Abstract
Objective
Nutrition therapy is a cornerstone of care for people with type 2 diabetes, yet starting new, healthy eating behaviors and sustaining them can be challenging. This decentralized, single-arm study assessed the impact of 28 days of home-delivered, pre-portioned meals (three meals per day) on continuous glucose monitoring (CGM)-derived glycemic control and quality of life.
Research design and methods
We enrolled 154 people with type 2 diabetes from across the United States. All participants were enrolled in a digital-first type 2 diabetes care center of excellence and had a time in range (TIR) <70% or a glucose management index (GMI) >7%. A total of 102 participants received another set of meals for a household member. Forty-four participants were excluded from CGM-based analysis because of sparse data in the baseline or intervention period.
Results
From the baseline through the intervention period, average TIR improved by 6.8% (95% CI 4.0–9.7, P <0.001), average GMI improved by 0.21% (95% CI 0.11–0.32, P <0.001), and participants’ odds of achieving ≥70% TIR increased (odds ratio 2.55 [95% CI 0.93–7.80, P = 0.051]). Although average TIR increased rapidly upon initiation of meal delivery, it regressed when the delivery period ended.
Conclusion
Home-delivered meals were associated with modest TIR and GMI improvements, but only in the short term. More research is needed to determine whether the effects of nutrition therapy can be extended by providing ongoing meal delivery or additional support such as behavioral intervention.
Nutrition therapy is a cornerstone of care for people with type 2 diabetes (1). Although it is important to personalize meal plans, there are common features across diabetes-friendly plans, including a preference for nonstarchy over starchy vegetables, minimized added sugars and refined grains, the choice of whole foods over highly processed foods, and a reduction in overall carbohydrate intake (2). Evidence supports a broad spectrum of nutritional strategies ranging from modest to intensive changes to successfully reduce glucose for people with type 2 diabetes (3) or even induce diabetes remission (4,5). Despite this variety of effective options, sustaining diabetes self-management strategies in the long term can be challenging (6).
Meal delivery is one promising strategy to reduce the friction of implementing and maintaining new eating behaviors for people with type 2 diabetes. Meal delivery involves delivering pre-portioned meals or ready-to-cook ingredients directly to people’s homes. Meal delivery companies usually focus on direct-to-consumer markets and often emphasize the convenience to the consumer rather than the ability to affect clinical outcomes. Although meal delivery is a growing commercial market, the application of this idea itself is not new; supporting people through home-delivered meals has existed in the United States since the 1950s, when the program now known as Meals on Wheels originated (7). In 2017, the Centers for Medicare & Medicaid Services included meal delivery on a limited basis as an allowed supplemental benefit for Medicare Advantage plans, and, starting in 2020, Medicare Advantage plans have been able to offer meals beyond a limited basis to members with one or more chronic conditions as so-called special supplemental benefits for the chronically ill. Several third-party payers now cover limited durations of home-delivered meals for select Medicare Advantage enrollees who meet facility discharge criteria (hospital or skilled nursing facility) or who have multiple chronic conditions (e.g., heart failure and end-stage renal disease) (8,9). Home-delivered meals are not the only unique care model for improving access to healthy foods; others include food pharmacies (10) and fresh vegetable prescriptions (11).
To date, research of meal delivery has generally been heterogenous in population, duration, and outcome, focusing predominantly on providing meals to vulnerable groups such as older adults (12,13), people experiencing food insecurity (14), those at risk for hospital readmissions or procedure complications (15,16), and those with multiple chronic conditions or complex comorbidities (17,18). Few research studies have explored meal delivery as a strategy for implementing healthy eating habits to improve outcomes for people with type 2 diabetes. We designed our study to better understand this potential by providing 28 days of home-delivered meals to people with type 2 diabetes, hypothesizing that provision of portion-controlled meals would reduce calorie and carbohydrate intake from baseline, leading to improved diabetes outcomes. Because food habits are often intertwined with cultural and social behaviors, we also gave participants the option of including a household member as a meal recipient as a strategy to increase engagement and adherence. To our knowledge, this is the first study assessing the impact of short-term meal delivery on quality of life and continuous glucose monitoring (CGM)-derived time in range (TIR) outcomes among people with type 2 diabetes.
Research Design and Methods
Study Design and Population
This was a decentralized, single-arm, prospective study of 4 weeks’ duration, ranging from June to September 2020. Participants were recruited from a population of people with type 2 diabetes from across the United States who were already enrolled in Level2, a digital-first type 2 diabetes care center of excellence (19). Level2 is an optional program offered to people with type 2 diabetes who are ≥18 years of age, meet certain clinical eligibility requirements, and have an insurance plan participating in Level2. Participants have the opportunity to wear a CGM sensor and/or fitness tracker, engage virtually with coaches and/or clinicians, and receive personalized activity recommendations to help manage diabetes.
Inclusion criteria for study participants included: currently wearing a CGM sensor, having at least 7 days of CGM data available during the eligibility period, and having an eligibility-period TIR <70% or a glucose management indicator (GMI) >7%. The study team contacted those eligible and offered them 4 weeks of home-delivered meals at no cost and an additional 4 weeks of meals for a household member. The study team screened interested participants and excluded those who self-reported any of the following: household size of more than two people, pregnancy, disinterest in wearing a CGM sensor for 4 weeks more, inability to receive meals at the same address for 4 weeks, or lack of access to a microwave or conventional oven to heat the meals.
As part of the enrollment call, all participants completed a brief telephone survey to assess their self-reported quality of life and diabetes self-care habits. Upon conclusion of the 4 weeks of meal delivery, participants completed (by phone or e-mail) a post-study survey that contained a psychosocial assessment, including the quality-of-life questions asked at enrollment, as well as measured opinions of the meal delivery program and a question on average weekly meal adherence.
This study was reviewed and approved by the UnitedHealth Group (UHG) Office of Human Research Affairs (OHRA) as minimal risk (UHG OHRA certificate of action #2020-0049). Subsequent review and approval for the publication of de-identified data were granted (UHG OHRA certificate of action #2020-0049-06). Per the protocol, anyone on short- or rapid-acting insulin was to be excluded from outreach; however, 25 people were ultimately enrolled despite an insurance claim for a prescription fill of a rapid- or short-acting insulin in the first 6 months of 2020 (UHG OHRA issues report #2020-0049). This deviation was reviewed by the UHG OHRA, which determined that no participants were placed at increased risk, and their data were subsequently included in the final analysis.
Intervention
Study participants received 21 home-delivered meals per week for 4 weeks, along with an additional 21 meals per week for those living with a household member who opted into the meals. The 4-week duration was selected as a minimum reasonable window to detect a clinically significant change in glucose and quality-of-life measures. The quantity was selected to account for participants’ total food intake during the 4 weeks; although participants were encouraged to exclusively eat the food provided in the study, full adherence was not a study requirement. The commercially available meals were packaged, prepared, refrigerated, and delivered by a meal-delivery vendor (Mom’s Meals, PurFoods, LLC, Ankeny, IA). Participants were able to select the meals they wanted from the vendor’s diabetes-friendly menu, which had 17 meal options averaging <500 calories per meal (<1,500 calories/day) and <75 g carbohydrate per meal when including the provided snacks. Notably, this calorie level represents a calorie restriction from most people’s typical daily intake and can reduce weight.
Composition of the meals was predominantly medium– to high–glycemic-index ingredients and represented an “American comfort food” style. The average macronutrient balance per meal was 68 g carbohydrate, 20 g protein, and 15 g fat. Example meals include homestyle meatloaf with herb pasta, mixed vegetables, and whole-wheat bread (555 calories, 67 g carbohydrate, 9 g fiber, 16 g sugar, 27 g protein, and 20 g fat) and beef taco filling with cheese, rice, and corn tortillas (447 calories, 65 g carbohydrate, 6 g fiber, 6 g sugar, 21 g protein, and 13 g fat). The vendor’s current seasonal menu describing the types of diabetes-friendly meals available for selection is publicly available online (20). Meals could be heated in a microwave or conventional oven and often contained a snack (e.g., bread, gelatin, or string cheese) and sometimes fruit juice. Participants wore CGM sensors (Dexcom G6, Dexcom, Inc., San Diego, CA), which take glucose readings every 5 minutes; these were worn throughout the 28-day meal-delivery period as part of ongoing participation in the Level2 program.
Outcomes
The primary outcomes specified in the protocol were change in patient-reported measures of quality of life, which came from pre- and post-study participant surveys. However, the primary concern of this analysis was change in the CGM-derived TIR metrics, a prespecified exploratory end point for which the study was adequately powered. Per the protocol, the study needed 73 participants to detect a five-point change in TIR with 80% power and a two-sided significance level of 0.05. Because a 10% change in TIR has been associated with a 0.8% change in A1C, a 5% TIR change was selected to serve as a clinically meaningful magnitude of change that may be reasonably obtained in 28 days (21). The study was not powered or designed to measure changes in medication regimen during the intervention.
CGM metrics of interest included:
TIR, the percentage of observed CGM readings between 70 and 180 mg/dL
GMI, an estimate of A1C as calculated from CGM data
Time above range (TAR)-1, the percentage of observed CGM readings >180 mg/dL
TAR2, the percentage of observed CGM readings >250 mg/dL
Time below range (TBR)-1, the percentage of observed CGM readings <70 mg/dL
TBR2, the percentage of observed CGM readings <54 mg/dL
Glycemic variability (GV), the coefficient of variation of observed CGM readings
Percentage of participants achieving and maintaining a TIR ≥70% or a GMI ≤7% (22).
The study team defined three periods of interest: eligibility, baseline, and intervention. The observed GMI and TIR from the eligibility period (26 May through 25 June 2021) determined whether a person was eligible for the study. The study’s baseline period extended from 26 June until the first meal delivery arrived at a participant’s home. The intervention period was the 28-day period after the initial meal delivery. The primary outcomes of interest were the changes from the baseline period through the intervention period for each of the CGM metrics listed above. Per the inclusion criteria specified a priori in the research protocol, a participant’s CGM data were only included in the final analysis if they had at least 7 days of CGM data in the baseline period and at least 20 days of CGM data in the intervention period (23). The analysis consisted of two-sample paired t tests and Fisher exact tests to measure the impact of the meal delivery program by comparing the baseline and intervention periods.
Synthetic Control
Given the backdrop of the coronavirus disease 2019 (COVID-19) pandemic, the study team created a post-hoc synthetic control group from the original eligibility list of 357 Level2 members who had declined to participate (2%), were not reached (44%), or were ineligible at screening (10%). The purpose of this synthetic control group was to adjust for any pandemic-related confounders that may have affected interpretation of any change from baseline TIR. Because the control group did not have an index date (i.e., first day of delivered meals), each control group member was matched to a study participant based on eligibility period TIR to define the three study periods; once matched, control group members were assigned the same index date as their matched counterpart.
Results
On the date that eligibility for the study was determined, 4,511 Level2 members were screened for eligibility based on CGM metrics; 357 members met the initial inclusion criteria. A total of 154 participants enrolled in the meal delivery study (77.8% of all successfully contacted people). The remaining 203 individuals comprised the synthetic control group. Enrollment flow is depicted in Figure 1.
Figure 1.
Enrollment flow diagram.
Table 1 summarizes study participants and the synthetic control group based on whether they had sufficient data to be included in the CGM analyses. Compared with members of the synthetic control group, a higher percentage of intervention participants (i.e., those receiving meal delivery) had sufficient CGM data. Although the synthetic control group was not randomized or matched to the study participants on anything other than eligibility period TIR, the two groups had similar demographic profiles, as seen in Table 1. This table also provides the percentage of intervention participants who reported eating an average of 4 or more nonadherent meals per week (∼16%), recorded at the end of the meal delivery period.
Table 1.
Characteristics of the Study Population by Group
| Meal Delivery Group | Synthetic Control Group | |||
|---|---|---|---|---|
| Total (n = 154) | With Sufficient CGM Data* (n = 110) | Total (n = 203) | With Sufficient CGM Data* (n = 100) | |
| Sex† Female Male |
83 (53.9) 71 (46.1) |
57 (51.8) 53 (48.2) |
90 (44.3) 111 (54.7) |
47 (47.0) 52 (52.0) |
| Age range, years 18–35 36–45 46–55 56–65 ≥66 |
7 (4.5) 15 (9.7) 46 (29.9) 77 (50.0) 9 (5.8) |
3 (2.7) 10 (9.1) 31 (28.2) 60 (54.5) 6 (5.5) |
4 (2.0) 20 (9.9) 76 (37.4) 92 (45.3) 11 (5.4) |
2 (2.0) 9 (9.0) 37 (37.0) 47 (47.0) 5 (5.0) |
| Census region† South Midwest Other‡ |
73 (47.4) 53 (34.4) 28 (18.1) |
50 (45.5) 39 (35.5) 21 (19.0) |
101 (49.8) 72 (35.5) 28 (13.8) |
50 (50.0) 34 (34.0) 15 (15.0) |
| Diabetes drugs by class Biguanide Sulfonylurea SGLT-2 inhibitor GLP-1 receptor agonist DPP-4 inhibitor Long- or intermediate-acting insulin Short- or rapid-acting insulin Any insulin or secretagogues§ |
89 (57.8) 32 (20.8) 32 (20.8) 43 (27.9) 15 (9.7) 43 (27.9) 25 (16.2) 66 (44.2) |
65 (59.1) 20 (18.2) 22 (20.0) 28 (25.5) 12 (10.9) 33 (30.0) 22 (20.0) 50 (45.4) |
113 (55.7) 56 (27.6) 54 (26.6) 50 (24.6) 23 (11.3) 55 (27.1) 28 (13.8) 100 (49.3) |
56 (56.0) 22 (22.0) 30 (30.0) 27 (27.0) 14 (14.0) 31 (31.0) 20 (20.0) 51 (51.0) |
| Diabetes drug classes, n 0 1 2 3 ≥4 |
39 (25.3) 26 (16.9) 32 (20.8) 38 (24.7) 19 (12.3) |
26 (23.6) 21 (19.1) 21 (19.1) 28 (25.5) 14 (12.7) |
51 (25.1) 27 (13.3) 44 (21.7) 50 (24.6) 31 (15.3) |
25 (25.0) 8 (8.0) 21 (21.0) 28 (28.0) 18 (18.0) |
| Household participation | 102 (66.2) | 71 (64.5) | — | — |
| Study completionǁ | 135 (87.7) | 101 (91.8) | — | — |
| Study adherence <4 Nonadherent meals/week ≥4 Nonadherent meals/week Missing |
68 (44.2) 25 (16.2) 61 (39.6) |
47 (42.7) 17 (15.5) 46 (41.8) |
— — — |
— — — |
| Age, years | 55.1 ± 9.1 | 55.9 ± 8.6 | 54.8 ± 7.7 | 55.2 ± 7.5 |
| TIR, % Eligibility period Baseline period |
53.7 ± 23.1 53.6 ± 24.1 |
56.6 ± 21.7 56.1 ± 22.7 |
54.5 ± 23.3 58.9 ± 22.9 |
58.9 ± 20.3 61.5 ± 20.4 |
| GMI, % Eligibility period Baseline period |
7.77 ± 0.92 7.77 ± 0.96 |
7.64 ± 0.77 7.67 ± 0.84 |
7.70 ± 0.76 7.57 ± 0.76 |
7.58 ± 0.72 7.49 ± 0.68 |
Data are n (%) or mean ± SD.
At least 7 days of CGM data in the baseline period and at least 20 days of CGM data in the intervention period.
The Synthetic Control Group gender and region columns do not sum to 100% due to unknown gender and region for two individuals in the Total and one individual in the Sufficient CGM Data group.
Other included West and Northeast and was reported in aggregate to abide by Centers for Medicare and Medicaid Services cell suppression policies for values <11.
Defined as any sulfonylurea or long-, intermediate-, short-, or rapid-acting insulin.
Defined as having all 4 weeks of meals successfully delivered. As self-reported by participants. Threshold of four meals per week was prespecified. DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon like peptide 1; SGLT-2, sodium–glucose cotransporter 2.
Table 2 shows the changes in CGM metrics from the baseline through the intervention period for the intervention and control groups, along with the difference in differences between the two groups. The P value for the treatment group reflects the unadjusted pre-study to post-study analysis, whereas the difference in differences P is adjusted based on the synthetic control group to account for population changes over time. Compared with their baseline data, the intervention group saw significant improvements in TIR (56.1 vs. 62.9%, P <0.001), TAR (43.5 vs. 36.7%, P <0.001), and GMI (7.67 vs. 7.45%, P <0.001). This improvement remained true after adjusting to account for the synthetic control group (control group baseline and intervention averages with differences in differences P value: TIR [61.5 vs. 61.9%, P = 0.003], TAR [38.2 vs. 37.9%, P = 0.004], GMI [7.49 vs. 7.46%, P = 0.025]). Of participants whose TIR was <70% in the baseline period, more were able to achieve a TIR ≥70% during the intervention period compared with the control group (26.0 vs. 12.1%, P = 0.051). Of those who already had TIR ≥70% at baseline, more were able to maintain a TIR ≥70% during the intervention period compared with the control group (94.6 vs. 66.7%, P = 0.002).
Table 2.
Summary of Change in CGM Metrics (Unadjusted and Adjusted) and Responses to Pre- and Post-Survey Quality-of-Life Questions (23)
| Metric | Change in Continuous Variables | ||||||
|---|---|---|---|---|---|---|---|
| Treatment Group | Control Group | Difference in Differences | |||||
| Mean ± SD | 95% CI | P | Mean ± SD | 95% CI | Mean (95% CI) | P | |
| TIR | 6.8 ± 15.0 | 4.0–9.7 | <0.001 | 0.3 ± 16.5 | −2.9 to 3.6 | 6.5 (2.2–10.8) | 0.003 |
| GMI | −0.21 ± 0.56 | −0.32 to −0.11 | <0.001 | −0.03 ± 0.61 | −0.15 to 0.09 | −0.18 (−0.34 to −0.02) | 0.025 |
| TAR1 | −6.8 ± 15.0 | −9.6 to −3.9 | <0.001 | −0.3 ± 16.7 | −3.6 to 3.0 | −6.4 (−10.8 to −2.1) | 0.004 |
| TAR2 | −2.8 ± 11.1 | −4.9 to −0.7 | 0.009 | −0.2 ± 11.6 | −2.5 to 2.1 | −2.6 (−5.7 to 0.5) | 0.100 |
| TBR1 | −0.1 ± 0.7 | −0.2 to 0.1 | 0.335 | 0.0 ± 0.6 | −0.1 to 0.1 | −0.1 (−0.2 to 0.1) | 0.495 |
| TBR2 | −0.1 ± 0.4 | − 0.1 to 0.0 | 0.070 | 0.0 ± 0.3 | −0.1 to 0.0 | 0.0 (−0.1 to 0.1) | 0.548 |
| GV | 0.00 ± 0.03 | − 0.01 to 0.00 | 0.174 | 0.00 ± 0.03 | −0.01 to 0.00 | 0.00 (−0.01 to 0.01) | 0.861 |
| Change in Categorical Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Improved From Baseline Through Intervention | Maintained From Baseline Through Intervention | |||||||
| Treatment, n/N (%) | Control, n/N (%) | Odds Ratio (95% CI) | P | Treatment n/N (%) | Control n/N (%) | Odds Ratio (95% CI) | P | |
| TIR ≥70% | 19/73 (26.0) | 7/58 (12.1) | 2.55 (0.93–7.80) | 0.051 | 35/37 (94.6) | 28/42 (66.7) | 8.54 (1.74–83.8) | 0.002 |
| GMI ≤7% | 20/90 (22.2) | 10/78 (12.8) | 1.94 (0.80–4.99) | 0.157 | 16/20 (80.0) | 15/22 (68.2) | 1.84 (0.37–10.41) | 0.491 |
| Quality of Life Metrics | ||||||
|---|---|---|---|---|---|---|
| Very Dissatisfied, n (%) | Dissatisfied, n (%) | Neither Dissatisfied nor Satisfied, n (%) | Satisfied, n (%) | Very Satisfied, n (%) | ||
| Health satisfaction | Pre-study | 6 (6.5) | 41 (44.6) | 19 (20.7) | 23 (25.0) | 3 (3.3) |
| Post-study | 7 (7.6) | 21 (22.8) | 25 (27.2) | 38 (41.3) | 1 (1.1) | |
| Sleep satisfaction | Pre-study | 9 (9.8) | 17 (18.5) | 12 (13.0) | 45 (48.9) | 9 (9.8) |
| Post-study | 5 (5.4) | 19 (20.7) | 12 (13.0) | 45 (48.9) | 11 (12.0) | |
| Support satisfaction | Pre-study | 1 (1.1) | 6 (6.6) | 7 (7.7) | 45 (49.5) | 32 (35.2) |
| Post-study | 2 (2.2) | 2 (2.2) | 14 (15.4) | 39 (42.9) | 34 (37.4) | |
| Not at All, n (%) | A Little, n (%) | Moderately, n (%) | Mostly, n (%) | Completely, n (%) | ||
|---|---|---|---|---|---|---|
| Energy | Pre-study | 2 (2.2) | 7 (7.7) | 24 (26.4) | 39 (42.9) | 19 (20.9) |
| Post-study | 4 (4.4) | 6 (6.6) | 19 (20.9) | 41 (45.1) | 21 (23.1) | |
Boldface type indicates statistical significance at threshold of P <0.05.
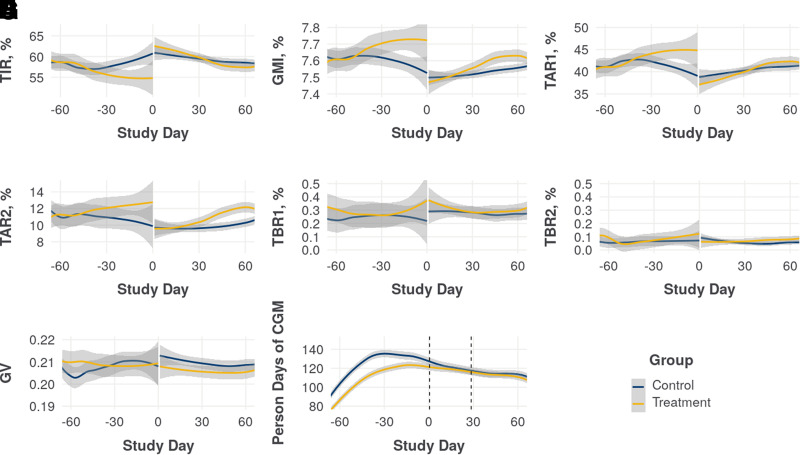
Figure 2 shows a Loess fit to the average daily CGM metrics for both the intervention and control groups. Sharp improvements in average TIR, GMI, and TAR are evident immediately upon initiation of meal delivery in the intervention group. Although there is no such change in the control group, their CGM metrics tended to improve over the course of the baseline period, whereas the intervention group’s CGM metrics did not. Some improvement in this period was expected from regression to the mean after enforcing GMI >7% or TIR <70% in the eligibility period. Additionally, although the intervention group experienced a sharp improvement in average glucose levels at the start of meal delivery, the effect diminished over the course of the 28-day study and continued to diminish after meal delivery stopped, dropping from an average TIR of 62.9% in the intervention period to 58.8% in the 14 days after the intervention period. Participants who also had meals delivered for a household member tended to have higher average TIR in the baseline period than those who did not (58.3 vs. 52.1%), but both groups saw similar increases in average TIR during the intervention period (6.4 vs. 7.6%).
Figure 2.
Loess fits to the daily group average CGM metrics over time, comparing those in the intervention group (N = 110) and the synthetic control group (N = 100) with sufficient CGM data by TIR (A), GMI (B), TAR1 (C), TAR2 (D), TBR1 (E), TBR2 (F), GV (G), and person-days of CGM (H). Plots represent the eligibility/baseline periods (study days −60 to 0), intervention period (study days 0–30), and post-intervention period (study days 30–60).
Table 2 summarizes results from the four quality-of-life questions on the pre- and post-study surveys (24). To allow for a fairer evaluation of the impact of meal delivery, the pre-study survey results are shown only for those who completed a post-study survey. Participants tended to be more satisfied with their health after completing the study, but they reported no detectable change in feelings about their sleep satisfaction, support, and energy.
Discussion
This work adds to a growing body of evidence for meal delivery and other novel nutrition-focused care models as promising strategies for improving outcomes for people with conditions such as type 2 diabetes. Our study observed a similar glycemic effect size to other meal-based intervention research among people with or at risk for type 2 diabetes (11); the effect size also generally aligns with the lower bound of observed A1C reductions for short courses (12 weeks) of low-dose, glucose-lowering pharmacologic agents (25). The study also demonstrates a lack of durable clinical benefit after the end of the meal-delivery period, similar to patterns observed in studies of vegetable prescriptions among people with obesity (26) or those seen in crossover studies that discontinue glucose-lowering drugs absent complementary longitudinal lifestyle changes (27). Notably, the provided meals were similar to American “comfort foods” and were relatively modest in their calorie and carbohydrate reduction. That they were still associated with glycemic improvements suggests an opportunity to achieve a more substantial clinical effect size with alternative meal profiles such as those with fewer grams of carbohydrate per meal. Additionally, this intervention was focused largely on the delivery mechanism of meals shipped directly to homes, and the addition of nutrition education, particularly individualized medical nutrition therapy with a registered dietitian nutritionist, may provide another opportunity to increase or lengthen the duration of the glycemic improvements (28).
This study is unique both in its option for participants to include a household member as a recipient of meals and by its virtual delivery across a decentralized population in the United States, such that participants were able to engage in the study without traveling for laboratory testing or food pick-up. Strengths of this study include the use of real-time CGM throughout the eligibility, baseline, and intervention periods, which provided a granular view of the impact of meal delivery on glycemic outcomes; the a priori enrollment targets to ensure adequate power to detect a clinically relevant five-point TIR change; and the heterogeneous range of type 2 diabetes treatment trajectories, as evidenced by the diverse use of diabetes drug classes among participants.
However, this study should be interpreted in light of several limitations. First, although we built a synthetic control group to validate the single-arm findings, this was not a randomized, controlled trial; thus, our ability to make causal statements about the role of home-delivered meals on outcomes is limited.
Second, the synthetic control group was not matched to the treatment group on clinical or demographic features beyond eligibility-period TIR and therefore was not able to account for the selection bias present in the treatment group. Notably, the control group saw a regression to the mean in TIR, TAR, and GMI in the baseline period that did not occur in the treatment group. One potential explanation for this difference is that opting into the study was a surrogate for additional participant factors contributing to worse baseline CGM metrics (e.g., social determinants of health factors such as food insecurity), although the authors cannot confirm this hypothesis with the available data. Regardless of the cause, the group differences raise further questions about selection bias and the generalizability of the results to a broader population.
Third, this intervention occurred during the COVID-19 pandemic, when many people’s daily routines—including eating and physical activities—were altered (29); the synthetic control group was designed, in part, to account for any population-wide temporal effects caused by the pandemic.
Fourth, this study was limited to households with one or two members, which may not be representative of a broader population of people with type 2 diabetes. Additionally, all study meals, which retail for $7–8 each (30), were provided at no cost to participants or their household member. Similar interventions requiring participant out-of-pocket cost for meals may limit uptake, adherence, or persistence.
Finally, this study was conducted alongside ongoing participation in a digital-first type 2 diabetes care center of excellence and included individuals already engaging in their care by regularly wearing a CGM sensor, which may have biased the results toward an engaged subset of insured adults with type 2 diabetes and may not be generalizable to other groups.
This study presents an opportunity to explore how pairing meal delivery with behavior change interventions could capitalize on the short-term clinical benefits seen in this study and extend those benefits beyond the delivery period by teaching participants sustainable skills. Additional research is also needed to understand the optimal cost-effective “dose” of meal delivery (e.g., number of meals per day or week, continuous vs. intermittent delivery, cost-sharing strategies, and so forth) and the ways in which engaging household members or family units might affect the effect size and post-intervention duration of improved outcomes conferred by meal delivery.
Conclusion
In summary, providing 28 days of home-delivered meals was associated with short-term improvements in TIR, GMI, and TAR among people with type 2 diabetes enrolled in a digital-first type 2 diabetes care center of excellence. These positive effects emerged quickly upon initiation of meals but diminished over time and upon completion of the study, suggesting that participants were not able to maintain their improved glycemic control without the meals and that the meals alone were not sufficient to alter participants’ longer-term eating behaviors. While this research demonstrates the utility of meal delivery interventions for short-term improvements in glycemic control, more research is needed to understand how to pair meal delivery to the right behavioral support such that the benefits can persist beyond the duration of the deliveries.
Article Information
Acknowledgments
The authors thank the participants in this study, without whom this work would not have been possible. They also thank Adam Seivert, a former OptumLabs employee, and Dan Levinson, a current OptumLabs employee, for their valuable support in the initiation of this work and the teams at OptumLabs and Level2 who supported it.
Funding
This research was funded by Optum Labs.
Duality of Interest
C.N.C., B.B.H., C.K.M., and J.M.D. are full-time employees, and G.B.W. is a former full-time employee of UnitedHealth Group, and all own stock in the company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
C.N.C., B.B.H., and G.B.W. designed the study. B.B.H. and C.K.M. performed the data analysis. J.M.D. oversaw study launch, recruitment, and execution. All authors interpreted the results of the analysis. C.N.C. and B.B.H. drafted the manuscript, and all authors reviewed and revised the manuscript and agreed to its publication. C.N.C. and B.B.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Parts of this study were presented at the American Diabetes Association’s virtual 81st Scientific Sessions, 25–29 June 2021.
Footnotes
C.N.C. and B.B.H. contributed equally as co-first authors.
References
- 1. American Diabetes Association . 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43 (Suppl. 1):S48–S65 [DOI] [PubMed] [Google Scholar]
- 2. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol 2018;33:157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–355 [DOI] [PubMed] [Google Scholar]
- 5. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol (Lausanne) 2019;10:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159–1171 [DOI] [PubMed] [Google Scholar]
- 7. Marcus JB. Global and US aging. In Aging, Nutrition and Taste. Marcus JB, Ed. London, U.K., Academic Press, 2019, p. 1–24 [Google Scholar]
- 8. UnitedHealthcare . 2019 Getting started guide: welcome to your Medicare Advantage plan. Available from https://trs.ky.gov/wp-content/uploads/2018/09/TRS_Getting_Started_Guide.pdf. Accessed 18 May 2021
- 9. Humana . Humana Well-Dine. Available from https://www.humana.com/provider/medical-resources/clinical/health-programs/well-dine. Accessed 18 May 2021
- 10. Hess A, Passaretti M, Coolbaugh S. Fresh Food Farmacy. Am J Health Promot 2019;33:830–832 [DOI] [PubMed] [Google Scholar]
- 11. Kerr D, Barua S, Glantz N, et al. Farming for life: impact of medical prescriptions for fresh vegetables on cardiometabolic health for adults with or at risk of type 2 diabetes in a predominantly Mexican-American population. BMJ Nutr Prev Health 2020;3:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Leary MF, Barreto M, Bowtell JL. Evaluating the effect of a home-delivered meals service on the physical and psychological wellbeing of a UK population of older adults: a pilot and feasibility study. J Nutr Gerontol Geriatr 2020;39:1–15 [DOI] [PubMed] [Google Scholar]
- 13. Denissen KF, Janssen LM, Eussen SJ, et al. Delivery of nutritious meals to elderly receiving home care: feasibility and effectiveness. J Nutr Health Aging 2017;21:370–380 [DOI] [PubMed] [Google Scholar]
- 14. Rabaut LJ. Medically tailored meals as a prescription for treatment of food-insecure type 2 diabetics. J Patient Cent Res Rev 2019;6:179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hummel SL, Karmally W, Gillespie BW, et al. Home-delivered meals postdischarge from heart failure hospitalization. Circ Heart Fail 2018;11:e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. IJmker-Hemink VE, Wanten GJA, de Nes LCF, van den Berg MGA. Effect of a preoperative home-delivered, protein-rich meal service to improve protein intake in surgical patients: a randomized controlled trial. JPEN J Parenter Enteral Nutr 2021;45:479–489 [DOI] [PubMed] [Google Scholar]
- 17. Berkowitz SA, Terranova J, Randall L, Cranston K, Waters DB, Hsu J. Association between receipt of a medically tailored meal program and health care use. JAMA Intern Med 2019;179:786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gurvey J, Rand K, Daugherty S, Dinger C, Schmeling J, Laverty N. Examining health care costs among MANNA clients and a comparison group. J Prim Care Community Health 2013;4:311–317 [DOI] [PubMed] [Google Scholar]
- 19. Level2 . Level2 homepage. Available from https://mylevel2.com. Accessed 14 February 2022
- 20. Mom’s Meals . Menu: diabetes-friendly. Available from https://www.momsmeals.com/webres/File/WaiverMenus/7802%20Diabetes%20Friendly%20Menu.pdf. Accessed 18 May 2021
- 21. Vigersky RA, McMahon C. The relationship of hemoglobin A1c to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21:81–85 [DOI] [PubMed] [Google Scholar]
- 22. Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonomi AE, Patrick DL, Bushnell DM, Martin M. Validation of the United States’ version of the World Health Organization Quality of Life (WHOQOL) instrument. J Clin Epidemiol 2000;53:1–12 [DOI] [PubMed] [Google Scholar]
- 25. Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care 2010;33:1859–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Casperson SL, Jahns L, Temple JL, Appleton KM, Duke SE, Roemmich JN. Consumption of a variety of vegetables to meet Dietary Guidelines for Americans’ recommendations does not induce sensitization of vegetable reinforcement among adults with overweight and obesity: a randomized controlled trial. J Nutr 2021;151:1665–1672 [DOI] [PubMed] [Google Scholar]
- 27. von Scholten BJ, Lajer M, Goetze JP, Persson F, Rossing P. Time course and mechanisms of the anti-hypertensive and renal effects of liraglutide treatment. Diabet Med 2015;32:343–352 [DOI] [PubMed] [Google Scholar]
- 28. Franz MJ, MacLeod J, Evert A, et al. Academy of Nutrition and Dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: systematic review of evidence for medical nutrition therapy effectiveness and recommendations for integration into the nutrition care process. J Acad Nutr Diet 2017;117:1659–1679 [DOI] [PubMed] [Google Scholar]
- 29. Ruiz-Roso MB, Knott-Torcal C, Matilla-Escalante DC, et al. COVID-19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients 2020;12:2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mom’s Meals . Self pay. Available from https://www.momsmeals.com/our-programs/self-pay/. Accessed 7 December 2021