 2001; Turk 2020). Foglio 2001 reported endpoint data, and Turk 2020 reported change from baseline data. The evidence is very uncertain regarding the effects of pulmonary rehabilitation on functional exercise capacity at follow‐up (MD 52.29 metres, 95% CI 0.7 to 103.88; I2 =72%; 2 studies; n = 42; very low certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Pulmonary rehabilitation vs usual care for adults with asthma (follow‐up), Outcome 2: Exercise performance: 6‐minute walk test distance at follow‐up
Asthma control
Five studies reported findings related to two different metrics of asthma control. Pooled data from two studies suggest that pulmonary rehabilitation may result in some improvement in asthma control as measured by the ACQ (MD −0.46, 95% CI −0.76 to −0.17; I2 = 0%; 2 studies; n = 93; low certainty evidence; Analysis 1.5; Figure 7). Turk 2020 reported ACQ scores as median values with interquartile ranges (IQRs) (25th to 75th percentiles). There was no difference in asthma control between pulmonary rehabilitation and usual care groups immediately following the intervention (median (IQR) change in ACQ score −0.67 (−1.42 to 0) for pulmonary rehabilitation and −0.25 (−0.66 to −0.63) for usual care; 24 participants; P = 0.113). For studies that reported ACQ score as an outcome, one study involved an intervention lasting ≤ 8 weeks (Toennesen 2017), and one study involved an intervention lasting > 8 weeks (Majd 2020). Subgroup analysis revealed no difference in ACQ score between subgroups on the basis of programme duration (test for subgroup differences: Chi2 = 0.10; df = 1; P = 0.75; I2 = 0%; Analysis 1.6). For studies that reported ACQ as an outcome, one study included participants with “non‐severe asthma” (Toennesen 2017), and one study included participants with “severe asthma”. Subgroup analyses revealed no difference in asthma control as measured by the ACQ between subgroups on the basis of disease severity (test for subgroup differences: Chi2 = 0.10; df = 1; P = 0.75; I2 = 0%; Analysis 1.7). The evidence is very uncertain regarding the effect of pulmonary rehabilitation on asthma control as measured by the ACT (MD 3.34, 95% CI −2.32 to 9.01; I2 = 91%; 2 studies; n = 442; very low certainty evidence; Analysis 1.8).
1.5. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 5: Asthma control: Asthma Control Questionnaire score at end‐intervention
7.

Analysis 1.6 Asthma control (Asthma Control Questionnaire) at end‐intervention.
1.6. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 6: Asthma control: Asthma Control Questionnaire score at end‐intervention (Subgroup: duration of pulmonary rehabilitation)
1.7. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 7: Asthma control: Asthma Control Questionnaire score at end‐intervention (Subgroup: asthma severity)
1.8. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 8: Asthma control: Asthma Control Test score at end‐intervention
Three studies reported findings related to asthma control as measured by the ACQ at follow‐up. We were able to pool data from two studies, with one study reporting results at nine months, Majd 2020, and one study reporting results at one year, Toennesen 2017. Pooled data suggest that pulmonary rehabilitation results in little to no difference in asthma control at follow‐up (MD 0.09, 95% CI −0.35 to 0.53; I2 =0%; 2 studies; n = 48; low certainty evidence; Analysis 2.3). Turk 2020 reported ACQ scores as median values with IQRs (25th to 75th percentiles) at 12 months, and at this time point ACQ scores were lower in the pulmonary rehabilitation group compared to the usual care group (β = −1.06, 95% CI −1.84 to −0.27, P = 0.011; 1 study; n = 24). The study of inpatient pulmonary rehabilitation by Schultz 2021 reported an adjusted MD between groups of 4.62 points (95% CI 3.78 to 5.46; 1 study; n = 412) on the ACT at three months following pulmonary rehabilitation.
2.3. Analysis.

Comparison 2: Pulmonary rehabilitation vs usual care for adults with asthma (follow‐up), Outcome 3: Asthma control: Asthma Control Questionnaire score at follow‐up
Health‐related quality of life
Eight studies reported findings related to four different metrics for health‐related quality of life. The evidence is very uncertain regarding the effect of pulmonary rehabilitation on AQLQ total score (MD 0.87, 95% CI −0.13 to 1.86; I2 = 88%; 2 studies; n = 442; very low certainty evidence; Analysis 1.9). Pulmonary rehabilitation may have little to no effect on AQLQ domain scores (Analysis 1.10) for activity (MD 0.80, 95% CI −0.26 to 1.85; I2 = 89%; 2 studies; n = 442); emotional function (MD 0.72, 95% CI −0.35 to 1.79; I2 =86%; 2 studies; n = 442); environment (MD 0.66, 95% CI −0.86 to 2.17; I2 = 89%; 2 studies; n = 442); and symptoms (MD 0.68, 95% CI −0.80 to 2.16; I2 = 96%; 2 studies; n = 442). Turk 2020 reported AQLQ scores as median values with IQRs (25th to 75th percentiles). There was no difference in AQLQ scores between pulmonary rehabilitation and usual care groups following the intervention by median change in AQLQ score 0.20 (IQR −0.33 to 0.84) for pulmonary rehabilitation and 0.12 (IQR −0.26 to 0.62) for usual care (1 study; n = 24; P = 0.758). In Toennesen 2017, there was a change in MiniAQLQ score following pulmonary rehabilitation compared to usual care (mean change 0.5, 95% CI 0.1 to 0.9; P < 0.01; 1 study; n = 63). For studies that reported on AQLQ as an outcome, one study involved an intervention lasting ≤ 8 weeks (Schultz 2021), and one study involved an intervention lasting > 8 weeks (Majd 2020). Subgroup analysis revealed a difference in AQLQ total score between subgroups on the basis of programme duration, favouring the shorter inpatient pulmonary rehabilitation programme (test for subgroup differences: Chi2 = 8.29; df = 1; P = 0.004; I2 = 87.9%; Analysis 1.11). For studies that reported AQLQ as an outcome, one study of inpatient pulmonary rehabilitation included participants with non‐severe asthma (Schultz 2021), and one study included participants with severe asthma (Majd 2020). Subgroup analysis revealed a difference in AQLQ total score between subgroups on the basis of disease severity, favouring participants with non‐severe asthma in the inpatient rehabilitation programme (test for subgroup differences: Chi2 = 8.29, df = 1; P = 0.004; I2 = 87.9%; Analysis 1.12). The findings of both subgroup analyses were strongly influenced (i.e. high weighting) by Schultz 2021, the study of inpatient pulmonary rehabilitation.
1.9. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 9: Health‐related quality of life: Asthma Quality of Life Questionnaire total score at end‐intervention
1.10. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 10: Health‐related quality of life: Asthma Quality of Life Questionnaire domain scores at end‐intervention
1.11. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 11: Health‐related quality of life: Asthma Quality of Life Questionnaire total score at end‐intervention (Subgroup: duration of pulmonary rehabilitation)
1.12. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 12: Health‐related quality of life: Asthma Quality of Life Questionnaire total score at end‐intervention (Subgroup: asthma severity)
Of the two studies that reported SGRQ scores at end intervention (Orooj 2020; Schultz 2021), the evidence suggests a large improvement in health‐related quality of life (SGRQ total score) immediately following pulmonary rehabilitation (MD −18.51, 95% CI −20.77 to −16.25; I2 = 0%; 2 studies; n = 440; moderate certainty evidence; Analysis 1.13) as well as SGRQ domain scores (Analysis 1.14) for symptoms (MD −20.50, 95% CI −23.78 to −17.22; I2 = 0%; 2 studies; n = 440); activity (MD −18.29, 95% CI −21.19 to −15.39; I2 = 0%; 2 studies; n = 440); and impact (MD −18.33, 95% CI −20.86 to −15.8; I2 = 0%; 2 studies; n = 442). We pooled CRQ domain scores from two studies for meta‐analysis (Analysis 1.15) (Cambach 1997; Majd 2020); the evidence suggests that pulmonary rehabilitation improves the CRQ domains of dyspnoea (standardised mean difference (SMD) 1.01, 95% CI 0.50 to 1.52) and fatigue (SMD 0.85, 95% CI 0.35 to 1.34), but not emotional function (SMD 0.43, 95% CI −0.66 to 1.52) or mastery (SMD 0.57, 95% CI −0.02 to 1.16). It is noteworthy that CRQ data from Cambach related to summed domain scores that were not divided by the number of domain items (conventional approach).
1.13. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 13: Health‐related quality of life: St George's Respiratory Questionnaire total score at end‐intervention
1.14. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 14: Health‐related quality of life: St George's Respiratory Questionnaire domain scores at end‐intervention
1.15. Analysis.

Comparison 1: Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention), Outcome 15: Health‐related quality of life: Chronic Respiratory Disease Questionnaire domain scores at end‐intervention
Data regarding the longer‐term effects of pulmonary rehabilitation on health‐related quality of life were available from three studies (Foglio 2001; Majd 2020; Schultz 2021). The evidence is very uncertain regarding the effect of pulmonary rehabilitation on quality of life as assessed by the AQLQ total score at follow‐up (MD 0.58, 95% CI −0.23 to 1.38; I2 = 78%; 2 studies; n = 435; very low certainty evidence; Analysis 2.4). The evidence suggests that pulmonary rehabilitation results in no difference in AQLQ domain scores (Analysis 2.5) related to activity (MD −0.03, 95% CI −2.02 to 1.97; I2 = 95%; 2 studies; n = 435); emotional function (MD −0.21, 95% CI −2.42 to 2.00; I2 = 94%; 2 studies; n = 435); environment (MD −0.22, 95% CI −2.23 to 1.8; I2 = 94%; 2 studies; n = 435); or symptoms (MD 0.49, 95% CI −0.78 to 1.77; I2 = 80%; 2 studies; n = 435) at follow‐up. Pulmonary rehabilitation may improve quality of life as assessed by the SGRQ total score at follow‐up, but the evidence is very uncertain (MD −13.4, 95% CI −15.93 to −10.88; I2 = 98%; 2 studies; n = 430; Analysis 2.6).
2.4. Analysis.

Comparison 2: Pulmonary rehabilitation vs usual care for adults with asthma (follow‐up), Outcome 4: Health‐related quality of life: Asthma Quality of Life Questionnaire total score at follow‐up
2.5. Analysis.

Comparison 2: Pulmonary rehabilitation vs usual care for adults with asthma (follow‐up), Outcome 5: Health‐related quality of life: Asthma Quality of Life Questionnaire domain scores at follow‐up
2.6. Analysis.

Comparison 2: Pulmonary rehabilitation vs usual care for adults with asthma (follow‐up), Outcome 6: Health‐related quality of life: St George's Respiratory Questionnaire total score at follow‐up
Secondary outcomes
Severe asthma exacerbations/hospitalisations
One study reported exacerbation rate (Turk 2020). No difference was observed between pulmonary rehabilitation and usual care groups in the proportion of participants who experienced an asthma exacerbation during the three‐month intervention (16.7% versus 55.6%; P = 0.16; 1 study; n = 24). At 12 months' follow‐up, a higher rate of exacerbations was observed in the usual care group compared to the pulmonary rehabilitation group (β (Poisson rate) = 0.839, 95% CI 0.116 to 1.563; P = 0.023; risk ratio (RR) 2.31, 95% CI 1.12 to 4.77). Foglio 2001 reported the number of exacerbations as well as the number of hospitalisations at one year following pulmonary rehabilitation in their mixed COPD/asthma study population, but data were not reported separately for those participants with asthma.
Mental health
Majd 2020 reported a mean difference for changes between the pulmonary rehabilitation and usual care groups of 0.4 (95% CI −1.3 to 2.1) points on the HADS anxiety subscale, and −0.3 (95% CI −1.9 to 1.3) points on the HADS depression subscale immediately following pulmonary rehabilitation (1 study; n = 30). There was a significant difference (P < 0.05) between groups at baseline for both domain scores. Schultz 2021 reported an adjusted mean difference (AMD) between pulmonary rehabilitation and usual care groups of −3.52 (95% CI −4.23 to −2.8; P < 0.001) points on the GAD‐7, and an AMD of −3.98 (95% CI −4.73 to −3.24; P < 0.001) on the PHQ‐9 immediately following pulmonary rehabilitation (1 study; n = 412). These benefits were maintained at three months' follow‐up for GAD‐7 (AMD −2.01, 95% CI −2.74 to −1.29; P < 0.001) and PHQ‐9 (AMD −1.96, 95% CI −2.7 to −1.23; P < 0.001).
Peripheral skeletal muscle force
Only one study reported measurement of peripheral muscle function (quadriceps muscle strength) (Majd 2020). Following the intervention, there was a mean difference between pulmonary rehabilitation and usual care groups of 22 (95% CI 4 to 40) Newtons (1 study; n = 30). The study was a feasibility study and was not powered to detect differences in the change of peripheral muscle skeletal force.
Levels of physical activity
One study reported physical activity levels (Turk 2020). There was no difference in daily step count (P = 0.100) or physical activity levels (P = 0.429) between pulmonary rehabilitation and usual care groups following the intervention (n = 24). Participants in the pulmonary rehabilitation group had a higher amount of daily steps compared to participants in the usual care group at 12 months' follow‐up (β coefficient = 3200, 95% CI 1256 to 5144; P = 0.005)
Inflammatory biomarkers: FeNO
Whilst inflammatory biomarkers of airway and systemic inflammation were reported in three studies (Majd 2020; Toennesen 2017; Turk 2020), meta‐analysis was not possible for any of these outcomes. There was no difference in FeNO levels following pulmonary rehabilitation between the pulmonary rehabilitation and usual care groups in the study by Toennesen 2017 (MD 6.7, 95% CI −7.22 to 20.62 parts per billion (ppb); 1 study; n = 63). FeNO levels appeared unchanged with pulmonary rehabilitation compared to usual care in the study by Majd 2020, with an MD of −4 (95% CI −14 to 5) ppb between groups following the 12‐week intervention. It was not clear in how many participants FeNO was measured following the intervention, therefore meta‐analysis was not possible. In Turk 2020 (n = 24), no differences were observed in FeNO levels following the intervention (median change −0.5 ppb in both groups; P = 0.113 between groups) or at 12 months' follow‐up (no data provided).
Inflammatory biomarkers: sputum eosinophils, sputum neutrophils
There was a small reduction in % sputum eosinophils (median (IQR) 7.8% (14.9)% pre‐intervention; 4.8% (13.1)% postintervention in the pulmonary rehabilitation group) in the study by Toennesen 2017; however, it did not reach statistical significance (44 participants). No differences were observed between pulmonary rehabilitation and usual care groups following the intervention for any sputum cell count outcomes. In Turk 2020, no differences were observed in % sputum neutrophils between the pulmonary rehabilitation and usual care groups following the intervention or at 12 months' follow‐up. There was no reduction in sputum eosinophil count after 12 weeks of pulmonary rehabilitation compared to usual care in the study by Majd 2020.
Inflammatory biomarkers: markers of systemic inflammation
There were no observed differences reported in blood eosinophils, serum levels of high‐sensitivity C‐reactive protein (hs‐CRP), and IL‐6 between the pulmonary rehabilitation and usual care groups following pulmonary rehabilitation in Toennesen 2017. No differences in CRP, blood eosinophils, or leucocytes were reported between the pulmonary rehabilitation and usual care groups immediately following pulmonary rehabilitation or at 12 months' follow‐up in Turk 2020.
Adverse events/side effects
Detailed data regarding adverse events were reported in one study only (Majd 2020). Thirteen serious adverse events were reported during the trial (11 related to asthma), and the proportion of adverse events in the intervention and usual care groups was similar. No adverse events were directly related to the intervention. Two serious adverse events were associated with CPET on a treadmill related to exercise‐induced bronchoconstriction. Data on adverse incidents in other studies were limited. Toennesen 2017 reported: “At no time point were the instructors in need to call for emergency assistance”, and Turk 2020 reported that the high‐intensity interval training modality employed was “generally ... well tolerated by the participants”, with muscle aches most frequently reported in the first weeks of training.
Sensitivity analyses
Orooj 2020 was the only study involving participants with ACO, contributing data to the outcomes of functional exercise tolerance (6‐minute walk distance) and quality of life (SGRQ). Exploratory sensitivity analyses related to disease type (asthma versus ACO) where this study was removed from all analyses revealed a negligible impact upon pooled effect estimates.
For analyses that demonstrated low statistical heterogeneity (I2), where a fixed‐effect model may have been justifiable, we compared these findings to our primary random‐effects model and found negligible differences in the magnitude, direction, or clinical interpretation of effect estimates.
Discussion
Summary of main results
This review included 10 studies comparing pulmonary rehabilitation to usual care in people with asthma, and was based on a published protocol (Osadnik 2019a). Pulmonary rehabilitation led to improvements in functional exercise capacity as assessed by the 6‐minute walk test distance (79.8 metres), which is a magnitude more than twice the minimally important difference (MCID) threshold for people with chronic respiratory disease (Holland 2014), and, more specifically, those with asthma (Zampogna 2021). Pulmonary rehabilitation also led to a small improvement in maximal exercise capacity as measured by % predicted VO2 max and VO2 peak, although the evidence is very uncertain in relation to VO2 peak. Determining the clinical relevance of these findings is challenging due to a lack of established MCID thresholds for these CPET‐derived physiological parameters. Insufficient data prevented us from accurately evaluate the longer‐term effects of pulmonary rehabilitation on functional and maximal exercise capacity in people with asthma.
The findings regarding the effect of pulmonary rehabilitation on asthma control and quality of life were positive, but more varied. Improvements in asthma control were noted on ACQ scores that were comparable to the MCID of 0.5 points (unsustained at 9‐ to 12‐month follow‐up) (Juniper 2005), but not on ACT scores. Large, clinically relevant improvements in quality of life that exceeded the MCID of 4 points were noted in SGRQ total scores immediately following pulmonary rehabilitation (sustained at long‐term follow‐up) (Jones 2005), but findings were more varied when evaluated via AQLQ or CRQ (across their respective domains). This large magnitude of benefit on quality of life appeared to be heavily influenced by the study of inpatient pulmonary rehabilitation by Schultz 2021, which is a less common model of rehabilitation in many countries.
The effect of pulmonary rehabilitation on asthma exacerbations was examined in a single study that demonstrated no difference between intervention and usual care groups during the intervention period (16.7% versus 55.6%, respectively), but higher rates of exacerbations in the usual care group one year later. Improvements in anxiety and depression following pulmonary rehabilitation were noted in one study and were maintained at three months' follow‐up. A single study demonstrated an improvement in quadriceps muscle strength following pulmonary rehabilitation. Physical activity was measured in one study only, with 12 months' follow‐up data demonstrating higher daily step counts in people who completed pulmonary rehabilitation compared to those who underwent usual care. Pulmonary rehabilitation appeared to have little to no effect on markers of airway and systemic inflammation based on data from three studies. No adverse effects directly related to pulmonary rehabilitation were reported.
Overall completeness and applicability of evidence
This review offers important insights regarding the extent of evidence that may underpin treatment decisions regarding pulmonary rehabilitation for adults affected by asthma. It yielded a modest evidence base underpinned by studies typically involving small sample sizes (only Nathell 2005 and Schultz 2021 included more than 100 participants, and of these two studies only Schultz 2021 contributed to meta‐analysis). Some important treatment effects on clinically relevant outcomes were observed; however, judicious interpretation and application of some findings appears indicated due to the heavy influence of the study by Schultz 2021, which involved inpatient pulmonary rehabilitation (which is not typical of many programme structures internationally). The review casts an important spotlight on overt inconsistencies between relevant studies regarding factors such as patient inclusion, programme duration, exercise components, priorities of education, instruments used to evaluate outcomes, and time points to conduct reassessments. This makes it challenging to draw firm conclusions to inform future clinical practice recommendations in many varied clinical settings, and reduces our confidence in the true observed treatment effects.
Overcoming this variability through standardisation procedures will prove to be important as the evidence matures, particularly given the dynamic clinical pulmonary rehabilitation landscape in recent times (including during the SARS‐CoV‐2 pandemic), which has witnessed rapid changes to the models of care offered to people. One programme was home based (Manzak 2020); however, most programmes were delivered in outpatient settings (two delivered in inpatient settings (Nathell 2005; Schultz 2021)), in accordance with typical clinical practice. This strong link to typical clinical care is a strength of the present review that has not been specifically addressed in other reviews (Carson 2013; Feng 2021). Pulmonary rehabilitation is not always considered an essential component of care for people with asthma, with guidelines recommending regular physical activity for general health benefits, rather than a supervised pulmonary rehabilitation programme (GINA 2021). It can be difficult to consider whether pulmonary rehabilitation is essential or desirable for people with asthma. Compared to people with other chronic lung diseases referred to pulmonary rehabilitation, people with asthma are often of younger age, and many maintain active employment. This makes attendance at 'traditional' centre‐based weekday programmes conducted during business hours difficult. Neither the ideal setting for delivery of self‐management education and skills training for people with asthma, nor the ideal composition of adjunct co‐interventions and relative contribution of such components to pulmonary rehabilitation outcomes, has been established.
Efforts to further define the optimal pulmonary rehabilitation structure and content for people with asthma may be required in order to advance knowledge in this important area. Only one study evaluated a pulmonary rehabilitation programme tailored specifically for the needs of people with asthma (Majd 2020). This is an interesting concept worthy of future attention, particularly for centres with sufficient patient numbers to allow such investigations. It is not, however, a model immune to logistical challenges associated with co‐ordinating separate disease‐specific programmes. Decision‐making regarding the value of such adaptations is likely to involve careful consideration of the expected benefits of a tailored approach (e.g. magnitude of responses on physiological, functional, and behavioural outcomes) against such pragmatic issues. The future design of interventions such as pulmonary rehabilitation that involve behavioural change and exercise are likely to benefit from input from the consumer (i.e. patient) perspective and qualitative research methodologies. An example of this being performed occurred in an RCT evaluating a yoga and mindfulness intervention in people with severe asthma (Hiles 2021). Qualitative interviews discussing barriers and facilitators to performing the twice‐weekly 12‐week programme highlighted that participants valued the social connections facilitated through the group setting, and enjoyed the asthma‐specific focus of the group, which allowed them to connect with a like‐minded community. However, they noted class scheduling during working hours was problematic.
The overall evidence base in asthma differs quite markedly to the consistent findings observed in larger bodies of evidence, such as that pertaining to people with COPD (McCarthy 2015). Whilst differences exist in the disease processes, pathophysiology, exposures, and lifestyle habits of people affected by asthma compared to those with COPD, several similarities also exist, including a known degree of overlap between the two conditions (asthma‐COPD overlap, ACO). We do not suspect that potential differences in treatment responses for some outcomes between asthma and COPD are likely to be explained by altered physiological adaptations in responses to an exercise stimulus, particularly within skeletal muscles. It is, however, possible that people affected by asthma have differing abilities to perform pulmonary rehabilitation components which may have some consequent impact on outcomes. For example, concerns regarding exercise‐induced bronchoconstriction may result in individuals with asthma performing at lower intensity levels than people with less risk of such adverse effects, thereby impacting apparent effectiveness. The lack of maintenance of treatment effects up to long‐term evaluations was one feature highly consistent with observations in other diseases such as COPD.
Obesity is a common extrapulmonary trait with a high prevalence in asthma (McDonald 2019), and is associated with poor outcome (McLoughlin 2021). A cluster analysis of extrapulmonary traits in severe asthma also indicates that the traits of obesity, physical inactivity, and anxiety and depression cluster together, and it is this cluster that is associated with the poorest outcomes compared to other clusters (Freitas 2021). This may assist in identifying an important asthma phenotype that may do well with a multicomponent intervention like pulmonary rehabilitation. The studies included in this systematic review did not explicitly aim to address this issue, and we propose this as an area of future research in asthma pulmonary rehabilitation. In COPD, whilst pulmonary rehabilitation is not specifically designed to target obesity, beneficial effects on body weight have been reported (Camillo 2015). Interventions in asthma that have combined a dietary weight loss intervention with exercise, compared to diet and a sham intervention, demonstrated greater weight loss in the diet and exercise group, as well as improvements in asthma outcomes (Freitas 2017), suggesting this may be an important treatment target that could be embedded in asthma pulmonary rehabilitation. Rehabilitation studies such as Freitas 2017 have also been conducted involving people with poorly controlled asthma and obesity and have shown promising findings. These were not included in this review, as they did not meet our definition of pulmonary rehabilitation. It is interesting to consider this patient subgroup, as they may be a group deemed highly likely to succeed with a multicomponent rehabilitation intervention (considering they may have the greatest room for improvement).
We planned to compare responses to treatment for outcomes of functional exercise capacity (6‐minute walk distance) and asthma control (ACQ score) between programmes of less than or greater than eight weeks' duration and those involving people with milder or more severe asthma disease. Subgroup differences were detected in favour of shorter programmes involving milder disease; however, we urge caution in interpreting this finding. This observation was unexpected, and differs to trends observed in diseases like COPD. These effects were likely driven by the study of Schultz 2021, which involved a high‐frequency but 'shorter' duration inpatient form of pulmonary rehabilitation, which is common in clinical practice in Germany. This study was considerably larger than all of the other studies, thus conferring significant weighting to any analyses. We were also interested in evaluating the potential impact of ACO on review findings; however, only one study involved such participants (Orooj 2020), thus limiting our ability to examine this important issue.
There was a small volume of data related to a number of secondary outcomes for this review, namely exacerbation rate, mental health, inflammatory biomarkers, peripheral muscle strength, and physical activity levels. Whilst pulmonary rehabilitation confers significant, clinically relevant benefits on anxiety and depression symptoms in people with COPD (Gordon 2019), only two studies included in this review examined mental health outcomes, demonstrating a need for additional research regarding the effectiveness of pulmonary rehabilitation in addressing this outcome in people with asthma. There is evidence that physical training may reduce airway inflammation in people with asthma (França‐Pinto 2015; Freitas 2017). Whilst inflammatory biomarkers of airway and systemic inflammation were reported in three included studies (Majd 2020; Toennesen 2017; Turk 2020), meta‐analysis was not possible for any of these outcomes. Only one study measured peripheral muscle strength, which was not adequately powered to detect changes in this outcome, and only one study measured physical activity levels despite strong clinical interest in this particular field. This lack of evidence does not equate to evidence of a lack of effect. Many factors likely contribute to the behaviour of physical activity, with data in COPD demonstrating inconsistent effects following pulmonary rehabilitation (Burge 2020; Ng 2011), and factors such as baseline exercise capacity identified as potential contributors to such observed heterogeneity of responses (Osadnik 2018). People with asthma may develop physical inactivity for reasons different to those with COPD (e.g. less likely attributable to skeletal muscle dysfunction and dyspnoea‐induced functional limitations), and they often present to pulmonary rehabilitation with better preservation of functional exercise tolerance. Further enquiry to examine whether this may result in differential responses to pulmonary rehabilitation appears indicated.
Quality of the evidence
This review had several sources of bias. One study was available in abstract form only, limiting the ability to adequately assess all risk of bias domains (Manzak 2020). Data that could be pooled for meta‐analysis ranged from two to five studies; however, most meta‐analyses were limited to two studies. Selection bias may have been present in five studies due to unclear reporting (Cambach 1997; Cochrane 1990; Foglio 2001; Manzak 2020; Orooj 2020). Given the physical nature of the intervention, it was assumed that no participants were blinded. We attributed high risk of bias to all studies using self‐reported outcome measures or a test of exercise capacity. Three studies reported blinding of the outcome assessor (Foglio 2001; Majd 2020; Toennesen 2017). Blinding of outcome assessors was not applied in studies by Schultz 2021 and Turk 2020. Three studies reported use of an ITT analysis (Nathell 2005; Schultz 2021; Turk 2020). Two studies reported that all participants completed the study and were included in the analysis (Cochrane 1990; Orooj 2020). Four full‐text studies had a published protocol or were listed in a trial registry, or both, and were deemed at low risk of reporting bias (Majd 2020; Schultz 2021; Toennesen 2017; Turk 2020).
We rated the overall certainty of evidence according to GRADE methodology as very low to moderate for different measures of exercise capacity; very low to low for different measures of asthma control; and very low to moderate for different measures of quality of life. This was mostly attributed to increased risk of bias due to lack of blinding of outcome measurement, imprecision, and inconsistency. Indirectness was observed once.
Potential biases in the review process
This review included a diverse array of studies involving differing interventions, co‐interventions, and time points for data measurement. We included studies published in abstract form, despite their obvious limitations in terms of the detail available to critique, which potentially affected bias ratings. In order to reduce this risk we attempted to source additional information from six study authors, with four providing additional information to clarify study characteristics or additional data, or both. This helped to refine the accuracy of our judgements, but may have given rise to apparent discrepancies in study characteristics between those offered by any other relevant review authors.
Agreements and disagreements with other studies or reviews
The current review pertains to a topic similar to a prior Cochrane Review 'Physical training in people with asthma' (Carson 2013); however, there are also notable differences. Firstly, we restricted eligibility criteria specifically to studies involving adult samples, unlike the Carson review, which included children. This was important, as children are very rarely referred to pulmonary rehabilitation programmes. The prior review also examined 'physical training', which is quite a liberal term relating to any aspects of physical activity involving structured and repetitive training components aimed at improving health. The current review focused only on interventions involving a minimum of two different components (i.e. physical exercise and education) in order to more accurately represent the nature of modern pulmonary rehabilitation programmes. This latter point is consistent with prior reviews involving pulmonary rehabilitation interventions (McCarthy 2015). It is also a point of distinction between the current review and a recently published systematic review 'Effects of exercise‐based pulmonary rehabilitation on adults with asthma' (Feng 2021), which included nine studies, of which only three were included in the current review (Cambach 1997; Cochrane 1990; Toennesen 2017). The main findings between the two reviews were fundamentally similar, with some differences noted regarding GRADE judgements and magnitudes of treatment effects.
Authors' conclusions
Implications for practice.
The evidence from this review suggests that pulmonary rehabilitation is likely to confer some benefits for people with asthma on outcomes such as exercise tolerance and quality of life. However, the precise magnitude of effect and resultant clinical relevance of such impact is difficult to ascertain. The effect of pulmonary rehabilitation on outcomes such as physical activity levels, inflammatory biomarkers, and mental health is not yet clear. We did not find sufficient data to formulate clear conclusions regarding the impact of pulmonary rehabilitation on adverse events for adults with asthma; however, findings from one study, Majd 2020, suggest close monitoring for symptoms of exercise‐induced bronchoconstriction may be indicated for patients completing maximal incremental treadmill tests. Judicious interpretation and application of findings is warranted in light of findings that were heavily influenced by the single large study of Schultz 2021, which involved inpatient pulmonary rehabilitation (which is not the typical model of care in many countries). As further research appears likely to influence review findings, there may be some challenges for healthcare policymakers in articulating the role of pulmonary rehabilitation for adults with asthma based on the current review findings.
Implications for research.
The modest amount of evidence synthesised in this review means that many opportunities exist to refine our understanding of the impact of pulmonary rehabilitation for adults with asthma. This is a notable distinction from the evidence base that currently exists for people with chronic obstructive pulmonary disease (COPD). For outcomes where we found data from multiple studies (e.g. exercise tolerance and asthma control), further research would assist in refining effect estimates and improving the certainty of the evidence. For outcomes where we found little or no data (e.g. inflammatory biomarkers, muscle strength, physical activity levels), further research would clearly be of benefit to guide future clinician judgement regarding the expected effects of treatment.
Whilst not the focus of this review, the heterogeneity of intervention components and programme types (e.g. asthma‐tailored versus conventional models) highlights the need for further work to refine the best model of pulmonary rehabilitation for people with asthma. This may not necessitate randomised controlled intervention trials; rather, there could be a valuable role to explore the consumer (i.e. patient) voice in designing future trials utilising qualitative enquiry and principles of co‐design.
The absence of robust comparisons between subgroups and sensitivity analyses planned in this review highlights an ongoing need for future large‐scale, definitive research involving outpatient pulmonary rehabilitation for adults with asthma in order to inform good health policy and practice. Such studies should ideally involve adequate sample sizes powered to detect changes in clinically important outcomes such as the primary outcomes used in this review. They should also involve careful consideration of important clinical features such as patient age, asthma severity, underlying clinical phenotypes (including distinguishing asthma from asthma‐COPD overlap (ACO)), and emerging pharmacological co‐interventions such as biologic therapies.
History
Protocol first published: Issue 11, 2019
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
The authors and Cochrane Airways editorial team are grateful to Candida Fenton (Cochrane Vascular, University of Edinburgh, UK) for peer‐reviewing the search strategy and to the following peer and consumer reviewers: Hilary Pinnock (UK), Dulce Estevao (Portugal), and Bernard McCarthy (Ireland) for their valuable comments on the review.
This project was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. Database search strategies
| Database/search platform/date of last search | Search strategy | Results (n) |
| Airways Register (via Cochrane Register of Studies) Date of most recent search: 11 May 2021 | 1 AST:MISC1 AND INSEGMENT 2 MeSH DESCRIPTOR Asthma Explode All AND INSEGMENT 3 asthma*:ti,ab AND INSEGMENT 4 #1 or #2 or #3 AND INSEGMENT 5 MESH DESCRIPTOR Physical Therapy Modalities EXPLODE ALL AND INSEGMENT 6 MESH DESCRIPTOR Physical Fitness EXPLODE ALL AND INSEGMENT 7 MESH DESCRIPTOR Physical Endurance EXPLODE ALL AND INSEGMENT 8 MESH DESCRIPTOR Rehabilitation AND INSEGMENT 9 MESH DESCRIPTOR Exercise Therapy EXPLODE ALL AND INSEGMENT 10 MESH DESCRIPTOR Physical Exertion AND INSEGMENT 11 MESH DESCRIPTOR Exercise Test EXPLODE ALL AND INSEGMENT 12 MESH DESCRIPTOR Exercise EXPLODE ALL AND INSEGMENT 13 ((pulmonary* or respiratory*) NEAR3 rehabilitation*):ti,ab AND INSEGMENT 14 exercis*:ti,ab AND INSEGMENT 15 (physical* NEAR3 (activit* or train* or fitness* or therap*)):ti,ab,kw AND INSEGMENT 16 interval train* AND INSEGMENT 17 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 AND INSEGMENT 18 #17 AND #4 AND INSEGMENT 19 INREGISTER 20 #18 AND #19 |
|
| CENTRAL (via Cochrane Register of Studies) Date of most recent search: 11 May 2021 | #1 AST:MISC1 AND CENTRAL:TARGET #2 MeSH DESCRIPTOR Asthma Explode All AND CENTRAL:TARGET #3 asthma*:ti,ab AND CENTRAL:TARGET #4 #1 or #2 or #3 AND CENTRAL:TARGET #5 MESH DESCRIPTOR Physical Therapy Modalities EXPLODE ALL AND CENTRAL:TARGET #6 MESH DESCRIPTOR Physical Fitness EXPLODE ALL AND CENTRAL:TARGET #7 MESH DESCRIPTOR Physical Endurance EXPLODE ALL AND CENTRAL:TARGET #8 MESH DESCRIPTOR Rehabilitation AND CENTRAL:TARGET #9 MESH DESCRIPTOR Exercise Therapy EXPLODE ALL AND CENTRAL:TARGET #10 MESH DESCRIPTOR Physical Exertion AND CENTRAL:TARGET #11 MESH DESCRIPTOR Exercise Test EXPLODE ALL AND CENTRAL:TARGET #12 MESH DESCRIPTOR Exercise EXPLODE ALL AND CENTRAL:TARGET #13 ((pulmonary* or respiratory*) NEAR3 rehabilitation*):ti,ab AND CENTRAL:TARGET #14 exercis*:ti,ab AND CENTRAL:TARGET #15 (physical* NEAR3 (activit* or train* or fitness* or therap*)):ti,ab,kw AND CENTRAL:TARGET #16 interval train* AND CENTRAL:TARGET #17 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 AND CENTRAL:TARGET #18 #17 AND #4 AND CENTRAL:TARGET #19 CENTRAL:TARGET #20 #18 AND #19 AND CENTRAL:TARGET |
|
| MEDLINE (Ovid) ALL Date of most recent search: 11 May 2021 | 1 exp Asthma/ 2 asthma$.ti,ab. 3 1 or 2 4 Physical Therapy Modalities/ 5 exp Physical Fitness/ 6 exp Physical endurance/ 7 exp Exercise Therapy/ 8 Physical Exertion/ 9 exp Exercise Test/ 10 exp Exercise/ 11 ((pulmonary or respiratory) adj3 rehabilitation$).ti,ab. 12 exercis$.ti,ab. 13 (physical$ adj3 (activit$ or train$ or fitness$ or therap$)).ti,ab. 14 interval train$.ti,ab. 15 or/4‐14 16 3 and 15 17 (controlled clinical trial or randomized controlled trial).pt. 18 (randomized or randomised).ab,ti. 19 placebo.ab,ti. 20 randomly.ab,ti. 21 trial.ab,ti. 22 groups.ab,ti. 23 or/17‐22 24 Animals/ 25 Humans/ 26 24 not (24 and 25) 27 23 not 26 28 16 and 27 |
|
| Embase (Ovid) Date of most recent search: 11 May 2021 | 1 exp asthma/ 2 asthma$.ti,ab. 3 1 or 2 4 exp physiotherapy/ 5 fitness/ 6 endurance/ 7 exp kinesiotherapy/ 8 exp exercise/ 9 exp exercise test/ 10 ((pulmonary or respiratory) adj3 rehabilitation$).ti,ab. 11 exercis$.ti,ab. 12 (physical$ adj3 (activit$ or train$ or fitness$ or therap$)).ti,ab. 13 interval train$.ti,ab. 14 or/4‐13 15 3 and 14 16 Randomized Controlled Trial/ 17 randomization/ 18 controlled clinical trial/ 19 Double Blind Procedure/ 20 Single Blind Procedure/ 21 Crossover Procedure/ 22 (clinica$ adj3 trial$).tw. 23 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 24 exp Placebo/ 25 placebo$.ti,ab. 26 random$.ti,ab. 27 ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 28 (crossover$ or cross‐over$).ti,ab. 29 or/16‐28 30 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 31 human/ or normal human/ or human cell/ 32 30 and 31 33 30 not 32 34 29 not 33 35 15 and 34 |
|
|
PEDro Date of most recent search: 11 May 2021 |
Title & abstract: asthma pulmonary rehabilitation Methods: clinical trial When searching: Match all terms (AND) |
|
| ClinicalTrials.gov Date of most recent search: 11 May 2021 | Study type: Interventional
Condition: asthma Intervention: pulmonary rehabilitation OR physiotherapy OR exercise |
|
| WHO trials portal Date of most recent search: 11 May 2021 | Condition: asthma Intervention: pulmonary rehabilitation OR physiotherapy OR exercise |
|
Data and analyses
Comparison 1. Pulmonary rehabilitation vs usual care for adults with asthma (end‐intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Exercise performance: % predicted VO2 max on incremental cardiopulmonary exercise test at end‐intervention | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 14.88 [9.66, 20.10] |
| 1.2 Exercise performance: Peak oxygen uptake (VO2 peak) on incremental cardiopulmonary exercise test at end‐intervention | 3 | 129 | Mean Difference (IV, Random, 95% CI) | 3.63 [1.48, 5.77] |
| 1.3 Exercise performance: 6‐minute walk test distance at end‐intervention | 5 | 529 | Mean Difference (IV, Random, 95% CI) | 79.79 [66.47, 93.11] |
| 1.4 Exercise performance: 6‐minute walk test distance at end‐intervention (Subgroup: duration of pulmonary rehabilitation) | 5 | 529 | Mean Difference (IV, Random, 95% CI) | 79.79 [66.47, 93.11] |
| 1.4.1 ≤ 8 weeks | 3 | 470 | Mean Difference (IV, Random, 95% CI) | 84.00 [69.18, 98.82] |
| 1.4.2 > 8 weeks | 2 | 59 | Mean Difference (IV, Random, 95% CI) | 62.07 [31.67, 92.47] |
| 1.5 Asthma control: Asthma Control Questionnaire score at end‐intervention | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.76, ‐0.17] |
| 1.6 Asthma control: Asthma Control Questionnaire score at end‐intervention (Subgroup: duration of pulmonary rehabilitation) | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.76, ‐0.17] |
| 1.6.1 ≤ 8 weeks | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.87, ‐0.13] |
| 1.6.2 > 8 weeks | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.90, 0.10] |
| 1.7 Asthma control: Asthma Control Questionnaire score at end‐intervention (Subgroup: asthma severity) | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.76, ‐0.17] |
| 1.7.1 Not severe | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.87, ‐0.13] |
| 1.7.2 Severe | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.90, 0.10] |
| 1.8 Asthma control: Asthma Control Test score at end‐intervention | 2 | 442 | Mean Difference (IV, Random, 95% CI) | 3.34 [‐2.32, 9.01] |
| 1.9 Health‐related quality of life: Asthma Quality of Life Questionnaire total score at end‐intervention | 2 | 442 | Mean Difference (IV, Random, 95% CI) | 0.87 [‐0.13, 1.86] |
| 1.10 Health‐related quality of life: Asthma Quality of Life Questionnaire domain scores at end‐intervention | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 Activity domain | 2 | 442 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.26, 1.85] |
| 1.10.2 Emotional Function domain | 2 | 442 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐0.35, 1.79] |
| 1.10.3 Environmental domain | 2 | 442 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.86, 2.17] |
| 1.10.4 Symptoms domain | 2 | 442 | Mean Difference (IV, Random, 95% CI) | 0.68 [‐0.80, 2.16] |
| 1.11 Health‐related quality of life: Asthma Quality of Life Questionnaire total score at end‐intervention (Subgroup: duration of pulmonary rehabilitation) | 2 | 442 | Mean Difference (IV, Random, 95% CI) | 0.87 [‐0.13, 1.86] |
| 1.11.1 ≤ 8 weeks | 1 | 412 | Mean Difference (IV, Random, 95% CI) | 1.32 [1.18, 1.46] |
| 1.11.2 > 8 weeks | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.38, 0.98] |
| 1.12 Health‐related quality of life: Asthma Quality of Life Questionnaire total score at end‐intervention (Subgroup: asthma severity) | 2 | 442 | Mean Difference (IV, Random, 95% CI) | 0.87 [‐0.13, 1.86] |
| 1.12.1 Not severe | 1 | 412 | Mean Difference (IV, Random, 95% CI) | 1.32 [1.18, 1.46] |
| 1.12.2 Severe | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.38, 0.98] |
| 1.13 Health‐related quality of life: St George's Respiratory Questionnaire total score at end‐intervention | 2 | 440 | Mean Difference (IV, Random, 95% CI) | ‐18.51 [‐20.77, ‐16.25] |
| 1.14 Health‐related quality of life: St George's Respiratory Questionnaire domain scores at end‐intervention | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.14.1 Symptoms domain | 2 | 440 | Mean Difference (IV, Random, 95% CI) | ‐20.50 [‐23.78, ‐17.22] |
| 1.14.2 Activity domain | 2 | 440 | Mean Difference (IV, Random, 95% CI) | ‐18.29 [‐21.19, ‐15.39] |
| 1.14.3 Impacts domain | 2 | 440 | Mean Difference (IV, Random, 95% CI) | ‐18.33 [‐20.86, ‐15.80] |
| 1.15 Health‐related quality of life: Chronic Respiratory Disease Questionnaire domain scores at end‐intervention | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.15.1 Dyspn o ea domain | 2 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 1.01 [0.50, 1.52] |
| 1.15.2 Emotional function domain | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.66, 1.52] |
| 1.15.3 Fatigue domain | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | 0.85 [0.35, 1.34] |
| 1.15.4 Mastery domain | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.02, 1.16] |
Comparison 2. Pulmonary rehabilitation vs usual care for adults with asthma (follow‐up).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Exercise performance: Peak oxygen uptake (VO2 peak) on incremental cardiopulmonary exercise test at follow‐up | 3 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐4.79, 3.42] |
| 2.2 Exercise performance: 6‐minute walk test distance at follow‐up | 2 | 42 | Mean Difference (IV, Random, 95% CI) | 52.29 [0.70, 103.88] |
| 2.3 Asthma control: Asthma Control Questionnaire score at follow‐up | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.35, 0.53] |
| 2.4 Health‐related quality of life: Asthma Quality of Life Questionnaire total score at follow‐up | 2 | 435 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐0.23, 1.38] |
| 2.5 Health‐related quality of life: Asthma Quality of Life Questionnaire domain scores at follow‐up | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 Activity domain | 2 | 435 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐2.02, 1.97] |
| 2.5.2 Emotional function domain | 2 | 435 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐2.42, 2.00] |
| 2.5.3 Environmental domain | 2 | 435 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐2.23, 1.80] |
| 2.5.4 Symptoms domain | 2 | 435 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.78, 1.77] |
| 2.6 Health‐related quality of life: St George's Respiratory Questionnaire total score at follow‐up | 2 | 430 | Mean Difference (IV, Fixed, 95% CI) | ‐13.40 [‐15.93, ‐10.88] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cambach 1997.
| Study characteristics | ||
| Methods |
Study design: Randomised cross‐over trial Total duration of study: 6 months Details of any run‐in period: None stated Number of study centres and location: Multicentre study. The Netherlands Study setting: Community‐based local physiotherapy practices Withdrawals: Overall 99 participants were randomised (including asthma and COPD). 46 randomised to intervention group (9 participants withdrew). 43 randomised to control group (14 participants withdrew). Not clear how many of the participants who withdrew had asthma diagnosis Date of study: June 1992 to July 1994 |
|
| Participants |
Number recruited: 89 participants with a diagnosis of asthma or COPD randomly assigned to intervention (n = 46) or control (n = 43) group. The number of participants with an asthma diagnosis who were randomly assigned to each group is not stated. Number completed: Intervention group (22). Control group (21) (asthma participants only) Mean (SD) age: Intervention group 40 (10) years. Control group 53 (15) years (asthma participants only) Age range: Not stated for asthma participants only Gender (M/F): Intervention group 4/18. Control group 7/14 (asthma participants only) Mean (SD) BMI: Not stated for asthma participants only Severity of condition: Not stated Diagnostic criteria: Complaints of dyspnoea occurring periodically with varying severity, at the present time or in the past, as well as an increase in FEV1 of at least 15% postbronchodilator, or a histamine provocation test producing a 20% fall in FEV1 (PC20) of < 8 mg/mL Baseline lung function: Mean (SD) % predicted FEV1 for asthma participants only: Intervention group 89 (17)%. Control group 84 (20)% Smoking history: Not stated for asthma participants only Asthma treatment: Not stated Inclusion criteria: Evidence of dyspnoea and decreased exercise tolerance due to obstructive lung disease. Age 18 to 75 years. Ability to travel independently to physiotherapy practice. Medication prescribed by a pulmonary physician. No manifest cardiac complaints or locomotor disabilities. Absence of hypercapnia or hypoxia, or both, during rest or maximal exercise testing. Motivation to self‐care. Informed consent Exclusion criteria: Hypoxaemia. Not meeting diagnostic criterion of “obstructive lung disease” |
|
| Interventions |
Intervention: 3‐month community‐based PRP consisting of upper and lower limb exercise training x 3 days per week x 90 minutes and education delivered by physiotherapists and district nurses Comparison: Medication alone Concomitant medications: Not stated Excluded medications: Not stated |
|
| Outcomes |
Primary outcomes: Exercise capacity (endurance cycle ergometer test, submaximal cycle ergometer test, 6MWD). Quality of life (CRQ) Secondary outcomes: None stated Time points reported: Baseline, 3 months, 6 months. 6‐month data not included in review, as cross‐over had occurred. Data reported as change from baseline. |
|
| Notes |
Funding: National Health Insurance Council subsidised and supported the study. Notable conflicts of interest: None stated Other: Data for asthma participants only at baseline and 3 months only before cross‐over occurred are included in this review. The number of participants analysed in each arm varied slightly according to outcome measure. Data from participants who did not return for 1 or more of the assessments or participants who were not measured within 3 weeks from baseline or programme completion were excluded from data analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear how the sequence was generated. “Within each physiotherapy practice, four out of eight patients were randomly allocated to group RC, and four patients to group CR (block randomization procedure; four closed envelopes for condition RC and four closed envelopes for condition CR).” |
| Allocation concealment (selection bias) | Unclear risk | Not clear how they decided which envelope to open, or whether envelopes were labelled. “Within each physiotherapy practice, four out of eight patients were randomly allocated to group RC, and four patients to group CR (block randomization procedure; four closed envelopes for condition RC and four closed envelopes for condition CR).” |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | Rehab programme‐ not able to blind participants or personnel delivering it. |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | Rehab programme ‐ not able to blind participants or personnel delivering it. |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | Unclear risk | No mention of blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | Unclear risk | No mention of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 99 patients recruited but data only reported for 66. No ITT data reported. “Data obtained from patients who did not return for one or more of the assessments (i.e. baseline (T0), after 3 months (T3) and/or after 6 months (T6)), or patients who were not measured within 3 weeks (from T0, T3 and T6) were excluded from data analysis.” |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No other suggestions of bias evident. |
Cochrane 1990.
| Study characteristics | ||
| Methods |
Study design: RCT Total duration of study: 3 months Details of any run‐in period: 6 week run‐in period Number of study centres and location: Single site. Scotland. Study setting: Hospital Withdrawals: None documented Date of study: Not stated |
|
| Participants |
Number recruited: Intervention group (18) Control group (18) Number completed: Intervention group (18) Control group (18) Mean (SD) age: Intervention group 27 (7) years. Control group 28 (8) years Age range: 16 to 40 years Gender (M/F): 14 male and 22 female participants Mean (SD) BMI: Not stated Severity of condition: Mild to moderate asthma Diagnostic criteria: Mild to moderate asthma was defined by a requirement for regular prophylactic treatment and reproducible airways obstruction when treatment was withdrawn. Baseline lung function: Mean (SD) % predicted postbronchodilator FEV1 Intervention Group 85 (16)% Control Group 89 (14)% Smoking history: All participants were non‐smokers. Asthma treatment: All participants were taking aerosolised sympathomimetic agents. 15 had also been prescribed inhaled sodium cromoglycate and 21 corticosteroid preparations for inhalation. 2 participants were dependent on long‐term oral steroids. Inclusion criteria: Not stated Exclusion criteria: Not stated |
|
| Interventions |
Intervention: 3‐month medically supervised indoor aerobic training programme (30 min x 3 days per week aerobic training at 75% predicted maximum HR). Educational sessions designed to encourage a greater understanding and to improve self‐management of asthma as well as description of the training programme principles Comparison: Attendance at education sessions designed to encourage a greater understanding and to improve self‐management of asthma. Concomitant medications: All participants were taking aerosolised sympathomimetic agents. 15 had also been prescribed inhaled sodium cromoglycate and 21 corticosteroid preparations for inhalation. 2 participants were dependent on long‐term oral steroids. Excluded medications: None stated |
|
| Outcomes |
Primary outcomes: No prespecified primary outcome, but the following were measured: Anthropometric characteristics. Spirometry. Provocative concentration of histamine causing a 20% fall in FEV1. Blood lipid profile. Incremental CPET (cycle ergometer): Oxygen consumption (VO2), Oxygen pulse, Breathlessness score (Borg), Blood lactate, Minute ventilation during submaximal exercise, Carbon dioxide production (VCO2), Dyspnoea index, Ventilatory anaerobic threshold. Secondary outcomes: No prespecified secondary outcomes Time points reported: Baseline and 3 months Data reported as endpoint rather than as change from baseline. |
|
| Notes |
Funding: Allen and Hanburys Ltd provided a clinical research fellowship for the first author. The Chest, Heart and Stroke Association (Scottish branch) funded the training programme. Notable conflicts of interest: None stated Other: All participants were “free from any concomitant illness”. Alteration in treatment: During the study period, 9 of the 36 participants (6 from intervention group and 3 from control group) had their treatment altered. 7 were changed from inhaled sodium cromoglycate to an inhaled steroid, and 2 participants had the dose of inhaled steroid increased. Number of training sessions: The mean number (range) of training sessions undertaken by participants in the intervention group was 36 (19 to 42) (22 (8 to 42) hospital sessions and 14 (0 to 36) home sessions). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified. “The patients were then randomly allocated to either the training or the control group” |
| Allocation concealment (selection bias) | Unclear risk | Not stated whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | No mention of blinding. |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | Unclear risk | No mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Protocol presumably not published, given the age of the study. |
| Other bias | Unclear risk | “During the study period, nine of the 36 study subjects (six of those undergoing training and three of the control subjects) had their treatment altered.” |
Foglio 2001.
| Study characteristics | ||
| Methods |
Study design: RCT. 1 year after completing an original PRP (Time point T2), participants with COPD and asthma were randomised to either Intervention Group (Repeat the PRP (termed PRP2)) or to Control Group (Do not repeat PRP). At the end of PRP2, participants in the intervention group only were assessed (Time point T3). 1 year after completing PRP2, participants in both intervention and control groups were assessed (Time point T4). Total duration of study: 1 year Details of any run‐in period: Participants were stable as assessed by stability in blood gas values and free from exacerbations in the 4 weeks prior to entry into the study. No changes made to routine therapy in the week preceding inclusion into the study. Number of study centres and location: Single site. Italy Study setting: Day hospital Withdrawals: 11 participants in intervention group and 10 participants in control group did not perform evaluations at 1 year (T4) (personal/transport/family reasons). 2 participants in intervention group and 2 participants in control group were excluded due to other pathologies. Not clear how many of these withdrawn participants had asthma diagnosis Date of study: Not stated |
|
| Participants |
Number recruited: Number of participants with asthma randomly assigned at T2 to intervention group 14, control group 17. Number completed: Data not available from information provided by authors regarding how many participants with asthma randomised at T2 completed PRP2 Mean (SD) age: Intervention group 58.8 (6.4) years, Control group 58.1 (7.6) years. (Data only available for the age of participants with asthma who had complete dataset at T2 and T4.) Age range: Intervention group 50 to 68 years, Control group 49 to 71 years. (Data only available for the age of participants with asthma who had complete dataset at T2 and T4.) Gender (M/F): Intervention group 3/5. Control group 3/7. (Data only available for the gender of participants with asthma who had complete dataset at T2 and T4.) Mean (SD) BMI: Not stated Severity of condition: Not stated Diagnostic criteria: “Asthma was characterised by dyspnoea with wheezing, variable airflow limitation with reversible obstruction and bronchial hyperresponsiveness in absence of smoking history.” Baseline lung function: Mean (SD) % predicted FEV1 asthma participants only: Intervention group 73.25 (17.28)%, Control group 92.4 (22.33)% Smoking history: Not stated Asthma treatment: All participants with asthma received inhaled steroids and bronchodilators. Inclusion criteria: Not specifically stated Exclusion criteria: Other organ failure or cancer. Unable to co‐operate |
|
| Interventions |
Intervention: Multidisciplinary outpatient 8‐week PRP which included optimisation of pharmacologic treatment, supervised exercise training (aerobic and resistance training), patient and family education, nutritional programme and psychological counselling when appropriate Comparison: Usual care Concomitant medications: All participants with asthma received inhaled steroids and bronchodilators. Excluded medications: None stated |
|
| Outcomes |
Primary outcomes: No specified primary outcomes. The following were measured: Exercise capacity (incremental CPET on cycle ergometer and 6MWD). Dyspnoea (BDI and TDI). Quality of life (SGRQ). Exacerbations. Hospitalisations. Mortality rate. Lung volumes and FRC. Arterial blood gas levels. Maximal inspiratory pressure. Secondary outcomes: No specified secondary outcomes Time points reported: Baseline (T2), completion of pulmonary rehabilitation (T3 ‐ intervention group only) and 1 year (T4) Data reported as endpoint rather than as change from baseline. |
|
| Notes |
Funding: None stated Notable conflicts of interest: None stated Other: Only data at T2 and T4 for asthma participants were eligible for the review. 1 of the authors (L Bianchi) provided us with all available raw data for all participants through email correspondence. Data for 6MWD represented as change from baseline, and corrected for error in original publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail provided on how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | Rehab programme‐ not able to blind participants or personnel delivering it. |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | Rehab programme‐ not able to blind participants or personnel delivering it. |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | Unclear risk | Not clear who collected questionnaire data. |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | Low risk | “The technicians who collected data were blinded to a patient’s allocation to PRP2 or the control group.” “All measurements were performed and recorded under the supervision of a nurse not involved in the study.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High numbers of dropouts and no ITT. “Eleven patients in group 1 and 10 patients in group 2 did not perform evaluations at T4 due to personal, transport, or familial problems.” |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Baseline imbalance in 6MWD at baseline between groups. Participants had already completed pulmonary rehabilitation a year previously, not clear how this affects generalisability |
Majd 2020.
| Study characteristics | ||
| Methods |
Study design: RCT. Participants were randomised 2:1 to asthma‐tailored pulmonary rehabilitation or usual care. Total duration of study: 12‐week intervention with follow‐up at 6 months Details of any run‐in period: Not stated Number of study centres and location: Single site. UK Study setting: Hospital Withdrawals: Retention rates were 62% for intervention group and 53% for control group. Date of study: Not stated |
|
| Participants |
Number recruited: 61 recruited. 51 randomised Number completed: Intervention group (21). Control group (9) Mean (SD) age: Data for all participants combined at baseline are presented. Mean (SD) age: 54 (14) years Age range: Not stated Gender (M/F): Data for all participants are combined. Total M/F: 13/38 Mean (SD) BMI: Data for all participants are combined. Mean (SD) BMI: 32 (6) kg/m2 Severity of condition: Severe asthma Diagnostic criteria: Not stated Baseline lung function: Data for all participants combined at baseline are presented. Mean (SD) FEV1: 1.95 (0.74) L. Mean (SD) FEV1/FVC: 69 (11)% Smoking history: Not stated Asthma treatment: Not stated. Inclusion criteria included “symptomatic asthma despite being on step 4–5 treatment of the SIGN/BTS guidelines (high‐dose inhaled corticosteroids (>1000 μg beclomethasone equivalent) plus a second controller and/or systemic corticosteroids”. Inclusion criteria: Symptomatic asthma despite being on step 4 to 5 treatment according to SIGN/BTS guidelines. Under the care of a difficult‐to‐treat asthma specialist for at least 6 months Exclusion criteria: Diagnosis of smoking‐related COPD. Having both fixed airflow obstruction (FEV1/FVC < 70%) and a smoking history of ≥ 10 pack years. Unable to exercise e.g. due to significant musculoskeletal or neurological abnormality. History of significant cardiac disease. Severe exacerbation of asthma in the preceding month prior to entry to the programme. Hospital admission due to an exacerbation of asthma within the last 3 months. An ITU admission involving intubation within the last year |
|
| Interventions |
Intervention: “Asthma tailored pulmonary rehabilitation” (based on a traditional PRP exclusively for severe asthma). 12‐week rolling programme. 2 supervised 1‐hour exercise sessions (combined endurance and strength training) per week and a home exercise programme. Two 1‐hour education sessions per week, half of the sessions facilitated by the multidisciplinary team and developed to be more specific to people with asthma, and the other half based on motivational consultation delivered by a health psychologist Comparison: Usual care: standard asthma management including disease education provided by experienced asthma nurse specialists and specific advice regarding participation in regular exercise. Offered standard PRP after participation in the trial Concomitant medications: None stated Excluded medications: None stated |
|
| Outcomes |
Primary outcomes: Recruitment rate, retention rate, incidence of adverse events Secondary outcomes: Exercise capacity (ISWT, ESWT, CPET on a treadmill and CPET on a cycle‐ergometer). Asthma control (ACQ). Quality of life (CRQ, AQLQ). Peripheral muscle force (quadriceps muscle strength). Inflammatory biomarkers (sputum eosinophil count, FeNO, inflammatory markers in the blood). Psychological morbidity (HADS). Body composition (BMI). Physical activity (triaxial accelerometer). Cost‐effectiveness (EuroQol) Time points reported: Baseline, postintervention (12 weeks), 9 months Data reported as endpoint rather than as change from baseline. |
|
| Notes |
Funding: Funded by the NIHR under its Research for Patient Benefit (RfPB) Programme Notable conflicts of interest: None declared. Other: VO2 peak data are from cycle ergometer testing. Amended data were obtained from study authors for AQLQ outcome (error detected in publication). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants will be randomised to either the ATPR group or UC group using randomisation codes as advised by our local clinical trials unit". |
| Allocation concealment (selection bias) | Low risk | “Randomisation allocation is sent by automated email to unblinded research team members”. |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | Participants and personnel not blinded but inherently challenging. |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | Participants and personnel not blinded but inherently challenging. |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | Low risk | “The outcome measures to assess the intervention will be collected at baseline, 12 weeks and 9 months by a blinded investigator”. |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | Low risk | “The outcome measures to assess the intervention will be collected at baseline, 12 weeks and 9 months by a blinded investigator”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 40% dropout, no ITT analysis. Primary outcome was feasibility. |
| Selective reporting (reporting bias) | Low risk | As per published protocol. |
| Other bias | Low risk | No other suggestions of bias evident |
Manzak 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Total duration of study: 8 weeks Details of any run‐in period: None stated Number of study centres and location: Single site. Turkey Study setting: Home based Withdrawals: Not stated Date of study: Commenced February 2019 |
|
| Participants |
Number recruited: Not stated Number completed: Intervention group (14). Control group (16) Mean (SD) age: Intervention group 42 (12.7) years. Control group 44.5 (13.2) years Age range: Not stated Gender (M/F): Not stated Mean (SD) BMI: Intervention group 28.1 (6.8) kg/m2. Control group 30.8 (8.6) kg/m2 Severity of condition: Not stated Diagnostic criteria: Not stated Baseline lung function: Not stated Smoking history: Not stated Asthma treatment: Not stated Inclusion criteria: Diagnosis of asthma. Age 18 to 65 years Exclusion criteria: Presence of an orthopaedic, neurological, or systemic disease that prevents exercise. Presence of mental, communicative, and behavioural disorders that may cause problems in understanding commands and questions or practising exercises. Exercising 3 or more days a week |
|
| Interventions |
Intervention: 8 weeks home‐based PRP consisting of stretching exercises, strengthening exercises for upper and lower extremities, breathing exercises, and regular physical activity such as walking. Minimum of 3 days per week, with 1 session supervised by a physiotherapist. Also provided with a pedometer and exercise diary Comparison: Booklets on breathing exercises and physical activity in addition to 1 education session on the course of the disease. Also provided with a pedometer and exercise diary Concomitant medications: Not stated Excluded medications: Not stated |
|
| Outcomes |
Primary outcomes: Functional capacity (Change in 6MWD from baseline to 8 weeks). Lung function (change in PEF and FEV1 from baseline to 8 weeks). Asthma control (change in ACT score from baseline to 8 weeks). Dyspnoea (change in MRCD score from baseline to 8 weeks) Secondary outcomes: Lower extremity strength and balance (change in 30‐second sit to stand test from baseline to 8 weeks). Health‐related quality of life (change in SGRQ from baseline to 8 weeks). Activities of daily living (change in LCADL score from baseline to 8 weeks) Time points reported: Baseline and 8 weeks |
|
| Notes |
Funding: Not stated Notable conflicts of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No detail on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | “Masking: None (open label)” |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | “Masking: None (open label)” |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | Unclear risk | Blinding not stated in trials registry. |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | Unclear risk | Blinding not stated in trials registry. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract data only. Dropouts not reported upon. |
| Selective reporting (reporting bias) | Unclear risk | Abstract data only. Dyspnea (primary outcome) and LCADL (secondary outcome) not reported. |
| Other bias | Unclear risk | Insufficient detail provided to accurately determine other bias (abstract only). Retrospectively registered ‐ study start date was before trial registration date on www.clinicaltrials.gov |
Nathell 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Total duration of study: 4‐week intervention with follow‐up up to 3 years Details of any run‐in period: None stated Number of study centres and location: Single site. Sweden Study setting: Inpatient clinic Withdrawals: Not stated Date of study: Not stated |
|
| Participants |
Number recruited: 197 (101 randomised to intervention group. 96 randomised to control group) Number completed: Not stated Mean (SD) age: Intervention group 40.8 years. Control group 42.9 years. No SD was provided for age. Age range: Not stated Gender (M/F): Intervention group 43/58. Control group 44/52 Mean (SD) BMI: Intervention group 26 kg/m2. Control group 26.6 kg/m2. No SD was provided for BMI. Severity of condition: Not stated Diagnostic criteria: Clinical examination Baseline lung function: Mean % predicted FEV1: Intervention group 89.6%. Control group 89.2%. No SD provided. Smoking history: 49.7% of participants were smokers, and 18.8% of participants were ex‐smokers at randomisation. Asthma treatment: Not stated Inclusion criteria: Not stated Exclusion criteria: None stated |
|
| Interventions |
Intervention: 4‐week inpatient PRP. The main components were education, pharmacological optimisation, physical training, and coping skill acquisition. Comparison: Usual care Concomitant medications: Intervention group: 50.5% of participants had used inhaled steroids, and 17.8% of participants had used oral steroids in year prior to randomisation. Control group: 54.2% of participants had used inhaled steroids, and 19.8% of participants had used oral steroids in year prior to randomisation. Excluded medications: Not stated |
|
| Outcomes |
Primary outcomes: Sick leave days (number of days with any type of sick leave in the 3 years after randomisation) Secondary outcomes: Use of inhaled steroids (questionnaire) and smoking habits (questionnaire) in the 3 years after randomisation Time points reported: 1, 2, and 3 years |
|
| Notes |
Funding: Financial funding received from the research department of AFA insurance company, Sweden. Notable conflicts of interest: None stated Other: No outcomes of interest to current review are reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A person outside the project, without knowledge of the subjects, executed a computerized randomization”. |
| Allocation concealment (selection bias) | Low risk | “A person outside the project, without knowledge of the subjects, executed a computerized randomization”. |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | Rehab programme‐ not able to blind participants or personnel delivering it. |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | Rehab programme‐ not able to blind participants or personnel delivering it. |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | Unclear risk | “The outcome assessor was not blinded to treatment allocation”. (Outcome: smoking status) |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | Unclear risk | “The outcome assessor was not blinded to treatment allocation”. (Outcome: sick days ‐ administrative data) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All analyses were based on intention to treat”. Data available on all but 2 of 197 participants. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available. |
| Other bias | Unclear risk | Sickness insurance scheme from which subjects were recruited is mainly for manual workers and includes only private workers. Persons living in “certain communities” were selected. Diagnosis of asthma made on clinical examination. The proportion of current smokers at randomization was very high (50% and 45% subjects still smoking at 3 year follow up |
Orooj 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Total duration of study: 6 weeks Details of any run‐in period: None stated Number of study centres and location: Single site. India Study setting: Hospital Withdrawals: None reported Date of study: Not stated |
|
| Participants |
Number recruited: Randomised to intervention group: 14. Randomised to control group: 14 Number completed: Completed intervention group: 14. Completed control group: 14 Mean (SD) age: Intervention group 66 (8.4) years. Control group 67 (6.29) years Age range: Not stated Gender (M/F): Not stated Mean (SD) BMI: Intervention group 24 (4.3) kg/m2. Control group 23 (5.1) kg/m2 Severity of condition: All participants had a diagnosis of ACO. Severity not stated. Diagnostic criteria: Participants were diagnosed with ACO according to syndromic and spirometric features from the GINA/GOLD joint documents. Baseline lung function: Mean (SD) % predicted FEV1: Intervention group 65.1 (26.7)%. Control group 62.8 (15.6)% Smoking history: Mean (SD) smoking pack year: Intervention group 11 (3.25). Control group 11.0 (2.9) Asthma treatment: Not stated Inclusion criteria: Diagnosis of ACO Exclusion criteria: History of myocardial infarction, angina, congestive heart failure. Orthopaedic or cognitive impairment that would interfere with participation in rehabilitation. History of thoracic surgical intervention |
|
| Interventions |
Intervention: 6‐week hospital‐based PRP. Exercise component supervised by physiotherapist. Endurance training on treadmill at 60% to 80% of VO2 peak 5 times per week. Resistance training of upper and lower limbs at 50% to 70% 1RM 3 times per week. Structured self‐management education programme including relaxation techniques, smoking cessation, and nutrition Comparison: Usual care Concomitant medications: Not stated Excluded medications: Not stated |
|
| Outcomes |
Primary outcomes: Exercise capacity (6MWD). Quality of life (SGRQ). Lung function (spirometry). Bode Index Secondary outcomes: Not stated Time points reported: Baseline and end of intervention (6 weeks) Data reported as endpoint rather than as change from baseline. SMD (95% CI) between groups at end of intervention also reported. |
|
| Notes |
Funding: No funding was received for the study. Notable conflicts of interest: No competing interests declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “patients were randomly allocated using computer‐generated block randomization to either the PR group or to the control group” |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | Rehabilitation intervention. No mention of blinding of participants or study personnel. |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | Rehabilitation intervention. No mention of blinding of participants or study personnel. |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | Unclear risk | No mention of blinding assessors. |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | Unclear risk | No mention of blinding assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. “All 28 participants enrolled in the investigation completed the study”. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. |
| Other bias | Unclear risk | No data on gender of participants provided. |
Schultz 2021.
| Study characteristics | ||
| Methods |
Study design: RCT Total duration of study: 5 months (plus additional 12 months follow‐up) Details of any run‐in period: Participants assessed at randomisation (T0) and 4 weeks later before commencing rehabilitation (T1). Number of study centres and location: Single site. Germany Study setting: Hospital Withdrawals: 12 participants from each group withdrew from the study. Date of study: June 2015 to August 2017 |
|
| Participants |
Number recruited: Randomised to intervention group: 214. Randomised to control group: 222 Number completed: Completed intervention group: 202. Completed control group: 210 Mean (SD) age: Intervention group 50.7 (8.8) years. Control group 51.6 (8.7) years. Age range: Not stated Gender (M/F): Intervention group 120/82. Control group 117/93 Mean (SD) BMI: Not stated Severity of condition: Not stated. Uncontrolled asthma ACT < 19 was an inclusion criterion. Diagnostic criteria: Physician diagnosis of asthma (ICD‐10; J45). Every asthma diagnosis was verified by a pulmonologist at admission to the rehabilitation unit. Baseline lung function: Mean (SD) % FEV1 postbronchodilator: Intervention group 87.5 (20.7)%. Control group 87.3 (20.5)% Smoking history: Current smokers: Intervention group 34 (16.8%). Control group 34 (16.2%) Asthma treatment: Not stated Inclusion criteria: Approved for pulmonary rehabilitation. Physician diagnosis of asthma (ICD‐10; J45). Uncontrolled asthma based on ACT < 20 Exclusion criteria: Cognitive impairment. Inadequate German language ability. Severe concomitant disease that might mask results of rehabilitation (e.g. cancer, orthopaedic, cardiac, psychiatric comorbidities). If initial diagnosis of asthma could not be confirmed by pulmonologist at admission to rehabilitation |
|
| Interventions |
Intervention: 3‐week inpatient PRP consisting of physical training (endurance training 5 units per week 45 to 60 min each time, strength training 3 sessions per week), education, respiratory physiotherapy and (if needed) psychosocial support, smoking cessation, nutritional counselling, inspiratory muscle training. Delivered by a multidisciplinary team Comparison: Waitlist control group Concomitant medications: Not stated Excluded medications: Not stated |
|
| Outcomes |
Primary outcomes: Asthma control (ACT) Secondary outcomes: Quality of life (SGRQ, AQLQ). Subjective health (Global Rating of Change). Symptoms (severity of dyspnoea cough sputum, pain on numerical rating scale). Fatigue (BFI). Depression and anxiety (PHQ‐9, GAD‐7). Illness representation (Brief IPQ). Subjective self‐management (Skill and Technique Acquisition Scale). Medication adherence and medical beliefs (MARS‐D). Lung function. Health‐related resource use (FIMA‐Lu questionnaire). Exercise capacity (6MWD). Work ability (work ability index) Time points reported: At randomisation (T0). 4 weeks after randomisation (T1). 7 weeks after randomisation (intervention group finish PR) (T2). 20 weeks after randomisation (intervention group 3 months after completing PR; control group begin PR) (T3). Also follow‐up at 6, 9, 12 months after inpatient rehabilitation in the intervention group, and 3, 6, 9 months after rehabilitation in the control group Data reported as endpoint rather than as change from baseline. Adjusted mean differences also reported. |
|
| Notes |
Funding: German Statutory Pension Insurance of South Bavaria. Funding covers costs for staff and materials and travelling expenses. Notable conflicts of interest: No competing interests declared. Other: Data at T2 and T3 extracted for review. 6MWD data were available pre‐ and post‐PRP for both intervention and control groups, with end of PRP occurring 3 months before the control group started PRP. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization list (with computer‐generated random numbers) is created by the Department of Medical Psychology and Psychotherapy, Medical Sociology and Rehabilitation Sciences at the University of Würzburg (concealed allocation).” |
| Allocation concealment (selection bias) | Low risk | The randomization list (with computer‐generated random numbers) is created by the Department of Medical Psychology and Psychotherapy, Medical Sociology and Rehabilitation Sciences at the University of Würzburg (concealed allocation).” |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | High for participants due to nature of intervention. Low for personnel blinding. "Patients themselves cannot be blinded due to the timepoint of the start of their inpatient rehabilitation. However, those who deliver the rehabilitation treatment are unaware whether the patient is a study participant, a participant of the IG or the CG, or a regular inpatient outside the study". |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | High for participants due to nature of intervention. Low for personnel blinding. "Patients themselves cannot be blinded due to the timepoint of the start of their inpatient rehabilitation. However, those who deliver the rehabilitation treatment are unaware whether the patient is a study participant, a participant of the IG or the CG, or a regular inpatient outside the study". |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | High risk | "Blinding was not possible" |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | High risk | "Blinding was not possible" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 436 subjects randomised. 412 subjects included in intention to treat analysis. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoints stated in protocol are reported upon. |
| Other bias | Low risk | No other suggestions of bias evident. |
Toennesen 2017.
| Study characteristics | ||
| Methods |
Study design: RCT parallel‐group design. 4 groups: (1) exercise group (2) diet group (3) exercise + diet group (4) control group. Results of exercise + diet group and control group have been extracted for this review. Total duration of study: 8 weeks with follow‐up at 1 year Details of any run‐in period: None stated Number of study centres and location: Single site. Denmark Study setting: Hospital Withdrawals: 37 participants randomised to exercise + diet (29 completed). 38 randomised to control (34 completed) Date of study: January 2015 to July 2016 |
|
| Participants |
Number recruited: Randomised to intervention (exercise + diet): 37. Randomised to control group: 38 Number completed: Completed intervention (exercise + diet): 29. Completed control: 34 Mean (SD) age: Intervention (exercise + diet) group: 43.7 (13.9) years. Control group: 38.2 (12.7) years Age range: Not stated Gender (M/F): Intervention (exercise + diet) group: 7/22. Control group: 8/26 Mean (SD) BMI: Intervention (exercise + diet) group: 26.1 (2.5) kg/m2. Control group: 25.5 (2.4) kg/m2 Severity of condition: Mild to moderate asthma. No participants received ICS in doses of greater than or equal to 1600 μg budesonide equivalents per day plus a second controller (indicating severe asthma) Diagnostic criteria: At least 1 positive diagnostic test demonstrating variable airflow obstruction (mannitol test, methacholine test, reversibility test) Baseline lung function: Mean (SD) % predicted FEV1: Intervention (exercise + diet) group: 82.6 (15.2)%. Control group: 81.9 (12.3)% Smoking history: Intervention (exercise + diet) group: non‐smoker 18 (62%); current smoker 1 (3%); former smoker 10 (34%). Control group: non‐smoker 19 (56%); current smoker 2 (6%); former smoker 13 (38%) Asthma treatment: Use of ICS: Intervention (exercise + diet) group: 17 (59%); control group: 23 (68%). Mean (SD) ICS dose (budesonide equivalents at entry in μg): Intervention (exercise + diet) group: 663 (370); control group: 739 (469) Inclusion criteria: Aged 18 to 65 years. BMI 20 to 30 kg/m2. ACQ score 1.0 or more. At least 1 positive diagnostic test demonstrating variable airflow obstruction. Either have been on stable prophylactic treatment regimen with inhaled ICS, ICS + LABA and/or leukotriene antagonist OR have had no prophylactic treatment at least 3 months before enrolment. Capable of exercising on bike. Sedentary (< 60 minutes of structured physical activity per week) Exclusion criteria: BMI > 30 kg/m2, BMI < 20.5 kg/m2. COPD. Pregnancy. Other inflammatory or metabolic diseases. Use of oral anti‐inflammatory medication or the use of antibiotic treatment during the last 8 weeks. Patients who were on oral corticosteroids or biological treatments were not included.* *Unclear if this was an exclusion criterion |
|
| Interventions |
Intervention: Exercise and education: 8 weeks of high‐intensity interval training using the “10‐20‐30” concept on indoor spinning bikes 3 times per week supervised by a trained spinning instructor, with 5 group counselling sessions and 1 individual counselling session with a dietician regarding a high‐protein, low GI diet Comparison: No intervention. Encouraged to maintain usual physical activity levels and diet Concomitant medications: Participants should either have been on stable prophylactic treatment regimen with inhaled ICS, ICS + LABA and/or leukotriene antagonist OR have had no prophylactic treatment at least 3 months before enrolment. Excluded medications: Patients who were on oral corticosteroids or biological treatments were not included.* *Unclear if this was an exclusion criterion |
|
| Outcomes |
Primary outcomes: Level of asthma control (ACQ) Secondary outcomes: Exercise capacity (VO2 max) on incremental cycle ergometer. Body composition (DEXA). Asthma‐related quality of life (MiniAQLQ). Lung function (spirometry). Sputum cell counts (sputum eosinophils, sputum neutrophils). Airway hyperresponsiveness (FeNO). Blood inflammatory markers (eosinophil count, serum levels of IL‐6, serum level of hs‐CRP). Urine urea excretion. Dietary GI Time points reported: Baseline, 8 weeks, 1 year Data reported as endpoint rather than as change from baseline. |
|
| Notes |
Funding: None stated Notable conflicts of interest: None stated Other: Data from exercise + diet group and data from control group used for this review. Not all outcomes reported at 1 year. Email correspondence with author (A Bentzon) for VO2 data in mL/min/kg at 1 year for exercise + diet group and control group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer‐generated block randomization method with a block size of 12, to ensure equal distribution of patients in treatment groups throughout the shifting seasons.” |
| Allocation concealment (selection bias) | Low risk | "The randomization was done using opaque sealed envelopes with a computer‐generated block randomization method with a block size of 12" |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | Rehabilitation intervention ‐ Not blinded but inherently challenging. |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | Rehabilitation intervention ‐ Not blinded but inherently challenging. |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | Low risk | “The investigators who carried out post intervention spirometry, mannitol tests, and handing out of questionnaires and all laboratory technicians were blinded to the randomization.” |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | Low risk | “The investigators who carried out post intervention spirometry, mannitol tests, and handing out of questionnaires and all laboratory technicians were blinded to the randomization.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT stated, but modest numbers of dropouts (<20%). Multiple imputation used but not clear what for. |
| Selective reporting (reporting bias) | Low risk | Almost all primary and secondary outcomes specified in clinical trial registry are reported on. |
| Other bias | Unclear risk | Considerable gender imbalance at baseline in one group – unclear if this may have affected outcomes, although appears to have been accounted for in analysis of primary outcome. No power calculation in the protocol, but trial registry suggests original target n = 200, and this was not achieved. |
Turk 2020.
| Study characteristics | ||
| Methods |
Study design: RCT parallel‐group design. 3 groups (PR only (PR), PR + self management support (PR + SMS), usual care). Results of PR only group and usual care group have been extracted for this review. Total duration of study: 1 year Details of any run‐in period: None stated Number of study centres and location: Single site. The Netherlands Study setting: General Hospital Withdrawals: 1 withdrawal from control group Date of study: January 2014 to December 2016 |
|
| Participants |
Number recruited: Randomised to intervention (PR group): 14. Randomised to control group: 11 Number completed: Completed intervention (PR group): 14. Completed control: 10 Mean (SD) age: Intervention (PR group) 41.5 (9.7) years. Control group 41.9 (8.5) years Age range: Not stated Gender (M/F): Intervention (PR group) 4/10. Control group 1/9 Mean (SD) BMI: Intervention (PR group) 36.72 (4.79) kg/m2. Control group 35.16 (3.86) kg/m2 Severity of condition: Median (IQR) ACQ scores at baseline: Intervention (PR group) 2.17 (1.46 to 2.5). Control group 2.09 (1.50 to 2.68) Diagnostic criteria: Asthma was diagnosed according to GINA guidelines. Baseline lung function: Mean (SD) % predicted FEV1: Intervention (PR group) 86.93 (9.35)%. Control group 82.4 (16.17)% Smoking history: Not stated Asthma treatment: In relation to the entire study population (all 3 groups) at baseline: all participants were using ICS and a LABA; 39% of participants were using a LTRA; and 23% of participants were using a LAMA. Inclusion criteria: Age 18 to 55 years. BMI > 30 kg/m2. Suboptimally controlled asthma (ACQ score > 0.75) despite optimal inhalation therapy (ICS and a LABA) Exclusion criteria: Current smoking or a smoking history of > 10 pack years. Asthma exacerbation (need of antibiotics/oral corticosteroids) in 6 weeks before inclusion. COPD or other pulmonary pathology apart from asthma, except for adequately treated OSAS with an apnoea‐hypopnoea index < 5.0. Any significant orthopaedic or neurologic problems |
|
| Interventions |
Intervention: High‐intensity interval training x 3 days per week x 12 weeks + nutritional intervention (3 clinical visits and 3 phone calls over 12 weeks) + psychological group sessions focusing on behavioural modification and motivational strategies (4 group sessions of 1‐hour duration) Comparison: Advised to lose weight and to exercise Concomitant medications: All participants were using ICS and a LABA. Excluded medications: None stated |
|
| Outcomes |
Primary outcomes: Difference of change in ACQ score between PR + SMS and PR only groups after 3 months Secondary outcomes: Difference of change in ACQ score between both PR groups and control group after 3 months. Difference of change in ACQ score between both PR groups and control group at 12 months. Difference of change between PR only group and control group and between PR + SMS group and PR group at 3 months and 12 months for quality of life (AQLQ), lung function, physical activity levels, exercise capacity (incremental CPET on cycle ergometer and 6MWD), body composition, airway inflammation, systemic inflammation and exacerbation rate Time points reported: Baseline, 3 months, 12 months Data reported as mean (SD) change from baseline for outcomes of exercise capacity and physical activity. Data reported as median (IQR) change from baseline for outcomes of asthma control, quality of life, step count, and inflammatory markers. |
|
| Notes |
Funding: None stated Notable conflicts of interest: None stated Other: Data from the PR only group and usual care group were extracted for review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned in a 1:1:1 ratio using a computer‐generated permuted‐block scheme”. |
| Allocation concealment (selection bias) | Low risk | “Allocation took place by an independent researcher after written consent had been obtained from all subjects and baseline data were collected, ensuring concealment of allocation.” |
| Blinding of participants and personnel (performance bias) Subjectively reported outcomes | High risk | "There was no blinding”. |
| Blinding of participants and personnel (performance bias) Not subjectively reported outcomes | High risk | "There was no blinding”. |
| Blinding of outcome assessment (detection bias) Subjectively reported outcomes | High risk | No mention of blinding of outcome assessor (Subjective measures: ACQ, AQLQ) |
| Blinding of outcome assessment (detection bias) Not subjectively assessed outcomes | High risk | No mention of blinding of outcome assessors. (Measures: Lung function, Physical activity level, Exercise capacity, Body composition, Airway inflammation, Systemic inflammation, Exacerbation rate) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “The data of the randomized subjects were analysed according to the intention to treat principle.” |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoints stated in trial registry are reported upon. |
| Other bias | Unclear risk | Did not achieve target sample size. |
Abbreviations: 6MWD: 6‐minute walk distance; ACO: asthma‐COPD overlap; ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; AQLQ: Asthma Quality of Life Questionnaire; BDI: baseline dyspnoea index; BFI: Brief Fatigue Inventory; BMI: body mass index; BTS: British Thoracic Society; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CPET: cardiopulmonary exercise test; CRQ: Chronic Respiratory Disease Questionnaire; DEXA: dual energy X‐ray absorptiometry; ESWT: endurance shuttle walk test; FeNO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in 1 second; FRC: functional residual capacity; FVC: forced vital capacity; GAD‐7: General Anxiety Disorder Assessment; GI: glycaemic index; GINA: Global Initiative for Asthma; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HADS: Hospital Anxiety and Depression Scale; HR: heart rate; hs‐CRP: high‐sensitivity C‐reactive protein; ICD‐10: International Statistical Classification of Diseases and Related Health Problems 10th Revision; ICS: inhaled corticosteroids; IL‐6: interleukin 6; IPQ: Illness Perception Questionnaire; IQR: interquartile range; ISWT: incremental shuttle walk test; ITU: intensive therapy unit; LABA: long‐acting beta agonist; LAMA: long‐acting muscarinic antagonist; LCADL: London Chest Activities of Daily Living Scale; LTRA: leukotriene receptor antagonist; MARS‐D: Medication Adherence Report Scale; MRCD: MEdical Research Council Dyspnea Scale; NIHR: National Institute for Health and Care Research; OSAS: obstructive sleep apnoea syndrome; PC20: concentration of inhaled methacholine that provokes a 20% decrease in FEV1; PEF: peak expiratory flow; PHQ‐9: Patient Health Questionnaire‐9; PR: pulmonary rehabilitation; PRP: pulmonary rehabilitation programme; RCT: randomised controlled trial; RM: repetition maximum; SD: standard deviation; SIGN: Scottish Intercollegiate Guidelines Network; SGRQ: St George's Respiratory Disease Questionnaire; SMD: standardised mean difference; TDI: transitional dyspnoea index; VO2 max: maximal oxygen uptake; VO2 peak: peak oxygen uptake.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd El‐Kader 2016 | Wrong intervention |
| ACTRN12617000991314 | Wrong intervention |
| Alison 2000 | Wrong study design |
| Astafieva 2011 | Wrong intervention |
| Bacon 2013 | Wrong intervention |
| Bacon 2015 | Wrong intervention |
| Boyd 2011 | Wrong intervention |
| Boyd 2012 | Wrong intervention |
| Cafarella 2001 | Wrong patient population |
| Carvalho 2014 | Wrong comparator |
| Carvalho 2014a | Wrong comparator |
| Cox 1991 | Wrong study design |
| Cox 1993 | Wrong study design |
| Da Silva 2015 | Wrong comparator |
| Del Giacco 2016 | Wrong study design |
| Didour 1998 | Wrong patient population |
| Didour 2002 | Wrong patient population |
| Dogra 2010 | Wrong intervention |
| Dogra 2010a | Wrong study design |
| Dogra 2011 | Wrong intervention |
| Emtner 2005 | Wrong comparator |
| Farid 2005 | Wrong intervention |
| Farid 2005a | Wrong intervention |
| Franca‐Pinto 2015 | Wrong comparator |
| Freitas 2014 | Wrong comparator |
| Freitas 2014a | Wrong comparator |
| Freitas 2014b | Wrong comparator |
| Freitas 2014c | Wrong comparator |
| Freitas 2015 | Wrong comparator |
| Freitas 2015a | Wrong comparator |
| Freitas 2015b | Wrong comparator |
| Freitas 2016 | Wrong comparator |
| Freitas 2016a | Wrong comparator |
| Freitas 2016b | Wrong comparator |
| Freitas 2017 | Wrong comparator |
| Freitas 2017a | Wrong comparator |
| Freitas 2017b | Wrong comparator |
| Freitas 2018 | Wrong comparator |
| Freitas 2018a | Wrong comparator |
| Freitas 2019 | Wrong comparator |
| Girodo 1992 | Wrong intervention |
| Goldman 1997 | Wrong patient population |
| Goncalves 2006 | Wrong comparator |
| Gonçalves 2008 | Wrong comparator |
| Greening 2014 | Wrong intervention |
| Hallstrand 2000 | Wrong study design |
| IRCT138811143270N1 | Wrong comparator |
| IRCT2015011420666N1 | Wrong intervention |
| IRCT2016052328028N1 | Wrong intervention |
| Jaakkola 2017 | Wrong intervention |
| Jaakkola 2019 | Wrong intervention |
| Jalbert 2018 | Wrong intervention |
| Kouznetsova 2007 | Wrong intervention |
| Lenz 2001 | Wrong study design |
| Lowe 2018 | Wrong intervention |
| Lowe 2018a | Wrong intervention |
| Ma 2010 | Wrong intervention |
| Ma 2015 | Wrong intervention |
| Mendes 2011 | Wrong comparator |
| Mendes 2013 | Wrong comparator |
| Mendes 2013a | Wrong comparator |
| Meyer 1999 | Wrong intervention |
| Meyer 2015 | Wrong intervention |
| Meyer 2015a | Wrong intervention |
| NCT00195117 (a) | Wrong intervention |
| NCT00195117 (b) | Wrong intervention |
| NCT00839137 | Wrong intervention |
| NCT00901095 | Wrong intervention |
| NCT00953342 | Wrong intervention |
| NCT00989365 | Wrong comparator |
| NCT01097473 | Wrong intervention |
| NCT02012400 | Wrong intervention |
| NCT02033122 | Wrong comparator |
| NCT02188940 | Wrong comparator |
| NCT03145883 | Wrong intervention |
| Pinto 2012 | Wrong comparator |
| Pinto 2012a | Wrong comparator |
| Pollart 2012 | Wrong intervention |
| Pollart 2012a | Wrong intervention |
| Postolache 2002 | Wrong study design |
| Refaat 2015 | Wrong intervention |
| Riegels‐Nielsen 2000 | Wrong intervention |
| Sampaio 2002 | Wrong intervention |
| Shaw 2011 | Wrong intervention |
| Shaw 2011a | Wrong intervention |
| Toennesen 2016 | Wrong intervention |
| Turner 2008 | Wrong intervention |
| Turner 2008a | Wrong intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Budnevsky 2018.
| Methods |
Study design: Unclear how participants were assigned to intervention/control groups Total duration of study: 5 weeks of education followed by 30 days of exercise Details of any run‐in period: None documented Number of study centres and location: Unclear. Voronezh State Medical University, Russia Study setting: Not explicitly stated Withdrawals: Not stated Date of study: Not stated |
| Participants |
Number recruited: Not stated Number completed: 60 (30 intervention group, 30 control group) Mean (SD) age: Intervention group 49.8 (1.11) years. Control group 49.94 (0.95) years Age range: Not stated Gender (M/F): Intervention group 8/22. Control group 7/23 Mean (SD) BMI: Intervention group 32.87 (0.37). Control group not stated Severity of condition: Assessment of asthma severity included the number of exacerbations, calls to emergency service, and hospital admissions in the past 12 months. Diagnostic criteria: Asthma diagnosis based on assessment of symptoms, medical history, health status, and spirometry parameters according to GINA Baseline lung function: Intervention group: mean (SD) FEV1 61.17 (0.84); mean (SD) FVC 65.40 (0.66). Control group: mean (SD) FEV1 59.92 (0.80); mean (SD) FVC 64.69 (2.67) Smoking history: Not stated Asthma treatment: Not stated Inclusion criteria: Aged 18 to 60 years. Diagnosis of asthma. Diagnosis of metabolic syndrome according to International Diabetes Federation (IDF) criteria Exclusion criteria: Not stated |
| Interventions |
Intervention: Standard pharmacologic therapy in addition to exercise and education. Education consisted of 5 x 1.5‐hour group seminars on the management and prevention of asthma and metabolic syndrome. Exercise was conducted daily for 30 days, after the education course. Comparison: Standard pharmacologic therapy Concomitant medications: Standard pharmacologic therapy Excluded medications: Not stated |
| Outcomes |
Primary outcomes: Not explicitly defined Secondary outcomes: Not explicitly defined The following outcomes are reported: asthma severity (exacerbation rate, emergency calls, hospital admissions), asthma symptoms (VAS), asthma control (ACT), spirometry, quality of life (SF‐36), anthropometrics (waist circumference, BMI), blood pressure, metabolic syndrome markers (fasting glucose, glucose tolerance test, lipid profile). Time points reported: Baseline and 12 months |
| Notes | We contacted study authors to clarify eligibility criteria, but have received no response. It is unclear: (1) how participants were assigned to the intervention or control groups; and (2) what type of physical exercise(s) the participants in the intervention group performed and the dosage of exercise (intensity, number of sessions per week, number of weeks). |
IRCT2014041617299N.
| Methods |
Study design: Randomised controlled trial Total duration of study: Not stated Details of any run‐in period: Not stated Number of study centres and location: Not stated. Iran Study setting: Not stated Withdrawals: Not stated (WHO ICTRP information only) Date of study: 2014 |
| Participants |
Number recruited: Not stated (WHO ICTRP information only) Number completed: Not stated (WHO ICTRP information only) Mean (SD) age: Not stated (WHO ICTRP information only) Age range: Not stated (WHO ICTRP information only) Gender (M/F): Not stated (WHO ICTRP information only) Mean (SD) BMI: Not stated (WHO ICTRP information only) Severity of condition: Not stated (WHO ICTRP information only) Diagnostic criteria: Not stated (WHO ICTRP information only) Baseline lung function: Not stated (WHO ICTRP information only) Smoking history: Not stated (WHO ICTRP information only) Asthma treatment: Not stated (WHO ICTRP information only) Inclusion criteria: Patients with asthma after approval by the relevant specialist. Age range 19 to 65 years. Has not participated in similar programmes in the previous 6 months. Absence of other chronic conditions that "requires special care and different programmes" Exclusion criteria: Musculoskeletal, orthopaedic, cardiovascular disease that may be incompatible with exercise. Pregnancy. Absence of 2 or more training sessions |
| Interventions |
Intervention: Supervised exercise: moderate‐intensity aerobic exercise 6 to 5 days per week x 30 minutes. Education: 4 to 6 sessions of 60 minutes for 1 month (depending on training needs of individual). Topics included understanding asthma, asthma medications, self‐care. Comparison: Routine care and education Concomitant medications: None stated Excluded medications: None stated |
| Outcomes |
Primary outcomes: Asthma control (ACT and peak flow measurement). Quality of life (AQLQ) Secondary outcomes: None stated Time points reported: Pre‐intervention, postintervention, and 2 months after the intervention |
| Notes | We contacted study authors to clarify eligibility criteria, but have received no response. It is unclear: (1) how many weeks supervised exercise training was undertaken; and (2) whether the education received by the control group differed from that received by the intervention group. We requested any data that may be in the public domain that they may be willing to share. |
NTR4398.
| Methods |
Study design: Randomised controlled trial Total duration of study: 12 weeks Details of any run‐in period: None stated Number of study centres and location: Not stated. The Netherlands Study setting: Not explicitly stated Withdrawals: Not stated Date of study: January 2014 to December 2016 |
| Participants |
Number recruited: Not stated (WHO ICTRP information only) Number completed: Not stated (WHO ICTRP information only) Mean (SD) age: Not stated (WHO ICTRP information only) Age range: Not stated (WHO ICTRP information only) Gender (M/F): Not stated (WHO ICTRP information only) Mean (SD) BMI: Not stated (WHO ICTRP information only) Severity of condition: Not stated (WHO ICTRP information only) Diagnostic criteria: Asthma diagnosis based on the presence of symptoms and bronchial hyperresponsiveness (provocative dose to achieve 20% reduction in FEV1% (PD20) metacholine < 1.76 mg) Baseline lung function: Not stated (WHO ICTRP information only) Smoking history: Not stated (WHO ICTRP information only) Asthma treatment: Not stated (WHO ICTRP information only) Inclusion criteria: Age > 18 and < 50 years. Acceptable operative risk. ACQ > 0.75 despite optimised medication use (long‐acting beta‐agonist and inhaled corticosteroid). BMI > 35 kg/m2 with a maximum weight of 150 kg. Ability to perform a reproducible lung function test. Ability to participate in pulmonary rehabilitation. Approval for 3‐, 6‐, and 12‐month follow‐up visits. Patient motivation to achieve the fullest benefit from pulmonary rehabilitation. Informed consent Exclusion criteria: Significant orthopaedic or neurologic problems that reduce mobility or co‐operation with physical training. COPD or other pulmonary pathology apart from asthma, except for adequately treated obstructive sleep apnoea with an apnoea‐hypopnoea index score < 5. Pregnancy. Asthma exacerbation in 6 weeks prior to screening requiring a course of oral steroids or antibiotics. Maintenance therapy with oral steroids. Current smoking (during pulmonary rehabilitation) or > 10 pack year history. Participation in pulmonary rehabilitation programme in 2 years before the study |
| Interventions |
Intervention: Pulmonary rehabilitation: training x 60 minutes x 3 times per week for 12 weeks under supervision of a physiotherapist, and with counselling of a psychologist and a dietician. Laparoscopic bariatric surgery: either a gastric sleeve gastrectomy or a Roux‐and‐Y gastric bypass surgery Comparison: Standard care Concomitant medications: Not stated Excluded medications: Maintenance therapy with oral steroids |
| Outcomes |
Primary outcomes: Symptom scores (ACQ), 3 months after bariatric surgery Secondary outcomes: BMI. Asthma‐related quality of life (AQLQ). Activity level (move‐monitor). Lung function (FEV1). Exercise capacity (6MWD). Postoperative complications. Cancelled surgeries. Inflammation (blood) Time points reported: After pulmonary rehabilitation and 3, 6, and 12 months after surgery |
| Notes |
Abbreviations: 6MWD: 6‐minute walk distance; ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; AQLQ: Asthma Quality of Life Questionnaire; BMI: body mass index; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GINA: Global Initiative for Asthma; SF‐36: 36‐item Short Form Health Survey; VAS: visual analogue scale; WHO ICTRP: World Health Organization International Clinical Trials Registry Platform.
Characteristics of ongoing studies [ordered by study ID]
NCT03630432.
| Study name | Pulmonary rehabilitation for uncontrolled asthma associated with elevated BMI |
| Methods |
Study design: Randomised controlled trial Total duration of study: 8 weeks intervention and follow‐up 40 weeks later Details of any run‐in period: None stated Number of study centres and location: Single site. Scotland Study setting: Hospital Withdrawals: No report of results found. |
| Participants |
Number recruited: No report of results found. Number completed: No report of results found. Mean (SD) age: No report of results found. Age range: No report of results found. Gender (M/F): No report of results found. Mean (SD) BMI: No report of results found. Severity of condition: Severe asthma Diagnostic criteria: Diagnosed as per GINA Guidelines 2015 with characteristic symptoms and at least 1 of the following.
Baseline lung function: No report of results found. Smoking history: No report of results found. Asthma treatment: Not stated Inclusion criteria: Adults aged 18 to 80 years (smokers, ex‐smokers, and non‐smokers). Confirmed asthma as per GINA Guidelines 2015. Difficult asthma defined as per SIGN/BTS Guideline 201. BMI ≥ 25 kg/m2. MRCD score ≥ 3/5 Exclusion criteria: ITU admission +/‐ mechanical ventilation in the previous year for asthma exacerbation. Respiratory tract infection requiring antibiotics or asthma exacerbation requiring corticosteroid boost in preceding 4 weeks. Significant respiratory or other comorbidity likely to influence the conduct of the study. Pregnancy and breastfeeding. Severe and/or unstable cardiac disease. Impaired mobility that impacts upon ability to participate in physical training. Commenced antifungal, biologic (omalizumab, lebrikizumab, mepolizumab), or Airsonett device within the preceding 6 months |
| Interventions |
Intervention: 8‐week rolling PRP. Once‐weekly 1‐hour session of supervised exercise (aerobic, resistance, and flexibility training) and once‐weekly 0.5‐hour education session (topics including what is asthma, treatments and inhaler technique, self‐management, importance of exercise and health promotion) Comparison: Usual care for 8 weeks, then enter PRP Concomitant medications: Not stated Excluded medications: Commencement within the preceding 6 months of antifungal, biologic (omalizumab, lebrikizumab, mepolizumab), or Airsonett device; eligible if on treatment for > 6 months or discontinued > 6 months ago |
| Outcomes |
Primary outcomes: Quality of life (AQLQ) Secondary outcomes: Asthma control (ACQ). Change in treatment burden (asthma medication use treatment chart). Change in healthcare usage (number of episodes of unscheduled care). MRCD score. BMI. Inflammation (changes in blood eosinophils and FeNO). Lung function (changes in FEV1 and FVC, lowest oxygen saturation). Exercise tolerance (change in 6MWD). Physical activity (change in actigraphy data). Hospital Anxiety and Depression Scale score Time points reported: Baseline, 8 weeks, 40 weeks |
| Starting date | May 2017 |
| Contact information | Douglas C Cowan: douglas.cowan@ggc.scot.nhs.uk Clare Ricketts: clare.ricketts@nhs.net |
| Notes |
Funding: None stated Notable conflicts of interest: None stated Other: Trial commenced May 2017 based on trial registry data. Emailed authors to determine whether any results available (without reply) |
Abbreviations: 6MWD: 6‐minute walk distance; ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; BMI: body mass index; BTS: British Thoracic Society; FeNO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GINA: Global Initiative for Asthma; ITU: intensive therapy unit; MRCD: MEdical Research Council Dyspnea Scale; PRP: pulmonary rehabilitation programme; SIGN: Scottish Intercollegiate Guidelines Network.
Differences between protocol and review
We included one randomised cross‐over study despite stating in our protocol that we would exclude cross‐over studies due to difficulties associated with washout periods and enduring effects of interventions that involve behaviour change. We made this decision in light of the small number of eligible studies that were retrieved from the database search, in the interest of providing clinically meaningful findings. In doing so, we only considered data that were reported pre‐cross‐over and those pertaining to effects immediately postintervention (no long‐term outcome data were used).
Contributions of authors
CR Osadnik was responsible for study conception. CR Osadnik, VM McDonald, and AE Holland all contributed equally to the methodological design, preparation, and write‐up of all aspects of the protocol. CR Osadnik and C Gleeson co‐ordinated citation screening, data extraction, risk of bias appraisal, data analysis, and manuscript write‐up, supported by VM McDonald and AE Holland. All authors approved the final version of the manuscript.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor) checked data prior to full review write‐up.
Sally Spencer (Co‐ordinating Editor) advised on content and edited the review.
Iain Crossingham (Contact Editor) edited the review.
Emma Dennett (Deputy Co‐ordinating Editor) advised on content, edited the review, and approved the review prior to publication.
Emma Jackson (Managing Editor) co‐ordinated the editorial process, conducted peer review, and edited the references and other sections in the review.
Elizabeth Stovold (Information Specialist) designed and conducted the searches and arranged for peer review of the search strategy.
Sources of support
Internal sources
-
Monash University, Australia
Salary
-
La Trobe University and Alfred Health, Australia
Salary
-
The University of Newcastle, Australia
Salary
External sources
-
Ciara Gleeson, Ireland
Ciara Gleeson was part supported by the Health Research Board (Ireland) and the HSC Public Health Agency (Grant number CBES‐2018‐001) through Evidence Synthesis Ireland/Cochrane Ireland.
Declarations of interest
CR Osadnik was recipient of a Lung Foundation Australia/Boehringer Ingelheim COPD Research Fellowship during 2016 to 2018 (unrelated to the present work). He has received fees from Novartis for non‐promotional speaking engagements (unrelated to the present work).
C Gleeson was supported by an Evidence Synthesis Ireland fellowship.
VM McDonald has participated in educational symposia funded by GlaxoSmithKline, AstraZeneca, and Menarini, and has participated on advisory boards for GlaxoSmithKline, Novartis, AstraZeneca, and Menarini (unrelated to the present work).
AE Holland has received fees from AstraZeneca and Boehringer Ingelheim for non‐promotional speaking engagements (unrelated to the present work).
To the best of all authors' knowledge, at the time of submitting this work, none of the named entities have any financial interest in the findings of this review and do not manufacture any such intervention or competing product(s).
New
References
References to studies included in this review
Cambach 1997 {published data only}
- Cambach W, Chadwick-Straver RV, Wagenaar RC, Keimpema AR, Kemper HC. The effects of a community-based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. European Respiratory Journal 1997;10(1):104-13. [DOI] [PubMed] [Google Scholar]
- Cambach W, Chadwick-Straver RVM, Wagenaar RC, Van Keimpema ARJ, Kemper HCG. The effects of a community-based pulmonary rehabilitation programme on exercise capacity and quality of life: a randomized controlled trial. European Respiratory Journal 1997;10(Suppl 25):394S. [DOI] [PubMed] [Google Scholar]
- Cambach W, Chadwick-Straver RVM, Wagenaar RC, Keimpema ARJ. Effectiveness of a rehabilitation programme for patients with asthma and COPD carried out in the first line health care. Nederlands Tijdschrift voor Fysiotherapie 1998;108:26-36. [Google Scholar]
Cochrane 1990 {published data only}
- Cochrane LM, Clark CJ. Benefits and problems of a physical training programme for asthmatic patients. Thorax 1990;45(5):345-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane LM, Clark CJ. Physical training improves perception of breathlessness during exercise in asthmatics. European Respiratory Journal 1988;1 Suppl 2:192s. [Google Scholar]
Foglio 2001 {published and unpublished data}
- Foglio K, Bianchi L, Ambrosino N. Is it really useful to repeat outpatient pulmonary rehabilitation programs in patients with chronic airway obstruction? A 2-year controlled study. Chest 2001;119(6):1696-704. [DOI] [PubMed] [Google Scholar]
Majd 2020 {published and unpublished data}
- Majd S, Apps L, Chantrell S, Hudson N, Eglington E, Hargadon B, et al. A feasibility study of a randomized controlled trial of asthma-tailored pulmonary rehabilitation compared with usual care in adults with severe asthma. Journal of Allergy and Clinical Immunology: In Practice 2020;8(10):3418-4327. [DOI] [PubMed] [Google Scholar]
- Majd S, Apps LD, Hudson N, Hewitt S, Eglinton E, Murphy A, et al. Protocol for a feasibility study to inform the development of a multicentre randomised controlled trial of asthma-tailored pulmonary rehabilitation versus usual care for individuals with severe asthma. BMJ Open 2016;6(3):e010574. [DOI: 10.1136/bmjopen-2015-010574] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majd S, Green R, Bradding P, Singh S, Evans R. A feasibility study of a multi-centre randomised controlled trial of asthma tailored pulmonary rehabilitation (AT-PR) versus usual care (UC) in individuals with severe asthma. Thorax 2017;72(Suppl 3):A50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majd S, Singh S, Bradding P, Hewitt S, Apps L, Murphy A, et al. Exercise training and airway inflammation in severe-asthma: results from a feasibility study. European Respiratory Journal 2018;52(Suppl 62):PA2050. [Google Scholar]
Manzak 2020 {published data only}
- Manzak A, Özyilmaz S, Atagün Güney P. Efficiency of home-based pulmonary rehabilitation in adults with asthma. European Respiratory Journal 2020;56(Suppl 64):5179. [Google Scholar]
- NCT04088669. Efficiency of home-based pulmonary rehabilitation in adults with asthma. clinicaltrials.gov/show/NCT04088669 (first received 13 September 2019).
Nathell 2005 {published data only}
- Nathell L. Effects on sick leave of an inpatient rehabilitation programme for asthmatics in a randomized trial. Scandinavian Journal of Public Health 2005;33(1):57-64. [DOI] [PubMed] [Google Scholar]
Orooj 2020 {published data only}
- Orooj M, Moiz JA, Mujaddadi A, Ali MS, Talwar D. Effect of pulmonary rehabilitation in patients with asthma COPD overlap syndrome: a randomized control trial. Oman Medical Journal 2020;25(3):e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schultz 2021 {published and unpublished data}
- Schultz K, Seidl H, Jelusic D, Wagner R, Wittmann M, Faller H, et al. Effectiveness of pulmonary rehabilitation for patients with asthma: study protocol of a randomized controlled trial (EPRA). BMC Pulmonary Medicine 2017;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K, Wittmann M, Lehbert N, Schwarzkopf L, Szentes B, Nowak D, et al. Asthma control (AC) and quality of life (QoL) one year after pulmonary rehabilitation (PR). European Respiratory Journal 2019;54:PA513. [Google Scholar]
- Schultz K, Wittmann M, Wagner R, Lehbert N, Schwarzkopf L, Szentes B, et al. Effectiveness of pulmonary rehabilitation for patients with asthma: ePRA-RCT. European Respiratory Journal 2018;52(Suppl 62):OA1620. [Google Scholar]
- Schultz K, Wittmann M, Wagner R, Lehbert N, Schwarzkopf L, Szentes B, et al. In-patient pulmonary rehabilitation to improve asthma control - a randomized controlled study (EPRA, Effectiveness of Pulmonary Rehabilitation for Patients with Asthma). Deutsches Ärzteblatt international 2021;118:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toennesen 2017 {published data only}
- Bentzon A, Loehde L, Toennesen L, Porsbjerg C, Hostrup M, Backer V. One-year follow-up of asthma patients in a randomised controlled trial of high-intensity training. Are there lasting effects? European Respiratory Journal 2018;52(Suppl 62):PA3986. [Google Scholar]
- Bentzon AK, Loehde LW, Backer V, Toennesen L. The long-term effect of an exercise and diet intervention in asthma patients: a 1-year follow-up on a randomised controlled trial. ERJ Open Research 2019;5(2):00032-2019. [DOI: 10.1183/23120541.00032-2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02355964. EFFORT Asthma: effects of diet and exercise in asthma. clinicaltrials.gov/ct2/show/NCT02355964 (first received 4 February 2015).
- Toennesen L, Meteran H, Hostrup M, Porsbjerg C, Geiker N, Jensen C, et al. Exercise and diet improve asthma control in non-obese asthma patients - a randomised controlled trial. European Respiratory Journal 2017;50(Suppl 61):OA4678. [Google Scholar]
- Toennesen LL, Meteran H, Hostrup M, Wium Geiker NR, Jensen CB, Porsbjerg C, et al. Effects of exercise and diet in nonobese asthma patients - a randomized controlled trial. Journal of Allergy and Clinical Immunology: In Practice 2017;6(3):803-11. [DOI] [PubMed] [Google Scholar]
Turk 2020 {published data only}
- NTR4322. Pulmonary rehabilitation and self-management in obese patients with asthma. trialregister.nl/trial/4163 (first received 17 December 2013).
- Türk Y, Theel W, Van Huisstede A, Birnie E, Hiemstra P, Taube C, et al. Long-term effects of a high intensity life style program in obese patients with asthma. European Respiratory Journal 2018;52(Suppl 62):PA650. [DOI] [PubMed] [Google Scholar]
- Türk Y, Theel W, Huisstede A, de Geijn G, Birnie E, Hiemstra P, et al. Short-term and long-term effect of a high intensity pulmonary rehabilitation programme in obese patients with asthma: a randomised controlled trial. European Respiratory Journal 2020;56(1):1901820. [DOI] [PubMed] [Google Scholar]
- Turk Y, Van Huisstede A, Van Der Geijn GJ, Hiemstra P, Taube C, Braunstahl GJ. Effect of a high intensity life style program on asthma control in obese patients with asthma. European Respiratory Journal 2017;50(Suppl 61):PA4904. [Google Scholar]
References to studies excluded from this review
Abd El‐Kader 2016 {published data only}
- Abd El-Kader SM, Al-Jiffri OH, Ashmawy EM, Gaowgzeh RAM. Treadmill walking exercise modulates bone mineral status and inflammatory cytokines in obese asthmatic patients with long term intake of corticosteroids. African Health Sciences 2016;16(3):798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12617000991314 {published data only}
- ACTRN12617000991314. The impact of varying exercise training intensity on clinical asthma outcomes and inflammation in adults with asthma. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372941 (first received 26 June 2017).
Alison 2000 {published data only}
- Alison J. Physical training improves asthmatic subjects' cardiopulmonary function. Australian Journal of Physiotherapy 2000;46(4):315. [Google Scholar]
Astafieva 2011 {published data only}
- Astafieva N, Kobzev D, Gamova I, Perfilova I, Udovichenko E. Physical rehabilitation in asthma management. European Respiratory Journal 2011;38(55):879s. [Google Scholar]
Bacon 2013 {published data only}
- Bacon SL, Lavoie KL, Bourbeau J, Ernst P, Maghni K, Gautrin D, et al. The effects of a multisite aerobic exercise intervention on asthma morbidity in sedentary adults with asthma: the ex-asthma study randomised controlled trial protocol. BMJ Open 2013;3(6):e003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bacon 2015 {published data only}
- Bacon S, Lavoie K, Bourbeau J, Lemiere C, Pepin V, Beland M. Impact of a 12-week supervised aerobic exercise program on asthma control in adult patients with asthma: preliminary results from the EX-ASTHMA behavioral RCT. Chest 2015;148(4 Suppl):640A. [Google Scholar]
Boyd 2011 {published data only}
- Boyd A, Estell K, Dransfield M, Schwiebert LM. The effect of aerobic exercise on asthma-related responses in adults. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl 1):AB211. [Google Scholar]
- Boyd AW, Estell K, Dransfield M, Schwiebert L. The effect of aerobic exercise on asthma-related responses in adults. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB223. [Google Scholar]
Boyd 2012 {published data only}
- Boyd A, Yang CT, Estell K, Tuggle C, Gerald LB, Dransfield M, et al. Feasibility of exercising adults with asthma: a randomized pilot study. Allergy, Asthma & Clinical Immunology 2012;8(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A, Yang CT, Estell K, Tuggle C, Gerald LB, Dransfield M, et al. Feasibility of exercising adults with asthma: a randomized pilot study. Allergy, Asthma & Clinical Immunology 2012;8(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cafarella 2001 {published data only}
- Cafarella P, Frith P. Patient completion of pulmonary rehabilitation reduces carer handicap. Respirology 2001;6(Suppl):A35.75. [Google Scholar]
Carvalho 2014 {published data only}
- Carvalho CRF, França-Pinto A, Mendes FAR, Stelmach R, Cukier A, Agondi RC. Aerobic training decrease bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomized controlled trial. European Respiratory Journal 2014;44(Suppl 58):1707. [DOI] [PubMed] [Google Scholar]
Carvalho 2014a {published data only}
- Carvalho CRF, França-Pinto A, Mendes FAR, Stelmach R, Cukier A, Agondi RC, et al. Aerobic training decrease bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomized controlled trial. European Respiratory Journal 2014;44(Suppl 58):1707. [Google Scholar]
Cox 1991 {published data only}
- Cox NJ, Hendriks J, Binkhorst RA, Herwaarden CL. Favorable effects of rehabilitation in patients with CARA. Nederlands Tijdschrift voor Geneeskunde 1991;135(22):987-91. [PubMed] [Google Scholar]
Cox 1993 {published data only}
- Cox NJ, Hendricks JC, Binkhorst RA, Herwaarden CL. A pulmonary rehabilitation program for patients with asthma and mild chronic obstructive pulmonary diseases (COPD). Lung 1993;171(4):235-44. [DOI] [PubMed] [Google Scholar]
Da Silva 2015 {published data only}
- Da Silva RA, De Freitas PD, Rocco PGL, Saccomani M, Grandi A, Evaristo KB. Comparison among different instruments to evaluate the clinical control in asthmatic patients submitted to aerobic training. European Respiratory Journal 2015;46(Suppl 59):PA3712. [Google Scholar]
Del Giacco 2016 {published data only}
- Del Giacco SR, Garcia-Larsen V. Aerobic exercise training reduces bronchial hyper-responsiveness and serum pro-inflammatory cytokines in patients with asthma. BMJ Evidence-Based Medicine 2016;21(2):70. [DOI] [PubMed] [Google Scholar]
Didour 1998 {published data only}
- Didour M, Pravosudov V, Sinitcina T. Effects of a short pulmonary rehabilitation in patients with asthma. European Respiratory Journal 1998;12(Suppl 28):404S. [Google Scholar]
Didour 2002 {published data only}
- Didour MD, Trophymov VI, Martchenko VI, Titova ON, Turkin YN, Rubakov DP. Effects of a pulmonary rehabilitation (PR) in outpatients with asthma. European Respiratory Journal 2002;20(Suppl 38):183s. [Google Scholar]
Dogra 2010 {published data only}
- Dogra S, Jamnik V, Baker J. Self-directed exercise improves perceived measures of health in adults with partly controlled asthma. Journal of Asthma 2010;47(9):972-7. [DOI] [PubMed] [Google Scholar]
Dogra 2010a {published data only}
- Dogra S. Regular Physical Activity Is Associated With Better Asthma Control in Adults [PhD thesis]. York University (Canada), 2010. [Google Scholar]
Dogra 2011 {published data only}
- Dogra S, Kuk JL, Baker J, Jamnik V. Exercise is associated with improved asthma control in adults. European Respiratory Journal 2011;37(2):318-23. [DOI] [PubMed] [Google Scholar]
Emtner 2005 {published data only}
- Emtner M, Johansson H, Holmback J. A randomised controlled trial of the effects of the quality of life physical capacity and exercise in adults with asthma after short term intervention. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1172. [Google Scholar]
Farid 2005 {published data only}
- Farid R, Azad FJ, Atri AE, Rahimi MB, Khaledan A, Talaei-Khoei M, et al. Effect of aerobic exercise training on pulmonary function and tolerance of activity in asthmatic patients. Iranian Journal of Allergy, Asthma & Immunology 2005;4(3):133-8. [PubMed] [Google Scholar]
Farid 2005a {published data only}
- Farid R, Jabbari Azad F, Ebrahimi Atri A, Rafatpanah H, Talei-Khoei M. Effect of aerobic exercise training on pulmonary function and tolerance of activity in asthmatic patients. In: XIX World Allergy Organization Congress; 2005 June 26-July 1; Munich, Germany. 2005:Abstract 1514. [PubMed]
Franca‐Pinto 2015 {published data only}
- Franca-Pinto A, Mendes FAR, De Carvalho-Pinto RM, Agondi RC, Cukier A, Stelmach R, et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax 2015;70(8):732-9. [DOI] [PubMed] [Google Scholar]
Freitas 2014 {published data only}
- Freitas PD, Ferreira PG, Analuci S, Stelmach R, Pinto RC, Sage JM. Effects of exercise training in a weight loss lifestyle intervention on clinical control, quality of life and psychosocial symptoms in obese asthmatics: a RCT. European Respiratory Journal 2014;44(Suppl 58):1710. [Google Scholar]
Freitas 2014a {published data only}
- Freitas PD, Ferreira PG, Silva AG, Cukier A, Pinto RC, Salge JM. Exercise training associated with a weight-loss lifestyle program improves daily physical activity in obese asthmatics. European Respiratory Journal 2014;44(Suppl 58):P3361. [Google Scholar]
Freitas 2014b {published data only}
- Freitas PD, Ferreira PG, Silva AG, Cukier A, Pinto RC, Salge JM, et al. Exercise training associated with a weight-loss lifestyle program improves daily physical activity in obese asthmatics. European Respiratory Journal 2014;44(Suppl 58):P3361. [Google Scholar]
Freitas 2014c {published data only}
- Freitas PD, Ferreira PG, Analuci S, Stelmach R, Pinto RC, Sage JM, et al. Effects of exercise training in a weight loss lifestyle intervention on clinical control, quality of life and psychosocial symptoms in obese asthmatics: a RCT. European Respiratory Journal 2014;44(Suppl 58):1710. [Google Scholar]
Freitas 2015 {published data only}
- Freitas PD, Ferreira PG, da Silva A, Trecco S, Stelmach R, Cukier A, et al. The effects of exercise training in a weight loss lifestyle intervention on asthma control, quality of life and psychosocial symptoms in adult obese asthmatics: protocol of a randomized controlled trial. BMC Pulmonary Medicine 2015;15(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freitas 2015a {published data only}
- Freitas PD, Ferreira PG, Silva AG, Cukier A, Stelmach R, Carvalho-Pinto R, et al. Increased fitness and weight-loss are associated with improvement in daily life physical activity and clinical control in obese asthmatics: a RCT. European Respiratory Journal 2015;46(Suppl 59):OA479. [Google Scholar]
Freitas 2015b {published data only}
- Freitas PD, Ferreira PG, Silva AG, Cukier A, Stelmach R, Carvalho-Pinto R. Exercise training is a determinant of weight-loss and improvement on asthma control, airway inflammation and psychosocial morbidity in obese asthmatics: a RCT. European Respiratory Journal 2015;46(Suppl 59):PA734. [Google Scholar]
Freitas 2016 {published data only}
- Freitas PD, Silva AG, Ferreira PG, da Silva A, Salge JM, Cukier A. The role of exercise training in a weight loss program on psychosocial morbidity, sleep quality and physical activity in obese asthmatics: a RCT. European Respiratory Journal 2016;48(Suppl 60):OA3048. [Google Scholar]
Freitas 2016a {published data only}
- Freitas P, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto R, Fernandes FLA, et al. Mechanisms underlying the role of exercise training as part of a weight loss program on asthma control in obese asthmatics. European Respiratory Journal 2016;48(Suppl 60):PA527. [Google Scholar]
Freitas 2016b {published data only}
- Freitas PD, Silva AG, Ferreira PG, da Silva A, Salge JM, Cukier A, et al. The role of exercise training in a weight loss program on psychosocial morbidity, sleep quality and physical activity in obese asthmatics: a RCT. European Respiratory Journal 2016;48(Suppl 60):OA3048. [Google Scholar]
Freitas 2017 {published data only}
- Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FLA, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma: a randomized controlled trial. American Journal of Respiratory & Critical Care Medicine 2017;195(1):32-42. [DOI] [PubMed] [Google Scholar]
Freitas 2017a {published data only}
- Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FLA. The role of exercise in a weight-loss program on clinical control in obese adults with asthma: a randomized controlled trial. American Journal of Respiratory & Critical Care Medicine 2017;195(1):32-42. [DOI] [PubMed] [Google Scholar]
Freitas 2017b {published data only}
- Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FLA, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma: a randomized controlled trial. American Journal of Respiratory & Critical Care Medicine 2017;195(1):32-42. [DOI] [PubMed] [Google Scholar]
Freitas 2018 {published data only}
- Freitas PD, Silva AG, Ferreira PG, Da Silva A, Salge JM, Carvalho-Pinto RM, et al. Exercise improves physical activity and comorbidities in obese adults with asthma. Medicine and Science in Sports and Exercise 2018;50(7):1367-76. [DOI] [PubMed] [Google Scholar]
Freitas 2018a {published data only}
- Freitas PD, Silva AG, Ferreira PG, Da Silva A, Salge JM, Carvalho-Pinto RM, et al. Exercise improves physical activity and comorbidities in obese adults with asthma. European Respiratory Journal 2018;52(Suppl 62):OA1619. [DOI] [PubMed] [Google Scholar]
Freitas 2019 {published data only}
- Freitas PD, Xavier RF, Passos NFP, Carvalho-Pinto RM, Cukier A, Martins MA, et al. Effects of a behaviour change intervention aimed at increasing physical activity on clinical control of adults with asthma: study protocol for a randomised controlled trial. BMC Sports Science, Medicine and Rehabilitation 2019;11(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Girodo 1992 {published data only}
- Girodo M, Ekstrand KA, Metivier GJ. Deep diaphragmatic breathing: rehabilitation exercises for the asthmatic patient. Archives of Physical Medicine and Rehabilitation 1992;73(8):717-20. [PubMed] [Google Scholar]
Goldman 1997 {published data only}
- Goldman J, Carr V, Dobson L, Jones S, Rowles R, Wallace L. A randomised controlled trial of pulmonary rehabilitation PR in a district general hospital. Thorax 1997;52:A10. [Google Scholar]
Goncalves 2006 {published data only}
- Goncalves RC, Nunes MPT, Cukier A, Stelmach R, Ribeiro M, Martins MA, et al. Comparison between breathing exercises and aerobic conditioning on symptoms, quality of life and exhaled nitric oxide in asthmatic adults. European Respiratory Journal 2006;28(Suppl 50):370s. [Google Scholar]
Gonçalves 2008 {published data only}
- Gonçalves RC, Nunes MPT, Cukier A, Stelmach R, Martins MA, Carvalho CRF. Effects of an aerobic physical training program on psychosocial characteristics, quality-of-life, symptoms and exhaled nitric oxide in individuals with moderate or severe persistent asthma. Brazilian Journal of Physical Therapy 2008;12(2):127-35. [Google Scholar]
Greening 2014 {published data only}
- Greening NJ, Williams JEA, Hussain SF, Harvey-Dunstan TC, Bankart MJ, Chaplin EJ, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014;349:g4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallstrand 2000 {published data only}
- Hallstrand TS, Bates PW, Schoene RB. Aerobic conditioning in mild asthma decreases the hyperpnea of exercise and improves exercise and ventilatory capacity. Chest 2000;118(5):1460-9. [DOI] [PubMed] [Google Scholar]
IRCT138811143270N1 {published data only}
- IRCT138811143270N1. The effects of exercising on patients with asthma. irct.ir/trial/3345 (first received 28 April 2010).
IRCT2015011420666N1 {published data only}
- IRCT2015011420666N1. The effect of resistance and specific training in patient with asthma. irct.ir/trial/18286 (first received 16 March 2015).
IRCT2016052328028N1 {published data only}
- IRCT2016052328028N1. Effect of aerobic exercise training on inflammatory markers and sex hormones in asthmatic women. irct.ir/trial/22831 (first received 5 January 2017).
Jaakkola 2017 {published data only}
- Jaakkola JJK, Heikkinen SAM, Hernberg S, Kiihamaki S-P, Jaakkola MS. Regular exercise and asthma control in adults: a randomized controlled trial. European Respiratory Journal 2017;50(Suppl 61):PA3563. [Google Scholar]
Jaakkola 2019 {published data only}
- Jaakkola JJK, Aalto SAM, Hernberg S, Kiihamaki SP, Jaakkola MS. Regular exercise improves asthma control in adults: a randomized controlled trial. Scientific Reports 2019;9(1):12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jalbert 2018 {published data only}
- Jalbert A-C, Lavoie K, Bacon S. Effects of aerobic exercise on asthma control in eosinophilic asthma patients: a randomized controlled trial. European Respiratory Journal 2018;52(Suppl 62):PA5041. [Google Scholar]
Kouznetsova 2007 {published data only}
- Kouznetsova N, Koutsenko M, Chuchalin A. Influence of groups physical exercises upon psychic and physical status of asthma patients. European Respiratory Journal 2007;30(Suppl 51):766s. [Google Scholar]
Lenz 2001 {published data only}
- Lenz S, Lachtermann E, Weber M, Pleyer K, Schmitz M, Jung K. Comparison of interval training at different intensities on rehabilitation of severe bronchial asthma and chronic obstructive pulmonary disease (COPD). Deutsche Zeitschrift fur Sportmedizin 2001;52(7/8):S16. [Google Scholar]
Lowe 2018 {published data only}
- Lowe AA, Garcia DO, Stern DA, Gerald LB, Bime C. Home-based exercise intervention versus remote asthma care guidance via telephone/text message in obese asthmatics: a pilot randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2018;197(Meeting Abstracts):A2715. [Google Scholar]
Lowe 2018a {published data only}
- Lowe AA, Garcia DO, Stern DA, Gerald LB, Bime C. Feasibility of a home-based exercise intervention with remote guidance for obese asthmatics. American Journal of Respiratory and Critical Care Medicine 2018;197(Meeting Abstracts):A4843. [Google Scholar]
Ma 2010 {published data only}
- Ma J, Strub P, Camargo CA, Xiao L, Ayala E, Gardner CD, et al. The Breathe Easier through Weight Loss Lifestyle (BE WELL) Intervention: a randomized controlled trial. BMC Pulmonary Medicine 2010;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2015 {published data only}
- Ma J, Strub P, Xiao L, Lavori PW, Camargo CA Jr, Wilson SR, et al. Behavioral weight loss and physical activity intervention in obese adults with asthma: a randomized trial. Annals of the American Thoracic Society 2015;12(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendes 2011 {published data only}
- Mendes FA, Almeida FM, Cukier A, Stelmach R, Jacob-Filho W, Martins MA, et al. Effects of aerobic training on airway inflammation in asthmatic patients. Medicine & Science in Sports & Exercise 2011;43(2):197-203. [DOI] [PubMed] [Google Scholar]
Mendes 2013 {published data only}
- Mendes F, França-Pinto A, Cukier A, Stelmach R, Agondi R, Martins MA. Aerobic training decreases bronchial hyperresponsiveness, serum chemokines and symptoms in asthmatic patients: randomized controlled trial. European Respiratory Journal 2013;42(Suppl 57):680s. [Google Scholar]
Mendes 2013a {published data only}
- Mendes F, Franca-Pinto A, Cukier A, Stelmach R, Agondi R, Martins MA, et al. Aerobic training decreases bronchial hyperresponsiveness, serum chemokines and symptoms in asthmatic patients: randomized controlled trial. European Respiratory Journal 2013;42(Suppl 57):680s. [Google Scholar]
Meyer 1999 {published data only}
- Meyer A, Gunther S, Volmer T, Keller A, Taube K, Pforte A. Weekly training improves physical capacity in older asthmatics. Pneumologie 1999;53(SH1):S22. [Google Scholar]
Meyer 2015 {published data only}
- Meyer A, Gunther S, Volmer T, Taube K, Baumann HJ. A 12-month, moderate-intensity exercise training program improves fitness and quality of life in adults with asthma: a controlled trial. BMC Pulmonary Medicine 2015;15(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyer 2015a {published data only}
- Meyer A, Gunther S, Volmer T, Taube K, Baumann HJ. A 12-month, moderate-intensity exercise training program improves fitness and quality of life in adults with asthma: a controlled trial. BMC Pulmonary Medicine 2015;15(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00195117 (a) {published data only}
- NCT00195117. A randomized trial of changing exercise and physical activity behavior in asthma patients. clinicaltrials.gov/ct2/show/NCT00195117 (first received 19 September 2005).
NCT00195117 (b) {published data only}
- NCT00195117. A randomised trial of changing exercise and physical activity behavior in asthma patients. clinicaltrials.gov/ct/show/NCT00195117 (first received 19 September 2005).
NCT00839137 {published data only}
- NCT00839137. Exercise therapy for asthma (ETA Trial). clinicaltrials.gov/ct2/show/NCT00839137 (first received 9 February 2009).
NCT00901095 {published data only}
- NCT00901095. Can diet- and exercise-induced weight loss improve asthma control in adults? clinicaltrials.gov/ct2/show/NCT00901095 (first received 13 May 2009).
NCT00953342 {published data only}
- NCT00953342. Impact of aerobic exercise on asthma morbidity. clinicaltrials.gov/ct2/show/NCT00953342 (first received 6 August 2009).
NCT00989365 {published data only}
- Mendes FA, Gonçalves RC, Nunes MP, Saraiva-Romanholo BM, Cukier A, Stelmach R, et al. Effects of aerobic training on psychosocial morbidity and symptoms in patients with asthma: a randomized clinical trial. Chest 2010;138(2):331-7. [DOI] [PubMed] [Google Scholar]
- NCT00989365. Effect of aerobic training on asthmatic patients. clinicaltrials.gov/ct2/show/NCT00989365 (first received 5 October 2009).
NCT01097473 {published data only}
- NCT01097473. Long term physical training in asthma. clinicaltrials.gov/ct2/show/NCT01097473 (first received 1 April 2010).
NCT02012400 {published data only}
- NCT02012400. Effects of regular exercise on adult asthma. clinicaltrials.gov/ct2/show/NCT02012400 (first received 16 December 2013).
NCT02033122 {published data only}
- NCT02033122. Effect of aerobic training on bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02033122 (first received 10 January 2014).
NCT02188940 {published data only}
- NCT02188940. Effects of exercise training in a weight-loss lifestyle intervention on clinical control and psychosocial morbidity in obese asthmatics: a randomized and controlled trial. clinicaltrials.gov/ct2/show/NCT02188940 (first received 14 July 2014).
NCT03145883 {published data only}
- NCT03145883. Exercise and asthma in obese adults. clinicaltrials.gov/ct2/show/NCT03145883 (first received 9 May 2017).
Pinto 2012 {published data only}
- Pinto A, Mendes F, Agondi R, Saraiva-Romanholo B, Stelmach R, Cukier A. Effect of aerobic exercise training on bronchial hyperresponsiveness, airway inflammation and health related quality of life in asthmatic patients: a pilot study. European Respiratory Journal 2012;40(Suppl 56):69s. [Google Scholar]
Pinto 2012a {published data only}
- Pinto A, Mendes F, Agondi R, Saraiva-Romanholo B, Stelmach R, Cukier A, et al. Effect of aerobic exercise training on bronchial hyperresponsiveness, airway inflammation and health related quality of life in asthmatic patients: a pilot study. European Respiratory Journal 2012;40(Suppl 56):69s. [Google Scholar]
Pollart 2012 {published data only}
- Pollart SM, Elward KS, Platts-Mills TAE. Improvements in quality of life measures in a structured exercise program for persistent asthma. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl 1):AB60. [Google Scholar]
Pollart 2012a {published data only}
- Pollart SM, Elward KS, Platts-Mills TAE. Improvements in quality of life measures in a structured exercise program for persistent asthma. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl 1):AB60. [Google Scholar]
Postolache 2002 {published data only}
- Postolache PA. Long-term benefits of pulmonary rehabilitation in patients with chronic asthma. European Respiratory Journal 2002;20(Suppl 38):454s. [Google Scholar]
Refaat 2015 {published data only}
- Refaat A, Gawish M. Effect of physical training on health-related quality of life in patients with moderate and severe asthma. Egyptian Journal of Chest Diseases and Tuberculosis 2015;64(4):761-6. [Google Scholar]
Riegels‐Nielsen 2000 {published data only}
- Riegels-Nielsen T, Sondergaard R, Jensen JI, Pedersen PK. Improved lung function and exercise tolerance in adult asthmatics following 7 weeks of strenuous bicycle training. Journal of Sports Sciences 2000;18(7):496-7. [Google Scholar]
Sampaio 2002 {published data only}
- Sampaio LMM, Jamami M, Pires VA, Silva AB, Costa D. Respiratory muscle strength in asthmatic patient submitted by respiratory muscle training and physical training. Revista de Fisioterapia da Universidade de Sao Paulo 2002;9(2):43-8. [Google Scholar]
Shaw 2011 {published data only}
- Shaw BS, Shaw I. Static standing posture and pulmonary function in moderate-persistent asthmatics following aerobic and diaphragmatic breathing training. Pakistan Journal of Medical Sciences Online 2011;27(3):549-52. [Google Scholar]
Shaw 2011a {published data only}
- Shaw BS, Shaw I. Pulmonary function and abdominal and thoracic kinematic changes following aerobic and inspiratory resistive diaphragmatic breathing training in asthmatics. Lung 2011;189(2):131-9. [DOI] [PubMed] [Google Scholar]
Toennesen 2016 {published data only}
- Toennesen LL, Meier N, Hostrup M, Porsbjerg C, Backer V. High-intensity interval training improves maximal oxygen consumption in untrained adult asthmatics. American Journal of Respiratory and Critical Care Medicine 2016;193(Meeting Abstracts):A2304. [Google Scholar]
Turner 2008 {published data only}
- Turner S, Eastwood P, Cook A, Thompson P, Jenkins SS. Exercise training improves quality of life in middle-aged and older adults with moderate to severe asthma. Respirology 2008;13(Suppl 2):A39. [Google Scholar]
Turner 2008a {published data only}
- Turner S, Eastwood P, Cook A, Thompson P, Jenkins S. Effects of exercise training on quality of life and 6 minute walk distance (6MWD) in adults with fixed airway obstruction asthma (FAOA). In: European Respiratory Society Annual Congress; 2008 October 4-8; Berlin, Germany. 2008:P4106.
References to studies awaiting assessment
Budnevsky 2018 {published data only}
- Budnevsky AV, Tribuntceva LV, Titova LA, Kozhevnikova SA, Ovsyannikov ES, Shkatova YS. Patient education and exercising in addition to standard therapy in asthma and metabolic syndrome. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2018;9(3):396-400. [Google Scholar]
IRCT2014041617299N {published data only}
- IRCT2014041617299N. Effect of care program on asthma control and quality of life [Comparing the effect of a designed care program by cases without intervention on control of asthma and quality of life in 60 patients with asthma referring to selected clinics of Shiraz University of Medical Sciences]. irct.ir/trial/15946 (first received 19 December 2014).
NTR4398 {published data only}
- NTR4398. Obesity and asthma: effect of training on top of surgery. trialregister.nl/trial/4262 (first received 14 January 2014).
References to ongoing studies
NCT03630432 {published data only}
- NCT03630432. Pulmonary rehabilitation for uncontrolled asthma associated with elevated BMI. clinicaltrials.gov/ct2/show/NCT03630432 (first received 14 August 2018).
Additional references
Aggarwal 2018
- Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. npj Primary Care Respiratory Medicine 2018;28:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alison 2017
- Alison JA, McKeough ZJ, Johnston K, McNamara RJ, Spencer LM, Jenkins SC, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology 2017;22(4):800-19. [DOI] [PubMed] [Google Scholar]
Bolton 2013
- Bolton CE, Bevan-Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013;68 Suppl 2:ii1-30. [DOI] [PubMed] [Google Scholar]
Burge 2020
- Burge AT, Cox NS, Abramson MJ, Holland AE. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD012626. [DOI: 10.1002/14651858.CD012626.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Camillo 2015
- Camillo CA, Osadnik CR, Janssens W, Troosters T. How to assess the benefits of pulmonary rehabilitation. In: Anzueto A, Heijdra Y, Hurst JR, editors(s). Controversies in COPD. Plymouth, UK: European Respiratory Society, 2015:328. [Google Scholar]
Carson 2013
- Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No: CD001116. [DOI: 10.1002/14651858.CD001116.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2014
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk P, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal 2014;43(2):343-73. [DOI] [PubMed] [Google Scholar]
Cochrane Airways 2019
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 7 May 2019).
Cordova‐Rivera 2018
- Cordova-Rivera L, Gibson PG, Gardiner PA, McDonald VM. A systematic review of associations of physical activity and sedentary time with asthma outcomes. Journal of Allergy and Clinical Immunology 2018;6(6):1968-81. e2. [DOI] [PubMed] [Google Scholar]
Cordova‐Rivera 2018a
- Cordova-Rivera L, Gibson PG, Gardiner PA, Powell H, McDonald VM. Physical activity and exercise capacity in severe asthma: key clinical associations. Journal of Allergy and Clinical Immunology 2018;6(3):814-22. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 14 November 2019. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Crapo 2000
- Crapo RO. Guidelines for methacholine and exercise challenge testing - 1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. American Journal of Respiratory and Critical Care Medicine 2000;161:309-29. [DOI] [PubMed] [Google Scholar]
da Silva 2016
- da Silva RA, Rocco PGL, Mazzucatto F, Cukier A, Stelmach R, Martins MA, et al. High intensity interval training increases daily life physical activity and quality of life in patients with moderate and severe asthma. European Respiratory Journal 2016;48:PA1338. [Google Scholar]
Dogra 2009
- Dogra S, Baker J, Ardern CI. The role of physical activity and body mass index in the health care use of adults with asthma. Annals of Allergy, Asthma and Immunology 2009;102(6):462-8. [DOI] [PubMed] [Google Scholar]
Dogra 2011
- Dogra S, Kuk JL, Baker J, Jamnik V. Exercise is associated with improved asthma control in adults. European Respiratory Journal 2011;37(2):318-23. [DOI] [PubMed] [Google Scholar]
Ebmeier 2017
- Ebmeier S, Thayabaran D, Braithwaite I, Bénamara C, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 2017;390(10098):935-45. [DOI] [PubMed] [Google Scholar]
Eisner 2000
- Eisner MD, Katz PP, Yelin EH, Shiboski SC, Blanc PD. Risk factors for hospitalization among adults with asthma: the influence of sociodemographic factors and asthma severity. Respiratory Research 2000;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2021
- Feng Z, Wang J, Xie Y, Li J. Effects of exercise-based pulmonary rehabilitation on adults with asthma: a systematic review and meta-analysis. Respiratory Research 2021;22(1):33. [DOI: 10.1186/s12931-021-01627-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
FIRS 2017
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease Second Edition. www.who.int/gard/publications/The_Global_Impact_of_Respiratory_Disease.pdf (accessed prior to 2 July 2019).
Foster 2017
- Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. European Respiratory Journal 2017;50(3):1700765. [DOI] [PubMed] [Google Scholar]
França‐Pinto 2015
- França-Pinto A, Mendes FAR, Carvalho-Pinto RM, Agondi RC, Cukier A, Stelmach R, et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax 2015;70(8):732-9. [DOI] [PubMed] [Google Scholar]
Freitas 2017
- Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FLA, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma. A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2017;195(1):32-42. [DOI] [PubMed] [Google Scholar]
Freitas 2021
- Freitas PD, Xavier RF, McDonald VM, Gibson PG, Cordova-Rivera L, Furlanetto KC, et al. Identification of asthma phenotypes based on extrapulmonary treatable traits. European Respiratory Journal 2021;57(1):2000240. [DOI: 10.1183/13993003.00240-2020] [DOI] [PubMed] [Google Scholar]
GAN 2018
- Global Asthma Network. The Global Asthma Report 2018. www.globalasthmareport.org/ (accessed prior to 2 July 2019).
Gibson 2015
- Gibson PG, McDonald VM. Asthma–COPD overlap 2015: now we are six. Thorax 2015;70(7):683-91. [DOI] [PubMed] [Google Scholar]
GINA 2021
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2021. ginasthma.org (accessed prior to 2 July 2022).
Gordon 2019
- Gordon CS, Waller JW, Cook RM, Cavalera SL, Lim WT, Osadnik CR. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta-analysis. Chest 2019;156(1):80-91. [DOI: 10.1016/j.chest.2019.04.009] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 4 April 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Hiles 2021
- Hiles SA, Urroz PD, Gibson PG, Bogdanovs A, McDonald VM. A feasibility randomised controlled trial of Novel Activity Management in severe ASthma-Tailored Exercise (NAMASTE): yoga and mindfulness. BMC Pulmonary Medicine 2021;21(1):71. [DOI: 10.1186/s12890-021-01436-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holland 2014
- Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. European Respiratory Journal 2014;44(6):1428. [DOI: 10.1183/09031936.00150314] [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2(1):75-9. [DOI] [PubMed] [Google Scholar]
Juniper 2005
- Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respiratory Medicine 2005;99(5):553-8. [DOI] [PubMed] [Google Scholar]
Lucas 2005
- Lucas SR, Platts-Mills TAE. Physical activity and exercise in asthma: relevance to etiology and treatment. Journal of Allergy and Clinical Immunology 2005;115(5):928-34. [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD003793. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane Reviews. In: Global Evidence Summit; 2017 September 13-16; Cape Town. 2017.
McDonald 2018
- McDonald VM, Hiles SA, Jones KA, Clark VL, Yorke J. Health‐related quality of life burden in severe asthma. Medical Journal of Australia 2018;209(S2):S28-33. [DOI] [PubMed] [Google Scholar]
McDonald 2019
- McDonald VM, Hiles SA, Godbout K, Harvey ES, Marks GB, Hew M, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology 2019;24(1):37-47. [DOI: 10.1111/resp.13389] [DOI] [PubMed] [Google Scholar]
McGowan 2016
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of Clinical Epidemiology 2016;75:40-6. [DOI: 10.1016/j.jclinepi.2016.01.021] [DOI] [PubMed] [Google Scholar]
McLoughlin 2021
- McLoughlin RF, McDonald VM. The management of extrapulmonary comorbidities and treatable traits; obesity, physical inactivity, anxiety and depression in adults with asthma. Frontiers in Allergy 2021;2:53. [DOI: 10.3389/falgy.2021.735030/full] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2011
- Ng C, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chronic Respiratory Disease 2011;9(1):17-26. [DOI: 10.1177/1479972311430335] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2018
- Noel-Storr AH, Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford. 2018.
Osadnik 2018
- Osadnik CR, Loeckx M, Louvaris Z, Demeyer H, Langer D, Rodrigues FM, et al. The likelihood of improving physical activity after pulmonary rehabilitation is increased in patients with COPD who have better exercise tolerance. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:3515-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osadnik 2019
- Osadnik CR, Singh S. Pulmonary rehabilitation for obstructive lung disease. Respirology 2019;24(9):1-8. [DOI: 10.1111/resp.13569] [DOI] [PubMed] [Google Scholar]
Parsons 2013
- Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. American Journal of Respiratory and Critical Care Medicine 2013;187(9):1016-27. [DOI] [PubMed] [Google Scholar]
Poulos 2014
- Poulos LM, Correll PK, Toelle BG, Reddel HK, Marks GB. Lung disease in Australia. www.cesphn.org.au/preview/chronic-disease-management-1/respiratory-1/1851-lung-disease-in-australia-report-final-22october14/file (accessed prior to 3 August 2022).
Reddel 2009
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations. American Journal of Respiratory and Critical Care Medicine 2009;180(1):59-99. [DOI] [PubMed] [Google Scholar]
Reddel 2015
- Reddel HK, Sawyer SM, Everett PW, Flood PV, Peters MJ. Asthma control in Australia: a cross‐sectional web‐based survey in a nationally representative population. Medical Journal of Australia 2015;202(9):492-6. [DOI] [PubMed] [Google Scholar]
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Ritz 2010
- Ritz T, Rosenfield D, Steptoe A. Physical activity, lung function, and shortness of breath in the daily life of individuals with asthma. Chest 2010;138(4):913-8. [DOI] [PubMed] [Google Scholar]
Sahin 2019
- Sahin H, Naz I. Comparing the effect of pulmonary rehabilitation in patients with uncontrolled and partially controlled asthma. Journal of Asthma 2019;56(1):87-94. [DOI] [PubMed] [Google Scholar]
Scott 2015
- Scott HA, Latham JR, Callister R, Pretto JJ, Baines K, Saltos N, et al. Acute exercise is associated with reduced exhaled nitric oxide in physically inactive adults with asthma. Annals of Allergy, Asthma and Immunology 2015;114(6):470-9. [DOI] [PubMed] [Google Scholar]
Spruit 2013
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188(8):e13-64. [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI] [PubMed] [Google Scholar]
Toennesen 2018
- Toennesen LL, Soerensen ED, Hostrup M, Porsbjerg C, Bangsbo J, Backer V. Feasibility of high-intensity training in asthma. European Clinical Respiratory Journal 2018;5(1):1468714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiler 2010
- Weiler JM, Anderson SD, Randolph C, Bonini S, Craig TJ, Pearlman DS, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Annals of Allergy, Asthma and Immunology 2010;105(6):S1-47. [DOI] [PubMed] [Google Scholar]
Winn 2018
- Winn CON, Mackintosh KA, Eddolls WTB, Stratton G, Wilson AM, Rance JY, et al. Perceptions of asthma and exercise in adolescents with and without asthma. Journal of Asthma 2018;55(8):868-76. [DOI] [PubMed] [Google Scholar]
Zampogna 2021
- Zampogna E, Ambrosino N, Centis R, Cherubino F, Migliori GB, Pignatti P, et al. Minimal clinically important difference of the 6-min walking test in patients with asthma. International Journal of Tuberculosis and Lung Disease 2021;25(3):215-21. [DOI: 10.5588/ijtld.20.0928] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Osadnik 2019a
- Osadnik CR, McDonald VM, Holland AE. Pulmonary rehabilitation for adults with asthma. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013485. [DOI: 10.1002/14651858.CD013485] [DOI] [PMC free article] [PubMed] [Google Scholar]