Abstract
Background
Prolonged shedding/relapse of COVID-19 infection has been reported, particularly in patients who received anti-CD20 agents (eg. rituximab). However, cases of occult COVID-19, in which SARS-CoV-2 persistence in lung parenchyma is diagnosed despite clearance from nasopharyngeal (NP) specimens, are uncommon.
Case summary
We describe two cases of occult COVID-19 in immunocompromised patients. Both patients had received rituximab previously. Both cases initially presented as ground-glass infiltrates on lung imaging; the diagnosis was originally not suspected due to repeated demonstration of negative SARS-CoV-2 from NP specimens, and alternative etiologies were originally considered. Persistence of SARS-CoV-2 in lung parenchyma, however, was demonstrated on bronchoalveolar lavage (BAL) specimens; additionally, isolation of viable SARS-CoV-2 virus and detection of SARS-CoV-2 nucleocapsid and spike-protein antigen in lung tissue on immunohistochemistry close to 3-months from primary infection strongly suggested ongoing viral persistence and replication as a driver of the lung parenchymal changes, which resolved after antiviral treatment.
Discussion
Occult COVID-19 can be a cause of unexplained ground-glass infiltrates on lung imaging; negative NP samples do not rule out SARS-CoV-2 persistence and invasive sampling must be considered. The unsuspected presence of viable virus on BAL, however, highlights that procedurists perfoming aerosol-generating-procedures during an ongoing pandemic wave must also practise appropriate infection-prevention precautions to limit potential exposure.
Keywords: SARS-CoV-2, COVID-19, Viral antigen, Relapse, Immunocompromise, Rituximab, Occult COVID-19
Introduction
Immunocompromised patients are at-risk for severe coronavirus disease 2019 (COVID-19). In particular, patients with secondary hypogammaglobulinemia after receipt of anti-CD20 treatment (eg.rituximab) tend to have more severe COVID-19 manifestations [1], [2], [3]. Amongst COVID-19 cases receiving concurrent rituximab, prolonged shedding or relapse has been encountered [4], [5]. Cases of occult COVID-19, in which SARS-CoV-2 relapse in lung parenchyma is diagnosed despite repeated demonstration of negative PCR from nasopharyngeal (NP) specimens, have been rarely reported in the literature [5]. We describe two patients who received rituximab and presented with ground-glass opacities (GGO) on lung imaging; COVID-19 relapse was originally not suspected due to negative NP swabs and diagnosed only after invasive sampling.
Methodology
Definitions
A COVID-19 relapse was defined as a clinical episode of symptoms consistent with acute COVID-19, accompanied by re-positive/persisting polymerase-chain-reaction (PCR) test for SARS-CoV-2 in respiratory samples, within 90 days of initial infection [4]. Occult COVID-19 was defined as COVID-19 relapse with negative PCR results on NP swab [5], with positive PCR on deeper respiratory specimens (endotracheal-aspirates [ETA] or bronchoalveolar- lavage [BAL]).
SARS-CoV-2 testing
At our institution, a tertiary hospital housing Singapore’s largest cancer centre, Cepheid Xpert Xpress-SARS-CoV-2 RT-PCR assay (targeting E/N2-gene targets) is used for diagnostic testing of respiratory samples whilst the TaqPath-Combo PCR kit (Thermo Fisher Scientific) is utilised to detect S/N/ORF1-gene targets in blood/tissue samples. Whole-genome-sequencing (WGS) was performed according to previously published protocols on a MinION MK1b system (Oxford Nanopore Technologies, Oxford, UK) in accordance with the ARTIC Network protocol v3 [6]. Serology test for SARS-CoV-2 IgG (RBD) is performed using the chemiluminescent-microparticle-immunoassay (CMIA) on the Architect i2000SR system (Abbott Laboratories) with ≥ 50 IU/ml defined as a positive result. Immunohistochemistry (IHC) staining for SARS-CoV-2 antigen is performed on formalin-fixed paraffin-embedded (FFPE) transbronchial-lung-biopsy (TBLB) samples according to previously published protocols [7]. FFPE tissue sections (4-μm thick) are labelled with antibodies targeting the SARS-CoV-2 nucleocapsid (Novus Biologicals, Cat# NB100-56576, Polyclonal) and spike-protein (GeneTex, Cat# GTX632604, 1A9). Respiratory viral culture is performed by inoculating samples into HeLa, HEp2, HEL, LLC-MK2 and MDCK cell cultures; cultures were observed for up to 21 days for cytopathic effect (CPE). Upon observation of CPE suspected for SARS-CoV-2, this was confirmed via PCR by an external BSL-3 laboratory.
Case description
Patient A
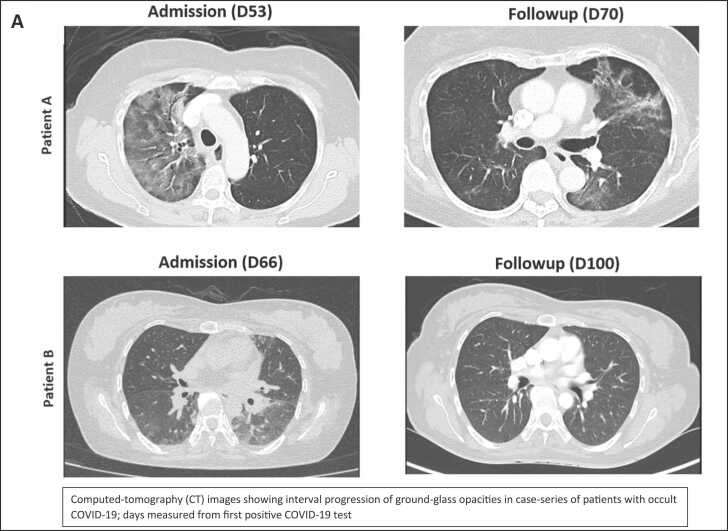
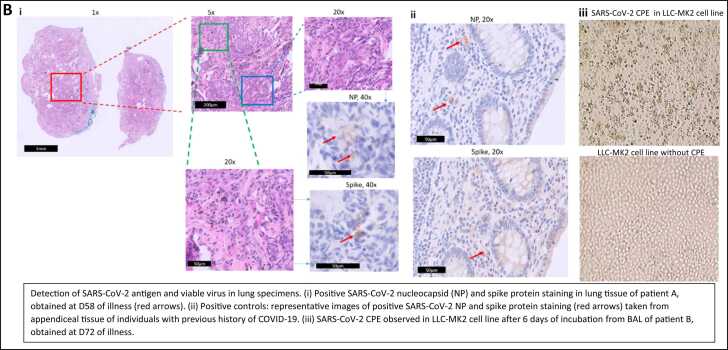
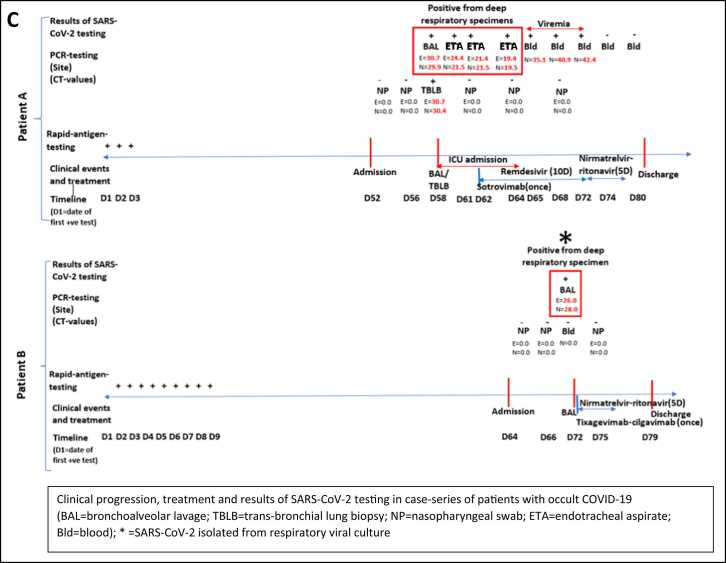
A was a 70-year-old female with diffuse large-b-cell-lymphoma in remission and currently on two-monthly maintenance rituximab (last administered 2-months prior to presentation). She had previously received 3 doses of BNT162b2 mRNA vaccine. The patient presented with a 2-week history of increased breathlessness and reduced effort tolerance, requiring supplemental oxygen on admission; initial computed-tomography-scan (CT-scan) of the thorax showed unilateral ground-glass changes (Fig. 1a). COVID-19 was not suspected initially as NP SARS-CoV-2 PCR on admission was repeatedly negative, and an alternative diagnosis of rituximab-induced pneumonitis was considered. The patient underwent BAL and biopsy a week after admission; BAL was SARS-CoV-2 PCR positive, confirming the diagnosis of occult COVID-19; viral culture was not performed. BAL and lung tissue were also SARS-CoV-2 PCR positive, with atypical lymphocytes seen on BAL fluid. Omicron variant was confirmed on WGS. Histopathology results showed a chronic interstitial pneumonitis-like pattern with positive immunohistochemistry staining for SARS-CoV-2 antigen (Fig. 1bi,ii). On directed questioning, the patient subsequently provided a history of having self-tested positive for SARS-CoV-2 at home using rapid-antigen-testing (RAT) 52 days prior to admission; the patient had not sought medical care previously as initial symptoms were mild (Fig. 1c). Post-bronchoscopy, the patient deteriorated and required intubation. Given persistence of SARS-CoV-2 in lung parenchyma, intravenous remdesivir was administered for 10 d; sotrovimab was also given as SARS-CoV-2 IgG (RBD) levels were < 50 IU/ml at D59. The patient was subsequently extubated a week later; however, as fever persisted, SARS-CoV-2 PCR was performed on blood, which was positive (Fig. 1c). Viremia persisted from D65-D72 even after 10 d of IV remdesivir, and only cleared after an additional 5 d of nirmatrelvir/ritonavir. Repeat CT-thorax (D70) showed resolution of initial ground-glass changes in the right lung (Fig. 1a) and she was discharged well at D80.
Fig. 1.
Clinical course, results of SARS-CoV-2 testing, imaging progression and histopathological assessment, in case-series of patients with occult COVID-19 (N = 2).
Patient B
B was a 42-year-old female with systemic-sclerosis on prednisolone 10 mg daily and received rituximab 3-months prior to presentation. She had previously received 1 dose of BNT162b2 mRNA vaccine. The patient presented with a 2-week history of increased breathlessness, reduced effort tolerance, and intermittent fever; initial CT-thorax showed bilateral ground-glass changes (Fig. 1a). Again, COVID-19 was not suspected initially as NP SARS-CoV-2 PCR on admission was repeatedly negative; given that blood PCR for cytomegalovirus (CMV) was positive at low levels, a diagnosis of CMV pneumonitis was considered. She underwent BAL a week after admission; BAL was SARS-CoV-2 PCR positive, confirming occult COVID-19; Omicron variant was confirmed on WGS. Atypical lymphocytes were also seen on BAL fluid and SARS-CoV-2 virus was isolated in LLC-MK2 cell culture after 6 days of incubation (Fig. 1biii), confirming the persistence of viable virus. CMV was also detected in BAL by PCR but was not isolated in viral cultures. On directed questioning, the patient subsequently provided a history of having self-tested positive for SARS-CoV-2 at home using RAT 64 days prior to admission; the patient had not sought medical care previously as initial symptoms were mild (Fig. 1c). The patient received 5 d of nirmatrelvir/ritonavir; tixagevimab/cilgavimab was also given as SARS-CoV-2 IgG (RBD) levels were < 50 IU/ml at D72 of illness. Fever lysed and the patient was discharged well (D79). CT-thorax (D100) showed resolution of lung changes (Fig. 1a).
Discussion
We present two cases of occult COVID-19 in immunocompromised hosts presenting as GGO on lung imaging, in which the diagnosis was originally not suspected due to repeated demonstration of negative SARS-CoV-2 from NP specimens, and alternative etiologies were originally considered. Persistence of SARS-CoV-2 in lung parenchyma, however, was demonstrated on BAL specimens; additionally, isolation of viable SARS-CoV-2 virus and detection of SARS-CoV-2 nucleocapsid and spike-protein antigen in lung tissue on immunohistochemistry close to 3-months from primary infection [7] strongly suggested ongoing viral persistence and replication as a driver of the lung parenchymal changes, which resolved after antiviral treatment. The hitherto unsuspected presence of viable virus on BAL also raised infection-prevention concerns, given the potential aerosol-generating nature of the procedure. At our institution, universal usage of N95 respirators was practised during the COVID-19 pandemic [8]; there was no evidence of onward transmission to potentially exposed healthcare workers post-procedure, despite intensive post-exposure surveillance and twice-weekly routine-rostered-testing for SARS-CoV-2 amongst HCWs [9]. Both patients did not seek medical care from the outset as their initial symptoms were mild and only self-tested for COVID-19 due to prevailing public health messaging strongly encouraging COVID-19 home testing for cases of mild flu-like illnesses; the only clue to the aetiology of their GGO, a history of positive RAT for SARS-CoV-2 in the preceding 2 months, could thus have been easily missed. While both cases had received BNT162b2 mRNA vaccine prior to infection, none produced detectable antibodies against SARS-CoV-2, possibly contributing to viral persistence. Patients treated with rituximab may have discordant humoral and cellular immune responses to COVID-19 vaccination; immunogenic non-response to mRNA vaccination is not uncommon in this patient population [10], [11]. Seroconversion rates of 17.5–40 % have been reported in the literature [10], [11]. Similarly, other cases of occult COVID-19 reported in the literature occurred in individuals who received anti-CD20 treatment and were unable to produce neutralising antibodies to SARS-CoV-2 [5], [12]. Both our cases made a full recovery after administration of antivirals and monoclonal antibodies directed against COVID-19; this was in keeping with emerging data suggesting good clinical outcomes in B-cell-depleted patients treated with anti-spike monoclonal antibodies [13]. Clinicians caring for such immunocompromised patients need to remain vigilant for occult COVID-19 in cases with unexplained ground-glass infiltrates on lung imaging and consider treatment with COVID-19 therapeutics. Negative NP samples do not rule out SARS-CoV-2 persistence and invasive sampling must be considered. The unsuspected presence of viable virus on BAL, however, highlights that procedurists perfoming aerosol-generating-procedures during an ongoing pandemic wave must also practise appropriate infection-prevention precautions to limit potential exposure.
CRediT authorship contribution statement
WLE: study design, data collections, data analysis, Writing – original draft, Writing – review & editing. TJY: data collection, Writing – original draft, Writing – review & editing. KKKK, WYW, DCML, LLEO, ATG, JPSY, AMTP, TKHL, EPC, IV: data collection. LW, TTT: Writing – original draft, Writing – review & editing, Supervision.
Funding
This work was not grant-funded.
Ethical approval
Ethics approval was not required for case reports under our institutional review board guidelines.
C. onsent
Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Avouac J., Drumez E., Hachulla E., Seror R., Georgin-Lavialle S., El Mahou S., et al. FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors; FAIR/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3(6):e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levavi H., Lancman G., Gabrilove J. Impact of rituximab on COVID-19 outcomes. Ann Hematol. 2021;100(11):2805–2812. doi: 10.1007/s00277-021-04662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cos Esquius M.L., López Montesinos I., Gimeno Martinez R., Eguía Núñez J., Caballero-Rabasco M.A., Sánchez González B., et al. Severe COVID-19 pneumonia in Good syndrome with a favorable outcome. Clin Immunol. 2022;235 doi: 10.1016/j.clim.2021.108789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderón-Parra J., Múñez-Rubio E., Fernández-Cruz A., García-Sánchez M.C., Maderuelo-González E., López-Dosil M., et al. Incidence, clinical presentation, relapses and outcome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients treated with anti-CD20 monoclonal antibodies. Clin Infect Dis. 2022;74(10):1786–1794. doi: 10.1093/cid/ciab700. [DOI] [PubMed] [Google Scholar]
- 5.Morel A., Imbeaud S., Scemla A., Péré H., Fourgeaud J., Amrouche L., et al. Severe relapse of SARS-CoV-2 infection in a kidney transplant recipient with negative nasopharyngeal SARS-CoV-2 RT-PCR after rituximab. Am J Transpl. 2022 doi: 10.1111/ajt.17000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko K.K.K., Yingtaweesittikul H., Tan T.T., Wijaya L., Cao D.Y., Goh S.S., et al. Emergence of SARS-CoV-2 spike mutations during prolonged infection in immunocompromised hosts. Microbiol Spectr. 2022 doi: 10.1128/spectrum.00791-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung C.C.L., Goh D., Lim X., Tien T.Z., Lim J.C.T., Lee J.N., et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2022;71(1):226–229. doi: 10.1136/gutjnl-2021-324280. [DOI] [PubMed] [Google Scholar]
- 8.Liang En W., Ko K.K., Conceicao E.P., Aung M.K., Oo A.M., Yong Y., et al. Enhanced infection-prevention measures including universal N95 usage and daily testing: the impact on SARS-CoV-2 transmission in cohorted hospital cubicles through successive Delta and Omicron waves. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wee L.E., Venkatachalam I., Sim X.Y.J., Tan K.B., Wen R., Tham C.K., et al. Containment of COVID-19 and reduction in healthcare-associated respiratory viral infections through a multi-tiered infection control strategy. Infect Dis Health. 2021;26(2):123–131. doi: 10.1016/j.idh.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Möller B., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3(11):e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furer V., Eviatar T., Zisman D., Peleg H., Braun-Moscovici Y., Balbir-Gurman A., et al. Predictors of immunogenic response to the BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases treated with rituximab. Vaccines. 2022;10(6):901. doi: 10.3390/vaccines10060901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancon A., Rizzo A., Mileto D., Grosso S., Foschi A., Cutrera M., et al. Viro-immunological evaluation in an immunocompromised patient with long-lasting SARS-CoV-2 infection. Emerg Microbes Infect. 2022;11(1):786–789. doi: 10.1080/22221751.2022.2045877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yetmar Z.A., Khodadadi R.B., Seville M.T., Brumble L., O'Horo J.C., Ganesh R., et al. Outcomes of B-cell-depleted patients with coronavirus disease 2019 treated with antispike monoclonal antibodies. Open Forum Infect Dis. 2022;9(7) doi: 10.1093/ofid/ofac204. [DOI] [PMC free article] [PubMed] [Google Scholar]