ABSTRACT
Preclinical data demonstrate that the gut microbiota can promote pancreatic ductal adenocarcinoma (PDAC), but mechanisms remain unclear. We hypothesized that intestinal microbiota alters anti-tumor innate immunity response to facilitate PDAC progression. Human PDAC L3.6pl cells were heterotopically implanted into Rag1−/− mice after microbiota depletion with antibiotics, while syngeneic murine PDAC Pan02 cells were implanted intrapancreatic into germ-free (GF) C57BL/6 J mice. Natural killer (NK) cells and their IFNγ expression were quantitated by flow cytometry. NK cells were depleted in vivo using anti-Asialo GM1 antibody to confirm the role of NK cells. Bacteria-free supernatant from SPF and GF mice feces was used to test its effect on NK-92MI cell anti-tumor response in vitro. SPF and ex-GF mice (reconstituted with SPF microbiota) developed larger PDAC tumors with decreased NK cell tumor infiltration and IFNγ expression versus GF-Rag1−/−. Microbiota-induced PDAC tumorigenesis was attenuated by antibiotic exposure, a process reversed following NK cell depletion in both Rag1−/− and C57BL/6 J mice. Compared to GF, SPF-Rag1−/− abiotic stool culture supernatant inhibited NK-92MI cytotoxicity, migration, and anti-cancer related gene expression. Gut microbiota promotes PDAC tumor progression through modulation of the intratumoral infiltration and activity of NK cells.
KEYWORDS: Pancreatic cancer, pancreatic ductal adenocarcinoma, microbiome, immune response, natural killer cells
Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for more than 85% of pancreatic cancer cases, is the third leading cause of cancer-related death in the United States, and has a dismal 5-year survival rate of <10% for all stages combined.1,2 Treatment difficulties likely arise from the highly immunosuppressive tumor microenvironment but the underlying mechanisms responsible for this phenomenon are unclear.3 The intestinal microbiota, composed of trillions of microorganisms, has both beneficial and deleterious roles in various human diseases, including pancreatic cancer.4,5 A recent study by Kartal et al. reported a fecal microbiota signature that can discriminate between healthy individuals and patients with PDAC, prior to their diagnosis. How this signature corresponds with the process of pancreatic carcinogenesis is an area of active research.6,7 Additionally, studies have reported that the gut microbiota can modulate the immunosuppressive intratumoral environment in PDAC through an adaptive immune response.8,9 These studies evaluated the adaptive immune system in PDAC with limited focus on the role of the innate immune system. Previously, we reported that the gut microbiota can enhance PDAC progression independent of adaptive immunity, suggesting that innate cells may also be involved in microbiota-dependent PDAC development.10
Natural killer (NK) cells, an important group of innate cytotoxic lymphocytes, were first identified for their tumor killing ability11,12 and are now known to be essential in host immunity against various malignancies,13 including PDAC.14 They elicit anti-tumor function through various mechanisms including activation receptor-based killing and cytokine production for immune modulation and recruitment.15,16 Once activated, NK cells induce target cell apoptosis through lytic granule-mediated pathways. Moreover, NK cells secrete numerous cytokines and chemokines, such as interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα),16 which trigger the activation and recruitment of other innate and adaptive immune cells. Prior studies have demonstrated that NK cell tumor infiltration and their subsequent activation positively correlate with PDAC patient survival.17,18 In advanced stages of PDAC, however, the anti-tumor effect of NK cells appears to be lost, as evidenced by decreased IFNγ expression, which is critical for NK cell activation and adaptive immune cell recruitment.17,19,20
While it is well documented that the intestinal microbiota interacts with tissue-resident immune cells,21 a microbial role for systemic immune modulation likely relies on signaling independent of a specific organ.22 Such crosstalk may be related to microbial-derived metabolites, cellular byproducts, or small molecules.23 These microbial components have the capacity to enter the host systemic circulation to modulate immune response thereby influencing cancer development.24 However, sparse information about the interplay between intestinal microbiota and NK cells in cancer is available. Herein, we report that tumor-infiltrating NK cells are important for the crosstalk between gut microbiota and PDAC progression. Bacterial-derived culture supernatant can modulate NK cell anti-tumor immunity. These data support a connection between intestinal microbiota and PDAC development through modulation of NK cell anti-tumor activity via microbial-derived components.
Results
Gut microbiota-enhanced pancreatic cancer progression associates with decreased intratumoral NK cell infiltration and activation
We previously reported that the gut microbiota promotes PDAC development independent of host adaptive immunity in a remote (i.e. independent from a tumor or organ-resident microbiome) manner.10 To determine which innate immune population may be involved in the remote gut microbiota-PDAC modulation, we first utilized our previously published RNAseq dataset.10 Utilizing the TIMER2.0 platform-based25–27 immune estimation through CIBERSORT algorithm, there was predicted decreased intratumoral NK cells in PDAC xenografts in microbiota-intact mice compared to mice with antibiotic-mediated depletion (Abx) of the gut microbiota (0 and 7.9% for control and Abx-treated NOD-SCID mice, respectively; Fig. S1A). In addition, a query of The Cancer Genome Atlas (TCGA), demonstrates longer overall survival in patients with high intratumoral NK cell infiltration compared to low (Fig. S1B, p = .02). Given emerging evidence of NK cells in cancer treatment28,29 and this unexplored area in the PDAC-microbiome literature, we pursued this line of investigation for our work. We therefore hypothesized that the gut microbiota inhibits tumor infiltrating NK cells and therefore promotes PDAC progression.
To test this hypothesis, we first utilized germ-free or Abx-exposed Rag1−/− mice, and heterotopically established PDAC xenografts in the flank of mice using human L3.6pl cells. 16S PCR of DNA isolated from stool just prior to the heterotopic establishment of PDAC xenografts confirmed microbiota depletion in representative examples of Abx-exposed mice (Fig. S2A) or absence in germ-free (GF) mice (Fig. S2B). At study endpoint of 28 days, tumors were harvested, weighed and measured, dissociated into single-cell suspensions, and flow cytometry performed to quantitate NK cell infiltration. Compared to Abx-Rag1−/− mice, SPF-Rag1−/− with an intact microbiota had a 57.4% reduction in intratumoral NK cell infiltration (p = .01; Figure 1a). To control for any potential confounding effects of Abx, L3.6pl xenografts were similarly established in GF, Ex-GF (GF mice gavaged with stool from SPF mice; Fig. S2B), and SPF-Rag1−/−. The presence of a microbiota in either SPF-Rag1−/− or the Ex-GF mice likewise resulted in a 64.4% (p = .005) and 78% (p = .008) reduced intratumoral NK cells infiltration compared to GF mice, respectively (Figure 1b). Finally, in order to account for NK cell interactions with the adaptive immune system,30 we extended our investigation to immunocompetent C57BL/6 J mice orthotopically implanted with the syngeneic PDAC cell line, Pan02. Ablation of the gut microbiota with antibiotics was accomplished as described above. In agreement with the previous models, presence of a microbiota attenuated intratumoral NK cell infiltration into the PDAC xenografts by 63.7% (p = .02; Figure 1c). In each of these models, PDAC xenograft volume and weight were greater in microbiota-intact mice (SPF-Rag1−/−, Ex-GF-Rag1−/−, SPF-C57BL/6 J; Fig. S3A-C, respectively) compared to microbiota-depleted (Abx-Rag1−/−, Abx-C57BL/6 J) or gnotobiotic (GF-Rag1−/−), confirming the inverse relationship between PDAC xenograft progression and anti-tumor NK cell infiltration. Consistent with Pushalkar et al.,8 gut microbiota also decreased adaptive intratumoral immune response in SPF-C57BL/6 J mice but was not statistically significant (Fig. S4A-B). Flow cytometry gating strategy to quantitate immune cell populations is illustrated in Fig. S5.
Figure 1.
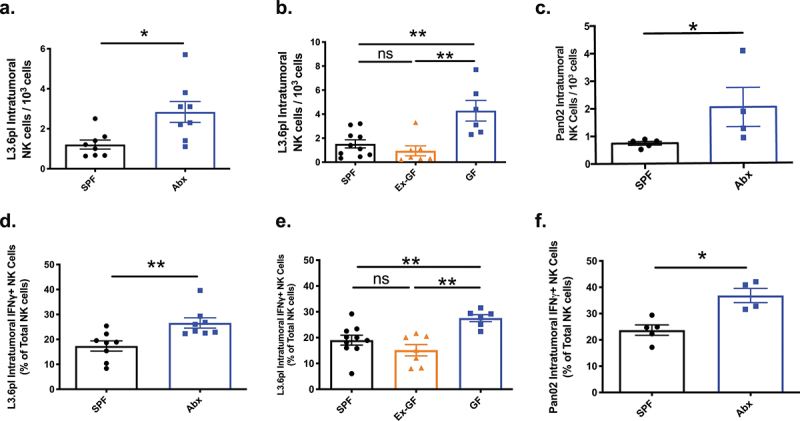
Gut microbiota mediates intratumoral NK cell infiltration and activity in immunocompromised and immunocompetent mice bearing pancreatic cancer xenografts. Heterotopic or orthotopic PDAC xenografts were established as described. At the time of xenograft harvest, tumors were dissociated into single cell suspensions and total NK cell infiltration relative to 104 total tumor cells dissociated were analyzed by flow cytometry. The Rag1−/− mice bearing human L3.6pl PDAC xenografts had decreased NK cell infiltration in specific pathogen-free (SPF) mice compared to those with microbiota depletion with antibiotics (Abx, A). Likewise, SPF-Rag1−/− mice had decreased intratumoral NK cell infiltration compared to germ-free (GF) Rag1−/− mice and the increased intratumoral NK cell infiltration seen in the gnotobiotic mice was reversed with reconstitution of the microbiota of GF-Rag1−/− mice with stool derived from SPF-Rag1−/− mice (“Ex-GF”, B). Finally, the presence of a microbiota in immunocompetent C57BL/6 J mice bearing orthotopic syngeneic Pan02 PDAC xenografts also resulted in decreased intratumoral NK cell infiltration compared to Abx-mediated microbiota depletion in C57BL/6 J mice (c). To determine if differences in NK cell infiltration into PDAC tumors also correlated with differences in activation as measured by interferon gamma (IFNγ) expression, flow cytometry for IFNγ was performed on the intratumoral NK cell population. This demonstrated that the presence of a microbiota also inhibited the activation of intratumoral NK cells in the SPF-Rag1−/− (d, e), Ex-GF-Rag1−/− (e), and SPF-C57BL/6 J models (f) compared to their Abx treated (d, f) or GF (E) cohorts. (*) p < .05, (**) p < .01.
A primary indicator of NK cell activation is IFNγ production.31 To elucidate if reduced intratumoral NK cell infiltration is also associated with decreased NK cell activation, intratumoral NK cell population was isolated and IFNγ expression determined by flow cytometry. In SPF-Rag1−/− mice bearing L3.6pl PDAC xenografts, there was a 34.7% reduction in NK cell IFNγ expression compared to microbiota-depleted Abx-Rag1−/− mice (p = .002; Figure 1d). Intratumoral NK cells of SPF-Rag1−/− and Ex-GF-Rag1−/− mice had 31% (p = .005) and 45% (p = .001) reduced IFNγ-expressing NK cells compared to GF-Rag1−/− mice, confirming the inhibitory capability of intestinal bacteria (Figure 1e). Finally, immunocompetent C57BL/6 J mice likewise demonstrated 35.7% (p = .016) decreased intratumoral NK cell activation by IFNγ(+) expression in the presence of an intestinal microbiota (SPF-C57BL/6 J) compared to microbiota-depleted Abx-C57BL/6 J mice (figure 1f). These findings indicate that gut microbiota-mediated pancreatic cancer progression is associated with decreased intratumoral infiltration and activation of NK cells regardless of the presence of adaptive immunity.
Anti-PDAC effect of gut microbiota depletion is dependent on NK cells
To establish the role of NK cells in microbiota-mediated PDAC progression in both immunodeficient and immunocompetent mouse models, we depleted these cells using an anti-ASGM1 antibody (Figure 2a). Flow cytometry analysis confirmed highly efficient depletion of the NK cell population (CD45+/CD3-/NK1.1+) in the anti-ASGM1 cohort compared to the anti-IgG isotype control cohort in both the spleen (0.97% vs. 35.1%, respectively) and L3.6pl xenograft (0.13% vs. 29.1%, respectively; Fig. S6A-B). Interestingly, the anti-PDAC effect of microbiota depletion was abolished following antibody-mediated NK cell depletion in Rag1−/− mice bearing L3.6pl xenografts (Figure 2b). Finally, to extend our observations to immunocompetent mice, the NK cell population was again depleted utilizing anti-ASGM1 antibody in C57BL/6 J mice bearing orthotopic syngeneic Pan02 tumors. NK cell depletion in this model also eliminated the anti-tumor phenotype of gut microbiota depletion (Figure 2c). These findings suggest that the anti-PDAC effect of gut microbiota depletion is dependent on NK cells in both PDAC models.
Figure 2.
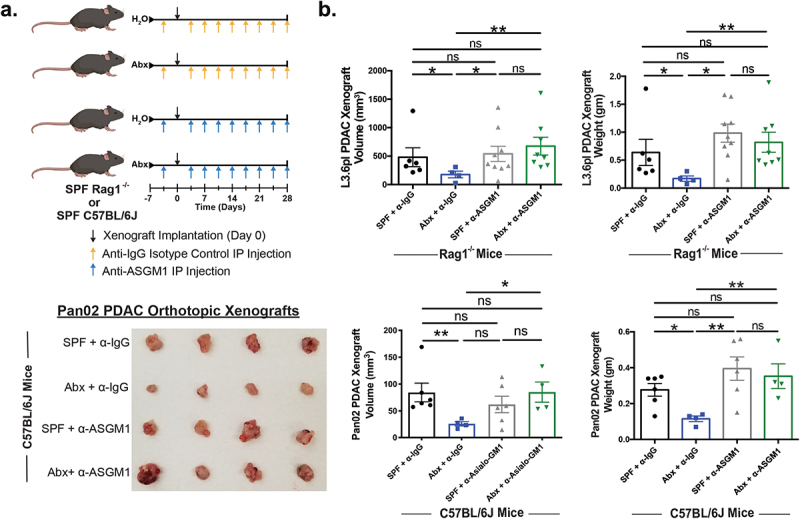
Anti-PDAC effect of gut microbiota depletion is dependent on NK cells. SPF-Rag1−/− (n = 10) or C57BL/6 J (n = 7) mice were given water or antibiotic cocktail (Abx) to deplete their gut microbiota (a). NK cells were depleted with twice weekly intraperitoneal (IP) injection of anti-ASGM1 antibody or anti-IgG isotype control antibody starting three days prior to PDAC xenograft implantation (Rag1−/−: subcutaneous L3.6pl; C57BL/6 J: intrapancreatic Pan02). At endpoint, NK cell depletion abrogated the anti-tumor effect of microbiota depletion in both Rag1−/− (b) and C57BL/6 J mice, representative Pan02 xenografts are shown (c). There was one premature death in the L3.6pl SPF + anti-IgG cohort and differences in cohort size for the remaining cohorts was due to failure of xenograft engraftment with no evaluable tumor to measure and were thus excluded from analysis. (*) p < .05, (**) p < .01.
Microbial-derived components alter NK cell anti-PDAC activity in vitro
While we, and others, have demonstrated intrapancreatic bacteria in human PDAC samples,8,10 the importance of this phenomenon is unknown since PDAC xenografts lacking intratumoral bacteria are still responsive to the state of the intestinal microbiota.10 We further confirmed, as previously reported,10 that xenografts from both heterotopic L3.6pl and orthotopic Pan02 are devoid of detectable bacteria by qPCR analysis (Fig. S7). Therefore, we hypothesized that the gut microbiota modulates NK cell anti-PDAC activity independent of direct microbial interaction within the tumor microenvironment. To test this hypothesis, we prepared abiotic supernatant from anaerobic and aerobic cultured SPF or GF-Rag1−/− mouse stool. Human NK-92MI cells were pre-treated with abiotic culture supernatant to determine cytotoxicity to L3.6pl and BxPC3 human PDAC cells in vitro (Figure 3a). When compared to the abiotic anaerobic culture supernatant from GF-Rag1−/− fecal pellets, the NK-92MI cells treated with abiotic anaerobic culture supernatant derived from SPF-Rag1−/− stool had a 51% reduced cytotoxicity against L3.6pl PDAC cells (p = .087) and 32.8% reduction against BxPC3 cells (p = .002, Figure 3b). There was no statistical difference in cytotoxicity between aerobic culture supernatant and GF-derived stool culture supernatant (data not shown). An essential part for NK cell anti-tumor function is the ability to migrate and infiltrate the tumor microenvironment. Thus, we next tested the effect of abiotic culture supernatant on NK cell migration. NK-92MI cells treated with abiotic culture supernatant from SPF-Rag1−/− stool had 26% decreased migration ability compared to treatment with abiotic supernatant derived from GF-Rag1−/− stool (p = .026, Figure 3c). This reduction in cytotoxicity and migration correlated with decreased NK-92MI cell activation as evidenced by a 33.1% reduction in IFNγ expression upon exposure to abiotic culture supernatant derived from SPF-Rag1−/− stool compared to GF-Rag1−/− stool (Figure 3d). Subsequently, qPCR analysis for NK cell killing pathways was performed and again demonstrated decreased IFNγ expression (p = .048) as well as Perforin (p = .015) in NK-92MI cells exposed to abiotic stool culture supernatant derived from SPF-Rag1−/− compared to GF-Rag1−/− mice. (Figure 3e). Finally, these findings were further confirmed by a qPCR array, which indicated that NK-92MI cells treated with abiotic culture supernatant from SPF-Rag1−/− stool had decreased gene expression of NK cell activation/migration pathways including FASLG, CCL18, IL-13, CXCR2, CCL4, and increased expression associated with inhibition of NK cell activity including CCR1 and IL-6 (figure 3f, Supplemental Table 1). These data suggest a modulatory impact of gut microbiota-derived components on NK cell activation and associated anti-tumor activities.
Figure 3.
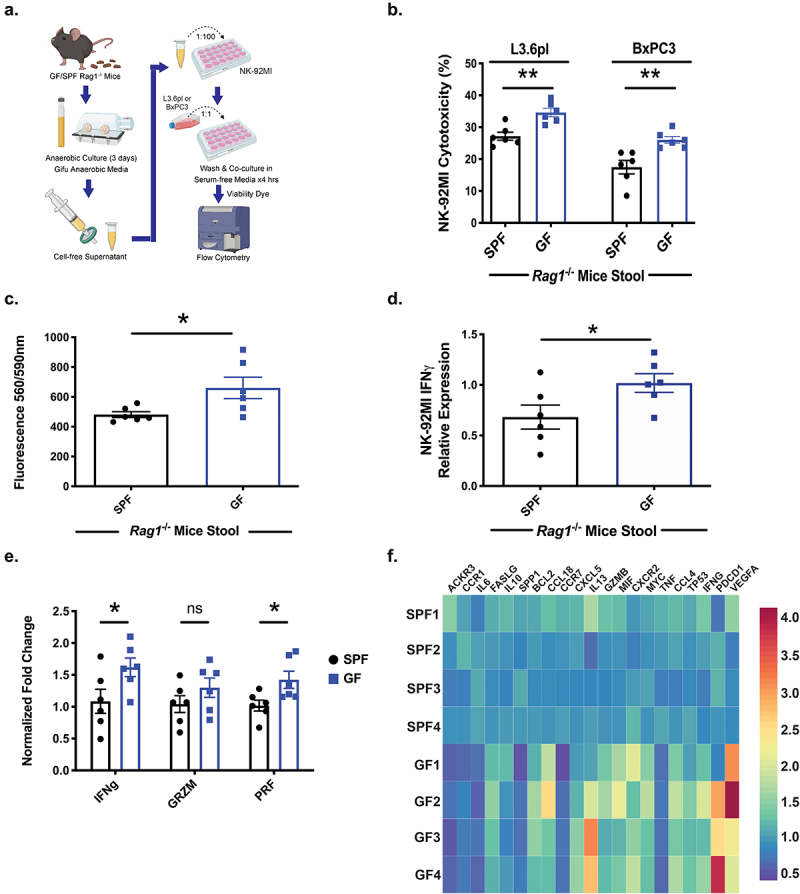
Abiotic bacterial culture supernatant modulates NK cell anti-PDAC activity in vitro. Stool was collected from germ-free (GF) and SPF-Rag1−/− mice and cultured anaerobically for 3 days. Abiotic culture supernatant was prepared after filtration and NK-92MI cells treated with 1% of this supernatant for 3 days. After exposure, NK-92MI cells were harvested and then co-cultured with the human PDAC cell line L3.6pl or BxPC3 for 4 hours and flow cytometry performed to determine cytotoxicity as described (a). NK cells exposed to abiotic culture supernatant from SPF-derived stool had decreased cytotoxicity to L3.6pl and BxPC3 compared to GF-derived abiotic supernatant in vitro (b). Furthermore, NK-92MI cells had a decreased migratory ability in a Boyden chamber assay when pre-treated with abiotic culture supernatant from SPF-Rag1−/− stool (c). These findings of decreased cytotoxicity and decreased migration correlated with decreased activation of NK-92MI cells as measured by flow cytometry for IFNγ(+) NK-92MI cells after exposure to abiotic culture supernatant from SPF-Rag1−/− stool (d). To interrogate pathways responsible for altered cytoxicity, qPCR of treated NK-92MI cells again demonstrated decreased NK cell activation (IFNγ+) but also decreased Perforin expression when exposed to abiotic culture supernatant from SPF stool (e). Furthermore, decreased NK cell cytotoxicity and migration pathways were found via qPCR array as well as increased expression of inhibitory NK cell pathways in NK-92MI cells treated with abiotic culture supernatant from SPF stool (f). (*) p < .05, (**) p < .01.
Discussion
In this study, we demonstrate that the gut microbiota inhibits intratumoral NK cell infiltration and activation with resultant increased PDAC progression. NK cells are important for gut microbiota-mediated PDAC progression in immunodeficient and immunocompetent murine models given that antibody-mediated NK cell depletion in both models resulted in advanced tumors despite the lack of a microbiota in antibiotic-treated mice.
Our findings that innate immunity is important for PDAC control is supported by numerous lines of evidence. In addition to our previous work with Nod-SCID mice,10 herein we utilized a second immunodeficient model, Rag1−/− mice, combined with two different microbiota conditions: GF and antibiotic-mediated microbiota depletion. While data has shown increased intratumoral Th1 cells in antibiotic-treated mice, which could result in increased IFNγ production,8 this potential confounder was addressed both by the use of Rag1−/− mice, which lack Th1 cells, and GF mice for which antibiotic microbiota depletion was not necessary. Both approaches demonstrated that innate immunity is important for microbiota-mediated PDAC development. Thus, in addition to the well-established anti-tumor role of the adaptive immune system in PDAC,32 our study demonstrates that innate immunity, specifically, the NK cell component, is also an important player in bacteria-mediated tumor immune response. Although Rag1−/− mice may raise the potential for altered NK cell response to PDAC tumors, the data generated using immunocompetent C57BL/6 J mice harboring a syngeneic PDAC cell line, in conjunction with depletion of NK cells in both immunocompromised and immunocompetent models, support the premise that bacteria influence PDAC NK cell infiltration and activation, and subsequently pancreatic cancer progression.
Although intratumoral bacteria may impact the tumor immune environment and cancer development,8,33 intratumoral bacteria were not detected in our xenograft models. This finding implies a mechanism connecting intestinal bacteria to distant organs, potentially through microbial-derived components.24,34 These microbial-derived components could have deleterious or beneficial impact on host anti-tumor immune response. For example, gut microbiome-mediated bile acid metabolism has been shown to decrease CXCL16 expression in liver sinusoidal endothelial cells, which inhibited CXCR6+ hepatic natural killer T (NKT) cells recruitment and activation.34 In contrast, microbiome-derived butyrate can increase liver NKT and Th17 cells but decrease Tregs, which subsequently has been shown to improve the anti-cancer immune response to colorectal cancer liver metastasis.35 Our abiotic supernatant assays suggest that microbial-derived component(s) impacts NK cell anti-tumor activities including migration and cytotoxicity, which could explain the pro-tumor effect of the gut microbiota. Such an impact of bacterial soluble components on NK cell anti-tumor function has a prescedent.36 For example, byproducts of microbial metabolism of soy isoflavones have been shown to decrease cytokine-induced NK cell function.37 Additionally, the gut commensals Bifidobacterium and Lactobacillus can synthesize folate, which has also been found to modulate NK cell cytotoxicity.38,39 Further studies would be necessary to identify specific bacteria species and its derived components that could crosstalk with tumor infiltrating NK cells and subsequent PDAC progression.
In summary, our study demonstrates that the gut microbiota mediates PDAC progression through NK cell modulation and that gut microbiota-derived supernatant can modulate anti-tumor NK cell activity. As such, harnessing the gut microbiota to modulate the innate immune system holds promise for the treatment of patients with pancreatic cancer and modulating the microbiome may be one way to facilitate the goal of improving survival in patients with this deadly disease.
Materials and methods
Animal husbandry
The University of Florida (UF) Institutional Animal Care and Use Committee approved all animal experiments and national guidelines were followed (Protocol #202008485). Mixed-gender mice aged 4–6 weeks old were used and housed in a single dedicated specific pathogen-free (SPF) room throughout each experiment unless indicated for gnotobiotic experiments. Rag1−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME) and acclimated in their SPF room for at least 1 week prior to utilization. In-house C57BL/6 J mouse breeding was performed by dedicated UF Animal Care Service personnel. Where indicated, gut microbiota depletion was accomplished by supplementing the animal drinking water with an antibiotic cocktail (Abx) consisting of 0.5 mg/L metronidazole, 1 gm/L neomycin, 0.5 gm/L vancomycin, and 0.125 gm/L ciprofloxacin. Microbiota depletion was confirmed by routine culture of murine stool and/or 16S quantitative PCR (qPCR) of fecal bacterial DNA. For gnotobiotic experiments, germ-free Rag1−/− mice (generous gift from Dr. Jeremiah Faith, Mount Sinai) were bred and maintained in gnotobiotic isolators by UF Animal Care Service personnel until ready for use at which time they were transferred into the Techniplast ISOcage P Bioexclusion system (Techniplast; West Chester, PA) and cages were sterilely changed every two weeks per manufacturer protocol.40,41
Bacterial manipulation
To assess the ability of specific microbes to modulate PDAC progression, bacteria were orally gavaged into GF-Rag1−/− mice at 1*108 colony forming units (CFU) at the time of transfer into the ISOcage system. After two weeks colonization, as confirmed by culture and PCR, 1*106 L3.6pl cells were subcutaneously injected as described. To maintain IsoCage experimental gnotobiotic status, xenografts were only measured at the study endpoint when the xenografts were harvested. The murine bacterial status (GF or microbiota-associated) was confirmed by routine bacterial culture of stool and qPCR of bacterial DNA isolated from stool at the time of each cage change. For fecal transplant experiments, fecal pellets (1 gm) from SPF-Rag1−/− mice were collected and resuspended in 5 mL sterile PBS. Subsequently, 200 μL of this fecal slurry was orally gavaged into GF mice to create the “Ex-GF” cohort. This cohort was then transferred to and remained in SPF housing for the duration of the experiment.
NK cell depletion
NK cell depletion in mice was achieved by intraperitoneal injection of 50ug anti-Asialo GM1 antibody (“anti-ASGM1”; Invitrogen-ThermoFisher Scientific; Waltham, MA) twice weekly starting one week before tumor implantation and continued until the experimental endpoint.42,43 Controls were treated with an equivalent amount of rabbit polyclonal anti-IgG isotype control antibody. NK cell depletion was confirmed by flow cytometry of single-cell isolations from mouse spleen.
Statistical analysis
Statistical comparison between two groups was performed using a two-tailed Mann–Whitney U-test while multi-group comparisons were performed by ANOVA. p ≤ .05 was considered statistically significant. Data were graphed and analyzed using GraphPad Prism 6 (GraphPad Software; San Diego, CA). Graphs depict mean data ±SEM, unless indicated otherwise.
Supplementary Material
Funding Statement
American Cancer Society Norma and Rich DiMarco Mentored Research Scholar Grant [MRSG-17-228-01-TBG] (RMT), UF Health Cancer Center Pilot Project Grant (RMT, RZG), UF Health Cancer Center A1-Accelerator Grant (RMT), NIH grant R01DK073338 (CJ), the University of Florida Health Cancer Center Funds (RZG, CJ), and University of Florida Department of Medicine Gatorade Fund (CJ).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The authors confirm that all data supporting the findings of this study are available within the article and its supplementary materials. Raw data that support the findings of this study are available from the corresponding author [RMT], upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2022.2112881
References
- 1.American Cancer Society . 2021. Cancer Facts & Figures 2021. Atlanta, GA, USA: American Cancer Society. [Google Scholar]
- 2.Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, Ng K, Chong C, Zheng Z-J, Wong MCS, et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology. 2021;160(3):744–10. doi: 10.1053/j.gastro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Zheng L, Xue J, Jaffee EM, Habtezion A.. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144(6):1230–1240. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsilimigras MCB, Fodor A, Jobin C. Carcinogenesis and therapeutics: the microbiota perspective. Nat Microbiol. 2017;2(3):17008. doi: 10.1038/nmicrobiol.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kartal E, Schmidt TSB, Molina-Montes E, Rodríguez-Perales S, Wirbel J, Maistrenko OM, Akanni WA, Alashkar Alhamwe B, Alves RJ, Carrato A, et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut. 2022;71(7):1359–1372. doi: 10.1136/gutjnl-2021-324755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newsome R, Jobin C. Finding clues in unexpected places: detection of pancreatic cancer through the faecal microbiome. Gut. 2022;71(7):1247–1248. doi: 10.1136/gutjnl-2021-326710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 2018;155(1):33–37.e6. doi: 10.1053/j.gastro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas RM, Gharaibeh RZ, Gauthier J, Beveridge M, Pope JL, Guijarro MV, Yu Q, He Z, Ohland C, Newsome R, et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis. 2018;39(8):1068–1078. doi: 10.1093/carcin/bgy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 12.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 13.S-Y W, Fu T, Jiang Y-Z, Shao Z-M. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Audenaerde JRM, Roeyen G, Darcy PK, Kershaw MH, Peeters M, Smits ELJ. Natural killer cells and their therapeutic role in pancreatic cancer: a systematic review. Pharmacol Ther. 2018;189:31–44. doi: 10.1016/j.pharmthera.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Muñoz ME, Valenzuela-Vázquez L, Sánchez-Herrera J, Santa‐Olalla Tapia J. From the “missing self” hypothesis to adaptive NK cells: insights of NK cell-mediated effector functions in immune surveillance. J Leukoc Biol. 2019;105(5):955–971. doi: 10.1002/JLB.MR0618-224RR. [DOI] [PubMed] [Google Scholar]
- 16.Konjević GM, Vuletić AM, Mirjačić Martinović KM, Larsen AK, Jurišić VB. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine. 2019;117:30–40. doi: 10.1016/j.cyto.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Lim SA, Kim J, Jeon S, Shin MH, Kwon J, Kim T-J, Im K, Han Y, Kwon W, Kim S-W, et al. Defective localization with impaired tumor cytotoxicity contributes to the immune escape of NK cells in pancreatic cancer patients. Front Immunol. 2019;10:496. doi: 10.3389/fimmu.2019.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis M, Conlon K, Bohac GC, Barcenas J, Leslie W, Watkins L, Lamzabi I, Deng Y, Li Y, Plate JMD, et al. Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J Immunother. 2012;35(8):629–640. doi: 10.1097/CJI.0b013e31826c8a4f. [DOI] [PubMed] [Google Scholar]
- 19.Jun E, Song AY, Choi J-W, Lee HH, Kim M-Y, Ko D-H, Kang HJ, Kim SW, Bryceson Y, Kim SC, et al. Progressive impairment of NK cell cytotoxic degranulation is associated with TGF-β1 deregulation and disease progression in pancreatic cancer. Front Immunol. 2019;10:1354. doi: 10.3389/fimmu.2019.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcon F, Zuo J, Pearce H, Nicol S, Margielewska-Davies S, Farhat M, Mahon B, Middleton G, Brown R, Roberts KJ, et al. NK cells in pancreatic cancer demonstrate impaired cytotoxicity and a regulatory IL-10 phenotype. Oncoimmunology. 2020;9(1):1845424. doi: 10.1080/2162402X.2020.1845424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomkovich S, Dejea CM, Winglee K, Drewes JL, Chung L, Housseau F, Pope JL, Gauthier J, Sun X, Mühlbauer M, et al. Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J Clin Invest. 2019;129(4):1699–1712. doi: 10.1172/JCI124196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-modulated metabolites at the interface of host immunity. J Immunol. 2017;198(2):572–580. doi: 10.4049/jimmunol.1601247. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Tang H, Chen P, Xie, H, Tao, Y. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther. 2019;4:41. doi: 10.1038/s41392-019-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Severson E, Pignon J-C, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 29.Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15(2):226–233. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 31.Mah AY, Cooper MA. Metabolic regulation of natural killer cell IFN-γ production. Crit Rev Immunol. 2016;36(2):131–147. doi: 10.1615/CritRevImmunol.2016017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang JH, Jiang Y, Pillarisetty VG. Role of immune cells in pancreatic cancer from bench to clinical application: an updated review. Medicine. 2016;95(49):e5541. doi: 10.1097/MD.0000000000005541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360(6391). doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma X, Zhou Z, Zhang X, Fan M, Hong Y, Feng Y, Dong Q, Diao H, Wang G. Sodium butyrate modulates gut microbiota and immune response in colorectal cancer liver metastatic mice. Cell Biol Toxicol. 2020;36(5):509–515. doi: 10.1007/s10565-020-09518-4. [DOI] [PubMed] [Google Scholar]
- 36.Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30(14):1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mace TA, Ware MB, King SA, Loftus S, Farren MR, McMichael E, Scoville S, Geraghty C, Young G, Carson WE, et al. Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function. Sci Rep. 2019;9(1):5068. doi: 10.1038/s41598-019-41687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim Y-I, Hayek M, Mason JB, Meydani SN. Severe folate deficiency impairs natural killer cell-mediated cytotoxicity in rats. J Nutr. 2002;132(6):1361–1367. doi: 10.1093/jn/132.6.1361. [DOI] [PubMed] [Google Scholar]
- 39.Qiu Y, Jiang Z, Hu S, Wang, L, Ma, X, Yang, X . Lactobacillus plantarum enhanced IL-22 production in natural killer (NK) cells that protect the integrity of intestinal epithelial cell barrier damaged by enterotoxigenic Escherichia coli. Int J Mol Sci . 2017;8(11):2409. doi: 10.3390/ijms18112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecht G, Bar-Nathan C, Milite G, Alon I, Moshe Y, Greenfeld L, Dotsenko N, Suez J, Levy M, Thaiss CA, et al. A simple cage-autonomous method for the maintenance of the barrier status of germ-free mice during experimentation. Lab Anim. 2014;48(4):292–297. doi: 10.1177/0023677214544728. [DOI] [PubMed] [Google Scholar]
- 41.Paik J, Pershutkina O, Meeker S, Yi JJ, Dowling S, Hsu C, Hajjar AM, Maggio-Price L, Beck DAC. Potential for using a hermetically-sealed, positive-pressured isocage system for studies involving germ-free mice outside a flexible-film isolator. Gut Microbes. 2015;6(4):255–265. doi: 10.1080/19490976.2015.1064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ludigs K, Jandus C, Utzschneider DT, Staehli F, Bessoles S, Dang AT, Rota G, Castro W, Zehn D, Vivier E, et al. NLRC5 shields T lymphocytes from NK-cell-mediated elimination under inflammatory conditions. Nat Commun. 2016;7(1):10554. doi: 10.1038/ncomms10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshino H, Ueda T, Kawahata M, Kobayashi K, Ebihara Y, Manabe A, Tanaka R, Ito M, Asano S, Nakahata T, et al. Natural killer cell depletion by anti-asialo GM1 antiserum treatment enhances human hematopoietic stem cell engraftment in NOD/Shi-scid mice. Bone Marrow Transplant. 2000;26(11):1211–1216. doi: 10.1038/sj.bmt.1702702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that all data supporting the findings of this study are available within the article and its supplementary materials. Raw data that support the findings of this study are available from the corresponding author [RMT], upon reasonable request.


