ABSTRACT
Background
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are highly effective in improving glycaemic control either as monotherapy or in combination with other hypoglycaemic drugs, and have low incidence of side effects, such as hypoglycaemia, nausea and weight gain, thus increasing patients' adherence to therapy.
Methods
In this review we report the most recent studies demonstrating the beneficial effects of GLP-1RAs on renal outcomes, and also discuss the direct and indirect mechanisms through which they confer kidney protection. Finally, we discuss the metabolic and anti-inflammatory effects of GLP-1RAs in diabetic patients with COVID-19 disease.
Results
GLP-1RAs have a nephroprotective action, which is expressed through both indirect (improvement of blood pressure and glycaemic control, weight loss) and direct (restoration of normal intrarenal haemodynamics, prevention of ischaemic and oxidative damage) effects. They have shown also metabolic and anti-inflammation beneficial effects in patients with COVID-19 disease.
Conclusions
GLP-1RAs prevent albuminuria and slow the decline of renal function towards end stage renal disease in patients with diabetic kidney disease. They might be an opportunity to break the typical inflammation processes of COVID-19 disease.
Keywords: albuminuria, COVID-19 disease, diabetic kidney disease, GLP-1 receptor agonists, nephroprotection
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is associated with micro- and macrovascular complications, which are often responsible for severe disability in affected patients, with high costs for health and social care. T2DM represents a major cause of chronic kidney disease (CKD) worldwide, and statistics show that the incidence of end-stage renal disease (ESRD) requiring renal replacement therapy varies from 0.04% to 1.8% per year [1]. Renal damage related to T2DM includes both structural (glomerular basement membrane thickening, mesangial expansion, interstitial fibrosis, loss of capillary architecture, hyalinosis of small and medium caliber arteries) and functional (dysfunction of the mitochondrial respiratory chain, over expression of proinflammatory cytokines such as interleukin (IL)-6, IL-8, IL-18, TNF-α, interferon (INF)-γ) alterations induced by hyperglycaemia [2–4]. The resulting clinical manifestations configure the so-called ‘diabetic kidney disease’ (DKD), characterized by selective and nonselective proteinuria, hypertension and progressive decline in renal function. Except for angiotensin-converting enzyme inhibitors and angiotensin-II receptor antagonists, for many years no drugs capable of improving renal outcomes in DKD patients have been available. Therefore, the development of new molecules that allow prevention of the onset and progression of kidney damage has become a priority. Current therapeutic strategies aim at optimizing glycaemic control through various modalities: (i) increasing the availability of circulating insulin (through the administration of exogenous insulin or drugs that promote the secretion of endogenous insulin), (ii) improving tissues sensitivity to insulin, (iii) delaying the absorption of carbohydrates in the gut and (iv) promoting the urinary excretion of glucose. Over the past 10 years, the advent of drugs such as glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs), dipeptidyl peptidase 4 (DPP-4) inhibitors and sodium-glucose co-transport 2 (SGLT-2) gave new impetus to the implementation of antidiabetic therapy, which today must be considered to all intents and purposes a topic of multidisciplinary interest. Emerging data support the efficacy of GLP-1RAs, in reducing the incidence of major adverse cardiovascular events (MACEs), preventing the onset of macroalbuminuria and slowing the progression of renal damage toward ESRD [5, 6], thus stimulating the interest of endocrinologists, cardiologists and nephrologists. Despite this evidence, the mechanisms by which GLP-1RAs confer nephroprotection are poorly understood and their use in clinical practice is still limited. The aim of this review is to analyse the latest evidence on the pharmacokinetics and pharmacodynamics of GLP-1 RAs, the direct and indirect mechanisms through which they confer nephroprotection, and the most recent studies on their efficacy in improving renal outcomes in diabetic patients. Finally, we discuss the metabolic and anti-inflammatory effects of GLP-1RAs in diabetic patients with coronavirus disease 2019 (COVID-19).
Physiology of incretins
At the same dose, glucose taken orally causes a greater insulin response than intravenous administration. This is due to the so-called ‘incretin effect’, which involves two peptide hormones produced in the gastrointestinal tract: glucose-dependent insulinotropic polypeptide (GIP) and GLP-1. After a meal, the glucose located inside the intestinal lumen increases the synthesis and release of GLP-1 into the bloodstream by stimulating the activity of SGLT-1 expressed on the enteroendocrine L cells membrane. GLP-1 interacts with its receptor (GLP-1R) expressed on pancreatic β and δ cells, where it promotes the biosynthesis and release of insulin and somatostatin, respectively. Somatostatin, in turn, is able to inhibit the secretion of glucagon by pancreatic α cells by means of the somatostatin receptor 2. In experimental models of diabetes, GLP-1 has been shown to inhibit apoptosis of β cells and promote their proliferation through the recruitment of cellular precursors, thus implementing the availability of functionally active β cells [7, 8]. In addition to acting on pancreatic cells, GLP-1 improves glycaemic control and tissues sensitivity to insulin through numerous indirect systemic effects. The activation of GLP-1R in the hypothalamic regulatory centres of hunger and satiety promotes weight loss and reduces food intake. The bidirectional communication between the central and the enteric nervous system is known as ‘gut–brain axis’. During the meal, GLP-1 stimulates the sensory fibers of the vagus nerve by interacting with GLP-1R in the intrahepatic tract of the portal vein. The afferent signal reaches the hindbrain, where efferent fibres originate from the nucleus of the solitary tract to go toward the liver (where they inhibit gluconeogenesis and reduce steatosis and fibrosis) and the digestive tract (where they slow the rate of gastric emptying and peristalsis of the small intestine). This results in increased satiety and a decreased appetite [9]. It has also been demonstrated that the link between GLP-1 and GLP-1R increases metabolism and energy consumption at the level of brown adipose tissue cells regardless of physical activity, and at the same time reduces lipid deposits in white adipose tissue through transduction pathways involving fibres of the sympathetic nervous system [10].
About 1–2 min after its release into the bloodstream, GLP-1 is rapidly degraded to an inactive peptide by the enzyme DPP-4. Thanks to its short half-life, the modulating action of GLP-1 on glycaemic control is calibrated and proportional to the glucose load introduced with the diet, thereby preventing dangerous hypoglycaemia. Figure 1 summarizes the physiological actions of GLP-1.
Figure 1:
Physiologic effects of GLP-1 (adapted from Granata et al. [11]).
Pharmacological properties of GLP-1RAs
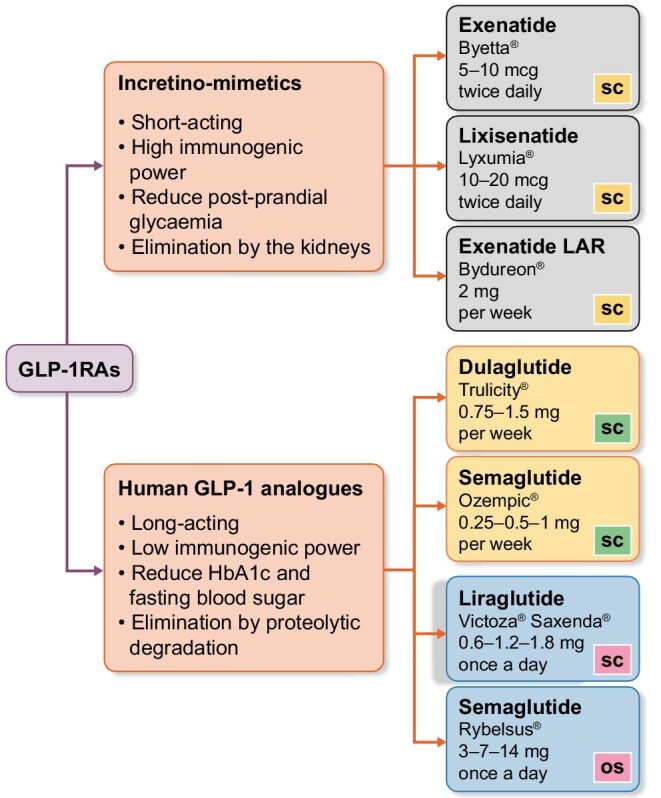
GLP-1RAs induce supra-physiological stimulation of GLP-1R, mimicking the mechanism of action of endogenous GLP-1 and amplifying both local and systemic effects without interfering in any way with GIP and without being degraded by endogenous DPP-4. A peculiar feature is the ability to increase insulin secretion in proportion to glycaemic values. This glucose-dependent insulinotropic mechanism explains the low incidence of hypoglycaemic events associated with GLP-1RAs therapy, both when used alone and in dual or triple therapy with metformin, pioglitazone or basal insulin. GLP-1RAs can be classified into two groups (shown in Figure 1):
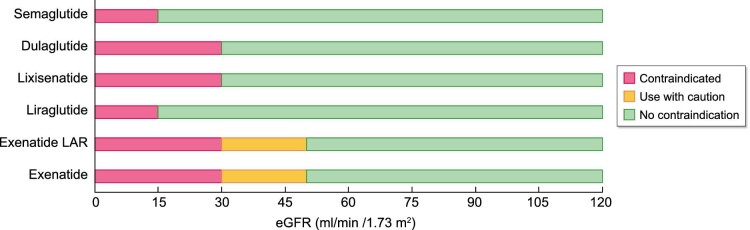
Incretino-mimetics: Exenatide (Byetta®), Exenatide long-acting release (LAR—Bydureon®), Lixisenatide (Lyxumia®). They derive from exendin-4, a peptide isolated from the saliva of the Gila Monster (Heloderma suspectum), a venomous lizard from southern Arizona. These drugs are resistant to rapid degradation by DPP-4, but since they have a structural analogy of only 52% compared with endogenous GLP-1, they have great immunogenic power with potential development of inactivating antibodies. With the exception of exenatide in LAR formulation, incretino-mimetics are defined as ‘short-acting’ drugs, as they are characterized by short-lasting plasma concentration peaks with intermittent periods of very low and almost close to zero plasma values, during which GLP-1Rs are not activated at all [12]. Thanks to these characteristics, they show a more marked effect on slowing gastric emptying, which translates into a greater reduction in post-prandial glycaemic increase, while they are less effective in controlling fasting blood glucose, basal insulin secretion and in maintaining stable values of glycated hemoglobin (HbA1c) [13–15]. The elimination of these drugs occurs mainly by glomerular filtration, tubular reabsorption and consequent proteolytic degradation. Therefore, their use is contraindicated in the presence of estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 (shown in Figure 2).
Figure 2:
Direct and indirect effects through which GLP-1RAs confer nephroprotection (adapted from Granata et al. [11]).
Human GLP-1 analogues: Liraglutide (Victoza®, Saxenda®), Dulaglutide (Trulicity®), Injectable Semaglutide (Ozempic®), Oral Semaglutide (Rybelsus®), Albiglutide (Eperzan®). They are also called ‘long-acting’ since they maintain elevated blood concentrations once the steady state is reached, allowing a continuous stimulation of GLP-1R and only minor fluctuations between administrations. These drugs have low immunogenic power due to a high structural analogy with endogenous GLP-1. Specific molecular characteristics, such as the covalent bond with albumin (albiglutide), with the Fc portion of human immunoglobulin (Ig) G4 (dulaglutide) or with specific fatty acids (liraglutide), give them a long half-life and prevent their elimination by the kidneys. As a result, human GLP-1 analogues can be safely used even at eGFR values to 15 mL/min/1.73 m2 (shown in Figure 2). Catabolism of these drugs occurs in target tissues in a similar way to large proteins, without an organ-specific main route of elimination. Contrary to short-acting GLP-1RAs, human GLP-1 analogues induce a more marked reduction in HbA1c and fasting blood sugar and decrease the incidence of side effects such as nausea and vomiting; moreover, they have shown greater efficacy on cardiovascular mortality and morbidity [19, 20].
GLP-1RAs generally require subcutaneous administration. Nowadays, devices for subcutaneous injection of GLP-1RAs have reached a remarkable technological evolution in terms of frequency of administration, size of needles and ease of self-inoculation of the drug using pre-filled pens. All of these aspects have had a significant impact on the quality of life of diabetic patients and significantly improved their therapeutic adherence. Starting from 2020, the first GLP-1RA (semaglutide—Rybelsus®) has been available in oral formulation and may be administered once daily. The ‘PIONEER’ series of trials [21–30] demonstrated that oral semaglutide has a positive impact on average HbA1c levels and weight reduction compared with several other drug classes (SGLT-2 inhibitor, DPP-4 inhibitor, another GLP-1RA, insulin and placebo). Based on the data collected so far, renal impairment, even severe, does not significantly affect the pharmacokinetics of oral semaglutide and to date it can be used in patients with eGFR to 15 mL/min/1.73 m2.
GLP-1RA and COVID-19
It is well known that acute respiratory distress syndrome represents the most severe form of COVID-19. The so-called ‘Cytokine Storm’ that happens in this syndrome is characterized by the highest levels of inflammatory cytokines which damage alveolar epithelial cells in the lung and inactivate pulmonary surfactant, resulting in the formation of the hyaline membrane and lung parenchyma breakdown. With this background, GLP-1RAs might provide an opportunity to break the remarkable inflammation process, exerting broad anti-inflammatory actions and reducing biomarkers of systemic inflammation in particular in human subjects with type 2 diabetes and people with obesity [31]. Preclinical studies showed that GLP-1RAs reduce cytokine production, attenuate pulmonary inflammation and preserve lung function in rats and mice with experimental lung injury [8, 32]. The stimulation of pulmonary vasodilators like atrial natriuretic peptide (ANP) and facilitation of surfactant protein A are documented actions. Moreover, GLP-1RAs exert the repression of the proinflammatory cytokine and the stimulation of endothelial nitric oxide synthase (eNOS)/soluble guanylate cyclase(sGC)/protein kinase G (PKG) signalling, and cause the inactivation of the Nuclear Factor kappa B (NF-κB) signalling. In addition, liraglutide attenuates the expression of key inflammasome components, such as the thioredoxin-interacting protein which is significantly increased after the administration of lipopolysaccharide along with cytokines and chemokine genes [33].
Although it is not possible at present to make recommendations on this specific use, the metabolic and anti-inflammation beneficial effects might identify GLP-1-based drugs as fundamental tools for treating COVID-19 patients [31].
GLP-1RAs and kidney: beyond cardioprotection
A growing literature supports the hypothesis that GLP-1RAs confer nephroprotection not only because they promote weight loss and improve glycaemic control but also through direct interaction with renal cells. Numerous human studies have shown the presence of GLP-1R both in the glomerulus and in the renal tubule. GLP-1RAs would appear to counteract glomerular hyperfiltration as they induce an increase in diuresis and natriuresis by phosphorylation and consequent direct inhibition of the sodium-hydrogen exchanger 3, located on the brush border of proximal tubular cells [34, 35]. Kim et al. [36] demonstrated that liraglutide promotes natriuresis also through an increased secretion of ANP by cardiomyocytes. These mechanisms explain, at least in part, the correlation between chronic GLP-1RAs intake and lowering of blood pressure. The increased sodium filtered load that reaches the macula densa restores the normal functioning of the tubulo-glomerular feedback, suppresses the overactivation of the renin–angiotensin–aldosterone system and lowers the serum concentration of angiotensin II. Furthermore, GLP-1RA inhibits mesangial expansion, reduces the endothelial expression of profibrotic molecules and increases the availability of intraglomerular nitric oxide, thus slowing the progression of DKD [37, 38].
The decline of glomerular filtrate and microalbuminuria in diabetic patients is part of a corollary of systemic signs and symptoms that share dyslipidemia and atherosclerosis as causative events. According to some authors [39–41], GLP-1RAs confer nephroprotection through various anti-atherogenic properties:
They reduce the production and secretion of intestinal chylomicrons with beneficial effects on plasma levels of total cholesterol, low-density lipoprotein (LDL) and triglycerides [39];
They minimize renal hypoxic-ischaemic damage as they regulate the mitochondrial activity of renal cells [40];
They prevent oxidative damage and the formation of oxygen free radicals (ROS) as they increase the levels of cAMP and the activity of protein kinase A, while they reduce the activity of NAD(P)H oxidase, interfere with the expression of receptors for advanced glycation products (AGEs) and suppress the NF-κB-mediated signalling pathway [41].
Finally, one fascinating hypothesis concerns the possible role of GLP-1RAs in modifying the composition of the intestinal microbiota, whose dysregulation is related to the onset of numerous pathological conditions, including CKD. An animal model study compared the effects of liraglutide and saxagliptin on gut microbiota composition. The authors [42] observed that liraglutide (but not saxagliptin) causes a lower expression of obesity-related philotypes (including Roseburia, Erysipelotrichaceae Incertae Sedis, Marvinbryantia and Parabacteroides), while on the contrary it promotes the growth of the philotypes Blautia and Coprococcus, which are related to a lower body mass index. A possible explanation is that liraglutide induces an increase in GLP-1 levels between 4 and 6 times higher than the DPP-4 inhibitor, with a more pronounced effect on the slowing of gastric emptying and intestinal transit. All this contributes to modifying the pH and the concentration of the different nutrients within the intestinal lumen. Mechanisms by which the influence of GLP-1RA on the intestinal microbiota can improve the clinical outcomes of diabetic patients have yet to be demonstrated.
GLP-1RAs and renal outcomes in diabetic patients: review of randomized controlled trials
Numerous studies have investigated the nephroprotective effects of GLP-1RA in diabetic patients (Table 1).
Table 1.
Review of the main clinical trials that analyzed the impact of GLP-IRA on renal outcomes (adapted from Granata et al. [11])
| Drug | Renal endpoint | Results | |
|---|---|---|---|
| LEADER | Liraglutide versus placebo |
- Macroalbuminuria - Doubling of sCreat - eGFR <45 mL/min/1.73 m2 - Need for dialysis - Death for renal causes |
A lower incidence of nephropathy was found in the group treated with liraglutide, due to a favourable impact on macroalbuminuria. The eGFR decline over time was slower in patients with moderate/severe CKD. |
| SCALE | Liraglutide versus placebo |
- Changes in UACR | A significant weight loss also but a decreased urinary albumin/creatinine ratio (UACR) was observed in both liraglutide arms compared with placebo (18.36, 10.79 and 2.34%, respectively). |
| LIRA-RENAL | Liraglutide versus placebo |
- Changes in eGFR - Changes in UACR |
There was no difference between liraglutide and placebo in terms of eGFR and UACR. This result could be partly due to the small size of the sample and the short observation period. |
| SUSTAIN–6 | Semaglutide versus placebo |
- Macroalbuminuria - Doubling of sCreat - eGFR <45 mL/min/1.73 m2 - Need for dialysis |
Semaglutide reduces the incidence of de novo macroalbuminuria. Nevertheless, no difference was found regarding the incidence of ESRD and death for renal causes. |
| ELIXA | Lixisenatide versus placebo |
- Changes in UACR | Lixisenatide slows the worsening of UACR over time regardless of basal albuminuria. No difference was found regarding eGFR decline rate. |
| EXSCEL | Exenatide LAR versus placebo |
- 40% eGFR decline - Need for dialysis - Death for renal causes - Macroalbuminuria de novo |
Exenatide LAR performs better than placebo regarding the composite renal outcome, with greater efficacy on the incidence of macroalbuminuria. |
| AWARD-7 | Dulaglutide versus glargine |
- Changes in eGFR and UACR from baseline | Dulaglutide was more effective than insulin glargine in attenuating the decline in renal function, while there were no statistically significant differences on the reduction of UACR. The authors did not observe any significant correlation between the variation of creatinine, cystatin C and body weight. |
| REWIND | Dulaglutide versus Placebo |
- Macroalbuminuria de novo - ≥30% eGFR decline from baseline - Need for dialysis |
Although the incidence of macroalbuminuria was lower in the dulaglutide group, the percentages of eGFR decline ≥30% and the need for dialysis showed an almost comparable trend in the two groups. Sensitivity analysis revealed that dulaglutide significantly reduces the worsening eGFR when it is defined as a reduction of ≥40% or ≥50%, rather than ≥30%. |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; sCreat, serum creatinine; UACR, urine albumin/creatinine ratio.
In the LEADER trial [6], 9340 diabetic patients at high risk or with known cardiovascular disease were randomly assigned to the liraglutide versus placebo group. The study included both patients already on oral hypoglycaemic and/or insulin therapy and naïve subjects. The primary endpoint was the time elapsed between randomization and the onset of MACEs (cardiovascular death, nonfatal myocardial infarction and/or nonfatal stroke). Other endpoints included percutaneous revascularization, hospitalization for unstable angina or heart failure, death from all causes, nephropathy (defined as occurrence of macroalbuminuria, doubling of creatinine, eGFR <45 mL/min/1.73 m2, need for dialysis or death from kidney causes) and retinopathy. After a mean follow-up period of 3.8 years, a mean reduction of 0.4% for HbA1c and 2.3 kg for body weight was achieved in the liraglutide group. The primary endpoint showed a lower incidence of MACEs in the liraglutide group (13%) compared with placebo (14.9%). Regarding the renal composite outcome, in the liraglutide group the incidence of nephropathy was 22% lower than in placebo with a favourable impact especially on macroalbuminuria, while no significant differences were observed about renal hard endpoints. The eGFR decline was slower in the liraglutide group; this effect was most evident in subcategories of patients with moderate (eGFR 30–59 mL/min/1.73 m2) or severe (eGFR <30 mL/min/1.73 m2) CKD.
The SCALE trial [43] evaluated the usefulness of liraglutide in the management of body weight in 846 overweight or obese diabetic patients. Patients were randomly assigned to receive 3 mg or 1.8 mg of liraglutide or placebo. At the end of the 56-week study period, a significant weight loss also but a decreased urinary albumin/creatinine ratio (UACR) was observed in both liraglutide arms compared with placebo (18.36, 10.79 and 2.34%, respectively).
Unlike the LEADER and SCALE trials, in the LIRA-RENAL trial liraglutide was found to be ineffective in improving renal outcomes in a sample of 279 diabetic patients with moderate CKD (eGFR 30–59 mL/min/1.73 m2). The authors found no difference in terms of eGFR and UACR compared with placebo after 26 weeks of treatment [44]. This result could be partly due to the limited sample size and the short observation period.
The ‘SUSTAIN’ series [5, 45–53] includes 10 randomized controlled trials aimed at evaluating the efficacy of weekly subcutaneous semaglutide on glycaemic control in patients with T2DM. Semaglutide was given as monotherapy or in combination with metformin, sulfonylurea and/or insulin, and compared with the most commonly used drugs for T2DM (sitagliptin, exenatide, insulin glargine, dulaglutide, canagliflozin and liraglutide). Recently, Mann et al. [54] conducted a post-hoc analysis of data from 8416 patients enrolled in SUSTAIN 1–7 trials in order to examine the effects of subcutaneous semaglutide on eGFR, UACR and renal adverse events. Although semaglutide is associated with a decline in eGFR in the first 12–16 weeks of treatment and then stabilized, the overall difference compared with other antidiabetic drugs and to placebo over the entire observation period was statistically insignificant; furthermore, UACR values showed a decreasing trend in the group of patients treated with semaglutide. The authors therefore concluded that treatment with semaglutide does not increase the incidence of adverse renal events compared with the other antidiabetic treatments. In particular, the SUSTAIN-6 trial [5] was designed to demonstrate the noninferiority of semaglutide compared with placebo in terms of cardiovascular safety on a sample of 3297 patients. Again, the primary endpoint was the incidence of MACEs. After 2.1 years of follow-up, the semaglutide arm performed better than placebo for MACEs (6.6% versus 8.9%), glycaemic control (mean HbA1c −1.1% versus −1.4%), weight loss (−3.6 vs -4.9 kg) and the onset or worsening of nephropathy (3.8% versus 6.1%). Similar to what emerged from the LEADER trial, the reduction in macroalbuminuria (up to 46%) appears to be the main mechanism by which semaglutide positively affects renal outcomes.
ELIXA [55] was a randomized, double-blind, parallel-group study designed to evaluate the impact of lixisenatide on cardiovascular risk compared with placebo in a population of 6068 diabetic adults with a recent episode of acute coronary syndrome. The primary composite endpoint, assessed for non-inferiority and superiority, included the incidence of MACEs. The mean observation period was 108 weeks. The study showed that lixisenatide is not inferior, although not superior, to placebo in cardiovascular safety. In a recent sub-analysis of the results of the ELIXA trial, Muskiet et al. [56] analysed the effects of lixisenatide on renal outcomes. The authors demonstrated that lixisenatide reduces the UACR variation in both microalbuminuric (−21%) and macroalbuminuric (−39%) patients at baseline and prevents the appearance of macroalbuminuria in initially normoalbuminuric subjects (−1.69%). On the other hand, eGFR decline in the two groups was not statistically significant regardless of the baseline albuminuria values.
In the EXSCEL trial [57], 14 752 diabetic subjects were randomly assigned to receive exenatide LAR at a dose of 2 mg weekly versus placebo for an observation period of 3.2 years. The results showed that exenatide is noninferior to placebo in terms of safety but is not superior in terms of efficacy in preventing MACEs. These findings were confirmed in all categories of patients with CKD of varying severity (baseline eGFR >60 or <60 mL/min/1.73 m2). Although exenatide did not produce any significant improvement in eGFR decline and incidence of ESRD and renal-related death in the EXSCEL trial, subsequent analysis of the data adjusted for baseline demographics and comorbidities revealed a significant improvement in the renal composite outcome, mainly mediated by a lower incidence of macroalbuminuria [58].
The AWARD-7 study [59] recruited and randomized 577 diabetic patients with CKD stages G3 and G4 in three arms: (i) dulaglutide, 1.5 mg weekly; (ii) dulaglutide, 0.75 mg weekly; and (iii) insulin glargine, all in combination with insulin lispro. The primary endpoint was the HbA1c changes at 26 weeks; secondary endpoints included changes in UACR and eGFR, the latter estimated using both creatinine and cystatin C. After 52 weeks of observation, the results demonstrated that dulaglutide effectively and safely improves glycaemic control in diabetic patients with advanced renal disease, with impact on HbA1c fluctuations comparable to basal insulin glargine therapy. Regarding secondary endpoints, dulaglutide was more effective than insulin glargine in attenuating the decline in renal function (eGFR reduction of −1.1, −1.5 and −2.9 mL/min/1.73 m2 in the three groups, respectively), while there were no statistically significant differences on the reduction of UACR. It is interesting to note that the authors did not observe any significant correlation between the variation of creatinine (whose serum concentrations are known to depend on the patient's muscle mass) of cystatin C (which is not influenced by muscle mass) and that of body weight. These data indirectly confirm that the weight loss recorded in patients treated with GLP-1RA is the result of a loss of fat mass and not of muscle mass.
The REWIND trial [60] was a multicentre, randomized, double-blind, placebo-controlled study in which 9901 diabetic patients with a previous cardiovascular event or with cardiovascular risk factors were randomly assigned (1:1) to a weekly subcutaneous injection of dulaglutide (1.5 mg) or placebo. The primary outcome was the incidence of MACEs with an intention-to-treat approach. Secondary outcomes included a composite of retinopathy, nephropathy (de novo macroalbuminuria, eGFR decline ≥30% from baseline, need for dialysis), single primary outcome events, hospitalization for unstable angina or heart failure, and death. During a median observation period of 5.4 years, the primary composite outcome occurred in 12% in the dulaglutide group versus 13.4% in the placebo group. All-cause mortality did not differ between groups. Although the incidence of macroalbuminuria was 8.9% versus 11.3% in the placebo group, the percentages of eGFR decline ≥30% and the need for dialysis showed an almost comparable trend in the two groups. This randomized controlled trial is the only one with a superiority study design.
The meta-analysis by Palmer et al. [61] grouped 764 randomized controlled trials that compared SGLT-2 inhibitors and GLP-1RA in order to evaluate their efficacy in diabetic patients. The authors concluded that both drugs, when combined with other antidiabetic treatments, reduce the incidence of nonfatal myocardial infarction and severe hypoglycaemia, prevent the development of CKD and lower mortality in proportion to the patient's cardiovascular and renal risk profile at baseline (very low, low, moderate, high and very high). Careful stratification of cardiovascular risk in diabetic patients is therefore a necessary condition in order to establish the most suitable therapeutic strategy. In accordance with this perspective, the latest American Diabetes Association (ADA) [62], Italian Society of Diabetology (SID) [63] and KDIGO guidelines [64] differentiate the pharmacological approach to T2DM not just based on the target glycaemic values but also taking into account the indicators of high-risk or established atherosclerotic cardiovascular disease, CKD and heart failure.
The re-evaluation of the REWIND trial data, conducted with the sensitivity analysis method, has shown that dulaglutide significantly reduces the worsening eGFR when it is defined as a reduction of ≥40% or ≥50%, rather than ≥30% as in the original study design. Similarly, a very recent meta-analysis [65] in which sensitivity analysis excluded the only trial that recruited patients with a recent episode of acute coronary syndrome (ELIXA) showed that GLP-1RAs not only reduce the incidence of MACEs, hospitalization for heart failure and all-cause mortality, but also improve the composite renal outcome in terms of eGFR decline over time. These data show that the nephroprotective action of GLP-1RAs is not exclusively linked to their impact on macroalbuminuria. In 2019, the FLOW trial (Effect of Semaglutide Versus Placebo on the Progression of Renal Impairment in Subjects With Type 2 Diabetes and Chronic Kidney Disease) [66] was launched. The authors recruited 3508 patients in order to evaluate the ability of semaglutide to reduce the incidence of the composite primary endpoint (eGFR decline ≥50% from baseline, need for dialysis, death from renal causes and death from cardiovascular disease) compared with placebo. The results of the study, expected in 2024, will help define the real role of GLP-1RAs as drugs capable of slowing the worsening of DKD in patients with T2DM.
CONCLUSIONS
GLP-1RAs have shown a positive impact on reducing cardiovascular risk with excellent safety profile. The clinical trials published so far agree in attributing to GLP-1RAs a nephroprotective action, which is expressed through both indirect (improvement of blood pressure and glycaemic control, weight loss) and direct (restoration of normal intrarenal haemodynamics, prevention of ischaemic and oxidative damage) effects. This results in reduced incidence of albuminuria and a slower decline of renal function. Although the high cost currently represents an important limitation for their use as a first therapeutic choice, these drugs could be advantageous compared with insulin treatment thanks to a lower rate of adverse side effects, better therapeutic adherence and to the positive effects on body weight. Further studies are needed in order to expand knowledge on the nephroprotective effects of GLP-1RAs and their ability to implement long-term cardiovascular and renal outcomes in diabetic patients.
Contributor Information
Antonio Granata, Nephrology Unit, “Cannizzaro” Hospital, Catania, Italy.
Rosario Maccarrone, Nephrology Unit, “Cannizzaro” Hospital, Catania, Italy.
Massimiliano Anzaldi, Endocrinology Unit, “Cannizzaro” Hospital, Catania, Italy.
Giuseppe Leonardi, SSD Severe Heart Failure, PO “G. Rodolico”, University of Catania, Italy.
Francesco Pesce, Nephrology, Dialysis and Transplantation Unit, Department of Emergency and Organ Transplantation, ‘Aldo Moro’ University of Bari, Piazza Giulio Cesare 11, Bari, Italy.
Francesco Amico, Cardiology Unit, “Cannizzaro” Hospital, Catania, Italy.
Loreto Gesualdo, Nephrology, Dialysis and Transplantation Unit, Department of Emergency and Organ Transplantation, ‘Aldo Moro’ University of Bari, Piazza Giulio Cesare 11, Bari, Italy.
Salvatore Corrao, Department of Internal Medicine, UOC Medicina Interna 2 iGR, National Relevance Hospital Trust, ARNAS Civico, Di Cristina e Benfratelli, Piazza Nicola Leotta, Palermo, Italy; Dipartimento di Promozione Della Salute, Materno Infantile, Medicina Interna e Specialistica di Eccellenza “G. D'Alessandro”, PROMISE, University of Palermo, Palermo, Italy.
ACKNOWLEDGEMENTS
All authors contributed to the study conception and design. Special thanks go to A.G., S.C., R.M. and M.A. for having performed material preparation and data analysis. Thanks to G.L., F.P., F.A. and L.G. for reviewing and editing the manuscript.
FUNDING
None declared.
AUTHORS’ CONTRIBUTIONS
All authors have read and approved the manuscript. All the authors meet all four criteria for ICMJE Criteria for Authorship.
CONFLICT OF INTEREST STATEMENT
The authors disclose no relationship with industry and financial associations and declare the absence of conflict of interest. No research involving human participants and/or animals.
DATA AVAILABILITY STATEMENT
No new data were created or analyzed in this study. All the data reported in this review derive from studies already published in the literature, which are available in the on-line PubMed biomedical database and are listed in the references.
REFERENCES
- 1. Koye DN, Shaw JE, Reid CMet al. . Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies. Diabet Med 2017; 34: 887–901 [DOI] [PubMed] [Google Scholar]
- 2. Gross JL, de Azevedo MJ, Silveiro SPet al. . Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005; 28: 164–176 [DOI] [PubMed] [Google Scholar]
- 3. Navarro JF, Mora C, Maca Met al. . Infiammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis 2003; 42: 53–61 [DOI] [PubMed] [Google Scholar]
- 4. Wolkow PP, Niewczas MA, Perkins Bet al. . Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol 2008; 19: 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marso SP, Bain SC, Consoli Aet al. . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844 [DOI] [PubMed] [Google Scholar]
- 6. Marso SP, Daniels GH, Brown-Frandsen Ket al. . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baggio LL, Drucker DJ.. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132: 2131–2157 [DOI] [PubMed] [Google Scholar]
- 8. Drucker DJ. Mechanisms of action and therapeutic application of Glucagon-like Peptide-1. Cell Metab 2018; 27: 740–756 [DOI] [PubMed] [Google Scholar]
- 9. Gutierrez-Aguilar R, Woods SC.. Nutrition and l and K-enteroendocrine cells. Curr Opin Endocrinol Diabetes Obes 2011; 18: 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nogueiras R, Pérez-Tilve D, Veyrat-Durebex Cet al. . Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J Neurosci 2009; 29: 5916–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granata A., Maccarrone R., La Rosa S.et al. . GLP-1 receptor agonists in the treatment of diabetes mellitus type 2: cardioprotection, and more!. G Ital Nefrol 2021; 38: 2021–vol6 [PubMed] [Google Scholar]
- 12. Gorgojo-Martínez JJ. New glucose-lowering drugs for reducing cardiovascular risk in patients with type2 diabetes mellitus. Hipertens Riesgo Vasc 2019; 36: 145–161 [DOI] [PubMed] [Google Scholar]
- 13. Kapitza C, Forst T, Coester HVet al. . Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab 2013; 15: 642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christensen M, Knop FK, Holst JJet al. . Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. IDrugs 2009; 12: 503–513 [PubMed] [Google Scholar]
- 15. Werner U, Haschke G, Herling AWet al. . Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 2010; 164: 58–64 [DOI] [PubMed] [Google Scholar]
- 16. Drucker DJ, Nauck MA.. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705 [DOI] [PubMed] [Google Scholar]
- 17. Jimenez-Solem E, Rasmussen MH, Christensen Met al. . Dulaglutide, a long-acting GLP-1 analog fused with an fc antibody fragment for the potential treatment of type 2 diabetes. Curr Opin Mol Ther 2010; 12: 790–797 [PubMed] [Google Scholar]
- 18. Lau J, Bloch P, Schäffer Let al. . Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem 2015; 58: 7370–7380 [DOI] [PubMed] [Google Scholar]
- 19. Buse JB, Nauck M, Forst Tet al. . Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomized, open-label study. Lancet 2013; 381: 117–124 [DOI] [PubMed] [Google Scholar]
- 20. Blevins T, Pullman J, Malloy Jet al. . DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: 1301–1310 [DOI] [PubMed] [Google Scholar]
- 21. Aroda VR, Rosenstock J, Terauchi Yet al. . PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care 2019; 42: 1724–1732 [DOI] [PubMed] [Google Scholar]
- 22. Rodbard HW, Rosenstock J, Canani LHet al. . Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care 2019; 42: 2272–2281 [DOI] [PubMed] [Google Scholar]
- 23. Rosenstock J, Allison D, Birkenfeld ALet al. . Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylureathe PIONEER 3 randomized clinical trial. JAMA 2019; 321: 1466–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pratley R., Amod A, Hoff STet al. . Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 2019; 394: 39–50 [DOI] [PubMed] [Google Scholar]
- 25. Mosenzon O, Blicher TM, Rosenlund Set al. . Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019; 7: 515–527 [DOI] [PubMed] [Google Scholar]
- 26. Husain M, Birkenfeld AL, Donsmark Met al. . Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019; 381: 841–851 [DOI] [PubMed] [Google Scholar]
- 27. Pieber TR, Bode B, Mertens Aet al. . Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019; 7: 528–539 [DOI] [PubMed] [Google Scholar]
- 28. Zinman B, Aroda VR, Buse JBet al. . Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care 2019; 42: 2262–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamada Y, Katagiri H, Hamamoto Yet al. . Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol 2020; 8: 377–391 [DOI] [PubMed] [Google Scholar]
- 30. Yabe D, Nakamura J, Kaneto Het al. . Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol 2020; 8: 392–406 [DOI] [PubMed] [Google Scholar]
- 31. Corrao S, Pinelli K, Vacca Met al. . Type 2 diabetes mellitus and COVID-19: a narrative review. Front Endocrinol (Lausanne) 2021; 12: 609470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raj VS, Mou H, Smits SLet al. . Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013; 495: 251–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin T, Liu M.. Letter to the editor: comment on GLP-1-based drugs and COVID-19 treatment. Acta Pharm Sin B 2020; 10: 1249–1250; Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crajoinas RO, Oricchio FT, Pessoa TDet al. . Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 2011; 301: F355–F363 [DOI] [PubMed] [Google Scholar]
- 35. Gutzwiller JP, Tschopp S, Bock Aet al. . Glucagon-like peptide-1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 2004; 89: 3055–3061 [DOI] [PubMed] [Google Scholar]
- 36. Kim M, Platt MJ, Shibasaki Tet al. . GLP-1 receptor activation and epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 2013; 19: 567–575 [DOI] [PubMed] [Google Scholar]
- 37. Mosterd C, Bjornstad P, van Raalte DHet al. . Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J Nephrol 2020; 33: 965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawanami D, Takashi Y.. GLP-1 receptor agonists in diabetic kidney disease: from clinical outcomes to mechanisms. Front Pharmacol 2020; 11: 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Farr S, Taher J, Adeli K.. Glucagon-like peptide-1 as a key regulator of lipid and lipoprotein metabolism in fasting and postprandial states. Cardiovasc Hematol Disord Drug Targets 2014; 14: 126–136 [DOI] [PubMed] [Google Scholar]
- 40. Wang C, Li L, Liu Set al. . GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis. PLoS One 2018; 13: e0193473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujita H, Morii T, Fujishima Het al. . The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 2014; 85: 579–589 [DOI] [PubMed] [Google Scholar]
- 42. Lin Wang L, Peicheng L, Zhaosheng Tet al. . Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep 2016; 6: 33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davies MJ, Bergenstal R, Bode Bet al. . Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015; 314: 687–699 [DOI] [PubMed] [Google Scholar]
- 44. Davies MJ, Bain SC, Atkin SLet al. . Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care 2016; 39: 222–230 [DOI] [PubMed] [Google Scholar]
- 45. Sorli C, Harashima SI, Tsoukas GMet al. . Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 251–260 [DOI] [PubMed] [Google Scholar]
- 46. Ahrén B, Masmiquel L, Kumar Het al. . Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017; 5: 341–354 [DOI] [PubMed] [Google Scholar]
- 47. Ahmann AJ, Capehorn M, Charpentier Get al. . Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care 2018; 41: 258–266 [DOI] [PubMed] [Google Scholar]
- 48. Aroda VR, Bain SC, Cariou Bet al. . Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 355–366 [DOI] [PubMed] [Google Scholar]
- 49. Rodbard HW, Lingvay I, Reed Jet al. . Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab 2018; 103: 2291–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pratley RE, Aroda VR, Lingvay Iet al. . Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018; 6: 275–286 [DOI] [PubMed] [Google Scholar]
- 51. Lingvay I, Catarig AM, Frias JPet al. . Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol 2019; 7: 834–844 [DOI] [PubMed] [Google Scholar]
- 52. Zinman B, Bhosekar B, Busch Ret al. . Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2019; 7: 356–367 [DOI] [PubMed] [Google Scholar]
- 53. Capehorn MS, Catarig AM, Furberg JKet al. . Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab 2020; 46: 100–109 [DOI] [PubMed] [Google Scholar]
- 54. Mann JFE, Hansen T, Idorn Tet al. . Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol 2020; 8: 880–893 [DOI] [PubMed] [Google Scholar]
- 55. Pfeffer MA, Claggett B, Diaz Ret al. . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257 [DOI] [PubMed] [Google Scholar]
- 56. Muskiet MHA, Tonneijck L, Huang Yet al. . Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2018; 6: 859–869 [DOI] [PubMed] [Google Scholar]
- 57. Holman RR, Bethel MA, Mentz RJet al. . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bethel MA., Mentz RJ, Merrill Pet al. . Microvascular and cardiovascular outcomes according to renal function in patients treated with once-weekly exenatide: insights from the EXSCEL trial. Diabetes Care 2020; 43: 446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tuttle KR, Lakshmanan MC, Rayner Bet al. . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gerstein HC, Colhoun HM, Dagenais GRet al. . Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019; 394: 131–138 [DOI] [PubMed] [Google Scholar]
- 61. Palmer SC, Tendal B, Mustafa RAet al. . Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021; 372: m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. American Diabetes Association . Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care 2021; 44: S111–S124 [DOI] [PubMed] [Google Scholar]
- 63. Available from: https://snlg.iss.it/?cat=6.
- 64. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98: S1–S115 [DOI] [PubMed] [Google Scholar]
- 65. Sattar N, Lee MMY, Kristensen SLet al. . Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021; 9: 653–662 [DOI] [PubMed] [Google Scholar]
- 66. Available from: https://clinicaltrials.gov/ct2/show/NCT038-19153.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. All the data reported in this review derive from studies already published in the literature, which are available in the on-line PubMed biomedical database and are listed in the references.