Highlights
-
•
CCA has a dismal prognosis, and it is usually diagnosed in advanced stage for which available treatments have limited efficacy.
-
•
CCA TME presents an abundant desmoplastic stroma and exhibits a high heterogeneity.
-
•
TME plays a central role in cancer development and in the resistance to treatments.
-
•
Treatments targeting the TME in association with cytotoxic agents could represent a promising therapeutic strategy.
Keywords: Cholangiocarcinoma, Tumor microenvironment, Cancer associated fibroblasts, Tumor infiltrating lymphocytes, Immunotherapy, Tumor associated macrophages
Abstract
Systemic treatments (e.g., chemotherapy and targeted therapies) have limited efficacy for patients with locally advanced – unresectable – and metastatic cholangiocarcinoma (CCA), with an overall survival of less than a year. Tumor microenvironment (TME) represents the ecosystem surrounding the tumor which comprises immune cells, fibroblasts, endothelial cells, and a wide range of soluble factors. CCA TME is characterized by an abundant desmoplastic stroma, exhibits a high heterogeneity and it plays a central role in cancer onset and progression. There is growing evidence suggesting that it is possible to target TME in association with other treatment modalities, such as cytotoxic chemotherapy or targeted therapies, paving the way to possible combination strategies with a synergistic effect. Herein, we describe the components of CCA TME – such as cancer-associated fibroblasts and other cells of pivotal importance - with their most relevant interactions, focusing on the preclinical rationale for the development of effective anticancer treatments.
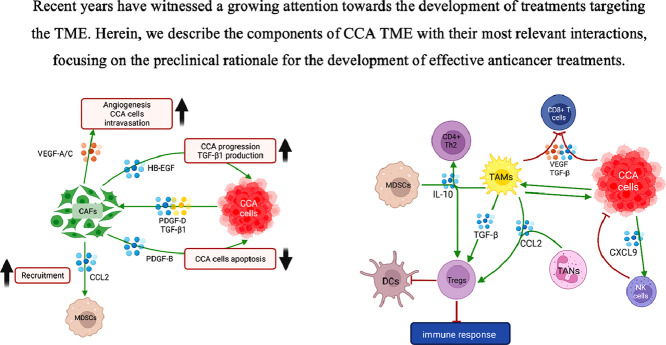
Graphical abstract
.
Introduction
Cholangiocarcinoma (CCA) includes a group of different tumors originating from the biliary tree and which are classically classified into intrahepatic (iCCA), perihilar (pCCA), and distal cholangiocarcinoma (dCCA), according to their anatomical location [1]. Surgery represents the only potentially curative treatment [2]; despite adjuvant chemotherapy, the recurrence rate after surgery remains high with more than 60% of resected patients presenting disease recurrence in the first five years after surgery [3]. Given the absence of specific symptoms, approximately 70% of patients are diagnosed with advanced disease, for which only palliative treatments are indicated. Chemotherapy with gemcitabine plus cisplatin and possibly the addition of durvalumab represents the first-line treatment for metastatic/advanced disease [4,5]. However, results provided by systemic treatments are still modest with overall survival (OS) of less than a year after gemcitabine plus cisplatin and only six months after second-line chemotherapy [6]. Recent years have witnessed the advent of molecular profiling in the advanced CCA, and new technologies have led to the identification of several molecular alterations in CCA. The discovery of molecular aberrations has led to the development of specific treatments, such as Fibroblast Growth Factor Receptor (FGFR) 2, Isocitrate Dehydrogenase 1 (IDH-1), and BRAF inhibitors. However, most of molecularly targeted therapies have shown limited efficacy and short-term responses in BTC, where acquired resistance to targeted therapies remain a major obstacle, and several questions regarding the effective role of these anticancer treatments remain unanswered [7].
Tumor microenvironment (TME) represents the ecosystem surrounding the tumor, which is composed by several types of cells, such as immune cells, fibroblasts, and endothelial cells, and comprises extracellular matrix, blood vessels and soluble factors. TME plays a central role in cancer development, and it has been suggested to have the potential to influence the response to treatments. Based on these premises, a growing attention has been paid to TME in order to develop effective anticancer treatments [8]. CCA TME is characterized by an abundant desmoplastic stroma which exhibits a high heterogeneity, as it has been showed by several studies [9,10]. Job and colleagues analyzed gene expression profile and cells composition of iCCA TME identifying four different TME subtypes, based on different cells composition and distinct mechanisms of immune dysfunction. The “immune desert phenotype” was the most frequently reported subtype displaying very few immune cells inside the tumor and a markedly reduced tumor and stromal immune signaling. By contrast, the “immunogenic subtype” (approximately 13% of cases) presented an inflammatory TME with a high number of infiltrating innate and adaptive immune cells with an overexpression of major histocompatibility complex and immune checkpoint molecules, such as cytotoxic T lymphocyte antigen 4 (CTLA-4) and PD‐L1. Finally, the “myeloid subtype” presented a relevant expression of the M2-macrophages signature, while the “mesenchymal subtype” showed a high expression of gene signatures of activated fibroblasts, with increased levels of tumorigenic soluble factors [10]. Interestingly, authors found significant difference in terms of prognosis between subgroups, with the immunogenic subtype showing the most favorable survival rates, while mesenchymal subtype had the worst prognosis.
Several studies, such as the aforementioned, showed an association between modifications in the TME and prognosis in CCA, and thus, underlined the central role of TME in this type of hepatobiliary tumor [11,12]. Of note, it is possible to target TME in association with other treatment modalities, such as cytotoxic chemotherapy or targeted therapies, paving the way to possible combination strategies with a synergistic effect. In this review, we will provide an overview of CCA TME describing the role of its main components and focusing on potential therapeutic targets.
Tumor microenvironment
Cancer associated fibroblasts (CAFs)
One of the most important types of stromal cells - for both their great number and their role in cancer progression - are cancer associated fibroblasts (CAFs). CAFs are a heterogeneous group of cells which express various phenotypic markers, such as α-smooth muscle actin (α-SMA) and platelet-derived growth factor receptor beta (PDGFRβ) [13,14]. CAFs play a central role in TME, as these cells can interact with both immune cells and CCA cells modifying and influencing their activity. The origin of CAFs remains unclear since it has been hypothesized their origin from portal fibroblasts, liver resident hepatic stellate cells, or from bone marrow-derived circulating mesenchymal cells [15]. Haga and colleagues showed that CCA cells release extracellular vesicles which induce fibroblastic differentiation of bone marrow mesenchymal stem cells, and thus, a possible origin of CAFs from circulating mesenchymal cells has been hypothesized [16]. In addition, it has been suggested that CAFs phenotype may be an independent prognostic factor for OS in resected iCCA [17]. Particularly, high expression of α-SMA on CAFs has been associated with larger tumor size and reduced OS. The same report also suggested that high α-SMA expressing CAFs determined an increased proliferation effect both on CCA cells and on non-cancer biliary epithelial cells, compared to normal liver fibroblasts with low α-SMA expression [18].
Overall, CCA cells recruit CAFs which, in turn, induce CCA progression. Via the production of several mediators, such as platelet-derived growth factor D (PDGF-D) and TGF-β1, CCA cells recruit CAFs [19,20]. In addition, PDGF-D, upon binding PDGF receptor (PDGFR)β on CAFs, stimulates the release of VEGF-C and VEGF-A determining an expansion of the lymphatic vasculature and tumor cell intravasation [21]. Notably, blocking PDGFRβ with imatinib markedly decreased PDGF-D-induced fibroblast migration and reduced the trans-endothelial migration of CCA cells by inhibiting the secretion of VEGF-A/C [19,21]. In addition, in CCA mice models the induction of CAFs apoptosis via navitoclax produced a significant decrease in the lymphatic microvascular density with a reduction of tumor growth and lymph node metastases [21,22]. On the other hand, CAFs induce the progression of CCA via the production of several mediators, such as TGFβ, CXCL12, heparin-binding epidermal growth factor (HB-EGF) and PDGF-B [20]. PDGF-B, a member of the PDGF family, upon binding to PDGFRβ on CCA cells, protects cancer cells from TRAIL-mediated apoptosis via a hedgehog signaling-dependent process [23]. In CCA mice models, hedgehog blockade inhibits CCA growth by inducing cancer cells apoptosis [23]. Preclinical studies showed that also PDGFRβ inhibition with imatinib may achieve the same result [24]. Despite this, clinical trials evaluating imatinib or sorafenib (a multikinase inhibitor which targets also PDGFRβ) in CCA patients showed only modest results, suggesting that combination with other agents, or different inhibitors should be considered [[25], [26], [27]]. HB-EGF binds epidermal growth factor receptor (EGFR) on CCA cells determining an activation of β-catenin pathway through extracellular signal-regulated kinase (ERK) 1/2 and signal transducer and activator of transcription 3 (STAT3), resulting in an enhanced migration and invasion of CCA cells. Moreover, HB-EGF binding on CCA cells induces the release of TGFβ which, in turn, promote further myofibroblast activation and release of HB-EGF [28] Despite this, EGFR inhibitors showed contrasting results in clinical trials [29,30]. CAFs seem to have a role in CCA resistance to EGFR inhibitors via the production of insulin-like grow factor (IGF) 2 which boosts the insulin receptor (IR) and IGF1 receptor signaling. Combined inhibition of EGFR and IR/IGF1R decreased cancer growth, also reducing the CAFs proliferation and activation [31]. Overall, EGFR remains an interesting potential therapeutic target, as it also have been recently demonstrated that EGFR inhibition could potentiate FGFR therapy in FGFR2 fusion–positive CCA [32].
CAFs, as previously mentioned, can interact with the immune system. Fibroblast activation protein (FAP) is a serine protease selectively expressed on a subset of CAFs which induces the release of CCL2 by CAFs in a STAT3-dependent manner. CCL2, mainly produced by FAP+ CAFs, induces tumor growth by recruiting myeloid-derived suppressor cells (MDSCs) [33]. It has been recently suggested that FAP+ CAFs-educated MDSCs promote stemness marker gene expression in iCCA via 5-lipoxygenase. Notably, inhibition of BLT2 (a receptor of LTB4, which is a downstream metabolite of 5-lipoxygenase) enhances gemcitabine efficacy in CCA mice models, while treatment with gemcitabine alone markedly increases the expression of stemness marker genes [34]. Given this, the 5-lipoxygenase / LTB4-BLT2 axis represents a promising therapeutic target [34]. Recently, in an in vivo study it has been suggested that the antifibrotic agent nintedanib may suppress proliferation of CAFs and their tumor-promoting effect by reducing the production of several cytokines such as IL-6 and IL-8, and thus, resulting in a reduced tumor growth in iCCA mice models [35]. Finally, an in vitro study suggested that everolimus may inhibit the pro-invasive effect of CAFs on CCA cells lines [36]. Following the promising results showed by liver transplantation in non-resectable early stage CCA, this study underlined how the choice of the immunosuppressive drugs could be relevant also for preventing CCA recurrence. Additionally, in the phase II RADiChol study, everolimus demonstrated a 5.5 months PFS and 48% DCR at 12 weeks further supporting the evaluation of the everolimus efficacy in larger advanced CCA cohorts. Interactions between CAFs, cancer cells and TME components are summarized in Fig. 1.
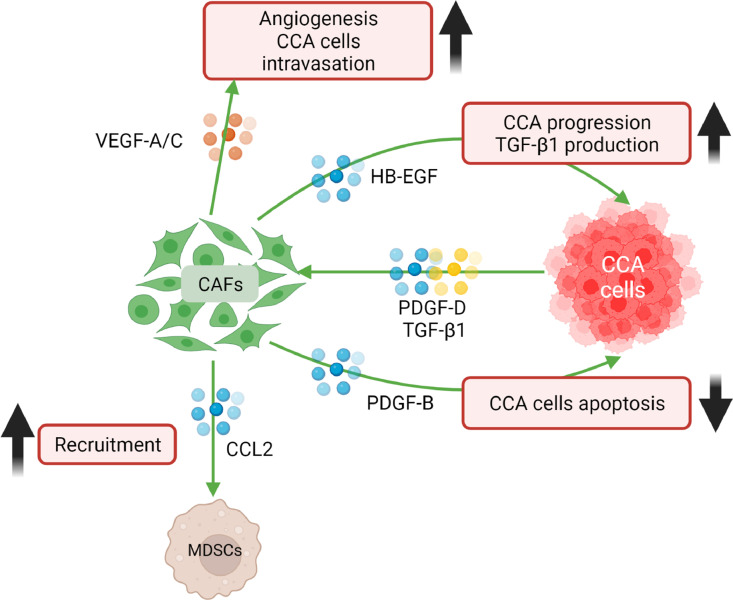
Fig. 1.
CCA cells recruit CAFs via the production of several mediators, such as PDGF-D and TGF-β1. In addition, PDGF-D, upon binding its receptor on CAFs, stimulates the release of VEGF-C and VEGF-A resulting in increased angiogenesis and CCA cells intravasation. CAFs promote tumor progression through the release of several mediators, such as HB-EGF and PDGF-B. HB-EGF binds EGFR determining an enhanced invasion of CCA cells and the release of TGF-β1. PDGF-B, upon binding its receptor on CCA cells, protects cancer cells from apoptosis. CAFs recruit MDSCs via the release of CCL2.
Immune cells in TME
CCA tumor immune microenvironment (TIME) is composed by several immune cell types. Tumor infiltrating lymphocytes (TILs) (e.g. T cells, B cells, and NK cells) represent one of the most important determinants of immune response against cancer cells [37]. T lymphocytes are the most common type of inflammatory cells in CCA, with a major proportion of CD8+ over CD4+, while it has been observed a modest number of NK cells and B lymphocytes, with the latter being the less represented [37]. Overall, TILs are associated with prognosis in both iCCAs and eCCAs [38].
CD8+, CD4,+ and CD3+ T lymphocytes seem to be mainly located in the peritumoral area both in iCCA and eCCA, while PD-1+ T cells are mainly localized in tumor [[37], [39], [40], [41], [42]]. A high number of CD8+ or CD4+ T cells has been positively correlated with prognosis and this effect does not seem to be related to lymphocyte spatial distribution [43,44]. NK cells play a central role in tumor immune surveillance, and this seems to be confirmed also in the context of CCA. Epidemiological data showed that patients with CCA had multiple alterations in several genes important for NK functioning (inhibitory killer cell immunoglobulin-like receptors and HLA gene loci), compared to control individuals, while polymorphisms in the natural killer cell receptor G2D gene were associated with an increased risk of CCA transformation in patients with primary sclerosing cholangitis [45,46]. Finally, a greater number of infiltrating NK cells was associated with longer OS and relapse-free survival (RFS) in resected iCCA [47]. Tumor associated macrophages (TAMs) are another cell type which plays a central role in the tumor immune contexture, remodeling the microenvironment and influencing cancer progression [48], with TAMs deriving from recruited macrophages supplemented by circulating monocytes, or from Kupfer cells [49]. TAMs are classically divided in two different subtypes: M1 macrophages with pro-inflammatory and phagocytic functions, and M2, which favor tumor progression. In CCA patients, a high density of M2 cells has been associated with the presence of metastases via the increased cell migration and N-cadherin expression suggesting a putative role in the epithelial-to-mesenchymal transition [50].
Dendritic cells (DCs) act as antigen-presenting cells and are essential for the activation of the adaptive immune response. In the TME, DCs capture, process and present tumor antigens determining an activation of the T cell response [51]. The presence of mature DCs at the tumor invasive margin has been positively correlated with an increased presence of CD4+ and CD8+ T cells in the peritumoral region of CCA patients. In addition, the presence of mature DCs was associated with an improved prognosis, with also a significantly lower incidence of lymph node metastasis [52]. However, cancer cells are able to transform DCs into an immature immunosuppressive phenotype and, specifically in CCA patients, the presence of immature DCs in the tumor region has been associated with a decreased number of tumor infiltrating CD4+ and CD8+ T cells [51,52]. The high-affinity IgE receptor FcεRI is essential for cross-presentation and priming of cytotoxic CD8+ T lymphocytes by DCs [53]. Martin-Sierra et al. observed a significant decrease in FcεRI+ monocytes and DCs in the peripheral blood of CCA patients, compared to control individuals [54]. Overall, these observations suggest that DCs are dysfunctional in CCA, and thus, DCs may represent a potential therapeutic target. Finally, tumor associated neutrophils (TANs) play also an important role in tumor development and progression, and it seems to be confirmed also in CCA [55]. A study conducted on 254 resected CCA showed that accumulation of TANs was associated with reduced DFS and OS, with their number being also an independent risk factor for OS [56]. However, further studies are needed to better understand their role in cancer progression and their use as a potential therapeutic target.
Functional role of cells in TME
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells which originate from bone marrow and exert a potent immunosuppressive effect in the TME [57]. The population of regulatory T cells (Tregs), a subpopulation of lymphocytes with immune-suppressive properties, is induced by MDSCs, TAMs and TANs via the production of IL-10 and CCL2 [58,59]. In addition, IL-10 induces a polarization of CD4+ T cells towards Th2 response instead of a Th1 response, which induces a cytotoxic T lymphocytes mediated killing of cancer cells [60]. Tregs, via the expression of CTLA-4, induce an immature immunosuppressive phenotype of DCs resulting in an impaired antigen presentation and determining an immune-tolerant microenvironment [61]. In this context, an important role in tumor progression is played by TGF-β which is produced by MDSCs and TAMs resulting in an enhanced activity of Tregs and the inhibition of CD8+ T cells [62,58].
By combining laser capture microdissection and gene expression profiling, Sulpice and colleagues studied transcriptomic alterations in stromal cells of iCCA [63]. The authors found 1073 nonredundant genes which were differentially expressed between tumor stroma and the non-tumor fibrous tissue in the adjacent liver. In particular, the study documented an overexpression of osteopontin and TGF-β2 which were correlated with a reduced OS [63], something that underlines the role of TGF-β pathway in CCA. In vitro experiments conducted on human DCs and CCA cell lines showed that inhibition of IL-10 and TGF-β receptors on DCs, via anti-IL-10 and anti-TGF-β receptor II (TGF-βRII) antibodies, increased IFN-γ levels and determined an enhanced activation of effector T cells against CCA cells. Thus, IL-10 and TGF-β could represent potential targets for therapy and their inhibition is fundamental for developing DC-activated effector T cells for adoptive T-cell therapy(65). TGF-β signaling has been suggested to promote tumor growth by exerting regulatory effects on both immune cells and cancer cells which are present in the TME as well as by inducing angiogenesis, fibrosis, and epithelial-mesenchymal transition. Bintrafusp alfa, a first-in-class, bifunctional fusion protein composed of a monoclonal antibody against PD-L1 fused with extracellular domain of two TGF-βRII molecules designed to bind TGF-β in the TME, is under evaluation in several clinical trials (NCT03833661, NCT04708067, NCT04066491) [64]. The first results from a phase I study in which 30 CCA patients were treated with Bintrafusp alfa after progression to first-line chemotherapy were encouraging, with a ORR of 20% independent of PD-L1 expression and microsatellite instability [65].
CCA cancer cells are not simply prone to TIME, but they can influence it by determining a more immune-tolerant microenvironment, and thus, favoring their own self-preservation and cancer progression. It has been demonstrated that CCA cells produce TGF-β which seems to further induce production of other TGF-β in an autocrine manner [66,67]. In addition, CCA cancer stem cells are able to induce TAMs polarization towards a tumor-promoting phenotype and periostin seems to play a key role in this process, with this cell adhesion protein suggested to represent a potential target to modify TIME [68,69]. On the other hand, in vitro experiments showed that activated TAMs could stimulate CCA cells proliferation via the Wnt / β-catenin pathway, which is pathologically activated in CCA. Interestingly, it has been demonstrated that suppression of β-catenin translation determines an inhibition of CCA cells growth [70]. Given these observations, it has been hypothesized that blocking TAMs recruitment would have inhibited tumor progression. However, Loeuillard et al. showed that blocking TAMs recruitment in mice models did not impair tumor growth as it determines the compensatory emergence of a subtype of ApoE-MDSCs with immunosuppressive properties [71]. ApoE-MDSCs could be inhibited via an anti-Ly6G antibody (Ly6G is a differentiation antigen expressed on ApoE-MDSCs) or via the activation of the liver-X receptor (LXR)/ApoE axis which has been implicated in MDSCs survival [72]. Notably, it has been demonstrated in CCA mice models that dual inhibition of TAMs and ApoE-MDSCs enhances the effect of anti-programmed cell death protein 1(PD-1) therapy [71]. Given poor results obtained by monotherapy with immune checkpoint inhibitors (ICIs) in CCA patients, this evidence supports the strategy of combining ICIs with MDSCs and TAMs inhibition. Currently, GW3965, an LXR/ApoE axis agonist, is under evaluation in a phase I study in advanced solid tumors (NCT02922764). CCA cells could also influence NK cells recruitment via the production of C-X-C motif ligand 9 (CXCL9). CXCL9 is a chemokine induced by IFN-γ, and high expression of CXCL9 in CCA is associated with less aggressive tumors, and improved OS and RFS of resected CCA patients. It has been demonstrated that tumor-derived CXCL9 could enhance NK cells recruitment, thus resulting in an improved immune response against cancer cells [47]. Of note, in breast and ovarian cancer models unselective cyclooxygenases (COX) inhibitors are able to increase cancer derived CXCL9 via the inhibition of prostaglandin E2 production, which negatively regulates CXCL9 expression [73,74]. Fukuda and colleagues showed that treatment with low-dose COX-2 inhibitor celecoxib determined an increased production of CXCL9 by CCA cells [47]. However, definitive results have not been published yet and in vivo experiments are needed to confirm the potential benefit of this therapeutic strategy. Additionally, celecoxib blocked the phosphorylation of Akt and induced apoptosis in a cell-line study, further supporting the exploration of COX-2 pathway as a therapeutic strategy for CCA [75].
The discovery of inhibitory checkpoint molecules led to the development of several immune checkpoint inhibitors (ICIs), such as anti-PD-1/PD-L1 and anti-CTLA-4 antibodies, which revolutionized the treatment of several solid tumors. Despite this, results obtained by monotherapy with ICIs in metastatic disease were disappointing, with a 5.8% ORR reported by the PD-1 inhibitor pembrolizumab in unselected biliary tract cancer patients in the Keynote-158 trial [76]. These modest results could partially be explained by the absence of reliable predictors of response to ICIs, as the role of PD-L1 expression or other candidates such as tumor mutational burden or high microsatellite instability in this context are still to be clarified [77]. The composition of TME represents a promising candidate as biomarker of response to ICIs. For example, in other cancer types, such as non-small-cell lung cancer or melanoma, the CD8+/CD4+ TILs ratio is able to predict response to anti-PD-1 treatment [78]. In a study by Yoon and colleagues, specimens from 121 advanced CCA patients were analyzed via targeted sequencing and immunohistochemical staining to identify predictive biomarkers of response. Forty-eight patients were treated with anti-PD-1/PD-L1 antibodies after progression to first-line chemotherapy, and among these patients, a high intratumoral TILs density was associated with response to ICIs [79]. One possible explanation for these findings is provided by a study conducted on specimens from 192 resected iCCA which showed a positive association between PD-L1 expression on cancer cells and the number of CD8+ T-cells, with these factors associated with a favorable prognosis [80].
However, further studies are needed to better understand the real potential of TILs as biomarker of response to ICIs in CCA. Immune-based combination strategies have also been investigated in order to increase ICIs efficacy. Recent results showed by the combination of ICIs with chemotherapy are particularly notable, with durvalumab (an anti-PD-L1 antibody) plus gemcitabine and cisplatin that will probably represent the new standard of care for treatment-naïve advanced CCA after first results of TOPAZ-1 phase III trial [81]. Another promising combination strategy which modifies the TIME is the association of ICIs with anti-VEGF agents. VEGF, which is produced by both CCA cells and TAMs, has an immunosuppressive effect on TIME, as it suppresses T cell response via the inhibition of DCs maturation and inducing the FasL-mediated killing of CD8+ T cells [[82], [83], [84]]. Currently, the association of bevacizumab plus atezolizumab with gemcitabine plus cisplatin is under evaluation in the phase II IMbrave151 trial (NCT04677504). In vitro experiments showed that adding cytotoxic-T lymphocytes, activated by DCs, to gemcitabine enhances CCA cells killing as gemcitabine seems to enhance cytotoxic activity of T lymphocytes [85]. However, gemcitabine increases also the expression of PD-L1 in CCA cell lines, which consequently reduces the activity of cytotoxic-T lymphocytes [85]. Given this, in a recently published study, Wathikthinnakon et al. evaluated the PD-L1xCD3 bispecific T cell engager, which is a chimeric protein that binds both PD-L1 on cancer cells and CD3 on T cells resulting in T cells activation, in association with gemcitabine. The authors hypothesized that blocking PD-1/PD-L1 axis would prevent its inhibitory effect on T cells activated by binding CD3, whose activity is enhanced by gemcitabine. Authors observed an enhanced T cells activity, resulting in a CCA cells killing, and suggested that the association of PD-L1xCD3 bispecific T cell engager and gemcitabine could represent a potential therapeutic strategy [86]. In another recent study it has been showed that stimulation of DCs and TAMs, via a CD40 agonist, may improve response to anti-PD-1 therapy with an increased number of activated CD4+ and CD8+ T cells in CCA mice models [87]. Currently, CDX-1140 (a CD40 agonist) is under evaluation in a phase I trial alone, or in association with other treatments (ICIs or chemotherapy) in advanced solid tumors (NCT03329950).
Inhibitory killer cell immunoglobulin-like receptors (KIRs) inhibit NK cells response and as mentioned above, their gene loci present several alterations in CCA [45]. Lirilumab (an anti-KIR antibody) in association with nivolumab (an anti-PD-1 antibody), with or without ipilimumab (an anti-CTLA-4 antibody), is now under evaluation in a phase I trial in advanced solid tumors including CCA (NCT03203876). Targeting PD-L1 and CTLA-4 with a DNA vaccine has also shown promising results in CCA mice models [88]. Components of the TIME and their interactions are summarized in Fig. 2, while Table 1 summarizes potential therapeutic strategies targeting the TME.
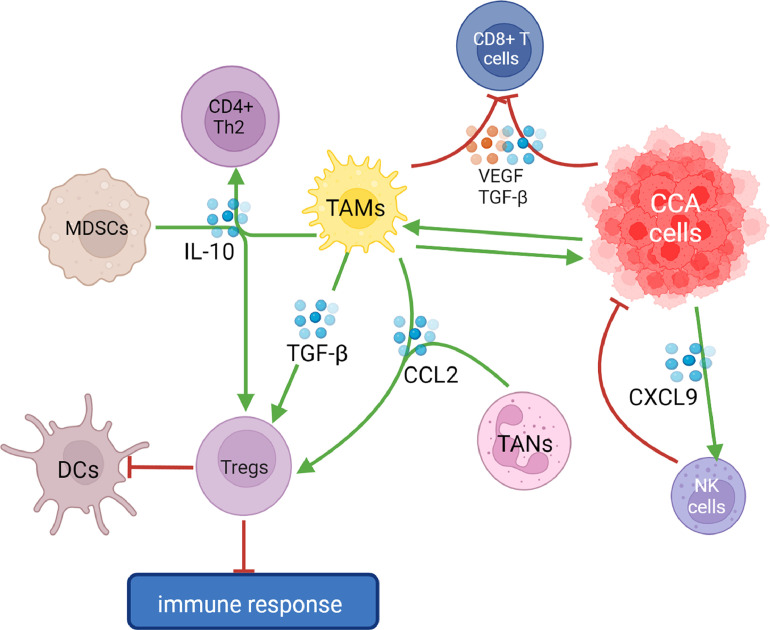
Fig. 2.
Tregs are induced by MDSCs, TAMs and TANs via the production of IL-10 and CCL2 They determine a polarization of DCs towards an immature immunosuppressive phenotype resulting in an impaired antigen presentation and determining an immune-tolerant microenvironment. In addition, IL-10 induces a polarization of CD4+ T cells towards Th2 response. TGF-β, which is produced by CCA cells and TAMs, play a central role in immunotolerance by enhancing activity of Tregs and via the inhibition of CD8+ T cells. TAMs-derived VEGF reduces CD8+ T cells activity by inducing their FasL-mediated killing. CCA cancer stem cells are able to induce TAMs polarization towards a tumor-promoting phenotype, on the other hand activated TAMs could stimulate CCA cells proliferation via the Wnt/β-catenin pathway. CCA-derived CXCL9 enhance NK cells recruitment, thus resulting in an improved immune response.
Table 1.
Potential therapeutic strategies targeting CCA TME.
| Refs/NCT | Target | Type of study | Study population | Treatment | Outcomes |
|---|---|---|---|---|---|
| Mertens et al. [22] | CAFs | Cell culture Animal model | / | Navitoclax | CAFs apoptosis, reduced tumor size and metastases |
| Vaquero et al. [31] | IR/IGF1R, EGFR | Cell culture Animal model | / | EGFR inhibitor (erlotinib) + IR/IGF1R inhibitor (BMS536924, linsitinib) | Reduced tumor growth and CAFs proliferation and activation |
| Lin et al. [34] | BLT2 | Animal model | / | Gemcitabine + BLT2 antagonist | Enhanced chemotherapeutic efficacy with reduced tumor growth |
| Yamanaka et al. [35] | CAFs | Cell culture Animal model | / | Nintedanib | Suppression of CAFs activation and secretion of cancer-promoting cytokines (IL-6 and IL-8) by CAFs. Reduced tumor growth |
| Heits et al. [36] | CAFs | Cell culture | / | Everolimus | Inhibiton of the pro-invasive effect of CAFs on CCA cells |
| Yoo et al. [65] | |||||
| (NCT02699515) | PD-L1, TGF-β | ||||
| Phase I clinical trial | Pretreated advanced CCA pts | Bintrafusp alfa | ORR 20% | ||
| Loeuillard et al. [71] | TAMs, MDSCs | Animal model | / | Anti-Ly6G ab + anti-CSF1R + anti–PD-1 ab | enhanced effect of anti- PD-1 therapy |
| NCT04677504 | PD-L1, VEGFR | Phase II clinical trial | Advanced CCA pts 1st line | Atezolizumab + bevacizuab + gemcitabine + cisplatin | NA |
| Wathikthinnakon et al. [86] | CD3+ T lymphocytes, PD-L1 | Cell culture | PD-L1xCD3 BiTE | Enhanced T cells activity against CCA cells | |
| Diggs L. P. | CD40+ APCs, PD-1 | Animal model | CD40 agonist + PD-1 ab | Increased number of activated CD4+ and CD8+ T cells with improved response to anti-PD-1 therapy | |
| NCT03203876 | KIR, PD-1 | Phase I clinical trial | Advanced solid tumors | Lirilumab + nivolumab +/- ipilimumab | NA |
| Pan et al. [88] | PD-L1, CTLA4 | Animal model | / | PD-L1-CTLA4 DNA vaccination | Increased number of CD8+ T cells, reduced tumor growth |
Abbreviations
CAFs: cancer associated fibroblasts; EGFR: epidermal growth factor receptor; IR: insulin receptor; IGF1R: insulin-like growth factor 1 receptor; IL-6: interleukin-6; IL-8: interleukin-8; CCA: cholangiocarcinoma; PD-L1: programmed cell death 1 ligand; TGF-β: Transforming growth factor beta; ORR: overall response rate; TAMs: tumor associated macrophages; MDSCs: myeloid-derived suppressor cells; PD-1: programmed cell death 1; ab: antibody; VEGFR: vascular endothelial growth factor receptor; pts: patients; NA: not available; APCs: antigen presenting cells; CSF1R: CSF1 receptor; BiTE: bispecific T cell engager; KIR: Inhibitory killer cell immunoglobulin-like receptor; CTLA4: cytotoxic T-lymphocyte-associated protein 4.
Future perspectives and conclusions
CCAs tend to be immune “cold” tumors, with single agent ICIs showing activity only in a small subset of patients for which there are no reliable predictors of response. Given this, immunotherapy with single agent ICIs showed unsatisfactory results in clinical trials [75,89,90]. Strategies aiming to increase ICIs efficacy via their association with other agents are of particular interest, a setting where a deeper understanding of TME would be fundamental. In this context, the results of the phase III TOPAZ-1 trial in which the association of durvalumab with gemcitabine plus cisplatin determined an increased OS and progression-free survival (PFS) compared to chemotherapy alone have been recently published [81]. However, the improvement in OS was modest compared to the significant improvements in other solid tumors and this issue pointed out a need for further efforts to improve patient selection as well as a need for combinations potentially targeting other pathways in the TME [[91], [92], [93]].
TME plays a central role in CCA progression and resistance to treatments, and thus, combining therapeutic strategies targeting cancer cells and TME represent a promising approach. In particular, targeting TME cells, for example suppressing CAFs proliferation via nintedanib, or stimulating DCs via CD40 agonists is an interesting strategy [87,35]. Targeting the crosstalk between CCA cells and TME components via the inhibition of secretion of cytokines, or their receptors, represent another promising research avenue. Our knowledge of CCA TME is mainly based on immunohistochemical studies on specimens of resected CCAs which provide a comprehensive view of main components and their distribution in the TME. By contrast, our understanding of the complex crosstalk between different components of TME and CCA cells remains limited. Further studies are warranted in this field in order to develop effective treatments targeting TME. Recent studies which take advantage of single-cell RNA-sequencing highlighted a wide heterogeneity of both cancer cells and TME cells, such as CAFs or immune cells [10,13].
The classification in four immune subtypes proposed by Job and colleagues provides several suggestions for treatment and study design [10]. For example, in the “immune desert subtype”, strategies aiming to convert “cold” tumors into inflamed tumors, such as the association of ICIs with local therapies or with agents targeting cellular DNA damage repair, could represent a promising approach [94]. The “immunogenic subtype” would probably benefit from ICIs, as its TME presents immune-stimulating factors, suggesting an effective anticancer immune response. Of note, this subtype seems to be particularly common among cirrhotic patients, suggesting a link between the immune activated TME and systemic inflammation related to cirrhosis [95]. Finally, the “mesenchymal subtype” appears as an interesting subset of CCAs in which test antifibrotic drugs. In our opinion, combining cytotoxic agents, such as chemotherapy or target therapies, with effective treatments targeting TME will probably represent one of the most promising strategies in CCA treatment.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of Competing Interest
All authors declared that there are no conflicts of interest.
Acknowledgment
None.
References
- 1.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17(9):557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewitt DB, Brown ZJ, Pawlik TM. Current perspectives on the surgical management of perihilar cholangiocarcinoma. Cancers (Basel) 2022;14(9):2208. doi: 10.3390/cancers14092208. Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi: 10.1016/S1470-2045(18)30915-X. May. [DOI] [PubMed] [Google Scholar]
- 4.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. Ann. Oncol. 2016;27:v28–v37. doi: 10.1093/annonc/mdw324. Sep 1. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo A, Ricci AD, Tober N, Nigro MC, Mosca M, Palloni A, et al. Second-line treatment in advanced biliary tract cancer: today and tomorrow. Anticancer Res. 2020;40(6):3013–3030. doi: 10.21873/anticanres.14282. Jun 1. [DOI] [PubMed] [Google Scholar]
- 7.Verlingue L, Hollebecque A, Boige V, Ducreux M, Malka D, Ferté C. Matching genomic molecular aberrations with molecular targeted agents: are biliary tract cancers an ideal playground? Eur. J. Cancer. 2017;81:161–173. doi: 10.1016/j.ejca.2017.05.006. Aug. [DOI] [PubMed] [Google Scholar]
- 8.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020;5(1):166. doi: 10.1038/s41392-020-00280-x. Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E, et al. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology. 2013;58(6):1992–2000. doi: 10.1002/hep.26577. Dec. [DOI] [PubMed] [Google Scholar]
- 10.Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72(3):965–981. doi: 10.1002/hep.31092. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabris L, Sato K, Alpini G, Strazzabosco M. The tumor microenvironment in cholangiocarcinoma progression. Hepatology. 2021;73(1):75–85. doi: 10.1002/hep.31410. JanSuppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guedj N, Blaise L, Cauchy F, Albuquerque M, Soubrane O, Paradis V. Prognostic value of desmoplastic stroma in intrahepatic cholangiocarcinoma. Mod. Pathol. 2021;34(2):408–416. doi: 10.1038/s41379-020-00656-y. Feb. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Yang H, Wan L, Wang Z, Wang H, Ge C, et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 2020;73(5):1118–1130. doi: 10.1016/j.jhep.2020.05.039. Nov. [DOI] [PubMed] [Google Scholar]
- 14.Nishihara Y, Aishima S, Hayashi A, Iguchi T, Fujita N, Taketomi A, et al. CD10+ fibroblasts are more involved in the progression of hilar/extrahepatic cholangiocarcinoma than of peripheral intrahepatic cholangiocarcinoma. Histopathology. 2009;55(4):423–431. doi: 10.1111/j.1365-2559.2009.03398.x. Oct. [DOI] [PubMed] [Google Scholar]
- 15.Vaquero J, Aoudjehane L, Fouassier L. Cancer-associated fibroblasts in cholangiocarcinoma. Curr. Opin. Gastroenterol. 2020;36(2):63–69. doi: 10.1097/MOG.0000000000000609. Mar. [DOI] [PubMed] [Google Scholar]
- 16.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumor cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J. Extracell. Vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XF, Dong M, Pan YH, Chen JN, Huang XQ, Jin Y, et al. Expression pattern of cancer-associated fibroblast and its clinical relevance in intrahepatic cholangiocarcinoma. Hum. Pathol. 2017;65:92–100. doi: 10.1016/j.humpath.2017.04.014. Jul. [DOI] [PubMed] [Google Scholar]
- 18.Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol. Rep. 2009;21(4):957–969. doi: 10.3892/or_00000309. Apr. [DOI] [PubMed] [Google Scholar]
- 19.Cadamuro M, Nardo G, Indraccolo S, Dall'olmo L, Sambado L, Moserle L, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58(3):1042–1053. doi: 10.1002/hep.26384. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentilini A, Pastore M, Marra F, Raggi C. The role of stroma in cholangiocarcinoma: the intriguing interplay between fibroblastic component, immune cell subsets and tumor epithelium. Int. J. Mol. Sci. 2018;19(10):2885. doi: 10.3390/ijms19102885. Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J. Hepatol. 2019;70(4):700–709. doi: 10.1016/j.jhep.2018.12.004. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73(2):897–907. doi: 10.1158/0008-5472.CAN-12-2130. Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, Cazanave SC, et al. Myofibroblast-derived PDGF-BB promotes hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54(6):2076–2088. doi: 10.1002/hep.24588. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fingas CD, Mertens JC, Razumilava N, Bronk SF, Sirica AE, Gores GJ. Targeting PDGFR-β in cholangiocarcinoma. Liver Int. 2012;32(3):400–409. doi: 10.1111/j.1478-3231.2011.02687.x. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan TT, Wang W, Jia WD, Xu GL. A single-center experience of sorafenib monotherapy in patients with advanced intrahepatic cholangiocarcinoma. Oncol. Lett. 2017;13(5):2957–2964. doi: 10.3892/ol.2017.5847. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo X, Jia W, Huang Z, Li X, Xing B, Jiang X, et al. Effectiveness and safety of sorafenib in the treatment of unresectable and advanced intrahepatic cholangiocarcinoma: a pilot study. Oncotarget. 2016;8(10):17246–17257. doi: 10.18632/oncotarget.12825. Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth A, Schleyer E, Schoppmeyer K, Kluge R, Wittekind C, Mössner J, et al. Imatinib mesylate for palliative second-line treatment of advanced biliary tract cancer: a bicentric phase II study. Onkologie. 2011;34(8–9):469–470. doi: 10.1159/000331065. [DOI] [PubMed] [Google Scholar]
- 28.Clapéron A, Mergey M, Aoudjehane L, Ho-Bouldoires THN, Wendum D, Prignon A, et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology. 2013;58(6):2001–2011. doi: 10.1002/hep.26585. Dec. [DOI] [PubMed] [Google Scholar]
- 29.Rizzo A, Frega G, Ricci AD, Palloni A, Abbati F, DE Lorenzo S, et al. Anti-EGFR monoclonal antibodies in advanced biliary tract cancer: a systematic review and meta-analysis. In Vivo. 2020;34(2):479–488. doi: 10.21873/invivo.11798. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahipal A, Kommalapati A, Tella SH, Lim A, Kim R. Novel targeted treatment options for advanced cholangiocarcinoma. Expert Opin. Investig. Drugs. 2018;27(9):709–720. doi: 10.1080/13543784.2018.1512581. Sep 2. [DOI] [PubMed] [Google Scholar]
- 31.Vaquero J, Lobe C, Tahraoui S, Clapéron A, Mergey M, Merabtene F, et al. The IGF2/IR/IGF1R pathway in tumor cells and Myofibroblasts mediates resistance to EGFR inhibition in cholangiocarcinoma. Clin. Cancer Res. 2018;24(17):4282–4296. doi: 10.1158/1078-0432.CCR-17-3725. Sep 1. [DOI] [PubMed] [Google Scholar]
- 32.Wu Q, Zhen Y, Shi L, Vu P, Greninger P, Adil R, et al. EGFR inhibition potentiates FGFR inhibitor therapy and overcomes resistance in FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2022;12(5):1378–1395. doi: 10.1158/2159-8290.CD-21-1168. May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Y, Li B, Yang X, Cai Q, Liu W, Tian M, et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia. 2019;21(12):1133–1142. doi: 10.1016/j.neo.2019.10.005. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y, Cai Q, Chen Y, Shi T, Liu W, Mao L, et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology. 2022;75(1):28–42. doi: 10.1002/hep.32099. Jan. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka T, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Hagiwara K, et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br. J. Cancer. 2020;122(7):986–994. doi: 10.1038/s41416-020-0744-7. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heits N, Heinze T, Bernsmeier A, Kerber J, Hauser C, Becker T, et al. Influence of mTOR-inhibitors and mycophenolic acid on human cholangiocellular carcinoma and cancer associated fibroblasts. BMC Cancer. 2016;16:322. doi: 10.1186/s12885-016-2360-8. May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, et al. Prognostic impact of tumor-infiltrating immune cells on biliary tract cancer. Br. J. Cancer. 2013;109(10):2665–2674. doi: 10.1038/bjc.2013.610. Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigano L, Soldani C, Franceschini B, Cimino M, Lleo A, Donadon M, et al. Tumor-infiltrating lymphocytes and macrophages in intrahepatic cholangiocellular carcinoma. impact on prognosis after complete surgery. J. Gastrointest. Surg. 2019;23(11):2216–2224. doi: 10.1007/s11605-019-04111-5. Nov. [DOI] [PubMed] [Google Scholar]
- 39.Tian L, Ma J, Ma L, Zheng B, Liu L, Song D, et al. PD-1/PD-L1 expression profiles within intrahepatic cholangiocarcinoma predict clinical outcome. World J. Surg. Oncol. 2020;18(1):303. doi: 10.1186/s12957-020-02082-5. Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Wei Y, Jian M, Lu H, Song Q, Hao L, et al. Clinicopathological and prognostic significance of immunoscore and PD-L1 in intrahepatic cholangiocarcinoma. OncoTargets Ther. 2021;14:39–51. doi: 10.2147/OTT.S288982. Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu YP, Zhou YQ, Zhao YJ, Zhao Y, Wang F, Huang XY, et al. High level of CD73 predicts poor prognosis of intrahepatic cholangiocarcinoma. J. Cancer. 2021;12(15):4655–4660. doi: 10.7150/jca.51038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu JC, Zeng HY, Sun QM, Meng QN, Huang XY, Zhang PF, et al. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics. 2019;9(16):4678–4687. doi: 10.7150/thno.36276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asahi Y, Hatanaka KC, Hatanaka Y, Kamiyama T, Orimo T, Shimada S, et al. Prognostic impact of CD8+ T cell distribution and its association with the HLA class I expression in intrahepatic cholangiocarcinoma. Surg. Today. 2020;50(8):931–940. doi: 10.1007/s00595-020-01967-y. Aug. [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Heij LR, Czigany Z, Dahl E, Lang SA, Ulmer TF, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022;41(1):127. doi: 10.1186/s13046-022-02340-2. Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cornillet M, Jansson H, Schaffer M, Hertwig L, Berglin L, Zimmer CL, et al. Imbalance of genes encoding natural killer immunoglobulin-like receptors and human leukocyte antigen in patients with biliary cancer. Gastroenterology. 2019;157(4):1067–1080. doi: 10.1053/j.gastro.2019.06.023. Octe9. [DOI] [PubMed] [Google Scholar]
- 46.Wadsworth CA, Dixon PH, Taylor-Robinson S, Kim JU, Zabron AA, Wong JH, et al. Polymorphisms in natural killer cell receptor protein 2D (NKG2D) as a risk factor for cholangiocarcinoma. J. Clin. Exp. Hepatol. 2019;9(2):171–175. doi: 10.1016/j.jceh.2018.06.521. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuda Y, Asaoka T, Eguchi H, Yokota Y, Kubo M, Kinoshita M, et al. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci. 2020;111(2):323–333. doi: 10.1111/cas.14267. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588–602. doi: 10.1016/j.ccell.2019.02.009. Apr 15e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dou L, Shi X, He X, Gao Y. Macrophage phenotype and function in liver disorder. Front. Immunol. 2020;28(10):3112. doi: 10.3389/fimmu.2019.03112. https://www.frontiersin.org/article/10.3389/fimmu.2019.03112 PMID: 32047496; PMCID: PMC6997484 [cited 2022 Apr 28];10. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thanee M, Loilome W, Techasen A, Namwat N, Boonmars T, Pairojkul C, et al. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac. J. Cancer Prev. 2015;16(7):3043–3050. doi: 10.7314/apjcp.2015.16.7.3043. [DOI] [PubMed] [Google Scholar]
- 51.Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis. J. Immunol. 2015;194(7):2985–2991. doi: 10.4049/jimmunol.1403134. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takagi S, Miyagawa SI, Ichikawa E, Soeda J, Miwa S, Miyagawa Y, et al. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum. Pathol. 2004;35(7):881–886. doi: 10.1016/j.humpath.2004.03.016. Jul. [DOI] [PubMed] [Google Scholar]
- 53.Platzer B, Elpek KG, Cremasco V, Baker K, Stout MM, Schultz C, et al. IgE/FcεRI-mediated antigen cross-presentation by dendritic cells enhances anti-tumor immune responses. Cell Rep. 2015;10(9):1487–1495. doi: 10.1016/j.celrep.2015.02.015. Mar 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martín-Sierra C, Martins R, Laranjeira P, Abrantes AM, Oliveira RC, Tralhão JG, et al. Functional impairment of circulating FcεRI+ monocytes and myeloid dendritic cells in hepatocellular carcinoma and cholangiocarcinoma patients. Cytom. B Clin. Cytom. 2019;96(6):490–495. doi: 10.1002/cyto.b.21777. Nov. [DOI] [PubMed] [Google Scholar]
- 55.Shaul ME, Fridlender ZG. Tumor-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019;16(10):601–620. doi: 10.1038/s41571-019-0222-4. Oct. [DOI] [PubMed] [Google Scholar]
- 56.Mao ZY, Zhu GQ, Xiong M, Ren L, Bai L. Prognostic value of neutrophil distribution in cholangiocarcinoma. World J. Gastroenterol. 2015;21(16):4961–4968. doi: 10.3748/wjg.v21.i16.4961. Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19(2):108–119. doi: 10.1038/s41590-017-0022-x. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Disis ML. Immune regulation of cancer. J. Clin. Oncol. 2010;28(29):4531–4538. doi: 10.1200/JCO.2009.27.2146. Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabris L, Perugorria MJ, Mertens J, Björkström NK, Cramer T, Lleo A, et al. The tumor microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39(1):63–78. doi: 10.1111/liv.14098. MaySuppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol. Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goeppert B, Frauenschuh L, Zucknick M, Roessler S, Mehrabi A, Hafezi M, et al. Major histocompatibility complex class I expression impacts on patient survival and type and density of immune cells in biliary tract cancer. Br. J. Cancer. 2015;113(9):1343–1349. doi: 10.1038/bjc.2015.337. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou W. Immunosuppressive networks in the tumor environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. Apr. [DOI] [PubMed] [Google Scholar]
- 63.Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E, et al. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology. 2013;58(6):1992–2000. doi: 10.1002/hep.26577. Dec. [DOI] [PubMed] [Google Scholar]
- 64.Lind H, Gameiro SR, Jochems C, Donahue RN, Strauss J, Gulley JL, et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000433. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000564. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum. Vaccines Immunother. 2018;14(6):1423–1431. doi: 10.1080/21645515.2018.1431598. Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu T, Yokomuro S, Mizuguchi Y, Kawahigashi Y, Arima Y, Taniai N, et al. Effect of transforming growth factor-β1 on human intrahepatic cholangiocarcinoma cell growth. World J. Gastroenterol. 2006;12(39):6316–6324. doi: 10.3748/wjg.v12.i39.6316. Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J. Hepatol. 2017;66(1):102–115. doi: 10.1016/j.jhep.2016.08.012. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng J, Liu Z, Sun S, Xie J, Cao L, Lv P, et al. Tumor-associated macrophages recruited by periostin in intrahepatic cholangiocarcinoma stem cells. Oncol. Lett. 2018;15(6):8681–8686. doi: 10.3892/ol.2018.8372. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loilome W, Bungkanjana P, Techasen A, Namwat N, Yongvanit P, Puapairoj A, et al. Activated macrophages promote Wnt/β-catenin signaling in cholangiocarcinoma cells. Tumor Biol. 2014;35(6):5357–5367. doi: 10.1007/s13277-014-1698-2. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J. Clin. Investig. 2020;130(10):5380–5396. doi: 10.1172/JCI137110. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tavazoie MF, Pollack I, Tanqueco R, Ostendorf BN, Reis BS, Gonsalves FC, et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell. 2018;172(4):825–840. doi: 10.1016/j.cell.2017.12.026. Feb 8e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bronger H, Kraeft S, Schwarz-Boeger U, Cerny C, Stöckel A, Avril S, et al. Modulation of CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase inhibitors in human breast cancer. Breast Cancer Res. 2012;14(1):R30. doi: 10.1186/bcr3115. Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bronger H, Singer J, Windmüller C, Reuning U, Zech D, Delbridge C, et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br. J. Cancer. 2016;115(5):553–563. doi: 10.1038/bjc.2016.172. Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu T, Leng J, Han C, Demetris AJ. The cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cells. Mol. Cancer Ther. 2004;3(3):299–307. MarPMID: 15026550. [PubMed] [Google Scholar]
- 76.Bang YJ, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J. Clin. Oncol. 2019;37(15_suppl):4079. May 20. [Google Scholar]
- 77.Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and other predictors of response to immune checkpoint inhibitors in biliary tract cancer. Cancers (Basel) 2021;13(3):558. doi: 10.3390/cancers13030558. Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uryvaev A, Passhak M, Hershkovits D, Sabo E, Bar-Sela G. The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma. Med. Oncol. 2018;35(3):25. doi: 10.1007/s12032-018-1080-0. Jan 31. [DOI] [PubMed] [Google Scholar]
- 79.Yoon JG, Kim MH, Jang M, Kim H, Hwang HK, Kang CM, et al. Molecular characterization of biliary tract cancer predicts chemotherapy and programmed death 1/programmed death-ligand 1 blockade responses. Hepatology. 2021;74(4):1914–1931. doi: 10.1002/hep.31862. [DOI] [PubMed] [Google Scholar]
- 80.Zhu Y, Wang XY, Zhang Y, Xu D, Dong J, Zhang Z, et al. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8+ T-cell immune responses. Cancer Manag. Res. 2018;10:4113–4123. doi: 10.2147/CMAR.S172719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022;40(4_suppl):378. Feb 1. [Google Scholar]
- 82.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J. Immunol. 1998;160(3):1224–1232. Feb 1. [PubMed] [Google Scholar]
- 83.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014;20(6):607–615. doi: 10.1038/nm.3541. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roy S, Glaser S, Chakraborty S. Inflammation and progression of cholangiocarcinoma: role of angiogenic and lymphangiogenic mechanisms. Front. Med. 2019;6:293. doi: 10.3389/fmed.2019.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sawasdee N, Thepmalee C, Sujjitjoon J, Yongpitakwattana P, Junking M, Poungvarin N, et al. Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int. Immunopharmacol. 2020;78 doi: 10.1016/j.intimp.2019.106006. Jan. [DOI] [PubMed] [Google Scholar]
- 86.Wathikthinnakon M, Luangwattananun P, Sawasdee N, Chiawpanit C, Lee VS, Nimmanpipug P, et al. Combination gemcitabine and PD-L1xCD3 bispecific T cell engager (BiTE) enhances T lymphocyte cytotoxicity against cholangiocarcinoma cells. Sci. Rep. 2022;12(1):6154. doi: 10.1038/s41598-022-09964-6. Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diggs LP, Ruf B, Ma C, Heinrich B, Cui L, Zhang Q, et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J. Hepatol. 2021;74(5):1145–1154. doi: 10.1016/j.jhep.2020.11.037. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan YR, Wu CE, Chen MH, Huang WK, Shih HJ, Lan KL, et al. Comprehensive evaluation of immune-checkpoint DNA cancer vaccines in a rat cholangiocarcinoma model. Vaccines (Basel) 2020;8(4):E703. doi: 10.3390/vaccines8040703. Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J. Hepatol. 2020;73(1):170–185. doi: 10.1016/j.jhep.2020.03.007. Jul. [DOI] [PubMed] [Google Scholar]
- 90.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24(5):541–550. doi: 10.1038/s41591-018-0014-x. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. Oct 5Epub 2017 Sep 11. Erratum in: N Engl J Med. 2018 Nov 29;379(22):2185. PMID: 28889792; PMCID: PMC5706778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ. Escudier B—CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. Apr 5Epub 2018 Mar 21. PMID: 29562145; PMCID: PMC5972549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. KEYNOTE-024 investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. Nov 10Epub 2016 Oct 8. PMID: 27718847. [DOI] [PubMed] [Google Scholar]
- 94.Appleton E, Hassan J, Chan Wah Hak C, Sivamanoharan N, Wilkins A, Samson A, et al. Kickstarting immunity in cold tumors: localized tumor therapy combinations with immune checkpoint blockade. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.754436. Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J. Hepatol. 2014;61(6):1385–1396. doi: 10.1016/j.jhep.2014.08.010. Dec. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.