Abstract
Background
Telemedicine has been increasingly integrated into chronic disease management through remote patient monitoring and consultation, particularly during the COVID-19 pandemic. We did a systematic review and meta-analysis of studies reporting effectiveness of telemedicine interventions for the management of patients with cardiovascular conditions.
Methods
In this systematic review and meta-analysis, we searched PubMed, Scopus, and Cochrane Library from database inception to Jan 18, 2021. We included randomised controlled trials and observational or cohort studies that evaluated the effects of a telemedicine intervention on cardiovascular outcomes for people either at risk (primary prevention) of cardiovascular disease or with established (secondary prevention) cardiovascular disease, and, for the meta-analysis, we included studies that evaluated the effects of a telemedicine intervention on cardiovascular outcomes and risk factors. We excluded studies if there was no clear telemedicine intervention described or if cardiovascular or risk factor outcomes were not clearly reported in relation to the intervention. Two reviewers independently assessed and extracted data from trials and observational and cohort studies using a standardised template. Our primary outcome was cardiovascular-related mortality. We evaluated study quality using Cochrane risk-of-bias and Newcastle-Ottawa scales. The systematic review and the meta-analysis protocol was registered with PROSPERO (CRD42021221010) and the Malaysian National Medical Research Register (NMRR-20–2471–57236).
Findings
72 studies, including 127 869 participants, met eligibility criteria, with 34 studies included in meta-analysis (n=13 269 with 6620 [50%] receiving telemedicine). Combined remote monitoring and consultation for patients with heart failure was associated with a reduced risk of cardiovascular-related mortality (risk ratio [RR] 0·83 [95% CI 0·70 to 0·99]; p=0·036) and hospitalisation for a cardiovascular cause (0·71 [0·58 to 0·87]; p=0·0002), mostly in studies with short-term follow-up. There was no effect of telemedicine on all-cause hospitalisation (1·02 [0·94 to 1·10]; p=0·71) or mortality (0·90 [0·77 to 1·06]; p=0·23) in these groups, and no benefits were observed with remote consultation in isolation. Small reductions were observed for systolic blood pressure (mean difference –3·59 [95% CI –5·35 to –1·83] mm Hg; p<0·0001) by remote monitoring and consultation in secondary prevention populations. Small reductions were also observed in body-mass index (mean difference –0·38 [–0·66 to –0·11] kg/m2; p=0·0064) by remote consultation in primary prevention settings.
Interpretation
Telemedicine including both remote disease monitoring and consultation might reduce short-term cardiovascular-related hospitalisation and mortality risk among patients with heart failure. Future research should evaluate the sustained effects of telemedicine interventions.
Funding
The British Heart Foundation.
Introduction
Digital health interventions (DHIs) have the potential to transform the diagnosis, monitoring, and management of chronic cardiovascular conditions. However, the DHI term covers a disparate group of technologies, ranging from electronic health record systems to wearable devices and remote consultation software. Many DHIs are widely deployed in health systems across the world, with adoption rapidly increasing in response to the COVID-19 pandemic. This adoption has not always been based on evidence of effectiveness in improving patient outcomes. In 2018, WHO provided a framework to classify DHIs into one of 28 categories to allow more transparent comparisons of technologies aiming to solve similar health-care challenges.1
Telemedicine is a major category of the WHO DHI classification with a direct impact on the delivery of clinical care.1 These technologies provide remote consultations between patients and clinicians, case management, remote monitoring of health data, and transmission of these data to health-care providers. Cardiovascular diseases are a leading cause of morbidity and mortality across the globe, accounting for nearly a third of global deaths in 2016.2 Conditions such as heart failure and management of high blood pressure are well suited to telemedicine approaches through the adaption of existing technologies for use outside of traditional health-care environments (eg, bluetooth and mobile transmission in blood pressure sphygmomanometers).3 The UK National Health Service is estimated to spend more than £9 billion per annum on direct health-care costs associated with cardiovascular disease, and an additional £19 billion might be attributed to lost economic activity from morbidity and premature mortality.2 Telemedicine approaches could improve the efficiency and effectiveness of disease management, particularly during COVID-19 pandemic restrictions. For example, in the early months of the global COVID-19 response, hospital presentations for acute coronary syndrome fell by 40–50% in North America and Europe, representing a sudden shift in the burden of active cardiac disease away from acute hospital facilities.4, 5, 6
Research in context.
Evidence before this study
We searched PubMed, Scopus, and Cochrane Library from inception to Jan 18, 2021, using terms for telemedicine interventions (eg, remote monitoring, wearables, and biosensors) and cardiac disease. We also searched the PROSPERO systematic review protocol database using the same criteria. We identified trials and observational studies in which technologies consistent with the WHO definition of telemedicine were tested in people with or at risk of cardiovascular disease and outcomes were reported. A single relevant meta-analysis reported the effect of a broad group of digital health interventions (not restricted to telemedicine) on a composite outcome of myocardial infarction, stroke, revascularisation, hospitalisations (admissions to hospital), and all-cause mortality. The interventions were associated with a 39% reduction in this outcome across nine randomised trials. However, studies included were published before January, 2014, and technologies with very different purposes were grouped in a single intervention, with notable heterogeneity in findings. This review predated WHO definitions for telemedicine (published in 2018). Therefore, we did a systematic review and meta-analysis to specifically address the effect of telemedicine interventions for cardiovascular populations, to include contemporary trial data, and to report relevant individual cardiovascular outcomes rather than using a composite endpoint.
Added value of this study
Our study used newer WHO classification of digital health interventions to focus on specific telemedicine approaches across 72 studies of patients with cardiovascular disease or risk factors. Meta-analysis was possible for 34 studies across groups of patients with heart failure, other known cardiovascular diseases, and primary prevention populations. In patients with heart failure, meta-analysis of available evidence showed reduced short-term risk of cardiovascular-related hospitalisation and mortality in patients receiving combined remote disease monitoring and consultation. This association was not observed by remote consultation alone. Small reductions in systolic blood pressure and body-mass index were also shown in smaller studies using telemedicine for risk factor modification.
Implications of all the available evidence
Telemedicine, including both remote disease monitoring and consultation, might reduce short-term cardiovascular-related mortality and hospitalisation risk among patients with heart failure. There was limited consistent evidence in other cardiovascular populations. Future research should evaluate the sustained effects of telemedicine interventions and generalisability of these technologies to populations in a non-trial setting.
The role of DHIs in monitoring and managing patients with cardiovascular disease was systematically reviewed for studies reported up to Jan 21, 2014, by Widmer and colleagues.7 The authors identified 40–49% relative risk (RR) reductions in a composite outcome including acute cardiovascular events, hospitalisations, and deaths for patients with existing cardiovascular diseases managed by a DHI. However, the technologies included in the review predated WHO classifications and were analysed as a single DHI entity despite covering vastly different approaches to patient management.1 As well as the newer WHO standardisation, there has been extensive progress in health-care technology during the last 7 years. In this systematic review and meta-analysis, we provide an updated synthesis of evidence on the effectiveness of telemedicine in the management of cardiovascular diseases.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we searched PubMed, Scopus, and Cochrane Library from inception to Jan 18, 2021. The search strategy is outlined in the appendix (pp 3–4), including terms for telemedicine interventions (eg, remote monitoring, wearables, and biosensors) and cardiac disease. We included any study that evaluated the effects of a telemedicine intervention on cardiovascular outcomes for people either at risk (primary prevention) of cardiovascular disease or with established (secondary prevention) cardiovascular disease. The comparator groups included patients who did not receive a telemedicine intervention. We included randomised controlled trials and observational or cohort studies published in English language peer-reviewed journals. Studies applying telemedicine interventions to mixed disease populations were included if patients with cardiovascular disease comprised more than 50% of the total study population. We excluded studies if there was no clear telemedicine intervention described or if cardiovascular or risk factor outcomes were not clearly reported in relation to the intervention. We manually searched the three most frequently identified journals (European Heart Journal, Journal of Telemedicine and Telecare, and Journal of Medical Internet Research) from our initial search and searched Google Scholar to identify additional relevant papers.
PXK, DKFY, and MAAR independently screened the titles and abstracts of retrieved citations to identify relevant studies. All screenings were completed by two researchers independently (PXK, DKFY, or MAAR), with disagreements resolved by consensus. PXK and DKFY reviewed the full-text articles, and any disagreements were resolved by consensus with MAAR serving as arbiter. The strength of agreement between the two initial reviewers was assessed by Cohen's Kappa. We contacted the primary authors for data verification and missing data in publications if the relationship between the telemedicine intervention and outcomes were qualitatively but not quantitatively expressed, with the aim to gain additional primary data for meta-analysis.
Data analysis
Our primary outcome was cardiovascular-related mortality. Our secondary outcomes included hospitalisation (ie, admission to hospital) secondary to cardiovascular causes, all-cause mortality, and all-cause hospitalisation. We also included studies that reported changes in cardiovascular risk factors such as blood pressure, lipid profile, and body-mass index (BMI). We used the duration of patient follow-up to separate studies into short-term (≤12 months) or long-term (>12 months) studies.
Telemedicine interventions were classified into one of four groups, as defined by WHO.1 These groups were remote consultations between patients and health-care providers (remote consultation), remote monitoring of patient health or remote monitoring of diagnostic data (remote monitoring), transmission of medical data to health-care providers (medical data transmission), and consultations for case management between health-care providers (remote case management).
For studies meeting our inclusion criteria, we did a full-text review and data extraction using a standardised template for outcome measures and study population demographics (study design, population size, age and sex distribution, duration of study, specifics of the telemedicine intervention, WHO telemedicine classification, and key findings). We did quantitative analyses using Cochrane systematic review software (ReviewManager version 5.4.1) to determine the effect size of each telemedicine intervention in the meta-analysis. Methods for extraction and analysis followed Cochrane guidance.8 We chose to only calculate meta-estimates in which there was comparable data available for at least three studies per outcome.9 The relative intervention effects were described by RR and standard mean difference along with the 95% CIs.
We generated the meta-analysis estimates for dichotomous outcomes using pooled RRs. For the continuous data, pooling was done by combining the differences in means or standardised differences in means with a random-effect model and displayed with forest plots. All grouped studies were analysed for variability with reported I 2 estimates of heterogeneity.
We used the Cochrane risk-of-bias tool for randomised controlled trials10 and Newcastle-Ottawa Scale quality assessment for studies that were not randomised controlled trials.11 We assessed publication bias was for the primary outcome by visual inspection of a funnel plot and Egger's regression test for asymmetry.12
This systematic review and meta-analysis followed PRISMA reporting guidelines and was prospectively registered with the PROSPERO (CRD42021221010) and the Malaysian National Medical Research Register (NMRR-20–2471–57236).
Role of the funding source
The funder had no role in the study design, data collection, analysis, interpretation of data, writing of the report, or the decision to submit for publication.
Results
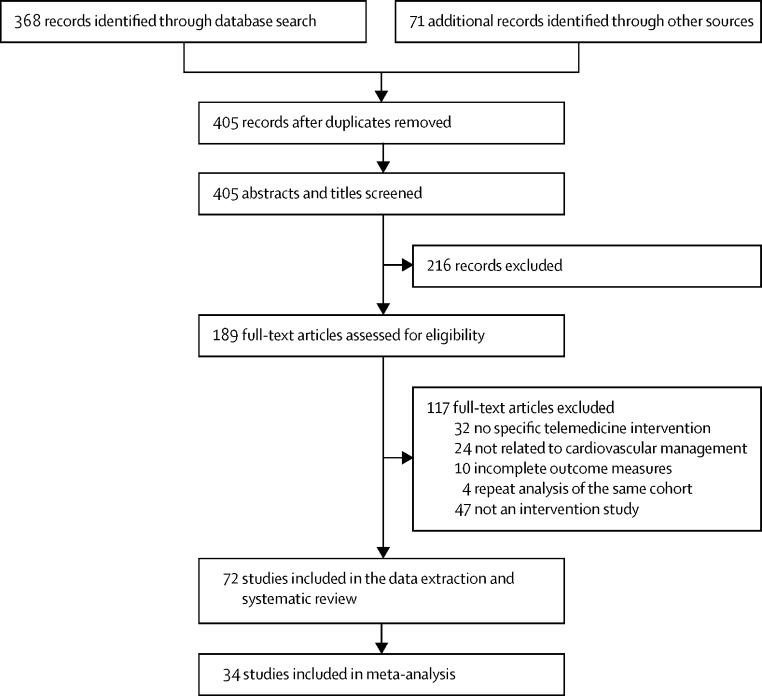
A summary of the screening process and exclusions is provided in a PRISMA flow diagram (figure 1 ). A total of 368 records were identified from the electronic databases with an additional 71 records through manually searching journals and searching Google Scholar. After removing duplicates, 405 records were screened for the titles and abstracts. Of these, we assessed 189 full-text articles for eligibility and included 72 studies in the data extraction and qualitative synthesis. The two independent reviewers had high agreement on study inclusion (κ=0·91 [95% CI 0·85–0·98]; p<0·0001). Five of 12 primary authors who were contacted provided verification and additional data.
Figure 1.
Study selection
The 72 studies included in the systematic review involved 127 869 participants, of whom 82 818 (65%) were male and 45051 (35%) were female (table and appendix pp 5–12). Only one study evaluated solely a female population,53 and the rest included participants of both sexes. All studies focused on adult populations except for one neonatal study.54 39 (54%) studies were done in Europe, 23 (32%) in North America, six (8%) in Asia, two (3%) in South America, one (1%) in Australia, and one (1%) in both Europe and Asia. The age of included participants could be aggregated across 59 studies (n=122 891) at 70 (SD 13) years. Four studies only reported the age ranges of participants between 41·5 and 90·0 years. The duration of the studies varied between 1 and 79 months, with 49 meeting our criteria for short-term follow-up (≤12 months; 68%) and 22 for long-term follow-up (>12 months; 31%). One study did not report follow-up time and could not be included in the metaanalysis. All trials included in the meta-analysis used individual patient randomisation.
Table.
Summary of heart failure studies
| Country | Study design | Total population (N) | Patient characteristics | Duration of follow-up (months) | Telemedicine group (n) | Telemedicine Intervention | WHO classification | Key findings | |
|---|---|---|---|---|---|---|---|---|---|
| Antonicelli et al (2008)13 | Italy | Randomised controlled trial | 57 | Mean age 78·0 (SD 7·1) years; 35 male participants | 12 | 28 | Telephone consultation and data monitoring (weekly electrocardiogram transmission, symptoms, adherence, blood pressure, heart rate, weight, and urine output) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Reduced mortality and hospitalisation rates in intervention group recruited after hospitalisation with heart failure, associated with better compliance with treatment than control (91% vs 43%); secondary reductions in blood pressure and cholesterol level, and better health perception reported than in controls |
| Antonicelli et al (2010)14 | Italy | Randomised controlled trial | 57 | Mean age 78·2 (SD 7·3) years; 33 male participants | 12 | 29 | Telephone consultation and data monitoring (weekly electrocardiogram transmission, symptoms, adherence, blood pressure, heart rate, weight, and urine output) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Home telemonitoring group recruited as outpatients had increased β blocker usage, lower mortality and hospital admissions, and better medication adherence than control group (89·7% vs 35·7%) |
| Böhm et al (2016)15 | Germany | Randomised controlled trial | 1002 | Mean age 66·3 (SD 10·4) years; 799 male participants | 22·9 | 505 | Text message alerts, telephone consultation, and data monitoring (fluid status) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Primary endpoint as death from any cause or first hospitalisation for cardiovascular disease was 45·0% in the intervention group and 48·1% in control group (p=0·13); all-cause death did not differ significantly between groups (p=0·52) |
| Boriani et al (2017)16 | Europe and Israel | Randomised controlled trial | 865 | Mean age 66·0 (SD 10·0) years; 133 male participants | 24 | 437 | Data monitoring (lung fluid accumulation and atrial tachyarrhythmia) | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | No difference in composite and individual endpoints of death and cardiovascular-related and device-related hospitalisation between groups; a significant reduction in a composite endpoint of health-care resource use of 38% in the telemedicine vs control groups (IRR 0·62 [95% CI 0·58–0·66]; p<0·001) |
| Chaudhry et al (2010)17 | USA | Randomised controlled trial | 1653 | Median age 61·0 (IQR 51·0–73·0) years; 959 male participants | 6 | 826 | Telephone-based interactive voice-response system (symptoms and weight monitoring) | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | The telemedicine group and the usual-care group did not differ significantly for all-cause mortality (11·1% in the telemonitoring group and 11·4% in the usual-care group; p=0·88) or hospital readmission (49·3% in the telemonitoring group and 47·4% in the usual-care group; p=0·45) |
| Dendale et al (2012)18 | Belgium | Randomised controlled trial | 160 | Mean age 76·0 (SD 10·0) years; 104 male participants | 6 | 80 | Bluetooth-enabled cell phone for automated data monitoring (blood pressure, weight, and heart rate), web-based, and email | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | The total number of follow-up days lost to hospitalisation, dialysis, or death was significantly lower in telemedicine group as compared to usual care group (13 days vs 30 days; p=0·02) |
| Dunagan et al (2005)19 | USA | Randomised controlled trial | 151 | Mean age 70·0 (SD 13·3) years; 66 male participants | 12 | 76 | Telephone consultation | Consultations between remote patient and health-care provider | Patients assigned to telemedicine had a reduced risk of any hospital attendance (HR 0·67 [95% CI 0·47–0·96]; p=0·029) or hospital readmission (0·67 [0·46–0·99]; p=0·045). There were no significant associations with heart failure-specific readmission, functional status, mortality, or satisfaction with care. |
| Frederix et al (2019)20 | Belgium | Randomised controlled trial | 160 | Mean age 76·0 (SD 10·0) years; 93 male participants | 79 | 80 | Email, telephone consultation, and data monitoring (weight, blood pressure, and heart rate) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Telemedicine was associated with reduced days lost to heart failure readmission compared with usual care (p=0·04), but without effect on all-cause mortality (HR 0·83 [95% CI 0·57–1·20]; p=0·32) |
| Gingele et al (2019)21 | Netherlands | Randomised controlled trial | 382 | Mean age 71·0 (SD 11·0) years; 226 male participants | 26 | 197 | Telephone consultation and electronic device for data monitoring (symptoms, knowledge, and behaviour) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Telemedicine associated with fewer heart failure-related hospitalisations [IRR 0·54 [95% CI 0·31–0·88]), but no difference in time to first heart failure-related hospital admission, all-cause mortality, or days alive and out of hospital |
| Giordano et al (2009)22 | Italy | Randomised controlled trial | 460 | Mean age 57·0 (SD 10·0) years; 391 male participants | 12 | 230 | Telephone consultation and data monitoring (electrocardiogram) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | 1 year home-based telemonitoring programme reduced hospital readmissions and significantly reduced the mean cost of hospital admissions by 35% among patients with chronic heart failure (€843 [SD 1733] in the intervention vs €1298 [2322] in usual care, p<0·01) |
| Guédon-Moreau et al (2013)23 | France | Randomised controlled trial | 433 | Mean age 61·6 (SD 12·5) years; 382 male participants | 24·2 | 221 | Data monitoring by implantable cardioverter-defibrillator holter (abnormal heart rhythm) | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | The telemedicine home monitored group had fewer inappropriate implantable cardiac defibrillator shocks than patients with usual ambulatory monitoring (5% vs 10% with usual care; p<0·05) with non-inferiority for major adverse events |
| Koehler et al (2011)24 | Germany | Randomised controlled trial | 710 | Mean age NA; 577 male participants | 24 | 354 | Telephone consultation, wireless Bluetooth device, personal digital assistant cell phone, and data monitoring (electrocardiogram, blood pressure, and weight) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | No significant effect of remote telemonitoring on all-cause mortality, cardiovascular death, or hospitalisation |
| Koehler et al (2018)25 | Germany | Randomised controlled trial | 1538 | Mean age 70·0 (SD 10·5) years; 1070 male participants | 59 | 765 | Telephone consultation and data monitoring (electrocardiogram, blood pressure, weight, and oxygen saturation) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Reduced proportion of days lost due to unplanned cardiovascular-related hospital admissions and all-cause death in a telemedicine management group compared with usual care (IRR 0·80 [95% CI 0·65–1·00]; p=0·046) |
| Kotooka et al (2018)26 | Japan | Randomised controlled trial | 181 | Mean age 66·2 (SD 14·3) years; 107 male participants | 31 | 90 | Telephone consultation, web-based, and data monitoring (blood pressure, pulse rate, weight, and body composition) | Consultations between remote patient and healthcare provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | There was no difference in the primary composite endpoint of all-cause death or rehospitalisation due to worsening heart failure between telemedicine and usual care groups (HR 0·95 [95% CI 0·55–1·65], p=0·57) |
| Lear et al (2014)27 | Canada | Randomised controlled trial | 78 | Age range 41·5–76·0 years; 66 male participants | 4 | 38 | Web-based cardiac rehabilitation, one-to-one chat consultation, email, and data monitoring (heart rate) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | The telemedicine cardiac rehabilitation programme was associated with no difference in exercise capacity (45·7 [95% CI 1·04–90·48] increase in Bruce protocol time in the intervention group versus baseline, but below the specified clinically relevant threshold of 60 s) |
| López-Liria et al (2019)28 | Spain | Randomised controlled trial | 50 | Mean age 75·0 (SD 12·0) years; 24 male participants | 12 | 25 | Web-based data monitoring from implantable cardiac devices | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | Following permanent pacemaker insertion, no difference observed between remote monitoring and control groups for emergency hospital visits and rehospitalisations (28% vs 32%; p=0·53); both groups showed statistically significant improvements in the baseline parameters based on the Minnesota Living with Heart Failure questionnaire |
| Lundgren et al (2016)29 | Sweden | Randomised controlled trial | 50 | Mean age 62·9 (SD 12·8) years; 29 male participants | 2 | 25 | Web-based and email consultations | Consultations between remote patient and health-care provider | No significant difference in depressive symptoms, cardiac anxiety, and quality of life for patients with heart failure between groups managed remotely using internet-based cognitive behavioural therapy and online moderated discussion forums |
| Lüthje et al (2015)30 | Germany | Randomised controlled trial | 176 | Mean age 65·9 (SD 12·0) years; 136 male participants | 15 | 87 | Data monitoring (fluid overload) | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | Remote monitoring of implantable cardiac devices in patients with heart failure was associated with no difference in heart failure-related hospitalisations (HR 1·23 [95% CI 0·62–2·44]; p=0·55) or all-cause mortality compared with controls (8·6% vs 4·6% usual care at 1 year; p=0·50) |
| Morgan et al (2017)31 | England | Randomised controlled trial | 1650 | Mean age 69·5 (SD 10·2) years; 1415 male participants | 24 | 824 | Telephone consultation and data monitoring from implantable electronic devices | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | No significant differences between remote monitoring group and controls for a primary outcome of all-cause mortality or unplanned cardiovascular hospitalisation (42·4% vs 40·8% in usual care; p=0·87); no differences were observed for secondary outcomes |
| Piette et al (2015)32 | USA | Randomised controlled trial | 372 | Mean age 67·9 (SD 10·2) years; 366 male participants | 12 | 189 | Email and telephone consultations, and data monitoring (systematic monitoring and tailored self-management education via interactive voice response) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Telemedicine intervention using a mobile health application was associated with less caregiving strain, and better engagement of care givers with patients with heart failure than in the control group |
| Piotrowicz et al (2015)33 | Poland | Randomised controlled trial | 107 | Mean age 56·7 (SD 11·9) years; 95 male participants | 2 | 75 | Data monitoring (electrocardiogram, weight, and blood pressure) | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | Significant improvement for peak oxygen uptake in the telemedicine-delivered exercise intervention group; however, there were no observed deaths or hospitalisations in either intervention or control groups |
| Rahimi et al (2020)34 | UK | Randomised controlled trial | 202 | Mean age 71·6 (SD 11·5) years; 145 male participants | 20 | 101 | Telephone consultation, tablet computer-enabled Bluetooth and app, and data monitoring (weight, blood pressure, and heart rate) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Physical wellbeing of participants did not differ significantly between telemedicine home monitoring of patients with heart failure and control groups |
| Riegel et al (2002)35 | USA | Randomised controlled trial | 358 | Mean age 72·0 (SD 12·0) years; 175 male participants | 6 | 130 | Telephone and email consultation | Consultations between remote patient and health-care provider | Significant reduction in the hospitalisation rate for heart failure (0·21 [SD 0·5] vs 0·41 [0·77 admissions per person in usual care, p=0·01), hospital days for heart failure, and multiple readmissions, and better patient satisfaction in the telemedicine intervention group than in the control group; cost savings for inpatient heart failure care were reported after deduction of the intervention costs |
| Rodríguez-Gázquez et al (2012)36 | Colombia | Randomised controlled trial | 63 | Mean age 70·0 (SD 10·5) years; 31 male participants | 9 | 33 | Telenursing sessions | Consultations between remote patient and health-care provider | Improvement in a self-care scale of at least 20% for patients managed by telemedicine compared with controls |
| Scherr et al (2009)37 | Austria | Randomised controlled trial | 120 | Median age 66·0 (IQR 62·0–72·0) years; 85 male participants | 6 | 54 | Telephone consultation, email, web-based, and data monitoring (blood pressure, heart rate, and weight) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Home telemonitoring following an episode of decompensated heart failure was associated with a non-significant trend towards a lower composite outcome of death or hospitalisation compared with controls (50% RR reduction, p=0·06) |
| Tajstra et al 202038 | Poland | Randomised controlled trial | 600 | Mean age 64·0 (SD NA) years; 487 male participants | 12 | 299 | Telephone consultation and data monitoring from remote monitoring devices | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Remote monitoring and guided care of implantable cardiac devices was associated with a reduction in the primary composite outcome of all-cause mortality or cardiovascular death compared with usual care (39·5% vs 48·5% in usual care; p=0·048) |
| Thorup et al (2016)39* | Denmark | Randomised controlled trial | 119 | Mean age 62·8 (SD 11·5) years; 51 male participants | 12 | 64 | Tablet and data monitoring (blood pressure, pulse rate, weight, and daily steps) | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | Increased walking from a mean of 5191 (SD 3198) to 7890 (SD 2629) steps per day among patients for cardiac diseases with remote monitoring; notably more among younger patients with better adherence to the pedometer |
| Weintraub et al (2010)40 | USA | Randomised controlled trial | 188 | Mean age 69·0 (SD 13·5) years; 124 male participants | 3 | 95 | Telephone consultation, data monitoring via automated health monitoring device (weight, blood pressure, and heart rate) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Remote telemedicine monitoring of bodyweight, blood pressure, heart rate, and self-reported health associated with a reduction in rate of heart failure hospitalisation compared with controls (risk rate 0·50 [95% CI 0·25–0·99]; p=0·05) |
| Dadosky et al (2018)41 | USA | Prospective non-randomised trial | 141 | Mean age 79·8 (SD 10·1) years; 105 male participants | 1 | 49 | Interactive tele-management video sessions and data monitoring via remote sensor (heart rate, respiration, body position, electrocardiogram, and weight) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Patients receiving the telemedicine intervention had lower rehospitalisation rates (17% vs 24%) than those receiving usual care, despite higher predicted rehospitalisation risk |
| Quinn (2006)42 | USA | Quasi-experimental study | 22 | Mean age 76·5 (SD NA) years; age range 49·0–90·0 years; 11 male participants | 3 | 22 | Telephone consultation | Consultations between remote patient and health-care provider | The frequency of reported symptoms decreased at the end of the telemedicine intervention; the hospitalisation rate was also lower than in a historical cohort with hospitalisation data available |
| Chen et al (2010)43 | Taiwan | Cohort study | 550 | Mean age 62·8 (SD 15·5) years; 387 male participants | 6 | 275 | Telephone consultation | Consultations between remote patient and health-care provider | A significantly lower all-cause admission rate per person (intervention group had 0·60 [SD 0·77] admissions per person; and usual care group had 0·96 [0·85] admissions per person), shorter length of hospital stay (reduced by 8 days per person), and lower total 6 month medical costs (reduced by US$2682 per patient) in the intervention group compared than in the usual care group. |
| Kurek et al (2017)44 | Germany | Cohort study | 574 | Median age for remote monitoring group 63·0 (IQR 56·0–69·0) years; median age for non-remote monitoring group 62·0 (IQR 53·0–70·0) years; 482 male participants | 36 | 574 | Data monitoring of implantable cardiac devices via remote monitoring online system | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | Significantly lower all-cause mortality in patients under remote monitoring compared with propensity-matched controls up to 3 years of follow-up (4·9% vs 22·3% in controls; p<0·0001) |
| Mittal et al (2016)45 | USA | Cross-sectional | 106 027 | Mean age 71·6 (SD 13·0) years; 68 159 male participants | 30 | 106 027 | Telephone consultation and data monitoring from cardiac implantable electronic device | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Comparisons made between early and later initiation of remote monitoring for implantable cardiac devices; prompt initiation of remote monitoring was associated with increased chance of survival (HR 1·18 [95% CI 1·13–1·22, p<0·001) |
| Martín-Lesende et al (2017)46 | Spain | Cohort study | 42 | Mean age 78·9 (SD 7·5) years; 19 male participants | 12 | 15 | Data monitoring from smartphones to web-platform (aided with alert system) | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | Home-based telemedicine application and alerting system associated with reduced hospitalisation days and emergency department attendances compared with patients who were not randomised from before the intervention (1·1 [SD 1·5] vs 2·6 [1·6] admissions per patient for usual care attendances) |
| Masella et al (2008)47 | Italy | Cohort study | 67 | Mean age 64·0 (SD 9·0) years; 58 male participants | 3 | 67 | Data monitoring from implantable cardioverter defibrillator | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | A remote telemonitoring service for implantable cardiac devices improved efficiency of care; only a small number of clinical events occurred in cohort study |
| Moore (2016)48 | USA | Cohort study | 22 | Median age 78·2 (IQR NA) years; 7 male participants | 4 | 22 | Telephone consultation and data monitoring (blood pressure, oxygen saturation, and weight) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Home-based telemonitoring supported by nurse practitioner reviews was associated with lower short-term admission rates to hospital compared with national average figures, but this was a small cohort |
| Nishii et al (2015)49 | Japan | Cohort study | 195 | Mean age 66·3 (SD 11·3) years; 149 male participants | 24 | 195 | Data monitoring (serum brain natriuretic peptide and fluid status) | Remote monitoring of patient health or remote monitoring of diagnostic data by provider | Device implanted to measure volume status by intrathoracic impedance triggering alerts; B-type natriuretic peptide concentrations and bodyweight were not significantly different from baseline in patients with alerts |
| Odeh et al (2015)50 | UK | Cohort study | 48 | Mean age 71·1 (SD 10·4) years; 19 male participants | 24 | 48 | Telehealth service | Consultations between remote patient and health-care provider | In a mixed observational cohort including patients with heart failure, telemedicine was associated with reduced hospital admissions compared with a pre-telemedicine period |
| Rosen et al (2017)51 | USA | Cohort study | 50 | Mean age 61·0 (SD 12·0) years; 14 male participants | 6 | 50 | Telehealth platform for daily, real-time reporting of health status, and video conferencing | Consultations between remote patient and health-care provider | Patients given telemedicine intervention did not have lower hospital admission rates compared with a previous period in this non-randomised study (37% vs 43%; p=0·32) |
| Scalvini et al (2006)52 | Italy | Cohort study | 438 | Mean age 68·2 (SD 14·8) years; 268 male participants | 12 | 226 | Teleassistance and data monitoring (electrocardiogram) | Consultations between remote patient and health-care provider; remote monitoring of patient health or remote monitoring of diagnostic data by provider | Patients with heart failure supported with a home-based telemonitoring system had more proactive health-care contacts than a comparator group managed by general practitioners; however, the cohorts differed widely in baseline risk |
HR=hazard ratio. IRR=incident rate ratio. NA=not available. OR=odds ratio. RR=risk ratio.
This study also included secondary cardiovascular prevention patients.
The majority of studies included patients with heart failure (n=39; table), and 19 studies addressed secondary prevention populations (appendix pp 5–9). A smaller number of studies included primary prevention strategies (n=12; appendix pp 10–12). An additional two studies included combinations of these groups (table and appendix pp 5–12). There were similar numbers of studies testing remote monitoring (n=19), remote consultation (n=22), and both strategies in combination (n=29). The other categories of telemedicine interventions were less common; only one study combined remote monitoring with remote case management, another combined remote consultation with remote case management, and none included the fourth category, medical data transmission.
A total of 34 studies involving 13 269 patients (8629 [65%] were male participants and 4640 [35%] were female participants) contributed data that were considered appropriate for meta-analysis. Of these participants, 6620 (50%) were assigned to a telemedicine intervention group and 6649 (50%) received usual clinical care. Follow-up ranged 3–79 months, with 21 (62%) studies reporting short-term outcomes within 12 months of telemedicine intervention. 22 (65%) of the 34 studies included patients with heart failure. Similar numbers tested remote consultation alone (12 [35%]) and combined remote monitoring and consultation (16 [47%]), with fewer studies focused on remote monitoring alone (six [18%]).
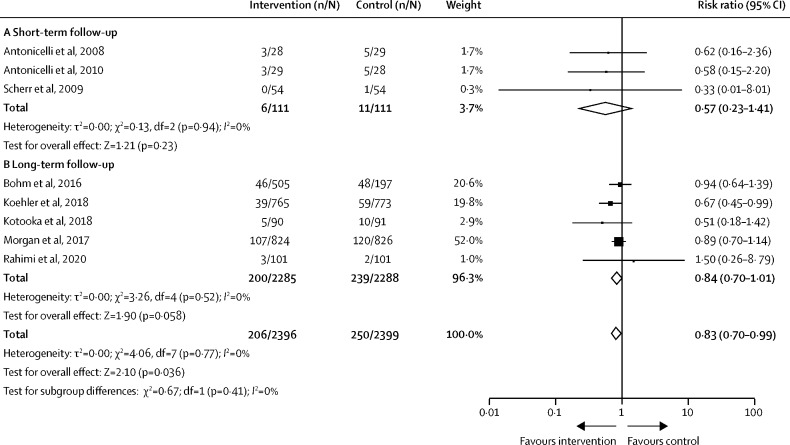
Only studies including patients with heart failure had sufficient homogeneity to be included in the meta-analysis for cardiovascular mortality. Combined remote monitoring and consultation was associated with a 17% reduction in the risk of cardiovascular mortality across eight studies that included 4795 patients with heart failure (RR 0·83 [95% CI 0·70–0·99]; p=0·036; figure 2 ). There was no significant difference in effect size between short-term (n=222; 0·57 [0·23–1·41]; p=0·23) and long-term (n=4573; 0·84 [0·70–1·01]; p=0·058) follow-up studies (p=0·41 for subgroup difference) and heterogeneity remained low (I 2=0%). No overall benefit in cardiovascular mortality risk was observed in three studies using remote consultation techniques alone (n=572; 0·97 [0·63–1·47]; p=0·87; appendix p 13).
Figure 2.
Risk of cardiovascular-related mortality in patients with heart failure studies
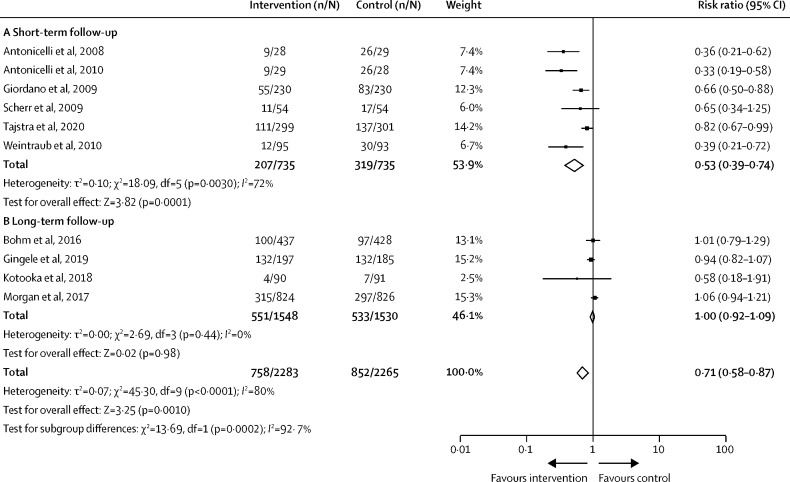
For hospitalisation secondary to cardiovascular disease, only studies focused on patients with heart failure were suitable for pooling within meta-analysis. Telemedicine interventions combining remote monitoring and consultation were associated with a reduction in the risk of cardiovascular hospitalisation across nine studies (n=4548; RR 0·71 [95% CI 0·58–0·87]; p=0·0002; figure 3 ). However, there was high heterogeneity in this result (I 2=80%), caused by a risk reduction in studies of short-term follow-up (n=1470; 0·53 [0·39–0·74]; p=0·0001; figure 3A), in which longer follow-up had no change in the risk of cardiovascular-related hospitalisation (n=3078; 1·00 [0·92–1·09]; p=0·98; figure 3B). Remote consultation alone was not associated with altered risk in three studies with high heterogeneity (n=572; 0·74 [0·37–1·47]; p=0·38; appendix p 14).
Figure 3.
Studies reporting risk of cardiovascular-related hospitalisation in patients with heart failure using combined remote monitoring and consultation
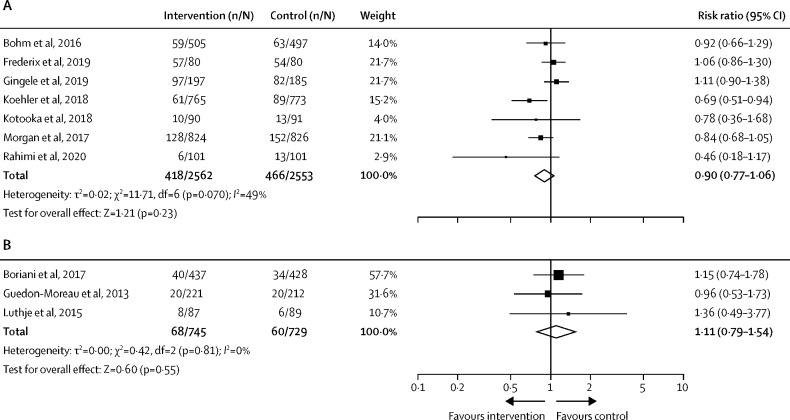
Seven long-term heart failure studies using combined remote monitoring and consultation reported all-cause mortality. A non-significant trend towards lower mortality was observed with the telemedicine intervention groups compared with standard care groups, but with moderate heterogeneity between studies (I 2=49%; n=5115; RR 0·90 [95% CI 0·77–1·06]; p=0·23; figure 4A ). Three long-term heart failure studies using remote monitoring alone were not associated with change in all-cause mortality risk (n=1474; 1·11 [0·79–1·54]; p=0·55; figure 4B).
Figure 4.
Studies reporting risk of all-cause mortality in patients with heart failure during long-term follow-up
(A) Remote monitoring and consultation for heart failure management. (B) Remote monitoring only for heart failure management.
Three studies in patients with heart failure showed no significant change in all-cause hospitalisation risk following a telemedicine remote monitoring intervention (n=1863; RR 1·02 [95% CI 0·94–1·10]; p=0·71; appendix p 15).
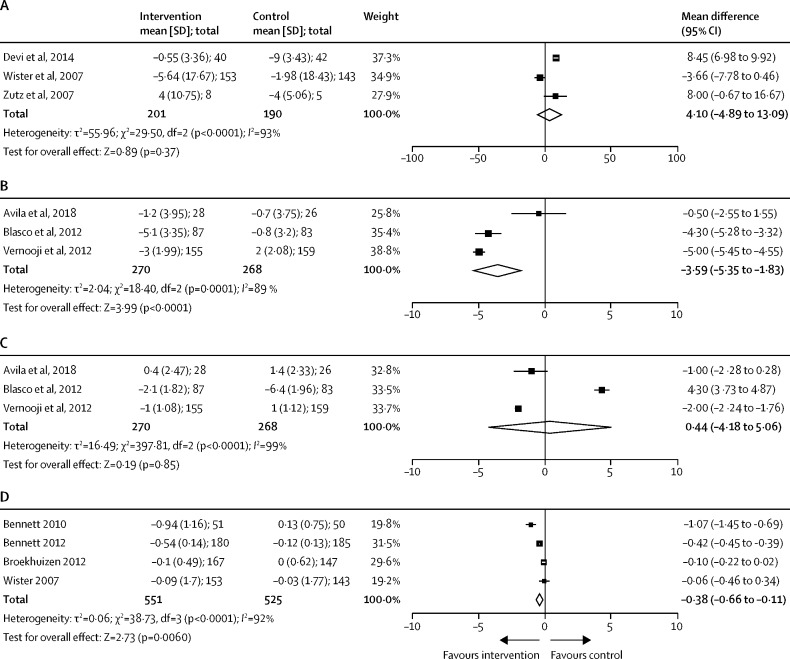
For cardiovascular risk factors, meta-analysis was possible for short-term follow-up studies measuring change in blood pressure and BMI in participants given a telemedicine intervention. In cardiovascular secondary prevention populations, the combination of remote monitoring and consultation was associated with a small but significant reduction in systolic blood pressure across three studies (n=538; mean systolic blood pressure difference –3·59 [95% CI –5·35 to –1·83] mm Hg; p<0·0001; figure 5 ). No effect was noted in diastolic blood pressure, or in studies using remote consultation without monitoring. For studies in primary prevention populations, telemedicine interventions did not have a significant effect on systolic or diastolic blood pressure (appendix p 16).
Figure 5.
Change in blood pressure and body-mass index during short-term follow-up
(A) Remote consultation only for secondary cardiovascular disease prevention (systolic blood pressure). (B) Remote monitoring and consultation for secondary cardiovascular disease prevention (systolic blood pressure). (C) Remote monitoring and consultation for secondary cardiovascular disease prevention (diastolic blood pressure). (D) Remote consultation only for primary cardiovascular disease prevention (body-mass index).
Four short-term follow-up studies used remote consultation targeting bodyweight as a primary prevention strategy for reducing cardiovascular risk, with an overall small effect on BMI (n=1076; mean BMI difference –0·38 [95% CI –0·66 to –0·11] kg/m2; p=0·0064; figure 5). No effect was shown in secondary prevention populations (appendix p 16).
A total of 38 studies in populations of patients with cardiovascular disease did not meet our criteria for quantitative meta-analysis (table and appendix pp 5–12). In populations of patients without heart failure, studies reported various outcome measures that could not be combined across more than two studies, such as physical activity and sedentary time, lipid concentrations, participation in cardiac rehabilitation, and peak oxygen uptake.
The major potential source of bias identified across all intervention trials was the inability to mask participants and practitioners in a telemedicine intervention, with the majority adopting an open-label design (appendix pp 17–18). The Newcastle-Ottawa Scale used for non-randomised studies showed the majority of studies to be of medium or high quality (16 [80%] studies), with four (20%) studies of low quality (appendix p 19). Visual assessment of a funnel plot for studies reporting cardiovascular mortality risk in heart failure did not suggest publication bias (appendix p 20) and this was supported by Egger's regression test for plot asymmetry (p=0·93).
Discussion
In this systematic review and meta-analysis, we found reduced cardiovascular-related mortality and hospitalisation for patients with heart failure who received combined remote telemedicine monitoring and consultation compared with usual care. This effect was not observed by simple remote access to a health-care professional without additional monitoring data. Reductions in cardiovascular-related hospitalisation were only observed in cohorts with heart failure short-term follow-up and were driven by early reductions within 12 months of telemedicine intervention. No telemedicine intervention was shown to alter risk of all-cause hospitalisation or mortality. Small improvements in systolic blood pressure were shown in secondary prevention populations and BMI in primary prevention populations with telemedicine. These findings suggest a definite role for telemedicine in the management of heart failure, particularly in early treatment optimisation, but the value is less clear for long-term management strategy and other cardiovascular diseases.
The average risk reduction of cardiovascular-related hospitalisation (28%) in patients with heart failure had notable heterogeneity, with the greatest reductions within 12 months of intervention but no effectiveness in larger studies with a longer follow-up. These results might suggest early gains from implementing telemedicine interventions in this group, but perhaps less success in patients with progressive or more advanced heart failure. An alternative explanation might be that patients have reduced responsiveness to monitoring prompts once the potential novelty of telemedicine interventions has diminished. This explanation is well recognised in the related area of activity tracking by wearable devices,55 but might be accentuated where active participation is required to measure trends, such as with patients with heart failure recording regular bodyweights for fluid status. Participation in telemedicine programmes is a complex area; a 2018 systematic review of barriers to telemedicine adoption identified 33 separate obstacles, including resistance to change and insufficient technological literacy of patients and health-care staff.56 These effects are even more likely to be observed in the real-world adoption of these tools outside of engaged clinical trial populations.
Although face-to-face clinician contact might be considered crucial for the delivery of traditional health care, our Article suggests isolated remote consultation does not improve hospitalisation or mortality risk in the absence of patient monitoring data. It is unsurprising that effectiveness was noted in cohorts of participants with heart failure, where medical therapy is proven for multiple therapeutic drugs in well conducted randomised trials. It is well recognised from registry data that many patients with heart failure do not receive optimised therapy, partly due to insufficient dose titration following drug initiation.57, 58 It is plausible that the remote monitoring data gave additional value to remote consultations for heart failure by allowing health-care professionals to review and titrate medical treatments with quicker cadence than might be possible using specialist review clinics. The high hospitalisation and mortality risk for patients with heart failure makes these endpoints easier to power for intervention trials. Our Article highlights a scarcity of standardised outcome measures for populations of patients without heart failure, which prevented meta-analyses. Plausible surrogate outcome measures of future risk (eg, physical activity levels) are important, but larger trials using mortality and hospitalisation as endpoints would advance the evidence base for telemedicine outside of heart failure.
In health-care settings where resources are constrained, or where patient access is challenging, equivalence of telemedicine with usual care might represent substantial benefit. However, most studies in this Article did not set out to show non-inferiority. As digital tracking and telemedicine become widespread, marginal, rather than transformative, gains from new technology are probable, akin to the small absolute risk reductions achieved by novel drug therapies.59 Improvements in access or engagement with health care are likely to become even more important outcome measures to demonstrate benefit.
Our results build on evidence from a previous systematic review and meta-analysis by Widmer and colleagues,7 which included studies reporting up to January, 2014. In the 8 years since this publication, we have identified an additional 22 studies to add to meta-analysis. Many recent trials used connected wearable technologies and automated mobile transmission of data such as blood pressure, heart rate, and oxygen saturations. These methods reflect very recent societal and technological advances that have normalised use of cost-effective personal telemedicine devices. The review by Widmer and colleagues7 also considered digital health interventions as a single entity, making the meta-estimate of relative risk less applicable to specific telemedicine methods. We have aligned our systematic review and meta-analysis with the newer WHO classification of DHIs published in 2018, highlighting differences between remote consultation and monitoring. These international classifications should form the basis of consistent comparisons in the future.1 Consistency and specificity is crucial for the synthesis of data in future meta-analyses; a 2021 review using a restrictive search strategy specific to the telemedicine term identified only four of the trials included in our systematic review.60 However, classification approaches are not without limitations in a world of rapidly evolving technologies and personal health-care devices. It is likely that the WHO classification will need regular review to recognise new approaches to telemedicine, such as robotics-assisted health care.61
An additional limitation of the previous systematic review and meta-analysis7 was the use of a composite cardiovascular outcome including myocardial infarction, stroke, revascularisation, hospitalisation, and mortality. The studies published since January, 2014, allow for more precise estimates using specific cardiovascular outcomes, although there is still some heterogeneity between studies. This heterogeneity is inevitable given the differences between telemedicine interventions. For example, two studies by Morgan and colleagues31 and Koehler and colleagues25 appeared to be providing similar remote interventions for patients with heart failure. However, there were marked differences in approach, with Morgan and colleagues31 using weekly data downloads from implantable cardiac devices to guide protocolised patient advice and Koehler and colleagues25 using a daily transmission of multiple measures including bodyweight, blood pressure, oxygen saturations, and self-reported health for immediate daily physician review and therapy modification. Both studies broadly implement a remote monitoring and consultation strategy and are grouped together for the purposes of the WHO classification.
The variation in technologies and evaluation methods highlights the challenge of estimating the broad efficacy of telemedicine as a therapeutic strategy; the variation in technology and implementation can be stark and is likely to increase with the rapid expansion of direct-to-consumer wearable tracking technologies that will form part of many pragmatic telemedicine interventions. These examples also highlight the important differences between efficacy in research settings and scalability to an unselected general population. It is crucial that pragmatic implementation studies that assess the acceptability, feasibility, and fidelity of telemedicine interventions are undertaken in addition to randomised trials of effectiveness.62 One systematic review and meta-analysis has suggested a trend towards improved EQ-5D quality of life scores among telemedicine users compared with usual care, but there are only a few small studies in this area.63 There is broader concern about replacing relationship-based clinical care with telemedicine-guided algorithmic pathways, which might not improve care for some patients. New care pathways must ensure additional support for groups at risk of digital exclusion.64 If done well, there is some evidence that provision of digital applications can be used to drive improvements in health literacy and behaviours in populations hard to reach using traditional medical approaches.65 Understanding the effect of socioeconomic advantage on the success of telemedicine is crucial to the real-world implementation of these systems.
There are some limitations to our approach. We adopted a focused search strategy for telemedicine digital health interventions to align with WHO classifications, which might have excluded some studies where these specific terms were not included. As discussed, the meta-estimates reported need to be interpreted with caution due to the high variation in the specific technologies and protocols implemented as well as differences in comparator groups with usual care. There were few studies reporting comparable data outside of cohorts of patients with heart failure. Although small reductions in bodyweight and systolic blood pressure were shown in primary and secondary cardiovascular prevention cohorts, the clinical impact and sustainability of these small changes were uncertain. The long-term benefits of telemedicine interventions were hard to assess, as many studies were of short duration, and even those with long-term follow-up did not necessarily continue the telemedicine intervention for the full period of reporting.
Our findings suggest a definite role for telemedicine in the management of heart failure, when combining both remote monitoring and patient consultation to optimise treatments and personalised advice for patients. Telemedicine was associated with reduced cardiovascular mortality and hospitalisation. Small gains in the management of cardiovascular risk factors might be achievable with telemedicine interventions, but there is less certainty for effectiveness in populations of patients with cardiovascular disease but without heart failure. Future research needs to address the application of these technologies to unselected populations and longer-term effectiveness.
Data sharing
As a meta-analysis, this study used data already reported in primary literature. Derived data used to calculate meta-estimates are available on request from the corresponding author.
Declaration of interests
NLM has acted as a consultant for Roche Diagnostics and LumiraDx, receiving personal payments; received honoraria and personal payments from Abbott Diagnostics and Siemens Healthineers; and received grants from Siemens Healthineers. AA has acted as a consultant for AbbVie and received personal fees. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the University of Edinburgh Masters in Clinical Trials Programme and the Director General of Health Malaysia for the permissions to publish this Article. NLM is supported by a Chair Award (CH/F/21/90010), a programme grant (RG/20/10/34966), and a Research Excellence Award (RE/18/5/34216) from the British Heart Foundation.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
PXK, WKC, NML, and AA were responsible for the concept and design. PXK, DKFY, and MAAR did the study selection. PXK, WKC, and DKFY did the data extraction, critical appraisal, and coding. PXK and WKC drafted the manuscript. All authors critically revised the manuscript for intellectually important content and had access to the data. PXK, WKC, DKFY, MAAR, and AA verified the underlying data.
Supplementary Material
References
- 1.WHO Classification of digital health intervention v1.0. 2018. https://www.isfteh.org/files/media/WHO-RHR-18.06-eng1.pdf
- 2.British Heart Foundation UK factsheet. January, 2021. https://www.bhf.org.uk/what-we-do/our-research/heart-statistics
- 3.Wongvibulsin S, Martin SS, Steinhubl SR, Muse ED. Connected health technology for cardiovascular disease prevention and management. Curr Treat Options Cardiovasc Med. 2019;21:29. doi: 10.1007/s11936-019-0729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon MD, McNulty EJ, Rana JS, et al. The COVID-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 5.Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Filippo O, D'Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during COVID-19 outbreak in Northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widmer RJ, Collins NM, Collins CS, West CP, Lerman LO, Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc. 2015;90:469–480. doi: 10.1016/j.mayocp.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.3. Cochrane. February, 2022. https://training.cochrane.org/handbook/current
- 9.Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010;35:215–247. [Google Scholar]
- 10.Cochrane Methods Bias RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. 2020. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
- 11.Wells GA, Shea B, O'Connell D, et al. Ottawa Hospital Research Institute; 2021. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14:300–305. doi: 10.1258/jtt.2008.071213. [DOI] [PubMed] [Google Scholar]
- 14.Antonicelli R, Mazzanti I, Abbatecola AM, Parati G. Impact of home patient telemonitoring on use of β-blockers in congestive heart failure. Drugs Aging. 2010;27:801–805. doi: 10.2165/11538210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Böhm M, Drexler H, Oswald H, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J. 2016;37:3154–3163. doi: 10.1093/eurheartj/ehw099. [DOI] [PubMed] [Google Scholar]
- 16.Boriani G, Da Costa A, Quesada A, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail. 2017;19:416–425. doi: 10.1002/ejhf.626. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dendale P, De Keulenaer G, Troisfontaines P, et al. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA-HF 1 (Telemonitoring in the Management of Heart Failure) study. Eur J Heart Fail. 2012;14:333–340. doi: 10.1093/eurjhf/hfr144. [DOI] [PubMed] [Google Scholar]
- 19.Dunagan WC, Littenberg B, Ewald GA, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Card Fail. 2005;11:358–365. doi: 10.1016/j.cardfail.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Frederix I, Vanderlinden L, Verboven AS, et al. Long-term impact of a six-month telemedical care programme on mortality, heart failure readmissions and healthcare costs in patients with chronic heart failure. J Telemed Telecare. 2019;25:286–293. doi: 10.1177/1357633X18774632. [DOI] [PubMed] [Google Scholar]
- 21.Gingele AJ, Brunner-la Rocca H, Ramaekers B, et al. Telemonitoring in patients with heart failure: is there a long-term effect? J Telemed Telecare. 2019;25:158–166. doi: 10.1177/1357633X17747641. [DOI] [PubMed] [Google Scholar]
- 22.Giordano A, Scalvini S, Zanelli E, et al. Multicenter randomised trial on home-based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int J Cardiol. 2009;131:192–199. doi: 10.1016/j.ijcard.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Guédon-Moreau L, Lacroix D, Sadoul N, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. 2013;34:605–614. doi: 10.1093/eurheartj/ehs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 25.Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047–1057. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 26.Kotooka N, Kitakaze M, Nagashima K, et al. The first multicenter, randomized, controlled trial of home telemonitoring for Japanese patients with heart failure: home telemonitoring study for patients with heart failure (HOMES-HF) Heart Vessels. 2018;33:866–876. doi: 10.1007/s00380-018-1133-5. [DOI] [PubMed] [Google Scholar]
- 27.Lear SA, Singer J, Banner-Lukaris D, et al. Randomized trial of a virtual cardiac rehabilitation program delivered at a distance via the internet. Circ Cardiovasc Qual Outcomes. 2014;7:952–959. doi: 10.1161/CIRCOUTCOMES.114.001230. [DOI] [PubMed] [Google Scholar]
- 28.López-Liria R, López-Villegas A, Enebakk T, Thunhaug H, Lappegård KT, Catalán-Matamoros D. Telemonitoring and quality of life in patients after 12 months following a pacemaker implant: the Nordland study, a randomised trial. Int J Environ Res Public Health. 2019;16 doi: 10.3390/ijerph16112001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundgren JG, Dahlström Ö, Andersson G, Jaarsma T, Kärner Köhler A, Johansson P. The effect of guided web-based cognitive behavioral therapy on patients with depressive symptoms and heart failure: a pilot randomized controlled trial. J Med Internet Res. 2016;18:e194. doi: 10.2196/jmir.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lüthje L, Vollmann D, Seegers J, Sohns C, Hasenfuß G, Zabel M. A randomized study of remote monitoring and fluid monitoring for the management of patients with implanted cardiac arrhythmia devices. Europace. 2015;17:1276–1281. doi: 10.1093/europace/euv039. [DOI] [PubMed] [Google Scholar]
- 31.Morgan JM, Kitt S, Gill J, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38:2352–2360. doi: 10.1093/eurheartj/ehx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piette JD, Striplin D, Marinec N, Chen J, Aikens JE. A randomized trial of mobile health support for heart failure patients and their informal caregivers: impacts on caregiver-reported outcomes. Med Care. 2015;53:692–699. doi: 10.1097/MLR.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piotrowicz E, Zieliński T, Bodalski R, et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: a randomised controlled study. Eur J Prev Cardiol. 2015;22:1368–1377. doi: 10.1177/2047487314551537. [DOI] [PubMed] [Google Scholar]
- 34.Rahimi K, Nazarzadeh M, Pinho-Gomes AC, et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. 2020;106:1573–1578. doi: 10.1136/heartjnl-2020-316773. [DOI] [PubMed] [Google Scholar]
- 35.Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162:705–712. doi: 10.1001/archinte.162.6.705. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Gázquez ML, Arredondo-Holguín E, Herrera-Cortés R. Effectiveness of an educational program in nursing in the self-care of patients with heart failure: randomized controlled trial. Rev Lat Am Enfermagem. 2012;20:296–306. doi: 10.1590/s0104-11692012000200012. [DOI] [PubMed] [Google Scholar]
- 37.Scherr D, Kastner P, Kollmann A, et al. Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial. J Med Internet Res. 2009;11:e34. doi: 10.2196/jmir.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tajstra M, Sokal A, Gadula-Gacek E, et al. Remote supervision to decrease hospitalization rate (RESULT) study in patients with implanted cardioverter-defibrillator. Europace. 2020;22:769–776. doi: 10.1093/europace/euaa072. [DOI] [PubMed] [Google Scholar]
- 39.Thorup C, Hansen J, Grønkjær M, et al. Cardiac patients' walking activity determined by a step counter in cardiac telerehabilitation: data from the intervention arm of a randomized controlled trial. J Med Internet Res. 2016;18:e69. doi: 10.2196/jmir.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weintraub A, Gregory D, Patel AR, et al. A multicenter randomized controlled evaluation of automated home monitoring and telephonic disease management in patients recently hospitalized for congestive heart failure: the SPAN-CHF II trial. J Card Fail. 2010;16:285–292. doi: 10.1016/j.cardfail.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Dadosky A, Overbeck H, Barbetta L, et al. Telemanagement of heart failure patients across the post-acute care continuum. Telemed J E Health. 2018;24:360–366. doi: 10.1089/tmj.2017.0058. [DOI] [PubMed] [Google Scholar]
- 42.Quinn C. Low-technology heart failure care in home health: improving patient outcomes. Home Healthc Nurse. 2006;24:533–540. doi: 10.1097/00004045-200609000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Chen YH, Ho YL, Huang HC, et al. Assessment of the clinical outcomes and cost-effectiveness of the management of systolic heart failure in Chinese patients using a home-based intervention. J Int Med Res. 2010;38:242–252. doi: 10.1177/147323001003800129. [DOI] [PubMed] [Google Scholar]
- 44.Kurek A, Tajstra M, Gadula-Gacek E, et al. Impact of remote monitoring on long-term prognosis in heart failure patients in a real-world cohort: results from all-comers COMMIT-HF Trial. J Cardiovasc Electrophysiol. 2017;28:425–431. doi: 10.1111/jce.13174. [DOI] [PubMed] [Google Scholar]
- 45.Mittal S, Piccini JP, Snell J, Prillinger JB, Dalal N, Varma N. Improved survival in patients enrolled promptly into remote monitoring following cardiac implantable electronic device implantation. J Interv Card Electrophysiol. 2016;46:129–136. doi: 10.1007/s10840-016-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martín-Lesende I, Orruño E, Mateos M, et al. Telemonitoring in-home complex chronic patients from primary care in routine clinical practice: impact on healthcare resources use. Eur J Gen Pract. 2017;23:135–142. doi: 10.1080/13814788.2017.1306516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masella C, Zanaboni P, Di Stasi F, Gilardi S, Ponzi P, Valsecchi S. Assessment of a remote monitoring system for implantable cardioverter defibrillators. J Telemed Telecare. 2008;14:290–294. doi: 10.1258/jtt.2008.080202. [DOI] [PubMed] [Google Scholar]
- 48.Moore JA. Evaluation of the efficacy of a nurse practitioner-led home-based congestive heart failure clinical pathway. Home Health Care Serv Q. 2016;35:39–51. doi: 10.1080/01621424.2016.1175992. [DOI] [PubMed] [Google Scholar]
- 49.Nishii N, Kubo M, Okamoto Y, et al. Decreased intrathoracic impedance associated with OptiVol alert can diagnose increased B-type natriuretic peptide—MOMOTARO (monitoring and management of OptiVol alert to reduce heart failure hospitalization) study. Circ J. 2015;79:1315–1322. doi: 10.1253/circj.CJ-15-0076. [DOI] [PubMed] [Google Scholar]
- 50.Odeh B, Kayyali R, Nabhani-Gebara S, Philip N, Robinson P, Wallace CR. Evaluation of a telehealth service for COPD and HF patients: clinical outcome and patients' perceptions. J Telemed Telecare. 2015;21:292–297. doi: 10.1177/1357633X15574807. [DOI] [PubMed] [Google Scholar]
- 51.Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326–1330. doi: 10.1016/j.amjmed.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Scalvini S, Zanelli E, Paletta L, et al. Chronic heart failure home-based management with a telecardiology system: a comparison between patients followed by general practitioners and by a cardiology department. J Telemed Telecare. 2006;12(suppl 1):46–48. doi: 10.1258/135763306777978461. [DOI] [PubMed] [Google Scholar]
- 53.Park MJ, Kim HS. Evaluation of mobile phone and internet intervention on waist circumference and blood pressure in post-menopausal women with abdominal obesity. Int J Med Inform. 2012;81:388–394. doi: 10.1016/j.ijmedinf.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Randolph GR, Hagler DJ, Khandheria BK, et al. Remote telemedical interpretation of neonatal echocardiograms: impact on clinical management in a primary care setting. J Am Coll Cardiol. 1999;34:241–245. doi: 10.1016/s0735-1097(99)00182-5. [DOI] [PubMed] [Google Scholar]
- 55.Shin G, Feng Y, Jarrahi MH, Gafinowitz N. Beyond novelty effect: a mixed-methods exploration into the motivation for long-term activity tracker use. JAMIA Open. 2018;2:62–72. doi: 10.1093/jamiaopen/ooy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018;24:4–12. doi: 10.1177/1357633X16674087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365–2383. doi: 10.1016/j.jacc.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 59.Fordyce CB, Roe MT, Ahmad T, et al. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol. 2015;65:1567–1582. doi: 10.1016/j.jacc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Ma X, Li J, Ren X. The efficacy of telemedical care for heart failure: a meta-analysis of randomized controlled trials. Am J Emerg Med. 2021;47:1–5. doi: 10.1016/j.ajem.2021.01.032. [DOI] [PubMed] [Google Scholar]
- 61.Bhaskar S, Bradley S, Sakhamuri S, et al. Designing futuristic telemedicine using artificial intelligence and robotics in the COVID-19 era. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.556789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subedi N, Rawstorn JC, Gao L, Koorts H, Maddison R. Implementation of telerehabilitation interventions for the self-management of cardiovascular disease: systematic review. JMIR Mhealth Uhealth. 2020;8 doi: 10.2196/17957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han X, Chen W, Gao Z, et al. Effectiveness of telemedicine for cardiovascular disease management: systematic review and meta-analysis. Ann Palliat Med. 2021;10:12831–12844. doi: 10.21037/apm-21-3626. [DOI] [PubMed] [Google Scholar]
- 64.Greenhalgh T, A'Court C, Shaw S. Understanding heart failure; explaining telehealth—a hermeneutic systematic review. BMC Cardiovasc Disord. 2017;17:156. doi: 10.1186/s12872-017-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Redfern J, Coorey G, Mulley J, et al. A digital health intervention for cardiovascular disease management in primary care (CONNECT) randomized controlled trial. NPJ Digit Med. 2020;3:117. doi: 10.1038/s41746-020-00325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As a meta-analysis, this study used data already reported in primary literature. Derived data used to calculate meta-estimates are available on request from the corresponding author.