Abstract
Copy number variation (CNV) is one of the main sources of variation between different individuals that has recently attracted much researcher interest as a major source for heritable variation in complex traits. The aim of this study was to identify CNVs in Afghan indigenous sheep consisting of three Arab, Baluchi, and Gadik breeds using genomic arrays containing 53,862 single nucleotide polymorphism (SNP) markers. Data were analyzed using the Hidden Markov Model (HMM) of PennCNV software. In this study, out of 45 sheep studied, 97.8% (44 animals) have shown CNVs. In total, 411 CNVs were observed for autosomal chromosomes and the entire sequence length of around 144 Mb was identified across the genome. The average number of CNVs per each sheep was 9.13. The identified CNVs for Arab, Baluchi, and Gadik breeds were 306, 62, and 43, respectively. After merging overlapped regions, a total of 376 copy number variation regions (CNVR) were identified, which are 286, 50, and 40 for Arab, Baluchi, and Gadik breeds, respectively. Bioinformatics analysis was performed to identify the genes and QTLs reported in these regions and the biochemical pathways involved by these genes. The results showed that many of these CNVRs overlapped with the genes or QTLs that are associated with various pathways such as immune system development, growth, reproduction, and environmental adaptions. Furthermore, to determine a genome-wide pattern of selection signatures in Afghan sheep breeds, the unbiased estimates of FST was calculated and the results indicated that 37 of the 376 CNVRs (~ 10%) have been also under selection signature, most of those overlapped with the genes influencing production, reproduction and immune system. Finally, the statistical methods used in this study was applied in an external dataset including 96 individuals of the Iranian sheep breed. The results indicated that 20 of the 114 CNVRs (18%) identified in Iranian sheep breed were also identified in our study, most of those overlapped with the genes influencing production, reproduction and immune system. Overall, this is the first attempts to develop the genomic map of loss and gain variation in the genome of Afghan indigenous sheep breeds, and may be important to shed some light on the genomic regions associated with some economically important traits in these breeds.
Subject terms: Evolution, Genetics, Molecular biology
Introduction
Livestock species play major socio-economic roles in the world, since they provide many goods and services to human populations. The sheep (Ovis aries) is one of the most important farm animal species, because of its high productive potential and its ability to adapt to harsh environments1,2. The breeding of sheep in Afghanistan dates back to about 9000 years ago when these valuable livestock species were domesticated3. The grassland in Afghanistan is around 30 million hectares, accounting for about 47% of Afghanistan's land area. The Hindu Kush slopes are a natural and permanent pasture for sheep breeding in Afghanistan and the production of sheep is of great importance in the livestock sector. Before the war and the recent drought, approximately 90% of the foreign exchange income of the livestock sector was derived from sheep, and livestock industry have a contribution up to 40% in country's exports3. Despite the importance of sheep production in Afghanistan, the lack of accurate pedigree and phenotypic records, are the main problems that limit the implementation of animal breeding programs.
Recently, the genome-wide association studies have successfully uncovered single-nucleotide polymorphisms (SNPs) associated with complex traits such as diseases and quantitative phenotypes4. However, various studies showed that these variations account for a small proportion of heritability5. With the development of high throughput techniques, abundant structural variations have been found in the genome, of which the main variations are copy number variations (CNVs). The CNVs are increasingly recognized as an important genetic source of different phenotypic traits6–8. CNVs have been shown to explain around 18% of genetic variation in the gene expression, having no overlap with that explained by SNPs9. Initial research on CNV traces to 1936, when C. Bridges found an association between the BAR “gene”, duplication of a part of a chromosome, and the eye size in Drosophila melanogaster10. Since 2006, several studies in humans and animals have identified the association between CNV polymorphisms and different complex phenotypic traits7. Several studies have shown the usefulness of CNVs data for describing the genetic basis of phenotypic variations in a variety of species like cattle11,12, dog13, chicken14,15, pig16,17, goat18,19, and rabbit20. As for sheep, Fontanesi et al.21 provided the first comparative map of CNVs in the sheep genome using a cross-species array comparative genome hybridization (aCGH). It has been reported that the duplication of the agouti signaling protein gene is associated with white coat color in sheep22, a gene duplication affecting expression of the ovine ASIP gene is responsible for white and black sheep22 and higher levels of KIF2A and PHKG2 expression in Gansu Morden sheep are associated with disease resistance23. These studies suggest that identifying the CNVs distributed across the sheep genome can be very important for finding the genomic regions associated with some economically important traits in this species.
The aim of this study was to identify the genome-wide copy number variations (CNV) in three Afghan sheep breeds, and to study the associations between these regions of interest with different biological pathways. The results of this study, as one of the first attempts to develop the genome-wide map of CNVRs in local Afghan sheep breeds, can provide a valuable information genomic regions associated with economically important traits in these breeds.
Materials and methods
Animal samples
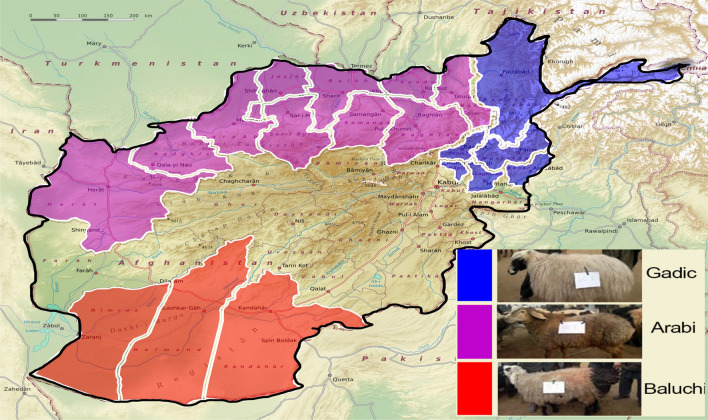
A total of 45 individuals, sampled from three breeds including 15 samples per each breeds of Arab, Baluchi and Gadik were collected for this study from different parts of Afghanistan (Fig. 1). The breeds used in this study are the most common and main indigenous sheep breeds reared in Afghanistan that have been prioritized over the past few years by animal breeding researches and private investment sectors24. Arab, Baluchi, and Gadik breeds account for around 13, 13, and 1% of total sheep reared in Afghanistan, respectively. Among these breeds, the Arabian sheep have a large body size and are the more capable for meat producing and known as a meat sheep, the Baluchi breed are well adapted to arid and desert environments and are known as dual purpose for wool and meat production and the Gadik breed as a wool sheep, is producing the best type of wool among other breeds24. The color of wools for each animal was collected at the time of blood sampling. While the color of wool for all Gadik sheep breed was white, a combination of different colors was observed in other two breeds specially in Baluchi. The white coats are favored in sheep for wool production that is easily dyed.
Figure 1.
The studied breeds and their geographic distribution in different regions of Afghanistan (the figure has been created by using Adobe Photoshop CC 2020 v.21.0.2, https://www.adobe.com/products/photoshop/).
DNA extraction, genotyping and quality control
The blood samples were collected via the jugular vein in the tubes containing EDTA (ethylene diamine tetraacetic acid) and the DNA was extracted using the GenElute Blood Genomic DNA Kit24. Genotyping was carried out in all animals using Illumina Ovine SNP 50K BeadChip, which contains 53,862 SNPs spanning the ovine genome. Quality control was implemented using the PLINK 1.90 software25. We removed the individuals and SNPs meeting any of the following criteria: individuals with a genotyping rate < 0.90; SNPs with > 0.05 missing data; and minor allele frequency (MAF) < 0.0226; SNPs with a P-value of < 0.000001 for the Fisher’s exact test of Hardy–Weinberg equilibrium (HWE). Finally, the SNPs without chromosomal or physical locations or located on the sex chromosome were excluded from final analysis. The markers located on the sex chromosomes were excluded from subsequent analyzes due to the different distribution of IBD (Identical by descent) pattern and the effective population size that affects identifying the CNVs27. This resulted in a data set of 45 individuals genotyped for 47,519, 46,430 and 47,711 autosomal SNPs in Arab, Baluchi and Gadik breeds, respectively.
Identification of CNV and CNVR
CNVs were inferred within each individual using PennCNV v.1.0.5 software28. A Hidden Markov Model (HMM) algorithm as employed in PennCNV incorporates multiple parameters, such as signal intensity ratios (log R Ratio: LRR) and allelic frequencies (B allele frequency: BAF) values of each marker in each individual, and the population frequency of B allele (PFB) of SNPs. The software extracts the signal intensity of each allele from the array genotyping data and then combines allelic frequency information and single nucleotide polymorphism distance to identify CNVs. For this purpose, the values of X and Y, which is the signal intensity of alleles A and B were used. R is calculated as the total signal intensity of two alleles, or R = X + Y. As a normalized measure of total signal intensity, the LRR value for each SNP is then calculated as LRR = log2(Robserved/RExpected), which Robserved is computed from linear interpolation of canonical genotype clusters29 obtained from a set of reference samples. The θ value measures the relative allelic intensity ratio of two alleles and is calculated from Eq. (1) which ranges from 0 to 1.
| 1 |
The B Allele Frequency (BAF) refers to a normalized measure of relative signal intensity ratio of the B and A alleles28:
| 2 |
in which θAA, θAB and θBB are the θ values for three canonical genotype clusters generated from external reference samples (such as HapMap samples). We retrieved signal intensity ratios (LRR) and allelic frequencies (BAF) for each SNP using the Illumina Genome Studio v1.0 software. To increase the confidence of the detected CNVs, quality control was performed by employing standard exclusions of the LRR (standard deviation of LRR) < 0.3, a BAF drift < 0.01 and a waviness factor < 0.05 (http://www.openbioinformatics.org).
CNV regions (CNVR) were determined by aggregating overlapped CNVs, identified in different animals, as reported previously30. For construction of the CNVR map, we classified the status of these CNVR into three categories, ‘Loss’ (CNVR containing deletion), ‘Gain’ (CNVR containing duplication) and ‘Both’ (CNVR containing both deletion and duplication). Chromosomal distribution of CNVRs in Afghan sheep breeds has been developed in R v4.0.2. The VENN diagram web tool was also used to create a Venn diagram showing the overlap between CNVRs identified in different breeds [https://bioinformatics.psb.ugent.be/webtools/Venn/ (accessed on July 3, 2021)].
Finally, to evaluate the CNVRs identified in the present study in more detail, we compared them to the CNVRs identified in an Iranian sheep breed. In particular, 96 animals belong to Iranian Baluchi sheep breed were used. This breed is rearing in eastern parts of Iran, close to the borders of Afghanistan, and genotyped using the Illumina Ovine SNP50k Beadchip that fully described previously by Gholizadeh et al.31. The same statistical methods used in this study was applied to detect CNV and CNVRs in Iranian Baluchi sheep.
Genome-wide detection of selection signatures
To determine a genome-wide pattern of selection signatures in Afghan sheep breeds, the unbiased estimates of FST (Theta) was calculated as described by Weir and Cockerham32. Three comparisons consisting Arabi vs Baluchi, Arabi vs Gadik and finally Baluchi vs Gadik was performed. The values can theoretically range from 0 (showing no differentiation) to 1 (indicating complete differentiation, i.e. populations are fixed for different alleles). For each set of 5 adjacent SNPs, the average of FST values was calculated and termed windowed FST. This is an approximate method of looking for regions where selection is apparent over multiple markers, rather than one-off high values. A window of 5 markers was chosen as it appeared to provide the better signal compare to other arbitrary window sizes26. The windowed FST values were then plotted against genome location. All scripts were written and performed in R v 4.0.2 (https://cloud.r-project.org/).
Gene and QTL content and Cytoscape analysis
Genes harbored in the previously identified CNVRs were obtained from the Ensembl Genes 64 Database using BioMart software (https://www.ensembl.org/biomart/) based on the Ovis aries gene sequence assembly (Oar_v3.1). To functionally annotate these CNVRs, the gene ontology analysis (GO)29 was conducted using the Database for Annotation, Visualization, and Integrated Discovery Cytoscape functional annotation tool (http://www.cytoscape.org/). Also, to better understand the functions of the genes within the detected CNVRs, the O. aries Ensembl gene IDs were converted to human ortholog Ensembl gene IDs using BioMart because annotation of the sheep genome was limited33. In addition, to study whether these CNVRs were associated with economically important traits, these CNVRs were compared with QTLs in the sheep QTL database (https://www.animalgenome.org/cgi-bin/QTLdb/OA/index, sheep QTL database, accessed on 10 May 2022).
Approval for animal experiments
The experimental protocols were approved by the Biomedical Ethics Committee of Arak University. All methods were carried out in accordance with relevant guidelines and regulations. The authors also complied with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Results and discussion
Genome-wide detection of CNVs
The large-scale CNV explorations was performed and the results identified 411 CNVs across 45 individuals from three breeds, where the total length was 144 Mb and the average length for each individual was 9.13 Mb (Table 1). Among them 306, 62 and 43 CNVs was observed in Arabian, Baluchi and Gadik sheep breeds, respectively. The length of CNVs compared to whole-genome length, per each animal was 1.13%. The average length of the CNVs was 352 kb, and the median length was 214 kb. We found that approximately 24% of the CNVs range from 20 to 100 kb, 55% of CNVs range from 100 to 500 kb in size, and 21% being larger fragment CNVs (> 500 kb) (Table 1).
Table 1.
Summary of CNVs identified in Afghan sheep breeds.
| Descriptive statistics | Arab | Baluchi | Gadik | All animals |
|---|---|---|---|---|
| Number of CNVs | 306 | 62 | 43 | 411 |
| Total length of CNV (kb) | 128,897 | 9864 | 5872 | 144,635 |
| The proportion of CNVs length divided by whole-genome length per each animal1 | 3.44 | 0.26 | 0.17 | 1.31 |
| The average length of CNVs (kb) | 421 | 159 | 137 | 352 |
| The median length of CNVs (kb) | 301 | 103 | 102 | 214 |
| Number (percentage) of CNVs range 20–100 kb | 51 (17%) | 28 (45%) | 21 (49%) | 100 (24%) |
| Number (percentage) of CNVs range 100–500 kb | 173 (56%) | 32 (52%) | 22 (51%) | 227 (55%) |
| Number (percentage) CNVs with the length of > 500 kb | 82 (27%) | 2 (3%) | 0 | 84 (21%) |
1The length of the sheep genome is considered to be 2500,000 kb (https://www.ensemble.org).
In this study, CNVs were observed in all autosomal chromosomes, that was in agreement with the results obtained in previous studies in Chinese sheep34, native Korean Hanwoo cattle35, Chicken14 and human36. The average of CNVs per each animal in the present study was 9.13. A variety of CNVs number per each individual have been reported, including less than 123 to 16.921 in sheep, 17.9 in goat18, 9.6 in chicken14, 3.1 for horse37, and 19.16 in Iranian native cattle38. The number and the length of CNVs are influenced by a variety of factors including the sample size, the statistical method, species, and markers density39,40. For instance, while 45 samples from three Afghan sheep breeds (15 samples per each breed) have been used in the present study, Fontanesi et al.21 studied 11 samples (1 up to 2 samples per each breeds) to develop the first comparative map of CNVs in Italian dairy or dual-purpose sheep breeds using a cross-species array comparative genome hybridization (aCGH). Ma et al.23 were identified the CNVs in the genomes of eight sheep breeds (20 samples per each breed) using the sheep SNP50k BeadChip genotyping array, where, a total of 173 CNVs were discovered by the average and median sizes of 123 kb and 100 kb, respectively. The number of CNVs obtained in Afghan sheep breeds were in the range of previously reported studies by Fontanesi et al.21 and Ma et al.23 and the differences may be caused due to different breeds of animal and the number of samples.
Genome-wide identification of autosomal CNVRs
CNVRs were determined by merging the overlapping CNVs identified in all samples, as reported previously6,30. A total of 376 autosomal CNVRs (125 losses, 242 gains, and 7 both event) were identified. The average and median sizes of CNVRs were 361 kb and 224 kb, respectively. Approximately 25% of the CNVRs ranged from 20 to 100 kb, 53% ranged from 100 to 500 kb and 22% of the CNVRs were larger than 500 kb (Table 2). Detailed information on CNVRs in Afghan sheep breeds is presented in Table 2.
Table 2.
Summary of CNVRs identified in Afghan sheep breeds.
| Descriptive statistics | Arab | Baluchi | Gadik | All |
|---|---|---|---|---|
| Number of CNVRs in deletion state | 50 | 44 | 31 | 125 |
| Number of CNVRs in duplication state | 233 | 3 | 8 | 242 |
| Number of CNVRs in deletion/duplication state | 3 | 3 | 1 | 7 |
| Total length of CNVR (kb) | 122,811 | 7667 | 5275 | 135,756 |
| Average of CNVRs length (kb) | 429 | 153 | 132 | 361 |
| Median of CNVRs length (kb) | 306 | 100 | 100 | 224 |
| Number (percentage) of CNVRs range 20–100 kb | 46 (16%) | 27 (54%) | 20 (50%) | 93 (25%) |
| Number (percentage) of CNVRs range 100–500 kb | 160 (56%) | 21 (42%) | 20 (50%) | 201 (53%) |
| Number (percentage) CNVRs with the length of > 500 kb | 80 (28%) | 2 (4%) | 0 | 82 (22%) |
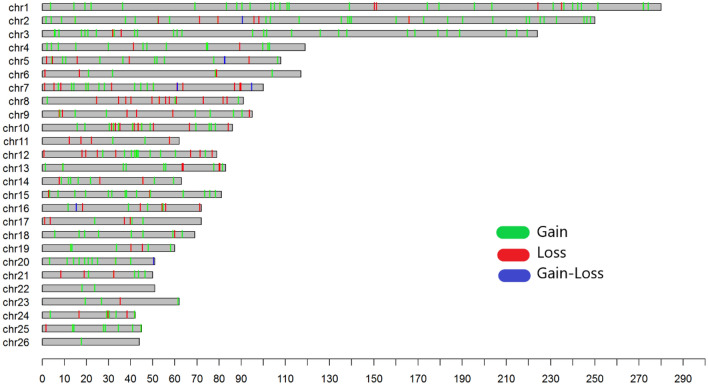
The map of the CNVRs distribution on autosomal chromosomes was created in total samples of Afghan sheep and has been shown in Fig. 2.
Figure 2.
Chromosomal distribution of copy number variant regions (CNVRs) in Afghan sheep breeds, developed using R v4.0.2 (https://cloud.r-project.org/): in this figure the chromosome length (Mb) is displayed on the X-axis and CNVR variations with deletion, duplication, and deletion/duplication state showed on different chromosomes on Y-axis.
Keeping in mind that the detection rate of CNVRs is affected by many factors41, the results of this study were compared with previous studies in sheep. Between 11142 and 348843 CNVRs have been detected in previous studies, with CNVR lengths of 10.5621 up to 120.5323 Mbs. The CNVRs identified in these studies were different to some extent, which may be due to the differences in sheep breeds, CNV detection platforms and CNV calling algorithms used. Similar findings were also observed in CNVR studies conducted in different mammals including pig44 dairy and beef cattle45 and goat breeds46. The use of different CNV calling algorithms has a substantial effect on the results of CNVR studies. Currently, three algorithms for CNV detection based on SNP arrays have been developed, which are available in different programs, including PennCNV, cnvPartition and QuantiSNP23. Compared to other algorithms, PennCNV provides a standard proven method that is more reliable for assessing the number of copies when using Illumina arrays because it incorporates the allelic intensity ratio at each SNP marker and the total signal intensity, the allele frequency of SNPs, the distance between neighboring SNPs, and the GC content to overcome biases47. Also, the software packages currently commonly used for CNV detection include PennCNV, CNVcaller, and CNVnator, each of them have their own advantages and disadvantages. PennCNV software has been extensively applied to Illumina chip data48 while, CNVcaller and CNVnator software are using read depth methods to detect CNVs in resequencing data33,49.
The results indicate that the number of CNVRs in the chromosomes varies from 1 to 39 (Additional file Table S1), with the top three highest rate of CNVRs (more than 30) located on chromosomes 2, 1, and 3, which contained 39, 34, and 31 CNVRs respectively. Chromosome 26 had the smallest number of CNVRs. This observation was in agreement with Yuan et al.50 where they reported that the chromosome length had a significant positive linear relationship with the number of CNVRs (R2 = 0.87) with the maximum found in chromosome 1 and the minimum found on 26. Conflicting results have also reported by Jenkins et al.43 in Texel, Coopworth, Perendale, Romania, and Merino sheep and Zhu et al.48, in the study of CNVR-containing genomic regions in Tibetan sheep, stated that chromosome 3 had the highest number of CNVRs. These results may be influenced by the number of samples and arrays used. For example, while 50k chips were used in the present study, Zhu et al.48 used the chips with a 600k marker density. However, the medium density of Illumina OvineSNP50 BeadChip array used in our study, provides a standard proven method to detect CNVs and has been widely used in CNV detection in different sheep breeds41,51–53. It was also observed in the present study that CNV duplication state is more than the deletion state, which may be because the deletions are under stronger purifying selection (which removes deleterious variants from the population) than duplications, therefore deletions should on average, be both less frequent and smaller than duplications30.
A comparison of the CNVRs among breeds is shown in the Venn diagram (Fig. 3). The highest number of shared CNVRs was observed between Arabi and Gadik (n = 8), and the lowest number, was shared between Baluchi and Gadik (n = 2). Long-term adaptation to different environmental conditions or different selection schemes increases the variation in CNVRs among breeds30. Similar environmental conditions, gene flow, and shared ancestral components may have led to an increase in shared CNVRs among breeds41. The more CNVRs identified in Arab sheep and the highest shared CNVRs observed between Arab and Gadik are in agreement with the distribution of these breeds in Afghanistan. Arab sheep breed is rearing in a wider geographic ranges and therefore, is adapted to different climatic conditions. Also, Arab and Gadik sheep breeds are rearing close to each other and gene flow may have occurred during last generations, leading to an increase in shared CNVRs among breeds (Fig. 1).
Figure 3.
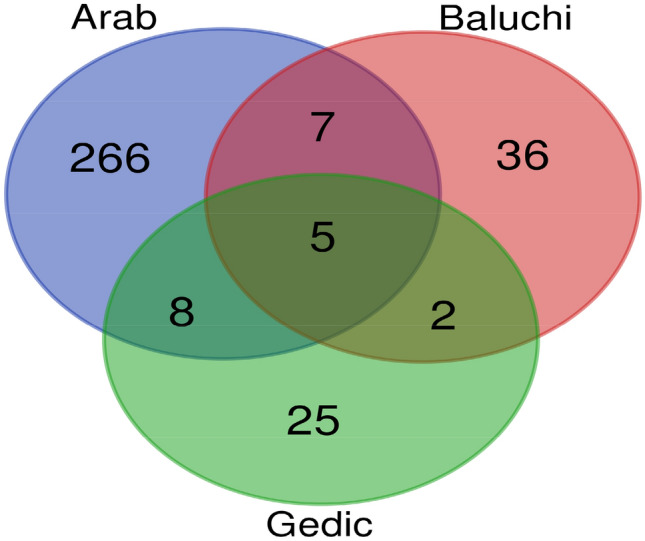
Venn diagram representing common and unique CNVRs found among the three Afghan sheep breeds. This figure was created using VENN diagram web tool (https://bioinformatics.psb.ugent.be/webtools/Venn/).
Gene content and functional annotation
The BioMart system in ENSEMBL was used to retrieve the gene content in the 376 CNVRs (125 losses, 242 gains, and 7 both event). Our results revealed that some CNVRs did not overlap with any annotated gene that was in agreement with the results reported in previous studies on cattle54 and sheep52. This may be due to the genome assembly version, especially in non-human species54, and also several studies have highlighted the genome-wide distribution of CNVs in regions covering non-coding sequences, thus affecting the regulation of distant target genes55. However, many genes identified in these regions had economically important biological functions including IL37 genes, affecting immune response, DCT gene with an important role in melanin synthesis and color expression in skin and feathers, CACNA1A gene influencing contraction process muscles and the TCF12 gene affecting skeletal muscle, thyroid, B and T cells (GeneCards). The IL37 gene is located on chromosome 3, which is involved in pro-inflammatory and anti-inflammatory reactions that it is one of the candidate genes influencing the immune response and resistance to mastitis in cattle56. The DCT gene is located on chromosome 10, which is involved in the production of the pigment Eomelanin and Feomelanin. In mammals, skin and hair color are depending on the relative ratio of Eomelanin and Feomelanin52. The CACNA1A gene on chromosome 5 involve in muscle contraction, hormone or neurotransmitter secretion, and gene expression (GeneCards). Zhu et al.57 have shown that this gene is associated with fat deposition in fat tailed sheep breeds. The TCF12 gene which is situated on chromosome 7, is expressed in many tissues, including skeletal muscle, thyroid, B, and T cells (GeneCards). Dunner et al.58 reported that this gene affects muscle fatty acid production in Bos Taurus cattle.
To determine the underlying biological functions of the genes overlapped with CNVRs, the Gene Ontology (GO) analysis was used and the genes were evaluated for their affects on biological process (BP), cellular component (CC), molecular function (MF), and immune system. Our results showed that the most of the genes were associated with sensory perception and immune system activity, interleukins, and growth performance (Table 3). The same pathways have previously been reported for the development of skeletal muscle and immune system in Altai and Tibetan sheep48 and in antigenic processes and immune responses by Fontanesi et al.21 and Hou et al.34. Liu et al.6 reported the genes that contributed in the adaptation to environmental conditions, which is consistent with the results of the present study.
Table 3.
Summary of the results from the ontology of genes identified in CNV regions in all samples.
| GOID | GO term | P-value |
|---|---|---|
| GO:0007600 | Sensory perception | 1.9E−6 |
| GO:0050907 | Detection of chemical stimulus involved in sensory perception | 9.5E−5 |
| GO:0050906 | Detection of stimulus involved in sensory perception | 1.4E−11 |
| GO:0007606 | Sensory perception of chemical stimulus | 1.8E−11 |
| GO:0007608 | Sensory perception of smell | 1.4E−13 |
| GO:0048588 | Developmental cell growth | 9.8E−3 |
| GO:0005149 | Interleukin-1 receptor binding | 4.5E−5 |
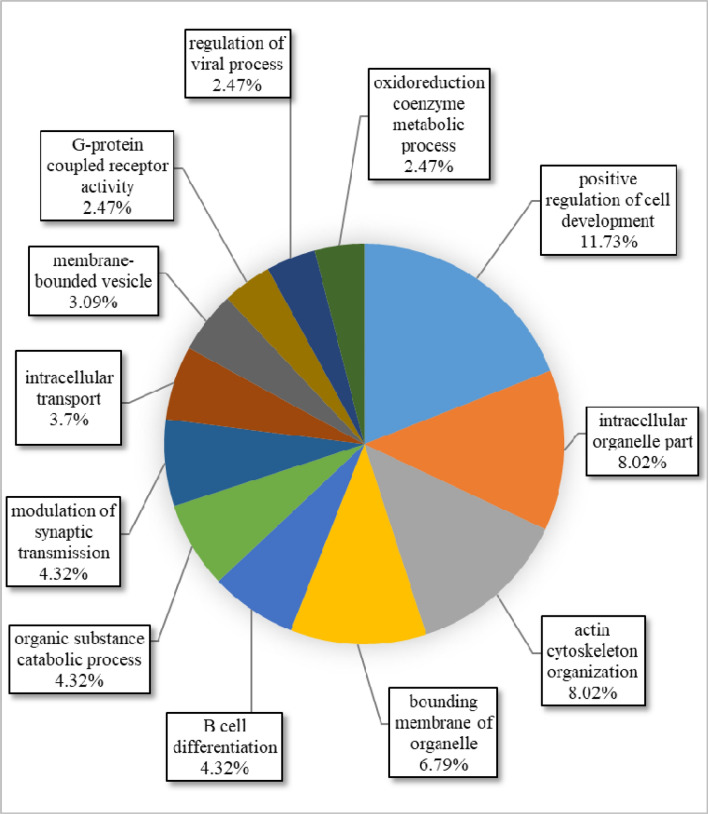
A circular diagram of the biological pathways involved by the genes reported in CNVR-containing regions of all Afghan sheep breeds has been shown in Fig. 4. The results showed that 11.73% of the genes affected the positive regulation of cell development. This pathway refers to any process that increases the speed, frequency, or rate of development of a cell from its formation into a mature structure (QuickGO). The ratios of 8.02, 8.02, and 6.79%, were also influencing the pathways of intracellular organelle part, actin cytoskeleton organization, and binding membrane of organelles, respectively (Fig. 4). The identification of GOTerms in this study and their comparisons with other studies showed a number of similar pathways. Most of these similar pathways include the G-Protein coupled receptor signaling pathway (GO:0007186)6,34, Sensory perception (GO:0007600)6, neurological system process (GO:0050877)34, Cation binding (GO:0043169)7, metal ion binding (GO:0046872)7 and enzyme binding (GO:0019899)23. The study of these GOTerms in other species also showed some reported pathways that are consistent with the present study, for instance, G-Protein coupled receptor signaling pathway (GO:0007186) in cattle59.
Figure 4.
Circular diagram of the biological pathways involved by the genes reported in CNV-containing regions of all Afghan sheep breeds produced by Cytoscape functional annotation tool (http://www.cytoscape.org/): in this figure, the name of each process and the degree of the gene participation have been reported. The pathways with a participation rate of less than 2% are not shown.
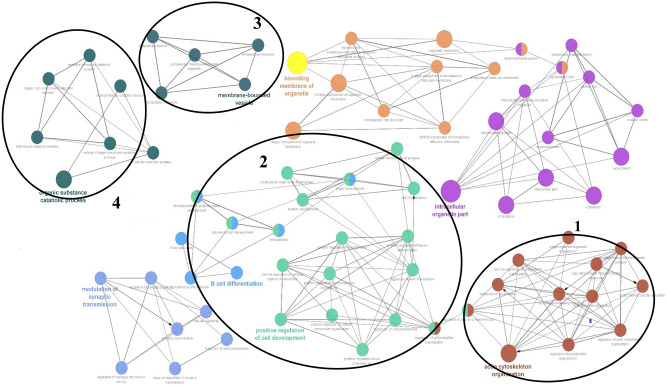
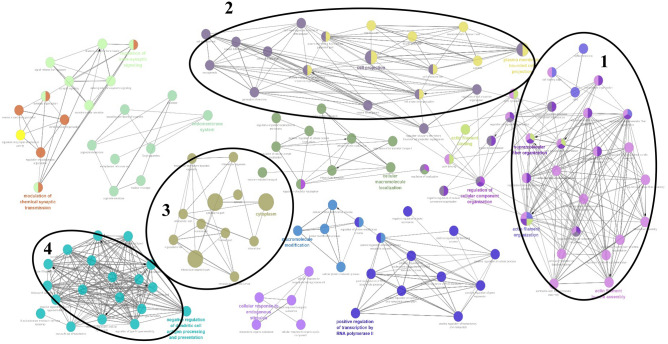
The gene networks involved by the genes identified in the present study are shown in Fig. 5. Four gene networks related to different traits were created and several co-associated genes in these networks are involved in biological processes previously found to be associated with production and reproduction performance traits. Network 1 in stance, included different genes, such as ZNF and CAPZB that were previously reported to be associated with muscle structure and meat production60. Genes in cluster 2 primarily included HINT2, TNFRSF19, CCIN, FNDC3A, SYCP2, TUBGCP3, and RECK, which were related with birth weight, body size, fertility, embryonic development, as well as skeletal morphology61,62. Cluster 3 included some genes such as ACAN and cluster 4 the genes like NID2 which were previously reported to be associated with skeletal development and cartilage structure36 and embryonic development63, respectively.
Figure 5.
Gene-network interactions between all the genes reported in the regions containing CNVRs in Afghan sheep breeds, produced by Cytoscape functional annotation tool (http://www.cytoscape.org/). Circles of different colors represent (biochemical pathways) and the lines between these circles indicate the interactions between these pathways with each other. Zone 1 include the genes that affecting the skeletal muscle, zone 2 influence the birth weight, fertility, embryonic development and skeletal morphology, zone 3 is containing the genes that effect on skeletal development, cartilage structure, and joint function and zone 4 plays a role in fetal development and development.
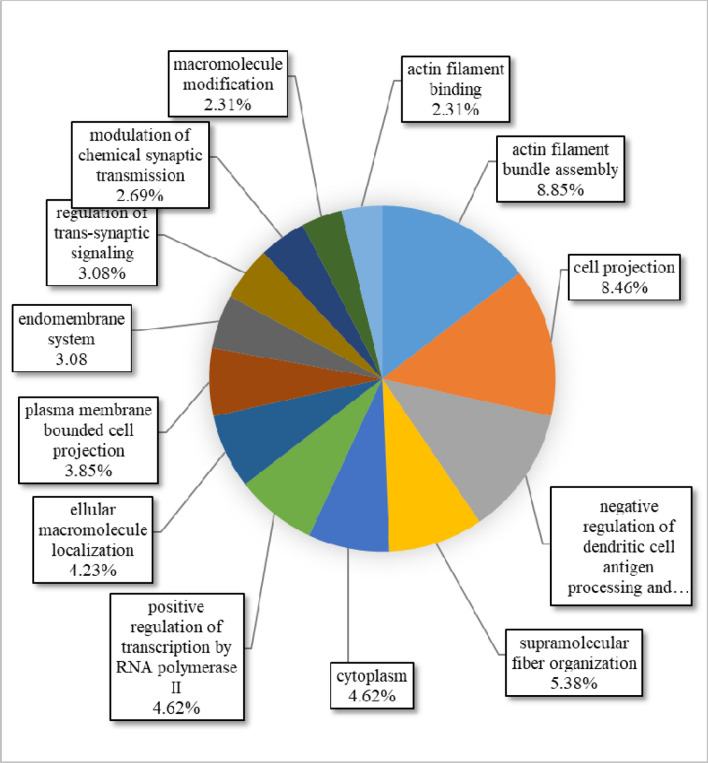
As the current annotation of the sheep genome is not as comprehensive as H. sapience26, the regions of interest in O. aries were compared to the corresponding areas in human (Fig. 6). The results of gene ontology showed that 8.85% of the genes that overlapped with CNV regions affected the actin filament bundle assembly pathway. This pathway consists a set of actin filament bands that are aligned in an axis with identical or opposite poles (approximately 30–60 nm apart) (QuickGO). Also, the ratios of 8.46, 7.31, and 5.38% were associated with cell projection, negative regulation of dendritic cell antigen presentation, and supramolecular fiber organization, respectively (Fig. 6). Given that actin and myosin are one of the most important components of muscle development, it seems that the development of the skeletal and muscle systems has been one of the most important targets during the evolution of the Afghan sheep. These pathways had a P-value of 3.4E−7, 3.2E−5 and 1.2E−11 in O. aries and 2.6E−3, 4.5E−5 and 6.6E−9 in H. sapience, respectively.
Figure 6.
Circular diagram of the biological pathways involved by the genes reported in orthologous regions containing CNVs in H. sapiens, created by Cytoscape functional annotation tool (http://www.cytoscape.org/): In this figure the name of each process and its percentage has been showed. Pathways with a contribution of less than 2% were removed from the graph.
Also, the gene networks developed by the genes identified in orthologous regions of H. sapience were also evaluated and the results revealed that some networks are associated with muscle skeletal, embryonic, reproductive stages, birth weight, melanocyte, pigmentation, enteric nerves, skeletal development, immune response, antigens and antibodies (Fig. 7). Network 1 in stance, included different genes, such as ROCK-1, ROCK-2 that were previously reported to be associated with muscle structure system in sheep64 and network 2 included PTGDR, HINT2, SPAG8, CCIN, TNFRSF19 and ACAN genes which were related with sex determination65, fertility, fetal development, birth and carcass weights66 and skeletal development and cartilage structure36. Cluster 3 included some genes such as SYCP2, NPR2, EDN3 influencing birth weight, skeletal morphology and body size67, and finally network 4 including the genes like PRSS55, CRISP2 that are relate with immune system, antigens and antibodies68.
Figure 7.
Gene-network interactions between all the genes reported in the orthologous areas of H. sapience, for the genomic regions containing CNVRs in Afghan sheep breeds [the figure developed by Cytoscape functional annotation tool (http://www.cytoscape.org/)]. Circles of different colors represent biochemical paths and the lines between these circles indicate the interactions between these paths with each other, whose intensity is a measure of the assurance of a functional partnership. Region 1 contains the genes affecting the muscle skeletal region, region 2, the sex determination of males and females, birth weight, carcass weight, fetal development, skeletal development and cartilage structure region 3 is associated with birth weight and skeletal morphology and body size, and finally, region 4, affect the immune response, antigens, and antibodies.
Due to the high conservation of the genes between humans and sheep69, genes that are known to be related to complex human traits may also be important for related traits in sheep. However, further research will be needed to formally test the functional relevance of these genes.
QTLs overlapping with identified CNVRs
To further reveal the CNVRs associated with sheep economic traits, the detected CNVRs were compared with quantitative trait loci (QTLs) in the sheep QTL database. We found 121 CNVRs overlapping with 171 QTLs, including milk production and quality, growth and carcass traits, reproduction performance, fleece yield, fecal egg counts, tail fat deposition, horn type and health-related traits (Additional file Table S1).
Many studies have shown that CNVRs contain QTLs associated with important economic traits in animals50,70. Therefore, the CNVRs detected in this study were compared with the QTLs reported in the sheep QTL database. The QTL categories found in this study were basically identical to those found in previously reported studies50,52,71. For example, the health-related QTLs found included fecal egg count QTLs, and worm count QTLs that previous studies have reported that worm disease infection rates in sheep can exceed 70% in many countries, causing huge losses to the livestock industry72. Relative to barn-fed livestock, grazing livestock are more likely to be infected with worms. These results indicate that CNVs, which are a critical type of genetic variation, may have an important effect on sheep health.
Comparison of identified CNVRs with the regions that have been under selection signatures
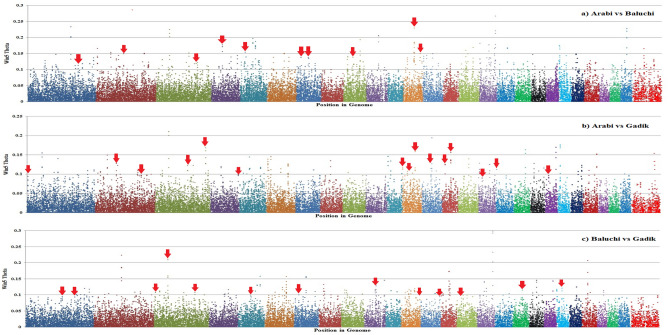
To identify loci that have been targets of selection across different Afghan sheep breeds, the windowed FST was plotted against genomic location (Fig. 8). Variants with unusually large FST values are typically interpreted as being the targets of local selective pressures due to the hitch-hiking effect26.
Figure 8.
Distribution of selective sweep signatures for different comparisons in Afghan sheep breeds by chromosome. SNP position in the genome is shown on the X-axis and each chromosome was highlighted in different colors, and windowed FST (Theta) is plotted on the Y-axis. The red arrows are highlighting the peaks that overlapped by CNV regions identified in our study. This figure was created using R v 4.0.2 (https://cloud.r-project.org/).
Comparison of identified CNVRs with the regions that have been under selection signatures indicated that, 37 of the 376 CNVRs (~ 10%) were also identified to be under selection pressure and confirmed by selective sweep signatures (Fig. 8 and Additional file Table S1). Almost all of these regions overlapped with the genes or QTLs that had previously been identified to be associated with production, reproduction and environmental adaptions traits in sheep (Additional file Table S1, Sheet 2). Zhu et al.57 identified the CNVs and selection signatures on the X chromosome in Chinese indigenous sheep breeds with different tail types. Their results revealed 20 common genes that were shared by CNVRs and selection signatures, and were associated with fat, energy metabolism, and immune system.
Comparison of identified CNVRs with Iranian sheep breed
Finally, to characterize the CNVRs identified in the present study in more detail, we compared them to the CNVRs identified in an Iranian sheep breed namely Baluchi. This breed is fat-tailed and dual-purpose breed (wooly and meaty), which is well adapted to the dry and hot climate conditions of eastern Iran. Iranian Baluchi sheep has the largest population than other breeds of sheep in Iran (about 30% sheep population) and have selected for this comparison since, it is mostly rearing in the regions of Iran that are close to the boarder of Afghanistan31. After quality control, 45,673 autosomal SNPs from 87 animals were used for CNVRs detection. The results indicated that 20 of the 114 CNVRs (18%) identified in Iranian sheep breed were overlapped or located close to the CNVRs identified in our study (Table 4). Study of the genes reported in these regions of interest revealed that most of those overlapped with the genes influencing production, reproduction and immune system (Table 4).
Table 4.
The list of CNVs overlapped between Afghan and Iranian sheep breeds and the genes and their functions reported in these locations.
| CNVs positions in Afghan sheep | CNVs positions in Iranian sheep | Distance (kb) | The genes reported in this location | Function of the gene | References |
|---|---|---|---|---|---|
| Chr1:242324429–242574074 | Chr1:241583858–241653162 | 671 | SLITRK3 | Milk production traits | 73 |
| Chr2:172582236–172616581 | Chr2:172272878–172424726 | 158 | MBD5 and EPC2 | Growth traits | 74 |
| Chr5:39479796–39659262 | Chr5:39400849–39585301 | 0 | COL23A1 | Fertility | 75 |
| Chr6:78371034–79041954 | Chr6:78371034–78600405 | 0 | EXOC1L | Reproduction | 76 |
| CEP135 | Milk production | 77 | |||
| AASDH | Metabolism | 78 | |||
| Chr6:78796013–79165093 | Chr6:79007752–79203136 | 0 | HOPX | Sperm traits | 79 |
| Chr8:52949829–52997226 | Chr8:52676280–52949829 | 0 | BACH2 | Immune system | 80 |
| Chr9:7615407–8155540 | Chr9:7838010–7948027 | 0 | |||
| Chr10:19347073–19637375 | Chr10:18607317–18670320 | 677 | RUBCNL | Vertebrate autophagy | 81 |
| LRCH1 | Immune system | 82 | |||
| HTR2A | Adaptive traits | 83 | |||
| Chr10:66683508–66747944 | Chr10:65063529–65212062 | 977 | SLITRK6 | Milk production traits | 73 |
| Chr10:83028244–85695176 | Chr10:85120520–85216381 | 0 | HS6ST3 | Growth traits | 84 |
| STK24 | Immune response | 85 | |||
| SLC15A1 | Development of growth | 75 | |||
| GPR18 | Immune system | 86 | |||
| TM9SF2 | Fertility | 87 | |||
| DOCK9 | Disease resistance | 88 | |||
| Chr12:27323634–27702125 | Chr12:27961834–28032557 | 260 | DUSP10 | Immunity inflammation | 89 |
| Chr14:12743159–13111196 | Chr14:13401157–13465959 | 290 | FOXC2 | Immune system | 90 |
| Chr15:75842476–75936079 | Chr15:76144260–76212569 | 208 | |||
| Chr16:47568518–47871748 | Chr16:46887875–47002471 | 566 | |||
| Chr17:23675899–23828049 | Chr17:23538968–23738050 | 0 | |||
| Chr17:23675899–23828049 | Chr17:23988299–24240466 | 0 | |||
| Chr21:18899984–19383548 | Chr21:18655157–18820162 | 80 | LRFN4 | Ovarian cancer | 91 |
| Chr21:46501772–46700862 | Chr21:46501772–46628225 | 0 | TENM4 | Nervous system development | 92 |
| NARS2 | Energy deficiency disorders | 93 | |||
| Chr:25:14234573:14644765 | Chr25:14561562–14644765 | 0 | PHYHIPL | Growth and wool traits | 94 |
| Chr:25:34269680:34689602 | Chr25:34845271–34945170 | 156 | KCNMA1 | Growth traits | 95 |
| DLG5 | Muscle growth | 96 | |||
| POLR3A | Immune response | 97 | |||
| RPS24 | Heat stress | 98 |
The CNVRs identified in different breeds have previously compared in a variety of species including sheep23, cattle99 and chicken100 to evaluate the reliability of the detected regions consisting CNVs. The high proportion of overlapped CNVRs observed in the current study between Iranian and Afghanistan sheep breeds may probably reflect this fact that these breeds have been influenced by similar evolutionary events, mostly due to their close geographic distribution and may some gene flow occurred between these breeds in the past.
In general, this study is an important step towards the generation of a CNV map and provides an important resource for studies of ovine genomic variation in indigenous sheep of Afghanistan. However, the number of animals used in the current study may be one of the limitations affecting the number of CNVs identified in this study. Although, it should be considered that this is the only genomic information available from Afghan local sheep breeds and most of the similar published reports have been performed with almost the same or lower sample size in different indigenous sheep breeds7,21,42,50,57,94,101, however, there were validation or phenotypes in most of the previous studies. For example, quantitative PCR (qPCR) has been used to confirm subsets of CNVs identified in different studies42,101. Given that no validation of our findings has been performed in the current study, the results presented in this study is purely exploratory and external validation will be necessary to confirm the findings. It seems by increasing the number of genotyped samples in the future researches, we would be able to shed some new lights on the domestication event and the history of Afghan sheep. Also, further research on phenotypic associations would help understanding the role of CNVs in the formation of the local sheep breeds. Our results offer meaningful genomic insights that will help to guide future research and to provide a preliminary basis for the future exploration of the relationship between CNVs and economically important phenotypes of sheep.
Conclusion
Overall, the first map of CNVs on the genome of Afghan sheep breeds was developed in this study using genome-wide information. A total number of 411 CNVs and 376 CNVRs were identified that cover 144 Mb of the genome. Since each CNV variant contains more length of the DNA, comparing to single nucleotide polymorphism (SNP) markers, and a variety of previously published studies suggest that these regions have been associated with economically important traits, it seems CNVs can provide better information for realizing the missing inheritance related to complex traits in Afghan sheep breeds. The results of this study showed that the genes reported in the genomic regions containing CNVRs in this study are associated with various metabolic pathways including reproductive traits, carcass characteristics, and body weight, immune and musculoskeletal system. The results of the present study provide valuable information for analyzing the association of CNVs with economically important phenotypes in Afghan sheep.
Supplementary Information
Acknowledgements
The authors gratefully acknowledge Dr. Nicolo Macciotta and Dr. Giustino Gaspa from Sassari University, Italy for providing the facilities to conduct the experimental work and genotyping the animal samples.
Author contributions
The data were collected by M.H.M. and M.O.K., and analyzed by M.H.M., R.M., and A.H.F. The paper was drafted by M.H.M. and R.M. All authors have contributed to the editing of the article, and approved the final manuscript.
Data availability
All CNVRs identified in Afghan sheep breeds have been deposited in figshare repository (https://www.figshare.com) with accession no. 10.6084/m9.figshare.16577954.v1. The genomic information used in this study belongs to private sheep breeding programs, so we do not have authorization to share it and are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-18571-4.
References
- 1.Kijas JW, et al. Genome-wide analysis of the world’s sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 2012;10:e1001258. doi: 10.1371/journal.pbio.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moradi MH, Khaltabadi-Farahani AH, Khodaei-Motlagh M, Kazemi-Bonchenari M, McEwan J. Genome-wide selection of discriminant SNP markers for breed assignment in indigenous sheep breeds. Ann. Anim. Sci. 2021;21:807–831. doi: 10.2478/aoas-2020-0097. [DOI] [Google Scholar]
- 3.Rashiq MH. Building carbohydrates from a livestock nutrition perspective. Kabul Univ. J. 1995;1:15–24. [Google Scholar]
- 4.Khanzadeh H, Ghavi Hossein-Zadeh N, Ghovvati S. Genome wide association studies, next generation sequencing and their application in animal breeding and genetics: A review. Iran. J. Appl. Anim. Sci. 2020;10:395–404. [Google Scholar]
- 5.Zhu H, Zhou X. Statistical methods for SNP heritability estimation and partition: A review. Comput. Struct. Biotechnol. J. 2020;18:1557–1568. doi: 10.1016/j.csbj.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, et al. Analysis of copy number variations in the sheep genome using 50K SNP BeadChip array. BMC Genomics. 2013;14:229. doi: 10.1186/1471-2164-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan J, et al. Genome-wide detection of autosomal copy number variants in several sheep breeds using Illumina OvineSNP50 BeadChips. Small Rumin. Res. 2017;155:24–32. doi: 10.1016/j.smallrumres.2017.08.022. [DOI] [Google Scholar]
- 8.Huang Y, et al. An atlas of CNV maps in cattle, goat and sheep. Sci. China Life Sci. 2021;64:1747–1764. doi: 10.1007/s11427-020-1850-x. [DOI] [PubMed] [Google Scholar]
- 9.Stranger BE, et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science. 2007;315:848–853. doi: 10.1126/science.1136678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridges CB. The bar “gene” a duplication. Science. 1936;83:210–211. doi: 10.1126/science.83.2148.210. [DOI] [PubMed] [Google Scholar]
- 11.Bae JS, et al. Identification of copy number variations and common deletion polymorphisms in cattle. BMC Genomics. 2010;11:1–10. doi: 10.1186/1471-2164-11-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butty AM, et al. Genome-wide association study between copy number variants and hoof health traits in Holstein dairy cattle. J. Dairy Sci. 2021;104:8050–8061. doi: 10.3168/jds.2020-19879. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez CE, Akey JM. Copy number variation in the domestic dog. Mamm. Genome. 2012;23:144–163. doi: 10.1007/s00335-011-9369-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Nahashon S, Feaster TK, Bohannon-Stewart A, Adefope N. An initial map of chromosomal segmental copy number variations in the chicken. BMC Genomics. 2010;11:1–10. doi: 10.1186/1471-2164-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes AC, et al. Genome-wide detection of CNVs and their association with performance traits in broilers. BMC Genomics. 2021;22:1–18. doi: 10.1186/s12864-021-07676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadista J, Nygaard M, Holm L-E, Thomsen B, Bendixen C. A snapshot of CNVs in the pig genome. PLoS One. 2008;3:e3916. doi: 10.1371/journal.pone.0003916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramayo-Caldas Y, et al. Copy number variation in the porcine genome inferred from a 60 k SNP BeadChip. BMC Genomics. 2010;11:1–10. doi: 10.1186/1471-2164-11-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontanesi L, et al. An initial comparative map of copy number variations in the goat (Capra hircus) genome. BMC Genomics. 2010;11:1–15. doi: 10.1186/1471-2164-11-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, et al. Genome-wide CNV analysis revealed variants associated with growth traits in African indigenous goats. Genomics. 2020;112:1477–1480. doi: 10.1016/j.ygeno.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Fontanesi L, et al. Exploring copy number variation in the rabbit (Oryctolagus cuniculus) genome by array comparative genome hybridization. Genomics. 2012;100:245–251. doi: 10.1016/j.ygeno.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Fontanesi L, et al. A first comparative map of copy number variations in the sheep genome. Genomics. 2011;97:158–165. doi: 10.1016/j.ygeno.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Norris BJ, Whan VA. A gene duplication affecting expression of the ovine ASIP gene is responsible for white and black sheep. Genome Res. 2008;18:1282–1293. doi: 10.1101/gr.072090.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Q, et al. Genome-wide detection of copy number variation in Chinese indigenous sheep using an ovine high-density 600 K SNP array. Sci. Rep. 2017;7:912. doi: 10.1038/s41598-017-00847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karimi, M. O. Investigation of genetic diversity and selection markers of genome level in some Afghani sheep breeds. PhD thesis, Ferdowsi University of Mashhad (2016).
- 25.Purcell S, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moradi MH, Nejati-Javaremi A, Moradi-Shahrbabak M, Dodds KG, McEwan JC. Genomic scan of selective sweeps in thin and fat tail sheep breeds for identifying of candidate regions associated with fat deposition. BMC Genet. 2012;13:10. doi: 10.1186/1471-2156-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curik I, Ferenčaković M, Karapandza N, Cubric-Curik V, Sölkner J. Estimation of inbreeding and effective population size in Istrian cattle using molecular information. Acta Agraria Kaposváriensis. 2014;18:30–34. [Google Scholar]
- 28.Wang K, et al. PennCNV: An integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res. 2007;17:1665–1674. doi: 10.1101/gr.6861907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peiffer DA, et al. High-resolution genomic profiling of chromosomal aberrations using Infinium whole-genome genotyping. Genome Res. 2006;16:1136–1148. doi: 10.1101/gr.5402306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redon R, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gholizadeh M, Rahimi-Mianji G, Nejati-Javaremi A, De Koning DJ, Jonas E. Genomewide association study to detect QTL for twinning rate in Baluchi sheep. J. Genet. 2014;93:489–493. doi: 10.1007/s12041-014-0372-1. [DOI] [PubMed] [Google Scholar]
- 32.Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, et al. CNVcaller: Highly efficient and widely applicable software for detecting copy number variations in large populations. GigaScience. 2017;6:gix115. doi: 10.1093/gigascience/gix115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou C-L, et al. Genome-wide analysis of copy number variations in Chinese sheep using array comparative genomic hybridization. Small Rumin. Res. 2015;128:19–26. doi: 10.1016/j.smallrumres.2015.04.014. [DOI] [Google Scholar]
- 35.Mohanty TR, et al. Molecular variation in pigmentation genes contributing to coat colour in native Korean Hanwoo cattle. Anim. Genet. 2008;39:550–553. doi: 10.1111/j.1365-2052.2008.01746.x. [DOI] [PubMed] [Google Scholar]
- 36.Weedon MN, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat. Genet. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzger J, et al. Analysis of copy number variants by three detection algorithms and their association with body size in horses. BMC Genomics. 2013;14:1–15. doi: 10.1186/1471-2164-14-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karimi K, Esmailizadeh A, Wu DD, Gondro C. Mapping of genome-wide copy number variations in the Iranian indigenous cattle using a dense SNP data set. Anim. Prod. Sci. 2017;58:1192–1200. doi: 10.1071/AN16384. [DOI] [Google Scholar]
- 39.Nosrati M, Tahmoursipour M. Identification of variation in genome copy number of 15 Italian sheep breeds using a 50 kb sheep chip. Iran. J. Anim. Sci. Res. 2016;8:489–501. [Google Scholar]
- 40.Pielberg GR, et al. A cis-acting regulatory mutation causes premature hair graying and susceptibility to melanoma in the horse. Nat. Genet. 2008;40:1004–1009. doi: 10.1038/ng.185. [DOI] [PubMed] [Google Scholar]
- 41.Di Gerlando R, et al. Identification of copy number variations and genetic diversity in Italian insular sheep breeds. Animals. 2022;12:217. doi: 10.3390/ani12020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Zhang Q, Lu Z, Zhao X, Zhang Y. Analysis of copy number variations by SNP50 BeadChip array in Chinese sheep. Genomics. 2015;106:295–300. doi: 10.1016/j.ygeno.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Jenkins GM, et al. Copy number variants in the sheep genome detected using multiple approaches. BMC Genomics. 2016;17:441. doi: 10.1186/s12864-016-2754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, et al. Enhancing genome-wide copy number variation identification by high density array CGH using diverse resources of pig breeds. PLoS One. 2014;9:e87571. doi: 10.1371/journal.pone.0087571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letaief R, et al. Identification of copy number variation in French dairy and beef breeds using next-generation sequencing. Genet. Sel. Evol. 2017;49:1–15. doi: 10.1186/s12711-017-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M, et al. Diversity of copy number variation in the worldwide goat population. Heredity. 2019;122:636–646. doi: 10.1038/s41437-018-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marenne G, et al. Assessment of copy number variation using the Illumina Infinium 1M SNP-array: A comparison of methodological approaches in the Spanish Bladder Cancer/EPICURO study. Hum. Mutat. 2011;32:240–248. doi: 10.1002/humu.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu C, et al. Genome-wide detection of CNVs in Chinese indigenous sheep with different types of tails using ovine high-density 600K SNP arrays. Sci. Rep. 2016;6:1–9. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abyzov A, Urban AE, Snyder M, Gerstein M. CNVnator: An approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 2011;21:974–984. doi: 10.1101/gr.114876.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan C, et al. A global analysis of CNVs in Chinese indigenous fine-wool sheep populations using whole-genome resequencing. BMC Genomics. 2021;22:78. doi: 10.1186/s12864-021-07387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goyache F, et al. Functional characterization of copy number variations regions in Djallonké sheep. J. Anim. Breed. Genet. 2021;138:600–612. doi: 10.1111/jbg.12542. [DOI] [PubMed] [Google Scholar]
- 52.Di Gerlando R, et al. Genome-wide association study between CNVs and milk production traits in Valle del Belice sheep. PLoS One. 2019;14:e0215204. doi: 10.1371/journal.pone.0215204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang L, et al. Diversity of copy number variation in a worldwide population of sheep. Genomics. 2018;110:143–148. doi: 10.1016/j.ygeno.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Prinsen R, et al. A genome wide association study between CNVs and quantitative traits in Brown Swiss cattle. Livest. Sci. 2017;202:7–12. doi: 10.1016/j.livsci.2017.05.011. [DOI] [Google Scholar]
- 55.Zhang F, Lupski JR. Non-coding genetic variants in human disease. Hum. Mol. Genet. 2015;24:R102–R110. doi: 10.1093/hmg/ddv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Usman T, et al. Novel SNPs in IL-17F and IL-17A genes associated with somatic cell count in Chinese Holstein and Inner-Mongolia Sanhe cattle. J. Anim. Sci. Biotechnol. 2017;8:5. doi: 10.1186/s40104-016-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu C, Li M, Qin S, Zhao F, Fang S. Detection of copy number variation and selection signatures on the X chromosome in Chinese indigenous sheep with different types of tail. Asian Australas. J. Anim. Sci. 2020;33:1378. doi: 10.5713/ajas.18.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunner S, et al. Genes involved in muscle lipid composition in 15 European Bos taurus breeds. Anim. Genet. 2013;44:493–501. doi: 10.1111/age.12044. [DOI] [PubMed] [Google Scholar]
- 59.da Silva VH, et al. Genome-wide detection of CNVs and their association with meat tenderness in Nelore cattle. PLoS One. 2016;11:e0157711. doi: 10.1371/journal.pone.0157711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taye M, et al. Whole genome scan reveals the genetic signature of African Ankole cattle breed and potential for higher quality beef. BMC Genet. 2017;18:1–14. doi: 10.1186/s12863-016-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei C, et al. Genome-wide analysis reveals population structure and selection in Chinese indigenous sheep breeds. BMC Genomics. 2015;16:1–12. doi: 10.1186/1471-2164-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meuwissen RL, et al. A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J. 1992;11:5091–5100. doi: 10.1002/j.1460-2075.1992.tb05616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forde N, et al. Effects of low progesterone on the endometrial transcriptome in cattle. Biol. Reprod. 2012;87:121–124. doi: 10.1093/biolreprod/87.s1.121. [DOI] [PubMed] [Google Scholar]
- 64.Levent A, Büyükafşar K. Expression of Rho-kinase (ROCK-1 and ROCK-2) and its substantial role in the contractile activity of the sheep ureter. Br. J. Pharmacol. 2004;143:431–437. doi: 10.1038/sj.bjp.0705961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pispa J, Mikkola ML, Mustonen T, Thesleff I. Ectodysplasin, Edar and TNFRSF19 are expressed in complementary and overlapping patterns during mouse embryogenesis. Gene Expr. Patterns. 2003;3:675–679. doi: 10.1016/S1567-133X(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 66.Li R, Tang X, Miao S, Zong S, Wang L. Regulation of the G2/M phase of the cell cycle by sperm associated antigen 8 (SPAG8) protein. Cell Biochem. Funct. Cell. Biochem. Modul. Act. Agents Dis. 2009;27:264–268. doi: 10.1002/cbf.1574. [DOI] [PubMed] [Google Scholar]
- 67.Zheng YH, et al. Expression pattern of meiosis associated SYCP family members during germline development in chickens. Reproduction. 2009;138:483. doi: 10.1530/REP-09-0163. [DOI] [PubMed] [Google Scholar]
- 68.Arangasamy A, Kasimanickam VR, DeJarnette JM, Kasimanickam RK. Association of CRISP2, CCT8, PEBP1 mRNA abundance in sperm and sire conception rate in Holstein bulls. Theriogenology. 2011;76:570–577. doi: 10.1016/j.theriogenology.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z, et al. Genome-wide detection of CNVs and association with body weight in sheep based on 600K SNP arrays. Front. Genet. 2020;11:558. doi: 10.3389/fgene.2020.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao X-K, et al. Integrating CNVs into meta-QTL identified GBP4 as positional candidate for adult cattle stature. Funct. Integr. Genomics. 2018;18:559–567. doi: 10.1007/s10142-018-0613-0. [DOI] [PubMed] [Google Scholar]
- 71.Hu L, et al. Genome-wide analysis of CNVs in three populations of Tibetan sheep using whole-genome resequencing. Mapp. Intim. 2021 doi: 10.21203/rs.3.rs-1145952/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raza MA, Younas M, Schlecht E. Prevalence of gastrointestinal helminths in pastoral sheep and goat flocks in the Cholistan Desert of Pakistan. J. Anim. Plant Sci. 2014;24:127–134. [Google Scholar]
- 73.Li C, et al. RNA-Seq reveals 10 novel promising candidate genes affecting milk protein concentration in the Chinese Holstein population. Sci. Rep. 2016;6:1–11. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, et al. Genome-wide association studies for growth and meat production traits in sheep. PLoS One. 2013;8:e66569. doi: 10.1371/journal.pone.0066569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu L, Liu H, Ning L, Li F. Rabbit SLC15A1, SLC7A1 and SLC1A1 genes are affected by site of digestion, stage of development and dietary protein content. Animal. 2019;13:326–332. doi: 10.1017/S1751731118001404. [DOI] [PubMed] [Google Scholar]
- 76.Osawa Y, et al. EXOC1 plays an integral role in spermatogonia pseudopod elongation and spermatocyte stable syncytium formation in mice. Elife. 2021;10:e59759. doi: 10.7554/eLife.59759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rui LIU, et al. Fine mapping QTLs affecting milk production traits on BTA6 in Chinese Holstein with SNP markers. J. Integr. Agric. 2013;12:110–117. doi: 10.1016/S2095-3119(13)60211-7. [DOI] [Google Scholar]
- 78.Ramayo-Caldas Y, et al. From SNP co-association to RNA co-expression: Novel insights into gene networks for intramuscular fatty acid composition in porcine. BMC Genomics. 2014;15:1–15. doi: 10.1186/1471-2164-15-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serrano M, et al. Genome-wide association studies for sperm traits in Assaf sheep breed. Animal. 2021;15:100065. doi: 10.1016/j.animal.2020.100065. [DOI] [PubMed] [Google Scholar]
- 80.Marroquí L, et al. BACH2, a candidate risk gene for type 1 diabetes, regulates apoptosis in pancreatic β-cells via JNK1 modulation and crosstalk with the candidate gene PTPN2. Diabetes. 2014;63:2516–2527. doi: 10.2337/db13-1443. [DOI] [PubMed] [Google Scholar]
- 81.Li Y, Liu R, Wu J, Li X. Self-eating: Friend or foe? The emerging role of autophagy in fibrotic diseases. Theranostics. 2020;10:7993. doi: 10.7150/thno.47826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavani L, et al. Genomic study of Babesia bovis infection level and its association with tick count in Hereford and Braford cattle. Front. Immunol. 2020;11:1905. doi: 10.3389/fimmu.2020.01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garza-Brenner E, et al. Influence of genetic markers on the feeding behavior of yearling bulls. Revista Colombiana de Ciencias Pecuarias. 2019;32:14–20. doi: 10.17533/udea.rccp.v32n1a02. [DOI] [Google Scholar]
- 84.Huang C, et al. Copy number variation of the HPGDS gene in the Ashidan yak and its associations with growth traits. Gene. 2021;772:145382. doi: 10.1016/j.gene.2020.145382. [DOI] [PubMed] [Google Scholar]
- 85.Hsu H-P, Wang C-Y, Hsieh P-Y, Fang J-H, Chen Y-L. Knockdown of serine/threonine-protein kinase 24 promotes tumorigenesis and myeloid-derived suppressor cell expansion in an orthotopic immunocompetent gastric cancer animal model. J. Cancer. 2020;11:213. doi: 10.7150/jca.35821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neumann A, et al. Computational investigations on the binding mode of ligands for the cannabinoid-activated G protein-coupled receptor GPR18. Biomolecules. 2020;10:686. doi: 10.3390/biom10050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gross N, Peñagaricano F, Khatib H. Integration of whole-genome DNA methylation data with RNA sequencing data to identify markers for bull fertility. Anim. Genet. 2020;51:502–510. doi: 10.1111/age.12941. [DOI] [PubMed] [Google Scholar]
- 88.Abied A, et al. Genomic diversity, population structure, and signature of selection in five Chinese native sheep breeds adapted to extreme environments. Genes. 2020;11:494. doi: 10.3390/genes11050494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiménez-Martínez M, Stamatakis K, Fresno M. The dual-specificity phosphatase 10 (DUSP10): Its role in cancer, inflammation, and immunity. Int. J. Mol. Sci. 2019;20:1626. doi: 10.3390/ijms20071626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liebl J, et al. Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2. Nat. Commun. 2015;6:1–13. doi: 10.1038/ncomms8274. [DOI] [PubMed] [Google Scholar]
- 91.Li H, Li M, Tang C, Xu L. Screening and prognostic value of potential biomarkers for ovarian cancer. Ann. Transl. Med. 2021;9:1007. doi: 10.21037/atm-21-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karimi K, Farid AH, Myles S, Miar Y. Detection of selection signatures for response to Aleutian mink disease virus infection in American mink. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-020-79139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Napierala JS, et al. Comprehensive analysis of gene expression patterns in Friedreich’s ataxia fibroblasts by RNA sequencing reveals altered levels of protein synthesis factors and solute carriers. Dis. Model. Mech. 2017;10:1353–1369. doi: 10.1242/dmm.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohammadi H, Rafat SA, Moradi Shahrbabak H, Shodja J, Moradi MH. Genome-wide association study and gene ontology for growth and wool characteristics in Zandi sheep. J. Livest. Sci. Technol. 2020;8:45–55. [Google Scholar]
- 95.Amaral AJ, Pavão AL, Gama LT. Genomic tools for the conservation and genetic improvement of a highly fragmented breed—The Ramo Grande cattle from the Azores. Animals. 2020;10:1089. doi: 10.3390/ani10061089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bloch M, et al. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. 2007;26:2525–2534. doi: 10.1038/sj.onc.1210036. [DOI] [PubMed] [Google Scholar]
- 97.Aliloo H, Mrode R, Okeyo AM, Gibson JP. Ancestral haplotype mapping for GWAS and detection of signatures of selection in admixed dairy cattle of Kenya. Front. Genet. 2020;11:544. doi: 10.3389/fgene.2020.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mehla K, et al. Genome-wide analysis of the heat stress response in Zebu (Sahiwal) cattle. Gene. 2014;533:500–507. doi: 10.1016/j.gene.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 99.Upadhyay M, et al. Distribution and functionality of copy number variation across European cattle populations. Front. Genet. 2017;8:108. doi: 10.3389/fgene.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gorla E, et al. Genomic variability in Mexican chicken population using copy number variants. BMC Genet. 2017;18:1–11. doi: 10.1186/s12863-017-0524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Igoshin AV, et al. Copy number variants in genomes of local sheep breeds from Russia. Anim. Genet. 2021;53:119–132. doi: 10.1111/age.13163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All CNVRs identified in Afghan sheep breeds have been deposited in figshare repository (https://www.figshare.com) with accession no. 10.6084/m9.figshare.16577954.v1. The genomic information used in this study belongs to private sheep breeding programs, so we do not have authorization to share it and are available from the corresponding authors upon reasonable request.