Abstract
Background
Knowing the level of active ingredients in an expired drug is a matter of concern irrespective of its final disposition. This is also a matter of national security and defense as it has important implications on the nation’s stockpile of prescription medications. Current literature has limited information about the strength of expired medications and any relevant trends.
Objective
To utilize high performance liquid chromatography (HPLC) to determine the strength of selected drugs for asthma and chronic obstructive pulmonary disease (COPD) as a class of therapeutic agents commonly used in free clinics.
Methods
Samples from expired lots of montelukast and albuterol pharmaceutical products were analyzed for their levels of their respective active ingredients. Two HPLC methods were developed, validated, and applied to achieve this goal. Quantitative analysis of each drug was performed using two different reversed phase C18 columns with a linear gradient of acetonitrile in 0.1% aqueous formic acid at a flow rate of 1 mL/min for both methods. Detection wavelength for montelukast and albuterol was 280 and 277 nm, respectively.
Results
Expiry dates of analyzed batches ranged from 2003 to 2019. Despite the extended time range beyond expiry dates, levels of both drugs were relatively consistent and exceeded 90% of the listed strength in most analyzed lots.
Conclusions
Our results introduce a new perspective towards reducing the financial burden resulting from disposal of expired medications with retained strength. They also offer supporting evidence to extend the use of out-of-date montelukast and albuterol preparations at home and in free clinics.
Keywords: Asthma, COPD, Albuterol, Montelukast, Expired medications, HPLC
Asthma; COPD; Albuterol; Montelukast; Expired medications; HPLC.
1. Introduction
Current good manufacturing practice (cGMP) for finished pharmaceuticals defines requirements for establishing stability data and expiry dates to ensure safety and efficacy of these products [1]. Expiration dates listed by manufacturers range from 1 to 5 years depending on nature and dosage form of pharmaceutical products [2]. Stability testing of drugs and biologics relies primarily on validated analytical procedures established per FDA and ICH guidelines [3]. Major analytical validation parameters include specificity, calibration curve linearity, accuracy, and precision. Acceptance range for pharmaceutical product strength is 90%–110% of listed dose [4]. As included in many USP-National Formulary monographs, high-performance liquid chromatography (HPLC) is a reliable analytical technique for strength and stability testing of pharmaceutical products [4].
Despite the obvious intent of setting expiration dates, one outcome is that nearly 800 million dollars of medications are wasted in the inpatient setting due to being out-of-date [5, 6]. Even more pharmaceuticals are wasted at long-term care facilities, pharmacies, and by consumers themselves [7, 8, 9]. Whether drug disposal is managed by drug take-back programs or individuals, the economic, environmental, and public health impact cannot be ignored [10, 11, 12]. While expiration dates are intended to protect the consumer, this term is indeed a misnomer. Often, the expiration date is interpreted as the date beyond which the medication is no longer effective. However, these dates instead refer to the longest period of time which the drug has shown potency and safety in pre-launch stability studies; i.e., the true “expiry” may be significantly longer than this [13, 14]. Thus, an obvious question to ask is: how long do drugs retain their potency after reaching their manufacturer-designated expiry dates?
Apart from a few recent reviews such as the one published by Zilker et al. [15], literature reports on the subject are scarce and the most significant ones are summarized here. Different medications that were expired 28–40 years and found in retail stores showed that 12 out of 14 active ingredients retained potency [16]. Out of a total of 40 epinephrine auto-injectors (EpiPen and EpiPen Jr) that were up to 50 months post-expiration, 24 (60%) were found to retain more than 90% of their listed strength [17]. In a similar study, 35 EpiPen injectors retained 84–95% of their listed potency up to 36-month post-expiration [18]. The British Antarctic Survey Medical Unit 5 analyzed expired drugs stored at simultaneously higher (tropics, 25–30 °C) and lower (Antarctic, -10 °C) temperatures and found they did indeed retain potency [19]. A study in outer space conducted at the International Space Station (NASA Human Research Program) found that 8 out of 9 medications met USP standards up to 5 months after their expiration date [20]. In the largest study of its kind, the US Military and FDA 2009 Shelf-Life Extension Program (SLEP) analyzed 122 medications as part of the national stockpile resulting in an average extension of potency up to 5 years based on analytical potency verification [2]. The performed potency extensions enabled the Department of Defense to significantly reduce the replacement costs by more than $100 for each $1 spent on analysis of a federally stockpiled critical medicine [2].
On a more individual level, most patients who visit free clinics cannot afford health insurance yet also do not qualify for state Medicaid. Accordingly, there exists additional financial challenge in providing medications to these patients. Donated medications and physician samples provide a partial solution to this problem. However, many of these donated medications may be close to or beyond their expiry dates. This presents a clinical dilemma for pharmacists, clinicians, and patients in terms of how, or even if, they should use these medications. Due to the significant prevalence of asthma and chronic obstructive pulmonary disease (COPD) in many communities, drugs to manage these conditions are among the most commonly used medications. Albuterol (also known as salbutamol), usually prescribed in oral inhaler format, is used in the management of asthma and COPD as well as acute asthma exacerbation. Albuterol is a short-acting beta-2 agonist which provides rapid bronchodilation. Accordingly, albuterol is a critical rescue medication and is included in the World Health Organization’s List of Essential Medicines [21]. Montelukast is a leukotriene receptor antagonist used for the prophylaxis and chronic treatment of asthma, chiefly as a smooth muscle relaxant and moderator of inflammation [22]. Both medications are therefore frequently prescribed for patients with asthma, and to some extent, COPD [23]. As part of our ongoing efforts to investigate out-of-date medication potency, we recently communicated a summary of our findings about medications containing albuterol and montelukast by using HPLC as a reliable method for pharmaceutical ingredient analysis [24]. In this report we provide a comprehensive background and full details of our approach to determine the post-expiration strength of expired albuterol and montelukast products. In doing so, the financial burden of disposal may be reduced and the use of these medications beyond expiration at free clinics could be justified.
2. Material and methods
2.1. Chemicals and samples
Standard albuterol and montelukast were purchased from Sigma-Aldrich (St. Louis, MO). Analytical solvents were purchased from Fisher Scientific (Waltham, MA). All analyzed batches were supplied by the Greater Milwaukee Free Clinic (GMFC, Milwaukee, WI) at the time of its closure in May 2019. These batches supplied to GMFC were donated by clinicians and individuals from the surrounding area throughout the course of its operation. During this time, samples were stored in the clinic at standard room conditions at all times with the exception of rare power outages affecting heating and cooling. All samples were sealed and unopened unless otherwise specifically stated in this manuscript.
2.2. Standard solutions
2.2.1. Albuterol standard stock solutions A & B
Two 100.0 mg aliquots of standard albuterol were transferred to separate 100-mL volumetric flasks labeled as AS-A and AS-B. Methanol was added to mark in each flask and ultrasonicated for 5 min before capping and storage at 4 °C. AS-A was used for generation of a calibration curve while AS-B was used for validation of accuracy and precision.
2.2.2. Montelukast standard stock solutions A & B
The above procedure was repeated with two 100.0 mg aliquots of montelukast to prepare MS-A and MS-B. These solutions were used for generation of a calibration curve and for validation of accuracy and precision, respectively
2.3. Analytical methods
An HPLC system (Prominence LC-2030, Shimadzu, Japan) with a quaternary pump, autosampler, and photodiode array (PDA) detector was used for all analytical procedures. The following gradient was applied at a flow rate of 1 mL/min for both drugs: 5% acetonitrile (solvent A) in 0.1% aqueous formic acid (solvent B) 1 min; 5–100% (A) 6 min; 100% (A) 1 min; 5% (A) 2 min. Column temperature was set at 25 °C and injection volume at 10 μL. For albuterol, a HyPurity® column was used (C18, 150 × 4.6 mm, 3μ, Thermo Scientific) and detection was achieved at 277 nm. For montelukast, a Kinetex® column was used (C18, 100 × 2.1 mm, 5μ, Phenomenex) and detection was achieved at 280 nm.
2.4. Method validation
The method developed for each drug was validated for selectivity, calibration curve linearity, accuracy, and precision following FDA/ICH guidelines for drugs and biologics [4, 25].
2.5. Selectivity
Many columns were tested to select the column with optimal retention time, peak shape, and specificity for each analyte. Peak purity was verified by UV spectral comparison at the beginning, middle and end of peak.
2.6. Calibration curve linearity
A multipoint calibration curve was generated for albuterol and montelukast (8 points for albuterol, 7 points for montelukast) by serially diluting AS-A and MS-A with methanol. Linearity of each curve was verified by determining the regression coefficient (R2) correlating nominal concentrations of calibration levels and their respective peak areas. The lowest concentration of each curve was considered as the limit of detection and quantitation (LOD & LOQ) for the respective drug.
2.7. Accuracy (trueness)
Three quality control levels were prepared from AS-B and MS-B by dilution with methanol. Each concentration was injected in triplicate to compare nominal values with experimental results which was reported as percent accuracy.
2.8. Precision
For each drug, intraday precision was determined by calculating the standard error (SE) obtained from triplicate injections at each concentration level of the three quality control samples used for determination of accuracy. Interday precision was similarly determined (as SE) from triplicate injections repeated for three days at each concentration level of the three quality control samples used for determination of accuracy.
2.9. Sample preparation and analysis
For tablet dosage forms, sample size was 30% of total tablets per lot number with a minimum of 3 and a maximum of 10 tablets. Based on total number of available tablets per lot number, 3–10 tablets were weighed to determine the average weight of one tablet. Weighed tablets were ground to a fine powder and the equivalent of one tablet was transferred to a 15-mL Falcon tube for analysis. The remaining powder was stored at 4 °C. Analysis samples were suspended in 3 mL of methanol and ultrasonicated for 5 min followed by centrifugation for 5 min. Supernatant was decanted in 10-mL volumetric flask. Extraction procedure was repeated two more times and all supernatants were collected in the same flask then brought to volume with methanol. Approximately 2 mL of the sample solution were passed through 0.45 μ filter into an HPLC vial before injection in triplicate on the column. Samples were diluted as needed to fit within the calibration range.
For granules and liquid dosage forms, 30% of samples (minimum 3 units/maximum 10 units) were diluted with deionized water to calibration range and analyzed in triplicates.
For inhaled albuterol, oral inhalers were primed by shaking and dispensing an initial puff of medication. A rubber balloon was affixed to the mouth of each inhaler and 4 puffs of medication were deposited into the balloon. The balloon was removed from the inhaler and 4 mL of 50% aqueous methanol was poured into the balloon. The balloon was then agitated by hand for 30 s and the solution was transferred to a Falcon tube. Samples were subsequently diluted and analyzed in the same fashion as liquid dosage forms.
3. Results
3.1. Method validation
Each of the developed methods was selective in detecting albuterol and montelukast at 3.7 and 5.2 min, respectively. Peaks of both drugs displayed full UV spectral overlap at the beginning, middle and end of each peak, which verified peak purity and lack of chromatographic interference.
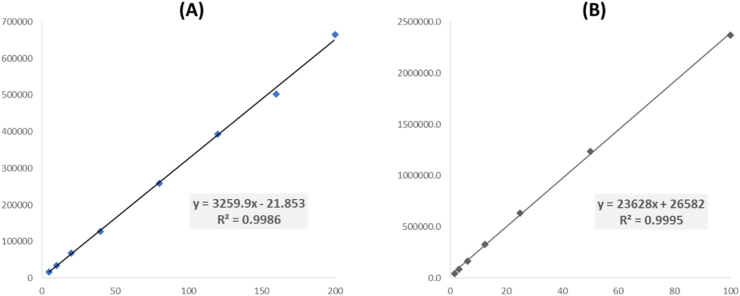
Calibration curves were generated for albuterol and montelukast by serial dilution of AS-A and MS-A, respectively. Both curves were linear across the calibration range (albuterol: 5.0–200.0 μg/mL, montelukast: 1.6–100.0 μg/mL) with regression coefficient (R2) above 0.99 for both curves (Figure 1). Signal-to-noise ratio (S/N) of the lowest concentration of each calibration standard was higher than 10. Thus, it was appropriate to define these concentrations as LOD and LOQ for albuterol (5.0 μg/mL) and montelukast (1.6 μg/mL).
Figure 1.
Standard calibration curves with line equations and regression coefficients (R2) of (A) albuterol and (B) montelukast.
Method accuracy and precision were validated by repeated analysis of quality control samples prepared at 3 concentrations of AS-B and MS-B. Accuracy was within ±5% of target concentrations of the quality control samples. Intraday and interday precision expressed as standard error were within ±5% of all quality control samples. Table 1 summarizes accuracy and precision data for both drugs.
Table 1.
Accuracy and precision validation of albuterol and montelukast standards.
| Albuterol QC (μg/mL) |
Montelukast QC (μg/mL) |
|||||
|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 23.5 | 47 | 94 | |
| Day 1 | 48.2 | 98.2 | 151.1 | 24.9 | 48.0 | 92.6 |
| 48.2 | 98.3 | 151.0 | 24.4 | 47.4 | 92.4 | |
| 48.1 | 98.2 | 151.0 | 23.6 | 47.9 | 91.6 | |
| Day 2 | 49.9 | 102.7 | 157.4 | 24.0 | 47.5 | 91.1 |
| 50.0 | 102.7 | 157.3 | 24.1 | 45.9 | 90.5 | |
| 49.9 | 102.5 | 157.2 | 23.9 | 46.0 | 90.3 | |
| Day 3 | 45.5 | 94.0 | 142.6 | 24.1 | 46.5 | 90.0 |
| 45.5 | 93.9 | 142.7 | 23.8 | 45.7 | 91.0 | |
| 45.6 | 93.8 | 142.6 | 23.3 | 45.4 | 88.1 | |
| Mean | 47.9 | 98.3 | 150.3 | 24.0 | 46.7 | 90.8 |
| SD | 1.9 | 3.8 | 6.4 | 0.5 | 1.0 | 1.4 |
| RSD (%) | 4.0 | 3.8 | 4.2 | 1.9 | 2.2 | 1.5 |
| Accuracy (%) | 95.8 | 98.3 | 100.2 | 102.2 | 99.4 | 96.6 |
3.2. Sample analysis
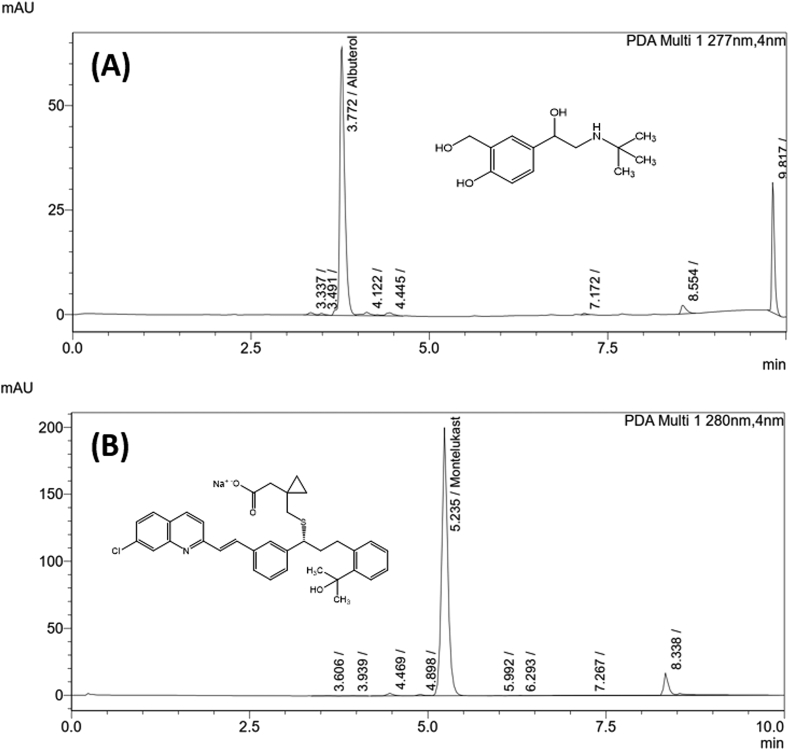
The analyzed samples included eleven out-of-date batches of albuterol nebulizer solutions, five batches of oral albuterol inhalers, and four batches of montelukast tablets and granules. An in-date sample was also analyzed for each drug product. No major degradation products were detected in any of the analyzed samples. Representative chromatograms are shown in Figure 2 for two analyzed products. Manufacturer expiration dates ranged from March 2001 to April 2019 for albuterol and from January 2003 to August 2009 for montelukast. As shown in Table 2, all montelukast samples showed drug levels above 90% of box label (mean 93.1%). Similar results were obtained for albuterol solutions except for two samples with drug levels at 80–90% of listed strength. However, variation was more significant in albuterol inhalers with product strength ranging from 73-103%.
Figure 2.
Chemical structures and representative chromatograms of out-of-date samples of (A) albuterol [Generic, Exp. Date: June 2003], and (B) montelukast [Singulair®, Exp. Date: Jan 2003].
Table 2.
HPLC-determined strength of out-of-date batches of albuterol and montelukast products relative to manufacturer-disclosed dose per unit.
| Product | Exp. Date | Manufacturer | Lot# | Dosage Form | Dose (mg/unit) | Strength (% of Dose) |
|---|---|---|---|---|---|---|
|
(A) Albuterol | ||||||
| Generic | March 2001 | Bausch & Lomb | 166071 | Solution | 5 | 88.4 ± 0.1 |
| Generic | August 2001 | Bausch & Lomb | 215981 | Solution | 5 | 92.0 ± 0.1 |
| Generic | June 2003 | Bausch & Lomb | 435071 | Solution | 5 | 92.9 ± 0.1 |
| Generic | June 2007 | Bausch & Lomb | 915631 | Solution | 5 | 85.3 ± 0.0 |
| Generic | May 2009 | Nephron | A6211C | Solution | 0.83 | 101.0 ± 0.0 |
| Generic | December 2013 | Watson | 1T06 | Solution | 0.83 | 97.6 ± 0.0 |
| Generic | October 2015 | Ritedose | 3P94 | Solution | 0.83 | 105.6 ± 0.0 |
| Generic | April 2016 | Mylan | 4D09 | Solution | 0.83 | 97.4 ± 0.0 |
| Generic | January 2019 | Nephron | 721211 | Solution | 0.83 | 97.6 ± 0.1 |
| Generic | March 2019 | Ritedose | 7CB5 | Solution | 0.83 | 101.7 ± 0.0 |
| Generic∗ | June 2021 | Nephron | 921481 | Solution | 0.83 | 96.7 ± 0.1 |
| Ventolin® | June 2009 | GSK | 8ZP8118 | Inhaler | 0.09 | 102.5 ± 0.0 |
| Proventil® | February 2012 | Schering-Plough | 100443 | Inhaler | 0.09 | 77.8 ± 0.1 |
| Proventil® | December 2012 | Schering-Plough | 110293 | Inhaler | 0.09 | 72.9 ± 0.0 |
| Proventil® | May 2015 | Schering-Plough | 130401 | Inhaler | 0.09 | 82.7 ± 0.0 |
| Ventolin® | December 2017 | GSK | 7L8E | Inhaler | 0.09 | 97.5 ± 0.0 |
| Ventolin®∗ |
January 2021 |
GSK |
8W9R |
Inhaler |
0.09 |
87.2 ± 0.1 |
|
(B) Montelukast | ||||||
| Singulair® | January 2003 | Merck | L5267 | Tablet | 10 | 97.2 ± 0.6 |
| Singulair® | April 2003 | Merck | M3448 | Tablet | 5 | 96.0 ± 0.2 |
| Singulair® | June 2003 | Merck | M3453 | Tablet | 10 | 95.6 ± 0.5 |
| Singulair® | August 2009 | Merck | F8279 | Granule | 4 | 94.3 ± 0.7 |
| Generic∗ | September 2019 | Torrent | BU65D024 | Tablet | 10 | 93.1 ± 1.0 |
In-date at time of analysis.
4. Discussion
The pharmaceutical industry implements FDA guidelines to establish drug stability and provide expiration dates disclosed on product packaging. Disclosed expiration dates state that the drug is guaranteed to retain its listed strength if stored as recommended and as long as it is used before its expiration date. However, the expiration date of a drug does not imply that the product loses its strength or efficacy once the expiration date is exceeded. Thus, it is hard to judge the strength of a pharmaceutical product within any time period post-‘expiration’. Moreover, out-of-date drugs are often retained for personal use or donated for use at free clinics for the uninsured. Healthcare professionals at these aforementioned sites are often faced with the dilemma of whether or not to use out-of-date medications, particularly when they are the only option available to provide to their patients. Based on these facts, it is imperative to provide physicians with data to guide their decisions to utilize or avoid out-of-date medications for specific disease conditions.
Due to availability of batches of many expired medications at free clinics in the Wisconsin Association of Free and Charitable Clinics, including the Greater Milwaukee Free Clinic, our approach was to categorize these medications based on indication and frequency of use. Samples of these high priority medications where then analyzed to determine levels of their active ingredients using HPLC methods. Additionally, we developed novel drug recovery and analytical HPLC methods in our lab for this study. The first disease conditions selected were asthma and chronic obstructive pulmonary disease (COPD) and the drugs selected for our study were albuterol and montelukast in different dosage forms. Two HPLC methods were developed and validated for selectivity, accuracy, and precision before being utilized to analyze the out-of-date samples. These methods also achieved high levels of sensitivity with LOQs of 5.0 and 1.56 ug/mL for albuterol and montelukast, respectively. Validation data assured method reliability to perform all necessary analyses and generate accurate values for drug strength.
The overall results of our study indicate that nearly all albuterol products retained more than 90% of their listed strength for more than 15 years post-expiration. The only exceptions were two lots (166071 and 915631) whose albuterol levels were at 85–88% of the listed concentration and several inhalers. These multiple use bottles of albuterol were unsealed and had already been used which likely had an impact on product stability as compared to newer ones packaged in single-use vials. This observation is supported by the relatively lower level of albuterol in the other two lots of multiple-use bottles (215981 and 435071) compared to all single-use vials. With regard to oral inhaler albuterol samples, we note a novel method to collect and measure albuterol concentrations from those delivery devices. It should be noted that this method is not a direct sampling of the solution within the pressurized cartridge and instead relies on assumptions that the volume of solution expelled in each puff is precise and that the method collects 100% of the expelled albuterol solution. Given the design of these inhaler devices it is possible, and in fact likely, that some albuterol solution is lost and therefore not captured. When accounting for this by normalizing all GSK-manufactured albuterol inhalers to the in-date sample, all expired lots had greater than 100% of expected levels (Table 3). Lots manufactured by Schering-Plough utilize a slightly different physical design which may account for their lower measured concentrations. Although normalization to a GSK-manufactured in-date sample is not entirely appropriate, doing so gives nearly 90% of expected levels for 66% of samples.
Table 3.
HPLC-determined strength of out-of-date batches of albuterol inhalers relative to in-date inhaler strength (0.09 mg/unit).
| Product | Exp. Date | Manufacturer | Lot# | Relative Strength (% of Dose) |
|---|---|---|---|---|
| Ventolin® | June 2009 | GSK | 8ZP8118 | 117.5% |
| Proventil® | February 2012 | Schering-Plough | 100443 | 89.2% |
| Proventil® | December 2012 | Schering-Plough | 110293 | 83.6% |
| Proventil® | May 2015 | Schering-Plough | 130401 | 94.9% |
| Ventolin® | December 2017 | GSK | 7L8E | 111.8% |
| Ventolin®∗ | January 2021 | GSK | 8W9R | 100% |
In-date at time of analysis.
Similarly, all analyzed montelukast products retained more than 90% of their listed strength for more than 15 years. Compared to albuterol, the analyzed montelukast products were formulated as solid dosage forms (tablets and granules). One in-date sample of each drug was also analyzed and their respective drug concentrations were above 90% of label value.
Using albuterol and montelukast as representative drugs for managing pulmonary conditions, our findings support the potential of utilizing out-of-date medications by healthcare professionals at free clinics. Provided storage conditions are acceptable, the length of time after listed expiration date may extend beyond 10 years for the aforementioned drug formulations. In fact, given that the analyzed samples were stored in less-than-ideal conditions for portions of their life, it is reasonable to consider that the stability data shown here represents an understatement of these drugs' true stability. The implications of these data are vast and affect the entire spectrum of clinical care including the medicine cabinet at home, the clinic, readiness of national security, and international medication availability. The financial burden of discarding expired medication may thus be reduced while health benefits for needy patients may still be achieved. Furthermore, these data provide patients and physicians with increased peace of mind. Albuterol in particular is a rescue medication, i.e., it is used as a first-line agent in the treatment of life-threatening asthma exacerbations. Accordingly, asthmatics rely heavily on albuterol in emergent situations. For this reason, asthmatics carry multiple inhalers of albuterol in multiple locations; the cost to replace all of these every 18 months can be restrictive for some patients. Studies such as ours add credence to the notion that medications retain their potency beyond their listed expiry dates. Informed utilization of these medications in free clinics may thus be warranted especially if incorporated into a database of similar information. From a national defense perspective, increasing the shelf life of medications in the national stockpile improves national readiness and response. Finally, the World Health Organization has very clear rules about drug donation across international borders. Indeed, medications can only be transported to countries in need if they will not expire within the next 6 months [26]. Our data therefore suggest that expired medications may provide relief to countries affected by war, strife, and natural disasters. As more studies are conducted on drug potency after expiration, it is possible to envision a “whitelist” of medications that could be acceptable for export beyond their listed expiration date.
5. Conclusions
Although earlier investigations of other pulmonary medications exist [17, 18, 27], ours is the first to demonstrate the retained strength of albuterol or montelukast products. Here, we introduce novel, highly accurate, and highly sensitive methods to measure concentrations of albuterol and montelukast using HPLC. Further, we introduce a novel method to collect and measure concentrations of albuterol in pre-packaged oral inhaler devices. Using these methods we show that both montelukast and albuterol retain their potency for many years beyond their listed “expiry” dates. These data may serve to guide pharmacists, physicians, and patients in the decision-making process of using “expired” medications at home, in free clinics, in national defense, and in international humanitarian efforts.
Declarations
Author contribution statement
Raman G. Kutty: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mackenzie Bevry: Performed the experiments; Analyzed and interpreted the data.
Paul Hoffmann: Contributed reagents, materials, analysis tools or data.
Ehab A. Abourashed: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Dr. George Schneider and Mrs. Kathy Schneider for their invaluable donation of all samples received from the Greater Milwaukee Free Clinic. We also thank Dr. Holly Schaack for her earlier contribution to the project and for preparing an inventory of all expired medications received from GMFC.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Sengupta P., Chatterjee B., Tekade R.K. Current regulatory requirements and practical approaches for stability analysis of pharmaceutical products: a comprehensive review. Int. J. Pharm. 2018;543(1-2):328–344. doi: 10.1016/j.ijpharm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Courtney B., et al. Maximizing state and local medical countermeasure stockpile investments through the Shelf-Life Extension Program. Biosecur. Bioterrorism. 2009;7(1):101–107. doi: 10.1089/bsp.2009.0011. [DOI] [PubMed] [Google Scholar]
- 3.Q1A(R2) Food and Drug Administration; Rockville, MD: 2003. Guidance for Industry: Q1A (R2) Stability Testing of New Drug Substances and Products. I.C.f.H. (ICH) [Google Scholar]
- 4.USP23-NF18 . 23 ed. United States Pharmacopeial Convention; 1994. The United States Pharmacopeia, USP 23. The national formulary, NF 18. [Google Scholar]
- 5.Vogler S., de Rooij R. Medication wasted – contents and costs of medicines ending up in household garbage. Res. Soc. Adm. Pharm. 2018;14(12):1140–1146. doi: 10.1016/j.sapharm.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Nelson R. The excessive waste of prescription drugs. Am. J. Nurs. 2015;115(6):19–20. doi: 10.1097/01.NAJ.0000466308.08390.7b. [DOI] [PubMed] [Google Scholar]
- 7.Alnahas F., et al. Expired medication: societal, regulatory and ethical aspects of a wasted opportunity. Int. J. Environ. Res. Publ. Health. 2020;17(3) doi: 10.3390/ijerph17030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeng D.D., Tom L.A., Wright E.A. Patient characteristics and healthcare utilization patterns associated with unused medications among medicare patients. Res. Soc. Adm. Pharm. 2017;13(6):1090–1094. doi: 10.1016/j.sapharm.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Tong A.Y., Peake B.M., Braund R. Disposal practices for unused medications around the world. Environ. Int. 2011;37(1):292–298. doi: 10.1016/j.envint.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Tchen J., Vaillancourt R., Pouliot A. Wasted medications, wasted resource. Can. Pharm. J. (Ott) 2013;146(4):181–182. doi: 10.1177/1715163513490654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray J., et al. Prescription disposal practices: a 2-year ecological study of drug drop box donations in appalachia. Am. J. Publ. Health. 2015;105(9):e89–94. doi: 10.2105/AJPH.2015.302689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bound J.P., Voulvoulis N. Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environ. Health Perspect. 2005;113(12):1705–1711. doi: 10.1289/ehp.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.21CFR211 . 2020. Code of Federal Regulations Title 21.https://www.ecfr.gov/cgi-bin/searchECFR?ob=r&idno=21&q1=&r=&SID=69737dcba57bb5c1d107db7ec4f7bc99&mc=true [cited 2021 May 25]; Available from: [Google Scholar]
- 14.EMEA . 2003. Guideline on Stability Testing: Stability Testing of Existing Active Substances and Related Finished Products.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-stability-testing-stability-testing-existing-active-substances-related-finished-products_en.pdf [cited 2021 May 25]; Available from: [Google Scholar]
- 15.Zilker M., Sorgel F., Holzgrabe U. A systematic review of the stability of finished pharmaceutical products and drug substances beyond their labeled expiry dates. J. Pharm. Biomed. Anal. 2019;166:222–235. doi: 10.1016/j.jpba.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Cantrell L., et al. Stability of active ingredients in long-expired prescription medications. Arch. Intern. Med. 2012;172(21):1685–1687. doi: 10.1001/archinternmed.2012.4501. [DOI] [PubMed] [Google Scholar]
- 17.Cantrell F.L., et al. Epinephrine concentrations in EpiPens after the expiration date. Ann. Intern. Med. 2017;166(12):918–919. doi: 10.7326/L16-0612. [DOI] [PubMed] [Google Scholar]
- 18.Rachid O., et al. Epinephrine doses contained in outdated epinephrine auto-injectors collected in a Florida allergy practice. Ann. Allergy Asthma Immunol. 2015;114(4):354–356 e1. doi: 10.1016/j.anai.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Browne E., et al. Expired drugs in the remote environment. Wilderness Environ. Med. 2019;30(1):28–34. doi: 10.1016/j.wem.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Wotring V.E. Chemical potency and degradation products of medications stored over 550 earth days at the international space station. AAPS J. 2016;18(1):210–216. doi: 10.1208/s12248-015-9834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth L., et al. Monographs for medicines on WHO's model list of essential medicines. Bull. World Health Organ. 2018;96(6):378–385. doi: 10.2471/BLT.17.205807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paggiaro P., Bacci E. Montelukast in asthma: a review of its efficacy and place in therapy. Ther. Adv. Chronic Dis. 2011;2(1):47–58. doi: 10.1177/2040622310383343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes, P.J., Pulmonary pharmacology. In: Brunton LL, Hilal-Dandan R, Knollmann BC. eds. Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 13 ed. McGraw-Hill; Accessed July 07, 2021. https://accesspharmacy.mhmedical.com/content.aspx?bookid=2189§ionid=172481165. (Accessed on 8/10/2022)
- 24.Abourashed E.A., et al. Retained strength of expired albuterol and montelukast pharmaceuticals. Ann. Allergy Asthma Immunol. 2021;128(2):222–223. doi: 10.1016/j.anai.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Analytical Procedures and Methods Validation for Drugs and Biologics-Guidance for Industry. July 2015. https://www.fda.gov/files/drugs/published/Analytical-Procedures-and-Methods-Validation-for-Drugs-and-Biologics.pdf [cited 2022 05/12/2022]; Available from: [Google Scholar]
- 26.Organization W.H. World Health Organization; 2011. Guidelines for Medicine Donations 2010. [Google Scholar]
- 27.Cantrell F.L. Epinephrine concentrations in EpiPens after the expiration date. Ann. Intern. Med. 2018;168(1):82. doi: 10.7326/L17-0496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.