Abstract
Background
This study aimed to explore the relationship between the Sirtuin 3 (SIRT3) gene and endothelial cell dysfunction, contributing to the progression of coronary atherosclerosis driven by hyperglycemia.
Methods
We measured serum SIRT3 levels using enzyme‐linked immunosorbent assay in 95 patients with type 2 diabetes mellitus (T2DM) who underwent diagnostic coronary angiography. The patients were divided into two groups according to the presence (n = 45) or absence (n = 50) of coronary artery disease (CAD). Human aortic endothelial cells (HAECs) grown in vitro in a medium with various concentrations of glucose (5.5, 11, 16.5, 22, 27.5, 33, and 38.5 mM) for 24 h were assessed for protein expression of SIRT3, peroxisome proliferator‐activated receptor alpha (PPAR‐α), endothelial nitric oxide (NO) synthase (eNOS), and inducible NO synthase (iNOS) using Western blot analysis. HAECs were subjected to SIRT3 overexpression or inhibition through SIRT3 adenovirus and siRNA transfection.
Results
Serum SIRT3 levels were significantly lower in T2DM patients with CAD than in those without CAD (p = 0.048). The in vitro results showed that HG significantly increased SIRT3, PPAR‐α, and eNOS protein expression in a concentration‐dependent manner. Moreover, iNOS expression was decreased in HAECs in response to HG. Reduced PPAR‐α and eNOS levels and increased iNOS levels were observed in SIRT3 silenced HAECs cells. In contrast, SIRT3 overexpression significantly improved PPAR‐α and eNOS expression and suppressed iNOS expression.
Conclusion
SIRT3 was associated with the progression of atherosclerosis in T2DM patients through upregulation of PPAR‐α and eNOS and downregulation of iNOS, which are involved in endothelial dysfunction under hyperglycemic conditions.
Keywords: atherosclerosis, coronary artery disease, diabetes, endothelial dysfunction, Sirtuin 3
In our manuscript, we explored the clinical role and potential mechanism of the SIRT3 contribute to the progression of coronary atherosclerosis in high glucose, and revealed SIRT3 may exert crucial effect on the upregulation of PPAR‐α, eNOS and downregulation of iNOS, which were associated with endothelial dysfunction under hyperglycemic conditions.
1. INTRODUCTION
Diabetes mellitus (DM), clinically characterized by hyperglycemia, is a metabolic disease and a major risk factor for cardiovascular morbidity and mortality. 1 Patients with type 2 DM (T2DM) are at higher risk for the development and progression of cardiovascular complications, such as atherosclerosis and myocardial infarction (MI), than those without diabetes. 2 , 3 Endothelial dysfunction occurring in the early stage of diabetes is recognized as a risk factor for the initiation and progression of atherosclerotic lesions, which are aggravated by hyperglycemia. 4 , 5 Molecular events linking hyperglycemia with endothelial dysfunction have been reported to be involved in reduced nitric oxide (NO) bioavailability, 6 , 7 increased generation of reactive oxygen species (ROS), 8 and increased inflammatory activation. 9
Sirtuin 3 (SIRT3), the mitochondrial nicotinamide adenine dinucleotide (+)‐dependent deacetylase, has emerged as an important contributor for improving obesity and diabetes by removing harmful ROS. 10 The deficiency of SIRT3 has been reported to result in a high risk of developing diabetes. 11 , 12 , 13 SIRT3 has also contributed to cardiovascular pathologies, such as endothelial dysfunction, atherosclerosis, oxidative stress, and inflammation. 13 , 14 However, different findings have been reported regarding the potential function of SIRT3 in atherosclerosis. A previous study reported that SIRT3 deficiency affected neither plaque burden nor fibrous cap thickness and necrotic core diameter in low‐density lipoprotein receptor knockout mice. 15 Another study indicated that loss of SIRT3 does not impact endothelial function in advanced atherosclerosis but may accelerate the progression of vascular calcification. 16 Reduced SIRT3 levels resulting from deoxyribonucleic acid sequence variants may contribute to the development of MI. 17 However, Emrullah et al. 18 reported that circulating SIRT3 levels were similar between patients with acute MI (AMI) and those with normal coronary findings, suggesting that no cardioprotective effect was found for SIRT3 in patients with AMI. Therefore, the role of SIRT3 in the progression of coronary heart disease associated with diabetes is not well established.
Herein, we used the serum of T2DM patients with coronary artery disease (CAD) and human aortic endothelial cells (HAECs) to explore the effect of human SIRT3 on endothelial cell dysfunction associated with atherosclerosis in diabetes.
2. MATERIALS AND METHODS
2.1. Study population
A total of 95 consecutive patients with T2DM who underwent diagnostic coronary angiography were recruited from the Second Hospital of Shandong University and divided into two groups: patients with CAD (15 females, n = 45) and patients without CAD (control patients, 22 females, n = 50). The diagnostic evidence of CAD was based on coronary angiography confirmed stenosis of >50% in one or more major coronary arteries, including the left main coronary artery, left anterior descending branch, left circumflex artery, right coronary artery, and larger diagonal branch. Individuals were excluded if they had a history of smoking, type 1 DM, gestational diabetes, acute ST‐elevation MI, acute heart failure, cerebrovascular and peripheral vascular disease, mental abnormalities, and chronic hepatic, renal, or any other serious disease. Informed consent was obtained from all participants, and the procedures were approved by the Ethics Committee of the Second Hospital of Shandong University and were in accordance with the Declaration of Helsinki.
2.2. Clinical measurements
The baseline demographics and clinical characteristics of all the patients were determined. The details of age, sex, body mass index (BMI), and systolic and diastolic blood pressure were recorded. Fasting blood samples were collected at baseline for glucose, glycosylated hemoglobin (HbA1c), total cholesterol, triglycerides, high‐density lipoprotein (HDL) cholesterol, and low‐density lipoprotein (LDL) cholesterol.
2.3. Enzyme‐linked immunosorbent assay (ELISA) analysis
Blood samples were obtained from all participants at admission, centrifuged at 4000 rpm for 10 min, and serum was separated and stored at −80°C. Serum SIRT3 levels were determined in all patients using a commercial human ELISA kit (Enzyme‐linked Biotechnology Co., Ltd., Shanghai, PRC) with a detection range of 62.5–2000 pg/ml. The typical sensitivity of the SIRT3 ELISA kit is <10 pg/ml. The optical density was measured at a wavelength of 450 nm.
2.4. Cell culture
Human aortic endothelial cells were grown in endothelial cell growth medium 2 containing 2% fetal calf serum, 10 ng/ml growth factors, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C, 5% CO2 in atmosphere, and 95% humidity.
2.5. ELISA analysis for SIRT3 in HAECs
SIRT3 levels in the culture supernatants of HAECs treated with different concentrations of glucose (5.5, 11, 16.5, 22, 27.5, 33, and 38.5 mM) for 24 h were determined using ELISA kits (Shanghai Enzyme‐linked Biotechnology Co., Ltd.), according to the manufacturer's instructions.
2.6. Western blot analysis
Confluent cells were incubated with various concentrations of glucose (5.5, 11, 16.5, 22, 27.5, 33 and 38.5 mM) for 24 h. Moreover, HAECs were maintained in 5.5 mM normal glucose as a control group. HAECs were washed twice with phosphate‐buffered saline, incubated on ice with 200 μl radioimmunoprecipitation assay buffer (Beijing Solarbio Science & Technology Co., Ltd.) and 2 μl phenylmethylsulfonyl fluoride for 10 min, and centrifuged at 12,000 × g for 15 min at 4°C. Protein concentrations were determined using a standard bicinchoninic acid assay (Beijing ComWin Biotech Co., Ltd.). Total cell lysates were electrophoresed on 10% sodium dodecyl sulfate‐poly acrylamide gels, followed by electroblotting onto polyvinylidene difluoride membranes. The membrane was blocked for 1 h in 5% nonfat dry milk in 1× Tris‐buffered saline with Tween™ 20 detergent (TBST) buffer and then incubated overnight with monoclonal antibodies. SIRT3, proliferator‐activated receptor alpha (PPAR‐α), endothelial NO synthase (eNOS), and inducible NO synthase (iNOS) protein expression was detected using rabbit anti‐human SIRT3 (1:1000, Abcam, England), rabbit anti‐human PPAR‐α (1:200, Wuhan Boster Biological Technology, Ltd.), rabbit anti‐human eNOS (1:1000, Sigma company, USA), and rabbit anti‐human iNOS (1:1000, Sigma company, USA) antibodies, as described previously. The membrane was washed thrice for 10 min each with 1× TBST buffer and then incubated in 5% milk with horseradish peroxidase‐conjugated goat anti‐rabbit immunoglobulin G for 1.5 h. The membrane was washed thrice for 12 min each with 1× TBST buffer and developed using a highly sensitive chemiluminescence reagent (Beijing ComWin Biotech Co., Ltd.). Anti‐β‐actin rabbit polyclonal antibody (Beijing ComWin Biotech Co., Ltd.) was used as the loading control. Images were captured with a camera (Alpha Innotech, USA), and densitometric measurements were performed using Image J software (Kodak, USA).
2.7. SIRT3 adenovirus transfection
Human aortic endothelial cells were plated onto six‐well culture plates and transfected with adenovirus overexpressing green fluorescent protein or SIRT3 (ViGene BioSciences). After 48 h of transfection, cells were harvested for Western blot analysis.
2.8. Short interfering ribonucleic acid (siRNA) transfection
Human aortic endothelial cells were plated onto six‐well culture plates and transfected with siRNA for SIRT3 knockdown using Lipofectamine iMAX (Life Technologies) according to the manufacturer's protocol. After 48 h of transfection, cells were harvested for Western blot analysis. The target sequences of siRNA were sense: GCUCAUGGGUCCUUUGUAUTT and antisense: AUACAAAGGACCCAUGAG CTT for SIRT3.
2.9. Statistical analysis
All data are expressed as the mean ± standard error of the mean. After testing for normal distribution of variables, data were analyzed using Student's t‐test and one‐way analysis of variance (ANOVA) followed by the least significant difference post hoc test, as appropriate. In all tests, p < 0.05 was considered statistically significant. Data analysis was performed using SPSS version 23.0 and GraphPad Prism software version 8.0.2.
3. RESULTS
3.1. SIRT3 was decreased in T2DM patients with CAD
The baseline and clinical characteristics of the T2DM subgroups are shown in Table 1. No differences were found in age, sex, BMI, blood pressure, blood lipids, glucose, creatinine, or smoking between the two subgroups. The distribution of serum SIRT3 levels was approximately a normal distribution. SIRT3 levels were significantly decreased in T2DM patients with CAD compared with those patients without CAD (284.21 ± 54.36 pg/ml vs. 315.28 ± 90.16 pg/ml, p = 0.048) (Figure 1).
TABLE 1.
Baseline and clinical characteristics of the T2DM subgroups
| Variables | Control patients | CAD patients | p value |
|---|---|---|---|
| Number | 50 | 45 | – |
| Sex (male/female) | 28/22 | 30/15 | 0.833 |
| Age (years) | 55.3 ± 14.4 | 60.0 ± 9.4 | 0.063 |
| BMI (kg/m2) | 26.11 ± 4.42 | 27.39 ± 3.60 | 0.128 |
| Systolic BP (mmHg) | 145.56 ± 20.53 | 140.36 ± 16.90 | 0.183 |
| Diastolic BP (mmHg) | 87.66 ± 10.33 | 84.44 ± 10.20 | 0.131 |
| Total cholesterol (mmol/L) | 4.58 ± 1.24 | 4.29 ± 1.21 | 0.251 |
| Triglyceride (mmol/L) | 1.60 ± 1.12 | 1.88 ± 2.08 | 0.431 |
| HDL cholesterol (mmol/L) | 1.08 ± 0.29 | 1.09 ± 0.25 | 0.985 |
| LDL cholesterol (mmol/L) | 2.67 ± 0.99 | 2.50 ± 0.97 | 0.399 |
| Glucose (mmol/L) | 8.91 ± 2.84 | 8.01 ± 2.63 | 0.115 |
| HbA1c (%) | 8.98 ± 1.84 | 8.40 ± 1.61 | 0.111 |
| Creatinine (μmol/L) | 72.39 ± 29.94 | 71.32 ± 20.63 | 0.842 |
| Smoking (n, %) | 20 (40%) | 25 (55.6%) | 0.153 |
Note: Values are mean ± SD.
FIGURE 1.
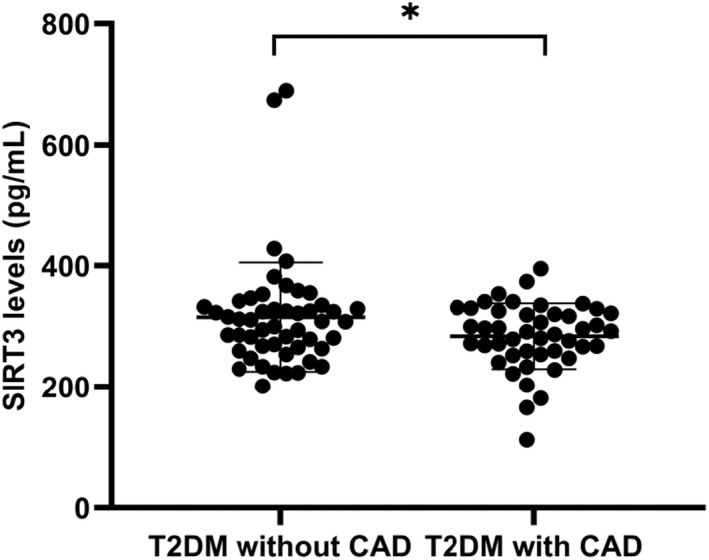
Serum levels of SIRT3 by ELISA in T2DM subgroups. The T2DM patients with CAD displayed significant decrease in SIRT3 levels when compared to those without CAD. *p = 0.048
3.2. The expression of SIRT3 is concentration‐dependent
In vitro experiments, we observed the expression of full‐length SIRT3, PPAR‐α, eNOS, and iNOS in HAECs incubated in various concentrations of glucose. No significant difference was observed in β‐actin protein expression in HAECs among groups treated with various concentrations of glucose. Although the presence of SIRT3 expression in endothelial function and human and animal atherosclerotic tissue is still debated, 15 , 16 , 17 , 18 we wanted to focus on the expression of SIRT3 in HAECs treated under hyperglycemic conditions. HAECs were treated with 5.5, 11, 16.5, 22, 27.5, 33, 38.5 mM glucose for 24 h. As presented in Figure 2, ELISA revealed that high glucose (HG) treatment significantly increased the protein expression of SIRT3 in the culture supernatants. We further measured protein levels of SIRT3 using Western blot analysis. Consistent with the ELISA results, distinct SIRT3 protein expression was observed in HAECs in a concentration‐dependent manner (all p < 0.05). The levels of SIRT3 reached a maximum at 27.5 mM glucose and decreased at 33 and 38.5 mM (Figure 3A,B).
FIGURE 2.
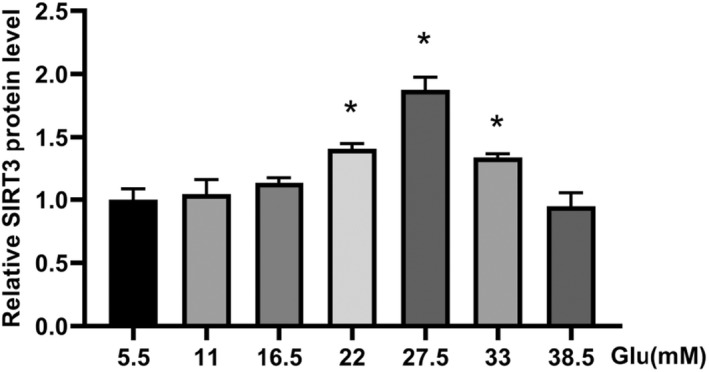
ELISA analysis of SIRT3 expression in the supernatant of HAECs exposed to different concentrations of glucose. *p < 0.05 vs. 5.5 mM Glucose
FIGURE 3.
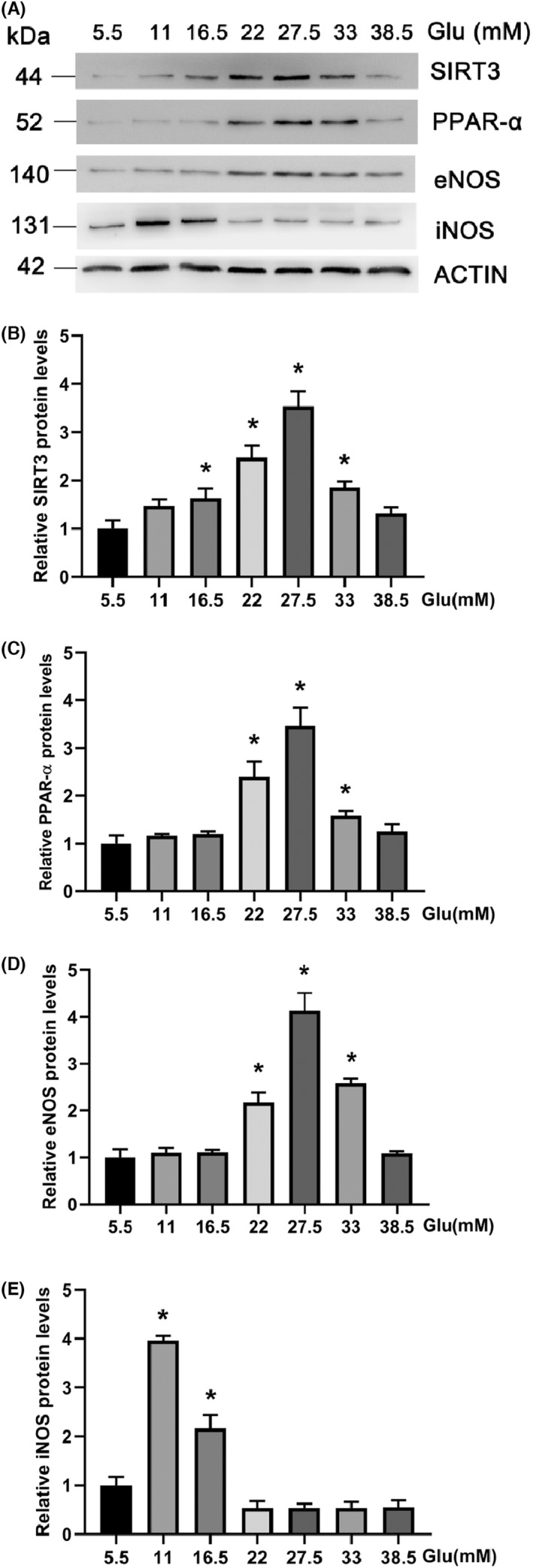
(A) Western blot analysis of SIRT3, PPAR‐α, eNOS and iNOS expression in HAECs with indicated concentrations of glucose. (B) Quantification of the SIRT3 level with Actin as a reference. (C) Quantification of the PPAR‐α level with Actin as a reference. (D) Quantification of the eNOS level with Actin as a reference. (E) Quantification of the iNOS level with Actin as a reference. *p < 0.05 vs. 5.5 mM Glucose
3.3. The expression of PPAR‐α is concentration‐dependent
PPAR‐α is known to play a role in atherosclerosis derived from inflammation as well as insulin resistance. 19 To identify whether PPAR‐α regulates endothelial cells, cells were treated with various concentrations of glucose to test PPAR‐α expression (Figure 3A,C). At the protein level, hyperglycemia caused a significant upregulation of PPAR‐α (all p < 0.05). The most dramatic response in PPAR‐α expression was observed at 27.5 mM glucose. These data show that the expression of PPAR‐α is consistent with SIRT3, attributable to hyperglycemia treatment in HAECs.
3.4. The expression of eNOS and iNOS is concentration‐dependent
Nitric oxide has a pivotal role in mediating vascular endothelial function. To understand how endothelial dysfunction is related to the antioxidant effects of SIRT3, eNOS and iNOS levels were also investigated (Figure 3A,D,E). Compared with the control, eNOS was upregulated in HAECs exposed to higher glucose concentrations (all p < 0.05). eNOS expression was prominent at the protein levels at 27.5 mM glucose. Meanwhile, downregulation of iNOS expression was demonstrated in a concentration‐ dependent manner in response to hyperglycemia, with the highest expression observed at 11 mM glucose, then decreased, with the lowest at 27.5 mM glucose compared with the control (all p < 0.05).
3.5. SIRT3 positively regulates the expression of PPAR‐α
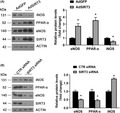
SIRT3 siRNA and adenovirus transfection were conducted to further investigate the effects of SIRT3 on hyperglycemia‐ or diabetes‐induced atherosclerosis. As shown in Figure 4A, SIRT3 overexpression aggravated PPAR‐α upregulation (p < 0.05). Consistently, siRNA‐mediated SIRT3 knockdown significantly suppressed PPAR‐α expression in HAECs (p < 0.05) (Figure 4B). Taken together, these results imply that SIRT3 is a positive regulator of PPAR‐α expression.
FIGURE 4.
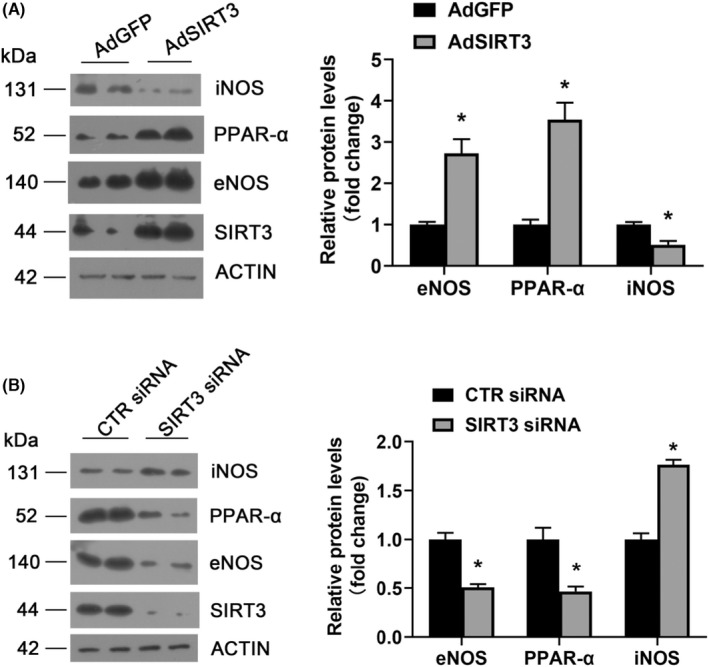
SIRT3 positively regulated PPAR‐α, eNOS, and negatively regulated iNOS. (A) Western blot analysis of SIRT3, PPAR‐α, eNOS, iNOS protein expression in cells infected with AdGFP or AdSIRT3. *p < 0.05 vs. AdGFP. (B) Western blot analysis of SIRT3, PPAR‐α, eNOS, iNOS protein expression in cells transfected with control or SIRT3 siRNA. *p < 0.05 versus CTR siRNA. AdGFP adenovirus GFP; AdSIRT3 adenovirus SIRT3; CTR siRNA control siRNA
3.6. SIRT3 regulates the expressions of eNOS and iNOS
Under similar conditions, as demonstrated in Figure 4A, SIRT3 overexpression significantly improved eNOS expression and suppressed iNOS expression (all p < 0.05). In contrast, reduced eNOS levels and increased iNOS levels were observed in SIRT3 silenced HAECs cells (all p < 0.05) (Figure 4B). These results indicate that SIRT3 positively regulates eNOS expression and negatively regulates iNOS expression.
4. DISCUSSION
Our results suggest that serum SIRT3 levels are significantly lower in diabetic patients with CAD than in those with normal coronary arteries. An in vitro study using a high‐glucose‐treated HAEC model demonstrated that SIRT3 could positively regulate the expression of PPAR‐α and eNOS while negatively regulating iNOS expression. Our study may help explain the potential mechanisms contributing to the progression of diabetes‐associated CAD.
In diabetes, hyperglycemia is identified as a major cause for pathological conditions such as vascular complications, endothelial dysfunction, and atherosclerosis. 20 Although the effect of hyperglycemia on the atherosclerotic process has been widely recognized, previous data have reported that some diabetic patients never develop long‐term hyperglycemia‐related vascular complications due to the autoregulatory mechanism. 21 , 22 , 23 The molecular mechanisms involving hyperglycemia in the pathogenesis of endothelial cell injury and atherosclerosis are not well established. Several studies have revealed that mitochondrial dysfunction and oxidative stress might be involved in the development of vascular complications in T2DM. 24 , 25 SIRT3 plays a crucial role in atherosclerosis by governing mitochondrial metabolism and ROS homeostasis. 26 Previous studies discovered that SIRT3 has an antioxidant effect in the pathogenesis of atherosclerosis. 27 , 28 However, human data on SIRT3 in cardiovascular disease are limited. In this study, we observed a marked decrease in circulating SIRT3 levels in T2DM patients with CAD. Our data support the notion that there are connections between SIRT3 and diabetes‐associated coronary atherosclerosis in humans, which is inconsistent with an earlier study by Emrullah et al. 18
Endothelial cell dysfunction induced by hyperglycemia is considered a hallmark of vascular complications in diabetes. 29 , 30 One recent report demonstrated that HG downregulated the expression of SIRT3 in human umbilical vein endothelial cells (HUVECs) stimulated by HG (30 mM) for 48 h. 31 In line with this, the expression levels of SIRT3 were decreased under HG conditions (20 mM) for 48 h in human monocytic cells (THP‐1). 32 Here, our results indicated that the pattern of SIRT3 expression was in a concentration‐dependent manner, with the highest level noted at 27.5 mM and decreased at 33 and 38.5 mM glucose. We speculate that the reduction in SIRT3 levels induced by HG (33 and 38.5 mM) might be associated with the upregulation of oxidative stress and endothelial injury in endothelial cells. 33 , 34 The analysis of SIRT3 expression in HAECs following HG stimulation was not completely consistent with previous findings. These discrepancies might be related to differences in multiple glucose conditions, exposure durations, or cell types.
PPAR‐α, eNOS, and iNOS may be involved in the effect of SIRT3 on endothelial dysfunction in this study. PPAR‐α, a ligand‐activated transcription factor, regulates glucose metabolism, fatty acid metabolism, lipid metabolism, and inflammation, especially in the pathogenesis of obesity, hypercholesterolemia, insulin resistance, and atherosclerosis. 19 , 35 , 36 In our study, we found that HG could stimulate PPAR‐α expression in HAECs. PPAR‐α levels almost paralleled the pattern of SIRT3 expression. Yang et al. 37 reported that elevated SIRT3 expression reduced oxidative stress in kidney tissues after green tea polyphenols treatment, which was mediated by PPAR‐α. In the present study, we also observed that the protein expression of PPAR‐α was decreased in SIRT3 silenced HAECs cells. On the contrary, SIRT3 overexpression upregulated PPAR‐α levels. These data indicated that SIRT3 plays a crucial role in positively regulating PPAR‐α expression. Our results identified potential regulatory links between SIRT3 and PPAR‐α in HG‐induced endothelial dysfunction.
The dysfunction of endothelial cells could be characterized by impaired carbohydrate metabolism, mitochondrial dysfunction, oxidative stress, and decreased NO production. 24 , 38 It is well established that NO plays an important role in maintaining vascular homeostasis and modulating oxidative stress. Endothelial NO synthase‐derived NO has been responsible for anti‐atherosclerotic effects in endothelial cells. 39 Excess NO derived by iNOS has been involved in cytostatic and cytotoxic effects. 40 SIRT3 siRNA could worsen the angiotensin II‐induced eNOS decrease and downregulate eNOS protein expression in HUVECs. 41 In this study, we discovered that SIRT3 could positively regulate the expression of eNOS and negatively regulate iNOS expression, implicating the key role of SIRT3 in improving endothelial cell dysfunction.
5. CONCLUSIONS
In conclusion, our study revealed that T2DM patients with CAD exhibited lower serum SIRT3 levels than those with normal coronary arteries. The expression levels of SIRT3, PPAR‐α, eNOS increased and iNOS decreased in a concentration‐ dependent manner in HG‐treated HAECs. Furthermore, SIRT3 positively regulated PPAR‐α and eNOS and negatively regulated iNOS expression. Our findings shed some light on how SIRT3 affects endothelial dysfunction involved in the pathogenesis of atherosclerosis under HG conditions.
AUTHOR CONTRIBUTIONS
XN Lyu, YM Du, and HP Gong were responsible for the study design. HP Gong analyzed the data and drafted the manuscript. XN Lyu and YM Du reviewed the manuscript. J Liu, ZW Xue, and WW Wang performed the medical records and blood sample collection. J Liu, ZW Xue, CC Li, and FF Xu performed the experiments.
CONFLICT OF INTEREST
All authors have read and approved the final version of the manuscript. The authors declare that they have no conflicts of interest.
Gong H, Liu J, Xue Z, et al. SIRT3 attenuates coronary atherosclerosis in diabetic patients by regulating endothelial cell function. J Clin Lab Anal. 2022;36:e24586. doi: 10.1002/jcla.24586
Funding information
This work was supported by the Key Science and Technology Program of Shandong Province, Grant Number: 2017G006029.
Contributor Information
Yimeng Du, Email: yimengd@163.com.
Xiaona Lyu, Email: 502661480@qq.com.
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article. The data are available from the corresponding authors on reasonable request.
REFERENCES
- 1. Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134‐1146. [DOI] [PubMed] [Google Scholar]
- 2. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 1993;16(2):434‐444. [DOI] [PubMed] [Google Scholar]
- 3. Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016;118(11):1771‐1785. [DOI] [PubMed] [Google Scholar]
- 4. Guangda X, Yuhua W. Apolipoprotein e4 allele and endothelium‐dependent arterial dilation in type 2 diabetes mellitus without angiopathy. Diabetologia. 2003;46(4):514‐519. [DOI] [PubMed] [Google Scholar]
- 5. Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335(1):165‐189. [DOI] [PubMed] [Google Scholar]
- 6. Noyman I, Marikovsky M, Sasson S, et al. Hyperglycemia reduces nitric oxide synthase and glycogen synthase activity in endothelial cells. Nitric Oxide. 2002;7(3):187‐193. [DOI] [PubMed] [Google Scholar]
- 7. Kemeny SF, Figueroa DS, Clyne AM. Hypo‐ and hyperglycemia impair endothelial cell Actin alignment and nitric oxide synthase activation in response to shear stress. PLoS One. 2013;8(6):e66176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1‐19. [DOI] [PubMed] [Google Scholar]
- 9. Knapp M, Tu X, Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin. 2019;40(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Xiang H, Liu J, Chen Y, He RR, Liu B. Mitochondrial Sirtuin 3: new emerging biological function and therapeutic target. Theranostics. 2020;10(18):8315‐8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou Y, Chung ACK, Fan R, et al. Sirt3 deficiency increased the vulnerability of pancreatic beta cells to oxidative stress‐induced dysfunction. Antioxid Redox Signal. 2017;27(13):962‐976. [DOI] [PubMed] [Google Scholar]
- 12. Xu H, Hertzel AV, Steen KA, Bernlohr DA. Loss of fatty acid binding protein 4/aP2 reduces macrophage inflammation through activation of SIRT3. Mol Endocrinol. 2016;30(3):325‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun W, Liu C, Chen Q, Liu N, Yan Y, Liu B. SIRT3: a new regulator of cardiovascular diseases. Oxid Med Cell Longev. 2018;2018:7293861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Zhang X, Wang P, et al. Sirt3 overexpression alleviates hyperglycemia‐induced vascular inflammation through regulating redox balance, cell survival, and AMPK‐mediated mitochondrial homeostasis. J Recept Signal Transduct Res. 2019;39(4):341‐349. [DOI] [PubMed] [Google Scholar]
- 15. Winnik S, Gaul DS, Preitner F, et al. Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development. Basic Res Cardiol. 2014;109(1):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roos CM, Hagler MA, Zhang B, Miller JD. Effects of SIRT3 deficiency on vasomotor function and atherosclerotic plaque composition in mice. FASEB J. 2016;30(S1):722.7‐727.7. [Google Scholar]
- 17. Yin X, Pang S, Huang J, Cui Y, Yan B. Genetic and functional sequence variants of the SIRT3 gene promoter in myocardial infarction. PLoS One. 2016;11(4):e0153815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kızıltunç E, Kösem A, Özkan C, et al. Serum Sirtuin 1, 3 and 6 levels in acute myocardial infarction patients. Arq Bras Cardiol. 2019;113(1):33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown JD, Plutzky J. Peroxisome proliferator‐activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115(4):518‐533. [DOI] [PubMed] [Google Scholar]
- 20. Sena CM, Pereira AM, Seiça R. Endothelial dysfunction ‐ a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832(12):2216‐2231. [DOI] [PubMed] [Google Scholar]
- 21. Riahi Y, Sin‐Malia Y, Cohen G, et al. The natural protective mechanism against hyperglycemia in vascular endothelial cells: roles of the lipid peroxidation product 4‐hydroxydodecadienal and peroxisome proliferator‐activated receptor delta. Diabetes. 2010;59(4):808‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National cholesterol education program adult treatment panel III guidelines. J Am Coll Cardiol. 2004;44(3):720‐732. [DOI] [PubMed] [Google Scholar]
- 23. National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) . Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143‐3421. [PubMed] [Google Scholar]
- 24. Yu Y, Lyons TJ. A lethal tetrad in diabetes: hyperglycemia, dyslipidemia, oxidative stress, and endothelial dysfunction. Am J Med Sci. 2005;330(5):227‐232. [DOI] [PubMed] [Google Scholar]
- 25. Wu N, Shen H, Liu H, Wang Y, Bai Y, Han P. Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro‐inflammatory cytokine expression in vivo. Cardiovasc Diabetol. 2016;15(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jing SH, Yu B, Qiao H. Correlation between endothelial cell apoptosis and SIRT3 gene expression in atherosclerosis rats. Eur Rev Med Pharmacol Sci. 2019;23(20):9033‐9040. [DOI] [PubMed] [Google Scholar]
- 27. Karnewar S, Vasamsetti SB, Gopoju R, et al. Mitochondria‐targeted esculetin alleviates mitochondrial dysfunction by AMPK‐mediated nitric oxide and SIRT3 regulation in endothelial cells: potential implications in atherosclerosis. Sci Rep. 2016;6:24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tao R, Coleman MC, Pennington JD, et al. Sirt3‐mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8(1):e54514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813‐820. [DOI] [PubMed] [Google Scholar]
- 31. Chen T, Ma C, Fan G, et al. SIRT3 protects endothelial cells from high glucose‐induced senescence and dysfunction via the p53 pathway. Life Sci. 2021;264:118724. [DOI] [PubMed] [Google Scholar]
- 32. Kim A, Lee W, Yun JM. Luteolin and fisetin suppress oxidative stress by modulating sirtuins and forkhead box O3a expression under in vitro diabetic conditions. Nutr Res Pract. 2017;11(5):430‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu J, Chen S, Biswas S, et al. Glucose‐induced oxidative stress and accelerated aging in endothelial cells are mediated by the depletion of mitochondrial SIRTs. Physiol Rep. 2020;8(3):e14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han WM, Chen XC, Li GR, Wang Y. Acacetin protects against high glucose‐induced endothelial cells injury by preserving mitochondrial function via activating Sirt1/Sirt3/AMPK signals. Front Pharmacol. 2020;11:607796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109(1):121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Werner CM, Schirmer SH, Gensch C, et al. The dual PPARα/γ agonist aleglitazar increases the number and function of endothelial progenitor cells: implications for vascular function and atherogenesis. Br J Pharmacol. 2014;171(10):2685‐2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang H, Zuo XZ, Tian C, et al. Green tea polyphenols attenuate high‐fat diet‐induced renal oxidative stress through SIRT3‐dependent deacetylation. Biomed Environ Sci. 2015;28(6):455‐459. [DOI] [PubMed] [Google Scholar]
- 38. Dhingra R, Vasan RS. Diabetes and the risk of heart failure. Heart Fail Clin. 2012;8(1):125‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10(1):4‐18. [DOI] [PubMed] [Google Scholar]
- 40. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731‐1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu H, Chen T, Li N, Wang S, Bu P. Role of SIRT3 in angiotensin II‐induced human umbilical vein endothelial cells dysfunction. BMC Cardiovasc Disord. 2015;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article. The data are available from the corresponding authors on reasonable request.


